Simulation of a Continuous Pyrolysis Reactor for a Heat Self-Sufficient Process and Liquid Fuel Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Biomass Characterization
2.3. Aspen Plus Schematics
2.3.1. Aspen Simulation Properties
2.3.2. Aspen Flow Sheet
2.3.3. External MS Excel Subroutine
Atomic Carbon Mass Balance
Hydrogen Mass Balance
Oxygen Mass Balance
Bio-Composition
3. Results and Discussion
3.1. Experimental Results
3.2. Model Validation
3.3. Aspen Plus Results
3.3.1. Char Composition
3.3.2. Bio-Oil Composition
4. Process Energy Balance
5. Techno-Economic Analysis
5.1. Incomes
5.1.1. Bio-Oil Cost
5.1.2. MSW Landfilled Tax
5.2. Expenses
5.3. Economic Parameters
5.4. Production Parameters
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments

Conflicts of Interest
Nomenclature
| Symbol | Unit | Description |
| - | Van Krevelen atomic ratio of O/C | |
| - | Van Krevelen atomic ratio of H/C | |
| kg/h | Carbon contained in the biomass | |
| kg/h | Carbon contained in the bio-oil | |
| kg/h | Carbon contained in the char | |
| kg/h | Carbon contained in the gas | |
| € | Net cash inflow during the period. | |
| € | Total initial investment cost. | |
| kg/h | Hydrogen contained in the biomass | |
| kg/h | Hydrogen contained in the bio-oil | |
| kg/h | Hydrogen contained in the char | |
| kg/h | Hydrogen contained in the gas | |
| i | % | Discount rate or return that could be earned in an alternative investment. |
| IRR | % | Internal Rate of Return |
| % | Net Present Value | |
| kg | Mass of the biomass in the fix-bed reactor | |
| kg | Mass of the condenser at the beginning of the pyrolysis | |
| kg | Mass of the condenser at the end of the pyrolysis | |
| kg | Mass of the non-condensable gases in the experimentation | |
| kg | Mass of the bio-oil and water in the experimentation | |
| kg | Mass of the reactor at the beginning of the pyrolysis | |
| kg | Mass of the reactor at the end of the pyrolysis | |
| kg | Mass of the char and ashes in the experimentation | |
| kg/h | Biomass flow rate = 10 | |
| kg/h | CH4 mass flow rate of a given stream | |
| kg/h | CO mass flow rate of a given stream | |
| kg/h | CO2 mass flow rate of a given stream | |
| kg/h | C2H4 mass flow rate of a given stream | |
| kg/h | C2H6 mass flow rate of a given stream | |
| kg/h | C3H8 mass flow rate of a given stream | |
| kg/h | H mass flow rate of a given stream | |
| kg/kmol | Molecular weight of carbon | |
| kg/kmol | Molecular weight of hydrogen | |
| kg/kmol | Molecular weight of oxygen | |
| kg/h | Oxygen contained in the biomass | |
| kg/h | Oxygen contained in the bio-oil | |
| kg/h | Oxygen contained in the char | |
| kg/h | Oxygen contained in the gas | |
| Years | Payback period | |
| kW | Heat duty produced by the burner | |
| kW | Heat duty required for the DECOMP block | |
| kW | Heat duty required for the pyrolysis process | |
| kW | Heat duty required for the PYRO-R block | |
| kW | Total Heat duty | |
| Rt | € | Net cash inflow minus outflows during a single period. |
| t | Number of periods. | |
| - | Mass fraction of the produced bio-oil (including water) | |
| - | Mass fraction of the produced bio-oil (without water) | |
| - | Mass fraction of the produced char | |
| - | Mass fraction of the produced gas |
References
- EPA. Global Greenhouse Gas Emissions Data. Available online: https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data (accessed on 17 July 2023).
- Chavando, J.A.M.; de Matos, E.C.J.; Silva, V.B.; Tarelho, L.A.; Cardoso, J.S. Pyrolysis characteristics of RDF and HPDE blends with biomass. Int. J. Hydrogen Energy 2021, 47, 19901–19915. [Google Scholar] [CrossRef]
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; Urban Development Series; World Bank: Washington, DC, USA, 2018. [Google Scholar]
- Kaur, S. Public preferences for setting up a biomass power plant to combat open-field burning of rice crop residues: A case study of district Sangrur, Punjab, India. Biomass Bioenergy 2020, 138, 105577. [Google Scholar] [CrossRef]
- University of Leeds. Open Burning of Solid Waste Is a Global Threat to Human Health and Safety That Requires Urgent Action. Available online: https://phys.org/news/2021-01-solid-global-threat-human-health.html (accessed on 19 April 2024).
- Wiedinmyer, C.; Yokelson, R.J.; Gullett, B.K. Global emissions of trace gases, particulate matter, and hazardous air pollutants from open burning of domestic waste. Environ. Sci. Technol. 2014, 48, 9523–9530. [Google Scholar] [CrossRef] [PubMed]
- Our World in Data. CO2 Emissions. Available online: https://ourworldindata.org/co2-emissions (accessed on 19 April 2024).
- The World Bank Group. Trends in Solid Waste Management. Available online: https://datatopics.worldbank.org/what-a-waste/trends_in_solid_waste_management.html (accessed on 8 July 2024).
- Li, P.; Wang, B.; Hu, J.; Zhang, Y.; Chen, W.; Chang, C.; Pang, S. Research on the kinetics of catalyst coke formation during biomass catalytic pyrolysis: A mini review. J. Energy Inst. 2023, 110, 101315. [Google Scholar] [CrossRef]
- EUR-Lex. Consolidated Text: Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2018.328.01.0082.01.ENG (accessed on 17 May 2024).
- Pacheco-López, A.; Lechtenberg, F.; Somoza-Tornos, A.; Graells, M.; Espuña, A. Economic and Environmental Assessment of Plastic Waste Pyrolysis Products and Biofuels as Substitutes for Fossil-Based Fuels. Front. Energy Res. 2021, 9, 676233. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, S.; Ouyang, Y. Reliable Biomass Supply Chain Design under Feedstock Seasonality and Probabilistic Facility Disruptions. Energies 2017, 10, 1895. [Google Scholar] [CrossRef]
- Mateus, M.M.; Cecílio, D.; Fernandes, M.C.; Correia, M.J.N. Refuse derived fuels as an immediate strategy for the energy transition, circular economy, and sustainability. Bus. Strat. Environ. 2023, 32, 3915–3926. [Google Scholar] [CrossRef]
- Shahbaz, M.; AlNouss, A.; Ghiat, I.; Mckay, G.; Mackey, H.; Elkhalifa, S.; Al-Ansari, T. A comprehensive review of biomass based thermochemical conversion technologies integrated with CO2 capture and utilisation within BECCS networks. Resour. Conserv. Recycl. 2021, 173, 105734. [Google Scholar] [CrossRef]
- IEA Bioenergy. Review of Waste to Energy Policies in South Africa and International Comparisons. Available online: https://www.ieabioenergy.com/wp-content/uploads/2023/05/Task-36-Waste-to-Energy-Policy-Review_2023final-2.pdf (accessed on 4 May 2024).
- Livingston, W.R.; Babcock, M. Technical and Economic Assessment of Energy Conversion Technologies for MSW; U.S. Department of Energy Office of Scientific and Technical Information: Oak Ridge, TN, USA, 2002. [Google Scholar]
- Ramos, A.; Monteiro, E.; Silva, V.; Rouboa, A. Co-gasification and recent developments on waste-to-energy conversion: A review. Renew. Sustain. Energy Rev. 2018, 81, 380–398. [Google Scholar] [CrossRef]
- Shah, H.H.; Amin, M.; Iqbal, A.; Nadeem, I.; Kalin, M.; Soomar, A.M.; Galal, A.M. A review on gasification and pyrolysis of waste plastics. Front. Chem. 2023, 10, 960894. [Google Scholar] [CrossRef]
- Rawat, J.; Saxena, J.; Sanwal, P. Biochar: A Sustainable Approach for Improving Plant Growth and Soil Properties. In Biochar—An Imperative Amendment for Soil and the Environment; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Santos, S.M.; Assis, A.C.; Gomes, L.; Nobre, C.; Brito, P. Waste Gasification Technologies: A Brief Overview. Waste 2022, 1, 140–165. [Google Scholar] [CrossRef]
- Solis, M.; Silveira, S. Technologies for chemical recycling of household plastics—A technical review and TRL assessment. Waste Manag. 2020, 105, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, T.Y.A.; Fahmy, Y.; Mobarak, F.; El-Sakhawy, M.; Abou-Zeid, R.E. Biomass pyrolysis: Past, present, and future. Environ. Dev. Sustain. 2020, 22, 17–32. [Google Scholar] [CrossRef]
- Gandidi, I.M.; Susila, M.D.; Mustofa, A.; Pambudi, N.A. Thermal—Catalytic cracking of real MSW into Bio-Crude Oil. J. Energy Inst. 2018, 91, 304–310. [Google Scholar] [CrossRef]
- Guo, M.; Wang, K.; Bing, X.; Cheng, J.; Zhang, Y.; Sun, X.; Guan, B.; Yu, J. Pyrolysis of plastics-free refuse derived fuel derived from municipal solid waste and combustion of the char products in lab and pilot scales: A comparative study. Fuel 2024, 359, 130335. [Google Scholar] [CrossRef]
- Zhang, Z.; Gora-Marek, K.; Watson, J.S.; Tian, J.; Ryder, M.R.; Tarach, K.A.; López-Pérez, L.; Martínez-Triguero, J.; Melián-Cabrera, I. Recovering waste plastics using shape-selective nano-scale reactors as catalysts. Nat. Sustain. 2019, 2, 39–42. [Google Scholar] [CrossRef]
- Elordi, G.; Olazar, M.; Lopez, G.; Artetxe, M.; Bilbao, J. Product yields and compositions in the continuous pyrolysis of high-density polyethylene in a conical spouted bed reactor. Ind. Eng. Chem. Res. 2011, 50, 6650–6659. [Google Scholar] [CrossRef]
- Dong, R.; Tang, Z.; Song, H.; Chen, Y.; Wang, X.; Yang, H.; Chen, H. Co-pyrolysis of vineyards biomass waste and plastic waste: Thermal behavior, pyrolytic characteristic, kinetics, and thermodynamics analysis. J. Anal. Appl. Pyrolysis 2024, 179, 106506. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Zhang, M.; Kaya, M.G.A.; Wang, F.; Tang, K. Co-pyrolysis of chrome-tanned leather shavings with wheat straw: Thermal behavior, kinetics and pyrolysis products. Energy 2024, 301, 131733. [Google Scholar] [CrossRef]
- Pio, D.; Tarelho, L.; Tavares, A.; Matos, M.; Silva, V. Co-gasification of refused derived fuel and biomass in a pilot-scale bubbling fluidized bed reactor. Energy Convers. Manag. 2020, 206, 112476. [Google Scholar] [CrossRef]
- Global Specs. CEN-EN 14774-1. Available online: https://standards.globalspec.com/std/1216262/en-14774-1 (accessed on 17 May 2024).
- Global Specs. BSI-BS EN 15414-3. Available online: https://standards.globalspec.com/std/1312754/bs-en-15414-3 (accessed on 17 May 2024).
- Global Specs. BSI-BS DD CEN/TS 14775. Available online: https://standards.globalspec.com/std/799079/bs-dd-cen-ts-14775 (accessed on 17 May 2024).
- Global Specs. BSI-BS EN 15403. Available online: https://standards.globalspec.com/std/1308310/bs-en-15403 (accessed on 17 May 2024).
- Global Specs. DIN CEN/TS 15148. Available online: https://standards.globalspec.com/std/1323516/din-cen-ts-15148 (accessed on 17 May 2024).
- Global Specs. BSI-BS EN 15402. Available online: https://standards.globalspec.com/std/1307862/bs-en-15402 (accessed on 17 May 2024).
- Haydary, J. Chemical Process Design and Aspen Plus and Aspen HYSYS Applications; General Procedure for Process Simulation; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 15–58. [Google Scholar]
- Wang, S.; Wang, Y.; Shi, Z.; Sun, K.; Wen, Y.; Niedzwiecki, L.; Pan, R.; Xu, Y.; Zaini, I.N.; Jagodzińska, K.; et al. Van Krevelen diagrams based on machine learning visualize feedstock-product relationships in thermal conversion processes. Commun. Chem. 2023, 6, 273. [Google Scholar] [CrossRef] [PubMed]
- Ben, H.; Wu, F.; Wu, Z.; Han, G.; Jiang, W.; Ragauskas, A.J. A Comprehensive Characterization of Pyrolysis Oil from Softwood Barks. Polymers 2019, 11, 1387. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Kong, L.; Cai, H.; Zhang, Q.; Bi, D.; Yi, W. Effects of alkali and alkaline earth metals on the co-pyrolysis of cellulose and high density polyethylene using TGA and Py-GC/MS. Fuel Process. Technol. 2019, 191, 71–78. [Google Scholar] [CrossRef]
- Voima, V. Pioneer of Biofuel Plants, Producer of Combined Heat and Power. Available online: https://www.vv.fi/wp-content/uploads/sites/8/2017/03/Vaskiluodon_Voima_ENG_FINAL.pdf (accessed on 17 May 2024).
- Li, L.; Bai, X.; Qu, C.; Zhou, K.; Sun, Y. Effects of Moisture on the Ignition and Combustion Characteristics of Lignite Particles: Modeling and Experimental Study. ACS Omega 2022, 7, 34912–34920. [Google Scholar] [CrossRef] [PubMed]
- Lestinsky, P.; Palit, A. Wood Pyrolysis Using Aspen Plus Simulation and Industrially Applicable Model. Geosci. Eng. 2016, 62, 11–16. [Google Scholar] [CrossRef]
- Puig-Gamero, M.; Pio, D.; Tarelho, L.; Sánchez, P.; Sanchez-Silva, L. Simulation of biomass gasification in bubbling fluidized bed reactor using aspen plus®. Energy Convers. Manag. 2021, 235, 113981. [Google Scholar] [CrossRef]
- No, S.Y. Bio-oil and pyrolytic oil. Green Energy Technol. 2019, 181–219. [Google Scholar] [CrossRef]
- Cai, W.; Wang, X.; Zhu, Z.; Kumar, R.; Amaniampong, P.N.; Zhao, J.; Hu, Z.-T. Synergetic effects in the co-pyrolysis of lignocellulosic biomass and plastic waste for renewable fuels and chemicals. Fuel 2023, 353, 129210. [Google Scholar] [CrossRef]
- Riedewald, F.; Patel, Y.; Wilson, E.; Santos, S.; Sousa-Gallagher, M. Economic assessment of a 40,000 t/y mixed plastic waste pyrolysis plant using direct heat treatment with molten metal: A case study of a plant located in Belgium. Waste Manag. 2021, 120, 698–707. [Google Scholar] [CrossRef]
- Procurement Resource. Pyrolysis Oil Prices: Latest Price, Pricing, Chart, Market Analysis. Available online: https://www.procurementresource.com/resource-center/pyrolysis-oil-price-trends (accessed on 1 May 2024).
- OPEC. OPEC Monthly Oil Market Report. 2023. Available online: https://www.opec.org/opec_web/static_files_project/media/downloads/publications/OPEC_MOMR_June_2023.pdf (accessed on 1 May 2024).
- EPA. Full Cost Accounting for Municipal Solid Waste Management: A Handbook; EPA: Washington, DC, USA, 1997. [Google Scholar]
- EEA. Early Warning Assessment Related to the 2025 Targets for Municipal Waste and Packaging Waste. June 2022. Available online: https://www.eea.europa.eu/publications/many-eu-member-states/early-warning-assessment-related-to (accessed on 2 January 2024).
- Statista. U.S. Biomass Feedstock Prices by Product 2023. Available online: https://www.statista.com/statistics/856660/biomass-feedstock-prices-in-the-us-by-product/ (accessed on 4 May 2024).
- Cardoso, J.S.; Silva, V.; Chavando, J.A.M.; Eusébio, D.; Hall, M.J.; Costa, M. Small-Scale Biomass Gasification for Green Ammonia Production in Portugal: A Techno-Economic Study. Energy Fuels 2021, 35, 13847–13862. [Google Scholar] [CrossRef]



| Technology | T °C | Advantages | Disadvantages | TRL | REF |
|---|---|---|---|---|---|
| Combustion | 1000–1300 |
|
| 9 | [16] |
| Gasification | 700–1200 |
|
| 6–9 | [20] |
| Pyrolysis | 300–700 |
|
| 6–9 | [21] |
| Description | RDF Pellets | Pine Chips |
|---|---|---|
| Proximate Analysis | ||
| Moisture (wt.%, wet basis) | 4.30 | 11.00 |
| Volatile Matter (wt.%, wet basis) | 75.20 | 77.90 |
| Fixed Carbon (wt.%, wet basis) | 7.10 | 10.80 |
| Ash (wt.%, wet basis) | 13.40 | 0.30 |
| Moisture (wt.%, dry basis) | 4.30 | 11.00 |
| Volatile Matter (wt.%, dry basis) | 78.58 | 87.53 |
| Fixed Carbon (wt.%, dry basis) | 7.42 | 12.13 |
| Ash (wt.%, dry basis) | 14.00 | 0.34 |
| Ultimate Analysis | ||
| Ash (wt.%, dry basis) | 14.00 | 0.34 |
| C (wt.%, dry basis) | 54.00 | 46.40 |
| H (wt.%, dry basis) | 7.40 | 6.60 |
| N (wt.%, dry basis) | 0.50 | 0.20 |
| O (wt.%, dry basis) | 24.10 | 46.46 |
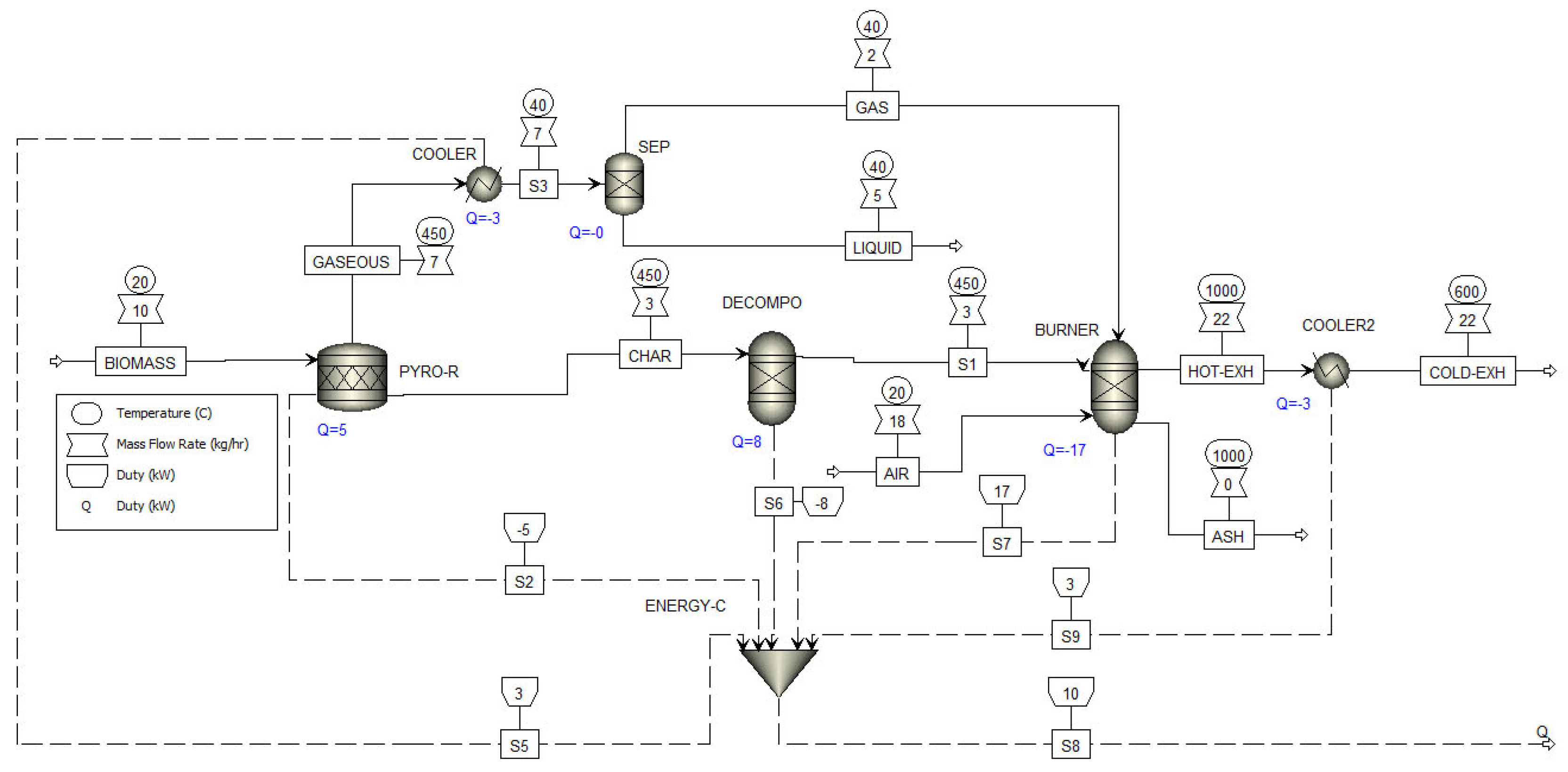
| Equipment | Type | Reactor |
|---|---|---|
| PYRO-R | Stoichiometric reactor | It is a stochiometric reactor that decomposed biomass into pyrolytic compounds using an Excel subroutine based on the obtained experimental data and a mass balance. Depending on the simulation, it operated at 450, 500, or 550 °C and 1 bar. |
| DECOMP | Ryield reactor | It decomposes the char in their elemental components C, H, O, and N. |
| BURNER | RGibbs | It produces a combustion reaction of the non-condensable gases and the decomposed char. This reactor solves its model by minimizing Gibbs free energy. |
| COOLER 1 | Heat exchanger | It reduces the temperature of the gases to be further separated into the non-condensable and condensable gases. |
| COOLER 2 | Heat exchanger | It reduces the temperature of hot gases produced in the combustion. |
| SEP | Separator | It separate the non-condensable gases from the condensable ones |
| ENERGY-C | Mixer | It combines energy streams to provide an energy balance |
| Run | Solid | Liquid | Gas | σ Solid | σ Liquid | σ Gas |
|---|---|---|---|---|---|---|
| E01-450°C-RDF0% | 27.91% | 54.40% | 17.69% | 0.7 | 1.2 | 1.8 |
| E03-450°C-RDF25% | 29.52% | 50.98% | 19.50% | 1.0 | 1.0 | 1.9 |
| E04-500°C-RDF0% | 24.73% | 58.16% | 17.11% | 0.6 | 2.3 | 1.9 |
| E06-500°C-RDF25% | 27.11% | 53.43% | 19.46% | 0.7 | 1.8 | 1.4 |
| E07-550°C-RDF0% | 24.57% | 56.98% | 18.46% | 0.9 | 1.0 | 1.1 |
| E09-550°C-RDF25% | 26.67% | 51.44% | 21.89% | 0.7 | 1.0 | 1.0 |
| Run | H2 | CH4 | CO | CO2 | C2H4 | C2H6 | C3H8 |
|---|---|---|---|---|---|---|---|
| E01-450°C-RDF0% | 0.67% | 7.70% | 40.89% | 49.01% | 0.59% | 0.85% | 0.29% |
| E03-450°C-RDF25% | 0.84% | 7.27% | 34.37% | 54.55% | 0.94% | 1.25% | 0.77% |
| E04-500°C-RDF0% | 1.20% | 9.44% | 40.04% | 47.15% | 0.71% | 1.10% | 0.36% |
| E06-500°C-RDF25% | 2.20% | 11.54% | 35.38% | 46.87% | 1.26% | 1.81% | 0.95% |
| E07-550°C-RDF0% | 3.55% | 13.41% | 39.33% | 41.06% | 0.82% | 1.42% | 0.40% |
| E09-550°C-RDF25% | 3.37% | 13.01% | 38.51% | 41.69% | 1.20% | 1.55% | 0.67% |
| Experiment | Moisture | VM | FC | Ashes |
|---|---|---|---|---|
| E01-450°C-RDF0% | 3.05% | 29.81% | 69.11% | 1.07% |
| E03-450°C-RDF25% | 2.53% | 28.51% | 60.51% | 10.98% |
| E04-500°C-RDF0% | 2.25% | 27.92% | 70.80% | 1.27% |
| E06-500°C-RDF25% | 2.45% | 22.93% | 63.93% | 13.14% |
| E07-550°C-RDF0% | 2.65% | 15.73% | 82.82% | 1.45% |
| E09-550°C-RDF25% | 2.66% | 19.95% | 68.49% | 11.56% |
| Experimental | Simulation | |||||||
|---|---|---|---|---|---|---|---|---|
| Run | Solid * | Liquid * | Gas * | Gas lhv MJ/m3 | Solid * | Liquid * | Gas * | Gas LHV MJ/m3 |
| E01-450°C-RDF0% | 27.91% | 54.40% | 17.69% | 8.43 | 27.91% | 54.40% | 17.69% | 9.51 |
| E03-450°C-RDF25% | 30.10% | 50.01% | 19.89% | 8.39 | 29.52% | 50.98% | 19.50% | 8.64 |
| E04-500°C-RDF0% | 24.73% | 58.16% | 17.11% | 9.23 | 24.73% | 58.16% | 17.11% | 9.51 |
| E06-500°C-RDF25% | 27.89% | 52.09% | 20.02% | 10.71 | 27.11% | 53.43% | 19.46% | 11.03 |
| E07-550°C-RDF0% | 24.57% | 56.98% | 18.46% | 10.98 | 24.57% | 56.98% | 18.46% | 11.31 |
| E09-550°C-RDF25% | 27.03% | 50.78% | 22.19% | 11.24 | 26.67% | 51.44% | 21.89% | 11.58 |
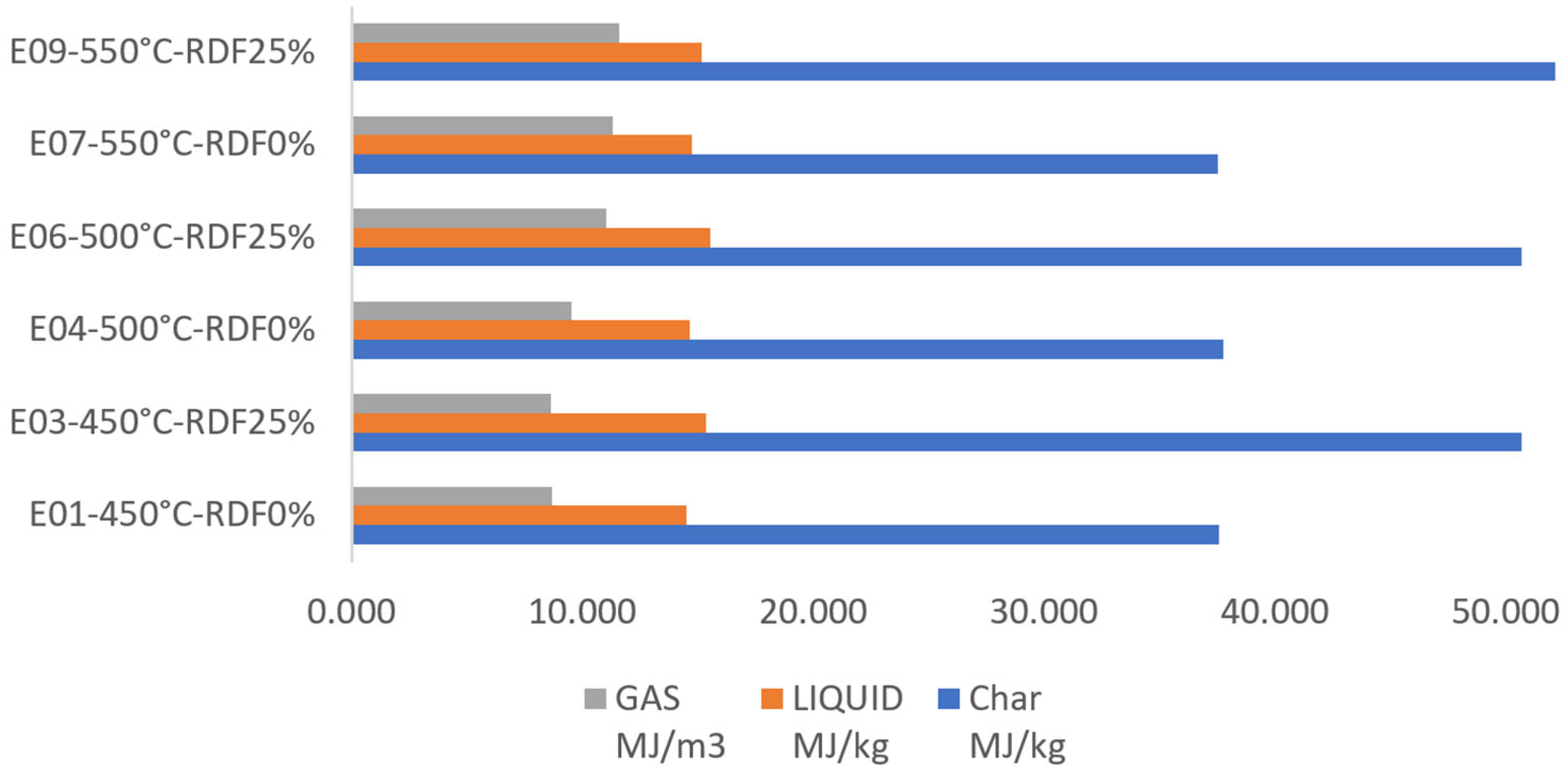
| Experiment | C | H | O | N | Ash | LHV MJ/kg |
|---|---|---|---|---|---|---|
| E01-450°C-RDF0% | 0.421 | 0.076 | 0.487 | 0.006 | 0.011 | 37.587 |
| E03-450°C-RDF25% | 0.550 | 0.083 | 0.240 | 0.008 | 0.119 | 50.703 |
| E04-500°C-RDF0% | 0.432 | 0.070 | 0.478 | 0.007 | 0.012 | 37.761 |
| E06-500°C-RDF25% | 0.554 | 0.076 | 0.233 | 0.009 | 0.128 | 50.703 |
| E07-550°C-RDF0% | 0.432 | 0.068 | 0.480 | 0.007 | 0.012 | 37.547 |
| E09-550°C-RDF25% | 0.541 | 0.080 | 0.237 | 0.009 | 0.132 | 52.703 |
| Experiment | H2O | C5H6O2 | C6H6 | C7H16 | LHV (MJ/kg) |
|---|---|---|---|---|---|
| E01-450°C-RDF0% | 0.350 | 0.526 | 0.044 | 0.080 | 14.52 |
| E03-450°C-RDF25% | 0.337 | 0.563 | 0.000 | 0.100 | 15.34 |
| E04-500°C-RDF0% | 0.350 | 0.551 | 0.017 | 0.082 | 14.66 |
| E06-500°C-RDF25% | 0.332 | 0.565 | 0.000 | 0.103 | 15.57 |
| E07-550°C-RDF0% | 0.350 | 0.563 | 0.004 | 0.083 | 14.73 |
| E09-550°C-RDF25% | 0.341 | 0.558 | 0.000 | 0.101 | 15.15 |
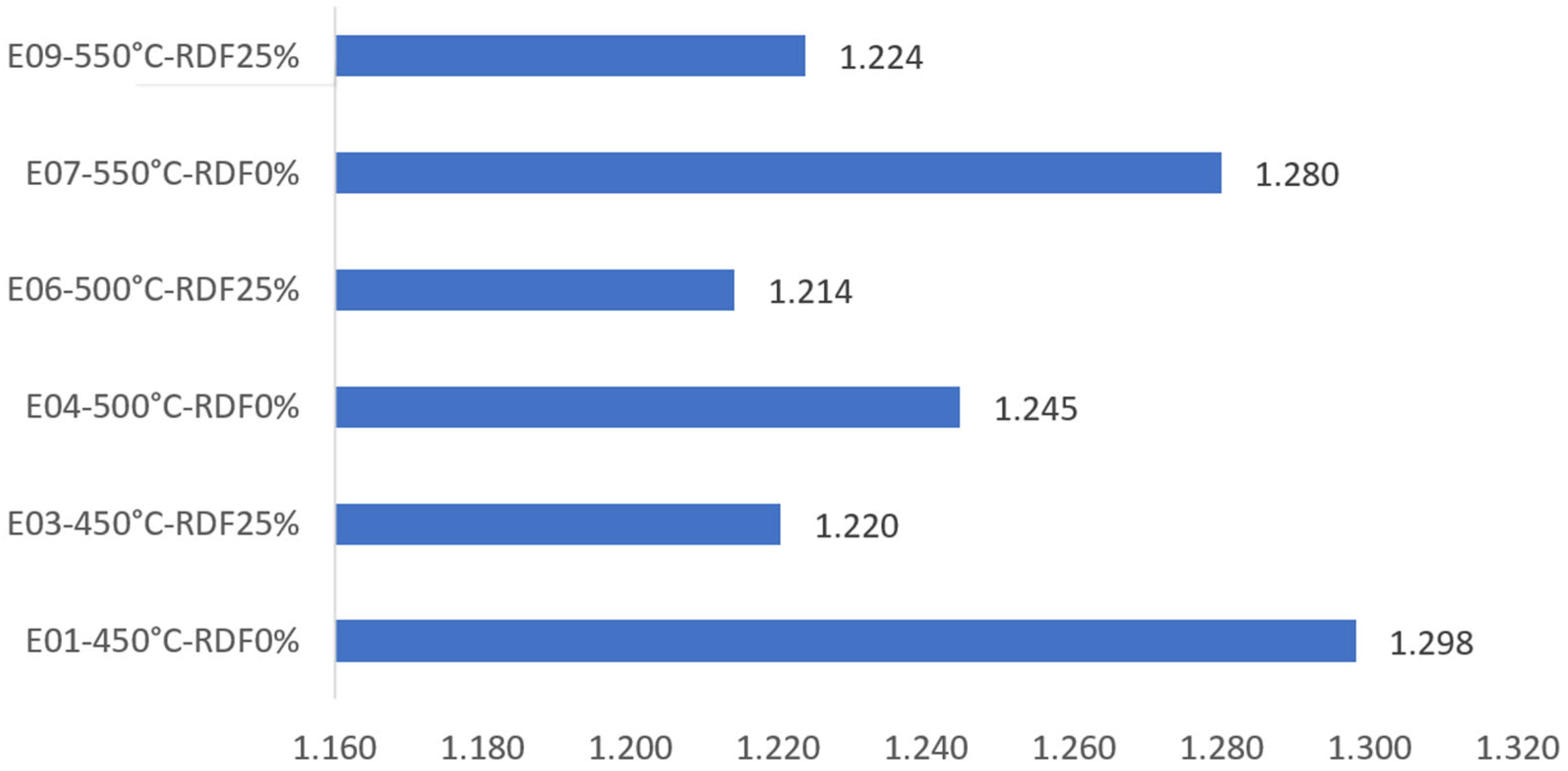
| Experiments * | Heat Duty Required by the Pyrolysis Process kW | Heat Duty ** Produced by the Burner. kW | kW | Energy Excess kW/kg |
|---|---|---|---|---|
| E01-450°C-RDF0% | 12.98 | −14.53 | −1.55 | −0.155 |
| E03-450°C-RDF25% | 12.20 | −17.50 | −5.30 | −0.530 |
| E04-500°C-RDF0% | 12.45 | −13.21 | −0.76 | −0.076 |
| E06-500°C-RDF25% | 12.14 | −16.57 | −4.43 | −0.443 |
| E07-550°C-RDF0% | 12.80 | −13.75 | −0.95 | −0.095 |
| E09-550°C-RDF25% | 12.24 | −17.03 | −4.79 | −0.479 |
| Parameter | Description | Scenario 1 Optimistic | Scenario 2 Conservative | Scenario 3 Tragic |
|---|---|---|---|---|
| Income | Bio-Oil price (€/ton) | 818 | 715 | 500 |
| MSW Landfilled Tax (€/ton) | 79 | 25 | 11 | |
| Expenses | CAPEX [46] | €20,190,000.00 | €20,190,000.00 | €20,190,000.00 |
| OPEX (per year) [46] | €3,425,414.00 | €3,425,414.00 | €3,425,414.00 | |
| Biomass cost (€/ton) | 40 | 50 | 60 | |
| Amortization (€/year) | 3,028,500 | 3,028,500 | 3,028,500 | |
| Economic parameters | Discount rate | 15 | 15 | 15 |
| Evaluation period (years) | 20 | 20 | 20 | |
| Production | Plant production (€/ton) | 40,000 | 40,000 | 40,000 |
| Bio-Oil Yield (Without water) | 0.352 | 0.352 | 0.352 |
| EU Country | Tax (€/ton Waste Landfilled) | MSW Generation 2020 | |||||
|---|---|---|---|---|---|---|---|
| Lower Level | Upper Level | Annual MSW Generation (Million Tons) | Annual kg/Person | Landfilled % | Incinerated % | Recycled, Composted/Digested % | |
| Denmark | 79 | 79 | 5.0 | 845 | 0.9 | 45.2 | 53.9 |
| Luxemburg | 0 | 0 | 0.5 | 790 | 3.8 | 43.2 | 53.0 |
| Malta | 0 | 0 | 0.3 | 643 | 82.5 | 0.0 | 10.5 |
| Germany | 0 | 0 | 52.6 | 632 | 0.0 | 33.0 | 67.0 |
| Cyprus | 0 | 0 | 0.5 | 609 | 67 | 1.5 | 31.5 |
| Finland | 70 | 70 | 3.0 | 596 | 0.5 | 57.9 | 41.6 |
| Austria ** | 30 | 30 | 5.2 | 588 | 2.0 | 38 | 58.0 |
| Ireland *** | 75 | 75 | 2.7 | 555 | 31.8 | 36.7 | 31.5 |
| Czechia | 40 | 40 | 5.8 | 543 | 47.7 | 12.6 | 39.7 |
| France | 25 | 42 | 36.1 | 537 | 18.1 | 38.1 | 42.7 |
| Netherlands | 37 | 37 | 9.0 | 534 | 1.4 | 41.8 | 56.8 |
| Greece ** | 25 | 25 | 5.6 | 524 | 77.7 | 1.3 | 21.0 |
| Portugal | 25 | 25 | 5.3 | 513 | 47 | 19.0 | 34.0 |
| Italy ** | 5 | 26 | 30.0 | 503 | 20.9 | 20.0 | 59.1 |
| Lithuania | 50 | 50 | 1.35 | 483 | 16.3 | 25.9 | 57.8 |
| Latvia | 95 | 95 | 0.9 | 478 | 52.8 | 3.0 | 44.2 |
| Slovenia | 11 | 11 | 1.0 | 457 | 6.7 | 13.1 | 73.7 |
| Spain | 30 | 40 | 21.5 | 455 | 52.0 | 12.0 | 36.0 |
| Slovakia | 11 | 33 | 2.4 | 433 | 49.7 | 7.9 | 42.2 |
| Sweden | 55 | 55 | 4.6 | 431 | 1.0 | 53.0 | 46.0 |
| Croatia | 0 | 0 | 1.7 | 418 | 55.7 | 0.0 | 44.3 |
| Belgium | 63 | 114 | 5.0 | 416 | 43.0 | 1.0 | 56.0 |
| Bulgaria * | 50 | 50 | 2.9 | 407 | 61 | 7.0 | 32.0 |
| Hungary | 17 | 17 | 3.9 | 403 | 54.0 | 0.0 | 46.0 |
| Estonia | 30 | 30 | 0.5 | 383 | 14.7 | 42.8 | 42.5 |
| Poland | 15 | 60 | 13.1 | 346 | 39.8 | 21.5 | 38.7 |
| EU Average | 505 | ||||||
| Financial Parameters | Scenario 1 Optimistic | Scenario 2 Conservative | Scenario 3 Tragic |
|---|---|---|---|
| PBP | 7.5 | - | - |
| NPV | EUR 8,944,505.51 | EUR −5,390,865.80 | EUR −27,093,219.89 |
| IRR | 22.67% | 9.96% | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavando, A.; Silva, V.B.; Tarelho, L.A.C.; Cardoso, J.S.; Eusebio, D. Simulation of a Continuous Pyrolysis Reactor for a Heat Self-Sufficient Process and Liquid Fuel Production. Energies 2024, 17, 3526. https://doi.org/10.3390/en17143526
Chavando A, Silva VB, Tarelho LAC, Cardoso JS, Eusebio D. Simulation of a Continuous Pyrolysis Reactor for a Heat Self-Sufficient Process and Liquid Fuel Production. Energies. 2024; 17(14):3526. https://doi.org/10.3390/en17143526
Chicago/Turabian StyleChavando, Antonio, Valter Bruno Silva, Luís A. C. Tarelho, João Sousa Cardoso, and Daniela Eusebio. 2024. "Simulation of a Continuous Pyrolysis Reactor for a Heat Self-Sufficient Process and Liquid Fuel Production" Energies 17, no. 14: 3526. https://doi.org/10.3390/en17143526





