Impact of Aromatic Hydrocarbons on Emissions in a Custom-Built High-Pressure Combustor
Abstract
:1. Introduction
1.1. Emissions
1.2. Synthetic Fuels
1.3. Selection of Aromatic Species
1.4. Research Aim and Objectives
2. Design Methodology
2.1. Fuel Atomiser and Ignitor Design
2.2. Baffle Plate Design
2.3. Fuel Preparation
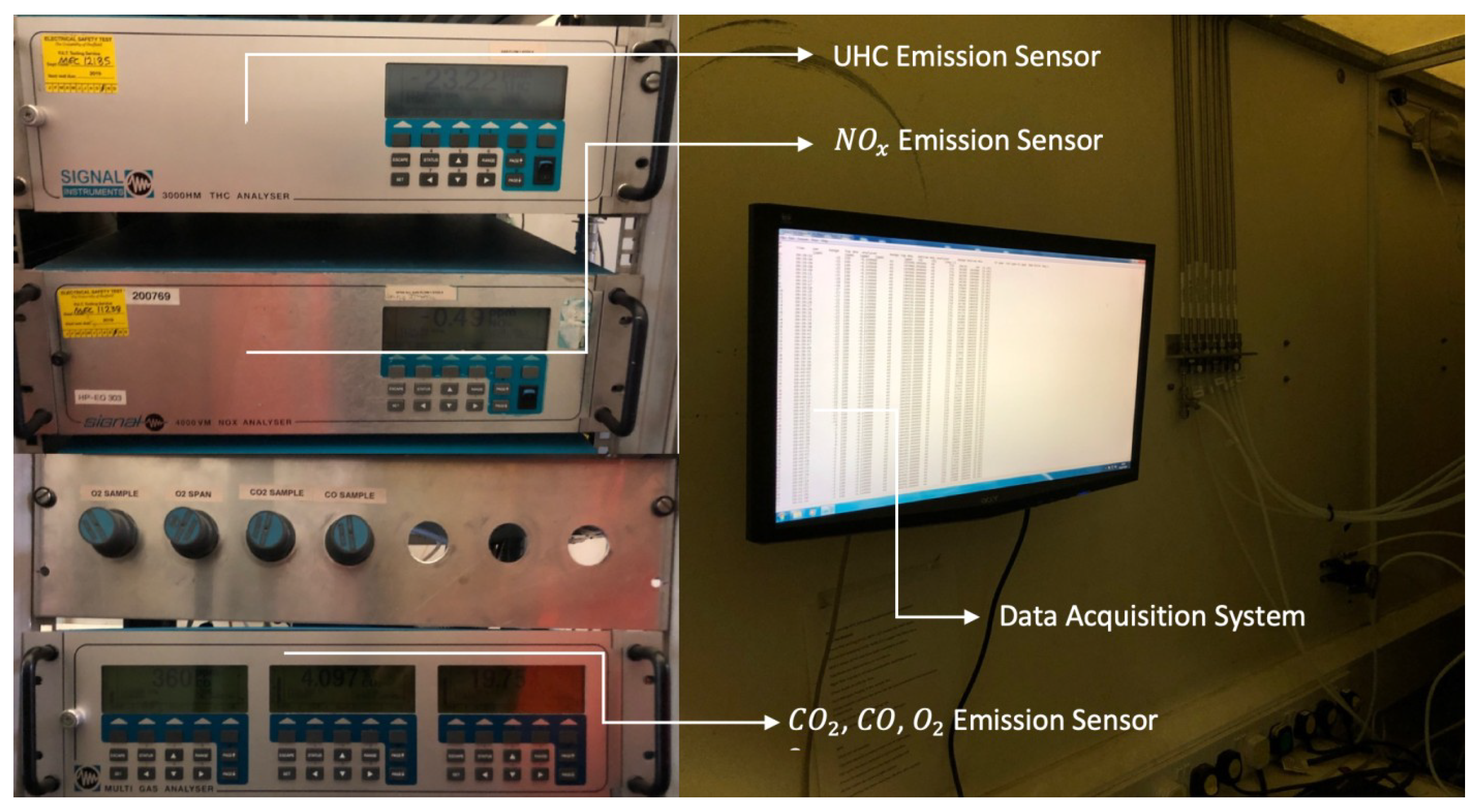
2.4. Data Acquisition Systems
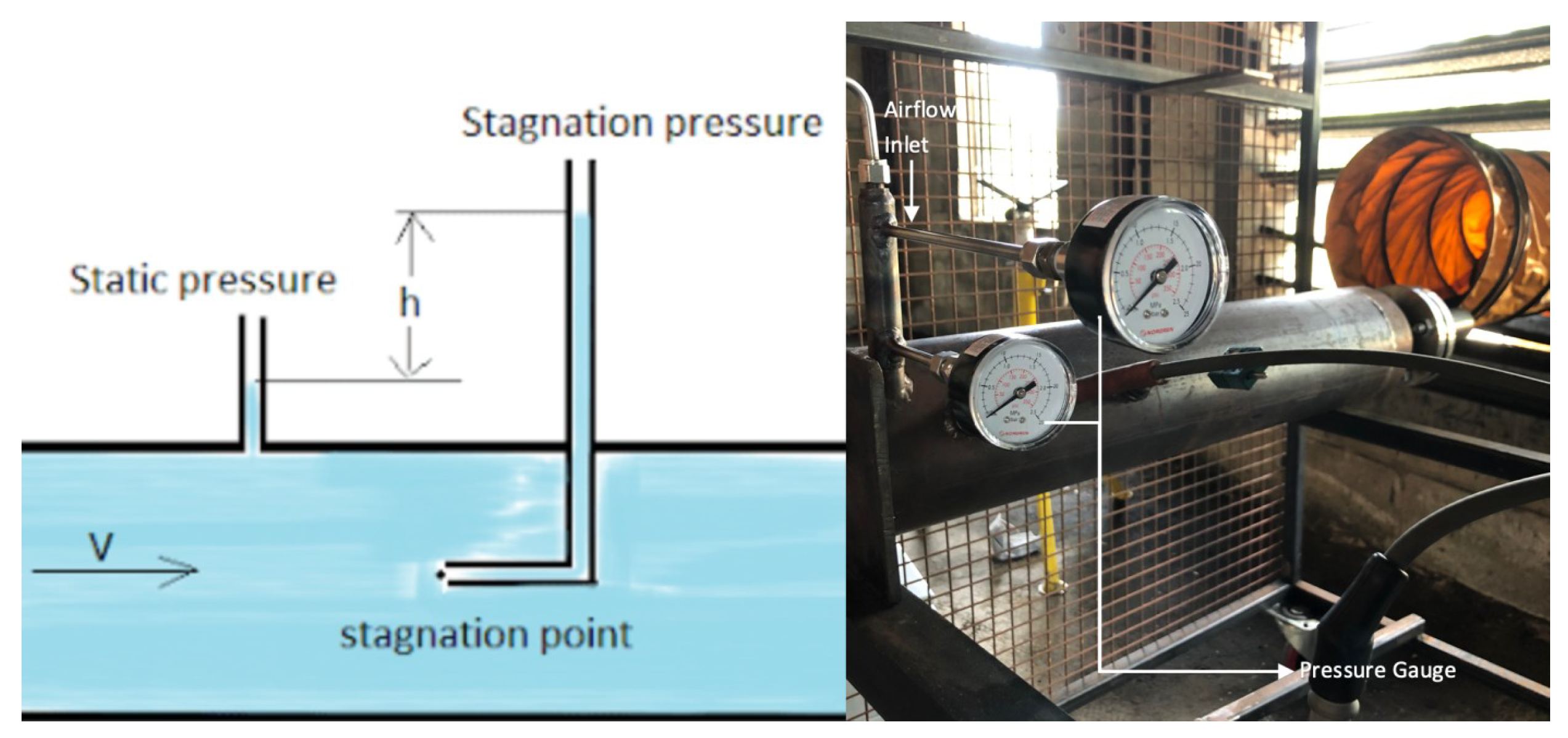
2.4.1. Temperature and Pressure
2.4.2. Smoke and Emissions
Measurement Techniques
Instrumentation and Data Collection
2.4.3. UHC, , , and Emissions
Measurement Techniques
Data Collection and Analysis
2.5. Error Analysis for Experimental Instrumentations and Data Cleaning
- Temperature Measurements: Thermocouples (K-type) used in high-temperature environments have a typical error of ±1.5 °C or 0.4% of the measured temperature, whichever is greater. Regular calibration and optimal positioning reduce errors [44]. Infrared thermometers have an accuracy of ±2% of the reading or ±3 °C, whichever is greater [45].
- Pressure Measurements: Pressure transducers used in the experiments have an accuracy of ±0.25% of full-scale reading. Vibration-damping fixtures and averaging multiple readings help minimise errors [46].
- Airflow and Mass Flow Rate Measurements: Differential pressure sensors used for air mass flow rate measurement have an accuracy of ±1% of full-scale reading [47]. Controlled conditions for temperature and pressure ensure accurate calculations.
- Emission Measurements: Laser-induced incandescence (LII-300) for PM emissions has an error margin of ±10% for soot volume fraction measurements [48]. The 3000HM THC Analyser (accuracy: ±1%), 4000VM Analyser (accuracy: ±0.5 ppm), and Multi-Gas Analyser (accuracy: ±2%) were regularly calibrated with certified gas mixtures to prevent drift [49].
- Data Acquisition and Processing: High-quality DAQ systems with noise filtering capabilities have an accuracy of ±0.1% of the reading. High sampling rates and data redundancy ensure accurate capture of transient phenomena [50].
- Calculation of Robust Statistical Metrics:
- Median and Interquartile Range (IQR):where and are the 25th and 75th percentiles of the data, respectively.
- Application of a Modified Z-Score Method:
- Modified Z-Score for Outlier Detection:This method is particularly useful for datasets with skewed distributions or those susceptible to influence from extreme values.
- Elimination of Outliers:
- Outliers were defined as any data points where the absolute value of the modified Z-score exceeded 3.5. An illustrative example using Toluene measurements, where 140 readings of UHCs during the combustion test were analyzed, demonstrates this approach:Data points with were removed from the dataset.
3. Results and Discussion
3.1. Testing Results for Jet−A1
Importance of Baseline Test
3.2. Testing Results for Jet−A1 Blended with Different Aromatic Species
3.2.1. Particulate () Emission Results
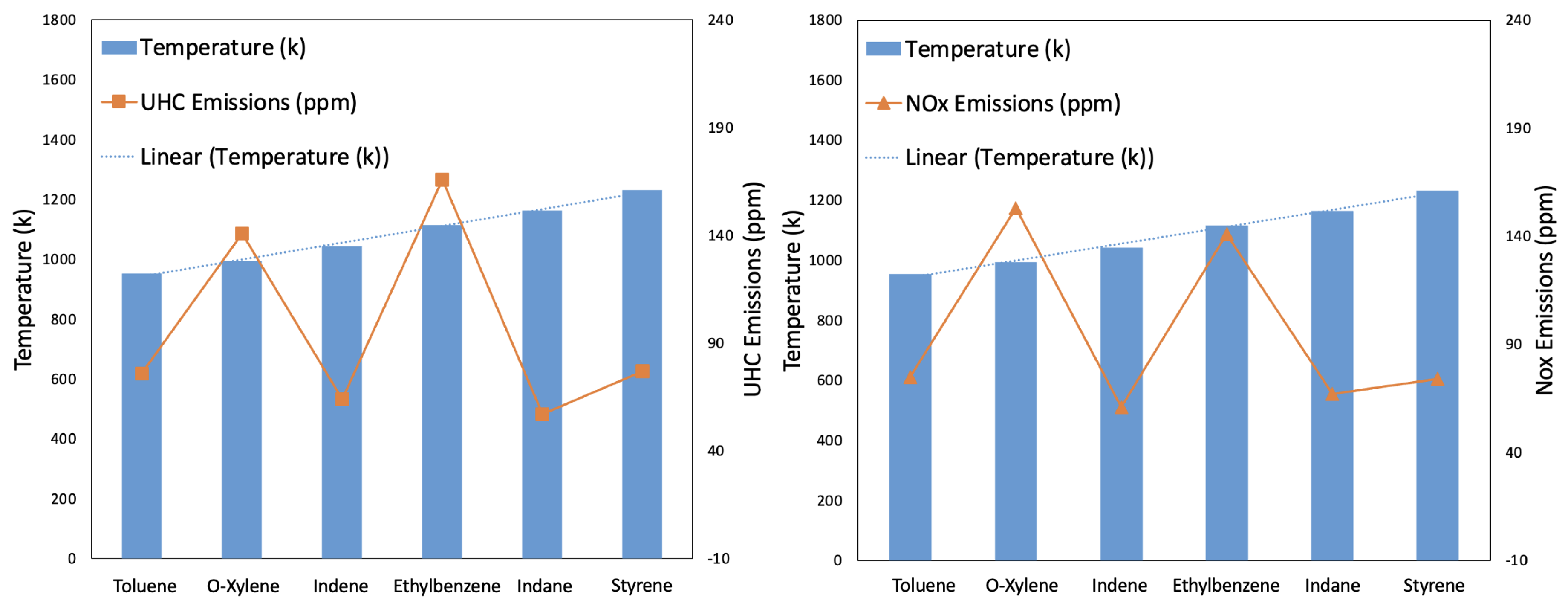
- Temperature and PM Emissions: A clear trend was observed where the combustion temperature influenced the PM emissions. As shown in Table 3, Toluene combustion, which had the lowest temperature, resulted in the highest PM emissions. Conversely, O−Xylene combustion, with the highest temperature, produced the lowest PM emissions. This inverse relationship suggests that higher combustion temperatures enhance the completeness of combustion, thereby reducing soot formation and PM emissions.
- Potential Impact of Aromatic Structure: The structure of the aromatic hydrocarbons significantly affects the emission outcomes. Studies have shown that the molecular structure of aromatics influences their combustion behaviour and the formation of soot particles. For instance, aromatic compounds with more rings and higher molecular weights tend to produce more soot [11]. One explanation for this phenomenon is that lower aromatic content in the fuel reduces the formation of soot precursors. Aromatic compounds are known to be significant contributors to soot formation due to their ring structures, which promote the polymerisation processes that form soot particles. The production decreases when the aromatic content is reduced or modified to include species less prone to soot formation. This is particularly evident with synthetic paraffinic kerosene (SPK) blends, which have been shown to reduce PM emissions due to their lower aromatic content and more complete combustion properties [31]. Furthermore, the specific types of aromatic species used in the blend play a crucial role. Not all aromatics contribute equally to soot formation; some have higher sooting tendencies than others. The overall PM emissions can be significantly reduced by selecting aromatic species with lower sooting propensities for blending. This selective blending improves combustion efficiency and helps achieve cleaner exhaust emissions, aligning with environmental regulations and standards.
- Role of Fuel Composition: The 13% mass blend composition of the aromatic species with Jet−A1 also plays a critical role in emission characteristics. The presence of aromatic compounds could alter the chemical kinetics of the combustion process, affecting the formation of intermediate species that lead to soot and PM. The findings align with those reported in the literature, where specific aromatic contents in fuel blends have been linked to variations in emission profiles [51].
3.2.2. UHC, , , and Emission Results
- Emissions: As shown in Figure 11, Ethylbenzene produced the highest UHC emissions, while Indane produced the lowest. This variation indicates that the chemical structure and properties of the aromatic hydrocarbons significantly influence the formation of unburned hydrocarbons.
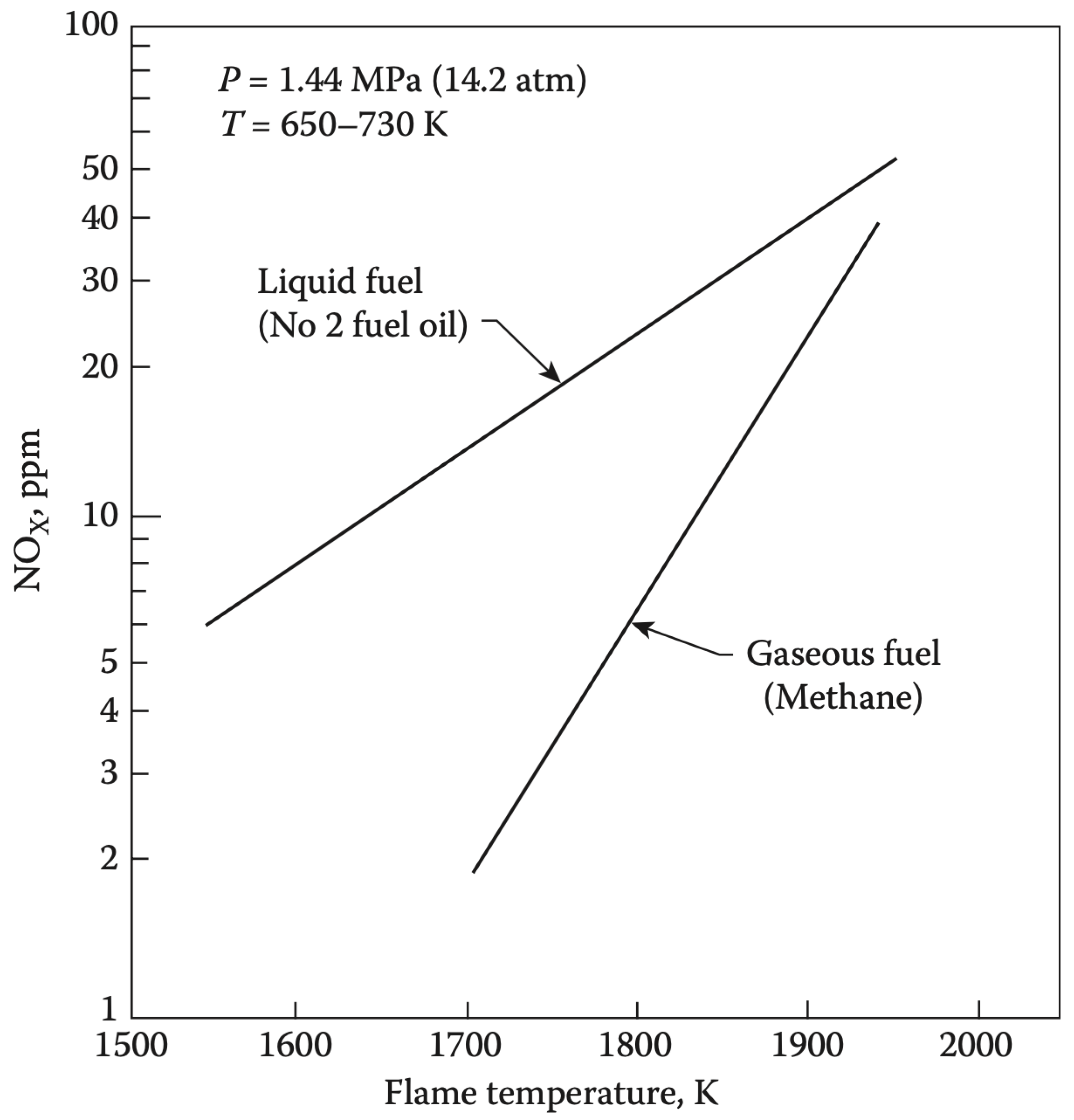
- Emissions: For emissions, Indane again produced the least amount, whereas O−Xylene produced the highest. formation is highly temperature-dependent, and the combustion temperature profiles indicate that variations in aromatic structure can lead to different thermal formation rates [2]. Higher combustion temperatures generally increase emissions due to the enhanced nitrogen and oxygen reaction rates [43].
- Emissions: Figure 12 reveals that emissions were relatively high for Ethylbenzene and low for Indane. High emissions typically indicate incomplete combustion, where insufficient oxygen or inadequate mixing prevents the complete oxidation of carbon to . The variation in CO emissions across different aromatics suggests differences in combustion efficiency and fuel oxidation processes [11].
- Emissions: emissions were found to be almost the same across different aromatics, with Ethylbenzene having the highest and Indane having the lowest emissions. is a primary combustion product, and its consistent levels across different fuels suggest that, despite variations in intermediate species like and UHC, the overall carbon conversion to remains fairly constant. This could imply a similar level of overall combustion efficiency across the tested aromatic fuels, albeit with different pathways and intermediate stages [41].
- Chemical Structure: The molecular structure of aromatics, including ring number and substituents, influences the combustion process and emission formation. More complex structures may lead to higher emissions due to incomplete combustion and soot formation;
- Combustion Temperature: Higher combustion temperatures generally reduce UHC and emissions but increase emissions. This inverse relationship underscores the importance of optimising combustion conditions to balance the reduction of all pollutants;
- Fuel Blending: The proportion of aromatics in the fuel blend affects the emission profile. Due to incomplete oxidation, Higher aromatic content could increase soot and UHC emissions.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Federal Aviation Administration. Aviation Emissions, Impacts & Mitigation: A Primer. Available online: https://www.faa.gov/sites/faa.gov/files/regulations_policies/policy_guidance/envir_policy/Primer_Jan2015.pdf (accessed on 2 March 2019).
- Lefebvre, A.; Ballal, D. Gas Turbine Combustion, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Saravanamuttoo, H. Gas Turbine Theory, 7th ed.; Licensing Agency Ltd.: London, UK, 2017. [Google Scholar]
- Jones, W.P.; Lindstedt, R.P. Global reaction schemes for hydrocarbon combustion. Combust. Flame 2008, 152, 170–195. [Google Scholar] [CrossRef]
- Ballal, D.R.; Lefebvre, A.H. Weak extinction limits of turbulent flowing mixtures. J. Eng. Power 1979, 343–348. [Google Scholar] [CrossRef]
- Turns, S.R. An Introduction to Combustion: Concepts and Applications, 2nd ed.; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Nathanson, J.A. Greenhouse Gases. Encyclopedia Britannica. 2019. Available online: https://www.britannica.com/science/air-pollution/Greenhouse-gases (accessed on 16 March 2019).
- Energy UK. Environmental Regulations. Available online: https://www.energy-uk.org.uk/insights/electricity-generation/ (accessed on 16 March 2019).
- Tikuisis, P.; Kane, D.; McLellan, T.; Buick, F.; Fairburn, S. Rate of formation of carboxyhemoglobin in exercising humans exposed to carbon monoxide. J. Appl. Physiol. 1992, 72, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Connellan, S. Lung diseases associated with hydrocarbon exposure. Respir. Med. 2017, 126, 46–51. [Google Scholar] [CrossRef]
- Zheng, L.; Singh, P.; Cronly, J.; Ubogu, E.A.; Ahmed, I.; Ling, C.; Zhang, Y.; Khandelwal, B. Impact of aromatic structures and content in formulated fuel for jet engine applications on particulate matter emissions. J. Energy Resour. Technol. 2021, 143, 122301. [Google Scholar] [CrossRef]
- Almohammadi, B.A.; Singh, P.; Sharma, S.; Kumar, S.; Khandelwal, B. Experimental investigation and correlation development for engine emissions with polycyclic aromatic blended formulated fuels. Fuel 2021, 303, 121280. [Google Scholar] [CrossRef]
- Chapman, J.; Singh, P.; Kumar, S.; Khandelwal, B. Optimization of aromatic species in formulated fuel for simultaneous reduction of PM and NOx emissions from combustion engines. J. Energy Inst. 2022, 103, 94–103. [Google Scholar] [CrossRef]
- Singh, P.; Khandelwal, B.; Almohammadi, B.A.; Sharma, S.; Kumar, S. Investigations on combustion and emissions characteristics of aromatic fuel blends in a distributed combustor. Fuel Process. Technol. 2021, 208, 106548. [Google Scholar]
- Dandajeh, H.A.; Talibi, M.; Ladommatos, N. Polycyclic aromatic hydrocarbon and soot emissions in a diesel engine and from a tube reactor. J. King Saud Univ.-Eng. Sci. 2022, 65, 435–444. [Google Scholar] [CrossRef]
- Talibi, M.; Hellier, P.; Ladommatos, N. Impact of increasing methyl branches in aromatic hydrocarbons on diesel engine combustion and emissions. Fuel 2018, 216, 579–588. [Google Scholar] [CrossRef]
- Chu, H.; Qi, J.; Feng, S.; Dong, W.; Hong, R.; Qiu, B.; Han, W. Soot formation in high-pressure combustion: Status and challenges. Fuel 2023, 345, 128236. [Google Scholar] [CrossRef]
- Liu, P.; Guo, J.J.; Quadarella, E.; Bennett, A.; Gubba, S.R. The effect of preheating temperature on PAH/soot formation in methane/air co-flow flames at elevated pressure. Fuel 2022, 313, 122656. [Google Scholar] [CrossRef]
- Kalvakala, K.C.; Aggarwal, S.K. PAHs and soot emissions in oxygenated ethylene diffusion flames at elevated pressures. In Proceedings of the ASME Turbo Expo: Power for Land, Sea, and Air, Oslo, Norway, 11–15 June 2018. [Google Scholar] [CrossRef]
- Liang, S.; Li, Z.; Gao, J.; Ma, X.; Xu, H.; Shuai, S. PAHs and soot formation in laminar partially premixed co-flow flames fuelled by PRFs at elevated pressures. Combust. Flame 2019, 206, 363–378. [Google Scholar] [CrossRef]
- Braun-Unkhoff, M.; Kathrotia, T.; Rauch, B.; Riedel, U. About the interaction between composition and performance of alternative jet fuels. CEAS Aeronaut. J. 2016, 7, 83–94. [Google Scholar] [CrossRef]
- Xue, X.; Hui, X.; Singh, P.; Sung, C.J. Soot formation in non-premixed counterflow flames of conventional and alternative jet fuels. Fuel 2017, 210, 343–351. [Google Scholar] [CrossRef]
- Lobo, P.; Christie, S.; Khandelwal, B.; Blakey, S.G.; Raper, D.W. Evaluation of Non-volatile Particulate Matter Emission Characteristics of an Aircraft Auxiliary Power Unit with Varying Alternative Jet Fuel Blend Ratios. Energy Fuels 2015, 29, 7705–7711. [Google Scholar] [CrossRef]
- Silverman, B. Effects of High Aromatic Aviation Fuel on Sealant Systems. SAE Tech. Pap. 1980, 89, 2646–2650. [Google Scholar]
- Chen, K.; Liu, H.; Xia, Z. The Impacts of Aromatic Contents in Aviation Jet Fuel on the Volume Swell of the Aircraft Fuel Tank Sealants. SAE Int. J. Aerosp. 2013, 6, 350–354. [Google Scholar] [CrossRef]
- Hamilton, J.; Khandelwal, B. Effects of Aromatic Blends on Seal Swell Rates Using Novel Seals Compatibility Test Rig. In Proceedings of the AIAA SCITECH 2023 Forum, National Harbor, MD, USA, 23–27 January 2023; p. 0596. [Google Scholar]
- Anuar, A.; Undavalli, V.K.; Khandelwal, B.; Blakey, S. Effect of fuels, aromatics and preparation methods on seal swell. Aeronaut. J. 2021, 125, 1542–1565. [Google Scholar] [CrossRef]
- Khandelwal, B.; Roy, S.; Lord, C.; Blakey, S. Comparison of vibrations and emissions of conventional jet fuel with stressed 100% SPK and fully formulated synthetic jet fuel. Aerospace 2014, 1, 52–66. [Google Scholar] [CrossRef]
- Ruslan, M.; Ahmed, I.; Khandelwal, B. Evaluating Effects of Fuel Properties on Smoke Emissions. In Proceedings of the ASME Turbo Expo 2016: Turbomachinery Technical Conference and Exposition, Seoul, Republic of Korea, 13–17 June 2016; p. V04AT04A046. [Google Scholar] [CrossRef]
- Leach, F.; Chapman, E.; Jetter, J.J.; Rubino, L.; Christensen, E.D.; St. John, P.C.; Fioroni, G.M.; McCormick, R.L. A review and perspective on particulate matter indices linking fuel composition to particulate emissions from gasoline engines. SAE Int. J. Fuels Lubr. 2022, 15, 3–28. [Google Scholar] [CrossRef]
- Schripp, T.; Anderson, B.E.; Bauder, U.; Rauch, B.; Corbin, J.C.; Smallwood, G.J.; Lobo, P.; Crosbie, E.C.; Shook, M.A.; Miake-Lye, R.C.; et al. Aircraft engine particulate matter emissions from sustainable aviation fuels: Results from ground-based measurements during the NASA/DLR campaign ECLIF2/ND-MAX. Fuel 2022, 325, 124764. [Google Scholar] [CrossRef]
- Tian, B.; Liu, A.; Chong, C.T.; Fan, L.; Ni, S.; Hull, A.; Rigopoulos, S.; Luo, K.H.; Hochgreb, S. Measurement and simulation of sooting characteristics by an ATJ-SKA biojet fuel and blends with Jet A-1 fuel in laminar non-premixed flames. Combust. Flame 2021, 233, 111582. [Google Scholar] [CrossRef]
- Durdina, L.; Brem, B.T.; Elser, M.; Schonenberger, D.; Siegerist, F.; Anet, J.G. Reduction of nonvolatile particulate matter emissions of a commercial turbofan engine at the ground level from the use of a sustainable aviation fuel blend. Environ. Sci. Technol. 2021, 55, 14576–14585. [Google Scholar] [CrossRef] [PubMed]
- Heeb, N.V.; Munoz, M.; Haag, R.; Wyss, S.; Schoonenberger, D.; Durdina, L.; Elser, M.; Siegerist, F.; Mohn, J.; Brem, B.T. Corelease of Genotoxic Polycyclic Aromatic Hydrocarbons and Nanoparticles from a Commercial Aircraft Jet Engine–Dependence on Fuel and Thrust. Environ. Sci. Technol. 2024, 58, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Longwell, J.P.; Chenevey, J.E.; Clark, W.W.; Frost, E.E. Flame Stabilization by Baffles in a High Velocity Gas Stream. Symp. Combust. Flame Explos. Phenom. 1984, 3, 40–44. [Google Scholar] [CrossRef]
- National Aerospace Laboratory. Design and Development of a High-Pressure Combustor for Aero Engines. ASME Digit. Collect. 2014, 100, 129–140. [Google Scholar]
- Baxter, M.R.; Lefebvre, A.H. Weak Extinction Limits of Large Scale Flame-holders. J. Eng. Gas Turbines Power 1991, 114, 776–782. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Polycyclic Aromatic Hydrocarbon Emissions from the Combustion of Alternative Fuels in a Gas Turbine Engine. Available online: https://www.researchgate.net/publication/224848815_Polycyclic_Aromatic_Hydrocarbon_Emissions_from_the_Combustion_of_Alternative_Fuels_in_a_Gas_Turbine_Engine (accessed on 16 March 2019).
- Gianinoni, I.; Rossi, A.; Villani, L. Optical Probe for the Turbine Inlet Temperature Measurement in Gas Turbine Plants. J. Eng. Gas Turbines Power 2020, 142, 450–459. [Google Scholar]
- Lauer, J.; Krause, S.; Will, S. Laser-Induced Incandescence for Soot Measurement: Recent Developments and Applications. Appl. Phys. B 2019, 125, 116. [Google Scholar]
- Santoro, R.J.; Shaddix, C.R. Laser-induced incandescence. Appl. Combust. Diagn. 2002, 26, 252–286. [Google Scholar]
- GE Vernova. Gas Turbine Emissions and Control. Available online: https://www.gevernova.com/content/dam/gepower-new/global/en_US/downloads/gas-new-site/resources/reference/ger-4211-gas-turbine-emissions-and-control.pdf (accessed on 16 March 2019).
- Environmental Protection Agency. Emission Standards for Stationary Gas Turbines. Available online: https://www.epa.gov/stationary-sources-air-pollution/stationary-gas-and-combustion-turbines-new-source-performance (accessed on 18 May 2019).
- Thermocouple Product Information. Available online: https://www.omega.com/prodinfo/thermocouples.html (accessed on 18 May 2024).
- Infrared Thermometer Specifications. Available online: https://www.thermoworks.com/ (accessed on 18 May 2024).
- Pressure Transducer Product Information. Available online: https://www.omega.com/prodinfo/pressuretransducers.html (accessed on 19 May 2024).
- Differential Pressure Sensors. Available online: https://www.omega.com/en-us/ (accessed on 20 May 2024).
- Laser-Induced Incandescence Information. Available online: https://www.combustioninstitute.org/news-and-events/news/ (accessed on 20 May 2024).
- Emissions Measurement Instruments. Available online: https://www.horiba.com/int/ (accessed on 20 May 2024).
- Data Acquisition Systems. Available online: https://www.ni.com/en-us/shop/data-acquisition.html (accessed on 20 May 2024).
- Hileman, J.I.; Ortiz, D.S.; Bartis, J.T.; Wong, H.M.; Donohoo, P.E.; Weiss, M.A.; Waitz, I.A. Near-Term Feasibility of Alternative Jet Fuels; Rand Corporation: Los Angeles, CA, USA, 2009. [Google Scholar]
- Ruan, R.; Wang, X.; Li, J.; Cui, B.; Lyu, Z.; Wang, X.; Tan, H. The effect of preheating temperature and combustion temperature on the formation characteristics of PM0.4 from preheating combustion of high-alkali lignite. Fuel 2023, 348, 128560. [Google Scholar] [CrossRef]
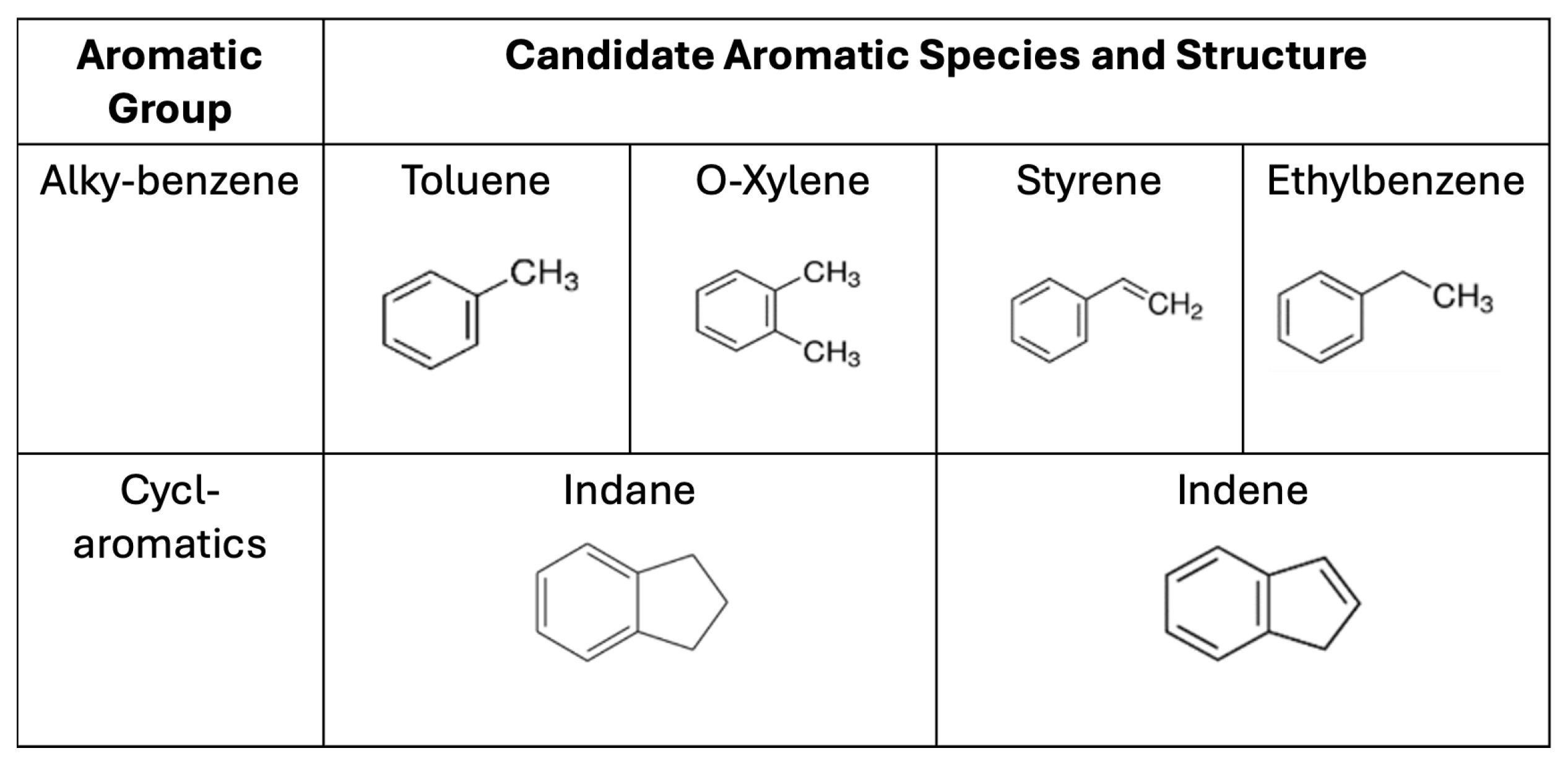

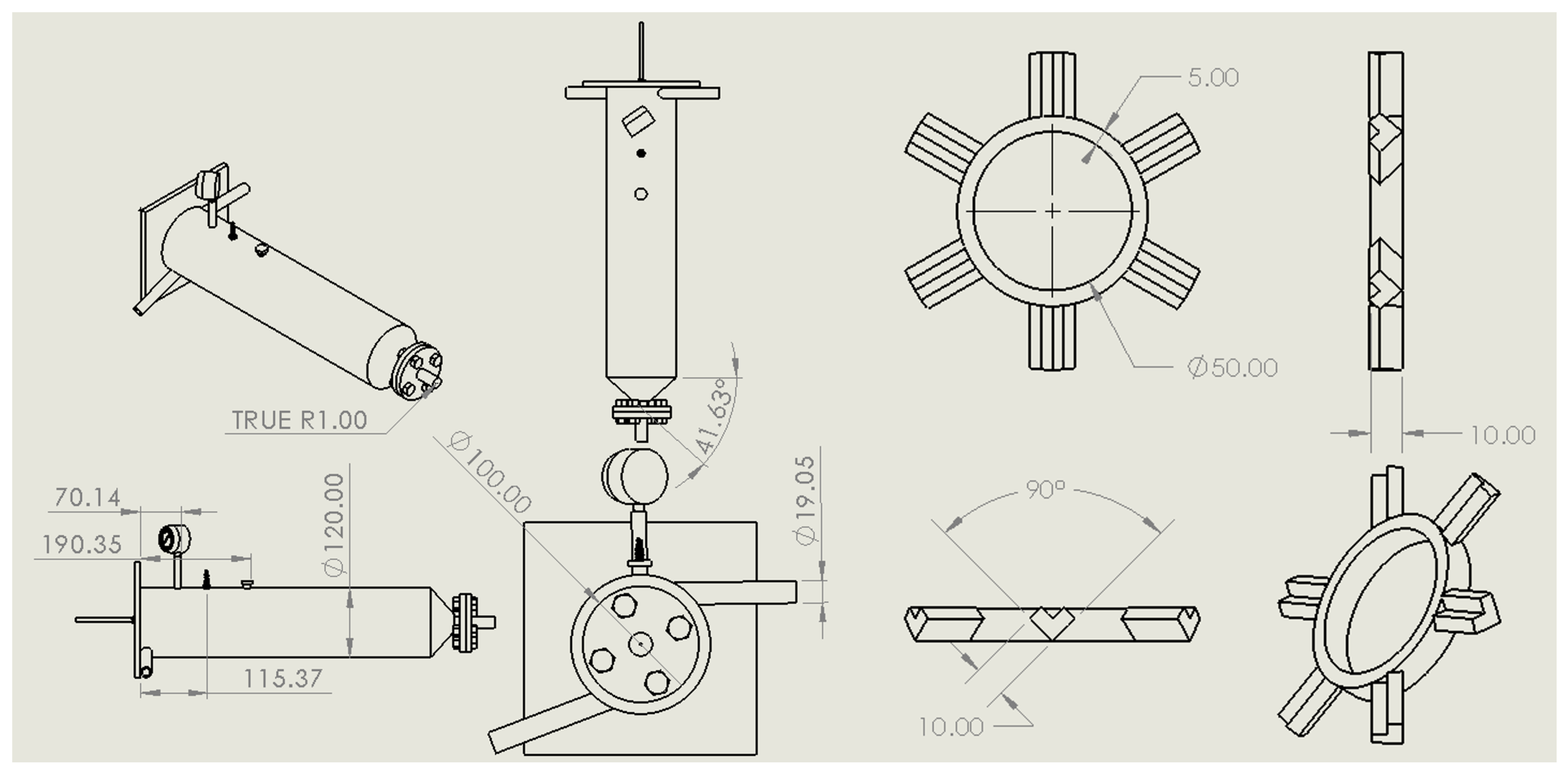

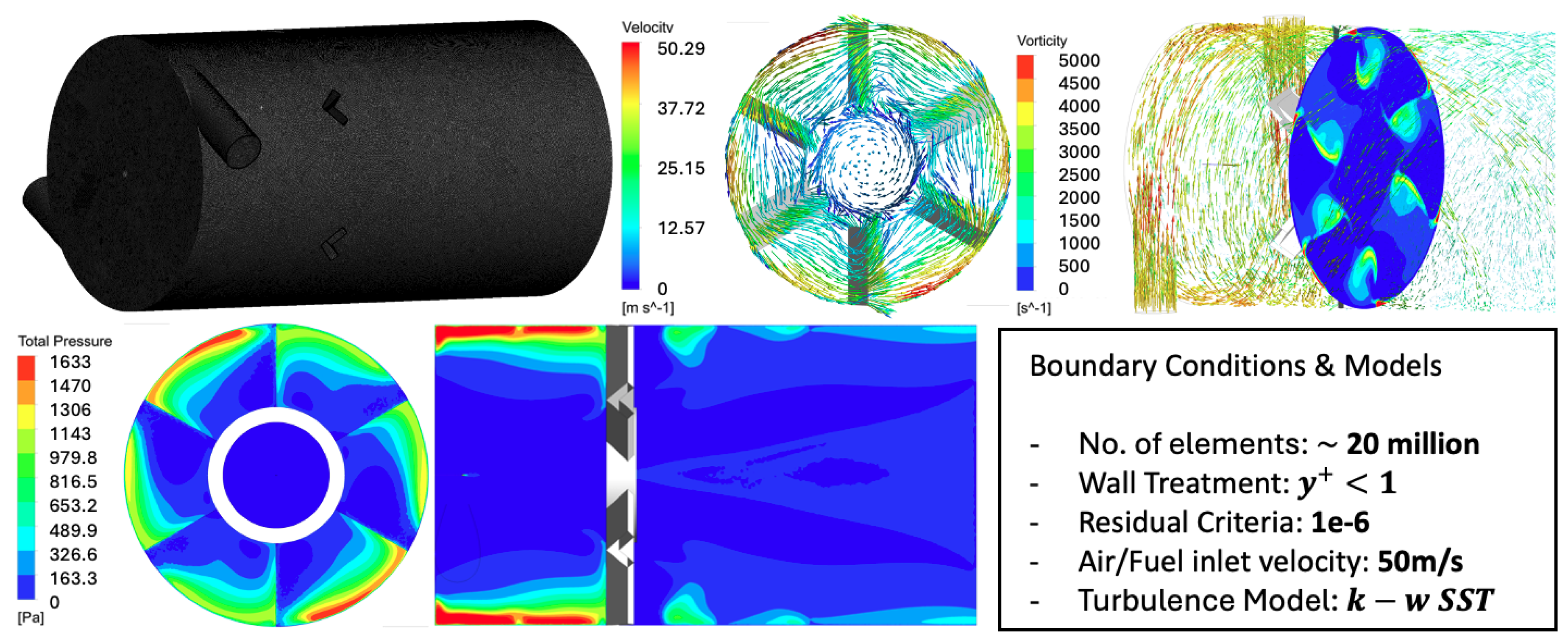



| Fuel | Chemical Formula | Auto Ignition Temp. (K) |
|---|---|---|
| Toluene | 753.15 | |
| O−Xylene | 1140.15 | |
| Styrene | 763.15 | |
| Ethylbenzene | 705.15 | |
| Indene | / | |
| Indane | / |
| Air Mass Flow Rate (kg/s) | Fuel Mass Flow Rate (kg/s) | Environmental Temperature (K) |
|---|---|---|
| ∼0.0209 | ∼0.00042 | ∼300 |
| Aromatic Type | (°C) | (mg/m3) |
|---|---|---|
| Toluene | 952.7–954.7 | 28–30 |
| O-Xylene | 1204.95–1257.35 | 6–7 |
| Styrene | 987.85–1002.45 | 12–14 |
| Ethylbenzene | 999.45–1231.85 | 10–11 |
| Indene | 989.45–1096.35 | 15–16 |
| Indane | 1130.75–1196.75 | 11–12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Q.; Khandelwal, B. Impact of Aromatic Hydrocarbons on Emissions in a Custom-Built High-Pressure Combustor. Energies 2024, 17, 3939. https://doi.org/10.3390/en17163939
Yu Q, Khandelwal B. Impact of Aromatic Hydrocarbons on Emissions in a Custom-Built High-Pressure Combustor. Energies. 2024; 17(16):3939. https://doi.org/10.3390/en17163939
Chicago/Turabian StyleYu, Qiming, and Bhupendra Khandelwal. 2024. "Impact of Aromatic Hydrocarbons on Emissions in a Custom-Built High-Pressure Combustor" Energies 17, no. 16: 3939. https://doi.org/10.3390/en17163939






