SO2 Emissions from Oil Shale Oxyfuel Combustion in a 60 kWth Circulating Fluidized Bed
Abstract
1. Introduction
2. Materials and Methods
2.1. Oil Shale
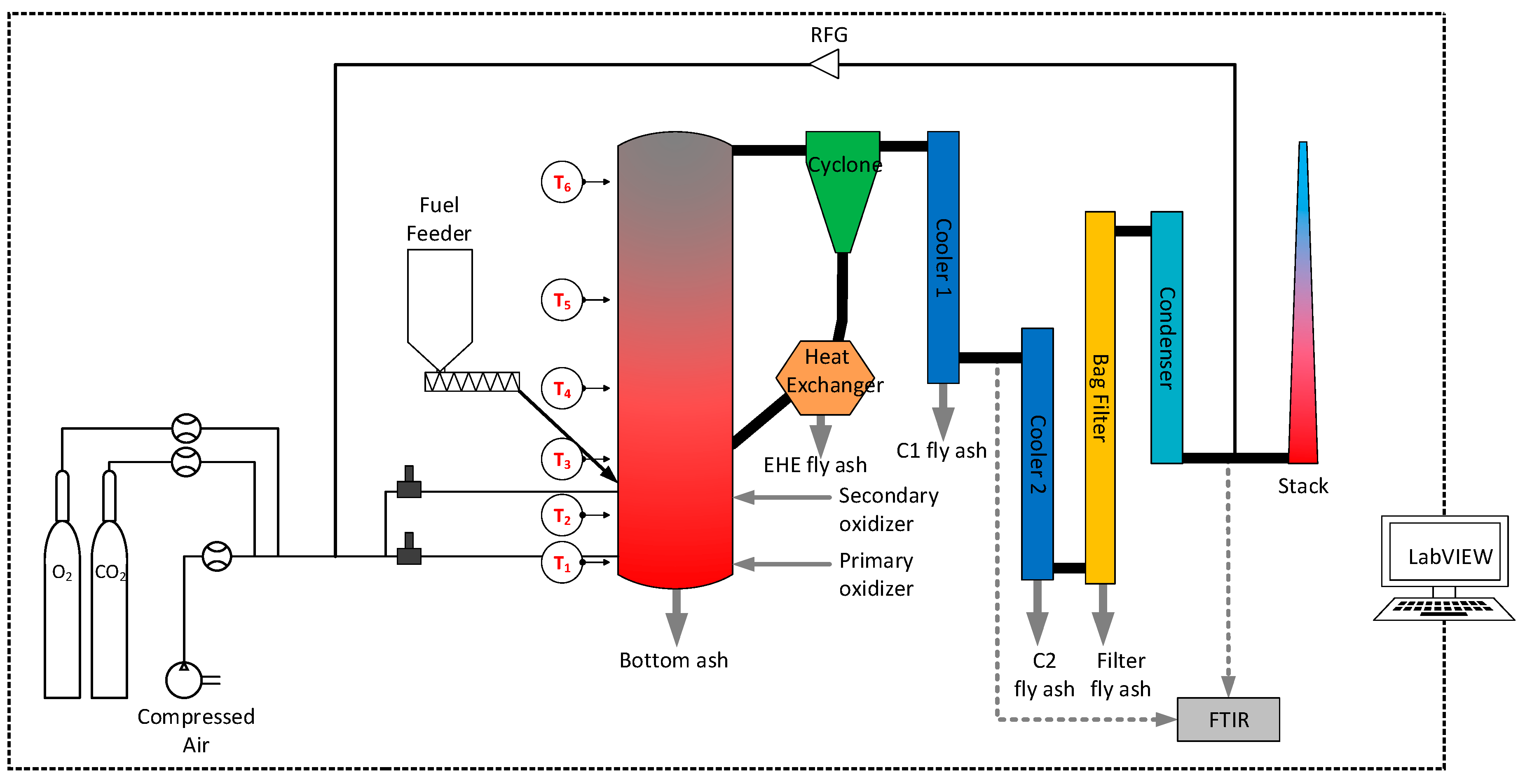
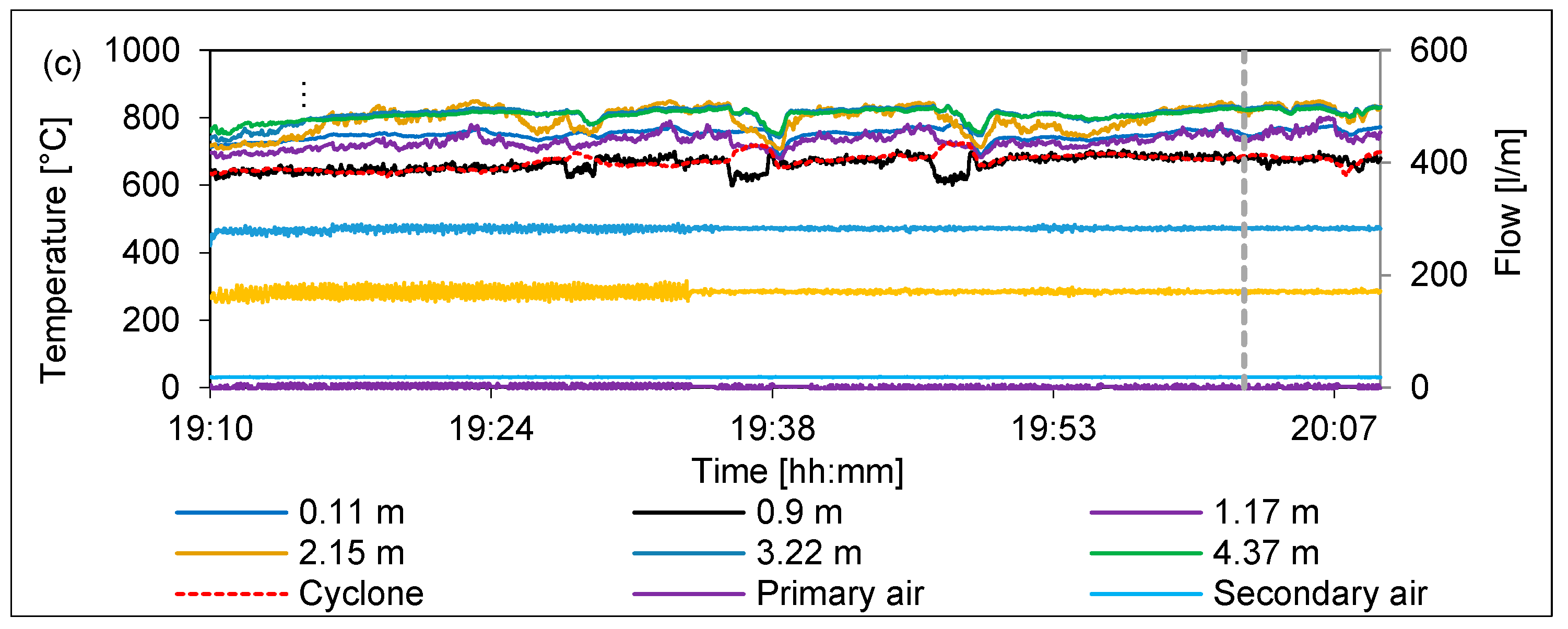
2.2. CFB Pilot Facility
3. Results and Discussion
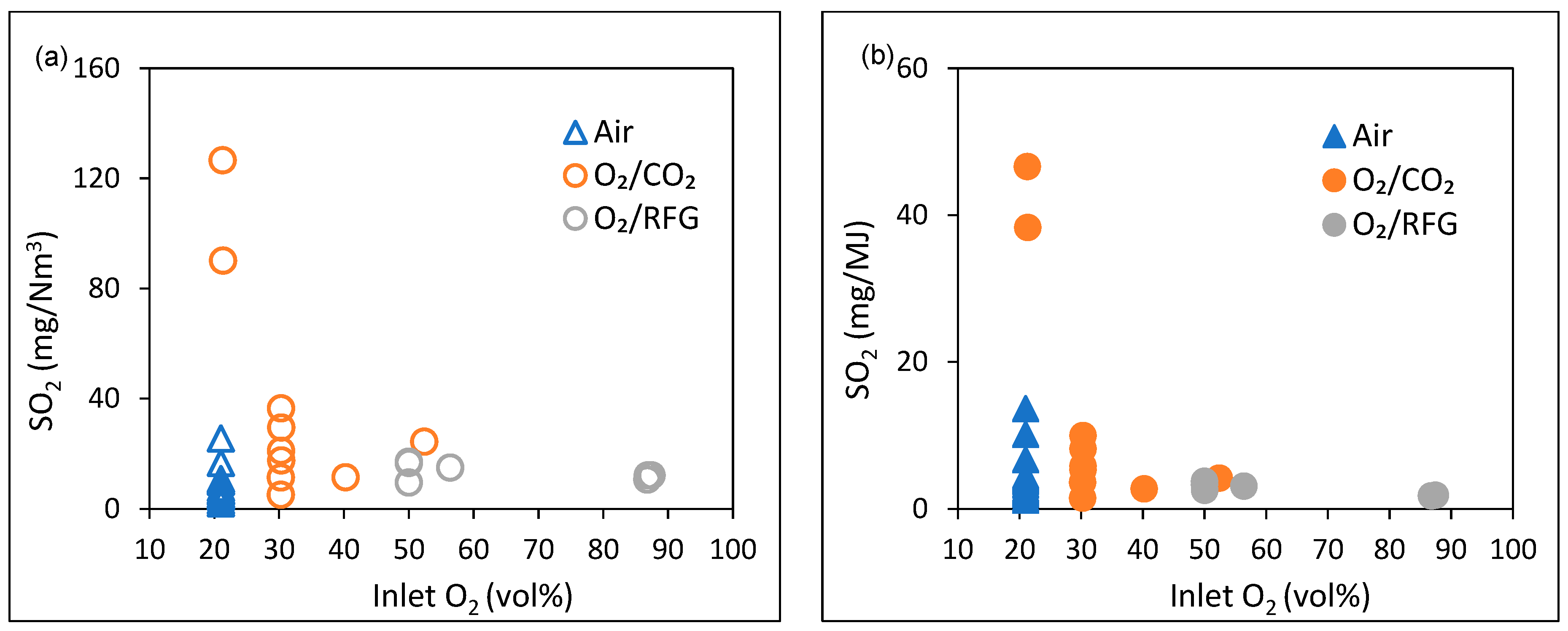
3.1. SO2 Emissions under Air and Oxyfuel Combustion Atmospheres
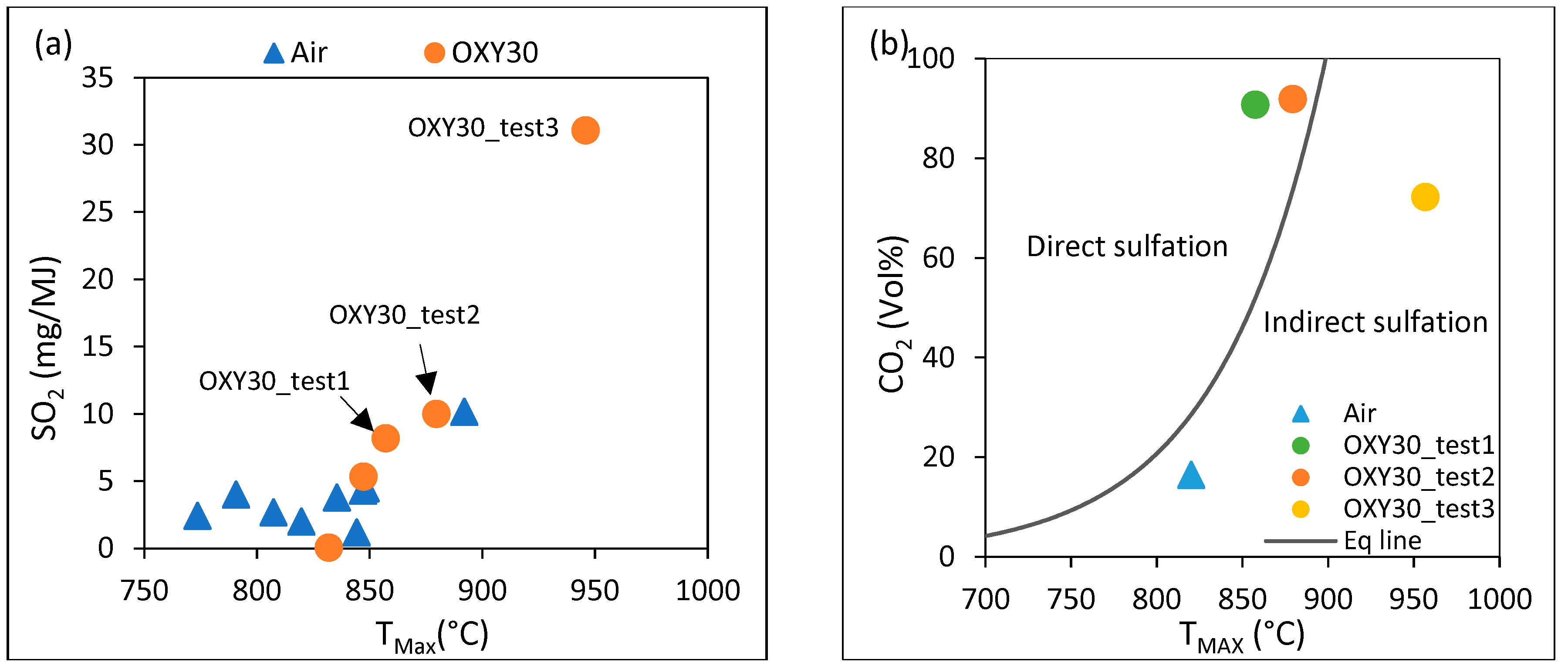
3.2. Effect of Temperature
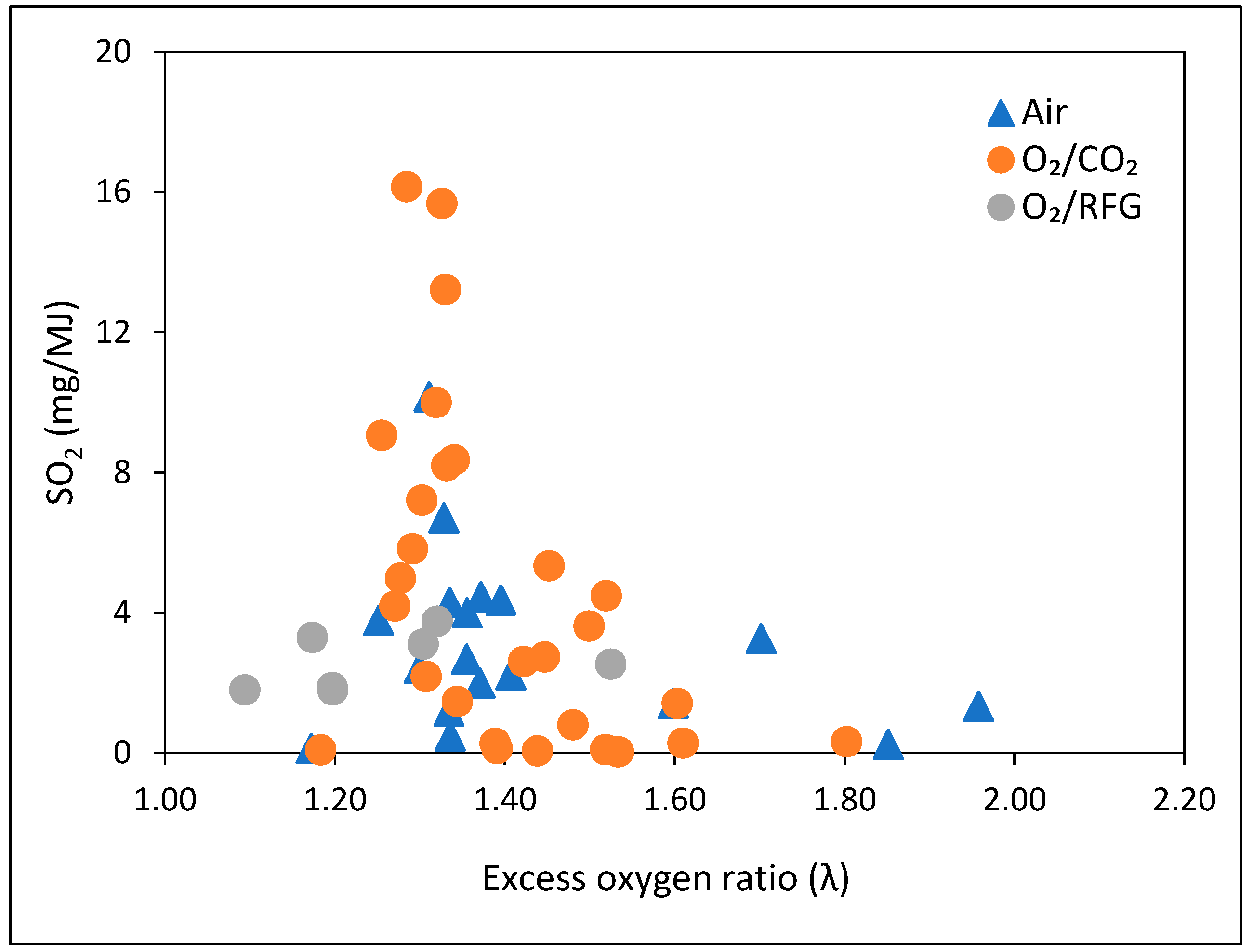
3.3. Effect of Excess Oxygen Ratio (λ)
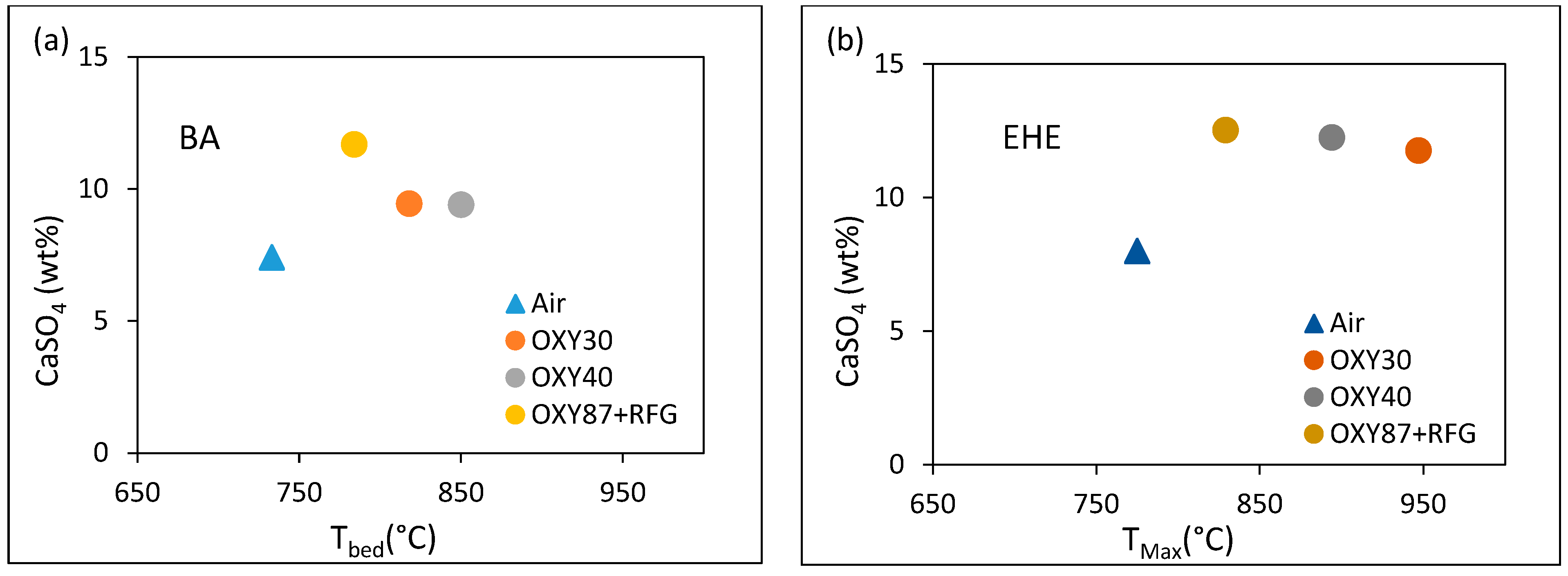
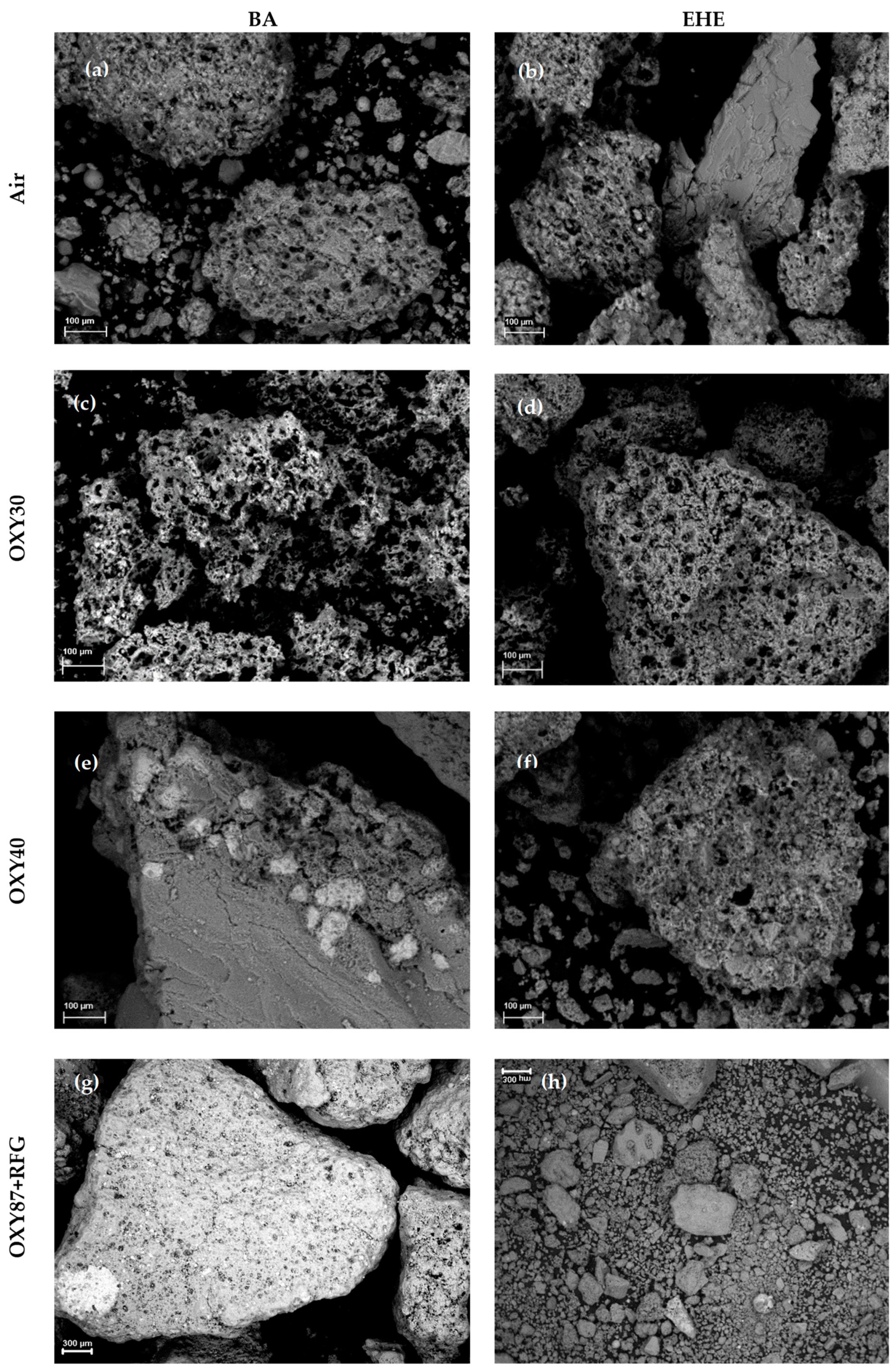
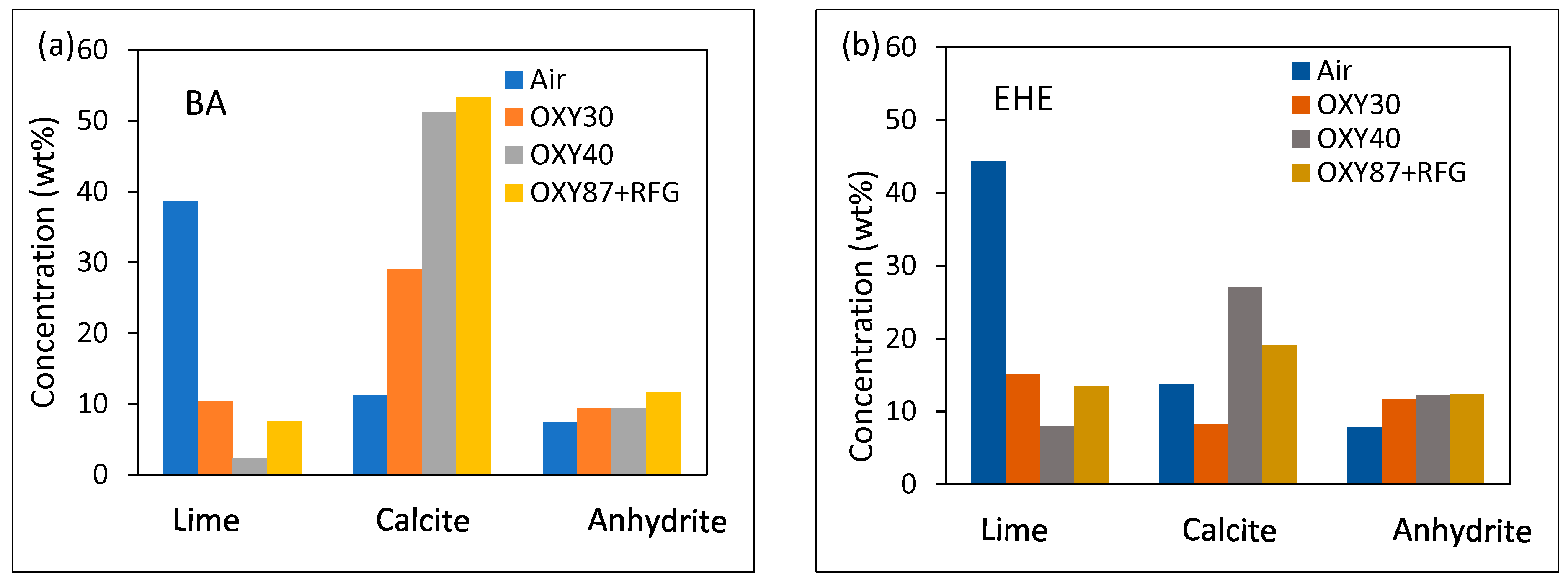
3.4. Sulfation and Ash Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Carbon, C. COP27 Carbon Capture and Storage. Available online: https://www.carbonclean.com/insights/time-for-growth-and-delivery (accessed on 26 June 2023).
- Stanger, R.; Wall, T.; Spörl, R.; Paneru, M.; Grathwohl, S.; Weidmann, M.; Scheffknecht, G.; McDonald, D.; Myöhänen, K.; Ritvanen, J.; et al. Oxyfuel combustion for CO2 capture in power plants. Int. J. Greenh. Gas Control 2015, 40, 55–125. [Google Scholar] [CrossRef]
- Yang, C.; Kim, Y.; Bang, B.; Jeong, S.; Moon, J.; Mun, T.Y.; Jo, S.; Lee, J.; Lee, U. Oxy-CFB combustion technology for use in power-generation applications. Fuel 2020, 267, 117206. [Google Scholar] [CrossRef]
- Seddighi, S. Design of large scale oxy-fuel fluidized bed boilers: Constant thermal power and constant furnace size scenarios. Energy 2017, 118, 1286–1294. [Google Scholar] [CrossRef]
- Yadav, S.; Mondal, S.S. A review on the progress and prospects of oxy-fuel carbon capture and sequestration (CCS) technology. Fuel 2022, 308, 122057. [Google Scholar] [CrossRef]
- Chen, G.; Shiyuan, L.; Linwei, W. Current investigation status of oxy-fuel circulating fluidized bed combustion. Fuel 2023, 342, 127699. [Google Scholar] [CrossRef]
- García-Labiano, F.; Abad, A.; de Diego, L.F.; Gayán, P.; Adánez, J. Calcination of calcium-based sorbents at pressure in a broad range of CO2 concentrations. Chem. Eng. Sci. 2002, 57, 2381–2393. [Google Scholar] [CrossRef]
- Baker, E.H. 87. The calcium oxide–carbon dioxide system in the pressure range 1–300 atmospheres. J. Chem. Soc. 1962, 60, 464–470. [Google Scholar] [CrossRef]
- Hill, K.J.; Winter, E.R.S. Thermal dissociation pressure of calcium carbonate. J. Phys. Chem. 1956, 60, 1361–1362. [Google Scholar] [CrossRef]
- Wang, C.; Jia, L.; Tan, Y.; Anthony, E.J. Carbonation of fly ash in oxy-fuel CFB combustion. Fuel 2008, 87, 1108–1114. [Google Scholar] [CrossRef]
- Basu, P. Combustion and Gasification in Fluidized Beds; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Chadwick, M.J.; Highton, N.H.; Lindman, N. Environmental Impacts of Coal Mining &Utilization: A Complete Revision of Environmental Implications of Expanded Coal Utilization; Elsevier: Amsterdam, The Netherlands, 1987; p. 674. [Google Scholar]
- Ja’baz, I.; Jiao, F.; Wu, X.; Yu, D.; Ninomiya, Y.; Zhang, L. Influence of gaseous SO2 and sulphate-bearing ash deposits on the high-temperature corrosion of heat exchanger tube during oxy-fuel combustion. Fuel Process. Technol. 2017, 167, 193–204. [Google Scholar] [CrossRef]
- Bläsing, M.; Melchior, T.; Müller, M. Influence of temperature on the release of inorganic species during high temperature gasification of Rhenish lignite. Fuel Process. Technol. 2011, 92, 511–516. [Google Scholar] [CrossRef]
- Tian, L.; Yang, W.; Chen, Z.; Wang, X.; Yang, H.; Chen, H. Sulfur behavior during coal combustion in oxy-fuel circulating fluidized bed condition by using TG-FTIR. J. Energy Inst. 2016, 89, 264–270. [Google Scholar] [CrossRef]
- Maaten, B.; Pikkor, H.; Konist, A.; Siirde, A. Determination of the total sulphur content of oil shale by using different analytical methods. Oil Shale 2018, 35, 144–153. [Google Scholar] [CrossRef]
- Belo, L.P.; Elliott, L.K.; Stanger, R.J.; Spörl, R.; Shah, K.V.; Maier, J.; Wall, T.F. High-temperature conversion of SO2 to SO3: Homogeneous experiments and catalytic effect of fly ash from air and oxy-fuel firing. Energy Fuels 2014, 28, 7243–7251. [Google Scholar] [CrossRef]
- Gu, Y.; Yperman, J.; Vandewijngaarden, J.; Reggers, G.; Carleer, R. Organic and inorganic sulphur compounds releases from high-pyrite coal pyrolysis in H2, N2 and CO2: Test case Chinese LZ coal. Fuel 2017, 202, 494–502. [Google Scholar] [CrossRef]
- Belo, L.P. Reactions, Transformations and Impacts of Sulfur Oxides during Oxy-Fuel Combustion by. Ph.D. Thesis, De La Salle University, Manila, Philippines, 2015. [Google Scholar] [CrossRef]
- Kiga, T.; Takano, S.; Kimura, N.; Omata, K.; Okawa, M.; Mori, T.; Kato, M. Characteristics of pulverized-coal combustion in the system of oxygen/recycled flue gas combustion. Energy Convers. Manag. 1997, 38, S129–S134. [Google Scholar] [CrossRef]
- Snow, M.J.H.; Longwell, J.P.; Sarofim, A.F. Direct sulfation of calcium carbonate. Ind. Eng. Chem. Res. 1988, 27, 268–273. [Google Scholar] [CrossRef]
- García-Labiano, F.; Rufas, A.; De Diego, L.F.; Obras-Loscertales, M.D.L.; Gayán, P.; Abad, A.; Adánez, J. Calcium-based sorbents behaviour during sulphation at oxy-fuel fluidised bed combustion conditions. Fuel 2011, 90, 3100–3108. [Google Scholar] [CrossRef]
- De Diego, L.F.; Rufas, A.; García-Labiano, F.; De Las Obras-Loscertales, M.; Abad, A.; Gayán, P.; Adánez, J. Optimum temperature for sulphur retention in fluidised beds working under oxy-fuel combustion conditions. Fuel 2013, 114, 106–113. [Google Scholar] [CrossRef]
- Tan, L.; Li, S.; Li, W.; Shou, E.; Lu, Q. Effects of Oxygen Staging and Excess Oxygen on O2/CO2 Combustion with a High Oxygen Concentration in a Circulating Fluidized Bed. Energy Fuels 2014, 28, 2069–2075. [Google Scholar] [CrossRef]
- Czakiert, T.; Sztekler, K.; Karski, S.; Markiewicz, D.; Nowak, W. Oxy-fuel circulating fluidized bed combustion in a small pilot-scale test rig. Fuel Process. Technol. 2010, 91, 1617–1623. [Google Scholar] [CrossRef]
- Anthony, E.J.; Granatstein, D.L. Sulfation phenomena in fluidized bed combustion systems. Prog. Energy Combust. Sci. 2001, 27, 215–236. [Google Scholar] [CrossRef]
- Anthony, E.J.; Bulewicz, E.M.; Jia, L. Reactivation of limestone sorbents in FBC for SO2 capture. Prog. Energy Combust. Sci. 2007, 33, 171–210. [Google Scholar] [CrossRef]
- Kang, S.Y.; Seo, S.B.; Go, E.S.; Kim, H.W.; Keel, S.I.; Park, Y.K.; Lee, S.H. Effect of particle size on in-situ desulfurization for oxy-fuel CFBC. Fuel 2021, 291, 120270. [Google Scholar] [CrossRef]
- De Las Obras-Loscertales, M.; De Diego, L.F.; García-Labiano, F.; Rufas, A.; Abad, A.; Gayán, P.; Adánez, J. Sulfur retention in an oxy-fuel bubbling fluidized bed combustor: Effect of coal rank, type of sorbent and O2/CO2 ratio. Fuel 2014, 137, 384–392. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Guo, X.; Yao, H.; Chen, J. Nitrogen and Sulfur Behavior during Oxy-Fuel Combustion and Its Retention; Zheng, C., Liu, Z., Eds.; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar] [CrossRef]
- Baqain, M.; Rüstü Yörük, C.; Nešumajev, D.; Järvik, O.; Konist, A. Ash characterisation formed under different oxy-fuel circulating fluidized bed conditions. Fuel 2023, 338, 127244. [Google Scholar] [CrossRef]
- Al-Makhadmeh, L.A.; Maier, J.; Batiha, M.A.; Scheffknecht, G. Oxyfuel technology: Oil shale desulfurization behavior during staged combustion. Fuel 2017, 190, 229–236. [Google Scholar] [CrossRef]
- Loo, L.; Konist, A.; Neshumayev, D.; Pihu, T.; Maaten, B.; Siirde, A. Ash and Flue Gas from Oil Shale Oxy-Fuel Circulating Fluidized Bed Combustion. Energies 2018, 11, 1218. [Google Scholar] [CrossRef]
- Saia, A.; Neshumayev, D.; Hazak, A.; Sander, P.; Järvik, O.; Konist, A. Techno-economic assessment of CO2 capture possibilities for oil shale power plants. Renew. Sustain. Energy Rev. 2022, 169, 112938. [Google Scholar] [CrossRef]
- Ots, A. Oil Shale Fuel Combustion; Tallinn University of Technology: Tallinn, Estonia, 2006. [Google Scholar]
- Kuusik, R.; Uibu, M.; Kirsimäe, K.; Mõtlep, R.; Meriste, T. Open-air deposition of Estonian oil shale ash: Formation, state of art, problems and prospects for the abatement of environmental impact. Oil Shale 2012, 29, 376–403. [Google Scholar] [CrossRef]
- Baqain, M.H.S. Oil Shale Oxyfuel CFB Combustion. Ph.D. Thesis, Tallinn University of Thechnology, Tallinn, Estonia, 2024. [Google Scholar]
- EVS-ISO 29541:2015; Solid Mineral Fuels—Determination of Total Carbon, Hydrogen and Nitrogen Content-Instrumental Method. Estonian Center for Standardisation and Accreditation: Tallinn, Estonia, 2015. Available online: https://www.evs.ee/en/evs-iso-29541-2015 (accessed on 10 September 2024).
- EVS-ISO 21654:2021; Solid Recovered Fuels-Determination of Calorific Value. Estonian Center for Standardisation and Accreditation: Tallinn, Estonia, 2021. Available online: https://www.evs.ee/en/evs-en-iso-21654-2021 (accessed on 10 September 2024).
- ISO 29581-2:2010; Cement—Test Methods—Part 2: Chemical Analysis by X-ray Fluorescence. ISO: Geneva, Switzerland, 2010. Available online: https://www.iso.org/standard/45567.html (accessed on 10 September 2024).
- De Las Obras-Loscertales, M.; Mendiara, T.; Rufas, A.; De Diego, L.F.; García-Labiano, F.; Gayán, P.; Abad, A.; Adánez, J. NO and N2O emissions in oxy-fuel combustion of coal in a bubbling fluidized bed combustor. Fuel 2015, 150, 146–153. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Xu, M.; Wang, X.; Li, H.; Lu, Q. The experimental study on nitrogen oxides and SO2 emission for oxy-fuel circulation fluidized bed combustion with high oxygen concentration. Fuel 2015, 146, 81–87. [Google Scholar] [CrossRef]
- Reinik, J.; Irha, N.; Steinnes, E.; Urb, G.; Jefimova, J.; Piirisalu, E.; Loosaar, J. Changes in trace element contents in ashes of oil shale fueled PF and CFB boilers during operation. Fuel Process. Technol. 2013, 115, 174–181. [Google Scholar] [CrossRef]
- Duan, L.; Zhou, W.; Li, H.; Chen, X.; Zhao, C. Sulfur fate during bituminous coal combustion in an oxy-fired circulating fluidized bed combustor. Korean J. Chem. Eng. 2011, 28, 1952–1955. [Google Scholar] [CrossRef]
- Baqain, M.; Neshumayev, D.; Konist, A. TG-MS analysis and kinetic study of co-combustion of ca-rich oil shale with biomass in air and oxy-like conditions. Carbon Capture Sci. Technol. 2024, 10, 100162. [Google Scholar] [CrossRef]
- Yörük, C.R.; Meriste, T.; Trikkel, A.; Kuusik, R. Thermo-oxidation characteristics of oil shale and oil shale char under oxy-fuel combustion conditions. J. Therm. Anal. Calorim. 2015, 121, 509–516. [Google Scholar] [CrossRef]
- Raheem, D.G.; Yilmaz, B.; Kayahan, U.; Özdoǧan, S. Effect of Recycled Flue Gas Ratio on Combustion Characteristics of Lignite Oxy-Combustion in a Circulating Fluidized Bed. Energy Fuels 2020, 34, 14786–14795. [Google Scholar] [CrossRef]
- Duan, L.; Sun, H.; Zhao, C.; Zhou, W.; Chen, X. Coal combustion characteristics on an oxy-fuel circulating fluidized bed combustor with warm flue gas recycle. Fuel 2014, 127, 47–51. [Google Scholar] [CrossRef]
- Wall, T.; Liu, Y.; Spero, C.; Elliott, L.; Khare, S.; Rathnam, R.; Zeenathal, F.; Moghtaderi, B.; Buhre, B.; Sheng, C.; et al. An overview on oxyfuel coal combustion-State of the art research and technology development. Chem. Eng. Res. Des. 2009, 87, 1003–1016. [Google Scholar] [CrossRef]
- De Diego, L.F.; de las Obras-Loscertales, M.; García-Labiano, F.; Rufas, A.; Abad, A.; Gayán, P.; Adánez, J. Characterization of a limestone in a batch fluidized bed reactor for sulfur retention under oxy-fuel operating conditions. Int. J. Greenh. Gas Control 2011, 5, 1190–1198. [Google Scholar] [CrossRef]
- Parve, T.; Ots, A.; Skrifars, B.-J.; Hupa, M. The sintering of Estonian oil shale ashes|Health & Environmental Research Online (HERO)|US EPA. Oil Shale 1995, 12, 341–356. [Google Scholar]
- Gómez, M.; Fernández, A.; Llavona, I.; Kuivalainen, R. Experiences in sulphur capture in a 30 MWth Circulating Fluidized Bed boiler under oxy-combustion conditions. Appl. Therm. Eng. 2014, 65, 617–622. [Google Scholar] [CrossRef]
- Baqain, M.; Neshumayev, D.; Konist, A. NOx and N2O emissions from Ca-rich fuel conversion in oxyfuel circulating fluidized bed combustion. Therm. Sci. Eng. Prog. 2023, 42, 101938. [Google Scholar] [CrossRef]
- Wang, C.; Song, G.; Chen, R.; Jiang, Y.; Lyu, Q. Influence of Operating Parameters on NOx and SO2 Emissions in Circulating Fluidized Bed with Post-Combustion. J. Therm. Sci. 2023, 32, 1858–1867. [Google Scholar] [CrossRef]
- De Las Obras-Loscertales, M.; Rufas, A.; De Diego, L.F.; García-Labiano, F.; Gayán, P.; Abad, A.; Adánez, J. Morphological analysis of sulfated Ca-based sorbents under conditions corresponding to oxy-fuel fluidized bed combustion. Fuel 2015, 162, 264–270. [Google Scholar] [CrossRef]
- Illerup, J.B.; Dam-Johansen, K.; Lundén, K. High-temperature reaction between sulfur dioxide and limestone—VI. The influence of high pressure. Chem. Eng. Sci. 1993, 48, 2151–2157. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Yrjas, P.; Hupa, M. Sulfur dioxide capture under PFBC conditions: The influence of sorbent particle structure. Fuel 1998, 77, 285–292. [Google Scholar] [CrossRef]
- Zheng, Z.; Wang, H.; Guo, S.; Luo, Y.; Du, Q.; Wu, S. Fly Ash Deposition during Oxy-fuel Combustion in a Bench-Scale Fluidized-Bed Combustor. Energy Fuels 2013, 27, 4609–4616. [Google Scholar] [CrossRef]


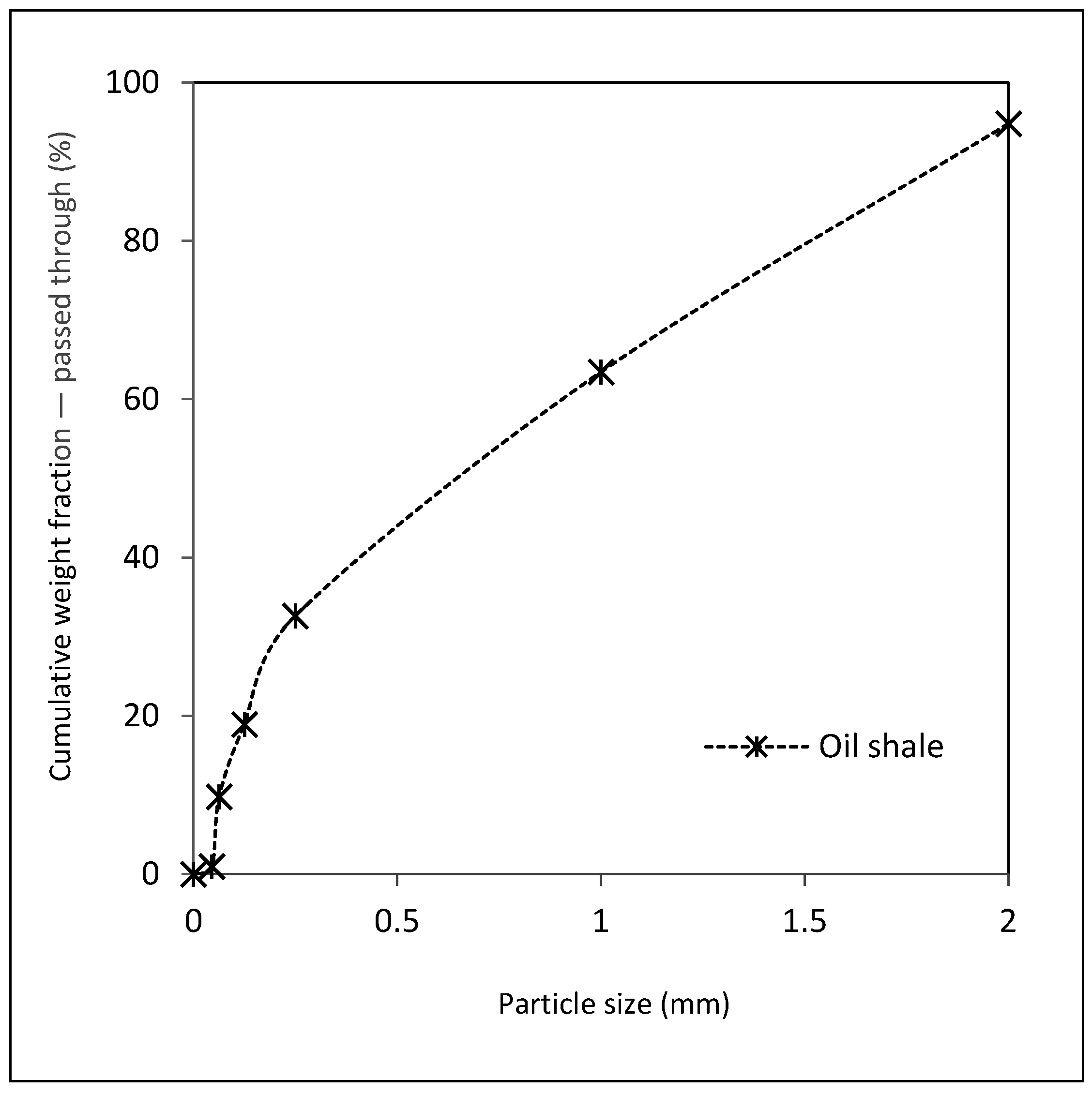


| Proximate Analysis d (wt.%) | Ultimate Analysis d (wt.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Moisture | Ash | LHV (MJ/kg) | C | H | aO | N | Stotal | (CO2)Mineral | |
| 0.18 | 52.05 | 9.76 | 27.38 | 2.68 | 0.36 | 0.07 | 1.57 | 18.9 | |
| Components | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | Na2O | K2O | SO3 | LOI950°C |
|---|---|---|---|---|---|---|---|---|---|
| Content, wt.% | 15.39 | 3.64 | 2.03 | 22.52 | 3 | 0.09 | 1.6 | 3.91 | 47.12 |
| Components | Quartz SiO2 | K-Feldspar KAISi3O8 | Calcite CaCO3 | Dolomite CaMg(CO3)2 | Chlorite ClO2 | Pyrite FeS2 | Illite |
|---|---|---|---|---|---|---|---|
| Content, wt.% | 11.6 | 6.5 | 43.1 | 20.3 | 3.5 | 1.9 | 13 |
| Working Parameter | Combustion Mode | ||
|---|---|---|---|
| Air | O2/CO2 | O2/RFG | |
| Inlet O2 ratio (%) | - | 21–59 | 50, 56, 87 |
| CO2 in flue gas, dry (%) | 13–18 | 76–93 | 42–73 |
| TMAX (°C) | 738–892 | 695–947 | 810–875 |
| Oil shale feed rate (kg/h) | 9.5 | 5–12 | 5–9 |
| Ash sampling point | BA, EHE | BA, EHE | BA, EHE |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baqain, M.; Neshumayev, D.; Konist, A. SO2 Emissions from Oil Shale Oxyfuel Combustion in a 60 kWth Circulating Fluidized Bed. Energies 2024, 17, 4567. https://doi.org/10.3390/en17184567
Baqain M, Neshumayev D, Konist A. SO2 Emissions from Oil Shale Oxyfuel Combustion in a 60 kWth Circulating Fluidized Bed. Energies. 2024; 17(18):4567. https://doi.org/10.3390/en17184567
Chicago/Turabian StyleBaqain, Mais, Dmitri Neshumayev, and Alar Konist. 2024. "SO2 Emissions from Oil Shale Oxyfuel Combustion in a 60 kWth Circulating Fluidized Bed" Energies 17, no. 18: 4567. https://doi.org/10.3390/en17184567
APA StyleBaqain, M., Neshumayev, D., & Konist, A. (2024). SO2 Emissions from Oil Shale Oxyfuel Combustion in a 60 kWth Circulating Fluidized Bed. Energies, 17(18), 4567. https://doi.org/10.3390/en17184567







