Investigation of Sewage Sludge–Derived Biochar for Enhanced Pollutant Adsorption: Effect of Particle Size and Alkali Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
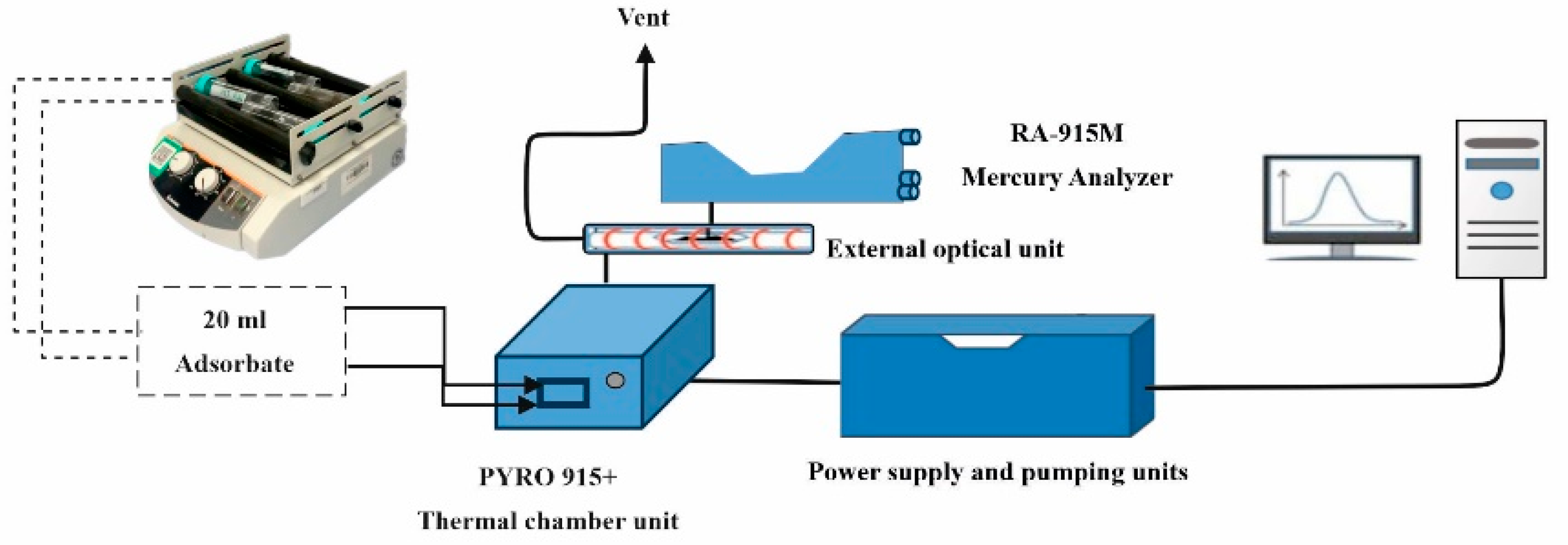
2.2. Pyrolysis Reactor
2.3. Modification of Biochar
2.4. Batch Adsorption Studies
2.5. Material Characterization
3. Results and Discussion
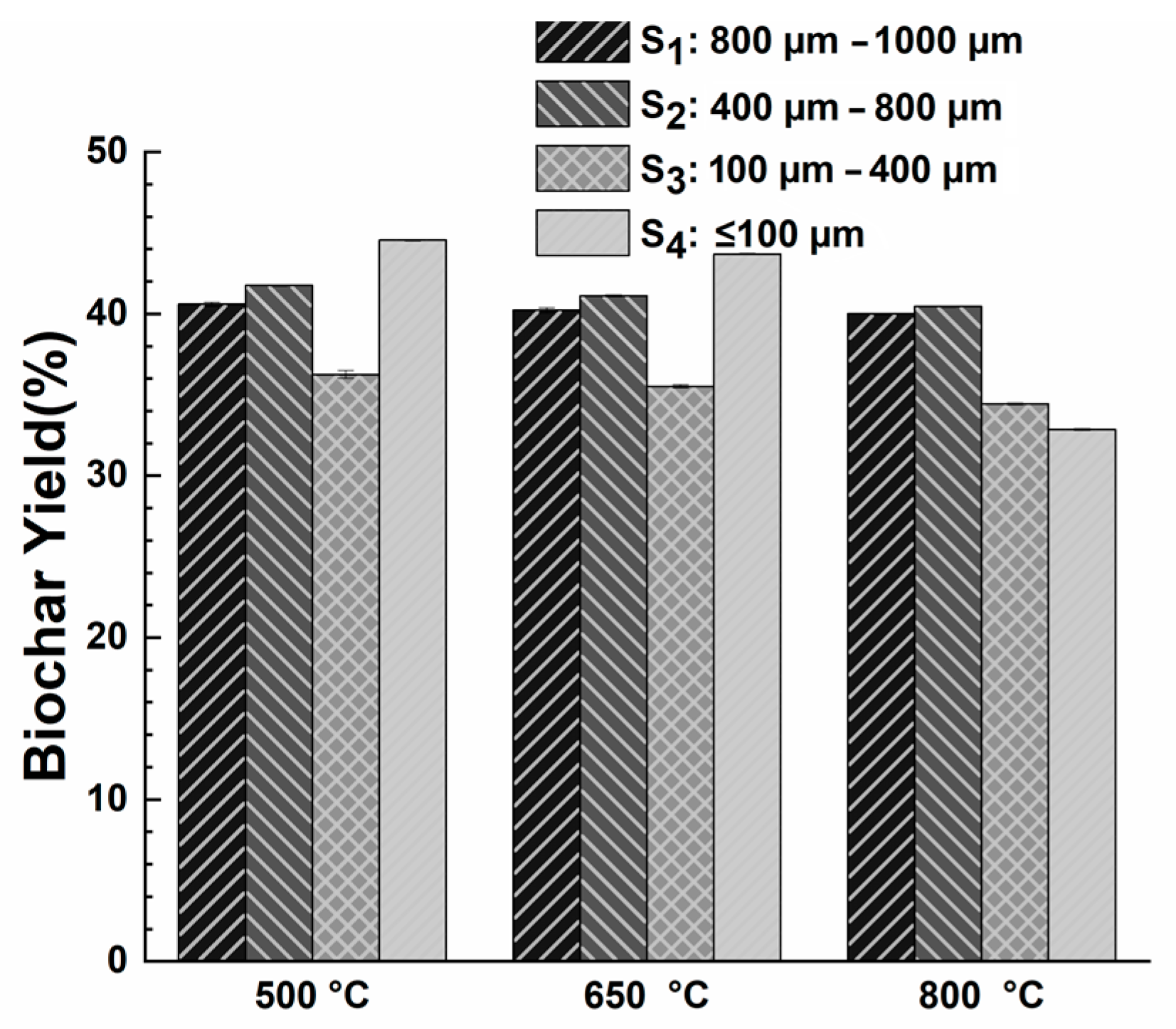
3.1. Effects of Pyrolysis Temperature on Biochar Yield with Different Particle Sizes
3.2. Characterization of Sludge-Derived Char
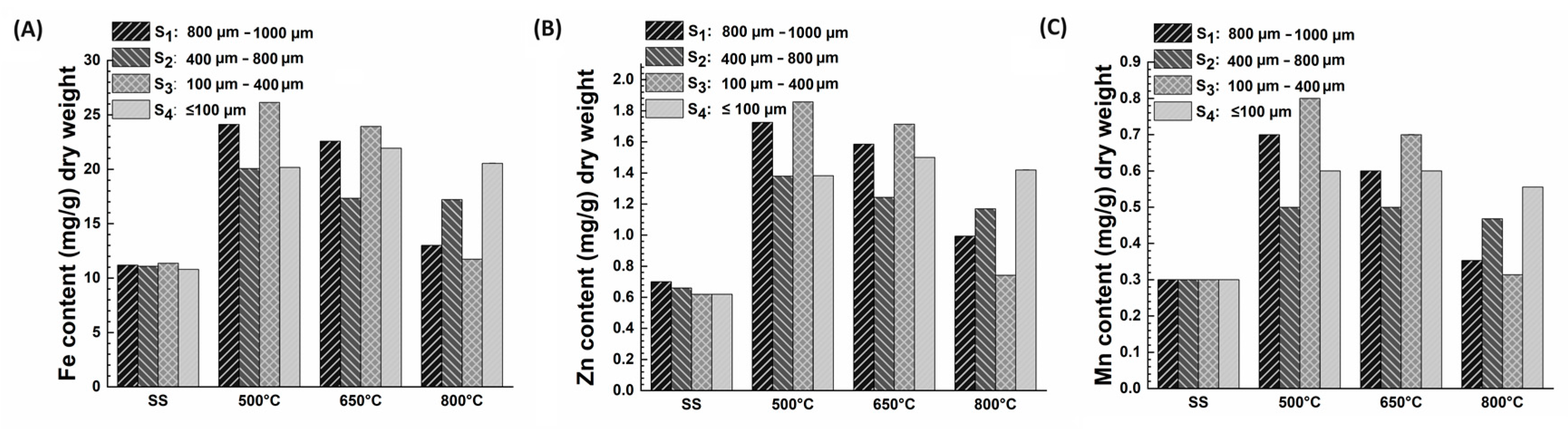
3.2.1. Heavy Metals Distribution in Different Particle Sizes of SS Biochar
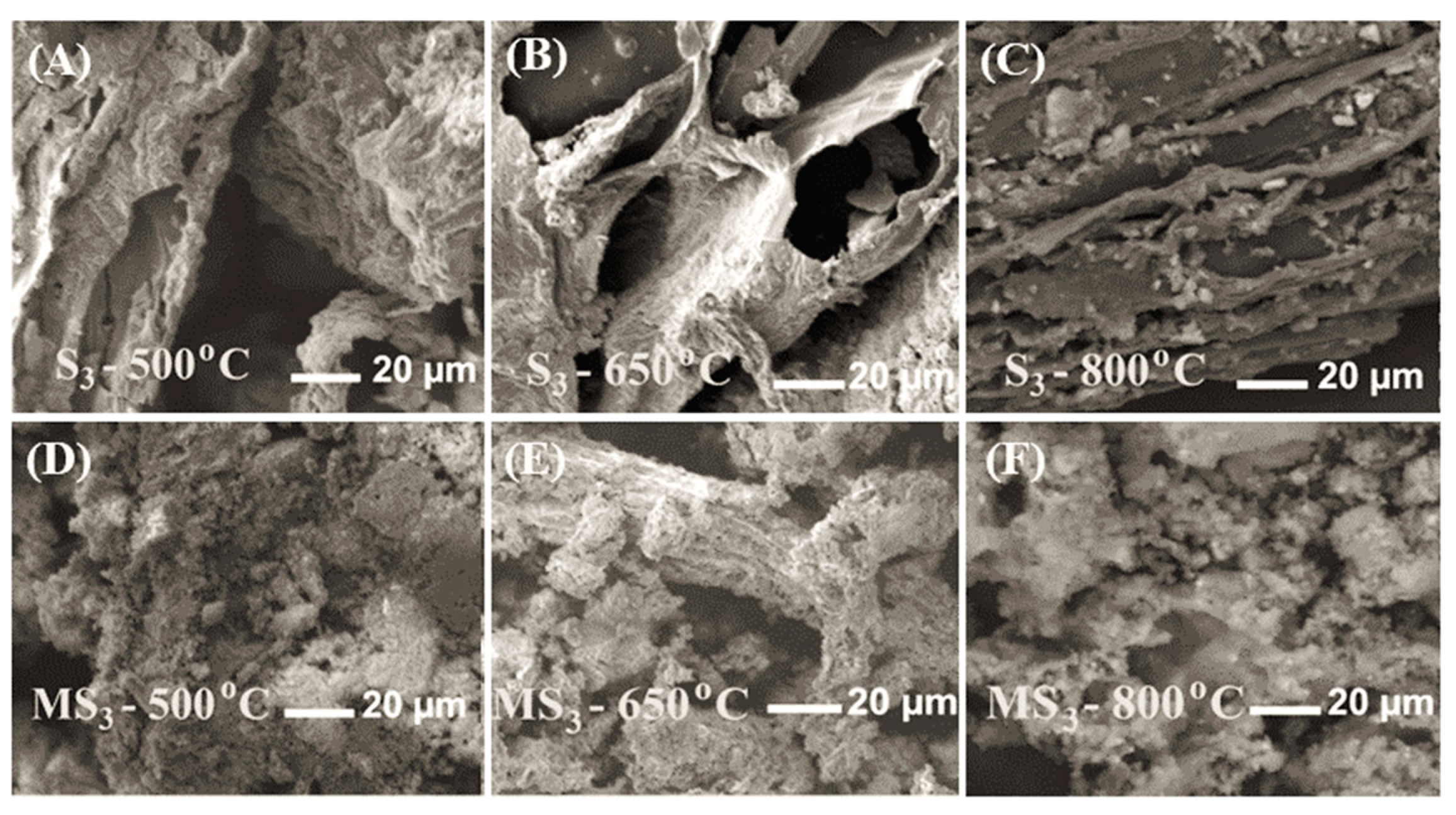
3.2.2. Surface Morphology of the SS Biochar and NaOH-Modified Biochar
3.2.3. Specific Surface Area of the SS-Derived Biochar
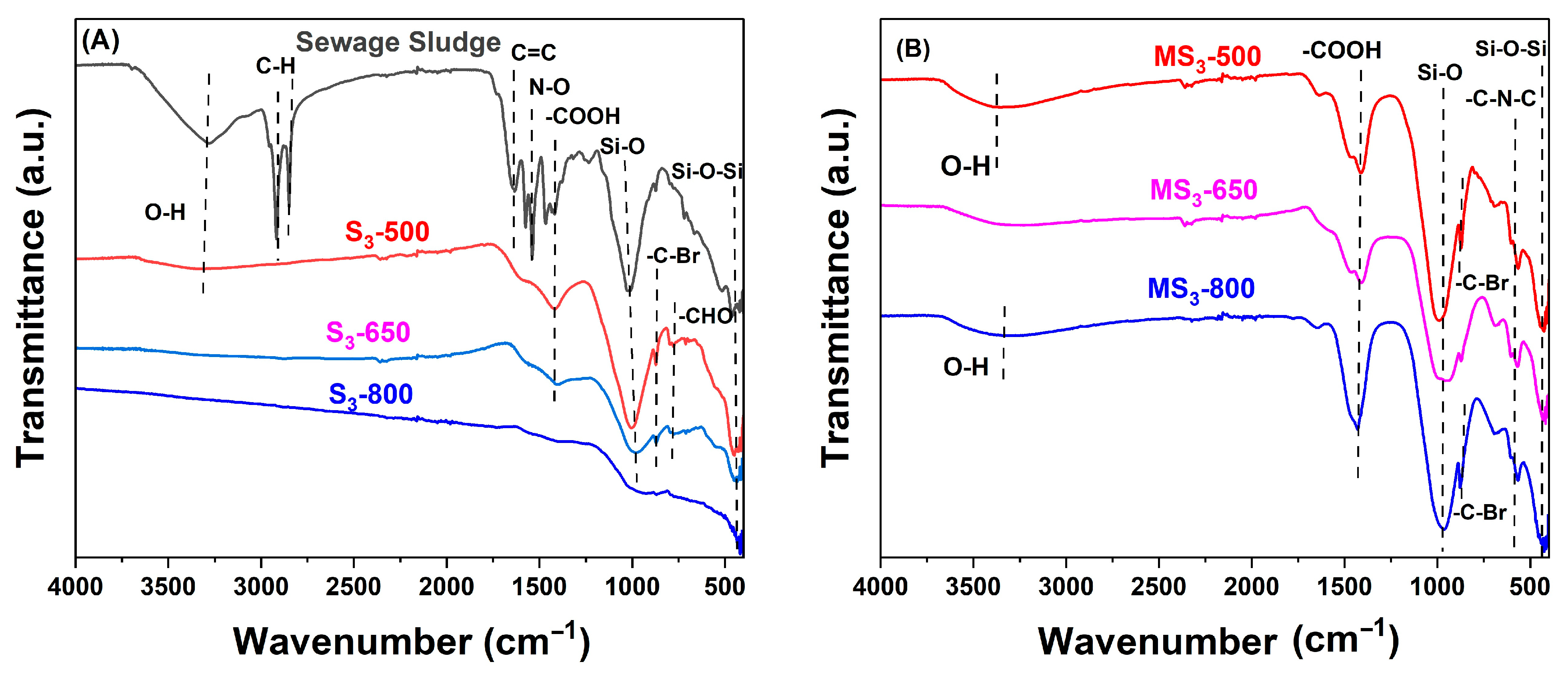
3.2.4. FTIR Spectrum of Sewage Sludge, Sewage Sludge–Derived Biochar, and Modified Biochar
3.3. Adsorption of Methylene Blue (MB) and Mercury (Hg2+)
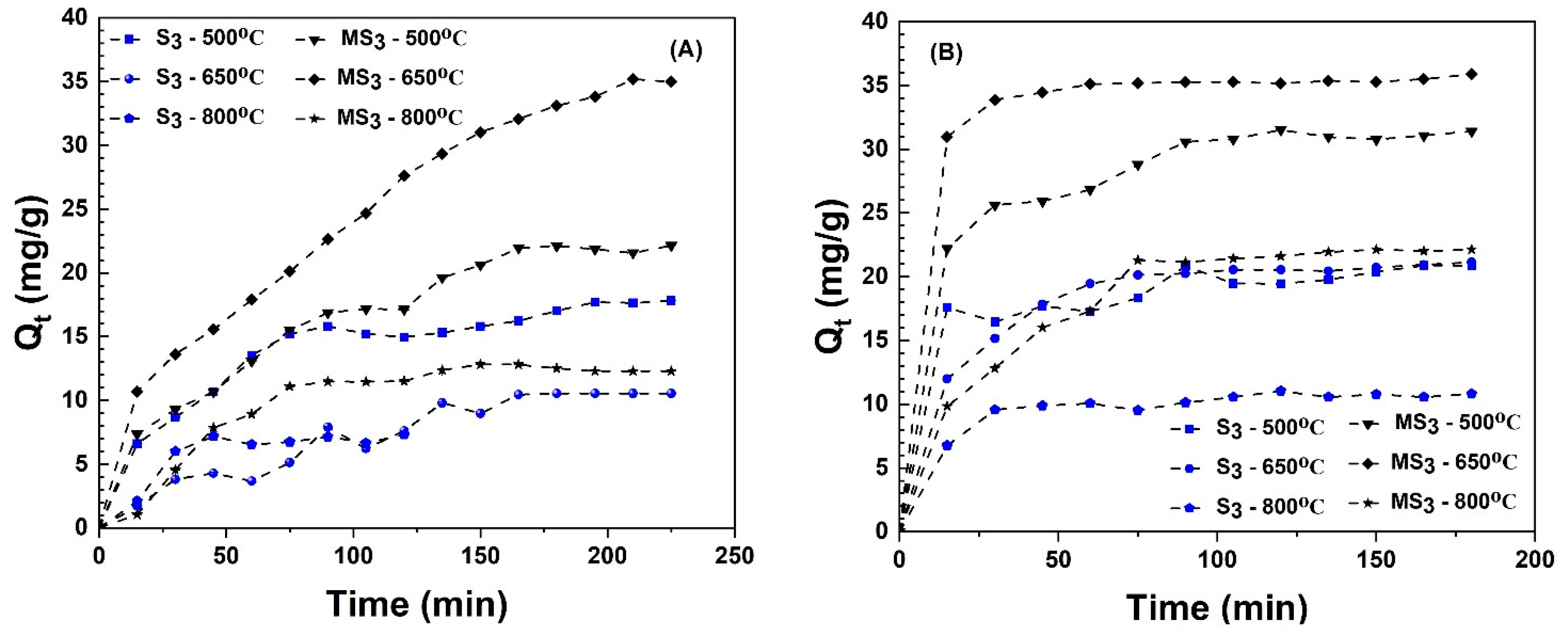
3.3.1. Influence of Biochar Particle Size on MB Adsorption
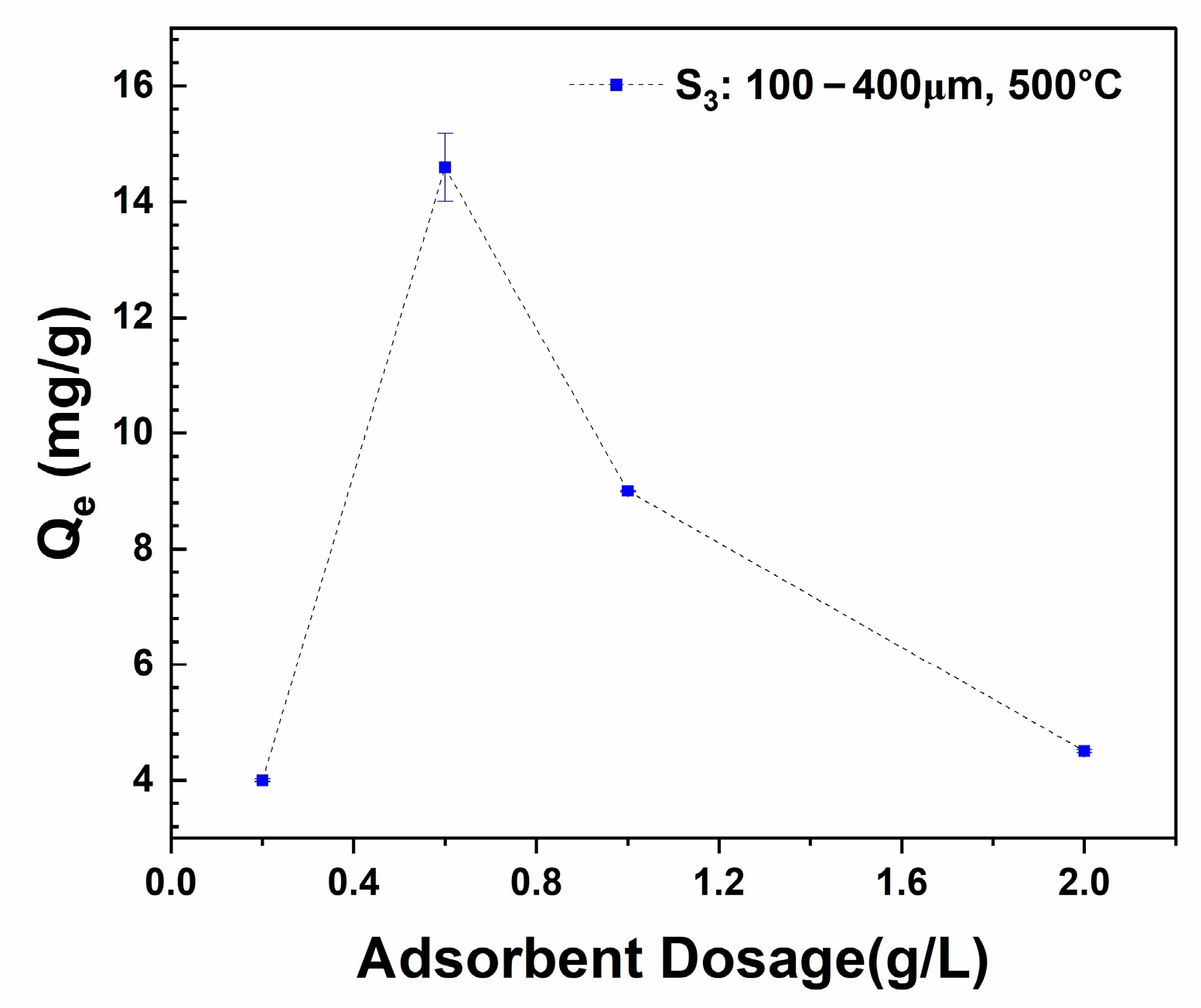
3.3.2. Effect of Adsorbent Dosages
3.3.3. Influence of Pyrolysis Temperature and Alkali Activation on Adsorption
3.3.4. Comparison with Other Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Du, Z.L.; Hu, A.B.; Wang, Q.D.; Ai, J.; Zhang, W.J.; Liang, Y.; Cao, M.X.; Wu, H.J.; Wang, D.S. Molecular composition and biotoxicity effects of dissolved organic matters in sludge- based carbon: Effects of pyrolysis temperature. J. Hazard. Mater. 2022, 424, 12734. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Zhang, W.; Liao, G.; Chen, F.; Wang, D. A novel waste activated sludge multistage utilization strategy for preparing carbon-based Fenton-like catalyst: Catalytic performance assessment and micro-interfacial mechanisms. Water Res. 2019, 150, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Ospanov, K.; Kuldeyev, E.; Kenzhaliyev, B.; Korotunov, A. Wastewater Treatment Methods and Sewage Treatment Facilities in Almaty, Kazakhstan. J. Ecol. Eng. 2022, 23, 240–251. [Google Scholar] [CrossRef]
- Temireyeva, A.; Zhunussova, K.; Aidabulov, M.; Venetis, C.; Sarbassov, Y.; Shah, D. Greenhouse Gas Emissions-Based Development and Characterization of Optimal Scenarios for Municipal Solid and Sewage Sludge Waste Management in Astana City. Sustainability 2023, 14, 15850. [Google Scholar] [CrossRef]
- Yuan, S.J.; Dai, X.H. Sewage sludge-based functional nanomaterials: Development and applications. Environ. Sci. Nano 2017, 4, 17–26. [Google Scholar] [CrossRef]
- NY525-2021; Water Soluble Fertilizers–Determination of Water Insoluble Matter Content and pH. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2021.
- El Ouadrhiri, F.; Elyemni, M.; Lahkimi, A.; Lhassani, A.; Chaouch Mehdi Taleb, M. Mesoporous Carbon from Optimized Date Stone Hydrochar by Catalytic Hydrothermal Carbonization Using Response Surface Methodology: Application to Dyes Adsorption. Int. J. Chem. Eng. 2021, 5555406. [Google Scholar] [CrossRef]
- Hu, M.; Ye, Z.; Zhang, H.; Chen, B.; Pan, Z.; Wang, J. Thermochemical conversion of sewage sludge for energy and resource recovery: Technical challenges and prospects. Environ. Pollut. Bioavailab. 2021, 33, 145–163. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Shao, Y.; Guo, H.; Liu, Z.; Hu, G.; Xiang, H.; Hu, J. Removal of elemental mercury using magnetic Fe-containing carbon prepared from sludge flocculated with ferrous sulfate by Zinc chloride activation. J. Energy Inst. 2021, 98, 98–106. [Google Scholar] [CrossRef]
- Zaini, M.A.A.; Zakaria, M.; Mohd-Setapar, S.H.; Che-Yunus, M.A. Sludge-adsorbents from palm oil mill effluent for methylene blue removal. J. Environ. Chem. Eng. 2013, 1, 1091–1098. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Najeeb, J.; Hussain, Z. Fundamentals and photocatalysis of methylene blue dye using various nanocatalytic assemblies- a critical review. J. Clean Prod. 2021, 298, 126567. [Google Scholar] [CrossRef]
- Ali, J.; Wang, H.; Ifthikar, J.; Khan, A.; Wang, T.; Zhan, K. Efficient, stable, and selective adsorption of heavy metals by the-functionalized layered double hydroxide in diverse types of water. Chem. Eng. J. 2018, 332, 387–397. [Google Scholar] [CrossRef]
- Ye, G.; Zhou, J.; Huang, R.; Ke, Y.; Peng, Y.; Zhou, Y. Magnetic sludge-based biochar derived from Fenton sludge as an efficient heterogeneous Fenton catalyst for degrading Methylene blue. J. Environ. Chem. Eng. 2022, 10, 107242. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Z.; Tang, Q.; Du, B.; Huang, X.; Mo, Y.; Fan, L.; Luo, H.; Chen, F. Assessment of a novel aminated magnetic adsorbent with excellent adsorption capacity for dyes and drugs. J. Environ. Manag. 2021, 293, 112809. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Chowdhary, P.; Bharagava, R.N. Conventional methods for the removal of industrial pollutants, their merits and demerits. In Emerging and Eco-Friendly Approaches for Waste Management; Springer: Singapore, 2018; pp. 1–31. [Google Scholar] [CrossRef]
- El Ouadrhiri, F.; Althomali, R.H.; Adachi, A.; Saleh, E.A.M.; Husain, K.; Lhassani, A.; Hassan, I.; Moharam, M.M.; Kassem, A.F.; Chaouch, M.; et al. Nitrogen and phosphorus co-doped carbocatalyst for efficient organic pollutant removal through persulfate- based advanced oxidation processes. J. Saudi Chem. Soc. 2023, 27, 101648. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, S.; Chen, D.; Dai, G.; Wei, D.; Shu, Y. Activation of persulfate with char for degradation of bisphenol A in soil. J. Chem. Eng. 2020, 381, 122637. [Google Scholar] [CrossRef]
- Lei, Y.; Guo, X.; Jiang, M.; Sun, W.; He, H.; Chen, Y.; Thummavichai, K.; Ola, O.; Zhu, Y.; Wang, N. Co-ZIF reinforced cow manure biochar (CMB) as an effective peroxymonosulfate activator for degradation of carbamazepine. Appl. Catal. B Environ. 2022, 319, 121932. [Google Scholar] [CrossRef]
- Fana, S.; Wanga, Y.; Wanga, Z.; Tanga, J.; Tanga, J.; Lia, X. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics, and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Park, J.H.; Wang, J.J.; Zhou, B.; Mikhael, J.E.R.; DeLaune, R.D. Removing mercury from aqueous solution using sulfurized biochar and associated mechanisms. Environ. Pollut. 2019, 244, 627–635. [Google Scholar] [CrossRef]
- Ren, J.; Cao, J.; Zhao, X.; Liu, Y. Fundamentals and applications of char in biomass tar reforming. Fuel Process Technol. 2021, 216, 106782. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Li, Z.; Yu, G.; Wang, Y. Effect of pyrolysis temperature on characteristics, chemical speciation, and risk evaluation of heavy metals in biochar derived from textile dyeing sludge. Ecotox. Environ. Safe 2019, 168, 45–52. [Google Scholar] [CrossRef]
- Jina, H.; Arazo, R.O.; Gaoe, J.; Capareda, S.; Changa, Z. Leaching of heavy metals from fast pyrolysis residues produced from different particle sizes of sewage sludge. J. Anal. Appl. Pyrolysis 2014, 109, 168–175. [Google Scholar] [CrossRef]
- Huang, Y.F.; Huang Chiueh, P.T.; Lo, S.L. Heterogeneous Fenton oxidation of trichloroethylene catalyzed by sewage sludge biochar: Experimental study and life cycle assessment. Chemosphere 2020, 249, 126139. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.H.; Chen, X.G.; Jeyaseelan, S.; Graham, N. Optimizing the preparation of activated carbon from digested sewage sludge and coconut husk. Chemosphere 2001, 44, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Quan, C.; Liu, B.; Li, Z.; Wu, C.; Li, A. Continuous Pyrolysis of sewage sludge in a screw-feeding reactor: Products characterization and ecological risk assessment of heavy metals. Energy Fuels 2017, 31, 5063–5072. [Google Scholar] [CrossRef]
- Huang, Y.X.; Sun, Y.R.; Xu, Z.H.; Luo, M.Y.; Zhu, C.L.; Li, L. Removal of aqueous oxalic acid by heterogeneous catalytic ozonation with MnOx/sewage sludge-derived activated carbon as catalysts. Sci. Total Environ. 2017, 575, 50–57. [Google Scholar] [CrossRef]
- Wang, X.P.; Gu, L.; Zhou, P.; Zhu, N.W.; Li, C.X.; Tao, H.; Wen, H.F.; Zhang, D.F. Pyrolytic temperature-dependent conversion of sewage sludge to carbon catalyst and their Performance in persulfate degradation of 2-Naphthol. Chem. Eng. J. 2017, 324, 203–215. [Google Scholar] [CrossRef]
- Huang, B.C.; Jiang, J.; Huang, G.X.; Yu, H.Q. Sludge biochar-based catalysts for improved pollutant degradation by activating peroxymonosulfate. J. Mater. Chem. 2018, 6, 8978–8985. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Ifthikar, J.; Shi, L.; Khan, A.; Chen, Z.; Chen, Z. Towards a better understanding on mercury adsorption by magnetic bio-adsorbents with gamma-Fe2O3 from pinewood sawdust derived hydrochar: Influence of atmosphere in heat treatment. Bioresour. Technol. 2018, 256, 269–276. [Google Scholar] [CrossRef]
- Wang, T.; Liu, J.; Zhang, Y.; Zhang, H.; Chen, W.; Norris, P.; Pan, W. Use of a non-thermal plasma technique to increase the number of chlorine active sites on biochar for improved mercury removal. J. Chem. Eng. 2018, 331, 536–544. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G.; Fu, B. Conversion of sewage sludge into environmental catalyst and microbial fuel cell electrode material: A review. Sci. Total Environ. 2019, 666, 525–539. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, N.; Giri, B.S.; Chowdhary, P.; Chaturvedi, P. Removal of methylene blue dye using rice husk, cow dung, and sludge biochar: Characterization, application, and kinetic studies. Bioresour. Technol. 2020, 306, 12320. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Deng, W.; Hu, M.; Chen, G.; Zhou, P.; Zhou, Y.; Su, Y. Preparation of binder-less activated char briquettes from pyrolysis of sewage sludge for liquid-phase adsorption of methylene blue. J. Environ. Manag. 2021, 299, 113601. [Google Scholar] [CrossRef] [PubMed]
- Lia, Y.H.; Chang, F.M.; Huang, B.; Song, Y.P.; Zhao, H.Y.; Wang, K.J. Activated carbon preparation from pyrolysis char of sewage sludge and its adsorption performance for organic compounds in sewage. Fuel 2020, 266, 117053. [Google Scholar] [CrossRef]
- Zeng, H.; Qi, W.; Zhai, L.; Wang, F.; Zhang, J.; Li, D. Preparation and Characterization of Sludge-Based Magnetic Biochar by Pyrolysis for Methylene Blue Removal. Nanomaterials 2021, 11, 2473. [Google Scholar] [CrossRef]
- Agrafioti, E.; Bouras, G.; Kalderis, D.; Diamadopoulos, E. Biochar production by sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 72–78. [Google Scholar] [CrossRef]
- Tursunov, O.; Suleimenova, B.; Kuspangaliyeva, B.; Inglezakis, V.J.; Anthony, E.J.; Sarbassov, Y. Characterization of tar generated from the mixture of municipal solid waste and coal pyrolysis at 800 °C. Energy Rep. 2020, 6, 147–152. [Google Scholar] [CrossRef]
- Kalampaliki, D.; Jayasinghe, G.D.T.M.; Avramiotis, E.; Manariotis, I.D.; Venier, D.; Poulopoulos, S.G.; Szpunar, J.; Vakros, J.; Mantzavinos, D. Application of KOH-activated biochar for the activation of persulfate and the degradation of sulfamethoxazole. J. Chem. Eng. Res. Des. 2023, 194, 306–317. [Google Scholar] [CrossRef]
- ASTM method D6722-11; Standard Test Method for Total Mercury in Coal and Coal Combustion Residues by Direct Combustion Analysis. ASTM: West Conshohocken, PA, USA, 2011.
- Zhai, Y.; Peng, W.; Zeng, G.; Fu, Z.; Lan, Y.; Chen, H.; Wang, C.; Fan, X. Pyrolysis characteristics and kinetics of sewage sludge for different sizes and heating rates. J. Therm. Anal. Calorim. 2012, 107, 1015–1022. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Vinu, R. Effects of Biomass Particle Size on Slow Pyrolysis Kinetics and Fast Pyrolysis Product Distribution. Waste Biomass Valorization 2018, 9, 465–477. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Wang, S.; Zhuang, L.; Yang, Y.; Qiu, R. Characterization of sewage sludge-derived biochars from different feedstocks and pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2013, 102, 137–143. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, Y.; Guo, Z.; Xiao, X.; Peng, C.; Zeng, P. Optimizing pyrolysis temperature of contaminated rice straw biochar: Heavy metal(loid) deportment, properties evolution, and Pb adsorption/immobilization. J. Saudi Chem. Soc. 2022, 26, 101439. [Google Scholar] [CrossRef]
- GB4284-84; Control Standards for Pollutants in Sludges from Agricultural Use. Ministry of Ecology and Environment of The People’s Republic of China: Beijing, China, 1984.
- Yuan, Y.; Yuan, T.; Wang, D.; Tang, J.; Zhou, S. Sewage sludge biochar as an efficient catalyst for oxygen reduction in a microbial fuel cell. Bioresour. Technol. 2013, 144, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, Y.; Cao, Y.; Han, L. Characteristics of tetracycline adsorption by cow manure biochar prepared at different pyrolysis temperatures. Bioresour. Technol. 2019, 285, 121348. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, G.; Li, G. The characteristics of pharmaceutical sludge-derived biochar and its application for the adsorption of tetracycline. Sci. Total Environ. 2020, 747, 141492. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Oleszczuk, P. The conversion of sewage sludge into biochar reduces polycyclic aromatic hydrocarbon content and ecotoxicity but increases trace metal content. Biomass Bioenergy 2015, 75, 235–244. [Google Scholar] [CrossRef]
- Peng, H.; Gao, P.; Chu, G.; Pan, B.; Peng, J.; Xing, B. Enhanced adsorption of Cu (II) and Cd (II) by phosphoric acid-modified biochars. Environ. Pollut. 2017, 229, 846–853. [Google Scholar] [CrossRef]
- Liu, X.Q.; Ding, H.S.; Wang, Y.Y.; Liu, W.J.; Jiang, H. Pyrolytic temperature dependent and ash catalyzed formation of sludge char with ultra-high adsorption to 1-naphthol. Environ. Sci. Technol. 2016, 50, 2602–2609. [Google Scholar] [CrossRef]
- Raj, A.; Yadav, A.; Arya, S.; Sirohi, R.; Kumar, S.; Rawat, A.P.; Thakur, R.S.; Patel, D.K.; Bahadur, L.; Pandey, A. Preparation, characterization and agri applications of biochar produced by pyrolysis of sewage sludge at different temperatures. Sci. Total Environ. 2021, 795, 148722. [Google Scholar] [CrossRef]
- Nematollahzadeh, A.; Seraj, S.; Mirzayi, B. Catecholamine-coated maghemite nanoparticles for the environmental remediation: Hexavalent chromium ions removal. Chem. Eng. J. 2015, 277, 21–29. [Google Scholar] [CrossRef]
- Khraisheh, M.; Al-Ghouti, M.; Allen, S.; Ahmad, M. Effect of O.H. and silanol groups in removing dyes from aqueous solution using diatomite. Water Res. 2005, 39, 922–932. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, C.; Cheng, W.; Wang, X. Simultaneous adsorption and reduction of U (VI) on reduced graphene oxide-supported nanoscale zerovalent iron. J. Hazard. Mater. 2014, 280, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.; Tseng, R.-L.; Juang, R.-S. Characteristics of Elovich equation used to analyze adsorption kinetics in dye-chitosan systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Hoslett, J.; Ghazzal, H.; Mohamad, N.; Johura, H. Removal of methylene blue from aqueous solutions by biochar prepared from the pyrolysis of mixed municipal discarded material. Sci. Total Environ. 2020, 714, 136832. [Google Scholar] [CrossRef] [PubMed]
- Ismaturrahmi, R.; Mustafa, I. Methylene blue removal from water using H2SO4 crosslinked magnetic chitosan nanocomposite beads. Microchem. J. 2019, 144, 397–402. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Bollinger, J.C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Ma dani, K.; Remini, H. Removal of methylene blue from aqueous solutions by adsorption on kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Kong, H.; He, J.; Gao, Y. Cosorption of phenanthrene and mercury (II) from aqueous solution by soybean stalk-based biochar. J. Agric. Food Chem. 2011, 59, 12116–12123. [Google Scholar] [CrossRef]
- Lu, X.; Jiang, J.; Sun, K. Influence of the pore structure and surface chemical properties of activated carbon on the adsorption of mercury from aqueous solutions. Mar. Pollut. Bull. 2014, 78, 69–76. [Google Scholar] [CrossRef]
- Tan, G.; Sun, W.; Xu, Y. Bioresource technology sorption of mercury (II) and atrazine by biochar, modified biochars and biochar based activated carbon in aqueous solution. Bioresour. Technol. 2016, 211, 727–735. [Google Scholar] [CrossRef]
- Xu, X.; Schierz, A.; Xu, N.; Cao, X. Comparison of the characteristics and mechanisms of Hg (II) sorption by biochars and activated carbon. J. Colloid Interface Sci. 2016, 463, 55–60. [Google Scholar] [CrossRef]
- Khoramzadeh, E.; Nasernejad, B.; Halladj, R. Mercury biosorption from aqueous solutions by sugarcane bagasse. J. Taiwan Inst. Chem. Eng. 2013, 44, 266–269. [Google Scholar] [CrossRef]
- Rao, M.M.; Reddy, D.H.K.K.; Venkateswarlu, P.; Seshaiah, K. Removal of mercury from aqueous solutions using activated carbon prepared from agricultural by-product/waste. J. Environ. Manag. 2009, 90, 634–643. [Google Scholar] [CrossRef] [PubMed]


| Sample | BET Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| S3-500 °C | 26.38 | 0.04 | 5.81 |
| S3-650 °C | 27.50 | 0.06 | 5.82 |
| S3-800 °C | 2.72 | 0.01 | 1.90 |
| MS3-500 °C | 42.30 | 0.14 | 13.54 |
| MS3-650 °C | 144.27 | 0.25 | 7.07 |
| MS3-800 °C | 27.78 | 0.14 | 1.90 |
| Adsorbent | Adsorption Capacity (mg/g) | Pollutant | pH | Initial Concentration (mg/L) | Adsorbent Dosage (g/L) | Surface Area (m2/g) | Particle Size | Ref. |
|---|---|---|---|---|---|---|---|---|
| MMDM-derived char | 7.2 | Methylene Blue | 5 | 100 | 5 | - | - | [58] |
| Chitosan nanocomposite | 20.49 | Methylene Blue | - | 7 | 10 | - | 800 μm | [59] |
| Kaolin | 52.76 | Methylene blue | 6 | 250 | 0.5 | 21.27 | 15 μm | [61] |
| Modified SS (MS3-650) | 35 | Methylene blue | 7 | 400 | 500 | 144.27 | 100–400 μm | * |
| Soybean stalk | 0.57 | Mercury | 7 | 0.25 | 0.333 | 250 | 250 μm | [61] |
| Coconut activated carbon | 2.23 | Mercury | 7 | 7.212 | 3.33 | 870 | <45 μm | [62] |
| Corn-straw biochar | 5.71 | Mercury | 6 | 0.5 | 20 | 32.85 | <1 mm | [63] |
| Bagasse/hickory chips (HCB) | 13 | Mercury | 6 | 5 | 0.75 | 15.3 | 0.84 mm | [64] |
| Sugarcane bagasse | 35.71 | Mercury | 4 | 76 | 5 | - | - | [65] |
| Activated carbon | 25.88 | Mercury | 6 | 140 | 6 | 521 | - | [66] |
| Modified SS (MS3-650) | 36 | Mercury | 6.5 | 400 | 500 | 144.27 | 100–400 μm | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agoe, A.K.; Poulopoulos, S.G.; Sarbassov, Y.; Shah, D. Investigation of Sewage Sludge–Derived Biochar for Enhanced Pollutant Adsorption: Effect of Particle Size and Alkali Treatment. Energies 2024, 17, 4554. https://doi.org/10.3390/en17184554
Agoe AK, Poulopoulos SG, Sarbassov Y, Shah D. Investigation of Sewage Sludge–Derived Biochar for Enhanced Pollutant Adsorption: Effect of Particle Size and Alkali Treatment. Energies. 2024; 17(18):4554. https://doi.org/10.3390/en17184554
Chicago/Turabian StyleAgoe, Andy Kofi, Stavros G. Poulopoulos, Yerbol Sarbassov, and Dhawal Shah. 2024. "Investigation of Sewage Sludge–Derived Biochar for Enhanced Pollutant Adsorption: Effect of Particle Size and Alkali Treatment" Energies 17, no. 18: 4554. https://doi.org/10.3390/en17184554








