A Doublet State Palladium(I) N-Heterocyclic Carbene Complex as a Dopant and Stabilizer for Improved Photostability in Organic Solar Cells
Abstract
:1. Introduction
1.1. Organic Photovoltaics (OPVs)
1.2. Mechanism for Generation of Photocurrent in Organic Solar Cells (OSCs)
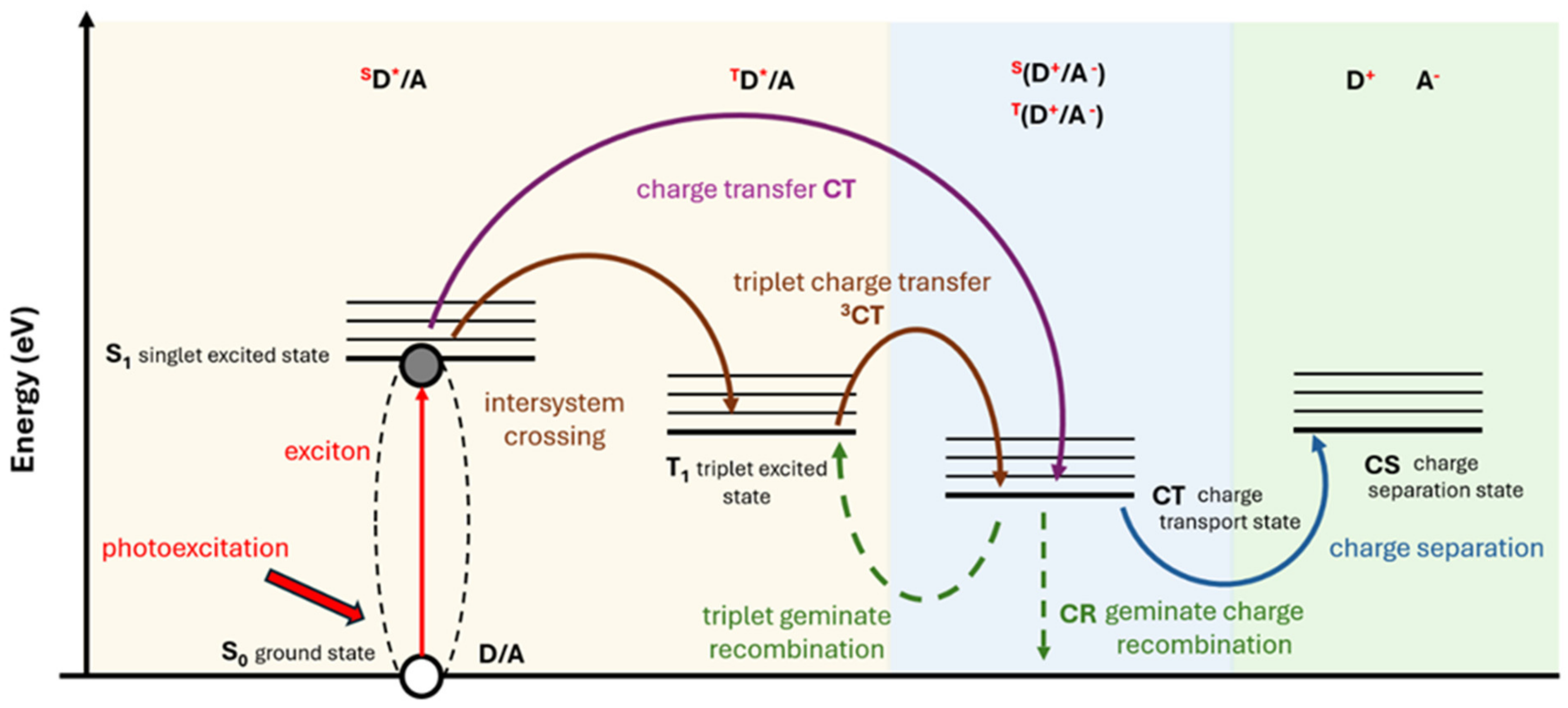
1.3. Effect of Singlet and Triplet States in Organic Solar Cells
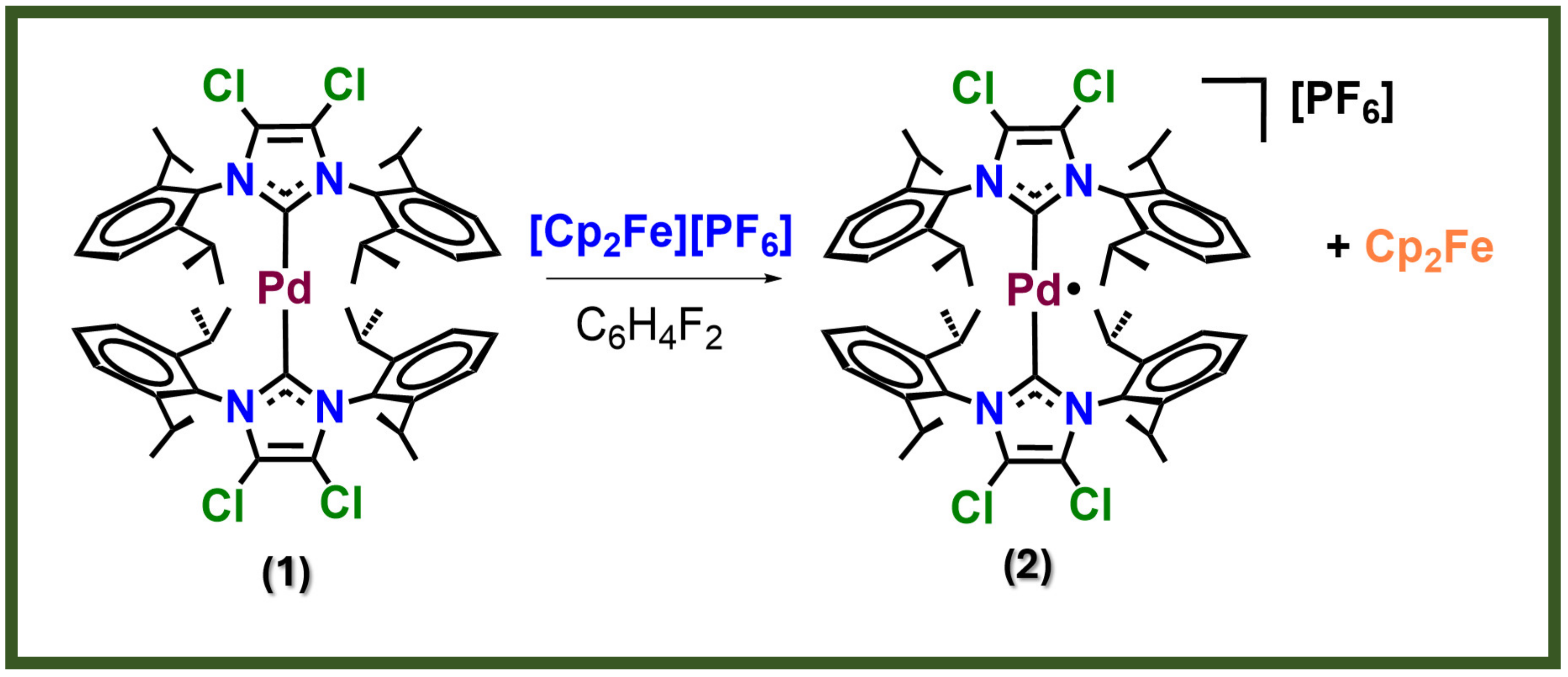
1.4. Organometallic Complexes in OSCs
1.5. Target of This Work
2. Materials and Methods
3. Results and Discussion
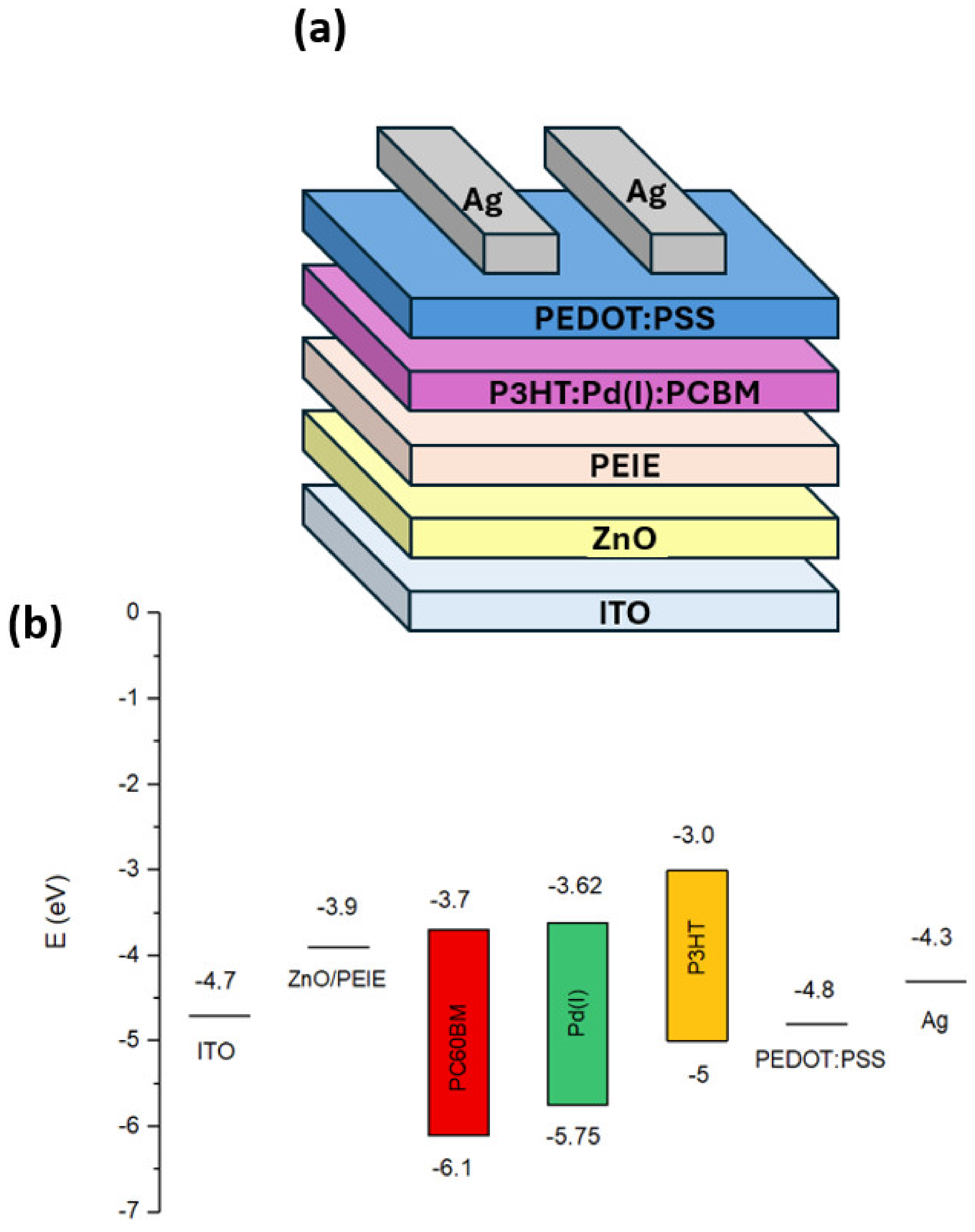
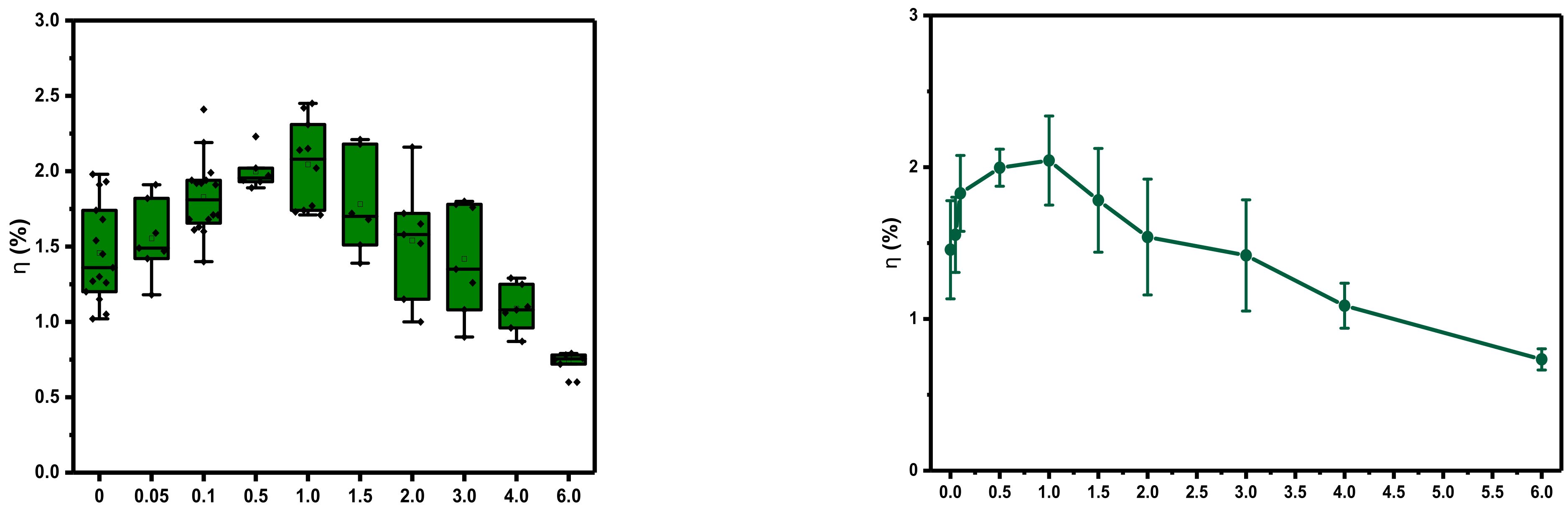
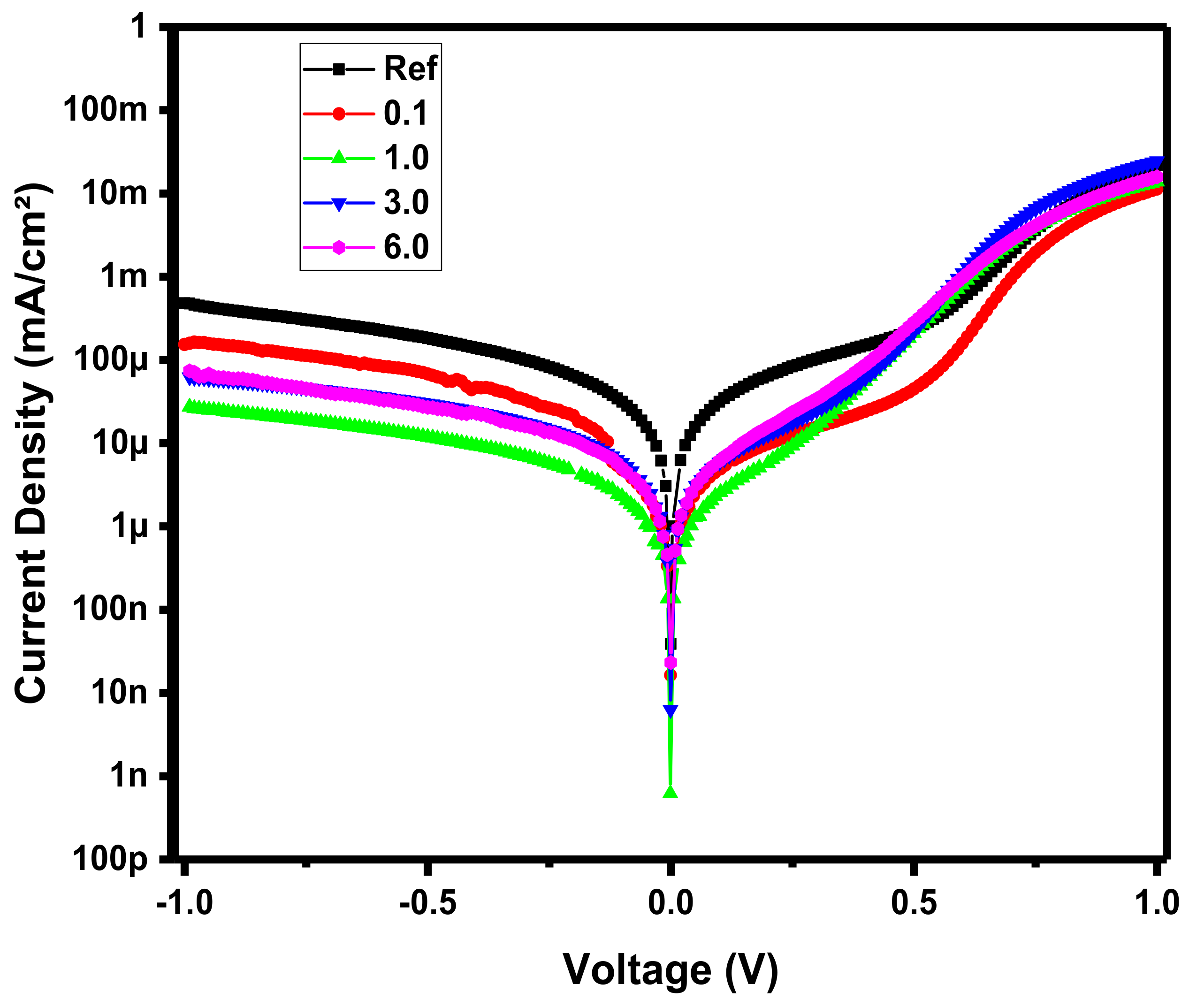
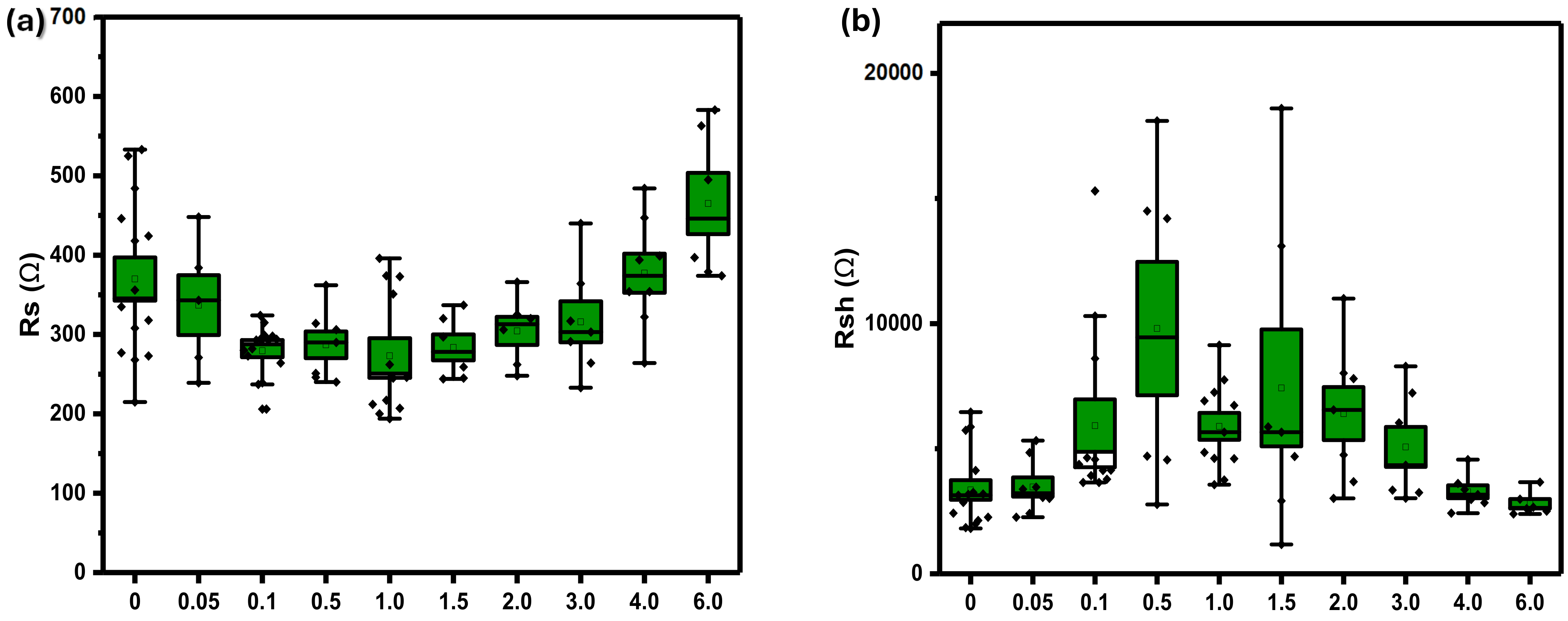
3.1. Device Optoelectronic Properties
3.2. Morphological Studies of the Active Layer
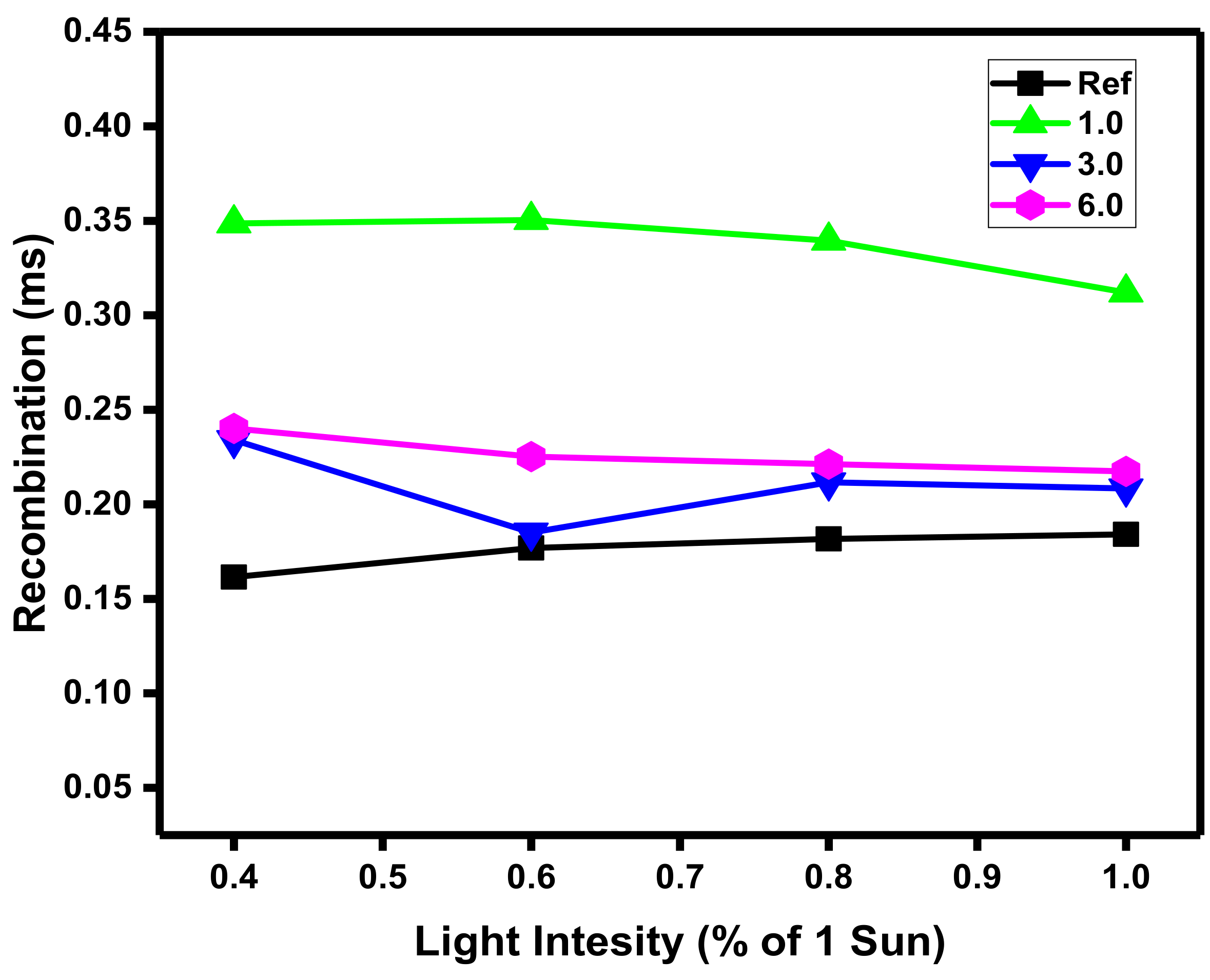
3.3. Recombination Analysis
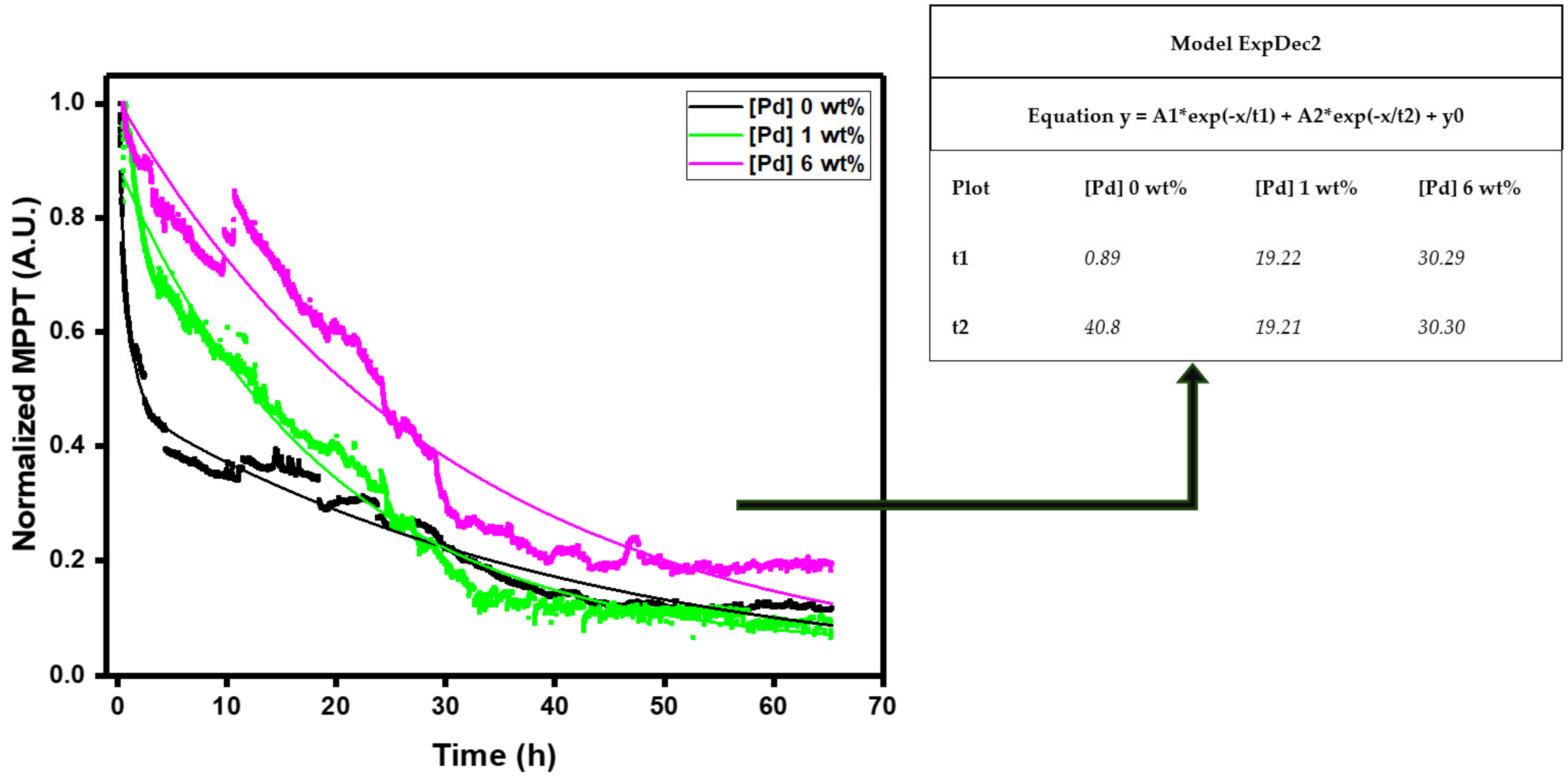
3.4. Photostability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ylikunnari, M.; Välimäki, M.; Väisänen, K.-L.; Kraft, T.M.; Sliz, R.; Corso, G.; Po, R.; Barbieri, R.; Carbonera, C.; Gorni, G.; et al. Flexible OPV modules for highly efficient indoor applications. Flex. Print. Electron. 2020, 5, 014008. [Google Scholar] [CrossRef]
- Sampaio, P.; González, M.; Ferreira, P.; Vidal, P.; Pereira, J.; Ferreira, H.; Oprime, P. Overview of printing and coating techniques in the production of organic photovoltaic cells. Int. J. Energy Res. 2020, 44, 9912–9931. [Google Scholar] [CrossRef]
- Koidis, C.; Logothetidis, S.; Ioakeimidis, A.; Laskarakis, A.; Kapnopoulos, C. Key factors to improve the efficiency of roll-to-roll printed organic photovoltaics. Org. Electron. 2013, 14, 1744–1748. [Google Scholar] [CrossRef]
- Lee, S.; Jeong, D.; Kim, C.; Lee, C.; Kang, H.; Woo, H.Y.; Kim, B.J. Eco-Friendly Polymer Solar Cells: Advances in Green-Solvent Processing and Material Design. ACS Nano 2020, 14, 14493–14527. [Google Scholar] [CrossRef]
- McDowell, C.; Bazan, G. Organic solar cells processed from green solvents. Curr. Opin. Green Sustain. Chem. 2017, 5, 49–54. [Google Scholar] [CrossRef]
- Xu, T.; Yu, L. How to design low bandgap polymers for highly efficient organic solar cells. Mater. Today 2014, 17, 11–15. [Google Scholar] [CrossRef]
- Krebs, F.C. (Ed.) Polymeric Solar Cells: Materials, Design, Manufacture; DEStech: Lancaster, PA, USA, 2010. [Google Scholar]
- Chen, B.; Zheng, X.; Bai, Y.; Padture, N.; Huang, J. Progress in Tandem Solar Cells Based on Hybrid Organic-Inorganic Perovskites. Adv. Energy Mater. 2017, 7, 1602400. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.; Meng, L.; Lien, S.-Y.; Gao, P. Perovskite-Based Tandem Solar Cells: Get the Most Out of the Sun. Adv. Funct. Mater. 2020, 30, 2001904. [Google Scholar] [CrossRef]
- Mauer, R.; Kastler, M.; Laquai, F. The Impact of Polymer Regioregularity on Charge Transport and Efficiency of P3HT: PCBM Photovoltaic Devices. Adv. Funct. Mater. 2010, 20, 2085–2092. [Google Scholar] [CrossRef]
- Yang, C.-M.; Wu, C.-H.; Liao, H.-H.; Lai, K.-Y.; Cheng, H.-P.; Horng, S.-F.; Meng, H.-F.; Shy, J.T. Enhanced photovoltaic response of organic solar cell by singlet-to-triplet exciton conversion. Appl. Phys. Lett. 2007, 90, 133509. [Google Scholar] [CrossRef]
- Çaldıran, Z.; Erkem, Ü.; Baltakesmez, A.; Biber, M. Effects of the PENTACENE as doping material on the power conversion efficiency of P3HT:PCBM based ternary organic solar cells. Phys. B Condens. Matter 2021, 607, 412859. [Google Scholar] [CrossRef]
- Barbour, L.W.; Hegadorn, M.; Asbury, J.B. Watching Electrons Move in Real Time: Ultrafast Infrared Spectroscopy of a Polymer Blend Photovoltaic Material. J. Am. Chem. Soc. 2007, 129, 15884–15894. [Google Scholar] [CrossRef]
- Tian, H.; Ni, Y.; Zhang, W.; Xu, Y.; Zheng, B.; Jeong, S.Y.; Wu, S.; Ma, Z.; Du, X.; Hao, X.; et al. Over 19.2% efficiency of layer-by-layer organic photovoltaics enabled by a highly crystalline material as an energy donor and nucleating agent. Energy Environ. Sci. 2024, 17, 5173–5182. [Google Scholar] [CrossRef]
- Xu, W.; Tian, H.; Ni, Y.; Xu, Y.; Zhang, L.; Zhang, F.; Wu, S.; Young Jeong, S.; Huang, T.; Du, X.; et al. Eco-friendly solvent-processed layer-by-layer ternary all-polymer solar cells exhibiting over 18.5% efficiency. Chem. Eng. J. 2024, 493, 152558. [Google Scholar] [CrossRef]
- Abbas, Z.; Ryu, S.U.; Haris, M.; Song, C.E.; Lee, H.K.; Lee, S.K.; Shin, W.S.; Park, T.; Lee, J.-C. Optimized vertical phase separation via systematic Y6 inner side-chain modulation for non-halogen solvent processed inverted organic solar cells. Nano Energy 2022, 101, 107574. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, L.; Wang, X.; Mao, H.; Zeng, L.; Tan, L.; Zhuang, X.; Chen, Y. Regulation of Crystallinity and Vertical Phase Separation Enables High-Efficiency Thick Organic Solar Cells. Adv. Funct. Mater. 2022, 32, 2202103. [Google Scholar] [CrossRef]
- Griffith, M.; Cooling, N.; Vaughan, B.; Elkington, D.; Hart, A.; Lyons, A.; Qureshi, S.; Belcher, W.; Dastoor, P. Combining Printing, Coating and Vacuum Deposition on the Roll-to-Roll Scale: A Hybrid Organic Photovoltaics Fabrication. IEEE J. Sel. Top. Quantum Electron. 2015, 22, 112–125. [Google Scholar] [CrossRef]
- Xue, R.; Zhang, J.; Li, Y.; Li, Y. Organic Solar Cell Materials toward Commercialization. Small 2018, 14, 1801793. [Google Scholar] [CrossRef]
- Brédas, J.-L.; Norton, J.; Cornil, J.; Coropceanu, V. Molecular Understanding of Organic Solar Cells: The Challenges. Acc. Chem. Res. 2009, 42, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Scharber, M.C.; Sariciftci, N.S. Efficiency of bulk-heterojunction organic solar cells. Prog. Polym. Sci. 2013, 38, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Wadsworth, A.; Hamid, Z.; Kosco, J.; Gasparini, N.; McCulloch, I. The Bulk Heterojunction in Organic Photovoltaic, Photodetector, and Photocatalytic Applications. Adv. Mater. 2020, 32, 2001763. [Google Scholar] [CrossRef]
- Yamanaka, T.; Nakanotani, H.; Adachi, C. Significant role of spin-triplet state for exciton dissociation in organic solids. Sci. Adv. 2022, 8, eabj9188. [Google Scholar] [CrossRef]
- Proctor, C.; Kuik, M.; Nguyen, T.-Q. Charge carrier recombination in organic solar cells. Prog. Polym. Sci. 2013, 38, 1941–1960. [Google Scholar] [CrossRef]
- Zhang, K.-N.; Jiang, Z.-N.; Wang, T.; Qiao, J.-W.; Feng, L.; Qin, C.-C.; Yin, H.; So, S.-K.; Hao, X.-T. Exploring the mechanisms of exciton diffusion improvement in ternary polymer solar cells: From ultrafast to ultraslow temporal scale. Nano Energy 2021, 79, 105513. [Google Scholar] [CrossRef]
- Tang, Z.; Tress, W.; Inganas, O. Light trapping in thin film organic solar cells. Mater. Today 2014, 17, 389–396. [Google Scholar] [CrossRef]
- Li, S.; Chen, Y.; Wang, Z.; Chen, J.; Zhang, J.; Nie, J.; Duan, Y.; Geng, Y.; Su, Z. Theoretical studies of new iridium-based terpolymer donors for high-efficiency triplet-material-based organic photovoltaics: Incorporation of different iridium(III) complexes. Mater. Chem. Phys. 2023, 302, 127780. [Google Scholar] [CrossRef]
- Bredas, J.-L. Molecular understanding of organic solar cells: The challenges. AIP Conf. Proc. 2013, 1519, 55–58. [Google Scholar] [CrossRef]
- Su, Y.-W.; Lan, S.-C.; Wei, K.-H. Organic photovoltaics. Mater. Today 2012, 15, 554–562. [Google Scholar] [CrossRef]
- Jungbluth, A.; Kaienburg, P.; Riede, M. Charge transfer state characterization and voltage losses of organic solar cells. J. Phys. Mater. 2022, 5, 024002. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, Y.; Liu, Y.; Xue, J.; Li, W.; Qiao, J.; Zhang, F. Limitations and Perspectives on Triplet-Material-Based Organic Photovoltaic Devices. Adv. Mater. 2019, 31, 1900690. [Google Scholar] [CrossRef] [PubMed]
- Clarke, T.M.; Durrant, J.R. Charge photogeneration in organic solar cells. Chem. Rev. 2010, 110, 6736–6767. [Google Scholar] [CrossRef]
- Winroth, G.; Podobinski, D.; Cacialli, F. Dopant optimization for triplet harvesting in polymer photovoltaics. J. Appl. Phys. 2011, 110, 124504. [Google Scholar] [CrossRef]
- Zhou, H.; Sun, Y.; Zhang, M.; Ni, Y.; Zhang, F.; Young Jeong, S.; Huang, T.; Li, X.; Young Woo, H.; Zhang, J.; et al. Over 18.2% efficiency of layer–by–layer all–polymer solar cells enabled by homoleptic iridium(III) carbene complex as solid additive. Sci. Bull. 2024, in press. [Google Scholar] [CrossRef]
- Gao, H.; Yu, R.; Ma, Z.; Gong, Y.; Zhao, B.; Lv, Q.; Tan, Z.A. Recent advances of organometallic complexes in emerging photovoltaics. J. Polym. Sci. 2021, 60, 865–916. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, X.; Zhang, H.; Zhu, L.; Xu, L.; Zhang, F.; Tsang, C.-S.; Lee, L.Y.S.; Woo, H.Y.; He, Z.; et al. Metallated terpolymer donors with strongly absorbing iridium complex enables polymer solar cells with 16.71% efficiency. Chem. Eng. J. 2022, 430, 132832. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, M.; Wang, X.; Xu, C.; Xu, W.; Gao, J.; Wang, J.; Wong, W.-Y.; Son, J.H.; Jeong, S.Y.; et al. Ternary polymer solar cells with iridium-based polymer PM6Ir1 as a donor and N3:ITIC-Th as an acceptor exhibiting over 17.2% efficiency. Sustain. Energy Fuels 2021, 5, 5825–5832. [Google Scholar] [CrossRef]
- Baranoff, E.; Yum, J.-H.; Graetzel, M.; Nazeeruddin, M. Cyclometallated iridium complexes for conversion of light into electricity and electricity into light. J. Organomet. Chem. 2009, 694, 2661–2670. [Google Scholar] [CrossRef]
- Marin-Beloqui, J.M.; Congrave, D.G.; Toolan, D.T.W.; Montanaro, S.; Guo, J.; Wright, I.A.; Clarke, T.M.; Bronstein, H.; Dimitrov, S.D. Generating Long-Lived Triplet Excited States in Narrow Bandgap Conjugated Polymers. J. Am. Chem. Soc. 2023, 145, 3507–3514. [Google Scholar] [CrossRef] [PubMed]
- Kober, E.M.; Meyer, T.J. Concerning the absorption spectra of the ions M(bpy)32+ (M = Fe, Ru, Os; bpy = 2,2′-bipyridine). Inorg. Chem. 1982, 21, 3967–3977. [Google Scholar] [CrossRef]
- Heindl, M.; Hongyan, J.; Hua, S.A.; Oelschlegel, M.; Meyer, F.; Schwarzer, D.; González, L. Excited-State Dynamics of [Ru((S-S)bpy)(bpy)(2)](2+) to Form Long-Lived Localized Triplet States. Inorg. Chem. 2021, 60, 1672–1682. [Google Scholar] [CrossRef]
- Lo, S.-C.; Burn, P.L. Development of Dendrimers: Macromolecules for Use in Organic Light-Emitting Diodes and Solar Cells. Chem. Rev. 2007, 107, 1097–1116. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Nozawa, S.; Tomita, A.; Hoshino, M.; Koshihara, S.-Y.; Fujii, H.; Adachi, S.-I. Coordination and Electronic Structure of Ruthenium(II)-tris-2,2′-bipyridine in the Triplet Metal-to-Ligand Charge-Transfer Excited State Observed by Picosecond Time-Resolved Ru K-Edge XAFS. J. Phys. Chem. C 2012, 116, 14232–14236. [Google Scholar] [CrossRef]
- Sun, Y.; Giebink, N.C.; Kanno, H.; Ma, B.; Thompson, M.E.; Forrest, S.R. Management of singlet and triplet excitons for efficient white organic light-emitting devices. Nature 2006, 440, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, M.; Ni, Y.; Xu, W.; Zhou, H.; Ke, S.; Tian, H.; Jeong, S.Y.; Woo, H.Y.; Wong, W.-Y.; et al. Over 17.1% or 18.2% Efficiency of Layer-by-Layer All-Polymer Solar Cells via Incorporating Efficient Pt Complexes as Energy Donor Additive. ACS Mater. Lett. 2024, 6, 2964–2973. [Google Scholar] [CrossRef]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Visbal, R.; Gimeno, M.C. N-heterocyclic carbene metal complexes: Photoluminescence and applications. Chem. Soc. Rev. 2014, 43, 3551–3574. [Google Scholar] [CrossRef] [PubMed]
- Henwood, A.F.; Lesieur, M.; Bansal, A.K.; Lemaur, V.; Beljonne, D.; Thompson, D.G.; Graham, D.; Slawin, A.M.Z.; Samuel, I.D.W.; Cazin, C.S.J.; et al. Palladium(0) NHC complexes: A new avenue to highly efficient phosphorescence. Chem. Sci. 2015, 6, 3248–3261. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Majumdar, S.; Fortman, G.C.; Cazin, C.S.J.; Slawin, A.M.Z.; Lhermitte, C.; Prabhakar, R.; Germain, M.E.; Palluccio, T.; Nolan, S.P.; et al. Oxygen Binding to [Pd(L)(L’)] (L= NHC, L’ = NHC or PR3, NHC = N-Heterocyclic Carbene). Synthesis and Structure of a Paramagnetic trans-[Pd(NHC)2(η1-O2)2] Complex. J. Am. Chem. Soc. 2011, 133, 1290–1293. [Google Scholar] [CrossRef] [PubMed]
- Maties, G.; Gómez-Sal, P.; Yebra, C.G.; Andrés, R.; de Jesús, E. Reversible Single-Electron-Transfer to Oxygen in a Stable N-Heterocyclic Carbene Palladium(I) Metalloradical. Inorg. Chem. 2023, 62, 19838–19842. [Google Scholar] [CrossRef]
- Maiti, S.; Siebbeles, L.D.A. Developments and Challenges Involving Triplet Transfer across Organic/Inorganic Heterojunctions for Singlet Fission and Photon Upconversion. J. Phys. Chem. Lett. 2023, 14, 11168–11176. [Google Scholar] [CrossRef]
- Adnan, M.; Irshad, Z.; Hussain, R.; Lee, W.; Kim, M.; Lim, J. Efficient ternary active layer materials for organic photovoltaics. Sol. Energy 2023, 257, 324–343. [Google Scholar] [CrossRef]
- Balasubramanian, S.; León-Luna, M.Á.; Romero, B.; Madsen, M.; Turkovic, V. Vitamin C for Photo-Stable Non-fullerene-acceptor-Based Organic Solar Cells. ACS Appl. Mater. Interfaces 2023, 15, 39647–39656. [Google Scholar] [CrossRef]
- Turkovic, V.; Prete, M.; Bregnhøj, M.; Inasaridze, L.; Volyniuk, D.; Obrezkov, F.A.; Grazulevicius, J.V.; Engmann, S.; Rubahn, H.-G.; Troshin, P.A.; et al. Biomimetic Approach to Inhibition of Photooxidation in Organic Solar Cells Using Beta-Carotene as an Additive. ACS Appl. Mater. Interfaces 2019, 11, 41570–41579. [Google Scholar] [CrossRef]
- Abdallaoui, M.; Sengouga, N.; Chala, A.; Meftah, A.F.; Meftah, A.M. Comparative study of conventional and inverted P3HT: PCBM organic solar cell. Opt. Mater. 2020, 105, 109916. [Google Scholar] [CrossRef]
- Baek, W.-H.; Yang, H.; Yoon, T.-S.; Kang, C.J.; Lee, H.H.; Kim, Y.-S. Effect of P3HT:PCBM concentration in solvent on performances of organic solar cells. Sol. Energy Mater. Sol. Cells 2009, 93, 1263–1267. [Google Scholar] [CrossRef]
- Zhao, D.W.; Kyaw, A.K.K.; Sun, X.W. Organic Solar Cells with Inverted and Tandem Structures. In Energy Efficiency and Renewable Energy through Nanotechnology; Zang, L., Ed.; Green Energy and Technology; Springer: London, UK, 2011; pp. 115–170. [Google Scholar]
- Saliba, M.; Etgar, L. Current Density Mismatch in Perovskite Solar Cells. ACS Energy Lett. 2020, 5, 2886–2888. [Google Scholar] [CrossRef]
- Wang, L.M.; Li, Q.; Liu, S.; Cao, Z.; Cai, Y.P.; Jiao, X.; Lai, H.; Xie, W.; Zhan, X.; Zhu, T. Quantitative Determination of the Vertical Segregation and Molecular Ordering of PBDB-T/ITIC Blend Films with Solvent Additives. ACS Appl. Mater. Interfaces 2020, 12, 24165–24173. [Google Scholar] [CrossRef]
- Walter, R.; Meyer, H.; Voss, S. Palladium(0)-Dibenzylidene Acetone Complexes. U.S. Patent 2010/0167408A1, 1 July 2010. [Google Scholar]
- Kapdi, A.R.; Whitwood, A.C.; Williamson, D.C.; Lynam, J.M.; Burns, M.J.; Williams, T.J.; Reay, A.J.; Holmes, J.; Fairlamb, I.J.S. The Elusive Structure of Pd2(dba)3. Examination by Isotopic Labeling, NMR Spectroscopy, and X-ray Diffraction Analysis: Synthesis and Characterization of Pd2(dba-Z)3 Complexes. J. Am. Chem. Soc. 2013, 135, 8388–8399. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Nolan, S.P. Efficient Cross-Coupling of Aryl Chlorides with Aryl Grignard Reagents (Kumada Reaction) Mediated by a Palladium/Imidazolium Chloride System. J. Am. Chem. Soc. 1999, 121, 9889–9890. [Google Scholar] [CrossRef]
- Jafarpour, L.; Stevens, E.D.; Nolan, S.P. A sterically demanding nucleophilic carbene: 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene). Thermochemistry and catalytic application in olefin metathesis. J. Organomet. Chem. 2000, 606, 49–54. [Google Scholar] [CrossRef]
- Connelly, N.G.; Geiger, W.E. Chemical Redox Agents for Organometallic Chemistry. Chem. Rev. 1996, 96, 877–910. [Google Scholar] [CrossRef] [PubMed]
- Tait, C.; Lehner, J.; Nehrkorn, J.; Krzyaniak, M.; Ho, A.; Stoll, S. EasySpin, Software Package for Spectral Simulation and Analysis in EPR, Version 5.2.25. 2014. Available online: http://www.easyspin.org (accessed on 18 November 2023).
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Etienne, E.; Le Breton, N.; Martinho, M.; Mileo, E.; Belle, V. SimLabel: A graphical user interface to simulate continuous wave EPR spectra from site-directed spin labeling experiments. Magn. Reson. Chem. 2017, 55, 714–719. [Google Scholar] [CrossRef]
- Arduengo, A.J., III; Krafczyk, R.; Schmutzler, R.; Craig, H.A.; Goerlich, J.R.; Marshall, W.J.; Unverzagt, M. Imidazolylidenes, imidazolinylidenes and imidazolidines. Tetrahedron 1999, 55, 14523–14534. [Google Scholar] [CrossRef]
- Zalesskiy, S.S.; Ananikov, V.P. Pd2(dba)3 as a precursor of soluble metal complexes and nanoparticles: Determination of palladium active species for catalysis and synthesis. Organometallics 2012, 31, 2302–2309. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016.
- Staroverov, V.N.; Scuseria, G.E.; Tao, J.; Perdew, J.P. Comparative Assessment of a New Nonempirical Density Functional: Molecules and Hydrogen-Bonded Complexes. J. Chem. Phys. 2003, 119, 12129–12137. [Google Scholar] [CrossRef]
- Tao, J.; Perdew, J.P.; Staroverov, V.N.; Scuseria, G.E. Climbing the Density Functional Ladder: Nonempirical Meta−Generalized Gradient Approximation Designed for Molecules and Solids. Phys. Rev. Lett. 2003, 91, 146401. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Andrae, D.; Haeussermann, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-Adjusted Ab Initio Pseudopotentials for the Second and Third Row Transition Elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Ehlers, A.W.; Bohme, M.; Dapprich, S.; Gobbi, A.; Hollwarth, A.; Jonas, V.; Kohler, K.F.; Stegmann, R.; Veldkamp, A.; Frenking, G. A Set of f-Polarization Functions for Pseudo-Potential Basis-Sets of the Transition-Metals Sc-Cu, Y-Ag and La-Au. Chem. Phys. Lett. 1993, 208, 111–114. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian-basis sets for molecular calculations. 1. 2nd row atoms, Z=11-18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Raghavachari, K.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. 20. Basis set for correlated wave-functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar]






| [Pd]/[P3HT:PC60BM] wt% | [Pd] in the Active Layer Solution (µg/mL) |
|---|---|
| 0 (Ref) | 0 |
| 0.05 | 10 |
| 0.10 | 20 |
| 0.50 | 100 |
| 1.00 | 200 |
| 1.50 | 300 |
| 2.00 | 400 |
| 3.00 | 600 |
| 4.00 | 800 |
| 6.00 | 1200 |
| Highest Occupied | Lowest Unoccupied | ||
|---|---|---|---|
| alpha | beta | alpha | beta |
| −5.75 | −5.94 | −1.38 | −3.62 |
| wt% [Pd] | Voc (V) | Jsc (mA cm−2) | FF (%) | Eff (%) |
|---|---|---|---|---|
| 0.00 (Ref) | 0.509 ± 0.049 | 6.11 ± 0.31 | 46.8 ± 6.4 | 1.46 ± 0.32 |
| 0.05 | 0.532 ± 0.043 | 6.13 ± 0.08 | 47.2 ± 3.7 | 1.55 ± 0.25 |
| 0.10 | 0.550 ± 0.033 | 6.43 ± 0.19 | 53.4 ± 4.4 | 1.83 ± 0.25 |
| 0.50 | 0.558 ± 0.011 | 6.40 ± 0.27 | 54.1 ± 3.1 | 2.00 ± 0.12 |
| 1.00 | 0.559 ± 0.023 | 6.54 ± 0.28 | 56.1 ± 9.5 | 2.04 ± 0.29 |
| 1.50 | 0.550 ± 0.029 | 5.84 ± 0.33 | 55.1 ± 5.4 | 1.78 ± 0.34 |
| 2.00 | 0.495 ± 0.068 | 6.01 ± 0.44 | 49.5 ± 4.3 | 1.54 ± 0.38 |
| 3.00 | 0.479 ± 0.039 | 6.04 ± 0.48 | 51.2 ± 8.9 | 1.42 ± 0.37 |
| 4.00 | 0.451 ± 0.024 | 5.40 ± 0.36 | 44.5 ± 2.2 | 1.09 ± 0.15 |
| 6.00 | 0.393 ± 0.020 | 4.65 ± 0.23 | 42.0 ± 2.7 | 0.73 ± 0.07 |
| wt% [Pd] | Jsc (mA/cm−2)—J–V Curve | J Integrated (mA/cm−2)—IPCE | Deviation Value (%) |
|---|---|---|---|
| 0.0 (Ref) | 6.11 ± 0.31 | 8.10 | 33 |
| 0.1 | 6.43 ± 0.19 | 7.93 | 23 |
| 1.0 | 6.54 ± 0.28 | 8.08 | 24 |
| 4.0 | 5.40 ± 0.36 | 6.19 | 15 |
| [Pd]/[P3HT:PC60BM] (wt%) | ITO/ZnO/PEIE/ PBDB-T:ITIC:Pd (nm) | ITO/ZnO/PEIE/ PBDB-T:ITIC: Pd/PEDOT:PSS (nm) |
|---|---|---|
| 0 (Ref) | 107 ± 3 | 126 ± 1 |
| 0.1 | 103 ± 4 | 119 ± 1 |
| 1.0 | 104 ± 4 | 124 ± 3 |
| 3.0 | 106 ± 2 | 117 ± 2 |
| 6.0 | 106 ± 2 | 116 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Astal-Quirós, A.; Carrarini, V.; Zarotti, F.; Rahman, A.U.; Lledós, A.; Yebra, C.G.; de Jesús, E.; Reale, A. A Doublet State Palladium(I) N-Heterocyclic Carbene Complex as a Dopant and Stabilizer for Improved Photostability in Organic Solar Cells. Energies 2024, 17, 3787. https://doi.org/10.3390/en17153787
El Astal-Quirós A, Carrarini V, Zarotti F, Rahman AU, Lledós A, Yebra CG, de Jesús E, Reale A. A Doublet State Palladium(I) N-Heterocyclic Carbene Complex as a Dopant and Stabilizer for Improved Photostability in Organic Solar Cells. Energies. 2024; 17(15):3787. https://doi.org/10.3390/en17153787
Chicago/Turabian StyleEl Astal-Quirós, Aliah, Valentina Carrarini, Francesca Zarotti, Atiq Ur Rahman, Agustí Lledós, Cristina G. Yebra, Ernesto de Jesús, and Andrea Reale. 2024. "A Doublet State Palladium(I) N-Heterocyclic Carbene Complex as a Dopant and Stabilizer for Improved Photostability in Organic Solar Cells" Energies 17, no. 15: 3787. https://doi.org/10.3390/en17153787








