Abstract
Hydrogen is undeniably one of the most promising options for producing energy with minimal environmental impact. However, current hydrogen production is still derived from carbon-intensive processes relying on fossil fuels. Biomass is a sustainable and versatile resource that can be converted into hydrogen through biological and thermochemical pathways from a large variety of feedstocks and technologies. This work reviews and compares existing biomass-to-hydrogen technologies, focusing on their characteristics, maturity level, benefits, limitations, and techno-economic and lifecycle environmental impacts. Less-developed biological conversion methods are characterized by low efficiencies and hydrogen productivity. More mature thermochemical routes enable higher efficiencies and hydrogen yields. Overall, while thermochemical processes suit centralized large-scale hydrogen production, biological pathways offer decentralized options, necessitating continued innovation for integration into future energy strategies. Some of these technologies, such as anaerobic digestion (best-case: 1.28 EUR/kgH2) and conventional gasification (best-case: 1.79 EUR/kgH2), emerge as promising, sustainable, and affordable alternatives for renewable hydrogen generation, offering production costs comparable to those of natural gas steam reforming (0.92–2.8 EUR/kgH2).
1. Introduction
Increasing global concern over issues related to energy security, energy availability, greenhouse gas (GHG) emissions, and climate change is prompting governments worldwide to implement programs that facilitate a shift to a low-carbon economy. In this sense, new strategies are being established and implemented to reduce the energy sector’s carbon intensity on a global scale and achieve a robust industry with low or zero carbon emissions. However, despite these policies, fossil fuels are likely to remain a significant part of the global energy supply for decades, especially in emerging economies where affordable and reliable energy is crucial for their technological and social advancement [1].
The least expensive form of energy production involves burning fossil fuels, which results in substantial CO2 emissions. Hydrogen (H2) presents an alternative to fossil fuels with the potential to contribute to achieving carbon neutrality. When burned, hydrogen only emits water, but its carbon neutrality depends on the method of production [2]. In this context, hydrogen has gained projection on the European and international agenda as a key factor in facilitating the European Union’s (EU) transition towards climate neutrality by 2050 and improving energy security.
The hydrogen economy is defined as a new industry based on hydrogen as a commercial fuel that has the potential to revolutionize the existing energy system [3,4]. Large-scale hydrogen production using accessible sustainable energy sources can replace the current fossil fuel-based energy economy. This transition involves the simultaneous development of hydrogen production, storage, transportation, and distribution, needing strategic political support. In the long term, hydrogen can reduce energy consumption and emissions while stimulating economic growth and creating new employment opportunities. This vision can become a reality if hydrogen can be produced economically and environmentally from renewable energy sources [3].
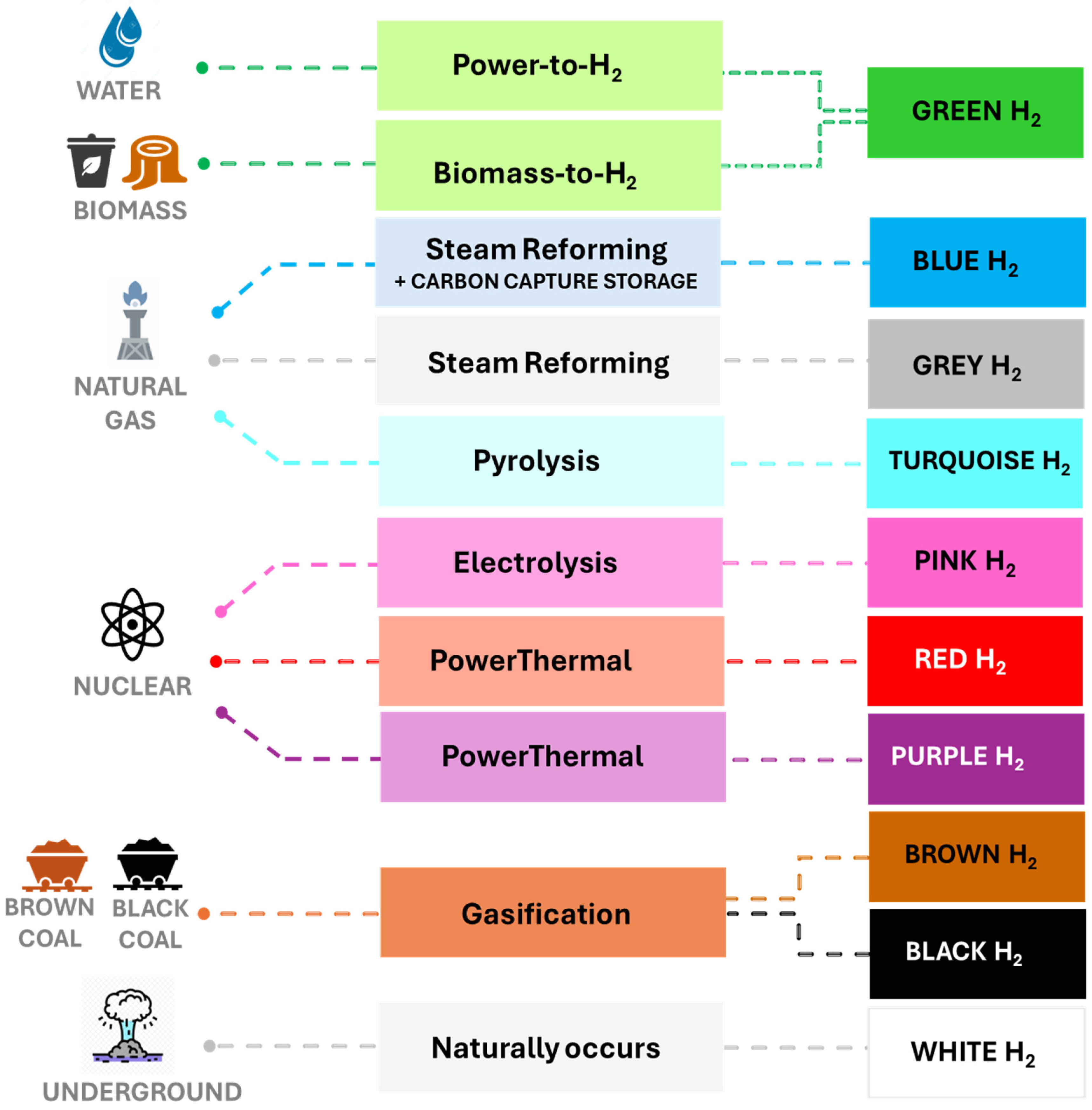
Hydrogen is categorized into several types based on the energy source and production process. A color code system is currently used to categorize hydrogen according to its production method (Figure 1) [5,6].
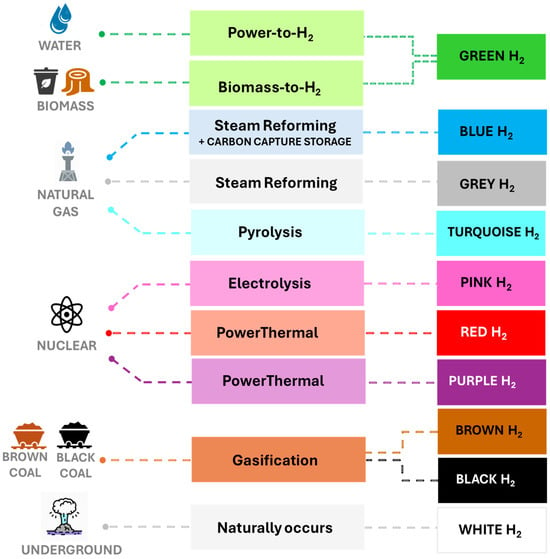
Figure 1.
The colors of hydrogen, considering the type of energy and technology used for its production.
Gray hydrogen is produced by steam reforming, partial oxidation, or the gasification of fossil fuels, such as natural gas, oil, and coal. This method is the most widely used for hydrogen production, accounting for 95% of global production in 2020 [7]. Carbon capture technologies can be combined with gray hydrogen-producing processes to reduce the carbon footprint, yielding blue hydrogen. On the other hand, renewable hydrogen (also known as green hydrogen) is a clean form of hydrogen produced from renewable energy sources, such as wind, solar, and biomass [8]. White hydrogen, also known as geological or natural hydrogen, refers to molecular hydrogen that forms and accumulates naturally on Earth, primarily through a geochemical process known as serpentinization. Recent studies have confirmed the presence of vast geological formations where hydrogen has accumulated over time, making white hydrogen a promising source with a key role in the planet’s energy transition [9].
Renewable hydrogen (renewable-H2) is essential for a sustainable industrial policy, as outlined in the EU’s Green Deal. It is considered a fundamental pillar in accelerating the reliance on fossil fuels and ensuring economic and social well-being. The Hydrogen Strategy for a Climate Neutral Europe, adopted in 2020 by the European Commission, encompasses the entire hydrogen supply chain, intending to create favorable conditions to increase supply and demand, improve industrial competitiveness, and ensure energy security in the EU [10]. This was also emphasized in the REPowerEU plan, which set ambitious targets to increase hydrogen production from renewable sources to 10 million tons of annual domestic production and an additional 10 million tons of annual imports by 2030 [11].
Currently, the most widely used method to produce renewable-H2 is water electrolysis using electricity from renewable sources. However, several other pathways exist for renewable-H2 production, including biological methods (e.g., bio-photolysis, photo-fermentation, dark fermentation, and a hybrid strategy combining dark fermentation and photosynthetic activities) and thermochemical methods (e.g., gasification, pyrolysis, and hydrothermal processes) [8,12]. As an endogenous and available renewable energy source, biomass enables the production of clean hydrogen from a wide array of feedstocks and technologies. Biomass-based methods are a complementary option to the existing power-to-hydrogen processes and a more viable alternative to these solutions in locations where renewable electricity from solar and wind energy have high production costs.
Several reviews have been published in the scientific literature over time, which have allowed the global community to follow the advances in renewable-H2 production. For instance, the production of hydrogen through biological processes has been discussed by Akhlaghi and Najafpour-Darzi [12], Mudhoo et al. [13], and Singh et al. [14]. Renewable-H2 generation using biomass thermochemical conversion technologies, such as steam reforming [15], pyrolysis [16,17,18,19,20], gasification [21,22,23,24,25], and supercritical water gasification [26,27,28,29], has also been addressed.
Other studies covered more specific details on biomass-to-hydrogen technologies. Shahbaz et al. [30] presented a comprehensive analysis of the application of biomass conversion methods and membrane separation systems for obtaining renewable hydrogen. Subsequently, Pal and coworkers [8] analyzed H2 production methods using biomass and its derivatives as raw materials, paying special attention to biological processes.
Despite the significant number of available scientific studies, there is still a need to address the operating principles and the technical–economic viability of renewable-H2 production from biomass-based methods. This review aims to contribute by providing an analysis of renewable hydrogen production from biomass by biological and thermochemical methods, including the main production pathways from both routes, process parameters and their influence on hydrogen yield, advantages and challenges for each technology, and an overview on techno-economic and life cycle aspects in each hydrogen production methodology.
2. Type of Biomass Used in Renewable-H2 Production
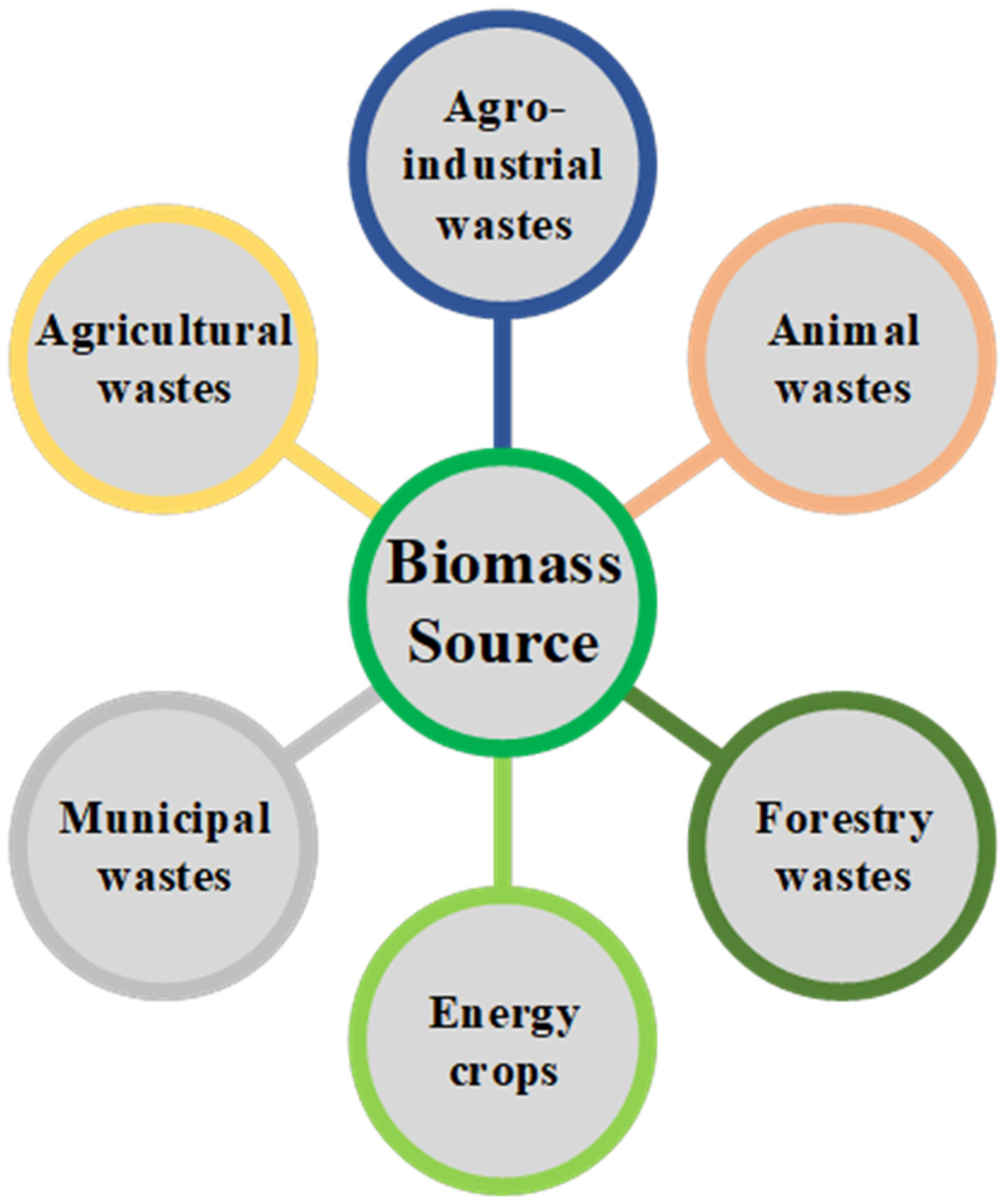
According to the United Nations Framework Convention on Climate Change (UNFCCC) [31], “biomass” denotes organic materials that are non-fossilized and biodegradable, originating from plants, animals, and microorganisms. This category includes by-products, residues, and waste from agriculture, forestry, and associated industries, along with non-fossilized and biodegradable organic fractions of industrial and municipal waste. Biomass also includes gases and liquids produced from the decomposition of non-fossilized and biodegradable organic matter. In contemporary times, biomass serves as a crucial renewable feedstock for hydrogen generation. A substantial quantity of biomass is available for this application, and it can be categorized into six types based on its source, as shown in Figure 2.
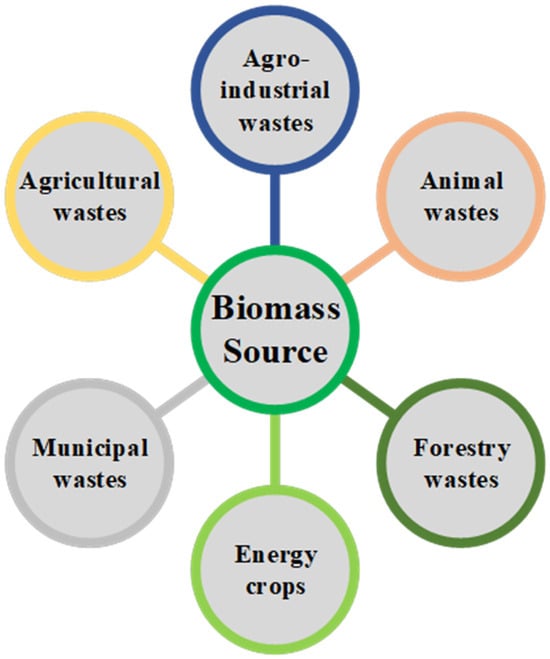
Figure 2.
Types of biomass with potential to be used in renewable-H2 production.
A key strength of biomass as feedstock for hydrogen generation is its versatility. It can be obtained and delivered locally, contributing to the development of regional economies and decreasing the carbon footprint of the process, with the potential to lead to negative emissions for some types of feedstocks. Moreover, there is a wide variety of biomass with the potential for hydrogen production through different technologies, including sewage, forestry and agro-industrial residues, and animal wastes, which lessens the negative effects related to the seasonal availability of some kinds of biomass.
Agricultural waste encompasses straw, husks, stems, leaves, seeds, and other by-products derived from different crops, such as corn, rice, and wheat. Typically, these wastes persist in the fields post-harvest. Agro-industrial waste primarily results from the industrial processing of crops, including, among others, wheat, corn, rice, soybeans, barley, rapeseed, sugar cane, sugar beets, and olives. Approximately 3.3 Gt/year of agricultural waste is generated, with China, the United States, Brazil, and India being its main producers. Some of this waste is utilized as animal feed, and others are employed in energy generation, albeit with low efficiency. The considerable abundance and availability of agricultural waste hold significant potential to update energy markets and gradually replace fossil fuels [32,33].
Forest wastes comprise branches, leaves, bark, and other wood fragments. Lignocellulosic biomass generally is composed of 35–55% cellulose, 25–40% hemicellulose, 15–25% lignin, 2–16% ash, and a lower proportion of extracts and proteins. Most woody biomass, without foreign matter, such as “dirt” (leaves and needles), has an ash content of less than 2%, while agricultural and agro-industrial waste can contain up to 20%. In terms of elemental composition, forest wastes tend to exhibit slightly higher amounts of carbon and hydrogen compared to agricultural and agro-industrial wastes. The heating value of these wastes can vary between 8 and 20 MJ/kg, depending on the ash and moisture contents [32,34].
Animal waste consists of excrement, urine, hair, feathers, blood, fur, etc. These wastes pose a source of pollution, a source of unpleasant odors, and a threat to health. Thus, relevant regulations order the adoption of appropriate management methods. An effective technique is anaerobic digestion, which yields biogas as a potential source for green hydrogen production or electricity production in power plants or internal combustion engines (ICE) [35].
Energy crops are grown specifically to provide biomass and are divided into two categories: herbaceous and woody. Herbaceous energy crops, such as switchgrass, miscanthus, bamboo, sweet sorghum, and others, are perennial grasses that are harvested annually after a two-to-three-year establishment period to achieve full productivity. On the other hand, short-rotation woody crops, including hybrid poplar, eucalyptus, hybrid willow, silver maple, and others, are fast-growing hardwood trees that can be harvested five-to-eight years after planting. The cultivation of these species can lead to improvements in water and soil quality, the enhancement of wildlife habitats, the diversification of income sources, and an overall increase in agricultural productivity compared to annual crops [35,36].
Municipal solid waste (MSW) constitutes a heterogeneous mixture, encompassing household waste in addition to tree pruning, garden waste, and other miscellaneous residues. Typically, MSW consists of 61.3% organic material, 16.3% plastic, 11.4% refuse-derived fuel (RDF), and 3.7% recyclable materials. Its organic fraction presents an average composition that includes carbohydrates (41–62%), proteins (15–25%), and lipids (13–30%), which can be effectively converted into eco-friendly biofuels and value-added products [37]. According to a study by Khan et al. [38], the annual generation of MSW is estimated to be approximately 2010 million tons, with 33% of this volume remaining unmanaged. This proportion is anticipated to increase in the coming years, particularly in developing countries, posing a significant challenge to environmental sustainability. Consequently, there is an urgent requirement for the implementation of effective strategies to address the escalating growth of MSW on a global scale [39]. Table 1 shows the lignocellulosic and elemental composition of examples of biomass wastes from each of the above-mentioned types.

Table 1.
The lignocellulosic composition and elemental analysis of various biomass wastes fitting into the types of biomass with the potential to be used in renewable-H2 production.
3. Methods for Renewable-H2 Production from Biomass
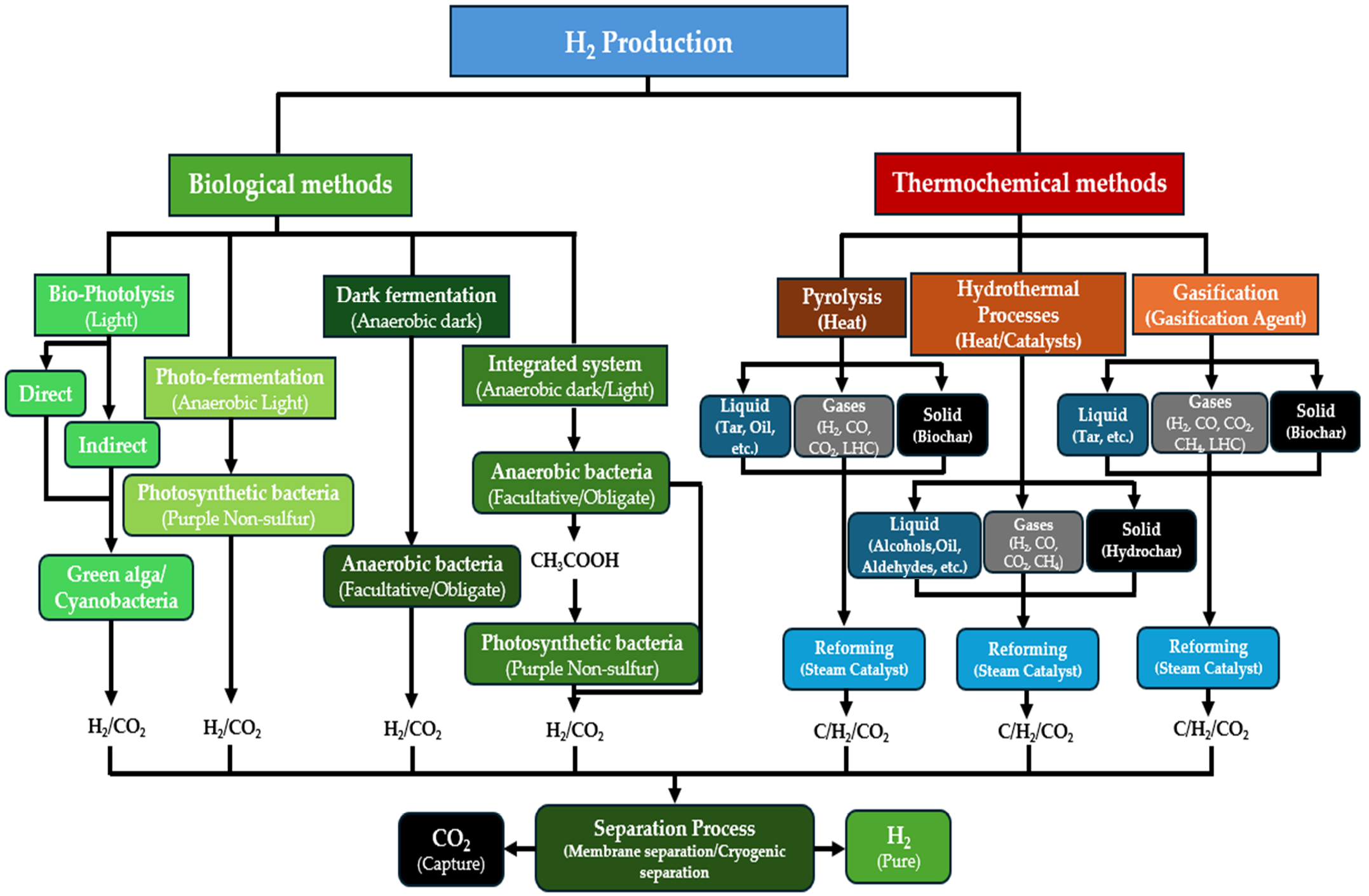
Despite hydrogen being the most abundant element in the universe and being widely distributed on our planet, it is not directly available in its pure form. Instead, it is typically bound to other chemical elements, and separating it requires energy-intensive conversion processes that generally cannot be accomplished in a single step. This is especially true for feedstocks derived from biomass, where the hydrogen must be separated from the other constituent elements. Essentially, two methods are used to separate the hydrogen from biomass, biological and thermochemical, as depicted in Figure 3. In this work, “renewable-H2” encompasses biohydrogen from microorganisms as well as hydrogen produced via the thermochemical conversion of biomass, so all biomass-derived hydrogen production technologies.
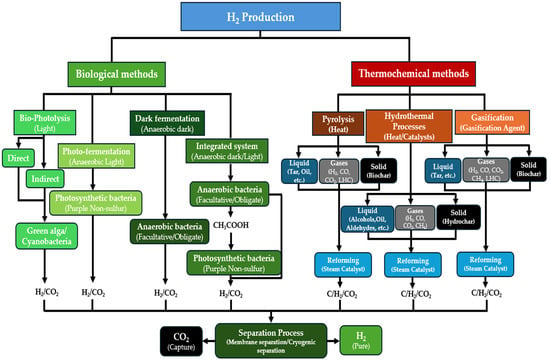
Figure 3.
The schematic diagram of the technological routes for the conversion of biomass into hydrogen.
3.1. Biological Methods
Hydrogen production through biological methods involves using microorganisms to produce hydrogen through metabolic processes such as fermentation or photosynthesis and is generally classified as a light-dependent or light-independent process [49]. Light-dependent methods include photo-fermentation and bio-photolysis (direct or indirect) using algae or microalgae, while dark fermentation takes place in a light-independent process using bacteria [48]. Hydrogen production from algae, microalgae, and bacteria has the advantage of producing high yields at low temperatures (usually between 20 and 45 °C) and being low cost compared to other production methods [50]. However, some secondary reactions that normally accompany the production of hydrogen, such as oxygen generated in photosynthesis and organic acids from the decomposition of organic compounds, inhibit hydrogen production and consequently lower yields [51,52]. Integrated systems, such as co-fermentation and photo-fermentation, offer possible solutions to these issues and constitute alternative methods for sustainable hydrogen production, increasing efficiency as well as the production rate and hydrogen yield [52]. Bio-photolysis (direct or indirect), photo-fermentation, dark fermentation, and integrated systems will be described in the following sections.
3.1.1. Bio-Photolysis
Hydrogen production via bio-photolysis is primarily based on photosynthetic oxygenic microorganisms, including green microalgae (e.g., Chlamydomonas reinhardtii, Chlorella vulgaris, Scenedesmus obliquus, and Chlorococcum minutum) and cyanobacteria (e.g., Nostoc sp., Cyanothece sp.) [53,54,55,56,57,58]. These microorganisms harness solar energy to drive water-splitting photosynthesis and transfer high-energy electrons to generate hydrogen, with the enzyme hydrogenase as a catalyst [59].
Bio-photolysis directs the reductants produced by splitting water for the evolution of hydrogen without entering the Calvin cycle or pentose phosphate pathway, which involves photochemical oxidation in the thylakoid membrane of algae and cyanobacteria. In these organisms, light-absorbing pigments are organized into two functional assemblies: photosystems (PS) I and II. Under anaerobic conditions or when the system absorbs a lot of energy, certain microorganisms direct excess electrons to hydrogenase, converting proton (H+) ions into renewable-H2. The electrons and protons generated in the process can be recombined to form 98% pure renewable-H2 using the chloroplast enzyme dehydrogenase. Bio-photolysis is classified into direct bio-photolysis (DbP) and indirect bio-photolysis (i-DbP) [60].
In the context of DbP, photoautotrophic organisms employ the catalytic activity of the enzyme hydrogenase to convert water molecules into molecular hydrogen and oxygen under anaerobic conditions, utilizing light absorbed by PSII. These organisms harness light energy to extract electrons and protons from water molecules. Subsequently, these extracted electrons and protons are used to reduce ferredoxin (Fd) and nicotinamide adenine dinucleotide phosphate (NADP) [61]. The general reactions characterizing DbP are outlined below in Equations (1) and (2).
i-DbP is a two-step process in which cyanobacteria and microalgae produce hydrogen and oxygen in separate reactions. In the first step, carbohydrates are synthesized using light energy, carbon dioxide is fixed, and oxygen is produced. In the second step, hydrogen is produced from the synthesized carbohydrates using cell metabolism under anaerobic conditions [62]. Cyanobacteria are preferred for i-DbP because of their ability to use the enzymes nitrogenase and hydrogenase for hydrogen evolution, while microalgae rely solely on hydrogenase. Nitrogenase has the advantage of acting unidirectionally, while hydrogenase acts bidirectionally. The general reaction for the formation of hydrogen by cyanobacteria can be represented by the following equations [12]:
The i-DbP begins when cyanobacteria fix carbon dioxide and use sunlight to produce cell substances and oxygen [63]. NADPH generated during metabolism is transferred to the plastoquinone pool (PQ) and PSII. The electrons produced by PSII are transported by ferredoxin through PSII and PSI to hydrogenase. Hydrogenase then catalyzes the reaction where H+ is converted into renewable-H2 [62]. Nitrogen (N2) fixation is performed by the hydrogenase uptake composed of small (hupS) and large (hupL) subunits. The hydrogen produced by nitrogenase can be reabsorbed by H2-capturing hydrogenase while, simultaneously, oxygen is removed by nitrogenase. This process protects O2-sensitive enzymes and indirectly improves hydrogen production. Some filamentous cyanobacteria, such as Anabaena, Calothrix, and Nostoc, have developed heterocysts, which are micro-anaerobic environments that aid in nitrogen fixation and can enhance hydrogen production [64]. Table 2 summarizes some works using bio-photolysis for hydrogen production.

Table 2.
Summary of relevant works using bio-photolysis for hydrogen production.
The optimization of operational conditions is essential for increasing hydrogen production through bio-photolysis using microalgae or cyanobacteria. Table 3 summarizes the main factors that affect hydrogen yield from bio-photolysis.

Table 3.
Summary of the main parameters affecting hydrogen yield in bio-photolysis.
3.1.2. Fermentation
In photo-fermentation, hydrogen is generated during the degradation of organic compounds by photosynthetic bacteria, primarily purple non-sulfur bacteria (e.g., Rhodobacter sphaeroides, Rhodobacter capsulatus, Rhodobacter sulfidophilus, Rhodopseudomonas palustris, and Rhodospirillum rubrum), which are known as PNS bacteria. This process is catalyzed by nitrogenase with the help of light energy [80]. The organic substrates are metabolized through the tricarboxylic acid (TCA) cycle (also known as the citric acid cycle), which provides the necessary reducing power and carbon intermediates for hydrogen production [81]. Hydrogen production by PNS bacteria via the TCA cycle follows a series of biochemical steps: Initially, the carbon substrate undergoes oxidation to produce carbon dioxide, H+ ions, and electrons. These electrons are then transferred to nitrogenase, facilitated by the oxidation/reduction of electron carriers such as NAD(P)H and ferredoxin (Fd). Simultaneously, the ATP required for nitrogenase activity is generated by PSI in the photosynthetic membrane utilizing light energy and through ATP synthase. Subsequently, nitrogenase reduces protons (H+) to produce molecular hydrogen (H2). Additionally, uptake hydrogenase enzymes play a role in the metabolism by catalyzing the conversion of hydrogen back into electrons and protons under certain conditions [12]. Notably, one molecule of hydrogen is produced per molecule of nitrogen fixed during ammonia production [82], as seen in Equation (5).
In the absence of nitrogen, nitrogenase loses its ability to fix nitrogen and instead catalyzes an alternative reaction that produces hydrogen, as shown in Equation (6).
Under these conditions, PNS bacteria typically utilize organic acids, such as acetic acid (C2H4O2), butyric acid (C4H8O2), or lactic acid (C3H6O3), to generate hydrogen [83]. They can also utilize monosaccharides, such as glucose and polysaccharides, such as starch, for hydrogen production [63]. However, this reaction requires a significant expenditure of intracellular energy in the form of ATP molecules. While this process is energetically demanding, it is highly efficient for hydrogen production since all available protons can be converted to renewable-H2 [82].
The efficiency of photo-fermentation in the production of hydrogen is influenced by various factors, including anaerobic conditions, light intensity, temperature, pH, wavelength, substrate concentration, and type. Nitrogenase is extremely sensitive to oxygen and becomes irreversibly inactivated by it [82]. Thus, maintaining anaerobic conditions by excluding oxygen from the reaction environment enhances hydrogen production [12]. To achieve efficient hydrogen production, it is necessary to maintain the temperature between 31 and 36 °C, light intensity between 6 and 10 klux, optimal pH between 6.8 and 7.5, and a wavelength range between 400 and 1000 nm. Another critical factor is the concentration and type of substrate [84]. Studies indicate that PNS bacteria exhibit higher hydrogen production rates with fatty acid substrates, such as short-chain fatty acids and volatile fatty acids (e.g., acetate, butyrate, lactate, malate, etc.), while hydrogen production is low for sugar substrates [55,56].
Dark fermentation primarily uses anaerobic microorganisms (e.g., Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Bacillus subtillis—facultative anaerobes; and Clostridium butyricum, Clostridium acetobutylicum, Clostridium thermocellum, and Thermoanaerobacterium thermosaccharolyticum—strict anaerobes) in the absence of light, typically at mesophilic temperatures (between 25 and 45 °C) or thermophilic temperatures (45–80 °C) and sometimes at extreme thermophilic temperatures (>80 °C). In addition to hydrogen, other gases, such as carbon dioxide, carbon monoxide, and hydrogen sulfide, can be released under these conditions. A primary source of hydrogen is the glucose molecules present in carbohydrates and other feedstocks [85].
The hydrogen yield and effluent composition from dark fermentation are highly dependent on the metabolic pathways used by microorganisms. The first pathway, used by facultative anaerobes, involves pyruvate formate lyase (PFL). The second pathway, used primarily by strict anaerobes, involves pyruvate ferredoxin oxidoreductase (PFOR) [86]. During hydrogen production via glucose, complex compounds are first hydrolyzed into simpler molecules like glucose. This glucose is then anaerobically degraded to produce NADH, pyruvate, and ATP [87]. In the PFL pathway, pyruvate is converted to formate and acetyl-CoA with the help of coenzyme A (CoA-H). Formate is then oxidized to carbon dioxide and hydrogen either via the formate-H2 lyase (NiFe-hydrogenase) pathway or a formate-dependent [FeFe] hydrogenase pathway. In the PFOR pathway, pyruvate is broken down to produce reduced ferredoxin and acetyl-CoA, facilitated by ferredoxin oxidase and CoA-H. Reduced ferrodoxin is then oxidized while producing hydrogen through a ferredoxin-dependent hydrogenase (Fd-[FeFe]) [88]. Additionally, hydrogen can be produced using NADH through the reduction of ferredoxin, the reduction of a hydrogenase (NADH-[FeFe]), or the oxidation of NADH by Fd-NADH-[FeFe]. Acetyl-CoA can be further converted into acetic acid, butyric acid, or ethanol using NADH, resulting in various dark fermentation liquid effluents. Other liquid effluents, such as propionate, butanol, and lactate, can also be produced [87].
Theoretical yield of renewable-H2 produced through dark fermentation is 4 mol H2/mol glucose, with acetic acid as the final product. However, this yield is reduced to 2 mol H2/mol glucose when butyric acid is the final product, as indicated by the reactions shown in Equations (7) and (8) [62,63].
Under anaerobic conditions, the thermodynamics of acid formation favors the production of both acetic and butyric acids, resulting in their presence among the metabolites of the final dark fermentation product. Consequently, the hydrogen yield is always less than 4 mol H2/mol glucose [87].
Table 4 summarizes works on hydrogen production using both photo- and dark fermentation, highlighting the used inocula, substrates, main operating conditions, and maximum hydrogen yields.

Table 4.
Summary of relevant works using photo- and dark fermentation for hydrogen production.
The maximum production of hydrogen through photo- and dark fermentation depends on several factors, which together lead to higher yields and the greater efficiency of fermentative bacteria. Table 5 summarizes the main factors that affect hydrogen yield from photo- and dark fermentation.

Table 5.
A summary of the main parameters affecting hydrogen yield in photo- and dark fermentation.
3.1.3. Integrated Systems
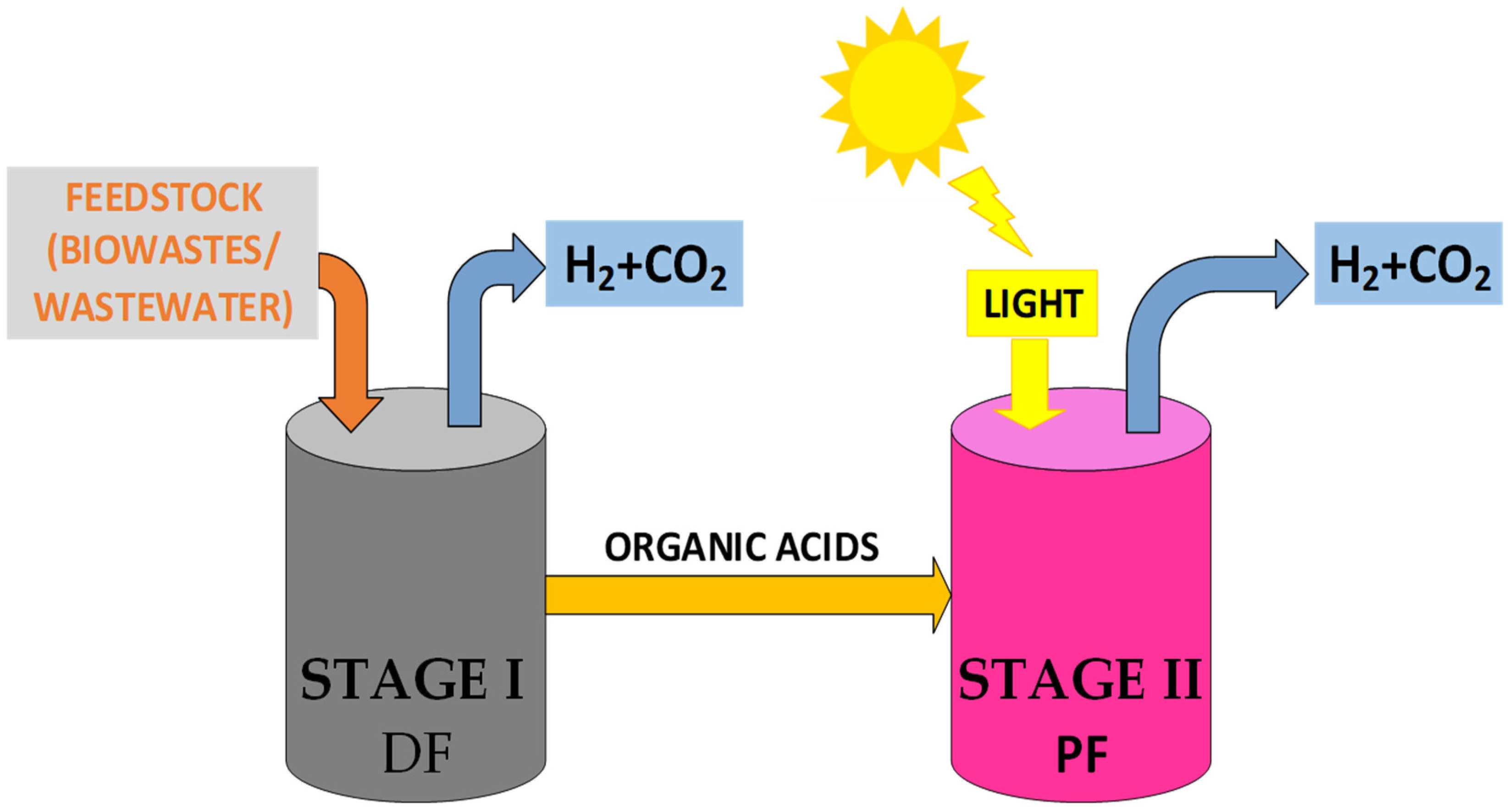
The combination of photo-fermentation and dark fermentation processes in an integrated system presents a promising technological option for hydrogen production. This approach allows for the optimal utilization of the intrinsic advantages of each process, leading to increased efficiency and sustainability. The integration of photo-fermentation and dark fermentation can occur as a single-step process, using the co-cultivation of H2-producing bacteria in dark fermentation and photosynthetic bacteria in the photo-fermentation process, or as a two-stage process, where the effluent from dark fermentation is used as a substrate for the photo-fermentation process, thereby enhancing renewable-H2 production. Due to the relatively low yields of the single-stage process, the two-stage process presents itself as more relevant. In this system, during stage I, dark fermentation bacteria break down glucose and produce hydrogen and intermediate products, mainly acetic acid (as seen in Equation (7)), which are then converted to hydrogen by photo-fermentative bacteria in stage II (Equation (9)) [78].
Based on the results of the integrated process equations, it is theoretically possible to achieve a maximum hydrogen yield of 12 mol H2/mol substrate, assuming glucose as the sole substrate in dark fermentation, where acetic acid is the predominant metabolite [87]. However, to optimize production efficiency, several parameters of the effluent from the dark fermentation process, such as concentration, composition, and pH, must be adjusted through pretreatments as well as the type of photo-fermentative bacteria used. A two-stage bioreactor with integrated dark fermentation (DF) and photo-fermentation (PF) systems is illustrated in Figure 4.
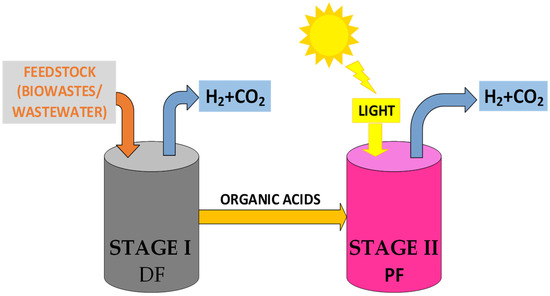
Figure 4.
A scheme of an integrated system bioreactor that combines dark fermentation (DF) and photo-fermentation (PF).
3.1.4. Advantages and Challenges of the Different Biological Methods for Renewable-H2 Production
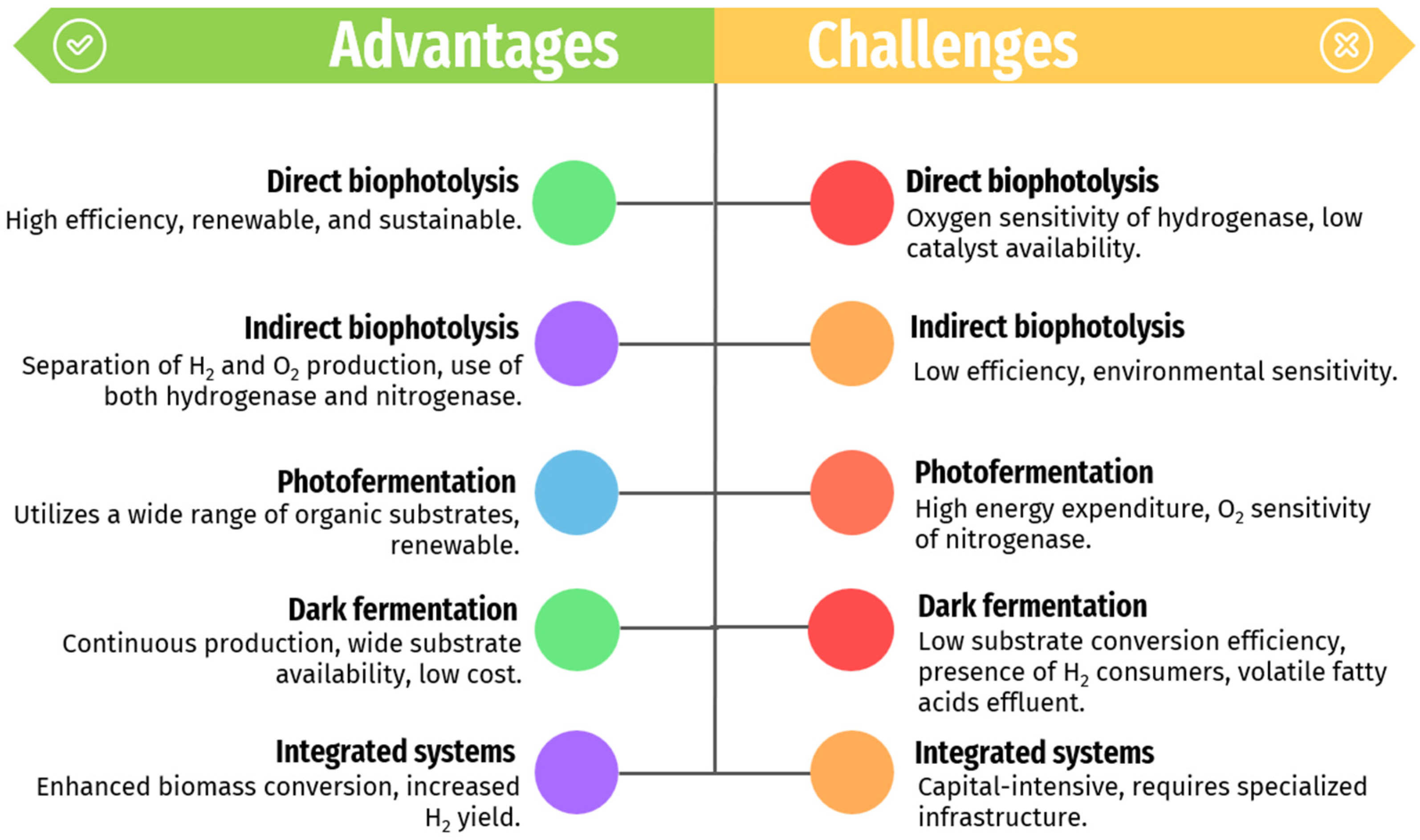
The comparison of biological methods for hydrogen production focuses on the major advantages and challenges. Each method—direct bio-photolysis (DbP), indirect bio-photolysis (i-DbP), photo-fermentation, dark fermentation, and integrated systems—offers distinct benefits and faces specific limitations (as seen in Figure 5).
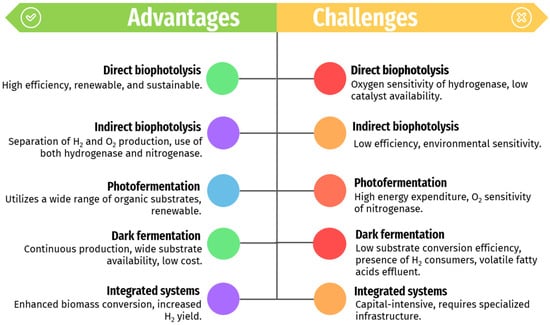
Figure 5.
The advantages and challenges related to the biological methods for renewable-H2 production mentioned in this work.
DbP demonstrates high energy efficiency, reaching up to 12.2% in green algae [107]. Comparatively, DbP generally outperforms i-DbP (max efficiency 4.1%), photo-fermentation (max efficiency 8.5%), and dark fermentation (max efficiency 12.0%) [88,107]. Despite its efficiency, DbP is hindered by oxygen produced during PSII, which strongly inhibits hydrogenases, thereby reducing hydrogen production [108]. Mitigation strategies such as inert gas purging, oxygen production blockers (e.g., copper), and sulfur deprivation have been explored [109,110].
i-DbP offers advantages such as renewability, sustainability, scalability, and integration potential with existing infrastructure. However, it suffers from low efficiency, limited catalyst availability, environmental sensitivity, and technological challenges, necessitating further research and development for improved performance and cost-effectiveness [14,59,61].
Dark fermentation is a well-established method for renewable-H2 production, using a wide range of substrate sources and operating continuously in the absence of light. It ensures stable and reliable hydrogen production at a lower cost [111]. Nevertheless, its efficiency in converting substrates into hydrogen is limited by competition from hydrogen-consuming microorganisms within the mixed microflora. These include homo-acetogens, hydrogenotrophic methanogens, sulfate-reducing bacteria, nitrate-reducing bacteria, and propionate producers, which decrease net hydrogen production. Moreover, dark fermentation generates effluents rich in volatile fatty acids (VFAs), necessitating costly treatment before discharge [87]. Integration with other biological methods like photo-fermentation offers potential solutions for enhancing overall hydrogen yield and mitigating effluent challenges. In that sense, integrated systems, which combine dark and photo-fermentation, show potential for maximizing hydrogen yield through synergistic effects [12]. However, further research and technological advancements are crucial to enhance their efficiency and scalability since implementing integrated systems can be capital-intensive and require specialized infrastructure as a primary drawback [63].
3.2. Thermochemical Methods
The thermochemical methods used for converting biomass to renewable-H2 involve applying heat and chemically transforming biomass to produce hydrogen. These methods can generate hydrogen directly from biomass or from intermediate products (e.g., methanol, ethanol) obtained from the depolymerization of biomass. Thermochemical methods include gasification, pyrolysis, and hydrothermal processes.
3.2.1. Gasification
Gasification is a thermochemical process wherein a carbonaceous substrate, such as biomass, is converted to a combustible gas at high temperatures (700 to 1200 °C). This process occurs in the presence of a gasification agent of limited concentration, including air, oxygen, steam, carbon dioxide, or their mixtures [112]. The primary product of gasification is syngas, a gaseous fuel composed predominantly of hydrogen, carbon monoxide, carbon dioxide, nitrogen, tars, char (a solid carbonaceous product), ash, and particles. Equation (10) illustrates the general equation for biomass steam gasification:
The composition and lower heating value (LHV) of syngas depend on the type of biomass and the gasification agent employed. Syngas produced using air as the gasification agent typically has an LHV ranging from 4 to 8 MJ/Nm3, while steam, oxygen, or their mixtures yield syngas with an LHV between 8 and 20 MJ/Nm3 [112].
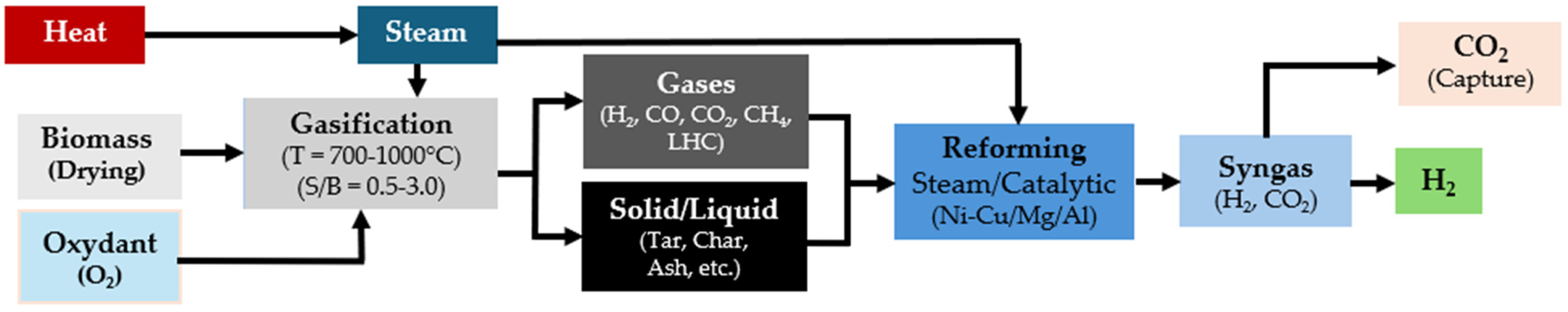
Gasification processes are classified based on the gasifier type and the heat source. Direct and autothermal gasification involves the use of air or oxygen, with a partial combustion of biomass providing process heat, while indirect and allothermal gasification uses steam with external heat sources. Indirect and allothermal gasification is preferred for hydrogen production due to its promotion of the steam reforming (SR) reaction within the syngas. This reaction enhances hydrogen yield compared to air gasification, which primarily promotes combustion and results in a high proportion of nitrogen in the syngas [113]. The SR reaction in gasification reduces the carbon-to-hydrogen (C/H) mass ratio in the syngas, improving its composition by decreasing the volume of light hydrocarbons and tar. This optimization not only increases hydrogen production but also mitigates issues such as pipe blockage and corrosion caused by the polymerization and condensation of tars [114]. Figure 6 depicts the mechanisms and stages of hydrogen production during biomass steam gasification.
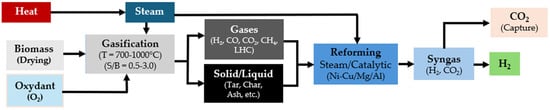
Figure 6.
Renewable-H2 production process from biomass gasification.
During steam gasification, several additional reactions occur. After the drying step, the biomass undergoes a pyrolysis reaction, which converts it into a gas rich in carbon monoxide, carbon dioxide, methane, light hydrocarbons (e.g., ethylene), char, and tar [115], as shown in Equation (11).
Tar is a complex, condensed mixture of polycyclic aromatic hydrocarbons (PAHs) and oxygenated molecules, including alcohols, phenols, and furans. Under steam gasification temperature conditions (700–1000 °C), homogeneous reactions such as cracking and steam reforming alter the structure of these oxygenated molecules. During reforming reactions, tar breaks down into carbon monoxide, hydrogen, char, and polycyclic aromatic hydrocarbons (PAH), as shown in Equation (12) [116]. PAH can further be converted to carbon monoxide and hydrogen by thermal cracking at extremely high temperatures (~1250 °C), as a part of heterogeneous reactions.
The reforming reactions occur in the presence of steam and carbon dioxide, converting light hydrocarbons like methane and ethylene into carbon monoxide and hydrogen, as illustrated in Equations (13)–(17):
During tar cracking, the light hydrocarbons and carbon oxides are generated, along with hydrogen. These compounds are stable under cracking/reforming operating conditions. Additionally, the water–gas shift (WGS) reaction converts carbon monoxide in the presence of water to a mixture of carbon dioxide and hydrogen, as shown in Equation (18):
The addition of steam to the gasification process enhances this reversible reaction by shifting it towards the production of hydrogen [21]. The WGS reaction is exothermic, and increasing the temperature shifts the equilibrium towards the reactants, resulting in the formation of carbon monoxide and water, according to Le Chatelier’s principle. Finally, heterogeneous reactions also occur, as described by Equations (19)–(22), including the oxidation of carbon and the Boudouard reaction, where carbon formed during pyrolysis is converted to carbon monoxide and carbon dioxide [114].
To enhance hydrogen production yield via steam gasification, various parameters must be optimized, including biomass characteristics, the operating temperature, the steam–biomass ratio (S/B), and catalysts [21]. Table 6 provides a summary of the impacts of these parameters.

Table 6.
A summary of the main parameters affecting hydrogen yield in biomass steam gasification.
3.2.2. Pyrolysis
Pyrolysis is a thermochemical process that involves the decomposition of biomass into solid, liquid, and gaseous fractions through the application of heat in an inert atmosphere [126]. During the pyrolysis process, the large hydrocarbon molecules present in biomass are broken down into smaller molecules through several reaction mechanisms. Hemicellulose is the first component of biomass to undergo decomposition, occurring within the temperature range of 220–315 °C, resulting in the production of acetic acid, other organic acids, sugars, and furans. Cellulose degradation occurs subsequently within the temperature range of 315–400 °C and leads to the formation of levoglucosan and other anhydrocelluloses [127]. Lignin, which contains multiple aromatic rings, is particularly challenging to degrade, and its decomposition occurs over a broad temperature range (200–900 °C), producing various compounds, including oligomers, monomers of polysubstituted phenols, hydrogen, and methane as the primary products [128].
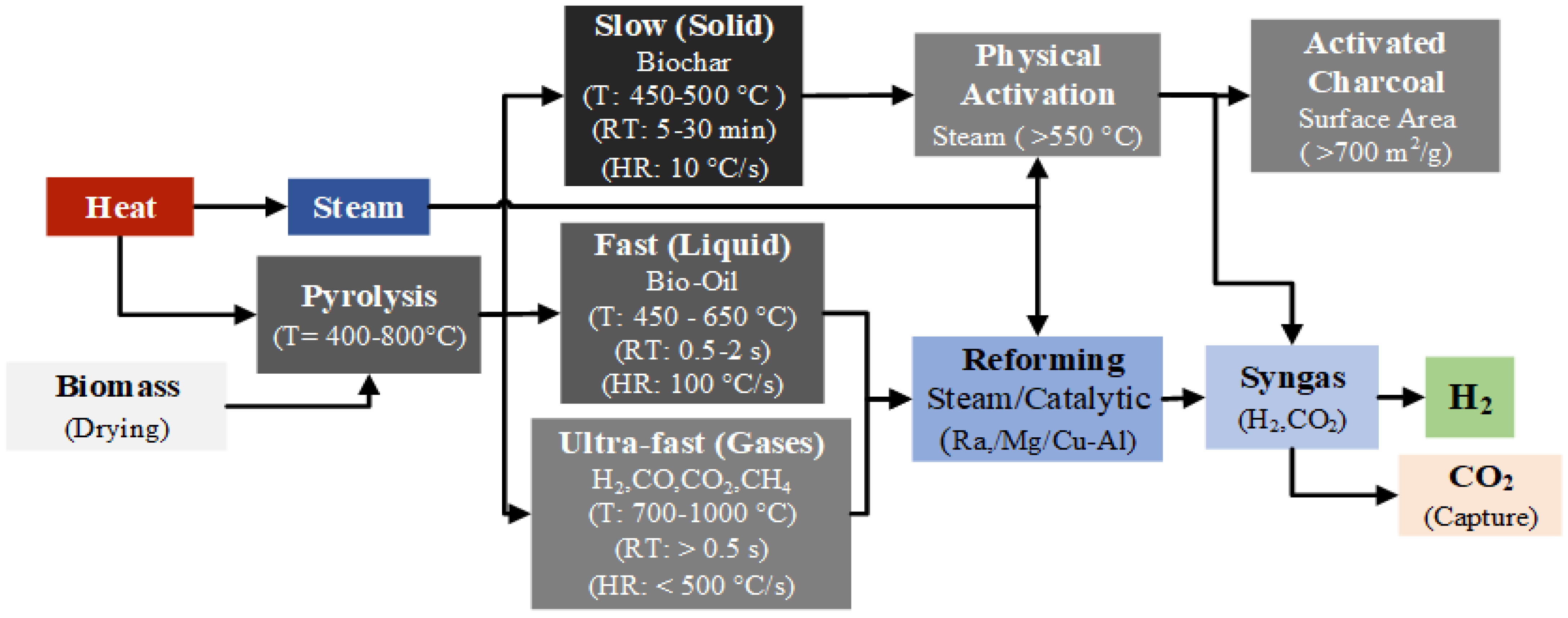
Pyrolysis can be carried out within the temperature range of 400–800 °C, and depending on the operating temperature, heating rate, and residence time, it can be classified into three types: slow pyrolysis, fast pyrolysis, and flash (ultra-fast) pyrolysis. Figure 7 shows the mechanisms and stages of hydrogen production via biomass pyrolysis, and Equation (23) presents the general pyrolysis reaction [114].
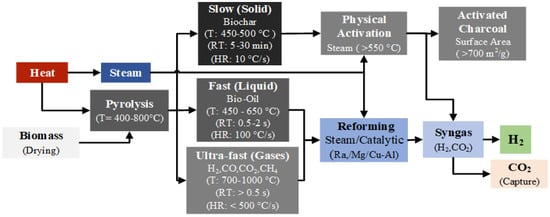
Figure 7.
Renewable-H2 and activated charcoal production process from biomass pyrolysis.
Optimizing various parameters is essential for improving hydrogen production yield through pyrolysis. These parameters include biomass characteristics, temperature, heating rate, residence time, and catalyst usage [21]. Table 7 presents the main parameters that affect hydrogen yield in the biomass pyrolysis process.

Table 7.
A summary of the main parameters affecting hydrogen yield in biomass pyrolysis.
3.2.3. Hydrothermal Processes
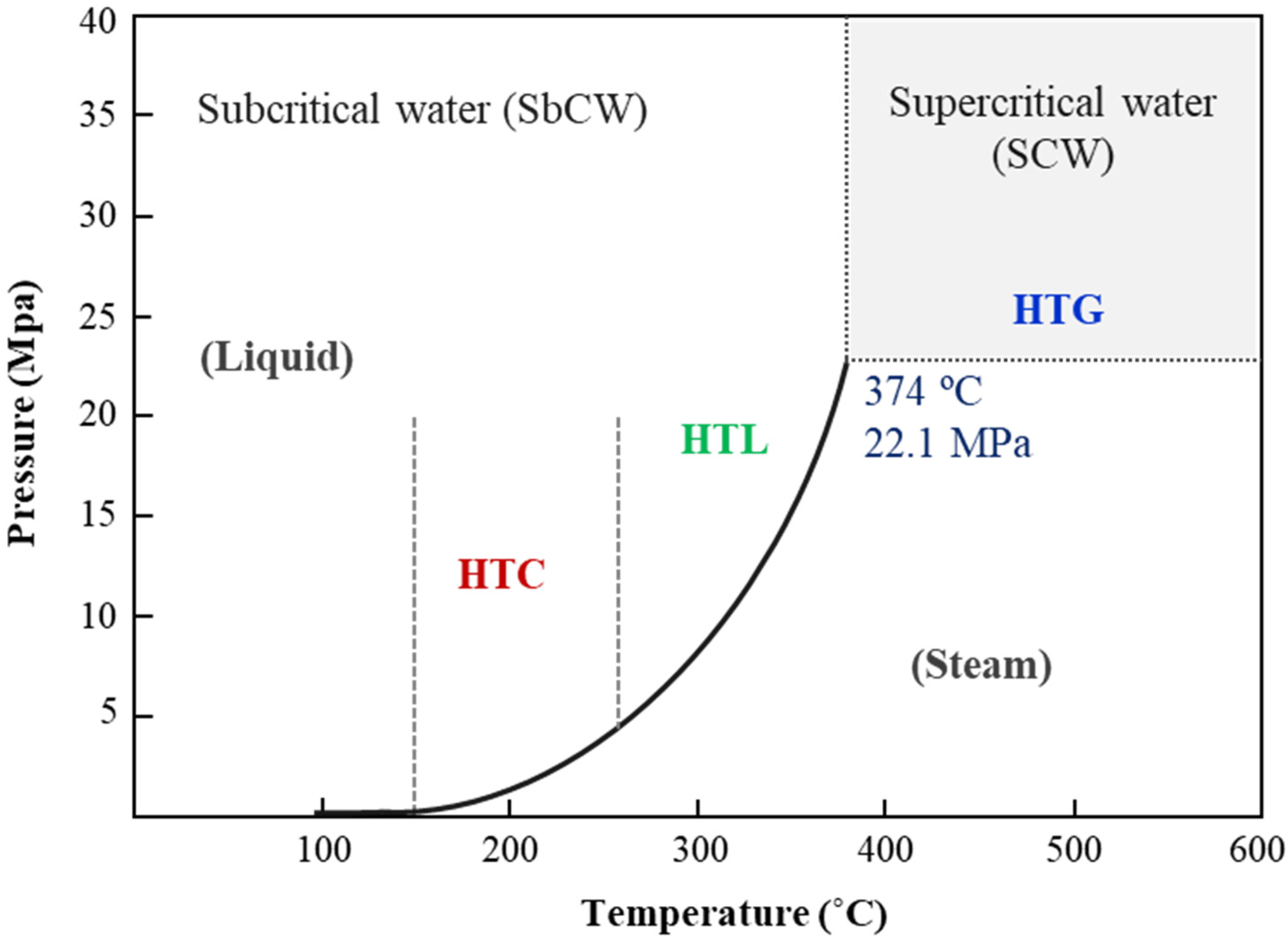
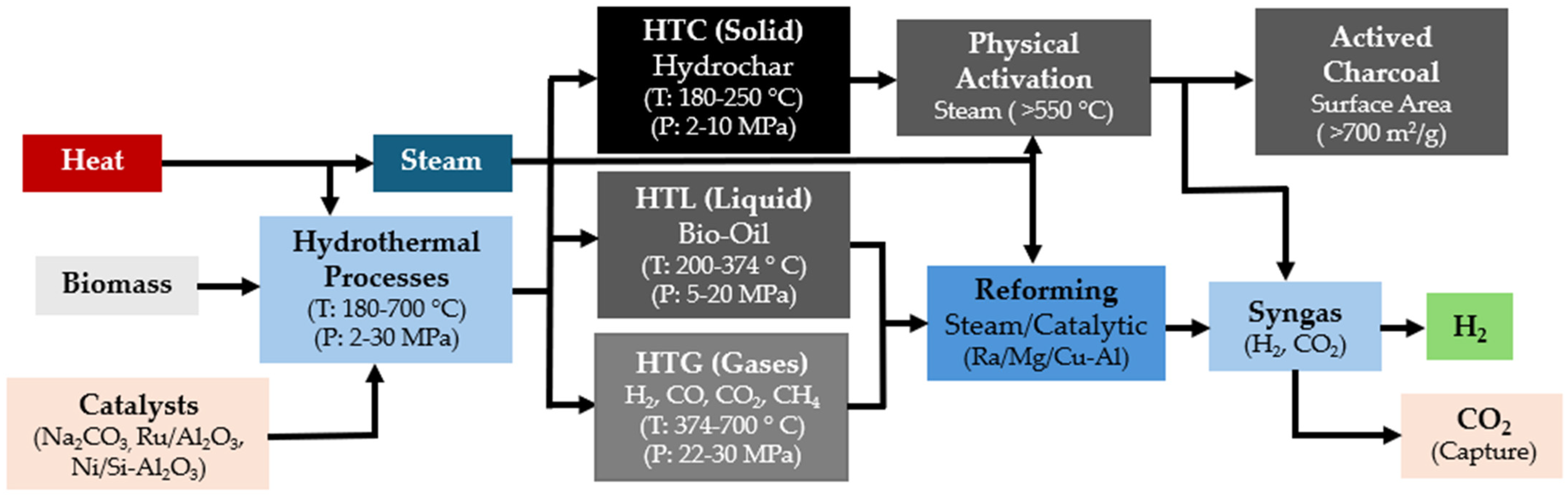
Hydrothermal processes are thermochemical methods that operate at high temperatures and pressures, exceeding the saturation pressure of water. This environment facilitates various reactions that modify the physicochemical and magnetic properties of water, including its density, dielectric constant, and ionic product. These reactions contribute to the production of energy-dense fuels and high-value chemicals. Researchers categorize hydrothermal processes into three main types based on temperature range and target products: hydrothermal carbonization (HTC), hydrothermal liquefaction (HTL), and hydrothermal gasification (HTG) [137] (Figure 8).
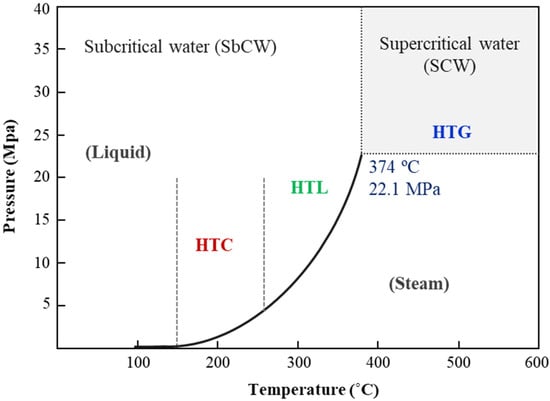
Figure 8.
Phase diagram of water illustrating the regions where hydrothermal processes occur, delineating the conditions under which hydrothermal carbonization (HTC), hydrothermal liquefaction (HTL), and hydrothermal gasification (HTG) take place.
Biomass HTC represents an efficient and ecologically favorable method for producing hydrochar, a carbon-rich solid fuel with high energy density. This process converts various biomass feedstocks into smokeless solid fuels, operating at pressures between 2 and 10 MPa and temperatures from 180 to 250 °C [138]. Hydrochar can be further processed to produce activated carbon by employing physical activation with steam at high temperatures (500–900 °C), achieving surface areas exceeding 700 m2/g [139].
On the other hand, HTL chemically transforms biomass mostly into biocrude, a type of crude oil like pyrolysis bio-oil, under conditions of high temperature (280–370 °C) and high pressure (10–25 MPa). This process also yields by-products in solid, aqueous, and gaseous phases characterized by their high energy content and enhanced heat recovery capabilities compared to other methods [140]. Further processing of biocrude through reforming techniques can convert it into biomethane and hydrogen [141].
Finally, HTG, also known as supercritical water gasification (SWG), leverages the unique properties of water in its supercritical state to function both as a solvent and a reactant. This process efficiently converts biomass into hydrogen and carbon dioxide through catalytic cracking and steam (T ≥ 374 °C, P ≥ 22 MPa) [142]. Under these conditions, water serves as an oxidant and engages in a steam reforming reaction with biomass, as depicted in Equation (24), producing hydrogen, methane, carbon monoxide, and carbon dioxide.
This initial reaction is followed by homogeneous gas–gas reactions, leading to the formation of hydrogen and carbon dioxide, as detailed in Equations (25)–(27) [142].
Figure 9 depicts the specific operating parameters essential for optimizing the conversion of biomass into these valuable gases and activated charcoal through hydrothermal processes. This figure highlights the critical temperature and pressure settings required to maximize efficiency and product yield in the biomass conversion process.
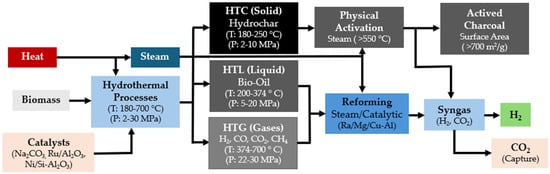
Figure 9.
Renewable-H2 production process from biomass hydrothermal processes.
As HTG (and the other hydrothermal processes) involves the use of water as both the reaction and reagent medium, it eliminates the need to dry the feedstock before the process. This results in significant reduction in energy consumption [143]. Consequently, this technique holds great promise for the gasification of biomass with high moisture content. Furthermore, it can achieve high biomass conversion rates (up to 100%) and high volumetric hydrogen content in final gaseous products (≥50%) and produce no tar or other by-products [144]. Achieving these high yields requires the optimization of several parameters, including the operating temperature, operating pressure, reagent concentration, and reaction time. Table 8 provides a summary of the effects of these key parameters.

Table 8.
A summary of the main parameters affecting hydrogen yield in biomass HTG.
3.2.4. Advantages and Challenges of the Different Thermochemical Methods for Renewable-H2 Production
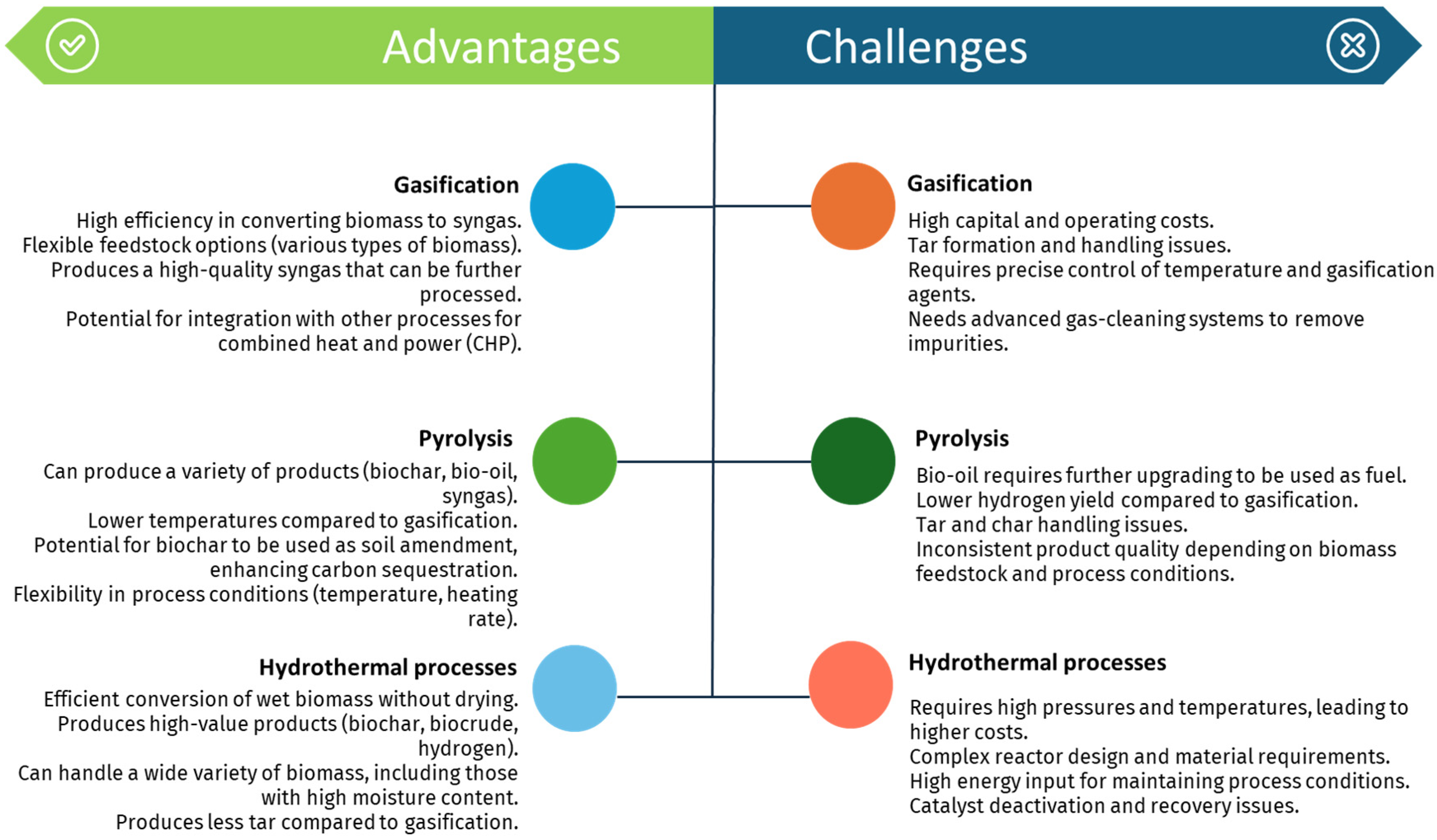
Thermochemical methods offer a versatile approach to converting biomass into renewable hydrogen, each with its unique advantages and challenges. Figure 10 summarizes the key benefits and obstacles associated with gasification, pyrolysis, and hydrothermal processes, highlighting their potential and limitations in sustainable hydrogen production.
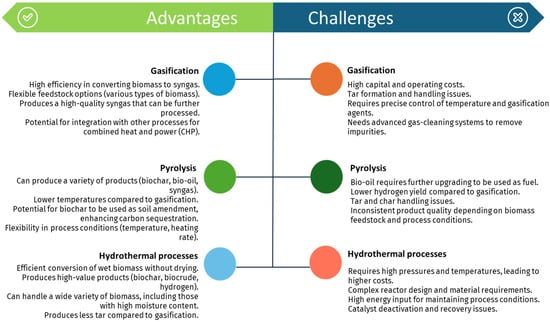
Figure 10.
Advantages and challenges related to the thermochemical methods for renewable-H2 production mentioned in this work.
Steam gasification represents an effective pathway to produce hydrogen from biomass with minimal environmental impact, capitalizing on the widespread abundance of biomass resources. This technology holds promise for sustainable hydrogen production. Nonetheless, its primary drawbacks encompass a substantial initial investment, limited energy efficiency, and significant tar formation within the syngas.
Similarly, pyrolysis, a well-established thermochemical process, has gained considerable attention for producing hydrogen through the thermal conversion of biomass. This process is highly promising for decarbonizing the economy by leveraging renewable and sustainable raw materials, offering high energy efficiency and scalability [126,148]. However, pyrolysis poses significant challenges, including issues related to hydrogen separation and purification from complex gas mixtures, minimizing GHG emissions, and managing the high costs and complexity associated with large-scale hydrogen production [149].
HTG has also emerged as a highly promising technology for hydrogen production, particularly due to its ability to directly gasify biomass with high moisture content. This method exhibits high reaction efficiency and yields substantial hydrogen production [150]. However, HTG has some drawbacks, such as high energy consumption rates and technological requirements for the equipment used, resulting in significant investment and maintenance costs [122]. As a result, scalability is limited, and no large-scale commercial system of this technology is currently known. Each of these technologies offers unique advantages and faces specific challenges, underscoring the need for continued research and development to enhance their feasibility for large-scale hydrogen production.
4. Techno-Economic Overview of Renewable-H2 Production Technologies
Table 9 presents a comparison between capital costs, hydrogen costs, production efficiencies, and Technology Readiness Levels (TRLs) for various hydrogen production technologies with varying feedstocks.

Table 9.
Comparison of different hydrogen production technologies.
The cost of producing renewable-H2 remains higher than that of grey hydrogen, potentially jeopardizing the goal of achieving net zero emissions by 2050. A comprehensive understanding of the techno-economics associated with renewable-H2 production is essential to identify the most favorable current scenarios and to improve less advantageous cases.
As seen in Table 9, renewable-H2 cost is associated with the production technology. In 2021, the levelized cost of hydrogen (LCOH) obtained from natural gas steam reforming ranged from 0.92 to 2.8 EUR/kg H2 [162]. According to the recent International Energy Agency’s hydrogen report (IEA, 2023), this route is still the cheapest option to generate hydrogen in most regions of the world compared, for instance, to the use of fossil fuels with carbon capture technologies (LCOH 1.4–3.3 EUR/kg H2) and low-carbon electricity (LCOH 3.1–11 EUR/kg H2). Although hydrogen from water electrolysis is out of the scope of this study, a techno-economic analysis (TEA) of electrolysis will also be presented below for comparative purposes since this is the state-of-the-art technology for renewable-H2 generation.
The LCOH from water electrolysis depends on the cost of renewable electricity, which, in turn, is related to the plant location. The White Paper [163] produced by the International Council on Clean Transportation (ICCT) in 2022, reported the cost of onsite hydrogen generation from electrolysis in the EU using mid-level and optimistic scenarios. According to this work, LCOH varies significantly across the EU, mostly due to different trends in the price of renewable electricity and tax fees. In 2050, green hydrogen could be produced at a cost of around 2.5–3.5 EUR/kg H2 in most of the EU countries (20 out of 26). However, for the same period, LCOH is expected to be higher in some EU member states, ranging from 4.2 EUR/kg H2 in Denmark to 5.2 EUR/kg H2 in Germany. Overall, countries with lower levelized electricity costs, such as Sweden, Finland, Portugal, and Spain, will enable lower hydrogen production prices.
Green hydrogen production costs are also related to the renewable energy source. Solar and wind hydrogen exhibit similar LCOH, always considering the best-case scenarios, and for the latter, onshore wind hydrogen production appears to be more cost-effective than offshore wind because of the lower capital expenditures (CAPEX) of onshore technologies [164]. For example, in 2020, the EU LCOH for solar hydrogen production ranged from 3.5 EUR/kg in Portugal to almost 15 EUR/kg in Norway. The LCOH for onshore wind-based hydrogen was 3–4 EUR/kg, while that coming from offshore wind ranged between 3.2 and 8.0 EUR/kg. The recent report by Hydrogen Europe [165] states average renewable hydrogen production costs in the EU in 2022 of 5.2 EUR/kg using solar PV (in Portugal) and 5.7 EUR/kg with wind power (in Austria). This document provides a good overview of the hydrogen production costs from various technologies in the EU, declaring that the production of renewable hydrogen in some European countries is already a cost-competitive alternative to traditional routes based on fossil fuels. For instance, renewable hydrogen could potentially be obtained from onshore wind at costs as low as 3.1 EUR/kg with onshore wind in Norway and Ireland and 4.4 EUR/kg from solar PV in Spain and Portugal. LCOH by methane steam reforming in the Euro-zone is estimated at 5.7 EUR/kg H2 in 2022.
The type of electrolysis technology, alkaline water electrolysis (AWE), proton exchange membrane (PEM) water electrolysis, and solid oxide electrolysis cells (SOEC), also has an impact on the LCOH. In 2022, Jang et al. [166] analyzed the cost of generating hydrogen from the three water electrolysis systems, and they calculated unit hydrogen production costs of 7.9, 7.0, and 6.6 EUR/kg H2 for PEM, AWE, and SOEC combined with waste heat, respectively.
4.1. Biological Methods
Han et al. [167] carried out a techno-economic analysis of a hydrogen production process combining solid-state fermentation and dark fermentation in 2016. This study demonstrated the economic feasibility of the proposed process with a hydrogen production cost of 2.1 EUR/m3 H2 for a payback period of 5 years and an internal rate of return (IRR) of 20.2%. In 2022, Hosseinzadeh et al. [168] estimated the average value of hydrogen production from a dark fermentation pilot plant with a processing capacity of 8–45 g H2/kg biomass at 2.1 EUR/kg H2. In 2024, Alam and Nayan [169] proposed a simulation model for renewable-H2 production via the dark fermentation of wastewater sludge. Their study showed that hydrogen can be produced from dark fermentation at 10.5 EUR/kg H2 for a biomass processing capacity of 23 t/day, which could be considerably lowered to 5.4 EUR/kg H2 by scaling up to a 500 t/day capacity.
Although anaerobic digestion (AD) is mostly used to produce biogas/biomethane, it can be also used to generate renewable-H2 through the reforming of the obtained biogas/biomethane. In 2019, Szima et al. [170] estimated an LCOH of 0.15 EUR/Nm3 for a flexible hydrogen and power co-generation plant based on the dry reforming of biomethane with low CO2 emissions. A similar technological path for the combined production of hydrogen and power was investigated by Cormos et al. [171] in 2022, showing LCOH costs of 58 EUR/MWh. The authors concluded that capital costs have the highest influence on LCOH, followed by biogas costs and plant availability factor, while operating costs have minor impacts.
Hydrogen production from cow manure through the integration of psychrophilic AD and dry methane reforming was studied by Hajizadeh et al. [172] in 2022. The process was optimized to reach the highest methane conversion and the lowest energy consumption. The economic analysis conducted by the authors indicated that hydrogen production cost depends on the hydrogen production rate, with higher production rates resulting in lower production costs. The best-case scenario was found for a plant capacity of 45.5 kg/h H2, yielding hydrogen production costs of 1.28 EUR/kg H2.
In 2023, Byun and Han [173] evaluated the economic viability of using the anaerobic digestion of food waste followed by biomethane steam reforming to generate renewable-H2. The capital cost of the biodigester is the major cost driver. This integrated process allowed for a hydrogen minimum selling price (MSP) of 24.2 EUR/kg H2, which was affected by the plant’s capacity. For the most favorable case of 2000 t H2/d processing capacity, hydrogen could be generated at an MSP of 5.5 EUR/kg H2, which is comparable with fossil-based hydrogen processes.
4.2. Thermochemical Methods
The economics of various biomass-to-biofuel scenarios were examined by Anex et al. [174] in 2010, and the authors assessed that hydrogen produced via biomass pyrolysis has the lowest production cost due to its low capital operating cost at 0.53 EUR/kg H2. In 2013, Brown et al. [175] modeled the techno-economics of corn stover fast pyrolysis for hydrogen generation and reported higher hydrogen production costs (1.9–2.8 EUR/kg H2) when compared to the previously described works. In 2014, Tan et al. [176], hydrogen MSP from biomass pyrolysis was found to be 1.64 EUR/kg H2. The research conducted by Lepage et al. [114] in 2021 compares the economic feasibility of several biomass-based technologies for hydrogen production. According to these authors, traditional natural gas steam reforming appeared to be the cheapest option, with hydrogen production costs below 0.92 EUR/kg H2, but biomass pyrolysis could yield renewable-H2 production costs going from 1.17 to 2.37 EUR/kg H2. In 2024, Li et al. [177] analyzed the production of hydrogen and biochar from corn straw pyrolysis. The authors reported that the costs related to biomass feedstock were the highest (68%), followed by catalyst costs (12%). Personnel wages and benefits accounted for 8% of the total costs, while electricity represented 4% of the total costs. Assuming an annual processing of 40,000 tons of corn straw, a maximum return of 805 million euros and an IRR of 22% in 7.3 years were estimated.
In 2018, Salkuyeh et al. [178] investigated the economic aspects associated with the production of hydrogen from biomass gasification in two reactor configurations, a fluidized bed (FB), and entrained flow (EF). The authors also explored the effect of implementing carbon capture and liquefaction units on hydrogen production costs. The FB gasification technology allowed for a more cost-effective hydrogen production process with a hydrogen MSP of 2.8 EUR/kg H2. The higher hydrogen price for the EF process (3.1 EUR/kg H2) was mostly due to the higher CAPEX of this type of gasifier. The integration of carbon capture and liquefaction systems led to increased hydrogen production costs by 3% and 11% for the FB and EF processes, respectively.
In 2022, Shaikh et al. [179] used an Aspen Plus simulation to assess the TEA of a combined cycle, biomass calcium looping gasification for the co-generation of hydrogen and electricity. The cost of hydrogen production was estimated to be 2.2 EUR/kg H2 with an IRR of 17.43% and a payback period of 7.35 years. The combined hydrogen–electricity process appeared to be more economically feasible than the production of electricity.
Other works also analyze the techno-economic feasibility of hydrogen from biomass gasification. For instance, in 2019, Wang et al. [180] reported production cost values of 0.9 EUR/kg H2, while in 2021, Lepage et al. [114] found higher hydrogen production costs (1.1–3.2 EUR/kg H2). Al-Qahtani et al. [181] compared the economics of hydrogen production from biomass gasification with and without carbon capture and storage (CCS) technology in 2021. The authors found that adding CCS resulted in an increased LCOH from 2.2 to 3.4 EUR/kg H2.
As for hydrothermal processes, the economic evaluation of hydrogen produced from the HTG of soybean straw identified an MSP of 1.79 EUR/kg H2 [182] in 2021. In 2024, Cook and Hagen [183] assessed the economics of three case studies (facilities) for hydrogen production via biomass gasification in the United States. Biomass supply costs and hydrogen transportation distance were pointed out as the most significant factors affecting the overall production costs. The best-case scenario showed the lowest transportation cost of hydrogen at 3.19 EUR/kg H2, which could increase up to 3.78 EUR/kg H2 in the less favorable scenario.
5. Environmental Impact Assessment Overview of Renewable-H2 Production Technologies
Life Cycle Assessment (LCA) is an essential tool for assessing the environmental impact of a product throughout its entire life cycle, allowing for the comparison of different production technologies. An ICCT White Paper [184] applies LCA methodology to calculate the GHG intensity of eight hydrogen production technologies, including biological pathways from various feedstocks as well as thermochemical and electrochemical routes. The study highlights that hydrogen produced from forestry biomass gasification and water electrolysis powered by renewable electricity results in lower GHG emissions. Conversely, hydrogen derived from biomethane reforming, sourced from wastewater sludge or manure anaerobic digestion, can significantly reduce GHG emissions depending on methane leakage during biomethane production. As expected, hydrogen produced from fossil fuels shows the highest GHG intensities among the analyzed technological routes, with values exceeding those of the fossil comparator.
Camacho et al. [185] carried out a detailed LCA of a combined dark fermentation/anaerobic digestion wastewater process for hydrogen generation. The authors studied different feedstocks, including wine molasses mixed with wastewater treatment plant (WWTP) sludge, cheese whey, and sugar beet molasses. For the determination of the system boundaries, cradle-to-gate and gate-to-gate approaches were applied and the following impact categories were analyzed: CC—climate change, FE—freshwater eutrophication, FRS—fossil resource scarcity, LU—land use, ME—marine eutrophication, TA—terrestrial acidification, TE—terrestrial ecotoxicity, and WC—water consumption. The authors demonstrated that the environmental impact depends on the feedstock used to obtain hydrogen. For the cradle-to-gate approach and the Midpoint analysis, sugar beet molasses exhibited the best environmental profile among the feedstocks considered, with a lower impact in four of the eight studied categories. In contrast, cheese whey showed the worst relative environmental profile. It was found that hydrogen obtained from wine molasses and WWTP sludge (9.13 kg CO2/Nm3 H2) and sugar beet molasses (3.56 kg CO2/Nm3 H2) led to a lower carbon footprint than fossil fuel hydrogen (12.08 kg CO2/Nm3 H2).
Barghash et al. [186] pointed out the beneficial effect of using solar energy in the reduction of the environmental footprint of the production of hydrogen from dark fermentation. The GHG emissions for the process carried out with and without solar energy were −1.12 × 104 kg CO2-eq and 3.13 × 104 kg CO2-eq, respectively. Zheng et al. [187] used LCA to analyze the environmental impacts of hydrogen production through the fermentation of food waste. The results indicated that electricity, hydrogen compression, and food waste transport were the main environmental contributors. The studied hydrogen production route showed lower GHG emissions (10.1 kg CO2-eq) when compared to conventional methane steam reforming (10.6 kg CO2-eq) and water electrolysis (28.4 kg CO2-eq). However, it presented a higher carbon footprint than the gasification of poplar wastes (1.5 kg CO2-eq). Nevertheless, it should be noted that biomass gasification LCA does not consider the emissions associated with hydrogen compression. Ma et al. [188] proved the positive effect of incorporating CCS technologies in hydrogen production from corn straw gasification, which can lead to negative GHG emissions. Chen et al. [189] applied LCA to estimate the environmental performance of a solar-assisted hydrothermal gasification of biomass in a pilot plant for hydrogen production. The authors detailed that system operation was the major environmental contributor, with the solar concentrator construction representing 78% of the total Global Warming Potential (GWP). The use of solar energy for heating instead of electricity allowed for a reduction in the environmental impact by 90%. Moreover, the environmental profile of biomass gasification combined with chemical looping for hydrogen generation has been recently evaluated by Wu et al. [190]. This integrated process appears to be a promising option to lower GHG emissions related to the production of renewable-H2, as evidenced by their negative GPW of −15.13 and −17.00 kg CO2-eq using air and oxygen as gasifying agents, respectively.
Overall, these studies underscore the importance of feedstock selection, energy sources, and technological integration in reducing the environmental impacts of hydrogen production from biomass. Biological methods, like dark fermentation, vary significantly based on feedstock, with some showing lower impacts. Thermochemical methods, such as biomass gasification, benefit greatly from technologies like carbon capture and solar assistance, which can reduce or even negate GHG emissions. Optimizing these factors is crucial for minimizing the environmental footprint of renewable hydrogen production.
6. Conclusions
The importance of hydrogen as a future fuel is increasingly acknowledged. Hydrogen derived from biomass represents a compelling alternative for sustainable and eco-friendly energy generation. This study reviews the predominant biological and thermochemical pathways employed for hydrogen production from biomass. Comparing different biomass-based hydrogen production is quite difficult, mostly due to the high diversity in technologies, feedstocks, operating conditions, scale, and the level of maturity.
The production of hydrogen through biomass wastes plays a crucial role in terms of the energy transition to sustainable sources and the reduction in CO2 and other greenhouse gases emissions. To achieve a 60% reduction in CO2 emissions by 2050, the combination of biological and thermochemical methods to produce H2 appears as a promising strategy to take advantage of the production potential associated with different biomass waste streams and the characteristics of each locality. Moreover, diverting organic waste streams from landfills or direct burning to H2 production decreases the carbon footprint and enhances the resource utilization efficiency. Biomass naturally captures and stores carbon dioxide, making it an effective way to remove it from the atmosphere. By valorizing biomass residues through biological and/or thermal methods, clean and renewable energy, and commercially valuable chemicals can be produced with neutral carbon dioxide emissions.
The most utilized biological methods encompass direct and indirect bio-photolysis, photo-fermentation, and dark fermentation. These methods offer a promising alternative to obtaining renewable hydrogen with low energy consumption since they occur at ambient pressure and temperature, also helping with organic waste recycling. Biological hydrogen production technologies are not so affected by plant size as thermochemical processes but face other limitations. Low hydrogen production rates and strict operating requirements of these processes, such as light intensity, temperature, water, pH, an absence of oxygen, substrate concentration, and type are the main drawbacks of biological routes. Adding metal-based nanoparticles, microbes’ immobilization, the genetic modification of microorganisms, and improved bioreactor designs are some of the considered approaches to circumvent these limitations and enhance the hydrogen productivity of biological processes. Despite the intensive research in this field, the scale-up of biological technologies for hydrogen production depends largely on developing innovative solutions to improve the efficiency and productivity of these processes.
One of the major factors influencing a thermochemical plant’s profitability is the type of biomass used since its composition, namely its energy content, directly impacts the amount of hydrogen produced. Clearly, the higher the energy content, the higher the hydrogen production and the lower the break-even point of the facility. Biomass purchase prices, which vary with the type and location of biomass or biomass mix, also affect the hydrogen production cost and, consequently, the project’s financial performance. Strategies to reduce costs and improve profitability and sustainability include using residual biomass and wastes locally sourced and highly available and identifying synergies with other sectors (e.g., agro-industrial, forestry, waste management).
Thermochemical biomass-to-hydrogen projects must also cope with economic constraints directly related to plant processing capacity. Depending on the technology, there will be a size plant ceiling, below which the project will not be economically viable. As a rule of thumb, economies of scale apply, turning more cost-effective, larger biomass thermochemical processing plants. However, these processes are context-sensitive; thus, case studies are always recommended to obtain a more realistic perspective on the techno-economic feasibility of the project.
Considering merely the economic factors, technological maturity level, and hydrogen production, conventional gasification is the most suitable way to obtain renewable hydrogen from biomass to date. Hydrothermal gasification is likely the best technology to produce hydrogen from biomass (particularly high moisture feedstocks) in the coming years, as it offers the benefits of high and cost-competitive hydrogen production combined with high efficiency.
Overall, thermochemical processes represent effective solutions for centralized hydrogen generation at a large scale, while biological pathways are more convenient for local production at a small scale.
Author Contributions
Conceptualization, J.R.C.R., P.B., and C.N.; methodology, J.R.C.R.; validation, C.M.-P., B.R., and C.N.; formal analysis, J.R.C.R., B.R., A.L., C.N., P.B., P.F., and C.M.-P.; investigation, J.R.C.R.; writing—original draft preparation, J.R.C.R., C.N., A.L., B.R., and C.M.-P.; writing—review and editing, J.R.C.R., C.N., A.L., B.R., and C.M.-P.; visualization, J.R.C.R., C.M.-P., and C.N.; supervision, P.B. and P.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by project RESIST (European Union’s Horizon Europe research and innovation program) under grant agreement no. 101093968 and by the Portuguese Foundation for Science and Technology (FCT) under project UIDB/05064/2020 (VALORIZA—Research Centre for Endogenous Resource Valorization).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Salahshoor, S.; Afzal, S. Subsurface Technologies for Hydrogen Production from Fossil Fuel Resources: A Review and Techno-Economic Analysis. Int. J. Hydrogen Energy 2022. [Google Scholar] [CrossRef]
- Gao, F.Y.; Yu, P.C.; Gao, M.R. Seawater Electrolysis Technologies for Green Hydrogen Production: Challenges and Opportunities. Curr. Opin. Chem. Eng. 2022, 36, 100827. [Google Scholar] [CrossRef]
- Koneczna, R.; Cader, J. Towards Effective Monitoring of Hydrogen Economy Development: A European Perspective. Int. J. Hydrogen Energy 2024, 59, 430–446. [Google Scholar] [CrossRef]
- Dou, Y.; Sun, L.; Ren, J.; Dong, L. Chapter 17—Opportunities and Future Challenges in Hydrogen Economy for Sustainable Development. In Hydrogen Economy, 2nd ed.; Scipioni, A., Manzardo, A., Ren, J., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 537–569. ISBN 978-0-323-99514-6. [Google Scholar]
- Hermesmann, M.; Müller, T.E. Green, Turquoise, Blue, or Grey? Environmentally Friendly Hydrogen Production in Transforming Energy Systems. Prog. Energy Combust. Sci. 2022, 90, 100996. [Google Scholar] [CrossRef]
- Eloffy, M.G.; Elgarahy, A.M.; Saber, A.N.; Hammad, A.; El-Sherif, D.M.; Shehata, M.; Mohsen, A.; Elwakeel, K.Z. Biomass-to-Sustainable Biohydrogen: Insights into the Production Routes, and Technical Challenges. Chem. Eng. J. Adv. 2022, 12, 100410. [Google Scholar] [CrossRef]
- Mosca, L.; Jimenez, J.A.M.; Wassie, S.A.; Gallucci, F.; Palo, E.; Colozzi, M.; Taraschi, S.; Galdieri, G.; Medrano Jimenez, J.A.; Wassie, S.A.; et al. Process Design for Green Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 7266–7277. [Google Scholar] [CrossRef]
- Pal, D.B.; Singh, A.; Bhatnagar, A. A Review on Biomass Based Hydrogen Production Technologies. Int. J. Hydrogen Energy 2022, 47, 1461–1480. [Google Scholar] [CrossRef]
- Blay-Roger, R.; Bach, W.; Bobadilla, L.F.; Reina, T.R.; Odriozola, J.A.; Amils, R.; Blay, V. Natural Hydrogen in the Energy Transition: Fundamentals, Promise, and Enigmas. Renew. Sustain. Energy Rev. 2024, 189, 113888. [Google Scholar] [CrossRef]
- Ciechanowska, M. Hydrogen Strategy for a Climate-Neutral Europe. Nafta-Gaz 2020, 2020, 951–954. [Google Scholar] [CrossRef]
- European Commission. REPowerEU: A Plan to Rapidly Reduce Dependence on Russian Fossil Fuels and Fast Forward the Green Transition; European Commission: Brussels, Belgium, 2022; pp. 1–11. [Google Scholar]
- Akhlaghi, N.; Najafpour-Darzi, G. A Comprehensive Review on Biological Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Mudhoo, A.; Forster-Carneiro, T.; Sánchez, A. Biohydrogen Production and Bioprocess Enhancement: A Review. Crit. Rev. Biotechnol. 2011, 31, 250–263. [Google Scholar] [CrossRef]
- Singh, A.; Sevda, S.; Reesh, I.M.A.; Vanbroekhoven, K.; Rathore, D.; Pant, D.; Abu Reesh, I.M.; Vanbroekhoven, K.; Rathore, D.; Pant, D. Biohydrogen Production from Lignocellulosic Biomass: Technology and Sustainability. Energies 2015, 8, 13062–13080. [Google Scholar] [CrossRef]
- Chong, C.C.; Cheng, Y.W.; Ng, K.H.; Vo, D.V.N.; Lam, M.K.; Lim, J.W. Bio-Hydrogen Production from Steam Reforming of Liquid Biomass Wastes and Biomass-Derived Oxygenates: A Review. Fuel 2022, 311, 122623. [Google Scholar] [CrossRef]
- Valle, B.; Aramburu, B.; Benito, P.L.; Bilbao, J.; Gayubo, A.G. Biomass to Hydrogen-Rich Gas via Steam Reforming of Raw Bio-Oil over Ni/La2O3-αAl2O3 Catalyst: Effect of Space-Time and Steam-to-Carbon Ratio. Fuel 2018, 216, 445–455. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Liang, D.T.; Zheng, C. Pyrolysis of Palm Oil Wastes for Enhanced Production of Hydrogen Rich Gases. Fuel Process. Technol. 2006, 87, 935–942. [Google Scholar] [CrossRef]
- Fahmy, T.Y.A.; Fahmy, Y.; Mobarak, F.; El-Sakhawy, M.; Abou-Zeid, R.E. Biomass Pyrolysis: Past, Present, and Future. Environ. Dev. Sustain. 2020, 22, 17–32. [Google Scholar] [CrossRef]
- Valliyappan, T.; Bakhshi, N.N.; Dalai, A.K. Pyrolysis of Glycerol for the Production of Hydrogen or Syn Gas. Bioresour. Technol. 2008, 99, 4476–4483. [Google Scholar] [CrossRef] [PubMed]
- Kaewpanha, M.; Guan, G.; Ma, Y.; Hao, X.; Zhang, Z.; Reubroychareon, P.; Kusakabe, K.; Abudula, A. Hydrogen Production by Steam Reforming of Biomass Tar over Biomass Char Supported Molybdenum Carbide Catalyst. Int. J. Hydrogen Energy 2015, 40, 7974–7982. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, K.S. Hydrogen Production from Steam Gasification of Biomass: Influence of Process Parameters on Hydrogen Yield—A Review. Renew. Energy 2014, 66, 570–579. [Google Scholar] [CrossRef]
- Tijmensen, M.J.A.; Faaij, A.P.C.; Hamelinck, C.N.; Van Hardeveld, M.R.M. Exploration of the Possibilities for Production of Fischer Tropsch Liquids and Power via Biomass Gasification. Biomass Bioenergy 2002, 23, 129–152. [Google Scholar] [CrossRef]
- Florin, N.H.; Harris, A.T. Enhanced Hydrogen Production from Biomass with In Situ Carbon Dioxide Capture Using Calcium Oxide Sorbents. Chem. Eng. Sci. 2008, 63, 287–316. [Google Scholar] [CrossRef]
- Shahbaz, M.; Yusup, S.; Inayat, A.; Patrick, D.O.; Partama, A. System Analysis of Poly-Generation of SNG, Power and District Heating from Biomass Gasification System. Chem. Eng. Trans. 2016, 52, 781–786. [Google Scholar] [CrossRef]
- Ghiat, I.; AlNouss, A.; McKay, G.; Al-Ansari, T. Biomass-Based Integrated Gasification Combined Cycle with Post-Combustion CO2 Recovery by Potassium Carbonate: Techno-Economic and Environmental Analysis. Comput. Chem. Eng. 2020, 135, 106758. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, C.; Lian, X.; Feng, H.; Sun, J.; Wang, L.; Jin, H. Catalytic Supercritical Water Gasification of Glucose with In-Situ Generated Nickel Nanoparticles for Hydrogen Production. Int. J. Hydrogen Energy 2019, 44, 21020–21029. [Google Scholar] [CrossRef]
- Sheikhdavoodi, M.J.; Almassi, M.; Ebrahimi-Nik, M.; Kruse, A.; Bahrami, H. Gasification of Sugarcane Bagasse in Supercritical Water; Evaluation of Alkali Catalysts for Maximum Hydrogen Production. J. Energy Inst. 2015, 88, 450–458. [Google Scholar] [CrossRef]
- K\ipçak, E.; Akgün, M. Hydrogen Production by Supercritical Water Gasification of Biomass. In Production of Hydrogen from Renewable Resources; Fang, Z., Smith, R.L., Jr., Qi, X., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 179–220. ISBN 978-94-017-7330-0. [Google Scholar]
- Reddy, S.N.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical Water Gasification of Biomass for Hydrogen Production. Int. J. Hydrogen Energy 2014, 39, 6912–6926. [Google Scholar] [CrossRef]
- Shahbaz, M.; Al-Ansari, T.; Aslam, M.; Khan, Z.; Inayat, A.; Athar, M.; Naqvi, S.R.; Ahmed, M.A.; McKay, G. A State of the Art Review on Biomass Processing and Conversion Technologies to Produce Hydrogen and Its Recovery via Membrane Separation. Int. J. Hydrogen Energy 2020, 45, 15166–15195. [Google Scholar] [CrossRef]
- UNFCCC. Clarifications on Definition of Biomass and Considerations of Changes in Carbon Pools Due to a CDN Project Activity; 2005; p. 1. Available online: https://cdm.unfccc.int/EB/020/eb20repan08.pdf (accessed on 10 July 2024).
- Chowdhury, R.; Ghosh, S.; Debnath, B.; Manna, D. Indian Agro-Wastes for 2G Biorefineries: Strategic Decision on Conversion Processes. In Sustainable Energy Technology and Policies: A Transformational Journey; De, S., Bandyopadhyay, S., Assadi, M., Mukherjee, D.A., Eds.; Springer: Singapore, 2018; Volume 1, pp. 353–373. ISBN 978-981-10-7188-1. [Google Scholar]
- Kalak, T. Potential Use of Industrial Biomass Waste as a Sustainable Energy Source in the Future. Energies 2023, 16, 1783. [Google Scholar] [CrossRef]
- Sivabalan, K.; Hassan, S.; Ya, H.; Pasupuleti, J. A Review on the Characteristic of Biomass and Classification of Bioenergy through Direct Combustion and Gasification as an Alternative Power Supply. J. Phys. Conf. Ser. 2021, 1831, 012033. [Google Scholar] [CrossRef]
- Tursi, A. A Review on Biomass: Importance, Chemistry, Classification, and Conversion. Biofuel Res. J. 2019, 6, 962–979. [Google Scholar] [CrossRef]
- Abreu, M.; Silva, L.; Ribeiro, B.; Ferreira, A.; Alves, L.; Paixão, S.M.; Gouveia, L.; Moura, P.; Carvalheiro, F.; Duarte, L.C.; et al. Low Indirect Land Use Change (ILUC) Energy Crops to Bioenergy and Biofuels—A Review. Energies 2022, 15, 4348. [Google Scholar] [CrossRef]
- Musharavati, F.; Ahmad, A.; Javed, M.H.; Sajid, K.; Nizami, A.S. Advancing Biohydrogen Production from Organic Fraction of Municipal Solid Waste through Thermal Liquefaction. Int. J. Hydrogen Energy 2024. [Google Scholar] [CrossRef]
- Khan, A.H.; López-Maldonado, E.A.; Alam, S.S.; Khan, N.A.; López, J.R.L.; Herrera, P.F.M.; Abutaleb, A.; Ahmed, S.; Singh, L. Municipal Solid Waste Generation and the Current State of Waste-to-Energy Potential: State of Art Review. Energy Convers. Manag. 2022, 267, 115905. [Google Scholar] [CrossRef]
- Ayeleru, O.O.; Okonta, F.N.; Ntuli, F. Municipal Solid Waste Generation and Characterization in the City of Johannesburg: A Pathway for the Implementation of Zero Waste. Waste Manag. 2018, 79, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Si, B.; Lu, J.; Watson, J.; Zhang, Y.; Liu, Z. Elemental Migration and Characterization of Products during Hydrothermal Liquefaction of Cornstalk. Bioresour. Technol. 2017, 243, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Watson, J.; Liu, Z.; Wu, Y. Elemental Migration and Transformation during Hydrothermal Liquefaction of Biomass. J. Hazard. Mater. 2022, 423, 126961. [Google Scholar] [CrossRef] [PubMed]
- Mathanker, A.; Das, S.; Pudasainee, D.; Khan, M.; Kumar, A.; Gupta, R. A Review of Hydrothermal Liquefaction of Biomass for Biofuels Production with a Special Focus on the Effect of Process Parameters, Co-Solvents, and Extraction Solvents. Energies 2021, 14, 4916. [Google Scholar] [CrossRef]
- Wang, F.; Chang, Z.; Duan, P.; Yan, W.; Xu, Y.; Zhang, L.; Miao, J.; Fan, Y. Hydrothermal Liquefaction of Litsea Cubeba Seed to Produce Bio-Oils. Bioresour. Technol. 2013, 149, 509–515. [Google Scholar] [CrossRef]
- Pio, D.T.; Tarelho, L.A.C. Empirical and Chemical Equilibrium Modelling for Prediction of Biomass Gasification Products in Bubbling Fluidized Beds. Energy 2020, 202, 117654. [Google Scholar] [CrossRef]
- Lu, J.; Li, H.; Zhang, Y.; Liu, Z. Nitrogen Migration and Transformation during Hydrothermal Liquefaction of Livestock Manures. ACS Sustain. Chem. Eng. 2018, 6, 13570–13578. [Google Scholar] [CrossRef]
- Cheikhwafa, J.; Glińska, K.; Torrens, E.; Bengoa, C. Effect of Temperature on Hydrothermal Liquefaction of High Lipids and Carbohydrates Content Municipal Primary Sludge. Heliyon 2024, 10, E24731. [Google Scholar] [CrossRef] [PubMed]
- Nazem, M.A.; Tavakoli, O. Bio-Oil Production from Refinery Oily Sludge Using Hydrothermal Liquefaction Technology. J. Supercrit. Fluids 2017, 127, 33–40. [Google Scholar] [CrossRef]
- Copa, J.R.; Tuna, C.E.; Silveira, J.L.; Boloy, R.A.M.; Brito, P.; Silva, V.; Cardoso, J.; Eusébio, D. Techno-Economic Assessment of the Use of Syngas Generated from Biomass to Feed an Internal Combustion Engine. Energies 2020, 13, 3097. [Google Scholar] [CrossRef]
- Kossalbayev, B.D.; Yilmaz, G.; Sadvakasova, A.K.; Zayadan, B.K.; Belkozhayev, A.M.; Kamshybayeva, G.K.; Sainova, G.A.; Bozieva, A.M.; Alharby, H.F.; Tomo, T.; et al. Biotechnological Production of Hydrogen: Design Features of Photobioreactors and Improvement of Conditions for Cultivating Cyanobacteria. Int. J. Hydrogen Energy 2024, 49, 413–432. [Google Scholar] [CrossRef]
- Trchounian, K.; Trchounian, A. Hydrogen Production from Glycerol by Escherichia coli and Other Bacteria: An Overview and Perspectives. Appl. Energy 2015, 156, 174–184. [Google Scholar] [CrossRef]
- Cheng, J.; Li, H.; Ding, L.; Zhou, J.; Song, W.; Li, Y.Y.; Lin, R. Improving Hydrogen and Methane Co-Generation in Cascading Dark Fermentation and Anaerobic Digestion: The Effect of Magnetite Nanoparticles on Microbial Electron Transfer and Syntrophism. Chem. Eng. J. 2020, 397, 125394. [Google Scholar] [CrossRef]
- Gutekunst, K.; Chen, X.; Schreiber, K.; Kaspar, U.; Makam, S.; Appel, J. The Bidirectional NiFe-Hydrogenase in Synechocystis sp. PCC 6803 Is Reduced by Flavodoxin and Ferredoxin and Is Essential under Mixotrophic, Nitrate-Limiting Conditions. J. Biol. Chem. 2014, 289, 1930–1937. [Google Scholar] [CrossRef]
- Márquez-Reyes, L.A.; del Pilar Sánchez-Saavedra, M.; Valdez-Vazquez, I. Improvement of Hydrogen Production by Reduction of the Photosynthetic Oxygen in Microalgae Cultures of Chlamydomonas gloeopara and Scenedesmus obliquus. Int. J. Hydrogen Energy 2015, 40, 7291–7300. [Google Scholar] [CrossRef]
- Torzillo, G.; Scoma, A.; Faraloni, C.; Ena, A.; Johanningmeier, U. Increased Hydrogen Photoproduction by Means of a Sulfur-Deprived Chlamydomonas reinhardtii D1 Protein Mutant. Int. J. Hydrogen Energy 2009, 34, 4529–4536. [Google Scholar] [CrossRef]
- Rashid, N.; Lee, K.; Mahmood, Q. Bio-Hydrogen Production by Chlorella vulgaris under Diverse Photoperiods. Bioresour. Technol. 2011, 102, 2101–2104. [Google Scholar] [CrossRef]
- Paramesh, K.; Chandrasekhar, T. Improvement of Photobiological Hydrogen Production in Chlorococcum minutum Using Various Oxygen Scavengers. Int. J. Hydrogen Energy 2020, 45, 7641–7646. [Google Scholar] [CrossRef]
- Mona, S.; Kaushik, A.; Kaushik, C.P. Hydrogen Production and Metal-Dye Bioremoval by a Nostoc linckia Strain Isolated from Textile Mill Oxidation Pond. Bioresour. Technol. 2011, 102, 3200–3205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sherman, D.M.; Shermana, L.A. The Uptake Hydrogenase in the Unicellular Diazotrophic Cyanobacterium Cyanothece sp. Strain PCC 7822 Protects Nitrogenase from Oxygen Toxicity. J. Bacteriol. 2014, 196, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, E.; Melis, A. Photobiological Hydrogen Production: Recent Advances and State of the Art. Bioresour. Technol. 2011, 102, 8403–8413. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.A.; Zafar, A.M.; Aly Hassan, A.; Zaidi, A.A.; Farooq, M.; El Badawy, A.; Lundquist, T.; Mohamed, M.M.A.; Al-Zuhair, S. The Role of Oxygen Regulation and Algal Growth Parameters in Hydrogen Production via Biophotolysis. J. Environ. Chem. Eng. 2022, 10, 107003. [Google Scholar] [CrossRef]
- Azwar, M.Y.; Hussain, M.A.; Abdul-Wahab, A.K. Development of Biohydrogen Production by Photobiological, Fermentation and Electrochemical Processes: A Review. Renew. Sustain. Energy Rev. 2014, 31, 158–173. [Google Scholar] [CrossRef]
- Das, D.; Veziroglu, T.N. Advances in Biological Hydrogen Production Processes. Int. J. Hydrogen Energy 2008, 33, 6046–6057. [Google Scholar] [CrossRef]
- Xu, X.; Zhou, Q.; Yu, D. The Future of Hydrogen Energy: Bio-Hydrogen Production Technology. Int. J. Hydrogen Energy 2022, 47, 33677–33698. [Google Scholar] [CrossRef]
- Jiménez-Llanos, J.; Ramírez-Carmona, M.; Rendón-Castrillón, L.; Ocampo-López, C. Sustainable Biohydrogen Production by Chlorella sp. Microalgae: A Review. Int. J. Hydrogen Energy 2020, 45, 8310–8328. [Google Scholar] [CrossRef]
- Song, W.; Rashid, N.; Choi, W.; Lee, K. Biohydrogen Production by Immobilized Chlorella sp. Using Cycles of Oxygenic Photosynthesis and Anaerobiosis. Bioresour. Technol. 2011, 102, 8676–8681. [Google Scholar] [CrossRef]
- Sengmee, D.; Cheirsilp, B.; Suksaroge, T.T.; Prasertsan, P. Biophotolysis-Based Hydrogen and Lipid Production by Oleaginous Microalgae Using Crude Glycerol as Exogenous Carbon Source. Int. J. Hydrogen Energy 2017, 42, 1970–1976. [Google Scholar] [CrossRef]
- Pyo Kim, J.; Duk Kang, C.; Hyun Park, T.; Sun Kim, M.; Jun Sim, S. Enhanced Hydrogen Production by Controlling Light Intensity in Sulfur-Deprived Chlamydomonas reinhardtii Culture. Int. J. Hydrogen Energy 2006, 31, 1585–1590. [Google Scholar] [CrossRef]
- Bala Amutha, K.; Murugesan, A.G. Biological Hydrogen Production by the Algal Biomass Chlorella vulgaris MSU 01 Strain Isolated from Pond Sediment. Bioresour. Technol. 2011, 102, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Chader, S.; Hacene, H.; Agathos, S.N. Study of Hydrogen Production by Three Strains of Chlorella Isolated from the Soil in the Algerian Sahara. Int. J. Hydrogen Energy 2009, 34, 4941–4946. [Google Scholar] [CrossRef]
- Hong, M.E.; Shin, Y.S.; Kim, B.W.; Sim, S.J. Autotrophic Hydrogen Photoproduction by Operation of Carbon-Concentrating Mechanism in Chlamydomonas reinhardtii under Sulfur Deprivation Condition. J. Biotechnol. 2016, 221, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Huesemann, M.H.; Hausmann, T.S.; Carter, B.M.; Gerschler, J.J.; Benemann, J.R. Hydrogen Generation Through Indirect Biophotolysis in Batch Cultures of the Nonheterocystous Nitrogen-Fixing Cyanobacterium Plectonema boryanum. Appl. Biochem. Biotechnol. 2010, 162, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Vargas, S.R.; dos Santos, P.V.; Giraldi, L.A.; Zaiat, M.; Calijuri, M.d.C. Anaerobic Phototrophic Processes of Hydrogen Production by Different Strains of Microalgae Chlamydomonas sp. FEMS Microbiol. Lett. 2018, 365, fny073. [Google Scholar] [CrossRef] [PubMed]
- Vargas, S.R.; Zaiat, M.; Calijuri, M.d.C. Influence of Culture Age, Ammonium and Organic Carbon in Hydrogen Production and Nutrient Removal by Anabaena sp. in Nitrogen-Limited Cultures. Int. J. Hydrogen Energy 2020, 45, 30222–30231. [Google Scholar] [CrossRef]
- Raksajit, W.; Satchasataporn, K.; Lehto, K.; Mäenpää, P.; Incharoensakdi, A. Enhancement of Hydrogen Production by the Filamentous Non-Heterocystous Cyanobacterium Arthrospira sp. PCC 8005. Int. J. Hydrogen Energy 2012, 37, 18791–18797. [Google Scholar] [CrossRef]
- Vargas, S.R.; Zaiat, M.; Calijuri, M.d.C. Chlamydomonas Strains Respond Differently to Photoproduction of Hydrogen and By-Products and Nutrient Uptake in Sulfur-Deprived Cultures. J. Environ. Chem. Eng. 2021, 9, 105930. [Google Scholar] [CrossRef]
- Kaushik, A.; Anjana, K. Biohydrogen Production by Lyngbya Perelegans: Influence of Physico-Chemical Environment. Biomass Bioenergy 2011, 35, 1041–1045. [Google Scholar] [CrossRef]
- Tiwari, A.; Pandey, A. Cyanobacterial Hydrogen Production—A Step towards Clean Environment. Int. J. Hydrogen Energy 2012, 37, 139–150. [Google Scholar] [CrossRef]
- Kossalbayev, B.D.; Tomo, T.; Zayadan, B.K.; Sadvakasova, A.K.; Bolatkhan, K.; Alwasel, S.; Allakhverdiev, S.I. Determination of the Potential of Cyanobacterial Strains for Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 2627–2639. [Google Scholar] [CrossRef]
- Tamburic, B.; Zemichael, F.W.; Maitland, G.C.; Hellgardt, K. Parameters Affecting the Growth and Hydrogen Production of the Green Alga Chlamydomonas reinhardtii. Int. J. Hydrogen Energy 2011, 36, 7872–7876. [Google Scholar] [CrossRef]
- Mishra, P.; Krishnan, S.; Rana, S.; Singh, L.; Sakinah, M.; Ab Wahid, Z. Outlook of Fermentative Hydrogen Production Techniques: An Overview of Dark, Photo and Integrated Dark-Photo Fermentative Approach to Biomass. Energy Strateg. Rev. 2019, 24, 27–37. [Google Scholar] [CrossRef]
- Koku, H.; Erolu, I.; Gündüz, U.; Yücel, M.; Türker, L. Aspects of the Metabolism of Hydrogen Production by Rhodobacter sphaeroides. Int. J. Hydrogen Energy 2002, 27, 1315–1329. [Google Scholar] [CrossRef]
- Ghosh, S.; Dairkee, U.K.; Chowdhury, R.; Bhattacharya, P. Hydrogen from Food Processing Wastes via Photofermentation Using Purple Non-Sulfur Bacteria (PNSB)—A Review. Energy Convers. Manag. 2017, 141, 299–314. [Google Scholar] [CrossRef]
- Sakurai, H.; Masukawa, H.; Kitashima, M.; Inoue, K. Photobiological Hydrogen Production: Bioenergetics and Challenges for Its Practical Application. J. Photochem. Photobiol. C Photochem. Rev. 2013, 17, 1–25. [Google Scholar] [CrossRef]
- Budiman, P.M.; Wu, T.Y. Role of Chemicals Addition in Affecting Biohydrogen Production through Photofermentation. Energy Convers. Manag. 2018, 165, 509–527. [Google Scholar] [CrossRef]
- Ahmad, A.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Banat, F. Biohydrogen Production through Dark Fermentation: Recent Trends and Advances in Transition to a Circular Bioeconomy. Int. J. Hydrogen Energy 2024, 52, 335–357. [Google Scholar] [CrossRef]
- Chandrasekhar, K.; Lee, Y.J.; Lee, D.W. Biohydrogen Production: Strategies to Improve Process Efficiency through Microbial Routes. Int. J. Mol. Sci. 2015, 16, 8266–8293. [Google Scholar] [CrossRef] [PubMed]
- Bundhoo, Z.M.A. Coupling Dark Fermentation with Biochemical or Bioelectrochemical Systems for Enhanced Bio-Energy Production: A Review. Int. J. Hydrogen Energy 2017, 42, 26667–26686. [Google Scholar] [CrossRef]
- Hallenbeck, P.C.; Abo-Hashesh, M.; Ghosh, D. Strategies for Improving Biological Hydrogen Production. Bioresour. Technol. 2012, 110, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Su, H.; Zhou, J.; Song, W.; Cen, K. Microwave-Assisted Alkali Pretreatment of Rice Straw to Promote Enzymatic Hydrolysis and Hydrogen Production in Dark- and Photo-Fermentation. Int. J. Hydrogen Energy 2011, 36, 2093–2101. [Google Scholar] [CrossRef]
- García-Sánchez, R.; Ramos-Ibarra, R.; Guatemala-Morales, G.; Arriola-Guevara, E.; Toriz-González, G.; Corona-González, R.I. Photofermentation of Tequila Vinasses by Rhodopseudomonas pseudopalustris to Produce Hydrogen. Int. J. Hydrogen Energy 2018, 43, 15857–15869. [Google Scholar] [CrossRef]
- Yue, T.; Sun, Y.; Zhang, Q.; Jiang, D.; Zhang, Z.; Zhang, H.; Li, Y.; Zhang, Y.; Zhang, T. Enhancement of Biohydrogen Production by Photo-Fermentation of Corn Stover via Visible Light Catalyzed Titanium Dioxide/Activated Carbon Fiber. Bioresour. Technol. 2024, 399, 130459. [Google Scholar] [CrossRef]
- Lu, C.; Wang, G.; Zhang, Q.; Yang, X.; Yu, J.; Liu, T.; Petracchini, F.; Zhang, Z.; Sun, Y.; Jiang, D.; et al. Comparison of Biorefinery Characteristics: Photo-Fermentation Biohydrogen, Dark Fermentation Biohydrogen, Biomethane, and Bioethanol Production. Appl. Energy 2023, 347, 121463. [Google Scholar] [CrossRef]
- Budiman, P.M.; Wu, T.Y.; Ramanan, R.N.; Jahim, J.M. Improving Photofermentative Biohydrogen Production by Using Intermittent Ultrasonication and Combined Industrial Effluents from Palm Oil, Pulp and Paper Mills. Energy Convers. Manag. 2017, 132, 110–118. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.H.; Kim, K.Y.; Shin, H.S. Experience of a Pilot-Scale Hydrogen-Producing Anaerobic Sequencing Batch Reactor (ASBR) Treating Food Waste. Int. J. Hydrogen Energy 2010, 35, 1590–1594. [Google Scholar] [CrossRef]
- Kumar, G.; Bakonyi, P.; Sivagurunathan, P.; Kim, S.H.; Nemestóthy, N.; Bélafi-Bakó, K.; Lin, C.Y. Enhanced Biohydrogen Production from Beverage Industrial Wastewater Using External Nitrogen Sources and Bioaugmentation with Facultative Anaerobic Strains. J. Biosci. Bioeng. 2015, 120, 155–160. [Google Scholar] [CrossRef]
- Yang, Y.; Bu, J.; Tiong, Y.W.; Xu, S.; Zhang, J.; He, Y.; Zhu, M.; Tong, Y.W. Enhanced Thermophilic Dark Fermentation of Hydrogen Production from Food Waste by Fe-Modified Biochar. Environ. Res. 2024, 244, 117946. [Google Scholar] [CrossRef]
- Zhao, B.; Yuan, A.; Cao, S.; Dong, Z.; Sha, H.; Song, Z. Advancing Two-Stage Hydrogen Production from Corn Stover via Dark Fermentation: Contributions of Thermally Modified Maifanite to Microbial Proliferation and PH Self-Regulation. Bioresour. Technol. 2024, 403, 130853. [Google Scholar] [CrossRef]
- Chantawan, N.; Moungprayoon, A.; Lunprom, S.; Reungsang, A.; Salakkam, A. High-Solid Dark Fermentation of Cassava Pulp and Cassava Processing Wastewater for Hydrogen Production. Int. J. Hydrogen Energy 2022, 47, 40672–40682. [Google Scholar] [CrossRef]
- Kazemi, R.; Mirmohamadsadeghi, S.; Amiri, H. Efficient Bio-Hydrogen Production by Dark Co-Fermentation of Food-Rich Municipal Solid Waste and Urban Pruning Wastes of Pine, Cypress, and Berry. Process Saf. Environ. Prot. 2024, 187, 398–407. [Google Scholar] [CrossRef]
- Ban, Q.; Wang, J.; Guo, P.; Yue, J.; Zhang, L.; Li, J. Improved Biohydrogen Production by Co-Fermentation of Corn Straw and Excess Sludge: Insights into Biochemical Process, Microbial Community and Metabolic Genes. Environ. Res. 2024, 256, 119171. [Google Scholar] [CrossRef]
- Hussien, M.; Jadhav, D.A.; Le, T.T.Q.; Jang, J.H.; Jang, J.K.; Chae, K.J. Tuning Dark Fermentation Operational Conditions for Improved Biohydrogen Yield during Co-Digestion of Swine Manure and Food Waste. Process Saf. Environ. Prot. 2024, 187, 1496–1507. [Google Scholar] [CrossRef]
- Srivastava, N.; Singh, R.; Lal, B.; Haque, S. Evaluation of Bioprocess Parameters for Pilot Scale Fermentative Biohydrogen Production Using Organic Waste: Environmental Remediation, Techno-Economic Challenges & Future Solutions. Int. J. Hydrogen Energy 2024. [Google Scholar] [CrossRef]
- Venkata Mohan, S.; Mohanakrishna, G.; Srikanth, S. Biohydrogen Production from Industrial Effluents. In Biofuels: Alternative Feedstocks and Conversion Processes; Academic Press: Cambridge, MA, USA, 2011; pp. 499–524. ISBN 9780123850997. [Google Scholar]
- Wang, J.; Yin, Y. Influencing Factors for Biohydrogen Production. In Biohydrogen Production from Organic Wastes; Green Energy and Technology; Springer: Berlin/Heidelberg, Germany, 2017; pp. 197–268. ISBN 9789811046759. [Google Scholar]
- Ananthi, V.; Bora, A.; Ramesh, U.; Yuvakkumar, R.; Raja, K.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A Review on the Technologies for Sustainable Biohydrogen Production. Process Saf. Environ. Prot. 2024, 186, 944–956. [Google Scholar] [CrossRef]
- Zhang, H.; Li, J.; Zhang, Q.; Zhu, S.; Yang, S.; Zhang, Z. Effect of Substrate Concentration on Photo-Fermentation Bio-Hydrogen Production Process from Starch-Rich Agricultural Leftovers under Oscillation. Sustainability 2020, 12, 2700. [Google Scholar] [CrossRef]
- Hallenbeck, P.C. Bioenergy from Microorganisms: An Overview. In Microbial BioEnergy: Hydrogen Production; Zannoni, D., De Philippis, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 3–21. ISBN 978-94-017-8554-9. [Google Scholar]
- Yang, D.; Zhang, Y.; Barupal, D.K.; Fan, X.; Gustafson, R.; Guo, R.; Fiehn, O. Metabolomics of Photobiological Hydrogen Production Induced by CCCP in Chlamydomonas reinhardtii. Int. J. Hydrogen Energy 2014, 39, 150–158. [Google Scholar] [CrossRef]
- Chen, W.; Li, T.; Ren, Y.; Wang, J.; Chen, H.; Wang, Q. Biological Hydrogen with Industrial Potential: Improvement and Prospection in Biohydrogen Production. J. Clean. Prod. 2023, 387, 135777. [Google Scholar] [CrossRef]
- McNeely, K.; Xu, Y.; Bennette, N.; Bryant, D.A.; Dismukes, G.C. Redirecting Reductant Flux into Hydrogen Production via Metabolic Engineering of Fermentative Carbon Metabolism in a Cyanobacterium. Appl. Environ. Microbiol. 2010, 76, 5032–5038. [Google Scholar] [CrossRef]
- Moon, C.; Jang, S.; Yun, Y.M.; Lee, M.K.; Kim, D.H.; Kang, W.S.; Kwak, S.S.; Kim, M.S. Effect of the Accuracy of PH Control on Hydrogen Fermentation. Bioresour. Technol. 2015, 179, 595–601. [Google Scholar] [CrossRef]
- Copa Rey, J.R.; Tamayo Pacheco, J.J.; António da Cruz Tarelho, L.; Silva, V.; Cardoso, J.S.; Silveira, J.L.; Tuna, C.E. Evaluation of Cogeneration Alternative Systems Integrating Biomass Gasification Applied to a Brazilian Sugar Industry. Renew. Energy 2021, 178, 318–333. [Google Scholar] [CrossRef]
- Briones-Hidrovo, A.; Copa, J.; Tarelho, L.A.C.; Gonçalves, C.; Pacheco da Costa, T.; Dias, A.C. Environmental and Energy Performance of Residual Forest Biomass for Electricity Generation: Gasification vs. Combustion. J. Clean. Prod. 2021, 289, 125680. [Google Scholar] [CrossRef]
- Lepage, T.; Kammoun, M.; Schmetz, Q.; Richel, A. Biomass-to-Hydrogen: A Review of Main Routes Production, Processes Evaluation and Techno-Economical Assessment. Biomass Bioenergy 2021, 144, 105920. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, J. Hydrogen Production from Biomass Steam Gasification: Experiment and Simulation. Chem. Eng. Sci. 2024, 292, 120011. [Google Scholar] [CrossRef]
- Shen, Y. Biomass Pretreatment for Steam Gasification toward H2-Rich Syngas Production—An Overview. Int. J. Hydrogen Energy 2024, 66, 90–102. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Huo, C.; Hu, M.; Guo, D.; Xiao, B. Effect of Bioleaching on Hydrogen-Rich Gas Production by Steam Gasification of Sewage Sludge. Energy Convers. Manag. 2015, 106, 1212–1218. [Google Scholar] [CrossRef]
- Pachapur, V.L.; Kutty, P.; Pachapur, P.; Brar, S.K.; Le Bihan, Y.; Galvez-Cloutier, R.; Buelna, G. Seed Pretreatment for Increased Hydrogen Production Using Mixed-Culture Systems with Advantages over Pure-Culture Systems. Energies 2019, 12, 530. [Google Scholar] [CrossRef]
- Gai, C.; Guo, Y.; Liu, T.; Peng, N.; Liu, Z. Hydrogen-Rich Gas Production by Steam Gasification of Hydrochar Derived from Sewage Sludge. Int. J. Hydrogen Energy 2016, 41, 3363–3372. [Google Scholar] [CrossRef]
- Niu, Y.; Han, F.; Chen, Y.; Lyu, Y.; Wang, L. Experimental Study on Steam Gasification of Pine Particles for Hydrogen-Rich Gas. J. Energy Inst. 2017, 90, 715–724. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.; Yang, Z.; Yan, Y.; Pu, G.; Guo, M. Hydrogen-Rich Gas Production from Wet Biomass Steam Gasification with CaO/MgO. Int. J. Hydrogen Energy 2015, 40, 8816–8823. [Google Scholar] [CrossRef]
- Cao, L.; Yu, I.K.M.; Xiong, X.; Tsang, D.C.W.; Zhang, S.; Clark, J.H.; Hu, C.; Ng, Y.H.; Shang, J.; Ok, Y.S. Biorenewable Hydrogen Production through Biomass Gasification: A Review and Future Prospects. Environ. Res. 2020, 186, 109547. [Google Scholar] [CrossRef]
- Chan, Y.H.; Cheah, K.W.; How, B.S.; Loy, A.C.M.; Shahbaz, M.; Singh, H.K.G.; Yusuf, N.R.; Shuhaili, A.F.A.; Yusup, S.; Ghani, W.A.W.A.K.; et al. An Overview of Biomass Thermochemical Conversion Technologies in Malaysia. Sci. Total Environ. 2019, 680, 105–123. [Google Scholar] [CrossRef]
- Barbuzza, E.; Buceti, G.; Pozio, A.; Santarelli, M.; Tosti, S. Gasification of Wood Biomass with Renewable Hydrogen for the Production of Synthetic Natural Gas. Fuel 2019, 242, 520–531. [Google Scholar] [CrossRef]
- Arregi, A.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Evaluation of Thermochemical Routes for Hydrogen Production from Biomass: A Review. Energy Convers. Manag. 2018, 165, 696–719. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, C.; Zhu, B.; Wang, C.; Jia, X.; Guan, G.; Zeng, X.; Hu, E.; Han, Z.; Xu, G. Pyrolysis of Biomass to Produce H-Rich Gas Facilitated by Simultaneously Occurring Magnesite Decomposition. Carbon Resour. Convers. 2024, 100265. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A Comprehensive Review on the Pyrolysis of Lignocellulosic Biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Chen, W.H.; Farooq, W.; Shahbaz, M.; Naqvi, S.R.; Ali, I.; Al-Ansari, T.; Saidina Amin, N.A. Current Status of Biohydrogen Production from Lignocellulosic Biomass, Technical Challenges and Commercial Potential through Pyrolysis Process. Energy 2021, 226, 120433. [Google Scholar] [CrossRef]
- Azizi, K.; Moraveji, M.K.; Najafabadi, H.A. A Review on Bio-Fuel Production from Microalgal Biomass by Using Pyrolysis Method. Renew. Sustain. Energy Rev. 2018, 82, 3046–3059. [Google Scholar] [CrossRef]
- Goyal, H.B.; Seal, D.; Saxena, R.C. Bio-Fuels from Thermochemical Conversion of Renewable Resources: A Review. Renew. Sustain. Energy Rev. 2008, 12, 504–517. [Google Scholar] [CrossRef]
- Al Arni, S. Comparison of Slow and Fast Pyrolysis for Converting Biomass into Fuel. Renew. Energy 2018, 124, 197–201. [Google Scholar] [CrossRef]
- Howe, D.; Westover, T.; Carpenter, D.; Santosa, D.; Emerson, R.; Deutch, S.; Starace, A.; Kutnyakov, I.; Lukins, C. Field-to-Fuel Performance Testing of Lignocellulosic Feedstocks: An Integrated Study of the Fast Pyrolysis–Hydrotreating Pathway. Energy Fuels 2015, 29, 3188–3197. [Google Scholar] [CrossRef]
- Yang, C.; Li, R.; Zhang, B.; Qiu, Q.; Wang, B.; Yang, H.; Ding, Y.; Wang, C. Pyrolysis of Microalgae: A Critical Review. Fuel Process. Technol. 2019, 186, 53–72. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Omairey, E.; Cai, J.; Gu, F.; Bridgwater, A.V. Intermediate Pyrolysis of Organic Fraction of Municipal Solid Waste and Rheological Study of the Pyrolysis Oil for Potential Use as Bio-Bitumen. J. Clean. Prod. 2018, 187, 390–399. [Google Scholar] [CrossRef]
- Banks, S.W.; Bridgwater, A.V. 14—Catalytic Fast Pyrolysis for Improved Liquid Quality. In Handbook of Biofuels Production, 2nd ed.; Luque, R., Lin, C.S.K., Wilson, K., Clark, J., Eds.; Woodhead Publishing: Sawston, UK, 2016; pp. 391–429. ISBN 978-0-08-100455-5. [Google Scholar]
- Abou Rjeily, M.; Gennequin, C.; Pron, H.; Abi-Aad, E.; Randrianalisoa, J.H. Pyrolysis-Catalytic Upgrading of Bio-Oil and Pyrolysis-Catalytic Steam Reforming of Biogas: A Review. Environ. Chem. Lett. 2021, 19, 2825–2872. [Google Scholar] [CrossRef]
- Teoh, R.H.; Mahajan, A.S.; Moharir, S.R.; Abdul Manaf, N.; Shi, S.; Thangalazhy-Gopakumar, S. A Review on Hydrothermal Treatments for Solid, Liquid and Gaseous Fuel Production from Biomass. Energy Nexus 2024, 14, 100301. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Baloch, H.A.; Griffin, G.J.; Mubarak, N.M.; Bhutto, A.W.; Abro, R.; Mazari, S.A.; Ali, B.S. An Overview of Effect of Process Parameters on Hydrothermal Carbonization of Biomass. Renew. Sustain. Energy Rev. 2017, 73, 1289–1299. [Google Scholar] [CrossRef]
- Pallarés, J.; González-Cencerrado, A.; Arauzo, I. Production and Characterization of Activated Carbon from Barley Straw by Physical Activation with Carbon Dioxide and Steam. Biomass Bioenergy 2018, 115, 64–73. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Kishore, N.; Gu, S. A Review on Hydrothermal Liquefaction of Biomass. Renew. Sustain. Energy Rev. 2018, 81, 1378–1392. [Google Scholar] [CrossRef]
- El Bast, M.; Allam, N.; Abou Msallem, Y.; Awad, S.; Loubar, K. A Review on Continuous Biomass Hydrothermal Liquefaction Systems: Process Design and Operating Parameters Effects on Biocrude. J. Energy Inst. 2023, 108, 101260. [Google Scholar] [CrossRef]
- Okolie, J.A.; Epelle, E.I.; Nanda, S.; Castello, D.; Dalai, A.K.; Kozinski, J.A. Modeling and Process Optimization of Hydrothermal Gasification for Hydrogen Production: A Comprehensive Review. J. Supercrit. Fluids 2021, 173, 105199. [Google Scholar] [CrossRef]
- Nanda, S.; Rana, R.; Hunter, H.N.; Fang, Z.; Dalai, A.K.; Kozinski, J.A. Hydrothermal Catalytic Processing of Waste Cooking Oil for Hydrogen-Rich Syngas Production. Chem. Eng. Sci. 2019, 195, 935–945. [Google Scholar] [CrossRef]
- Lee, C.S.; Conradie, A.V.; Lester, E. Review of Supercritical Water Gasification with Lignocellulosic Real Biomass as the Feedstocks: Process Parameters, Biomass Composition, Catalyst Development, Reactor Design and Its Challenges. Chem. Eng. J. 2021, 415, 128837. [Google Scholar] [CrossRef]
- Okolie, J.A.; Rana, R.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical Water Gasification of Biomass: A State-of-the-Art Review of Process Parameters, Reaction Mechanisms and Catalysis. Sustain. Energy Fuels 2019, 3, 578–598. [Google Scholar] [CrossRef]
- Nanda, S.; Reddy, S.N.; Hunter, H.N.; Dalai, A.K.; Kozinski, J.A. Supercritical Water Gasification of Fructose as a Model Compound for Waste Fruits and Vegetables. J. Supercrit. Fluids 2015, 104, 112–121. [Google Scholar] [CrossRef]
- Abdol Ghaffar Ebadi Hikmat Hisoriev, M.Z.; Ahmadi, H. Hydrogen and Syngas Production by Catalytic Gasification of Algal Biomass (Cladophora glomerata L.) Using Alkali and Alkaline-Earth Metals Compounds. Environ. Technol. 2019, 40, 1178–1184. [Google Scholar] [CrossRef]
- Matamba, T.; Tahmasebi, A.; Yu, J.; Keshavarz, A.; Abid, H.R.; Iglauer, S. A Review on Biomass as a Substitute Energy Source: Polygeneration Influence and Hydrogen Rich Gas Formation via Pyrolysis. J. Anal. Appl. Pyrolysis 2023, 175, 106221. [Google Scholar] [CrossRef]
- Obiora, N.K.; Ujah, C.O.; Asadu, C.O.; Kolawole, F.O.; Ekwueme, B.N. Production of Hydrogen Energy from Biomass: Prospects and Challenges. Green Technol. Sustain. 2024, 2, 100100. [Google Scholar] [CrossRef]
- Rodriguez Correa, C.; Kruse, A. Supercritical Water Gasification of Biomass for Hydrogen Production—Review. J. Supercrit. Fluids 2018, 133, 573–590. [Google Scholar] [CrossRef]
- Zang, G.; Graham, E.J.; Mallapragada, D. H2 Production through Natural Gas Reforming and Carbon Capture: A Techno-Economic and Life Cycle Analysis Comparison. Int. J. Hydrogen Energy 2024, 49, 1288–1303. [Google Scholar] [CrossRef]
- Katebah, M.; Al-Rawashdeh, M.; Linke, P. Analysis of Hydrogen Production Costs in Steam-Methane Reforming Considering Integration with Electrolysis and CO2 Capture. Clean. Eng. Technol. 2022, 10, 100552. [Google Scholar] [CrossRef]
- Law, L.C.; Mastorakos, E.; Othman, M.R.; Trakakis, A. A Thermodynamics Model for the Assessment and Optimisation of Onboard Natural Gas Reforming and Carbon Capture. Emiss. Control Sci. Technol. 2024, 10, 52–69. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J. Biofuel Production from Supercritical Water Gasification of Sustainable Biomass. Energy Convers. Manag. X 2022, 14, 100164. [Google Scholar] [CrossRef]
- Oni, A.O.; Anaya, K.; Giwa, T.; Di Lullo, G.; Kumar, A. Comparative Assessment of Blue Hydrogen from Steam Methane Reforming, Autothermal Reforming, and Natural Gas Decomposition Technologies for Natural Gas-Producing Regions. Energy Convers. Manag. 2022, 254, 115245. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Vuppaladadiyam, S.S.V.; Awasthi, A.; Sahoo, A.; Rehman, S.; Pant, K.K.; Murugavelh, S.; Huang, Q.; Anthony, E.; Fennel, P.; et al. Biomass Pyrolysis: A Review on Recent Advancements and Green Hydrogen Production. Bioresour. Technol. 2022, 364, 128087. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bastardo, N.; Schlögl, R.; Ruland, H. Methane Pyrolysis for Zero-Emission Hydrogen Production: A Potential Bridge Technology from Fossil Fuels to a Renewable and Sustainable Hydrogen Economy. Ind. Eng. Chem. Res. 2021, 60, 11855–11881. [Google Scholar] [CrossRef]
- Kayfeci, M.; Keçebaş, A.; Bayat, M. Chapter 3—Hydrogen Production. In Solar Hydrogen Production; Calise, F., D’Accadia, M.D., Santarelli, M., Lanzini, A., Ferrero, D., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 45–83. ISBN 978-0-12-814853-2. [Google Scholar]
- AlZohbi, G.; AlShuhail, L.; Almoaikel, A. An Estimation of Green Hydrogen Generation from Wind Energy: A Case Study from KSA. Energy Rep. 2023, 9, 262–267. [Google Scholar] [CrossRef]
- Ozcan, H.; El-Emam, R.S.; Amini Horri, B. Thermochemical Looping Technologies for Clean Hydrogen Production—Current Status and Recent Advances. J. Clean. Prod. 2023, 382, 135295. [Google Scholar] [CrossRef]
- Boretti, A.; Nayfeh, J.; Al-Maaitah, A. Hydrogen Production by Solar Thermochemical Water-Splitting Cycle via a Beam Down Concentrator. Front. Energy Res. 2021, 9, 666191. [Google Scholar] [CrossRef]
- International Energy Agency. Global Hydrogen Review 2023; International Energy Agency: Paris, France, 2023. [Google Scholar]
- Zhou, Y.; Searle, S. Cost of Renewable Hydrogen Produced Onsite at Hydrogen Refueling Stations in Europe; International Council on Clean Transportation: Washington, DC, USA, 2022. [Google Scholar]
- Janssen, J.L.L.C.C.; Weeda, M.; Detz, R.J.; van der Zwaan, B. Country-Specific Cost Projections for Renewable Hydrogen Production through off-Grid Electricity Systems. Appl. Energy 2022, 309, 118398. [Google Scholar] [CrossRef]
- Hydrogen Europe. Clean Hydrogen Monitor 2023. 2023. Available online: https://hydrogeneurope.eu/wp-content/uploads/2023/10/Clean_Hydrogen_Monitor_11-2023_DIGITAL.pdf (accessed on 10 July 2024).
- Jang, D.; Kim, J.; Kim, D.; Han, W.B.; Kang, S. Techno-Economic Analysis and Monte Carlo Simulation of Green Hydrogen Production Technology through Various Water Electrolysis Technologies. Energy Convers. Manag. 2022, 258, 115499. [Google Scholar] [CrossRef]
- Han, W.; Yan, Y.; Gu, J.; Shi, Y.; Tang, J.; Li, Y. Techno-Economic Analysis of a Novel Bioprocess Combining Solid State Fermentation and Dark Fermentation for H2 Production from Food Waste. Int. J. Hydrogen Energy 2016, 41, 22619–22625. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Zhou, J.L.; Li, X.; Afsari, M.; Altaee, A. Techno-Economic and Environmental Impact Assessment of Hydrogen Production Processes Using Bio-Waste as Renewable Energy Resource. Renew. Sustain. Energy Rev. 2022, 156, 111991. [Google Scholar] [CrossRef]
- Alam, M.; Nayan, N.F.; Fatema Nayan, N. Techno-Economic Assessment of Biohydrogen Production from Dark Fermentation of Wastewater Sludge. 2024. Available online: https://www.researchgate.net/publication/378002057_Techno-Economic_Assessment_of_Biohydrogen_Production_from_Dark_Fermentation_of_Wastewater_Sludge (accessed on 10 July 2024).
- Szima, S.; Cormos, C.C. Techno—Economic Assessment of Flexible Decarbonized Hydrogen and Power Co-Production Based on Natural Gas Dry Reforming. Int. J. Hydrogen Energy 2019, 44, 31712–31723. [Google Scholar] [CrossRef]
- Cormos, C.C.; Cormos, A.M.; Petrescu, L.; Dragan, S. Techno-Economic Assessment of Decarbonized Biogas Catalytic Reforming for Flexible Hydrogen and Power Production. Appl. Therm. Eng. 2022, 207, 118218. [Google Scholar] [CrossRef]
- Hajizadeh, A.; Mohamadi-Baghmolaei, M.; Cata Saady, N.M.; Zendehboudi, S. Hydrogen Production from Biomass through Integration of Anaerobic Digestion and Biogas Dry Reforming. Appl. Energy 2022, 309, 118442. [Google Scholar] [CrossRef]
- Byun, J.; Han, J.-h. Economic Feasible Hydrogen Production System from Carbohydrate-Rich Food Waste. Appl. Energy 2023, 340, 121044. [Google Scholar] [CrossRef]
- Anex, R.P.; Aden, A.; Kazi, F.K.; Fortman, J.; Swanson, R.M.; Wright, M.M.; Satrio, J.A.; Brown, R.C.; Daugaard, D.E.; Platon, A.; et al. Techno-Economic Comparison of Biomass-to-Transportation Fuels via Pyrolysis, Gasification, and Biochemical Pathways. Fuel 2010, 89, S29–S35. [Google Scholar] [CrossRef]
- Brown, T.R.; Thilakaratne, R.; Brown, R.C.; Hu, G. Techno-Economic Analysis of Biomass to Transportation Fuels and Electricity via Fast Pyrolysis and Hydroprocessing. Fuel 2013, 106, 463–469. [Google Scholar] [CrossRef]
- Tan, E.C.D.; Marker, T.L.; Roberts, M.J. Direct Production of Gasoline and Diesel Fuels from Biomass via Integrated Hydropyrolysis and Hydroconversion Process—A Techno-Economic Analysis. Environ. Prog. Sustain. Energy 2014, 33, 609–617. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Liu, P.; Wang, Z.; Sun, T.; Wu, S.; Wu, Y.; Lei, T. Oriented Pyrolysis of Biomass for Hydrogen-Rich Gas and Biochar Production: An Energy, Environment, and Economic Assessment Based on Life Cycle Assessment Method. Int. J. Hydrogen Energy 2024, 62, 979–993. [Google Scholar] [CrossRef]
- Salkuyeh, Y.K.; Saville, B.A.; MacLean, H.L. Techno-Economic Analysis and Life Cycle Assessment of Hydrogen Production from Different Biomass Gasification Processes. Int. J. Hydrogen Energy 2018, 43, 9514–9528. [Google Scholar] [CrossRef]
- Shaikh, A.R.; Wang, Q.; Han, L.; Feng, Y.; Sharif, Z.; Li, Z.; Cen, J.; Kumar, S. Techno-Economic Analysis of Hydrogen and Electricity Production by Biomass Calcium Looping Gasification. Sustainability 2022, 14, 2189. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Liu, Z.; Cui, P.; Zhu, Z.; Yang, S. Techno-Economic Analysis of Biomass-to-Hydrogen Process in Comparison with Coal-to-Hydrogen Process. Energy 2019, 185, 1063–1075. [Google Scholar] [CrossRef]
- Al-Qahtani, A.; Parkinson, B.; Hellgardt, K.; Shah, N.; Guillen-Gosalbez, G. Uncovering the True Cost of Hydrogen Production Routes Using Life Cycle Monetisation. Appl. Energy 2021, 281, 115958. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Techno-Economic Evaluation and Sensitivity Analysis of a Conceptual Design for Supercritical Water Gasification of Soybean Straw to Produce Hydrogen. Bioresour. Technol. 2021, 331, 125005. [Google Scholar] [CrossRef]
- Cook, B.; Hagen, C. Techno-Economic Analysis of Biomass Gasification for Hydrogen Production in Three US-Based Case Studies. Int. J. Hydrogen Energy 2024, 49, 202–218. [Google Scholar] [CrossRef]
- Zhou, Y.; Swidler, D.; Searle, S.; Baldino, C. Life-Cycle Greenhouse Gas Emissions of Biomethane and Hydrogen Pathways in the European Union; International Council on Clean Transportation: Washington, DC, USA, 2021. [Google Scholar]
- Camacho, C.I.; Estévez, S.; Conde, J.J.; Feijoo, G.; Moreira, M.T. Dark Fermentation as an Environmentally Sustainable WIN-WIN Solution for Bioenergy Production. J. Clean. Prod. 2022, 374, 134026. [Google Scholar] [CrossRef]
- Barghash, H.; AlRashdi, Z.; Okedu, K.E.; Desmond, P. Life-Cycle Assessment Study for Bio-Hydrogen Gas Production from Sewage Treatment Plants Using Solar PVs. Energies 2022, 15, 8056. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, J.; Huang, J.; Xu, X.; Tang, J.; Hou, P.; Han, W.; Li, H. Environmental Impact Assessment of a Combined Bioprocess for Hydrogen Production from Food Waste. Waste Manag. 2024, 173, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Liu, X.; Li, G.; Qiu, X.; Yao, D.; Zhu, Z.; Wang, Y.; Gao, J.; Cui, P. Energy Consumption, Environmental Performance, and Techno-Economic Feasibility Analysis of the Biomass-to-Hydrogen Process with and without Carbon Capture and Storage. J. Environ. Chem. Eng. 2021, 9, 106752. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Zuo, H.; Wu, X.; Jiaqiang, E.; Wang, T.; Zhang, F.; Lu, N. System Development and Environmental Performance Analysis of a Solar-Driven Supercritical Water Gasification Pilot Plant for Hydrogen Production Using Life Cycle Assessment Approach. Energy Convers. Manag. 2019, 184, 60–73. [Google Scholar] [CrossRef]
- Wu, D.; Gao, Z.; Wu, S.; Xiao, R. Negative Net Global Warming Potential Hydrogen Production through Biomass Gasification Combined with Chemical Looping: Environmental and Economic Assessments. Int. J. Hydrogen Energy 2024, 66, 24–32. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).