Photocatalytic Hydrogen Production Enhancement of NiTiO3 Perovskite through Cobalt Incorporation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pure and Co-Doped NiTiO3 Synthesis
2.3. Characterization
2.4. Hydrogen Evolution Test
3. Results and Discussion
3.1. Thermogravimetric and Differential Scanning Calorimetry
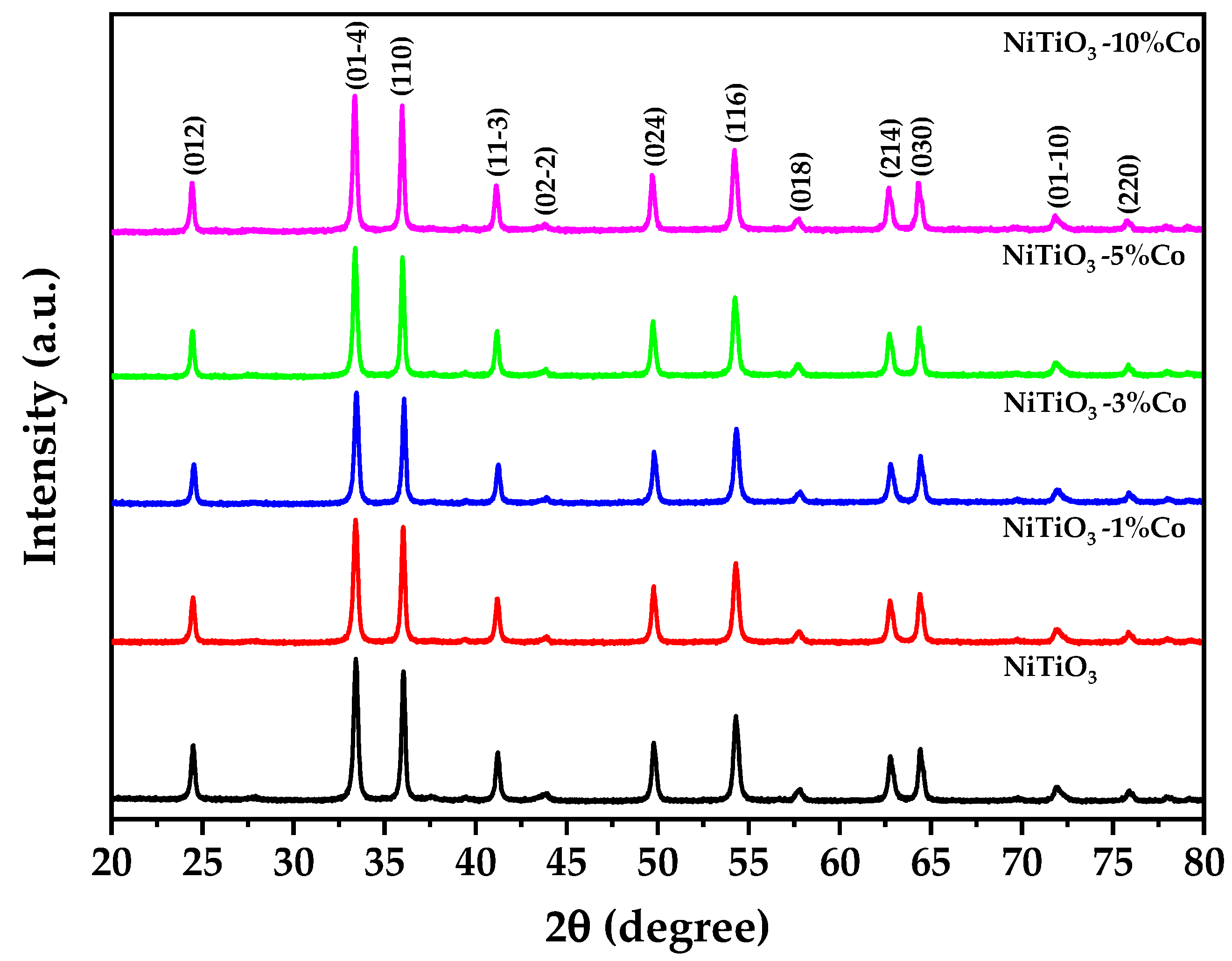
3.2. X-ray Diffraction
3.3. Raman Spectroscopy
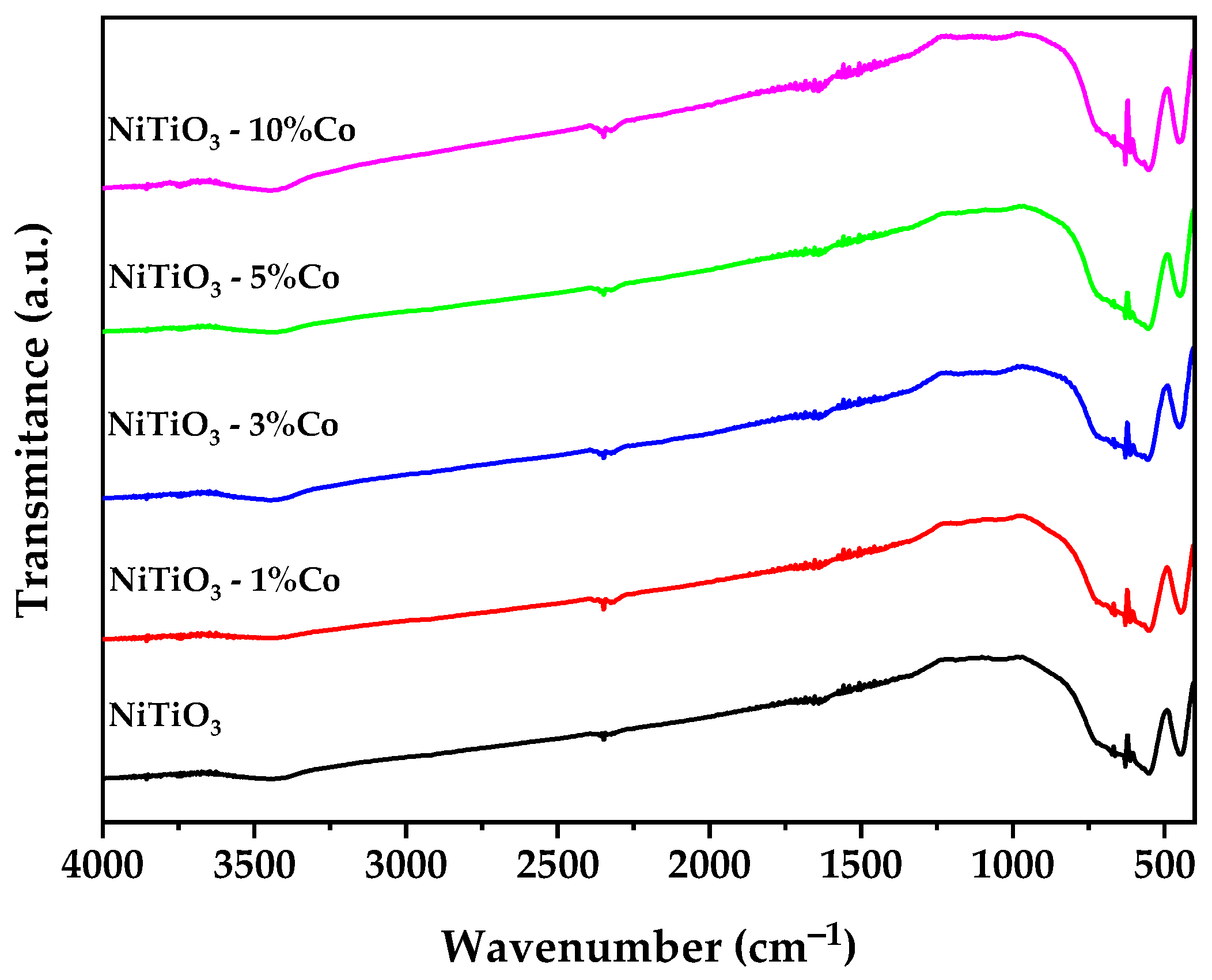
3.4. Fourier-Transform Infrared Spectroscopy (FTIR)
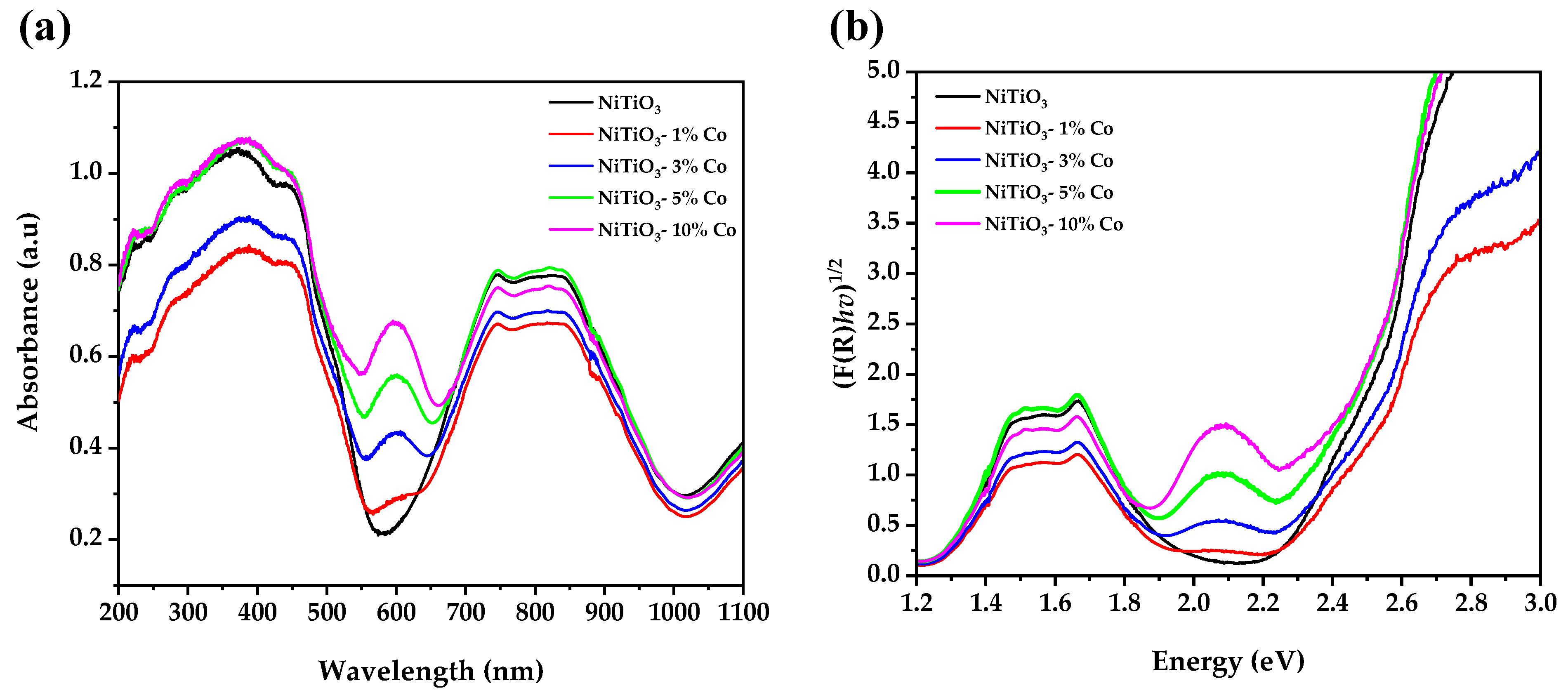
3.5. UV–Visible Spectroscopy
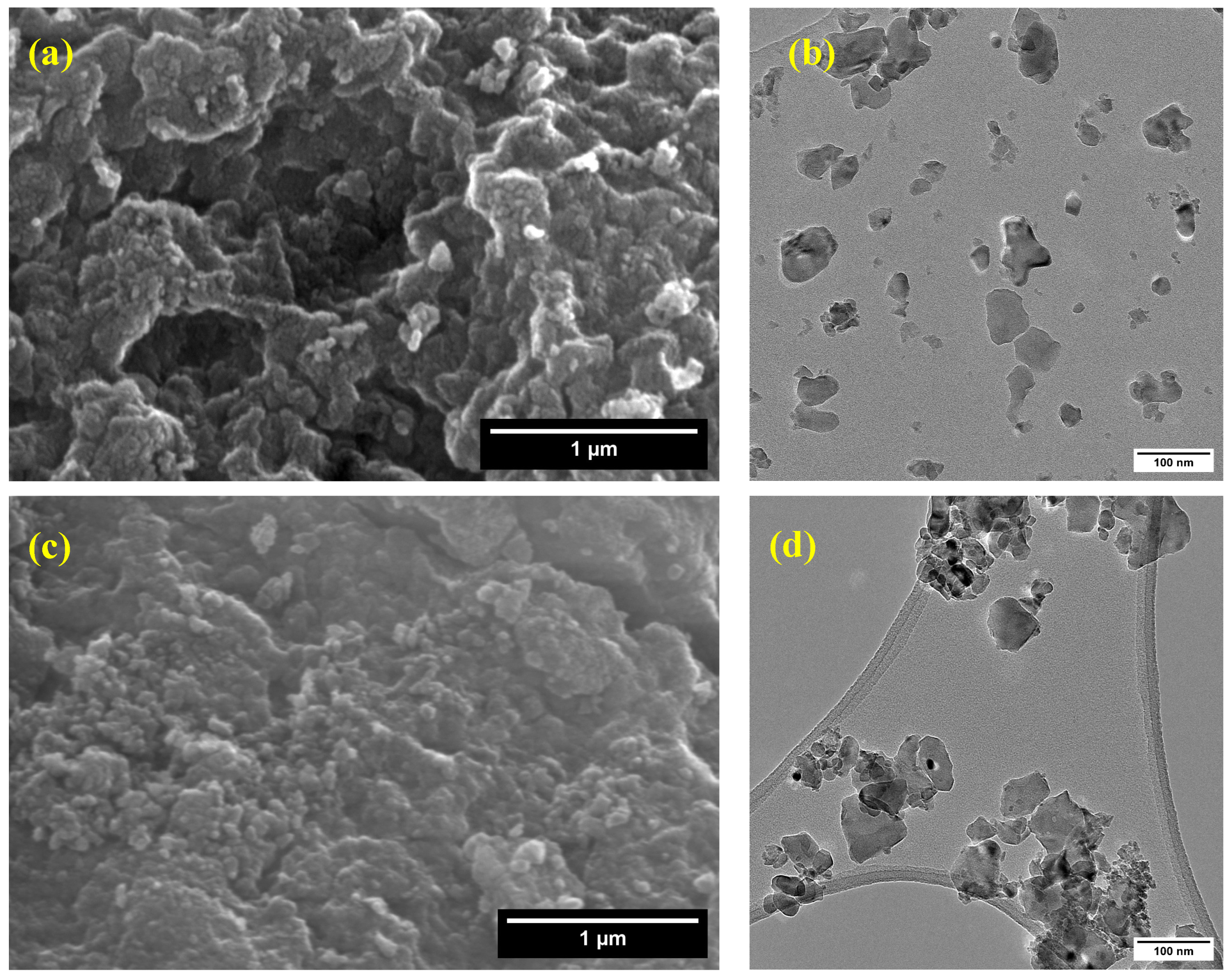
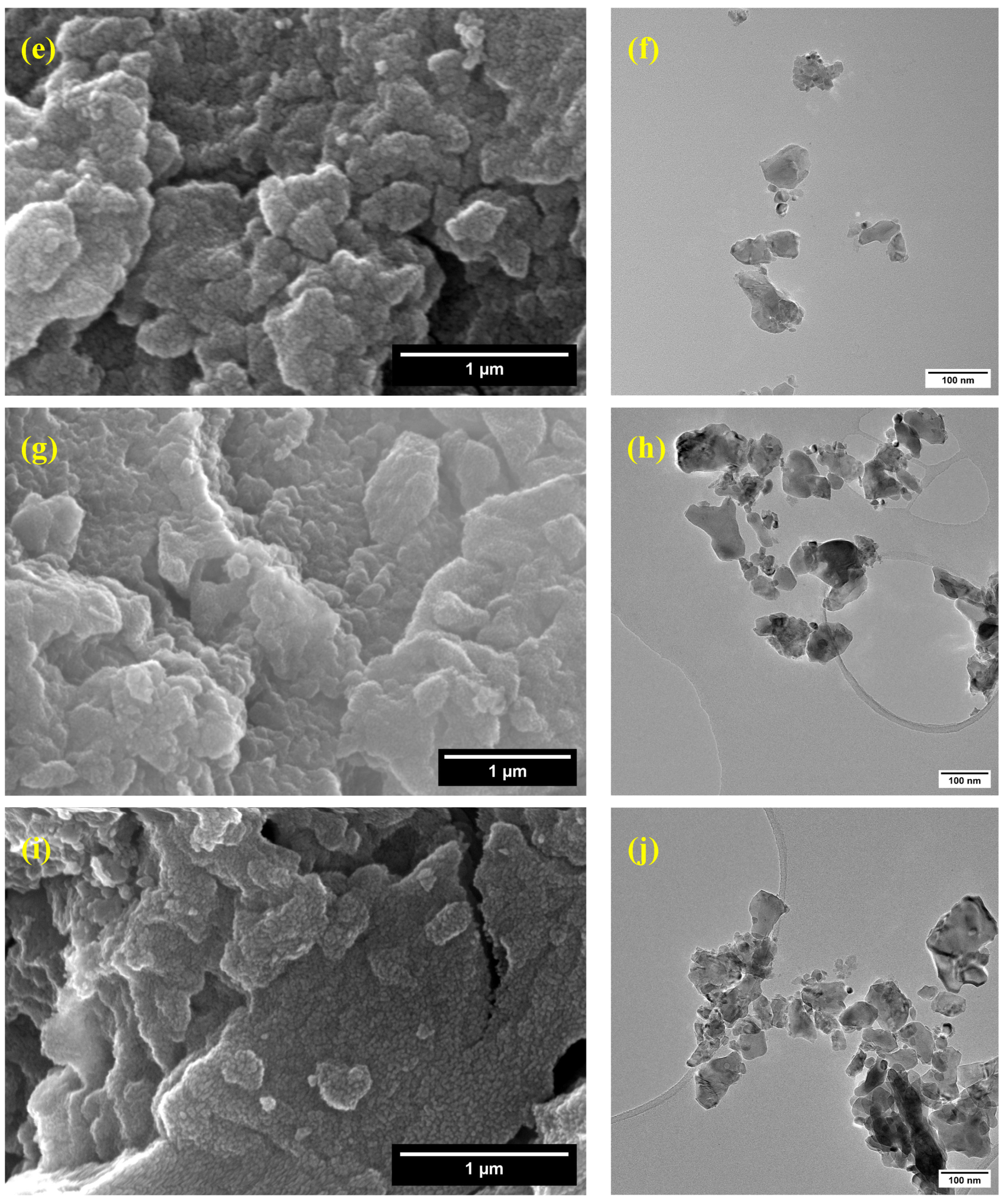
3.6. Scanning and Transmission Electron Microscopy
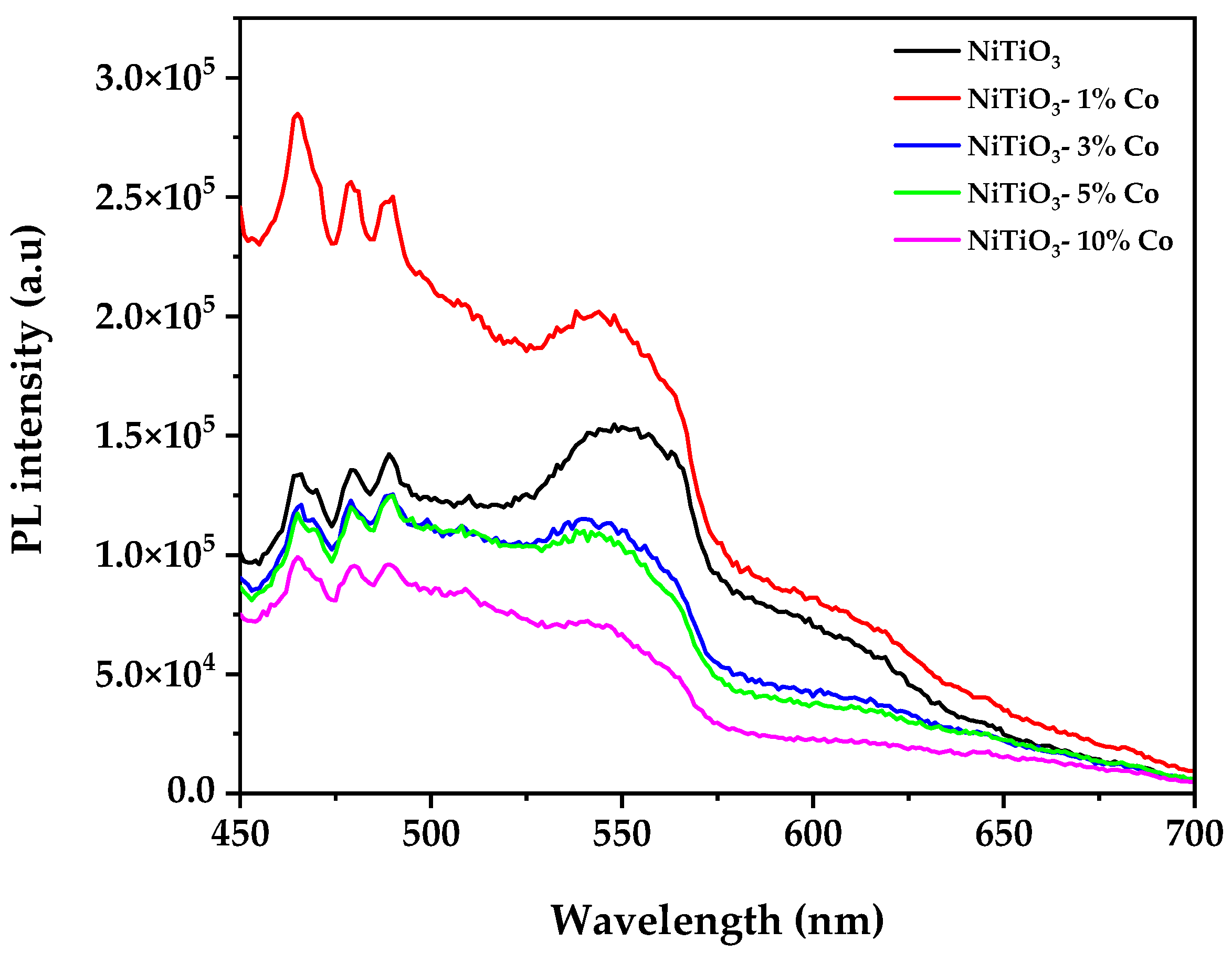
3.7. Photoluminiscence Spectroscopy
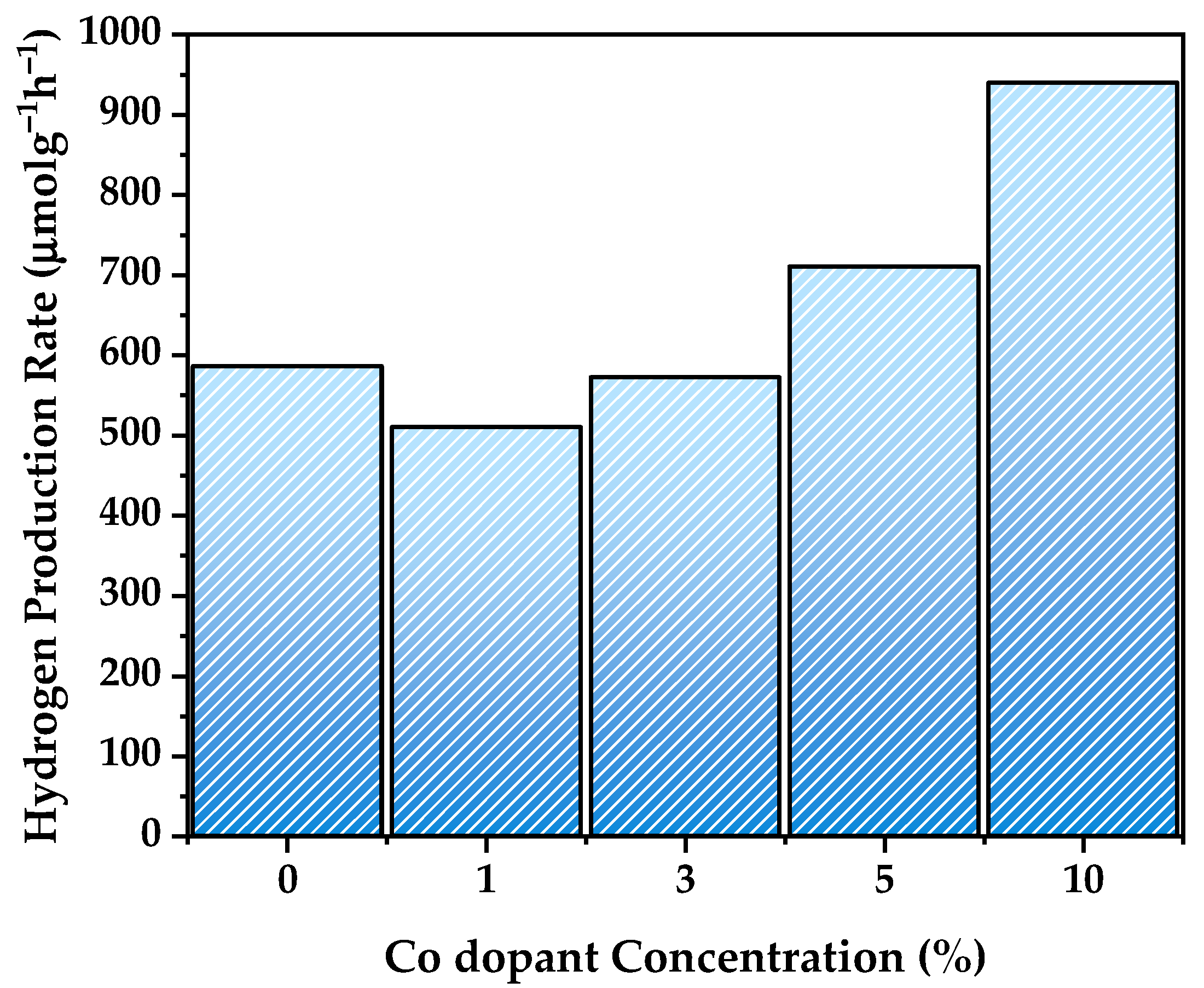
3.8. Hydrogen Generation Evaluation
3.9. Proposed Mechanism of Photocatalytic H2 Evolution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, L.; Lin, J.; Wu, N.; Xie, S.; Meng, C.; Zheng, Y.; Wang, X.; Zhao, Y. Review and outlook on the international renewable energy development. Energy Built Environ. 2022, 3, 139–157. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based photocatalytic hydrogen generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Tachibana, Y.; Vayssieres, L.; Durrant, J.R. Artificial photosynthesis for solar water-splitting. Nat. Photonics 2012, 6, 511–518. [Google Scholar] [CrossRef]
- Mazhar, F.; Kausar, A.; Iqbal, M. Photocatalytic hydrogen generation using TiO2: A state-of-the-art review. Zeitschrift Phys. Chem. 2022, 236, 1697–1728. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Lu, D.; Takata, T.; Saito, N.; Inoue, Y.; Domen, K. Photocatalyst releasing hydrogen from water. Nature 2006, 440, 295. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Do, H.H.; Le Nguyen, D.T.; Nguyen, X.C.; Le, T.-H.; Nguyen, T.P.; Trinh, Q.T.; Ahn, S.H.; Vo, D.-V.N.; Kim, S.Y.; van Le, Q. Recent progress in TiO2-based photocatalysts for hydrogen evolution reaction: A review. Arab. J. Chem. 2020, 13, 3653–3671. [Google Scholar] [CrossRef]
- Zhang, M.; Salvador, P.A.; Rohrer, G.S. Influence of particle size and shape on the rate of hydrogen produced by Al-doped SrTiO3 photocatalysts. J. Am. Ceram. Soc. 2022, 105, 5336–5346. [Google Scholar] [CrossRef]
- Karthik, K.V.; Reddy, C.V.; Reddy, K.R.; Ravishankar, R.; Sanjeev, G.; Kulkarni, R.V.; Shetti, N.P.; Raghu, A.V. Barium titanate nanostructures for photocatalytic hydrogen generation and photodegradation of chemical pollutants. J. Mater. Sci. Mater. Electron. 2019, 30, 20646–20653. [Google Scholar] [CrossRef]
- Li, H.; Wang, G.; Gong, H.; Jin, Z. Phosphated 2D MoS2 nanosheets and 3D NiTiO3 nanorods for efficient photocatalytic hydrogen evolution. ChemCatChem 2020, 12, 5492–5503. [Google Scholar] [CrossRef]
- Xie, P.; Yang, F.; Li, R.; Ai, C.; Lin, C.; Lin, S. Improving hydrogen evolution activity of perovskite BaTiO3 with Mo doping: Experiments and first-principles analysis. Int. J. Hydrogen Energy 2019, 44, 11695–11704. [Google Scholar] [CrossRef]
- Mishra, A.; Parangusan, H.; Bhadra, J.; Ahmed, Z.; Mallick, S.; Touati, F.; Al-Thani, N. Synthesis and photoelectrochemical performance of Co doped SrTiO3 nanostructures photoanode. Environ. Prog. Sustain. Energy 2023, 42, e14186. [Google Scholar] [CrossRef]
- Yan, T.; Liu, H.; Jin, Z. Graphdiyne Based Ternary GD-CuI-NiTiO3 S-Scheme Heterjunction Photocatalyst for Hydrogen Evolution. ACS Appl. Mater. Interfaces 2021, 13, 24896–24906. [Google Scholar] [CrossRef]
- Diab, K.R.; El-Maghrabi, H.H.; Nada, A.A.; Youssef, A.M.; Hamdy, A.; Roualdes, S.; Abd El-Wahab, S. Facile fabrication of NiTiO3/graphene nanocomposites for photocatalytic hydrogen generation. J. Photochem. Photobiol. A Chem. 2018, 365, 86–93. [Google Scholar] [CrossRef]
- Guo, H.; Wan, S.; Wang, Y.; Ma, W.; Zhong, Q.; Ding, J. Enhanced photocatalytic CO2 reduction over direct Z-scheme NiTiO3/g-C3N4 nanocomposite promoted by efficient interfacial charge transfer. Chem. Eng. J. 2021, 412, 128646. [Google Scholar] [CrossRef]
- Qu, Y.; Zhou, W.; Ren, Z.; Du, S.; Meng, X.; Tian, G.; Pan, K.; Wang, G.; Fu, H. Facile preparation of porous NiTiO3 nanorods with enhanced visible-light-driven photocatalytic performance. J. Mater. Chem. 2012, 22, 16471. [Google Scholar] [CrossRef]
- Pham, T.-T.; Shin, E.W. Influence of g-C3N4 Precursors in g-C3N4/NiTiO3 Composites on Photocatalytic Behavior and the Interconnection between g-C3N4 and NiTiO3. Langmuir 2018, 34, 13144–13154. [Google Scholar] [CrossRef]
- El-Maghrabi, H.H.; Younis, S.A.; Ali, H.R.; Nada, A.A. Solar-driven photocatalytic transformation of toluene to benzoic acid over perovskite-type NiTiO3 decorated with reduced GO and g-C3N4 nanosheets. J. Environ. Chem. Eng. 2023, 11, 109477. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, Y.; Chen, J.; Jiang, Y.; Kiani, M.; Li, B.; Wang, R. Fabrication of high-activity hybrid NiTiO3/g-C3N4 heterostructured photocatalysts for water splitting to enhanced hydrogen production. Ceram. Int. 2016, 42, 12297–12305. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, P.; Peng, T.; Liu, Q.; Chen, W.; Liu, B.; Yuan, Y.; Zhang, W.; Song, F.; Gu, J.; et al. Mechanically alloyed NiTiO3/transition metal heterostructures: Introducing oxygen vacancies for exceptionally enhanced hydrogen evolution reaction activity. J. Mater. Chem. A 2020, 8, 14908–14914. [Google Scholar] [CrossRef]
- Zan, L.; Zhang, H.; Bo, X.; Zhao, Y.; Tian, H.; Chen, H.; Wei, Q.; Tang, H.; Fu, F. Investigation of the synergistic effect on cobalt oxide modified silver surface for electrocatalytic hydrogen evolution reaction. J. Alloys Compd. 2021, 869, 159324. [Google Scholar] [CrossRef]
- Dat, T.T. Effects of Co doping on properties of ilmenite NiTiO3 ceramics. Vietnam J. Sci. Technol. 2018, 56, 119. [Google Scholar] [CrossRef]
- Jiang, K.; Pham, T.-T.; Kang, S.G.; Men, Y.; Shin, E.W. Modification of the structural properties of NiTiO3 materials by transition metal dopants: The dopant size effect. J. Alloys Compd. 2018, 739, 393–400. [Google Scholar] [CrossRef]
- Wu, M.; Ke, S.; Chen, W.; Zhang, S.; Zhu, M.; Zhang, Y.; Foo, M.L.; Tang, L. Optimization of the facet structure of cobalt oxide catalysts for enhanced hydrogen evolution reaction. Catal. Sci. Technol. 2020, 10, 1040–1047. [Google Scholar] [CrossRef]
- Hang, R.; Liu, S.; Liu, Y.; Zhao, Y.; Bai, L.; Jin, M.; Zhang, X.; Huang, X.; Yao, X.; Tang, B. Preparation, characterization, corrosion behavior and cytocompatibility of NiTiO3 nanosheets hydrothermally synthesized on biomedical NiTi alloy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 97, 715–722. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, B.; Li, R.; Fan, C.; Liang, Z.; Han, P. Structural, electronic and optical properties of Ilmenite ATiO3(A=Fe, Co, Ni). Mater. Sci. Semicond. Process. 2015, 39, 6–16. [Google Scholar] [CrossRef]
- Nguyen, P.A.; Nguyen, T.; Ha, C.A.; Hoang, T.C.; van Nguyen, T.T.; Nguyen, D.T.; Luu, C.L. Exceptional photodecomposition activity of heterostructure NiTiO3–TiO2 catalyst. J. Sci. Adv. Mater. Devices 2022, 7, 100407. [Google Scholar] [CrossRef]
- Chanda, A.; Joshi, S.R.; Akshay, V.R.; Varma, S.; Singh, J.; Vasundhara, M.; Shukla, P. Structural and optical properties of multilayered un-doped and cobalt doped TiO2 thin films. Appl. Surf. Sci. 2021, 536, 147830. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Luminescence characteristics of cobalt doped TiO2 nanoparticles. J. Lumin. 2012, 132, 178–184. [Google Scholar] [CrossRef]
- Jiang, K.; Men, Y.; Liu, S.; Wang, J.; An, W.; Yu, H.; Shin, E.W. Highly stable and selective CoxNiyTiO3 for CO2 methanation: Electron transfer and interface interaction. J. CO2 Util. 2021, 53, 101743. [Google Scholar] [CrossRef]
- Dao, D.Q.; Nguyen, T.K.A.; Pham, T.-T.; Shin, E.W. Synergistic Effect on Photocatalytic Activity of Co-Doped NiTiO3/g-C3N4 Composites under Visible Light Irradiation. Catalysts 2020, 10, 1332. [Google Scholar] [CrossRef]
- Böer, K.W. Doping and junction formation. In Survey of Semiconductor Physics; Böer, K.W., Ed.; Springer: Dordrecht, The Netherlands, 1992; pp. 963–997. ISBN 978-94-010-5293-1. [Google Scholar]
- Thoan, N.H.; Hung, P.P.; Dung, D.D.; Ngoc, T.V.; Bac, L. Effect of copper dopant on microstructural and optical properties of NiTiO3 ilmenite materials synthesized by citrate method. J. Nanostructures 2020, 10, 769–778. [Google Scholar] [CrossRef]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quirós, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; Le Bail, A. Crystallography Open Database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2012, 40, D420–D427. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Gupta, H.; Chitrarasu, K.; Chellasamy, V.; Thangadurai, P. Influence of Nb ion doping on the electrical properties of nanocrystalline NiTiO3 ceramics and their universal behavior. Ionics 2020, 26, 939–952. [Google Scholar] [CrossRef]
- Hung, P.P.; Dat, T.T.; Dung, D.D.; Trung, N.N.; Hanh, M.H.; Toan, D.N.; Bac, L.H. Effect of Annealing Temperature on Structural, Optical and Visible-Light Photocatalytic Properties of NiTiO3 Nanopowders. J. Electron. Mater. 2018, 47, 7301–7308. [Google Scholar] [CrossRef]
- Qi, W.; Mattursun, A.; Gao, M.; Hushur, A.; Zhang, H. Lattice dynamics of NiTiO3 under high pressure: Raman evidence under two pressure-transmitting mediums. Results Phys. 2022, 43, 106114. [Google Scholar] [CrossRef]
- Stella, C.; Prabhakar, D.; Prabhu, M.; Soundararajan, N.; Ramachandran, K. Oxygen vacancies induced room temperature ferromagnetism and gas sensing properties of Co-doped TiO2 nanoparticles. J. Mater. Sci. Mater. Electron. 2016, 27, 1636–1644. [Google Scholar] [CrossRef]
- Barsoum, M.W. Fundamentals of Ceramics; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9781498708166. [Google Scholar]
- Ben Saber, N.; Mezni, A.; Alrooqi, A.; Altalhi, T. Facile one-pot solvothermal approach to produce inorganic binary TiO2@NiTiO3 and ternary Au-TiO2@NiTiO3 yellow nano-pigment for environmental and energy use. Mater. Res. Express 2021, 8, 45016. [Google Scholar] [CrossRef]
- Yang, B.; Bai, X.; Wang, J.; Fang, M.; Wu, X.; Liu, Y.; Huang, Z.; Lao, C.-Y.; Min, X. Photocatalytic Performance of NiO/NiTiO3 Composite Nanofiber Films. Catalysts 2019, 9, 561. [Google Scholar] [CrossRef]
- Yuvaraj, S.; Nithya, V.D.; Fathima, K.S.; Sanjeeviraja, C.; Selvan, G.K.; Arumugam, S.; Selvan, R.K. Investigations on the temperature dependent electrical and magnetic properties of NiTiO3 by molten salt synthesis. Mater. Res. Bull. 2013, 48, 1110–1116. [Google Scholar] [CrossRef]
- Chellasamy, V.; Thangadurai, P. Structural and electrochemical investigations of nanostructured NiTiO3 in acidic environment. Front. Mater. Sci. 2017, 11, 162–170. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Chang, Y.-H.; Yang, W.-D.; Tsai, B.-S. Synthesis and characterization of ilmenite NiTiO3 and CoTiO3 prepared by a modified Pechini method. J. Non Cryst. Solids 2006, 352, 789–794. [Google Scholar] [CrossRef]
- Enhessari, M.; Sakhaei, M.; Salehabadi, A.; Etemad, L. CeO2/NiTiO3 nanocomposites; synthesis, photoluminescence and magnetic behavior. Mater. Sci. Pol. 2017, 35, 275–282. [Google Scholar] [CrossRef]
- Doeuff, S.; Henry, M.; Sanchez, C.; Livage, J. Hydrolysis of titanium alkoxides: Modification of the molecular precursor by acetic acid. J. Non Cryst. Solids 1987, 89, 206–216. [Google Scholar] [CrossRef]
- Li, M.-W.; Yuan, J.-P.; Gao, X.-M.; Liang, E.-Q.; Wang, C.-Y. Structure and optical absorption properties of NiTiO3 nanocrystallites. Appl. Phys. A 2016, 122, 725. [Google Scholar] [CrossRef]
- Radtke, G.; Lazar, S.; Botton, G.A. High-resolution EELS investigation of the electronic structure of ilmenites. Phys. Rev. B 2006, 74, 155117. [Google Scholar] [CrossRef]
- Lacz, A.; Lach, R.; Drozdz, E. Effect of nickel addition on the physicochemical properties of SrTiO3-based materials. Ceram. Int. 2022, 48, 24906–24914. [Google Scholar] [CrossRef]
- Díez-García, M.I.; Monllor-Satoca, D.; Vinoth, V.; Anandan, S.; Lana-Villarreal, T. Electrochemical Doping as a Way to Enhance Water Photooxidation on Nanostructured Nickel Titanate and Anatase Electrodes. ChemElectroChem 2017, 4, 1429–1435. [Google Scholar] [CrossRef]
- Ruiz Preciado, M.A.; Kassiba, A.; Morales-Acevedo, A.; Makowska-Janusik, M. Vibrational and electronic peculiarities of NiTiO3 nanostructures inferred from first principle calculations. RSC Adv. 2015, 5, 17396–17404. [Google Scholar] [CrossRef]
- Moghtada, A.; Shahrouzianfar, A.; Ashiri, R. Facile synthesis of NiTiO3 yellow nano-pigments with enhanced solar radiation reflection efficiency by an innovative one-step method at low temperature. Dye. Pigment. 2017, 139, 388–396. [Google Scholar] [CrossRef]
- Huang, H.; Liu, X.; Li, F.; He, Q.; Ji, H.; Yu, C. In situ construction of a 2D CoTiO3/g-C3N4 photocatalyst with an S-scheme heterojunction and its excellent performance for CO2 reduction. Sustain. Energy Fuels 2022, 6, 4903–4915. [Google Scholar] [CrossRef]
- El Mragui, A.; Logvina, Y.; Da Pinto Silva, L.; Zegaoui, O.; Da Esteves Silva, J.C.G. Synthesis of Fe- and Co-Doped TiO2 with Improved Photocatalytic Activity Under Visible Irradiation Toward Carbamazepine Degradation. Materials 2019, 12, 3874. [Google Scholar] [CrossRef]
- Fu, C.; Liu, L.; Wei, Y.; Huang, W.; Zhao, G. Linking the Doping-Induced Trap States to the Concentration of Surface-Reaching Photoexcited Holes in Transition-Metal-Doped TiO2 Nanoparticles. J. Phys. Chem. Lett. 2024, 15, 6504–6511. [Google Scholar] [CrossRef]
- Lakshmi, T.; Hajarabeevi, N.; Mishra, T.; Aman, N. Synthesis of cobalt doped nickel titanate nanomaterials for enhanced photocatalytic reduction of chromium. Optik 2024, 298, 171561. [Google Scholar] [CrossRef]
- Alam, U.; Pandey, A.; Verma, N. An anthraquinone-integrated S-scheme-based NiTiO3-g-C3N4 composite with enhanced hydrogen production activity. Int. J. Hydrogen Energy 2023, 48, 2532–2541. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef]



| Structural Parameter | Sample | ||||
|---|---|---|---|---|---|
| NiTiO3 | NiTiO3-1% Co | NiTiO3-3% Co | NiTiO3-5% Co | NiTiO3-10% Co | |
| Crystal system | Hexagonal | Hexagonal | Hexagonal | Hexagonal | Hexagonal |
| Space group | R-3 | R-3 | R-3 | R-3 | R-3 |
| a = b (nm) | 5.0276 | 5.0261 | 5.0284 | 5.0288 | 5.0306 |
| c (nm) | 13.8124 | 13.8122 | 13.8174 | 13.817 | 13.8212 |
| α = β (°) | 90 | 90 | 90 | 90 | 90 |
| γ (°) | 120 | 120 | 120 | 120 | 120 |
| ρ (g/cm3) | 5.1 | 5.09 | 5.09 | 5.09 | 5.08 |
| D (nm) | 39.34 | 39.97 | 41.15 | 43.64 | 44.52 |
| Rexp (%) | 6.8476 | 6.7381 | 6.8672 | 6.4237 | 5.7861 |
| Rp (%) | 4.0572 | 3.8981 | 4.0959 | 3.4486 | 2.8391 |
| Rwp (%) | 5.8159 | 5.4628 | 5.9956 | 4.9764 | 4.0642 |
| GOF | 0.8493 | 0.8107 | 0.8735 | 0.7747 | 0.7024 |
| Element | Sample | ||||
|---|---|---|---|---|---|
| NiTiO3 | NiTiO3-1%Co | NiTiO3-3%Co | NiTiO3-5%Co | NiTiO3-10%Co | |
| Ni (Atom %) | 15.60 | 14.84 | 22.00 | 37.02 | 21.95 |
| Ti (Atom %) | 14.10 | 16.37 | 21.77 | 17.25 | 22.61 |
| O (Atom %) | 70.30 | 68.72 | 55.78 | 44.16 | 53.07 |
| Co (Atom %) | - | 0.07 | 0.45 | 1.57 | 2.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quispe Cohaila, A.B.; Sacari Sacari, E.J.; Lanchipa Ramos, W.O.; Tamayo Calderón, R.M.; Medina Salas, J.P.; Gamarra Gómez, F.; Viswanathan Mangalaraja, R.; Rajendran, S. Photocatalytic Hydrogen Production Enhancement of NiTiO3 Perovskite through Cobalt Incorporation. Energies 2024, 17, 3704. https://doi.org/10.3390/en17153704
Quispe Cohaila AB, Sacari Sacari EJ, Lanchipa Ramos WO, Tamayo Calderón RM, Medina Salas JP, Gamarra Gómez F, Viswanathan Mangalaraja R, Rajendran S. Photocatalytic Hydrogen Production Enhancement of NiTiO3 Perovskite through Cobalt Incorporation. Energies. 2024; 17(15):3704. https://doi.org/10.3390/en17153704
Chicago/Turabian StyleQuispe Cohaila, Alberto Bacilio, Elisban Juani Sacari Sacari, Wilson Orlando Lanchipa Ramos, Rocío María Tamayo Calderón, Jesús Plácido Medina Salas, Francisco Gamarra Gómez, Ramalinga Viswanathan Mangalaraja, and Saravanan Rajendran. 2024. "Photocatalytic Hydrogen Production Enhancement of NiTiO3 Perovskite through Cobalt Incorporation" Energies 17, no. 15: 3704. https://doi.org/10.3390/en17153704








