Goat Manure Potential as a Substrate for Biomethane Production—An Experiment for Photofermentation
Abstract
1. Introduction
- (1)
- Temperature; optimum temperature for various types of bacteria: psychrophilic fermentation 20–25 °C, mesophilic 35–37 °C and thermophilic 55–60 °C [11]. The increase in temperature has a positive effect on the metabolic activity of microorganisms and the stability and efficiency of production CH4 [12,13]. Thermophilic fermentation is more difficult to control the process conditions and is also more energy intensive. Most biogas installations in the world operate using mesophilic technologies [14], including in Poland [15].
- (2)
- pH; this is one of the most important factors influencing the fermentation process. The optimal pH range is different for each stage of CH4 fermentation. For bacteria responsible for hydrolysis and acid-forming bacteria, the optimal pH range is 4.5–6.3 [4]. According to Kallistova et al. (2014) [16], these are values in the range of 5.5–6.5. The most favorable pH range for the development of bacteria responsible for acetogenesis and metanogenesis includes values from 6.8 to 7.5 [4].
- (3)
- The ratio of carbon (C) to nitrogen (N), as shown in Table 1; in order to ensure the proper growth and activity of the bacteria responsible for fermentation, it is necessary to provide them with the appropriate amount of nutrients, macro- and microelements [17]. When the C to N value is too high, microorganisms may not process the total amount of carbon, while a value that is too low causes the formation of NH3 [18]. According to Puñal et al. (2000) [19], the optimal C:N value is 10–30, while according to Jadhav et al. (2023) [20], it is C:N 20–30.
- (1)
- Biomass in the form of animal excrements, straw, natural substances from agricultural or forestry production and other substrates that do not pose a threat to human health, animals or the environment, exclusively of biological origin, e.g., plant biomass from the maintenance of green areas, only obtained directly from the biomass producer and then directly transferred to the biogas producer, non-municipal waste, animal by-products, pulp, pomace, post-fermentation sludge, food that is not suitable for consumption or further processing.
- (2)
- Waste, including waste plant mass, animal excrements, waste animal tissue, waste from the sugar industry, waste from the dairy industry, waste from the baking and confectionery industry and waste from the production of alcoholic and non-alcoholic beverages [32]. Available technologies are being improved and new solutions are being sought [33,34]. Research is being carried out to increase the share of by-products and waste among these materials, which constitute a raw material for the production of biogas and energy in the process. These activities are consistent with the goals of a circular economy and contribute to reducing the share of fossil fuels in energy production and reduce the adverse impact of their use on the environment [22,35].
Testing Manure before Using It for Biogas Production
- (1)
- Determination of dry mass and dry organic matter.
- (2)
- Determination of total nitrogen and NH3 using the Kjeldahl method.
- (3)
- Measurement of pH, conductivity and redox potential.
- (4)
- Elementary analysis.
- -
- immobilization on the goat manure bed (depending on the research material collected), which allows for demonstrating the activity of the fermentation bacterial flora, thus influencing the amount of biogas (biomethane) produced in the reactor.
- (1)
- The time of collecting research material;
- (2)
- The mineralization and elemental composition of research materials;
- (3)
- The % composition of individual biogas components for a given biogas flow;
- (4)
- The course of changes in individual biogas components depending on the temperature and time of biogas production.
2. Materials and Methods
- (a)
- Material A stored for 1 month (fresh—wet sample);
- (b)
- Material B stored for 12 months (old—wet sample);
- (c)
- Material C stored for 12 months (old sample—dry).
- (1)
- Determination of dry matter at 105 °C by gravimetric method—ISO 11465:1994 [52];
- (2)
- (3)
- Mineralization of vegetation samples and natural fertilizers in concentrated mineral acids for nitrogen—PB/31/09:2014* [56];
- (4)
- Determination of phosphorus and nitrogen—according to the SKALAR methodology [57];
- (5)
- (6)
- Determination of Fe, Mn, Zn, Cu (ASA)—PN-ISO 8288:2002 [60].* research procedure Research Laboratory of Environmental Chemistry.
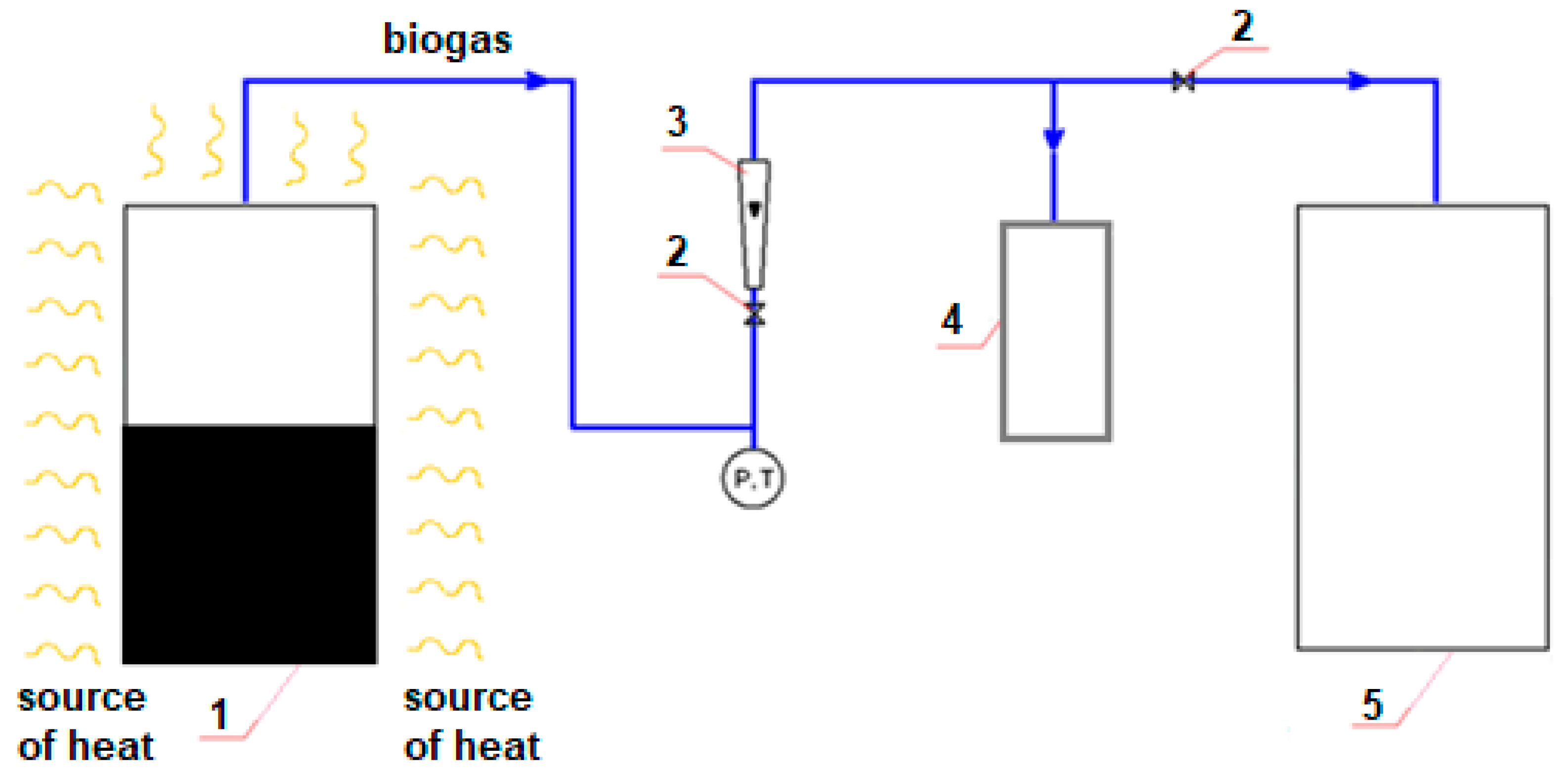
2.1. Experimental Stand
- (1)
- Material A;
- (2)
- Material B;
- (3)
- Material C.
- (a)
- Natural heat source (ambient heat from the Sun);
- (b)
- Thermometer for measuring heat;
- (c)
- Gas analyzer for checking the composition and physical parameters of naturally occurring gas mixtures, with a particular purpose for testing biogas.
2.2. Scope and Research Methodology
- (1)
- Determining the mineralization and elemental composition of research materials;
- (2)
- Establishing the conditions of the biogas (biomethane) production process depending on the adopted process criteria.
3. Results and Discussion
3.1. Evaluation of Goat Manure Research—Process Aspects
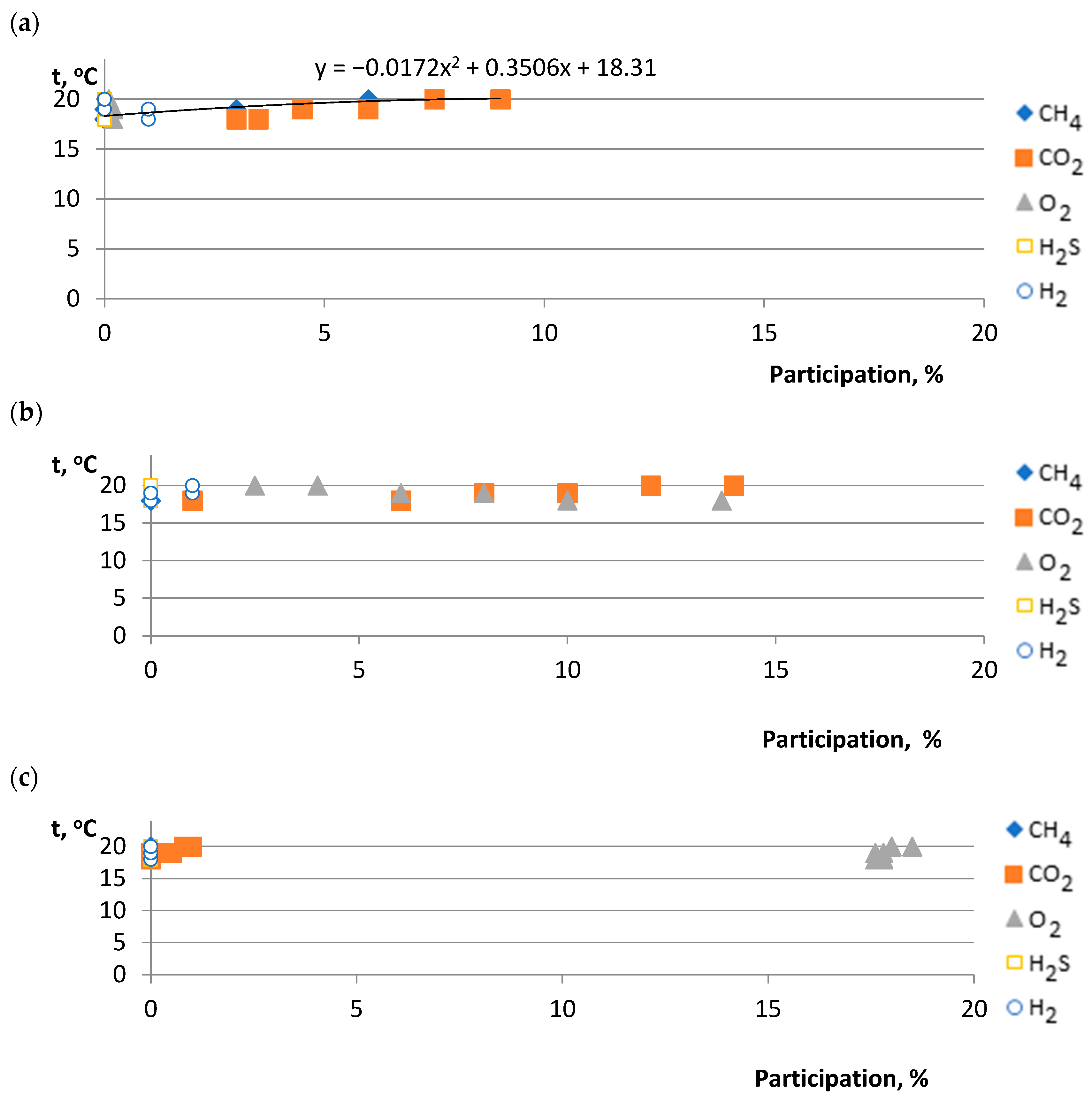
3.2. Assessment of Biogas (Biomethane) Production in the Reactor (Photodigester)
3.3. Technological Trends and Future Prospects for the Biogas Sector
4. Conclusions
- (1)
- It indicated that for 6 months, depending on the deposit and its condition due to gas permeability, the functionality of the microorganisms contained in the compact deposit has a decisive influence.
- (2)
- The elemental composition of the goat manure bed has a significant influence, thus causing inhibition of the process under photofermentation conditions.
- (3)
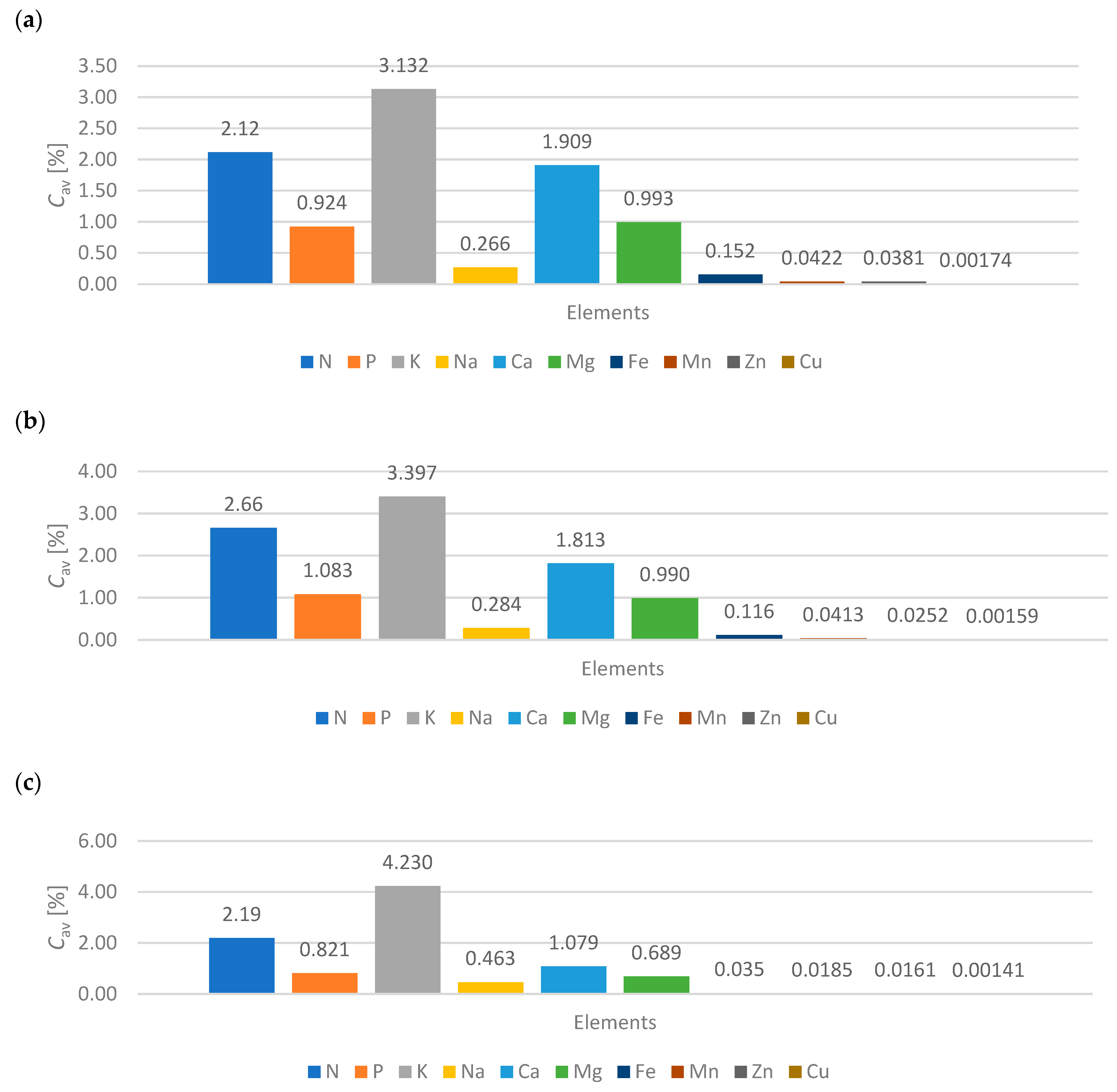
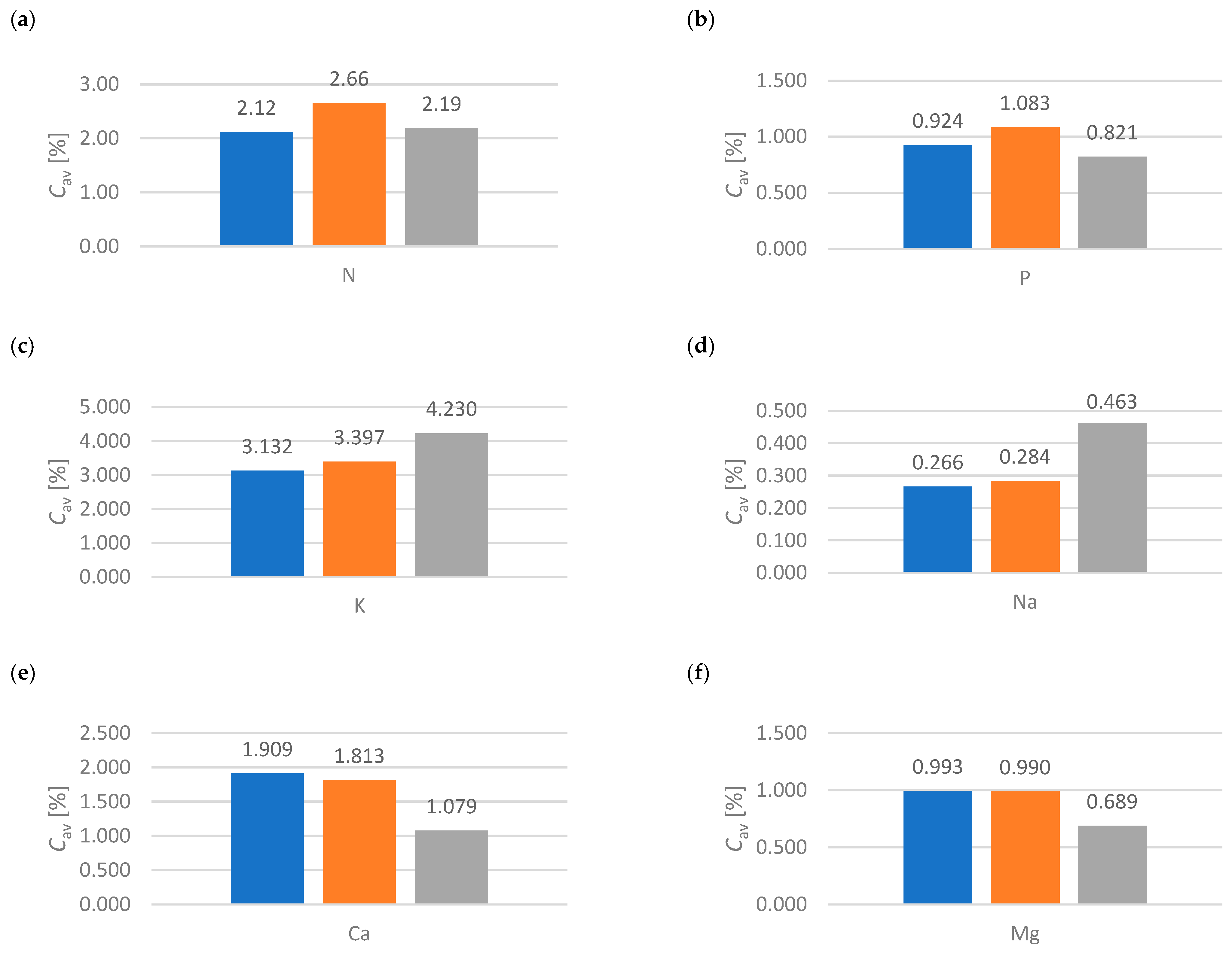
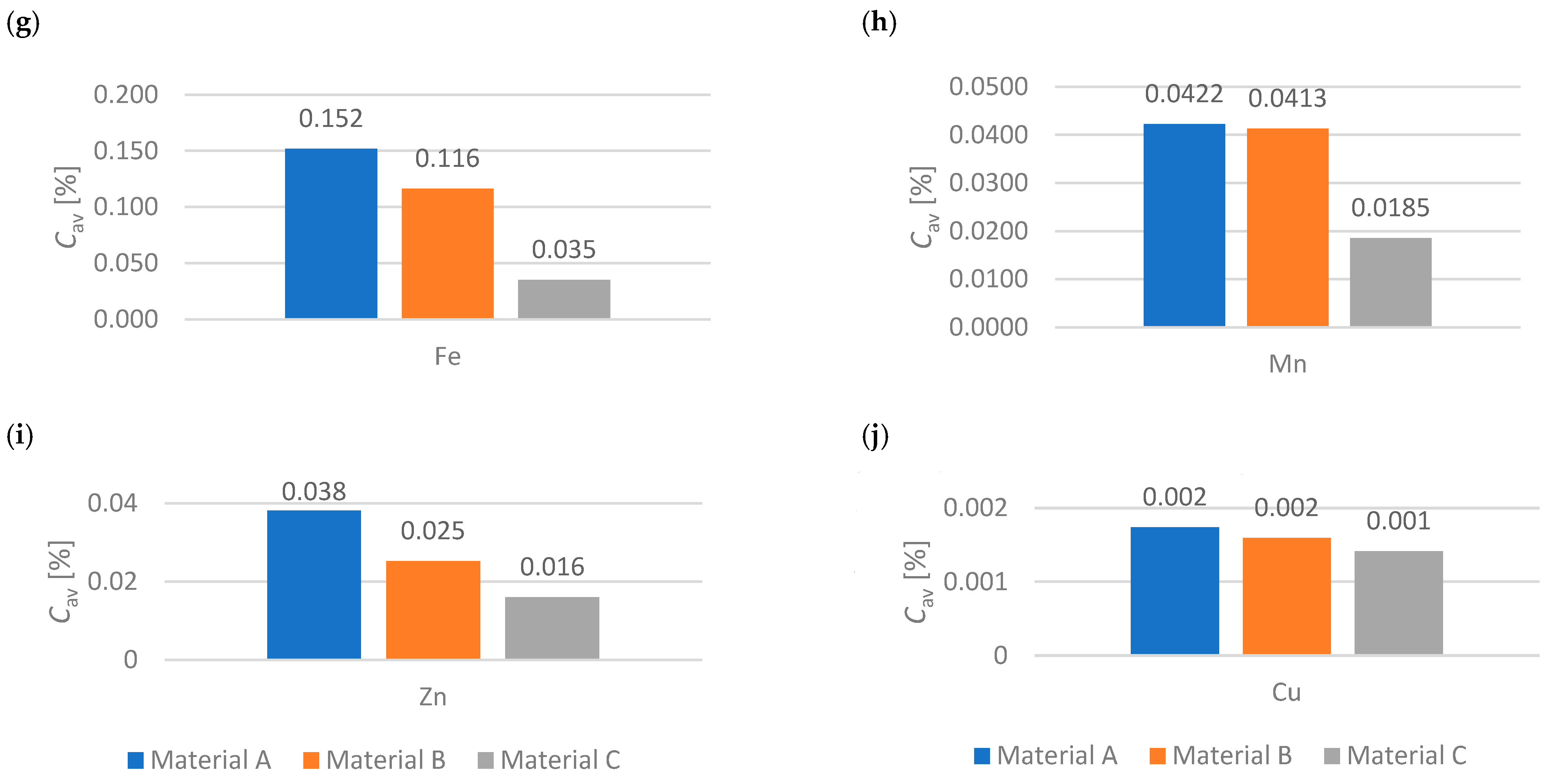
- A scientific curiosity was observed, when comparing goat manure, active group (compact bed), it should be noted that K 3.132%, Na 0.266%, Ca 1.909% and Mg 0.993% are lower values compared to the material with values K 3.397%, Na 0.284%, Ca 1.813% and Mg 0.990% which are higher. This is undoubtedly due to the presence of nutrients in the deposit that support the biomethane production process.
- (4)
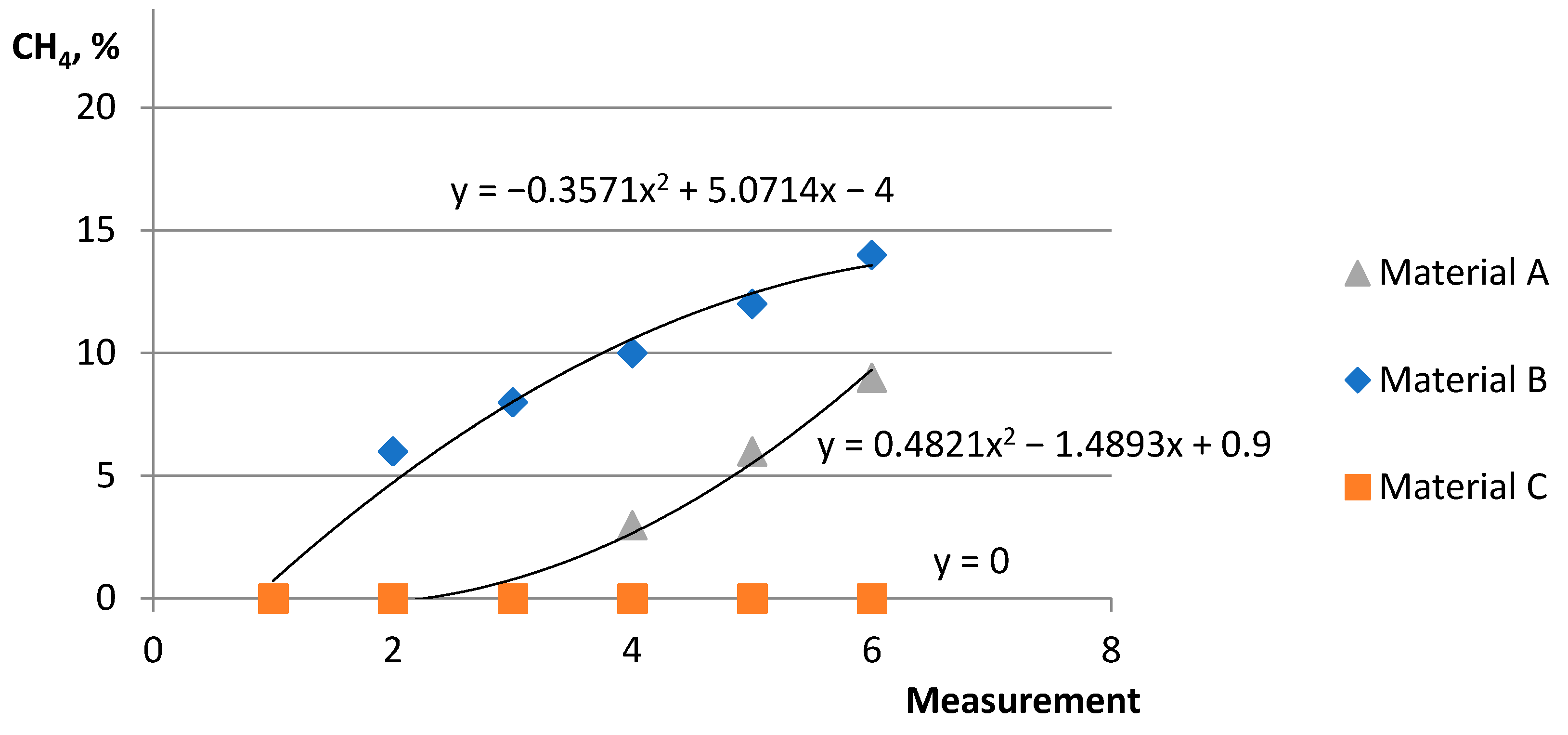
- Active group (compact bed) material A shows a dynamic increase in biomethane production with lower nutrient values. However, material B, having a higher percentage of ingredients, shows a stabilization of biomethane production after the sixth month of the process.
- (5)
- It was indicated that for 6 months (from December to May), depending on the deposit and its condition due to gas permeability, the functionality of the microorganisms contained in the compact deposit has a decisive influence. For goat manure—material A and material B—biomethane production occurs more so than in material C.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wałowski, G. Multi-phase flow assessment for the fermentation process in mono-substrate reactor with skeleton bed. J. Water Land Dev. 2019, 42, 150–156. [Google Scholar] [CrossRef][Green Version]
- Jarosz, Z.; Kapłan, M.; Klimek, K.; Dybek, B.; Herkowiak, M.; Wałowski, G. An Assessment of the Development of a Mobile Agricultural Biogas Plant in the Context of a Cogeneration System. Appl. Sci. 2023, 13, 12447. [Google Scholar] [CrossRef]
- Głodek, E. Obtaining and Using Agricultural Biogas for Energy Purposes; Silesian Institute: Opole, Poland, 2007. [Google Scholar]
- Werle, S. Thermal Processing of Waste Biomass as an Element of the Circular Economy; Silesian University of Technology Publishing House: Gliwice, Poland, 2021. [Google Scholar]
- Arthur, R.; Baidoo, M.F.; Antwi, E. Biogas as a potential renewable energy source: A Ghanaian case study. Renew. Energy 2011, 36, 1510–1516. [Google Scholar] [CrossRef]
- Paterson, M.; Amrozy, M.; Berutto, R.; Bijnagte, J.W.; Bonhomme, S.; Gysen, M.; Kayesr, K.; Majewski, E.; Parola, F. Implementation Guide for Small-Scale Biogas Plants. In Bioenergy Farm II Publication; Kuratorium für Technik und Bauwesen in der Landwirtschaft e. V. (KTBL): Kranichstein, Germay, 2016; Volume 1.2. [Google Scholar]
- Dublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources: An Introduction; WILEY-VCH Verlag GmbH & Co. KgaA: Weinheim, Germay, 2008. [Google Scholar]
- Czerwińska, E.; Kalinowska, K. Conditions of Conducting of Methane Fermentation in Biogas Plant; Technika Rolnicza Ogrodnicza: Leśna, Poland, 2014; pp. 12–14. Available online: https://bibliotekanauki.pl/articles/882912 (accessed on 28 May 2024).
- Wałowski, G. Assessment of polydisperse substrate flow in a fermentor for computational fluid dynamics modelling. J. Water Land Dev. 2022, 1–7. [Google Scholar] [CrossRef]
- Mohrmann, S.; Otter, V. Categorisation of Biogas Plant Operators in Germany with Regards to Their Intention to Use Straw Pellets as Innovative and Sustainable Substrate Alternative. Energies 2023, 16, 5. [Google Scholar] [CrossRef]
- Kim, J.K.; Oh, B.R.; Chun, Y.N.; Kim, S.W. Effects of temperature and hydraulic retention time on anaerobic digestion of food waste. J. Biosci. Bioeng. 2006, 102, 328–332. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Kumaran, P.; Hephzibah, D.; Sivasankari, R.; Saifuddin, N.; Shamsuddin, A.H. A review on industrial scale anaerobic digestion systems deployment in Malaysia: Opportunities and challenges. Renew. Sustain. Energy Rev. 2015, 56, 929–940. [Google Scholar] [CrossRef]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef] [PubMed]
- Czekała, W.; Kaniewski, J. Prospects for the Development of Biogas Plants in the Rural Areas of the Silesian Province; Wydaw. Akademia Słońca Krzysztof Frąszczak: Poznań, Poland, 2015; ISBN 978-83-944103-0-8. [Google Scholar]
- Kallistova, A.Y.; Goel, G.; Nozhevnikova, A.N. Microbial diversity of methanogenic communities in the systems for anaerobic treatment of organic waste. Microbiology 2014, 83, 462–483. [Google Scholar] [CrossRef]
- de Vrieze, J.; Hennebel, T.; Boon, N.; Verstraete, W. Methanosarcina: The rediscovered methanogen for heavy duty biomethanation. Bioresour. Technol. 2012, 112, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Arshad, M.; Anjum, M.; Mahmood, T.; Dawson, L. The anaerobic digestion of solid organic waste. Waste Manag. 2011, 31, 8. [Google Scholar] [CrossRef] [PubMed]
- Puñal, A.; Trevisan, M.; Rozzi, A.; Lema, J.M. Influence of C:N ratio on the start-up of up-flow anaerobic filter reactors. Water Res. 2000, 34, 2614–2619. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Dutta, S.; Sherpa, K.C.; Jayaswal, K.; Saravanabhupathy, S.; Mohanty, K.T.; Banerjee, R.; Kumar, J.; Rajak, R.C. Chapter 7—Co-digestion processes of waste: Status and perspective. In Bio-Based Materials and Waste for Energy Generation and Resource Management; Hussain, C.M., Kushwaha, A., Bharagava, R.N., Goswami, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 207–241. Available online: https://www.sciencedirect.com/science/article/abs/pii/B9780323911498000107 (accessed on 23 May 2024).
- Siddique, M.N.I.; Wahid, Z.A. Achievements and perspectives of anaerobic co-digestion: A review. J. Clean. Prod. 2018, 194, 359–371. [Google Scholar] [CrossRef]
- Kaur, H.; Kommalapati, R.R. Optimizing anaerobic co-digestion of goat manure and cotton gin trash using biochemical methane potential (BMP) test and mathematical modeling. SN Appl. Sci. 2021, 3, 724. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Bhatia, A.; Kubota, K.; Rajpal, A.; Ahmed, B.; Khan, A.A.; Kumar, M. Microbial community dynamics in anaerobic digesters treating organic fraction of municipal solid waste. Environ. Technol. Innov. 2021, 21, 101303. [Google Scholar] [CrossRef]
- Mlaik, N.; Sayadi, S.; Masmoudi, M.A.; Yaacoubi, D.; Loukil, S.; Khoufi, S. Optimization of anaerobic co-digestion of fruit and vegetable waste with animal manure feedstocks using mixture design. Biomass Conv. Bioref. 2024, 14, 4007–4016. [Google Scholar] [CrossRef]
- Bernat, K.; Wojnowska-Baryła, I.; Kasiński, S.; Szatkowski, M. Methods of Preliminary Preparation of Lignocellulosic Biomass for Methane Fermentation. Gas Water Sanit. Technol. 2014, 269–273. Available online: https://www.uwm.edu.pl/cbeo/sites/default/files/u260/metody-wstepnego-przygotowania-biomasy-lignocelulozowej-do-fermentacji-metanowej.pdf (accessed on 23 May 2024).
- Lindner, J.; Zielonka, S.; Oechsner, H.; Lemmer, A. Effects of mechanical treatment of digestate after anaerobic digestion on the degree of degradation. Bioresour. Technol. 2014, 178, 194–200. [Google Scholar] [CrossRef]
- Ruffino, B.; Campo, G.; Genon, G.; Lorenzi, E.; Novarino, D.; Scibilia, G.; Zanetti, M. Improvement of anaerobic digestion of sewage sludge in a wastewater treatment plant by means of mechanical and thermal pre-treatments: Performance, energy and economical assessment. Bioresour. Technol. 2015, 175, 298–308. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zicari, S.M.; Liu, G.Q.; Li, Y.Q.; Zhang, R.H. Pretreatment of wheat straw with potassium hydroxide for increasing enzymatic and microbial degradability. Bioresour. Technol. 2015, 185, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.B.; Chen, T.B.; Gao, D.; Zheng, G.D.; Chen, J.; Pan, T.H.; Liu, H.T.; Gu, R.Y. Simulation of water removal process and optimization of aeration strategy in sewage sludge composting. Bioresour. Technol. 2014, 171, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Reilly, M.; Dinsdale, R.; Guwy, A. Enhanced biomethane potential from wheat straw by low temperature alkaline calcium hydroxide pre-treatment. Bioresour. Technol. 2015, 189, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Angelidaki, I.; Ahring, B.K. Thermophilic anaerobic digestion of livestock waste: The effect of ammonia. Appl. Microbiol. Biotechnol. 1993, 38, 560–564. [Google Scholar] [CrossRef]
- Regulation of the Minister of Agriculture and Rural Development on a Detailed List of Substrates That Can Be Used in Agricultural Biogas Plants; Journal Laws of the Republic of Poland of October 17, 2023, item 2230; Minister of Agriculture and Rural Development: Poland, Warszawa, 2023.
- Wałowski, G. Development of biogas and biorafinery systems in Polish rural communities. J. Water Land Dev. 2021, 49, 156–168. [Google Scholar] [CrossRef]
- Rabii, A.; El Sayed, A.; Ismail, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. Optimizing the Mixing Ratios of Source-Separated Organic Waste and Thickened Waste Activated Sludge in Anaerobic Co-Digestion: A New Approach. Processes 2024, 12, 794. [Google Scholar] [CrossRef]
- Otobrise, C.; Udubor, C.W.; Osabohien, E. Kinetics of Biogas Production from Goat Dung and Pawpaw Seed. Orient. J. Chem. 2022, 38, 914–923. [Google Scholar] [CrossRef]
- Konkol, I.; Świerczek, L.; Cenian, A. Chicken Manure Pretreatment for Enhancing Biogas and Methane Production. Energies 2023, 16, 5442. [Google Scholar] [CrossRef]
- PN-EN ISO 17034:2017-03; General Requirements for the Competence of Producers of Reference Materials. Polish Center for Accreditation: Warszawa, Poland, 2017.
- Lądkiewicz, K.; Wszędyrówny-Nast, M.; Jaśkiewicz, K. Comparison of various methods for determining the content of organic substances. Sci. Rev.—Environ. Eng. Manag. 2017, 26, 99–107. [Google Scholar]
- Huang, P.T.; Patel, M.; Santagata, M.C.; Bobet, A. Classification of Organic Soil; Final Report, FHWA/IN/JTRP-2008/2. Project No. C-36-36TT; Joint Transportation Research Program, Indiana Department of Transporation and Purdue University: West Lafayette, IN, USA, 2009. [Google Scholar]
- Journal Laws of the Republic of Poland of February 14, 2020, Item 243, Regulation of the Council of Ministers on the Adoption of the Action Program Aimed at Reducing Water Pollution with Nitrates from Agricultural Sources and Preventing Further Pollution. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20200000243/O/D20200243.pdf (accessed on 28 May 2024).
- A Guide to Managing Barn Manure on Dairy Goat Farms. Dairy Goat Co-Operative 2010. Available online: https://www.waikatoregion.govt.nz/assets/WRC/managing-barn-manure-on-dairy-goat-farms.pdf (accessed on 28 May 2024).
- Hermans, T.; Jeurissen, L.; Hackert, V.; Hoebe, C. Land-Applied Goat Manure as a Source of Human Q-Fever in the Netherlands, 2006–2010. PLoS ONE 2014, 9, e96607. [Google Scholar] [CrossRef]
- Arricau-Bouvery, N.; Rodolakis, A. Is Q fever an emerging or re-emerging zoonosis? Vet. Res. 2005, 36, 327–349. [Google Scholar] [CrossRef] [PubMed]
- van den Brom, R.; Roest, H.-J.; de Bruin, A.; Dercksen, D.; Santman-Berends, I.; van der Hoek, W. A Probably Minor Role for Land-Applied Goat Manure in the Transmission of Coxiella burnetii to Humans in the 2007–2010 Dutch Q Fever Outbreak. PLoS ONE 2015, 10, e0121355. [Google Scholar] [CrossRef]
- Lung, A.J.; Lin, C.M.; Kim, J.M.; Marshall, M.R.; Nordstedt, R.; Thompson, N.P.; Wei, C.I. Destruction of Escherichia coli O157:H7 and Salmonella enteritidis in cow manure composting. J. Food. Prot. 2001, 64, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Stefaniuk, M.; Bartmiński, P.; Różyło, K.; Dębicki, R.; Oleszczuk, P. Ecotoxicological assessment of residues from different biogas production plants used as fertilizer for soil. J. Hazard. Mater. 2015, 298, 195–202. [Google Scholar] [CrossRef]
- Viaene, J.; Agneessens, L.; Capito, C.; Ameloot, N.; Reubens, B.; Willekens, K.; Vandecasteele, B.; De Neve, S. Co-ensiling, co-composting and anaerobic co-digestion of vegetable crop residues: Product stability and effect on soil carbon and nitrogen dynamics. Sci. Hortic. 2017, 220, 214–225. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, C.; Ferrer-Costa, A.; Carrasco, L.; Cegarra, J.; Abad, M.; Bernal, M.P. Assessment of the fertiliser potential of digestates from farm and agroindustrial residues. Biomass Bioenergy 2012, 40, 181–189. [Google Scholar] [CrossRef]
- Elgarahy, A.M.; Eloffy, M.G.; Priya, A.K.; Yogeshwaran, V.; Yang, Z.; Elwakeel, K.Z.; Lopez-Maldonado, E.A. Biosolids management and utilizations: A review. J. Clean. Prod. 2024, 451, 141974. [Google Scholar] [CrossRef]
- Nag, R.; Auer, A.; Markey, B.K.; Whyte, P.; Nolan, S.; O’Flaherty, V.; Russell, L.; Bolton, D.; Fenton, O.; Richards, K.; et al. Anaerobic digestion of agricultural manure and biomass—Critical indicators of risk and knowledge gaps. Sci. Total. Environ. 2019, 690, 460–479. [Google Scholar] [CrossRef]
- van Midden, C.; Harris, J.; Shaw, L.; Sizmur, T.; Pawlett, M. The impact of anaerobic digestate on soil life: A review. Appl. Soil Ecol. 2023, 191, 105066. [Google Scholar] [CrossRef]
- ISO 11465:1994; Soil Quality—Determination of Dry Matter and Water Content on a Mass Basis—Gravimetric Method. Technical Corrigendum 1, Edition 1. 1994. Available online: https://www.iso.org/standard/23695.html (accessed on 23 May 2024).
- PB/31/12:2014; Mineralization of Plant Samples and Natural Fertilizers in Concentrated Mineral Acids into General Components (Macro- and Microelements). The Research Procedure of the Environmental Chemistry Research Laboratory Falenty, 05-090 Raszyn, Poland Consists in Adopting Routine Procedures, Appropriate to the Working Conditions in the Outdoor Laboratory, with Expected Characteristics (Composition), Applicable to Specific Determinations, within a Specified Concentration Range. Available online: https://www.itp.edu.pl/bip/index.html (accessed on 23 May 2024).
- PB/31/14:2014; Mineralization of Plant Samples and Natural Fertilizers in Concentrated Mineral Acids into General Components (Macro- and Microelements). The Research Procedure of the Environmental Chemistry Research Laboratory Falenty, 05-090 Raszyn, Poland Consists in Adopting Routine Procedures, Appropriate to the Working Conditions in the Outdoor Laboratory, with Expected Characteristics (Composition), Applicable to Specific Determinations, within a Specified Concentration Range. Available online: https://www.itp.edu.pl/bip/index.html (accessed on 23 May 2024).
- PN-91/R-04014; Mineralization of Plant Samples and Natural Fertilizers in Concentrated Mineral Acids into General Components (Macro- and Microelements). The Research Procedure of the Environmental Chemistry Research Laboratory Falenty, 05-090 Raszyn, Poland Consists in Adopting Routine Procedures, Appropriate to the Working Conditions in the Outdoor Laboratory, with Expected Characteristics (Composition), Applicable to Specific Determinations, within a Specified Concentration Range. Available online: https://www.itp.edu.pl/bip/index.html (accessed on 23 May 2024).
- PB/31/09:2014; Mineralization of Vegetation Samples and Natural Fertilizers in Concentrated Mineral Acids for Nitrogen. The Research Procedure of the Environmental Chemistry Research Laboratory Falenty, 05-090 Raszyn, Poland Consists in Adopting Routine Procedures, Appropriate to the Working Conditions in the Outdoor Laboratory, with Expected Characteristics (Composition), Applicable to Specific Determinations, within a Specified Concentration Range. Available online: https://www.itp.edu.pl/bip/index.html (accessed on 23 May 2024).
- Determination of Phosphorus and Nitrogen—According to the Skalar Methodology. The Research Procedure of the Environmental Chemistry Research Laboratory Falenty, 05-090 Raszyn, Poland Consists in Adopting Routine Procedures, Appropriate to the Working Conditions in the Outdoor Laboratory, with Expected Characteristics (Composition), Applicable to Specific Determinations, within a Specified Concentration Range. Available online: https://www.itp.edu.pl/bip/index.html (accessed on 23 May 2024).
- PN-ISO 9964-1:1994; Water Quality—Determination of Sodium and Potassium—Determination of Sodium by Atomic Absorption Spectrometry. Available online: https://katalog.ubb.edu.pl/integro/572300185072/normy/pn-iso-9964-11994-jakosc-wody-oznaczanie-sodu-i-potasu-oznaczanie-sodu-metoda-absorpcyjnej?internalNav=1&bibFilter=57 (accessed on 23 May 2024).
- PN-ISO 9964-2/AK:1997; Water Quality—Determination of Sodium and Potassium—Determination of Potassium in Wastewater by Atomic Absorption Spectrometry (Country Sheet). Available online: https://katalog.ubb.edu.pl/integro/572300185076/normy/pn-iso-9964-2ak1997-jakosc-wody-oznaczanie-sodu-i-potasu-oznaczanie-potasu-w-sciekach-metoda?internalNav=1&bibFilter=57 (accessed on 23 May 2024).
- PN-ISO 8288:2002; Water Quality—Determination of Cobalt, Nickel, Copper, Zinc, Cadmium and Lead—Atomic Absorption Spectrometry Methods with Flame Atomization. Available online: https://bibliotekanauki.pl/articles/1366456 (accessed on 23 May 2024).
- Barałkiewicz, D.; Bulska, E. Chemical Speciation—Problems and Possibilities; Malamut Publishing House: Warsaw, Poland, 2009. [Google Scholar]
- European Union. Study on Variation of Manure N Efficiency throughout Europe. Final Report. Available online: https://op.europa.eu/en/publication-detail/-/publication/0df506b1-c2df-4561-98e3-7f86a82a35e8 (accessed on 23 May 2024).
- Thompson, L.M.; Troeh, F.R. Soil and Its Fertility; State Agricultural and Forestry Publishing House: Warsaw, Poland, 1978. [Google Scholar]
- Zhang, H. Managing Phosphorus from Animal Manure. Available online: https://extension.okstate.edu/fact-sheets/managing-phosphorus-from-animal-manure.html (accessed on 23 May 2024).
- Lityński, T.; Jurkowska, H. Soil Fertility and Plant Nutrition; National Scientific Publishing House: Warsaw, Poland, 1982. [Google Scholar]
- Szmeja, J. A Guide to Aquatic Vegetation Research; Publishing House of the University of Gdansk: Gdansk, Poland, 2006. [Google Scholar]
- Greenwood, N.N.; Earnshaw, A. Chemistry of the Elements, 2nd ed.; Butterworth-Heinemann: London, UK, 1997. [Google Scholar]
- Ochoa-Hueso, R.; Delgado-Baquerizo, M.; King, P.; Benham, M.; Arca, V.; Power, S.A. Ecosystem type and resource quality are more important than global change drivers in regulating early stages of litter decomposition. Soil Biol. Biochem. 2019, 129, 144. [Google Scholar] [CrossRef]
- Kering, M.K. Manganese Nutrition and Photosynthesis in NAD-Malic Enzyme C4 Plants. Ph.D. Thesis, University of Missouri-Columbia, Columbia, MO, USA, 2008. [Google Scholar]
- Subbarao, G.V.; Ito, O.; Berry, W.L.; Wheeler, R.M. Sodium—A Functional Plant Nutrient. Crit. Rev. Plant Sci. 2003, 22, 391. [Google Scholar]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.fertilizantesyabonos.com/english/agricultural-calcium (accessed on 23 May 2024).
- Available online: https://icl-growingsolutions.com/agriculture/categories/calcium-based-fertilizers (accessed on 23 May 2024).
- Szweykowska, A. Plant Physiology; AMU Scientific Publishing House: Poznan, Poland, 1997. [Google Scholar]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L.; Clarke, N.D.; Szweykowska-Kulińska, Z.; Jarmołowski, A.; Augustyniak, H. Biochemistry; PWN Scientific Publishing House: Warsaw, Poland, 2007. [Google Scholar]
- Yunta, F.; García-Marco, S.; Lucena, J.J.; Gómez-Gallego, M.; Alcázar, R.; Sierra, M.A. Chelating agents related to ethylenediamine bis(2-hydroxyphenyl)acetic acid (EDDHA): Synthesis, characterization, and equilibrium studies of the free ligands and their Mg2+, Ca2+, Cu2+, and Fe3+ chelates. Inorg. Chem. 2003, 42, 5412. [Google Scholar] [CrossRef] [PubMed]
- Yunta, F.; García-Marco, S.; Lucena, J.J. Theoretical speciation of Ethylenediamine-N-(o-hydroxyphenylacetic)-N’-(p-hydroxyphenylacetic) acid (o,p-EDDHA) in agronomic conditions. J. Agric. Food Chem. 2003, 51, 5391. [Google Scholar] [CrossRef]
- Available online: https://www.pthorticulture.com/en/training-center/role-of-manganese-in-plant-culture (accessed on 23 May 2024).
- Dau, H.; Haumann, M. Eight steps preceding O–O bond formation in oxygenic photosynthesis—A basic reaction cycle of the Photosystem II manganese complex. Biochim. Et Biophys. Acta 2007, 1767, 472. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Zinc in Soils and Crop Nutrition, 2nd ed.; International Fertilizer Industry Association: Paris, France; International Zinc Association: Brussels, Belgium, 2008. [Google Scholar]
- Broadley, M.R.; White, P.J.; Hammond, J.P.; Zelko, I.; Lux, A. Zinc in plants. New Phytol. 2007, 173, 677. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://herograespeciales.com/en/copper-and-its-importance-in-agriculture (accessed on 23 May 2024).
- Available online: https://kochagronomicservices.com/knowledge-center/the-key-role-of-copper-in-crops (accessed on 23 May 2024).
- Haque, A.; Kabir, A.; Hashem, A.; Azad, A.K.; Kamruj, M.; Bhuyian, J.; Rahmann, M. Efficacy of biogas production from different types of livestock manures. Int. J. Smart Grid 2021, 5, 158–166. [Google Scholar]
- Hanafiah, M.M.; Mohamed Ali, M.Y.; Abdul Aziz, N.I.H.; Ashraf, M.A.; Halim, A.A.; Lee, K.E.; Idris, M. Biogas production from goat and chicken manure in Malaysia. Appl. Ecol. Environ. Res. 2016, 15, 529–535. [Google Scholar] [CrossRef]
- Opurum, C.C.; Nweke, C.O.; Nwanyanwu, C.E.; Nwachukwu, I.N. Kinetic study oa anaerobic digestion of goat manure with poultry dropping and plantain peels for biogas production. Int. J. Eng. Apllied Sci. 2019, 6, 22–28. [Google Scholar]
- Grimsby, L.K.; Gulbrandsen, L.; Eik, L.O.; Msalya, G.; Kifaro, G.C. The prospect of biogas among small-holder dairy goat farmers in the Uluguru Montains, Tanzania, African. J. Food Agric. Nutr. Dev. 2016, 16, 10723–10737. [Google Scholar]
- Jorgensen, P.J. Biogas-Green Energy. 2009. Available online: http://lemvigbiogas.com/BiogasPjjuk.pdf (accessed on 23 May 2024).
- Osman, G.A.M.; Elhasan, H.E.; Hassan, A.B. Effect of cow rumen fluid concentration on biogas production from goat manure. Sudan. J. Agric. Sci. 2015, 2, 1–7. [Google Scholar]
- Harjinder, K.; Kommalapati, R.R. Biochemical methane potential and kinetic parameters of goat manure at various inoculum to substrate ratios. Sustainability 2021, 13, 12806. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, L.; Song, Z.; Ren, G.; Feng, Y.; Han, X.; Yang, G. Biogas production by co-digestion of goat manure with three crop residues. PLoS ONE 2013, 8, e66845. [Google Scholar] [CrossRef] [PubMed]
- Nikosi, S.M.; Lupuleza, I.; Sithole, S.N.; Zelda, Z.R.; Matheri, A.N. Renewable energy potential of anaerobic mono- and co-digestion of chicken manure, goat manure, potato peels and maize pap in South Africa. S. Afr. J. Sci. 2021, 117, 10362. [Google Scholar] [CrossRef] [PubMed]
- Alham, N.A.; Prabowo, B.D.; Siregar, I.R.S.; Margono, W.C.; Azhari, U.F.; Larasati, T.D. Utilization of goat manure towards PTLB (biogas) prototypes in Simple way. In Proceedings of the 5th International Confefence for Tropic Studies and Its Applications, Universitas Mulawarman, Kota Samarinda, Indonesia, 5–6 October 2021. [Google Scholar]
- Mohamed, H.H.; Morsy, M.I. Study the effect of dry fermentation of goat manure in optimization of biogas production and minimization of costs. Misr J. Agric. Eng. 2018, 35, 1149–1164. [Google Scholar] [CrossRef]
- Jarosz, Z.; Kapłan, M.; Klimek, K.; Anders, D.; Dybek, B.; Herkowiak, M.; Hołaj-Krzak, J.T.; Syrotyuk, S.; Korobka, S.; Syrotyuk, H.; et al. Evaluation of Biohydrogen Production Depending on the Substrate Used—Examples for the Development of Green Energy. Energies 2024, 17, 2524. [Google Scholar] [CrossRef]
- Moritz, M. Biological methods of obtaining hydrogen. Chemist 2012, 66, 827–834. [Google Scholar]
- Gest, H.; Kamen, M.D. Photoproduction of molecular hydrogen by Rhodospirillum rubrum. Science 1949, 109, 558. [Google Scholar] [CrossRef] [PubMed]
- Eroğlu, E.; Eroğlu, İ.; Gündüz, U.; Türker, L.; Yücel, M. Biological hydrogen production from olive mill wastewater with twostage processes. Int. J. Hydrogen Energy 2006, 31, 1527. [Google Scholar] [CrossRef]
- Urbaniec, K.; Grabarczyk, R. Directions of Research on Biological Methods of Obtaining Hydrogen as an Energy Carrier. In Inżynieria Systemów Bioagrotechnicznych 5(14); Powierża, L., Ed.; Department of Systems Engineering, Warsaw University of Technology in Płock: Plock, Poland, 2005; pp. 209–214. ISBN 83-915395-3-9. [Google Scholar]
- Renewables 2016 Global Status Report; Paris: REN21 Secretariat. Available online: https://www.ren21.net/gsr-2016/ (accessed on 12 January 2022).
- United Nations Development Programme. Available online: https://www.undp.org/sustainable-development-goals (accessed on 23 January 2022).
- Petersson, A.; Wellinger, A. Biogas Upgrading Technologies–Developments and Innovations. Task 37: Energy from Biogas and Lanfill Gas. Paris, France. 2009. Available online: http://groengas.nl/wp-content/uploads/2013/07/2009-10-00-Biogas-upgrading-technologies-developments-and-innovations.pdf (accessed on 23 May 2024).
- Zhang, X.; Xiong, W.; Peng, L.; Wu, Y.; Hu, X. Highly Selective Absorption Separation of H2S and CO2 from CH4 by Novel Azole-Based Protic Ionic Liquids. AIChE J. 2020, 66, e16936. [Google Scholar] [CrossRef]
- Wang, L.; Xu, Y.; Li, Z.; Wei, Y.; Wei, J. CO2/CH4 and H2S/CO2 Selectivity by Ionic Liquids in Natural Gas Sweetening. Energy Fuels 2018, 32, 10–23. [Google Scholar] [CrossRef]
- Akhmetshina, A.I.; Petukhov, A.N.; Gumerova, O.R.; Vorotyntsev, A.V.; Nyuchev, A.V.; Vorotyntsev, I.V. Solubility of H2S and CO2 in Imidazolium-Based Ionic Liquids with Bis(2-Ethylhexyl) Sulfosuccinate Anion. J. Chem. Thermodyn. 2019, 130, 173–182. [Google Scholar] [CrossRef]
- Choi, W.-J.; Cho, K.-C.; Lee, S.-S.; Shim, J.-G.; Hwang, H.-R.; Park, S.-W.; Oh, K.-J. Removal of Carbon Dioxide by Absorption into Blended Amines: Kinetics of Absorption into Aqueous AMP/HMDA, AMP/MDEA, and AMP/Piperazine Solutions. Green Chem. 2007, 9, 594–598. [Google Scholar] [CrossRef]
- Lin, P.-H.; Wong, D.S.H. Carbon Dioxide Capture and Regeneration with Amine/Alcohol/Water Blends. Int. J. Greenh. Gas Control 2014, 26, 69–75. [Google Scholar] [CrossRef]
- Guo, R.; Zhu, C.; Yin, Y.; Fu, T.; Ma, Y. Mass Transfer Characteristics of CO2 Absorption into 2-Amino-2-Methyl-1-Propanol Non-Aqueous Solution in a Microchannel. J. Ind. Eng. Chem. 2019, 75, 194–201. [Google Scholar] [CrossRef]
- Gómez-Díaz, D.; López, M.; Navaza, J.M.; Villarquide, L. Influence of Organic Co-Solvents upon Carbon Dioxide Chemical Absorption. J. Taiwan Inst. Chem. Eng. 2018, 91, 413–419. [Google Scholar] [CrossRef]
- Xu, H.-J.; Zhang, C.-F.; Zheng, Z.-S. Solubility of Hydrogen Sulfide and Carbon Dioxide in a Solution of Methyldiethanolamine Mixed with Ethylene Glycol. Ind. Eng. Chem. Res. 2002, 41, 6175–6180. [Google Scholar] [CrossRef]
- Heldebrant, D.J.; Yonker, C.R.; Jessop, P.G.; Phan, L. Organic Liquid CO2 Capture Agents with High Gravimetric CO2 Capacity. Energy Environ. Sci. 2008, 1, 487–493. [Google Scholar] [CrossRef]
- Barbarossa, V.; Barzagli, F.; Mani, F.; Lai, S.; Stoppioni, P.; Vanga, G. Efficient CO2 Capture by Non-Aqueous 2-Amino-2-Methyl-1-Propanol (AMP) and Low Temperature Solvent Regeneration. RSC Adv. 2013, 3, 12349–12355. [Google Scholar] [CrossRef]
- Théobald, O. Task 37—France Country Report 2019; IEA Bionergy: Paris, France, 2019; pp. 31–32. Available online: http://task37.ieabioenergy.com/ (accessed on 12 December 2021).
- Lukehurst, C.; Banks, C. Task 37—United Kingdom Country Report 2019; IEA Bionergy: London, UK, 2019; pp. 62–71. Available online: http://task37.ieabioenergy.com/ (accessed on 12 December 2021).
- Baier, U. Task 37—Switzerland Country Report 2019; IEA Bioenergy: Geneva, Switzerland, 2019; pp. 55–58. Available online: http://task37.ieabioenergy.com/ (accessed on 12 December 2021).
- Grande, C.A.; Morence, D.G.B.; Bouzga, A.M.; Andreassen, K.A. Silica Gel as a Selective Adsorbent for Biogas Drying and Upgrading. Ind. Eng. Chem. Res. 2020, 59, 10142–10149. [Google Scholar] [CrossRef]
- Abd, A.A.; Othman, M.R.; Helwani, Z. Unveiling the Critical Role of Biogas Compositions on Carbon Dioxide Separation in Biogas Upgrading Using Pressure Swing Adsorption. Biomass Convers. Biorefinery 2022, 13, 13827–13840. [Google Scholar] [CrossRef]
- Guan, Z.; Wang, Y.; Yu, X.; Shen, Y.; He, D.; Tang, Z.; Li, W.; Zhang, D. Simulation and Analysis of Dual-Reflux Pressure Swing Adsorption Using Silica Gel for Blue Coal Gas Initial Separation. Int. J. Hydrogen Energy 2020, 46, 683–696. [Google Scholar] [CrossRef]
- Chen, X.Y.; Vinh-Thang, H.; Ramirez, A.A.; Rodrigue, D.; Kaliaguine, S. Membrane Gas Separation Technologies for Biogas Upgrading. RSC Adv. 2015, 5, 24399–24448. [Google Scholar] [CrossRef]
- Yang, Y.; Li, A.; Wen, C. Optimization of Static Vanes in a Supersonic Separator for Gas Purification. Fuel Process. Technol. 2016, 156, 265–270. [Google Scholar] [CrossRef]
- Wen, C.; Karvounis, N.; Walther, J.H.; Yan, Y.; Feng, Y.; Yang, Y. An Efficient Approach to Separate CO2 Using Supersonic Flows for Carbon Capture and Storage. Appl. Energy 2019, 238, 311–319. [Google Scholar] [CrossRef]
- Rajaee Shooshtari, S.H.; Shahsavand, A. Reliable Prediction of Condensation Rates for Purification of Natural Gas via Supersonic Separators. Sep. Purif. Technol. 2013, 116, 458–470. [Google Scholar] [CrossRef]

| Substrats | C:N |
|---|---|
| Cow dung | 16–25 |
| Pig manure | 6–14 |
| Slaughterhouse waste | 22–37 |
| Fallen leaves | 50–53 |
| Algae | 75–100 |
| Poultry manure | 5–15 |
| Sheep dung | 30–33 |
| Goat manure | 10–17 |
| Wheat straw | 50–150 |
| Corn stalks/straw | 50–56 |
| Sugar beet/sugar foliage | 35–40 |
| Fruits and vegetable waste | 7–35 |
| Reference | Inhibitor | Concentration mg∙(dm3)−1 | |||
|---|---|---|---|---|---|
| [8] [31] | Ammonia | From 4000 From 1500 | |||
| Stimulation | Uninfluenced | Inhibition at Ph 7.4–7.6 | Toxic | ||
| [31] | Ammonium nitrogen: | 500–2000 | 200–1000 | 1500–3000 | >3000 |
| [8] | Hydrogen sulfide | From 50 | - | - | - |
| [31] | Sulfur | From 50 | 100 | 160 | 1000 |
| [31] | Heavy metals: | In free ionic form | In carbonate form | ||
| Ni | From 10 | - | |||
| Cu | From 40 | From 170 | |||
| Cr | From 130 | From 530 | |||
| Pb | From 340 | - | |||
| Zn | From 400 | From 160 | |||
| Cd | - | From 180 | |||
| Fe | - | From 1750 | |||
| [31] | Sodium | 6000–30,000 | |||
| Potassium | From 3000 | ||||
| Calcium | From 2800 | ||||
| Magnesium | From 2400 | ||||
| Fatty acids | Isobutyric acid: inhibitory effect from 50 | ||||
| Parameter | Goat Manure | Inoculum |
|---|---|---|
| Proximate analysis | ||
| Moisture [%] | 37.7 ± 0.3 | 97.2 ± 0.3 |
| TS [%] | 62.3 ± 0.3 | 2.8 ± 0.3 |
| VS [%] | 52.8 ± 0.4 | 1.5 ± 0 |
| VS [%-TS] | 84.7 ± 0.2 | |
| Ash [%] | 10.0 ± 0 | 1.5 ± 0.4 |
| Ultimate analysis | ||
| N [%-TS] | 2.8 ± 0.1 | |
| C [%-TS] | 43.9 ± 0.3 | |
| H [%-TS] | 1.5 ± 0.2 | |
| O [%-TS] | 51.3 ± 0.2 | |
| S [%-TS] | 0.6 ± 0 | |
| C:N | 15.7 ± 0.7 | |
| Elemental formula | C365.9H123.0O320.4N20.2S1.9 | |
| Compositional analysis | ||
| Cellulose + Hemi-cellulose [%-TS] | 72.4 | |
| Lignin [%-TS] | 17.6 | |
| Chemical properties | ||
| pH | 7.9 ± 0.1 | 7.5 ± 0.2 |
| VFA [mg/L] | 539.5 ± 75.7 | |
| Alkalinity [CaCO3 mg/L] | 3965.0 ± 120.2 | |
| NO3−–N [mg/L] | 12.7 ± 1.1 | |
| NH4+–N [mg/L] | 398.0 ± 9.9 | |
| PO4- [mg/L] | 1230 ± 0.4 | |
| Total N [mg/L] | 429.5 ± 78.5 | |
| NO3− + NO2− [mg/L] | 14.2 ± 0.1 | |
| TKN [mg/L] | 415 ± 77.8 | |
| Elemental Formula | C365.9H123.0O320.4N20.2S1.9 |
|---|---|
| C:N | 15.7 ± 0.7 |
| Theoretical biomethane potential [mL/gvs] * | 290.0 |
| Experimental biomethane potential | 274.1 ± 7.8 * |
| Biodegradability | 94.5 ± 2.7 * |
| Technological Group | Deep Litter Housing System | |
|---|---|---|
| Production [t∙year−1] | Nitrogen Content [kg N∙t−1] | |
| Mother goats | 1.2 | 8.4 |
| Goat kids up to 3.5 months old | 0.4 | 9.4 |
| Goat kids over 3.5 months old up to 1.5 year old | 0.8 | 6.9 |
| Others | 1.0 | 8.0 |
| Storage Time [Months] | N | P | K | S | Dry Matter [%] |
|---|---|---|---|---|---|
| [kg∙t−1 sm] | |||||
| 0 to 1 | 11.8 | 2.5 | 9.1 | 5.8 | 34 |
| 1 to 6 | 8.2 | 2.7 | 8.8 | 1.5 | 33 |
| 6 to 12 | 5 | 2.2 | 8.2 | 6.6 | 37 |
| >12 | 5.4 | 2.4 | 4 | 2.8 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hołaj-Krzak, J.T.; Konieczna, A.; Borek, K.; Gryszkiewicz-Zalega, D.; Sitko, E.; Urbaniak, M.; Dybek, B.; Anders, D.; Szymenderski, J.; Koniuszy, A.; et al. Goat Manure Potential as a Substrate for Biomethane Production—An Experiment for Photofermentation. Energies 2024, 17, 3967. https://doi.org/10.3390/en17163967
Hołaj-Krzak JT, Konieczna A, Borek K, Gryszkiewicz-Zalega D, Sitko E, Urbaniak M, Dybek B, Anders D, Szymenderski J, Koniuszy A, et al. Goat Manure Potential as a Substrate for Biomethane Production—An Experiment for Photofermentation. Energies. 2024; 17(16):3967. https://doi.org/10.3390/en17163967
Chicago/Turabian StyleHołaj-Krzak, Jakub T., Anita Konieczna, Kinga Borek, Dorota Gryszkiewicz-Zalega, Ewa Sitko, Marek Urbaniak, Barbara Dybek, Dorota Anders, Jan Szymenderski, Adam Koniuszy, and et al. 2024. "Goat Manure Potential as a Substrate for Biomethane Production—An Experiment for Photofermentation" Energies 17, no. 16: 3967. https://doi.org/10.3390/en17163967
APA StyleHołaj-Krzak, J. T., Konieczna, A., Borek, K., Gryszkiewicz-Zalega, D., Sitko, E., Urbaniak, M., Dybek, B., Anders, D., Szymenderski, J., Koniuszy, A., & Wałowski, G. (2024). Goat Manure Potential as a Substrate for Biomethane Production—An Experiment for Photofermentation. Energies, 17(16), 3967. https://doi.org/10.3390/en17163967









