A Review of Non-Destructive Testing for Lithium Batteries
Abstract
:1. Introduction
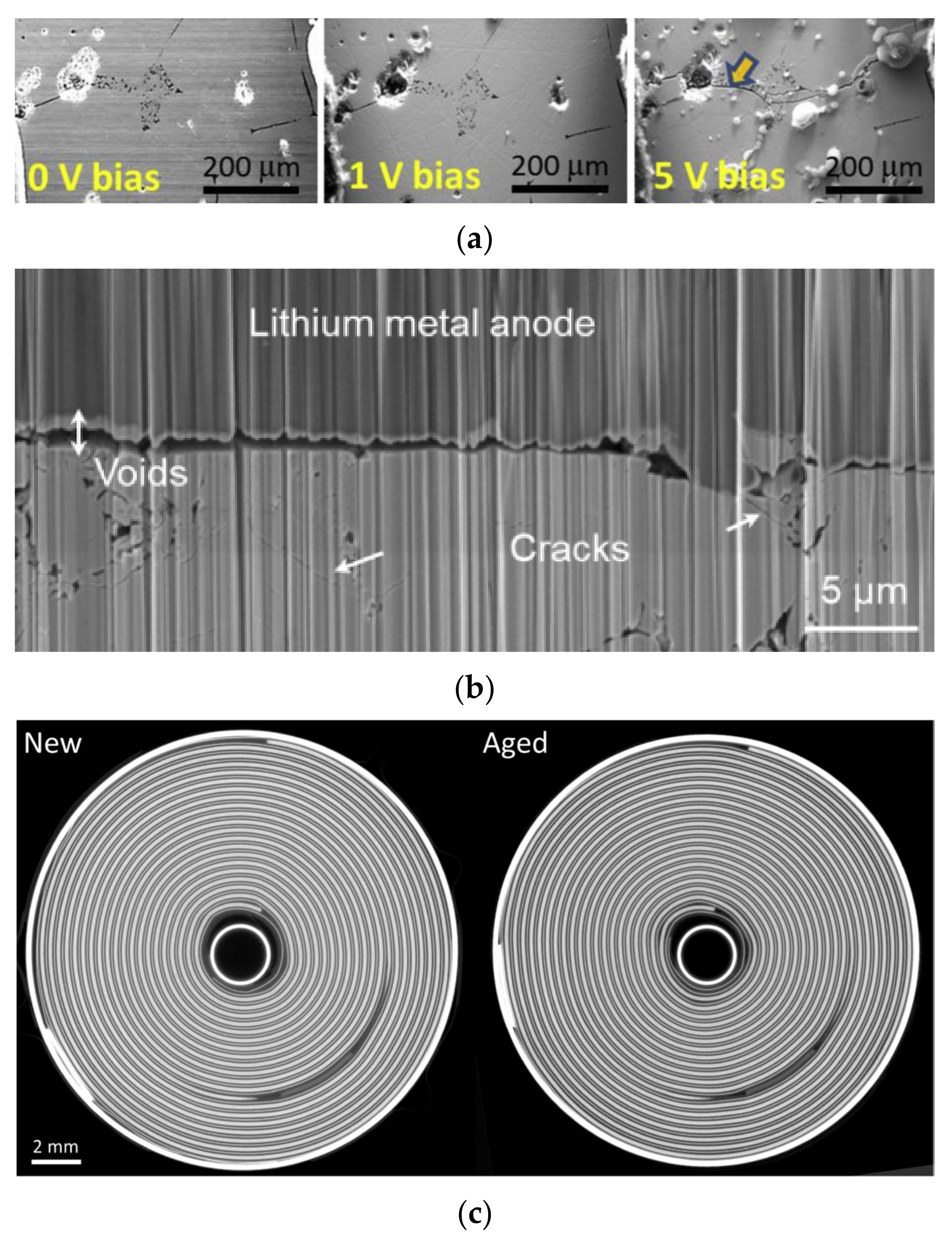
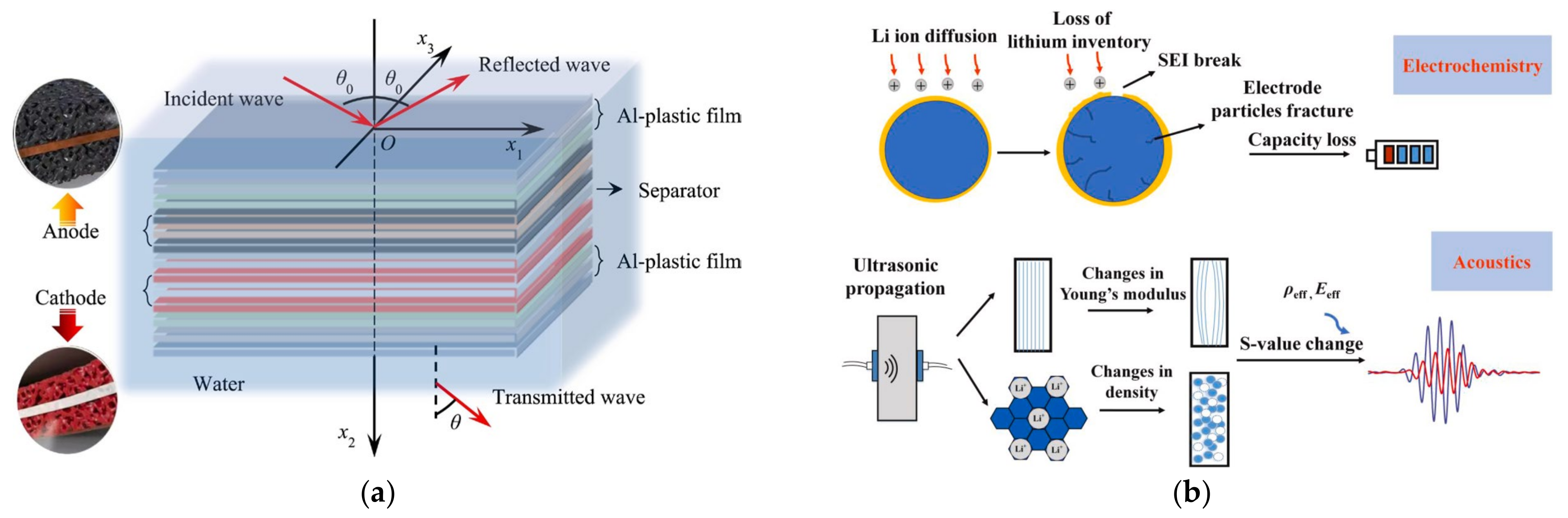
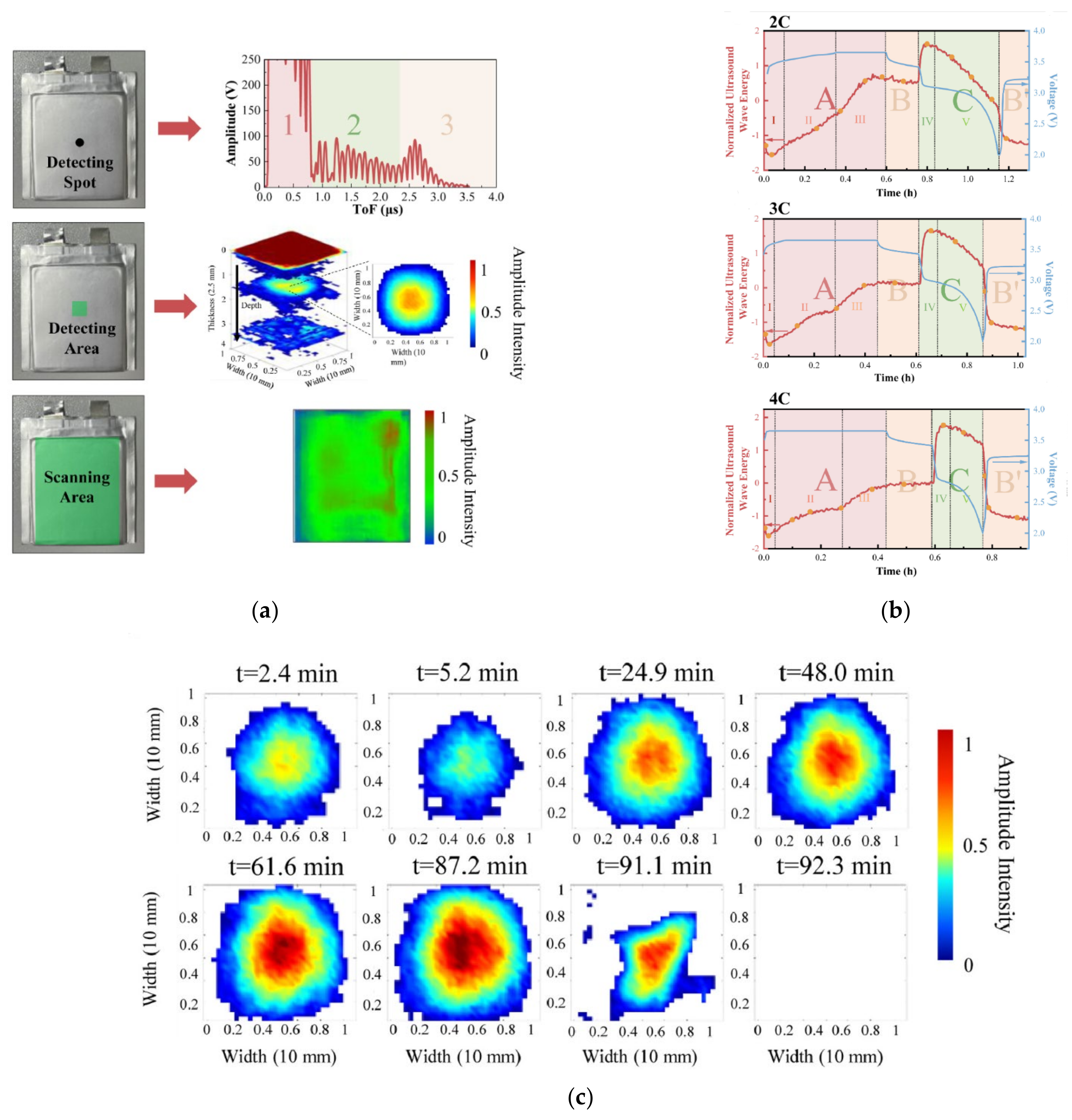
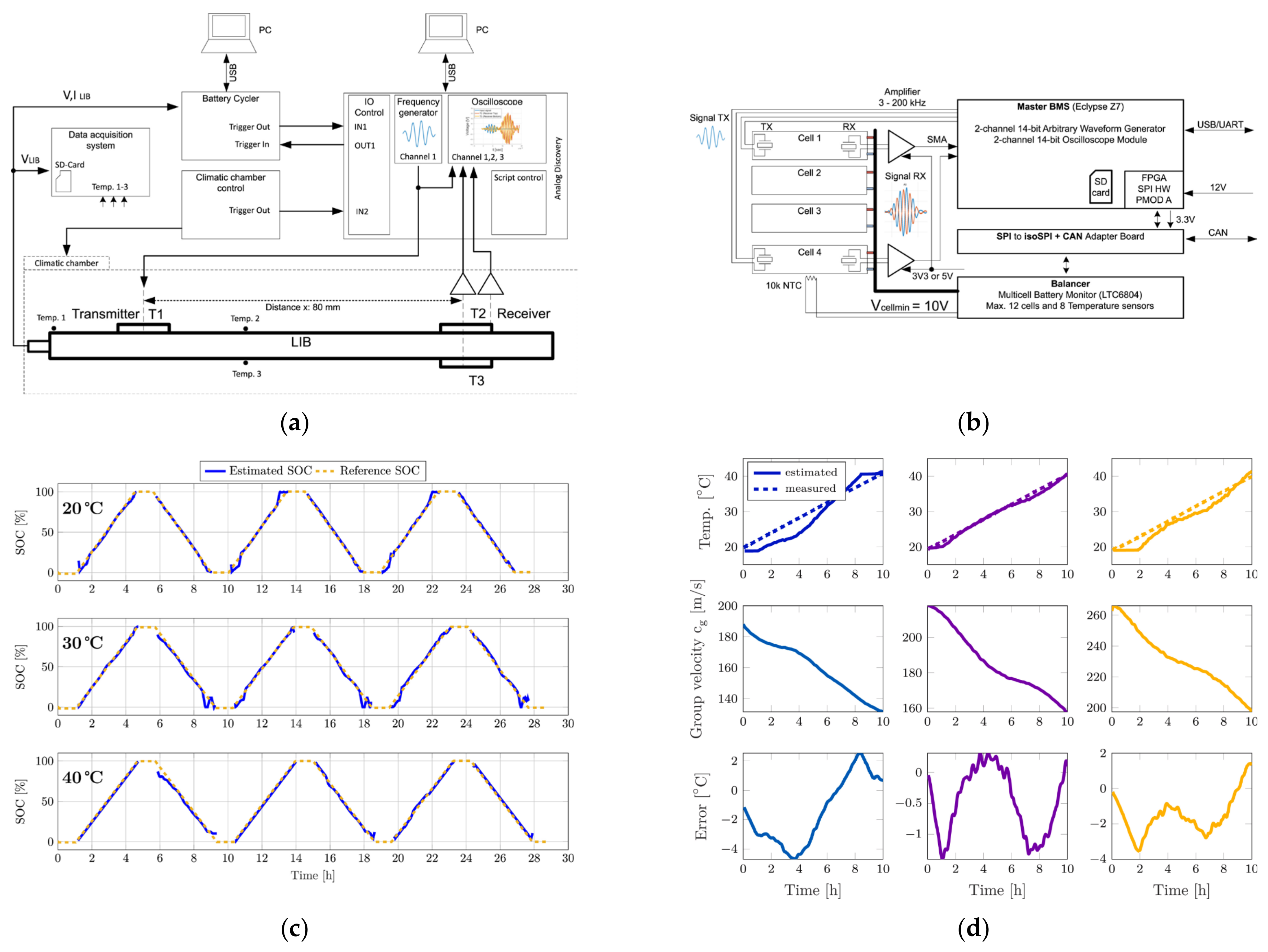
2. Ultrasonic Testing of Lithium Batteries
2.1. Development of Ultrasonic Non-Destructive Testing for Lithium Batteries
2.2. Ultrasonic Testing for the SOC and SOH of Lithium Batteries
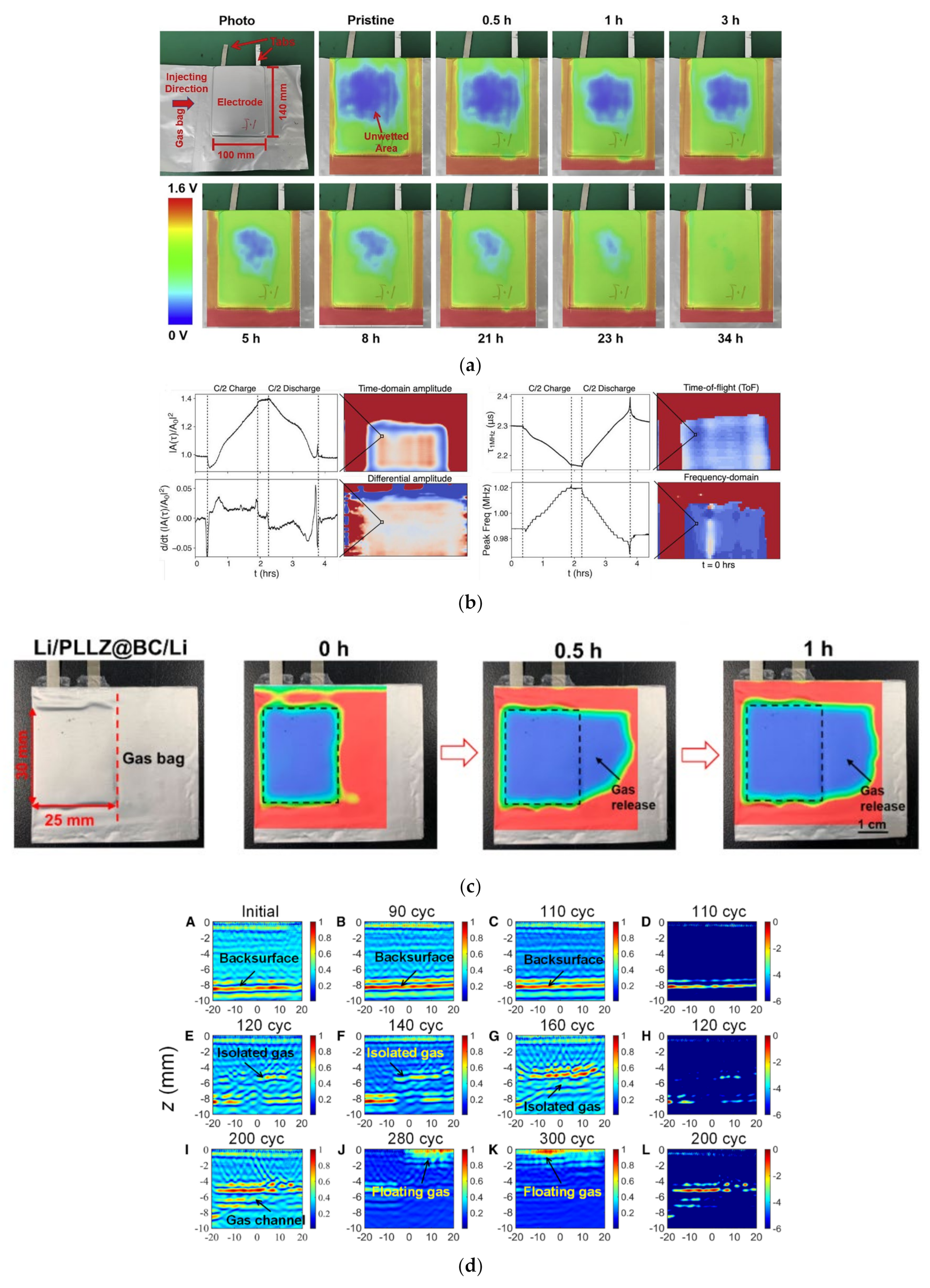
2.3. Ultrasonic Testing of Mechanical Defects in Lithium Batteries
2.4. Other Methods Based on Ultrasonic Testing of Lithium Batteries
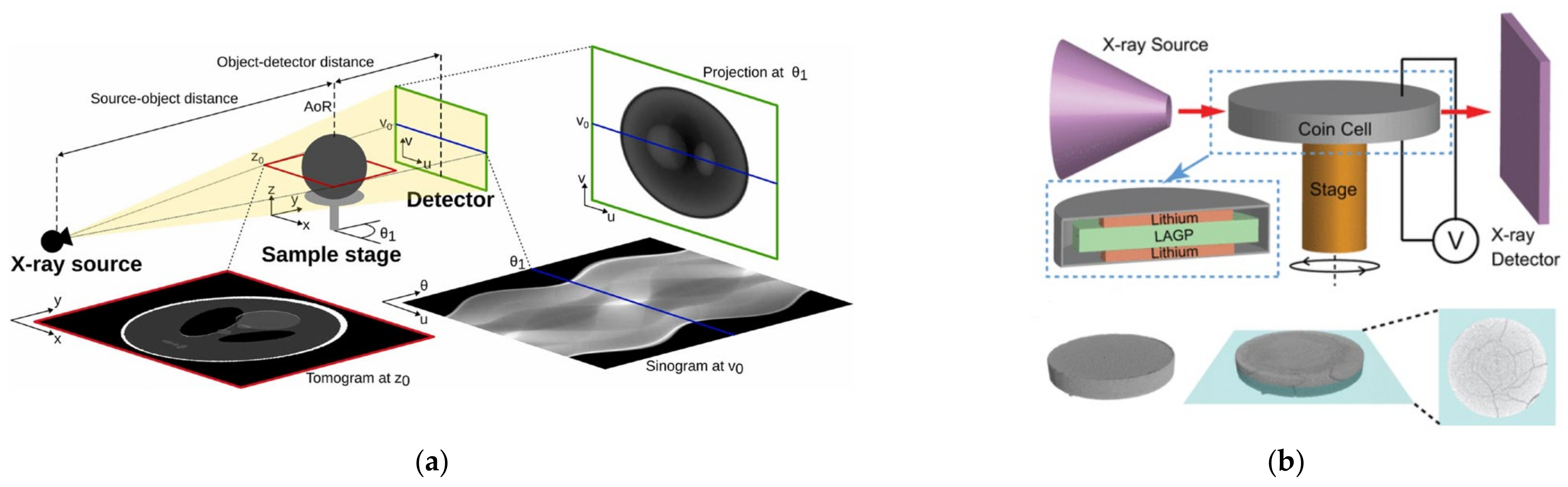
3. Computer Tomography of Lithium Batteries
3.1. Principle of X-ray CT Detection for Lithium Batteries
3.2. X-ray CT Detection of Overcharging and Over-Discharging of Lithium Batteries
3.3. X-ray CT Detection of Mechanical Damage in Lithium Batteries


3.4. Application of X-ray CT in Commercial Lithium Batteries
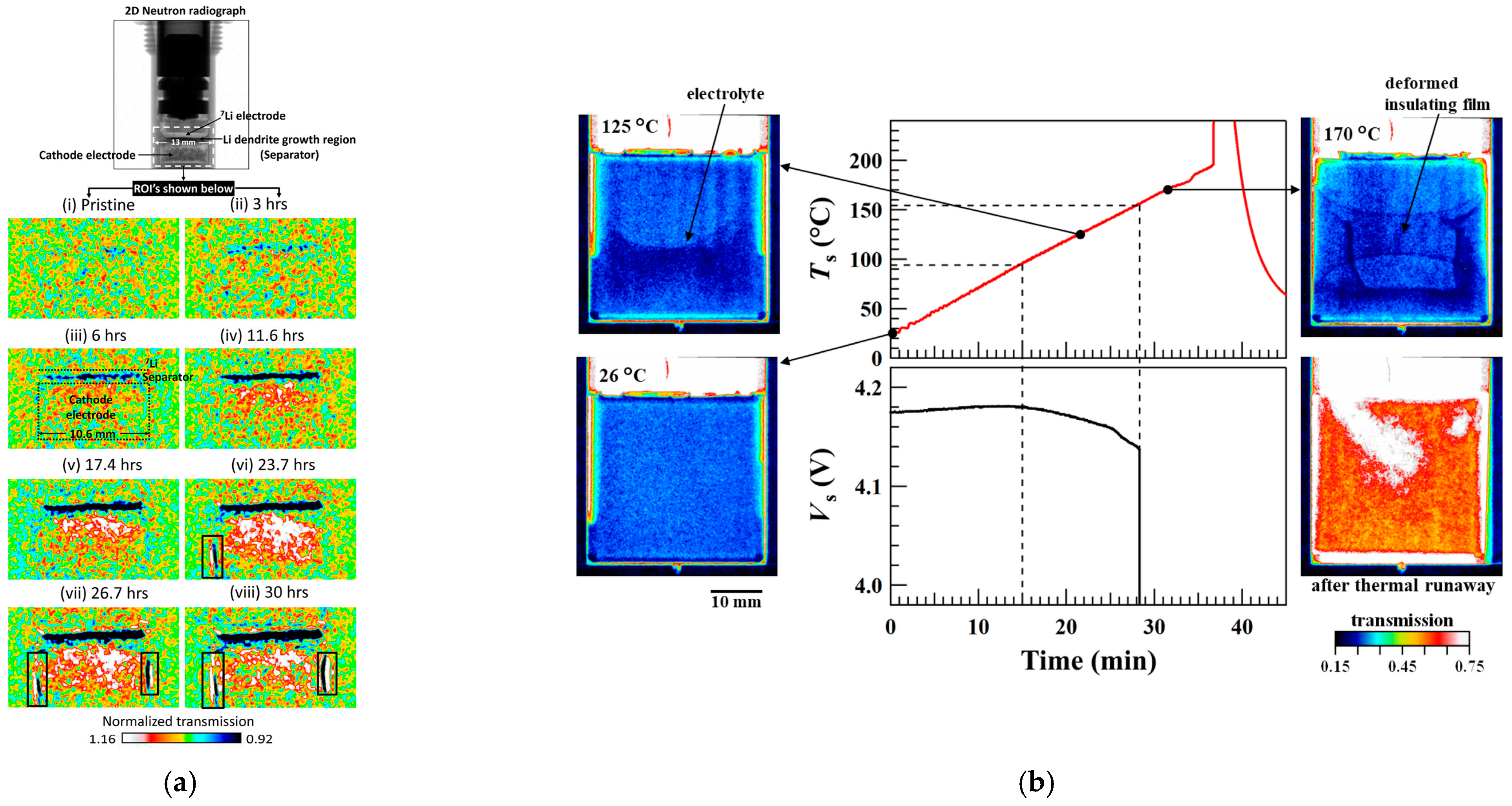
3.5. Neutron Tomography for Lithium Battery
4. Lithium Battery Non-Destructive Test Using Magnetic Resonance Detection
4.1. Principle of Nuclear Magnetic Resonance Detection for Lithium Batteries
4.2. In Situ Nuclear Magnetic Resonance
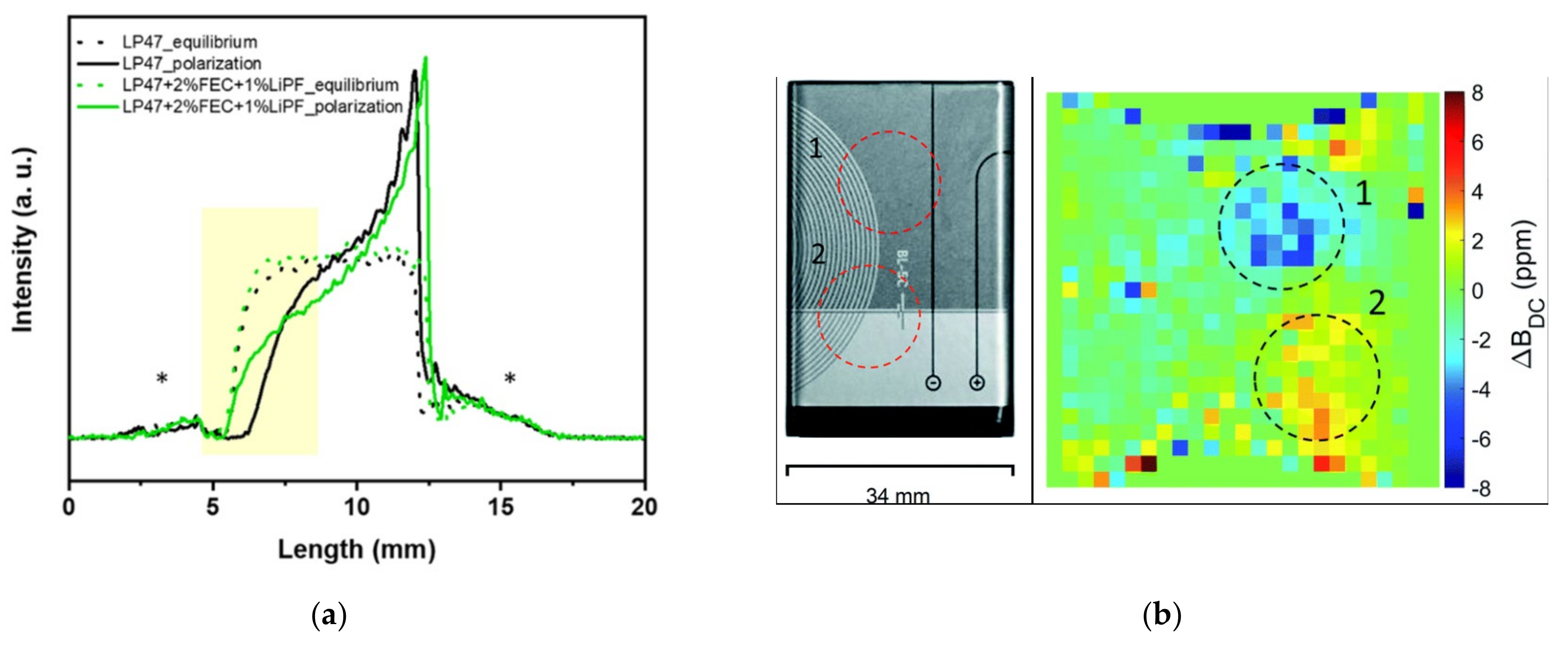
4.3. Ex Situ Nuclear Magnetic Resonance
4.4. Other Methods of Nuclear Magnetic Resonance
5. Summary
- (i)
- Could NDT techniques for some new electrode materials of lithium batteries (such as graphene, carbon silicon composite materials, etc.) also provide accurate detection?
- (ii)
- Could we obtain more accurate predictions about the health status and lifespan of lithium batteries, as well as effective warnings before battery failures occur?
- (iii)
- CT technology can already be precise to the microscale and nanoscale defects in lithium batteries. However, could ultrasound microscopy technology be applied to the detection of microscale defects in lithium batteries?
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Liu, Y.; Li, D.; Cui, X.; Wang, L.; Li, L.; Wang, K. Electrochemical Impedance Spectroscopy: A New Chapter in the Fast and Accurate Estimation of the State of Health for Lithium-Ion Batteries. Energies 2023, 16, 1599. [Google Scholar] [CrossRef]
- Owen, R.E.; Robinson, J.B.; Weaving, J.S.; Pham, M.T.M.; Tranter, T.G.; Neville, T.P.; Billson, D.; Braglia, M.; Stocker, R.; Tidblad, A.A.; et al. Operando Ultrasonic Monitoring of Lithium-Ion Battery Temperature and Behaviour at Different Cycling Rates and under Drive Cycle Conditions. J. Electrochem. Soc. 2022, 169, 040563. [Google Scholar] [CrossRef]
- Rahman, A.; Lin, X.; Wang, C. Li-Ion Battery Anode State of Charge Estimation and Degradation Monitoring Using Battery Casing via Unknown Input Observer. Energies 2022, 15, 5662. [Google Scholar] [CrossRef]
- Zou, B.; Zhang, L.; Xue, X.; Tan, R.; Jiang, P.; Ma, B.; Song, Z.; Hua, W. A Review on the Fault and Defect Diagnosis of Lithium-Ion Battery for Electric Vehicles. Energies 2023, 16, 5507. [Google Scholar] [CrossRef]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Z.; Bai, J. Influences of multi factors on thermal runaway induced by overcharging of lithium-ion battery. J. Energy Chem. 2022, 70, 531–541. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Fu, L.; Zhen, D.; Gu, F.; Ball, A.D. A review on rapid state of health estimation of lithium-ion batteries in electric vehicles. Sustain. Energy Technol. Assess. 2023, 60, 103457. [Google Scholar] [CrossRef]
- Choi, S.; Liu, P.; Yi, K.; Sampath, S.; Sohn, H. Noncontact laser ultrasonic inspection of weld defect in lithium-ion battery cap. J. Energy Storage 2023, 73, 108838. [Google Scholar] [CrossRef]
- Manalastas, W.; Rikarte, J.; Chater, R.J.; Brugge, R.; Aguadero, A.; Buannic, L.; Llordés, A.; Aguesse, F.; Kilner, J. Mechanical failure of garnet electrolytes during Li electrodeposition observed by in-operando microscopy. J. Power Sources 2019, 412, 287–293. [Google Scholar] [CrossRef]
- Duan, J.; Fuchs, T.; Mogwitz, B.; Minnmann, P.; Zuo, T.-T.; Henss, A.; Janek, J. Solid electrolyte cracking due to lithium filament growth and concept of mechanical reinforcement—An operando study. Mater. Today 2023, 70, 33–43. [Google Scholar] [CrossRef]
- Blazek, P.; Westenberger, P.; Erker, S.; Brinek, A.; Zikmund, T.; Rettenwander, D.; Wagner, N.P.; Keckes, J.; Kaiser, J.; Kazda, T.; et al. Axially and radially inhomogeneous swelling in commercial 18650 Li-ion battery cells. J. Energy Storage 2022, 52, 104563. [Google Scholar] [CrossRef]
- Chacón, X.C.; Laureti, S.; Ricci, M.; Cappuccino, G. A Review of Non-Destructive Techniques for Lithium-Ion Battery Performance Analysis. World Electr. Veh. J. 2023, 14, 305. [Google Scholar] [CrossRef]
- Wang, Y.; Lai, X.; Chen, Q.; Han, X.; Lu, L.; Ouyang, M.; Zheng, Y. Progress and challenges in ultrasonic technology for state estimation and defect detection of lithium-ion batteries. Energy Storage Mater. 2024, 69, 103430. [Google Scholar] [CrossRef]
- Gao, J.; Zhang, L.; Lyu, Y.; Shi, F.; Wu, B.; He, C. Ultrasonic guided wave measurement and modeling analysis of the state of charge for lithium-ion battery. J. Energy Storage 2023, 72, 108384. [Google Scholar] [CrossRef]
- Cai, Z.; Pan, T.; Jiang, H.; Li, Z.; Wang, Y. State-of-charge estimation of lithium-ion batteries based on ultrasonic detection. J. Energy Storage 2023, 65, 107264. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Z. Numerical Simulation and Experimental Study of Fluid-Solid Coupling-Based Air-Coupled Ultrasonic Detection of Stomata Defect of Lithium-Ion Battery. Sensors 2019, 19, 2391. [Google Scholar] [CrossRef]
- Li, X.; Hua, W.; Wu, C.; Zheng, S.; Tian, Y.; Tian, J. State estimation of a lithium-ion battery based on multi-feature indicators of ultrasonic guided waves. J. Energy Storage 2022, 56, 106113. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Xu, W. Analysis of Gas Production in Overcharged Lithium Battery by X-Ray Computed Tomography. J. Electrochem. Energy Convers. Storage 2020, 18, 021013. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Li, S. Research on Overdischarge Lithium-Ion Battery Based on X-Ray Computed Tomography. J. Electrochem. Energy Convers. Storage 2022, 20, 041004. [Google Scholar] [CrossRef]
- Hao, S.; Daemi, S.R.; Heenan, T.M.M.; Du, W.; Tan, C.; Storm, M.; Rau, C.; Brett, D.J.L.; Shearing, P.R. Tracking lithium penetration in solid electrolytes in 3D by in-situ synchrotron X-ray computed tomography. Nano Energy 2021, 82, 105744. [Google Scholar] [CrossRef]
- Ziesche, R.F.; Kardjilov, N.; Kockelmann, W.; Brett, D.J.L.; Shearing, P.R. Neutron imaging of lithium batteries. Joule 2022, 6, 35–52. [Google Scholar] [CrossRef]
- Haworth, A.R.; Cook, C.W.; Griffin, J.M. Solid-state NMR studies of coatings and interfaces in batteries. Curr. Opin. Colloid Interface Sci. 2022, 62, 101638. [Google Scholar] [CrossRef]
- Li, H.; Guo, S.; Zhou, H. In-situ/operando characterization techniques in lithium-ion batteries and beyond. J. Energy Chem. 2021, 59, 191–211. [Google Scholar] [CrossRef]
- Volkov, V.I.; Yarmolenko, O.V.; Chernyak, A.V.; Slesarenko, N.A.; Avilova, I.A.; Baymuratova, G.R.; Yudina, A.V. Polymer Electrolytes for Lithium-Ion Batteries Studied by NMR Techniques. Membranes 2022, 12, 416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Lyu, Y.; Gao, J.; Song, G.; Lee, Y.-C.; He, C.; Song, W.; Chen, H. Ultrasonic reflection/transmission characteristics for state of charge of li-ion battery. Appl. Acoust. 2023, 214, 109687. [Google Scholar] [CrossRef]
- Song, G.; Li, Y.; Lyu, Y.; Chen, H.; Song, W.; Gao, J.; He, C. Ultrasonic reflection characteristics of Lithium-ion battery based on Legendre orthogonal polynomial method. Ultrasonics 2022, 124, 106736. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhang, C.; Xu, Z.; Liu, S.; Yang, Q. Ultrasonic diagnosis of the nonlinear aging characteristics of lithium-ion battery under high-rate discharge conditions. J. Power Sources 2023, 567, 232921. [Google Scholar] [CrossRef]
- Wei, Y.; Yan, Y.; Zhang, C.; Meng, K.; Xu, C. State estimation of lithium-ion batteries based on the initial rise time feature of ultrasonic signals. J. Power Sources 2023, 581, 233497. [Google Scholar] [CrossRef]
- Meng, K.; Chen, X.; Zhang, W.; Chang, W.; Xu, J. A robust ultrasonic characterization methodology for lithium-ion batteries on frequency-domain damping analysis. J. Power Sources 2022, 547, 232003. [Google Scholar] [CrossRef]
- Sun, H.; Muralidharan, N.; Amin, R.; Rathod, V.; Ramuhalli, P.; Belharouak, I. Ultrasonic nondestructive diagnosis of lithium-ion batteries with multiple frequencies. J. Power Sources 2022, 549, 232091. [Google Scholar] [CrossRef]
- Sood, B.; Osterman, M.; Pecht, M. Health monitoring of lithium-ion batteries. In Proceedings of the 2013 IEEE Symposium on Product Compliance Engineering (ISPCE), Austin, TX, USA, 7–9 October 2013; pp. 1–6. [Google Scholar]
- Hsieh, A.G.; Bhadra, S.; Hertzberg, B.J.; Gjeltema, P.J.; Goy, A.; Fleischer, J.W.; Steingart, D.A. Electrochemical-acoustic time of flight: In operando correlation of physical dynamics with battery charge and health. Energy Environ. Sci. 2015, 8, 1569–1577. [Google Scholar] [CrossRef]
- Gold, L.; Bach, T.; Virsik, W.; Schmitt, A.; Müller, J.; Staab, T.E.M.; Sextl, G. Probing lithium-ion batteries’ state-of-charge using ultrasonic transmission—Concept and laboratory testing. J. Power Sources 2017, 343, 536–544. [Google Scholar] [CrossRef]
- Shen, Y.; Zou, B.; Zhang, Z.; Xu, M.; Wang, S.; Li, Q.; Li, H.; Zhou, M.; Jiang, K.; Wang, K. In situ detection of lithium-ion batteries by ultrasonic technologies. Energy Storage Mater. 2023, 62, 102915. [Google Scholar] [CrossRef]
- Li, X.; Wu, C.; Fu, C.; Zheng, S.; Tian, J. State Characterization of Lithium-Ion Battery Based on Ultrasonic Guided Wave Scanning. Energies 2022, 15, 6027. [Google Scholar] [CrossRef]
- Zhao, G.; Liu, Y.; Liu, G.; Jiang, S.; Hao, W. State-of-charge and state-of-health estimation for lithium-ion battery using the direct wave signals of guided wave. J. Energy Storage 2021, 39, 102657. [Google Scholar] [CrossRef]
- Robinson, J.B.; Owen, R.E.; Kok, M.D.R.; Maier, M.; Majasan, J.; Braglia, M.; Stocker, R.; Amietszajew, T.; Roberts, A.J.; Bhagat, R.; et al. Identifying Defects in Li-Ion Cells Using Ultrasound Acoustic Measurements. J. Electrochem. Soc. 2020, 167, 120530. [Google Scholar] [CrossRef]
- Huang, M.; Kirkaldy, N.; Zhao, Y.; Patel, Y.; Cegla, F.; Lan, B. Quantitative characterisation of the layered structure within lithium-ion batteries using ultrasonic resonance. J. Energy Storage 2022, 50, 104585. [Google Scholar] [CrossRef]
- Hwang, Y.-I.; Park, J.; Munir, N.; Kim, H.-J.; Song, S.-J.; Kim, K.-B. Discrimination of Poor Electrode Junctions within Lithium-Ion Batteries by Ultrasonic Measurement and Deep Learning. Batteries 2022, 8, 21. [Google Scholar] [CrossRef]
- Cho, H.; Kil, E.; Jang, J.; Kang, J.; Song, I.; Yoo, Y. Air-Coupled Ultrasound Sealing Integrity Inspection Using Leaky Lamb Waves in a Simplified Model of a Lithium-Ion Pouch Battery: Feasibility Study. Sensors 2022, 22, 6718. [Google Scholar] [CrossRef]
- Bruder, D.D.; McGovern, M.E.; James, R.; Rinker, T.J.; Gattani, V. Assessment of Laser-Generated Ultrasonic Total Focusing Method for Battery Cell Foil Weld Inspection. Res. Nondestruct. Eval. 2023, 34, 83–100. [Google Scholar] [CrossRef]
- Zheng, B.; Liu, X.; Zhu, J.; Zhao, J.; Zhong, G.; Xiang, Y.; Wang, H.; Zhao, W.; Umeshbabu, E.; Wu, Q.-H.; et al. Unraveling (electro)-chemical stability and interfacial reactions of Li10SnP2S12 in all-solid-state Li batteries. Nano Energy 2020, 67, 104252. [Google Scholar] [CrossRef]
- Carter, R.; Huhman, B.; Love, C.T.; Zenyuk, I.V. X-ray computed tomography comparison of individual and parallel assembled commercial lithium iron phosphate batteries at end of life after high rate cycling. J. Power Sources 2018, 381, 46–55. [Google Scholar] [CrossRef]
- Li, X.; Yu, X.; Zheng, S.; Tian, Y.; Tian, J. Study on Ultrasonic Transmission Characteristics and Failure Modes of a Lithium-Ion Battery. In Proceedings of the 5th International Conference on Energy Storage and Intelligent Vehicles (ICEIV 2022), Singapore, 3–4 December 2022; pp. 850–856. [Google Scholar]
- Koller, M.; Glanz, G.; Jaber, R.; Bergmann, A. Ultrasonic Battery Management System for Lamb wave mode tracking on Lithium-ion pouch cells. J. Energy Storage 2023, 74, 109347. [Google Scholar] [CrossRef]
- McGee, T.M.; Neath, B.; Matthews, S.; Ezekoye, O.A.; Haberman, M.R. Ultrasonic inspection of lithium-ion pouch cells subjected to localized thermal abuse. J. Power Sources 2023, 583, 233542. [Google Scholar] [CrossRef]
- Deng, Z.; Huang, Z.; Shen, Y.; Huang, Y.; Ding, H.; Luscombe, A.; Johnson, M.; Harlow, J.E.; Gauthier, R.; Dahn, J.R. Ultrasonic Scanning to Observe Wetting and “Unwetting” in Li-Ion Pouch Cells. Joule 2020, 4, 2017–2029. [Google Scholar] [CrossRef]
- Chang, W.; Steingart, D. Operando 2D Acoustic Characterization of Lithium-Ion Battery Spatial Dynamics. ACS Energy Lett. 2021, 6, 2960–2968. [Google Scholar] [CrossRef]
- Zheng, S.; Jiang, S.; Luo, Y.; Xu, B.; Hao, W. Guided wave imaging of thin lithium-ion pouch cell using scanning laser Doppler vibrometer. Ionics 2021, 27, 643–650. [Google Scholar] [CrossRef]
- Huo, H.; Huang, K.; Luo, W.; Meng, J.; Zhou, L.; Deng, Z.; Wen, J.; Dai, Y.; Huang, Z.; Shen, Y.; et al. Evaluating Interfacial Stability in Solid-State Pouch Cells via Ultrasonic Imaging. ACS Energy Lett. 2022, 7, 650–658. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Y.; Shi, F.; Li, L.; Wen, F.; Chen, Q. Ultrasonic phased array imaging of gas evolution in a lithium-ion battery. Cell Rep. Phys. Sci. 2023, 4, 101579. [Google Scholar] [CrossRef]
- Chen, W.; Chen, X.; Chen, W.; Jiang, Z. In Situ Atomic Force Microscopy and X-ray Computed Tomography Characterization of All-Solid-State Lithium Batteries: Both Local and Overall. Energy Technol. 2023, 11, 2201372. [Google Scholar] [CrossRef]
- Bond, T.; Gauthier, R.; Gasilov, S.; Dahn, J.R. In-Situ Computed Tomography of Particle Microcracking and Electrode Damage in Cycled NMC622/Graphite Pouch Cell Batteries. J. Electrochem. Soc. 2022, 169, 080531. [Google Scholar] [CrossRef]
- Zemek, M.; Šalplachta, J.; Zikmund, T.; Omote, K.; Takeda, Y.; Oberta, P.; Kaiser, J. Automatic marker-free estimation methods for the axis of rotation in sub-micron X-ray computed tomography. Tomogr. Mater. Struct. 2023, 1, 100002. [Google Scholar] [CrossRef]
- Villarraga-Gómez, H.; Begun, D.L.; Bhattad, P.; Mo, K.; Norouzi Rad, M.; White, R.T.; Kelly, S.T. Assessing rechargeable batteries with 3D X-ray microscopy, computed tomography, and nanotomography. Nondestruct. Test. Eval. 2022, 37, 519–535. [Google Scholar] [CrossRef]
- Tippens, J.; Miers, J.C.; Afshar, A.; Lewis, J.A.; Cortes, F.J.Q.; Qiao, H.; Marchese, T.S.; Di Leo, C.V.; Saldana, C.; McDowell, M.T. Visualizing Chemomechanical Degradation of a Solid-State Battery Electrolyte. ACS Energy Lett. 2019, 4, 1475–1483. [Google Scholar] [CrossRef]
- Finegan, D.P.; Scheel, M.; Robinson, J.B.; Tjaden, B.; Di Michiel, M.; Hinds, G.; Brett, D.J.L.; Shearing, P.R. Investigating lithium-ion battery materials during overcharge-induced thermal runaway: An operando and multi-scale X-ray CT study. Phys. Chem. Chem. Phys. 2016, 18, 30912–30919. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wei, Y.; Zhao, Z.; Zou, Y.; Luo, D. Investigation of the swelling failure of lithium-ion battery packs at low temperatures using 2D/3D X-ray computed tomography. Electrochim. Acta 2019, 305, 65–71. [Google Scholar] [CrossRef]
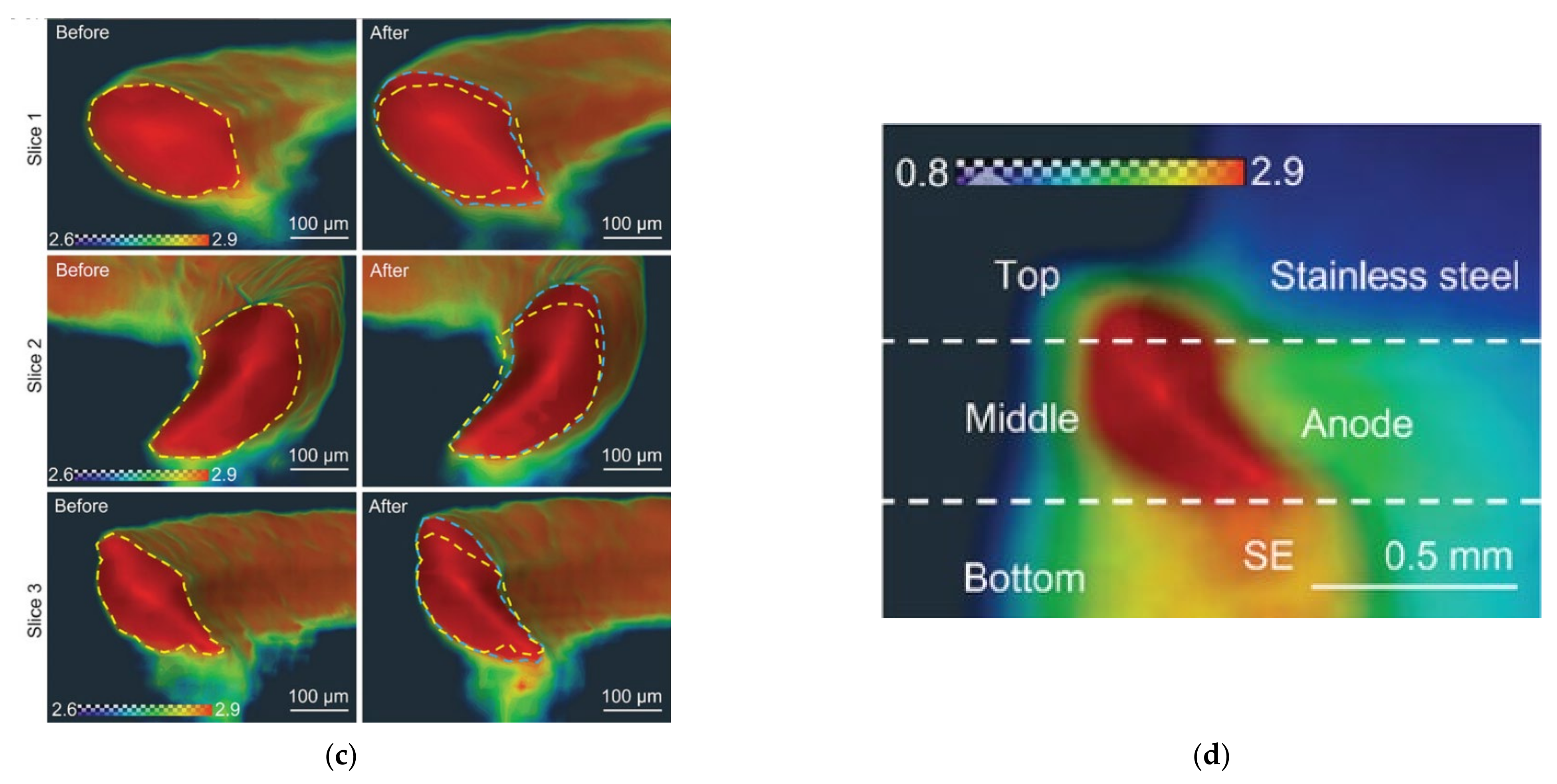
- Hao, S.; Bailey, J.J.; Iacoviello, F.; Bu, J.; Grant, P.S.; Brett, D.J.L.; Shearing, P.R. 3D Imaging of Lithium Protrusions in Solid-State Lithium Batteries using X-Ray Computed Tomography. Adv. Funct. Mater. 2020, 31, 2007564. [Google Scholar] [CrossRef]
- Finegan, D.P.; Tudisco, E.; Scheel, M.; Robinson, J.B.; Taiwo, O.O.; Eastwood, D.S.; Lee, P.D.; Di Michiel, M.; Bay, B.; Hall, S.A.; et al. Quantifying Bulk Electrode Strain and Material Displacement within Lithium Batteries via High-Speed Operando Tomography and Digital Volume Correlation. Adv. Sci. 2016, 3, 1500332. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.; Llewellyn, A.V.; Heenan, T.M.M.; Tan, C.; Brett, D.J.L.; Jervis, R.; Shearing, P.R. First Cycle Cracking Behaviour Within Ni-Rich Cathodes During High-Voltage Charging. J. Electrochem. Soc. 2023, 170, 070513. [Google Scholar] [CrossRef]
- Wu, Y.; Saxena, S.; Xing, Y.; Wang, Y.; Li, C.; Yung, W.K.C.; Pecht, M. Analysis of Manufacturing-Induced Defects and Structural Deformations in Lithium-Ion Batteries Using Computed Tomography. Energies 2018, 11, 925. [Google Scholar] [CrossRef]
- Ziegler, A.; Oeser, D.; Hein, T.; Montesinos-Miracle, D.; Ackva, A. Run to Failure: Aging of Commercial Battery Cells beyond Their End of Life. Energies 2020, 13, 1858. [Google Scholar] [CrossRef]
- Pfrang, A.; Kersys, A.; Kriston, A.; Sauer, D.U.; Rahe, C.; Käbitz, S.; Figgemeier, E. Long-term cycling induced jelly roll deformation in commercial 18650 cells. J. Power Sources 2018, 392, 168–175. [Google Scholar] [CrossRef]
- Heenan, T.M.M.; Jnawali, A.; Kok, M.; Tranter, T.G.; Tan, C.; Dimitrijevic, A.; Jervis, R.; Brett, D.J.L.; Shearing, P.R. Data for an Advanced Microstructural and Electrochemical Datasheet on 18650 Li-ion Batteries with Nickel-Rich NMC811 Cathodes and Graphite-Silicon Anodes. Data Brief 2020, 32, 106033. [Google Scholar] [CrossRef]
- Diao, W.; Xu, B.; Pecht, M. Charging induced electrode layer fracturing of 18650 lithium-ion batteries. J. Power Sources 2021, 484, 229260. [Google Scholar] [CrossRef]
- Matras, D.; Ashton, T.E.; Dong, H.; Mirolo, M.; Martens, I.; Drnec, J.; Darr, J.A.; Quinn, P.D.; Jacques, S.D.M.; Beale, A.M.; et al. Emerging chemical heterogeneities in a commercial 18650 NCA Li-ion battery during early cycling revealed by synchrotron X-ray diffraction tomography. J. Power Sources 2022, 539, 231589. [Google Scholar] [CrossRef]
- Liu, J.; Duan, Q.; Peng, W.; Feng, L.; Ma, M.; Hu, S.; Sun, J.; Wang, Q. Slight overcharging cycling failure of commercial lithium-ion battery induced by the jelly roll destruction. Process Saf. Environ. Prot. 2022, 160, 695–703. [Google Scholar] [CrossRef]
- Cao, D.; Zhang, Y.; Ji, T.; Zhu, H. In operando neutron imaging characterizations of all-solid-state batteries. MRS Bull. 2023, 48, 1257–1268. [Google Scholar] [CrossRef]
- Yusuf, M. The In-situ Characterization of Fast-charging Degradation Modes in Li-ion Batteries Using High-resolution Neutron Imaging. Electrochem. Soc. Interface 2022, 31, 38. [Google Scholar] [CrossRef]
- Song, B.; Dhiman, I.; Carothers, J.C.; Veith, G.M.; Liu, J.; Bilheux, H.Z.; Huq, A. Dynamic Lithium Distribution upon Dendrite Growth and Shorting Revealed by Operando Neutron Imaging. ACS Energy Lett. 2019, 4, 2402–2408. [Google Scholar] [CrossRef]
- Nozaki, H.; Kondo, H.; Shinohara, T.; Setoyama, D.; Matsumoto, Y.; Sasaki, T.; Isegawa, K.; Hayashida, H. In situ neutron imaging of lithium-ion batteries during heating to thermal runaway. Sci. Rep. 2023, 13, 22082. [Google Scholar] [CrossRef] [PubMed]
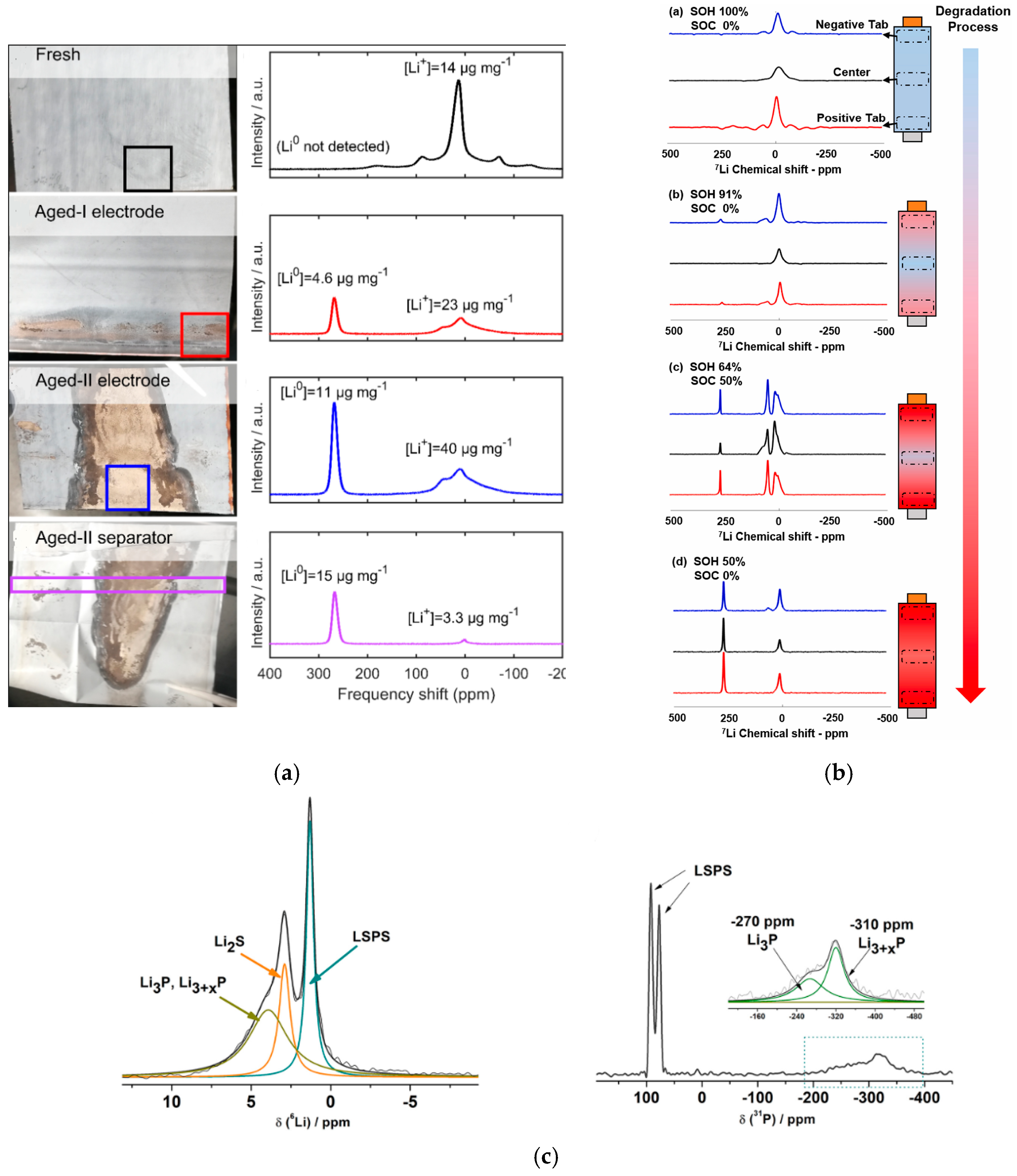
- Cao, D.; Zhang, K.; Li, W.; Zhang, Y.; Ji, T.; Zhao, X.; Cakmak, E.; Zhu, J.; Cao, Y.; Zhu, H. Nondestructively Visualizing and Understanding the Mechano-Electro-chemical Origins of “Soft Short” and “Creeping” in All-Solid-State Batteries. Adv. Funct. Mater. 2023, 33, 2307998. [Google Scholar] [CrossRef]
- Bagheri, K.; Deschamps, M.; Salager, E. Nuclear magnetic resonance for interfaces in rechargeable batteries. Curr. Opin. Colloid Interface Sci. 2023, 64, 101675. [Google Scholar] [CrossRef]
- Pecher, O.; Carretero-González, J.; Griffith, K.J.; Grey, C.P. Materials’ Methods: NMR in Battery Research. Chem. Mater. 2017, 29, 213–242. [Google Scholar] [CrossRef]
- Hu, J.Z.; Jaegers, N.R.; Hu, M.Y.; Mueller, K.T. In situ and ex situ NMR for battery research. J. Phys. Condens. Matter 2018, 30, 463001. [Google Scholar] [CrossRef] [PubMed]
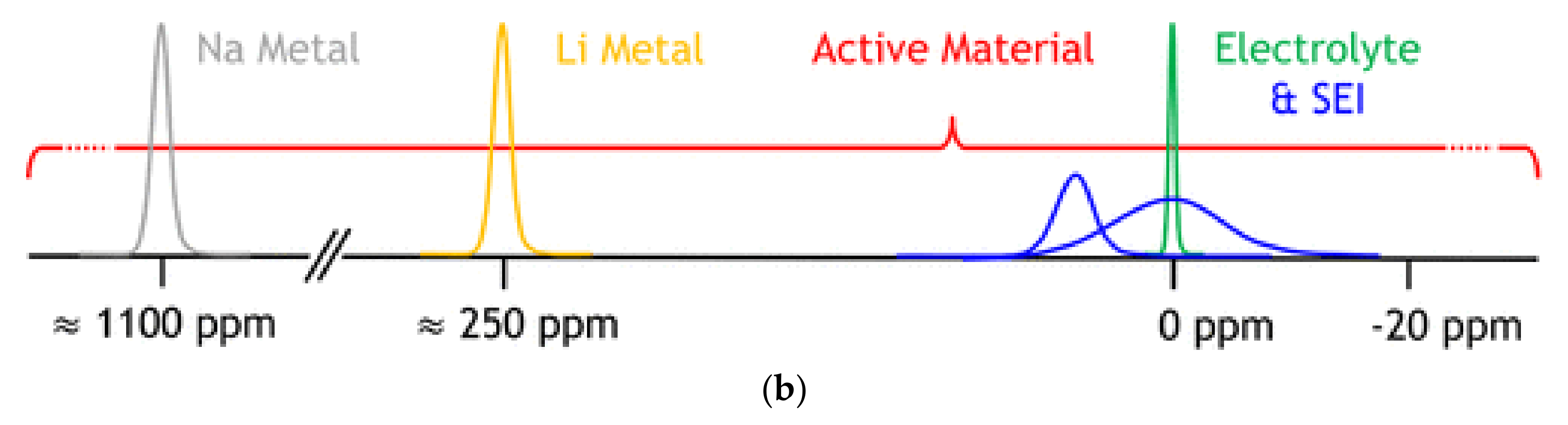
- Liu, X.; Liang, Z.; Xiang, Y.; Lin, M.; Li, Q.; Liu, Z.; Zhong, G.; Fu, R.; Yang, Y. Solid-State NMR and MRI Spectroscopy for Li/Na Batteries: Materials, Interface, and In Situ Characterization. Adv. Mater. 2021, 33, e2005878. [Google Scholar] [CrossRef]
- Gunnarsdóttir, A.B.; Vema, S.; Menkin, S.; Marbella, L.E.; Grey, C.P. Investigating the effect of a fluoroethylene carbonate additive on lithium deposition and the solid electrolyte interphase in lithium metal batteries using in situ NMR spectroscopy. J. Mater. Chem. A 2020, 8, 14975–14992. [Google Scholar] [CrossRef]
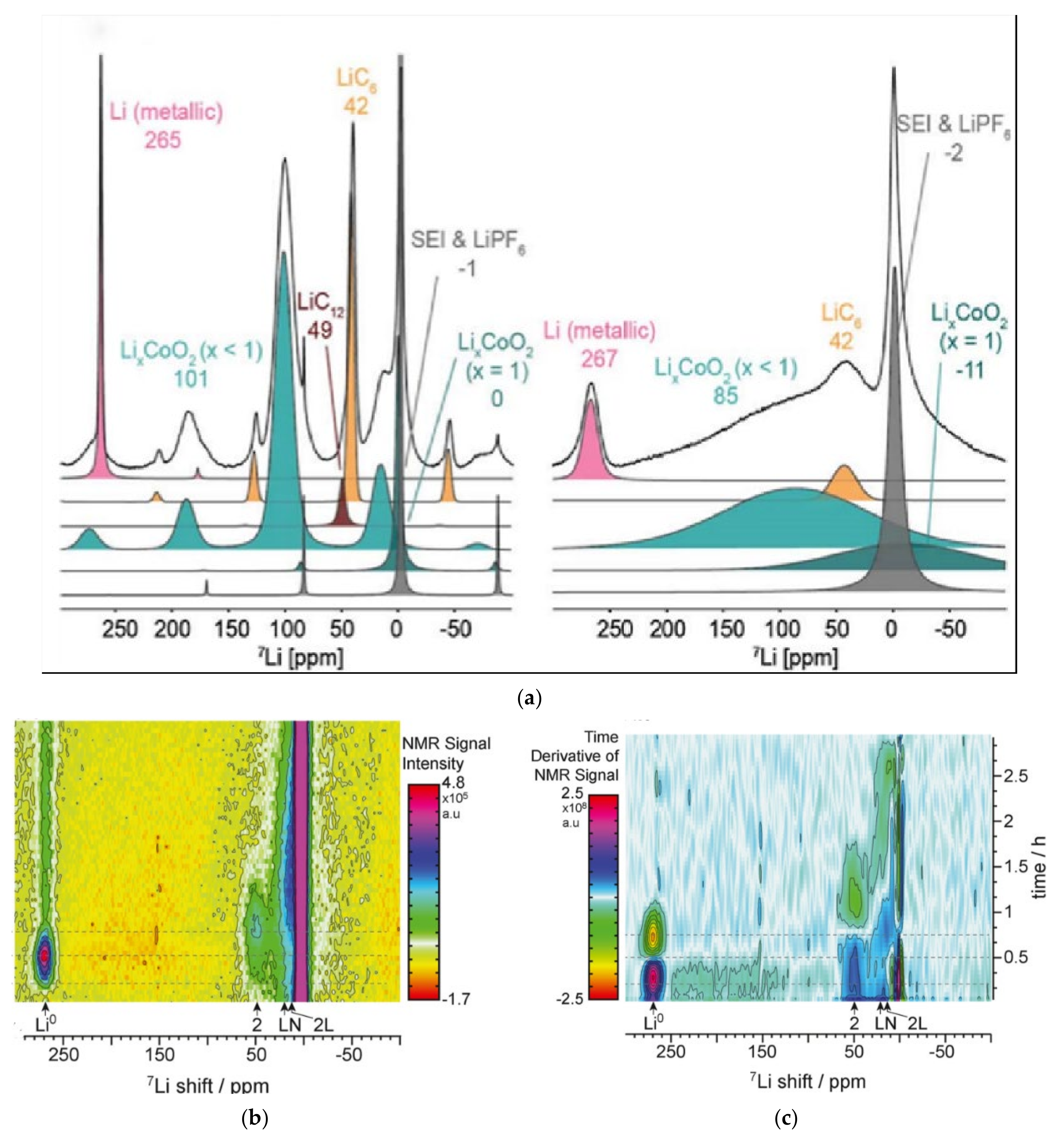
- Freytag, A.I.; Pauric, A.D.; Krachkovskiy, S.A.; Goward, G.R. In Situ Magic-Angle Spinning 7Li NMR Analysis of a Full Electrochemical Lithium-Ion Battery Using a Jelly Roll Cell Design. J. Am. Chem. Soc. 2019, 141, 13758–13761. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, K.; Yamakami, T.; Nishimura, I.; Kometani, H.; Ando, H.; Hashi, K.; Shimizu, T.; Ishida, H. Mechanisms for overcharging of carbon electrodes in lithium-ion/sodium-ion batteries analysed by operando solid-state NMR. J. Mater. Chem. A 2020, 8, 14472–14481. [Google Scholar] [CrossRef]
- Sanders, K.J.; Aguilera, A.R.; Keffer, J.R.; Balcom, B.J.; Halalay, I.C.; Goward, G.R. Transient lithium metal plating on graphite: Operando 7Li nuclear magnetic resonance investigation of a battery cell using a novel RF probe. Carbon 2022, 189, 377–385. [Google Scholar] [CrossRef]
- Fang, Y.; Smith, A.J.; Lindström, R.W.; Lindbergh, G.; Furó, I. Quantifying lithium lost to plating and formation of the solid-electrolyte interphase in graphite and commercial battery components. Appl. Mater. Today 2022, 28, 101527. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, S.; Li, R.; Ren, D.; Yi, M.; Xu, C.; Han, X.; Lu, L.; Friess, B.; Offer, G.; et al. Inhomogeneous degradation induced by lithium plating in a large-format lithium-ion battery. J. Power Sources 2022, 542, 231753. [Google Scholar] [CrossRef]
- Hsieh, Y.-C.; Thienenkamp, J.H.; Huang, C.-J.; Tao, H.-C.; Rodehorst, U.; Hwang, B.J.; Winter, M.; Brunklaus, G. Revealing the Impact of Film-Forming Electrolyte Additives on Lithium Metal Batteries via Solid-State NMR/MRI Analysis. J. Phys. Chem. C 2021, 125, 252–265. [Google Scholar] [CrossRef]
- Romanenko, K.; Jerschow, A. Observation of memory effects associated with degradation of rechargeable lithium-ion cells using ultrafast surface-scan magnetic resonance imaging. J. Mater. Chem. A 2021, 9, 21078–21084. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, J.; Chen, J.; He, Y.; Liu, C.; Liang, X.; Feng, J. Li Plating on Carbon Electrode Surface Probed by Low-Field Dynamic Nuclear Polarization 7Li NMR. Chin. Phys. Lett. 2021, 38, 126801. [Google Scholar] [CrossRef]
- Tao, M.; Chen, X.; Lin, H.; Jin, Y.; Shan, P.; Zhao, D.; Gao, M.; Liang, Z.; Yang, Y. Clarifying the Temperature-Dependent Lithium Deposition/Stripping Process and the Evolution of Inactive Li in Lithium Metal Batteries. ACS Nano 2023, 17, 24104–24114. [Google Scholar] [CrossRef]
- Schleker, P.P.M.; Eichel, R.-A.; Granwehr, J. Revealing the Equilibrium of Lithium Cations Across a Solid–Electrolyte Interface by T1 NMR Relaxation. Appl. Magn. Reson. 2023, 54, 1463–1480. [Google Scholar] [CrossRef]




| Method | Advantages | Limitations |
|---|---|---|
| UT | Detect cracks, delamination, electrolyte loss. | Cannot be used for analyzing elemental changes. |
| CT | Display the location and type of battery defects. | Cannot be used for detecting electrolyte loss. |
| NMR | Detect elemental changes and electrolyte loss. | Cannot be used to evaluate SOC and SOH. |
| EIS | Estimate the resistance and capacity related to SOC. | Cannot be used for detecting gas generation and electrolyte loss. |
| IRT | Evaluate the surface temperature and thermal runway of the batteries. | Cannot be used to evaluate lithium plating and electrode delamination. |
| Presenter | Time (Year) | Theory |
|---|---|---|
| Bhanu [31] | 2013 | Real-time measurement with ultrasonic transducers can be used to update degradation models on battery management systems. |
| Hsieh [32] | 2015 | A framework was proposed to link the sound speed change with the SOC and SOH of batteries. |
| Gold [33] | 2017 | Linking the actual SOC with ultrasonic propagation pulses and Biot’s theory. |
| Li [16] | 2019 | Establishing a fluid–solid coupling model for stomata defects in lithium-ion batteries using pressure acoustics and solid mechanics. |
| Aim | Conclusion | Reference |
|---|---|---|
| Whether the deformation is induced by changes in the thickness of the anode and cathode films during charging–discharging cycles | As the cathode electrode is thickened, additional pressure is generated around the area, resulting in the release of mechanical stress through deformation | [64] |
| Assess the consequences of overcharging 18650 cells | X-ray CT analysis shows that overcharging causes the electrode to rupture, which extends from the outermost layer to the middle electrode layer, including the anode and cathode | [66] |
| Study on the circulating stability of 18650 cells | Ex situ XRD-CT measurement of batteries indicates that during the battery discharge (de-lithiation) process, the behaviors of uniform lithiation region, delayed lithiation region, and inactive-to-lithiation region are similar | [67] |
| Research on faults caused by slight overcharging cycles in lithium-ion batteries | Overcharging failures are caused by damage to the jelly roll. The lithium plating accumulation leads to lithium dendrites. It will cause local internal short circuits and damage to the anode. | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Wang, S.; Hao, F. A Review of Non-Destructive Testing for Lithium Batteries. Energies 2024, 17, 4030. https://doi.org/10.3390/en17164030
Gao J, Wang S, Hao F. A Review of Non-Destructive Testing for Lithium Batteries. Energies. 2024; 17(16):4030. https://doi.org/10.3390/en17164030
Chicago/Turabian StyleGao, Junfu, Sikai Wang, and Feng Hao. 2024. "A Review of Non-Destructive Testing for Lithium Batteries" Energies 17, no. 16: 4030. https://doi.org/10.3390/en17164030




