Investigation of Alternative Substances for Replacing Hydrogen in Methanation
Abstract
:1. Introduction
2. Numerical Method
2.1. Chemical Equilibrium Calculation by NASA-CEA
- TP mode (temperature and pressure);
- HP mode (enthalpy and pressure);
- SP mode (entropy and pressure);
- TV mode (temperature and volume);
- UV mode (internal energy and volume);
- SV mode (entropy and volume).
2.2. Definition of Methane Yield
3. Results
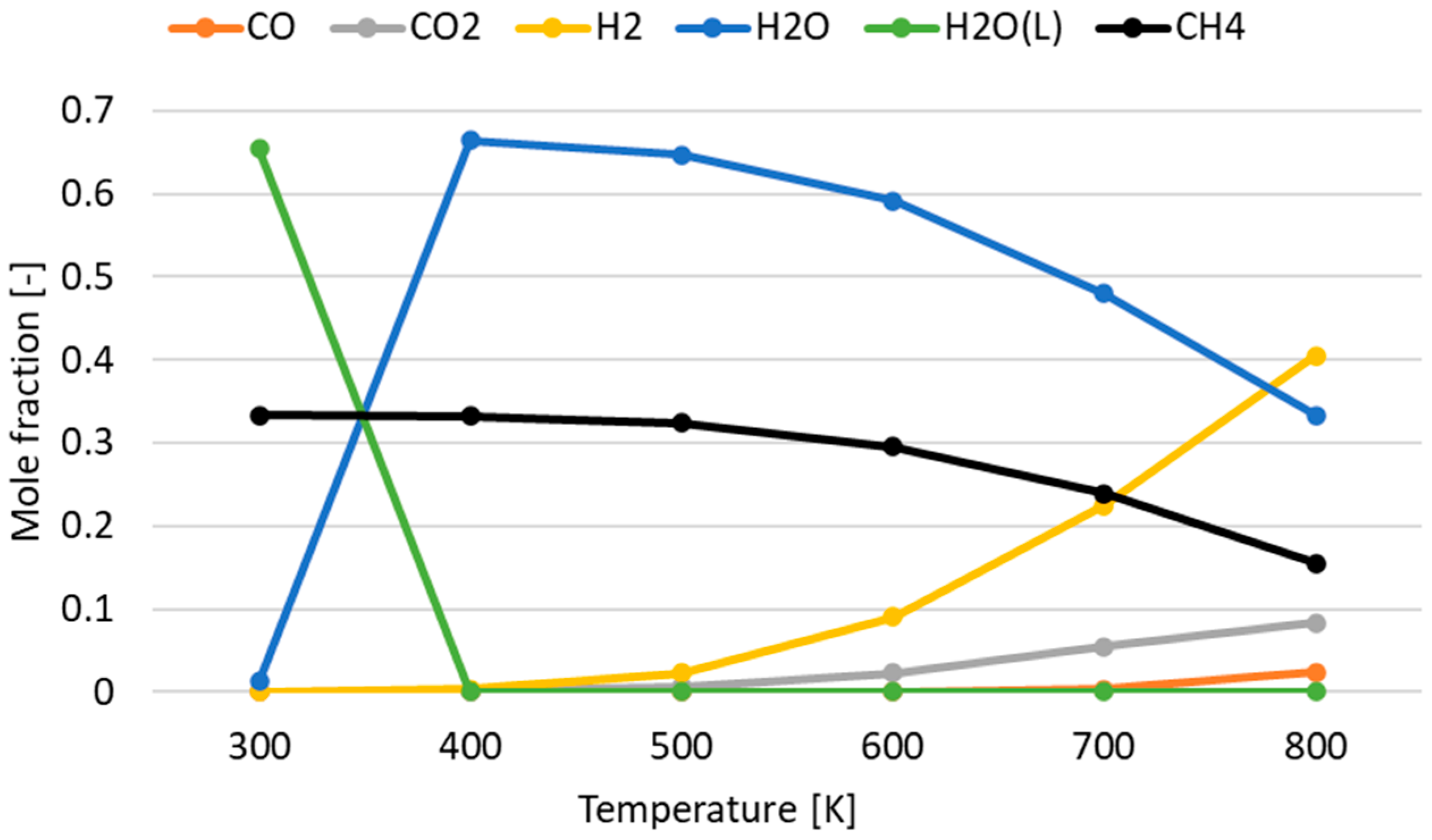
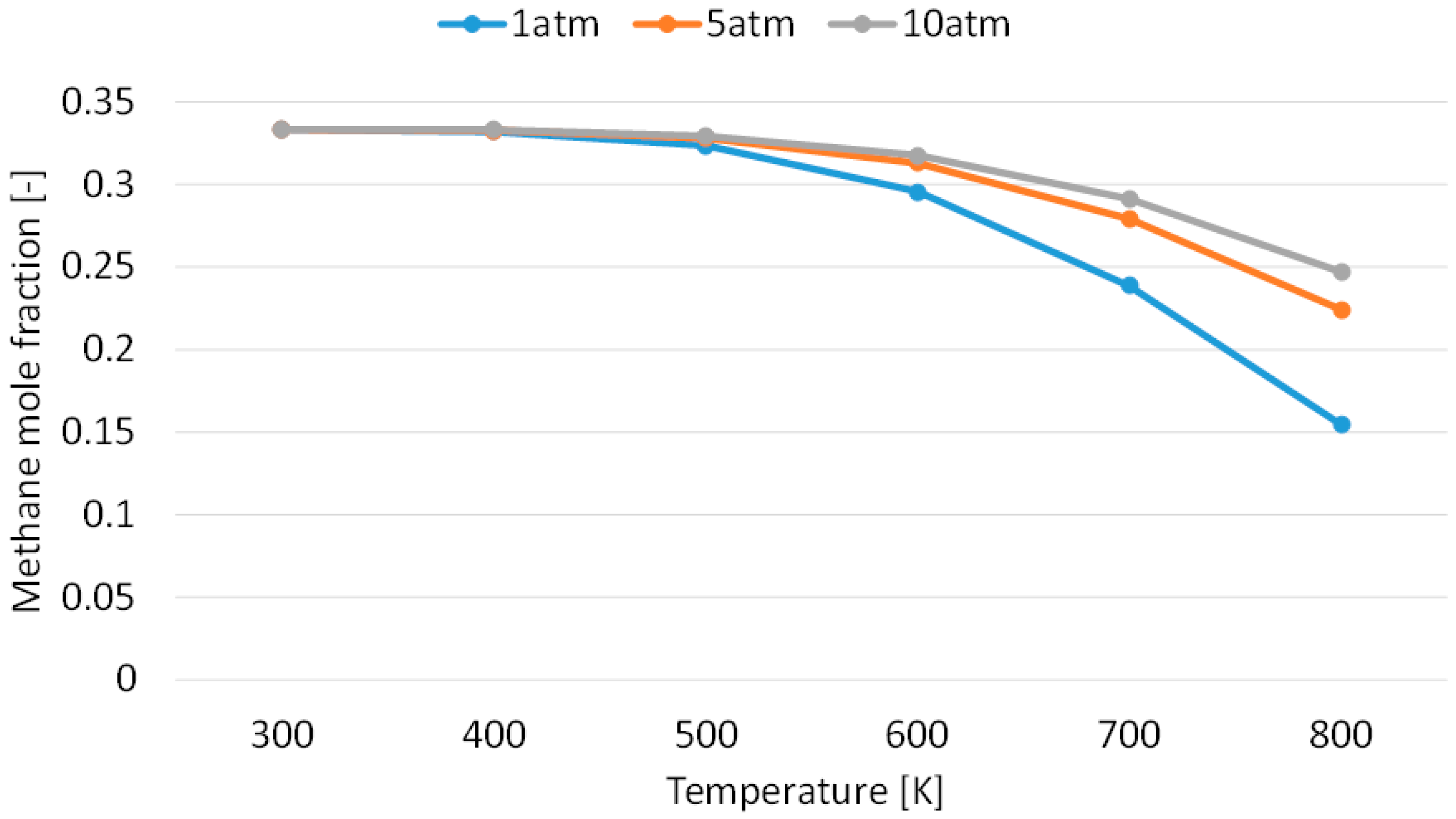
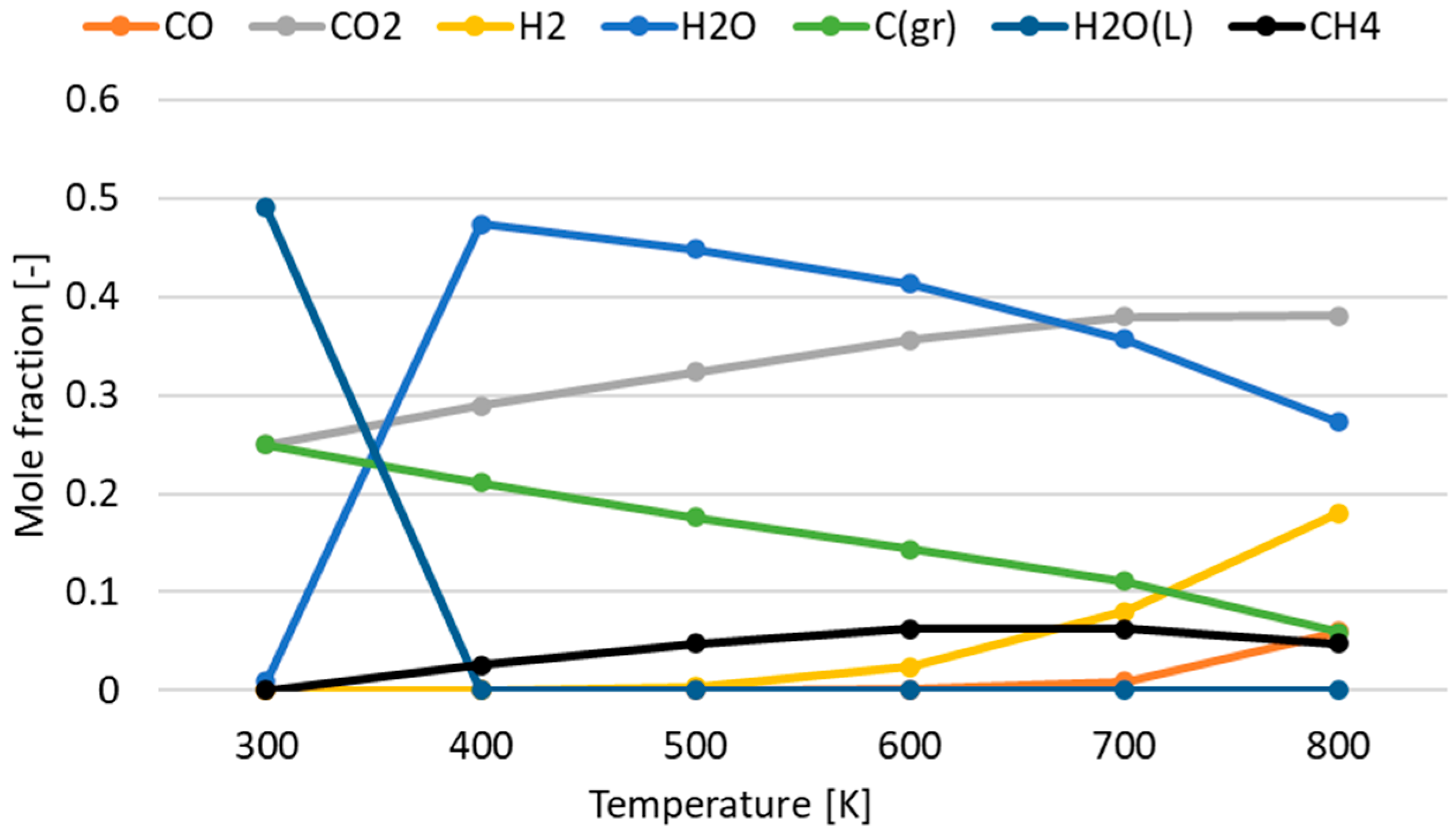
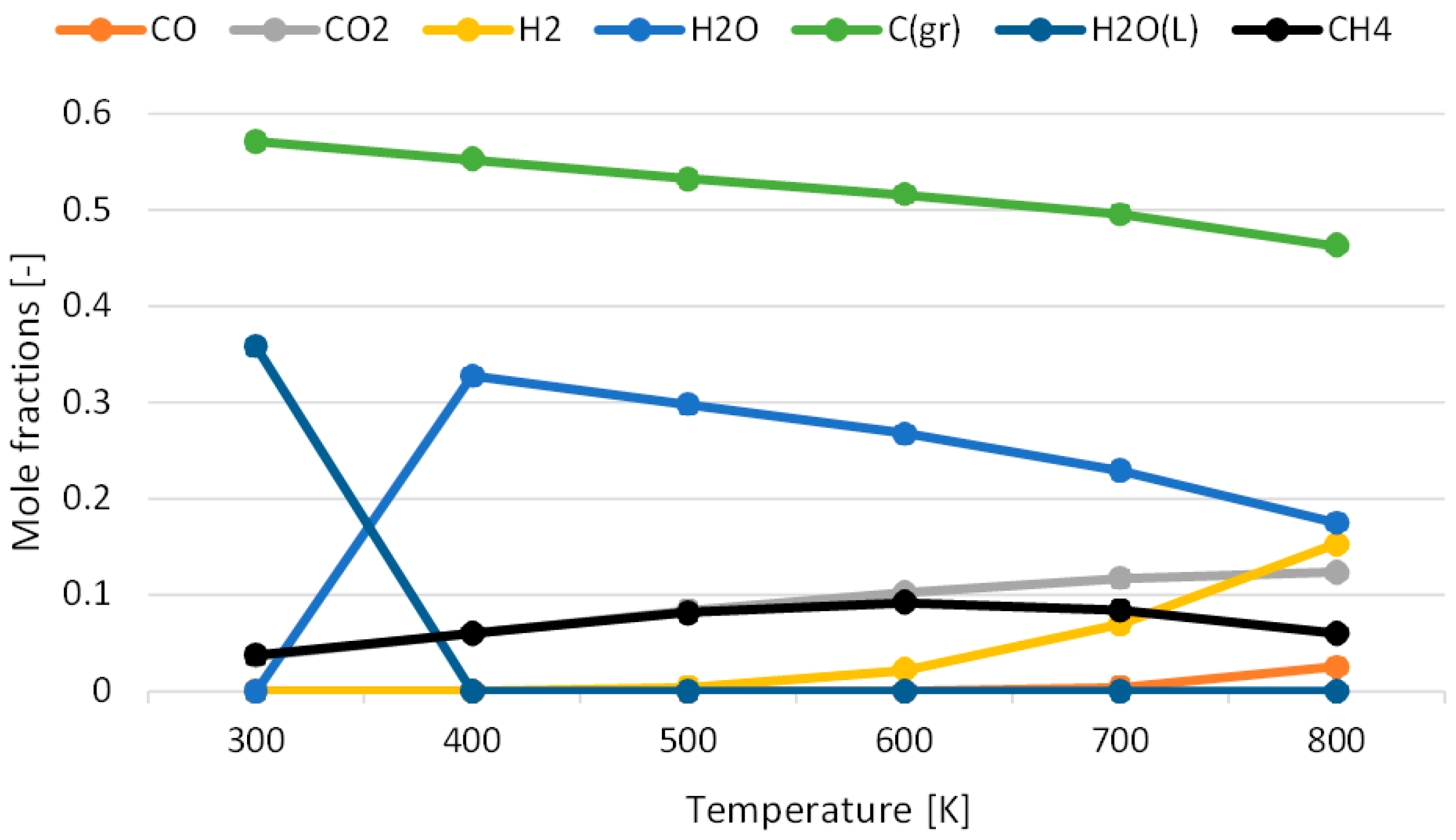
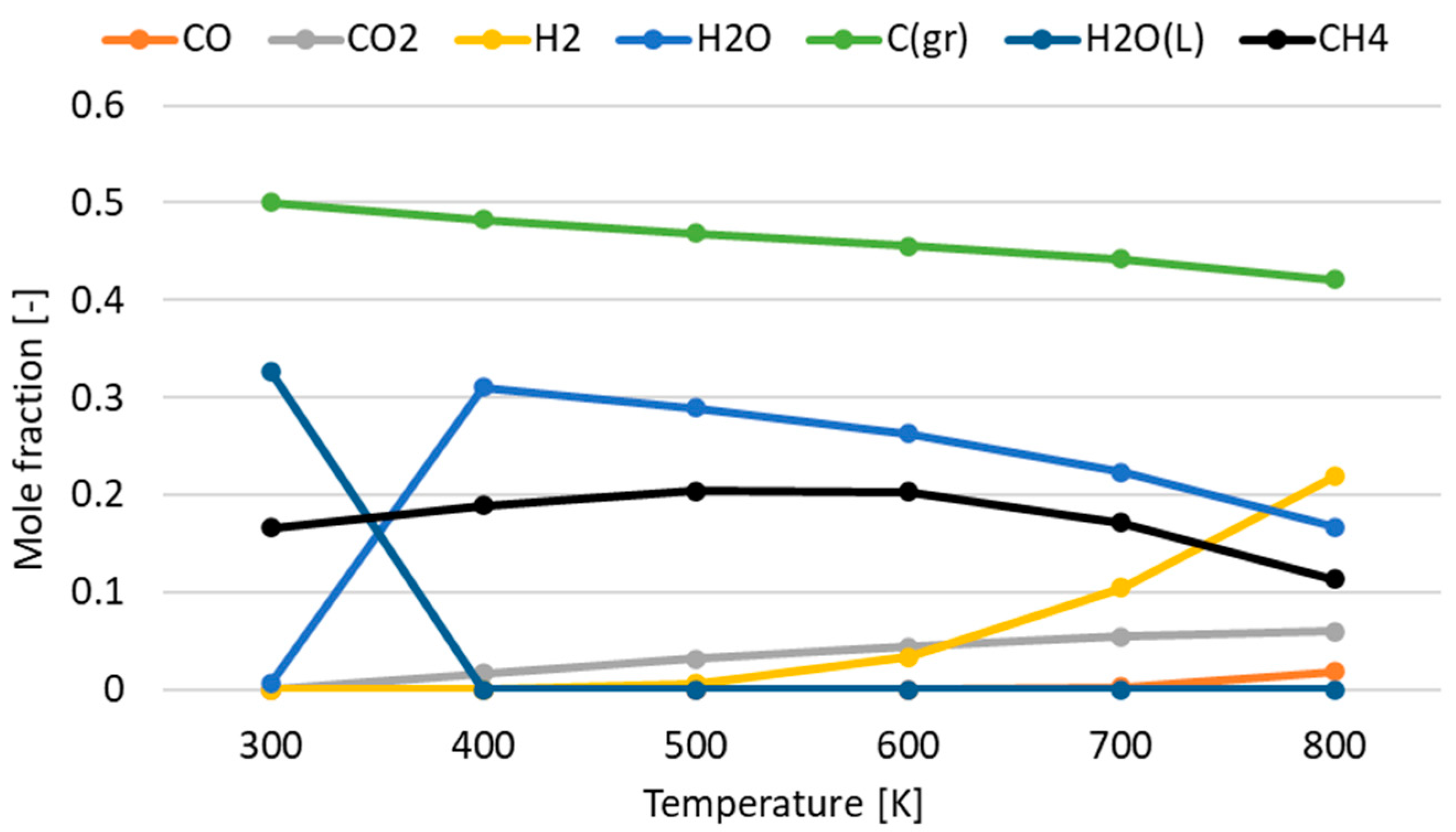
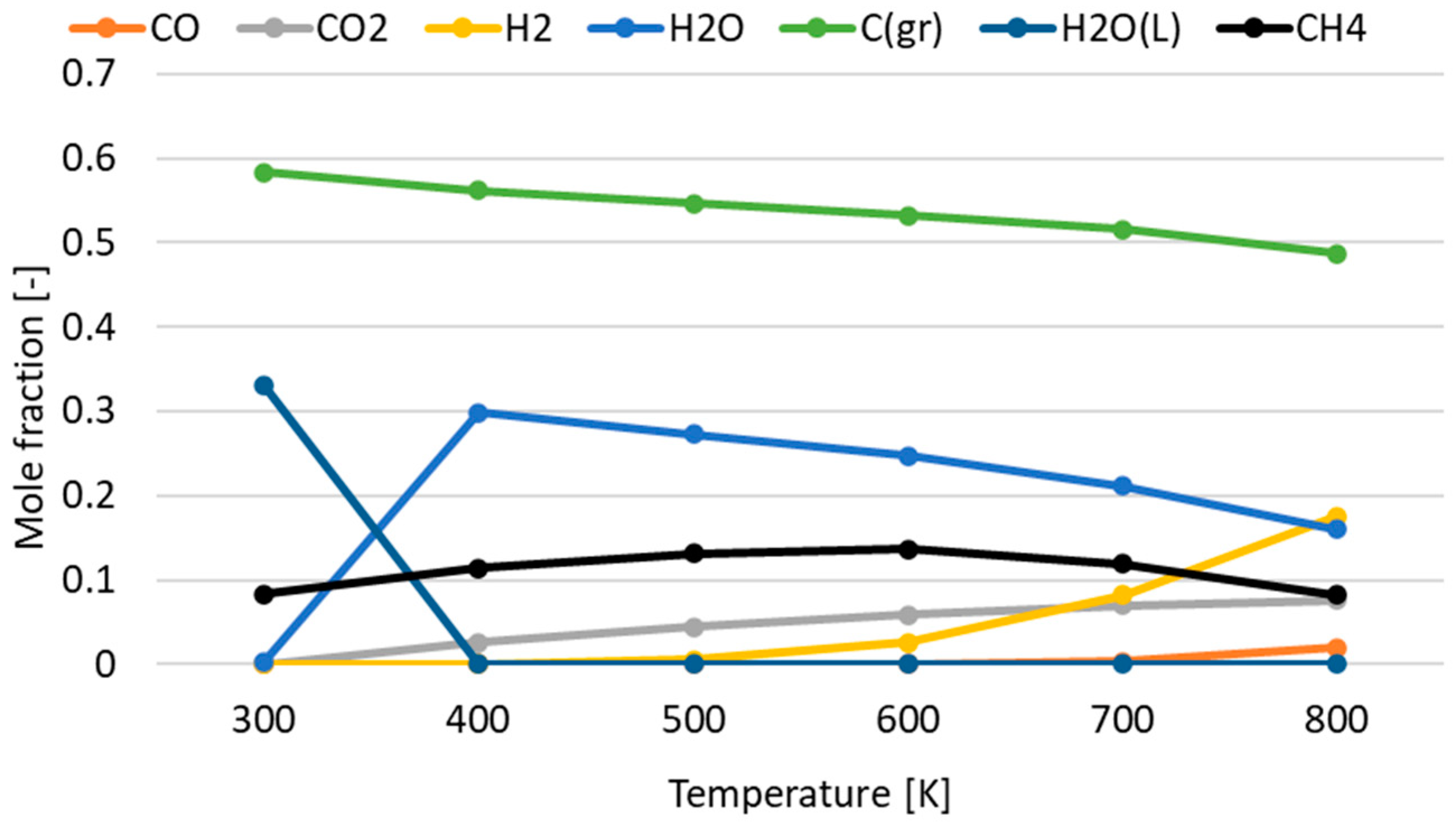
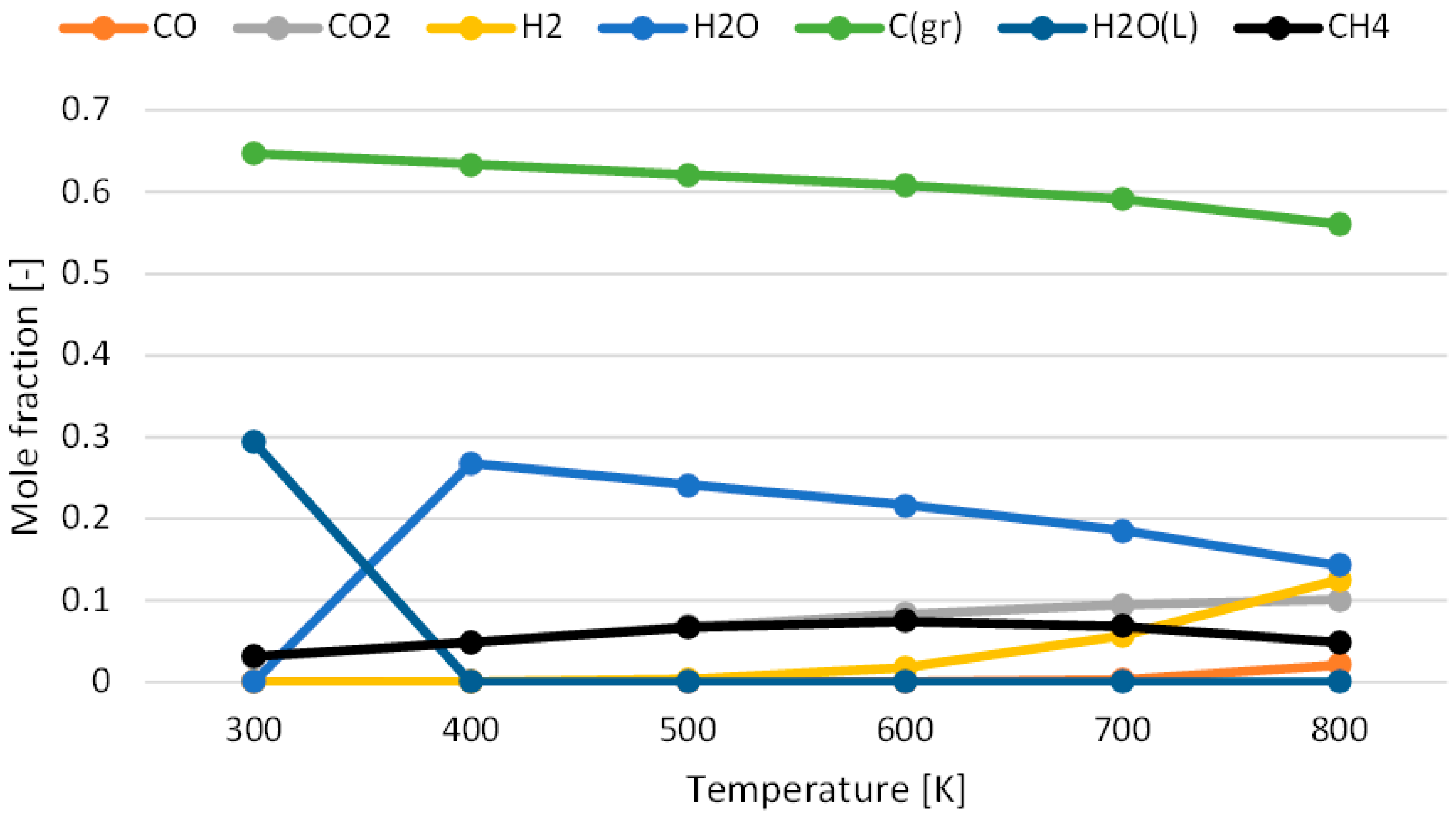
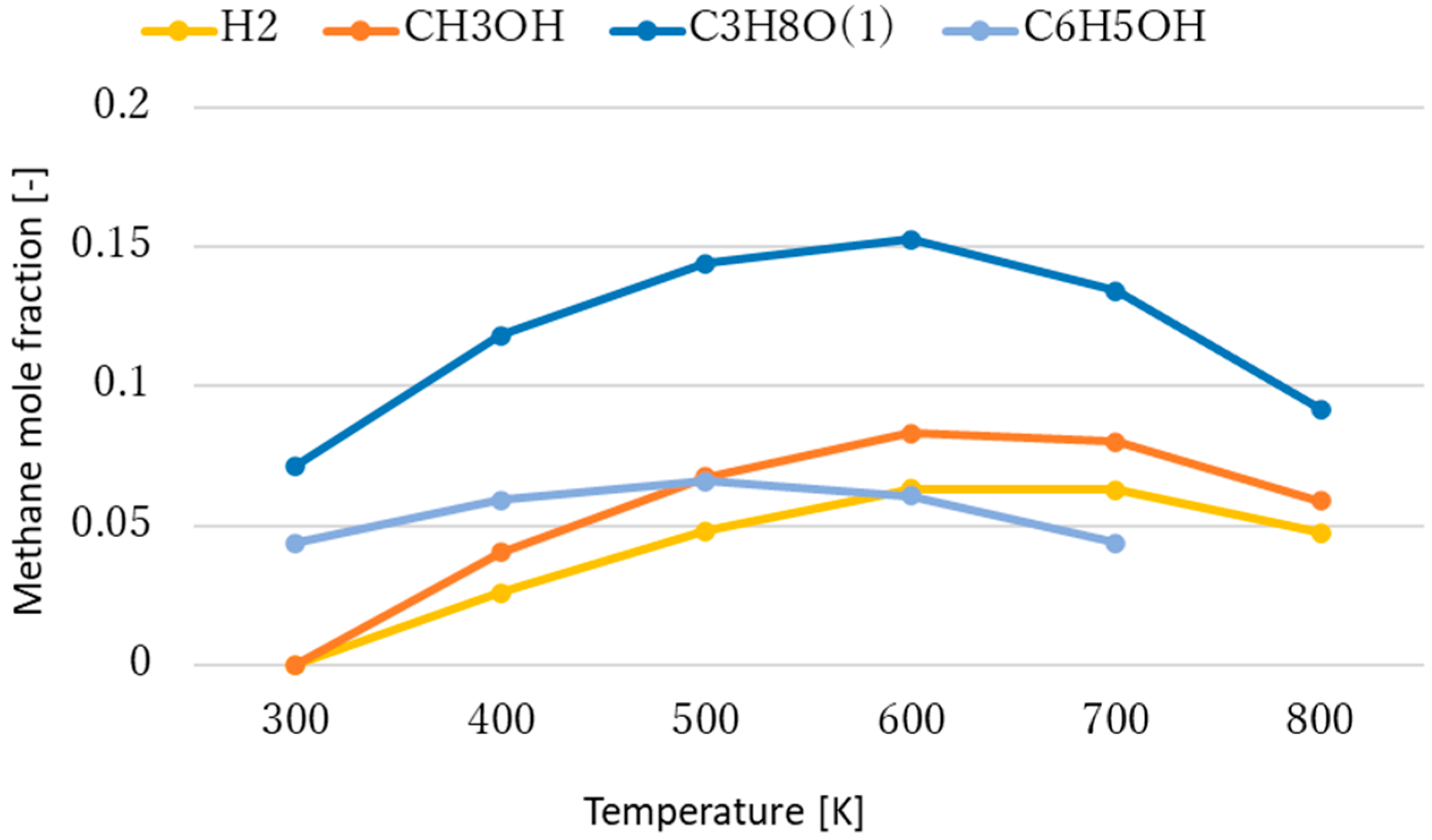
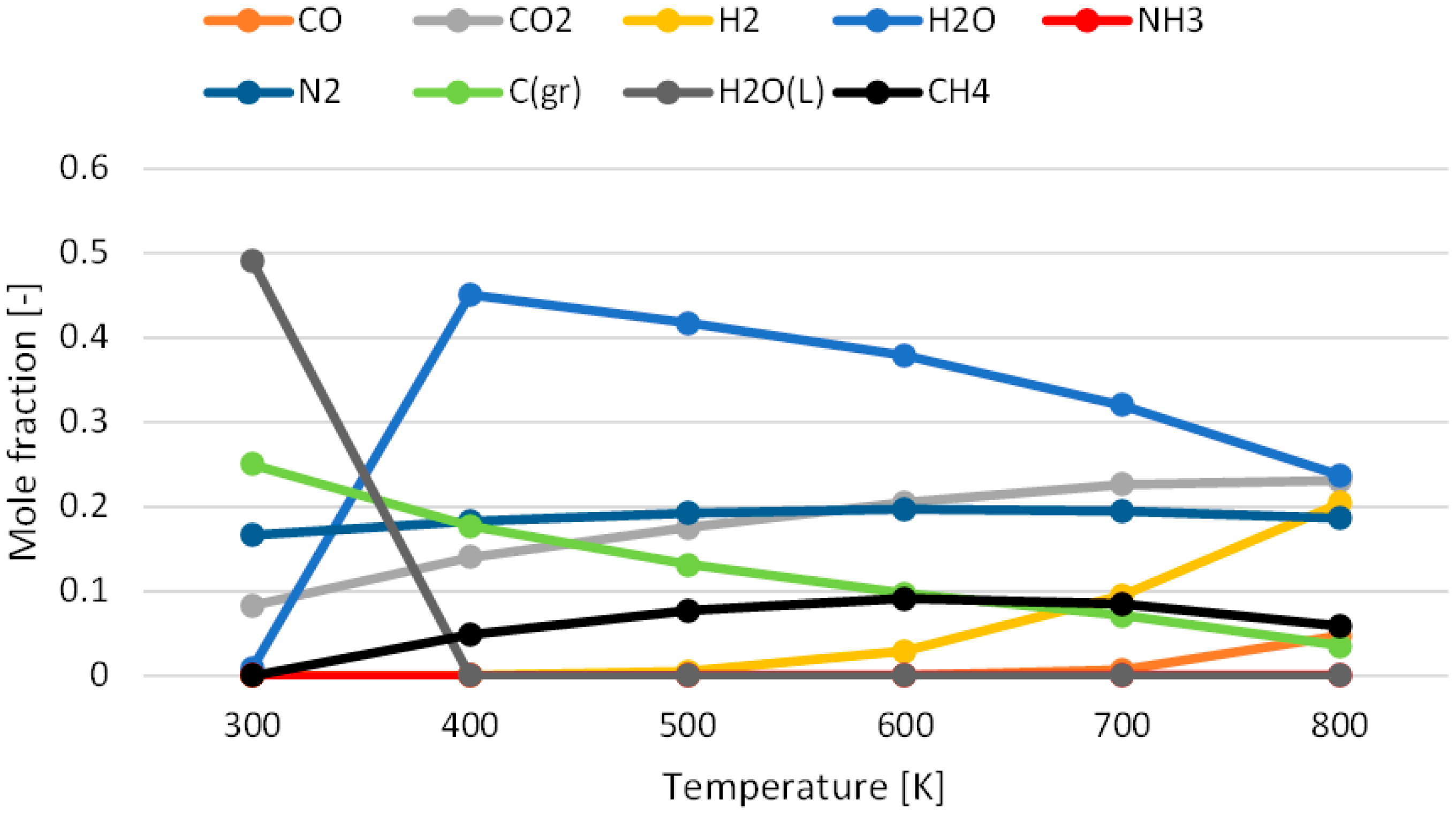
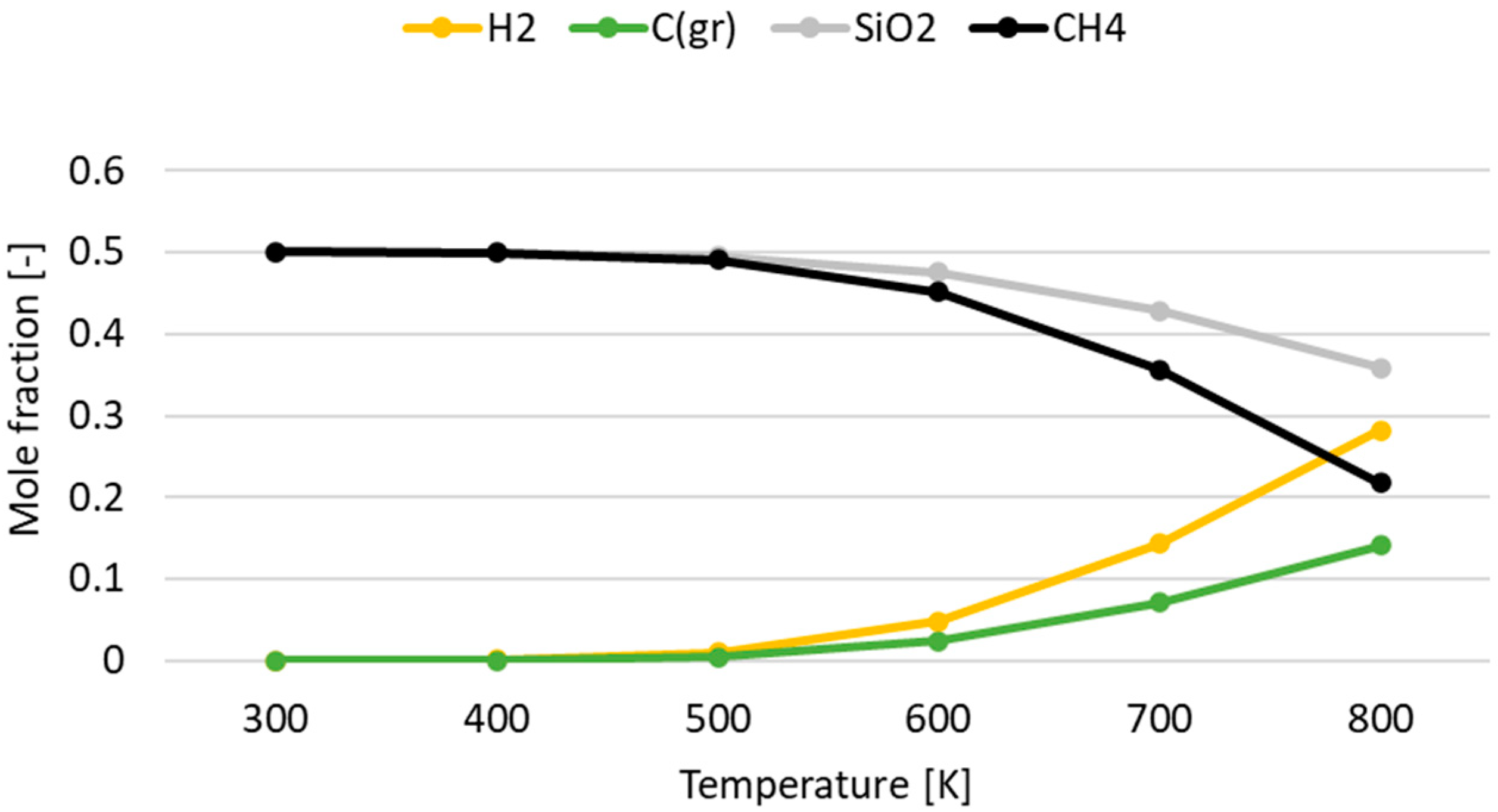
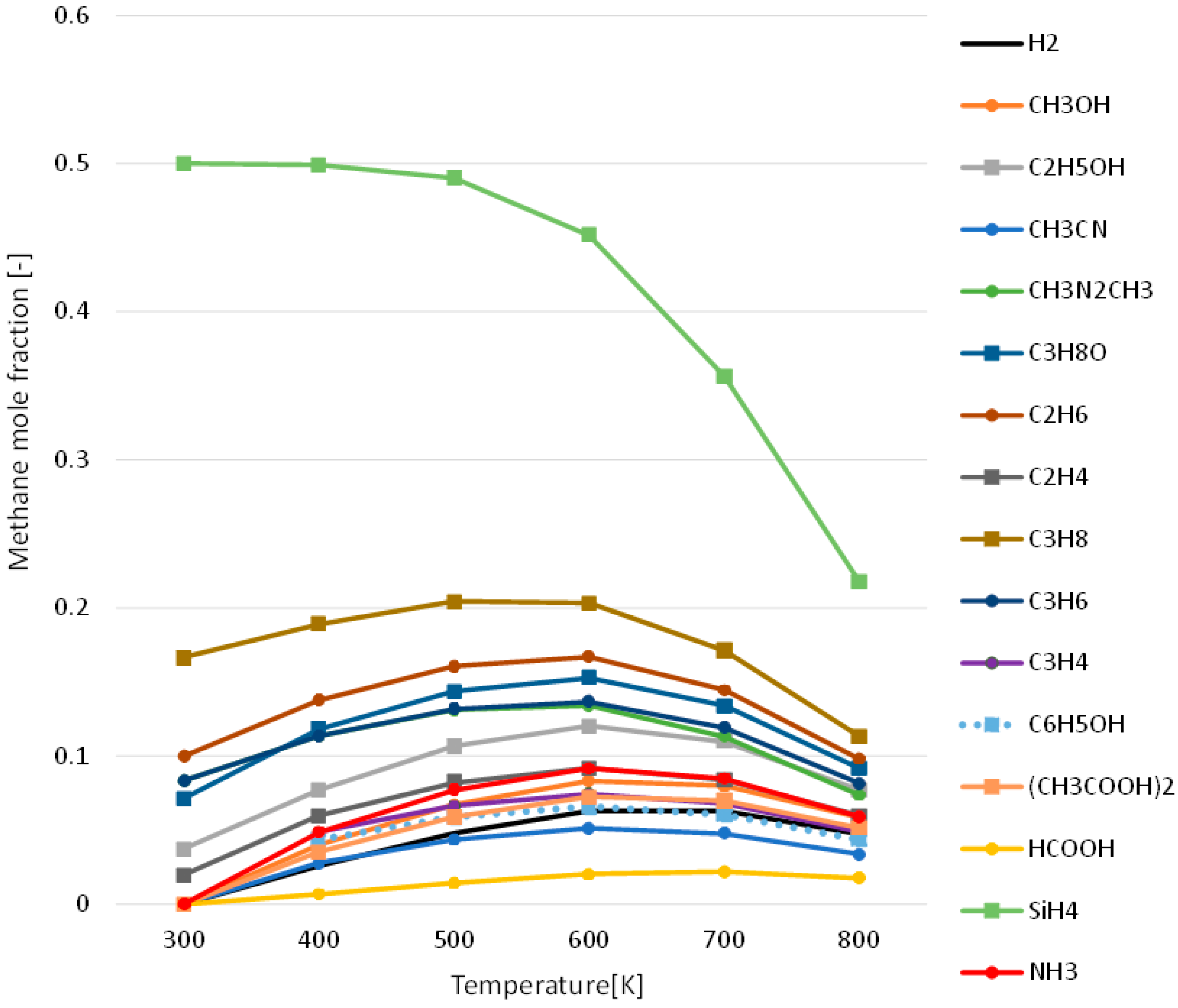
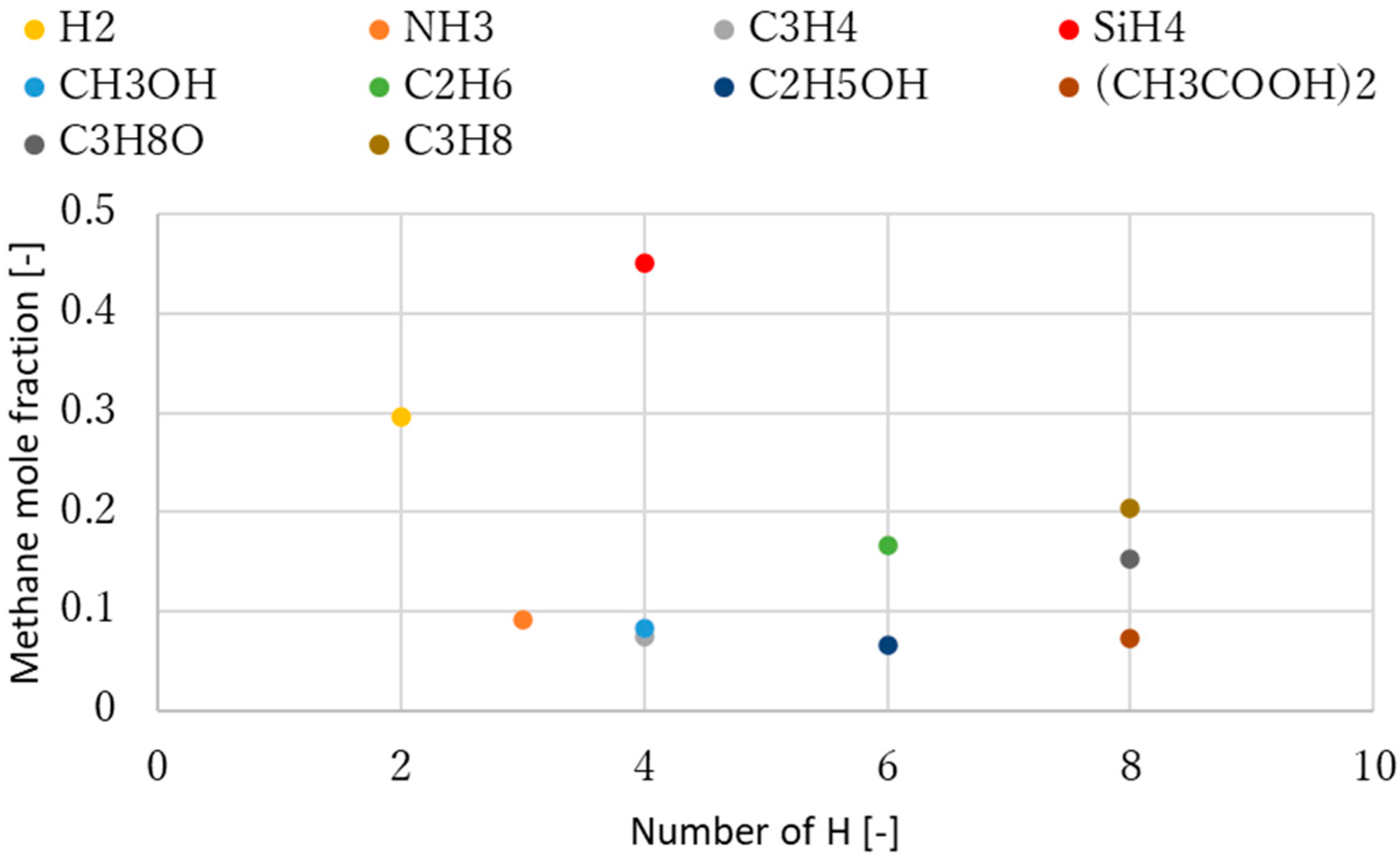
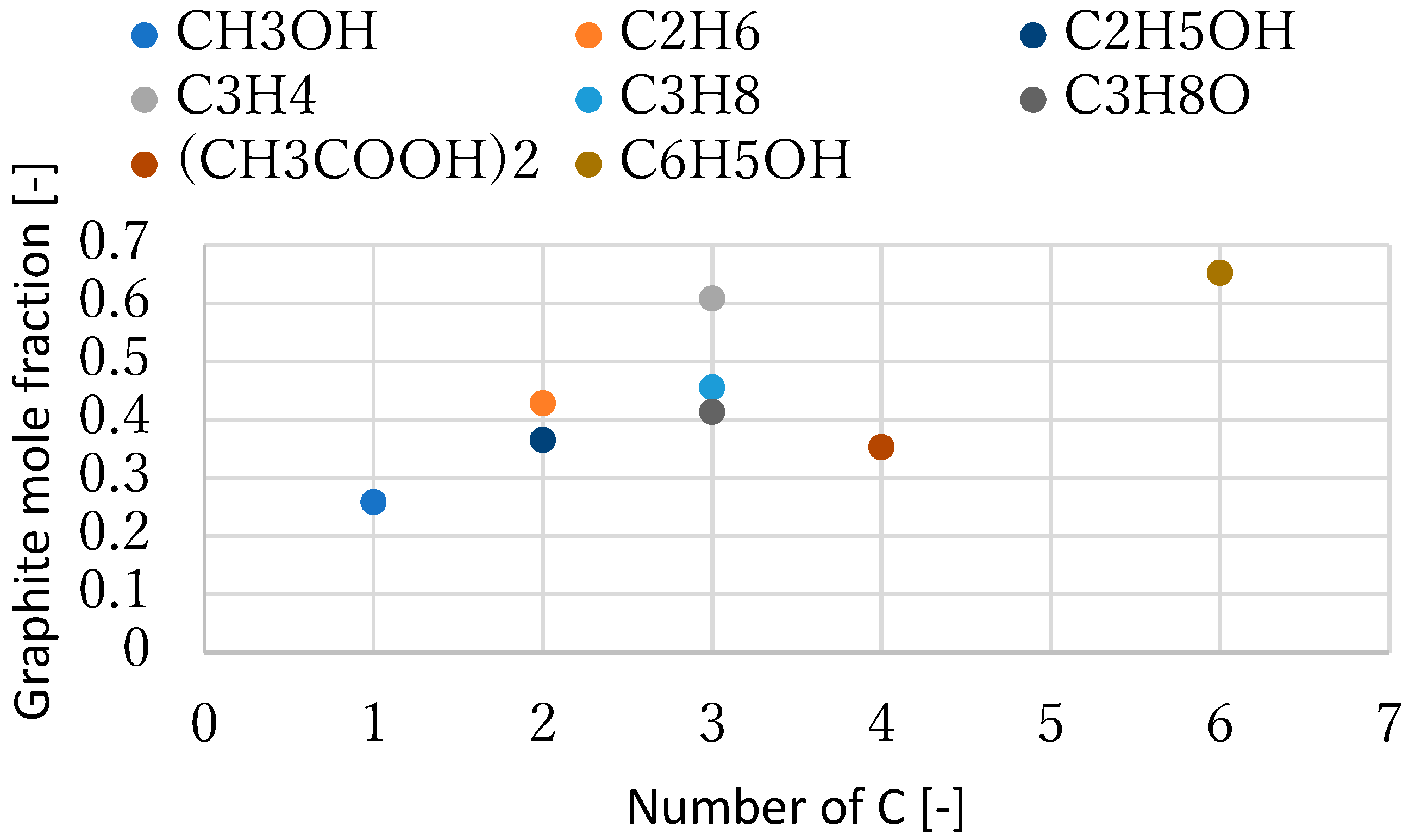
3.1. Effect of Temperature for Each Hydrogen Alternative
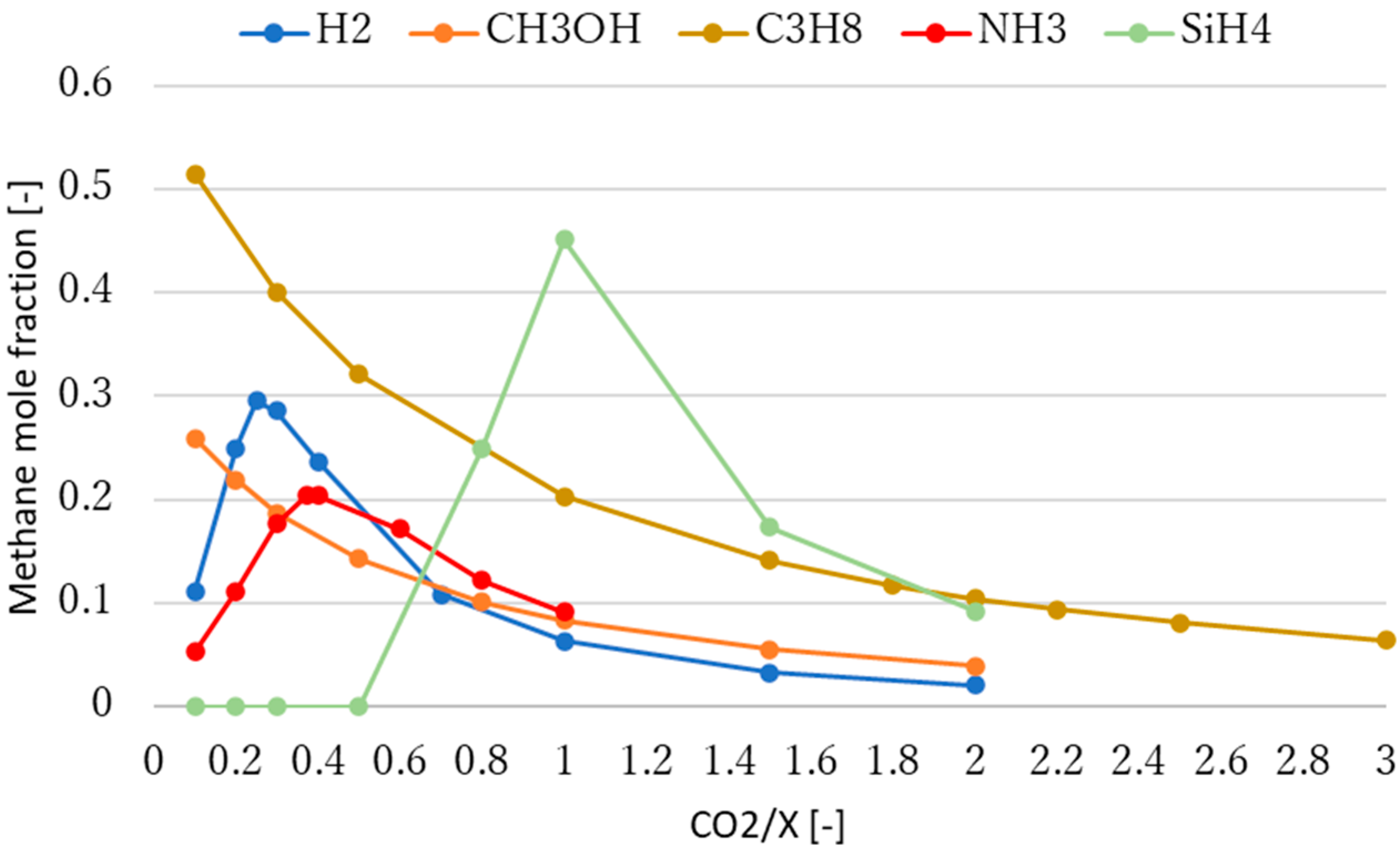
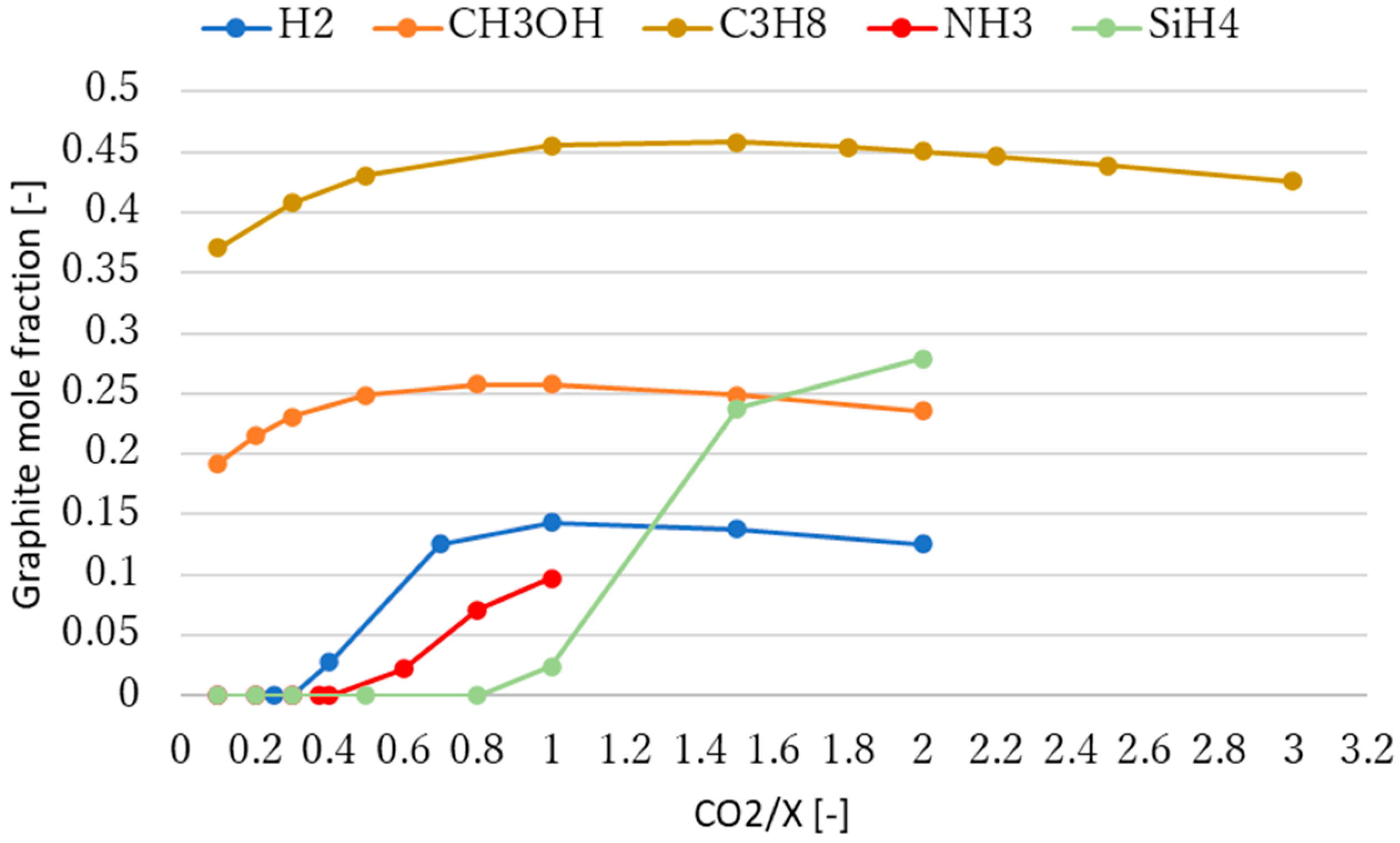
3.2. Effect of Molar Ratio of Reactants on Methane Production
4. Discussion
5. Conclusions and Future Work
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lees, F. Lee’s Loss Prevention in the Process Industries, 4th ed.; Sam Mannan: College Station, TX, USA, 2012. [Google Scholar]
- Turakulov, Z.; Kamolov, A.; Norkobilov, A.; Variny, M.; Díaz-Sainz, G.; Gómez-Coma, L.; Fallanza, M. Assessing various CO2 utilization technologies: A brief comparative review. J. Chem. Technol. Biotechnol. 2024, 99, 1291–1307. [Google Scholar] [CrossRef]
- Koytsoumpa, E.; Bergins, C.; Buddenberg, T.; Wu, S.; Sigurbjörnsson, Ó.; Tran, K.C.; Kakaras, E. The challenge of energy storage in Europa: Focus on power to fuel. J. Energy Resour. Technol. 2016, 138, 2049–2061. [Google Scholar] [CrossRef]
- Wulf, C.; Linßen, J.; Zapp, P. Review of power-to-gas projects in Europe. Energy Procedia 2018, 155, 367–378. [Google Scholar] [CrossRef]
- Moriyama, T.; Kimura, W.; Asai, H.; Yamamoto, K. OH chemiluminescence of methane-hydrogen premixed flames. J. Therm. Sci. Technol. 2021, 16, JTST0032. [Google Scholar] [CrossRef]
- Moriyama, T.; Yamamoto, K. Numerical simulation of methane-hydrogen premixed flames on a Bunsen burner. J. Therm. Sci. Technol. 2022, 17, 22–00129. [Google Scholar] [CrossRef]
- Nakahara, M.; Kido, H.; Shirasuna, T.; Hirakata, K. Effect of stretch on local burning velocity of premixed turbulent flames. J. Therm. Sci. Technol. 2007, 2, 268–280. [Google Scholar] [CrossRef]
- Nakahara, M.; Shirasuna, T.; Hashimoto, J. Experiment study on local flame properties of hydrogen added hydrocarbon premixed turbulent flames. J. Therm. Sci. Technol. 2009, 4, 190–201. [Google Scholar] [CrossRef]
- Zhen, H.S.; Leung, C.W.; Cheung, C.S.; Huang, Z.H. Characterization of biogas-hydrogen premixed flames using Bunsen burner. Int. J. Hydrogen Energy 2014, 39, 13292–13299. [Google Scholar] [CrossRef]
- Nilsson, E.J.K.; van Sprang, A.; Larfeldt, J.; Konnov, A.A. The comparative and combined effects of hydrogen addition on the laminar burning velocities of methane and its blends with ethane and propane. Fuel 2017, 189, 369–376. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Zirwes, T.; Zhang, F.; Bockhorn, H.; Chen, Z. Heat release rate markers for highly stretched premixed CH4/Air and CH4/H2/Air flames. Energy Fuels 2021, 35, 13349–13359. [Google Scholar] [CrossRef]
- de Vries, H.; Mokhov, A.V.; Levinsky, H.B. The impact of natural gas/hydrogen mixtures on the performance of end-use equipment: Interchangeability analysis for domestic appliances. Appl. Energy 2017, 208, 1007–1019. [Google Scholar] [CrossRef]
- Isaac, T. HyDeploy: The UK’s first hydrogen blending deployment project. Clean Energy 2019, 3, 114–125. [Google Scholar] [CrossRef]
- Inoue, K.; Miyamoto, K.; Domen, S.; Tamura, I.; Kawakami, T.; Tanimura, S. Development of hydrogen and natural gas co-firing gas turbine. Mitsubishi Heavy Ind. Tech. Rev. 2018, 55, 1–6. [Google Scholar]
- Bailera, M.; Lisbona, P.; Romeo, L.M.; Espatolero, S. Power to gas projects review: Lab, pilot and demo plants for storing renewable energy and CO2. Renew. Sustain. Energy Rev. 2017, 69, 292–312. [Google Scholar] [CrossRef]
- Inkeri, E.; Tynjälä, T.; Karjunen, H. Significance of methanation reactor dynamics on the annual efficiency of power-to-gas -system. Renew. Energy 2021, 163, 1113–1126. [Google Scholar] [CrossRef]
- Tada, S.; Shimizu, T.; Kameyama, H.; Haneda, T.; Kikuchi, R. Ni/CeO2 catalysts with high CO2 methanation activity and high CH4 selectivity at low temperatures. Int. J. Hydrogen Energy 2012, 37, 5527–5531. [Google Scholar] [CrossRef]
- Gahleitner, G. Hydrogen from renewable electricity an international review of power-to-gas pilot plants for stationary applications. Int. J. Hydrogen Energy 2013, 38, 2039–2061. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, M.S.; Lee, S.H.; Kim, T.W.; Park, E.D. CO and CO2 methanation over supported Ni catalysts. Catal. Today 2017, 293–294, 89–96. [Google Scholar] [CrossRef]
- Gordon, S.; McBride, B.J. Computer program for calculation of complex chemical equilibrium composition and application, Part I Analysis. NASA Ref. Publ. 1994, 1311, 1–58. [Google Scholar]
- Kamps, L.; Hirai, S.; Nagata, H. Hybrid rockets as post-boost stages and kick motors. Aerospace 2021, 8, 253. [Google Scholar] [CrossRef]
- Nguyen, C.; Thomas, J.C. Performance of additively manufactured fuels for hybrid rockets. Aerospace 2023, 10, 500. [Google Scholar] [CrossRef]
- Barat, R.B. Simple rate expression for catalyzed ammonia decomposition for fuel cells. Molecules 2023, 28, 6006. [Google Scholar] [CrossRef]
- Ghotkar, R.; Milcarek, R.J. Modeling of the kinetic factors in flame-assisted fuel cells. Sustainability 2022, 14, 4121. [Google Scholar] [CrossRef]
- Łysień, K.; Jarosz, T.; Głosz, K.; Stolarczyk, A. Elucidating the mechanisms of reactions in energetic materials: A critical methodology review. Fire 2024, 7, 99. [Google Scholar] [CrossRef]
- Kiewidt, L.; Thöming, J. Predicting optimal temperature profiles in single-stage fixed-bed reactors for CO2-methanation. Chem. Eng. Sci. 2015, 132, 59–71. [Google Scholar] [CrossRef]
- Yamamoto, K.; Sakaguchi, K. Hydrogen reactivity factor and effects of oxygen on methane conversion rate by chemical equilibrium calculation. Int. J. Thermofluids 2022, 15, 100186. [Google Scholar] [CrossRef]
- Leal, A.M.M.; Blunt, M.J.; LaForce, T.C. A robust and efficient numerical method for multiphase equilibrium calculations: Application to CO2–brine–rock systems at high temperatures, pressures and salinities. Adv. Water Resour. 2013, 62, 409–430. [Google Scholar] [CrossRef]
- Franz, R.; Kühlewind, T.; Shterk, G.; Abou-Hamad, E.; Parastaev, A.; Uslamin, E.; Hensen, E.J.M.; Kapteijn, F.; Gascon, J.; Pidko, E.A. Impact of small promoter amounts on coke structure in dry reforming of methane over Ni/ZrO2. Catal. Sci. Technol. 2020, 10, 3965–3974. [Google Scholar] [CrossRef]
- Yang, Q.; Zhou, C.; Ni, J.; Guan, X. Methane dry reforming in a coking- and sintering free liquid alloy-salt catalytic system. Sustain. Energy Fuels 2020, 4, 2768–2774. [Google Scholar] [CrossRef]
- Eley, D.D.; Selwood, P.W.; Weiss, P.B. Advances in Catalysis; Academic Press: New York, NY, USA, 1959. [Google Scholar]
- Schmider, D.; Maier, L.; Deutschmann, O. Reaction kinetics of CO and CO2 methanation over Nickel. Ind. Eng. Chem. Res. 2021, 60, 5792–5805. [Google Scholar] [CrossRef]
- Kakoee, A.; Gharehghani, A. Carbon oxides methanation in equilibrium; a thermodynamic approach. Int. J. Hydrogen Energy 2020, 45, 29993–30008. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamamoto, K.; Nakayama, R. Investigation of Alternative Substances for Replacing Hydrogen in Methanation. Energies 2024, 17, 3690. https://doi.org/10.3390/en17153690
Yamamoto K, Nakayama R. Investigation of Alternative Substances for Replacing Hydrogen in Methanation. Energies. 2024; 17(15):3690. https://doi.org/10.3390/en17153690
Chicago/Turabian StyleYamamoto, Kazuhiro, and Ryosuke Nakayama. 2024. "Investigation of Alternative Substances for Replacing Hydrogen in Methanation" Energies 17, no. 15: 3690. https://doi.org/10.3390/en17153690



