Hydrogen Storage Properties of Metal-Modified Graphene Materials
Abstract
:1. Introduction
2. Systems and Strategies
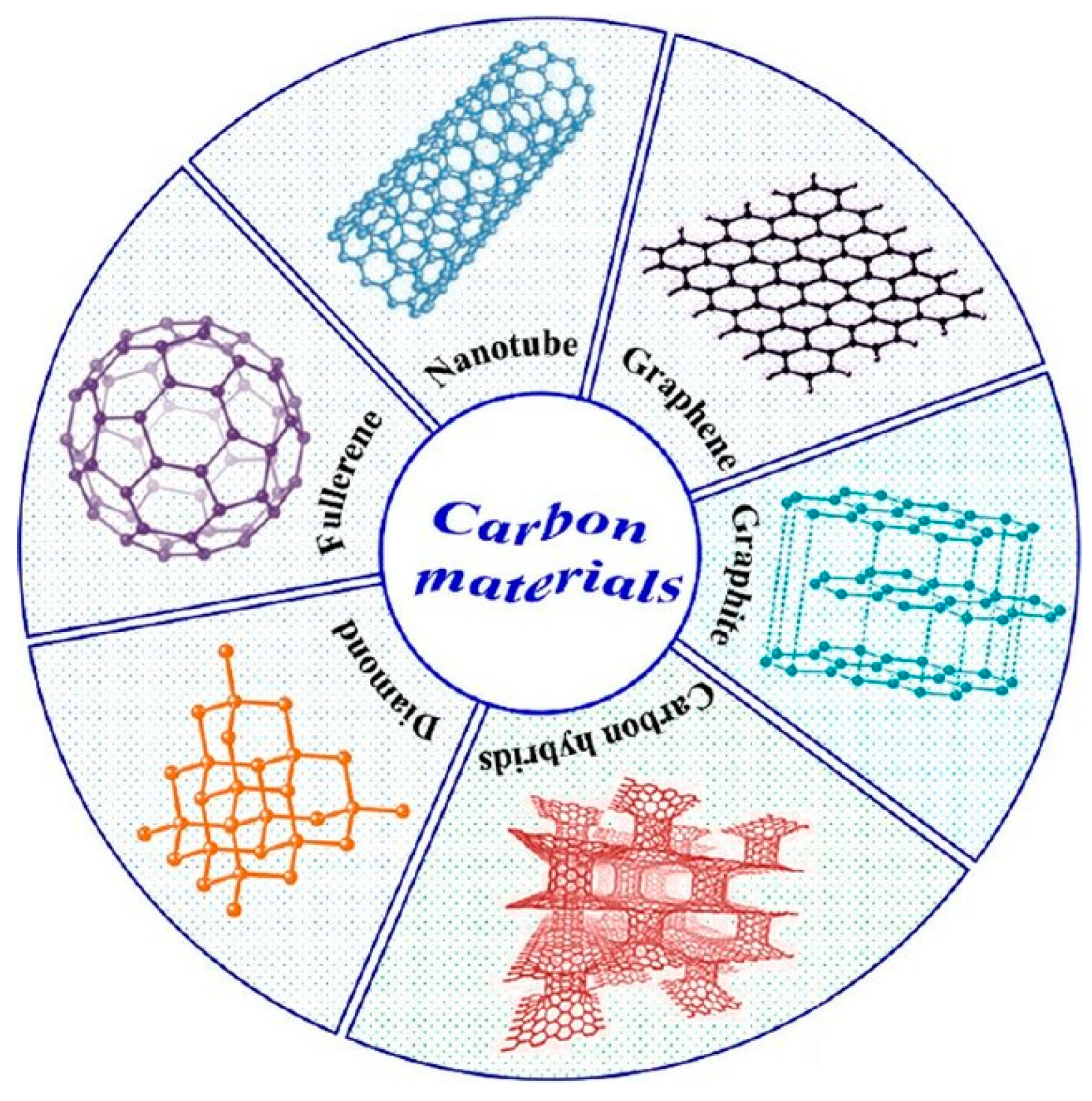
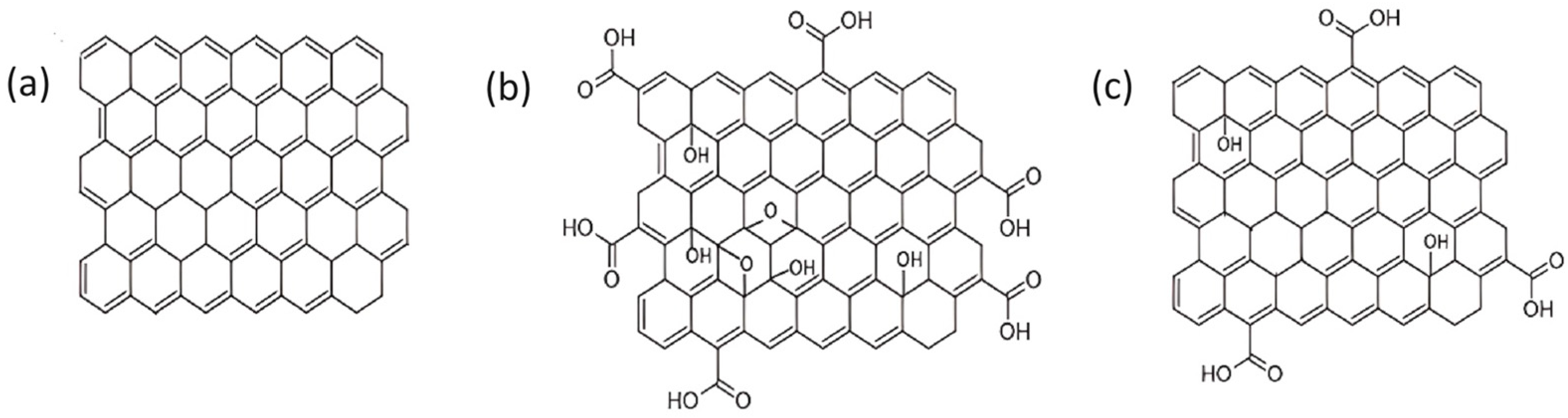
2.1. Carbon-Based Materials
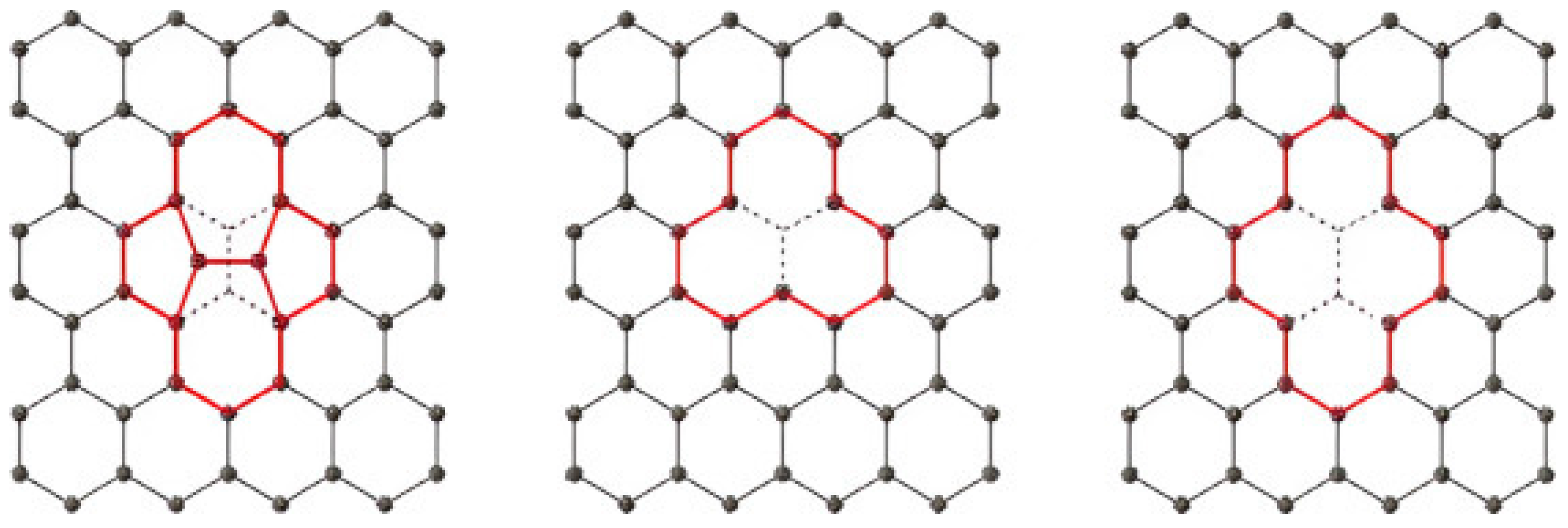
2.2. Chemical Modification and Dopant Introduction
2.3. Alkali and Alkaline Earth Metal Decoration
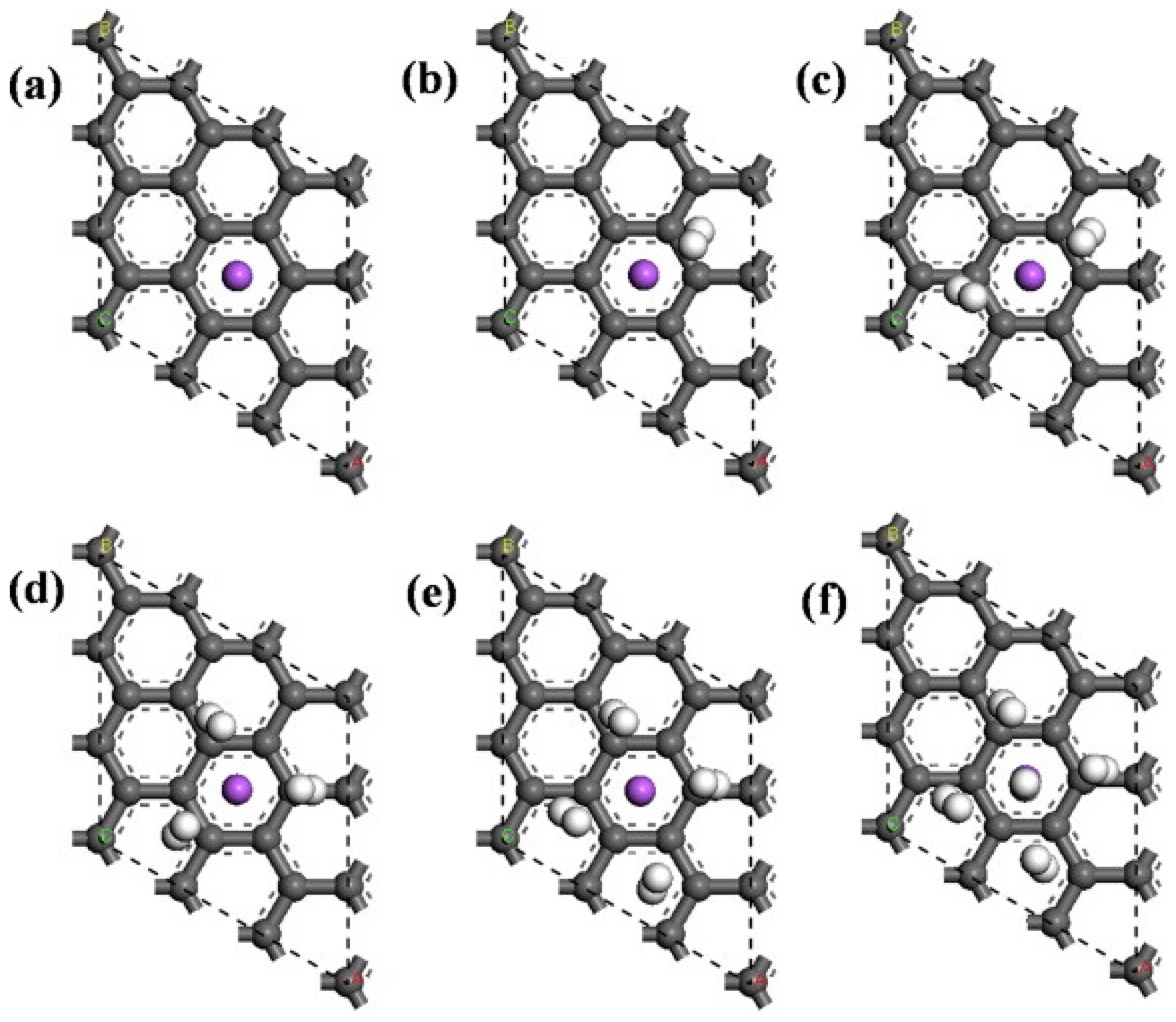
2.3.1. Lithium
2.3.2. Sodium
2.3.3. Potassium
2.3.4. Calcium
2.3.5. Strontium
2.3.6. Beryllium and Magnesium
2.4. Transition Metal Decoration
2.4.1. Scandium
2.4.2. Titanium
2.4.3. Chromium
2.4.4. Iron
2.4.5. Nickel
2.4.6. Yttrium
2.4.7. Zirconium
2.4.8. Ruthenium
2.4.9. Palladium
2.4.10. Platinum
2.5. Other Metal Dopants
Aluminum
2.6. Heteroatom Dopants
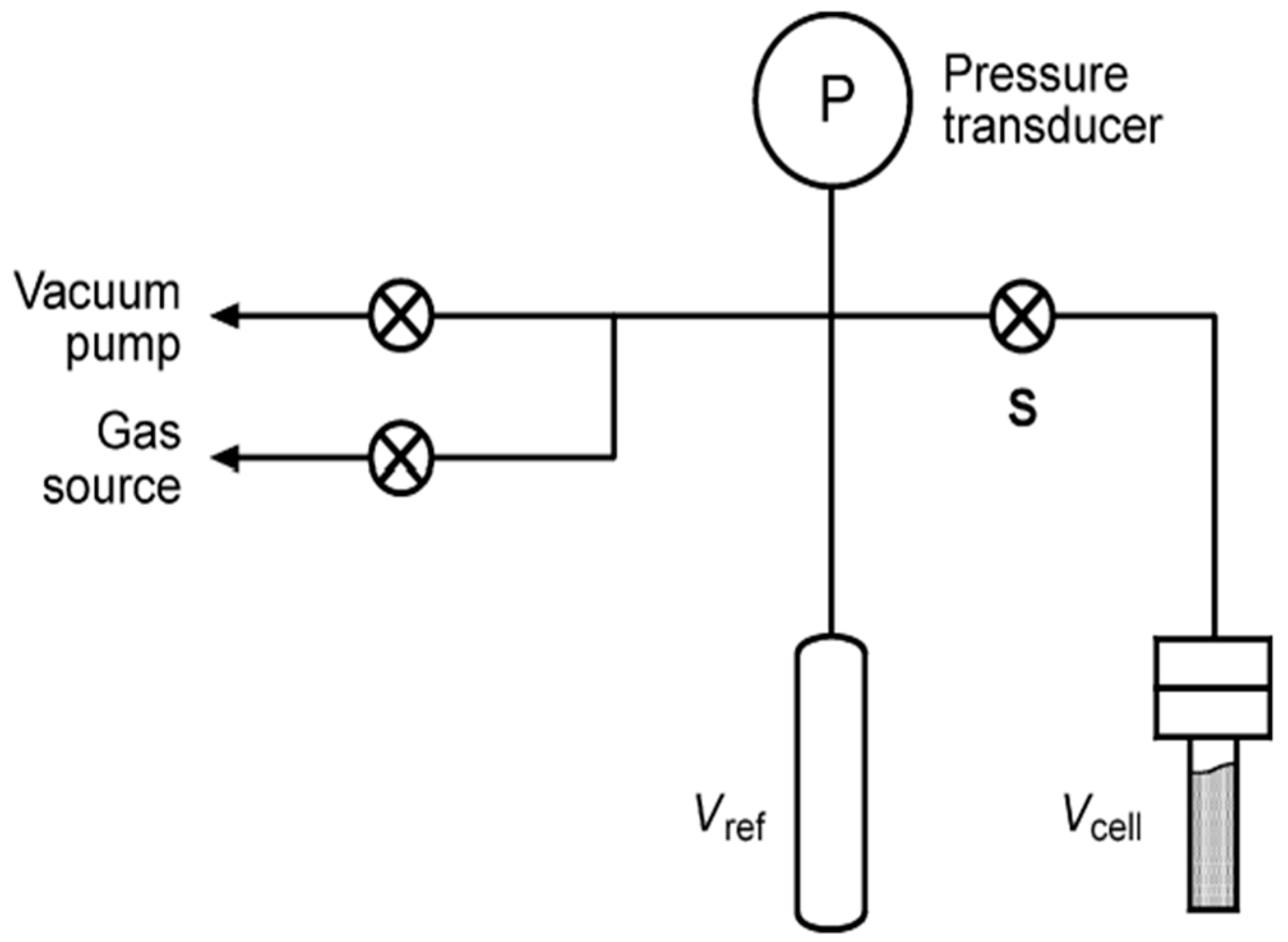
2.7. Hydrogen Storage Capacity Characterization
3. Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fossil Fuels. Available online: https://www.eesi.org/topics/fossils-fuels/description#:~:text=Overview,percent%20of%20the%20world%27s%20energy (accessed on 1 May 2023).
- Sources of Greenhouse Gas Emissions. Available online: https://www.epa.gov/ghgemissions/sources-greenhouse-gas-emissions#transportation (accessed on 1 May 2023).
- Kosonen, I. Intermittency of Renewable Energy; Review of Current Solutions and Their Sufficiency; Lappeenranta University of Technology: Lappeenranta, Finland, 2018. [Google Scholar]
- Hydrogen Storage. U.S. Department of Energy Office of Energy Efficiency and Renewable Energy. Available online: https://www.energy.gov/eere/fuelcells/hydrogen-storage (accessed on 1 May 2023).
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen Energy Systems: A Critical Review of Technologies, Applications, Trends and Challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar] [CrossRef]
- Jain, V.; Kandasubramanian, B. Functionalized Graphene Materials for Hydrogen Storage. J. Mater. Sci. 2020, 55, 1865–1903. [Google Scholar] [CrossRef]
- Morris, R.E.; Wheatley, P.S. Gas Storage in Nanoporous Materials. Angew. Chem. Int. Ed. 2008, 47, 4966–4981. [Google Scholar] [CrossRef]
- Hydrogen and Fuel Cell Technologies Office. DOE Technical Targets for Onboard Hydrogen Storage for Light-Duty Vehicles. U.S. Department of Energy Office of Energy Efficiency and Renewable Energy. Available online: https://www.energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydrogen-storage-light-duty-vehicles (accessed on 1 May 2023).
- Yang, J.; Sudik, A.; Wolverton, C.; Siegel, D.J. High Capacity Hydrogen Storage Materials: Attributes for Automotive Applications and Techniques for Materials Discovery. Chem. Soc. Rev. 2010, 39, 656–675. [Google Scholar] [CrossRef]
- Daulbayev, C.; Lesbayev, B.; Bakbolat, B.; Kaidar, B.; Sultanov, F.; Yeleuov, M.; Ustayeva, G.; Rakhymzhan, N. A Mini-Review on Recent Trends in Prospective Use of Porous 1D Nanomaterials for Hydrogen Storage. S. Afr. J. Chem. Eng. 2022, 39, 52–61. [Google Scholar] [CrossRef]
- Schaefer, S.; Fierro, V.; Szczurek, A.; Izquierdo, M.T.; Celzard, A. Physisorption, Chemisorption and Spill-over Contributions to Hydrogen Storage. Int. J. Hydrogen Energy 2016, 41, 17442–17452. [Google Scholar] [CrossRef]
- Bilić, A.; Gale, J.D. Chemisorption of Molecular Hydrogen on Carbon Nanotubes: A Route to Effective Hydrogen Storage? J. Phys. Chem. C 2008, 112, 12568–12575. [Google Scholar] [CrossRef]
- Ding, F.; Yakobson, B.I. Challenges in Hydrogen Adsorptions: From Physisorption to Chemisorption. Front. Phys. 2011, 6, 142–150. [Google Scholar] [CrossRef]
- Srinivasan, S.; Demirocak, D.E.; Kaushik, A.; Sharma, M.; Chaudhary, G.R.; Hickman, N.; Stefanakos, E. Reversible Hydrogen Storage Using Nanocomposites. Appl. Sci. 2020, 10, 4618. [Google Scholar] [CrossRef]
- Yang, R.T.; Wang, Y. Catalyzed Hydrogen Spillover for Hydrogen Storage. J. Am. Chem. Soc. 2009, 131, 4224–4226. [Google Scholar] [CrossRef]
- Hu, C.; Qu, J.; Xiao, Y.; Zhao, S.; Chen, H.; Dai, L. Carbon Nanomaterials for Energy and Biorelated Catalysis: Recent Advances and Looking Forward. ACS Cent. Sci. 2019, 5, 389–408. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Tian, W.; Zhang, Y.; Liu, T.; Wang, Y.; Shan, P.; Chen, Y.; Yuan, H. Study on the Hydrogen Storage Performance of Graphene(N)–Sc–Graphene(N) Structure. Int. J. Hydrogen Energy 2020, 45, 33789–33797. [Google Scholar] [CrossRef]
- Masika, E.; Mokaya, R. Hydrogen Storage in High Surface Area Carbons with Identical Surface Areas but Different Pore Sizes: Direct Demonstration of the Effects of Pore Size. J. Phys. Chem. C 2012, 116, 25734–25740. [Google Scholar] [CrossRef]
- Avornyo, A.; Chrysikopoulos, C.V. Applications of Graphene Oxide (GO) in Oily Wastewater Treatment: Recent Developments, Challenges, and Opportunities. J. Environ. Manag. 2024, 353, 120178. [Google Scholar] [CrossRef]
- Al-Hamdani, Y.S.; Zen, A.; Michaelides, A.; Alfè, D. Mechanisms of Adsorbing Hydrogen Gas on Metal Decorated Graphene. Phys. Rev. Mater. 2023, 7, 035402. [Google Scholar] [CrossRef]
- Gavallas, P.; Savvas, D.; Stefanou, G. Mechanical Properties of Graphene Nanoplatelets Containing Random Structural Defects. Mech. Mater. 2023, 180, 104611. [Google Scholar] [CrossRef]
- Tozzini, V.; Pellegrini, V. Prospects for Hydrogen Storage in Graphene. Phys. Chem. Chem. Physics PCCP 2012, 15, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Torsi, R.; Younas, R.; Hinkle, C.L.; Rigosi, A.; Hill, H.M.; Zhang, K.; Huang, S.; Shuck, C.E.; Chen, C.; et al. Recent Advances in 2D Material Theory, Synthesis, Properties, and Applications. ACS Nano 2023, 17, 9694–9747. [Google Scholar] [CrossRef]
- Mao, X.; Zhang, L.; Kour, G.; Zhou, S.; Wang, S.; Yan, C.; Zhu, Z.; Du, A. Defective Graphene on the Transition-Metal Surface: Formation of Efficient Bifunctional Catalysts for Oxygen Evolution/Reduction Reactions in Alkaline Media. ACS Appl. Mater. Interfaces 2019, 11, 17410–17415. [Google Scholar] [CrossRef]
- Bhatt, M.D.; Kim, H.; Kim, G. Various Defects in Graphene: A Review. RSC Adv. 2022, 12, 21520–21547. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Qing, M.; Wang, Y.; Chen, S. Defects in Graphene: Generation, Healing, and Their Effects on the Properties of Graphene: A Review. J. Mater. Sci. Technol. 2015, 31, 599–606. [Google Scholar] [CrossRef]
- Ullah, S.; Shi, Q.; Zhou, J.; Yang, X.; Ta, H.Q.; Hasan, M.; Ahmad, N.M.; Fu, L.; Bachmatiuk, A.; Rümmeli, M.H. Advances and Trends in Chemically Doped Graphene. Adv. Mater. Interfaces 2020, 7, 2000999. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Gopalakrishnan, K.; Govindaraj, A. Synthesis, Properties and Applications of Graphene Doped with Boron, Nitrogen and Other Elements. Nano Today 2014, 9, 324–343. [Google Scholar] [CrossRef]
- Xu, H.; Ma, L.; Jin, Z. Nitrogen-Doped Graphene: Synthesis, Characterizations and Energy Applications. J. Energy Chem. 2018, 27, 146–160. [Google Scholar] [CrossRef]
- Pasquini, L.; Callini, E.; Brighi, M.; Boscherini, F.; Montone, A.; Jensen, T.R.; Maurizio, C.; Vittori Antisari, M.; Bonetti, E. Magnesium Nanoparticles with Transition Metal Decoration for Hydrogen Storage. J. Nanopart. Res. 2011, 13, 5727–5737. [Google Scholar] [CrossRef]
- Spyrou, K.; Gournis, D.; Rudolf, P. Hydrogen Storage in Graphene-Based Materials: Efforts Towards Enhanced Hydrogen Absorption. ECS J. Solid State Sci. Technol. 2013, 2, M3160–M3169. [Google Scholar] [CrossRef]
- Liu, C.; Fan, Y.Y.; Liu, M.; Cong, H.T.; Cheng, H.M.; Dresselhaus, M.S. Hydrogen Storage in Single-Walled Carbon Nanotubes at Room Temperature. Science 1999, 286, 1127–1129. [Google Scholar] [CrossRef]
- Seenithurai, S.; Pandyan, R.K.; Kumar, S.V.; Saranya, C.; Mahendran, M. Li-Decorated Double Vacancy Graphene for Hydrogen Storage Application: A First Principles Study. Int. J. Hydrogen Energy 2014, 39, 11016–11026. [Google Scholar] [CrossRef]
- Huang, S.; Miao, L.; Xiu, Y.; Wen, M.; Li, C.; Zhang, L.; Jiang, J. Lithium-Decorated Oxidized Porous Graphene for Hydrogen Storage by First Principles Study. J. Appl. Phys. 2012, 112, 124312. [Google Scholar] [CrossRef]
- Wang, F.D.; Wang, F.; Zhang, N.N.; Li, Y.H.; Tang, S.W.; Sun, H.; Chang, Y.F.; Wang, R.S. High-Capacity Hydrogen Storage of Na-Decorated Graphene with Boron Substitution: First-Principles Calculations. Chem. Phys. Lett. 2013, 555, 212–216. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, W.; Wang, R.; Hao, L.; Jiao, W. Hydrogen Storage Using Na-Decorated Graphyne and Its Boron Nitride Analog. Int. J. Hydrogen Energy 2014, 39, 12757–12764. [Google Scholar] [CrossRef]
- Tokarev, A.; Avdeenkov, A.V.; Langmi, H.; Bessarabov, D.G. Modeling Hydrogen Storage in Boron-Substituted Graphene Decorated with Potassium Metal Atoms. Int. J. Energy Res. 2015, 39, 524–528. [Google Scholar] [CrossRef]
- Shams, M.; Reisi-Vanani, A. Potassium Decorated γ-Graphyne as Hydrogen Storage Medium: Structural and Electronic Properties. Int. J. Hydrogen Energy 2019, 44, 4907–4918. [Google Scholar] [CrossRef]
- Yoon, M.; Yang, S.; Hicke, C.; Wang, E.; Geohegan, D.; Zhang, Z. Calcium as the Superior Coating Metal in Functionalization of Carbon Fullerenes for High-Capacity Hydrogen Storage. Phys. Rev. Lett. 2008, 100, 206806. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J. Hydrogen Storage Materials Research and Development Work at Brookhaven National Laboratory; Brookhaven National Lab.: Upton, NY, USA, 1979. [Google Scholar]
- Guidotti, R.A.; Atkinson, G.B.; Wong, M.M. Hydrogen Absorption by Rare Earth-Transition Metal Alloys. J. Less Common Met. 1977, 52, 13–28. [Google Scholar] [CrossRef]
- Kubas, G.J. Fundamentals of H2 Binding and Reactivity on Transition Metals Underlying Hydrogenase Function and H2 Production and Storage. Chem. Rev. 2007, 107, 4152–4205. [Google Scholar] [CrossRef]
- Mahamiya, V.; Shukla, A.; Garg, N.; Chakraborty, B. High-Capacity Reversible Hydrogen Storage in Scandium Decorated Holey Graphyne: Theoretical Perspectives. Int. J. Hydrogen Energy 2022, 47, 7870–7883. [Google Scholar] [CrossRef]
- Kim, G.; Jhi, S.-H.; Park, N.; Louie, S.G.; Cohen, M.L. Optimization of Metal Dispersion in Doped Graphitic Materials for Hydrogen Storage. Phys. Rev. B 2008, 78, 085408. [Google Scholar] [CrossRef]
- Luo, Z.; Fan, X.; Pan, R.; An, Y. A First-Principles Study of Sc-Decorated Graphene with Pyridinic-N Defects for Hydrogen Storage. Int. J. Hydrogen Energy 2017, 42, 3106–3113. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Vaidyanathan, A.; Kandasamy, M.; Ramaniah, L.M.; Wagh, V.; Chakraborty, B. Hydrogen Storage in Sc-Decorated Ψ-Graphene via Density Functional Theory Simulations. Int. J. Hydrogen Energy 2024, 52, 376–389. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, L.; He, Y.; Cheng, H.-P. Titanium-Decorated Graphene for High-Capacity Hydrogen Storage Studied by Density Functional Simulations. J. Phys. Condens. Matter 2010, 22, 445301. [Google Scholar] [CrossRef] [PubMed]
- Mashoff, T.; Convertino, D.; Miseikis, V.; Coletti, C.; Piazza, V.; Tozzini, V.; Beltram, F.; Heun, S. Increasing the Active Surface of Titanium Islands on Graphene by Nitrogen Sputtering. Appl. Phys. Lett. 2015, 106, 083901. [Google Scholar] [CrossRef]
- Yuan, L.; Kang, L.; Chen, Y.; Wang, D.; Gong, J.; Wang, C.; Zhang, M.; Wu, X. Hydrogen Storage Capacity on Ti-Decorated Porous Graphene: First-Principles Investigation. Appl. Surf. Sci. 2018, 434, 843–849. [Google Scholar] [CrossRef]
- Xiang, C.; Li, A.; Yang, S.; Lan, Z.; Xie, W.; Tang, Y.; Xu, H.; Wang, Z.; Gu, H. Enhanced Hydrogen Storage Performance of Graphene Nanoflakes Doped with Cr Atoms: A DFT Study. RSC Adv. 2019, 9, 25690–25696. [Google Scholar] [CrossRef] [PubMed]
- Ferbel, L.; Veronesi, S.; Vlamidis, Y.; Rossi, A.; Sabattini, L.; Coletti, C.; Heun, S. Platinum-Decorated Graphene: Experimental Insight into Growth Mechanisms and Hydrogen Adsorption Properties. FlatChem 2024, 45, 100661. [Google Scholar] [CrossRef]
- Goharibajestani, Z.; Yürüm, A.; Yürüm, Y. Effect of Transition Metal Oxide Nanoparticles on Gas Adsorption Properties of Graphene Nanocomposites. Appl. Surf. Sci. 2019, 475, 1070–1076. [Google Scholar] [CrossRef]
- Moradi, S.E. Enhanced Hydrogen Adsorption by Fe3O4–Graphene Oxide Materials. Appl. Phys. A 2015, 119, 179–184. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Wang, T. Enhanced Hydrogen Storage Performance of Three-Dimensional Hierarchical Porous Graphene with Nickel Nanoparticles. Int. J. Hydrogen Energy 2018, 43, 11120–11131. [Google Scholar] [CrossRef]
- Flamina, A.; Raghavendra, R.M.; Gupta, A.; Subramaniam, A. Hydrogen Storage in Nickel Dispersed Boron Doped Reduced Graphene Oxide. Appl. Surf. Sci. Adv. 2023, 13, 100371. [Google Scholar] [CrossRef]
- Ismail, N.; Madian, M.; El-Shall, M.S. Reduced Graphene Oxide Doped with Ni/Pd Nanoparticles for Hydrogen Storage Application. J. Ind. Eng. Chem. 2015, 30, 328–335. [Google Scholar] [CrossRef]
- Chakraborty, B.; Modak, P.; Banerjee, S. Hydrogen Storage in Yttrium-Decorated Single Walled Carbon Nanotube. J. Phys. Chem. C 2012, 116, 22502–22508. [Google Scholar] [CrossRef]
- Yadav, A.; Chakraborty, B.; Gangan, A.; Patel, N.; Press, M.R.; Ramaniah, L.M. Magnetic Moment Controlling Desorption Temperature in Hydrogen Storage: A Case of Zirconium-Doped Graphene as a High Capacity Hydrogen Storage Medium. J. Phys. Chem. C 2017, 121, 16721–16730. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.T. Hydrogen Storage Properties of Carbons Doped with Ruthenium, Platinum, and Nickel Nanoparticles. J. Phys. Chem. C 2008, 112, 12486–12494. [Google Scholar] [CrossRef]
- Verdinelli, V.; Juan, A.; German, E. Ruthenium Decorated Single Walled Carbon Nanotube for Molecular Hydrogen Storage: A First-Principle Study. Int. J. Hydrogen Energy 2019, 44, 8376–8383. [Google Scholar] [CrossRef]
- Liu, P.; Liang, J.; Xue, R.; Du, Q.; Jiang, M. Ruthenium Decorated Boron-Doped Carbon Nanotube for Hydrogen Storage: A First-Principle Study. Int. J. Hydrogen Energy 2019, 44, 27853–27861. [Google Scholar] [CrossRef]
- Huang, C.-C.; Pu, N.-W.; Wang, C.-A.; Huang, J.-C.; Sung, Y.; Ger, M.-D. Hydrogen Storage in Graphene Decorated with Pd and Pt Nano-Particles Using an Electroless Deposition Technique. Sep. Purif. Technol. 2011, 82, 210–215. [Google Scholar] [CrossRef]
- Parambhath, V.B.; Nagar, R.; Ramaprabhu, S. Effect of Nitrogen Doping on Hydrogen Storage Capacity of Palladium Decorated Graphene. Langmuir 2012, 28, 7826–7833. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.-N.; Wu, S.-Y.; Chen, H.-T. Hydrogen Storage in N- and B-Doped Graphene Decorated by Small Platinum Clusters: A Computational Study. Appl. Surf. Sci. 2018, 441, 607–612. [Google Scholar] [CrossRef]
- Tan, H.L.; Du, A.; Amal, R.; Ng, Y.H. Decorating Platinum on Nitrogen-Doped Graphene Sheets: Control of the Platinum Particle Size Distribution for Improved Photocatalytic H2 Generation. Chem. Eng. Sci. 2019, 194, 85–93. [Google Scholar] [CrossRef]
- Ao, Z.M.; Jiang, Q.; Zhang, R.Q.; Tan, T.T.; Li, S. Al Doped Graphene: A Promising Material for Hydrogen Storage at Room Temperature. J. Appl. Phys. 2009, 105, 074307. [Google Scholar] [CrossRef]
- Akbari, F.; Reisi-Vanani, A.; Darvishnejad, M.H. DFT Study of the Electronic and Structural Properties of Single Al and N Atoms and Al-N Co-Doped Graphyne toward Hydrogen Storage. Appl. Surf. Sci. 2019, 488, 600–610. [Google Scholar] [CrossRef]
- Ghotia, S.; Rimza, T.; Singh, S.; Dwivedi, N.; Srivastava, A.K.; Kumar, P. Hetero-Atom Doped Graphene for Marvellous Hydrogen Storage: Unveiling Recent Advances and Future Pathways. J. Mater. Chem. A 2024, 12, 12325–12357. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Wang, P.; Li, C.; Yue, Q.; Cui, W.-G.; Wang, X.; Yang, Y.; Gao, F.; Zhang, M.; et al. Solid-State Hydrogen Storage Origin and Design Principles of Carbon-Based Light Metal Single-Atom Materials. Adv. Funct. Mater. 2024, 34, 2316368. [Google Scholar] [CrossRef]
- Blach, T.P.; Gray, E.M. Sieverts Apparatus and Methodology for Accurate Determination of Hydrogen Uptake by Light-Atom Hosts. J. Alloys Compd. 2007, 446–447, 692–697. [Google Scholar] [CrossRef]
- Broom, D.P. The Accuracy of Hydrogen Sorption Measurements on Potential Storage Materials. Int. J. Hydrogen Energy 2007, 32, 4871–4888. [Google Scholar] [CrossRef]
- Modak, P.; Chakraborty, B.; Banerjee, S. Study on the Electronic Structure and Hydrogen Adsorption by Transition Metal Decorated Single Wall Carbon Nanotubes. J. Phys. Condens. Matter 2012, 24, 185505. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, T.; Ciraci, S. Titanium-Decorated Carbon Nanotubes as a Potential High-Capacity Hydrogen Storage Medium. Phys. Rev. Lett. 2005, 94, 175501. [Google Scholar] [CrossRef]
- Cohesive Energy|The Elements Handbook at KnowledgeDoor. KnowledgeDoor. Available online: https://www.knowledgedoor.com/2/elements_handbook/cohesive_energy.html (accessed on 6 May 2024).
- Hwang, H.T.; Varma, A. Hydrogen Storage for Fuel Cell Vehicles. Curr. Opin. Chem. Eng. 2014, 5, 42–48. [Google Scholar] [CrossRef]

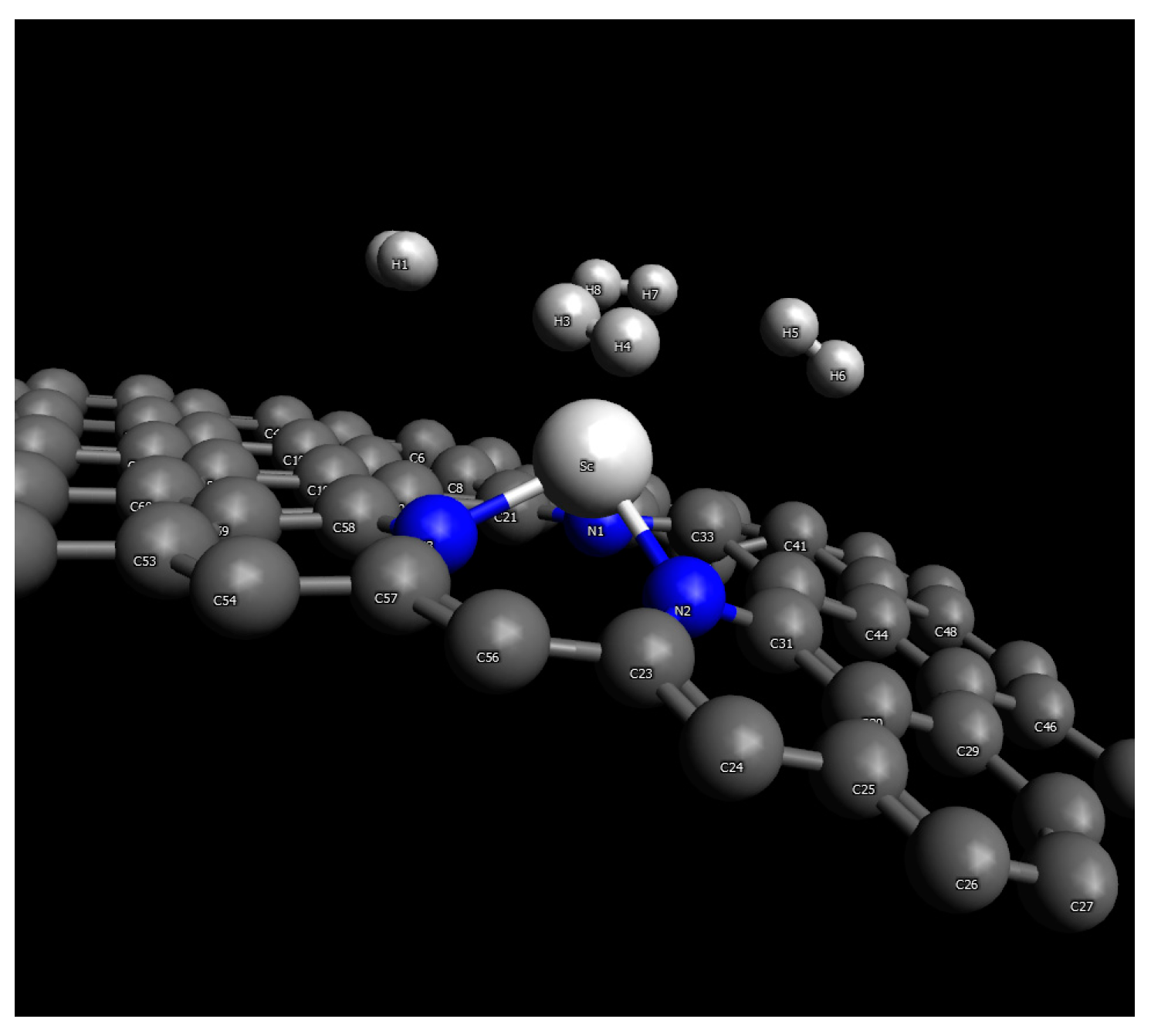
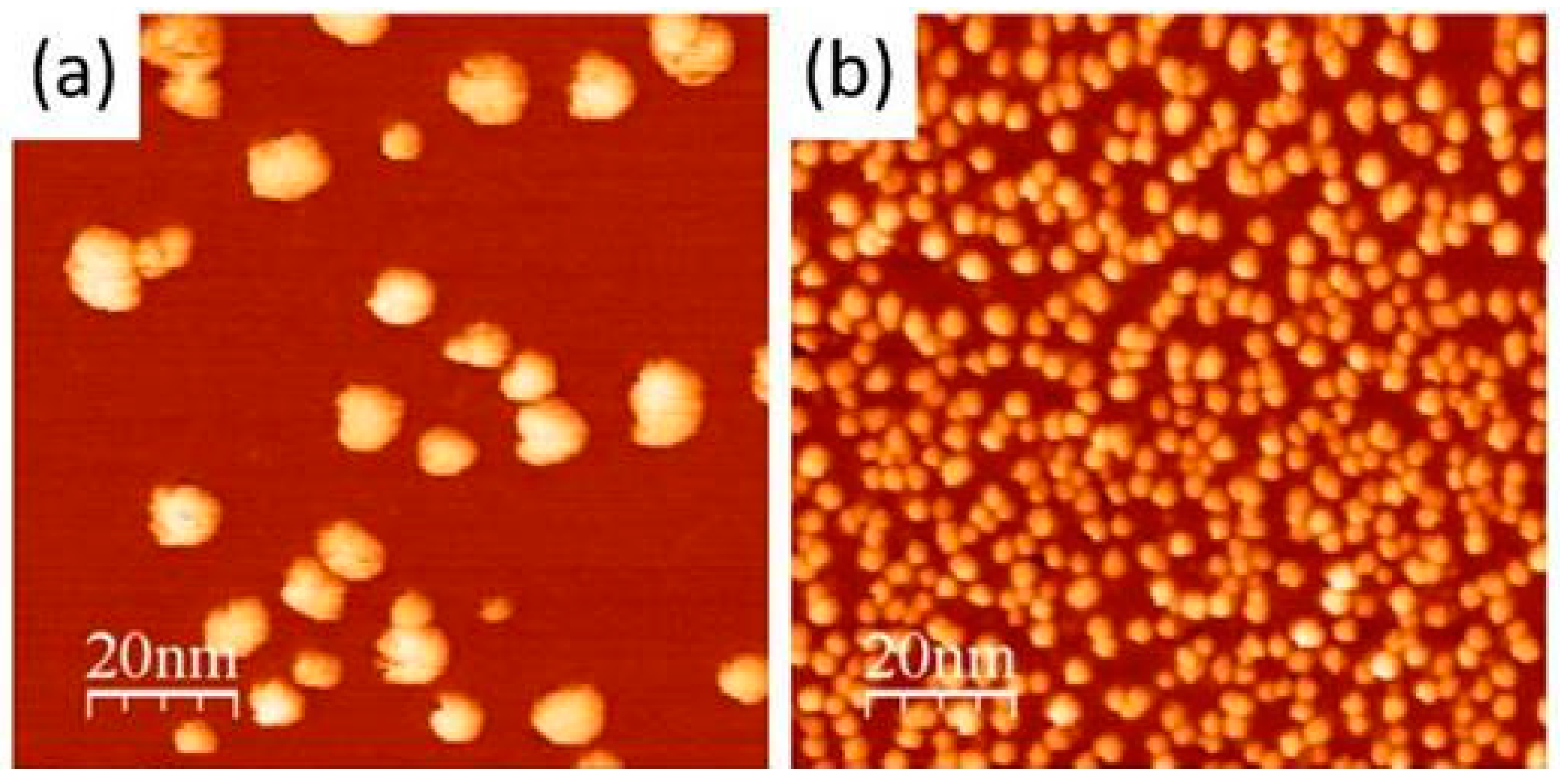
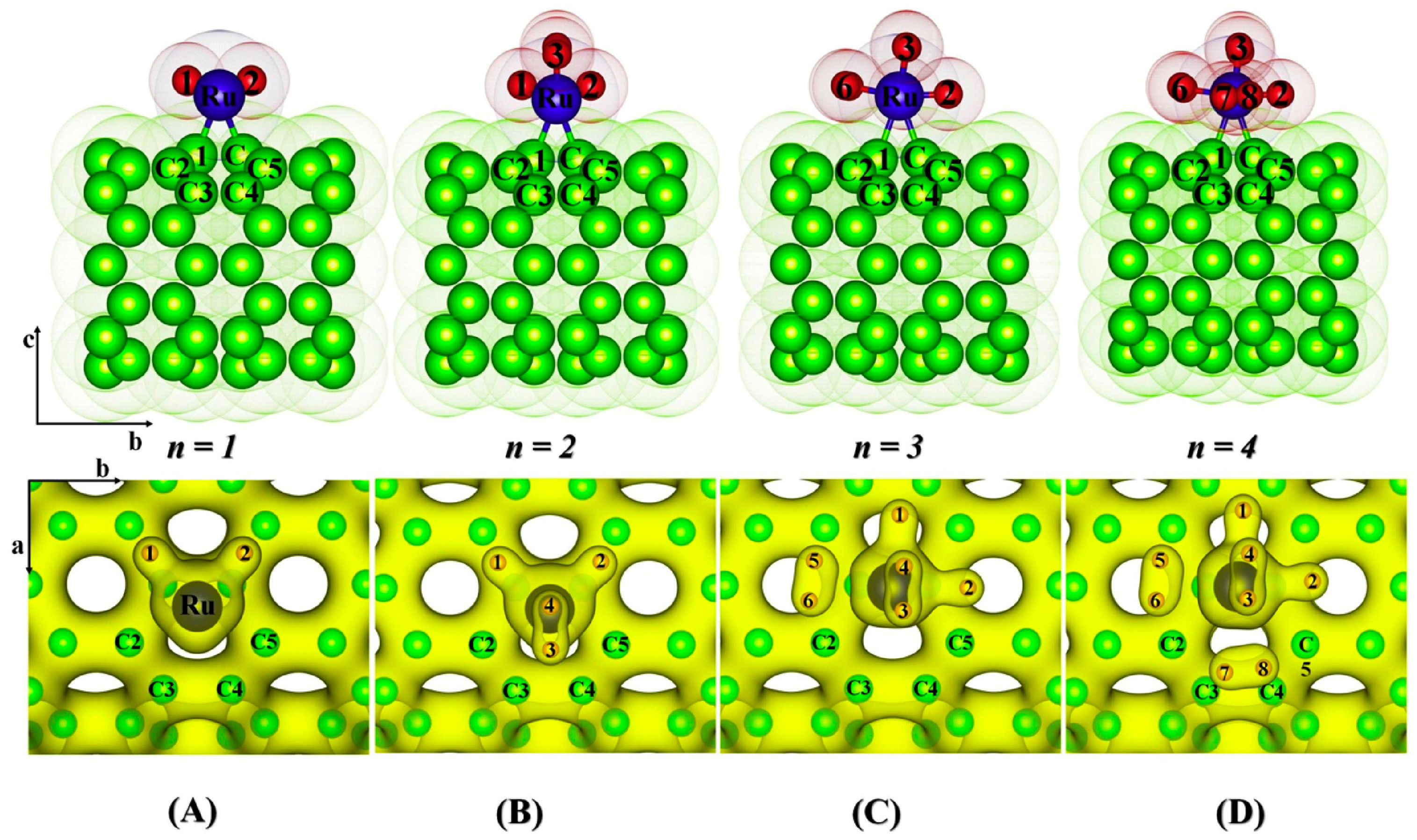
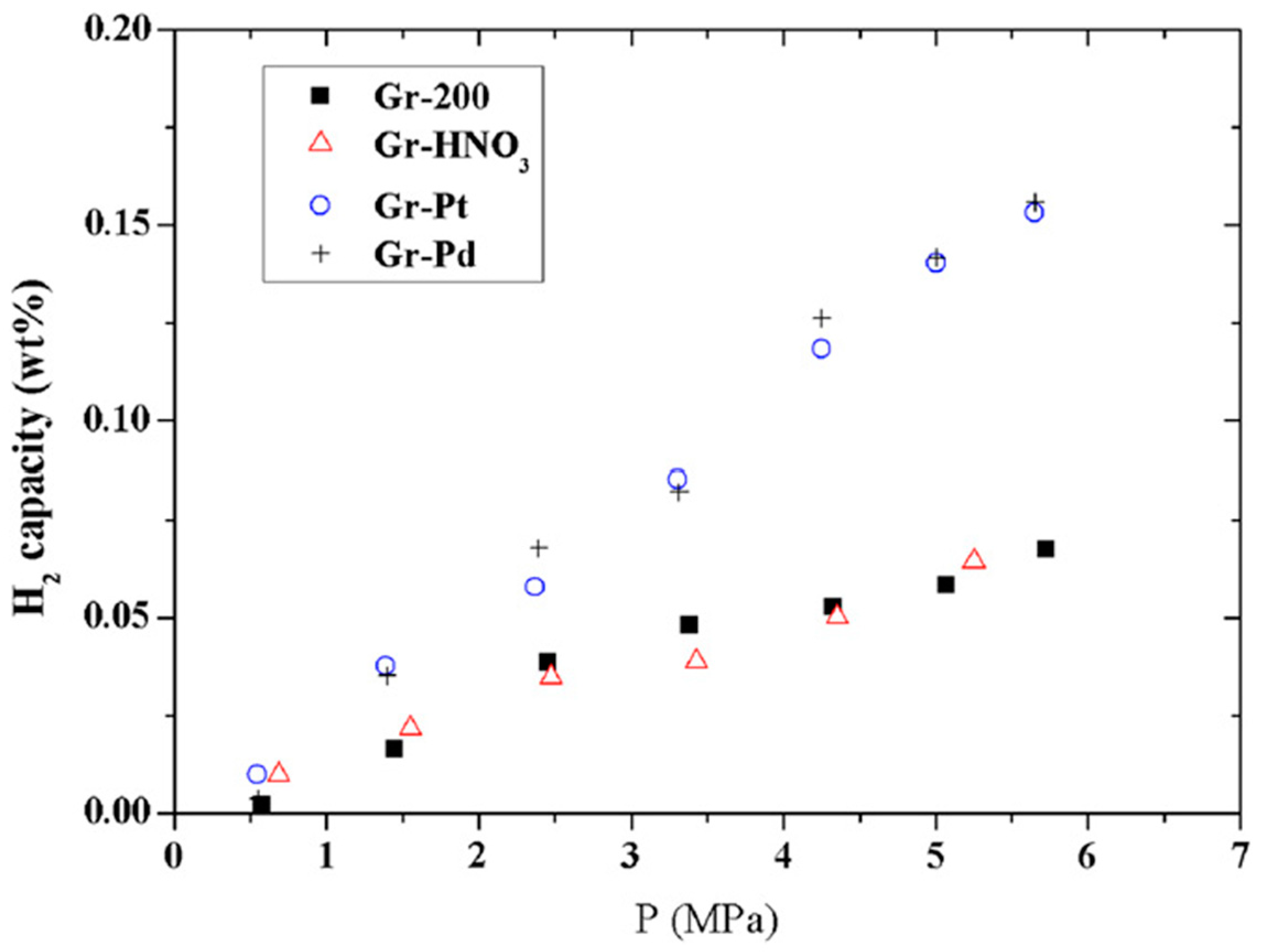

| Material | H2 Storage Capacity (wt.%) | Bond Length (Å) | Binding Energy (eV) | Conditions | Reference |
|---|---|---|---|---|---|
| Pristine graphene | 2.7 | Room temperature, 25 bar | [19] | ||
| Li@graphene | 4.32 | 0.30 | [26] | ||
| Li@CNT | 4.2 | Room temperature, 10 MPa | [25] | ||
| Li@oxidized porous graphene | 9.43 | [27] | |||
| Li@MV graphene | 2.64 | [25] | |||
| Li@DV graphene | 2.61 | [25] | |||
| Single-sided Na@graphene | 8.1 | [28] | |||
| Double-sided Na@graphene | 12.8 | [28] | |||
| Na@B-graphene | 11.7 | [28] | |||
| Single-sided Na@graphyne | 3.49 | [29] | |||
| Double-sided Na@graphyne | 5.98 | [29] | |||
| Double-sided Na@BN-yne | 5.84 | [29] | |||
| K@B-graphene | 22.5 | [30] | |||
| K@graphyne | 8.95 | −0.212 | [31] | ||
| Two-sided K@graphyne | 13.95 | [31] | |||
| Ca32C60 | >8.4 | ~0.2 | [32] | ||
| Double-sided Ca@B-graphene | 8.38 | 0.46 | [32] | ||
| Sr@C60 | 0.191 | [32] | |||
| Be@C60, Mg@C60 | 3.5 | [32] | |||
| Ti@graphene | 7.8 | [41] | |||
| Ti-PG | 6.11 | −0.457 | [6] | ||
| Ti-PG | 2.5 | [41] | |||
| Cr@GNF | −0.574 | [43] | |||
| Cr@pentagraphene | −0.25 | [43] | |||
| Fe3O4@RGO | 0.4 | 298 K, 9 bar | [45] | ||
| Fe3O4@RGO | 2.1 | 77 K, atmospheric pressure | [46] | ||
| 3DHPG-Ni | 4.22 | 77 K | [47] | ||
| 3DHPG-Ni | 1.95 | 298 K | [47] | ||
| Ni-B-rGO | 6.9 | 77 K | [48] | ||
| Ni-B-rGO | 0.16 | 298 K | [48] | ||
| rGO | 9.8 | 77 K | [48] | ||
| Ni@rGO | 2.7 | 80 K | [49] | ||
| Y@SWCNT | 6.1 | [50] | |||
| Zr@graphene | 11 | 0.34 | [51] | ||
| Ru@TC | 1.56 | 298 K, 10.3 MPa | [52] | ||
| Gr-HNO3-Pd | 0.156 | 303 K, 5.7 MPa | [55] | ||
| Pd-N-HEG | 4.40 | 25 °C, 4 MPa | [56] | ||
| PdCo-D(100)-G | 4.83 | 25 °C, 20 bar | [56] | ||
| Pt@graphene | 0.15 | 303 K, 5.7 MPa | [55] | ||
| Pt@B-graphene | 0.84 | [57] | |||
| Pt@N-graphene | 0.23 | [57] | |||
| Al@graphene | −0.427 | [59] | |||
| Pristine graphyne | 5.26 | [60] | |||
| Al-doped graphyne | 10.17 | [60] | |||
| N-doped graphyne | 8.75 | [60] | |||
| Al-N co-doped graphyne | 16.67 | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sotsky, L.; Castillo, A.; Ramos, H.; Mitchko, E.; Heuvel-Horwitz, J.; Bick, B.; Mahajan, D.; Wong, S.S. Hydrogen Storage Properties of Metal-Modified Graphene Materials. Energies 2024, 17, 3944. https://doi.org/10.3390/en17163944
Sotsky L, Castillo A, Ramos H, Mitchko E, Heuvel-Horwitz J, Bick B, Mahajan D, Wong SS. Hydrogen Storage Properties of Metal-Modified Graphene Materials. Energies. 2024; 17(16):3944. https://doi.org/10.3390/en17163944
Chicago/Turabian StyleSotsky, Leela, Angeline Castillo, Hugo Ramos, Eric Mitchko, Joshua Heuvel-Horwitz, Brian Bick, Devinder Mahajan, and Stanislaus S. Wong. 2024. "Hydrogen Storage Properties of Metal-Modified Graphene Materials" Energies 17, no. 16: 3944. https://doi.org/10.3390/en17163944





