Modeling and CFD Simulation of Macroalgae Motion within Aerated Tanks: Assessment of Light-Dark Cycle Period
Abstract
:1. Introduction
2. Theoretical Background
2.1. Multiphase Flow
2.2. Discrete Element Method
2.3. Mechanical Properties of Seaweed
2.4. Light Regime and Euler vs. Lagrange Approach of Photobioreactor Modeling
3. Material and Methods
3.1. Experimental Setup
3.2. Seaweed Ulva ohnoi Cultivation Experiment
3.3. CFD Setup
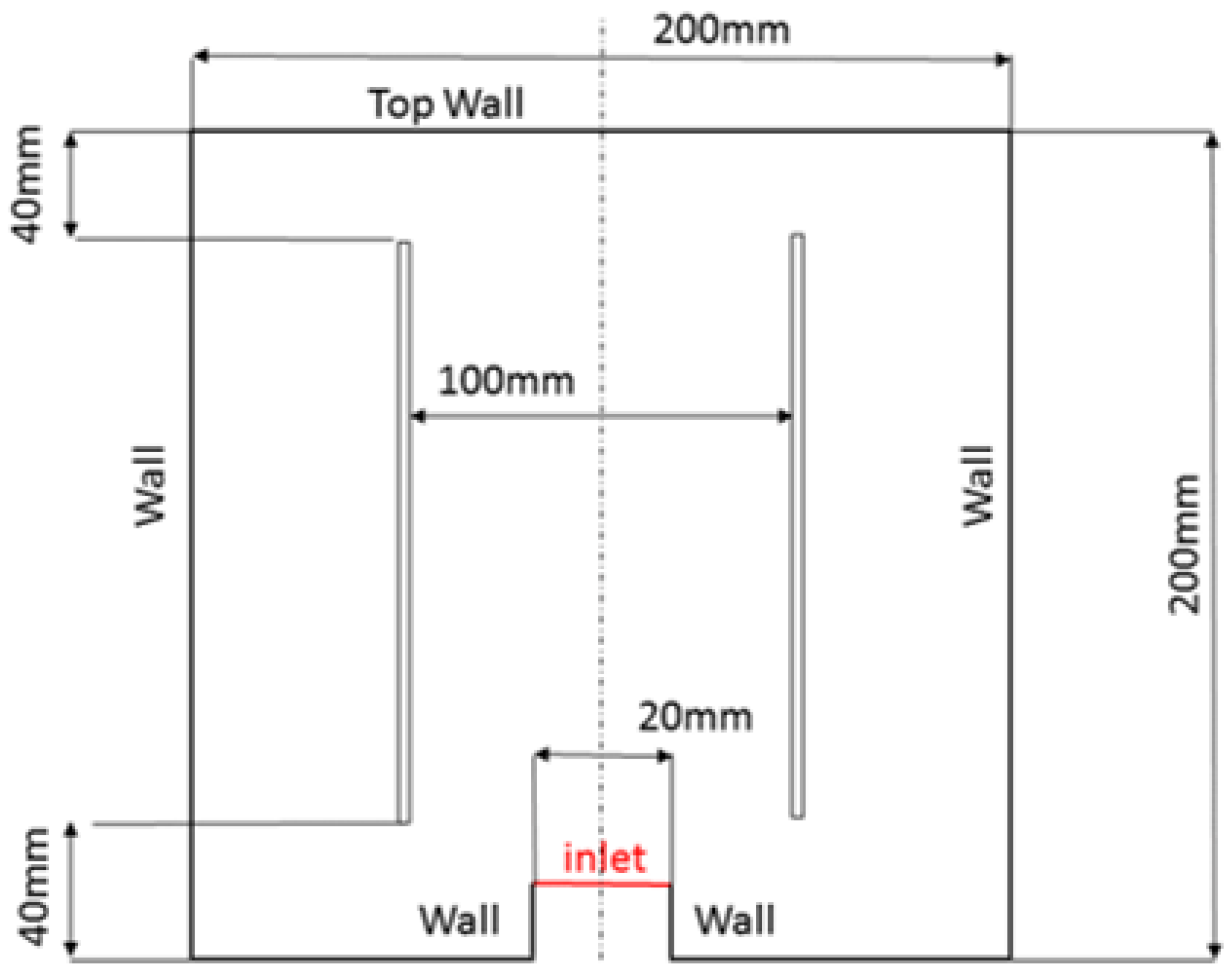
3.3.1. Geometry, Mesh Generation
3.3.2. Boundary Conditions for the 2D and 3D Models
3.3.3. CFD–DEM Coupling
- The macroalgae geometry is approximated with chains of spheres; the details are shown in Figure 4.
- 2.
- The diameter of the spheres is set to 3 mm to maintain a low computational time.
- 3.
- To prove the DEM concept, firstly, the 2D model was created and tested. Then, the 3D model was created and launched to verify the data from the 2D models.
- 4.
- It is assumed that the particle (solid) movement will not change the flow pattern of the liquid phase, and the interaction between the gas and solid phases can be neglected (single-phase flow).
- 5.
- The material properties for the CFD model have been set to ensure particle cohesion, flexibility, and a low computational time. The solid phase density is 1000 kg/m3, the elasticity modulus 10 kPa, and Poisson’s ratio .
- 6.
- The densities of the liquid and gaseous phases, i.e., water and air, are set to 1000 kg/m3 and 1.18 kg/m3, respectively. The dynamic viscosity of water is set to 0.9 Pa·s.
- 7.
- The flow field is steady and does not change over time.
3.3.4. CFD Solver Settings
4. CFD Simulation Results and Discussion
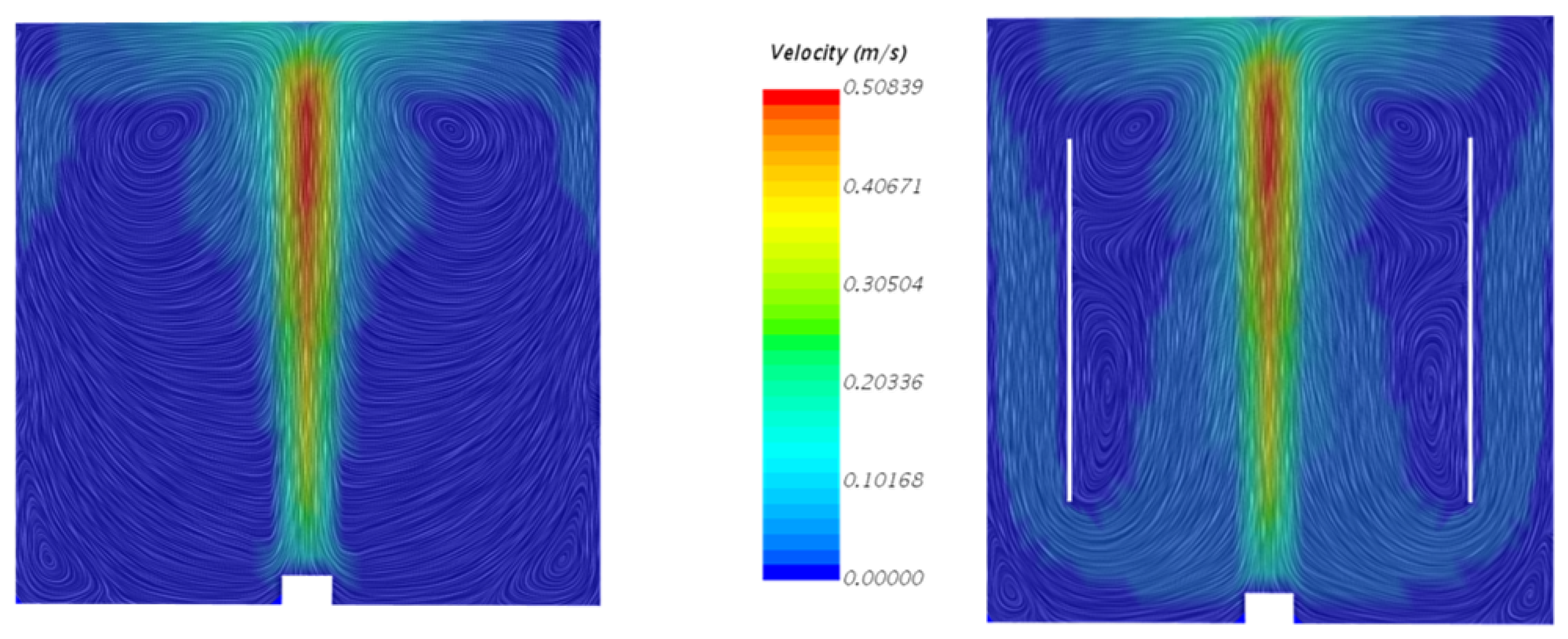
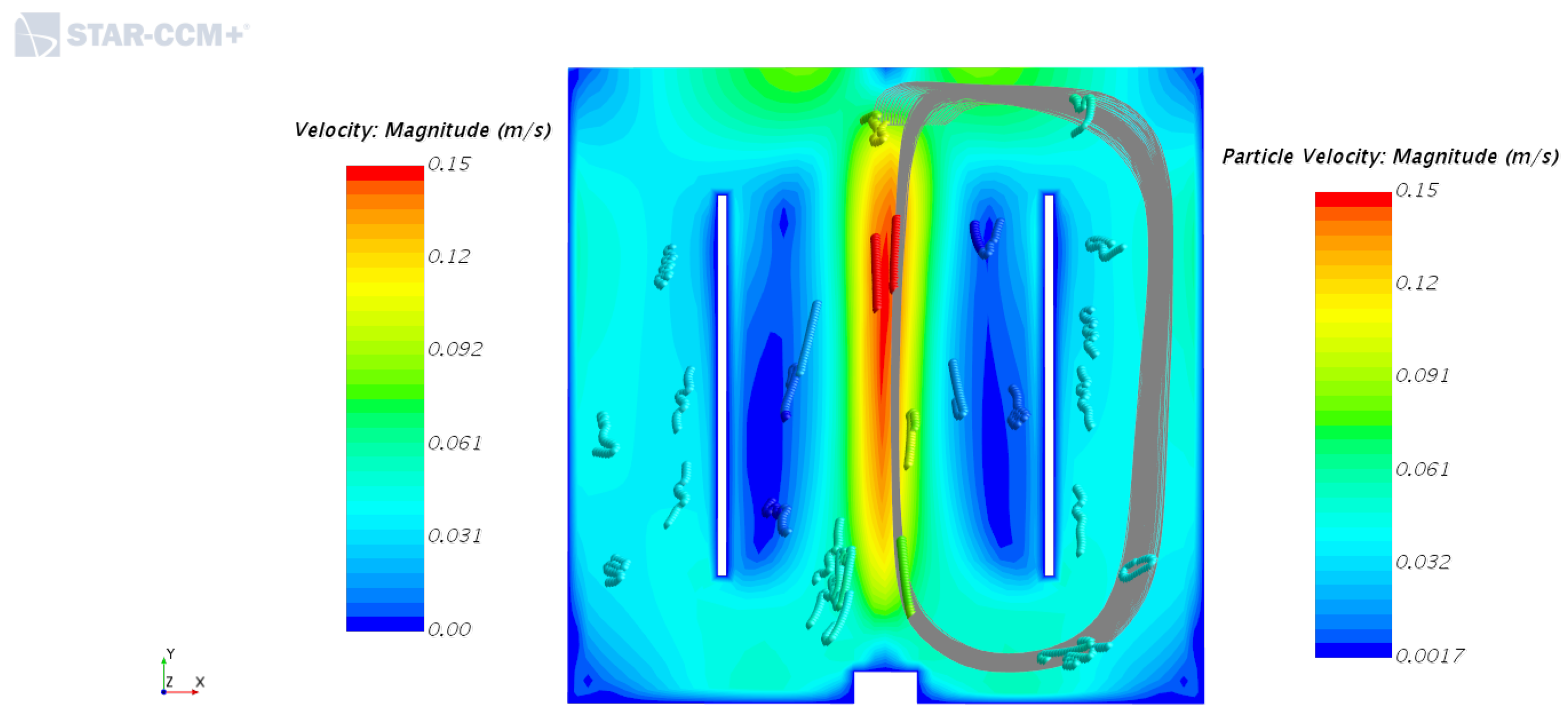
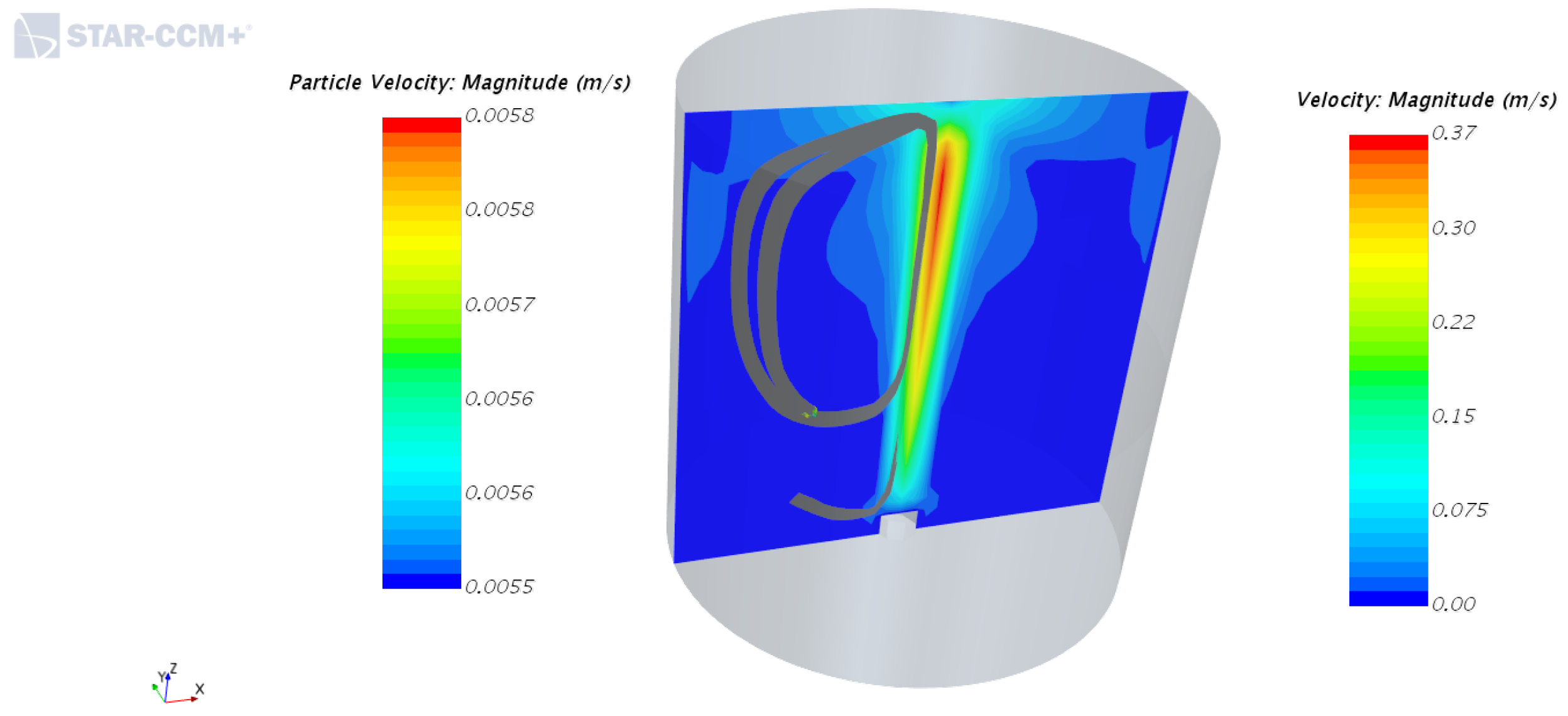
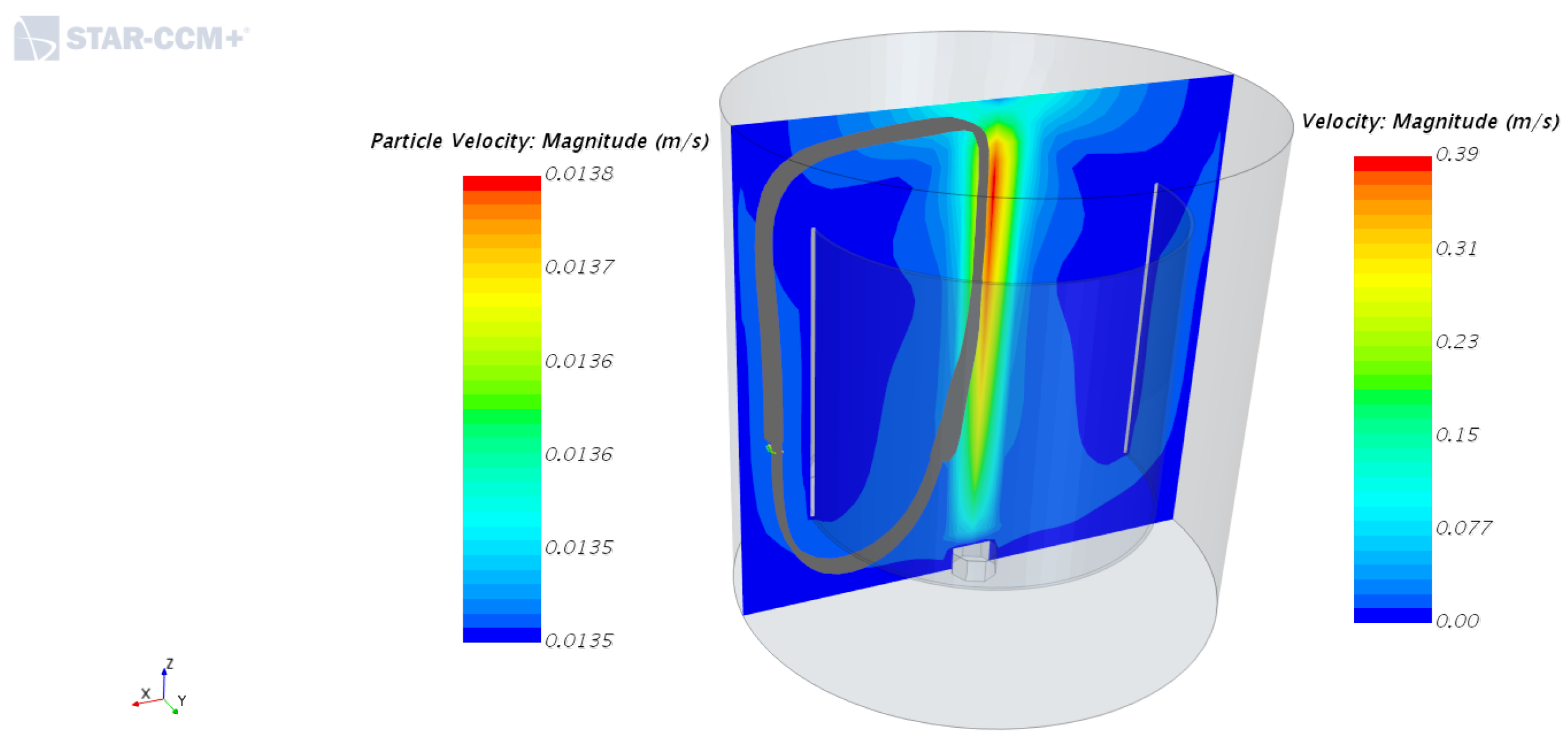
4.1. Velocity Fields of Liquid Phase with and without the Inner Cylinder Assembly
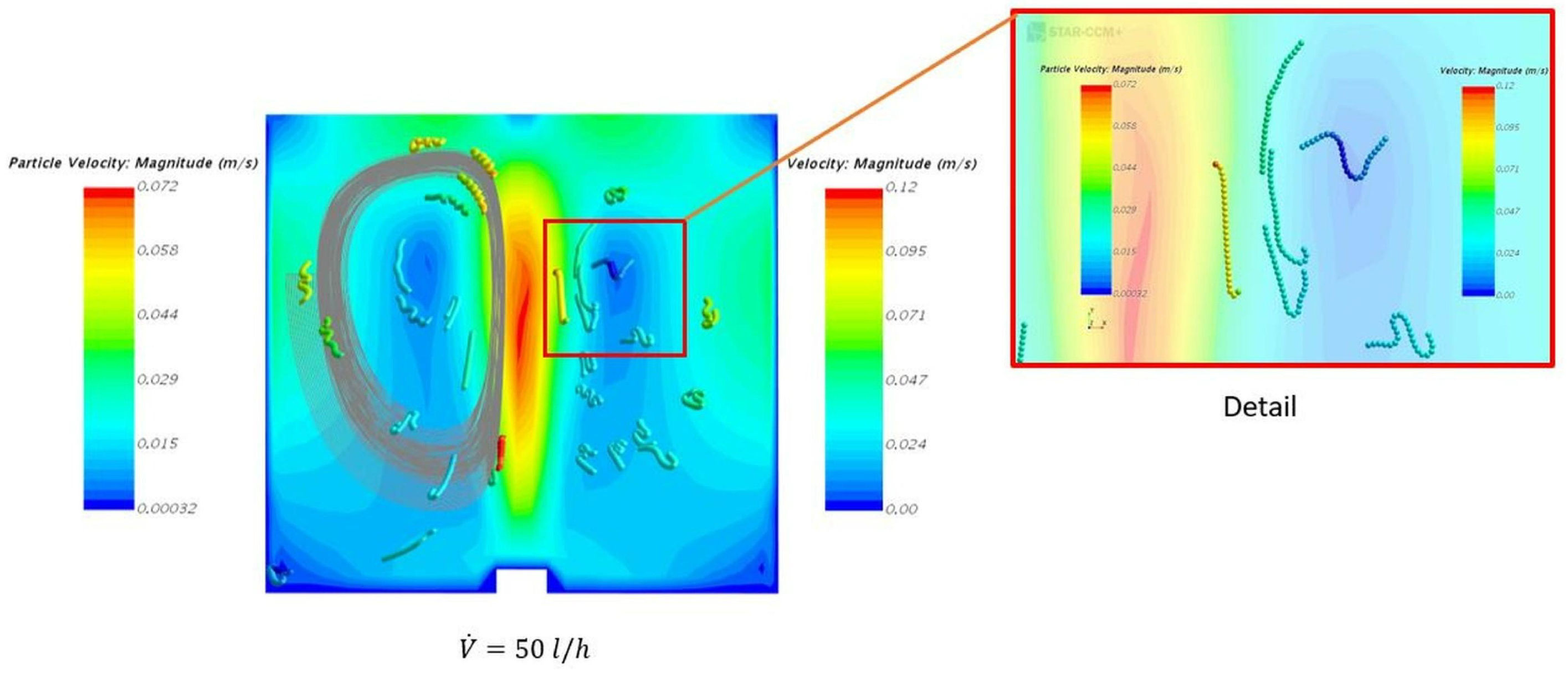
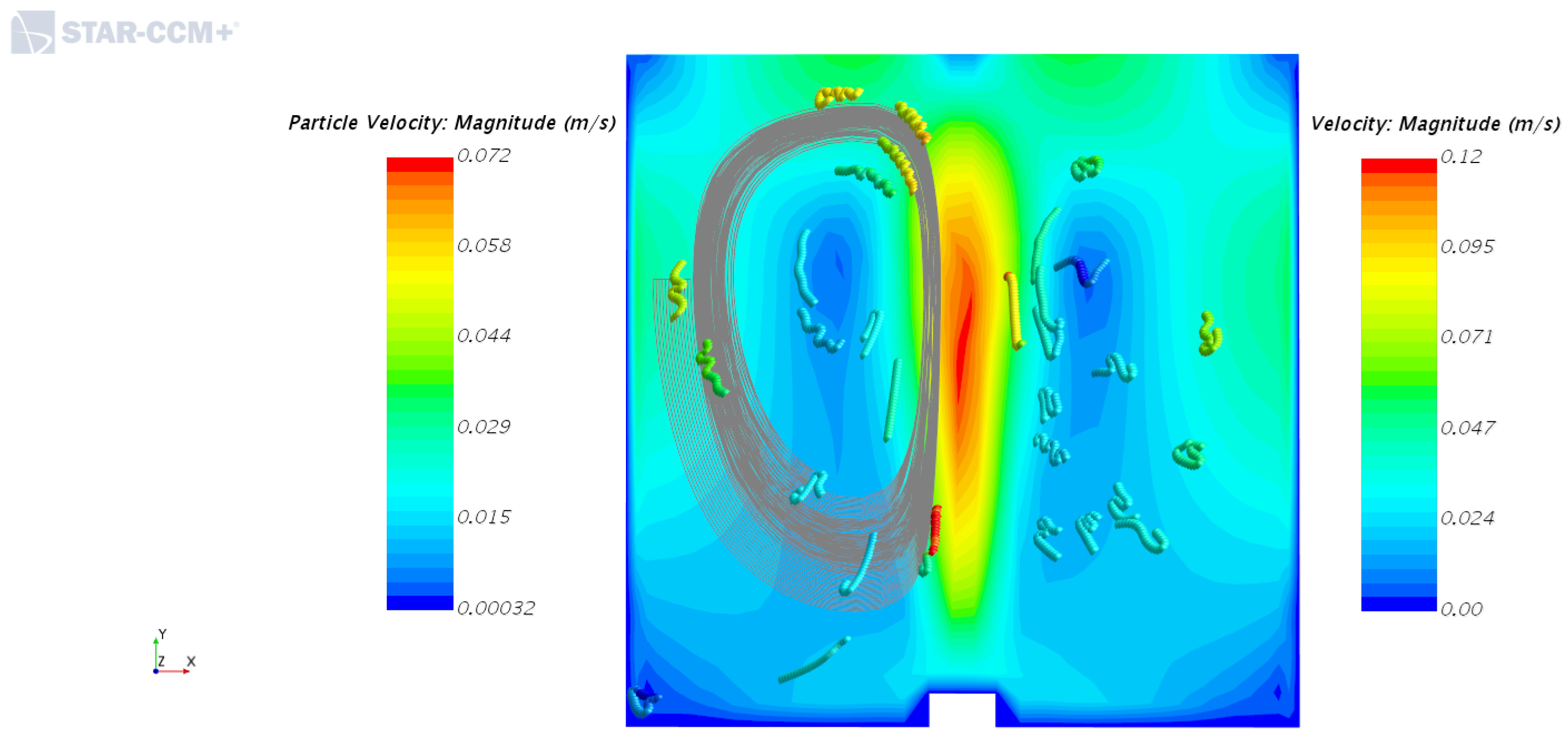
4.2. Solid Particle Tracking—Solid Phase Motion with and without the Inner Cylinder Assembly
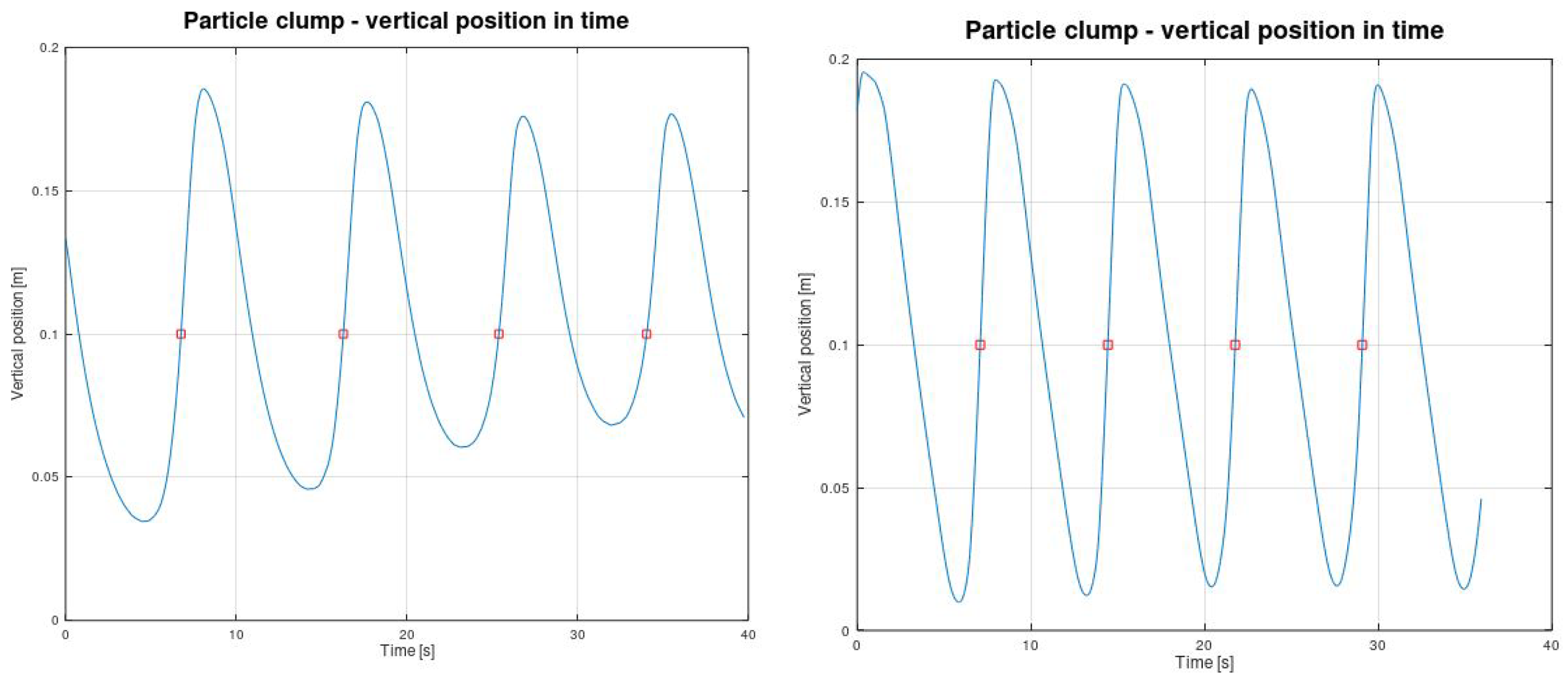
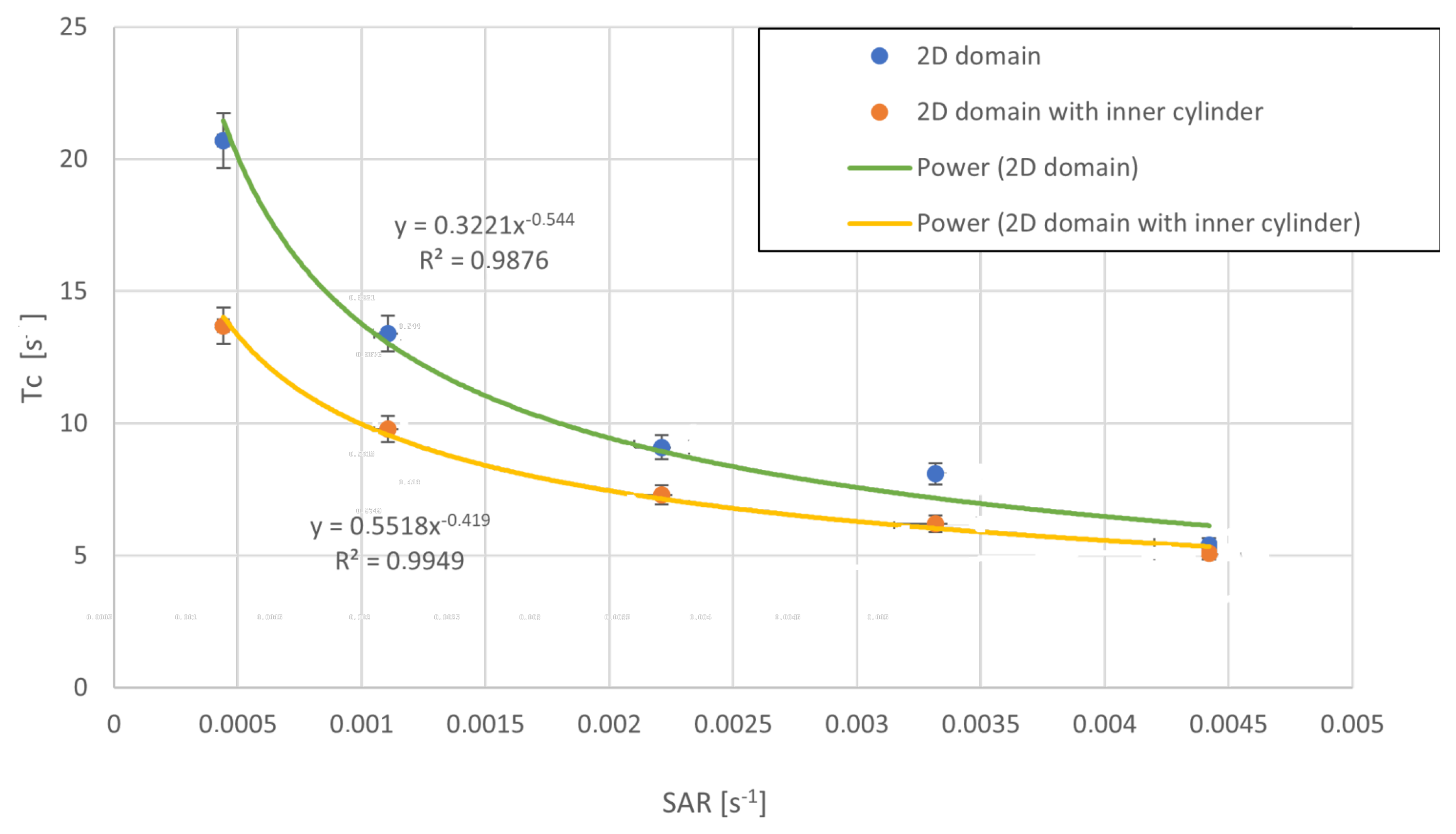
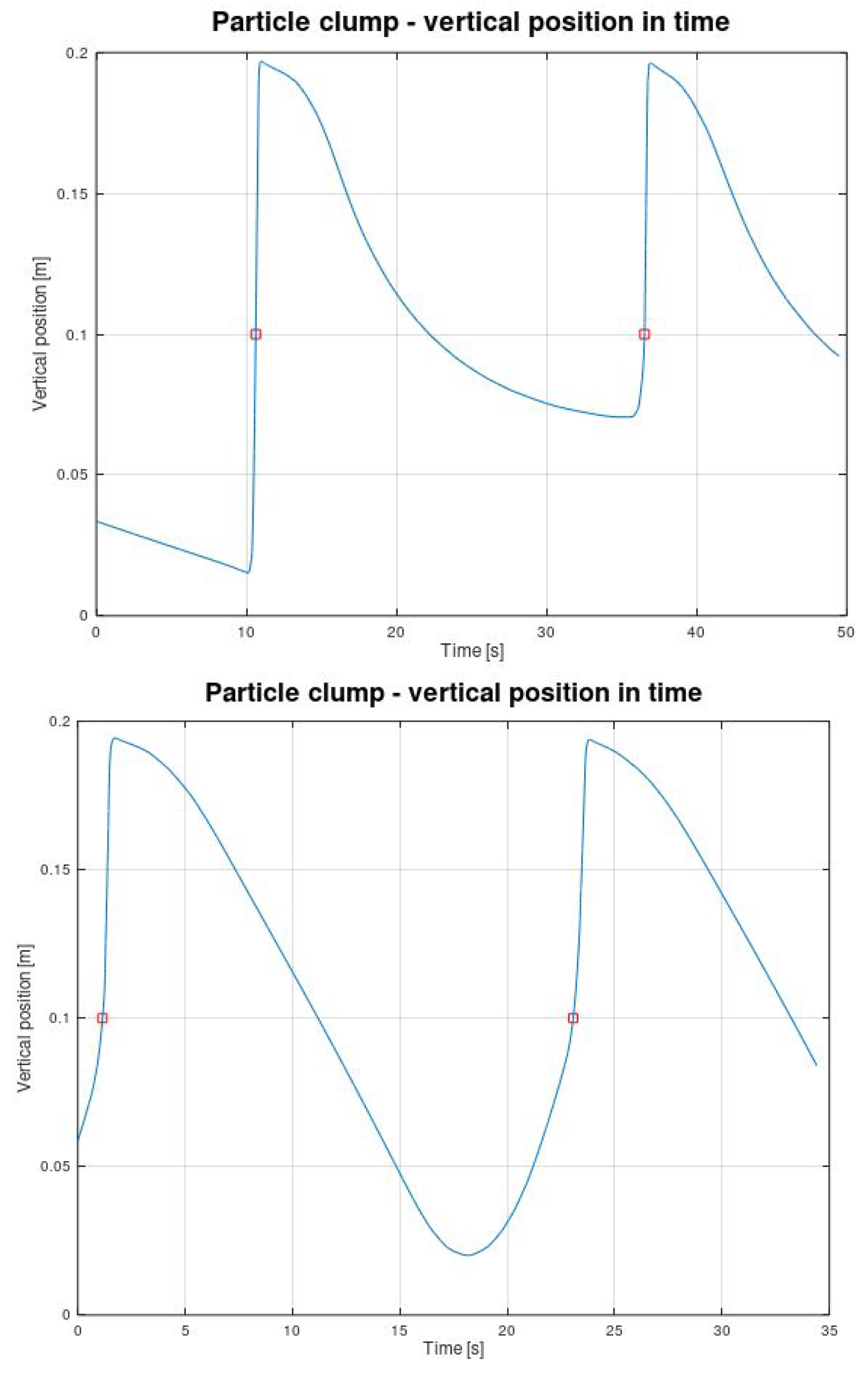
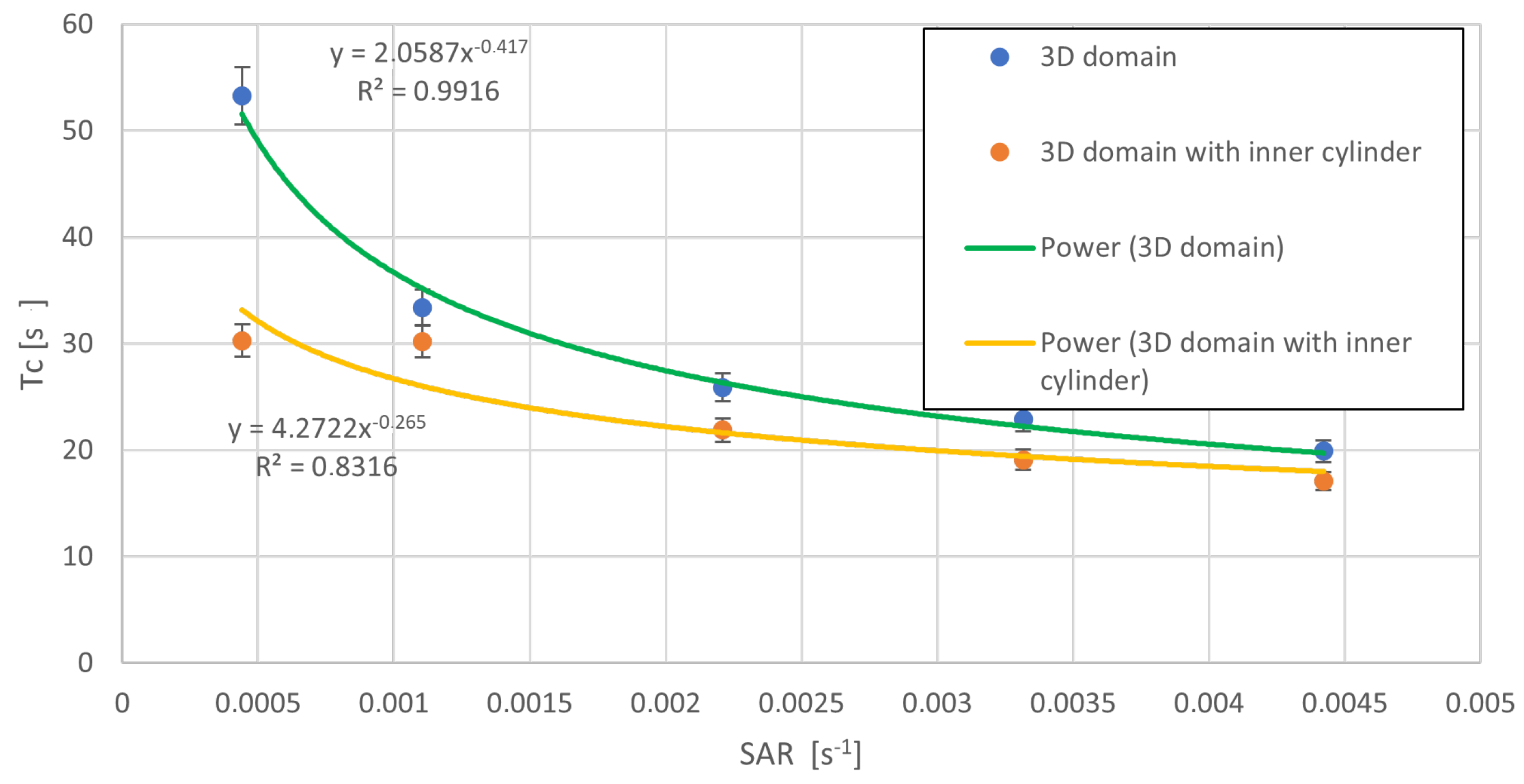
4.3. Post-Processing of Clump Trajectories
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neori, A.; Chopin, T.; Troell, M.; Buschmann, A.H.; Kraemer, G.P.; Halling, C.; Shpigel, M.; Yarish, C. Integrated aquaculture: Rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 2004, 231, 361–391. [Google Scholar] [CrossRef]
- Neori, A. Essential role of seaweed cultivation in integrated multi-trophic aquaculture farms for global expansion of mariculture: An analysis. J. Appl. Phycol. 2008, 20, 567–570. [Google Scholar] [CrossRef]
- Neori, A.; Shpigel, M.; Guttman, L.; Israel, A. Development of polyculture and integrated multi-trophic aquaculture (IMTA) in Israel: A review. Isr. J. Aquac. 2017, 69, 1–19. [Google Scholar] [CrossRef]
- Wu, X.; Merchuk, J.C. Simulation of algae growth in a bench-scale bubble column reactor. Biotechnol. Bioeng. 2002, 80, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Petera, K.; Papáček, Š.; González, C.I.; Fernández-Sevilla, J.M.; Acién Fernández, F.G. Advanced Computational Fluid Dynamics Study of the Dissolved Oxygen Concentration within a Thin-Layer Cascade Reactor for Microalgae Cultivation. Energies 2021, 14, 7284. [Google Scholar] [CrossRef]
- Inostroza, C.; Papáček, S.; Fernández-Sevilla, J.; Acién, F.G. Optimization of thin-layer photobioreactors for the production of microalgae by integrating fluid-dynamic and photosynthesis rate aspects. J. Appl. Phycol. 2023, 35, 2111–2123. [Google Scholar] [CrossRef]
- Martínez, E.C.; Kim, J.W.; Barz, T.; Cruz Bournazou, M.N. Probabilistic Modeling for Optimization of Bioreactors using Reinforcement Learning with Active Inference. In ESCAPE-31, Proceedings of the 31st European Symposium on Computer Aided Process Engineering, Istanbul, Turkey, 6–9 June 2021; Türkay, M., Gani, R., Eds.; Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2021; Volume 50, pp. 419–424. [Google Scholar] [CrossRef]
- Martins, C.; Eding, E.; Verdegem, M.; Heinsbroek, L.; Schneider, O.; Blancheton, J.; d’Orbcastel, E.R.; Verreth, J. New developments in recirculating aquaculture systems in Europe: A perspective on environmental sustainability. Aquac. Eng. 2010, 43, 83–93. [Google Scholar] [CrossRef]
- Edwards, P. Aquaculture environment interactions: Past, present and likely future trends. Aquaculture 2015, 447, 2–14. [Google Scholar] [CrossRef]
- Hadley, S.; Wild-Allen, K.; Johnson, C.; Macleod, C. Modeling macroalgae growth and nutrient dynamics for integrated multi-trophic aquaculture. J. Appl. Phycol. 2015, 27, 901–916. [Google Scholar] [CrossRef]
- Knowler, D.; Chopin, T.; Martínez-Espiñeira, R.; Neori, A.; Nobre, A.; Noce, A.; Reid, G. The economics of Integrated Multi-Trophic Aquaculture: Where are we now and where do we need to go? Rev. Aquac. 2020, 12, 1579–1594. [Google Scholar] [CrossRef]
- Oca, J.; Cremades, J.; Jiménez, P.; Pintado, J.; Masaló, I. Culture of the seaweed Ulva ohnoi integrated in a Solea senegalensis recirculating system: Influence of light and biomass stocking density on macroalgae productivity. J. Appl. Phycol. 2019, 31, 2461–2467. [Google Scholar] [CrossRef]
- Msuya, F.; Kyewalyanga, M.; Salum, D. The performance of the seaweed Ulva reticulata as a biofilter in a low-tech, low-cost, gravity generated water flow regime in Zanzibar, Tanzania. Aquaculture 2006, 254, 284–292. [Google Scholar] [CrossRef]
- Kok, B. Experiments on photosynthesis by Chlorella in flashing light. In Algal Culture from Laboratory to Pilot Plant; Burlew, J., Ed.; Carnegie Institute: Washington, DC, USA, 1953; Volume 600, pp. 63–75. [Google Scholar]
- Terry, K.L. Photosynthesis in modulated light: Quantitative dependence of photosynthetic enhancement on flashing rate. Biotechnol. Bioeng. 1986, 28, 988–995. [Google Scholar] [CrossRef]
- Grobbelaar, J.; Nedbal, L.; Tichý, V. Influence of high frequency light/dark fluctuations on photosynthetic characteristics of microalgae photoacclimated to different light intensities and implications for mass algal cultivation. J. Appl. Phycol. 1996, 8, 335–343. [Google Scholar] [CrossRef]
- Nedbal, L.; Tichý, V.; Xiong, F.; Grobbelaar, J. Microscopic green algae and cyanobacteria in high-frequency intermittent light. J. Appl. Phycol. 1996, 8, 325–333. [Google Scholar] [CrossRef]
- Ginovart, M.; Pintado, J.; Del Olmo, G.; Cremades, J.; Jiménez, P.; Masaló, I. Light distribution in tanks with the green seaweed Ulva ohnoi: Effect of stocking density, incident irradiance and chlorophyll content. J. Appl. Phycol. 2023, 35, 1995–2006. [Google Scholar] [CrossRef]
- Luo, H.P.; Kemoun, A.; Al-Dahhan, M.H.; Sevilla, J.; Sánchez, J.; Camacho, F.; Grima, E. Analysis of photobioreactors for culturing high-value microalgae and cyanobacteria via an advanced diagnostic technique: CARPT. Chem. Eng. Sci. 2003, 58, 2519–2527. [Google Scholar] [CrossRef]
- Oca, J.; Machado, S.; Jimenez de Ridder, P.; Cremades, J.; Pintado, J.; Masaló, I. Comparison of two water agitation methods in seaweed culture tanks: Influence of the rotating velocity in the seaweed growth and energy requirement. In Proceedings of the EAS2016—Food for Thought, Edinburgh, UK, 20–23 September 2016; pp. 716–717. [Google Scholar]
- Traugott, H.; Zollmann, M.; Cohen, H.; Chemodanov, A.; Liberzon, A.; Golberg, A. Aeration and nitrogen modulated growth rate and chemical composition of green macroalgae Ulva sp. cultured in a photobioreactor. Algal Res. 2020, 47, 101808. [Google Scholar] [CrossRef]
- Bitog, J.; Lee, I.B.; Lee, C.G.; Kim, K.S.; Hwang, H.S.; Hong, S.W.; Seo, I.H.; Kwon, K.S.; Mostafa, E. Application of Computational Fluid Dynamics for Modeling and Designing Photo-Bioreactors: For Microalgae Production: A Review. Comput. Electron. Agric. 2011, 76, 131–147. [Google Scholar] [CrossRef]
- Park, S.; Li, Y. Integration of biological kinetics and computational fluid dynamics to model the growth of Nannochloropsis salina in an open channel raceway. Biotechnol. Bioeng. 2015, 112, 923–933. [Google Scholar] [CrossRef]
- Ranganathan, P.; Pandey, A.K.; Sirohi, R.; Tuan Hoang, A.; Kim, S.H. Recent advances in computational fluid dynamics (CFD) modelling of photobioreactors: Design and applications. Bioresour. Technol. 2022, 350, 126920. [Google Scholar] [CrossRef] [PubMed]
- ANSYS Fluent. ANSYS Fluent Theory Guide; ANSYS, Inc.: Canonsburg, PA, USA, 2017. [Google Scholar]
- Siemens Digital Industries Software. Simcenter STAR-CCM+ Product Documentation. 2018. Available online: https://www.plm.automation.siemens.com/global/en/products/simcenter/STAR-CCM.html (accessed on 16 May 2024).
- Hurd, C.L. Water Motion, Marine Macroalgal Physiology, and Production. J. Phycol. 2000, 36, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Koehl, M.A.R. Seaweeds in Moving Water: Form and Function. In On the Economy of Plant Form and Function; Givnish, T.J., Ed.; Cambridge University Press: Cambridge, UK, 1986; pp. 603–634. [Google Scholar]
- Spatz, H.C.; Köhler, L.; Niklas, K.J. Mechanical behaviour of plant tissues: Composite materials or structures? J. Exp. Biol. 1999, 202, 3269–3272. [Google Scholar] [CrossRef] [PubMed]
- Burnett, N.P.; Koehl, M.A.R. Mechanical properties of the wave-swept kelp Egregia menziesii change with season, growth rate and herbivore wounds. J. Exp. Biol. 2019, 222, jeb190595. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.J.; Cyrus, M.D.; Brand, M.J.; Joubert, M.; Macey, B.M. Why grow Ulva? Its potential role in the future of aquaculture. Perspect. Phycol. 2016, 3, 113–120. [Google Scholar] [CrossRef]
- Subramaniam, S. Lagrangian–Eulerian methods for multiphase flows. Prog. Energy Combust. Sci. 2013, 39, 215–245. [Google Scholar] [CrossRef]
- Farivar, F.; Zhang, H.; Tian, Z.F.; Gupte, A. CFD-DEM simulation of fluidization of multisphere- modelled cylindrical particles. Powder Technol. 2020, 360, 1017–1027. [Google Scholar] [CrossRef]
- Zhu, H.; Zhou, Z.; Yang, R.; Yu, A. Discrete particle simulation of particulate systems: A review of major applications and findings. Chem. Eng. Sci. 2008, 63, 5728–5770. [Google Scholar] [CrossRef]
- Johnson, K. Contact Mechanics; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Rayleigh, L. On Waves Propagated along the Plane Surface of an Elastic Solid. Proc. Lond. Math. Soc. 1885, s1–17, 4–11. [Google Scholar] [CrossRef]
- Timoshenko, S.; Timoshenko, S.; Goodier, J. Theory of Elasticity; Timoshenko, S., Goodier, J.N., Eds.; McGraw-Hill Book Company: New York, NY, USA, 1951. [Google Scholar]
- Merchuk, J.C.; Garcia-Camacho, F.; Molina-Grima, E. Photobioreactor Design and Fluid Dynamics. Chem. Biochem. Eng. Q. 2007, 21, 345–355. [Google Scholar]
- Papáček, Š.; Štumbauer, V.; Štys, D.; Petera, K.; Matonoha, C. Growth impact of hydrodynamic dispersion in a Couette–Taylor bioreactor. Math. Comput. Model. 2011, 54, 1791–1795. [Google Scholar] [CrossRef]
- Gao, X.; Kong, B.; Dennis Vigil, R. Comprehensive computational model for combining fluid hydrodynamics, light transport and biomass growth in a Taylor vortex algal photobioreactor: Eulerian approach. Algal Res. 2017, 24, 1–8. [Google Scholar] [CrossRef]
- Masojídek, J.; Koblížek, M.; Torzillo, G. Photosynthesis in Microalgae. In Handbook of Microalgal Culture; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2003; Chapter 2; pp. 20–39. [Google Scholar] [CrossRef]
- Papáček, Š.; Jablonsky, J.; Petera, K. Advanced integration of fluid dynamics and photosynthetic reaction kinetics for microalgae culture systems. BMC Syst. Biol. 2018, 12, 93. [Google Scholar] [CrossRef]
- Molina, E.; Acién Fernández, F.G.; García Camacho, F.; Camacho Rubio, F.; Chisti, Y. Scale-up of Tubular Photobioreactors. J. Appl. Phycol. 2000, 12, 355–368. [Google Scholar] [CrossRef]
- Burlew, J.S. Algal Culture from Laboratory to Pilot Plant; Carnegie Institution of Washington: Washington, DC, USA, 1953. [Google Scholar]
- Celik, I.; Ghia, U.; Roache, P.; Freitas, C.; Coleman, H.; Raad, P. Procedure for Estimation and Reporting of Uncertainty Due to Discretization in CFD Applications. J. Fluids Eng. 2008, 130, 078001. [Google Scholar] [CrossRef]
- Bekkozhayeva, D.; Cisar, P. Image-Based Automatic Individual Identification of Fish without Obvious Patterns on the Body (Scale Pattern). Appl. Sci. 2022, 12, 5401. [Google Scholar] [CrossRef]
- Burnett, B. Coupled Fluid-Structure Interaction Modeling of a Parafoil. Master Thesis, Embryo-Riddle Aeronautical University, Daytona Beach, FL, USA, 2016. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filip, R.; Masaló, I.; Papáček, Š. Modeling and CFD Simulation of Macroalgae Motion within Aerated Tanks: Assessment of Light-Dark Cycle Period. Energies 2024, 17, 3555. https://doi.org/10.3390/en17143555
Filip R, Masaló I, Papáček Š. Modeling and CFD Simulation of Macroalgae Motion within Aerated Tanks: Assessment of Light-Dark Cycle Period. Energies. 2024; 17(14):3555. https://doi.org/10.3390/en17143555
Chicago/Turabian StyleFilip, Radomír, Ingrid Masaló, and Štěpán Papáček. 2024. "Modeling and CFD Simulation of Macroalgae Motion within Aerated Tanks: Assessment of Light-Dark Cycle Period" Energies 17, no. 14: 3555. https://doi.org/10.3390/en17143555
APA StyleFilip, R., Masaló, I., & Papáček, Š. (2024). Modeling and CFD Simulation of Macroalgae Motion within Aerated Tanks: Assessment of Light-Dark Cycle Period. Energies, 17(14), 3555. https://doi.org/10.3390/en17143555






