Opportunities and Threats for Supercapacitor Technology Based on Biochar—A Review
Abstract
1. Introduction
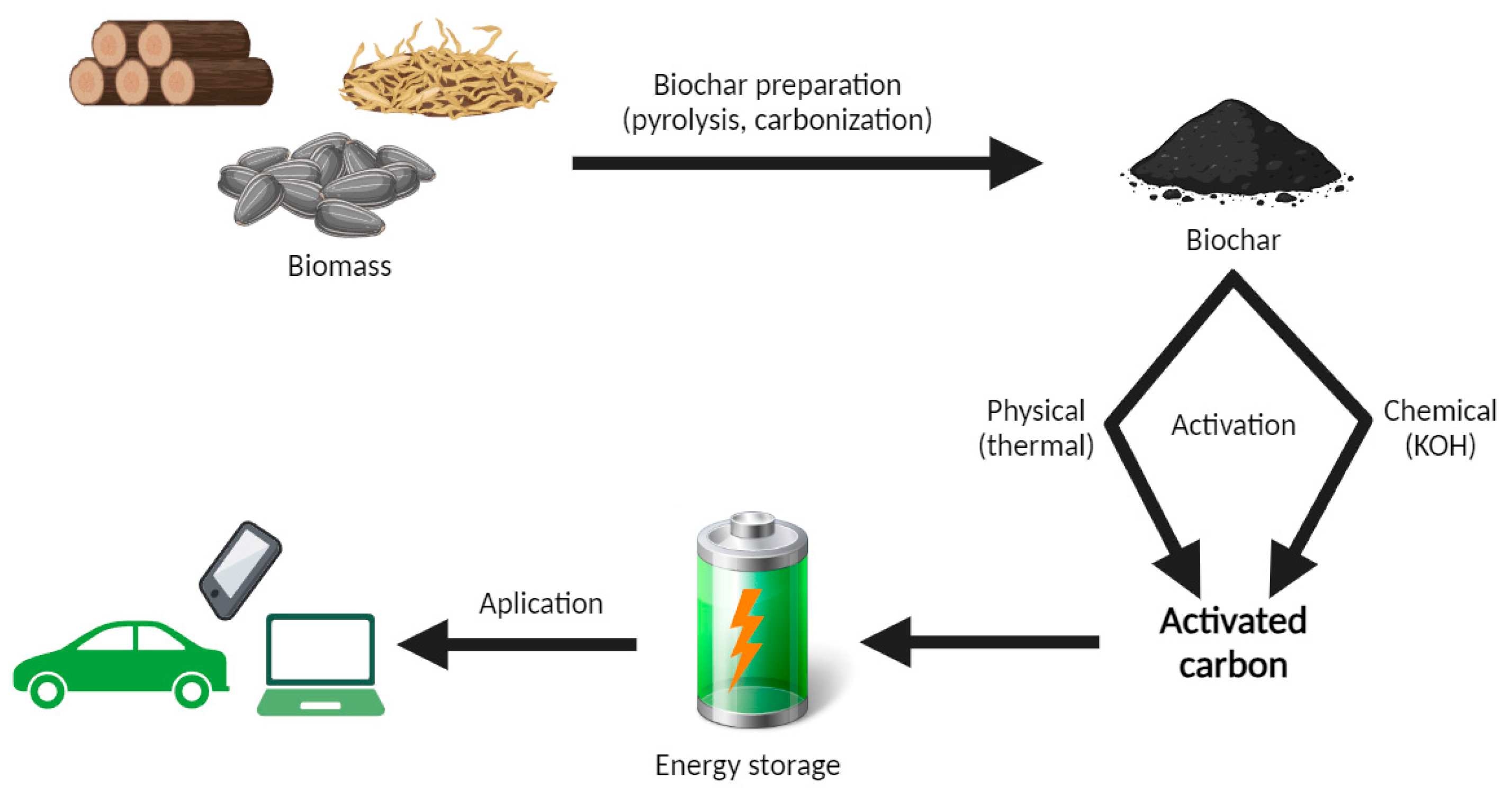
2. Biochar
2.1. Production and Use
2.2. Biochar in Supercapacitors
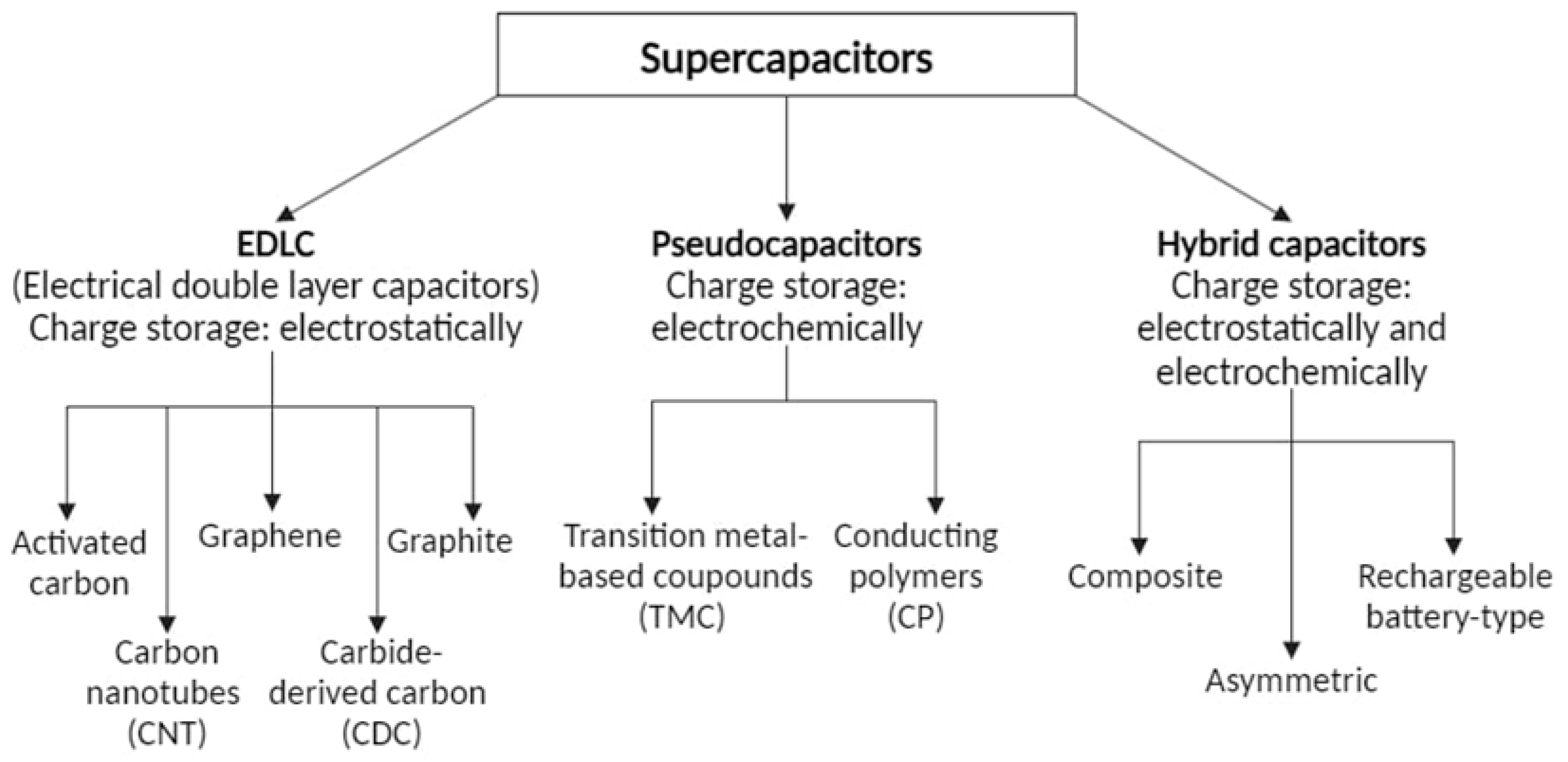
3. Division of Supercapacitors and Their Construction
Lifetime, Aging, and Recycling of Supercapacitors
4. Opportunities and Threats
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gajdzik, B.; Wolniak, R.; Nagaj, R.; Žuromskaitė-Nagaj, B.; Grebski, W.W. The Influence of the Global Energy Crisis on Energy Efficiency: A Comprehensive Analysis. Energies 2024, 17, 947. [Google Scholar] [CrossRef]
- Triguero-Ruiz, F.; Avila-Cano, A.; Trujillo Aranda, F. Measuring the diversification of energy sources: The energy mix. Renew. Energy 2023, 216, 119096. [Google Scholar] [CrossRef]
- Kołatek, R. Unijne regulacje prawne w zakresie odnawialnych źródeł energii (OZE) jako narzędzie transformacji energetycznej państw Unii Europejskiej. Kwart. Prawa Publicznego 2024, 21, 7–23. [Google Scholar] [CrossRef]
- Ahmed, B.; Al Mubarak, M.; Khouj, M. Renewable Technologies: Solar Power and Wind Power Energy Utilization—Advantages and Disadvantages. In Technological Sustainability and Business Competitive Advantage; Internet of Things; Al Mubarak, M., Hamdan, A., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 507–519. ISBN 978-3-031-35524-0. [Google Scholar] [CrossRef]
- Deshmukh, M.K.G.; Sameeroddin, M.; Abdul, D.; Abdul Sattar, M. Renewable energy in the 21st century: A review. Mater. Today Proc. 2023, 80, 1756–1759. [Google Scholar] [CrossRef]
- Yang, Y. Research on the proportion of solar energy replacing conventional energy under the development trend of green building. E3S Web Conf. 2024, 490, 01010. [Google Scholar] [CrossRef]
- Blakers, A.; Stocks, M.; Lu, B.; Cheng, C. A review of pumped hydro energy storage. Prog. Energy 2021, 3, 022003. [Google Scholar] [CrossRef]
- Chen, F.; Ji, Y.; Deng, Y.; Ren, F.; Tan, S.; Wang, Z. Ultrasonic-assisted fabrication of porous carbon materials derived from agricultural waste for solid-state supercapacitors. J. Mater. Sci. 2020, 55, 11512–11523. [Google Scholar] [CrossRef]
- Senthil, C.; Lee, C.W. Biomass-derived biochar materials as sustainable energy sources for electrochemical energy storage devices. Renew. Sustain. Energy Rev. 2021, 137, 110464. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Liu, B.; Su, Z. A critical review on the application and recent developments of post-modified biochar in supercapacitors. J. Clean. Prod. 2021, 310, 127428. [Google Scholar] [CrossRef]
- Ahuja, V.; Palai, A.K.; Kumar, A.; Patel, A.K.; Farooque, A.A.; Yang, Y.-H.; Bhatia, S.K. Biochar: Empowering the future of energy production and storage. J. Anal. Appl. Pyrolysis 2024, 177, 106370. [Google Scholar] [CrossRef]
- Li, C.; He, D.; Huang, Z.-H.; Wang, M.-X. Hierarchical Micro-/Mesoporous Carbon Derived from Rice Husk by Hydrothermal Pre-Treatment for High Performance Supercapacitor. J. Electrochem. Soc. 2018, 165, A3334–A3341. [Google Scholar] [CrossRef]
- European Commission. Commission Delegated Regulation (EU) 2021/2088 of 7 July 2021 Amending Annexes II, III and IV to Regulation (EU) 2019/1009 of the European Parliament and of the Council for the Purpose of Adding Pyrolysis and Gasification Materials as a Component Material Category in EU Fertilising Products (Text with EEA Relevance); European Commission: Brussels, Belgium, 2021; Volume 427. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU) 2021/1165 of 15 July 2021 Authorising Certain Products and Substances for Use in Organic Production and Establishing Their Lists (Text with EEA Relevance); European Commission: Brussels, Belgium, 2021; Volume 253. [Google Scholar]
- European Commission. Commission Delegated Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003 (Text with EEA Relevance); European Commission: Brussels, Belgium, 2019; Volume 170. [Google Scholar]
- Fisher, T.; Hajaligol, M.; Waymack, B.; Kellogg, D. Pyrolysis behavior and kinetics of biomass derived materials. J. Anal. Appl. Pyrolysis 2002, 62, 331–349. [Google Scholar] [CrossRef]
- Jahirul, M.; Rasul, M.; Chowdhury, A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis—A Technological Review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Saffari, N.; Hajabbasi, M.A.; Shirani, H.; Mosaddeghi, M.R.; Mamedov, A.I. Biochar type and pyrolysis temperature effects on soil quality indicators and structural stability. J. Environ. Manag. 2020, 261, 110190. [Google Scholar] [CrossRef]
- Uslu, A.; Faaij, A.P.C.; Bergman, P.C.A. Pre-treatment technologies, and their effect on international bioenergy supply chain logistics. Techno-economic evaluation of torrefaction, fast pyrolysis and pelletisation. Energy 2008, 33, 1206–1223. [Google Scholar] [CrossRef]
- Arfan, M.; Eriksson, O.; Wang, Z.; Soam, S. Life cycle assessment and life cycle costing of hydrogen production from biowaste and biomass in Sweden. Energy Convers. Manag. 2023, 291, 117262. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Mofijur, M.; Rasul, M.G.; Mahlia, T.M.I.; Ashrafur, S.M. An Overview of Recent Developments in Biomass Pyrolysis Technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef]
- Zou, J.; Hu, H.; Xue, Y.; Li, C.; Li, Y.; Yellezuome, D.; He, F.; Zhang, X.; Maksudur Rahman, M.; Cai, J. Exploring kinetic mechanisms of biomass pyrolysis using generalized logistic mixture model. Energy Convers. Manag. 2022, 258, 115522. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Gouws, S.M.; Carrier, M.; Bunt, J.R.; Neomagus, H.W.J.P. Lumped chemical kinetic modelling of raw and torrefied biomass under pressurized pyrolysis. Energy Convers. Manag. 2022, 253, 115199. [Google Scholar] [CrossRef]
- Phuakpunk, K.; Chalermsinsuwan, B.; Assabumrungrat, S. Pyrolysis kinetic parameters investigation of single and tri-component biomass: Models fitting via comparative model-free methods. Renew. Energy 2022, 182, 494–507. [Google Scholar] [CrossRef]
- Wang, K.; Remón, J.; Jiang, Z.; Ding, W. Recent Advances in the Preparation and Application of Biochar Derived from Lignocellulosic Biomass: A Mini Review. Polymers 2024, 16, 851. [Google Scholar] [CrossRef]
- Holtzer, M.; Kmita, A.; Roczniak, A. Processes of pyrolysis and their effect on cast quality and working conditions. Trans. Foundry Res. Inst. 2016, 93, 175–192. [Google Scholar] [CrossRef]
- Kufka, D.; Igo, P.-I.; Wrocławski, U. Adaptacja modułowego reaktora ciśnieniowego do testów pirolitycznej konwersji biomasy. Górnictwo Odkryw. 2015, 56, 43–46. [Google Scholar]
- Li, L.; Rowbotham, J.S.; Christopher Greenwell, H.; Dyer, P.W. An Introduction to Pyrolysis and Catalytic Pyrolysis: Versatile Techniques for Biomass Conversion. In New and Future Developments in Catalysis; Elsevier: Amsterdam, The Netherlands, 2013; pp. 173–208. ISBN 978-0-444-53878-9. [Google Scholar] [CrossRef]
- Fahmy, T.Y.A.; Fahmy, Y.; Mobarak, F.; El-Sakhawy, M.; Abou-Zeid, R.E. Biomass pyrolysis: Past, present, and future. Environ. Dev. Sustain. 2020, 22, 17–32. [Google Scholar] [CrossRef]
- Mohan, D.; Sarswat, A.; Ok, Y.S.; Pittman, C.U. Organic and inorganic contaminants removal from water with biochar, a renewable, low cost and sustainable adsorbent—A critical review. Bioresour. Technol. 2014, 160, 191–202. [Google Scholar] [CrossRef]
- Kalina, M.; Sovova, S.; Hajzler, J.; Kubikova, L.; Trudicova, M.; Smilek, J.; Enev, V. Biochar Texture—A Parameter Influencing Physicochemical Properties, Morphology, and Agronomical Potential. Agronomy 2022, 12, 1768. [Google Scholar] [CrossRef]
- Saletnik, B.; Saletnik, A.; Zaguła, G.; Bajcar, M.; Puchalski, C. The Use of Wood Pellets in the Production of High Quality Biocarbon Materials. Materials 2022, 15, 4404. [Google Scholar] [CrossRef]
- Safarian, S. Performance analysis of sustainable technologies for biochar production: A comprehensive review. Energy Rep. 2023, 9, 4574–4593. [Google Scholar] [CrossRef]
- Čespiva, J.; Niedzwiecki, L.; Wnukowski, M.; Krochmalny, K.; Mularski, J.; Ochodek, T.; Pawlak-Kruczek, H. Torrefaction and gasification of biomass for polygeneration: Production of biochar and producer gas at low load conditions. Energy Rep. 2022, 8, 134–144. [Google Scholar] [CrossRef]
- Bajcar, M.; Saletnik, B.; Zaguła, G.; Puchalski, C. Analysis of the Effect of the Biomass Torrefaction Process on Selected Parameters of Dust Explosivity. Molecules 2020, 25, 3525. [Google Scholar] [CrossRef] [PubMed]
- Erses Yay, A.S.; Birinci, B.; Açıkalın, S.; Yay, K. Hydrothermal carbonization of olive pomace and determining the environmental impacts of post-process products. J. Clean. Prod. 2021, 315, 128087. [Google Scholar] [CrossRef]
- Dermawan, D.; Febrianti, A.N.; Setyawati, E.E.P.; Pham, M.-T.; Jiang, J.-J.; You, S.-J.; Wang, Y.-F. The potential of transforming rice straw (Oryza sativa) and golden shower (Cassia fistula) seed waste into high-efficiency biochar by atmospheric pressure microwave plasma. Ind. Crops Prod. 2022, 185, 115122. [Google Scholar] [CrossRef]
- Potnuri, R.; Surya, D.V.; Rao, C.S.; Yadav, A.; Sridevi, V.; Remya, N. A review on analysis of biochar produced from microwave-assisted pyrolysis of agricultural waste biomass. J. Anal. Appl. Pyrolysis 2023, 173, 106094. [Google Scholar] [CrossRef]
- Ibitoye, S.E.; Loha, C.; Mahamood, R.M.; Jen, T.-C.; Alam, M.; Sarkar, I.; Das, P.; Akinlabi, E.T. An overview of biochar production techniques and application in iron and steel industries. Bioresour. Bioprocess. 2024, 11, 65. [Google Scholar] [CrossRef]
- DeLuca, T.H.; MacKenzie, M.D.; Gundale, M.J.; Holben, W.E. Wildfire-Produced Charcoal Directly Influences Nitrogen Cycling in Ponderosa Pine Forests. Soil Sci. Soc. Am. J. 2006, 70, 448–453. [Google Scholar] [CrossRef]
- Yanai, Y.; Toyota, K.; Okazaki, M. Effects of charcoal addition on N2O emissions from soil resulting from rewetting air-dried soil in short-term laboratory experiments. Soil Sci. Plant Nutr. 2007, 53, 181–188. [Google Scholar] [CrossRef]
- Wang, S.; Chai, Y.; Wang, Y.; Luo, G.; An, S. Review on the Application and Development of Biochar in Ironmaking Production. Metals 2023, 13, 1844. [Google Scholar] [CrossRef]
- Saletnik, B.; Fiedur, M.; Kwarciany, R.; Zaguła, G.; Bajcar, M. Pyrolysis as a Method for Processing of Waste from Production of Cultivated Tobacco (Nicotiana tabacum L.). Sustainability 2024, 16, 2749. [Google Scholar] [CrossRef]
- Saletnik, B.; Zaguła, G.; Bajcar, M.; Tarapatskyy, M.; Bobula, G.; Puchalski, C. Biochar as a Multifunctional Component of the Environment—A Review. Appl. Sci. 2019, 9, 1139. [Google Scholar] [CrossRef]
- Major, J.; Rondon, M.; Molina, D.; Riha, S.J.; Lehmann, J. Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 2010, 333, 117–128. [Google Scholar] [CrossRef]
- Singhal, S. Biochar as a cost-effective and eco-friendly substitute for binder in concrete: A review. Eur. J. Environ. Civ. Eng. 2023, 27, 984–1009. [Google Scholar] [CrossRef]
- Pravina Kamini, G.; Tee, K.F.; Gimbun, J.; Chin, S.C. Biochar in cementitious material—A review on physical, chemical, mechanical, and durability properties. AIMS Mater. Sci. 2023, 10, 405–425. [Google Scholar] [CrossRef]
- Amen, R.; Yaseen, M.; Mukhtar, A.; Klemeš, J.J.; Saqib, S.; Ullah, S.; Al-Sehemi, A.G.; Rafiq, S.; Babar, M.; Fatt, C.L.; et al. Lead and cadmium removal from wastewater using eco-friendly biochar adsorbent derived from rice husk, wheat straw, and corncob. Clean. Eng. Technol. 2020, 1, 100006. [Google Scholar] [CrossRef]
- Ren, S.; Song, N.; Zhang, Y.; Wang, C.; Lu, X. Controllable fabrication of cobalt molybdate nanofibers with oxygen vacancies induced by calcinable polymer for peroxymonosulfate activation towards water treatment. Appl. Surf. Sci. 2024, 674, 160965. [Google Scholar] [CrossRef]
- Yan, H.; Lai, C.; Liu, S.; Wang, D.; Zhou, X.; Zhang, M.; Li, L.; Ma, D.; Xu, F.; Huo, X.; et al. Insight into the selective oxidation behavior of organic pollutants via Ni-N4-C mediated electron transfer pathway. Chem. Eng. J. 2023, 473, 145253. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gopinath, A.; Ranjith, N.; Praveen Akre, A.; Sreedharan, V.; Suresh Kumar, M. Potential role of biochar in advanced oxidation processes: A sustainable approach. Chem. Eng. J. 2021, 405, 126582. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, H.; Huang, Y.; Wang, W.; Wei, S. Hierarchical porous carbon obtained from animal bone and evaluation in electric double-layer capacitors. Carbon 2011, 49, 838–843. [Google Scholar] [CrossRef]
- Zhou, X.; Zeng, Z.; Zeng, G.; Lai, C.; Xiao, R.; Liu, S.; Huang, D.; Qin, L.; Liu, X.; Li, B.; et al. Persulfate activation by swine bone char-derived hierarchical porous carbon: Multiple mechanism system for organic pollutant degradation in aqueous media. Chem. Eng. J. 2020, 383, 123091. [Google Scholar] [CrossRef]
- Pontiroli, D.; Scaravonati, S.; Magnani, G.; Fornasini, L.; Bersani, D.; Bertoni, G.; Milanese, C.; Girella, A.; Ridi, F.; Verucchi, R.; et al. Super-activated biochar from poultry litter for high-performance supercapacitors. Microporous Mesoporous Mater. 2019, 285, 161–169. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, R.; Li, A.; Ji, G. In-situ self-activation strategy toward highly porous biochar for supercapacitors: Direct carbonization of marine algae. J. Electroanal. Chem. 2021, 882, 114986. [Google Scholar] [CrossRef]
- Jiang, C.; Yakaboylu, G.A.; Yumak, T.; Zondlo, J.W.; Sabolsky, E.M.; Wang, J. Activated carbons prepared by indirect and direct CO2 activation of lignocellulosic biomass for supercapacitor electrodes. Renew. Energy 2020, 155, 38–52. [Google Scholar] [CrossRef]
- Yuan, R.; Yu, S.; Shen, Y. Pyrolysis and combustion kinetics of lignocellulosic biomass pellets with calcium-rich wastes from agro-forestry residues. Waste Manag. 2019, 87, 86–96. [Google Scholar] [CrossRef]
- Charoensook, K.; Huang, C.-L.; Tai, H.-C.; Lanjapalli, V.V.K.; Chiang, L.-M.; Hosseini, S.; Lin, Y.-T.; Li, Y.-Y. Preparation of porous nitrogen-doped activated carbon derived from rice straw for high-performance supercapacitor application. J. Taiwan Inst. Chem. Eng. 2021, 120, 246–256. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, S.-C.; Sun, G.-T.; Kang, K.; Zhu, M.-Q.; Geng, Z.-C. Comparison of activated carbons prepared by one-step and two-step chemical activation process based on cotton stalk for supercapacitors application. Energy 2021, 215, 119144. [Google Scholar] [CrossRef]
- Ozpinar, P.; Dogan, C.; Demiral, H.; Morali, U.; Erol, S.; Samdan, C.; Yildiz, D.; Demiral, I. Activated carbons prepared from hazelnut shell waste by phosphoric acid activation for supercapacitor electrode applications and comprehensive electrochemical analysis. Renew. Energy 2022, 189, 535–548. [Google Scholar] [CrossRef]
- Gurten Inal, I.I.; Aktas, Z. Enhancing the performance of activated carbon based scalable supercapacitors by heat treatment. Appl. Surf. Sci. 2020, 514, 145895. [Google Scholar] [CrossRef]
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo De Medeiros, G.; Do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Akar, S.T.; Yilmazer, D.; Celik, S.; Balk, Y.Y.; Akar, T. Effective biodecolorization potential of surface modified lignocellulosic industrial waste biomass. Chem. Eng. J. 2015, 259, 286–292. [Google Scholar] [CrossRef]
- Duan, D.; Chen, D.; Huang, L.; Zhang, Y.; Zhang, Y.; Wang, Q.; Xiao, G.; Zhang, W.; Lei, H.; Ruan, R. Activated carbon from lignocellulosic biomass as catalyst: A review of the applications in fast pyrolysis process. J. Anal. Appl. Pyrolysis 2021, 158, 105246. [Google Scholar] [CrossRef]
- Pallarés, J.; González-Cencerrado, A.; Arauzo, I. Production and characterization of activated carbon from barley straw by physical activation with carbon dioxide and steam. Biomass Bioenergy 2018, 115, 64–73. [Google Scholar] [CrossRef]
- Luo, M.; Chen, J.; Li, Q.; Wang, Y. Cotton-Based Activated Carbon Fiber with High Specific Surface Area Prepared by Low-Temperature Hydrothermal Carbonization with Urea Enhancement. Ind. Eng. Chem. Res. 2023, 62, 8744–8753. [Google Scholar] [CrossRef]
- Cruz, O.F., Jr.; Serafin, J.; Azar, F.-Z.; Casco, M.E.; Silvestre-Albero, J.; Hotza, D.; Rambo, C.R. Microwave-Assisted hydrothermal carbonization and characterization of Amazonian biomass as an activated carbon for methane adsorption. Fuel 2024, 358, 130329. [Google Scholar] [CrossRef]
- Azeez, M.O.; Tanimu, A.; Alhooshani, K.; Ganiyu, S.A. Synergistic effect of nitrogen and molybdenum on activated carbon matrix for selective adsorptive Desulfurization: Insights into surface chemistry modification. Arab. J. Chem. 2022, 15, 103454. [Google Scholar] [CrossRef]
- Tibor, S.T.; Grande, C.A. Industrial production of activated carbon using circular bioeconomy principles: Case study from a Romanian company. Clean. Eng. Technol. 2022, 7, 100443. [Google Scholar] [CrossRef]
- Awitdrus; Siregar, G.M.G.; Agustino; Saktioto; Iwantono; Syahputra, R.F.; Farma, R. KOH Activation with Microwave Irradiation and its Effect on the Physical Properties of Orange Peel Activated Carbon. J. Phys. Conf. Ser. 2021, 2049, 012025. [Google Scholar] [CrossRef]
- Taher, T.; Maulana, S.; Mawaddah, N.; Munandar, A.; Rianjanu, A.; Lesbani, A. Low-temperature hydrothermal carbonization of activated carbon microsphere derived from microcrystalline cellulose as carbon dioxide (CO2) adsorbent. Mater. Today Sustain. 2023, 23, 100464. [Google Scholar] [CrossRef]
- Li, C.; Feng, Y.; Zhong, F.; Deng, J.; Yu, T.; Cao, H.; Niu, W. Optimization of microwave-assisted hydrothermal carbonization and potassium bicarbonate activation on the structure and electrochemical characteristics of crop straw-derived biochar. J. Energy Storage 2022, 55, 105838. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, B.; Liu, L.; Cao, L.; Yuan, Q.; Tian, J.; Huang, Z.; Zong, Z.; Zhang, P.; Lin, Z.; et al. Tobacco Waste Biomass for Electrochemical Energy Storage Application. J. Phys. Conf. Ser. 2022, 2160, 012052. [Google Scholar] [CrossRef]
- Andrade, T.S.; Vakros, J.; Mantzavinos, D.; Lianos, P. Biochar obtained by carbonization of spent coffee grounds and its application in the construction of an energy storage device. Chem. Eng. J. Adv. 2020, 4, 100061. [Google Scholar] [CrossRef]
- Husain, Z.; Shakeelur Raheman, A.R.; Ansari, K.B.; Pandit, A.B.; Khan, M.S.; Qyyum, M.A.; Lam, S.S. Nano-sized mesoporous biochar derived from biomass pyrolysis as electrochemical energy storage supercapacitor. Mater. Sci. Energy Technol. 2022, 5, 99–109. [Google Scholar] [CrossRef]
- Wang, K.; Xu, M.; Gu, Z.; Ahrenkiel, P.; Lee, J.; Gibbons, W.; Croat, J.; Fan, Q. Pyrrole modified biomass derived hierarchical porous carbon as high performance symmetrical supercapacitor electrodes. Int. J. Hydrogen Energy 2016, 41, 13109–13115. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Cuña, A.; Da Silva, E.L.; Amaral-Labat, G.; Lenz E Silva, G.F.B.; Bouafif, H.; Koubaa, A. The conversion of wood residues, using pilot-scale technologies, into porous activated biochars for supercapacitors. J. Porous Mater. 2020, 27, 537–548. [Google Scholar] [CrossRef]
- Wang, X.; Yun, S.; Fang, W.; Zhang, C.; Liang, X.; Lei, Z.; Liu, Z. Layer-Stacking Activated Carbon Derived from Sunflower Stalk as Electrode Materials for High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 11397–11407. [Google Scholar] [CrossRef]
- Shu, Y.; Bai, Q.; Fu, G.; Xiong, Q.; Li, C.; Ding, H.; Shen, Y.; Uyama, H. Hierarchical porous carbons from polysaccharides carboxymethyl cellulose, bacterial cellulose, and citric acid for supercapacitor. Carbohydr. Polym. 2020, 227, 115346. [Google Scholar] [CrossRef]
- EL-Ouahabi, H.; Khaddor, M.; Lillo-Rodenas, M.A.; Roman Martinez, M.D.C.; Elmouwahidi, A.; Pérez-Cadenas, A.F.; Ouzzine, M. Bio-Waste Argan Nut Shell-Derived Porous Activated Carbon for High Performance Supercapacitor Electrode Material. ECS Meet. Abstr. 2023, MA2023-01, 1098. [Google Scholar] [CrossRef]
- Bui, T.A.N.; Huynh, T.V.; Tran, H.L.; Doong, R. Erbium-Doped GQD-Embedded Coffee-Ground-Derived Porous Biochar for Highly Efficient Asymmetric Supercapacitor. Nanomaterials 2022, 12, 1939. [Google Scholar] [CrossRef]
- Rawat, S.; Boobalan, T.; Sathish, M.; Hotha, S.; Thallada, B. Utilization of CO2 activated litchi seed biochar for the fabrication of supercapacitor electrodes. Biomass Bioenergy 2023, 171, 106747. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Órfão, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Azargohar, R.; Dalai, A.K. Steam and KOH activation of biochar: Experimental and modeling studies. Microporous Mesoporous Mater. 2008, 110, 413–421. [Google Scholar] [CrossRef]
- Hoseinzadeh Hesas, R.; Arami-Niya, A.; Wan Daud, W.M.A.; Sahu, J.N. Microwave-assisted production of activated carbons from oil palm shell in the presence of CO2 or N2 for CO2 adsorption. J. Ind. Eng. Chem. 2015, 24, 196–205. [Google Scholar] [CrossRef]
- Anto, S.; Sudhakar, M.P.; Shan Ahamed, T.; Samuel, M.S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. Activation strategies for biochar to use as an efficient catalyst in various applications. Fuel 2021, 285, 119205. [Google Scholar] [CrossRef]
- Sumangala Devi, N.; Hariram, M.; Vivekanandhan, S. Modification techniques to improve the capacitive performance of biocarbon materials. J. Energy Storage 2021, 33, 101870. [Google Scholar] [CrossRef]
- Gámiz, B.; Hall, K.; Spokas, K.A.; Cox, L. Understanding Activation Effects on Low-Temperature Biochar for Optimization of Herbicide Sorption. Agronomy 2019, 9, 588. [Google Scholar] [CrossRef]
- Tan, X.; Liu, S.; Liu, Y.; Gu, Y.; Zeng, G.; Hu, X.; Wang, X.; Liu, S.; Jiang, L. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef]
- Mendhe, A.; Panda, H.S. A review on electrolytes for supercapacitor device. Discov. Mater. 2023, 3, 29. [Google Scholar] [CrossRef]
- Ma, C.; Tang, L.; Cheng, H.; Li, Z.; Li, W.; He, G. Biochar for supercapacitor electrodes: Mechanisms in aqueous electrolytes. Battery Energy 2024, 3, 20230058. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Ramavath, J.N.; Raja, M.; Kumar, S.; Kothandaraman, R. Mild acidic mixed electrolyte for high-performance electrical double layer capacitor. Appl. Surf. Sci. 2019, 489, 867–874. [Google Scholar] [CrossRef]
- Ho, J.; Jow, T.R.; Boggs, S. Historical introduction to capacitor technology. IEEE Electr. Insul. Mag. 2010, 26, 20–25. [Google Scholar] [CrossRef]
- Naoi, K.; Simon, P. New Materials and New Configurations for Advanced Electrochemical Capacitors. Electrochem. Soc. Interface 2008, 17, 34–37. [Google Scholar] [CrossRef]
- Zhao, J.; Burke, A.F. Review on supercapacitors: Technologies and performance evaluation. J. Energy Chem. 2021, 59, 276–291. [Google Scholar] [CrossRef]
- Thiagarajan, K.; Theerthagiri, J.; Senthil, R.A.; Madhavan, J. Simple and low cost electrode material based on Ca2V2O7/PANI nanoplatelets for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2017, 28, 17354–17362. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Masrur Ahmed, A.A.; Bailek, N.; Abualigah, L.; Bouchouicha, K.; Kuriqi, A.; Sharifi, A.; Sareh, P.; Al Khatib, A.M.G.; Mishra, P.; Colak, I.; et al. Global control of electrical supply: A variational mode decomposition-aided deep learning model for energy consumption prediction. Energy Rep. 2023, 10, 2152–2165. [Google Scholar] [CrossRef]
- Song, Z.; Hou, J.; Hofmann, H.; Li, J.; Ouyang, M. Sliding-mode and Lyapunov function-based control for battery/supercapacitor hybrid energy storage system used in electric vehicles. Energy 2017, 122, 601–612. [Google Scholar] [CrossRef]
- Saikia, B.K.; Benoy, S.M.; Bora, M.; Tamuly, J.; Pandey, M.; Bhattacharya, D. A brief review on supercapacitor energy storage devices and utilization of natural carbon resources as their electrode materials. Fuel 2020, 282, 118796. [Google Scholar] [CrossRef]
- Berrueta, A.; Ursua, A.; Martin, I.S.; Eftekhari, A.; Sanchis, P. Supercapacitors: Electrical Characteristics, Modeling, Applications, and Future Trends. IEEE Access 2019, 7, 50869–50896. [Google Scholar] [CrossRef]
- Jalal, N.I.; Ibrahim, R.I.; Oudah, M.K. A review on Supercapacitors: Types and components. J. Phys. Conf. Ser. 2021, 1973, 012015. [Google Scholar] [CrossRef]
- Halper, M.S.; Ellenbogen, J.C. Supercapacitors: A Brief Overview; The MITRE Corporation: McLean, VA, USA, 2006. [Google Scholar]
- Czagany, M.; Hompoth, S.; Keshri, A.K.; Pandit, N.; Galambos, I.; Gacsi, Z.; Baumli, P. Supercapacitors: An Efficient Way for Energy Storage Application. Materials 2024, 17, 702. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, C.; Cho, S.; Moon, G.D.; Kim, B.; Chang, H.; Jang, H.D. High capacitance and energy density supercapacitor based on biomass-derived activated carbons with reduced graphene oxide binder. Carbon 2018, 132, 16–24. [Google Scholar] [CrossRef]
- Bello, A.; Barzegar, F.; Momodu, D.; Dangbegnon, J.; Taghizadeh, F.; Manyala, N. Symmetric supercapacitors based on porous 3D interconnected carbon framework. Electrochim. Acta 2015, 151, 386–392. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hou, H.; Xu, W.; Duan, G.; He, S.; Liu, K.; Jiang, S. Recent progress in carbon-based materials for supercapacitor electrodes: A review. J. Mater. Sci. 2021, 56, 173–200. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520. [Google Scholar] [CrossRef]
- Phiri, J.; Dou, J.; Vuorinen, T.; Gane, P.A.C.; Maloney, T.C. Highly Porous Willow Wood-Derived Activated Carbon for High-Performance Supercapacitor Electrodes. ACS Omega 2019, 4, 18108–18117. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Vermisoglou, E.C.; Giannouri, M.; Todorova, N.; Giannakopoulou, T.; Lekakou, C.; Trapalis, C. Recycling of typical supercapacitor materials. Waste Manag. Res. J. Sustain. Circ. Econ. 2016, 34, 337–344. [Google Scholar] [CrossRef]
- Seo, D.H.; Han, Z.J.; Kumar, S.; Ostrikov, K. (Ken) Structure-Controlled, Vertical Graphene-Based, Binder-Free Electrodes from Plasma-Reformed Butter Enhance Supercapacitor Performance. Adv. Energy Mater. 2013, 3, 1316–1323. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor Devices Based on Graphene Materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Krüner, B.; Odenwald, C.; Tolosa, A.; Schreiber, A.; Aslan, M.; Kickelbick, G.; Presser, V. Carbide-derived carbon beads with tunable nanopores from continuously produced polysilsesquioxanes for supercapacitor electrodes. Sustain. Energy Fuels 2017, 1, 1588–1600. [Google Scholar] [CrossRef]
- Zhong, W.; Sun, H.; Pan, J.; Zhang, Y.; Yan, X.; Guan, Y.; Shen, W.; Cheng, X. Hierarchical porous TiO2/carbide-derived carbon for asymmetric supercapacitor with enhanced electrochemical performance. Mater. Sci. Semicond. Process. 2021, 127, 105715. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent advances in utilization of biochar. Renew. Sustain. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Gao, P.-C.; Tsai, W.-Y.; Daffos, B.; Taberna, P.-L.; Pérez, C.R.; Gogotsi, Y.; Simon, P.; Favier, F. Graphene-like carbide derived carbon for high-power supercapacitors. Nano Energy 2015, 12, 197–206. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, G.; Lu, L.; Wang, T.; Xu, H.; Yu, C.; Li, H.; Tian, W. Improving the electrochemical performances of active carbon-based supercapacitors through the combination of introducing functional groups and using redox additive electrolyte. J. Saudi Chem. Soc. 2018, 22, 908–918. [Google Scholar] [CrossRef]
- Bai, X.; Hu, X.; Zhou, S.; Yan, J.; Sun, C.; Chen, P.; Li, L. In situ polymerization and characterization of grafted poly (3,4-ethylenedioxythiophene)/multiwalled carbon nanotubes composite with high electrochemical performances. Electrochim. Acta 2013, 87, 394–400. [Google Scholar] [CrossRef]
- Yang, M.; Cheng, B.; Song, H.; Chen, X. Preparation and electrochemical performance of polyaniline-based carbon nanotubes as electrode material for supercapacitor. Electrochim. Acta 2010, 55, 7021–7027. [Google Scholar] [CrossRef]
- Zhai, Z.; Zhang, L.; Du, T.; Ren, B.; Xu, Y.; Wang, S.; Miao, J.; Liu, Z. A review of carbon materials for supercapacitors. Mater. Des. 2022, 221, 111017. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D.; Long, J.W. To Be or Not To Be Pseudocapacitive? J. Electrochem. Soc. 2015, 162, A5185–A5189. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839. [Google Scholar] [CrossRef]
- Majumdar, D.; Maiyalagan, T.; Jiang, Z. Recent Progress in Ruthenium Oxide-Based Composites for Supercapacitor Applications. ChemElectroChem 2019, 6, 4343–4372. [Google Scholar] [CrossRef]
- Lou, S.; Cheng, X.; Wang, L.; Gao, J.; Li, Q.; Ma, Y.; Gao, Y.; Zuo, P.; Du, C.; Yin, G. High-rate capability of three-dimensionally ordered macroporous T-Nb2O5 through Li+ intercalation pseudocapacitance. J. Power Sources 2017, 361, 80–86. [Google Scholar] [CrossRef]
- Li, J.; Yuan, X.; Lin, C.; Yang, Y.; Xu, L.; Du, X.; Xie, J.; Lin, J.; Sun, J. Achieving High Pseudocapacitance of 2D Titanium Carbide (MXene) by Cation Intercalation and Surface Modification. Adv. Energy Mater. 2017, 7, 1602725. [Google Scholar] [CrossRef]
- Kurra, N.; Jiang, Q.; Syed, A.; Xia, C.; Alshareef, H.N. Micro-Pseudocapacitors with Electroactive Polymer Electrodes: Toward AC-Line Filtering Applications. ACS Appl. Mater. Interfaces 2016, 8, 12748–12755. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, Q.; Liu, L.; Manasa, P.; Kang, L.; Ran, F. Vanadium nitride for aqueous supercapacitors: A topic review. J. Mater. Chem. A 2020, 8, 8218–8233. [Google Scholar] [CrossRef]
- Fleischmann, S.; Mitchell, J.B.; Wang, R.; Zhan, C.; Jiang, D.; Presser, V.; Augustyn, V. Pseudocapacitance: From Fundamental Understanding to High Power Energy Storage Materials. Chem. Rev. 2020, 120, 6738–6782. [Google Scholar] [CrossRef] [PubMed]
- Afif, A.; Rahman, S.M.; Tasfiah Azad, A.; Zaini, J.; Islan, M.A.; Azad, A.K. Advanced materials and technologies for hybrid supercapacitors for energy storage—A review. J. Energy Storage 2019, 25, 100852. [Google Scholar] [CrossRef]
- Khan, M.A.; Zeb, K.; Sathishkumar, P.; Ali, M.U.; Uddin, W.; Hussain, S.; Ishfaq, M.; Khan, I.; Cho, H.-G.; Kim, H.-J. A Novel Supercapacitor/Lithium-Ion Hybrid Energy System with a Fuzzy Logic-Controlled Fast Charging and Intelligent Energy Management System. Electronics 2018, 7, 63. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145. [Google Scholar] [CrossRef]
- Liu, S.; Wei, L.; Wang, H. Review on reliability of supercapacitors in energy storage applications. Appl. Energy 2020, 278, 115436. [Google Scholar] [CrossRef]
- Saha, P.; Dey, S.; Khanra, M. Second-life applications of supercapacitors: Effective capacitance prognosis and aging. J. Power Sources 2021, 496, 229824. [Google Scholar] [CrossRef]
- European Commission. Directive 2012/19/EU of the European Parliament and of the Council of 4 July 2012 on Waste Electrical and Electronic Equipment (WEEE) (Recast); Text with EEA Relevance; European Commission: Brussels, Belgium, 2012; Volume 197. [Google Scholar]
- Jiang, G.; Pickering, S.J. Recycling supercapacitors based on shredding and mild thermal treatment. Waste Manag. 2016, 48, 465–470. [Google Scholar] [CrossRef]
- Jiang, G.; Pickering, S.J. Recycling Graphene from Supercapacitor Electrodes as Reinforcing Filler for Epoxy Resins. Waste Biomass Valorization 2019, 10, 215–221. [Google Scholar] [CrossRef]
- Yanshyna, O.; Weissman, H.; Rybtchinski, B. Recyclable electrochemical supercapacitors based on carbon nanotubes and organic nanocrystals. Nanoscale 2020, 12, 8909–8914. [Google Scholar] [CrossRef] [PubMed]
- Deep, A.; Gupta, A.; Singh, S.; Khatoon, N.; Gupta, G. Overview of Supercapacitors: A Comprehensive Review. Preprints 2024. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, V. Current Technology of Supercapacitors: A Review. J. Electron. Mater. 2020, 49, 3520–3532. [Google Scholar] [CrossRef]
- Jing, W.; Hung Lai, C.; Wong, S.H.W.; Wong, M.L.D. Battery-supercapacitor hybrid energy storage system in standalone DC microgrids: Areview. IET Renew. Power Gener. 2017, 11, 461–469. [Google Scholar] [CrossRef]
- Şahin, M.E.; Blaabjerg, F. A Hybrid PV-Battery/Supercapacitor System and a Basic Active Power Control Proposal in MATLAB/Simulink. Electronics 2020, 9, 129. [Google Scholar] [CrossRef]
- Glavin, M.E.; Hurley, W.G. Optimisation of a photovoltaic battery ultracapacitor hybrid energy storage system. Sol. Energy 2012, 86, 3009–3020. [Google Scholar] [CrossRef]
- Manandhar, U.; Ukil, A.; Gooi, H.B.; Tummuru, N.R.; Kollimalla, S.K.; Wang, B.; Chaudhari, K. Energy Management and Control for Grid Connected Hybrid Energy Storage System Under Different Operating Modes. IEEE Trans. Smart Grid 2019, 10, 1626–1636. [Google Scholar] [CrossRef]
- Logerais, P.-O.; Riou, O.; Camara, M.A.; Durastanti, J.-F. Study of Photovoltaic Energy Storage by Supercapacitors through Both Experimental and Modelling Approaches. J. Sol. Energy 2013, 2013, 659014. [Google Scholar] [CrossRef]
- Supercapacitor Market Analysis, Size, Share & Trends|2031. Available online: https://www.skyquestt.com/report/supercapacitor-market (accessed on 19 June 2024).
- Maxwell Technologies Products—Mouser Poland. Available online: https://www.mouser.pl/c/?m=Maxwell%20Technologies (accessed on 19 June 2024).
- Wang, Y.; Wu, X.; Han, Y.; Li, T. Flexible supercapacitor: Overview and outlooks. J. Energy Storage 2021, 42, 103053. [Google Scholar] [CrossRef]
- Zhao, Z.; Xia, K.; Shao, W.; Wang, X.; Zhang, Q.; Hou, Y.; Ye, Z.; Zheng, Z.; Lu, J. Ultrathin Tape-Supercapacitor with High Capacitance and Durable Flexibility via Repeated In-situ Polymerizations of Polyaniline. Chem. Eng. J. 2023, 471, 144721. [Google Scholar] [CrossRef]
- Bi, Z.; Kong, Q.; Cao, Y.; Sun, G.; Su, F.; Wei, X.; Li, X.; Ahmad, A.; Xie, L.; Chen, C.-M. Biomass-derived porous carbon materials with different dimensions for supercapacitor electrodes: A review. J. Mater. Chem. A 2019, 7, 16028–16045. [Google Scholar] [CrossRef]
- Şahin, M.; Blaabjerg, F.; Sangwongwanich, A. A Comprehensive Review on Supercapacitor Applications and Developments. Energies 2022, 15, 674. [Google Scholar] [CrossRef]
- Kumar, N.; Kim, S.-B.; Lee, S.-Y.; Park, S.-J. Recent Advanced Supercapacitor: A Review of Storage Mechanisms, Electrode Materials, Modification, and Perspectives. Nanomaterials 2022, 12, 3708. [Google Scholar] [CrossRef]
- Shang, W.; Yu, W.; Xiao, X.; Ma, Y.; He, Y.; Zhao, Z.; Tan, P. Insight into the self-discharge suppression of electrochemical capacitors: Progress and challenges. Adv. Powder Mater. 2023, 2, 100075. [Google Scholar] [CrossRef]
- ScienceDirect.com|Science, Health and Medical Journals, Full Text Articles and Books. Available online: https://www.sciencedirect.com/ (accessed on 16 July 2024).
- European Commission. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions; The European Green Deal; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Ånensen, K.R. Biochar—A Market Analysis and Predictions for the Future. Master Thesis, University of Stavanger, Stavanger, Norway, 2023. [Google Scholar]
- Biochar Market Size Is Expanding around USD 633.31 Mn by 2032. Available online: https://www.precedenceresearch.com/biochar-market (accessed on 22 July 2024).
- European Commission. Directive 2003/87/EC of the European Parliament and of the Council of 13 October 2003 Establishing a Scheme for Greenhouse Gas Emission Allowance Trading within the Community and Amending Council Directive 96/61/EC (Text with EEA Relevance); European Commission: Brussels, Belgium, 2003; Volume 275. [Google Scholar]
- Lin, Y.; Li, F.; Zhang, Q.; Liu, G.; Xue, C. Controllable preparation of green biochar based high-performance supercapacitors. Ionics 2022, 28, 2525–2561. [Google Scholar] [CrossRef]
- Yang, C.; Wu, H.; Cai, M.; Zhou, Y.; Guo, C.; Han, Y.; Zhang, L. Valorization of Biomass-Derived Polymers to Functional Biochar Materials for Supercapacitor Applications via Pyrolysis: Advances and Perspectives. Polymers 2023, 15, 2741. [Google Scholar] [CrossRef] [PubMed]
- Mian, M.M.; Kamana, I.M.L.; An, X.; Abbas, S.C.; Ahommed, M.S.; He, Z.; Ni, Y. Cellulose nanofibers as effective binders for activated biochar-derived high-performance supercapacitors. Carbohydr. Polym. 2023, 301, 120353. [Google Scholar] [CrossRef]
- Ma, Z.-W.; Liu, H.-Q.; Lü, Q.-F. Porous biochar derived from tea saponin for supercapacitor electrode: Effect of preparation technique. J. Energy Storage 2021, 40, 102773. [Google Scholar] [CrossRef]
- Gao, M.; Wang, W.-K.; Zheng, Y.-M.; Zhao, Q.-B.; Yu, H.-Q. Hierarchically porous biochar for supercapacitor and electrochemical H2O2 production. Chem. Eng. J. 2020, 402, 126171. [Google Scholar] [CrossRef]
- Lima, R.M.A.P.; Dos Reis, G.S.; Thyrel, M.; Alcaraz-Espinoza, J.J.; Larsson, S.H.; De Oliveira, H.P. Facile Synthesis of Sustainable Biomass-Derived Porous Biochars as Promising Electrode Materials for High-Performance Supercapacitor Applications. Nanomaterials 2022, 12, 866. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, R.; Khoja, A.H.; Naqvi, S.R.; Gao, N.; Amin, N.A.S. A Review on Production and Surface Modifications of Biochar Materials via Biomass Pyrolysis Process for Supercapacitor Applications. Catalysts 2022, 12, 798. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, P.; Jia, J.; Liu, Z.; Huo, L.; Zhao, L.; Zhao, Y.; Niu, W.; Yao, Z. Machine learning in clarifying complex relationships: Biochar preparation procedures and capacitance characteristics. Chem. Eng. J. 2024, 485, 149975. [Google Scholar] [CrossRef]
- Khedulkar, A.P.; Dang, V.D.; Thamilselvan, A.; Doong, R.; Pandit, B. Sustainable high-energy supercapacitors: Metal oxide-agricultural waste biochar composites paving the way for a greener future. J. Energy Storage 2024, 77, 109723. [Google Scholar] [CrossRef]
- Meng, L.-Y.; Ma, M.-G.; Ji, X.-X. Preparation of Lignin-Based Carbon Materials and Its Application as a Sorbent. Materials 2019, 12, 1111. [Google Scholar] [CrossRef]
- Zhang, M.; Duan, Y.; Chen, T.; Qi, J.; Xu, T.; Du, H.; Si, C. Lignocellulosic materials for energy storage devices. Ind. Crops Prod. 2023, 203, 117174. [Google Scholar] [CrossRef]
- Rawat, S.; Wang, C.-T.; Lay, C.-H.; Hotha, S.; Bhaskar, T. Sustainable biochar for advanced electrochemical/energy storage applications. J. Energy Storage 2023, 63, 107115. [Google Scholar] [CrossRef]
- Khedulkar, A.P.; Pandit, B.; Dang, V.D.; Doong, R. Agricultural waste to real worth biochar as a sustainable material for supercapacitor. Sci. Total Environ. 2023, 869, 161441. [Google Scholar] [CrossRef]
- Liu, H.; Liu, S.; Liu, L. Biomass-Derived Porous Carbon: Synthesis and Application for Energy Conversion and Storage. Adv. Mater. Sci. Technol. 2023, 5, 11809. [Google Scholar] [CrossRef]
- Lu, W.; Si, Y.; Zhao, C.; Chen, T.; Li, C.; Zhang, C.; Wang, K. Biomass-derived carbon applications in the field of supercapacitors: Progress and prospects. Chem. Eng. J. 2024, 495, 153311. [Google Scholar] [CrossRef]




| Pyrolysis Type | Temperature (°C) | Time (s) |
|---|---|---|
| Slow pyrolysis | 300–700 | 450–550 |
| Fast pyrolysis | 600–1000 | 0.5–10 |
| Flash pyrolysis | 800–1000 | 0.5 |
| Origin of Biomass | Example | Source |
|---|---|---|
| Forest biomass | Willow | [57] |
| Spruce | ||
| Sawmill waste | Sawdust | [58] |
| Wood scraps | ||
| Agricultural biomass | Seed residues | [44,59,60,61,62] |
| Rice straw | ||
| Cotton stalk | ||
| Hazelnut shells | ||
| Waste tea | ||
| Tobacco waste | ||
| Agricultural waste | Cattle manure | [63] |
| Sugar cane waste | ||
| Waste from corn | ||
| Industrial waste | Paper pulp | [64] |
| Paper sludge |
| Biomass Used | Electrolyte Used | Energy Density (A g−1) | Supercapacitor Capacitance (F g−1) | Stability (%) and the Number of Cycles | Source |
|---|---|---|---|---|---|
| Rice husks | 6 M KOH | 1 | 302.2 | 88.5/5000 | [12] |
| Garden waste pellets (800 °C) | 1 M H2SO4 | 1 | 228 | 88/5000 | [76] |
| Dried mushrooms | 6 M KOH | 1 | 235 | >100/1000 | [77] |
| White birch | 1 M H2SO4 | 1 | 350 | 98/1000 | [78] |
| White birch | 1 M Na2SO4 | 1 | 118 | 95/1000 | [78] |
| Sunflower stalk | 6 M KOH | 0.5 | 263 | 93/15,000 | [79] |
| Carbon composite material * | 6 M KOH | 0.5 | 350 | 96.1/10,000 | [80] |
| Argan nut shells | 1 M H2SO4 | 0.125 | 320 | 92/2500 | [81] |
| Coffee beans | 2 M KOH | 1 | 337 | - | [82] |
| Lychee seeds | 1 M H2SO4 | 1 | 493 | 90/10,000 | [83] |
| Type of Carbon-Based Materials | Specific Surface Area (m2 g−1) | Supercapacitor Capacitance (F g−1) | Stability (%) | Number of Cycles | Source |
|---|---|---|---|---|---|
| Activated carbon from biomass | 1000–3500 | 100–400 | 93–98 | 1000–15,000 | [78,79,110,111] |
| Graphene | <3100 | 100–264 | 90–99 | 1200–1500 | [112,113,114,115] |
| Carbide-derived carbon (CDC) | 1800–3000 | 80–173 | 90 | - | [116,117,118,119] |
| Carbon nanotubes (CNTs) | 120–500 | 2–200 | 97 | 1000 | [110,120,121,122,123] |
| Advantages | Source |
|---|---|
| High specific capacity | [152] |
| Higher power density compared to batteries | [153] |
| Larger operating temperature range compared to batteries | [153] |
| Long life cycle | [153] |
| Stability of the cycle | [152] |
| High charging/discharging speed | [152] |
| Disadvantages | |
| High diversity associated with the difficulty of applying large-scale production | [154] |
| Self-discharge | [155] |
| Advantages | Source |
|---|---|
| Porous structure to increase specific surface area | [164,165,166,167] |
| High specific capacity | [162,167] |
| High energy storage capacity | [168] |
| High conductivity | [169] |
| Short diffusion path of electrolyte ions | [170] |
| Long life cycle | [167] |
| Wide operating temperature range | [171] |
| Environmentally friendly | [172,173] |
| Disadvantages | |
| Relatively low conductivity compared to other carbon materials | [141] |
| Irregular morphology, translating into difficulties with mass production, repeatability of the number of pores and their shape in electrodes | [174] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwarciany, R.; Fiedur, M.; Saletnik, B. Opportunities and Threats for Supercapacitor Technology Based on Biochar—A Review. Energies 2024, 17, 4617. https://doi.org/10.3390/en17184617
Kwarciany R, Fiedur M, Saletnik B. Opportunities and Threats for Supercapacitor Technology Based on Biochar—A Review. Energies. 2024; 17(18):4617. https://doi.org/10.3390/en17184617
Chicago/Turabian StyleKwarciany, Radosław, Marcin Fiedur, and Bogdan Saletnik. 2024. "Opportunities and Threats for Supercapacitor Technology Based on Biochar—A Review" Energies 17, no. 18: 4617. https://doi.org/10.3390/en17184617
APA StyleKwarciany, R., Fiedur, M., & Saletnik, B. (2024). Opportunities and Threats for Supercapacitor Technology Based on Biochar—A Review. Energies, 17(18), 4617. https://doi.org/10.3390/en17184617






