Hydroprocessing Microbial Oils for Advanced Road Transportation, Aviation, and Maritime Drop-In Fuels: Industrially Relevant Scale Validation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Scope and Procedure
2.2. Feeds and Catalyst
2.3. Storage Stability Study
2.4. Testing Infrastructure
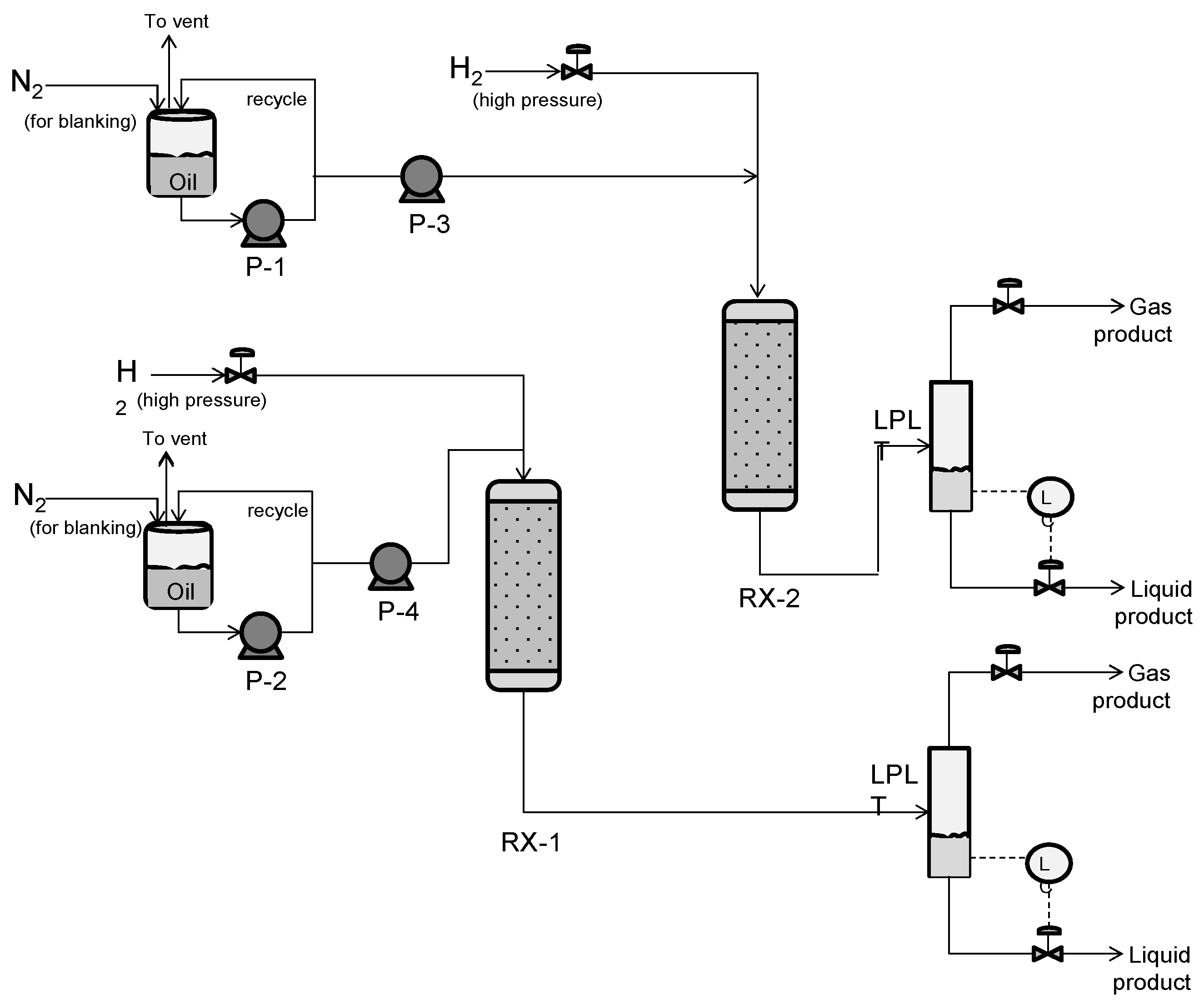
2.4.1. Hydroprocessing Equipment (TRL 4)
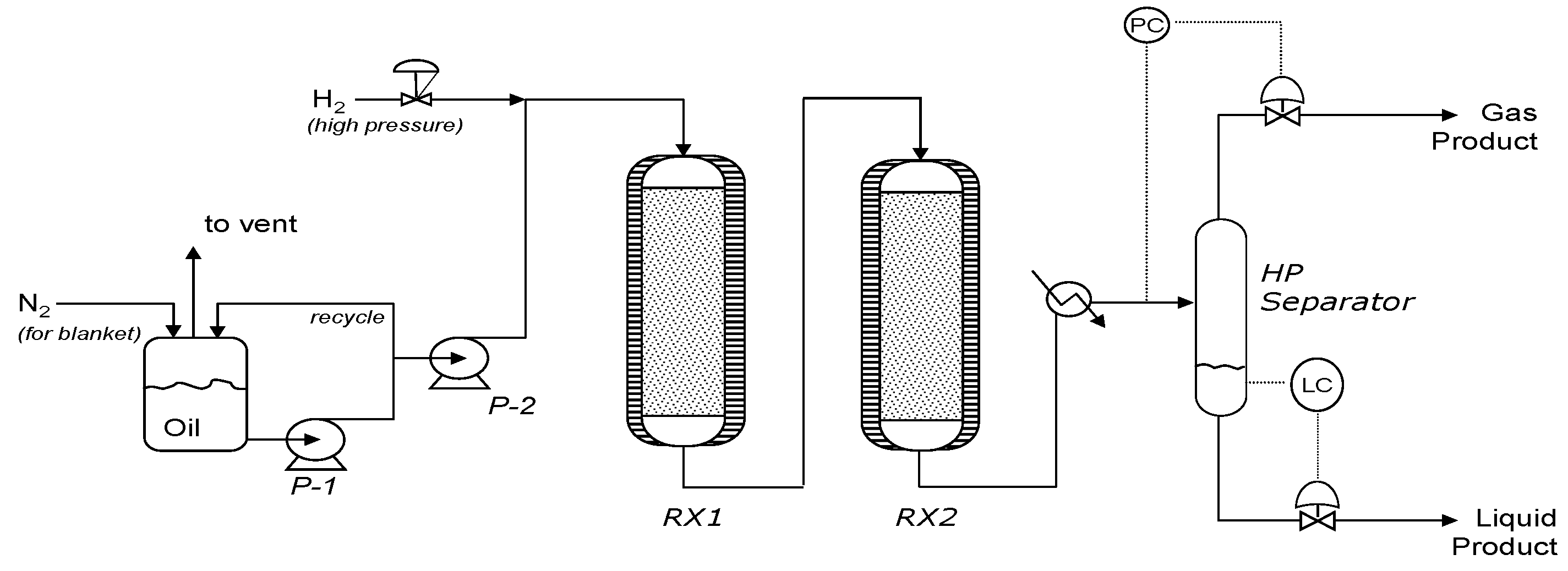
2.4.2. Hydroprocessing Equipment (TRL 5)
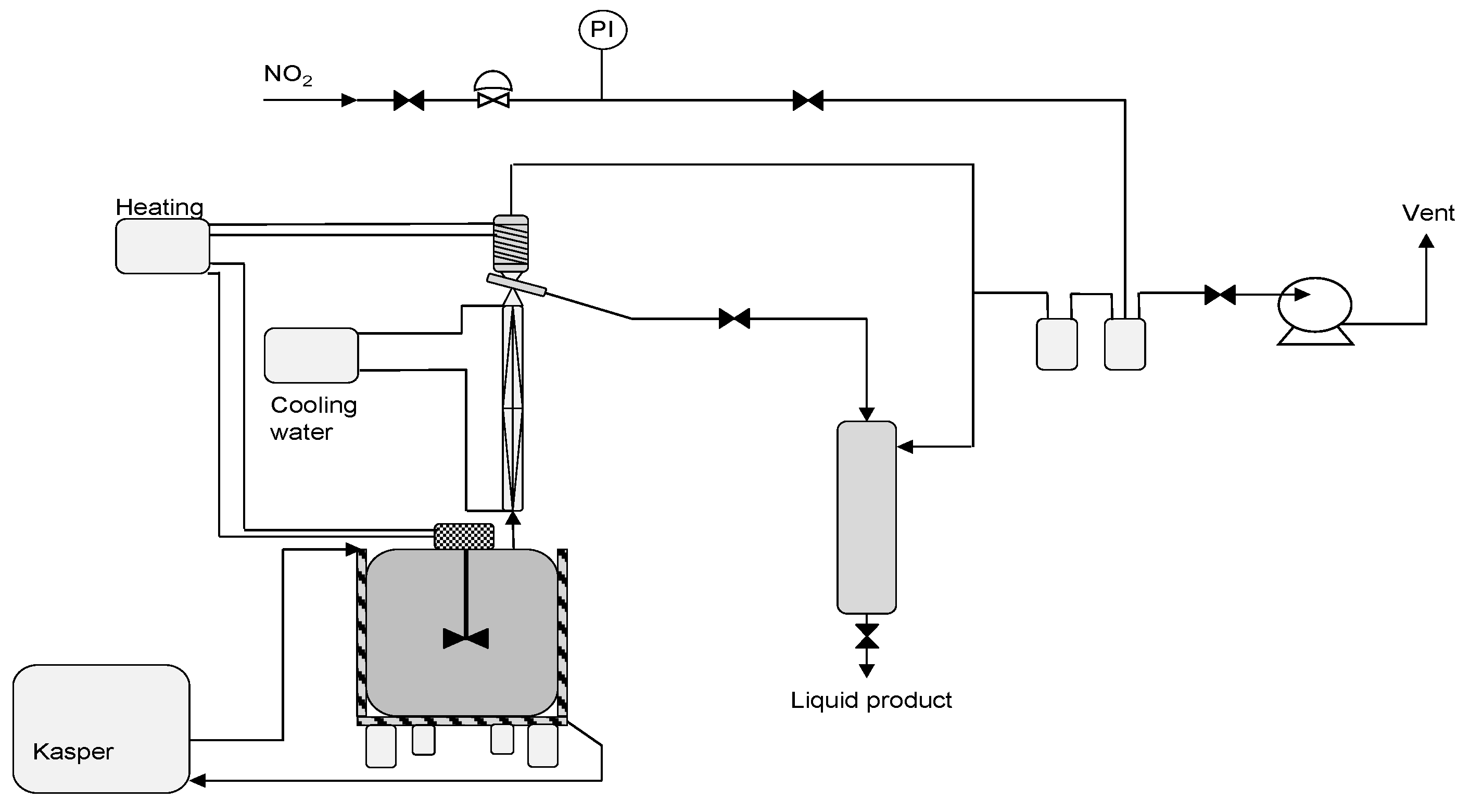
2.4.3. Fractionation Equipment
2.5. Analysis
3. Results
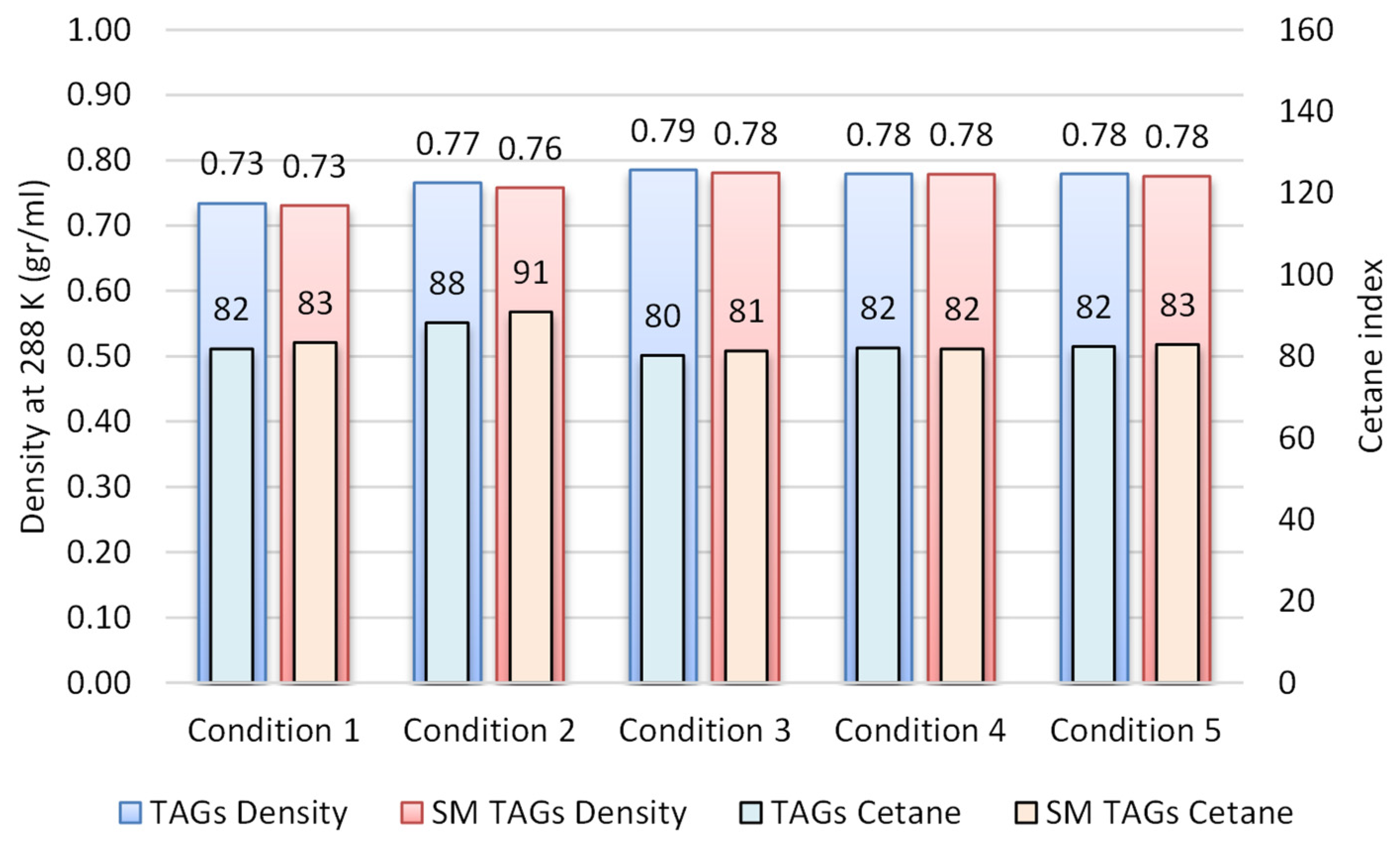
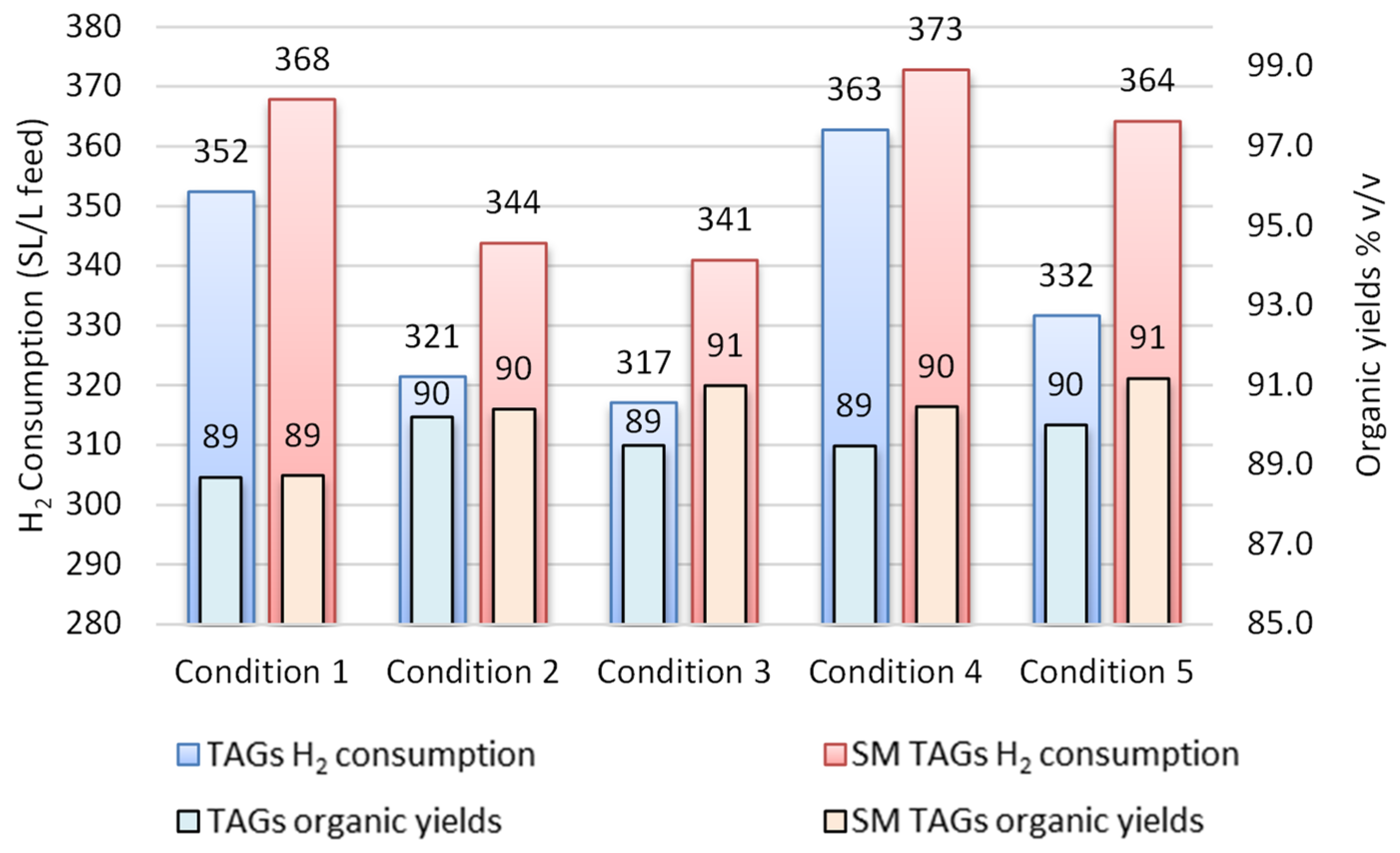
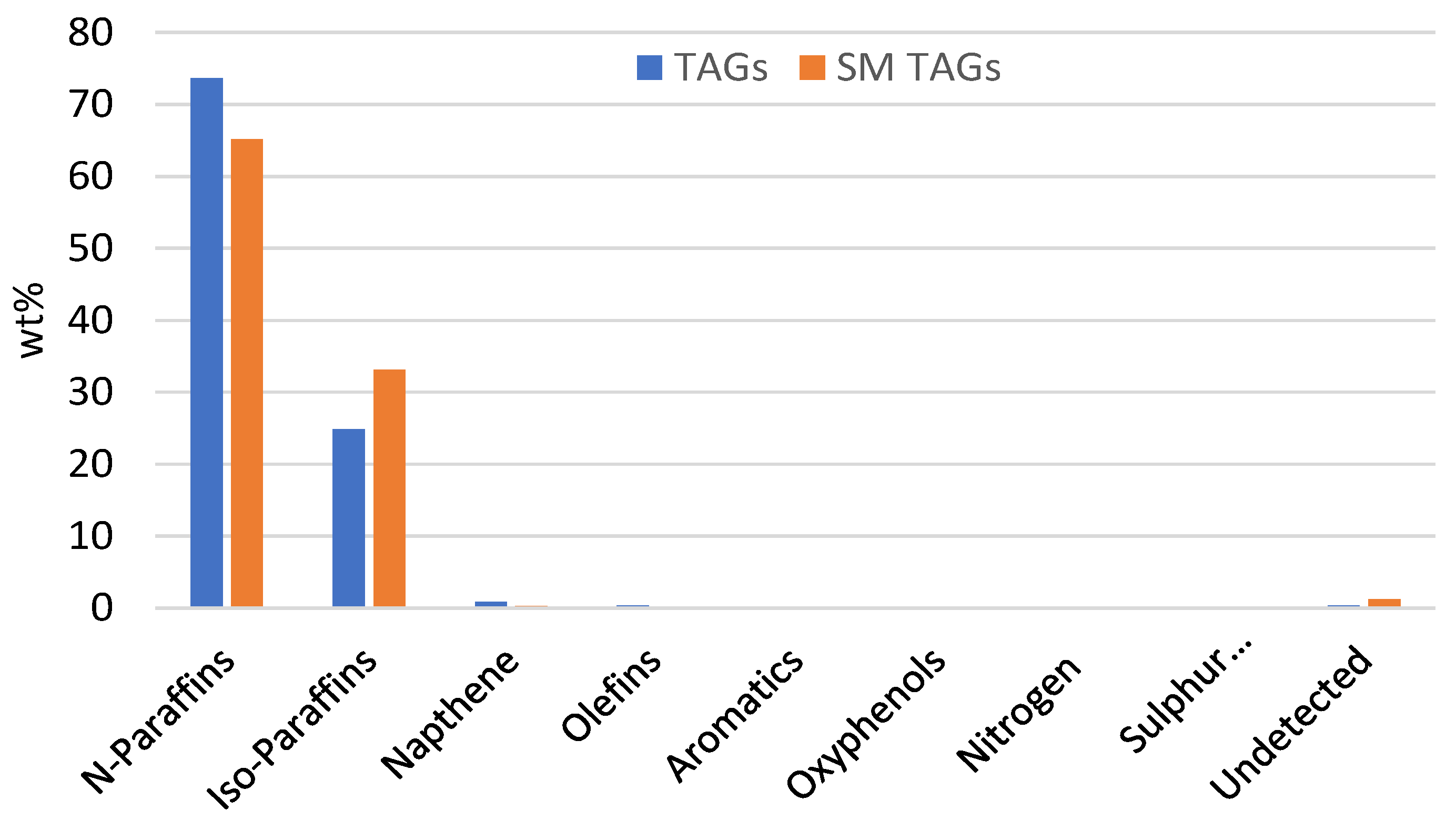
3.1. Simulated Feedstock Evaluation
3.2. Operating Process Evaluation
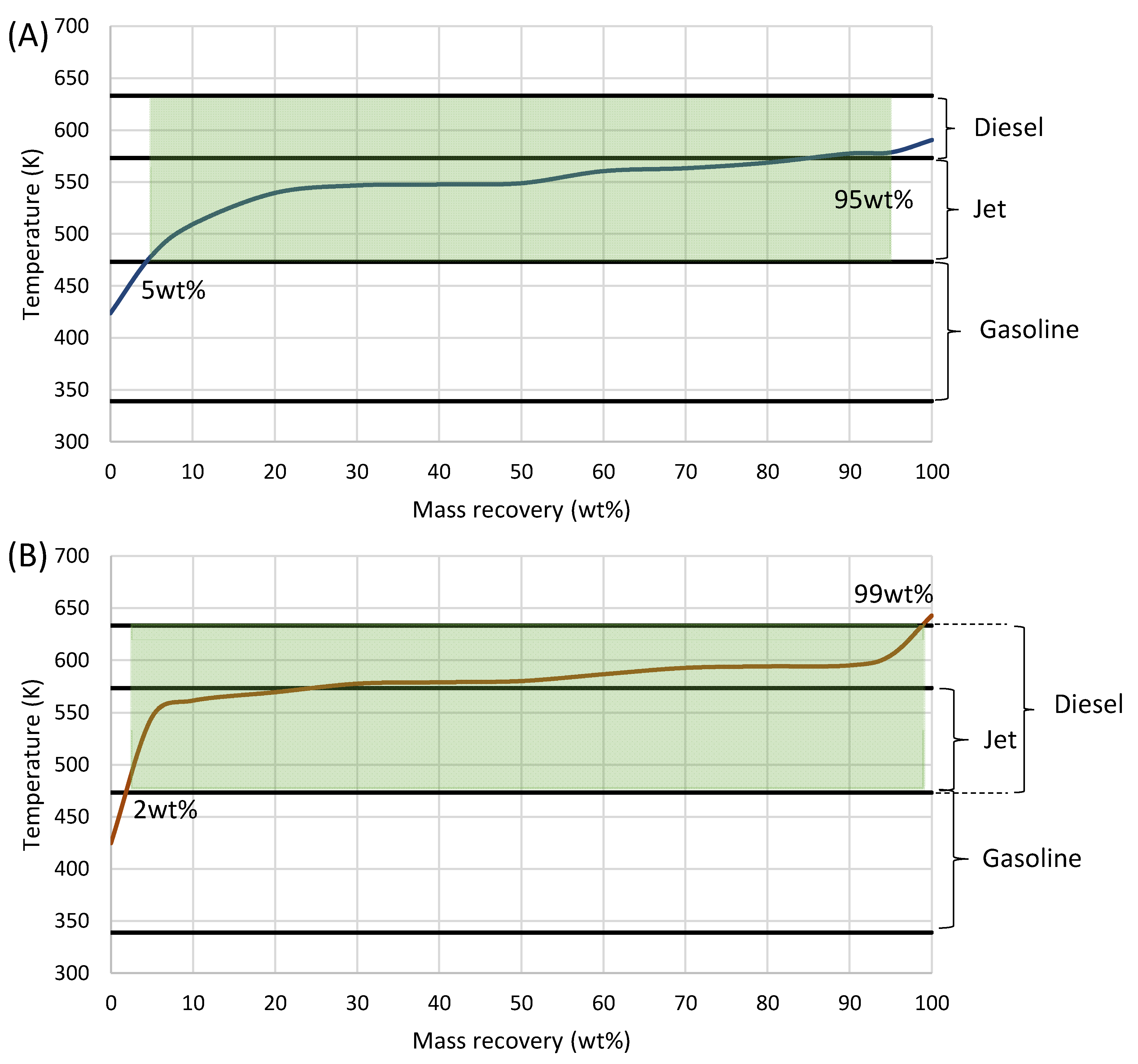
3.3. TRL 5 Scale-Up—Production
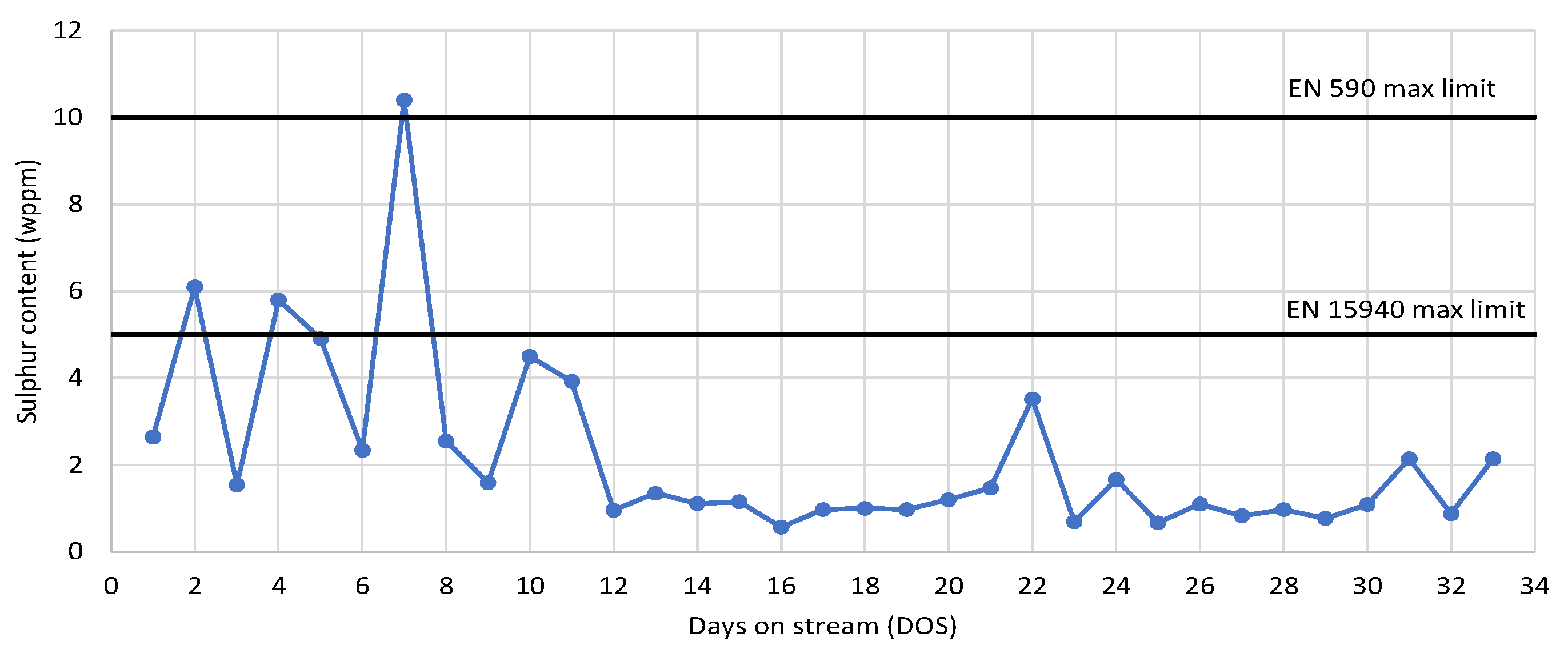
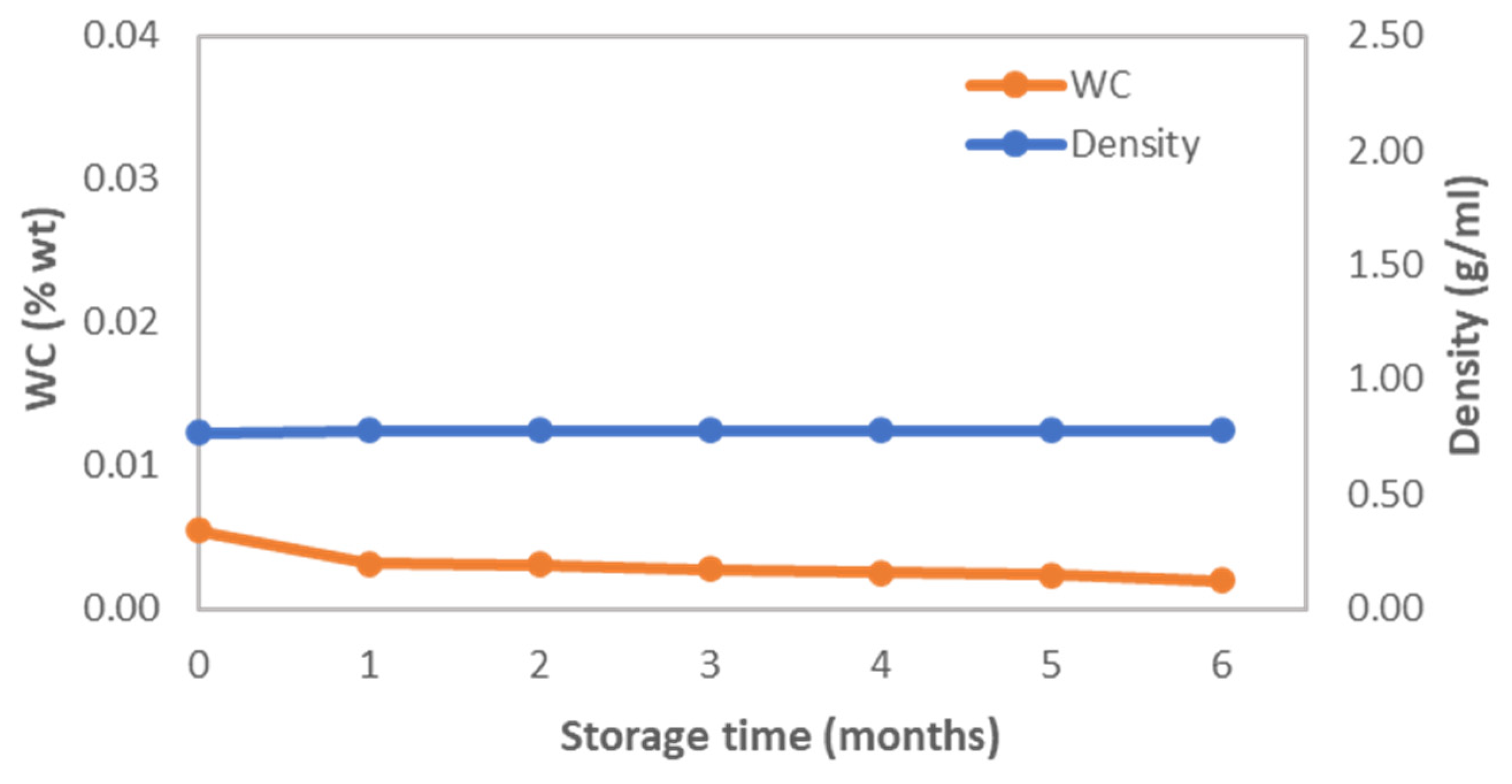
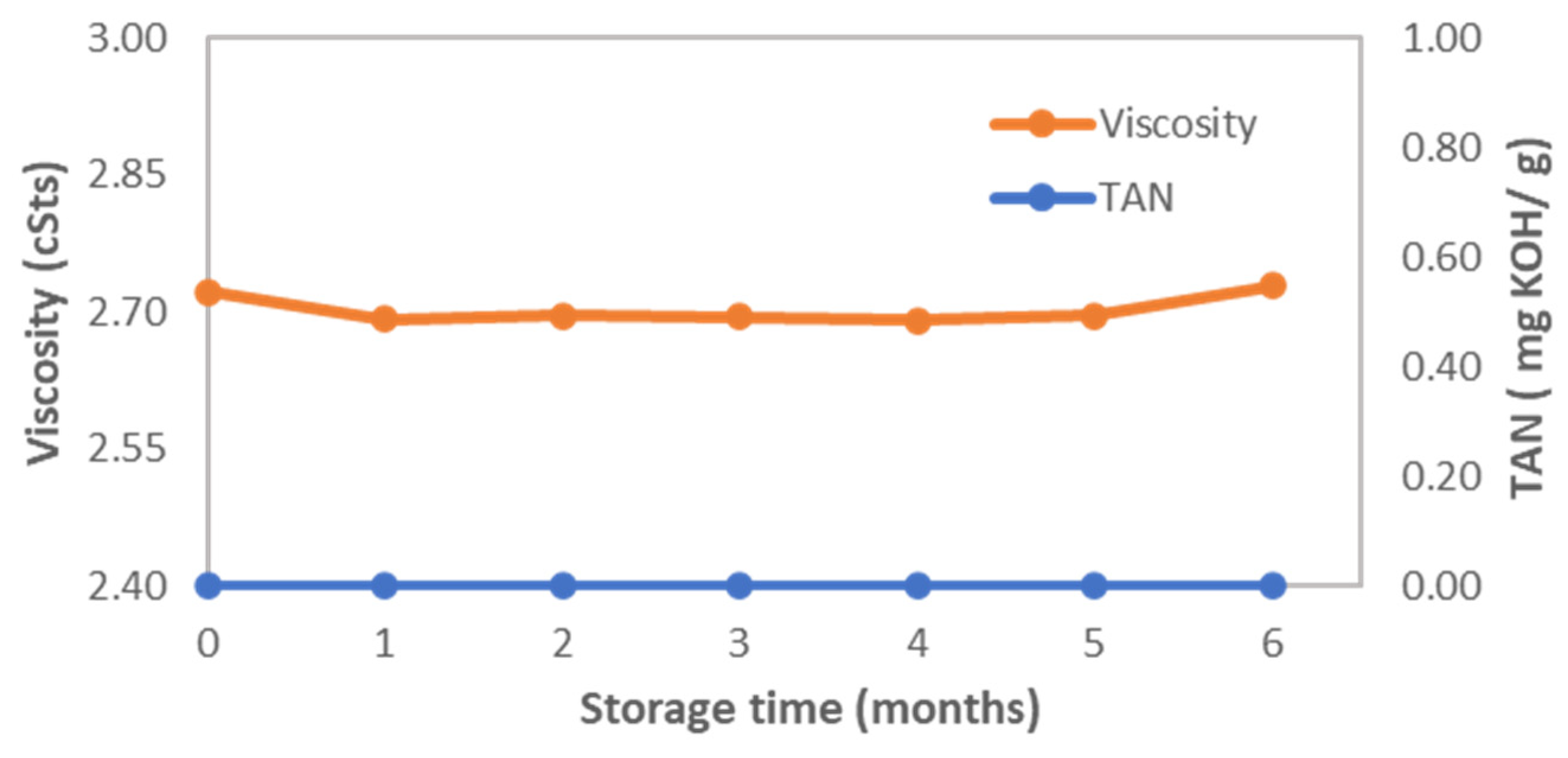
3.4. Storage Stability Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviation
| CERTH | Centre for Research & Technology Hellas |
| CPERI | Chemical Process & Energy Resources Institute |
| DFB | Dual fluidized bed |
| DMDS | Dimethyl-disulphide |
| DOS | Days on stream |
| DP | Drop pressure |
| FAME | Fatty acid methyl ester |
| GC | Gas chromatograph |
| HDO | Hydrodeoxygenation |
| HDT | Hydrotreating |
| HHV | Higher heating value |
| HVO | Hydrotreated vegetable oils |
| LAGO | Light atmospheric gas oil |
| LHSV | Liquid hourly space velocity |
| NDA | Nondisclosure agreement |
| SM TAGs | Simulated triacylglycerides |
| TAGs | Triacylglycerides |
| TAN | Total acid number |
| TBA | Tetra-butyl-amine |
| TRL | Technology readiness level |
| WC | Water content |
References
- Li, X.; Mupondwa, E.; Tabil, L. Technoeconomic analysis of biojet fuel production from camelina at commercial scale: Case of Canadian prairies. Bioresouce Technol. 2018, 249, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, S.M.; Pateraki, C.; Ladakis, D.; Papapostolou, H.; Tsakona, M.; Vlysidis, A.; Kookos, I.K.; Koutinas, A. Sustainable production of bio-based chemicals and polymers via integrated biomass refining and bioprocessing in a circular bioeconomy context. Bioresour. Technol. 2020, 307, 123093. [Google Scholar] [CrossRef] [PubMed]
- Perin, G.; Jones, P.R. Economic feasibility and long-term sustainability criteria on the path to enable a transition from fossil fuels to biofuels. Curr. Option Biotechnol. 2019, 57, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Tirlangi, S.; Kumar, A.; Imran, M.; Pillai HP, J.S.; Koshariya, A.K.; Sathish, T.; Ubaidullah, M.; Ayub, R.; Reddy, V.R.M.; et al. A review on the contribution of nanotechnology for biofuel production from algal biomass: A bridge to the reduction of carbon footprint. Sustain. Energy Technol. Assess. 2023, 60, 103498. [Google Scholar] [CrossRef]
- Elgharbawy, B.S.; Sadik, W.A.; Sadek, O.M.; Kasaby, M.A. A review on biodiesel feedstocks and production technologies. J. Chil. Chem. Soc. 2021, 66, 5098–5109. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.-Y.; Aziz, A.; Raman, A.; Ibrahim, S. Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232. [Google Scholar] [CrossRef]
- Zhou, Y.; Remon, J.; Jiang, Z.; Matharu, A.S.; Hu, C. Tuning the selectivity of natural oils and fatty acids/esters deoxygenation to biofuels and fatty alcohols: A review. Green Energy Environ. 2023, 8, 722–743. [Google Scholar] [CrossRef]
- Carmona-Cabello, M.; Garcia, I.L.; Papadaki, A.; Tsouko, E.; Joutinas, A.; Dorado, M.P. Biodiesel production using microbial lipids derived from food waste discarded by catering services. Bioresour. Technol. 2021, 323, 124597. [Google Scholar] [CrossRef]
- Detsios, N.; Theodoraki, S.; Maragoudaki, L.; Atsonios, K.; Grammelis, P.; Orfanoudakis, N.G. Recent Advances on Alternative Aviation Fuels/Pathways: A Critical Review. Energies 2023, 16, 1904. [Google Scholar] [CrossRef]
- El-Sawy, M.S.; Hanafi, S.A.; Ashour, F.; Aboul-Fotouh, T.M. Co-hydroprocessing and hydrocracking of alternative feed mixture (vacuum gas oil/waste lubricating oil/waste cooking oil) with the aim of producing high quality fuels. Fuel 2020, 269, 117437. [Google Scholar] [CrossRef]
- Wang, J.; Singer, S.D.; Souto, B.A.; Asomaning, J.; Ullah, A.; Bressler, D.C.; Chen, G. Current progress in lipid-based biofuels: Feedstocks and production technologies. Bioresour. Technol. 2022, 351, 127020. [Google Scholar] [CrossRef]
- Liu, H.; Han, J.; Huang, Q.; Shen, H.; Lei, L.; Huang, Z.; Zhang, Z.; Zhao, Z.K.; Wang, F. Catalytic hydrodeoxygenation of methyl stearate and microbial lipids to diesel-range alkanes over Pd/HPA-SiO2 catalysts. Ind. Eng. Chem. Res. 2020, 59, 17440–17450. [Google Scholar] [CrossRef]
- ASTM D1655-2024; Standard Specification for Aviation Turbine Fuels. ASTM International: West Conshohocken, PA, USA, 2024.
- ISO 8217:2017; Fuel Standard for Marine Distillate Fuels. ISO: Geneva, Switzerland, 2017.
- SIST EN 590:2022; Automotive Fuels-Diesel-Requirements and Test Methods. SIST: Ljubljana, Slovenian, 2022.
- SIST EN 15940:2023; Automotive Fuels—Paraffinic Diesel Fuel from Synthesis or Hydrotreatment—Requirements and Test Methods. SIST: Ljubljana, Slovenian, 2023.
- Detsios, N.; Maragoudaki, L.; Atsonios, K.; Grammelis, P.; Orfanoudakis, N.G. Design considerations of an integrated thermochemical/biochemical route for aviation and maritime biofuel production. Biomass Convers. Biorefinery 2023. [CrossRef]
- HoraceK, J.; Kubicka, D. Bio-oil hydrotreating over conventional CoMo & NiMo catalysts: The role of reaction conditions and additives. Fuel 2017, 198, 49–57. [Google Scholar] [CrossRef]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- ASTM D4052-22; Standard Test Method for Density, Relative Density, and API Gravity of Liquids by Digital Density Meter. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D7169-23; Standard Test Method for Boiling Point Distribution of Samples with Residues such as Crude Oils and Atmospheric and Vacuum Residues by High Temperature Gas Chromatography. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM D5291-21; Standard Test Methods for Instrument Determination of Carbon, Hydrogen, and Nitrogen in Petroleum Products and Lubricants. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D4294-21; Standard Test Method for Sulfur in Petroleum and Petroleum Products by Energy Dispersive X-ray Fluorescence Spectrometry. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D6304-20; Standard Test Method for Determination of Water in Petroleum Products, Lubricating Oils, and Additives by Coulometric Karl Fischer Titration. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM E203-16; Standard Test Method for Water Using Volumetric Karl Fischer Titration. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D664-18E02; Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration. ASTM International: West Conshohocken, PA, USA, 2024.
- ASTM D445-21; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity). ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D976-06; Standard Test Method for calculated cetane index of distillate fuels. ASTM International: West Conshohocken, PA, USA, 2006.
- SIST EN 15751:2014; Automotive Fuels—Fatty Acid Methyl Ester (FAME) Fuel and Blends with Diesel Fuel—Determination of Oxidation Stability by Accelerated Oxidation Method. SIST: Ljubljana, Slovenian, 2014.
- ASTM D97-17; Standard Test Method for Pour Point of Petroleum Products. ASTM International: West Conshohocken, PA, USA, 2022.
- Lin, C.H.; Wang, W.C. Direct conversion of glyceride-based oil into renewable jet fuels. Renew. Sustain. Energy Rev. 2020, 132, 110109. [Google Scholar] [CrossRef]
- Xu, J.; Long, F.; Jiang, J.; Li, F.; Zhai, Q.; Wang, F.; Liu, P.; Li, J. Integrated catalytic conversion of waste triglycerides to liquid hydrocarbons for aviation biofuels. J. Clean. Prod. 2019, 222, 784–792. [Google Scholar] [CrossRef]
- Maki-Arvela, P.; Martinez-Klimov, M.; Murzin, D.Y. Hydroconversion of fatty acids and vegetable oils for production of jet fuels. Fuel 2021, 306, 121673. [Google Scholar] [CrossRef]
- Feng, F.; Niu, X.; Wang, L.; Zhang, X.; Wang, Q. TEOS-modified Ni/ZSM-5 nanosheet catalysts for hydroconversion of oleic acid to high-performance aviation fuel: Effect of acid spatial distribution. Microporous Mesoporous Mater. 2020, 291, 109705. [Google Scholar] [CrossRef]
- Li, X.; Chen, Y.; Hao, Y.; Zhang, X.; Du, J.; Zhang, A. Optimization of aviation kerosene from one-step hydrotreatment of catalytic Jatropha oil over SDBS-Pt/SAPO-11 by response surface methodology. Renew. Energy 2019, 139, 551–559. [Google Scholar] [CrossRef]
- Dimitriadis, A.; Chrysikou, L.P.; Kokkalis, A.I.; Doufas, L.I.; Bezergianni, S. Animal fats valorization to green transportations fuels: From concept to industrially relevant scale validation. Waste Manag. 2022, 143, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.K.; Hsieh, C.H.; Wang, W.C. The production of renewable aviation fuel from waste cooking oil. Part II: Catalytic hydro-cracking/isomerization of hydro-processed alkanes into jet fuel range products. Renew. Energy 2020, 15, 731–740. [Google Scholar] [CrossRef]
- Kubička, D.; Tukač, V. Hydrotreating of Triglyceride Based Feedstocks in Refineries. In Advances in Chemical Engineering; Academic Press: Cambridge, MA, USA, 2013; Volume 42. [Google Scholar] [CrossRef]
- Dimitriadis, A.; Dimaratos, A.; Doulgeris, S.; Bezergianni, S.; Samaras, Z. Emissions Optimization Potential of a Diesel Engine Running on HVO: A Combined Experimental and Simulation Investigation; SAE Technical Paper No. 2019-24-0039; SAE International: Warrendale, PA, USA, 2019. [Google Scholar] [CrossRef]
- Dimitriadis, A.; Natsios, I.; Dimaratos, A.; Katsaounis, D.; Samaras, Z.; Bezergianni, S.; Lehto, K. Evaluation of a Hydrotreated Vegetable Oil (HVO) and Effects on Emissions of a Passenger Car Diesel Engine. Front. Mech. Eng. 2018, 4, 7. [Google Scholar] [CrossRef]

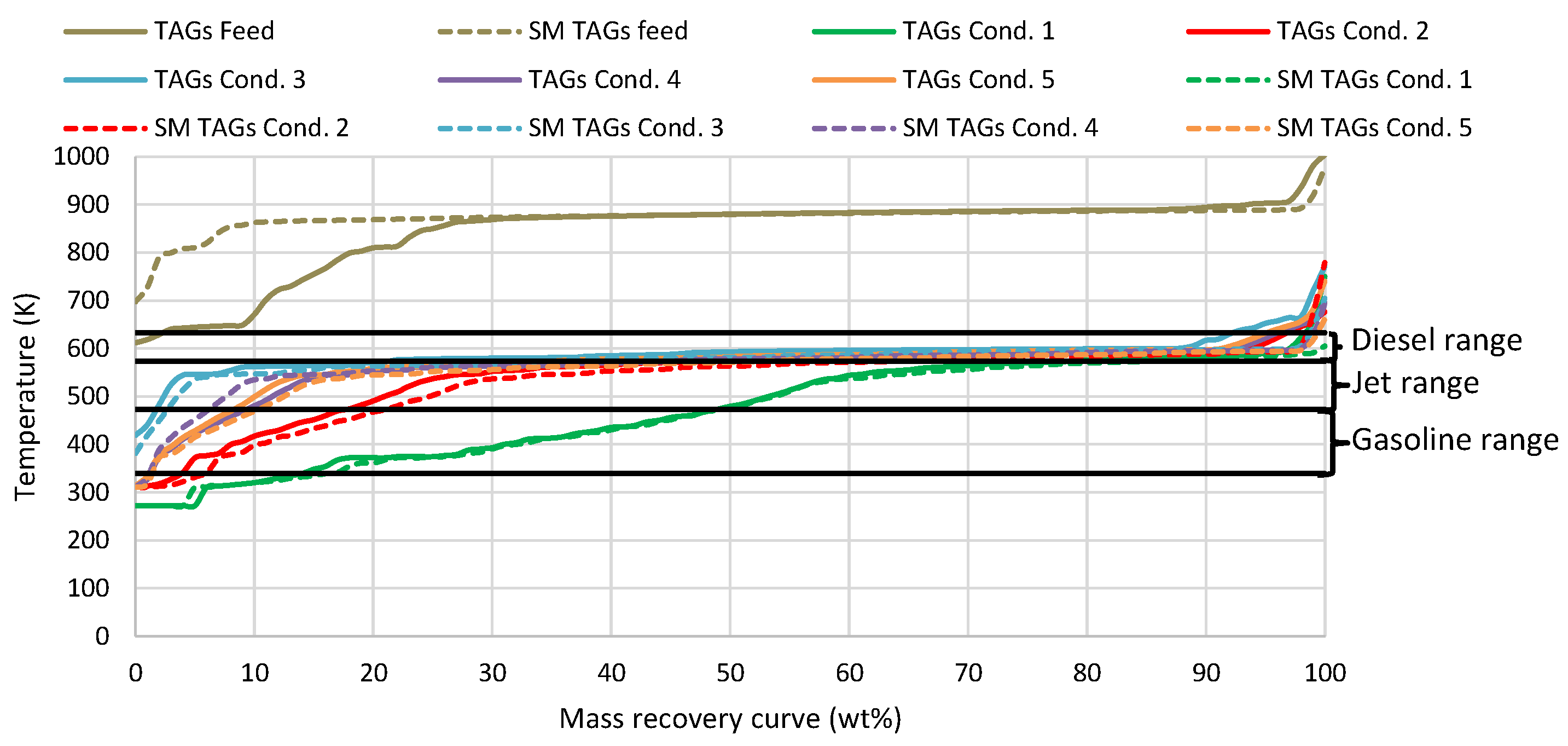
| Temperature (K) | Pressure (MPa) | LHSV (h−1) | H2/Oil Ratio (scfb) | |
|---|---|---|---|---|
| Condition 1 | 663 | 13.78 | 0.5 | 5000 |
| Condition 2 | 663 | 13.78 | 1 | 5000 |
| Condition 3 | 643 | 13.78 | 1 | 5000 |
| Condition 4 | 643 | 13.78 | 0.5 | 5000 |
| Condition 5 | 643 | 10.34 | 0.5 | 5000 |
| Acids | Formula | Units | Microbial Oil (TAGs) | Palm Oil | Flaxseed Oil | Olive Oil | Pumpkin Oil |
|---|---|---|---|---|---|---|---|
| Lauric | C12:0 | wt% | 0.00 | 0.21 | 0.00 | 0.00 | 0.00 |
| Myristic | C14:0 | wt% | 0.00 | 0.95 | 0.04 | 0.00 | 0.07 |
| Palmitic | C16:0 | wt% | 24.00 | 41.95 | 5.46 | 11.63 | 9.80 |
| Palmitoleic | C16:1 | wt% | 3.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Stearic | C18:0 | wt% | 14.00 | 3.21 | 3.55 | 1.52 | 5.60 |
| Oleic | C18:1 | wt% | 48.00 | 40.36 | 24.08 | 74.31 | 30.59 |
| Linoleic | C18:2 | wt% | 9.00 | 10.20 | 15.13 | 8.90 | 52.23 |
| Linolenic | C18:3 | wt% | 2.00 | 0.23 | 49.08 | 0.64 | 0.08 |
| Arachidic | C20:0 | wt% | 0.00 | 0.46 | 0.28 | 0.69 | 0.42 |
| Properties | Units | TAGs | SM TAGs |
|---|---|---|---|
| Density at 288 K | g/mL | 0.9159 | 0.9188 |
| Sulfur | wppm | 65.4 | 2.84 |
| Sulfur + DMDS | wppm | 863.3 | 854.1 |
| Hydrogen | wt% | 11.75 | 11.86 |
| Carbon | wt% | 75.37 | 76.90 |
| Nitrogen | wt% | 87.60 | 83.20 |
| Oxygen | wt% | 12.86 | 11.15 |
| Water dissolved | wt% | 0.373 | 0.026 |
| TAN | mgKOH/g | 32.21 | 0.51 |
| Viscosity at 315 K | cSts | 46.24 | 35.46 |
| H/C ratio | - | 0.155 | 0.154 |
| O/C ratio | - | 0.170 | 0.137 |
| HHV * | MJ/kg | 40.01 | 40.82 |
| Simulated distil. curve | |||
| IBO | K | 611 | 697 |
| 10 | K | 667 | 862 |
| 30 | K | 871 | 873 |
| 50 | K | 877 | 879 |
| 70 | K | 883 | 884 |
| 90 | K | 894 | 887 |
| 95 | K | 901 | 888 |
| FBP | K | 1012 | 976 |
| TAGs | SM TAGs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conditions | Conditions | |||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| Hydrogen | 88.612 | 88.597 | 90.828 | 93.067 | 90.605 | 81.276 | 86.807 | 89.792 | 89.263 | 88.567 |
| Methane | 2.132 | 1.586 | 1.025 | 1.174 | 1.118 | 4.151 | 2.484 | 1.461 | 2.242 | 1.642 |
| Propane | 2.862 | 2.756 | 2.323 | 1.778 | 2.575 | 5.095 | 3.645 | 2.966 | 3.222 | 3.343 |
| Isobutane | 0.000 | 0.576 | 0.110 | 0.000 | 0.200 | 0.000 | 0.644 | 0.078 | 0.151 | 0.306 |
| N-Butane | 0.788 | 0.476 | 0.127 | 0.106 | 0.138 | 1.103 | 0.439 | 0.068 | 0.114 | 0.203 |
| Isopentane | 0.521 | 0.854 | 0.294 | 0.082 | 0.106 | 0.816 | 0.394 | 0.082 | 0.095 | 0.173 |
| N-Pentane | 0.261 | 0.450 | 0.187 | 0.047 | 0.064 | 0.397 | 0.222 | 0.064 | 0.056 | 0.086 |
| Carbon dioxide | 0.112 | 0.517 | 0.596 | 0.140 | 0.580 | 0.322 | 0.763 | 0.796 | 0.370 | 0.801 |
| Carbon monoxide | 0.000 | 0.037 | 0.034 | 0.000 | 0.041 | 0.025 | 0.048 | 0.044 | 0.024 | 0.049 |
| Nitrogen | 0.055 | 0.050 | 0.045 | 0.041 | 0.060 | 0.054 | 0.052 | 0.050 | 0.055 | 0.050 |
| Ethane | 0.404 | 0.000 | 0.000 | 0.000 | 0.000 | 0.412 | 0.000 | 0.000 | 0.000 | 0.000 |
| C6+ | 0.262 | 0.479 | 0.396 | 0.190 | 0.167 | 0.355 | 0.437 | 0.352 | 0.187 | 0.180 |
| Units | Mass Flows | |
|---|---|---|
| Liquid feed | g/h | 344.5 |
| Hydrogen feed | g/h | 30.7 |
| Liquid organic product | g/h | 299.8 |
| Aqueous liquid product | g/h | 33.3 |
| Gas product | g/h | 31.4 |
| Mass balance | % | 97.1 |
| Properties | Units | Jet A1 Specs | EN590 Road Diesel Specs | DMA Marine Specs | HVO 15,940 Specs | Jet Fraction | Diesel Fraction |
|---|---|---|---|---|---|---|---|
| Density at 288 K | kg/m3 | 775–840 | 820–845 | <890 | 765–800 | 775 | 785 |
| Viscosity at 313 K | mm2s−1 | <8 * | 1.9–4.1 | 2.0–6.0 | 2–4.5 | 2.53 | 2.53 |
| Cetane number | - | - | >40 | >40 | >70 | - | 78.9 |
| Oxidation stability at 383 K | gm−3 | - | <25 | <25 | <25 | - | - |
| Aromatics | v/v% | <25 | - | - | <1.0 | 0.0 | - |
| S content | wt% | <0.3 | <0.0005 | <1.5 | <5 | 0.0004 | 0.0004 |
| Flash point | K | >311 | >325 | >333 | >323 | 373 | 391 |
| Lubricity at 333 K | μm | - | <520 | <520 | - | - | - |
| Ash | wt% | - | <0.01 | <0.01 | <0.01 | - | - |
| Water and sediment | v/v% | - | <0.05 | - | - | - | 0.0028 |
| Calorific value | MJ/kg | >42.8 | 43.8 | - | - | 46.93 | 46.37 |
| Carbon residue | wt% | - | - | <0.3 | <0.3 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitriadis, A.; Chrysikou, L.P.; Kosma, I.; Tourlakidis, N.; Bezergianni, S. Hydroprocessing Microbial Oils for Advanced Road Transportation, Aviation, and Maritime Drop-In Fuels: Industrially Relevant Scale Validation. Energies 2024, 17, 3854. https://doi.org/10.3390/en17153854
Dimitriadis A, Chrysikou LP, Kosma I, Tourlakidis N, Bezergianni S. Hydroprocessing Microbial Oils for Advanced Road Transportation, Aviation, and Maritime Drop-In Fuels: Industrially Relevant Scale Validation. Energies. 2024; 17(15):3854. https://doi.org/10.3390/en17153854
Chicago/Turabian StyleDimitriadis, Athanasios, Loukia P. Chrysikou, Ioanna Kosma, Nikos Tourlakidis, and Stella Bezergianni. 2024. "Hydroprocessing Microbial Oils for Advanced Road Transportation, Aviation, and Maritime Drop-In Fuels: Industrially Relevant Scale Validation" Energies 17, no. 15: 3854. https://doi.org/10.3390/en17153854





