A Novel Biogenic Silicon-Based Anode Material for Lithium-Ion Batteries: A Review
Abstract
1. Introduction
2. Silicon Anodes
3. Recent Developments in Research on Silicon Anodes
| Electrode | Capacity (mA h/g) | Cycles (mA h/g) | ICE (%) | Ref. |
|---|---|---|---|---|
| Si-bPOD | 1065 | 800 | 99.5 | [46] |
| Si-PAPA | 2312 | 100 | 94 | [47] |
| Si@N-P-LiPN | 2021 | 500 | 93.18 | [48] |
| S5 | 759 | 1300 | 31 | [49] |
| Si-M1 | 2522.6 | 100 | [50] |
4. Strategies to Overcome Pulverisation
5. Future Perspectives
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, W.; Favors, Z.; Ionescu, R.; Ye, R.; Bay, H.H.; Ozkan, M.; Ozkan, C.S. Monodisperse Porous Silicon Spheres as Anode Materials for Lithium Ion Batteries. Sci. Rep. 2015, 5, srep08781. [Google Scholar] [CrossRef]
- Liu, N.; Huo, K.; McDowell, M.T.; Zhao, J.; Cui, Y. Rice husks are used as a sustainable source of nanostructured silicon for high-performance Li-ion battery anodes. Sci. Rep. 2013, 3, 1919. [Google Scholar] [CrossRef]
- Morita, T.; Takami, N. The nano-Si cluster-SiOxC composite material is used as high-capacity anode material for rechargeable lithium batteries. J. Electrochem. Soc. 2006, 153, A425. [Google Scholar] [CrossRef]
- Wang, M.S.; Fan, L.Z. Silicon/carbon nanocomposite pyrolyzed from phenolic resin as anode materials for lithium-ion batteries. J. Power Sources 2013, 244, 570–574. [Google Scholar] [CrossRef]
- Park, C.M.; Choi, W.; Hwa, Y.; Kim, J.H.; Jeong, G.; Sohn, H.J. Characterisations and electrochemical behaviours of disproportionately silica O and its compound for rechargeable Li-ion batteries. J. Mater. Chem. 2010, 20, 4854–4860. [Google Scholar] [CrossRef]
- Khan, M.; Ding, X.; Zhao, H.; Wang, Y.; Zhang, N.; Chen, X.; Xu, J. SiO2-Based Lithium-Ion Battery Anode Materials: A Brief Review. J. Electron. Mater. 2022, 51, 3379–3390. [Google Scholar] [CrossRef]
- Lee, J.; Lee, K.T.; Cho, J.; Kim, J.; Choi, N.; Park, S. Chemical-Assisted Thermal Disproportionation of Porous Silicon Monoxide into Silicon-Based Multicomponent Systems. Angew. Chem. 2012, 124, 2821–2825. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, F.; Li, X.; Luo, W.; Li, L.; Zhang, H.; Wang, L.; Wang, Y.; Jiang, W.; Liu, H.K.; et al. Engineering the Distribution of Carbon in Silicon Oxide Nanospheres at the Atomic Level for Highly Stable Anodes. Angew. Chem. Int. Ed. 2019, 58, 6669–6673. [Google Scholar] [CrossRef]
- Lee, J.I.; Choi, N.S.; Park, S. Highly stable Si-based multicomponent anodes for practical use in lithium-ion batteries. Energy Environ. Sci. 2012, 5, 7878–7882. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, Y.; Wu, C.; Chu, R.; Zhang, J.; Jiang, H.; Zeng, Y.; Zhang, Y.; Guo, H. Double-carbon protected silicon anode for high performance lithium-ion batteries. J. Alloys Compd. 2020, 812, 151848. [Google Scholar] [CrossRef]
- Ryu, J.; Choi, S.; Bok, T.; Park, S. Nanotubular structured Si-based multicomponent anodes for high-performance lithium-ion batteries with controllable pore size via coaxial electro-spinning. Nanoscale 2015, 7, 6126–6135. [Google Scholar] [CrossRef]
- Hamedani, A.A.; Ow-Yang, C.W.; Soytas, S.H. Silicon nanocrystals-embedded carbon nanofibers from hybrid poly-acrylonitrile–TEOS precursor as high-performance lithium-ion battery anodes. J. Alloys Compd. 2022, 909, 164734. [Google Scholar] [CrossRef]
- Lee, J.C.; Ko, Y.; Shin, M.; Song, H.K.; Choi, N.S.; Kim, M.G.M.; Park, S. High-performance silicon-based multicomponent battery anodes are produced via synergistic coupling of multifunctional coating layers. Energy Environ. Sci. 2015, 8, 2075–2084. [Google Scholar] [CrossRef]
- Yan, Z.; Yi, S.; Li, X.; Jiang, J.; Yang, D.; Du, N. A scalable silicon/graphite anode with high silicon content for high-energy lithium-ion batteries. Mater. Today Energy 2023, 31, 101225. [Google Scholar] [CrossRef]
- Du, Z.; Ellis, S.N.; Dunlap, R.A.; Obrovac, M.N. NixSi1-x alloys prepared by mechanical milling as negative electrode materials for lithium-ion batteries. J. Electrochem. Soc. 2015, 163, A13. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, S.; Kalinina, M.; Zheng, L.; Li, L.; Zhu, M.; Obrovac, M.N. Lithium insertion in nanostructured Si1-xTix alloys. J. Electrochem. Soc. 2017, 164, A3006. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, J.; Li, H.; Nuli, Y.; Wang, J. Electrolytes for advanced lithium ion batteries using silicon-based anodes. J. Mater. Chem. A 2019, 7, 9432–9446. [Google Scholar] [CrossRef]
- Gordon, I.J.; Grugeon, S.; Takenouti, H.; Tribollet, B.; Armand, M.; Davoisne, C.; Débart, A.; Laruelle, S. Electrochemical Impedance Spectroscopy response study of a commercial graphite-based negative electrode for Li-ion batteries as function of the cell state of charge and ageing. Electrochim. Acta 2017, 223, 63–73. [Google Scholar] [CrossRef]
- Quach, L.; Adhitama, E.; Göldner, V.; Das, A.; Demelash, F.; Winter, M.; Karst, U.; Placke, T.; Glorius, F. Molecular Design of Film-Forming Additives for Lithium-Ion Batteries: Impact of Molecular Substrate Parameters on Cell Performance. ACS Appl. Energy Mater. 2023, 6, 9837–9850. [Google Scholar] [CrossRef]
- Jin, L.; Shen, C.; Wu, Q.; Shellikeri, A.; Zheng, J.; Zhang, C.; Zheng, J.P. Pre-lithiation strategies for next-generation practical lithium-ion batteries. Adv. Sci. 2021, 8, 2005031. [Google Scholar] [CrossRef]
- Su, K.; Wang, Y.; Yang, B.; Zhang, X.; Wu, W.; Lang, J.; Yan, X. A Review: Pre-lithiation Strategies Based on Cathode Sacrificial Lithium Salts for Lithium-Ion Capacitors. Energy Environ. Mater. 2023, 6, 12506. [Google Scholar] [CrossRef]
- Jin, L.; Shen, C.; Shellikeri, A.; Wu, Q.; Zheng, J.; Andrei, P.; Zhang, J.G.; Zheng, J.P. Progress and perspectives on pre-lithiation technologies for lithium ion capacitors. Energy Environ. Sci. 2020, 13, 2341–2362. [Google Scholar] [CrossRef]
- Qu, H. Developing and Investigating a Fast and Controllable Prelithiation Method for Lithium-Ion Batteries. Ph.D. Thesis, The University of Wisconsin-Milwaukee, Milwaukee, MI, USA, 2022. [Google Scholar]
- Zhan, R.; Wang, X.; Chen, Z.; Seh, Z.W.; Wang, L.; Sun, Y. Promises and Challenges of the Practical Implementation of Prelithiation in Lithium-Ion Batteries. Adv. Energy Mater. 2021, 11, 2101565. [Google Scholar] [CrossRef]
- Ge, M.; Lu, Y.; Ercius, P.; Rong, J.; Fang, X.; Mecklenburg, M.; Zhou, C. Large-scale fabrication, 3D tomography, and lithium-ion battery application of porous silicon. Nano Lett. 2014, 14, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Zhang, J.; Luo, P.; Yang, J.; Feng, X.; Zheng, Y.; Shen, Y.; Li, X.; Yang, Z.; Huang, R. Designing an Al-Rich In Situ Coating for Stabilizing High-Energy-Density Li Metal Battery Electrodes via Electrolyte Modulation. ACS Appl. Energy Mater. 2023, 6, 3452–3459. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, H.; Ji, W.; Zheng, D.; Ding, T.; Qiu, D.; Qu, D. An electrode-level prelithiation of SiO anodes with or-ganolithium compounds for lithium-ion batteries. J. Power Sources 2020, 478, 229067. [Google Scholar] [CrossRef]
- Kuo, C.Y.; Hsu, H.P.; Lan, C.W. Scalable chemical prelithiation of SiO/C anode material for lithium-ion batteries. J. Power Sources 2023, 558, 232599. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, X.; Liu, M.; Ding, T.; Qu, H.; Qiu, D.; Zheng, D.; Qu, D. High-performance all-solid-state Li–S batteries enabled by an all-electrochem-active prelithiated Si anode. Energy Storage Mater. 2022, 53, 613–620. [Google Scholar] [CrossRef]
- Hakari, T.; Hayashi, A.; Tatsumisago, M. Li2S-based solid solutions as positive electrodes with full utilization and superlong cycle life in all-solid-state Li/S batteries. Adv. Sustain. Syst. 2017, 1, 1700017. [Google Scholar] [CrossRef]
- Zhang, X.; Qu, H.; Ji, W.; Zheng, D.; Ding, T.; Abegglen, C.J.; Qiu, D.; Qu, D. Fast and Controllable Prelithiation of Hard Carbon Anodes for Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 11589–11599. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, J.; Yang, H.; Zhong, F.; Ai, X. Chemically prelithiated hard-carbon anode for high power and high capacity Li-ion batteries. Small 2020, 16, 1907602. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Cao, Y.; Wu, W.; Wang, G.; Qu, D. Prelithiation Bridges the Gap for Developing Next-Generation Lithium-Ion Batteries/Capacitors. Small Methods 2022, 6, e2200411. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Yang, M.; Zhou, X.; Zhou, Z. Structural design for anodes of lithium-ion batteries: Emerging horizons from materials to electrodes. Mater. Horiz. 2015, 2, 553–566. [Google Scholar] [CrossRef]
- Yin, S.; Zhao, D.; Ji, Q.; Xia, Y.; Xia, S.; Wang, X.; Wang, M.; Ban, J.; Zhang, Y.; Metwalli, E. Si/Ag/C nanohybrids with in situ incorporation of supersmall silver nanoparticles: Tiny amount, huge impact. ACS Nano 2018, 12, 861–875. [Google Scholar] [CrossRef]
- Fu, L.; Xu, A.; Song, Y.; Ju, J.; Sun, H.; Yan, Y.; Wu, S. Pinecone-like Silicon@Carbon Microspheres Covered by Al2O3 nano-petals for lithium-ion battery anode under high temperature. Electrochim. Acta 2021, 387, 138461. [Google Scholar] [CrossRef]
- Gao, R.; Tang, J.; Yu, X.; Tang, S.; Ozawa, K.; Sasaki, T.; Qin, L.C. In situ synthesis of MOF-derived carbon shells for silicon anode with improved lithium-ion storage. Nano Energy 2020, 70, 104444. [Google Scholar] [CrossRef]
- Li, Y.; Tian, Y.; Duan, J.; Xiao, P.; Zhou, P.; Pang, L.; Li, Y. Multi-functional double carbon shells coated boron-doped porous Si as anode materials for high-performance lithium-ion batteries. Electrochim. Acta 2023, 462, 142712. [Google Scholar] [CrossRef]
- Pinilla, S.; Park, S.-H.; Fontanez, K.; Márquez, F.; Nicolosi, V.; Morant, C. 0D-1D Hybrid Silicon Nanocomposite as Lithium-Ion Batteries Anodes. Nanomaterials 2020, 10, 515. [Google Scholar] [CrossRef]
- Tang, J.; Wu, F.; Dai, X.; Zhou, J.; Pang, H.; Duan, X.; Xiao, B.; Li, D.; Long, J. Robust MXene adding enables the stable interface of silicon anodes for high-performance Li-ion batteries. Chem. Eng. J. 2023, 452, 139139. [Google Scholar] [CrossRef]
- Tang, J.; Zhou, J.; Duan, X.; Yang, Y.; Dai, X.; Wu, F. Constructing the bonding between conductive agents and active materials/binders stabilizes silicon anode in Lithium-ion batteries. J. Energy Chem. 2023, 80, 23–31. [Google Scholar] [CrossRef]
- Bi, X.; Tang, T.; Shi, X.; Ge, X.; Wu, W.; Zhang, Z.; Wang, J. One-step synthesis of multicore-void@shell structured silicon anode for high-performance lithium-ion batteries. Small 2022, 18, 2200796. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, L.; Liu, S.; Chen, Y.; Sun, L.; Liu, C.; Zhu, Y.; Wang, X. A novel design idea of high-stability silicon anodes for lithium-ion batteries: Building in-situ “high-speed channels” while reserving space. Chem. Eng. J. 2023, 472, 144991. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, C.; Jiang, Y.; Zhu, J.; Zhao, Y.; Liang, S.; Wang, K.; Zhou, Y.; Liu, Y.; Zhang, J.; et al. Sponge-Like Porous-Conductive Polymer Coating for Ultrastable Silicon Anodes in Lithium-Ion Batteries. Small 2023, 19, 2303779. [Google Scholar] [CrossRef] [PubMed]
- Malik, Y.T.; Shin, S.Y.; Jang, J.I.; Kim, H.M.; Cho, S.; Do, Y.R.; Jeon, J.W. Self-repairable silicon anodes using a multi-functional binder for high-performance lithium-ion batteries. Small 2023, 19, 2206141. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Liu, T.; Gao, X.; Li, S.; Ling, M.; Liang, C.; Zheng, J.; Lin, Z. Silicon anode with high initial coulombic efficiency by modulated trifunctional binder for high-areal-capacity lithium-ion batteries. Adv. Energy Mater. 2020, 10, 1903110. [Google Scholar] [CrossRef]
- Ng, S.; Wang, J.; Wexler, D.; Konstantinov, K.; Guo, Z.; Liu, H. Highly Reversible Lithium Storage in Spheroidal Carbon-Coated Silicon Nanocomposites as Anodes for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2006, 45, 6896–6899. [Google Scholar] [CrossRef] [PubMed]
- Gattu, B.; Epur, R.; Jampani, P.H.; Kuruba, R.; Datta, M.K.; Kumta, P.N. Hollow nanotubular configuration with silicon-carbon core-shell high-performance lithium-ion anodes. J. Phys. Chem. C 2017, 121, 9662–9671. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, M.; Shi, W.; Liu, X.; Wu, L.; Hu, Z.Y.; Chen, L.; Li, Y.; Su, B.L. Chelation-Assisted formation of carbon nanotubes interconnected Yolk-Shell Silicon/Carbon anodes for High-Performance Lithium-ion batteries. J. Colloid Interface Sci. 2023, 641, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Q.; Chai, J.; Wang, Y.; Du, J.; Chen, Z.; Rui, Y.; Jiang, L.; Tang, B. Si-based Anode Lithium-Ion Batteries: A Comprehensive Review of Recent Progress. ACS Mater. Lett. 2023, 5, 2948–2970. [Google Scholar] [CrossRef]
- Zhou, Y.; Feng, W.; Xu, Y.; Liu, X.; Wang, S.; Lv, Z.; Li, X.; Burcar, E.; Wang, Z.; Yang, Z. Development of Silicon-Based Anode for Lithium-Ion Batteries and Its Application in Solid-State Electrolytes. Eng. Sci. 2023, 28, 1060. [Google Scholar] [CrossRef]
- Chen, X.; Fu, C.; Wang, Y.; Yan, J.; Ma, Y.; Huo, H.; Zuo, P.; Yin, G.; Gao, Y. Recent advances of silicon-based solid-state lithium-ion batteries. eTransportation 2023, 19, 100310. [Google Scholar] [CrossRef]
- Sayed, S.Y.; Kalisvaart, W.P.; Luber, E.J.; Olsen, B.C.; Buriak, J.M. Stabilizing Tin Anodes in Sodium-Ion Batteries by Alloying with Silicon. ACS Appl. Energy Mater. 2020, 3, 9950–9962. [Google Scholar] [CrossRef]
- Sekar, S.; Ahmed, A.T.A.; Kim, D.Y.; Lee, S. One-Pot Synthesized Biomass C-Si Nanocomposites as an Anodic Material for High-Performance Sodium-Ion Battery. Nanomaterials 2020, 10, 1728. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.M.; Tian, Y.R.; Liu, Y.X.; Wang, S.; Hu, C.Q.; Wang, B.; Wang, K.M. Alloy anodes for sodium-ion batteries. Rare Met. 2021, 40, 272–289. [Google Scholar] [CrossRef]
- Kalisvaart, W.P.; Olsen, B.C.; Luber, E.J.; Buriak, J.M. Sb–Si Alloys and Multilayers for Sodium-Ion Battery Anodes. ACS Appl. Energy Mater. 2019, 2, 2205–2213. [Google Scholar] [CrossRef]
- Yang, K.; Rao, L.; Hu, L.; Pan, F.; Yin, Q.; Chen, Y. Flexible, efficient and adaptive modular impact-resistant metamaterials. Int. J. Mech. Sci. 2023, 239, 107893. [Google Scholar] [CrossRef]
- Yang, K.; Qin, Q.; Zhai, Z.; Qiao, C.; Chen, Y.; Yang, J. Dynamic response of self-locked energy absorption system under impact loadings. Int. J. Impact Eng. 2018, 122, 209–227. [Google Scholar] [CrossRef]

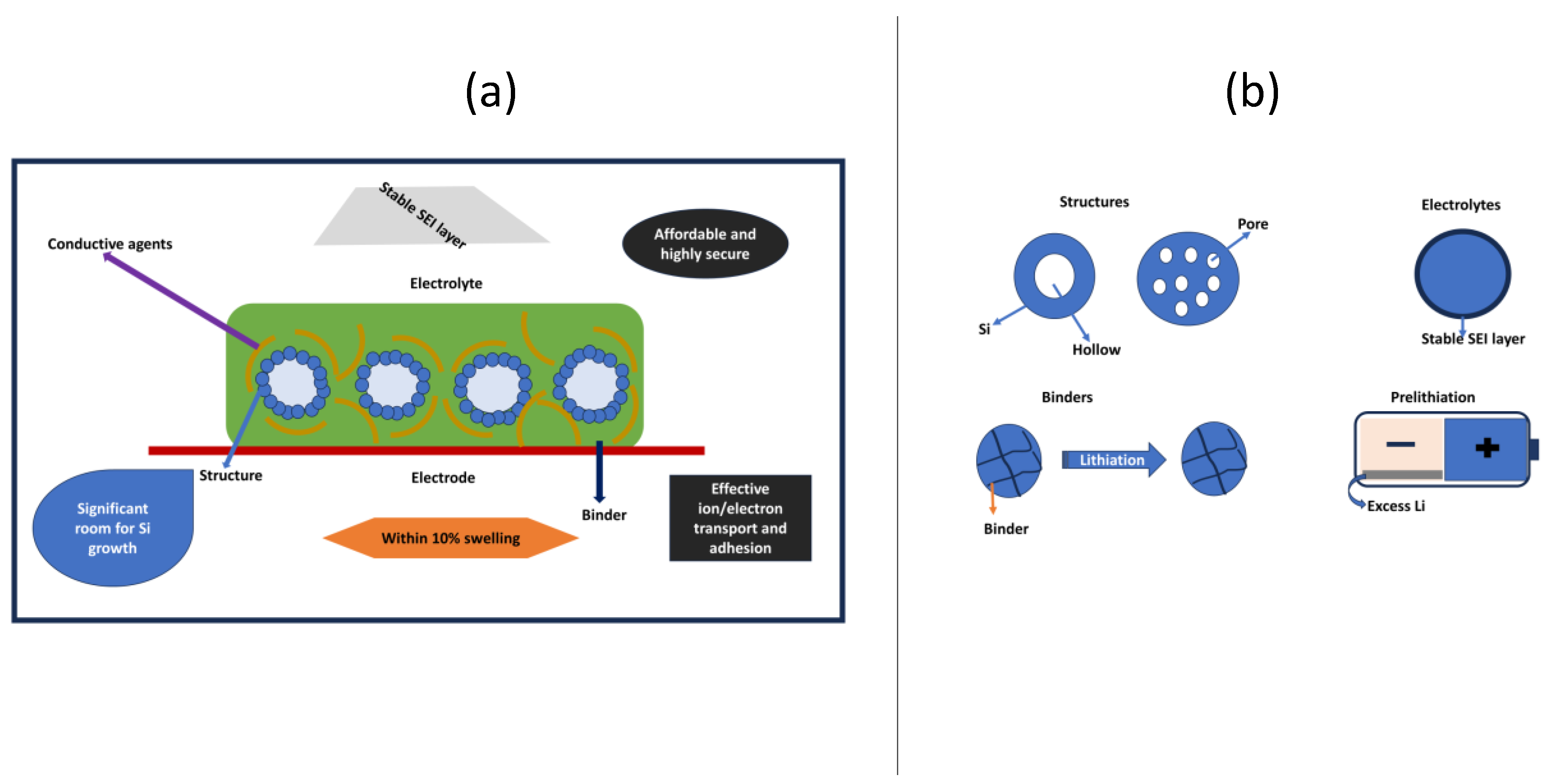

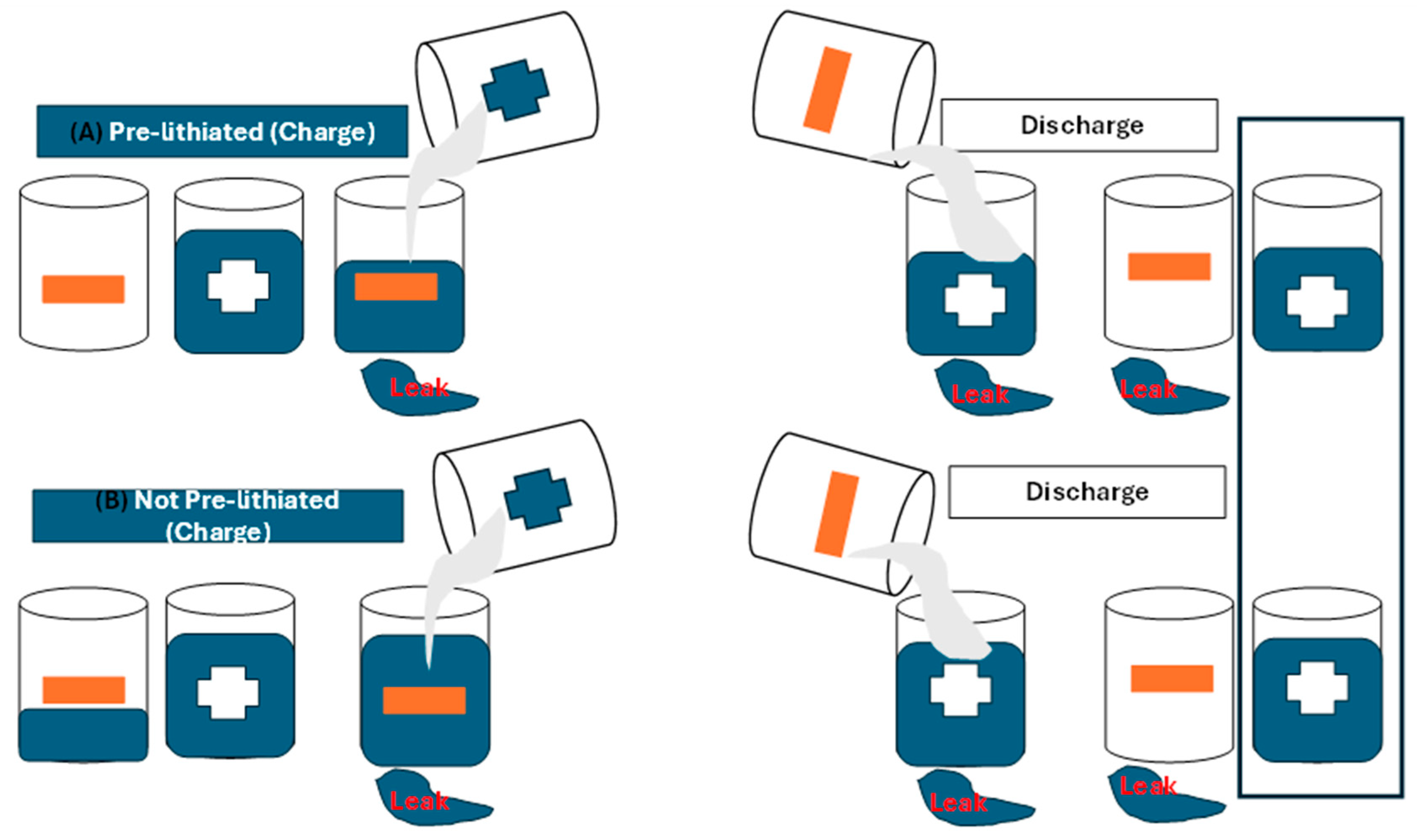
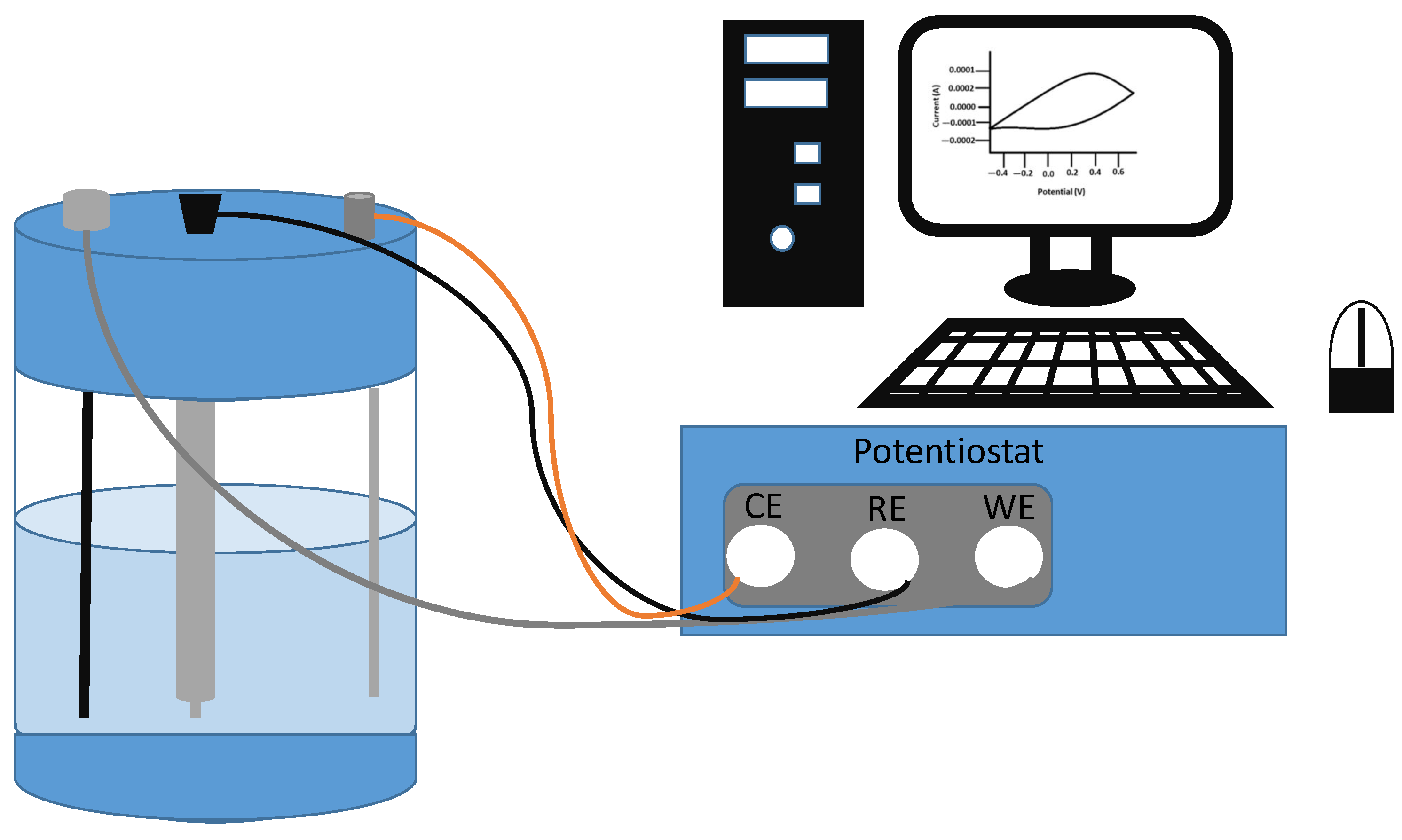
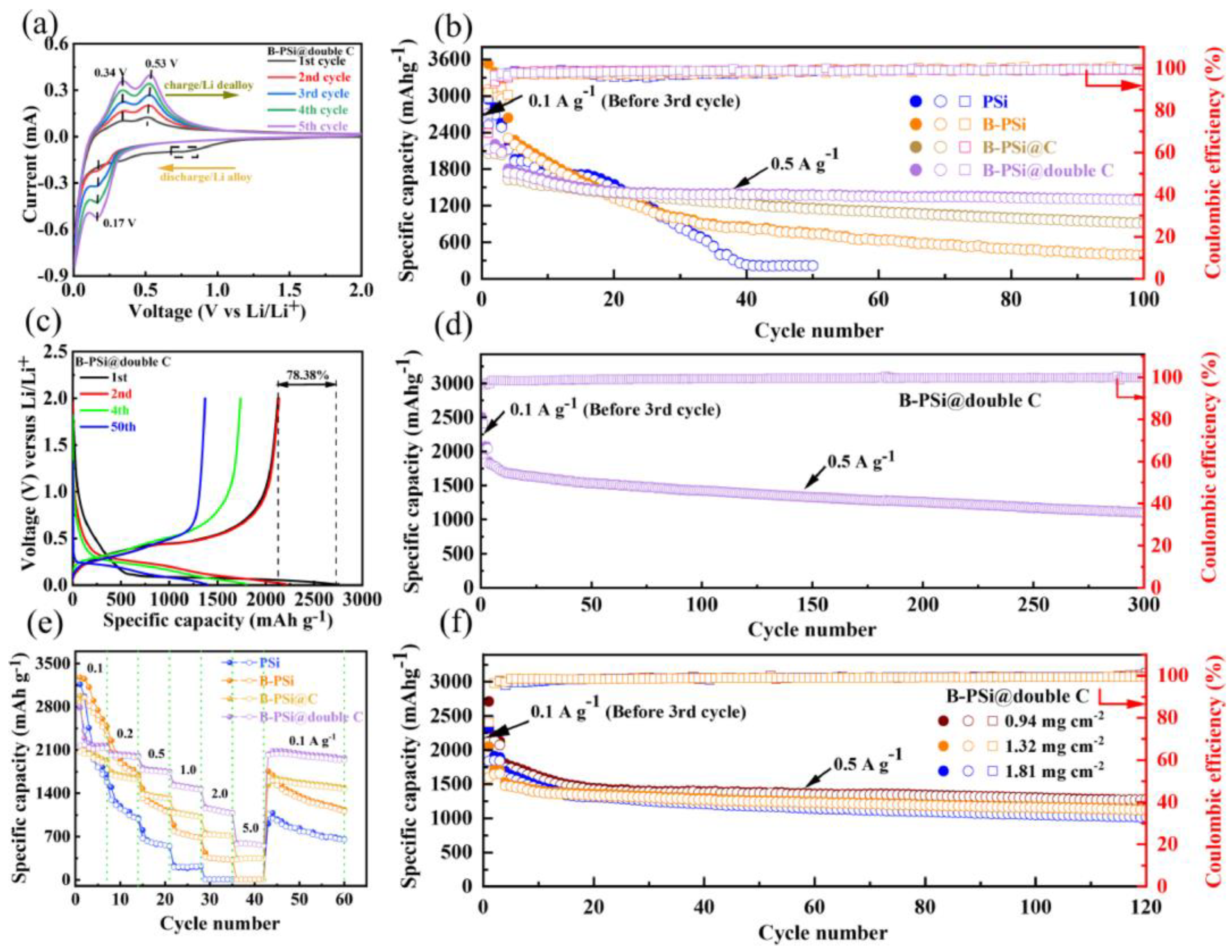
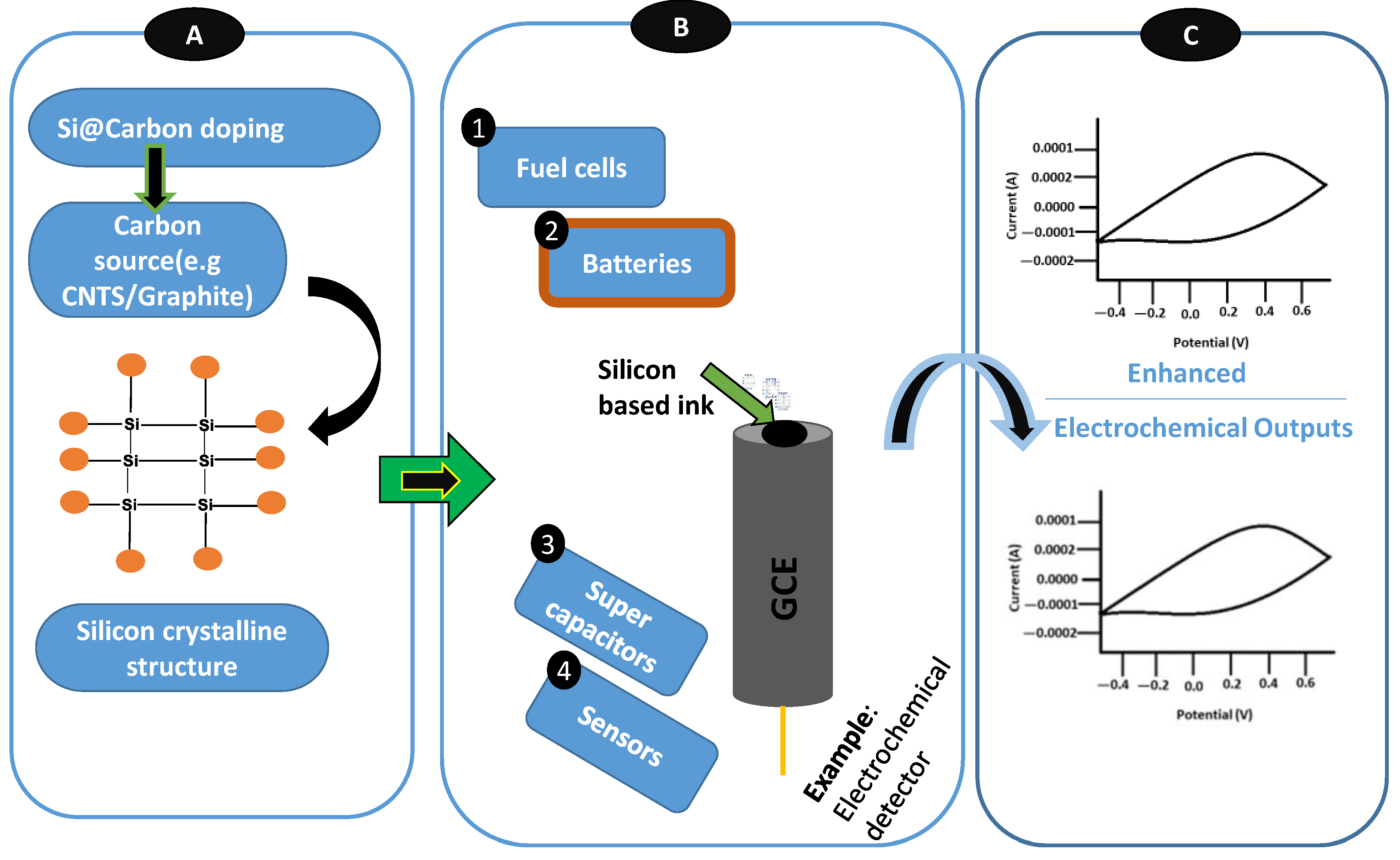
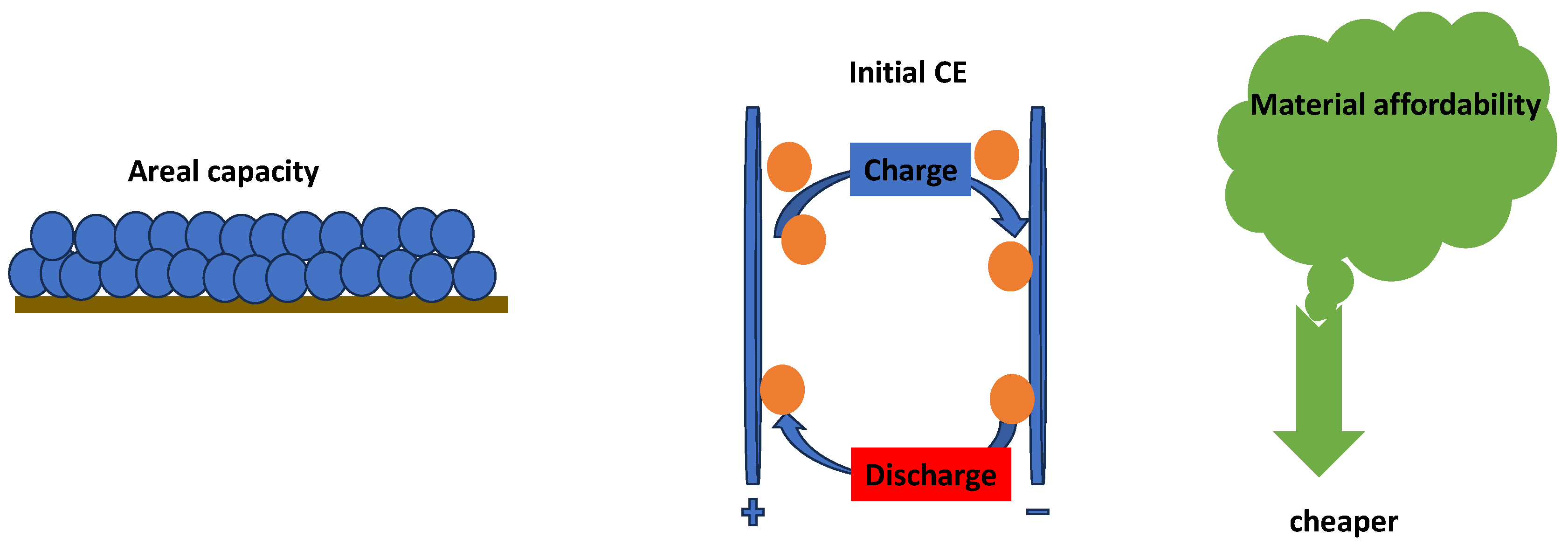
| Electrode | Synthesis Route | Capacity [mAh g−1] | Cycle no. | Current [mA g−1] | Ref. |
|---|---|---|---|---|---|
| Silicon composites | Disproportionation and polymerisation of furfuryl alcohol | 700 | 200 | 1 | [31] |
| Silicon mono-oxide composites | Disproportion with graphite | 1061 | 100 | 100 | [32] |
| 3D porous silicon mono-oxide composites | Chemical and thermal-assisted disproportionation | 1600 | 100 | 0.1 | [33] |
| Silicon multicomponent | High-temperature annealing process | 1280 | 200 | 0.2 | [34] |
| Structured nanotubular silicon multicomponent | Coaxial electrospinning technique and, metallothermic reduction reaction | 765 | 280 | 0.5 | [35] |
| Nanostructured silicon composites | Sol–gel process | 650 | 1000 | 1 | [36] |
| Nickel–silicon alloys | Spray drying and facile pyrolysis treatment | 1261 | 50 | 210 | [37] |
| Electrode | Anode (%) | Cathode (%) | Ref |
|---|---|---|---|
| HCMR (High-capacity manganese-rich) | - | ~15% | [34] |
| NCM (Mickel Cobalt Manganese) | - | ~10% | [35] |
| LFP (Lithium Iron Phosphate) | - | ~10% | [36] |
| LCO (Lithium Cobalt Oxide) | - | <3% | [37] |
| SiOx | ~30% | - | [38] |
| LTO (Lithium Titanium Oxide) | <3% | - | [39] |
| Carbon | >10% | - | [40] |
| Graphite | ~7% | - | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seroka, N.S.; Luo, H.; Khotseng, L. A Novel Biogenic Silicon-Based Anode Material for Lithium-Ion Batteries: A Review. Energies 2024, 17, 3520. https://doi.org/10.3390/en17143520
Seroka NS, Luo H, Khotseng L. A Novel Biogenic Silicon-Based Anode Material for Lithium-Ion Batteries: A Review. Energies. 2024; 17(14):3520. https://doi.org/10.3390/en17143520
Chicago/Turabian StyleSeroka, Ntalane Sello, Hongze Luo, and Lindiwe Khotseng. 2024. "A Novel Biogenic Silicon-Based Anode Material for Lithium-Ion Batteries: A Review" Energies 17, no. 14: 3520. https://doi.org/10.3390/en17143520
APA StyleSeroka, N. S., Luo, H., & Khotseng, L. (2024). A Novel Biogenic Silicon-Based Anode Material for Lithium-Ion Batteries: A Review. Energies, 17(14), 3520. https://doi.org/10.3390/en17143520








