Experimental Investigation of Physicochemical Properties of the Produced Biodiesel from Waste Frying Oil and Its Blend with Diesel Fuel
Abstract
:1. Introduction
2. State of the Knowledge Review of Biodiesels Produced from Waste Oils
3. Materials and Methods
3.1. Production of Biodiesel from Waste Frying Oil
3.2. Research Equipment
4. Results and Discussion
5. Conclusions
- The utilisation of proprietary technology and a transesterification reactor and model has permitted the production of biodiesel that meets the specifications set forth in EN-14214.
- The initial distillation temperature of subsequent quantities of WFOME biodiesel is demonstrably higher than that of diesel. This can result in a deterioration of the engine’s cold starting capabilities, particularly at low ambient temperatures. Concurrently, WFOME distilled completely to 360 °C, signifying that the most substantial fuel fractions should be incinerated within the engine and will not accumulate as carbon deposits on combustion chamber components. Furthermore, the diesel fully evaporated, reaching a temperature of 360 °C.
- The course of the distillation process of mixtures of WFOME biodiesel and DF diesel oil, with volumes of biodiesel content in the mixtures of 10, 20, and 30% (v/v), does not differ significantly from the distillation process of pure diesel oil. The use of B10, B20, and B30 fuels will not have a significant impact on engine starting and auto-ignition delay.
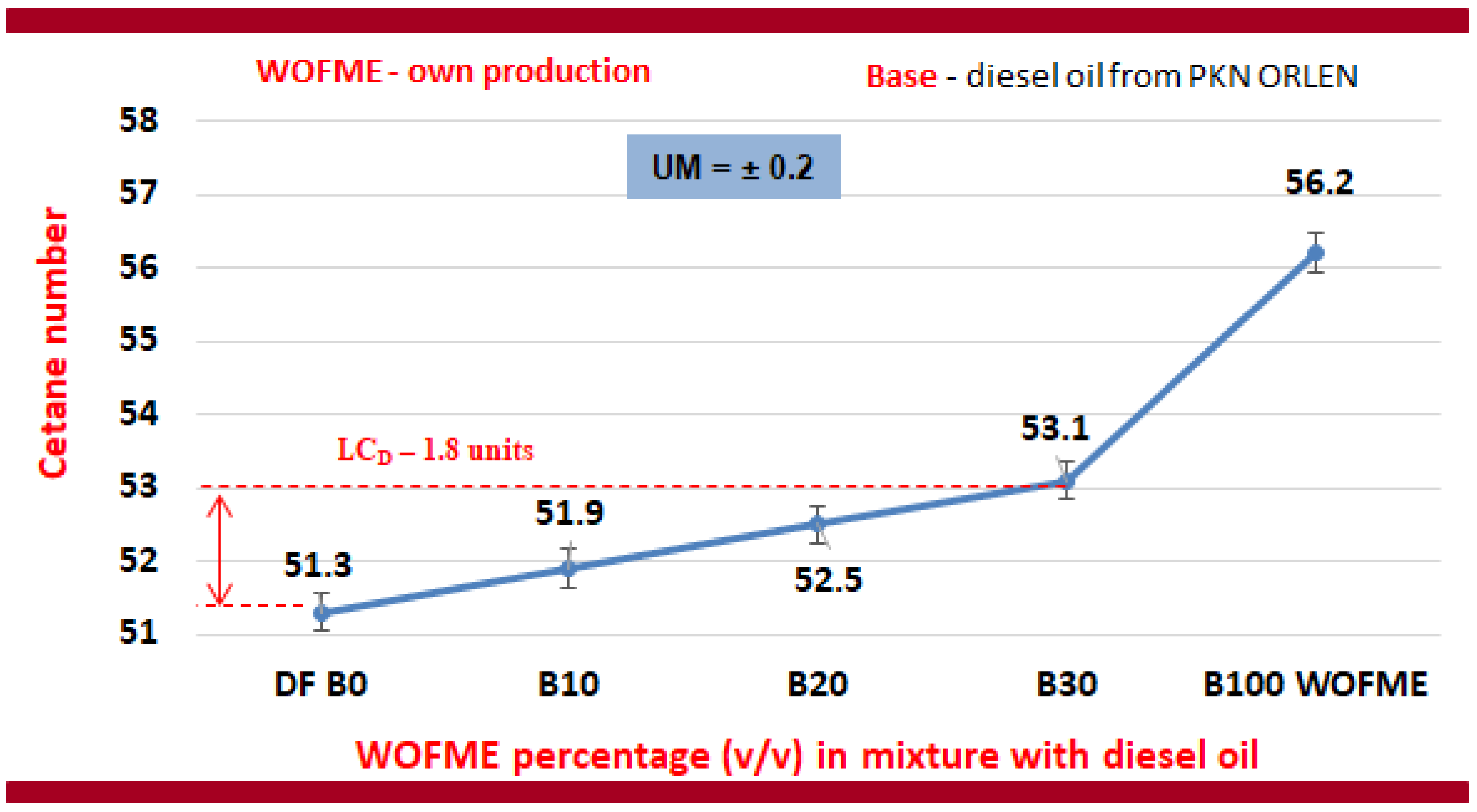
- The cetane number of diesel oil was determined to be 51.3 units. The addition of WFOME resulted in an enhancement of the cetane number. Increasing the content to 30% (v/v) WFOME to diesel resulted in a 1.8-unit increase in CN value, or 3.5%.
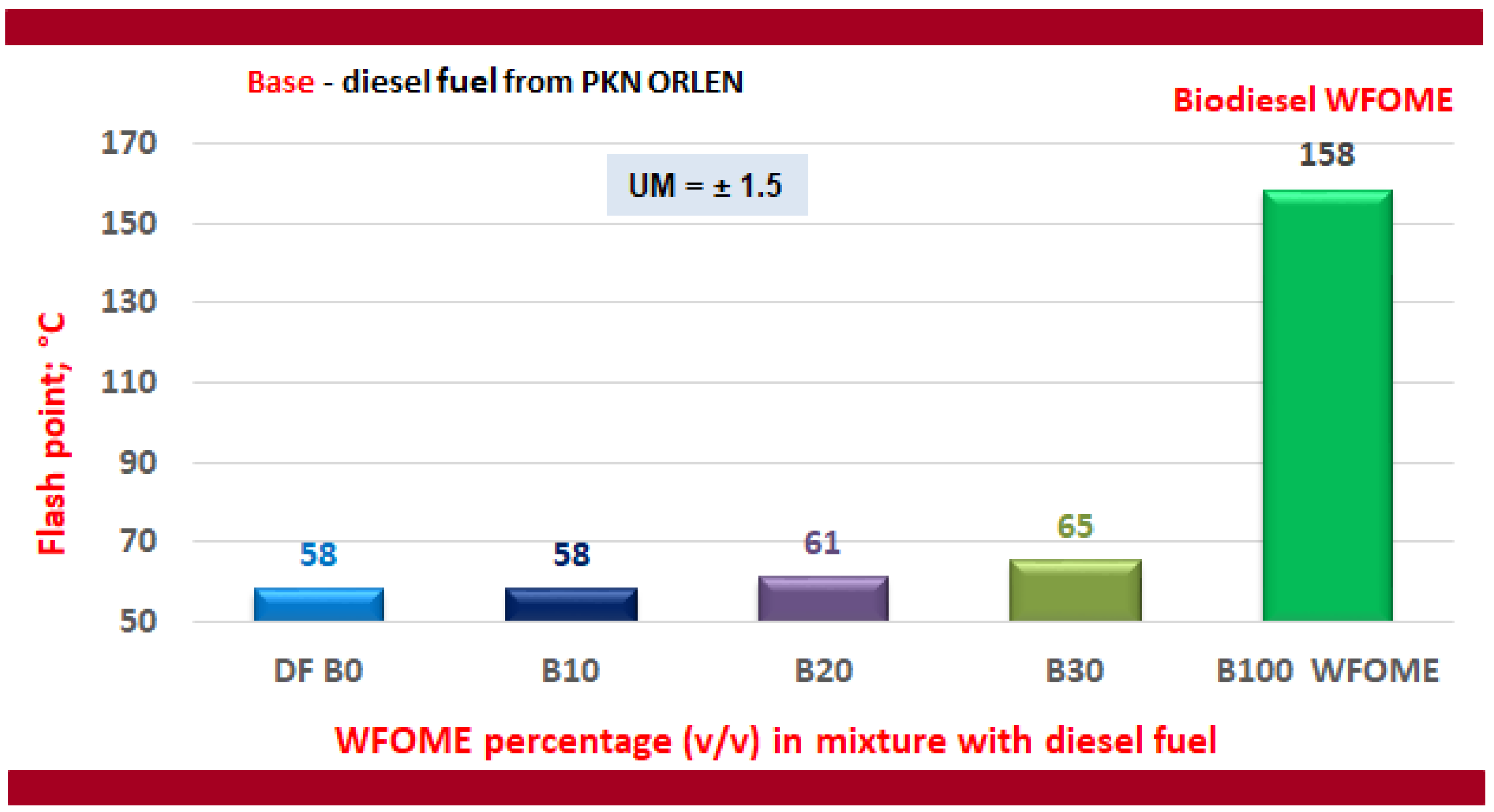
- The flash point of WFOME biodiesel is demonstrably higher than that of DF, which is a favourable feature from the point of view of the safety of storage, transport, and use of the fuel but is a disadvantageous feature from the point of view of the combustion process.
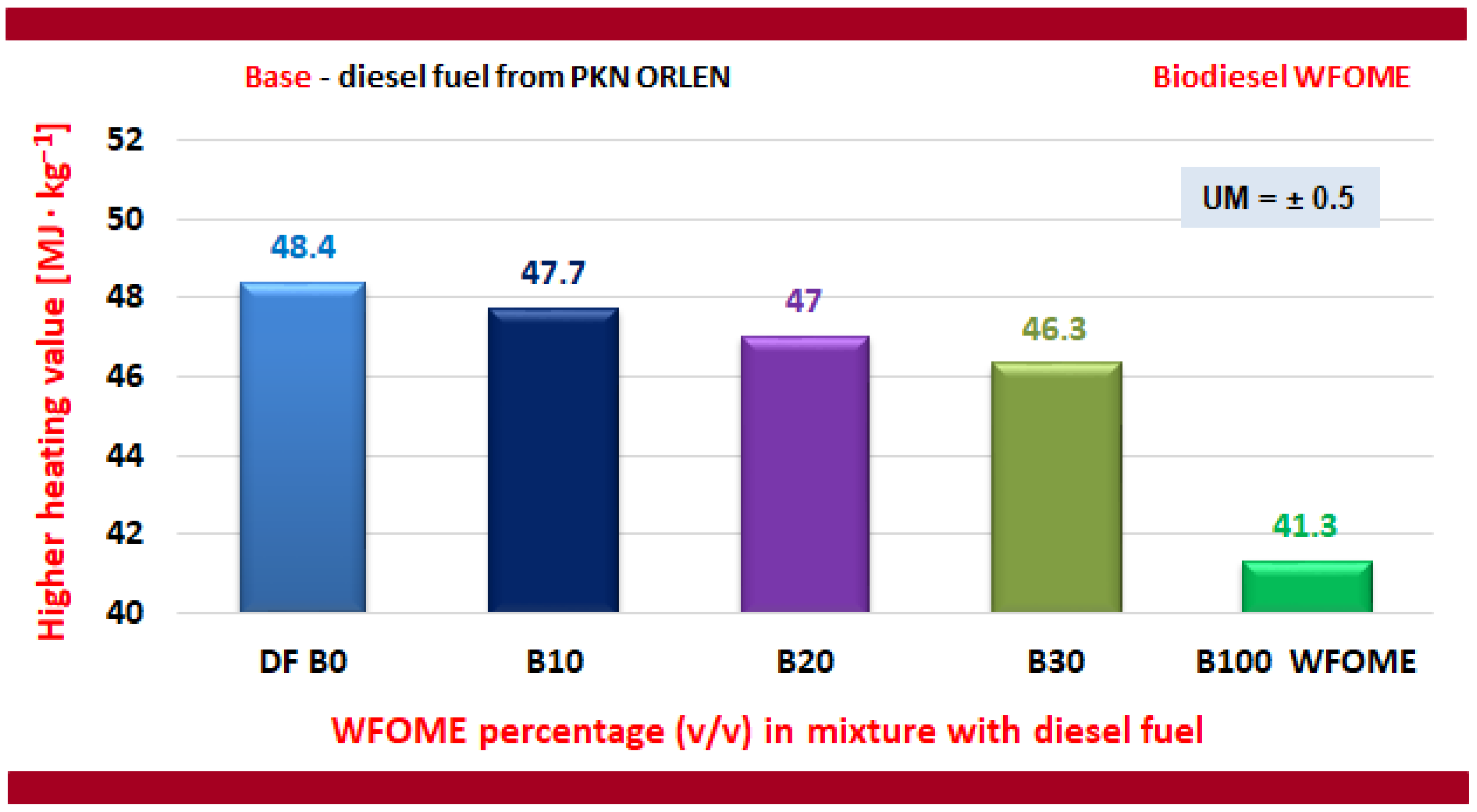
- The higher heating value and lower heating value of WFOME biodiesel exhibited slightly lower values than those determined for diesel. However, this was to be anticipated, given that oxygen is present in the structure of vegetable oils and FAME.
- An increase in the volume proportion of WFOME fuel in DF diesel resulted in an increase in dynamic viscosity.
- The kinematic viscosity of fuels: DF, B10, B20, and B30 meets the requirements of the PN-EN-590 standard, while the kinematic viscosity of WFOME biodiesel meets the requirements of the PN-EN 14214 standard.
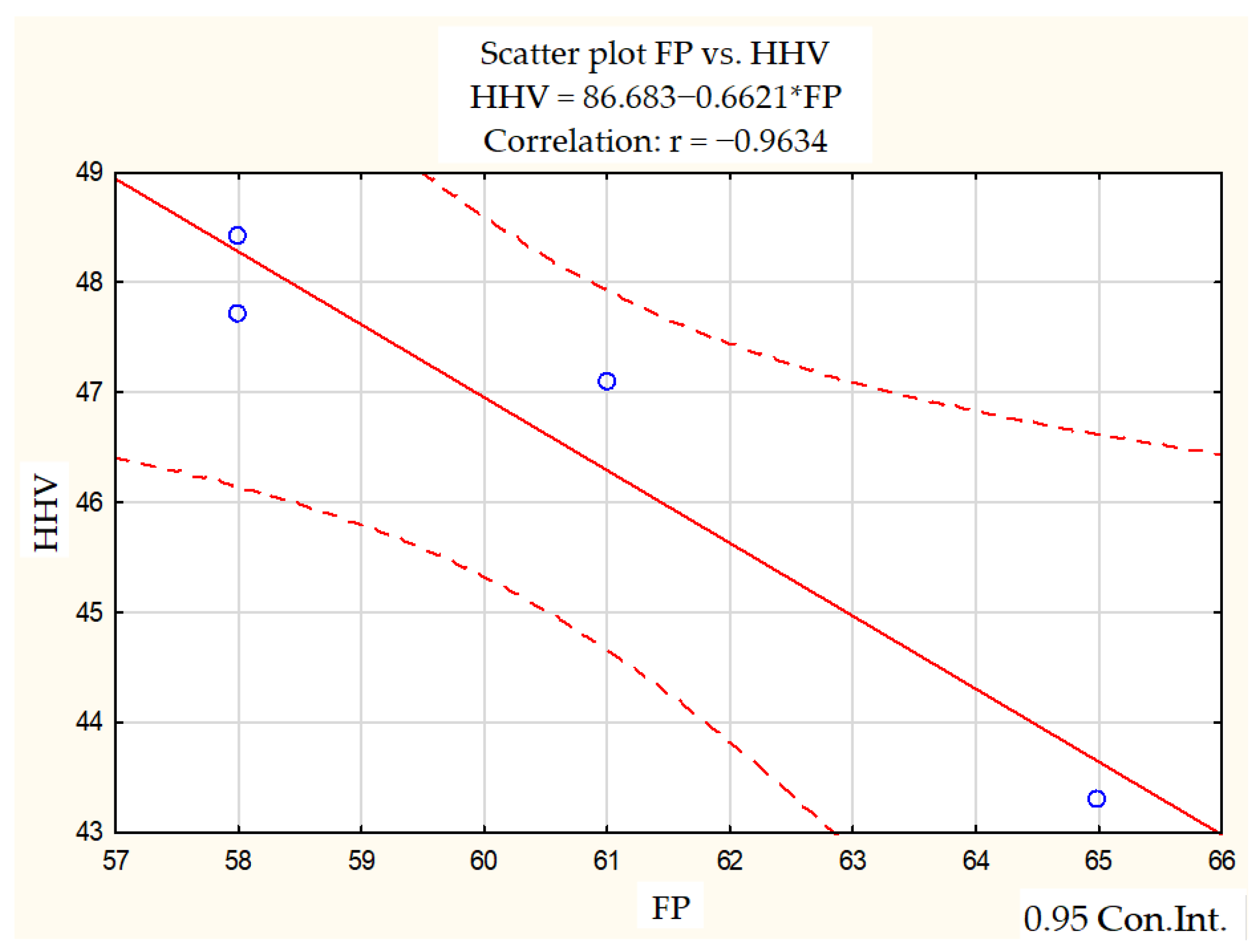
- A negative correlation was identified between flash point and higher heating value, lower heating value and cetane number. The correlation coefficient (r) for the fuels tested between cetane number and lower heating value was r = −0.9980, and that between flash point and the higher heating value was r = −0.9634, indicating a very strong linear relationship between the variables.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| AFME | animal fats methyl esters |
| AFBE | animal fats butyl esters |
| AO | ambadi seed oil |
| Al2O3 | diglinium trioxide |
| B0 | diesel fuel |
| B10 | fuel composed of 10% (v/v) biodiesel and 90% (v/v) diesel fuel |
| B20 | fuel composed of 20% (v/v) biodiesel and 80% (v/v) diesel fuel |
| B30 | fuel composed of 30% (v/v) biodiesel and 70% (v/v) diesel fuel |
| B50 | fuel composed of 50% (v/v) biodiesel and 50% (v/v) diesel fuel |
| B80 | fuel composed of 80% (v/v) biodiesel and 20% (v/v) diesel fuel |
| B100 | biodiesel |
| B10WFOME | fuel composed of 10% (v/v) WFOME and 90% (v/v) DF |
| B20WFOME | fuel composed of 20% (v/v) WFOME and 90% (v/v) DF |
| B30WFOME | fuel composed of 30% (v/v) WFOME and 90% (v/v) DF |
| B100WFOME | 100% waste frying oil methyl esters |
| BP10 | fuel composed of 10% (v/v) n-pentanol and 90% (v/v) biodiesel |
| BP20 | fuel composed of 20% (v/v) n-pentanol and 80% (v/v) biodiesel |
| BP30 | fuel composed of 30% (v/v) n-pentanol and 70% (v/v) biodiesel |
| BTE | brake thermal efficiency |
| CH3OH | methyl alcohol |
| CH3OK | potassium methanolate |
| CKOME | waste chicken oil methyl esters |
| CN | cetan number |
| CNTs | carbon nano tubes particles |
| CO | carbon monoxide |
| LHV | lower heating value |
| DF | diesel fuel |
| FP | flash point |
| HC | hydrocarbons |
| HHV | higher heating value |
| KOH | potassium hydroxide |
| NOx | nitrogen oxides |
| POME | palm oil methyl esters |
| Con.Int. | confidence interval |
| TBK | triglycerides of modified structure |
| T10 | distillation temperature of 10% by volume of fuel, °C |
| T50 | distillation temperature of 50% by volume of fuel, °C |
| T95 | distillation temperature of 95% of the fuel volume, °C |
| UCO | used cooking oil |
| TiO2 | titanium oxide nano particles |
| v/v | volumetric share |
| WCO | waste cooking oil |
| WCOME | waste cooking oil methyl esters |
| WFOME | waste frying oil methyl esters |
| w/w | mass share |
References
- OECD; FAO. OECD-FAO Agricultural Outlook 2023–2032; OECD Publishing: Paris, France, 2023. [Google Scholar] [CrossRef]
- Rosiak, E.; Łopaciuk, W.; Krzemiski, M. Produkcja Biopaliw i Jej Wpływ na Światowy Rynek Zbóż Oraz Roślin Oleistych i Tłuszczów Roślinnych; Instytut Ekonomiki Rolnictwa i Gospodarki Żywnościowej—Państwowy Instytut Badawczy IERiGŻ-PIB: Warszawa, Poland, 2011. [Google Scholar]
- Wong, P.K.; Ghadikolaei, M.A.; Chen, S.H.; Fadairo, A.A.; Ng, K.W.; Lee, S.M.Y.; Xu, J.C.; Lian, Z.D.; Li, S.; Wong, H.C.; et al. Physicochemical and cell toxicity properties of particulate matter (PM) from a diesel vehicle fueled with diesel, spent coffee ground biodiesel, and ethanol. Sci. Total Environ. 2022, 824, 153873. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Li, X.; Wang, S.; Ma, C.; Wang, X. Catalytic effect of diesel PM derived ash on PM oxidation activity. Chemosphere 2022, 299, 134445. [Google Scholar] [CrossRef]
- Katekaew, S.; Suiuay, C.; Senawong, K.; Seithtanabutara, V.; Intravised, K.; Laloon, K. Optimization of performance and exhaust emissions of single-cylinder diesel engines fueled by blending diesel-like fuel from yang-hard resin with waste cooking oil biodiesel via response surface methodology. Fuel 2021, 304, 121434. [Google Scholar] [CrossRef]
- Sayyed, S.; Das, R.K.; Kulkarni, K. Experimental investigation for evaluating the performance and emission characteristics of DICI engine fueled with dual biodiesel-diesel blends of Jatropha, Karanja, Mahua, and Neem. Energy 2022, 238, 121787. [Google Scholar] [CrossRef]
- Kurczyński, D.; Łagowski, P. Performance indices of a common rail-system CI engine powered by diesel oil and biofuel blends. J. Energy Inst. 2019, 92, 1897–1913. [Google Scholar] [CrossRef]
- Kurczyński, D.; Łagowski, P.; Wcisło, G. Experimental study of fuel consumption and exhaust gas composition of a diesel engine powered by biodiesel from waste of animal origin. Energies 2021, 14, 3472. [Google Scholar] [CrossRef]
- Yadav, A.K.; Khan, M.E.; Dubey, A.M.; Pal, A. Performance and emission characteristics of a transportation diesel engine operated with non-edible vegetable oils biodiesel. Case Stud. Therm. Eng. 2016, 8, 236–244. [Google Scholar] [CrossRef]
- Veljkovic, V.B.; Lakicevic, S.H.; Stamenkovic, O.S.; Todorovic, Z.B.; Lazic, M.L. Biodiesel production from tobacco (Nicotiana tabacum L.) seed oil with a high content of free fatty acids. Fuel 2006, 85, 2671–2675. [Google Scholar] [CrossRef]
- Kurczyński, D.; Łagowski, P.; Wcisło, G. Experimental study into the effect of the second-generation BBuE biofuel use on the diesel engine parameters and exhaust composition. Fuel 2021, 284, 118982. [Google Scholar] [CrossRef]
- And Do You Recycle Your Used Cooking Oil at Home? Available online: https://www.greenea.com/publication/and-do-you-recycle-your-used-cooking-oil-at-home/ (accessed on 9 August 2024).
- Production of Biocomponents in 2023. Available online: https://www.gov.pl/web/kowr/produkcja-biokomponentow-w-2023r (accessed on 9 August 2024).
- Ospina, G.; Selim, M.Y.; Al Omari, S.A.; Ali, M.I.H.; Hussien, A.M. Engine roughness and exhaust emissions of a diesel engine fueled with three biofuels. Renew. Energy 2019, 134, 1465–1472. [Google Scholar] [CrossRef]
- Mehregan, M.; Moghiman, M. Effects of nano-additives on pollutants emission and engine performance in a urea-SCR equipped diesel engine fueled with blended-biodiesel. Fuel 2018, 222, 402–406. [Google Scholar] [CrossRef]
- Varuwel, E.G.; Mrad, M.; Tazerout, M.; Aloui, F. Experimental Analysis Of Biofuel As An Alternative Fuel For Diesel Engines. Appl. Energy 2012, 94, 224–231. [Google Scholar] [CrossRef]
- Pattanaik, B.P.; Misra, R.D. Effect of reaction pathway and operating parameters on the deoxygenation of vegetable oils to produce diesel range hydrocarbon fuels: A review. Renew. Sustain. Energy Rev. 2017, 73, 545–557. [Google Scholar] [CrossRef]
- Wcisło, G. Determination of the Rheological Properties of Biofuels Containing SBME Biocomponent. Teka. Comm. Mot. Energetics Agric. 2014, 14, 185–190. [Google Scholar]
- Wcisło, G.; Pracuch, B. Determination of the distillation parameters of the milesPLUS® diesel fuel comprising a bio-component in the form of methyl esters of corn oil. Teka. Comm. Mot. Energetics Agric. 2016, 16, 83–86. [Google Scholar]
- Rajam, C.V.; Patil, G.V.; Sakri, M.I.; Jeeragal, R.N.; Adimurthy, M. Thermal and vibration analysis of CI engine using diesel and waste cooking oil biodiesel blends. Appl. Therm. Eng. 2023, 231, 120949. [Google Scholar] [CrossRef]
- Zhang, Y.; Lou, D.; Tan, P.; Hu, Z.; Fang, L. Effects of waste-cooking-oil biodiesel blends on diesel vehicle emissions and their reducing characteristics with exhaust after-treatment system. J. Clean. Prod. 2022, 381, 135190. [Google Scholar] [CrossRef]
- Kukana, R.; Jakhar, O.P. Performance, combustion and emission characteristics of a diesel engine using composite biodiesel from waste cooking oil—Hibiscus Cannabinus oil. J. Clean. Prod. 2022, 372, 133503. [Google Scholar] [CrossRef]
- Thompson, W.; Meyer, S. Second generation biofuels and food crops: Co-products or competitors? Glob. Food Secur. 2013, 2, 89–96. [Google Scholar] [CrossRef]
- Man, X.J.; Cheung, C.S.; Ning, Z.; Wei, L.; Huang, Z.H. Influence of engine load and speed on regulated and unregulated emissions of a diesel engine fueled with diesel fuel blended with waste cooking oil biodiesel. Fuel 2016, 180, 41–49. [Google Scholar] [CrossRef]
- Meng, J.; Xu, W.; Meng, F.; Wang, B.; Zhao, P.; Wang, Z.; Ji, H.; Yang, Y. Effects of waste cooking oil biodiesel addition on combustion, regulated and unregulated emission characteristics of common-rail diesel engine. Process Saf. Environ. Prot. 2023, 178, 1094–1106. [Google Scholar] [CrossRef]
- Adhikesavan, C.; Ganesh, D.; Augustin, V.C. Effect of quality of waste cooking oil on the properties of biodiesel, engine performance and emissions. Clean. Chem. Eng. 2022, 4, 100070. [Google Scholar] [CrossRef]
- Balasubramanian, D.; Hoang, A.T.; Venugopal, I.P.; Shanmugam, A.; Gao, J.; Wongwuttanasatian, T. Numerical and experimental evaluation on the pooled effect of waste cooking oil biodiesel/diesel blends and exhaust gas recirculation in a twin-cylinder diesel engine. Fuel 2021, 287, 119815. [Google Scholar] [CrossRef]
- Lin, Y.F.; Wu, Y.P.G.; Chang, C.T. Combustion characteristics of waste-oil produced biodiesel/diesel fuel blends. Fuel 2007, 86, 1772–1780. [Google Scholar] [CrossRef]
- Chuepeng, S.; Komintarachatb, C.; Klinkaewc, N.; Maithomklangd, S.; Sukjit, E. Utilization of waste-derived biodiesel in a compression ignition engine. Energy Rep. 2022, 8, 64–72. [Google Scholar] [CrossRef]
- Gad, M.S.; Abdel Azizb, M.M.; Kayed, H. Impact of different nano additives on performance, combustion, emissions and exergetic analysis of a diesel engine using waste cooking oil biodiesel. Propuls. Power Res. 2022, 11, 209–223. [Google Scholar] [CrossRef]
- Sharma, V.; Hossain, A.K.; Ahmed, A.; Rezk, A. Study on using graphene and graphite nanoparticles as fuel additives in waste cooking oil biodiesel. Fuel 2022, 328, 125270. [Google Scholar] [CrossRef]
- PN-EN 590:2022-08; Automotive Fuels-Diesel Fuels-Requirements and Test Methods. The Polish Committee for Standardization: Warsaw, Poland, 2022.
- PN-EN 14214+A2:2019-05; Liquid Petroleum Products-Fatty Acid Methyl Esters (FAME) for Use in Automotive Compression-Ignition (Diesel) Engines and Heating Applications-Requirements and Test Methods. The Polish Committee for Standardization: Warsaw, Poland, 2019.
- PN-EN ISO 3170:2006; Liquid Petroleum Products—Manual Sampling. The Polish Committee for Standardization: Warsaw, Poland, 2006.
- Kumbhar, V.; Pandey, A.; Sonawane, C.R.; El-Shafay, A.S.; Panchal, H.; Chamka, A.J. Statistical analysis on prediction of biodiesel properties from its fatty acid composition. Case Stud. Therm. Eng. 2022, 30, 101775. [Google Scholar] [CrossRef]
- Fernando, S.; Karra, P.; Hernandez, R.; Jha, S.K. Effect of incompletely converted soybean oil on biodiesel quality. Energy 2007, 32, 844–851. [Google Scholar] [CrossRef]
- Sanford, S.D.; White, J.M.; Shah, P.S.; Wee, C.; Valverde, M.A.; Meier, G.R. Feedstock and Biodiesel Characteristics Report; Renewable Energy Group: Ames, IA, USA, 2009. [Google Scholar]
- Wcisło, G. Analysis of the Impact of Rapeseed Varieties on the Properties of RME Biofuels and Diesel Engine Operation Parameters; FALL Publishing House: Krakow, Poland, 2013. [Google Scholar]
- Wcisło, G.; Kurczyński, D.; Łagowski, P.; Leśniak, A.; Kozak, M.; Pracuch, B. Determination of the impast of the secondo generation biodiesel BBuE addition to diesel oil on selected parameters of “B” fuels. Energies 2023, 16, 6999. [Google Scholar] [CrossRef]
- Girardi, J.C.; Bariccatti, R.A.; de Souza, S.N.M.; do Amaral, C.Z.; Guedes, C.L.B. Natural compounds as antifreeze additives to improve babassu biodiesel. Fuel 2021, 289, 119746. [Google Scholar] [CrossRef]
- Lapuerta, M.; Rodriguez-Fernandez, J.; Fernandez-Rodriguez, D.; Patino-Camino, R. Modeling viscosity of butanol and ethanol blends with diesel and biodiesel fuels. Fuel 2017, 199, 332–338. [Google Scholar] [CrossRef]
- Cisek, J.; Mruk, A.; Hlavna, V. The properties of a HDV Diesel engine fuelled by crude rapeseed oil. Teka Kom. Motoryz. I Energetyki Rol. 2011, 11, 29–39. [Google Scholar]
- Cisek, J.; Mruk, A. Characteristic of a diesel engine fuelled by natural rape oil. Proc. Inst. Veh. Wars. Univ. Technol. 2012, 1, 5–16. [Google Scholar]
- Jakóbiec, J.; Ambrozik, A. Ageing processes of rape-seed oil fatty acid methyl esters. Inż. Rol. 2009, 5, 85–90. [Google Scholar]
- Szlachta, Z. Zasilanie Silników Wysokoprężnych Paliwami Rzepakowymi; Wydawnictwa Komunikacji i Łączności WKŁ: Warsaw, Poland, 2002. [Google Scholar]
- Kurczyński, D.; Wcisło, G.; Leśniak, A.; Kozak, M.; Łagowski, P. Production and Testing of Butyl and Methyl Esters as New Generation Biodiesels from Fatty Wastes of the Leather Industry. Energies 2022, 15, 8744. [Google Scholar] [CrossRef]
- Wcisło, G. Determination of the impact of FAME biocomponent on the fraction composition of diesel engine fuels. Combust. Engine 2013, 154, 1098–1103. [Google Scholar]
- Thapa, S.; Indrawan, N.; Bhoi, P.R. An overview on fuel properties and prospects of Jatropha biodiesel as fuel for engines Environ. Environ. Technol. Innov. 2018, 9, 210–219. [Google Scholar] [CrossRef]
- Marshal, E.L.; Owen, K. Motor Gasoline. The College of Petroleum and Energy Studies; The Royal Society of Chemistry: Oxford, UK, 1995. [Google Scholar] [CrossRef]
- Gouveia, L.; Oliveira, A.C.; Congestri, R.; Bruno, L.; Soares, A.T.; Menezes, R.S.; Tzovenis, I. Biodiesel from microalgae. In Microalgae-Based Biofuels and Bioproducts. From Feedstock Cultivation to End-Products, 1st ed.; Muñoz, R., Gonzalez-Fernandez, C., Eds.; Woodhead Publishing: Sawston, UK, 2018; Volume 10, pp. 235–258. [Google Scholar] [CrossRef]
- Ateeq, E.A. Biodiesel Viscosity on Flash Point Determination. Master’s Thesis, An-Najah National University, Nablus, Palestine, 2015. [Google Scholar]
- Nisar, N.; Mehmood, S.; Nisar, H.; Jamil, S.; Ahmad, Z.; Ghani, N.; Oladipo, A.A.; Qadri, R.W.; Latif, A.A.; Ahmad, S.R.; et al. Brassicaceae family oil methyl esters blended with ultra-low sulphur diesel fuel (ULSD): Comparison of fuel properties with fuel standards. Renew. Energy 2018, 117, 393–403. [Google Scholar] [CrossRef]
- Foroutana, R.; Esmaeilib, H.; Mousavic, S.M.; Hashemic, S.A.; Yeganeh, G. The Physical Properties of Biodiesel-Diesel Fuel Produced via Transesterification Process from Different Oil Sources. Phys. Chem. Res. 2019, 7, 415–424. [Google Scholar] [CrossRef]
- Semorile, N.F.; Alviso, D.; Romano, S.D. Flash point and refractive index measurements of diesel and biodiesel, and their binary blends with n-butanol and n-pentanol. J. Braz. Soc. Mech. Sci. Eng. 2023, 45, 28. [Google Scholar] [CrossRef]
- Mattos, R.A.; Bastos, F.A.; Tubino, M. Correlation Between the Composition and Flash Point of Diesel-Biodiesel Blends. J. Braz. Chem. Soc. 2015, 26, 393–395. [Google Scholar] [CrossRef]
- Anwar, F.; Rashid, U.; Ashraf, S.; Al-Resayes, S.I.; Mehmood, M.A.; Nehdi, I.A.; Ibrahim, M.; Hanif, M.A. Biodiesel synthesis from Brassica napus seed oil using statistical optimization approach. J. Renew. Sustain. Energy 2017, 9, 013103. [Google Scholar] [CrossRef]
- Llamas, A.; Al-al, A.-M.; García Martínez, M.-J.; Ortega, M.F.; Llamas, J.F.; Lapuerta, M.; Canoira, L. Polycyclic aromatic hydrocarbons (PAHs) produced in the combustion of fatty acid alkyl esters from different feedstocks: Quantification, statistical analysis and mechanisms of formation. Sci. Total Environ. 2017, 586, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Imtenan, S.; Masjuki, H.H.; Varman, M.; Fattah, I.M.R.; Sajjad, H.; Arbab, M.I. Effect of n-butanol and diethyl ether as oxygenated additives on combustion–emission-performance characteristics of a multiple cylinder diesel engine fuelled with diesel–jatropha biodiesel blend. Energy Convers. Manag. 2015, 94, 84–94. [Google Scholar] [CrossRef]
- Szabados, G.; Bereczky, A. Evaluation analysis of particulate relevant emission of a diesel engine running on fossil diesel 1 and different biofuels. Energy 2018, 161, 1139–1153. [Google Scholar] [CrossRef]
- Bharathwaaj, R.; Nagarajan, P.K.; Kabeel, A.E.; Madhu, B.; Mageshbabu, D.; Sathyamurthy, R. Formation, characterization and theoretical evaluation of combustion of biodiesel obtained from wax esters of A. Mellifera. Alex. Eng. J. 2018, 57, 1205–1215. [Google Scholar] [CrossRef]
- Mohamed, M.; Tan, C.-K.; Fouda, A.; Gad, M.S.; Abu-Elyazeed, O.; Hashem, A.-F. Diesel Engine Performance, Emissions and Combustion Characteristics of Biodiesel and Its Blends Derived from Catalytic Pyrolysis of Waste Cooking Oil. Energies 2020, 13, 5708. [Google Scholar] [CrossRef]
- Raj, F.R.M.S.; Sahayaraj, J.W. A comparative study over alternative fuel (biodiesel) for environmental friendly emission. In Proceedings of the Recent Advances in Space Technology Services and Climate Change 2010 (RSTS & CC-2010), Chennai, India, 13–15 November 2010; pp. 80–86. [Google Scholar] [CrossRef]
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Pinzi, S.; Garcia, I.L.; Gimenez, F.J.L.; Castro, M.D.L.; Dorado, G.; Dorado, M.P. The ideal vegetable oil-based biodiesel composition: A review of social, economical and technical implications. Energy Fuels 2009, 23, 2325–2341. [Google Scholar] [CrossRef]
- Knothe, G. A comprehensive evaluation of the cetane numbers of fatty acid methyl esters. Fuel 2014, 119, 6–13. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Ladommatos, N.; Goacher, J. Equations for predicting the cetane number of diesel fuels from their physical properties. Fuel 1995, 74, 1083–1093. [Google Scholar] [CrossRef]
- Saxena, P.; Jawale, S.; Joshipura, M.H. A review on prediction of properties of biodiesel and blends of biodiesel. Procedia Eng. 2013, 51, 395–402. [Google Scholar] [CrossRef]
- El-Hagar, M.M.E.-G. Effect of fuel cetane numbers on reducing the ignition delay period and exhaust emissions from DI diesel engine. Wseas Trans. Heat Mass Transf. 2020, 99–105. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 169, 2070–2093. [Google Scholar] [CrossRef]
- Canakci, M.; Sanli, H. Biodiesel production from various feedstocks and their effects on the fuel properties. J. Ind. Microbiol. Biotechnol. 2008, 35, 431–441. [Google Scholar] [CrossRef]
- Guo, S.; Yang, Z.; Gao, Y. Effect of Adding Biodiesel to Diesel on the Physical and Chemical Properties and Engine Performance of Fuel Blends. J. Biobased Mater. Bioenergy 2016, 10, 34–43. [Google Scholar] [CrossRef]
- Kaisan, M.U.; Anafi, F.O.; Nuszkowski, J.; Kulla, D.M.; Umaru, S. Calorific value, flash point and cetane number of biodiesel from cotton, jatropha and neem binary and multi-blends with diesel. Biofuels 2017, 11, 321–327. [Google Scholar] [CrossRef]
- Heywood, J.B. Internal Combustion Engine Fundamentals; McGraw-Hill Inc.: New York, NY, USA, 1988. [Google Scholar]
- Xu, W.; Meng, J.; Wang, Z.; Chen, Z.; Wang, X.; Zhang, Z.; Zheng, B. Effects of n-pentanol/biodiesel blend fuels on combustion and conventional and unconventional emission characteristics of diesel engine. Environ. Sci. Pollut. Res. 2023, 30, 124204–124214. [Google Scholar] [CrossRef]


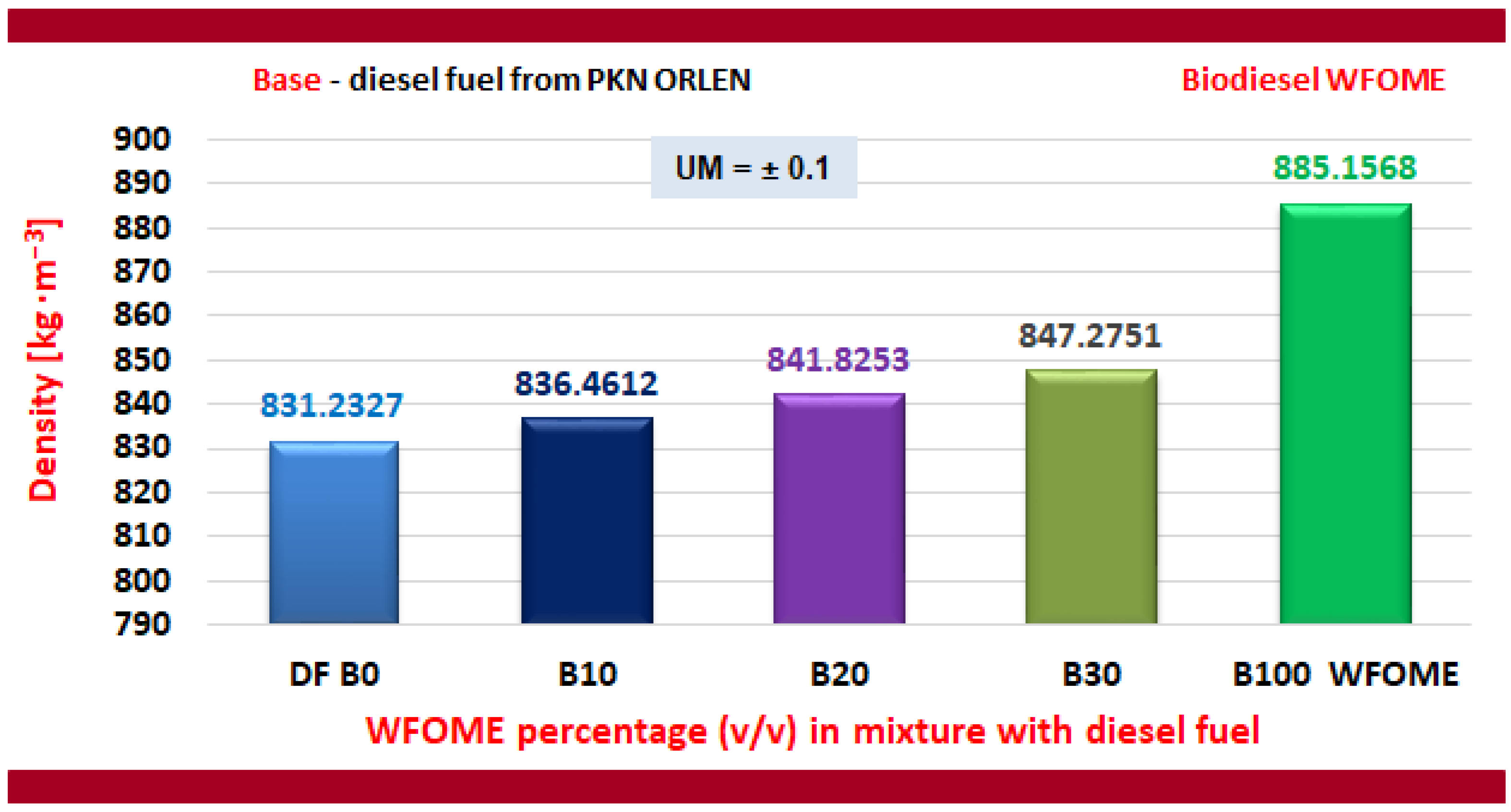
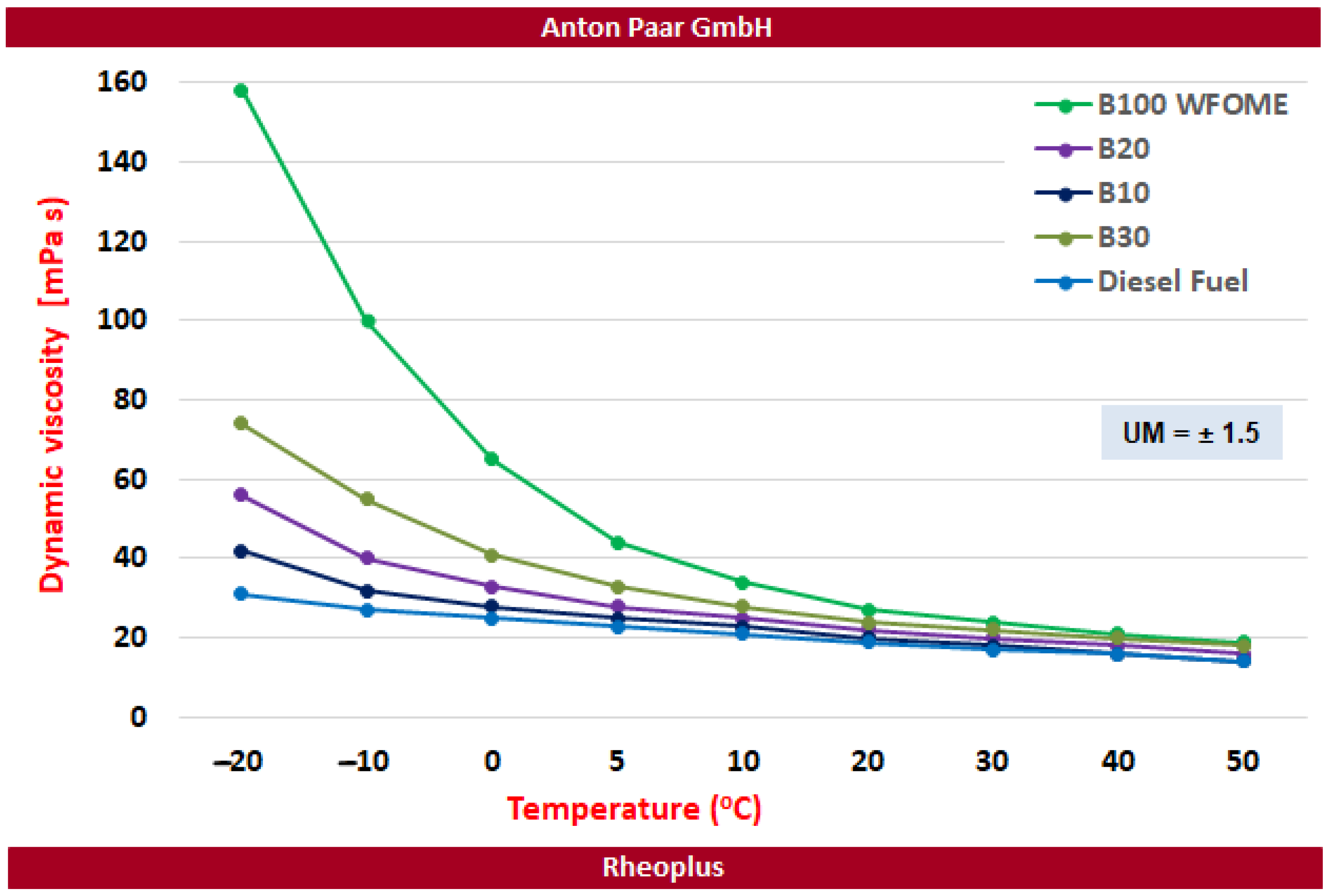
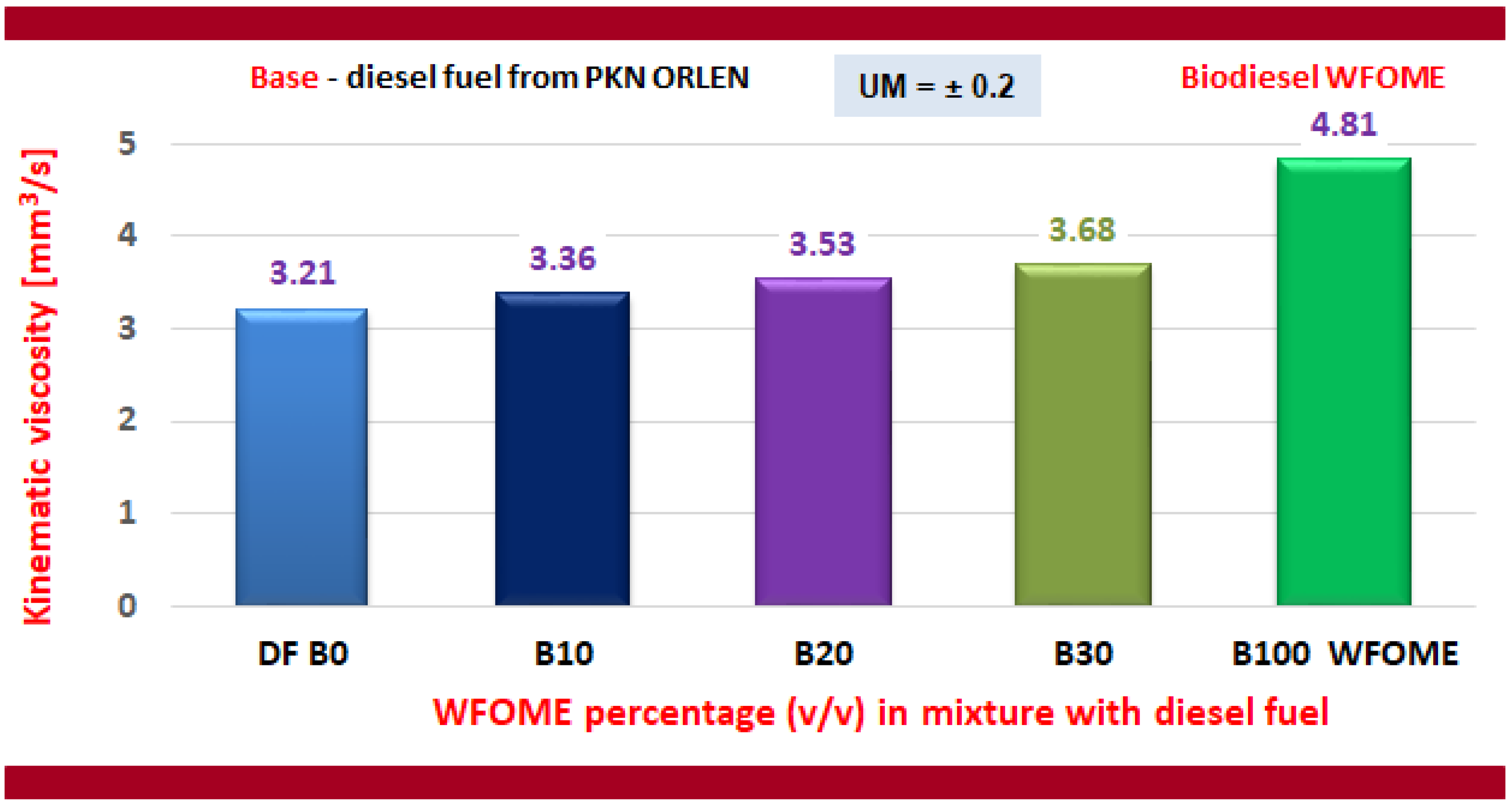
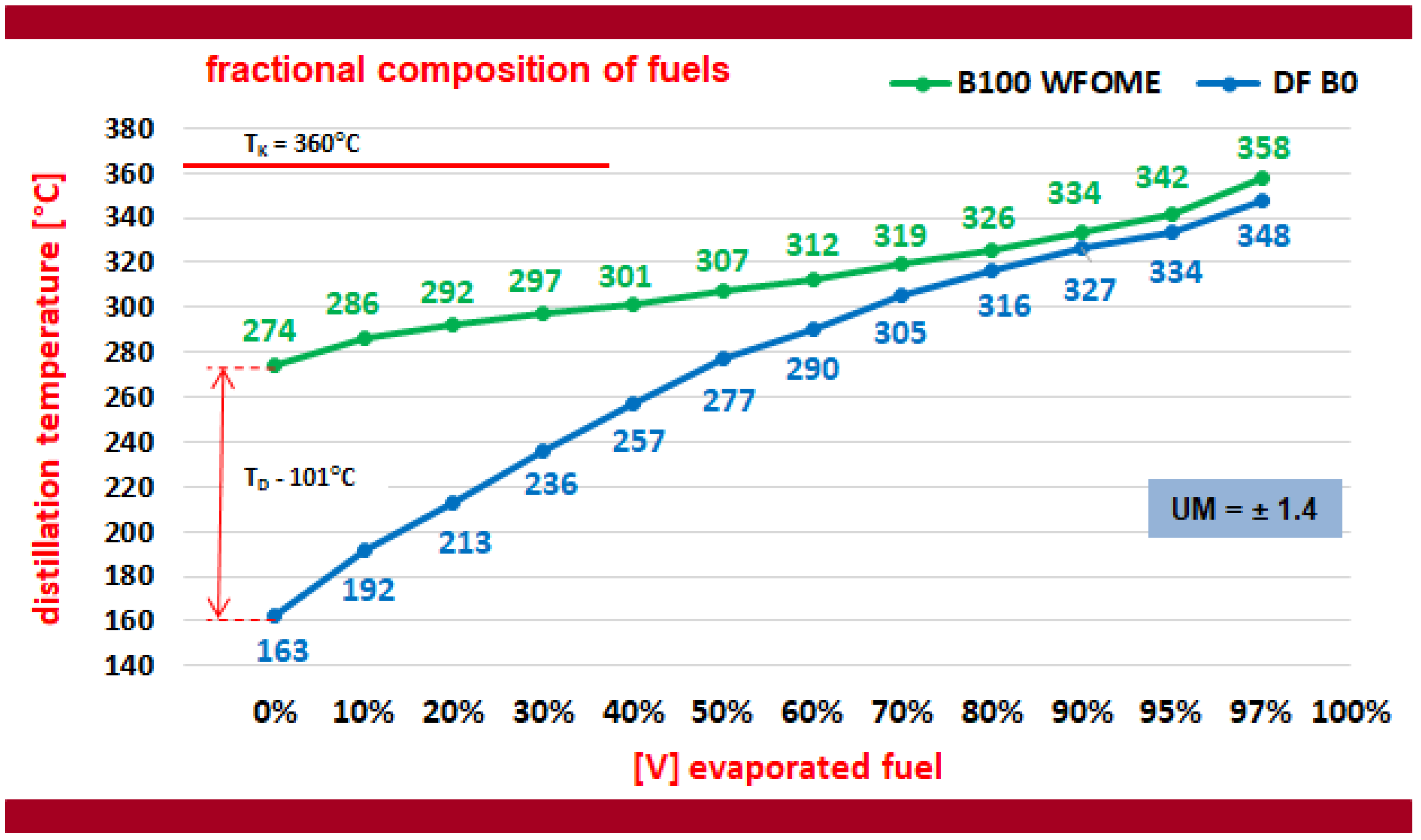



| Measured Parameter | Unit | Value |
|---|---|---|
| Supply voltage | V | 230 |
| Rated power | kWh | 5.15 |
| Production time per cycle | h/cycle | 1.5 |
| Efficiency per cycle | dm3/cycle | 50 |
| Type of catalyst | - | Basic/acidic |
| Process | - | Periodic or semi-continuous |
| Type of process | - | Single-stage or two-stage |
| Distillation Process Parameter | WFOME | DF | WFOME-DF |
|---|---|---|---|
| Temperature of onset of distillation TD; °C | 274 | 163 | 111 |
| Distillation temperature of 10% of the fuel sample volume T10; °C | 286 | 192 | 94 |
| Distillation temperature of 50% of the fuel sample volume T50; °C | 307 | 277 | 30 |
| Distillation temperature of 90% of the fuel sample volume T90; °C | 334 | 327 | 7 |
| Distillation temperature of 95% of the fuel sample volume T95; °C | 342 | 334 | 8 |
| Fuel distillation end temperature (T97); °C | 358 | 348 | 10 |
| Distillation Process Parameter | B10 WFOME | B20 WFOME | B30 WFOME |
|---|---|---|---|
| Temperature of onset of distillation TD; °C | 163 | 163 | 163 |
| Distillation temperature of 10% of the fuel sample volume T10; °C | 197 | 199 | 201 |
| Distillation temperature of 50% of the fuel sample volume T50; °C | 273 | 278 | 284 |
| Distillation temperature of 90% of the fuel sample volume T90; °C | 329 | 331 | 334 |
| Distillation temperature of 95% of the fuel sample volume T95; °C | 343 | 345 | 347 |
| Fuel distillation end temperature (T97); °C | 358 | 358 | 358 |
| Parameter | Standard Deviation | r(X,Y) | r2 | t | p |
|---|---|---|---|---|---|
| FP CN | 3.3166 0.7745 | 0.9342 | 0.8727 | 3.7033 | 0.06580 |
| FP HHV | 3.3166 2.2794 | −0.9634 | 0.9281 | −5.0824 | 0.03660 |
| FP LHV | 3.3166 0.7632 | −0.9415 | 0.8865 | −3.9524 | 0.05846 |
| CN HHV | 0.7745 2.2794 | −0.9005 | 0.8109 | −2.9289 | 0.09948 |
| CN LHV | 0.7745 0.7632 | −0.9980 | 0.9960 | −22.2999 | 0.00201 |
| HHV LHV | 2.2794 0.7632 | 0.9230 | 0.8520 | 3.3934 | 0.07695 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wcisło, G.; Leśniak, A.; Kurczyński, D.; Pracuch, B. Experimental Investigation of Physicochemical Properties of the Produced Biodiesel from Waste Frying Oil and Its Blend with Diesel Fuel. Energies 2024, 17, 4175. https://doi.org/10.3390/en17164175
Wcisło G, Leśniak A, Kurczyński D, Pracuch B. Experimental Investigation of Physicochemical Properties of the Produced Biodiesel from Waste Frying Oil and Its Blend with Diesel Fuel. Energies. 2024; 17(16):4175. https://doi.org/10.3390/en17164175
Chicago/Turabian StyleWcisło, Grzegorz, Agnieszka Leśniak, Dariusz Kurczyński, and Bolesław Pracuch. 2024. "Experimental Investigation of Physicochemical Properties of the Produced Biodiesel from Waste Frying Oil and Its Blend with Diesel Fuel" Energies 17, no. 16: 4175. https://doi.org/10.3390/en17164175






