Feasibility of Food Organics and Garden Organics as a Promising Source of Biomethane: A Review on Process Optimisation and Impact of Nanomaterials
Abstract
1. Introduction
2. Foundational Concepts in Anaerobic Digestion of Organic Waste
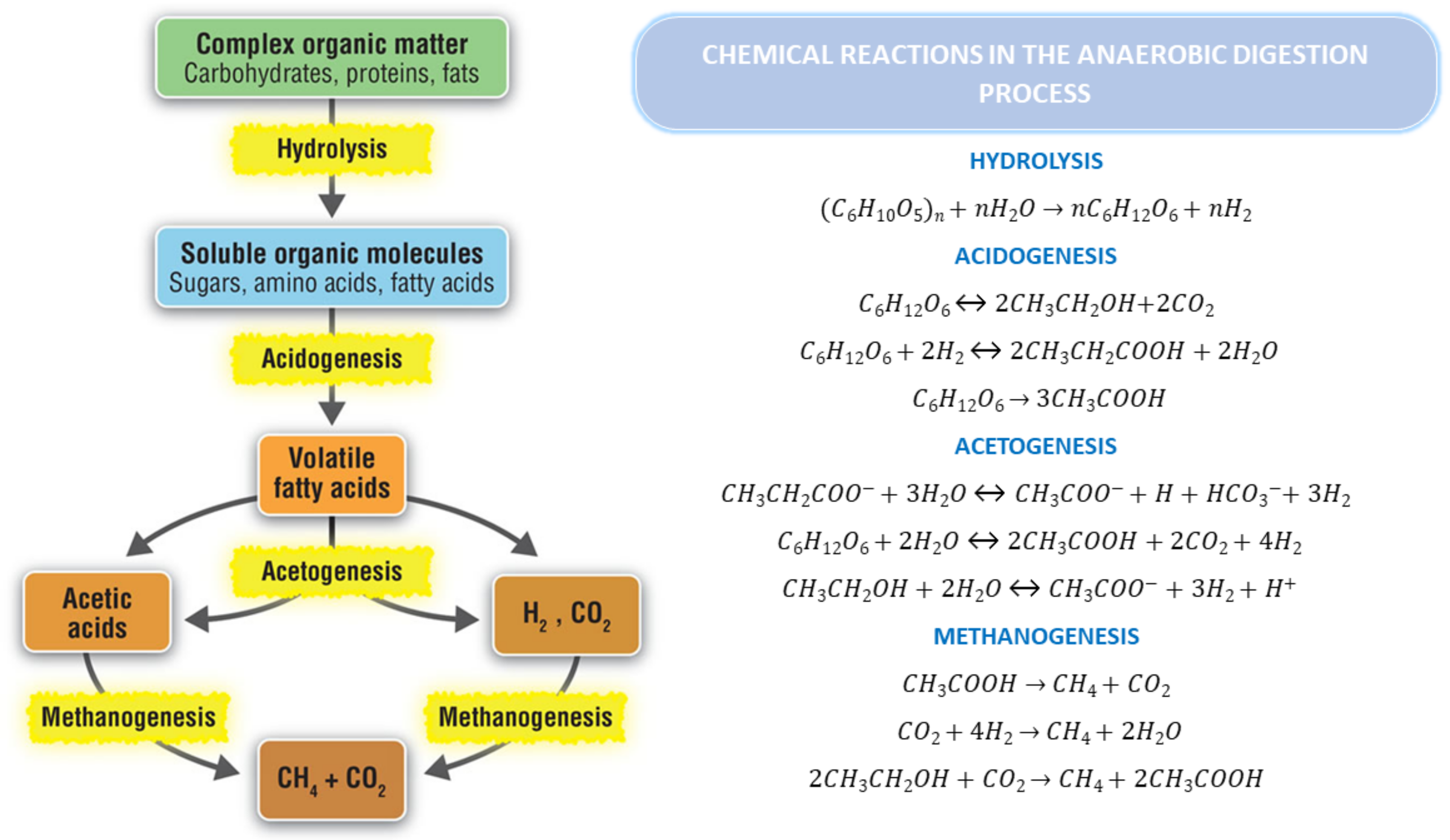
2.1. Anaerobic Digestion
2.1.1. Hydrolysis
2.1.2. Acidogenesis
2.1.3. Acetogenesis
2.1.4. Methanogenesis
2.2. Factors Affecting Anaerobic Digestion
2.2.1. Chemical Composition of the Substrate
2.2.2. Inoculum-to-Substrate Ratio (ISR)
2.2.3. C/N Ratio
2.2.4. Operation pH
2.2.5. Operating Temperature
2.2.6. Trace Elements
2.2.7. Organic Loading Rate (OLR)
- OLR = Organic loading rate (kg VSin/L/day);
- Q = Flow rate of the feed (L/day);
- VSin = Volatile solids concentration in the feed (kg/L);
- VSremoval = Volatile solids concentration in the digestate (kg/L);
- VSout = Volatile solids concentration in the digestate (kg/L);
- V = Volume of the digester (L);
- VSR = Volatile solid removal (%);
- MPR = Methane production rate (NL-CH4/day);
- SMY = Specific methane yields (NL-CH4/kg VSin);
- VFAaccumulation = Rate of VFA accumulation;
- VFAproduction = Rate of VFA production;
- VFAconsumed = Rate of VFA consumed.
2.2.8. Toxicity
2.2.9. Hydraulic Retention Time (HRT)
3. Feasibility of FOGO as a Promising Substrate for Biomethane Production
3.1. Characteristics of FOGO

| Substrate | Co-Substrate | Mixing Ratio | Mode of AD | Operating Conditions | Biogas Yield | Comments | Reference |
|---|---|---|---|---|---|---|---|
| FW | Pig Manure (PM) | PM:KW = 1:0, 4:1, 3:2, 2:3, 1:4, 0:1. | Batch | OLR—5.2 g VS, Temp—37 °C HRT—8 days | 0.521 m3/kg VS | Ideal mixing ratio = 1:4, no VFA or NH3 inhibition | [142] |
| FW | De-oiled grease trap waste | _ | CSTR: −3 system: single-stage, two-stage | HRT—30 days | 0.60 m3/kg VS | 19% increase in biogas yield, lipid–lipid/TS breakdown at 40% | [139] |
| FW: canteen waste | Straw from maize, sorgos, and wheat | 5:1 | Batch | HRT—8 days, OLR—5 g VS/L, Temp—35 °C | 0.392 m3 CH4/kg VS | Increased CH4 yields by 39.5% and 149.7%, respectively. | [143] |
| KW: canteen waste | CM | 1:1 | Batch | HRT—45 days, OLR—TS 8%, Temp—35 °C | 0.859 m3/kg VS | The effects of the initial pH were investigated, with 6.0 causing digester failure and 7.5 recommended. | [93] |
| FW: canteen waste | Rice husk | Combined to provide a 28 C/N ratio | Plug flow pilot plant single-stage, | OLR—6 kg VS/m3d, HRT—25 days Temp—37 °C | 0.446 m3 biogas/kg VS | With an increase in OLR and a drop in HRT, VS removal efficiency reduced; instability caused by VFA and high alkalinity (0.94) at OLR of 9 kg VS/m3d. | [144] |
| Yard waste (YW) | FW | 100% YW; YW:FW = 9:1; YW:FW = 4:1 | Batch | OLR—0.0–166 g/L, Temp—36 ± 1 °C HRT—30 days | 8.6 Lmethane/Lwork | When FW increased from 0% to 10%, the CH4 yield climbed by two times, at 20%, induced VFA inhibition, and CH4 output fell by 9.7 times. | [145] |
| FW | Microwave-treated YW | 1.5 | Batch | Temp—30 °C HRT—30 days | 431 mL/gVSadded | Max CH4 yield at an F/M ratio of 1.5. Highest nett energy gain of 6.5 kJ/gVS at F/M ratio of 1.0, suggesting the possibility of field application at 1.0 instead of 1.5. | [146] |
| YW | Sewage Sludge (SS) and FW | YW:FW:SS = 9:3:4; 6:6:4; 3:9:4 | Batch | Temp—37 °C HRT—60 days | 314.9 ± 17.1 mL/gVS | AcoD of SS (25%, VS basis) with YW boosted the CH4 yield by 2.04 times | [147] |
| OFMSW (FW, GW) | _ | _ | Batch | Temp—37 °C HRT—21 days | 126 mL CH4/(gVSadded) | S/I > 1.0 based on TS, accumulation of inhibitory intermediates can result in system failure due to mass transfer constraint under low moisture conditions. | [148] |
| FW | GW | 4:1 | Pilot scale reactors (500 L) | OLR—0.24 kgVS m−3d−1, Temp—40 °C HRT—40 days | 0.47 LCH4 gVS−1 | Garden waste promoted growth of micro-organisms as a sustaining medium for biofilms, delaying the reactor’s acidification | [149] |
| FW | GW, WAS (waste-activated sludge) | FW/YW/WAS = 0.8:1.7:0.5FW/YW/WAS = 1:1:1 | 4.7 L semi-continuous biodigester | HRT—28 days, Temp—35 °C | 186 mL/gVS | Compared to mixes with FW/YW/WAS = 1:1:1, mixtures with FW/YW/WAS = 0.8:1.7:0.5 had greater CH4 yields (134 ± 15 mL CH4/gVS) but took longer (10 days) to recover from volatile fatty acid inhibition. | [150] |
| FW: university canteen | CM | _ | Batch | Temp—35 °C HRT—28 days | 0.388 m3 CH4/kg VS | AcoD increased CH4 generation by 41.1% in batch mode and by 55.2% in semi-continuous mode at optimal mixing at 2. | [140] |
3.2. Methods of Biomethane Production from FOGO
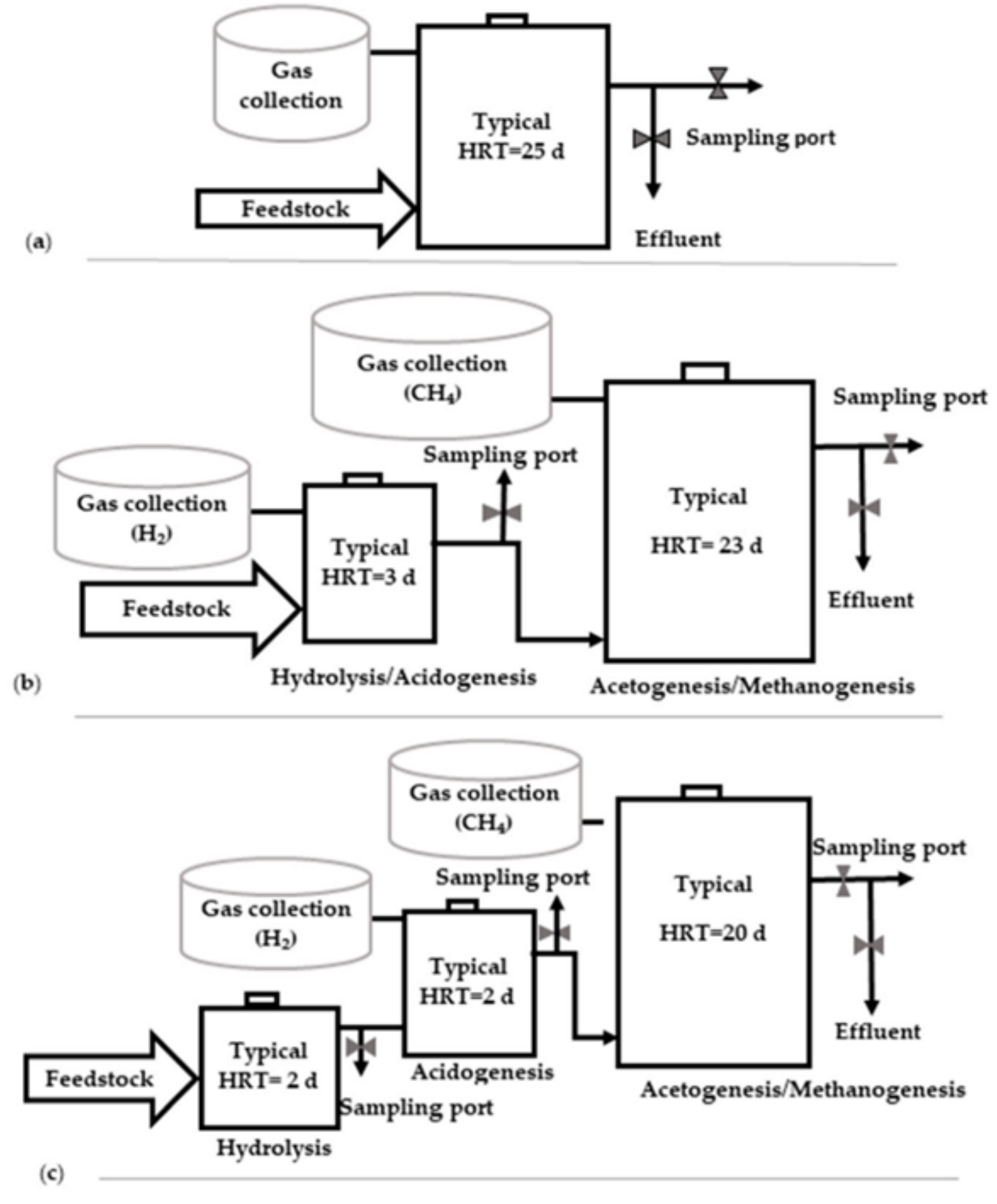
3.2.1. Comparison between Single-Stage and Multi-Stage Process
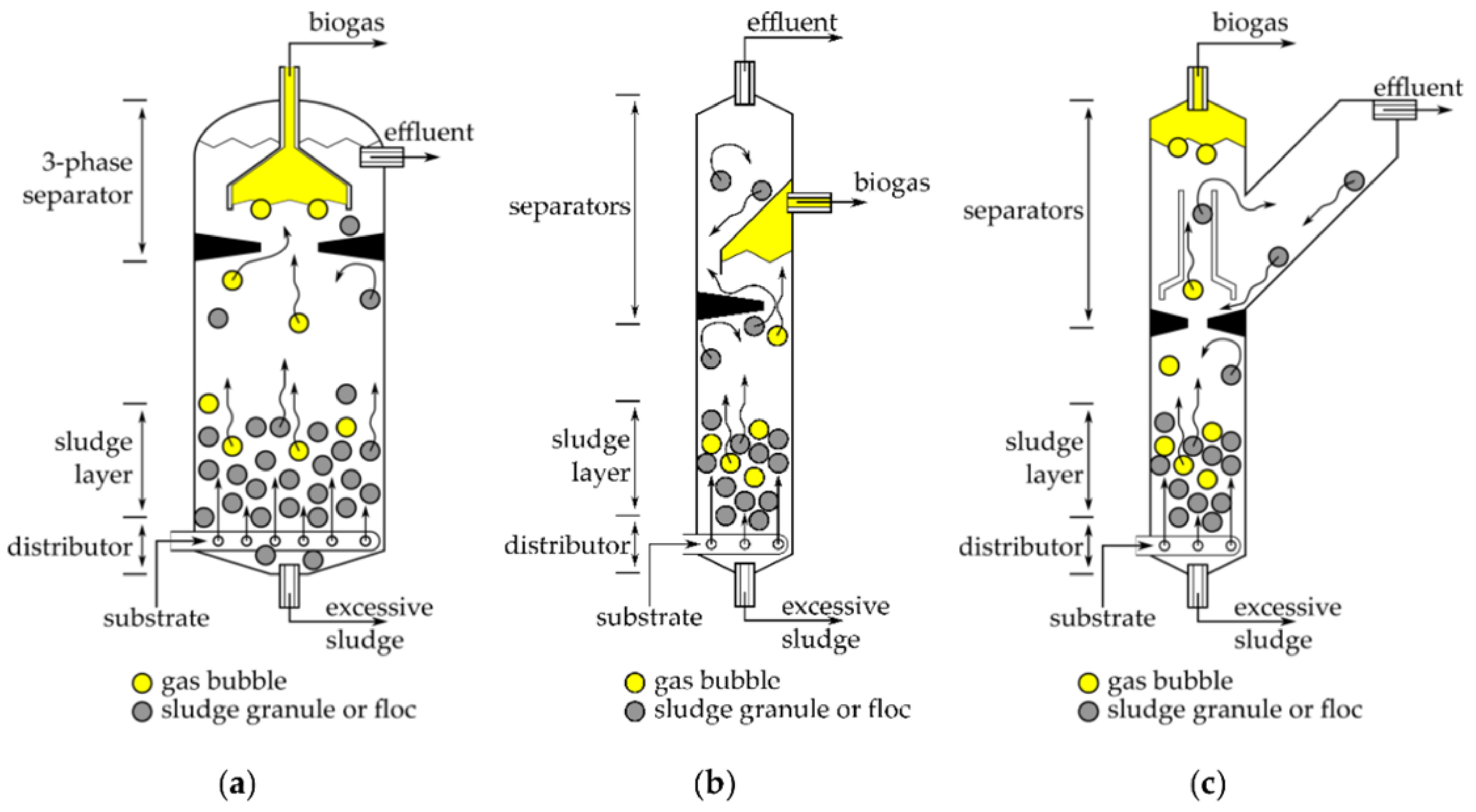
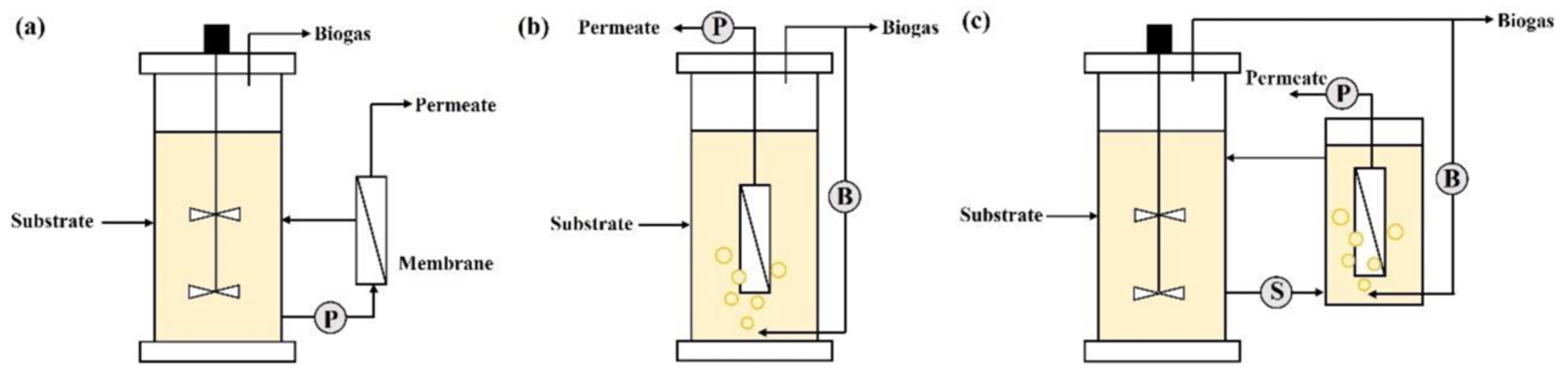
3.2.2. Reactor Configuration
Batch Reactors
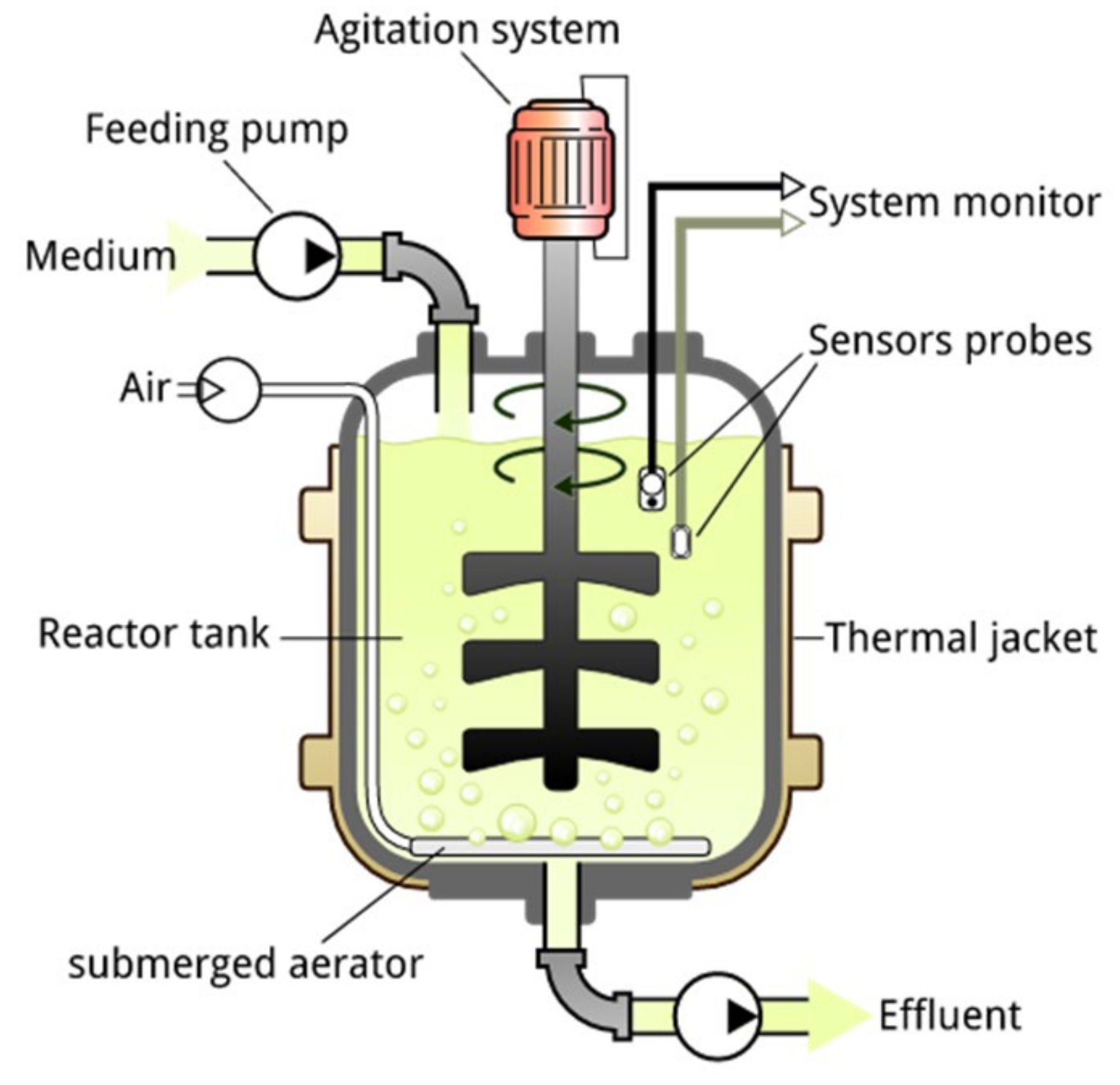
Continuously Stirred-Tank Reactor
4. Application of Nanotechnology on Enhancement of AD
4.1. Factors Involved in Enhancement of AD Performance
| Trace Elements | Metabolic Activity | Threshold for Enhancement of CH4 Conc. (mg/L) | Threshold for Inhibition of CH4 Conc. (mg/L) | References |
|---|---|---|---|---|
| Cu | Microbial community, methanogenic activity, cellulase activity, and volatile fatty acid concentration | 5 | 130 | [198] |
| Fe | Cellulase activity | 0–1000 | 20,010 | [199] |
| Ni | Methanogenic activity, cellulase activity | 0–20 | 32 | [200] |
| Zn | Methanogenic activity | 0–100 | _ | [201] |
| Molybdenum | CO2 reduction in methylotrophic pathways | 0.1–0.3 | 1 | [202] |
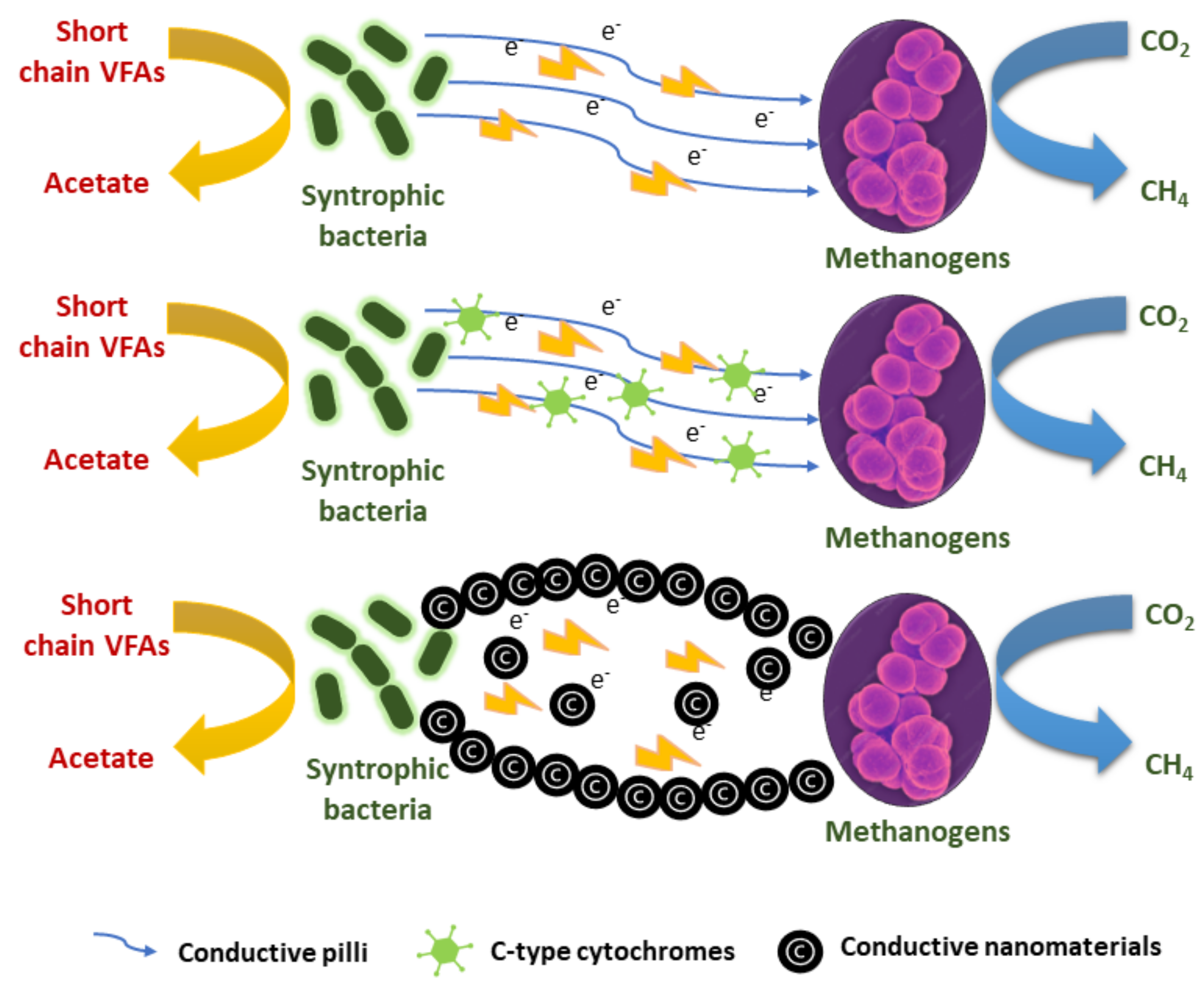
4.2. Impact of Nanomaterials on Biological Performances
- (i)
- Reduced energy consumption;
- (ii)
- No requirement for redox mediators to promote electron exchange;
- (iii)
- The avoidance of hazardous volatile fatty acid accumulation in the event of a process standstill;
- (iv)
- Redox mediators are produced, consumed, and diffused without the need for laborious enzymatic procedures, which have thermodynamic benefits.
4.3. Types of Nanomaterials and Their Applications on AD of FOGO
| NP Type | Size | Substrate | Temp | HRT | Dosage | Effect | References |
|---|---|---|---|---|---|---|---|
| Fe oxide | – | Seed sludge | 36 °C | 96 h | 750 mg/L | 38.2% enhancement in CH4 production | [234] |
| ZVI | 9 nm | Cattle dung slurry | 37 °C | 50 days | 5–20 mg/L | 43.75–45.37% increase in biogas production | [235] |
| Fe3O4 | 7 nm | 5–20 mg/L | 63.03–65.62% enhancement in biogas yield | ||||
| Fe3O4 | 7 nm24 nm | Municipal waste | 37 °C | 60 days | 100 ppm | 93.24% increase in biogas formation No enhancement | [217] |
| ZVI | 50 nm | Dewatered sludge | 37 °C | 12 days | 0.75 g 1.5 g | 45.77% increase in CH4 production 29.66% decrease in CH4 production | [218] |
| Fe3O4 | 20 nm | 0.75 g 1.5 g | 25.61% increase in CH4 production 11.51% decrease in CH4 production | ||||
| Fe3O4 | 10–35 nm | Municipal solid waste | 37 °C | 60 days | 50 mg/L 75 mg/L | 65.3–72.09% enhancement in CH4 yield | [221] |
| 100 mg/L | 44.22% increase in CH4 yield | ||||||
| 125 mg/L | 42.54% increase in CH4 yield | ||||||
| ZVI | 45 nm | Waste-activated sludge | 37 °C | 14 days | 1000 mg/L | Highest CH4 content is 88% | [236] |
| Zeolite | 7.13 µm | 4 g/L | No effect | ||||
| Mixture of zeolite and nZVI | – | 4 g/L and 500 mg/L | The production of biogas first grew before abruptly declining | ||||
| nZVI coated with zeolite | 24.1 µm | 500 mg/L 1000 mg/L | Biogas output decreased until the eighth day when it abruptly rose | ||||
| Fe | 200 nm | Activated sludge | 37 °C | 14 days | 50–3000 mg/L | 19–105% increase in biogas generation | [237] |
| Bimetallic Cu-Fe nanoparticle | 100 nm | 50–3000 mg/L | Increase in biogas production by 47.16–108.29% | ||||
| Fe3O4 | 20–30 nm | Waste sludge | 37 °C | 12 days | 20–200 mg/L | CH4 content increased from 58.5% to 65.5% But at 200 mg/L, ethane content reduced to 52.3% | [238] |
| ZVI | 60 nm | Anaerobic activated sludge | 37 °C | 14 days | 50 mg/L | 13.54% decline in biogas yield | [239] |
| ZVI | – | Sludge from municipal wastewater treatment | 37 °C | 30 days | 0.10% | 3.1% increase in CH4 content | [240] |
| Fe powder | – | Slaughterhouse sludge | 35 °C | 30 days | 1.60% | 11.6% increase in CH4 content | [241] |
| Fe powder | – | Slaughterhouse sludge | 35 °C | 30 days | 1.60% | 11.6% increase in CH4 content | [241] |
| Biosynthesized Fe | 20–40 nm | 3–9 mg/L | 32.9–33.3% rise in biogas yield | ||||
| Fe2O3 | 30–60 nm | Waste activated sludge | 37 °C | 48 days | 15–500 mg/g | 4–28.9% decline in CH4 yield | [242] |
| ZVI Fe oxide | 60 nm 20–40 nm | Anaerobic activated sludgeDairy manure | 37 °C 38 °C | 72 h 30 days | 5–1000 mg/g | 1.4–8.7% enhancement in biogas generation | [239] [243] |
| 100–1000 mg/L | 18.14–56.89% increase in CH4 yield | ||||||
| ZVI | 50 nm 20 nm | Dewatered sludge | 35 °C | 100 days | 0.5–4 g/L | 21.65% increase in methane yield | [244] |
| Fe3O4 | – | Slaughterhouse sludge | 35 °C | 30 days | 1.60% | 11.6% increase in CH4 content | [241] |
| Biosynthesized Fe | 20–40 nm | 0.5–4 g/L | 24.4% increase in methane yield | ||||
| Fe2NiO4 Fe4NiO4Zn | <50 nm <100 nm | Anaerobic sludge | 30 °C | 9 days | 1–100 mg/L | 30% enhancement in CH4 generation | [245] |
| 72 h | 1–100 mg/L | 65.6% decline in CH4 generation | |||||
| NiFe | 0.31 nm | Cow manure | 37 °C | 35 days | 20–130 mg/L | 30.8% increase in biogas production | [226] |
| NiFe | 0.57 nm 28 nm 17 nm | Dewatered sludge Livestock manure | 35 °C37 °C | 100 days 50 days | 20–130 mg/L 0.5–2 mg/L | 32.9% rise in biogas yield 64.12% rise in biogas yield | [244] [222] |
| CoNickel | |||||||
| 0.5–2 mg/L | 74.26% rise in biogas yield | ||||||
| Co | 100 nm | Microalgae Poultry litter | 37 °C 35 °C | 160 h 66 days | 0.4 mg/g | 9% rise in biogas production | [101] [246] |
| Co | 30–50 nm | 0.6 mg/g | 29.7% rise in biogas production | ||||
| Nickel | <100 nm | Green Algae | 37 °C | 264 h | 1.34 mg/g VS | 31.73% increase in biogas production | [247] |
| Titanium di oxide | 7.5 nm (spherical) | 0.84 mg/mL | Zero or low toxicity | ||||
| Gold | 20 nm (spherical) | Livestock manure | 37 °C | 50 days | 0.075 mg/mL | Zero or low toxicity | [222] |
| Silver | 30 nm (spherical) | 0.13 mg/mL | 33% inhibition on methanogenesis | ||||
| Fe2O3 | 20–40 nm | Cattle manure | 38 °C | 30 days | 20 mg/L 100 mg/L | 10.5% increase in CH4 production 19.1% increase in CH4 production | [248] |
| Titanium di oxide | 25 nm | 100 mg/L 500 mg/L | 9.7% increase in CH4 production | ||||
| 21.3% increase in CH4 production | |||||||
| Fe2O3 + TiO2 | – | (20–100) mg/L + 500 mg/L | 13.3% rise in CH4 production15% increase in CH4 production | ||||
| Titanium di oxide | 4–8 nm | Anaerobic sludge | 35 °C | 28 days | 500–2000 mg/L | 14.9% rise in CH4 production | [249] |
| Al2O3 | 40–50 nm | Waste activated sludge | 37 °C | 48 days | 50–500 mg/g | 14.8% enhancement in CH4 generation | [242] |
| Graphene | – | Waste sludge | 35 °C | 12 days | 0.5–2 g/L | 25% enhancement in CH4 generation | [250] |
| Nanosized biochar | 100–600 nm | FW and SS | 55 °C | 81 days | 7.5 g/L | 117% increase in CH4 yield | [251] |
| Fe3O4 NPs | 15 to 21 nm | FW | (37 ± 0.5 °C) | 60 days | 50, 75, 100, 125 mg/L | Improved DIET, reduced lag phase, higher CH4 yields | [252] |
| TiO2 NPs | – | Food and green waste co-digestion | 37 °C | 45 days | 0.5 to 2.5 mg/L | No significant effect | [67] |
| Biochar supported nZVI | – | FW | 35 ± 1 ℃ | 18 days | 1, 2, and 3 g/L | Compared with the control group, the abundance of acetoclastic methanogenesis increased by 1.92% in the BC-nZVI reactors | [253] |
5. Conclusions
- (i)
- Investigating the impact of varying feedstock ratios (e.g., food waste to kitchen waste or garden waste) derived from realistic kerbside FOGO bins on biogas production can provide valuable insights.
- (ii)
- Optimizing AD process parameters (e.g., temperature, pH, retention time) is essential. Research should explore how variations in these parameters impact biogas production from FOGO. Reactor studies should be conducted to examine the factors and conditions leading to inhibition and poisoning.
- (iii)
- Significant research is important to overcome reactor failures and enhancement of biogas yield from FOGO.
- (iv)
- To improve and synchronise the reaction rates of the multi-step AD process, two- and three-stage AD bioreactors can be studied. It is anticipated that multi-stage anaerobic bioreactors, which are becoming more and more significant in research, will soon be available for commercialization.
- (v)
- Nanotechnology offers promising techniques for incorporating additives into the feedstock. In anaerobic digesters for FOGO, nanomaterials can serve as effective immobilisation matrices for DIET and aid in determining the predominant metabolic routes of microbial communities. Therefore, future studies ought to concentrate on investigations that aid in determining which compounds are best for augmenting FOGO’s AD process.
- (vi)
- Integrating and harmonising different improvement methods is a difficult but necessary undertaking to maximise the efficiency of anaerobic digesters and ensure the continuous conversion of FOGO into renewable energy. This should also include clarification of the implications and potential for expanding large-scale AD strategies in an industrial context.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brusselaers, J.; Van Der Linden, A. Bio-Waste in Europe—Turning Challenges into Opportunities; European Environment Agency: Luxembourg, 2020. [Google Scholar]
- Department of Sustainability, Environment, Water, Population and Communities. Population and Communities Food And Garden Organics. Best Practice Collection Manual. Available online: https://www.dcceew.gov.au/sites/default/files/documents/collection-manual.pdf (accessed on 1 May 2024).
- Change, I.C. Mitigation of climate change. Contrib. Work. Group III Fifth Assess. Rep. Intergov. Panel Clim. Change 2014, 1454, 147. [Google Scholar]
- Awasthi, M.K.; Sarsaiya, S.; Patel, A.; Juneja, A.; Singh, R.P.; Yan, B.; Awasthi, S.K.; Jain, A.; Liu, T.; Duan, Y.; et al. Refining biomass residues for sustainable energy and bio-products: An assessment of technology, its importance, and strategic applications in circular bio-economy. Renew. Sustain. Energy Rev. 2020, 127, 109876. [Google Scholar] [CrossRef]
- Awasthi, S.K.; Sarsaiya, S.; Awasthi, M.K.; Liu, T.; Zhao, J.; Kumar, S.; Zhang, Z. Changes in global trends in food waste composting: Research challenges and opportunities. Bioresour. Technol. 2020, 299, 122555. [Google Scholar] [CrossRef]
- Wu, L.; Wei, W.; Song, L.; Woźniak-Karczewska, M.; Chrzanowski, Ł.; Ni, B.-J. Upgrading biogas produced in anaerobic digestion: Biological removal and bioconversion of CO2 in biogas. Renew. Sustain. Energy Rev. 2021, 150, 111448. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, H.; He, S.; Zhao, Q.; Wei, L. A review of biochar in anaerobic digestion to improve biogas production: Performances, mechanisms and economic assessments. Bioresour. Technol. 2021, 341, 125797. [Google Scholar] [CrossRef]
- Rehl, T.; Müller, J. Life cycle assessment of biogas digestate processing technologies. Resour. Conserv. Recycl. 2011, 56, 92–104. [Google Scholar] [CrossRef]
- Cuéllar, A.D.; Webber, M.E. Cow power: The energy and emissions benefits of converting manure to biogas. Environ. Res. Lett. 2008, 3, 034002. [Google Scholar] [CrossRef]
- Tambone, F.; Scaglia, B.; D’Imporzano, G.; Schievano, A.; Orzi, V.; Salati, S.; Adani, F. Assessing amendment and fertilizing properties of digestates from anaerobic digestion through a comparative study with digested sludge and compost. Chemosphere 2010, 81, 577–583. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, S.; Wang, Y.; Wang, R. Advantages of the integrated pig-biogas-vegetable greenhouse system in North China. Ecol. Eng. 2005, 24, 175–183. [Google Scholar] [CrossRef]
- Jiang, X.; Sommer, S.G.; Christensen, K.V. A review of the biogas industry in China. Energy Policy 2011, 39, 6073–6081. [Google Scholar] [CrossRef]
- Department of Industry, Science, Energy, and Resources. Table O, Australian Energy Statistics; Australian Bureau of Statistics: Canberra, Australia, 2022. [Google Scholar]
- Awasthi, S.K.; Joshi, R.; Dhar, H.; Verma, S.; Awasthi, M.K.; Varjani, S.; Sarsaiya, S.; Zhang, Z.; Kumar, S. Improving methane yield and quality via co-digestion of cow dung mixed with food waste. Bioresour. Technol. 2018, 251, 259–263. [Google Scholar] [CrossRef]
- Kasinath, A.; Fudala-Ksiazek, S.; Szopinska, M.; Bylinski, H.; Artichowicz, W.; Remiszewska-Skwarek, A.; Luczkiewicz, A. Biomass in biogas production: Pretreatment and codigestion. Renew. Sustain. Energy Rev. 2021, 150, 111509. [Google Scholar] [CrossRef]
- Myszograj, S.; Stadnik, A.; Płuciennik-Koropczuk, E. The influence of trace elements on anaerobic digestion process. Civ. Environ. Eng. Rep. 2018, 28, 105–115. [Google Scholar] [CrossRef]
- Veraart, A.J.; Steenbergh, A.K.; Ho, A.; Kim, S.Y.; Bodelier, P.L. Beyond nitrogen: The importance of phosphorus for CH4 oxidation in soils and sediments. Geoderma 2015, 259–260, 337–346. [Google Scholar] [CrossRef]
- Liu, H.; Kumar, V.; Jia, L.; Sarsaiya, S.; Kumar, D.; Juneja, A.; Zhang, Z.; Sindhu, R.; Binod, P.; Bhatia, S.K.; et al. Biopolymer poly-hydroxyalkanoates (PHA) production from apple industrial waste residues: A review. Chemosphere 2021, 284, 131427. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, M.; Liang, C.; Chui, C.; Wang, N.; Shi, J.; Liu, L. Multivariate insights into enhanced biogas production in thermophilic dry anaerobic co-digestion of food waste with kitchen waste or garden waste: Process properties, microbial communities and metagenomic analyses. Bioresour. Technol. 2022, 361, 127684. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, K.; Devanand, J.; Kumar, P.S.; Soundarajan, K.; Sivasubramanian, V.; Sindhu, J.; Vo, D.-V.N. A review on nano-catalysts and biochar-based catalysts for biofuel production. Fuel 2021, 306, 121632. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, J.; Wang, X. Effects of alkalinity sources on the stability of anaerobic digestion from food waste. Waste Manag. Res. 2015, 33, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Serna-Maza, A.; Heaven, S.; Banks, C. Biogas stripping of ammonia from fresh digestate from a food waste digester. Bioresour. Technol. 2015, 190, 66–75. [Google Scholar] [CrossRef]
- De la Rubia, M.Á.; Walker, M.; Heaven, S.; Banks, C.J.; Borja, R. Preliminary trials of in situ ammonia stripping from source segregated domestic food waste digestate using biogas: Effect of temperature and flow rate. Bioresour. Technol. 2010, 101, 9486–9492. [Google Scholar] [CrossRef]
- Serna-Maza, A.; Heaven, S.; Banks, C.J. Ammonia removal in food waste anaerobic digestion using a side-stream stripping process. Bioresour. Technol. 2014, 152, 307–315. [Google Scholar] [CrossRef]
- Walker, M.; Iyer, K.; Heaven, S.; Banks, C. Ammonia removal in anaerobic digestion by biogas stripping: An evaluation of process alternatives using a first order rate model based on experimental findings. Chem. Eng. J. 2011, 178, 138–145. [Google Scholar] [CrossRef]
- Siddique, M.N.I.; Wahid, Z.A. Achievements and perspectives of anaerobic co-digestion: A review. J. Clean. Prod. 2018, 194, 359–371. [Google Scholar] [CrossRef]
- Zamri, M.; Hasmady, S.; Akhiar, A.; Ideris, F.; Shamsuddin, A.; Mofijur, M.; Fattah, I.R.; Mahlia, T. A comprehensive review on anaerobic digestion of organic fraction of municipal solid waste. Renew. Sustain. Energy Rev. 2021, 137, 110637. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, L.; Wang, W.; Wang, Q.; Bai, R.; Zhuang, X.; Guo, Y.; Qi, W.; Yuan, Z. Impact of blending on hydrolysis and ethanol fermentation of garden wastes. J. Clean. Prod. 2018, 190, 36–43. [Google Scholar] [CrossRef]
- Jayanth, T.; Mamindlapelli, N.K.; Begum, S.; Arelli, V.; Juntupally, S.; Ahuja, S.; Dugyala, S.K.; Anupoju, G.R. Anaerobic mono and co-digestion of organic fraction of municipal solid waste and landfill leachate at industrial scale: Impact of volatile organic loading rate on reaction kinetics, biogas yield and microbial diversity. Sci. Total. Environ. 2020, 748, 142462. [Google Scholar]
- Borth, P.L.B.; Perin, J.K.H.; Torrecilhas, A.R.; Pan, N.C.; Kuroda, E.K.; Fernandes, F. Biochemical methane potential of food and garden waste co-digestion with variation in solid content and inoculum: Substrate ratio. J. Mater. Cycles Waste Manag. 2021, 23, 1974–1983. [Google Scholar] [CrossRef]
- Panigrahi, S.; Sharma, H.B.; Dubey, B.K. Optimization of F/M Ratio During Anaerobic Codigestion of Yard Waste with Food Waste: Biogas Production and System Stability. In Treatment and Disposal of Solid and Hazardous Wastes; Sengupta, D., Dubey, B.K., Goel, S., Eds.; Springer International Publishing: Cham, Germany, 2022; pp. 185–192. [Google Scholar]
- Zhang, S.; Liang, C.; Xiao, M.; Chui, C.; Wang, N.; Ji, Y.; Wang, Z.; Shi, J.; Liu, L. Metagenomic characterization of the enhanced performance of multicomponent synergistic thermophilic anaerobic co-digestion of food waste utilizing kitchen waste or garden waste as co-substrate. Water Res. 2023, 244, 120457. [Google Scholar] [CrossRef]
- Nithin Raja, C.; Purushothaman, P. Impact of sequential hybrid pretreatment in anaerobic digestion of food waste and garden waste co-digestion on waste characteristics and biogas production. J. Mater. Cycles Waste Manag. 2023, 25, 2937–2950. [Google Scholar]
- Arif, S.; Liaquat, R.; Adil, M. Applications of materials as additives in anaerobic digestion technology. Renew. Sustain. Energy Rev. 2018, 97, 354–366. [Google Scholar] [CrossRef]
- Khalid, A.; Arshad, M.; Anjum, M.; Mahmood, T.; Dawson, L. The anaerobic digestion of solid organic waste. Waste Manag. 2011, 31, 1737–1744. [Google Scholar] [CrossRef] [PubMed]
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic digestion of lignocellulosic biomass: Challenges and opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef]
- Zhen, G.; Lu, X.; Kato, H.; Zhao, Y.; Li, Y.-Y. Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: Current advances, full-scale application and future perspectives. Renew. Sustain. Energy Rev. 2017, 69, 559–577. [Google Scholar] [CrossRef]
- Lu, D.; Liu, X.; Apul, O.G.; Zhang, L.; Ryan, D.K.; Zhang, X. Optimization of biomethane production from anaerobic Co-digestion of microalgae and septic tank sludge. Biomass Bioenergy 2019, 127, 105266. [Google Scholar] [CrossRef]
- Khanal, S.K.; Tirta Nindhia, T.G.; Nitayavardhana, S. Chapter 11—Biogas From Wastes: Processes and Applications. In Sustainable Resource Recovery and Zero Waste Approaches; Taherzadeh, M.J., Bolton, K., Wong, J., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 165–174. [Google Scholar]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.K.; Suja, F.B.; Zain, S.M.; Pramanik, B.K. The anaerobic digestion process of biogas production from food waste: Prospects and constraints. Bioresour. Technol. Rep. 2019, 8, 100310. [Google Scholar] [CrossRef]
- Dahiya, S.; Sarkar, O.; Swamy, Y.; Mohan, S.V. Acidogenic fermentation of food waste for volatile fatty acid production with co-generation of biohydrogen. Bioresour. Technol. 2015, 182, 103–113. [Google Scholar] [CrossRef]
- Drake, H.L. Acetogenesis; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Fenchel, T.; King, G.M.; Blackburn, T.H. Chapter 1—Bacterial Metabolism. In Bacterial Biogeochemistry, 3rd ed.; Fenchel, T., King, G.M., Blackburn, T.H., Eds.; Academic Press: Boston, MA, USA, 2012; pp. 1–34. [Google Scholar]
- Wu, C.; Huang, Q.; Yu, M.; Ren, Y.; Wang, Q.; Sakai, K. Effects of digestate recirculation on a two-stage anaerobic digestion system, particularly focusing on metabolite correlation analysis. Bioresour. Technol. 2018, 251, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Thauer, R.K. Methyl (alkyl)-coenzyme M reductases: Nickel F-430-containing enzymes involved in anaerobic methane formation and in anaerobic oxidation of methane or of short chain alkanes. Biochemistry 2019, 58, 5198–5220. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, X.; Xia, W.; Li, Z.; Wang, L.; Chen, Z.; Ge, S. Effect of extreme pH conditions on methanogenesis: Methanogen metabolism and community structure. Sci. Total Environ. 2023, 877, 162702. [Google Scholar] [CrossRef]
- Sun, M.; Liu, B.; Yanagawa, K.; Ha, N.T.; Goel, R.; Terashima, M.; Yasui, H. Effects of low pH conditions on decay of methanogenic biomass. Water Res. 2020, 179, 115883. [Google Scholar] [CrossRef]
- Ni, R.; Xu, C.; Shi, X.; Yang, S.; Li, L.; Peng, X.; Song, L. Acetoclastic methanogenesis pathway stability despite the high microbial taxonomic variability in the transition from acidogenesis to methanogenesis during food waste anaerobic digestion. J. Clean. Prod. 2022, 372, 133758. [Google Scholar] [CrossRef]
- Greene, P. Managing Digester Feedstocks. Available online: https://www.biocycle.net/managing-digester-feedstocks/ (accessed on 1 May 2024).
- Chojnacka, K.; Moustakas, K.; Witek-Krowiak, A. Bio-based fertilizers: A practical approach towards circular economy. Bioresour. Technol. 2020, 295, 122223. [Google Scholar] [CrossRef]
- Santos, C.A.; Reis, A. Microalgal symbiosis in biotechnology. Appl. Microbiol. Biotechnol. 2014, 98, 5839–5846. [Google Scholar] [CrossRef]
- Mao, C.; Feng, Y.; Wang, X.; Ren, G. Review on research achievements of biogas from anaerobic digestion. Renew. Sustain. Energy Rev. 2015, 45, 540–555. [Google Scholar] [CrossRef]
- Cioabla, A.E.; Ionel, I.; Dumitrel, G.-A.; Popescu, F. Comparative study on factors affecting anaerobic digestion of agricultural vegetal residues. Biotechnol. Biofuels 2012, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Wintsche, B.; Glaser, K.; Sträuber, H.; Centler, F.; Liebetrau, J.; Harms, H.; Kleinsteuber, S. Trace Elements Induce Predominance among Methanogenic Activity in Anaerobic Digestion. Front. Microbiol. 2016, 7, 2034. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Li, A. Enhanced anaerobic digestion of food waste by trace metal elements supplementation and reduced metals dosage by green chelating agent [S, S]-EDDS via improving metals bioavailability. Water Res. 2015, 84, 266–277. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Browne, J.D.; Murphy, J.D. The impact of increasing organic loading in two phase digestion of food waste. Renew. Energy 2014, 71, 69–76. [Google Scholar] [CrossRef]
- Pagés-Díaz, J.; Pereda-Reyes, I.; Taherzadeh, M.J.; Sárvári-Horváth, I.; Lundin, M. Anaerobic co-digestion of solid slaughterhouse wastes with agro-residues: Synergistic and antagonistic interactions determined in batch digestion assays. Chem. Eng. J. 2014, 245, 89–98. [Google Scholar] [CrossRef]
- Ohemeng-Ntiamoah, J.; Datta, T. Evaluating analytical methods for the characterization of lipids, proteins and carbohydrates in organic substrates for anaerobic co-digestion. Bioresour. Technol. 2018, 247, 697–704. [Google Scholar] [CrossRef]
- Mao, C.; Wang, X.; Xi, J.; Feng, Y.; Ren, G. Linkage of kinetic parameters with process parameters and operational conditions during anaerobic digestion. Energy 2017, 135, 352–360. [Google Scholar] [CrossRef]
- Li, N.; He, J.; Yan, H.; Chen, S.; Dai, X. Pathways in bacterial and archaeal communities dictated by ammonium stress in a high solid anaerobic digester with dewatered sludge. Bioresour. Technol. 2017, 241, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, D.; Yan, J.; Qiao, W.; Wang, W.; Zhu, T. Effects of lipid concentration on anaerobic co-digestion of municipal biomass wastes. Waste Manag. 2014, 34, 1025–1034. [Google Scholar] [CrossRef]
- Cirne, D.G.; Paloumet, X.; Björnsson, L.; Alves, M.M.; Mattiasson, B. Anaerobic digestion of lipid-rich waste—Effects of lipid concentration. Renew. Energy 2007, 32, 965–975. [Google Scholar] [CrossRef]
- Wagner, A.O.; Lins, P.; Malin, C.; Reitschuler, C.; Illmer, P. Impact of protein-, lipid- and cellulose-containing complex substrates on biogas production and microbial communities in batch experiments. Sci. Total. Environ. 2013, 458–460, 256–266. [Google Scholar]
- Cook, S.M.; Skerlos, S.J.; Raskin, L.; Love, N.G. A stability assessment tool for anaerobic codigestion. Water Res. 2017, 112, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Paritosh, K.; Mathur, S.; Pareek, N.; Vivekanand, V. Feasibility study of waste (d) potential: Co-digestion of organic wastes, synergistic effect and kinetics of biogas production. Int. J. Environ. Sci. Technol. 2018, 15, 1009–1018. [Google Scholar] [CrossRef]
- Méndez-Acosta, H.O.; Campos-Rodríguez, A.; González-Álvarez, V.; García-Sandoval, J.P.; Snell-Castro, R.; Latrille, E. A hybrid cascade control scheme for the VFA and COD regulation in two-stage anaerobic digestion processes. Bioresour. Technol. 2016, 218, 1195–1202. [Google Scholar] [CrossRef]
- Jiang, Y.; Dennehy, C.; Lawlor, P.G.; Hu, Z.; Zhan, X.; Gardiner, G.E. Inactivation of enteric indicator bacteria and system stability during dry co-digestion of food waste and pig manure. Sci. Total. Environ. 2018, 612, 293–302. [Google Scholar] [CrossRef]
- Chinellato, G.; Cavinato, C.; Bolzonella, D.; Heaven, S.; Banks, C.J. Biohydrogen production from food waste in batch and semi-continuous conditions: Evaluation of a two-phase approach with digestate recirculation for pH control. Int. J. Hydrogen Energy 2013, 38, 4351–4360. [Google Scholar] [CrossRef]
- Ebner, J.H.; Labatut, R.A.; Lodge, J.S.; Williamson, A.A.; Trabold, T.A. Anaerobic co-digestion of commercial food waste and dairy manure: Characterizing biochemical parameters and synergistic effects. Waste Manag. 2016, 52, 286–294. [Google Scholar] [CrossRef]
- Hübner, M.; Oechsner, H.; Koch, S.; Seggl, A.; Hrenn, H.; Schmiedchen, B.; Wilde, P.; Miedaner, T. Impact of genotype, harvest time and chemical composition on the methane yield of winter rye for biogas production. Biomass Bioenergy 2011, 35, 4316–4323. [Google Scholar] [CrossRef]
- Bouzas, A.; Gabaldón, C.; Marzal, P.; Penya-roja, J.M.; Seco, A. Fermentation of Municipal Primary Sludge: Effect of Srt and Solids Concentration on Volatile Fatty Acid Production. Environ. Technol. 2002, 23, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Cremonez, P.A.; Sampaio, S.C.; Teleken, J.G.; Meier, T.W.; Dieter, J.; Teleken, J. Influence of inoculum to substrate ratio on the anaerobic digestion of a cassava starch polymer. Ind. Crops Prod. 2019, 141, 111709. [Google Scholar] [CrossRef]
- Zhang, W.; Li, L.; Xing, W.; Chen, B.; Zhang, L.; Li, A.; Li, R.; Yang, T. Dynamic behaviors of batch anaerobic systems of food waste for methane production under different organic loads, substrate to inoculum ratios and initial pH. J. Biosci. Bioeng. 2019, 128, 733–743. [Google Scholar] [CrossRef]
- Cordoba, V.; Fernandez, M.; Santalla, E. The effect of substrate/inoculum ratio on the kinetics of methane production in swine wastewater anaerobic digestion. Environ. Sci. Pollut. Res. 2018, 25, 21308–21317. [Google Scholar] [CrossRef] [PubMed]
- Latifi, P.; Karrabi, M.; Danesh, S. Anaerobic co-digestion of poultry slaughterhouse wastes with sewage sludge in batch-mode bioreactors (effect of inoculum-substrate ratio and total solids). Renew. Sustain. Energy Rev. 2019, 107, 288–296. [Google Scholar] [CrossRef]
- Pastor-Poquet, V.; Papirio, S.; Trably, E.; Rintala, J.; Escudié, R.; Esposito, G. High-solids anaerobic digestion requires a trade-off between total solids, inoculum-to-substrate ratio and ammonia inhibition. Int. J. Environ. Sci. Technol. 2019, 16, 7011–7024. [Google Scholar] [CrossRef]
- Divya, D.; Gopinath, L.; Christy, P.M. A review on current aspects and diverse prospects for enhancing biogas production in sustainable means. Renew. Sustain. Energy Rev. 2015, 42, 690–699. [Google Scholar] [CrossRef]
- Naik, L.; Gebreegziabher, Z.; Tumwesige, V.; Balana, B.B.; Mwirigi, J.; Austin, G. Factors determining the stability and productivity of small scale anaerobic digesters. Biomass Bioenergy 2014, 70, 51–57. [Google Scholar] [CrossRef]
- Karthikeyan, O.P.; Visvanathan, C. Effect of C/N ratio and ammonia-N accumulation in a pilot-scale thermophilic dry anaerobic digester. Bioresour. Technol. 2012, 113, 294–302. [Google Scholar]
- Wang, J. Decentralized biogas technology of anaerobic digestion and farm ecosystem: Opportunities and challenges. Front. Energy Res. 2014, 2, 10. [Google Scholar] [CrossRef]
- Kwietniewska, E.; Tys, J. Process characteristics, inhibition factors and methane yields of anaerobic digestion process, with particular focus on microalgal biomass fermentation. Renew. Sustain. Energy Rev. 2014, 34, 491–500. [Google Scholar] [CrossRef]
- Nyoman, W.I.; Seno, J. The kinetic of biogas production rate from cattle manure in batch mode. Int. J. Chem. Biol. Eng. 2010, 3, 39–45. [Google Scholar]
- Zhang, T.; Liu, L.; Song, Z.; Ren, G.; Feng, Y.; Han, X.; Yang, G. Biogas production by co-digestion of goat manure with three crop residues. PLoS ONE 2013, 8, e66845. [Google Scholar] [CrossRef]
- Mnkeni, P.; Austin, L. Fertiliser value of human manure from pilot urine-diversion toilets. Water SA 2009, 35, 133–138. [Google Scholar] [CrossRef]
- Muzenda, E. Bio-methane generation from organic waste. In Proceedings of the World Congress on Engineering and Computer Science, San Francisco, CA, USA, 22–24 October 2014. [Google Scholar]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Kallistova, A.Y.; Goel, G.; Nozhevnikova, A. Microbial diversity of methanogenic communities in the systems for anaerobic treatment of organic waste. Microbiology 2014, 83, 462–483. [Google Scholar] [CrossRef]
- Arslan, C.; Sattar, A.; Changying, J.; Nasir, A.; Ali Mari, I.; Zia Bakht, M. Impact of pH management interval on biohydrogen production from organic fraction of municipal solid wastes by mesophilic thermophilic anaerobic codigestion. BioMed Res. Int. 2015, 2015, 590753. [Google Scholar] [CrossRef]
- Ward, A.J.; Hobbs, P.J.; Holliman, P.J.; Jones, D.L. Optimisation of the anaerobic digestion of agricultural resources. Bioresour. Technol. 2008, 99, 7928–7940. [Google Scholar] [CrossRef]
- Dai, B.; Xu, J.; He, Y.; Xiong, P.; Wang, X.; Deng, Y.; Wang, Y.; Yin, Z. Acid inhibition during anaerobic digestion of biodegradable kitchen waste. J. Renew. Sustain. Energy 2015, 7, 023118. [Google Scholar] [CrossRef]
- Zhai, N.; Zhang, T.; Yin, D.; Yang, G.; Wang, X.; Ren, G.; Feng, Y. Effect of initial pH on anaerobic co-digestion of kitchen waste and cow manure. Waste Manag. 2015, 38, 126–131. [Google Scholar] [CrossRef]
- Normak, A.; Suurpere, J.; Suitso, I.; Jogi, E.; Kokin, E.; Pitk, P. Improving ADM1 model to simulate anaerobic digestion start-up with inhibition phase based on cattle slurry. Biomass Bioenergy 2015, 80, 260–266. [Google Scholar] [CrossRef]
- Souza, T.S.; Carvajal, A.; Donoso-Bravo, A.; Peña, M.; Fdz-Polanco, F. ADM1 calibration using BMP tests for modeling the effect of autohydrolysis pretreatment on the performance of continuous sludge digesters. Water Res. 2013, 47, 3244–3254. [Google Scholar] [CrossRef] [PubMed]
- Monou, M.; Pafitis, N.; Kythreotou, N.; Smith, S.R.; Mantzavinos, D.; Kassinos, D. Anaerobic co-digestion of potato processing wastewater with pig slurry and abattoir wastewater. J. Chem. Technol. Biotechnol. 2008, 83, 1658–1663. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Schnurer, A.; Jarvis, A. Microbiological handbook for biogas plants. Swed. Waste Manag. U 2010, 2009, 1–74. [Google Scholar]
- Qiang, H.; Niu, Q.; Chi, Y.; Li, Y. Trace metals requirements for continuous thermophilic methane fermentation of high-solid food waste. Chem. Eng. J. 2013, 222, 330–336. [Google Scholar] [CrossRef]
- Kelly, C.R.; Switzenbaum, M.S. Anaerobic treatment: Temperature and nutrient effects. Agric. Wastes 1984, 10, 135–154. [Google Scholar] [CrossRef]
- Zaidi, A.A.; RuiZhe, F.; Shi, Y.; Khan, S.Z.; Mushtaq, K. Nanoparticles augmentation on biogas yield from microalgal biomass anaerobic digestion. Int. J. Hydrogen Energy 2018, 43, 14202–14213. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. Trace element requirements of agricultural biogas digesters during biological conversion of renewable biomass to methane. Biomass Bioenergy 2011, 35, 992–998. [Google Scholar] [CrossRef]
- Müller, B.; Sun, L.; Schnürer, A. First insights into the syntrophic acetate-oxidizing bacteria—A genetic study. MicrobiologyOpen 2013, 2, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Birošová, L.; Mackuľak, T.; Bodík, I.; Ryba, J.; Škubák, J.; Grabic, R. Pilot study of seasonal occurrence and distribution of antibiotics and drug resistant bacteria in wastewater treatment plants in Slovakia. Sci. Total. Environ. 2014, 490, 440–444. [Google Scholar] [CrossRef]
- Dhar, H.; Kumar, P.; Kumar, S.; Mukherjee, S.; Vaidya, A.N. Effect of organic loading rate during anaerobic digestion of municipal solid waste. Bioresour. Technol. 2016, 217, 56–61. [Google Scholar] [CrossRef]
- Singh, P.K.; Mohanty, P.; Mishra, S.; Adhya, T.K. Food waste valorisation for biogas-based bioenergy production in circular bioeconomy: Opportunities, challenges, and future developments. Front. Energy Res. 2022, 10, 903775. [Google Scholar] [CrossRef]
- Sarker, S.; Lamb, J.J.; Hjelme, D.R.; Lien, K.M. A review of the role of critical parameters in the design and operation of biogas production plants. Appl. Sci. 2019, 9, 1915. [Google Scholar] [CrossRef]
- Bajpai, P.; Bajpai, P. Process parameters affecting anaerobic digestion. In Anaerobic Technology in Pulp and Paper Industry; Springer: Berlin/Heidelberg, Germany, 2017; pp. 13–27. [Google Scholar]
- Rocha-Meneses, L.; Zannerni, R.; Inayat, A.; Abdallah, M.; Shanableh, A.; Ghenai, C.; Kamil, M.; Kikas, T. Current progress in anaerobic digestion reactors and parameters optimization. Biomass Convers. Biorefinery 2022, 1–24. [Google Scholar] [CrossRef]
- Liu, X.; Wang, W.; Shi, Y.; Zheng, L.; Gao, X.; Qiao, W.; Zhou, Y. Pilot-scale anaerobic co-digestion of municipal biomass waste and waste activated sludge in China: Effect of organic loading rate. Waste Manag. 2012, 32, 2056–2060. [Google Scholar] [CrossRef]
- Morken, J.; Gjetmundsen, M.; Fjørtoft, K. Determination of kinetic constants from the co-digestion of dairy cow slurry and municipal food waste at increasing organic loading rates. Renew. Energy 2018, 117, 46–51. [Google Scholar] [CrossRef]
- Gou, C.; Yang, Z.; Huang, J.; Wang, H.; Xu, H.; Wang, L. Effects of temperature and organic loading rate on the performance and microbial community of anaerobic co-digestion of waste activated sludge and food waste. Chemosphere 2014, 105, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Kobayashi, T.; Qi, W.; Oshibe, H.; Xu, K.-Q. Effect of temperature and organic loading rate on siphon-driven self-agitated anaerobic digestion performance for food waste treatment. Waste Manag. 2018, 74, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, W.; Anwar, N.; Ma, Z.; Liu, G.; Zhang, R. Effect of Organic Loading Rate on Anaerobic Digestion of Food Waste under Mesophilic and Thermophilic Conditions. Energy Fuels 2017, 31, 2976–2984. [Google Scholar] [CrossRef]
- Guo, X.; Wang, C.; Sun, F.; Zhu, W.; Wu, W. A comparison of microbial characteristics between the thermophilic and mesophilic anaerobic digesters exposed to elevated food waste loadings. Bioresour. Technol. 2014, 152, 420–428. [Google Scholar] [CrossRef]
- Conn, E.; Stumpf, P. Outlines of Biochemistry; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Cabirol, N.; Barragán, E.; Durán, A.; Noyola, A. Effect of aluminium and sulphate on anaerobic digestion of sludge from wastewater enhanced primary treatment. Water Sci. Technol. 2003, 48, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Gerardi, M.H. The Microbiology of Anaerobic Digesters; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production. Renew. Sustain. Energy Rev. 2012, 16, 1462–1476. [Google Scholar] [CrossRef]
- Singh, S.P.; Prerna, P. Review of recent advances in anaerobic packed-bed biogas reactors. Renew. Sustain. Energy Rev. 2009, 13, 1569–1575. [Google Scholar] [CrossRef]
- Thanh, N.T.; Watari, T.; Thao, T.P.; Hatamoto, M.; Tanikawa, D.; Syutsubo, K.; Fukuda, M.; Tan, N.M.; Anh, T.K.; Yamaguchi, T.; et al. Impact of aluminum chloride on process performance and microbial community structure of granular sludge in an upflow anaerobic sludge blanket reactor for natural rubber processing wastewater treatment. Water Sci. Technol. 2016, 74, 500–507. [Google Scholar] [CrossRef]
- Jiménez, A.M.; Borja, R.; Martín, A. A comparative kinetic evaluation of the anaerobic digestion of untreated molasses and molasses previously fermented with Penicillium decumbens in batch reactors. Biochem. Eng. J. 2004, 18, 121–132. [Google Scholar] [CrossRef]
- Rodríguez-Abalde, Á.; Flotats, X.; Fernández, B. Optimization of the anaerobic co-digestion of pasteurized slaughterhouse waste, pig slurry and glycerine. Waste Manag. 2017, 61, 521–528. [Google Scholar] [CrossRef]
- Raynal, J.; Delgenès, J.P.; Moletta, R. Two-phase anaerobic digestion of solid wastes by a multiple liquefaction reactors process. Bioresour. Technol. 1998, 65, 97–103. [Google Scholar] [CrossRef]
- Bouallagui, H.; Touhami, Y.; Ben Cheikh, R.; Hamdi, M. Bioreactor performance in anaerobic digestion of fruit and vegetable wastes. Process. Biochem. 2005, 40, 989–995. [Google Scholar] [CrossRef]
- Peng, S.; Colosi, L.M. Anaerobic digestion of algae biomass to produce energy during wastewater treatment. Water Environ. Res. 2016, 88, 29–39. [Google Scholar] [CrossRef]
- Amon, T.; Amon, B.; Kryvoruchko, V.; Zollitsch, W.; Mayer, K.; Gruber, L. Biogas production from maize and dairy cattle manure—Influence of biomass composition on the methane yield. Agric. Ecosyst. Environ. 2007, 118, 173–182. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Belostotskiy, D.E.; Ilinskaya, O.N.; Boulygina, E.A.; Grigoryeva, T.V.; Ziganshin, A.M. Effect of the Organic Loading Rate Increase and the Presence of Zeolite on Microbial Community Composition and Process Stability During Anaerobic Digestion of Chicken Wastes. Microb. Ecol. 2015, 70, 948–960. [Google Scholar] [CrossRef]
- Durán-Barrantes, M.M.; Álvarez-Mateos, P.; Jiménez-Rodriguez, A.; Romero-Guzmán, F.; Fiestas-Ros De Ursinos, J.A. Anaerobic treatment of swine wastewater in semicontinuous clayey support reactors. Chem. Biochem. Eng. Q. 2009, 23, 385–391. [Google Scholar]
- Jiang, Y.; Heaven, S.; Banks, C. Strategies for stable anaerobic digestion of vegetable waste. Renew. Energy 2012, 44, 206–214. [Google Scholar] [CrossRef]
- Woon, K.S.; Lo, I.M. A proposed framework of food waste collection and recycling for renewable biogas fuel production in Hong Kong. Waste Manag. 2016, 47, 3–10. [Google Scholar] [CrossRef]
- Zhang, L.; Lee, Y.-W.; Jahng, D. Anaerobic co-digestion of food waste and piggery wastewater: Focusing on the role of trace elements. Bioresour. Technol. 2011, 102, 5048–5059. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Tan, T. Batch and semi-continuous anaerobic digestion of food waste in a dual solid–liquid system. Bioresour. Technol. 2013, 145, 10–16. [Google Scholar] [CrossRef]
- Zhang, R.; El-Mashad, H.M.; Hartman, K.; Wang, F.; Liu, G.; Choate, C.; Gamble, P. Characterization of food waste as feedstock for anaerobic digestion. Bioresour. Technol. 2007, 98, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ge, Y.; Wang, K.; Li, X.; Pang, Y. Characteristics and anaerobic digestion performances of kitchen wastes. Kezaisheng Nengyuan/Renew. Energy Resour. 2010, 28, 76–80. [Google Scholar]
- Oleszek, M.; Król, A.; Tys, J.; Matyka, M.; Kulik, M. Comparison of biogas production from wild and cultivated varieties of reed canary grass. Bioresour. Technol. 2014, 156, 303–306. [Google Scholar] [CrossRef]
- Zou, H.; Chen, Y.; Shi, J.; Zhao, T.; Yu, Q.; Yu, S.; Shi, D.; Chai, H.; Gu, L.; He, Q.; et al. Mesophilic anaerobic co-digestion of residual sludge with different lignocellulosic wastes in the batch digester. Bioresour. Technol. 2018, 268, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Rowan, M.; Umenweke, G.C.; Epelle, E.I.; Afolabi, I.C.; Okoye, P.U.; Gunes, B.; Okolie, J.A. Anaerobic co-digestion of food waste and agricultural residues: An overview of feedstock properties and the impact of biochar addition. Digit. Chem. Eng. 2022, 4, 100046. [Google Scholar] [CrossRef]
- Wu, L.-J.; Kobayashi, T.; Kuramochi, H.; Li, Y.-Y.; Xu, K.-Q. Improved biogas production from food waste by co-digestion with de-oiled grease trap waste. Bioresour. Technol. 2016, 201, 237–244. [Google Scholar] [CrossRef]
- Zhang, C.; Xiao, G.; Peng, L.; Su, H.; Tan, T. The anaerobic co-digestion of food waste and cattle manure. Bioresour. Technol. 2013, 129, 170–176. [Google Scholar] [CrossRef]
- Melbourne and Taupo. 2019 Parkville Waste Audit. Available online: https://sustainablecampus.unimelb.edu.au/reduce-reuse-recycle/waste-audits (accessed on 1 May 2024).
- Dennehy, C.; Lawlor, P.G.; Croize, T.; Jiang, Y.; Morrison, L.; Gardiner, G.E.; Zhan, X. Synergism and effect of high initial volatile fatty acid concentrations during food waste and pig manure anaerobic co-digestion. Waste Manag. 2016, 56, 173–180. [Google Scholar] [CrossRef]
- Yong, Z.; Dong, Y.; Zhang, X.; Tan, T. Anaerobic co-digestion of food waste and straw for biogas production. Renew. Energy 2015, 78, 527–530. [Google Scholar] [CrossRef]
- Jabeen, M.; Yousaf, S.; Haider, M.R.; Malik, R.N. High-solids anaerobic co-digestion of food waste and rice husk at different organic loading rates. Int. Biodeterior. Biodegrad. 2015, 102, 149–153. [Google Scholar] [CrossRef]
- Brown, D.; Li, Y. Solid state anaerobic co-digestion of yard waste and food waste for biogas production. Bioresour. Technol. 2013, 127, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, S.; Sharma, H.B.; Dubey, B.K. Anaerobic co-digestion of food waste with pretreated yard waste: A comparative study of methane production, kinetic modeling and energy balance. J. Clean. Prod. 2020, 243, 118480. [Google Scholar] [CrossRef]
- Mu, L.; Zhang, L.; Zhu, K.; Ma, J.; Ifran, M.; Li, A. Anaerobic co-digestion of sewage sludge, food waste and yard waste: Synergistic enhancement on process stability and biogas production. Sci. Total Environ. 2020, 704, 135429. [Google Scholar] [CrossRef]
- Dixon, P.J.; Ergas, S.J.; Mihelcic, J.R.; Hobbs, S.R. Effect of substrate to inoculum ratio on bioenergy recovery from food waste, yard waste, and biosolids by high solids anaerobic digestion. Environ. Eng. Sci. 2019, 36, 1459–1465. [Google Scholar] [CrossRef]
- Borth, P.L.B.; Perin, J.K.H.; Torrecilhas, A.R.; Lopes, D.D.; Santos, S.C.; Kuroda, E.K.; Fernandes, F. Pilot-scale anaerobic co-digestion of food and garden waste: Methane potential, performance and microbial analysis. Biomass Bioenergy 2022, 157, 106331. [Google Scholar] [CrossRef]
- Lee, E.; Bittencourt, P.; Casimir, L.; Jimenez, E.; Wang, M.; Zhang, Q.; Ergas, S.J. Biogas production from high solids anaerobic co-digestion of food waste, yard waste and waste activated sludge. Waste Manag. 2019, 95, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Wainaina, S.; Lukitawesa Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef]
- Wang, K.; Yun, S.; Xing, T.; Li, B.; Abbas, Y.; Liu, X. Binary and ternary trace elements to enhance anaerobic digestion of cattle manure: Focusing on kinetic models for biogas production and digestate utilization. Bioresour. Technol. 2021, 323, 124571. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, X.; Chen, X.; Ren, L. Effects of bentonite on antibiotic resistance genes in biogas slurry and residue from thermophilic and mesophilic anaerobic digestion of food waste. Bioresour. Technol. 2021, 336, 125322. [Google Scholar] [CrossRef]
- Wang, Y.; Jing, Y.; Lu, C.; Kongjan, P.; Wang, J.; Awasthi, M.K.; Tahir, N.; Zhang, Q. A syntrophic co-fermentation model for bio-hydrogen production. J. Clean. Prod. 2021, 317, 128288. [Google Scholar] [CrossRef]
- Yadav, M.; Joshi, C.; Paritosh, K.; Thakur, J.; Pareek, N.; Masakapalli, S.K.; Vivekanand, V. Organic waste conversion through anaerobic digestion: A critical insight into the metabolic pathways and microbial interactions. Metab. Eng. 2021, 69, 323–337. [Google Scholar] [CrossRef]
- Aziz, M.M.A.; Kassim, K.A.; ElSergany, M.; Anuar, S.; Jorat, M.E.; Yaacob, H.; Ahsan, A.; Imteaz, M.A. Recent advances on palm oil mill effluent (POME) pretreatment and anaerobic reactor for sustainable biogas production. Renew. Sustain. Energy Rev. 2020, 119, 109603. [Google Scholar] [CrossRef]
- Qin, S.; Wainaina, S.; Liu, H.; Soufiani, A.M.; Pandey, A.; Zhang, Z.; Awasthi, M.K.; Taherzadeh, M.J. Microbial dynamics during anaerobic digestion of sewage sludge combined with food waste at high organic loading rates in immersed membrane bioreactors. Fuel 2021, 303, 121276. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Kumar, V.; Yadav, V.; Sarsaiya, S.; Awasthi, S.K.; Sindhu, R.; Binod, P.; Kumar, V.; Pandey, A.; Zhang, Z. Current state of the art biotechnological strategies for conversion of watermelon wastes residues to biopolymers production: A review. Chemosphere 2021, 290, 133310. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Wang, C.; He, Z.; Qin, Y.; Li, Y.-Y. Enhanced biomethanation of lipids by high-solid co-digestion with food waste: Biogas production and lipids degradation demonstrated by long-term continuous operation. Bioresour. Technol. 2022, 348, 126750. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Lukitawesa, L.; Duan, Y.; Taherzadeh, M.J.; Zhang, Z. Bacterial dynamics during the anaerobic digestion of toxic citrus fruit waste and semi-continues volatile fatty acids production in membrane bioreactors. Fuel 2022, 319, 123812. [Google Scholar] [CrossRef]
- Baek, G.; Kim, J.; Lee, C. A review of the effects of iron compounds on methanogenesis in anaerobic environments. Renew. Sustain. Energy Rev. 2019, 113, 109282. [Google Scholar] [CrossRef]
- De Vrieze, J.; Smet, D.; Klok, J.; Colsen, J.; Angenent, L.T.; Vlaeminck, S.E. Thermophilic sludge digestion improves energy balance and nutrient recovery potential in full-scale municipal wastewater treatment plants. Bioresour. Technol. 2016, 218, 1237–1245. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Sarsaiya, S.; Wainaina, S.; Rajendran, K.; Kumar, S.; Quan, W.; Duan, Y.; Awasthi, S.K.; Chen, H.; Pandey, A.; et al. A critical review of organic manure biorefinery models toward sustainable circular bioeconomy: Technological challenges, advancements, innovations, and future perspectives. Renew. Sustain. Energy Rev. 2019, 111, 115–131. [Google Scholar] [CrossRef]
- Ndayisenga, F.; Yu, Z.; Zheng, J.; Wang, B.; Liang, H.; Phulpoto, I.A.; Habiyakare, T.; Zhou, D. Microbial electrohydrogenesis cell and dark fermentation integrated system enhances biohydrogen production from lignocellulosic agricultural wastes: Substrate pretreatment towards optimization. Renew. Sustain. Energy Rev. 2021, 145, 111078. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Sindhu, R.; Sirohi, R.; Kumar, V.; Ahluwalia, V.; Binod, P.; Juneja, A.; Kumar, D.; Yan, B.; Sarsaiya, S.; et al. Agricultural waste biorefinery development towards circular bioeconomy. Renew. Sustain. Energy Rev. 2022, 158, 112122. [Google Scholar] [CrossRef]
- Rabii, A.; Aldin, S.; Dahman, Y.; Elbeshbishy, E. A Review on Anaerobic Co-Digestion with a Focus on the Microbial Populations and the Effect of Multi-Stage Digester Configuration. Energies 2019, 12, 1106. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.; Guwy, A.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef]
- Reshmy, R.; Philip, E.; Madhavan, A.; Sindhu, R.; Pugazhendhi, A.; Binod, P.; Sirohi, R.; Awasthi, M.K.; Tarafdar, A.; Pandey, A. Advanced biomaterials for sustainable applications in the food industry: Updates and challenges. Environ. Pollut. 2021, 283, 117071. [Google Scholar]
- Awasthi, M.K.; Wainaina, S.; Mahboubi, A.; Zhang, Z.; Taherzadeh, M.J. Methanogen and nitrifying genes dynamics in immersed membrane bioreactors during anaerobic co-digestion of different organic loading rates food waste. Bioresour. Technol. 2021, 342, 125920. [Google Scholar]
- Qin, S.; Giri, B.S.; Patel, A.K.; Sar, T.; Liu, H.; Chen, H.; Juneja, A.; Kumar, D.; Zhang, Z.; Awasthi, M.K.; et al. Resource recovery and biorefinery potential of apple orchard waste in the circular bioeconomy. Bioresour. Technol. 2021, 321, 124496. [Google Scholar] [CrossRef]
- Maria, G.; Şcoban, A.G. Optimal Operating Policy of a Fluidized Bed Bioreactor used for Mercury Uptake from Wastewaters by Using Immobilized, P. Putida Cells. Curr. Trends Biomed. Eng. Biosci. 2017, 2, 66–72. [Google Scholar] [CrossRef]
- Pererva, Y.; Miller, C.D.; Sims, R.C. Approaches in Design of Laboratory-Scale UASB Reactors. Processes 2020, 8, 734. [Google Scholar] [CrossRef]
- Service and R&D of Innovative Water Technology. Anaerobic Fluidized Bed (AFB) Process. Available online: https://www.itriwater.org.tw/Eng/technology/More?id=106 (accessed on 2 April 2024).
- Li, Y.; Ren, Y.; Ji, J.; Li, Y.-Y.; Kobayashi, T. Anaerobic Membrane Bioreactors for Municipal Wastewater Treatment, Sewage Sludge Digestion and Biogas Upgrading: A Review. Sustainability 2023, 15, 15129. [Google Scholar] [CrossRef]
- Borowski, S.; Boniecki, P.; Kubacki, P.; Czyżowska, A. Food waste co-digestion with slaughterhouse waste and sewage sludge: Digestate conditioning and supernatant quality. Waste Manag. 2018, 74, 158–167. [Google Scholar] [CrossRef]
- Guven, H.; Ersahin, M.E.; Dereli, R.K.; Ozgun, H.; Isik, I.; Ozturk, I. Energy recovery potential of anaerobic digestion of excess sludge from high-rate activated sludge systems co-treating municipal wastewater and food waste. Energy 2019, 172, 1027–1036. [Google Scholar] [CrossRef]
- Orellana, E.; Guerrero, L.D.; Davies-Sala, C.; Altina, M.; Pontiggia, R.M.; Erijman, L. Extracellular hydrolytic potential drives microbiome shifts during anaerobic co-digestion of sewage sludge and food waste. Bioresour. Technol. 2022, 343, 126102. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.-S.; Han, Y.; Cao, S.; Wang, X.C. Effects of long-term acclimatization on the optimum substrate mixture ratio and substrate to inoculum ratio in anaerobic codigestion of food waste and cow manure. Bioresour. Technol. 2020, 317, 123994. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, Y.; Zhang, M.; Wu, S.; Zhou, L. A collaborative strategy for enhanced anaerobic co-digestion of food waste and waste activated sludge by using zero valent iron and ferrous sulfide. Bioresour. Technol. 2022, 347, 126420. [Google Scholar] [CrossRef]
- Chuenchart, W.; Logan, M.; Leelayouthayotin, C.; Visvanathan, C. Enhancement of food waste thermophilic anaerobic digestion through synergistic effect with chicken manure. Biomass Bioenergy 2020, 136, 105541. [Google Scholar] [CrossRef]
- Helenas Perin, J.K.; Biesdorf Borth, P.L.; Torrecilhas, A.R.; Santana da Cunha, L.; Kuroda, E.K.; Fernandes, F. Optimization of methane production parameters during anaerobic co-digestion of food waste and garden waste. J. Clean. Prod. 2020, 272, 123130. [Google Scholar] [CrossRef]
- Li, W.; Loh, K.-C.; Zhang, J.; Tong, Y.W.; Dai, Y. Two-stage anaerobic digestion of food waste and horticultural waste in high-solid system. Appl. Energy 2018, 209, 400–408. [Google Scholar] [CrossRef]
- Qin, Y.; Li, L.; Wu, J.; Xiao, B.; Hojo, T.; Kubota, K.; Cheng, J.; Li, Y.-Y. Co-production of biohydrogen and biomethane from food waste and paper waste via recirculated two-phase anaerobic digestion process: Bioenergy yields and metabolic distribution. Bioresour. Technol. 2019, 276, 325–334. [Google Scholar] [CrossRef]
- Xu, F.; Okopi, S.I.; Jiang, Y.; Chen, Z.; Meng, L.; Li, Y.; Sun, W.; Li, C. Multi-criteria assessment of food waste and waste paper anaerobic co-digestion: Effects of inoculation ratio, total solids content, and feedstock composition. Renew. Energy 2022, 194, 40–50. [Google Scholar] [CrossRef]
- Casallas-Ojeda, M.; Soto-Paz, J.; Alfonso-Morales, W.; Oviedo-Ocaña, E.R.; Komilis, D. Optimization of Operational Parameters during Anaerobic Co-digestion of Food and Garden Waste. Environ. Process. 2021, 8, 769–791. [Google Scholar] [CrossRef]
- Panigrahi, S.; Dubey, B.K. A critical review on operating parameters and strategies to improve the biogas yield from anaerobic digestion of organic fraction of municipal solid waste. Renew. Energy 2019, 143, 779–797. [Google Scholar] [CrossRef]
- Taheri, M.E.; Salimi, E.; Saragas, K.; Novakovic, J.; Barampouti, E.M.; Mai, S.; Malamis, D.; Moustakas, K.; Loizidou, M. Effect of pretreatment techniques on enzymatic hydrolysis of food waste. Biomass Convers. Biorefinery 2021, 11, 219–226. [Google Scholar] [CrossRef]
- Canul Bacab, F.; España Gamboa, E.; Ruiz Espinoza, J.E.; Leal-Bautista, R.M.; Tapia Tussell, R.; Domínguez Maldonado, J.; Canto Canché, B.; Alzate-Gaviria, L. Two phase anaerobic digestion system of municipal solid waste by utilizing microaeration and granular activated carbon. Energies 2020, 13, 933. [Google Scholar] [CrossRef]
- Mahmood, Z.; Cheng, H.; Tian, M. A critical review on advanced anaerobic membrane bioreactors (AnMBRs) for wastewater treatment: Advanced membrane materials and energy demand. Environ. Sci. Water Res. Technol. 2022, 8, 2126–2144. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; He, Y.; Wang, Y.; Wang, S.; Zheng, Z.; Wang, S.; Xu, J.; Cai, Y.; Ying, H. A Comprehensive Review of the Strategies to Improve Anaerobic Digestion: Their Mechanism and Digestion Performance. Methane 2024, 3, 227–256. [Google Scholar] [CrossRef]
- Garcia-Peña, E.I.; Parameswaran, P.; Kang, D.; Canul-Chan, M.; Krajmalnik-Brown, R. Anaerobic digestion and co-digestion processes of vegetable and fruit residues: Process and microbial ecology. Bioresour. Technol. 2011, 102, 9447–9455. [Google Scholar] [CrossRef] [PubMed]
- Bardi, M.J.; Vinardell, S.; Astals, S.; Koch, K. Opportunities and challenges of micronutrients supplementation and its bioavailability in anaerobic digestion: A critical review. Renew. Sustain. Energy Rev. 2023, 186, 113689. [Google Scholar] [CrossRef]
- Rahimieh, A.; Nosrati, M. A review on biochemistry, microbiology and thermodynamic aspects of propionate: The key intermediate in the anaerobic digestion and wastewater treatment. Desalination Water Treat. 2024, 317, 100191. [Google Scholar] [CrossRef]
- Nozhevnikova, A.; Russkova, Y.I.; Litti, Y.V.; Parshina, S.; Zhuravleva, E.; Nikitina, A. Syntrophy and interspecies electron transfer in methanogenic microbial communities. Microbiology 2020, 89, 129–147. [Google Scholar] [CrossRef]
- Ajay, C.M.; Mohan, S.; Dinesha, P.; Rosen, M.A. Review of impact of nanoparticle additives on anaerobic digestion and methane generation. Fuel 2020, 277, 118234. [Google Scholar] [CrossRef]
- Dehhaghi, M.; Tabatabaei, M.; Aghbashlo, M.; Panahi, H.K.S.; Nizami, A.-S. A state-of-the-art review on the application of nanomaterials for enhancing biogas production. J. Environ. Manag. 2019, 251, 109597. [Google Scholar] [CrossRef]
- Eduok, S.; Ferguson, R.; Jefferson, B.; Villa, R.; Coulon, F. Aged-engineered nanoparticles effect on sludge anaerobic digestion performance and associated microbial communities. Sci. Total. Environ. 2017, 609, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Tian, Z.; Lv, Z.; Chen, Z.; Liu, Y.; Yong, X.; Zhou, J.; Xie, X.; Jia, H.; Wei, P. Effects of copper salts on performance, antibiotic resistance genes, and microbial community during thermophilic anaerobic digestion of swine manure. Bioresour. Technol. 2020, 300, 122728. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Niu, J.; Zhang, W.; Liu, J.; Yuan, J.; Li, H.; Yue, X. Mini art review for zero valent iron application in anaerobic digestion and technical bottlenecks. Sci. Total. Environ. 2021, 791, 148415. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Awais, M.; Javed, H.M.A.; Mustafa, M.S.; Tlili, I.; Khan, S.U.; Safdari Shadloo, M. Photo-catalytic pretreatment of biomass for anaerobic digestion using visible light and Nickle oxide (NiOx) nanoparticles prepared by sol gel method. Renew. Energy 2020, 154, 128–135. [Google Scholar] [CrossRef]
- Pang, L.; Xu, K.; Qi, L.; Chatzisymeon, E.; Liu, X.; Yang, P. Response behavior of antibiotic resistance genes to zinc oxide nanoparticles in cattle manure thermophilic anaerobic digestion process: A metagenomic analysis. Bioresour. Technol. 2022, 347, 126709. [Google Scholar] [CrossRef]
- Thanh, P.M.; Ketheesan, B.; Yan, Z.; Stuckey, D. Trace metal speciation and bioavailability in anaerobic digestion: A review. Biotechnol. Adv. 2016, 34, 122–136. [Google Scholar] [CrossRef]
- Zhang, M.; Zang, L. A Review of Interspecies Electron Transfer in Anaerobic Digestion; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2019; p. 042026. [Google Scholar]
- Park, J.-H.; Kang, H.-J.; Park, K.-H.; Park, H.-D. Direct interspecies electron transfer via conductive materials: A perspective for anaerobic digestion applications. Bioresour. Technol. 2018, 254, 300–311. [Google Scholar] [CrossRef]
- Baek, G.; Kim, J.; Kim, J.; Lee, C. Role and potential of direct interspecies electron transfer in anaerobic digestion. Energies 2018, 11, 107. [Google Scholar] [CrossRef]
- Cheng, Q.; Call, D.F. Hardwiring microbes via direct interspecies electron transfer: Mechanisms and applications. Environ. Sci. Process. Impacts 2016, 18, 968–980. [Google Scholar] [CrossRef]
- Hussein, A.K. Applications of nanotechnology in renewable energies—A comprehensive overview and understanding. Renew. Sustain. Energy Rev. 2015, 42, 460–476. [Google Scholar] [CrossRef]
- Lu, X.; Wang, H.; Ma, F.; Zhao, G.; Wang, S. Enhanced Anaerobic Digestion of Cow Manure and Rice Straw by the Supplementation of an Iron Oxide–Zeolite System. Energy Fuels 2017, 31, 599–606. [Google Scholar] [CrossRef]
- Huangfu, X.; Xu, Y.; Liu, C.; He, Q.; Ma, J.; Ma, C.; Huang, R. A review on the interactions between engineered nanoparticles with extracellular and intracellular polymeric substances from wastewater treatment aggregates. Chemosphere 2019, 219, 766–783. [Google Scholar] [CrossRef]
- Qiang, H.; Lang, D.-L.; Li, Y.-Y. High-solid mesophilic methane fermentation of food waste with an emphasis on Iron, Cobalt, and Nickel requirements. Bioresour. Technol. 2012, 103, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Z.; Lu, T.; Liu, J.; Wang, Y.; Shen, P.; Wei, Y. Response and mechanisms of the performance and fate of antibiotic resistance genes to nano-magnetite during anaerobic digestion of swine manure. J. Hazard. Mater. 2019, 366, 192–201. [Google Scholar] [CrossRef]
- Kato, S.; Hashimoto, K.; Watanabe, K. Methanogenesis facilitated by electric syntrophy via (semi) conductive iron-oxide minerals. Environ. Microbiol. 2012, 14, 1646–1654. [Google Scholar] [CrossRef]
- Jing, Y.; Wan, J.; Angelidaki, I.; Zhang, S.; Luo, G. iTRAQ quantitative proteomic analysis reveals the pathways for methanation of propionate facilitated by magnetite. Water Res. 2017, 108, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Baek, G.; Kim, J.; Cho, K.; Bae, H.; Lee, C. The biostimulation of anaerobic digestion with (semi) conductive ferric oxides: Their potential for enhanced biomethanation. Appl. Microbiol. Biotechnol. 2015, 99, 10355–10366. [Google Scholar] [CrossRef]
- Baek, G.; Jung, H.; Kim, J.; Lee, C. A long-term study on the effect of magnetite supplementation in continuous anaerobic digestion of dairy effluent–magnetic separation and recycling of magnetite. Bioresour. Technol. 2017, 241, 830–840. [Google Scholar] [CrossRef]
- Yin, Q.; Miao, J.; Li, B.; Wu, G. Enhancing electron transfer by ferroferric oxide during the anaerobic treatment of synthetic wastewater with mixed organic carbon. Int. Biodeterior. Biodegrad. 2017, 119, 104–110. [Google Scholar] [CrossRef]
- Casals, E.; Barrena, R.; García, A.; González, E.; Delgado, L.; Busquets-Fité, M.; Font, X.; Arbiol, J.; Glatzel, P.; Kvashnina, K. Programmed iron oxide nanoparticles disintegration in anaerobic digesters boosts biogas production. Small 2014, 10, 2801–2808. [Google Scholar] [CrossRef] [PubMed]
- Suanon, F.; Sun, Q.; Mama, D.; Li, J.; Dimon, B.; Yu, C.-P. Effect of nanoscale zero-valent iron and magnetite (Fe3O4) on the fate of metals during anaerobic digestion of sludge. Water Res. 2016, 88, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, J.; Hu, Z. Impact of nano zero valent iron (NZVI) on methanogenic activity and population dynamics in anaerobic digestion. Water Res. 2013, 47, 6790–6800. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Shah, T.A.; Afzal, A.; Tabassum, R. Exploring lignocellulosic biomass for bio-methane potential by anaerobic digestion and its economic feasibility. Energy Environ. 2018, 29, 742–751. [Google Scholar] [CrossRef]
- Juntupally, S.; Begum, S.; Allu, S.K.; Nakkasunchi, S.; Madugula, M.; Anupoju, G.R. Relative evaluation of micronutrients (MN) and its respective nanoparticles (NPs) as additives for the enhanced methane generation. Bioresour. Technol. 2017, 238, 290–295. [Google Scholar] [CrossRef]
- Abdelsalam, E.; Samer, M.; Attia, Y.; Abdel-Hadi, M.; Hassan, H.; Badr, Y. Effects of Co and Ni nanoparticles on biogas and methane production from anaerobic digestion of slurry. Energy Convers. Manag. 2017, 141, 108–119. [Google Scholar] [CrossRef]
- Ali, A.; Mahar, R.B.; Soomro, R.A.; Sherazi, S.T.H. Fe3O4 nanoparticles facilitated anaerobic digestion of organic fraction of municipal solid waste for enhancement of methane production. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 1815–1822. [Google Scholar] [CrossRef]
- Zhang, L.; He, X.; Zhang, Z.; Cang, D.; Nwe, K.A.; Zheng, L.; Li, Z.; Cheng, S. Evaluating the influences of ZnO engineering nanomaterials on VFA accumulation in sludge anaerobic digestion. Biochem. Eng. J. 2017, 125, 206–211. [Google Scholar] [CrossRef]
- Chen, J.L.; Steele, T.W.; Stuckey, D.C. The effect of Fe2NiO4 and Fe4NiO4Zn magnetic nanoparticles on anaerobic digestion activity. Sci. Total Environ. 2018, 642, 276–284. [Google Scholar] [CrossRef]
- Abdallah, M.S.; Hassaneen, F.Y.; Faisal, Y.; Mansour, M.S.; Ibrahim, A.; Abo-Elfadl, S.; Salem, H.; Allam, N.K. Effect of Ni-Ferrite and Ni-Co-Ferrite nanostructures on biogas production from anaerobic digestion. Fuel 2019, 254, 115673. [Google Scholar] [CrossRef]
- Elreedy, A.; Ibrahim, E.; Hassan, N.; El-Dissouky, A.; Fujii, M.; Yoshimura, C.; Tawfik, A. Nickel-graphene nanocomposite as a novel supplement for enhancement of biohydrogen production from industrial wastewater containing mono-ethylene glycol. Energy Convers. Manag. 2017, 140, 133–144. [Google Scholar] [CrossRef]
- Mansour, M.S.; Abdallah, M.S.; Allam, N.K.; Ibrahim, A.; Khedr, A.M.; Al-Bulqini, H.M.; Zayed, M.F. Biogas production enhancement using nanocomposites and its combustion characteristics in a concentric flow slot burner. Exp. Therm. Fluid Sci. 2020, 113, 110014. [Google Scholar] [CrossRef]
- Zhang, G.; Shi, Y.; Zhao, Z.; Wang, X.; Dou, M. Enhanced two-phase anaerobic digestion of waste-activated sludge by combining magnetite and zero-valent iron. Bioresour. Technol. 2020, 306, 123122. [Google Scholar] [CrossRef]
- Hassaneen, F.Y.; Abdallah, M.S.; Ahmed, N.; Taha, M.M.; Abd ElAziz, S.M.M.; El-Mokhtar, M.A.; Badary, M.S.; Allam, N.K. Innovative nanocomposite formulations for enhancing biogas and biofertilizers production from anaerobic digestion of organic waste. Bioresour. Technol. 2020, 309, 123350. [Google Scholar] [CrossRef]
- Chen, J.; Yun, S.; Shi, J.; Wang, Z.; Abbas, Y.; Wang, K.; Han, F.; Jia, B.; Xu, H.; Xing, T.; et al. Role of biomass-derived carbon-based composite accelerants in enhanced anaerobic digestion: Focusing on biogas yield, fertilizer utilization, and density functional theory calculations. Bioresour. Technol. 2020, 307, 123204. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.M.; Thorat, N.D.; Shete, P.B.; Bedge, P.A.; Gavde, S.; Joshi, M.G.; Tofail, S.A.; Bohara, R.A. Comprehensive cytotoxicity studies of superparamagnetic iron oxide nanoparticles. Biochem. Biophys. Rep. 2018, 13, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, S.; Biscoff, R.; Enweremadu, C. A meta-analysis of iron-based additives on enhancements of biogas yields during anaerobic digestion of organic wastes. J. Clean. Prod. 2020, 269, 122449. [Google Scholar] [CrossRef]
- Ambuchi, J.J.; Zhang, Z.; Feng, Y. Biogas enhancement using iron oxide nanoparticles and multi-wall carbon nanotubes. Int. J. Chem. Mol. Nucl. Mat. Metall. Eng 2016, 10, 1239–1246. [Google Scholar]
- Abdelsalam, E.; Samer, M.; Attia, Y.; Abdel-Hadi, M.; Hassan, H.; Badr, Y. Influence of zero valent iron nanoparticles and magnetic iron oxide nanoparticles on biogas and methane production from anaerobic digestion of manure. Energy 2017, 120, 842–853. [Google Scholar] [CrossRef]
- Amen, T.W.; Eljamal, O.; Khalil, A.M.; Matsunaga, N. Biochemical methane potential enhancement of domestic sludge digestion by adding pristine iron nanoparticles and iron nanoparticles coated zeolite compositions. J. Environ. Chem. Eng. 2017, 5, 5002–5013. [Google Scholar] [CrossRef]
- Amen, T.W.M.; Eljamal, O.; Khalil, A.M.E.; Sugihara, Y.; Matsunaga, N. Methane yield enhancement by the addition of new novel of iron and copper-iron bimetallic nanoparticles. Chem. Eng. Process.-Process. Intensif. 2018, 130, 253–261. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, L.; Wang, Y.; Zhao, Y.; She, Z.; Gao, M.; Guo, Y. Application of iron oxide (Fe3O4) nanoparticles during the two-stage anaerobic digestion with waste sludge: Impact on the biogas production and the substrate metabolism. Renew. Energy 2020, 146, 2724–2735. [Google Scholar]
- Amen, T.W.; Eljamal, O.; Khalil, A.M.; Matsunaga, N. Evaluation of nano zero Valent Iron Effects on Fermentation of Municipal Anaerobic Sludge and Inducing Biogas Production; IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2017; p. 012004. [Google Scholar]
- Suanon, F.; Sun, Q.; Li, M.; Cai, X.; Zhang, Y.; Yan, Y.; Yu, C.-P. Application of nanoscale zero valent iron and iron powder during sludge anaerobic digestion: Impact on methane yield and pharmaceutical and personal care products degradation. J. Hazard. Mater. 2017, 321, 47–53. [Google Scholar] [CrossRef]
- Yazdani, M.; Ebrahimi-Nik, M.; Heidari, A.; Abbaspour-Fard, M.H. Improvement of biogas production from slaughterhouse wastewater using biosynthesized iron nanoparticles from water treatment sludge. Renew. Energy 2019, 135, 496–501. [Google Scholar] [CrossRef]
- Ünşar, E.K.; Perendeci, N.A. What kind of effects do Fe2O3 and Al2O3 nanoparticles have on anaerobic digestion, inhibition or enhancement? Chemosphere 2018, 211, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Farghali, M.; Andriamanohiarisoamanana, F.J.; Ahmed, M.M.; Kotb, S.; Yamamoto, Y.; Iwasaki, M.; Yamashiro, T.; Umetsu, K. Prospects for biogas production and H2S control from the anaerobic digestion of cattle manure: The influence of microscale waste iron powder and iron oxide nanoparticles. Waste Manag. 2020, 101, 141–149. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, Z.; Zhang, Y.; Xu, R.; Zheng, Y.; Hu, J.; Li, X.; Jia, M.; Xiong, W.; Cao, J. Influence of nanoscale zero-valent iron and magnetite nanoparticles on anaerobic digestion performance and macrolide, aminoglycoside, β-lactam resistance genes reduction. Bioresour. Technol. 2019, 294, 122139. [Google Scholar] [CrossRef]
- Chen, R.; Konishi, Y.; Nomura, T. Enhancement of methane production by Methanosarcina barkeri using Fe3O4 nanoparticles as iron sustained release agent. Adv. Powder Technol. 2018, 29, 2429–2433. [Google Scholar] [CrossRef]
- Hassanein, A.; Lansing, S.; Tikekar, R. Impact of metal nanoparticles on biogas production from poultry litter. Bioresour. Technol. 2019, 275, 200–206. [Google Scholar] [CrossRef]
- Zaidi, A.A.; RuiZhe, F.; Malik, A.; Khan, S.Z.; Bhutta, A.J.; Shi, Y.; Mushtaq, K. Conjoint effect of microwave irradiation and metal nanoparticles on biogas augmentation from anaerobic digestion of green algae. Int. J. Hydrogen Energy 2019, 44, 14661–14670. [Google Scholar] [CrossRef]
- Farghali, M.; Andriamanohiarisoamanana, F.J.; Ahmed, M.M.; Kotb, S.; Yamashiro, T.; Iwasaki, M.; Umetsu, K. Impacts of iron oxide and titanium dioxide nanoparticles on biogas production: Hydrogen sulfide mitigation, process stability, and prospective challenges. J. Environ. Manag. 2019, 240, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Avilés, P.; Ida, J.; Toda, T.; Cuevas-Rodríguez, G. Effects and fate of TiO2 nanoparticles in the anaerobic treatment of wastewater and waste sludge. J. Environ. Manag. 2018, 222, 227–233. [Google Scholar] [CrossRef]
- Lin, R.; Cheng, J.; Zhang, J.; Zhou, J.; Cen, K.; Murphy, J.D. Boosting biomethane yield and production rate with graphene: The potential of direct interspecies electron transfer in anaerobic digestion. Bioresour. Technol. 2017, 239, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, F.; Tsui, T.-H.; Yoh, K.; Sun, J.; Loh, K.-C.; Wang, C.-H.; Dai, Y.; Tong, Y.W. Microbial succession analysis reveals the significance of restoring functional microorganisms during rescue of failed anaerobic digesters by bioaugmentation of nano-biochar-amended digestate. Bioresour. Technol. 2022, 352, 127102. [Google Scholar] [CrossRef]
- Ali, A.; Mahar, R.B.; Abdelsalam, E.M.; Sherazi, S.T.H. Kinetic Modeling for Bioaugmented Anaerobic Digestion of the Organic Fraction of Municipal Solid Waste by Using Fe3O4 Nanoparticles. Waste Biomass Valorization 2019, 10, 3213–3224. [Google Scholar] [CrossRef]
- Li, Y.; Wang, P.; Zhao, L.; Yang, X.; Ren, L. Microbial mechanism underlying the effect of biochar supported nano zero-valent iron on the anaerobic digestion of food waste. J. Environ. Chem. Eng. 2023, 11, 111286. [Google Scholar] [CrossRef]





| Uses of Biogas | Benefits | References |
|---|---|---|
| Green energy production | [8] | |
| Electricity | ||
| Heat | ||
| Vehicle fuel | ||
| Tri-generation | ||
| Environmental protection | Pathogen reduction through sanitation | [9,10] |
| Less nuisance from insect flies | ||
| Air and water pollution reduction | ||
| Forest vegetation conservation | ||
| Replacing inorganic fertilizer | ||
| Eutrophication and acidification reduction | ||
| Biogas-linked agrosystem | Livestock–biogas–fruit system | [11] |
| Biogas–livestock and poultry farms system | ||
| Pig–biogas–vegetable greenhouse system | ||
| Organic waste disposal | Municipal solid wastes | [12] |
| Agricultural residues | ||
| Industrial waste | ||
| Household waste | ||
| Organic waste mixtures | ||
| Greenhouse gas emission reduction | Providing renewable energy alternatives for conventional energy sources | [9] |
| Substrates with Low C/N Ratio | Substrates with High C/N Ratio | ||
|---|---|---|---|
| Substrates | C/N Ratio | Substrates | C/N Ratio |
| Slaughterhouse waste | 22–37 | Sugar cane bagasse | 140–150 |
| Fruits and vegetable waste | 7–35 | Corn stalks/straw | 50–56 |
| Kitchen waste | 25–29 | Garden waste | 50–53 |
| Cow dung | 16–25 | Seaweed | 70–79 |
| Poultry manure | 5–15 | Sugar beet/sugar foliage | 35–40 |
| Pig manure | 6–14 | Rice straw | 51–67 |
| Horse manure | 20–25 | Algae | 75–100 |
| Food waste | 3–17 | Sawdust | 200–500 |
| Peanut shoots/hulls | 20–31 | Potatoes | 35–60 |
| Waste cereals | 12–16 | Oat straw | 48–50 |
| Grass/grass trimmings | 12–16 | Wheat straw | 50–150 |
| alfalfa | 12–17 | ||
| Mixed food wastes | 15–32 | ||
| Goat manure | 10–17 | ||
| Sheep dung | 30–33 | ||
| Type of Waste | Substrate | Characteristics | Inhibiting Factors | Reference |
|---|---|---|---|---|
| Agricultural waste | Potato peelings | TS = 119.2 (g/kg), VS = 105.5, COD = 126, Cellulose = 16.1 | High cellulose content, COD/N ratio imbalance | [124] |
| Fruit and vegetable waste | TS = 90.4 (g/kg), VS = 82.9, COD = 104.5, Cellulose = 9.2, Lignin = 4.5 | Recalcitrant indigestible cellulose and lignin, rapid acidification to volatile fatty acids (VFAs), and methanogenesis inhibition | [125] | |
| Algal biomass (S. dimorphus) | TS = 12.2, VS = 11.3 | NH3 toxicity, recalcitrant algal cell walls (cellulose), a high nitrogen-to-carbon content ratio, and serum ion toxicity increased | [126] | |
| Green peas and carrots | TS = 179.4 (g/kg), VS = 171, COD = 185, Cellulose = 16.1, = 5.5. | High cellulose content, COD/N ratio imbalance | [124] | |
| Industrial Waste | Wastewater from raw natural rubber processing | COD= 25–30 (g/L), VS= 15, TVFA = 40–50, TKN = 7–8 | Residual organic matter, NH3 (for natural latex preservation), and latex suspended solids (SS) washout of retained sludge | [121] |
| Vinasses, stillages, distillery slops | pH = 4–5, COD = 50–100 (g/L), TVFA = 8.5, TKN = 1.8 | Low pH 4–5, high organic content, salinity, and phenolic compounds | [122] | |
| Manure | Cattle manure | pH = 6.5–7, Cellulose = 160–230 g/kg DM, Lignin = 120–190 g/kg DM, protein = 150–300 g/kg DM | High cellulose and lignin contents | [127] |
| Poultry manure | pH = 6.9, VS = 89.15%, TVFA = 1.3 (g/L) | Excessive levels of uric acid and undigested proteins produce harmful free NH3 and ammonium ions (NH4+). | [128] | |
| Swine wastewater | pH = 7.7, COD = 25.0 (g/L), TVFA = 0.5, NH4+-N = 1.6 | high total solids content (25–50 kg m–3) and high NH4+ inhibitory concentration | [129] |
| Parameters/References | Zhang, L. et al. [132] | Zhang, C. et al. [133] | Zhang, R. et al. [134] | Li, R. et al. [135] |
|---|---|---|---|---|
| TS (%, w/w) | 18.1 (0.6) | 23.1 (0.3) | 30.90 (0.07) | 24 |
| VS (%, w/w) | 17.1 (0.6) | 21.0 (0.3) | 26.35 (0.14) | 232 |
| VS/TS (%) | 0.94 (0.01) | 90.9 (0.2) | 85.30 (0.65) | 94.1 |
| pH | 6.5 (0.2) | 4.2 (0.2) | – | – |
| Carbohydrate (% TS) | 61.9 | – | – | 55.2 |
| Protein (% TS) | – | – | 15 | |
| Fat (% TS) | 23.3 (0.45) | – | – | 23.9 |
| Oil (% TS) | – | 4.6 (0.5) | – | – |
| C (% TS) | 46.67 | 56.3 (1.1) | 46.78 (1.15) | 54 |
| N (% TS) | 3.54 | 2.3 (0.3) | 3.16 (0.22) | 2.4 |
| C/N | 13.2 | 24.5 (1.1) | 14.8 | 22.5 |
| S (mg/L TS) | 0.33 | – | 2508 (87) | 8.6 |
| P (mg/L) | 1.49 (0.09) | – | – | 88 |
| Na (% TS) | 0.84 | 3.45 (0.2) | – | 2.24 |
| K (% TS) | 0.3 | 2.30 (0.04) | 0.90 (0.11) | – |
| Ca (% TS) | 0.07 | 0.4 (0.01) | 2.16 (0.29) | 2.44 |
| Mg (% TS) | 0.03 | 0.16 (0.01) | 0.14 (0.01) | – |
| Fe (mg/L) | 3.17 | 100 (23) | 766 (402) | – |
| Cu (mg/L) | 3.06 | – | 31 (1) | – |
| Zn (mg/L) | 8.27 | 160 (30) | 76 (22) | – |
| Al (mg/L) | 4.31 | – | 1202 (396) | – |
| Mn (mg/L) | 0.96 | 110 (95) | 60 (30) | – |
| Cr (mg/L) | 0.17 | – | <1 | – |
| Ni (mg/L) | 0.19 | – | 2 (1) | – |
| Co-Substrate Types | Reactor Types | Optimum Operating Conditions | Methane Yields | VS Removal | Reference |
|---|---|---|---|---|---|
| FW and slaughterhouse waste | Pilot-scale semi-continuously reactor | Temp = 35 °C, mixing ratio = 7:3 (TS), SRT = 25 day | 630 dm3/kg VS No inhibition of VFA | 73.14% | [175] |
| FW and SS | Pilot-scale continuously reactor | Temp = 37 °C, OLR = 11.4 kg COD m−3 d−1, SRT = 90 day | 224 L CH4·kg−1 VSadded 50% increase in CH4 yield in co-digestion | 59% | [176] |
| Lab-scale semi-continuously reactor | Temp = 35 °C, OLR = 1.5 g VS L−1 d−1, SRT = 125 day | 683.7 ± 65.6 mL·g VS−1 | - | [177] | |
| FW and cow manure | Full-scale mesophilic anaerobic reactor at the brewery plant | Temp = 39 °C, mixing ratio = 2.5:1 (VS), SRT = 550 day | 653.4 mL/g VS | 87.3% | [178] |
| FW and cattle manure | Batch semi-continuous stirred tank reactors | Temp = 38 °C, mixing ratio = 3:1 (VS), SRT = 100 day | 467.3–507.6 mL/g VS | - | [179] |
| FW and chicken manure | Batch semi-continuous stirred tank reactors | Temp = 55 °C, mixing ratio = 7:3 (VS), SRT = 200 day | 0.795 m3CH4kg−1VSfed No VFA or NH3 inhibition | 82.81 to 63.01% with the increment in OLR from 1 to 4 kg VS·m−3·d−1 | [180] |
| FW and yard waste | Batch semi-continuous stirred tank reactors | Temp = 35 °C, mixing ratio = 75:25 (VS), SRT = 28 day | 314.9 ± 17.1 mL/g VS Effective improvement of C/N ratio and reactor stability | - | [147] |
| Food and garden waste | Pilot scale semi-continuous reactor | Temp =35 °C, OLR = 0.24 to 0.54 kg VS m−3d−1, HRT = 40 days, Feed ratio: FW:GW = 4:1 (VS) | CH4 yield (0.47 L g VS−1) and CH4 content (67%) food and garden waste co-digestion improved compared to only food waste AD | 83% | [181] |
| FW, chicken manure, and grass | Two-stage batch reactors | Mixing ratio = 4:4:5, Temp = 35 °C, S/I = 4 | 113.4 mL/gVS 83.25% higher than single stage | 57.30% Higher than single stage | [182] |
| FW and GW | Pilot scale reactor 500 mL | OLR—0.24 kgVS m−3d−1, Temp—40 °C | Specific CH4 yield of 0.47 LCH4 gVS−1, a biogas production rate of 86 L d−1 | 83% | [149] |
| FW and paper waste | Batch semi-continuous stirred tank reactors | Temp = 35 °C, mixing ratio = 1:1 (TS), SRT = 267 day | 426 CH4/kg VSfed | 84.9% | [183] |
| Batch semi-continuous stirred tank reactors | Temp = 37 °C, mixing ratio = 75:25 (TS), SRT = 40 day | 533–536 L/kg VSfeed | - | [184] | |
| FW and GW | Batch reactor (250 mL) | FW:GW = 80:20, 70:30 and 60:40 (w/w), ISR 1, Temp = 35 °C | 270 mL CH4/g VS 26% increase compared to monodigestion of FW | - | [185] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitra, S.; Kaparaju, P. Feasibility of Food Organics and Garden Organics as a Promising Source of Biomethane: A Review on Process Optimisation and Impact of Nanomaterials. Energies 2024, 17, 4198. https://doi.org/10.3390/en17164198
Mitra S, Kaparaju P. Feasibility of Food Organics and Garden Organics as a Promising Source of Biomethane: A Review on Process Optimisation and Impact of Nanomaterials. Energies. 2024; 17(16):4198. https://doi.org/10.3390/en17164198
Chicago/Turabian StyleMitra, Shweta, and Prasad Kaparaju. 2024. "Feasibility of Food Organics and Garden Organics as a Promising Source of Biomethane: A Review on Process Optimisation and Impact of Nanomaterials" Energies 17, no. 16: 4198. https://doi.org/10.3390/en17164198
APA StyleMitra, S., & Kaparaju, P. (2024). Feasibility of Food Organics and Garden Organics as a Promising Source of Biomethane: A Review on Process Optimisation and Impact of Nanomaterials. Energies, 17(16), 4198. https://doi.org/10.3390/en17164198








