FTIR Analysis for Determining Stability of Methanol–HVO Blends for Non-Road Engine Application
Abstract
:1. Introduction
1.1. Methanol–HVO Blends in NRMM
1.2. InfraRed Spectroscopy for Fuel Analytics
2. Materials and Methods
ATR–FTIR Spectroscopy as Experimental Method
3. Results
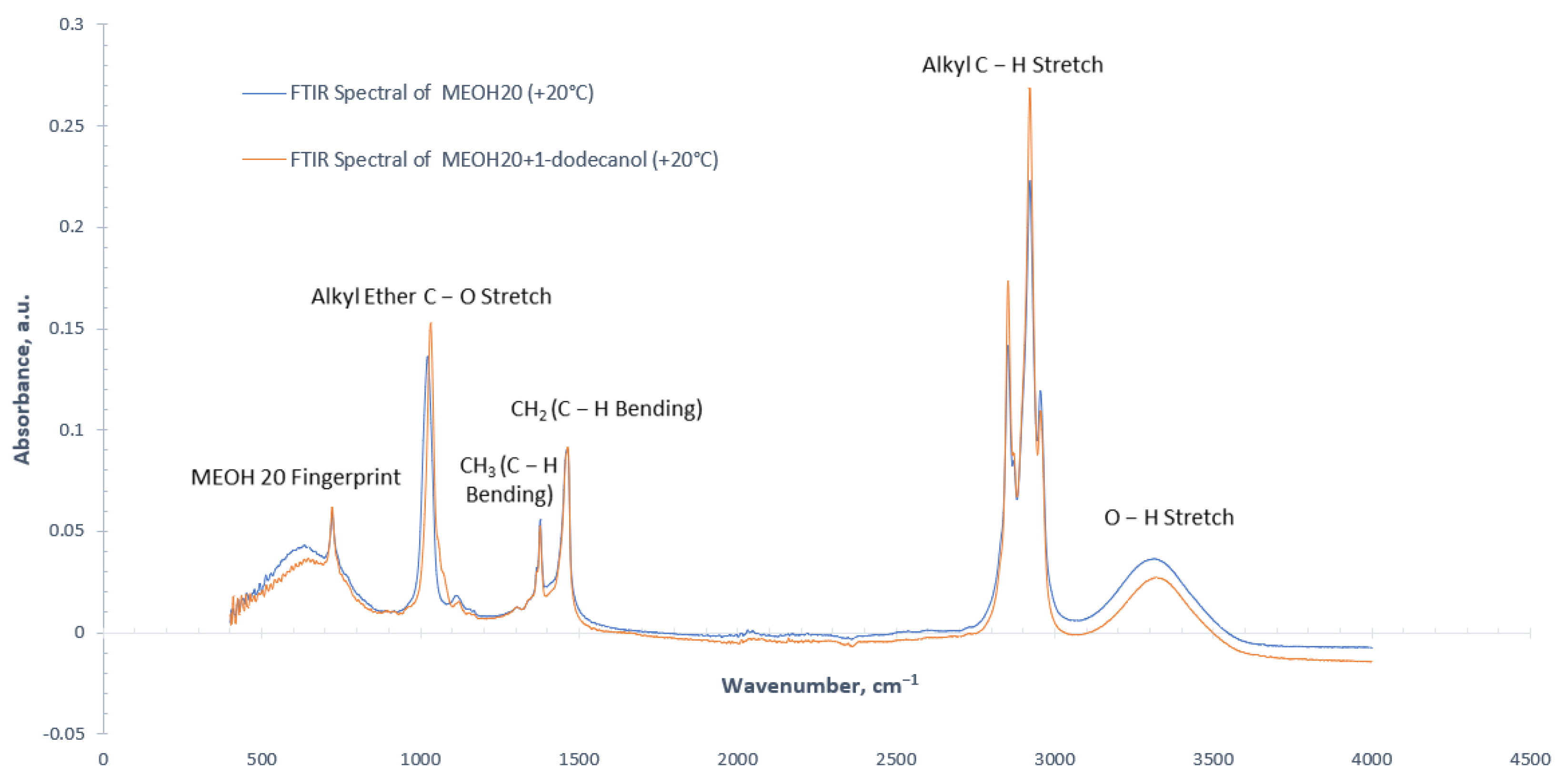
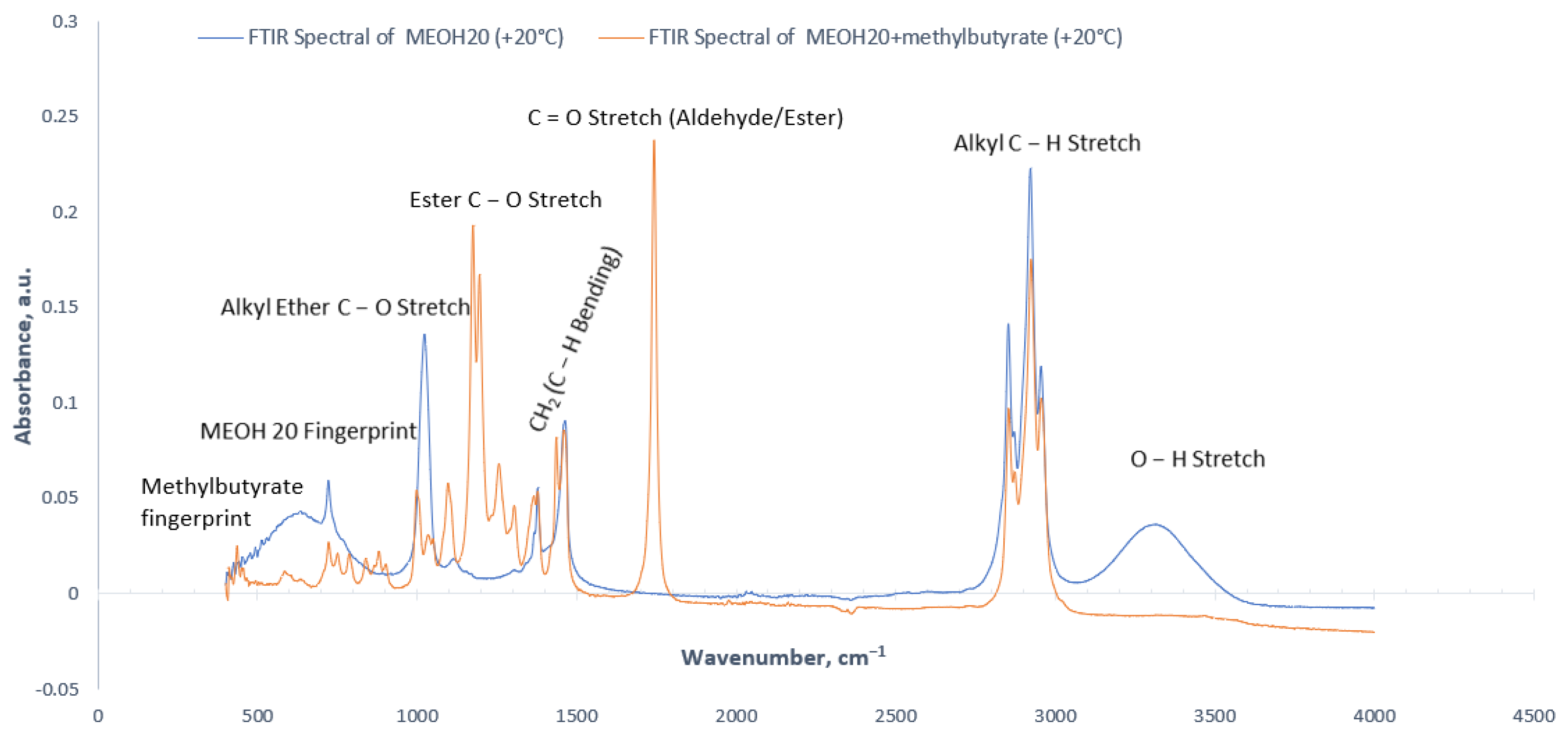
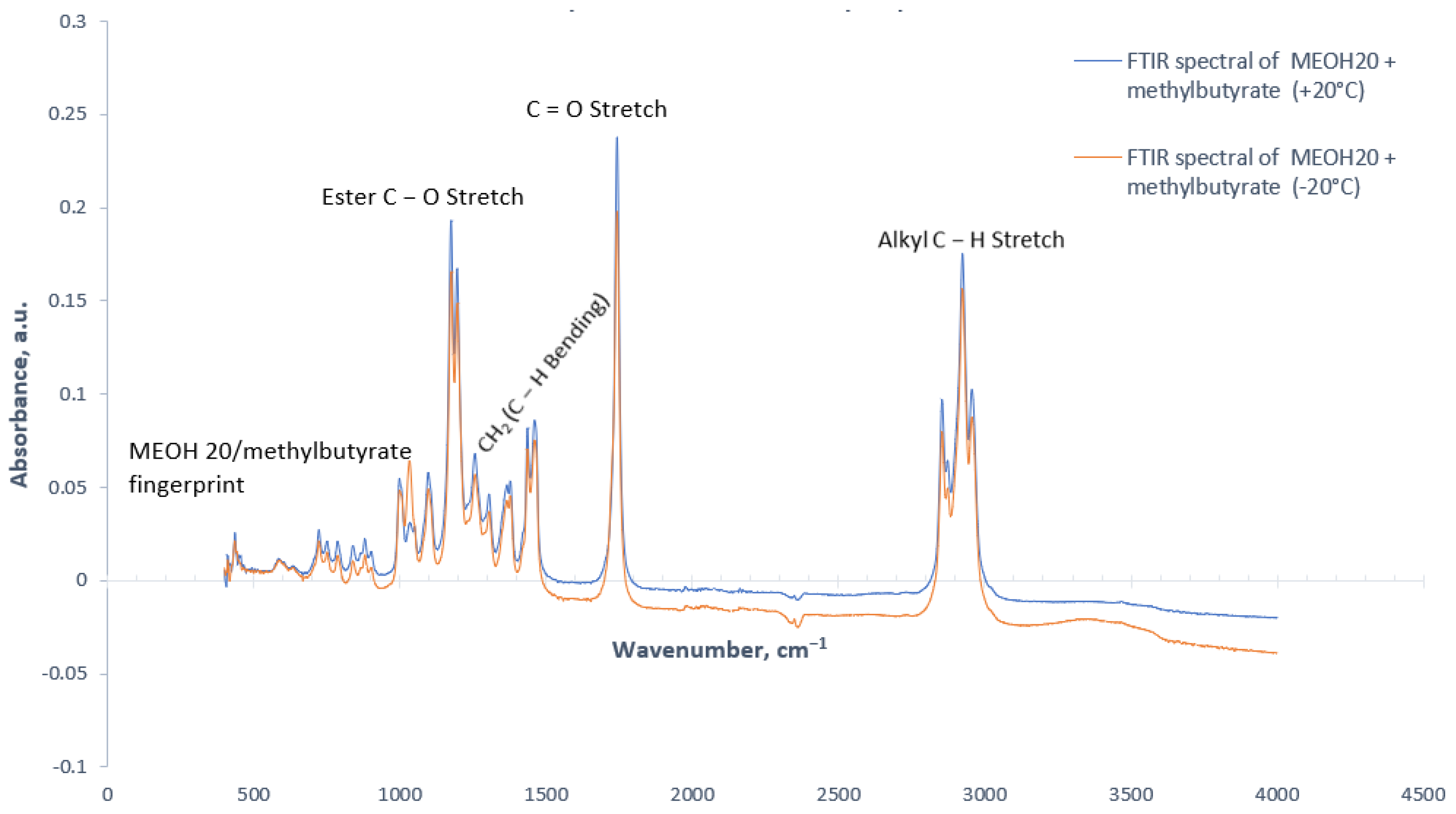
3.1. Effect of Stabilizing Agent on FTIR Spectra
(Methanol) + (HVO) = (AlkylEther) + (Water) + (Hydrogen)
(Methanol) + (HVO) + (Dodecanol) = (AlkylEther) + (Decanol) + (Hydrogen)
(Methanol) + (HVO) + (methyl butyrate) = (Ester) + (Hydrogen)
3.2. Effect of Temperature on FTIR Spectra
(Methanol) + (HVO) + (dodecanol) = (AlkylEther) + (dodecanol) + (Hydrogen)
4. Discussion
- MEOH/HVO blends with stabilizing agents form stable homogenous fuel blends at room temperature for at least 6 weeks of shelf−life.
- MEOH/HVO blend with 1−dodecanol solidifies at cold temperature but melted in approximately five minutes of standing at room temperature
- MEOH20 and methyl butyrate formed separate layers of fuels at cold temperatures.
- MEOH/HVO blend with 1−dodecanol results in stable homogenous alkyl ether fuels
- MEOH/HVO blend with methyl butyrate results in stable homogenous ester fuels.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stančin, H.; Mikulčić, H.; Wang, X.; Duić, N. A Review on Alternative Fuels in Future Energy System. Renew. Sustain. Energy Rev. 2020, 128, 109927. [Google Scholar] [CrossRef]
- Pramanik, A.; Sinha, A.; Chaubey, K.K.; Hariharan, S.; Dayal, D.; Bachheti, R.K.; Bachheti, A.; Chandel, A.K. Second−Generation Bio−Fuels: Strategies for Employing Degraded Land for Climate Change Mitigation Meeting United Nation−Sustainable Development Goals. Sustainability 2023, 15, 7578. [Google Scholar] [CrossRef]
- Karwacka, M.; Ciurzyńska, A.; Lenart, A.; Janowicz, M. Sustainable Development in the Agri−Food Sector in Terms of the Carbon Footprint: A Review. Sustainability 2020, 12, 6463. [Google Scholar] [CrossRef]
- EPA Emission Standards for Nonroad Engines and Vehicles. US EPA. Available online: https://www.epa.gov/emission-standards-reference-guide/epa-emission-standards-nonroad-engines-and-vehicles (accessed on 25 May 2024).
- De Poures, M.V.; Sathiyagnanam, A.P.; Rana, D.; Babu, R.K.; Subramani, S.; Sethuramasamyraja, B.; Damodharan, D. Using Renewable N−Octanol in a Non−Road Diesel Engine with Some Modifications. Energy Sources A 2018, 41, 1194–1208. [Google Scholar] [CrossRef]
- Heidari−Maleni, A.; Gundoshmian, T.M.; Karimi, B.; Jahanbakhshi, A.; Ghobadian, B. A Novel Fuel Based on Biocompatible Nanoparticles and Ethanol−Biodiesel Blends to Improve Diesel Engines Performance and Reduce Exhaust Emissions. Fuel 2020, 276, 118079. [Google Scholar] [CrossRef]
- Hunicz, J.; Mikulski, M.; Shukla, P.C.; Gęca, M.S. Partially Premixed Combustion of Hydrotreated Vegetable Oil in a Diesel Engine: Sensitivity to Boost and Exhaust Gas Recirculation. Fuel 2022, 307, 121910. [Google Scholar] [CrossRef]
- Fact Sheets & Guidelines. Home. Available online: https://www.etipbioenergy.eu/fact-sheets (accessed on 25 May 2024).
- EN590:2009; Automotive Fuel-Diesel-Requirements and Test Methods. 2009. Available online: https://standards.iteh.ai/catalog/standards/cen/c3d8c75c-ea89-4b8a-afc3-0fb533afbc14/en-590-2009 (accessed on 10 November 2023).
- Holzer, A.; Günthner, M.; Jung, P. Performance of Pure OME and Various HVO–OME Fuel Blends as Alternative Fuels for a Diesel Engine. Automot. Engine Technol. 2022, 7, 369–383. [Google Scholar] [CrossRef]
- EN228:2012+A1:2017; Automotive Fuels—Unleaded Petrol—Requirements and Test Methods. 2017. Available online: https://standards.iteh.ai/catalog/standards/cen/81cde377-aac3-4a49-9bde-bb71e02c4585/en-228-2012a1-2017 (accessed on 10 November 2023).
- Nashte, A.; J, S.P.S.; Rathod, D. Investigations of Emission Reduction Potential of Diesel−Methanol Blends in a Heavy−Duty Genset Engine. In Symposium on International Automotive Technology; SAE International: Warrendale, PA, USA, 2021. [Google Scholar] [CrossRef]
- Domínguez, V.M.; Hernández, J.J.; Ramos, Á.; Giménez, B.; Rodríguez-Fernández, J. Exploring the Effect of Methanol and Ethanol on the Overall Performance and Substitution Window of a Dual-Fuel Compression-Ignition Engine Fueled with HVO. Fuel 2024, 359, 130529. [Google Scholar] [CrossRef]
- Wang-Alho, H.; Sirviö, K.; Hissa, M.; Mikulski, M.; Niemi, S. Methanol-HVO Blends for Efficient Low-Temperature Combustion: Analytical Research on Fuel Properties. Agron. Res. 2023, 21, 994–1004. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. US EPA. 2015. Available online: https://www.epa.gov/sites/default/files/2015-07/documents/emission-factors_2014.pdf (accessed on 25 May 2024).
- ICNIRP. Infrared (780 Nm-1mm). ICNIRP. 2023. Available online: https://www.icnirp.org/en/frequencies/infrared/infrared.html (accessed on 25 May 2024).
- NIR Spectroscopic Method vs. FTIR/FT-NIR|Allied Scientific Pro. Allied Scientific Pro|Global Leader in Photonics Solutions. 2023. Available online: https://www.alliedscientificpro.com/blog/welcome-to-our-blogs-1/comparing-the-nir-spectroscopic-method-withftir-ft-nir-132 (accessed on 25 May 2024).
- Guide to FT-IR Spectroscopy. High-Value Life Science and Material Research and Diagnostics Solutions. Bruker. 2023. Available online: https://www.bruker.com/en/products-and-solutions/infrared-and-raman/ft-ir-routine-spectrometer/what-is-ft-ir-spectroscopy.html (accessed on 25 May 2024).
- Standard Test Method for Determination of Biodiesel (Fatty Acid Methyl Esters) Content in Diesel Fuel Oil Using Mid Infrared Spectroscopy (FTIR-ATR-PLS Method). ASTM International—Standards Worldwide. 2023. Available online: https://www.astm.org/standards/d7371 (accessed on 25 May 2024).
- Faraguna, F.; Racar, M.; Jukić, A. Test Method for Determination of Different Biodiesels (Fatty Acid Alkyl Esters) Content in Diesel Fuel Using FTIR-ATR. Renew. Energy 2019, 133, 1231–1235. [Google Scholar] [CrossRef]
- Gomes, T.; Ramos, R.; Garcia, F.; Sousa, D. Atr-Ftir and Uv-Vis as Techniques for Methanol Analysis in Biodiesel-Washing Wastewater. Quim. Nova 2023, 46, 698–705. [Google Scholar] [CrossRef]
- Xia, Q.; Yuan, L.-M.; Chen, X.; Meng, L.; Huang, G. Analysis of Methanol Gasoline by ATR-FT-IR Spectroscopy. Appl. Sci. 2019, 9, 5336. [Google Scholar] [CrossRef]
- Liu, S.; Wang, S.; Hu, C.; Bi, W. Determination of Alcohols-Diesel Oil by Near Infrared Spectroscopy Based on Gramian Angular Field Image Coding and Deep Learning. Fuel 2022, 309, 122121. [Google Scholar] [CrossRef]
- Czechlowski, M.; Marcinkowski, D.; Golimowska, R.; Berger, W.A.; Golimowski, W. Spectroscopy Approach to Methanol Detection in Waste Fat Methyl Esters. Spectrochim. Acta Part A 2019, 210, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Fořt, J.; Trník, A.; Pavlíková, M.; Pavlík, Z.; Černý, R. Fabrication of Dodecanol/Diatomite Shape-Stabilized PCM and Its Utilization in Interior Plaster. Int. J. Thermophys. 2018, 39, 137. [Google Scholar] [CrossRef]
- Cunliffe, S.; Martin, P.; Baker, M.; Mihailova, O.; Martin, P. Near Infrared Absorption Spectroscopy for the Quantification of Unsulfated Alcohol in Sodium Lauryl Ether Sulfate. J. Near Infrared Spectrosc. 2020, 29, 11–23. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Ozaki, Y.; Czarnecki, M.A.; Huck, C.W. Simulated NIR Spectra as Sensitive Markers of the Structure and Interactions in Nucleobases. Sci. Rep. 2019, 9, 17398. [Google Scholar] [CrossRef] [PubMed]
- Nicolet™ Summit™ FTIR Spectrometer. Access Denied. 2023. Available online: https://www.thermofisher.com/order/catalog/product/912A0972 (accessed on 25 May 2024).
- Ensing, B.; Tiwari, A.; Tros, M.; Hunger, J.; Domingos, S.R.; Pérez, C.; Smits, G.; Bonn, M.; Bonn, D.; Woutersen, S. On the Origin of the Extremely Different Solubilities of Polyethers in Water. Nat. Commun. 2019, 10, 2893. [Google Scholar] [CrossRef] [PubMed]
- Libretexts. Infrared Spectroscopy Absorption Table. Chemistry LibreTexts, 30 November 2014. Available online: https://chem.libretexts.org/Ancillary_Materials/Reference/Reference_Tables/Spectroscopic_Reference_Tables/Infrared_Spectroscopy_Absorption_Table (accessed on 25 May 2024).
- Libretexts. 11.5: Infrared Spectra of Some Common Functional Groups. Chemistry LibreTexts, 9 February 2016. Available online: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Map:_Organic_Chemistry_(Wade)_Complete_and_Semesters_I_and_II/Map:_Organic_Chemistry_(Wade)/11:_Infrared_Spectroscopy_and_Mass_Spectrometry/11.05:_Infrared_Spectra_of_Some_Common_Functional_Groups (accessed on 25 May 2024).
- Libretexts. Properties of Esters. Chemistry LibreTexts, 3 October 2013. Available online: https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Supplemental_Modules_(Organic_Chemistry)/Esters/Properties_of_Esters (accessed on 25 May 2024).
- Semelsberger, T.A.; Borup, R.L.; Greene, H.L. Dimethyl Ether (DME) as an Alternative Fuel. J. Power Sources 2006, 156, 497–511. [Google Scholar] [CrossRef]
- Oppong, F.; Xu, C.; Li, X.; Luo, Z. Esters as a Potential Renewable Fuel: A Review of the Combustion Characteristics. Fuel Process. Technol. 2022, 229, 107185. [Google Scholar] [CrossRef]

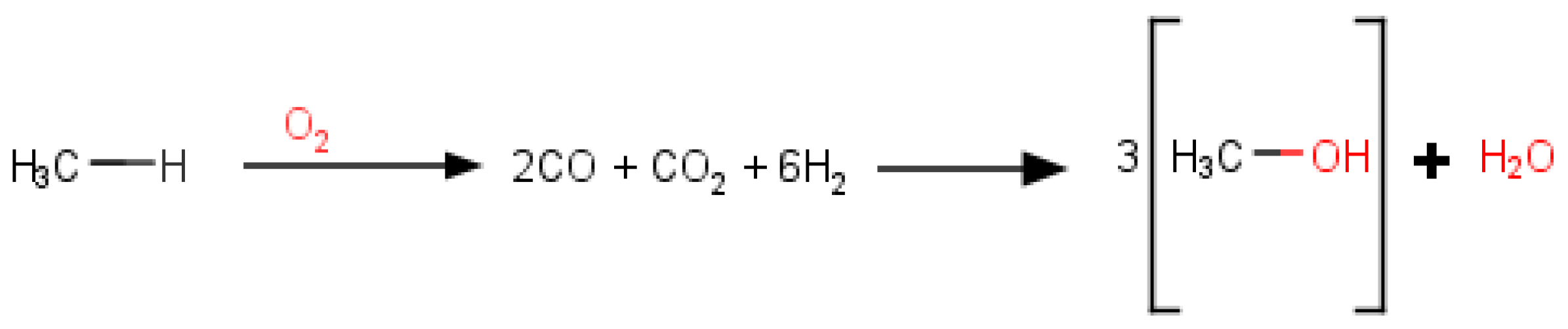



| Fuel Properties | Units | Fossil Diesel 1 | HVO 1 | Gasoline 1 | Methanol 1 | MeOH20/HVO 2 |
|---|---|---|---|---|---|---|
| Cetane number | – | 50 | >80 | – | – | – |
| Octane number | – | – | – | 92 | >110 | – |
| Oxidative stability | – | >48 h | 25 g m–3 | >60 h | – | – |
| Density (20 °C) | kg L–1 | 0.83 | 0.78 | 0.74 | 0.79 | 0.78 |
| Lower heating value | MJ kg−1 | 43.1 | 44.4 | 43.9 | 19.7 | – |
| IBP | °C | 162 | 217 | 160 | 65 | 63 |
| flash point | °C | 67.5 | 82 | −45 | 9 | 9 |
| Kinematic viscosity (40 °C) | mm2 s–1 | 3.33 | 3.14 | 0.7 | 0.55 | 2.64 |
| GHG 3 | kgCO2eq gallon–1 | 10.21 | 3.5 | 8.78 | 4.11 | – |
| Molecular weight | g mol–1 | ~203 | ~222 | ~100 | ~32 | – |
| NIR | FTIR |
|---|---|
| No sample preparation | Requires sample preparation for immiscible or solid samples |
| Inexpensive detectors | More expensive detectors that respond to variations in temperature |
| Excellent for samples with food samples and other samples with high water content | Highly sensitive to water signals, muddling up other useful spectral information in samples with high water content |
| Non-destructive penetration into bulky samples (solids) | Cannot probe beyond the surface of samples |
| Ideal for heterogeneous samples | Not ideal for heterogeneous samples |
| Accurate. Takes static and representative measurements. | Stronger absorption peaks than those in the NIR spectral. It can be used for chemical reaction monitoring |
| Parameter | Specification |
|---|---|
| Detector type | Thermoelectrically cooled DTGS for maximum detector response linearity |
| Source type | Standard: single-point source with a non-migrating hotspot for unmatched data reproducibility |
| Spectral range | 8000–350 cm−1 optimized, mid-infrared KBr beamsplitter |
| Spectral resolution | Better than 0.45 cm−1 |
| Analysis software | OMNIC Paradigm Software Version v2.5 |
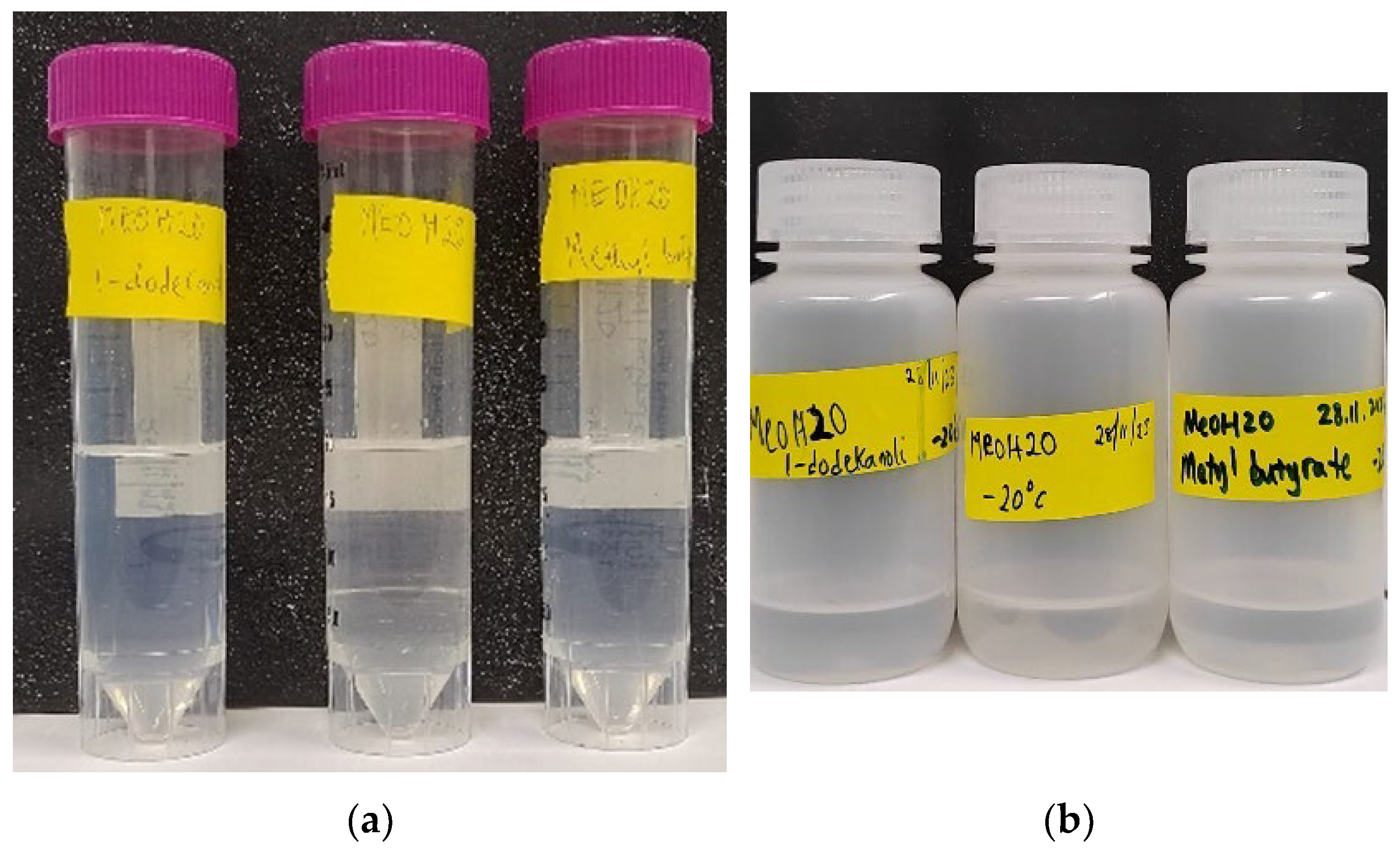
| Samples | +20 °C | −20 °C |
|---|---|---|
| MEOH20 | Formed separate liquid layer (baseline sample), No temperature effect | Did not solidify |
| No temperature effect, formed separate layer still | ||
| MEOH20 + 1–dodecanol | Formed a stable, single-phase fuel mixture. Slightly soapy after mix (presence of a surfactant) | Solidified at this temperature but went to a molten state within five minutes at room temperature. Formed a stable, single-phase liquid fuel mixture after being left standing at room temperature |
| MEOH20 + methyl butyrate | Formed a stable, single-phase liquid fuel mixture | Did not solidify |
| Formed separate liquid layer | ||
| Formed a single-phase liquid fuel after an additional three days of storage at room temperature |
| Wavenumber (cm−1) | Absorbance Signal Type | Molecule | Vibration Type | Functional Group |
|---|---|---|---|---|
| 3500−3200 | medium/broad | O−H | stretching | alcohol/phenol |
| 3000−2840 | medium/sharp | C−H | stretching | alkane |
| 1750−1735 | strong/sharp | C=O | stretching | ester |
| 1465 | medium | C−H | bending | methylene (alkane) |
| 1450−1375 | medium | C−H | bending | methyl (alkane) |
| 1275−1200, 1075−1020 | strong | C−O | stretching | alkyl ether |
| 1210−1163 | strong | C−O | stretching | ester |
| <1300 | weak | − | − | Sample fingerprints |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balogun, F.; Wang-Alho, H.; Sirviö, K.; Mikulski, M. FTIR Analysis for Determining Stability of Methanol–HVO Blends for Non-Road Engine Application. Energies 2024, 17, 3921. https://doi.org/10.3390/en17163921
Balogun F, Wang-Alho H, Sirviö K, Mikulski M. FTIR Analysis for Determining Stability of Methanol–HVO Blends for Non-Road Engine Application. Energies. 2024; 17(16):3921. https://doi.org/10.3390/en17163921
Chicago/Turabian StyleBalogun, F., H. Wang-Alho, K. Sirviö, and M. Mikulski. 2024. "FTIR Analysis for Determining Stability of Methanol–HVO Blends for Non-Road Engine Application" Energies 17, no. 16: 3921. https://doi.org/10.3390/en17163921









