Evaluation of Changes in the Chemical Composition of Grasses as a Result of the Methane Fermentation Process and Biogas Production Efficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Collection
2.2. Chemical Composition
- -
- Cellulose was determined according to Seifert using dioxane and acetylacetone [13];
- -
- Lignin was determined according to Tappi using 72% sulfuric acid [14];
- -
- Holocellulose was determined using sodium chlorite [15];
- -
- Extractives were determined in a Soxhlet apparatus using 96% ethanol [16];
- -
- Ash was determined according to DIN 51731;
- -
- Hemicelluloses were calculated arithmetically based on the difference between holocellulose and cellulose. Hemicellulose content was calculated based on the difference between the contents of holocellulose and cellulose.
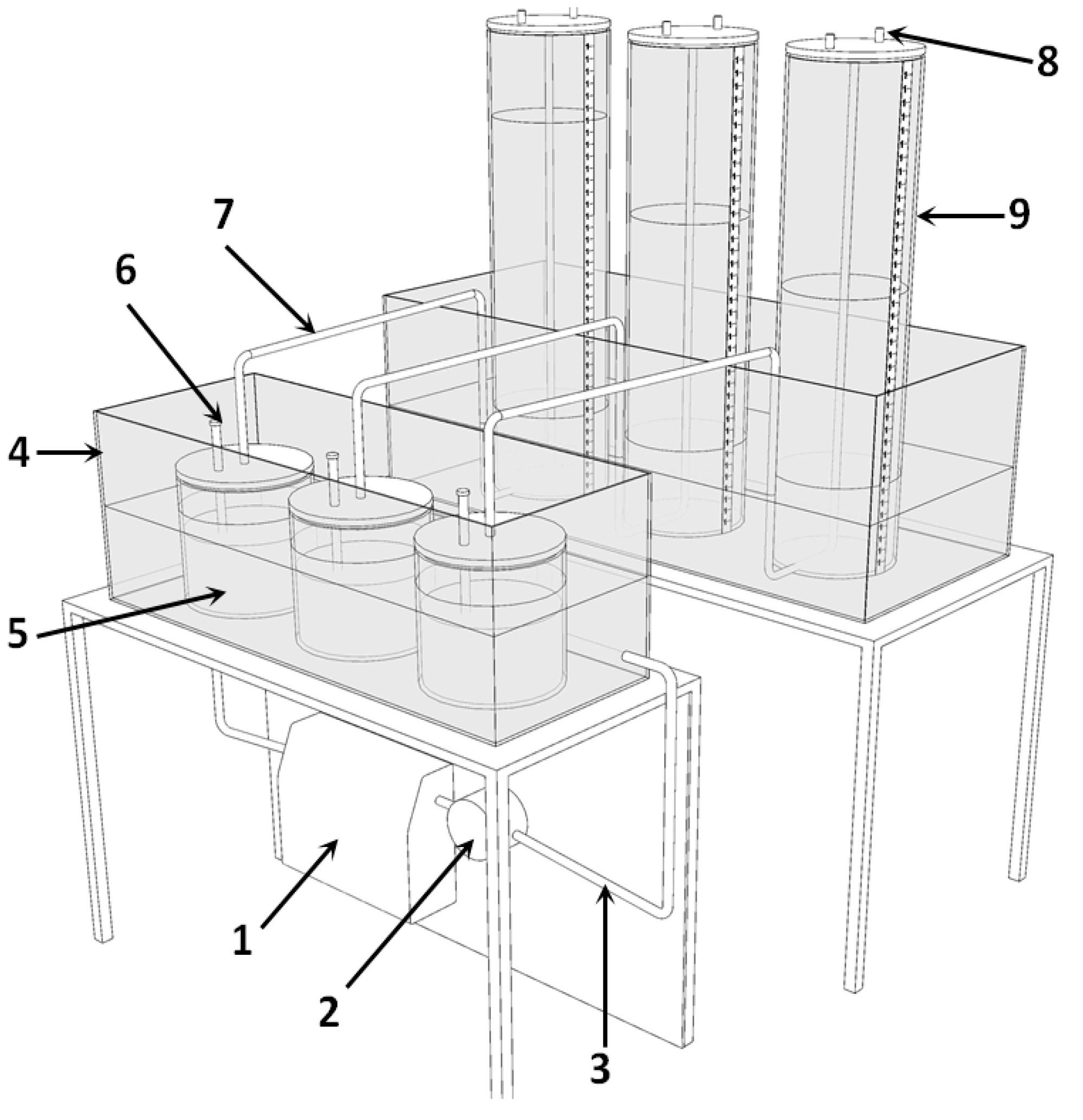
2.3. Batch Test
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richter, F.; Fricke, T.; Wachendorf, W. Utilization of semi-natural grassland through integrated generation of solid fuel and biogas from biomass. III. Effects of hydrothermal conditioning and mechanical dehydration on solid fuel properties and on energy and greenhouse gas balances. Grass Forage Sci. 2010, 65, 185–199. [Google Scholar] [CrossRef]
- Czekała, W. Solid Fraction of Digestate from Biogas Plant as a Material for Pellets Production. Energies 2021, 14, 5034. [Google Scholar] [CrossRef]
- Vázquez-Rowe, I.; Golkowska, K.; Lebuf, V.; Vaneeckhaute, C.; Michels, E.; Meers, E.; Benetto, E.; Koster, D. Environmental assessment of digestate treatment technologies using LCA methodology. Waste Manag. 2015, 43, 442–459. [Google Scholar] [CrossRef] [PubMed]
- Sobczak, A.; Chomać-Pierzecka, E.; Kokiel, A.; Różycka, M.; Stasiak, J.; Soboń, D. Economic Conditions of Using Biodegradable Waste for Biogas Production, Using the Example of Poland, and Germany. Energies 2022, 15, 5239. [Google Scholar] [CrossRef]
- Gao, X.; Tang, X.; Zhao, K.; Balan, V.; Zhu, Q. Biogas Production from Anaerobic Co-Digestion of Spent Mushroom Substrate with Different Livestock Manure. Energies 2021, 14, 570. [Google Scholar] [CrossRef]
- Weiland, P. Biomass digestion in agriculture: A successful pathway for the energy production and waste treatment in Germany. Eng. Life Sci. 2006, 6, 302–309. [Google Scholar] [CrossRef]
- Dena, H.M.; Kontges, A.; Rostek, S. Biogaspartner—A Joint Initiative. Biogas Grid Injection in Germany and Europe-Market, Technology and Players; German Energy Agency, HP Druck: Berlin, Germany, 2009. [Google Scholar]
- Prochnow, A.; Heiermann, M.; Plochl, M.; Linke, B.; Idler, C.; Amon, T.; Hobbs, P.J. Bioenergy from permanent grassland-A review: 1. Biogas. Bioresour. Technol. 2009, 100, 4931–4944. [Google Scholar] [CrossRef] [PubMed]
- Korres, N.E.; Singh, A.; Nizami, A.S.; Murphy, J.D. Is grass biomethane a sustainable transport biofuel? Biofuels Bioprod. Biorefin. 2010, 4, 310–325. [Google Scholar] [CrossRef]
- Pilarski, K.; Pilarska, A.A.; Boniecki, P.; Niedbała, G.; Witaszek, K.; Piekutowska, M.; Idzior-Haufa, M.; Wawrzyniak, A. Degree of Biomass Conversion in the Integrated Production of Bioethanol and Biogas. Energies 2021, 14, 7763. [Google Scholar] [CrossRef]
- Esteves, B.; Sen, U.; Pereira, H. Influence of Chemical Composition on Heating Value of Biomass: A Review and Bibliometric Analysis. Energies 2023, 16, 4226. [Google Scholar] [CrossRef]
- Korres, N.E.; Thamsiriroj, T.; Smyth, B.M.; Nizami, A.S.; Singh, A.; Murphy, J.D. Grass Biomethane for Agriculture and Energy. In Genetics, Biofuels and Local Farming Systems; Lichtfouse, E., Ed.; Springer Science+Business Media B.V.: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Seifert, K. Zur Frage der Cellulose-Schnellbestimmung nach der Acetylaceton-Methode. Das Pap. 1960, 14, 104–106. [Google Scholar]
- TAPPI T 222 om-06; Acid-Insoluble Lignin in Wood and Pulp. Technical Association of the Pulp and Paper Industry (TAPPI): Atlanta, GA, USA, 2006.
- TAPPI T 9 wd-75; Holocellulose in Wood. Technical Association of the Pulp and Paper Industry (TAPPI): Atlanta, GA, USA, 2006.
- TAPPI T 204 cm-07; Solvent Extractives of Wood and Pulp. Technical Association of the Pulp and Paper Industry (TAPPI): Atlanta, GA, USA, 2007.
- Cieślik, M.; Dach, J.; Lewicki, A.; Smurzyńska, A.; Janczak, D.; Pawlicka-Kaczorowska, J.; Boniecki, P.; Cyplik, P.; Czekała, W.; Jóźwiakowski, K. Methane fermentation of the maize straw silage under meso- and thermophilic conditions. Energy 2016, 115, 1495–1502. [Google Scholar] [CrossRef]
- Lewicki, A.; Pilarski, K.; Janczak, D.; Czekała, W.; Rodríguez Carmona, P.C.; Cieślik, M.; Witaszek, K.; Zbytek, Z. The biogas production from herbs and waste from the herbal industry. J. Res. Appl. Agric. Eng. 2013, 58, 114–117. [Google Scholar]
- de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research, R Package Version 1.3-7; 2023. Available online: https://cran.r-project.org/web/packages/agricolae/agricolae.pdf (accessed on 20 April 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 20 April 2024).
- Tapia-Maruri, D.; Evangelista-Lozano, S.; Alamilla-Beltrán, L.; Camacho-Díaz, B.H.; Ávila-Reyes, S.V.; Villalobos-Espinosa, J.d.C.; Jiménez-Aparicio, A.R. Comparative Evaluation of the Thermal, Structural, Chemical and Morphological Properties of Bagasse from the Leaf and Fruit of Bromelia hemisphaerica Lam. Delignified by Organosolv. Appl. Sci. 2022, 12, 3761. [Google Scholar] [CrossRef]
- Rahaman, T.; Biswas, S.; Ghorai, S.; Bera, S.; Dey, S.; Guha, S.; Maity, D.; De, S.; Ganguly, J.; Das, M. Integrated application of morphological, anatomical, biochemical, and physico-chemical methods to identify superior, lignocellulosic grass feedstocks for bioenergy purposes. Renew. Sustain. Energy Rev. 2023, 187, 113738. [Google Scholar] [CrossRef]
- Herrmann, C.; Plogsties, V.; Willms, M.; Hengelhaupt, F.; Eberl, V.; Eckner, J.; Strauß, C.; Idler, C.; Heiermann, M. Methane production potential of various crop species grown in energy crop rotations. Landtechnik 2016, 71, 194–208. [Google Scholar]
- Tilman, D.; Hill, J.; Lehman, C. Carbon-negative biofuels from low-input high diversity grassland biomass. Science 2006, 314, 1598–1600. [Google Scholar] [CrossRef]
- Gobena, G.; Urge, M.; Hundie, D.; Kumsa, D. Identification and evaluation of agro-ecological variation in dry matter yield and nutritional values of local grasses used as livestock feed in Adola Reedde. Guji Zone. Ethiopia. J. Appl. Anim. Res. 2022, 50, 369–379. [Google Scholar] [CrossRef]
- Waliszewska, B.; Grzelak, M.; Gaweł, E.; Spek-Dźwigała, A.; Sieradzka, A.; Czekała, W. Chemical Characteristics of Selected Grass Species from Polish Meadows and Their Potential Utilization for Energy Generation Purposes. Energies 2021, 14, 1669. [Google Scholar] [CrossRef]
- Platače, R.; Adamovičs, A. The evaluation of ash content in grass biomass used for energy production. Energy Prod. Manag. 21st Century 2014, 2, 1057–1065. [Google Scholar]
- Amaleviciute-Volunge, K.; Slepetiene, A.; Butkute, B. Methane yield of perennial grasses with as affected by the chemical composition of their biomass. Zemdirb.-Agric. 2020, 107, 243–248. [Google Scholar] [CrossRef]
- Chiumenti, A.; Boscaro, D.; Da Borso, F.; Sartori, L.; Pezzuolo, A. Biogas from Fresh Spring and Summer Grass: Effect of the Harvesting Period. Energies 2018, 11, 1466. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Kasulla, S.; Malik, S.J.; Yadav, A.; Kathpal, G. Potential of Biogas Generation from Hybrid Napier Grass. Int. J. Trend Sci. Res. Dev. 2022, 6, 277–281. [Google Scholar]
- Amon, T.; Amon, B.; Kryvoruchko, V.; Machmuller, A.; Hopfner-Sixt, K.; Bodiroza, V.; Hrbek, R.; Friedel, J.; Potsch, E.; Wagentristl, H.; et al. Methane production through anaerobic digestion of various energy crops grown in sustainable crop rotations. Bioresour. Technol. 2007, 98, 3204–3212. [Google Scholar] [CrossRef] [PubMed]
- Schittenhelm, S. Chemical composition and methane yield of maize hybrids with contrasting maturity. Eur. J. Agron. 2008, 29, 72–79. [Google Scholar] [CrossRef]

| Grass Species | Cellulose [%] | Lignin [%] | Holocellulose [%] | Hemicellulose [%] | ||||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | Before | After | |
| Methane Fermentation | ||||||||
| Festuca arundinacea | 35.56 cd ± 0.11 | 31.11 b ± 0.46 | 17.28 ab ± 0.36 | 44.86 a ± 0.18 | 67.43 bc ± 0.43 | 44.56 ab ± 0.54 | 31.87 b ± 0.51 | 13.45 ab ± 0.61 |
| Bromus inermis | 38.65 a ± 0.53 | 31.36 b ± 0.14 | 14.89 e ± 0.34 | 44.62 a ± 0.22 | 68.40 b ± 0.44 | 42.67 bc ± 0.56 | 29.75 bc ± 0.71 | 11.31 bcd ± 0.70 |
| Lolium perenne | 34.28 e ± 0.14 | 33.35 a ± 0.27 | 15.58 de ± 0.18 | 41.50 b ± 1.32 | 65.48 bcd ± 1.35 | 43.36 bc ± 1.20 | 31.20 b ± 1.23 | 10.01 cd ± 1.47 |
| Lolium westerwoldicum | 31.34 f ± 0.02 | 30.41 bc ± 0.19 | 16.12 bcde ± 0.39 | 44.55 a ± 0.06 | 59.77 e ± 0.63 | 44.44 ab ± 0.69 | 28.43 c ± 0.62 | 14.03 a ± 0.73 |
| Festuca pratensis | 35.17 d ± 0.12 | 29.96 c ± 0.23 | 15.90 cde ± 0.24 | 44.26 a ± 0.72 | 63.69 d ± 0.68 | 41.66 bcd ± 0.15 | 28.52 c ± 0.60 | 11.70 abc ± 0.38 |
| Alopecurus pratensis | 36.42 bc ± 0.26 | 29.48 c ± 0.14 | 17.70 a ± 0.92 | 44.72 a ± 0.25 | 68.97 bc ± 2.19 | 41.20 cd ± 0.77 | 32.55 b ± 2.02 | 11.72 abc ± 0.78 |
| Poa pratensis | 36.13 bc ± 0.03 | 31.37 b ± 0.73 | 16.71 abcd ± 0.15 | 45.32 a ± 0.15 | 71.42 a ± 0.23 | 42.37 bc ± 0.64 | 35.29 a ± 0.20 | 11.00 bcd ± 1.25 |
| Phleum pretense | 36.92 b ± 0.52 | 31.28 b ± 0.50 | 17.02 abc ± 0.25 | 39.67 c ± 0.49 | 65.48 cd ± 0.60 | 40.06 d ± 2.60 | 28.56 c ± 0.78 | 8.78 d ± 1.18 |
| Bromus hordeaceus | 36.39 bc ± 0.45 | 33.67 a ± 0.44 | 12.44 f ± 0.22 | 44.74 a ± 0.41 | 72.93 a ± 0.62 | 47.01 a ± 0.41 | 36.54 a ± 0.57 | 13.34 ab ± 0.78 |
| Grass Species | Extraction Substances [%] | Ash [%] | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| Methane Fermentation | ||||
| Festuca arundinacea | 15.95 cd ± 0.90 | 6.32 bc ± 0.27 | 5.39 f ± 0.02 | 44.40 a ± 0.47 |
| Bromus inermis | 12.02 f ± 0.62 | 6.24 c ± 0.07 | 8.57 b ± 0.02 | 44.61 a ± 0.01 |
| Lolium perenne | 17.04 c ± 0.50 | 6.55 bc ± 0.22 | 6.14 e ± 0.01 | 34.21 d ± 0.33 |
| Lolium vesterwoldicum | 25.04 a ± 0.35 | 6.61 bc ± 0.35 | 9.46 a ± 0.02 | 41.08 b ± 0.20 |
| Festuca pratensis | 20.77 b ± 0.14 | 6.71 bc ± 0.30 | 7.40 c ± 0.54 | 41.97 b ± 0.26 |
| Alopecurus pratensis | 14.19 de ± 0.25 | 6.83 bc ± 0.26 | 4.51 g ± 0.01 | 38.77 c ± 0.61 |
| Poa pratensis | 16.01 c ± 0.34 | 7.63 a ± 0.12 | 4.21 g ± 0.02 | 38.10 c ± 0.24 |
| Phleum pretense | 15.76 c ± 0.30 | 6.91 b ± 0.03 | 6.33 e ± 0.02 | 30.60 e ± 0.14 |
| Bromus hordeaceus | 12.46 ef ± 0.81 | 5.61 d ± 0.11 | 6.93 d ± 0.06 | 45.12 a ± 0.14 |
| Grass Species | DM [%] | ODM [%] |
|---|---|---|
| Festuca arundinacea | 91.21 | 93.00 |
| Bromus inermis | 90.80 | 94.61 |
| Lolium perenne | 92.98 | 87.10 |
| Lolium westerwoldicum | 90.61 | 92.30 |
| Festuca pratensis | 92.07 | 94.73 |
| Alopecurus pratensis | 91.16 | 94.93 |
| Poa pratensis | 89.85 | 91.65 |
| Phleum pretense | 90.96 | 92.35 |
| Bromus hordeaceus | 91.24 | 92.38 |
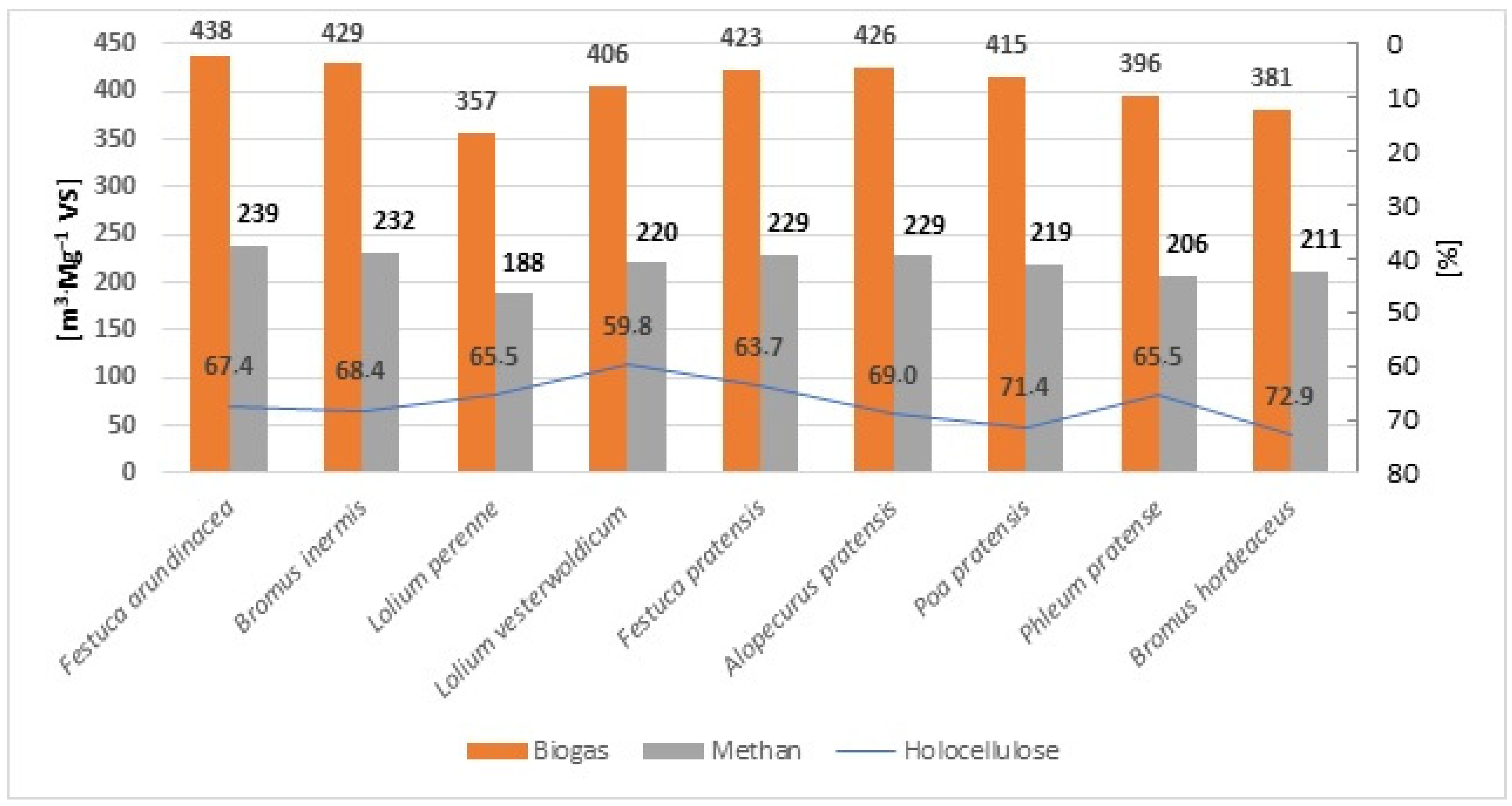
| Grass Species | Methane Yield [m3·Mg−1 VS] | Biogas Yield [m3·Mg−1 VS] |
|---|---|---|
| Festuca arundinacea | 239.36 | 437.89 |
| Bromus inermis | 232.45 | 429.24 |
| Lolium perenne | 187.80 | 356.94 |
| Lolium westerwoldicum | 220.62 | 406.15 |
| Festuca pratensis | 229.56 | 423.08 |
| Alopecurus pratensis | 229.16 | 425.97 |
| Poa pratensis | 219.34 | 415.40 |
| Phleum pretense | 205.62 | 396.58 |
| Bromus hordeaceus | 211.00 | 381.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Waliszewska, B.; Waliszewska, H.; Grzelak, M.; Majchrzak, L.; Gaweł, E.; Murawski, M.; Sieradzka, A.; Vaskina, I.; Spek-Dźwigała, A. Evaluation of Changes in the Chemical Composition of Grasses as a Result of the Methane Fermentation Process and Biogas Production Efficiency. Energies 2024, 17, 4100. https://doi.org/10.3390/en17164100
Waliszewska B, Waliszewska H, Grzelak M, Majchrzak L, Gaweł E, Murawski M, Sieradzka A, Vaskina I, Spek-Dźwigała A. Evaluation of Changes in the Chemical Composition of Grasses as a Result of the Methane Fermentation Process and Biogas Production Efficiency. Energies. 2024; 17(16):4100. https://doi.org/10.3390/en17164100
Chicago/Turabian StyleWaliszewska, Bogusława, Hanna Waliszewska, Mieczysław Grzelak, Leszek Majchrzak, Eliza Gaweł, Maciej Murawski, Agnieszka Sieradzka, Iryna Vaskina, and Agnieszka Spek-Dźwigała. 2024. "Evaluation of Changes in the Chemical Composition of Grasses as a Result of the Methane Fermentation Process and Biogas Production Efficiency" Energies 17, no. 16: 4100. https://doi.org/10.3390/en17164100
APA StyleWaliszewska, B., Waliszewska, H., Grzelak, M., Majchrzak, L., Gaweł, E., Murawski, M., Sieradzka, A., Vaskina, I., & Spek-Dźwigała, A. (2024). Evaluation of Changes in the Chemical Composition of Grasses as a Result of the Methane Fermentation Process and Biogas Production Efficiency. Energies, 17(16), 4100. https://doi.org/10.3390/en17164100








