Biogas-to-Power Systems Based on Solid Oxide Fuel Cells: Thermodynamic Analysis of Stack Integration Strategies
Abstract
1. Introduction
1.1. Small-Size High-Efficiency CHP with Fuel Cells
1.2. Advantages and Disadvantages of Biogas SOFC-CHP: A Short Review
- premix with air: atmospheric air is conditioned (O2 and N2) and admixed to biogas at the SOFC inlet; this choice is aimed at achieving an internal partial oxidation that consumes methane and delivers steam to further favour methane steam reforming.
- cold anode off-gases recirculation: after water condensation and removal, a bleeding of the anode off-gases (dry CO2 + unreacted H2, CO, and CH4) is recirculated back to the SOFC inlet and mixed with fresh biogas; it further promotes internal dry reforming.
- hot anode off-gases recirculation: a bleeding of wet anode off-gases (H2O, CO2 + unreacted H2, CO, and CH4) is recirculated back to the SOFC inlet and mixed with fresh biogas; it promotes both internal dry and steam reforming.
1.3. Scope
2. Methods
2.1. Gas Mixtures Definition
2.2. Modelling Methodology
3. Results
4. Discussion
- (a)
- Carbon dioxide is the only pure gas that can be either removed or added to the mixture. The separation of CO2 from biogas is a well-known and state-of-the-art technology. The most diffused technological solutions are membrane and PSA separation. Both technologies require an energy input, thereby impacting the system’s energy performances. Looking at the process quality, the amount of CO2 separated varies from dry biogas to dry methane in a high-risk carbon deposition area, as reported in Figure 2. The introduction of additional CO2 in compositions m9 and m10 can be obtained by recycling the SOFC anode gas exhausts. In this case, depending on the system design, CO2 has to be separated from hydrogen and steam with a cooling and separation process based on the previously listed technologies.
- (b)
- Air can be easily obtained from the ambient atmosphere with the only requirement in terms of filter and compression with a blower. Note that the SOFC already has an air feeding line for the cathode that can be partially used for the anode also.
- (c)
- Water can be partially, or completely, recovered from the anode exhausts after the condensation of the outlet gas stream. Then, water steam can be produced with a steam generator recovering heat from the exhausts or with an additional heat duty for the system.
- (d)
- Looking at hydrogen, two separate strategies can be implemented. On the one hand, hydrogen can be recycled from the gas exhausts. As for CO2, separation from the other species is necessary. In the case of both CO2 and H2, an integrated strategy can be implemented with an optimised tuning for the requested composition. On the other hand, hydrogen can be produced with an additional energy technology. The most interesting option is to consider an electrolyser fed with a renewable energy source, such as solar photovoltaic. In this case, and depending on the electrolyser technology, additional scenarios in terms of energy storage and system integration strategies can be considered—for instance, the water necessary for the electrolysis can be an additional external feedstock requirement or can be recovered from the anode exhausts as in the steam case.
- (e)
- Oxygen can be produced from the separation of air or the electrolysis of water. The latter option seems more interesting in the case hydrogen is also required.
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
References
- European Commission. Directive (Eu) 2023/2413 of the European Parliament and of the Council; European Commission: Brussels, Belgium; Luxembourg, 2023. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions REPowerEU Plan; European Commission: Brussels, Belgium; Luxembourg, 2022. [Google Scholar]
- Kaur, G.; Basak, N.; Kumar, S. State-of-the-art techniques to enhance biomethane/biogas production in thermophilic anaerobic digestion. Process Saf. Environ. Prot. 2024, 186, 104–117. [Google Scholar] [CrossRef]
- Tricase, C.; Lombardi, M. State of the art and prospects of Italian biogas production from animal sewage: Technical-economic considerations. Renew. Energy 2009, 34, 477–485. [Google Scholar] [CrossRef]
- International Energy Agency IEA. Outlook for Biogas and Biomethane: Prospects for Organic Growth—World Energy Outlook Special Report. 2022. Available online: https://www.iea.org/reports/outlook-for-biogas-and-biomethane-prospects-for-organic-growth (accessed on 15 January 2024).
- Sun, Q.; Li, H.; Yan, J.; Liu, L.; Yu, Z.; Yu, X. Selection of appropriate biogas upgrading technology-a review of biogas cleaning, upgrading and utilisation. Renew. Sustain. Energy Rev. 2015, 51, 521–532. [Google Scholar] [CrossRef]
- Bauer, F.; Hulteberg, C.; Persson, T.; Tamm, D. Biogas Upgrading—Review of Commercial Technologies (Biogasuppgradering—Granskning av Kommersiella Tekniker); Svenskt Gastekniskt Center (SGC): Malmoe, Sweden, 2013. [Google Scholar]
- Gkotsis, P.; Kougias, P.; Mitrakas, M.; Zouboulis, A. Biogas upgrading technologies—Recent advances in membrane-based processes. Int. J. Hydrogen Energy 2023, 48, 3965–3993. [Google Scholar] [CrossRef]
- Klavon, K.H.; Lansing, S.A.; Mulbry, W.; Moss, A.R.; Felton, G. Economic analysis of small-scale agricultural digesters in the United States. Biomass Bioenergy 2013, 54, 36–45. [Google Scholar] [CrossRef]
- The World Bank. Promoting Biogas as Sustainable Clean Cooking Fuel for Rural Households in Kenya Project; Proj. Rep., No. Report No: 101650-KE; The World Bank: Washington, DC, USA, 2016. [Google Scholar]
- Simader, G.R.; Krawinkler, R.; Trnka, G. Micro CHP Systems: State-of-the-Art; Austrian Energy Agency: Vienna, Austria, 2006. [Google Scholar]
- Baldinelli, A.; Barelli, L.; Bidini, G.; Cinti, G. Micro-cogeneration based on solid oxide fuel cells: Market opportunities in the agriculture/livestock sector. Int. J. Hydrogen Energy 2021, 46, 10036–10048. [Google Scholar] [CrossRef]
- Boldrin, P.; Millan-Agorio, M.; Brandon, N.P. Effect of sulfur- and tar-contaminated syngas on solid oxide fuel cell anode materials. Energy Fuels 2015, 29, 442–446. [Google Scholar] [CrossRef]
- Baldinelli, A.; Barelli, L.; Bidini, G. On the feasibility of on-farm biogas-to-electricity conversion: To what extent is solid oxide fuel cells durability a threat to break even the initial investment? Int. J. Hydrogen Energy 2018, 43, 16971–16985. [Google Scholar] [CrossRef]
- Bae, J. Fuel Processor Lifetime and Reliability in Solid Oxide Fuel Cells; Elsevier Ltd.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Tjaden, B.; Gandiglio, M.; Lanzini, A.; Santarelli, M.; Ja, M. Small-Scale Biogas-SOFC Plant: Technical Analysis and Assessment of Different Fuel Reforming Options. Energy Fuels 2014, 28, 4216–4232. [Google Scholar] [CrossRef]
- Trendewicz, A.A.; Braun, R.J. Techno-economic analysis of solid oxide fuel cell-based combined heat and power systems for biogas utilization at wastewater treatment facilities. J. Power Sources 2013, 233, 380–393. [Google Scholar] [CrossRef]
- Kupecki, J.; Skrzypkiewicz, M.; Wierzbicki, M.; Stepien, M. Analysis of a Micro-CHP Unit with in-series SOFC Stacks Fed by Biogas. Energy Procedia 2015, 75, 2021–2026. [Google Scholar] [CrossRef]
- Sulzer Hexis SOFC Systems for Biogas and Heating Oil. Available online: https://www.osti.gov/etdeweb/biblio/20375397 (accessed on 5 April 2024).
- BIOCELL—Energy Self-Sustaining and Environmental Footprint Reduction on Wastewater Treatment Plants via Fuel Cells. Available online: https://webgate.ec.europa.eu/life/publicWebsite/index.cfm?fuseaction=search.dspPage&n_proj_id=3279 (accessed on 8 February 2024).
- Jahn, M.; Heddrich, M. Brennstoffzellen für Biogas: Aufbau und Betrieb Eines Sofc-Systems. In Biotechnologische Energieumwandlung: Gegenwärtige Situation, Chancen und Künftiger Forschungsbedarf; Bley, T., Ed.; Springer: Berlin, Heidelberg, 2009. [Google Scholar] [CrossRef]
- Kushi, T. Performance and durability evaluation of dry reforming in solid oxide fuel cells. Int. J. Hydrogen Energy 2016, 41, 17567–17576. [Google Scholar] [CrossRef]
- Lin, Y.; Zhan, Z.; Barnett, S.A. Improving the stability of direct-methane solid oxide fuel cells using anode barrier layers. J. Power Sources 2006, 158, 1313–1316. [Google Scholar] [CrossRef]
- Ma, S.; Loreti, G.; Wang, L.; Maréchal, F.; Van Herle, J.; Dong, C. Comparison and optimization of different fuel processing options for biogas-fed solid-oxide fuel cell plants. Int. J. Hydrogen Energy 2022, 47, 551–564. [Google Scholar] [CrossRef]
- Santarelli, M.; Quesito, F.; Novaresio, V.; Guerra, C.; Lanzini, A.; Beretta, D. Direct reforming of biogas on Ni-based SOFC anodes: Modelling of heterogeneous reactions and validation with experiments. J. Power Sources 2013, 242, 405–414. [Google Scholar] [CrossRef]
- Pillai, M.; Lin, Y.; Zhu, H.; Kee, R.J.; Barnett, S.A. Stability and coking of direct-methane solid oxide fuel cells: Effect of CO2 and air additions. J. Power Sources 2010, 195, 271–279. [Google Scholar] [CrossRef]
- Saadabadi, S.A.; Illathukandy, B.; Aravind, P.V. Direct internal methane reforming in biogas fuelled solid oxide fuel cell; the influence of operating parameters. Energy Sci. Eng. 2021, 9, 1232–1248. [Google Scholar] [CrossRef]
- Baldinelli, A.; Subotic, V.; Mondi, F.; Di Michele, A.; Barelli, L.; Bidini, G. Quick degradation detection on biogas-fuelled SOFCs. ECS Trans. 2019, 91, 1571. [Google Scholar] [CrossRef]
- Baldinelli, A.; Barelli, L.; Bidini, G.; Di Michele, A.; Vivani, R. SOFC direct fuelling with high-methane gases: Optimal strategies for fuel dilution and upgrade to avoid quick degradation. Energy Convers. Manag. 2016, 124, 492–503. [Google Scholar] [CrossRef]
- The Biogas. Available online: http://www.biogas-renewable-energy.info/biogas_composition.html (accessed on 5 April 2024).
- Makaruk, A.; Miltner, M.; Harasek, M. Membrane biogas upgrading processes for the production of natural gas substitute. Sep. Purif. Technol. 2010, 74, 83–92. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Pérez-Fortes, M.; Aubin, P.; Lin, T.E.; Yang, Y.; Maréchal, F. Reversible solid-oxide cell stack based power-to-x-to-power systems: Comparison of thermodynamic performance. Appl. Energy 2020, 275, 115330. [Google Scholar] [CrossRef]
- Baldinelli, A.; Staffolani, A.; Nobili, F.; Barelli, L. Detailed experimental analysis of solid oxide cells degradation due to frequent fuel cell/electrolyser switch. J. Energy Storage 2023, 73, 109117. [Google Scholar] [CrossRef]
- NETL. Fuel Cell Handbook, 7th ed.; NETL: Morgantown, WV, USA, 2004. [Google Scholar]
- Hanna, J.; Lee, W.Y.; Shi, Y.; Ghoniem, A.F. Fundamentals of electro- and thermochemistry in the anode of solid-oxide fuel cells with hydrocarbon and syngas fuels. Prog. Energy Combust. Sci. 2014, 40, 74–111. [Google Scholar] [CrossRef]
- Thermfact/CRCT and GTT-Technologies. Fact Sage. Available online: https://factsage.com/ (accessed on 8 February 2024).
- Sasaki, K.; Teraoka, Y. Equilibria in Fuel Cell Gases. J. Electrochem. Soc. 2003, 150, A885. [Google Scholar] [CrossRef]
- Barelli, L.; Bidini, G.; Cinti, G. Operation of a Solid Oxide Fuel Cell Based Power System with Ammonia as a Fuel: Experimental Test and System Design. Energies 2020, 13, 6173. [Google Scholar] [CrossRef]
- Cinti, G.; Liso, V.; Araya, S.S. Design improvements for ammonia-fed SOFC systems through power rating, cascade design and fuel recirculation. Int. J. Hydrogen Energy 2023, 48, 15269–15279. [Google Scholar] [CrossRef]
- Peters, R.; Deja, R.; Engelbracht, M.; Frank, M.; Blum, L.; Stolten, D. Efficiency analysis of a hydrogen-fueled solid oxide fuel cell system with anode off-gas recirculation. J. Power Sources 2016, 328, 105–113. [Google Scholar] [CrossRef]

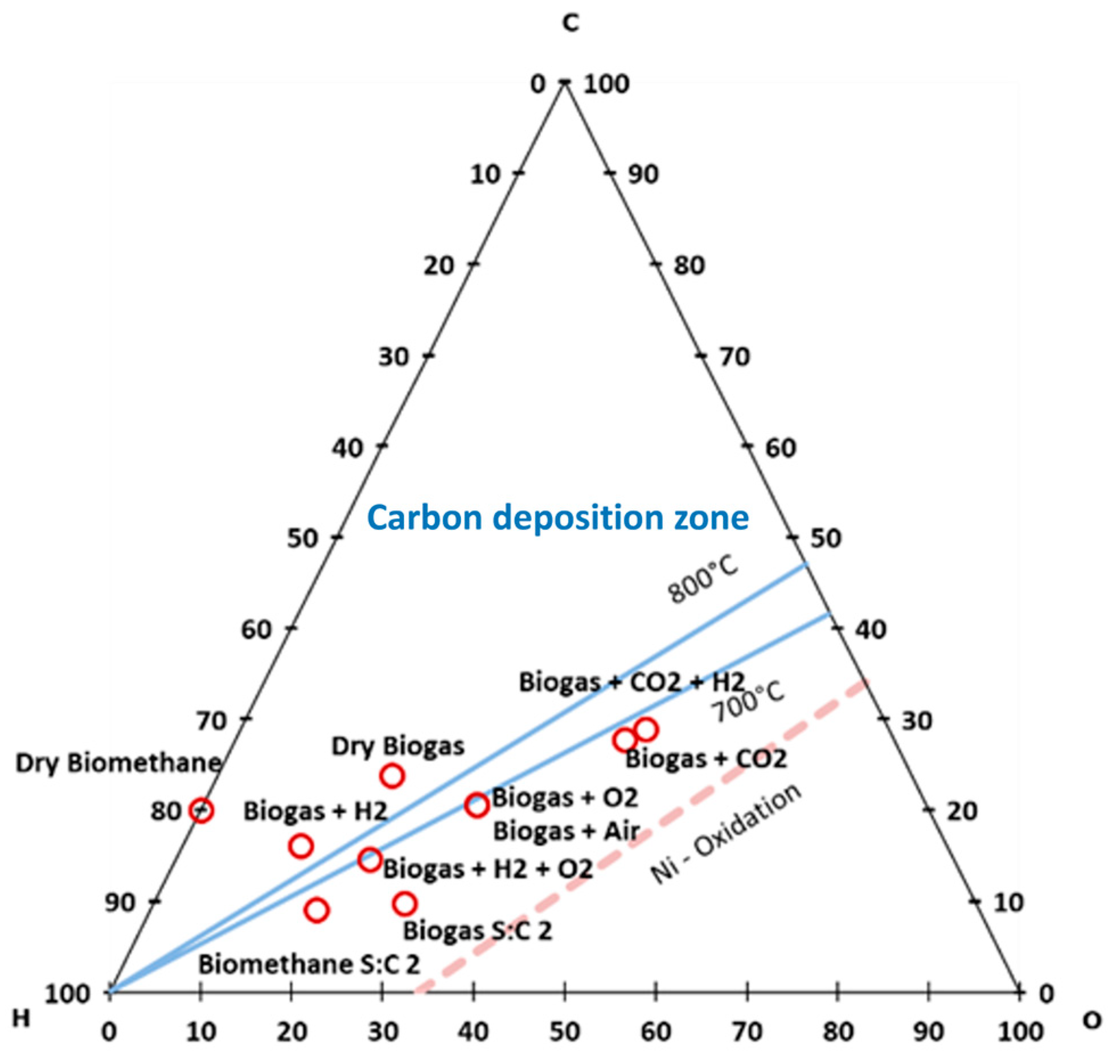
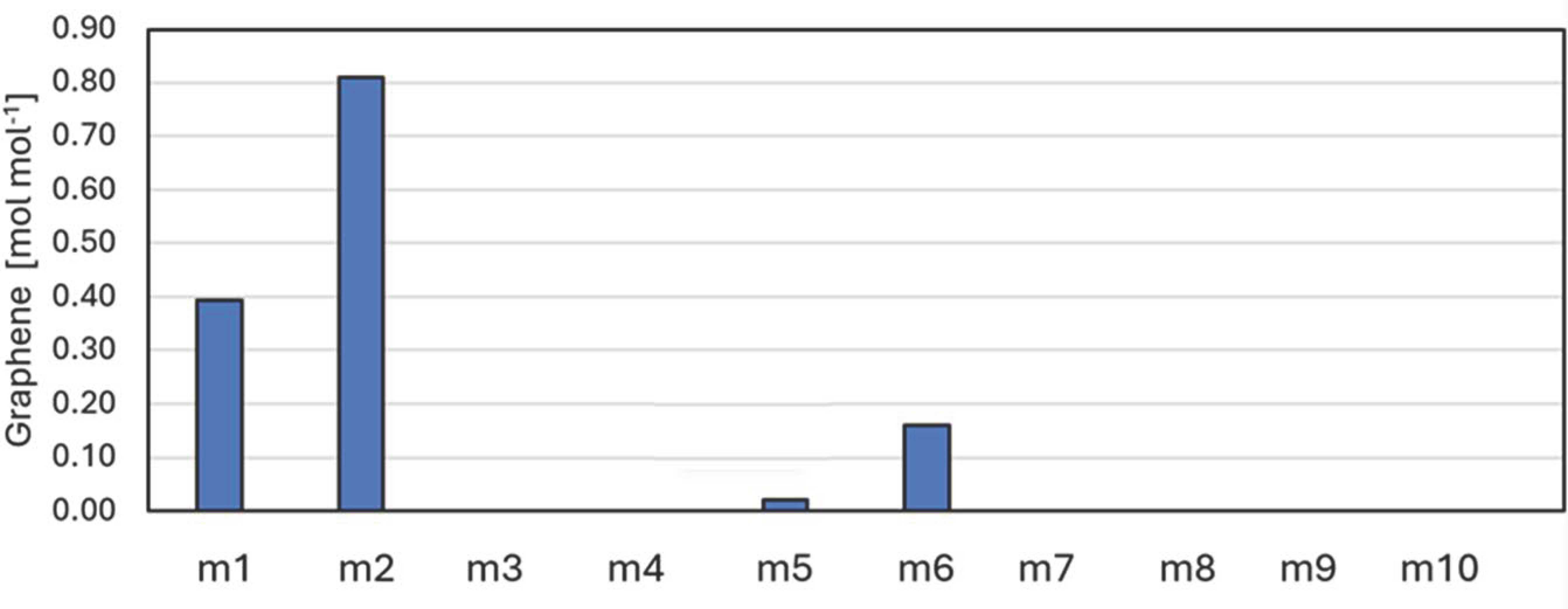
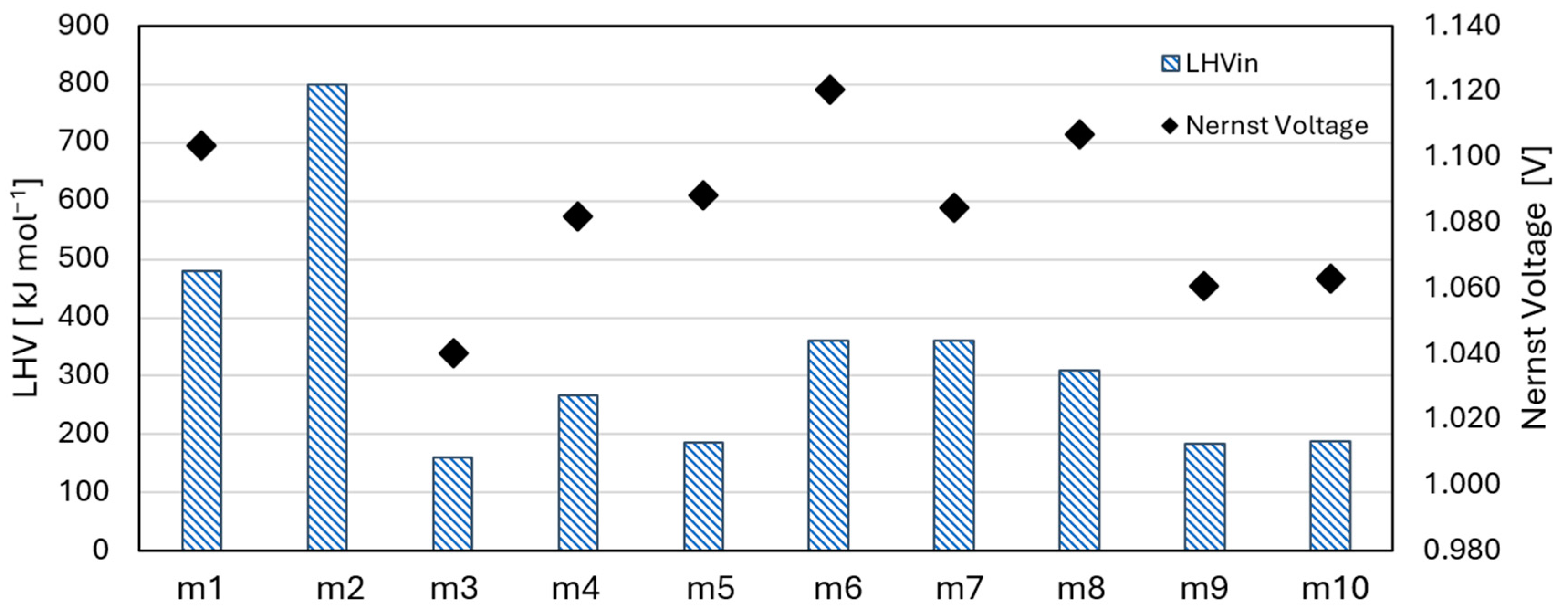
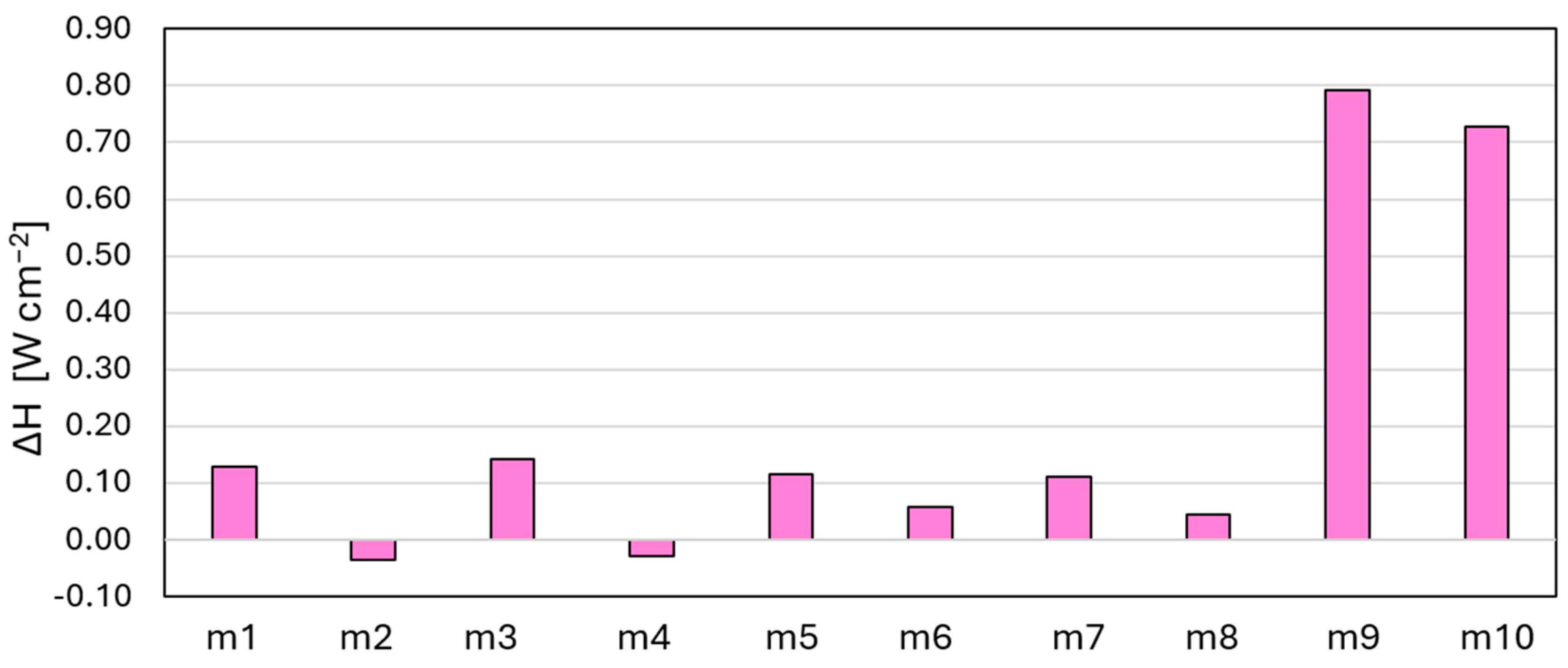
| # | Name | CH4 | CO2 | H2 | H2O | N2 | O2 |
|---|---|---|---|---|---|---|---|
| m1 | Dry Biogas | 60.0% | 40.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| m2 | Dry Biomethane | 100.0% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% |
| m3 | Biogas S:C 2 | 20.0% | 13.3% | 0.0% | 66.7% | 0.0% | 0.0% |
| m4 | Biomethane S:C 2 | 33.3% | 0.0% | 0.0% | 66.7% | 0.0% | 0.0% |
| m5 | Biogas + Air | 23.3% | 15.6% | 0.0% | 0.0% | 48.3% | 12.8% |
| m6 | Biogas + H2 | 30.0% | 20.0% | 50.0% | 0.0% | 0.0% | 0.0% |
| m7 | Biogas + O2 | 45.1% | 30.1% | 0.0% | 0.0% | 0.0% | 24.8% |
| m8 | Biogas + H2 + O2 | 25.7% | 17.1% | 42.9% | 0.0% | 0.0% | 14.3% |
| m9 | Biogas + CO2 | 23.1% | 76.9% | 0.0% | 0.0% | 0.0% | 0.0% |
| m10 | Biogas + CO2 + H2 | 21.5% | 71.7% | 6.7% | 0.0% | 0.0% | 0.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldinelli, A.; Desideri, U.; Fantozzi, F.; Cinti, G. Biogas-to-Power Systems Based on Solid Oxide Fuel Cells: Thermodynamic Analysis of Stack Integration Strategies. Energies 2024, 17, 3614. https://doi.org/10.3390/en17153614
Baldinelli A, Desideri U, Fantozzi F, Cinti G. Biogas-to-Power Systems Based on Solid Oxide Fuel Cells: Thermodynamic Analysis of Stack Integration Strategies. Energies. 2024; 17(15):3614. https://doi.org/10.3390/en17153614
Chicago/Turabian StyleBaldinelli, Arianna, Umberto Desideri, Francesco Fantozzi, and Giovanni Cinti. 2024. "Biogas-to-Power Systems Based on Solid Oxide Fuel Cells: Thermodynamic Analysis of Stack Integration Strategies" Energies 17, no. 15: 3614. https://doi.org/10.3390/en17153614
APA StyleBaldinelli, A., Desideri, U., Fantozzi, F., & Cinti, G. (2024). Biogas-to-Power Systems Based on Solid Oxide Fuel Cells: Thermodynamic Analysis of Stack Integration Strategies. Energies, 17(15), 3614. https://doi.org/10.3390/en17153614









