Abstract
The global increase in energy consumption, driven by population growth and improved living standards, has led to a heavy reliance on fossil fuels, causing significant environmental concerns. This has prompted a shift toward sustainable energy sources, with biomass, especially lignocellulosic forest biomass, emerging as a key alternative due to its abundance and carbon-neutral potential. Microwave-assisted pyrolysis (MAP) is an efficient method for converting forest biomass into valuable bioproducts and bioenergy with reduced energy use. This review introduces biomass types, focusing on forest biomass and its role in global energy production. It compares MAP to conventional pyrolysis, highlighting the benefits of rapid, uniform heating and improved product yields. Key operational conditions, such as temperature, microwave power, biomass size, and catalyst ratios, are discussed in relation to their impact on product quality and yield. Despite its advantages, MAP faces challenges, particularly in temperature control, which can affect bio-oil yield and quality. High temperatures may cause unwanted secondary reactions, while low temperatures can lead to incomplete decomposition. Research into biomass dielectric properties and process modeling is essential in order to optimize MAP and scale it up for industrial use. Addressing bio-oil quality issues through catalytic upgrading is also critical for broader adoption.
Keywords:
microwave-assisted pyrolysis; forest biomass; feedstocks; absorbers; temperature; power; catalyst 1. Introduction
The substantial population increase and improved quality of life have significantly increased energy consumption [1]. Historically, fossil fuels have met around 80% of the world’s energy demand [2,3], generating their intensive usage, which has raised carbon emissions [4] and amplified global warming [5]. Consequently, the reliance on fossil fuels not only depletes natural resources [6], but also possesses severe environmental concerns. Major economies have pledged to achieve net-zero emissions by 2050 or 2060, facing the challenge of supporting advanced technologies like microwave-assisted pyrolysis, at minimal costs to produce economic biofuels and high value products. Although challenging, achieving net-zero emissions will bring economic and social benefits [7]. Additionally, the energy crisis has accelerated the shift towards a clean energy economy and increased investments in transforming the energy system [3]. For these reasons, ensuring energy security with a sustainable approach and reducing the dependency on fossil fuels is essential [8].
Interest in discovering new, affordable energy sources includes alternatives such as solar, wind, hydro, geothermal and biomass. One of the renewable resources which has the highest potential is biomass due to its energy recovery possibilities, and its valuable components [9]. Integrating biotechnological processes and biorefinery methods for its valorization are considered key aspects in the production of bioproducts (chemicals and biofuels) and bioenergy (electrical energy and heat) [10,11]. Lignocellulosic biomass, composed of cellulose, hemicellulose, and lignin [12], is the most sustainable, abundant, and cost-effective form of biomass [13] which has emerged as one of the most promising feedstocks. This biomass that refers to plant biomass [14] has an enormous potential to generate sustainable products with zero net carbon emissions. This kind of biomass derives from a wide range of sources such as municipal solid wastes, industrial wastes, agricultural residues, and forest biomass [15]. In recent years, interest in the use of lignocellulosic agricultural crops and forest residues as feedstocks for biofuels and bioproducts due to their abundant availability and non-food nature has grown [16,17]. Forest biomass (branches, trees, bark, needles, roots, trunks, leaves, etc.) is the primary biomass source in Europe that does not compete with the food supply, and its high demand as both a material and energy source has generated a competition between industries and the need of circularity improvement and resource efficiency to enhance sustainable development [18,19].
A clean and efficient use of lignocellulosic biomass to produce value-added products or energy requires the development of suitable conversion techniques [20]. These techniques have being investigated for years, with pyrolysis as one of them [21]. Pyrolysis is a thermochemical process that takes place in an inert atmosphere or with a low oxygen concentration, particularly appealing due to its low pollutant emissions and the variety of products it can produce [8,22]. Pyrolysis products include non-condensable gases, bio-oil, and biochar [23,24]. Biomass pyrolysis can be divided into different categories (flash, fast, and slow) depending on the heating rate and residence time; each category primarily aims to maximize either non-condensable gases, bio-oil, or biochar yields [25]. Slow heating rates (slow pyrolysis) generates bio-oil with a high water content. In contrast, high heating rates lead to a higher bio-oil yield and better biochar quality [26]. It has been detected that products’ specificity requires more efficient or targeted heating methods and improvements [27]. For instance, microwave-assisted pyrolysis has appeared as a different method of heating which is easily operated through instant on/off control, improving product quality and yield [5,28]. This technique seems to be interesting due to its lower energy use and process time [29]. Although biomass is generally a poor microwave absorber, its microwave absorption capacity can be improved through inorganic substances or high humidity. The use of microwave absorbers (MWAs) enhances the pyrolysis temperature using low microwave power. MWAs can heat the surrounding biomass which modifies the quality and product yield. The simultaneous use of catalysts and MWAs can adapt the product distribution, increasing the concentration of specific components in non-condensable gases, bio-oil, and biochar, as well as the energy efficiency of MAP [30].
This paper reviews microwave-assisted pyrolysis (MAP), examining its characteristics and the forest biomass capacity to produce value-added products. This article also compares the products derived from different feedstocks and MAP operation conditions (microwave power, absorbers and catalysts use, temperature, residence time, and biomass size), as well as future directions in MAP use.
2. Biomass
Energy security, energy prices, health (emergence of diseases sensitive to global warming, and famine), and environmental concerns (climate change, global biodiversity loss, and soil degradation) [31,32,33,34,35] have turned towards bioenergy in a crucial part of different countries’ strategy to reduce the dependence on fossil fuels and their products. Additionally, the low prices of forest products have favored the production of a wide range of high-value bioproducts such as bioenergy, biochemicals, biomaterials, and other marketable products [36] that can substitute similar products from fossil fuels which can help diminish GHG emissions and the dependency on energy imports, bring about efficient waste management, and contribute to bioeconomy development, while reducing the risk of fires [37,38,39,40,41]. On the other hand, biofuels emerge as the most economically viable alternative for replacing fossil fuels [42], especially in the shipping and aviation sectors [39,43,44], as well as a flexible option to electrify areas of the heat sector [31,39]. Biomass-based systems enhance energy accessibility in rural regions, which provides new opportunities for socio-economic development [45].
2.1. Biomass Types
Biomass, often referred to as organic waste, can be categorized into different groups: forest-woody residues, agri-food residues, animal residues, industrial biomass, and municipal solid residues. The management of biomass possesses a significant challenge in numerous countries. Inadequate collection and disposal practices contribute to social, economic, and environmental issues. Therefore, a suitable utilization of biomass is crucial. Since biomass comes from a diverse range of sources, their valorization strategy should be based on the composition, chemical characteristics, source, and quantity of these residues [46,47].
Within this framework, lignocellulosic biomass emerges as a leading resource, being the most common and renewable biomass on Earth [48]. Within this framework, lignocellulosic biomass, whose worldwide yield is around 200 billion tons, of which 8.2 billion tons/year are being used to fulfill society’s needs (heat, energy, food, etc.) [49], emerges as a leading resource, being the most common and renewable biomass on Earth [48]. In 2017, approximately 1 billion tons of biomass were obtained in the EU; almost 95% of this biomass supply was lignocellulosic biomass (forestry, grazed biomass, agricultural crops, and their collected wastes) [50]. Moreover, lignocellulosic biomass is attracting increasing attention as a renewable feedstock for various applications (in the energy, food, and chemical industry). It can be converted into bioenergy through thermochemical and biochemical processes and is a key resource for biofuel production in transport. Additionally, it has the potential to replace petroleum-based plastics and petrochemicals, with applications in additive manufacturing, environmental remediation, and medical fields [51]. Softwood, hardwood, and grasses are the main kinds of lignocellulosic biomass. Although all these types of lignocellulosic biomass contain the same materials (cellulose, hemicellulose, and lignin) [52], as well as a variety of minor components (lipids, water, simple sugars, ash, proteins, starches, hydrocarbons, and extractives), depending on the plant species, growth stage, storage circumstances, cultivation conditions, soil minerals, fertilizers, biological type, or origin, the quantities may vary [15,25]. Subtle variations in the composition of softwood, hardwood, and grasses possess noticeable effects on the properties of their resulting high-value products when they are pyrolyzed. Cellulose comprises both high-ordered (crystalline) and low-ordered (amorphous) regions [53], with the ratio between these regions dependent on various factors, as previously explained. It undergoes decomposition within the range of 240–350 °C, yielding levoglucosan and anhydrocellulose. Hemicellulose, a shorter and amorphous polymer composed of various sugars, decomposes at 200–260 °C, resulting in a higher production of volatiles, less tar, and less char than cellulose. Lignin, an aromatic polymer, decomposes at 280–500 °C, yielding phenols. Lignin decomposition produces more char in comparison with cellulose [54].
Lignocellulosic biomass originates from a variety of sources such as paper, wood, and pulp industries, organic solid waste from recycling stations, or agricultural and forest residues [51].
2.2. Forest Biomass
Forests encompass approximately 30% of the Earth’s land area, offering economic, social, ecological, and health benefits. They provide resources such as wood, energy, food, and medicine, while also acting as central pillars within their communities. Moreover, forests make possible climate change mitigation and adaptation, erosion prevention, air and water purification, and biodiversity conservation. Globally, around 1.6 billion people need forests for their sustenance. Thus, the sustainable management of forests is imperative. In view of the forest role, they must be considered comprehensively and integratively. They have to be protected, restored, and used in a sustainable way, promoting their governance and enhancing forest-based value chains, encouraging a sustainable trade of forest products which contribute to fulfillment of international commitments [55].
Forest residues and secondary forest products include portions obtained during wood processing activities, industrial manufacturing processes, and logging or sawmilling processes (sawdust, chips, planer shavings, black liquor, tops, trunk, bark, branches, leaves, roots, low-value trees, plywood, and particleboard) [18,40,56].
Forest residue use would provide environmental, economic, and health benefits in many countries because they are generated in huge quantities worldwide [18,34,39,41,56,57,58]. Wood, wood residues, and forest residues collectively contribute to 73% of biomass sources utilized in bioenergy, with wood representing 67% of the biomass, wood residues 5%, and forest residues 1% [45]; consequently, the forestry sector holds the predominant stake in biomass energy [34]. The forest bioeconomy from wastes allows the conversion of forestry residues, which would otherwise be disposed of [45,59], into higher value bioproducts. The transformation of forest residues into a circular economy is an appealing aim towards reaching long-term sustainability [45].
2.3. Conversion Technologies
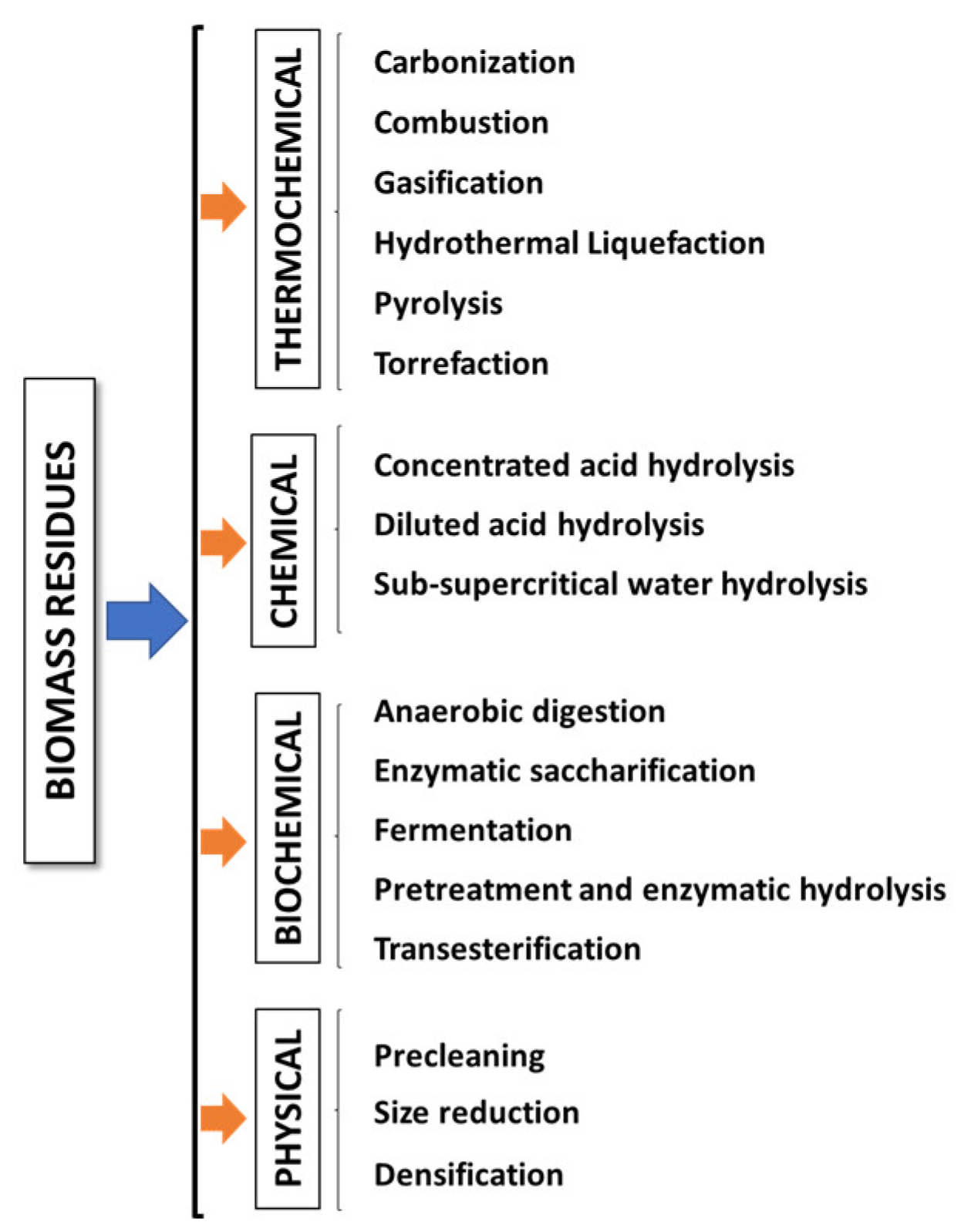
The biomass valorization to produce different fuels for energy production and other commodities is crucial for mitigating the disposal of biowastes into the environment and replacing petroleum-derived chemicals within the framework of the circular economy [47]. There are different conversion technologies to convert biomass into useful secondary energy carriers and high-value-added bioproducts (chemicals, biopolymers, enzymes, etc.) with a low carbon footprint [41]. These technologies can be divided into different categories (Figure 1) [15,22,45,60,61,62,63]. In thermochemical conversions, either external or internal energy triggers the biomass transformation effectively into fuels and chemicals in short periods in comparison with biochemical technologies [64]. During carbonization, which is similar to a slow pyrolysis, biomass is heated usually in an oxygen-limited rather than an oxygen-free environment to produce a highly carbonaceous material. The controlled presence of oxygen facilitates the partial combustion of the biomass, hence providing the necessary heat for the pyrolysis reactions. Moreover, some carbonization procedures operate at elevated pressures, reaching up to 1 MPa [65]. Combustion is a complete oxidation process in which biomass undergoes decomposition in the presence of oxygen to obtain heat that can be used for industrial and domestic heating and power generation. During this process, the carbon and hydrogen constituents of the biomass are converted into carbon dioxide (CO2) and water vapor (H2O), while inorganic ash remains as a solid byproduct [64,66]. Gasification involves the oxidation of biomass at high temperatures (800–1600 °C) using a gasifying agent such as air, steam, limited oxygen, or carbon dioxide. This process generates syngas, primarily composed of hydrogen (H2) and carbon monoxide (CO), along with small proportions of carbon dioxide and methane. Additionally, gasification produces a liquid fraction containing oil and tar, as well as a solid fraction known as biochar. Gasification can be classified as either direct or indirect, depending on the utilization of a limited amount or no oxidant at all, respectively [15,22,60]. Hydrothermal liquefaction, also known as hydrous pyrolysis, is a thermochemical depolymerization process conducted within a sealed reactor in the presence of a suitable solvent (commonly water). Wet biomass converts into biocrude oil and facilitates the extraction of water-soluble organics from the biomass at moderate temperatures (200–400 °C) and high pressures (5–25 MPa) [64,67]. Pyrolysis, executed in the absence of oxygen within the temperature range of 250–600 °C, yields three distinct products: solid coal (biochar), condensable heavy-molecular-weight compounds (bio-oil), and non-condensable light-molecular-weight gaseous products containing syngas based on operational parameters and biomass characteristics [15,60,64]. Biomass torrefaction is commonly conducted in a non-oxidative environment at mild temperatures (200–300 °C) and moderate residence times (30–60 min), resulting in torrefied biomass (which typically exhibits an enhanced energy value) as the primary product [22].
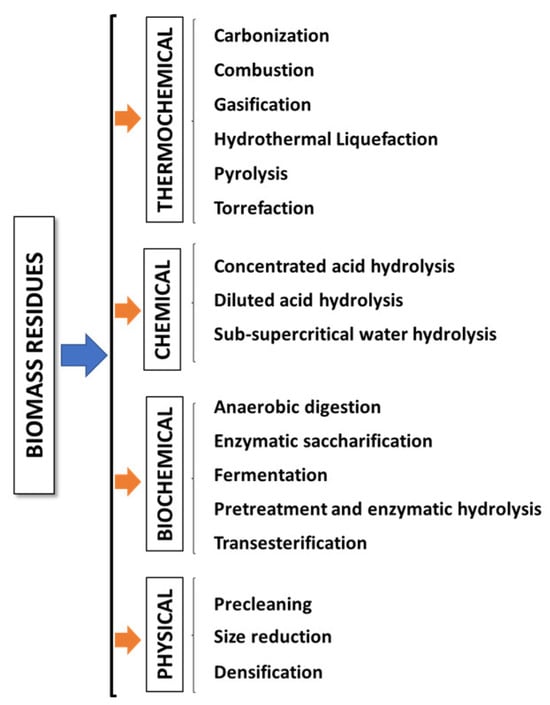
Figure 1.
Biomass conversion technologies.
Biochemical conversions entail the use of microorganisms, enzymes, and bacteria to decompose biomass into gaseous (biogas) or liquid fuels (bioethanol). Fermentation, an anaerobic process, commences with sucrose undergoing hydrolysis by enzymes, leading to its conversion into fructose and glucose. Subsequently, fermentation, distillation, and dehydration are carried out to yield bioethanol. The remaining solid residue from the fermentation process can serve as cattle-feed [41,68]. Anaerobic digestion is a series of biological processes in which biomass undergoes degradation by microorganisms in the absence of oxygen to produce biogas, containing methane and carbon dioxide, which can be used to generate both electricity and heat [41]. Enzymatic saccharification involves converting liberated polymeric sugars into soluble monosugars. Bridging the gap between these stages requires comprehensive pretreatment processes (physical, chemical, or biological) to disrupt the lignin matrix, facilitating improved enzyme interaction with the bound polymeric sugars [69]. Enzymatic hydrolysis stands as the pivotal technology in a typical biochemical conversion process. Preceded by pretreatment and succeeded by microbial or inorganic catalyst conversion, enzymatic hydrolysis is the key process that releases monomeric sugars from the structural carbohydrates, cellulose, and hemicellulose in lignocellulosic biomass [70]. Transesterification is the process in which triacylglycerides are transformed into fatty acid methyl esters (biodiesel) with a catalyst in the presence of alcohol, typically methanol or ethanol [71]. In chemical conversion technologies, the biomass structure is altered using a suitable reactant to obtain valuable products [15,64]. Concentrated acid hydrolysis involves the hydrolysis of hemicelluloses and celluloses within lignocellulosic biomass using strong mineral acids like sulfuric, hydrochloric, nitric, or phosphoric acids in aqueous solutions at moderate temperatures. Diluted acid hydrolysis is conducted at elevated temperatures with a low concentration of acid [72]. Sub- and supercritical water hydrolysis emerge as clean and fast hydrolysis methods that uses water, either supercritical or subcritical, as the reaction medium to transform biomass into fermentable sugars. This technique does not produce solid residues [73]. Physical treatments focus on cleaning and reducing the size to increase the porosity of the biomass which serves as a preliminary step toward improving the efficiency of subsequent conversion processes. Densification is the typical mechanical processing method which involves compressing biomass using compaction forces with or without the use of binders to enhance the properties of solid fuels [45].
Thermochemical and biochemical conversion technologies are the two main approaches for biomass valorization into useful products such as bioenergy, biofuels, and bioproducts. Thermochemical processes can handle a wider variety of biomass, while biochemical methods offer a higher product selectivity and flexibility under mild conditions, making them suitable for producing diverse biofuels and biogas. Biochemical methods are frequently used in agricultural waste management, transportation fuel production, and bio-based chemical manufacturing [74]. Nevertheless, thermochemical processes are appropriated for large-scale energy production, converting biomass into heat, electricity, and biofuels, being commonly applied in industrial and power generation.
In most biochemical processes, the raw material must undergo various pretreatment stages to produce suitable intermediate products for microorganisms. As a result, different fractions of the feedstock are often discarded as waste or unutilized, leading to increased losses in the process and reducing the overall productivity per kilogram of raw material [75]. Thermochemical processes typically have a much faster response time compared to biochemical processes. Moreover, they are more efficient at breaking down organic compounds and generally involve lower purification and catalyst costs than biochemical methods, which supports their consideration as commercially viable. Although thermochemical processes offer faster conversion, they require complex operational parameters and cope with issues such as water content and energy costs. Conversely, biochemical processes are slower but more sustainable, producing fewer emissions and volatile compounds. However, they often deal with challenges like high costs in hydrogen production and algae growth. The integration of both methods could overcome individual limitations, although optimizing parameters (temperature and pressure) and reducing costs remain critical challenges for large-scale biomass valorization [76].
Pyrolysis is a thermal depolymerization of any organic material (carbon-based) in the absence of oxygen, which can be carried out on both pure and a mixture of materials (co-pyrolysis) [77]. In this thermochemical process, a multitude of reactions occur simultaneously and sequentially. It is widely recognized that biomass pyrolysis comprises three primary stages: (i) the evaporation of water or moisture from biomass, and (ii) the primary decomposition of lignocellulosic components through complex mechanisms, followed by (iii) secondary reactions involving cracking and recondensation/repolymerization to produce a stable solid [22]. The decomposition of biomass primarily occurs during the primary decomposition phase, leading to solid char formation (200–400 °C). Subsequently, secondary reactions continue within the solid matrix as the temperature increases. Hemicellulose decomposition, mainly represented by xylan, occurs between 250 and 350 °C, followed by cellulose decomposition (325–400 °C), yielding levoglucosan as the main pyrolysis product. Lignin, being the most stable component, undergoes decomposition at 300–550 °C [24]. Pyrolysis often yields products of distinct and frequently superior quality compared to the original residue. These products generally include solid residues like charcoal or bio-char, a condensable heavy-molecular-weight compound referred to as bio-oil, and a non-condensable, light-molecular-weight gaseous product containing gases such as hydrogen (H2), carbon monoxide (CO), and carbon dioxide (CO2) [60]. Pyrolysis conditions can be tailored according to the desired product (biochar, bio-oil, or non-condensable fraction). It has been found that high temperatures and short residence times tend to promote the formation of condensable products, whereas high temperatures and longer residence times favor the production of non-condensable gaseous products, largely due to the occurrence of secondary reactions. Solid products, on the other hand, are typically enhanced at lower temperatures. Thus, by adjusting parameters such as temperature and residence time, the composition of pyrolysis products can be modified towards desirable products [23]. For all these reasons, pyrolysis offers a unique opportunity to transform low-energy-density compounds into high-energy-density fuels, thereby enabling the efficient utilization of resources. Additionally, pyrolysis facilitates the recovery of valuable products from a wide range of waste, a task that is often challenging. This ability to extract value from waste materials underscores the versatility and potential of pyrolysis as a sustainable solution for resource recovery and energy production [78].
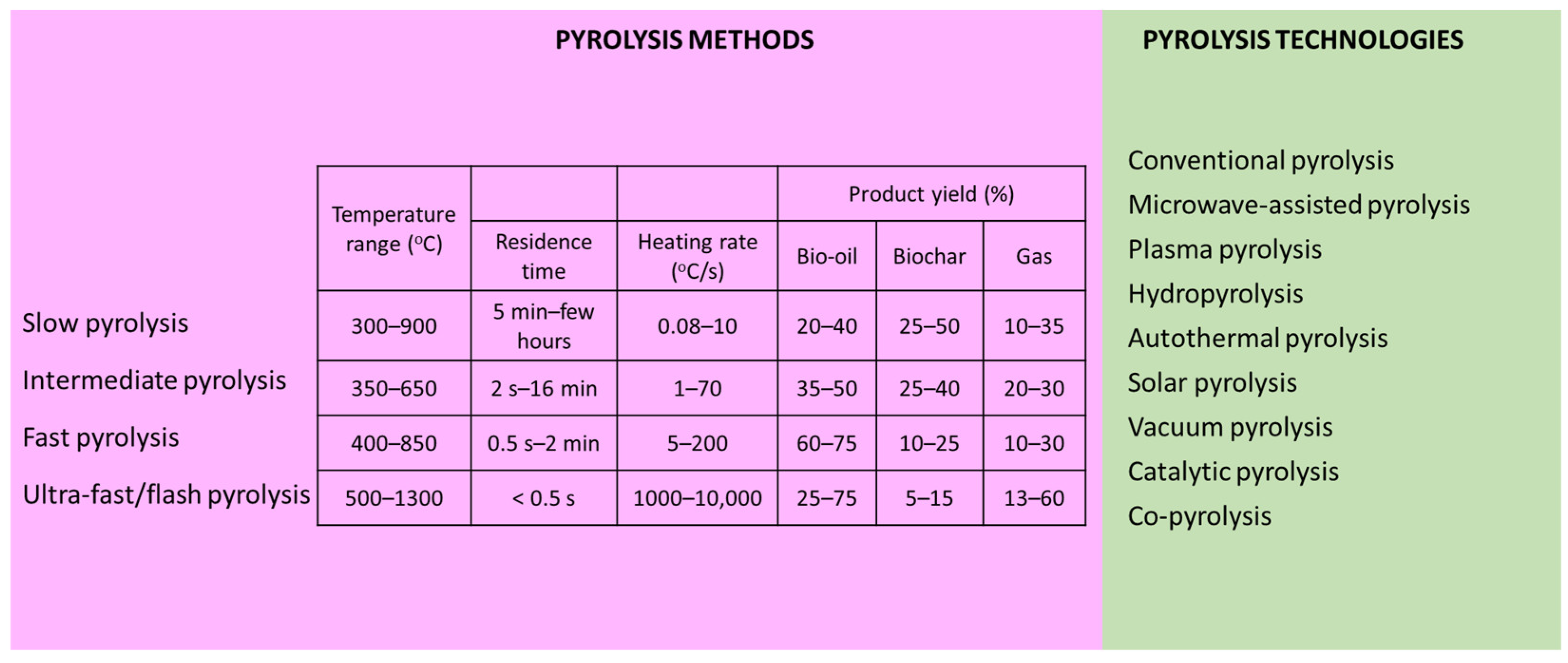
There are different technologies of biomass pyrolysis, and distinct methods, based on the process conditions for the desired product, can be implemented (Figure 2).
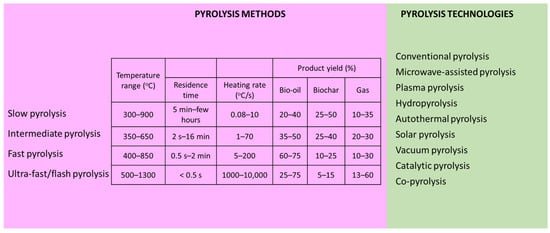
Figure 2.
Pyrolysis methods and technologies [22,60,78,79,80,81].
Pyrolysis methods can be categorized as slow, intermediate, fast, and flash pyrolysis. Slow pyrolysis yields biochar as its principal product, facilitated by moderate temperatures, low heating rates, and prolonged residence time. The carbonization of feedstock occurs during slow pyrolysis at a temperature of between 300 and 900 °C and a heating rate of 0.08–10 °C/s. Fast pyrolysis operates at temperatures of 400–850 °C, aiming for the comprehensive transfer of thermal energy to the carbonaceous feedstock. The rapid removal of pyrolytic vapors characterizes this method due to its high heating rate (5–200 °C/s) within the initial few seconds. The predominant product of fast pyrolysis is oil, comprising up to 75% of the output, typically of superior quality and quantity compared to slow pyrolysis. Flash pyrolysis, executed at temperatures of 400–850 °C, features an exceptionally high heating rate exceeding 1000 °C/s, making it suitable for the production of liquid products while minimizing gas and biochar generation. Intermediate pyrolysis operates within a temperature range of 350–650 °C, with a heating rate of 1–70 °C/s. The residence time of pyrolytic vapor in this method spans from 2 s to 16 min, resulting in product yields similar to slow pyrolysis [22,60,78,79,80,81].
Distinct pyrolysis technologies exist, including conventional pyrolysis, microwave-assisted pyrolysis, plasma pyrolysis, hydropyrolysis, autothermal pyrolysis, solar pyrolysis, vacuum pyrolysis, catalytic pyrolysis, and co-pyrolysis. In conventional pyrolysis, heat is transferred from the surface to the core of the material. This heat transfer process occurs through conduction and convection, which are relatively slow and energy-inefficient methods. Due to the non-selective heating nature of conventional pyrolysis, it often requires longer periods and more energy to complete. Additionally, achieving the desired pyrolysis temperature necessitates a higher temperature gradient within the sample. Common feedstocks used in conventional pyrolysis plants include agricultural and forestry residues, scrap tires, and waste plastics. Through pyrolysis, these waste materials undergo thermal decomposition to produce non-condensable gases, bio-oil, and biochar [82]. The challenge with conventional pyrolysis lies in its endothermic nature, requiring a high heat flux from an external source. Autothermal pyrolysis addresses this by utilizing part of the heat from feedstock or pyrolysis products to meet the heat demand, allowing for process scale-up. By introducing limited oxygen into the reaction zone, chemical reactions, rather than the available heat, control the process. This method alters pyrolysis products, with vapor products often combusting to meet heat requirements. Autothermal pyrolysis, using air as the fluidizing gas, achieves a several-fold process intensification by providing energy through the partial oxidation of pyrolysis products, simplifying the reactor design and reducing capital costs [80]. A distinct pyrolysis approach is represented by microwave pyrolysis in which microwaves serve as the primary heat source, enabling uniform heating throughout the material. By inducing molecular rotation, microwaves efficiently heat polar compounds like water molecules, reducing the activation energy required for thermal decomposition. This method offers advantages over conventional pyrolysis, facilitating the quick and consistent heating of irregular waste fragments from within, without the need for agitation or controllability. Microwave heating is energy-effective and allows for rapid startup and shutdown, making it increasingly favored for waste decomposition. However, its applicability depends on the microwave absorption capacity of the materials, with some requiring additives known as susceptors (Al, Fe, CaCO3, SiC, MgO, Fe3O4, KOH, CaO, Na2CO3, etc.) to enhance absorption [78,82]. Plasma pyrolysis, an emerging technique, offers a unique approach to pollutant degradation by generating ionized materials. This method has garnered significant attention for its potential in reducing pollution from waste decomposition and minimizing the emission of hazardous and toxic substances. Plasma technology is broadly categorized into two main groups, low-temperature (gas discharge plasma) and high-temperature (fusion plasma), with the latter being predominantly employed in waste treatment applications. Despite its numerous advantages, including the reduced emissions of hazardous compounds, rapid heating rates, and robust installation, plasma pyrolysis faces certain limitations such as high-power consumption, limited economic efficiency, and reduced endurance, which hinder its widespread adoption in the industry [78]. Hydropyrolysis, a distinct method from conventional pyrolysis, employs pressurized hydrogen (>10 MPa) to convert biomass into pyrolytic oil of higher quality. It inhibits char formation, resulting in bio-oil with a low oxygen content and infused hydrogen. The hydrocarbons produced exhibit enhanced stability, free from unwanted olefins and polynuclear aromatics. The presence of hydrogen facilitates effective bond cleavage within biomass components, enabling the production of fungible hydrocarbons. Unlike conventional pyrolysis, hydropyrolysis does not require a separation into organic and aqueous phases, allowing for the generation of a variety of specialty chemicals. Recently, the focus has shifted to fast hydropyrolysis, performed at high heating rates in a hydrogen atmosphere, yielding two liquid phases: an organic phase containing hydrocarbons, and an aqueous phase with char and permanent gases. The process, catalytic or non-catalytic, can be enhanced with a hydrotreating unit for deoxygenation, resulting in upgraded volatiles [23,81,83]. Solar pyrolysis utilizes concentrated solar energy (high-powered dish receivers or concentrators that enables the rapid attainment of the initial pyrolysis temperature) as the heat source, resulting in shorter heating times compared to fossil fuel heating methods. Moreover, the heating rate can be controlled effectively. Solar pyrolysis exhibits high reactivity due to the presence of functional sites, which reduces the residence time and shortens condensation reactions. Consequently, the biochar contains higher levels of oxygen and hydrogen and a lower carbon content. This method is conducted over a wide range of temperatures (150–2000 °C), heating rates (5–450 °C/s), and heat flux intensities (0.01–12 MW/m2). The primary products of the solar pyrolysis of biomass include bio-oil (25–78 wt.%) and syngas (1.4–63 wt.%), with biochar (8–29 wt.%) being a minor product. Vacuum pyrolysis is conducted under reduced below atmospheric pressure to replicate an inert environment, eliminating the need for sparging inert gases like nitrogen or argon. Typically, vacuum pyrolysis operates at pressures of 0.5–50 kPa and moderate temperatures (400–600 °C). Prior to pyrolysis, a vacuum pump evacuates air from the reactor. During pyrolysis, the released volatiles rapidly diffuse towards the pump due to the pressure gradient between the reactor and the pump. Compared to conventional pyrolysis, vacuum pyrolysis consumes less energy as there is no need to heat inert gas. Vacuum pyrolysis converts biomass waste into bio-oil and biochar with a considerably high heating value (22.4–40 MJ/kg). The bio-oil obtained from the vacuum pyrolysis of biomass primarily consists of polycyclic macromolecular compounds [22]. Catalytic pyrolysis involves the use of catalysts to facilitate the process. These catalysts improve product quality, reduce process temperatures, and minimize energy requirements. By increasing the speed of cracking reactions, catalysts promote the production of lighter compounds and enhance gas generation. Catalytic pyrolysis may lead to a reduction in bio-oil production; nevertheless, its quality is typically higher [26]. The effectiveness of each catalyst depends on its specific catalytic properties (acidity, surface area, pore volume, and pore size). A diverse range of catalysts has been employed including metal oxides (CaO, NiO, Ni2O3, MgO, γ-Al2O3, Fe2O3, etc.), zeolites (ZSM-5, HZSM-5, EDTA-HZSM-5, FeZSM-5, zeolite-β, natural zeolite, etc.), carbon materials (activated carbon), transition metals, and other catalysts like red mud, CO–Mo/Z, Ca(OH)2, and Al(OH)3 [78,84]. Co-pyrolysis is a variation of the pyrolysis process which involves organic compounds introduced to enhance the products quality [85]. Waste pyrolysis often produces components with low hydrogen-to-carbon ratios, making them unsuitable as fuel. Adding substances with higher hydrogen-to-carbon ratios, like biomass, which is rich in hydrogen, improves the hydrogen content of products [60]. This leads to a lower activation energy, better yield, and higher product quality, and reduces environmental pollutants’ emissions during pyrolysis [78,86].
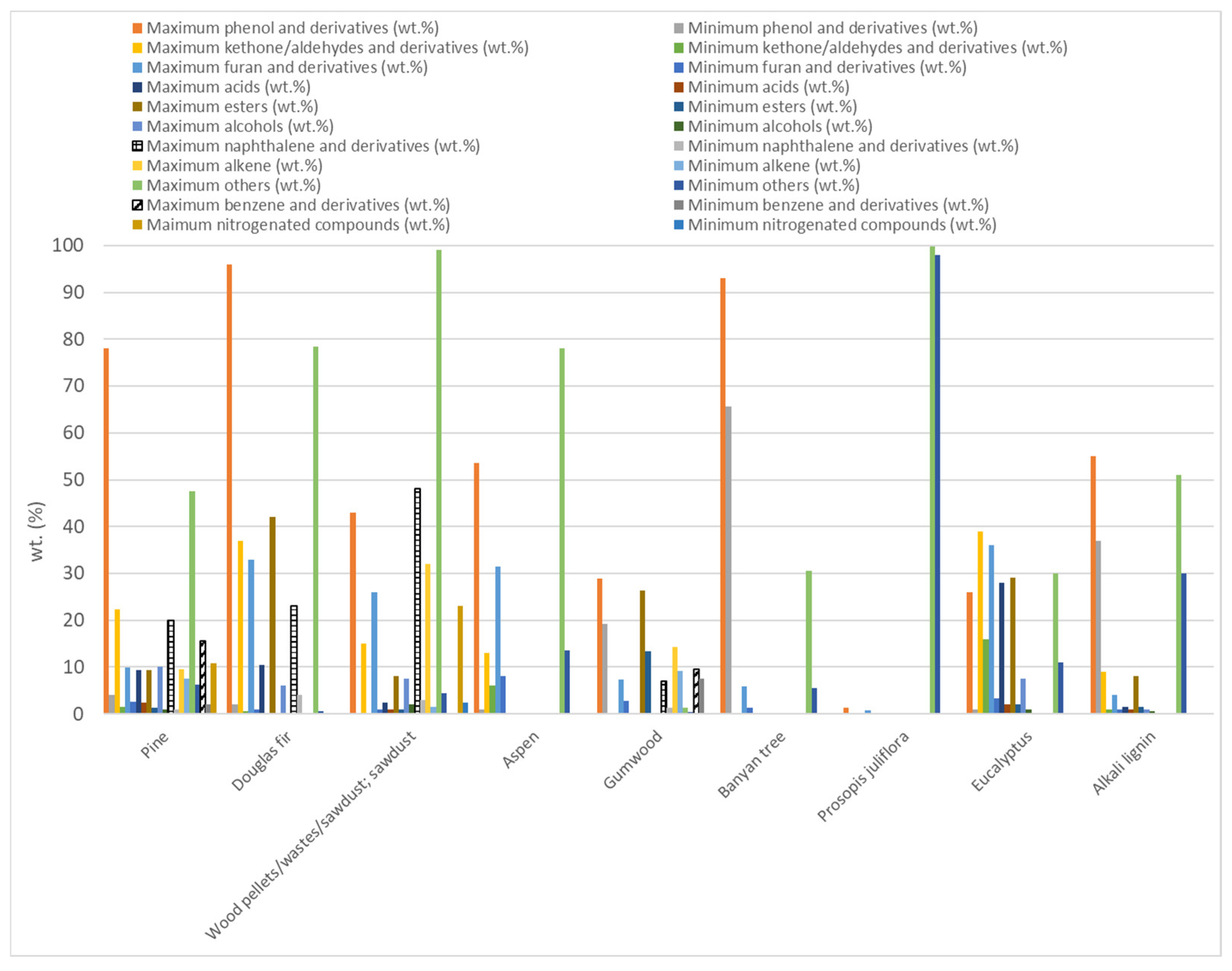
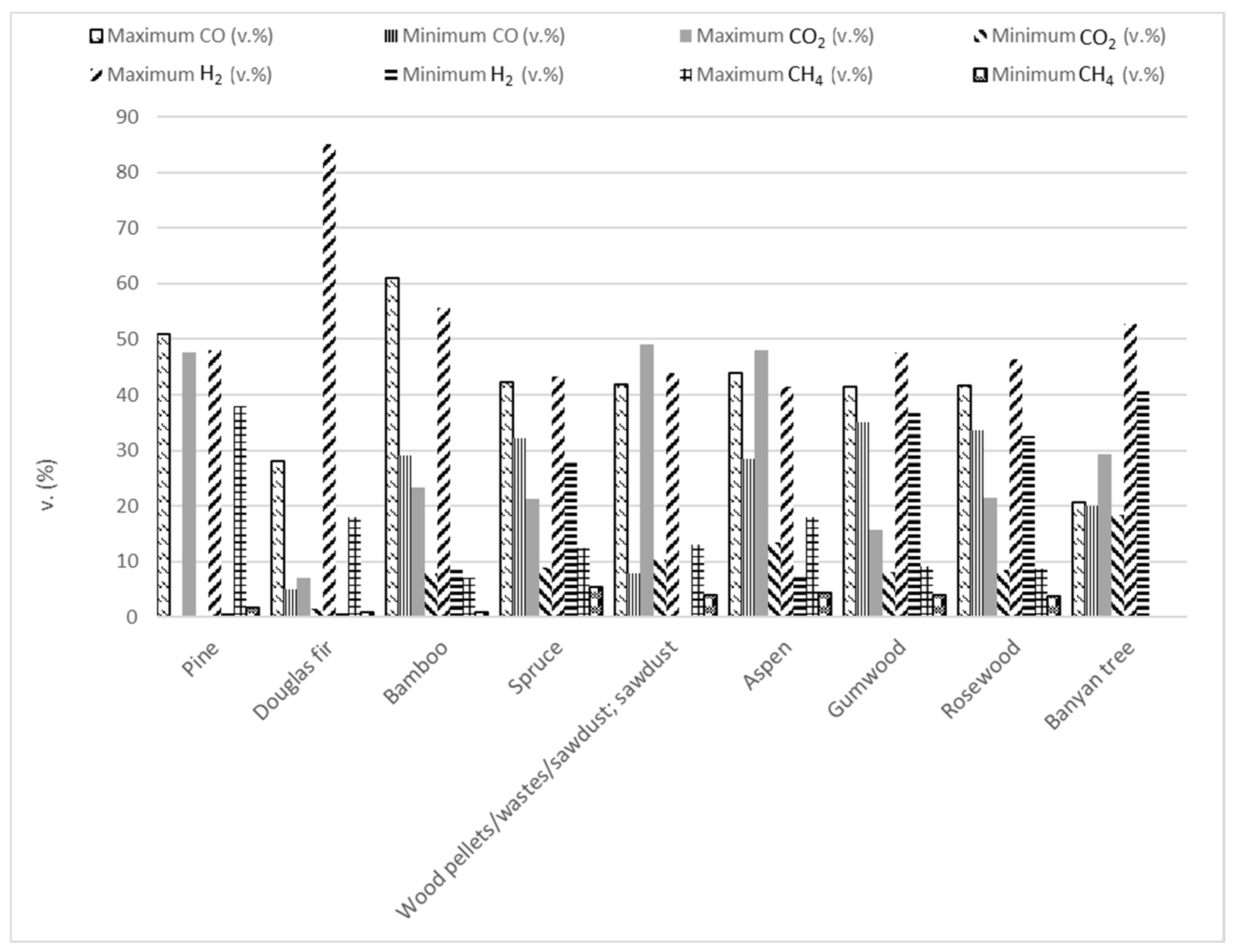
Biomass pyrolysis is a complex process in which three distinct fractions can be yielded: solid, liquid, and gas, as explained above. The solid fraction is a carbonaceous residue with a fine structure and a large specific surface area (biochar) [84] generated during primary and secondary pyrolysis reactions. However, the resulting biochar often possesses low quality due to the presence of ash and other impurities within its micropores [87]. Enhancing the structure of biochar to refine it makes possible that this can be used in several applications such as solid fuels to produce electricity and heat, raw materials for gasification, activated carbon or carbon nanofilaments fabrication, the removal of contaminants (heavy metals, dyes, pharmaceuticals, detergents, polyaromatic hydrocarbons, herbicides, and pesticides), catalysis, energy storage, gas adsorption, soil conditioner and enrichment, potential electrode material in biosensors, and food processing [8,22,23,30,61,62,88,89,90]. Biochar’s composition includes ash with minerals like Al, Ca, Cl, K, Mg, P, and Si, while its formation involves the decomposition of tar into methane, hydrogen, water, and other hydrocarbons [1]. A low temperature, prolonged residence time, and slow heating rate enhance the biochar generation. These operational conditions facilitate the breakdown of weaker bonds while preserving stronger ones. Consequently, the rearrangement reaction is encouraged, leading to the creation of a solid biochar with structural stability. This process inhibits the formation of volatile compounds and enables the retention of a significant amount of energy within the biochar [88]. Char properties (physical, chemical, and mechanical characteristics) are influenced by both the type of feedstock used and the conditions under which pyrolysis occurs [24]. Bio-oil, derived from biomass pyrolysis, because of the inherent moisture in the raw material and the water generated from secondary reactions during bio-oil storage, results in two separate phases: an aqueous phase and an organic phase. This liquid fraction also contains remnants of cellulose, hemicellulose, and lignin, which either vaporized in the pyrolysis environment or were carried as small droplets entrained from the reactor [23]. Bio-oil is a dark brown fluid, in which the moisture content is typically 15–35 wt.% [24], alongside a really complex mixture of more than 230 organic chemicals [91] characterized by highly oxygenated organic compounds including C1–C4 light oxygenates, furans, pyrans, anhydrosugars, phenols, benzenes, PAHs, aldehydes, ketones, organic acids, esters, ethers, alcohols, alkenes, nitrogen compounds, miscellaneous oxygenates, aliphatic hydrocarbons, and solid particles [1,5,52,92]. This fluid finds extensive application as a transportation fuel, for power generation, and in various industries such as the production of bio-plastics, phenolic resins, polyurethane materials [93,94], food (flavoring), pharmaceutical, paint industries, wood preservation [84], and flavoring [23]. The complex composition of bio-oil, as well as its physical properties, including low pH, high oxygen content, and low viscosity, contribute to their instability, creating challenges for direct utilization and necessitating upgrading before commercial use [95]. Achieving a high oil yield is insufficient; understanding and optimizing the bio-oil composition are crucial [12]. Efforts in the catalytic upgrading of bio-oils aim to enhance their quality. Upgrading bio-oils to reduce the oxygen content and enhance the similarity to crude oil is crucial for broader industrial adoption, highlighting their potential as a renewable and versatile resource. Finally, the non-condensable gases’ fraction in pyrolysis primarily originates from the cracking and decomposition of large molecules formed during the early stages of pyrolysis. It includes carbon dioxide, carbon monoxide, hydrogen, low-carbon hydrocarbons, nitrogen oxides, sulfur oxides, and other compounds [66]. The pyrolytic gases’ composition of lignocellulosic biomass depends on feedstock components (cellulose, hemicellulose, and lignin, with approximate weight percentages of 35–50%, 25–30%, and 15–30%, respectively). Hemicellulose, due to its higher carboxyl content, yields more CO2 during pyrolysis. Cellulose, on the other hand, yields higher amounts of CO, primarily due to the thermal cracking of carbonyl and carboxyl groups. Lignin, with its higher presence of aromatic rings and methoxyl functional groups, releases more H2 and CH4 during pyrolysis. Additionally, non-condensable gases can be recycled into the reactor to aid fluidization or utilized for process heat in large-scale operations (autothermal pyrolysis) [23,96].
3. Microwave-Assisted Pyrolysis (MAP)
Microwave-assisted pyrolysis utilizes microwaves to heat biomass wastes, offering rapid, targeted, and energy-efficient heating compared to conventional methods like furnace heating, which has drawn significant interest, supported by a series of reviews outlined in Zhang et al.’s study [97]. In conventional pyrolysis, heat is generated externally and transferred to the material through convection, conduction, and radiation, limiting efficiency due to surface temperature constraints and material properties. Heat moves from the surface to the core, creating an inward-flow temperature gradient [88]. In contrast, microwave pyrolysis combines microwave radiation with conventional pyrolysis to convert electromagnetic energy into kinetic energy. Unlike conventional pyrolysis, microwave pyrolysis generates heat from the interior outward, ensuring a uniform heat distribution within the sample particles. Additionally, microwave pyrolysis leads to the formation of micro-plasma, containing hot spots with significantly higher temperatures than the average, which stimulates catalytic reactions and increases hydrogen production. Moreover, microwave power induces non-thermal effects that reduce the activation energy of reactions, promoting chemical cracking processes and enhancing operational efficiency compared to conventional pyrolysis [98]. Because microwave pyrolysis’ ability to reach high temperatures and heating rates, it is considered a fast, energy-efficient, and time-saving process compared to conventional methods [28], reducing production costs and increasing the product’s yield [22,99] and quality [77].
Microwaves (MWs) are non-ionizing electromagnetic waves with frequencies between 300 MHz to 300 GHz and wavelengths ranging from 0.001 to 1 m. The potential of microwave (MW) heating is constrained by the inability to adjust the frequency over a broad range in standard microwave generators, such as magnetrons. The generation frequency of magnetrons is strictly determined by the strength of their magnetic field and their geometric dimensions [100]. Additionally, the Federal Communications Commission (FCC) has reserved the frequencies of 915 MHz and 2450 MHz for scientific, industrial, and medical aims, preventing interference with cellular phone and telecommunications frequencies [61]. Nevertheless, most researchers rely on commercial 2.45 GHz magnetrons [100]. Microwave heating occurs through the dissipation of electromagnetic waves within the irradiated material. The amount of power dissipated depends on the dielectric properties of the material and the local time-averaged strength of the electric field. Although microwaves generate volumetric heating, the energy distribution within the irradiated material is uneven due to the non-uniform electric field distribution. This unevenness depends on the geometry of the object and its dielectric properties [101]. MW-assisted heating involves three main mechanisms: dipolar reorientation, ionic conduction, and interfacial polarization. Dipolar molecules like water and some organic compounds in biomass attempt to realign with the rapidly alternating electrical field generated by microwaves. As the applied field oscillates, the dipolar molecules continuously rotate to follow these oscillations, causing the conversion of electromagnetic energy into heat within the material through dielectric loss and molecular friction [102]. In ionic conduction, ions contained in a material (such as salts or minerals in biomass) move back and forth due to the changing electric field, leading to an electric current which encounters internal resistance due to collisions between the charged species and neighboring molecules or atoms. This ionic conduction has a significantly higher impact on heat production compared to dipolar polarization [103]. Interfacial polarization, combining dipolar reorientation and ionic conduction, creates positive and negative charges at material interfaces, altering the field distribution and facilitating heating [60]. Different materials interact uniquely with MWs: insulators (glass, ceramics, and plastics) that transmit MWs, conductors or reflective materials (bulk metals) that reflect MWs, and dielectric or absorbers (carbonaceous materials, metal powders, SiC, and water) that absorb MWs to generate heat [28]. The critical electrical parameter governing the interaction between a material and electromagnetic field is its complex permittivity. This permittivity consists of two components: the dielectric constant, which reflects the material’s ability to store electric field energy, and the relative loss factor, which indicates how much energy is dissipated as heat. Power converted from microwave energy to heat depends on the relative loss factor, the frequency, and the local electric field intensity. As microwaves travel through a material, their intensity decreases due to energy dissipation as heat [102]. This decay is characterized by the penetration depth (dp) which is a crucial property used to categorize materials exposed to MWs. It refers to the depth at which the incident radiation inside the material decreases to 37% of its surface value. The penetration depth magnitude (dp) indicates whether the material reflects (dp ≈ 1–7 μm), transmits (dp ≈ 3–300 m), or absorbs (dp ≈ 0.1–10 cm) microwaves [64]. Furthermore, the penetration depth depends on both the loss factor and the dielectric constant. Different materials interact uniquely with MWs: insulators (glass, ceramics, and plastics) that transmit MWs, conductors or reflective materials (bulk metals) that reflect MWs, and dielectric or absorbers (carbonaceous materials, metal powders, SiC, and water) that absorb MWs to generate heat [28]. The dielectric properties of a material, including dielectric loss (tan δ) and dielectric constant, are crucial in determining its ability to convert electromagnetic energy into heat (insulators (tan δ < 0.1), reflectors (tan δ 0.1–0.5), and absorbers (tan δ > 0.5)) [2]. The complexity of microwave heating arises from the fact that these dielectric properties change with temperature and moisture [30] and these variables change significantly during MAP. In contrast to conventional heating, MW heating operates in a volumetric mode; heating uniformly warms entire volumes of solids, suspensions, or liquids on an industrial scale, ensuring a consistent distribution of electromagnetic energy [62]. Consequently, MW radiation offers notable advantages, including the following: (a) rapid heat transfer and shorter reaction times, (b) selective and uniformly distributed volumetric heating, (c) straightforward operation and energy efficiency, (d) reduced degradation or formation of side products, and (e) increased safety and automation levels [14,66].
Because of the distinctive heating mechanism of microwaves, various limitations and advantages have been reported in comparison with conventional pyrolysis (Table 1).

Table 1.
Microwave-assisted pyrolysis vs. conventional pyrolysis [22,28,30,97,104].
Table 1 shows that temperature measurement during MAP is relatively difficult. Contact methods like thermocouples are commonly used for temperature measurement; however, their use is limited by several factors. To avoid interference from the MW electric field, thermocouples must be positioned perpendicular to the field vector and shielded with a grounded covering. This shielding prevents current flow along the conductors and reduces the risk of discharges [105,106,107]. Nevertheless, this requirement makes thermocouples suitable primarily for single-mode systems where the electric field direction is predictable. The presence of conductive thermocouple elements distorts the electric field and, due to their high thermal conductivity, can affect the heating of the sample by dissipating heat. Thermocouples are best suited for measuring large sample temperatures, but accurate contact with the material being measured is required, which is challenging for low-density materials. Additionally, metal wires can interfere with the MW field, potentially causing sparks or discharges and affecting temperature measurements by reflecting MW radiation and altering the thermal field. These factors can result in measurement errors and unreliable data [100]. There are authors [108] who demonstrated that positioning thermocouples at the bottom of the cavity to avoid direct exposure to microwave (MW) irradiation significantly reduced interference. This setup provided more reliable and accurate temperature readings with minimal noise, as well as improved the biomass material’s absorbance due to a uniform electric field distribution. Their study emphasized the need to shield metallic thermocouples and avoid direct exposure to the MW electric field. Optical methods for measuring temperature rely on fiber-optic lines and various physical principles, including amplitude and phase-based techniques [109]. Common methods include optical pyrometry, light absorption, and interferometry. However, fiber-optic sensors suffer from a time lag of 8–10 s, which complicates real-time monitoring and control. In addition, these methods require calibration to ensure accuracy, as external factors like the surrounding atmosphere and the low thermal conductivity of quartz tubes can affect temperature readings. Calibration methods involve comparing optical sensor readings with conventional thermocouples or using reference materials to improve accuracy. Fiber-optic sensors are immune to electromagnetic interference but typically measure temperatures only up to 300 °C. Therefore, calibration compares fiber-optic measurements to infrared pyrometer data. Additionally, gases present during MW heating can affect the apparent emissivity of the sample, influencing infrared-based temperature readings. Overall, the proper calibration and consideration of environmental factors are essential for accurate temperature measurement in MW systems [100]. Other methods used to obtain the temperature during MAP include non-contact pyrometry which is a common method for measuring temperature by detecting thermal radiation from an object, typically in the near-infrared and visible ranges. Its advantage is that it does not require direct contact with the sample, avoiding interference with the electric field and temperature. Pyrometry is based on the thermal radiation of a blackbody, and real objects deviate from this due to their emissivity, which often must be measured experimentally. Two main types of pyrometry exist: radiation pyrometry, which measures infrared radiation intensity using devices like infrared thermometers, and optical pyrometry, which relies on the color of radiation, typically used in brightness and color pyrometry methods. Brightness pyrometry measures radiation intensity at a specific wavelength, while color pyrometry uses the ratio of intensities at two wavelengths to estimate the temperature [100]. Pyrometry measurements only provide surface temperatures, limiting their effectiveness to detect hot and cold spots within a material’s core. A single-spot measurement may be misleading if significant inhomogeneity is suspected, making pyrometer data less suitable for automated temperature-controlled microwave processes. Pyrometers are best for controlling homogeneous temperatures or at predefined critical points. Adding more pyrometers and combining their data through interpolation can offer a cost-effective alternative to thermal imaging. For systems with inhomogeneous heating like MAP, single-spot measurements provide limited information and require calibration based on factors like material and surface conditions. Despite these limitations, pyrometers allow fast, non-invasive temperature measurement, making them useful in automated microwave applications [110,111]. Spectral pyrometry, a more advanced technique, measures the full radiation spectrum over many wavelengths, improving accuracy even when the object’s emissivity is unknown. This method is beneficial in MW systems where optical fibers transmit radiation data to small spectrometers. However, the low signal level in spectral pyrometers, caused by the distribution of radiation across thousands of elements, results in longer exposure times or larger observation areas compared to brightness pyrometers. This makes it complicated to establish what temperature is measured when there are significant temperature variations or non-uniform distribution within the observation area, which is what happens during MAP [112]. It is observed that new approaches, which combine the best qualities of known methods or innovative solutions, are required in order to improve temperature measurement during MAP.
One of the advantages of microwave pyrolysis as gathered from Table 1 is this type of pyrolysis can lead to increased product yields or improved product quality. For instance, bio-oils produced via microwave pyrolysis contain light hydrocarbons, lack polycyclic aromatic hydrocarbons (PAHs), and are rich in phenols, unlike the hydrophilic and corrosive oxygen-rich liquid produced in traditional pyrolysis. The gas products of microwave pyrolysis mainly consist of hydrogen (H2) and carbon dioxide (CO2), serving as a foundation for chemical product synthesis such as ammonia, alcohol, and acetic acid [98]. Furthermore, biochar obtained from microwave pyrolysis exhibits higher fixed carbon content compared to that from conventional pyrolysis. Additionally, microwave-produced biochar typically possesses a greater surface area, enhancing its suitability for adsorbing contaminants, retaining water, and other environmental applications [8,15]. There are studies that have shown that incorporating microwave heating into the pyrolysis process has a more negative environmental impact compared to conventional pyrolysis [113], whereas other authors have obtained a reduction in energy consumption when MAP was implemented in comparison with conventional processes [114]. Increased yields or improved product quality obtained during MAP can deal with the reduced attractiveness of MAP compared to conventional pyrolysis. Co-pyrolysis, catalyst use, and the integration of renewable energy sources like solar energy in MAP have further shown decreased potential emissions [115,116]. Despite the higher capital costs of MAP compared to conventional pyrolysis, techno-economic analyses have suggested that MAP processes have promising economic potential, particularly in bio-oil production. Studies have reported reduced bio-oil production costs. As microwave technologies mature, the economic viability of MAP is expected to improve, especially with the potential for higher biochar prices due to the enhanced quality. On the other hand, it should be highlighted that most techno-economic analyses for MAP rely on data from process simulations and lab-scale experiments, leading to uncertainty, particularly regarding the cost of MAP reactor systems, as there are few commercial systems for benchmarking. Product yields and conversion efficiency may change as the process is scaled up. Additionally, electricity plays a critical role in MAP economics, making cheap electricity essential for its viability [117].
Most biomass resources have a low microwave absorption capacity; consequently, enhancing heating rates is required during the microwave pyrolysis of biomass, adding microwave absorbers [118]. Microwave absorbers, also referred to as susceptors or receptors, are materials with a high loss tangent value (>0.5), enabling the efficient conversion of radiant energy into heat [64]. Different compounds such as Fe3O4, CuO, V2O5, and carbon can undergo significant heating under microwave radiation, reaching temperatures exceeding 700 °C in less than a minute [54]. One approach to utilizing microwaves for biomass pyrolysis involves mixing the biomass with an efficient microwave receptor such as water, SiC, K3PO4, NaOH, KOH, zeolites, clays, fly ash, metal powder (Fe, Co, Ni, Cu, Al, steel slag), powdered metal oxides (MgO, NiO, CaO, CuO, Fe2O3, and Al2O3), SiO2, bauxite residue, metal salts (MgCl2, ZnCl2, FeCl3, CaCO3, Na2CO3, K2CO3, Na2HPO4, and NaH2PO3), or carbon-based materials (biochar, activated carbon, coke, and graphite) [52,53,84,104,119,120,121,122]. In addition to serving as an energy transfer medium, the presence of microwave absorbers in microwave-assisted pyrolysis can play, in some cases, a catalytic role, enhancing the selectivity towards desired products in the reaction [84,92,123]. The catalyst introduction can be carried out either in an in situ or ex situ configuration. In the in situ catalytic microwave pyrolysis, biomass and catalyst samples are mixed directly beforehand in the reactor before the experiment, enabling simultaneous biomass decomposition and vapor upgrading. In the ex situ setup, a catalytic reactor, independently controlled, is positioned downstream of the microwave pyrolysis reactor for pre-upgrading vapors [7,60]. Different catalysts have been investigated to be incorporated into microwave-assisted pyrolysis systems, giving rise to microwave-assisted catalytic pyrolysis [7]. These catalysts can be categorized into four groups: zeolites (clinoptilolite, Zeolite Socony Mobil-5 (ZSM-5) that can be synthesized with different Si/Al ratios, HZSM-5, EDTA-HZSM-5, SIO2-HZSM-5, and FeZSM-5), metal oxides/salts/alckaline (γ-Al2O3, CaO, MgO, CuO, Fe2O3, NiO, Ni2O3, ZnO, ZrO2, TiO2, MgCl2, AlCl3, CoCl2, ZnCl2, Na2HPO4, K2PO4, Fe2(SO4)3, NaOH, KOH, K2Cr2O7, and H3BO3), carbonaceous materials (activated carbon, graphite, and char), and clays (their interlamellar cations (Na+, K+, and Ca2+…) can be readily substituted by other cations or molecules) [64,84,118].
The microwave-assisted pyrolysis of biomass is a complex process influenced by different factors such as reactor design (type of reactor, type of microwave, and catalyst position), reaction material (feedstock type, susceptor, catalyst, feedstock composition, susceptor-to-feedstock ratio, catalyst-to-feedstock ratio, feedstock particle size, pre-treatment conditions, and type of co-processing feedstocks), and reaction conditions (microwave power, heating rate, temperature, residence time, carrier gas, flow rate of purging gas, microwave power switching frequency, mixing intensity, and pressure) [6,77,98,104,124,125]. It has been found that not all of these factors influencing the microwave-assisted pyrolysis possess the same importance [125]. The reactor design should be tailored to the desired products (fixed-bed configurations enhance gas yield and fluidized bed reactors facilitate liquid products) and catalyst location requires us to consider the cost and desired products. Furthermore, the temperature has a critical role and its best value depends on the type of feedstock; generally, a temperature increase produces a significant gain in gas yield, whereas an opposite trend occurs for the bio-liquid yield [60]. A raised microwave power and residence time increase gas yield, which is also favored by strong polar materials like NaOH. Additionally, the catalyst ratio adjustment improves the catalytic effects [98]. It has been demonstrated that the most effective strategy to optimize the MAP process is implementing strategies that consider the interaction between several independent factors. A well-optimized MAP reduces the energy, catalysts, and absorbent needs, and improves the quality and quantity of the desired product (biochar, bio-liquid, or non-condensable gases) [6].
MWs are not only used in pyrolysis, but also in pretreatments of biomass (microwave drying, and microwave-assisted Organosolv pretreatment) for enhancing pyrolytic yields [126,127,128,129], because it produces autohydrolysis, causing the separation of hemicellulose and lignin from cellulose [130,131]. Moreover, MWs have gained attention as a potential alternative to conventional activation methods due to its advantages, including the reduced activation time, uniform interior heating, high heating rate, selective heating, precise control over the process, absence of direct contact between the heating source and materials, and decreased equipment size and waste [89,132].
4. MAP of Forest Biomass
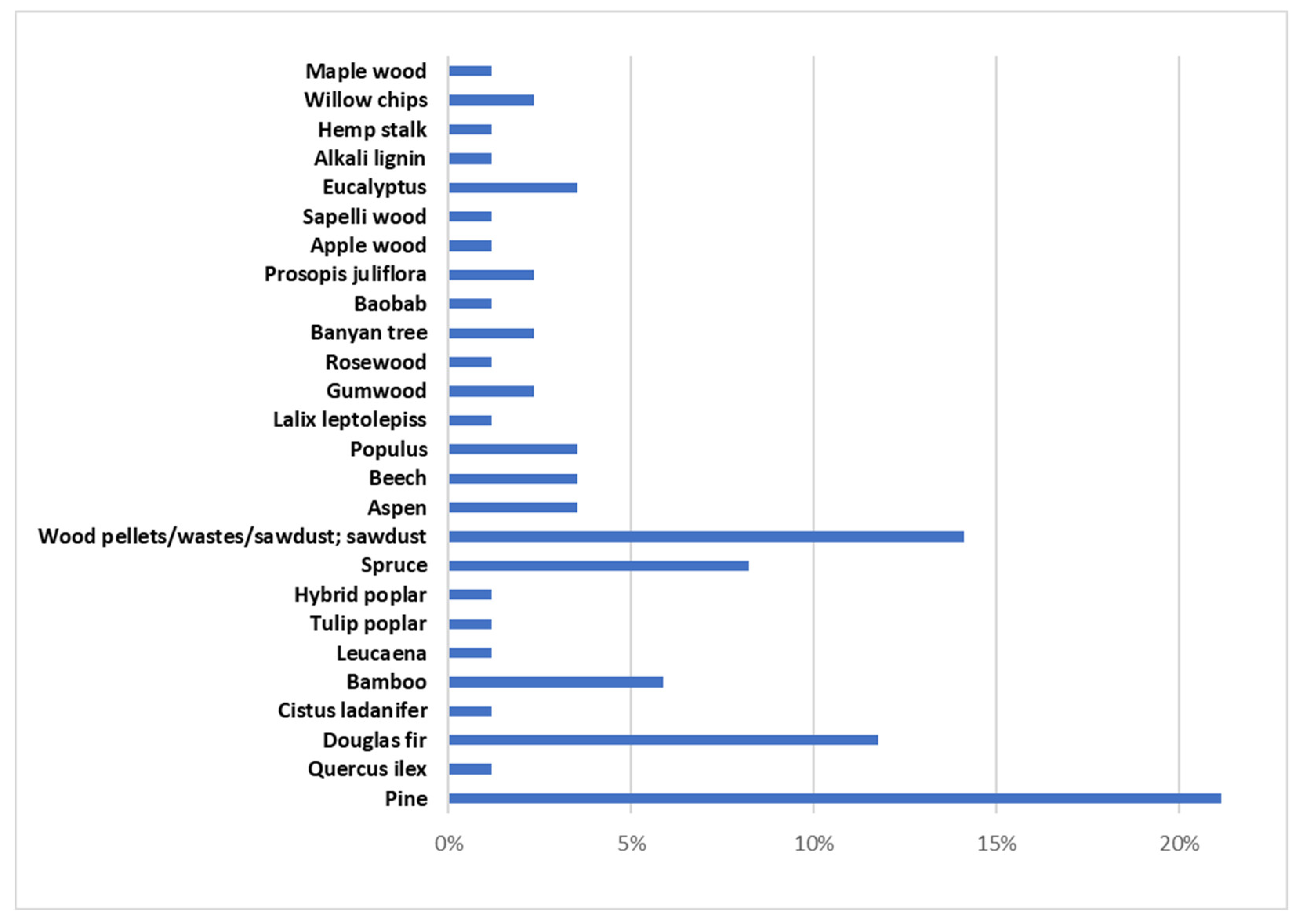
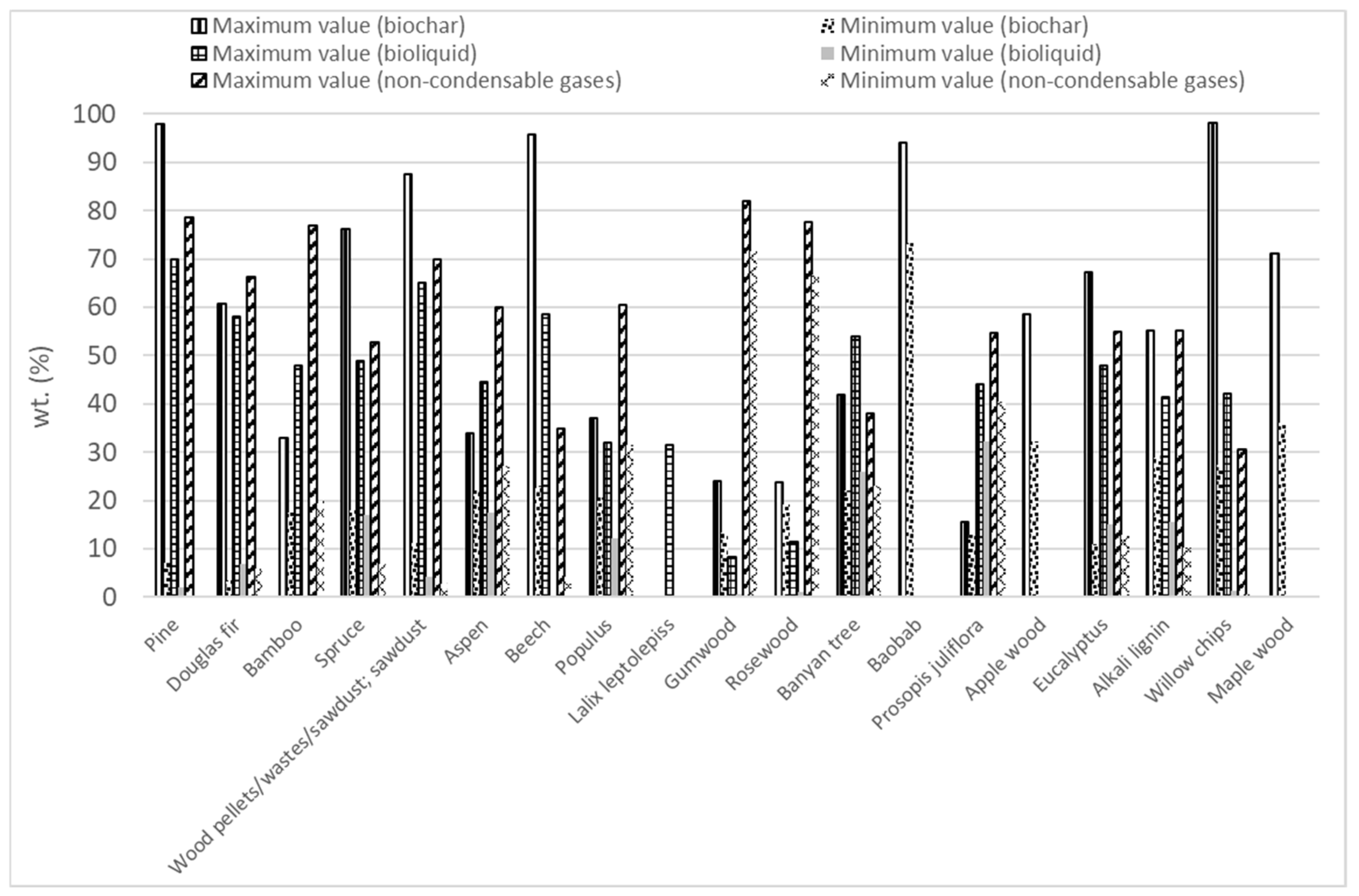
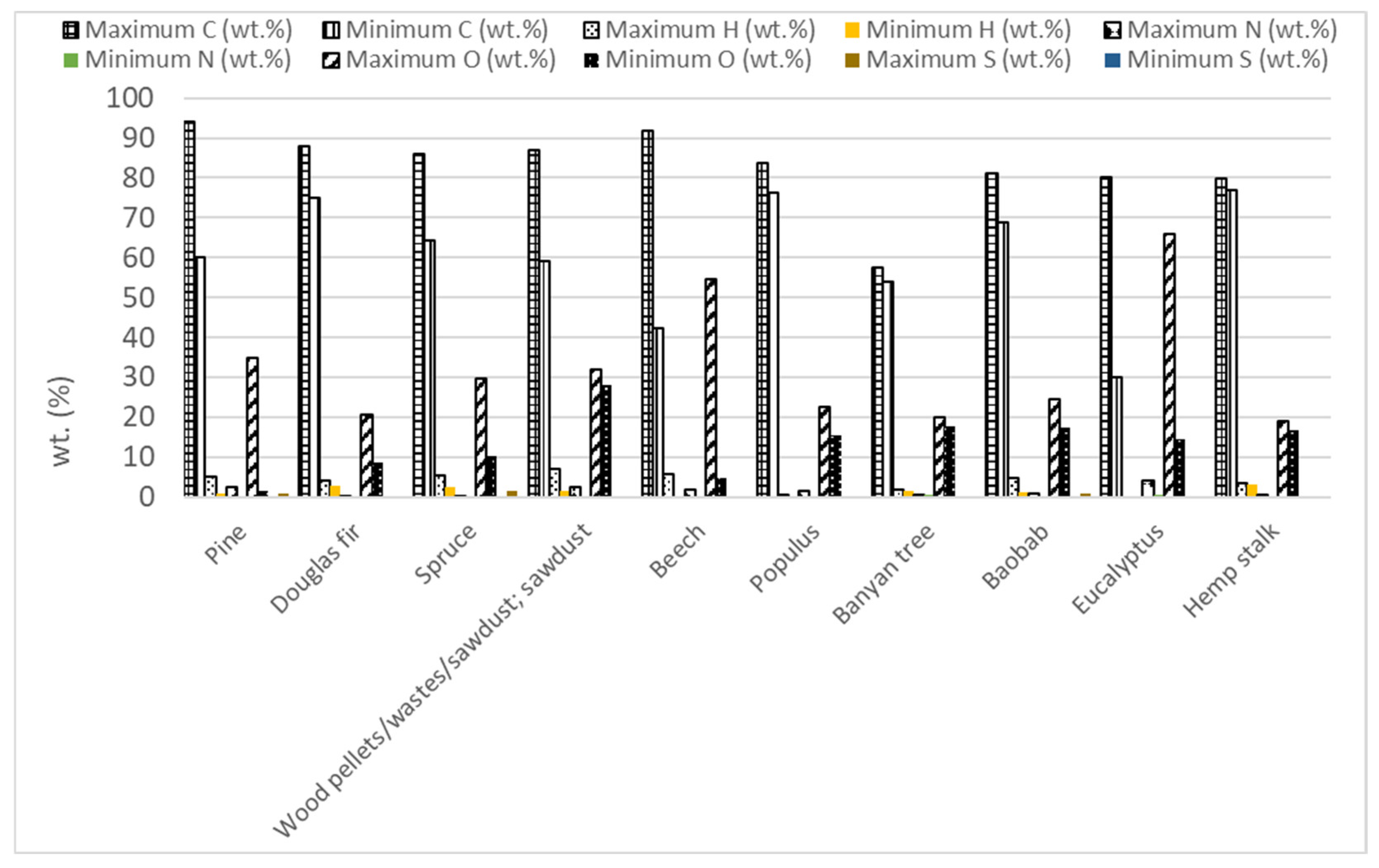
Forest biomass (FB) consists of lignocellulosic biomass containing three main components, hemicellulose (10.5–44.4 wt.%), cellulose (17.0–62.9 wt.%), and lignin (11.2–48.40 wt.%), along with minor amounts of ash and extractives [2,64,133,134,135,136,137,138,139,140,141,142]. Different types of FB have been used in microwave-assisted pyrolysis as Figure 3 shows. Considering the works that were evaluated in this review, approximately 20% have studied the microwave pyrolysis efficiency of biomass from pine. Secondly, the biomass most analyzed (14% of articles) was named wood pellets/wastes/sawdust without specifying the FB species. Douglas fir (12%) and spruce (9%) were the other two forest biomass species whose microwave-assisted pyrolysis has been most studied.
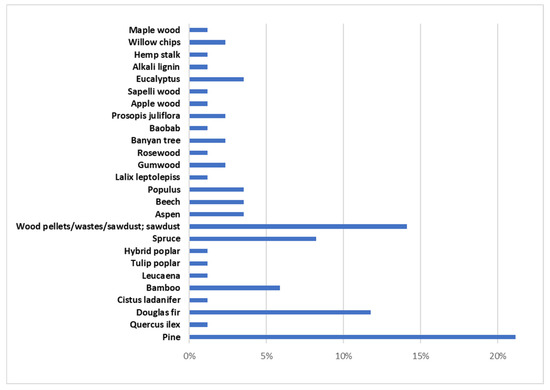
Figure 3.
Forestry biomass that has been studied in bibliography.
Product yields from the microwave-assisted pyrolysis of FB are influenced by various operation conditions, including microwave power, biomass/absorber–catalyst ratio, temperature, biomass size, and residence time.
Using regression analysis, a linear relationship between the heating rate and microwave power level was identified by Huang et al. [143], concluding that a higher microwave power results in a faster heating rate. Heating rates significantly impact biomass pyrolysis, as demonstrated by Dong and Xiong [144], who analyzed the pyrolysis kinetics of bamboo using both conventional and microwave heating methods. Their experiments showed that heating rates significantly impact biomass pyrolysis, with microwave pyrolysis requiring a much lower activation energy compared to conventional methods, which suggests that microwave heating, due to its volumetric heating style, is more efficient and could be a more promising technology for biomass pyrolysis. Huang et al. [143] also established a logarithmic relationship between the maximum temperature and the microwave power used during pyrolysis. The implementation of distinct power levels during microwave-assisted pyrolysis not only impacts the maximum temperature, but also product yields. Martin et al. [145] concluded that microwave power influences product yields in MAP. These authors utilized six distinct power levels (1000, 500, 400, 300, 200, and 100 W) to pyrolyze a mix of biomass (Pinus pinaster) and 5% of absorber (activated carbon), finding that, at 200 and 100 W, the microwave-assisted pyrolysis did not take place. Furthermore, biochar production diminished as microwave power increased being lower than 20% at 1000 W. On the other hand, the gas yield rose when microwave power grows, reaching a yield superior to 40% at 1000 W. Finally, liquid production achieved its maximum value (58%) at 400 W, and, from that, the power suffered a decrease as microwave power rose. Parvez et al. [146] also implemented different microwave powers (2700, 2400, and 2100 W) to carry out MAP of pine wood at different temperatures (600, 500, and 400 °C), obtaining an increasing gas yield (from 47.3% to 71.4%) with rising pyrolytic powers (from 2100 W to 2700 W). Conversely, char (33.1% to 19.3%) and bio-oil (19.6% to 9.3%) yields went down with power growth (2100 W to 2700W). Nhuchhen et al. [147] conducted experiments at three different powers (3000, 2500, and 2000 W), as well. However, they studied the behavior of wood pellets from spruce sawdust, proving that the product yield distribution was affected by microwave power. The biochar yield decreased as the microwave power increased, with the reduction being more significant at a higher power. That behavior can be attributed to the high heating rates observed when higher microwave powers are implemented. The microwave power levels of 500, 400, and 300 W were applied during microwave pyrolysis experiments carried out by Huang et al. [139]. In that work, the three-phase product distribution was also affected by the power level. For instance, it was seen that, during the microwave pyrolysis at 300 W of bamboo leaves, around 33 wt.% of the products were solid-phase, 47 wt.% were liquid-phase, and 20 wt.% were non-condensable gases. Nonetheless, when the microwave power was 500 W, the solid, liquid, and gas yields were 22 wt.%, 42 wt.%, and 36 wt.% respectively. As those authors concluded [139], this change of product distribution indicates that higher microwave powers enhance the non-condensable gas yield, possibly due to the self-gasification of the solid phase produced during pyrolysis. Sellamuthu et al. [148] evaluated the effect of microwave power (550, 650, and 750 W) on the bio-char yield percentage when Adansonia kilima wood chips were pyrolyzed. They showed that the power augmentation of microwave-assisted pyrolysis resulted in a considerable decrease in the biochar yield. Du et al. [135], who pyrolyzed sawdust and rice straw with ionic liquids at five different power levels (160–800 W) under microwave irradiation, found that, when the microwave power was lower than 480 W, a small yield of bio-oil was produced. Nevertheless, when the microwave power was increased, the bio-oil production rose from less than 10% to more than 30%. The bio-oil yield, obtained in the microwave-assisted pyrolysis of wood from Banyan tree trunks, also grew significantly when heating power rose from 600 to 1200 W [149]. The bio-oil production increased from 26.3 to 36.4 wt.%, as well as the non-condensable gas yield, which was augmented from 31.2 to 34 wt.%, whereas the biochar one diminished from 42.5 to 29.6 wt.%. A comparable effect of increasing microwave power (from 100 to 800 W) on non-condensable gas and bio-oil yields was observed by Khelfa et al. [150]. The highest oil and gas yields during the microwave-assisted pyrolysis of pine wood sawdust were achieved at the maximum studied power (800 W), whereas the char production systematically diminished with increasing power. Those results were in agreement with the ones obtained by Wu et al. [151] who saw an increase in bio-oil and non-condensable gas yields from 43.23 to 47.10 wt.% and from 8.39 to 9.68 wt.%, respectively, with increasing microwave power (600 to1200 W) during wood biomass pyrolysis. Lin et al. [152] focused their study on the influence of plasma power (1000, 900, and 800 W) on the obtained gases from banyan leaves which were pyrolyzed into a microwave plasma system. The gaseous products which were produced consisted of H2, N2, CO2, and CO, with smaller amounts of CH4 and formaldehyde. As the microwave power increased, the volume fraction of H2 increased and CO diminished. Specifically, the H2 production rates were 18.48, 20.05, and 20.44 mg-H2/g-biomass for power levels of 800, 900, and 1000 W, respectively, reflecting a roughly 10% increase in hydrogen production as the microwave power rises by 25%. The assessment of all these works shows that the three phases (biochar, bio-oil, and non-condensable gases) obtained during microwave-assisted pyrolysis are significantly influenced by microwave power which can be simply managed, allowing us to easily optimize the reaction requisites of microwave-assisted pyrolysis.
There are authors who have studied the impact of pyrolysis temperatures on product yields instead of microwave power levels, which influence heating rates and maximum temperatures, altering primary and secondary reactions. For instance, Zhang et al. [153] examined the effect of temperature (400, 500, 600, 700, and 800 °C) on the product distribution during the microwave-assisted pyrolysis of Aspen wood. They concluded that a pyrolysis temperature of 500 °C allowed them to maximize bio-oil production. They also deduced that high pyrolysis temperatures promoted pore formation in biochar. Furthermore, they saw that the compositions of bio-oil and non-condensable gases were influenced by the operation temperature. The phenol concentration diminished as the temperature rose, while the hydrocarbon and ketone concentration increased. In the case of the composition of non-condensable gases, it was noticed that, as the pyrolysis temperature rose, the hydrogen concentration increased as well, while the CO2 concentration decreases. Additionally, the CO concentration reached a minimum at 600 °C and the methane concentration was the maximum at 700 °C. Similar results of the temperature effect on non-condensable gases were gathered by Wang et al. [154]. These authors studied the behavior of Douglas fir when it was pyrolyzed, considering different operation temperatures (721, 700, 650, 600, and 579 °C) and biochar catalyst/Douglas fir mass ratios (4.4, 4, 3, 2, and 1.6). They noted that higher temperatures promoted an increase in gas yield and a drop in bio-oil production. Additionally, these authors found that the hydrogen concentration increased significantly with temperature, although it was constrained by thermodynamic factors. Huo et al. [155] also studied the behavior of Douglas fir during microwave-assisted pyrolysis. However, these authors investigated MgO and activated carbon (from corncob using phosphoric acid for its thermochemical activation) catalysts. They considered the impact of the experimental temperature (600, 550, 500, 450, and 400 °C), activated carbon/Douglas fir ratio, and MgO/activated carbon ratio on product yields. In this study, it was observed that the bio-oil yield reached a maximum yield of 53.0% at 500 °C, while char and coke yields decreased at temperatures between 500 °C and 600 °C, whereas gas yield increased. These behaviors were seen when the MgO/activated carbon ratio was 0.8 and the activated carbon/biomass ratio was 1:1. Moreover, it was detected that the bio-oil composition varied with temperature. Phenols increased from 38.7% to 42.2% as the temperature increased. The selectivity of cellulose-derived furans peaked at 450 °C and then declined. The main target gas (CO) of gaseous products increased from 5.8% to 10.4% at the highest temperature. Wallace et al. [156] evaluated the consequences of temperature (348.4 ± 33.9, 398.8 ± 38.7, 427.5 ± 31.2, 528.7 ± 13.2, 604.2 ± 10.8 and 659.8 ± 60.0) on biochar characteristics when hemp-stalk and a mix of spruce and fir softwood chip were pyrolyzed, applying microwave radiation. The experiments demonstrated that, as the pyrolysis temperature increased, the biochar carbon content also grew, whereas the char yield was reduced due to the accelerated volatile release. Higher temperatures also lower the surface pore size in biochar. Moreover, it was found that the temperature and heating rate were key factors influencing the mechanical properties of biochar. Dutta et al. [157], who also assessed the impact of pyrolysis temperature (250, 290, and 330 °C) on biochar properties and production, pyrolyzed maple wood using microwave-assisted pyrolysis. They used as the absorber char from willow wood, studying the dopant ratio effect on biochar yield and its properties. Furthermore, the influence of the operation time on the pyrolysis process was tested. These authors found that biochar production was also reduced with increasing temperature. Moreover, it was demonstrated that only the temperature significantly affected the biochar yield in comparison with the doping ratio and time, whose impact was minimal. On the other hand, it was concluded that the quadratic term of the doping ratio and temperature are critical factors affecting both the exothermic energy and volatile matter content in biochar. Zhou et al. [158] conducted microwave-assisted pyrolysis on wood sawdust and characterized the obtained char. They evaluated the influence of the processing temperature (750, 650, 550, and 450 °C) on biochar production. Char yield decreased from 36 to 26 wt.% as temperature rose, while bio-oil yield dropped sharply from 36.13% to 14.85 wt.% and the non-condensable gas production rose from 9.10% to 28.07 wt.%. Moreover, it was concluded that higher temperatures led to an increased carbon content in biochars. Additionally, it was inferred that the process temperature during pyrolysis affected the adsorption capacities of chars, with the optimal performance observed at specific temperatures for methylene blue (MB), crystal violet (CV), and iodine. Shi and Wang [159] assessed the pyrolysis process of cellulose, xylan, a mixture of cellulose and lignin from pine wood, and pine wood, considering the effect of pyrolysis temperature (300, 500, and 700 °C) on the three phase products’ distribution. At 700 °C, char yields were 16.7% for xylan, 16.1% for cellulose, 18.6% for the mixture, and 20.3% for pine wood, whereas, at 350 °C, these char yields were higher, particularly for the mixture and pine wood with minimal gas production from cellulose. Wang et al. [160] pyrolyzed pine sawdust at different temperatures (400, 500, 600, 700, and 800 °C) using conventional pyrolysis and microwave-assisted pyrolysis. The results showed that, during MAP, less biochar and more hydrogen and carbon monoxide in a non-condensable fraction were produced. As it has been observed in other works, higher temperatures during MAP led to a reduced biochar yield. These authors also found that MAP chars had smaller pores and smoother surfaces at higher temperatures, resulting in a lower reactivity compared to conventional pyrolysis chars. Additionally, the study observed that MAP could produce high-quality liquid products at lower temperatures by inhibiting secondary reactions, containing unique volatile compounds not found in conventional pyrolysis. Nzediegwu et al. [161] also compared the impact of conventional pyrolysis and microwave-assisted pyrolysis; however, they studied the differences in biochar properties from sawdust of white spruce that had been pyrolyzed at three distinct temperatures (500, 400, and 300 °C). At 500 °C, the biochar yields from both methods became similar because conventional pyrolysis required more time for heat transfer through conduction. MAP biochars generally had a higher pH than those from conventional pyrolysis. Nevertheless, the higher heating effects of MAP did not significantly alter the Gross Calorific Value (GCV) of the biochars. It was also detected that the thermal stability of biochar increased with pyrolysis temperature, leading to a higher carbon content. Biochar produced at 500 °C possessed a higher carbon stability and energy content, whereas those at 300 °C had higher energy yields. The biochar yield using MAP varied significantly with pyrolysis temperature, ranging from 45.3% at 300 °C to 24.7% at 500 °C.
Reaction time has been also selected as independent variable, which can impact product distribution in microwave-assisted pyrolysis, by several authors [95,148,162,163,164,165,166,167,168,169,170]. For instance, Bu et al. studied the impact of reaction time and temperature on the behavior of Douglas fir during MAP [162,166]. The microwave power was established at 700 W and a commercial activated carbon (GAC 830 PLUS) was used. These authors obtained the maximum gas and liquid yields when the reaction time was 12 min. Although it was found that the retention time impacts products, its influence was not as critical as the catalyst/biomass ratio and reaction temperature. Yang et al. pyrolyzed Douglas fir sawdust, taking into account four retention times (41, 34, 27, and 20 min), as well as different microwave powers and preparation conditions of activated carbon from corn stover with phosphoric acid [167]. These authors noted that the reaction time, as well as phosphoric-acid-to-corn-stover ratio, significantly impacted the final temperature. On the other hand, the irradiation time enhanced biomass devolatilization, which influenced the activated carbon yield negatively. Finally, these authors obtained the optimal conditions to maximize the phenolic compound production, which required that they control the reaction time, microwave power, and activated carbon preparation. Ren et al. evaluated five different reaction times during the MAP of Douglas fir pellets. They conclude that syngas and bio-oil yields increased with higher temperatures and longer retention times, achieving maximum yields of syngas and bio-oil at 471 °C and 15 min. Moreover, the control of the reaction time and temperature allowed them to achieve the maximum yield of specific phenolic chemicals [165]. Some years later, these authors pyrolyzed Douglas fir pellets again but employing as catalyst a commercial activated carbon (GAC 830 PLUS) impregnated with iron powder [95]. The pyrolysis in that work was carried out at 700 W, and the reaction time as well as temperature were chosen as independent variables. They studied different retention times (13.66, 12, 8, 4, and 2.34 min), obtaining the maximum bio-oil yield (37.1 wt.%) at 450 °C and 8 min as the reaction time. The syngas yield (from 31–44 wt.%) increased with longer retention times and higher temperatures, peaking at 550 °C and 12 min, whereas the biochar yield (16.33 wt.%) was the lowest at those conditions. Furthermore, coking on the catalyst decreased with higher temperatures and longer reaction times, likely due to coke decomposition. Sellamuthu et al. pyrolyzed Adansonia kilima wood chips using microwave heating to synthesize the high-quality activated carbon used for lead (II) cation removal from wastewater [148]. They evaluated the influence of three process variables: reaction time (10, 12.5, and 15 min), concentration of an activating agent of K2CO3, and microwave power. In that work [148], it was concluded that all the process variables that had been considered impacted the removal percentages of Pb (II) cations obtained by the activated carbon produced. Miura et al. [164] pyrolyzed wood blocks using microwave technology. They used two different ovens and evaluated yields considering diverse irradiation times (3, 6, 7, 9, 10, 11, 12, 12.5, 15, and 18 min). They found that the microwave irradiation time influences char and volatile (tar) productions as well as char properties (pore radius and specific surface area). They found that there was a specific irradiation time in which the tar yield was maximized; however, it was diminished when different times to that one were applied. Additionally, it was concluded that the pore radius decreased, and the specific surface area grew with rising radiation time until reaching a stabilization time, after which char properties were not altered.
Even though microwave-assisted pyrolysis can deal with larger feedstocks than conventional pyrolysis, not having strict particle size constraints, size particle control can effectively modify the final product distribution of microwave-assisted pyrolysis, as well as its energy efficiency. For instance, Miura et al. [164] studied cylindrical wood blocks of Lalix leptolepiss of different sizes (diameters and heights of 300, 100, 80, and 60 mm) to evaluate the impact of wood size on microwave-assisted pyrolysis. It was concluded that the char yield was correlated with an equation where the electric power consumption per weight was inversely proportional to the diameter square, which means that a smaller wood block possesses a higher electric power consumption per unit weight than a higher one during microwave-assisted pyrolysis [164]. Higher specific power consumptions when biomass with smaller sizes is pyrolyzed were also reported by Vorhauer-Huget et al. [171]. The influence of feedstock size on MAP was also examined by Fricler et al. [85]. These authors tested different particle sizes (2000, 1000, 800, 250, and 140 µm) of pine sawdust mixed with straw, rice husk, or wheat bran, which were pyrolyzed in a microwave reactor at 700 W. It was concluded that there was a relationship between the particle size and pyrolyzed gas composition that increased the concentration of CO2 and combustible gases as the biomass size increased from 140 to 800 µm. Smaller particles (140 µm) had a lower porosity and higher bulk density, obstructing the volatiles’ liberation and limiting thermal decomposition, which produced the highest char yield and the lowest bio-oil and gas yields. The highest gas yield was achieved by pyrolyzing 250 µm particles, while 800 µm particles produced the highest bio-oil yield and a better gas quality (higher CO and lower CO2 concentrations). Increasing the particle size to 1000–2000 µm slowed the oil and gas liberation, increased the char yield, and ended up in an incomplete pyrolysis, obtaining more residual coke. Consequently, Fricler et al. [85] verified that the biomass size allows us to define the pyrolysis product distribution. Nonetheless, there were authors such as Klinger et al. [172] who pyrolyzed large pellets (with a thickness of 5–6 mm) of thirty different biomass materials (residue, herbaceous, woody, waste, and blended materials) and smaller pellets (with thicknesses of 2–3 mm), concluding that the sample size studied in their work had a minimal influence on the liquid yields.
Forestry biomass has poor microwave absorption due to its low dielectric properties. To improve heat generation and transfer (by conduction) during the microwave pyrolysis of biomass, materials with higher dielectric properties named absorbers are added. Most of the articles analyzed in this review that use absorbents choose activated carbon [95,140,145,150,162,166,167,172,173,174,175,176,177,178], SiC [135,140,174,176,177,179,180,181,182,183], or biochar [145,146,147,154,156,157,160,174,181,184], with the biochar being from different biomass depending on the authors. For instance, Martin et al. [145] utilized as absorbent the biochar that had been produced in their continuous pilot plant from Pinus pinaster at 960 W. Furthermore, these authors also evaluated the impact of a different absorbent (DARCO G-60) which is a commercial activated carbon. Their results showed that the biochar generates lower liquid and gas yields in comparison with those obtained using activated carbon. Consequently, more biochar had to be added to induce pyrolysis reactions as effectively as activated carbon. Dong et al. [181] also employed biochar as a microwave absorber. They produced two different biochar that were obtained from rice husk and rice husk combined with Fe(NO3)3·9H2O. Additionally, these authors selected SiC as the absorber for the control group. In that work, it was found that both biochar selected were able to achieve 400 °C (pyrolysis temperature used in the study) in a time that was approximately six times less than the one needed by SiC. Shang et al. [174] demonstrated that strong absorbers such as K2CO3, SiC, activated carbon, NaOH, and the produced coke from the microwave process influence the fractional yields, acting as catalysts. It was found that K2CO3 and NaOH generated the products primarily consisting of gases, whereas SiC resulted in a higher solid product yield and lower liquid and gas yields. In the work carried out by Dutta et al. [157], biochar from willow wood was used as the absorber. These authors concluded that the ratio of biochar (16–32%) did not significantly affect the biochar yield obtained during microwave-assisted pyrolysis. However, they had concluded previously that biochar use increased biochar yield compared to microwave-assisted pyrolysis without biochar as an absorbent. The impact of biochar on biomass MAP was also analyzed by Ellison et al. [184], who pyrolyzed biomass with a specified amount of biochar from tallow tree and cane bagasse obtained in induction pyrolysis. They observed that pyrolysis temperatures were not achieved without a microwave absorber. Biochar use significantly affected the absorbed power and, consequently, pyrolysis temperatures, which increased four times when the biochar content grew from 0% to 10%. Nevertheless, a rise in biochar from 10% to 20% did not show a significant difference in temperatures. In their work, Wang et al. [154] concluded that, at the same temperature, the biochar (from the microwave-assisted pyrolysis of nanocellulose powder) ratio had effects on product yields in comparison with the MAP without absorber, increasing bio-oils and reducing gas yields. Nonetheless, as in the work carried out by Ellison et al. [184], it was found that the biochar/biomass ratio variable does not have a significant effect on production yields. Some authors such as Undri et al. [185] have assessed the impact of the blending procedure between biomass and microwave absorbers on the final products’ properties. They compared three different mixing methods between two absorbers (Fe and carbon) and wood pellets, resulting in different heat distributions and thus affecting the final product characteristics only when Fe was used.
Not only has the effect of absorbers on microwave-assisted pyrolysis been studied, but also the impact of catalysts on the distribution and composition of products (biochar, liquid phase, and non-condensable gases). Different catalysts have been tested in MAP. For instance, Lestinsky et al. [186] pyrolyzed spruce sawdust using microwave radiation and catalysts (char from sawdust, and sawdust char doped with metal ions of nickel, cobalt, and iron). The pyrolysis was carried out in a microwave reactor at 400 W for 20 min. These authors found that the use of metallic catalysts, particularly cobalt and nickel, reduced the liquid yield, while the non-condensable fraction rose from 31.21 wt.% to more than 50 wt.%. Additionally, the pyrolysis gas composition was influenced by the type of catalyst, doubling the hydrogen content in comparison with the char alone when cobalt and nickel catalysts were used. These catalysts also reduced the concentrations of CO2, CxHy, and CH4. On the other hand, catalysts significantly reduced the amount of oil-phase in the liquid product compared to pyrolysis without using catalysts while the liquid composition remained largely unchanged. Li et al. [187] tested the influence of iron additives as well; nonetheless, they investigated the catalytic effect of Fe(OH)3, Fe2(SO4)3, and Fe2O3. The biomass utilized for microwave pyrolysis was pine sawdust. The pyrolysis was carried out at 480 W for 60 min and the biochar was obtained from microwave-assisted pyrolysis without additives and with iron additives. The results evidenced that Fe2(SO4)3 and Fe2O3 promoted pyrolysis at lower temperatures, indicating less catalytic activity and a higher required heat for pyrolysis. Additionally, it was found that the Fe2O3 encouraged non-condensable gas production by 6.37% due to its catalytic properties, while Fe2(SO4)3 enhanced bio-oil yield by 80%. The Fe(OH)3 increased biochar yield by 23.05%. An iron additive (Fe(NO3)3) was also implemented during the microwave pyrolysis of wood wastes by Guo et al. [188], who also studied the influence of ZnCl2 at distinct pyrolysis temperatures (800, 700, 600, 500, and 400 °C). These authors concluded that the pyrolysis temperature significantly affects the product distribution as has been verified by other authors. Guo et al. [188] observed that the bio-oil yield peaked at 600 °C but decreased at higher temperatures, while the biochar yield declined with increasing temperature and the content of gas phase increases up to 800 °C. On the other hand, the Fe(NO3)3 and ZnCl2 additives enhanced biochar production. ZnCl2 also favored the condensation of aromatic hydrocarbons into macromolecules, while Fe(NO3)3 increased biochar yield and decreased bio-oil yield. ZnCl2 was noted for enhancing furfural production at low temperatures. Furthermore, the presence of Fe(NO3)3 and ZnCl2 affected the production of other compounds like ethylbenzene, p-xylene, and m-xylene, which are important for chemical applications. Finally, it was found that the use of Fe(NO3)3 and ZnCl2 generated the existence of Fe3O4 and ZnO in the biochar, indicating potential applications in photocatalysis and adsorption. Different additives were used by Li et al. [189] who investigated the microwave-assisted pyrolysis of Eucalyptus wood combined with different ratios of MoO3 (3:1, 2:1, and 1:1) and distinct nitrogen sources (ammonium chloride, soybean straw, and food waste digestate) to produce nitrogen-rich biochar and bio-oil. The addition of MoO3 increased gas yields and decreased solid yields, with the highest liquid yield achieved at a 2:1 Eucalyptus-wood-to-MoO3 ratio. Soybean straw at 20% increased liquid yield, while excessive food waste digestate led to more solid products. The effect of the catalysts on microwave-assisted pyrolysis has also been analyzed through the activation energy because it can define the variation of the reaction rate parameters during the pyrolysis process, as found by Liu et al. [190]. Recent research has increasingly focused on the interaction between microwaves and metals, confirming that microwave–metal interactions can reduce energy consumption and intensify chemical processes. In studies like the one carried out by Li et al. [191], the interaction between microwave radiation and iron-based needle metals, during microwave-assisted pyrolysis of forestry wastes, has been evaluated. The presence of iron-based needle metals reduced the initial temperature for pyrolysis by 141 °C and increased reaction rates. The addition of more than one iron-based needle metal had a diminishing return on the heating efficiency and pyrolysis rate. An increased microwave power also elevated bed temperatures and heating rates, further accelerating the pyrolysis process. Finally, it is necessary to note that it has been tested that, under particular conditions, like the presence of metal catalysts and chlorine and low pyrolysis temperatures during microwave-assisted pyrolysis, dibenzofurans, polychlorinated dibenzo-p-dioxins, and naphthalenes are generated [192]. That study verified that feedstock composition as well as MAP conditions altered the production of these chemicals; consequently, it is possible to more safely conduct microwave pyrolysis if variables impacting product yields are studied in detail.
Different feedstocks combined with a varied range of additives and operation conditions cause distinct effects on the product yields, as can be gathered from Figure 4.
Figure 4.
Impact of feedstock and operation conditions on biochar, bio-oil, and non-condensable gas yields.
It can be observed that the biochar production generated in the articles that have been considered in this review varies from 3.37% to 98%. The forest species whose yields are over 90% combining different operation conditions have been pine, beech, baobab, and willow, with the highest obtained production (98.1%) being the one using biomass from willow. On the other hand, the MAP of biomass from Douglas fir has generated the lowest biochar yield (3.37%) under specific conditions. Additionally, it has been seen that the MAP of biomass from pine has generated a biochar production of 7% [184], whereas different conditions in the MAP of biomass from pine have increased biochar productions to 98% [150]. This variability in production underlines the need for more detailed studies of the influence of operation conditions, which have been demonstrated to have a decisive impact on the biochar yields from the same forest species. Similar behaviors have been detected for the bioliquid fraction. The productions found range from 0% (beech and bamboo) to 70% (pine). The MAP of biomass from pine has generated bio-oil yields from 2% [150] to 70% [172]. Significant production variations in the bioliquid phase have also been obtained with biomass from beech, obtaining values as low as 0% [185] and as high as 58% [129]. By comparison with the biochar productions, it can be concluded that, up to now, the maximum bio-oil productions are lower under the analyzed conditions. Finally, non-condensable gas productions have also experienced huge variations from 0% (biomass from pine) to 82.1% (gumwood), depending on the operation conditions and feedstocks. Non-condensable gas yields during the MAP of biomass from pine have fluctuated from 0% [150] to 78.5% [140]; similar tendencies have been seen on the feedstock denominated as wood pellets/wastes/sawdust without specifying the species, where the gas fraction changes from 3% [183] to 70% [179].
MAP operation conditions not only impact yields, but also the biochar, bio-liquid, and non-condensable gas composition. Figure 5 displays the composition of biochars from different feedstocks. It is noticed that biochars possess carbon contents ranging from 29.98% (eucalyptus) to 93.96% (pine). It has been also detected that the biochars that have shown the highest variation of carbon content are those from eucalyptus in which the carbon concentration fluctuates from 29.98% to 80.24% [189].
Figure 5.
Impact of feedstock and operation conditions on biochar composition.
Oxygen has turned out to be the component present in biochar with the second highest concentrations, with its contents ranging from 1.59% (biomass from pine) to 65.97% (biomass from eucalyptus). Nitrogen and hydrogen contents in biochars are low, being less than 4.05% and 7.17% respectively.
Although the bio-oil composition is complex, the different chemicals that can been detected are usually classified in different groups such as phenol and derivatives, kethone/aldehydes and derivatives, furan and derivatives, acids, esters, alcohols, naphthalene and derivatives, alkene, benzene and derivatives, nitrogenated compounds, and others. Figure 6 shows that phenols, kethone/aldehydes, and furans are the main types of chemicals in bio-oil from MAP. However, the content of these chemicals can vary enormously depending on operation conditions and feedstocks. For instance, the content of phenol and derivatives has fluctuated from 4% [159] to 78% [93] during the MAP of pine, while, in the MAP of Douglas fir, it varied from 2% [175] to 96% [154]. Variations in kethone/aldehydes (0.5–37%) [95,165] and furans (1–33%) [155,175], when operation conditions are modified, have also been found; however, these variations are lower.
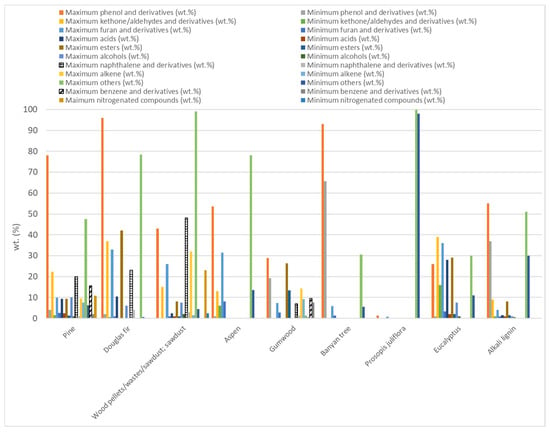
Figure 6.
Impact of feedstock and operation conditions on bio-liquid composition.
The non-condensable gases’ fraction of MAP mainly consists of hydrogen (H2), carbon monoxide (CO), and carbon dioxide (CO2). As it can be seen in Figure 7 the highest hydrogen content has been produced in the MAP of Douglas fir, in which a concentration of 85% was measured [154]. Nevertheless, when changing operation conditions, the hydrogen content during the MAP of Douglas fir was only 1% [155]. Significant variations in the CO concentration (0.07–51%) were also obtained during the MAP of pine [85,159] and feedstock denominated as wood pellets/wastes/sawdust, in which they were 7.85–41.79% [151,174]. Similar differences were measured in CO2 content when biomass from pine (0.1–47.6%) [159,172] and feedstock denominated as wood pellets/wastes/sawdust (10.3–49%) [151,188] were pyrolyzed.
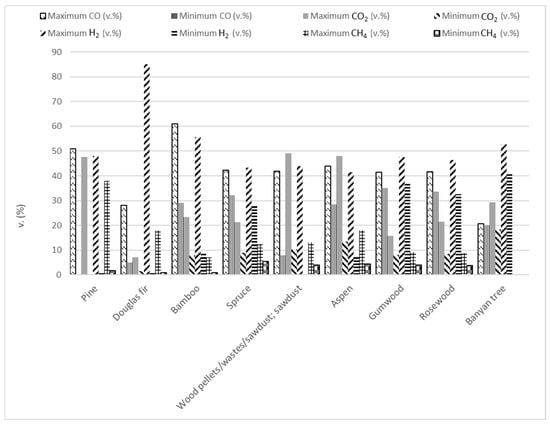
Figure 7.
Impact of feedstock and operation conditions on non-condensable gas composition.
Studying the variations of biochar, bioliquid, and non-condensable gas yields, as well as in their composition, the highest differences have usually been found in those biomasses from forest species that have been the most studied. Consequently, more detailed studies about the combination of different additives, feedstocks, and specific operation conditions are required to reach desired yields and compositions using different feedstocks.
5. Future Directions
Despite the fact that MAP has emerged as an effective solution for biomass waste conversion, offering rapid, uniform heating and improved product quality by minimizing secondary reactions, it faces challenges such as temperature control and measurement, which are critical for optimizing pyrolysis performance. High temperatures can cause secondary reactions, breaking down condensable gases into non-condensable ones, while low temperatures may result in incomplete biomass decomposition, reducing the bio-oil yield. Accurate temperature measurement and control are difficult due to environmental interference, nonlinearity, and parameter uncertainty. Intelligent optimization algorithms have shown better performance than traditional controllers in managing these challenges. Additionally, as biomass materials lose moisture at high temperatures, they become less effective at absorbing microwave energy, so further research is needed on the dielectric properties of materials at elevated temperatures to enhance heating efficiency [22]. Furthermore, bio-oils have several drawbacks that obstruct their use as fuel, including a high moisture content, low heating value, thermal instability, and corrosiveness due to low pH and oxygenated compounds. The moisture and suspended char in bio-oil can lead to separation, filtration issues, and increased viscosity, which, in turn, cause operational challenges like equipment erosion, combustion inefficiencies, and pipeline blockages. The study and implementation of techniques such as hot vapor filtration and catalytic upgrading are needed to address these issues, improve combustion properties, and reduce the viscosity and other problematic challenges that have been found during bio-oil use [23]. The comprehensive process modeling of various conversion pathways is essential in order to scale up MAP for industrial use. This requires extensive research on parameters and kinetics, especially since existing studies on the dielectric properties of different forestry biomass feedstocks are limited. For instance, Vorhauer-Huget et al. analyzed the dielectric properties of several wood samples before and after pyrolysis. Measurements were conducted at room temperature in the 2–3 GHz range. Their results emphasized the importance of understanding secondary reactions and the reduced microwave penetration depth in samples with high loss factors [171]. McKeown et al. [193] measured the dielectric properties of pine and hardwood pellets at microwave frequencies across a temperature range of 10 °C to 50 °C and moisture contents varying from 4.9% to 16.0%. Wang et al. [194] presented a method for accurately determining the dielectric properties of different biomass (bamboo, poplar wood, rice straw, and corn straw) using paraffin wax and a dielectric mixing rule. Ellison et al. [195] characterized the dielectric properties of the pulverized biomass of pine sawdust, Chinese tallow tree wood, live oak, and energy cane bagasse, and biochar mixtures in the range from 0.5 GHz to 20 GHz at room temperature. These authors concluded that dielectric loss and constant suffer a quadratic rise with increasing biochar content. Although important, these results are not precise enough to establish the dielectric behavior of those feedstocks during microwave-assisted pyrolysis, in which higher temperatures are implemented. This was shown by Luo et al. [93], who highlighted how the dielectric properties of wood sawdust changed with temperature, affecting the microwave heating behavior and altering reaction pathways. Consequently, studies such as those carried out in agriculture biomass on dielectric properties [196,197] has to be carried out by studying forestry biomass. Expanding the research to include a variety of feedstocks will provide valuable data for accurate modeling. Optimizing key factors such as microwave power, reaction duration, temperature, pressure, and catalyst loading can enhance heating efficiency and reduce energy consumption. Understanding the dielectric properties of biomass and fine-tuning these parameters will promote more sustainable MAP by minimizing the release of undesirable substances and maximizing resource and energy utilization without compromising overall efficiency. Additionally, identifying the catalytic mechanisms is crucial for determining the suitability of different microwave absorbers/catalysts for various conversion processes on a larger scale [60]. Conducting more techno-economic assessments of MAP for FB is needed in order to assess the economic viability of the process. The primary products intended for sale are bio-oil and biochar, while syngas is utilized for power generation, contributing to the self-sustainability of the operation. Additionally, the search for new applications for the products from the MAP of forestry biomass as the purification of residual streams might promote the full-scale implementation of this technology, contributing to the bio economy.
6. Conclusions
The microwave-assisted pyrolysis (MAP) bioconversion route of forestry biomass is reviewed in this paper, as an attempt to discover the main species that have been studied and variables influencing this method. It was concluded that around 20% of the reviewed articles focused on evaluating the efficiency of MAP using biomass derived from pine, 14% concentrated on biomass categorized as wood pellets/wastes/sawdust without specifying the species, 12% studied biomass from Douglas fir, and 8% of the works evaluated biomass from spruce, with those being the most studied forestry biomass. Additionally, it was found that MAP is significantly influenced by various operation conditions such as microwave power, temperature, biomass size, and the use of absorbers/catalysts. Research has shown that increasing microwave power leads to higher heating rates, faster pyrolysis, and varied product yields. Higher microwave power typically results in increased gas yields and decreased biochar production, with optimal bio-oil yields observed at moderate power levels. Similarly, the pyrolysis temperature plays a crucial role, with higher temperatures generally promoting an increase in gas yield and reducing biochar yield. The composition of bio-oil and non-condensable gases is also temperature-dependent, with specific compounds being favored at different temperatures. Reaction time is another critical factor, with longer times enhancing gas and bio-oil yields while reducing biochar yield. Additionally, biomass particle size affects energy efficiency and product distribution, with smaller particles leading to higher char yields due to a limited volatile release. The use of absorbers, such as activated carbon, SiC, and biochar, improves microwave absorption and heat generation, further influencing the efficiency and outcomes of the pyrolysis process. Overall, the choice of operating conditions in MAP can be tailored to optimize the yields of desired products, making it a versatile and efficient method for biomass conversion. MAP feasibility, although promising due to its faster heating rate, shorter reaction time, lower energy consumption, higher product quality, and lower production cost compared to conventional pyrolysis, still presents some challenges such as precise temperature measurement and control; more detailed studies of the dielectric properties of forestry biomass at high temperatures are needed in order to improve efficiency during microwave heating.
Author Contributions
Conceptualization, I.F. and S.F.P.; writing—original draft preparation, I.F. and S.F.P.; writing—review and editing, J.F.-F. and T.L.; funding acquisition, S.F.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Government of Cantabria through “Contrato Programa” which provides funding for the implementation of “Bridge Projects 2023” (Development of forestry bio economy through microwave-assisted stepwise pyrolysis, project PID2022-138142OB-I00).
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sridevi, V.; Surya, D.V.; Reddy, B.R.; Shah, M.; Gautam, R.; Kumar, T.H.; Puppala, H.; Pritam, K.S.; Basak, T. Challenges and opportunities in the production of sustainable hydrogen from lignocellulosic biomass using microwave-assisted pyrolysis: A review. Int. J. Hydrogen Energy 2023, 52 Pt A, 507–531. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nižetić, S.; Ong, H.C.; Mofijur, M.; Ahmed, S.F.; Ashok, B.; Bui, V.T.V.; Chau, M.Q. Insight into the recent advances of microwave pretreatment technologies for the conversion of lignocellulosic biomass into sustainable biofuel. Chemosphere 2021, 281, 130878. [Google Scholar] [CrossRef] [PubMed]
- International Energy Agency. World Energy Outlook 2023|Enhanced Reader. 2023. Available online: https://www.oecd-ilibrary.org/docserver/827374a6-en.pdf?expires=1727372493&id=id&accname=guest&checksum=D09E08F6E8794E5772E10F1FABA66EE0 (accessed on 25 September 2024).
- Motasemi, F.; Ani, F. A review on microwave-assisted production of biodiesel. Renew. Sustain. Energy Rev. 2012, 16, 4719–4733. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Bilal, M.; Salama, E.S.; Periyasamy, S.; Fattah, I.M.R.; Ruan, R.; Awasthi, M.K.; Leng, L. Phenolic-rich bio-oil production by microwave catalytic pyrolysis of switchgrass: Experimental study, life cycle assessment, and economic analysis. J. Clean. Prod. 2022, 366, 132668. [Google Scholar] [CrossRef]
- Mutsengerere, S.; Chihobo, C.H.; Musademba, D.; Nhapi, I. A review of operating parameters affecting bio-oil yield in microwave pyrolysis of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2019, 104, 328–336. [Google Scholar] [CrossRef]
- Zhu, H.; Saddler, J.; Bi, X. An economic and environmental assessment of biofuel produced via microwave-assisted catalytic pyrolysis of forest residues. Energy Convers. Manag. 2022, 263, 115723. [Google Scholar] [CrossRef]
- Selvam, S.M.; Paramasivan, B. Microwave assisted carbonization and activation of biochar for energy-environment nexus: A review. Chemosphere 2022, 286, 131631. [Google Scholar] [CrossRef]
- Zhang, J.; Tahmasebi, A.; Omoriyekomwan, J.E.; Yu, J. Direct synthesis of hollow carbon nanofibers on bio-char during microwave pyrolysis of pine nut shell. J. Anal. Appl. Pyrolysis 2018, 130, 142–148. [Google Scholar] [CrossRef]
- Makepa, D.C.; Chihobo, C.H.; Manhongo, T.T.; Musademba, D. Life-cycle assessment of microwave-assisted pyrolysis of pine sawdust as an emerging technology for biodiesel production. Results Eng. 2023, 20, 101480. [Google Scholar] [CrossRef]
- Arias, A.; Costa, C.E.; Feijoo, G.; Moreira, M.T.; Domingues, L. Process modeling, environmental and economic sustainability of the valorization of whey and eucalyptus residues for resveratrol biosynthesis. Waste Manag. 2023, 172, 226–234. [Google Scholar] [CrossRef]
- Farag, S.; Fu, D.; Jessop, P.G.; Chaouki, J. Detailed compositional analysis and structural investigation of a bio-oil from microwave pyrolysis of kraft lignin. J. Anal. Appl. Pyrolysis 2014, 109, 249–257. [Google Scholar] [CrossRef]
- Penín, L.; López, M.; Santos, V.; Alonso, J.L.; Parajó, J.C. Technologies for Eucalyptus wood processing in the scope of biorefineries: A comprehensive review. Bioresour. Technol. 2020, 311, 123528. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Yaashikaa, P.R.; Kumar, P.S.; Thamarai, P.; Deivayanai, V.C.; Rangasamy, G. A comprehensive review on techno-economic analysis of biomass valorization and conversional technologies of lignocellulosic residues. Ind. Crop. Prod. 2023, 200, 116822. [Google Scholar] [CrossRef]
- Iglesias, S.P.; Miyazaki, M.R.; Mariano, A.P.; Franco, T.T. Techno-economic assessment of bio-oil produced from Eucalyptus forestry residues. Ind. Crop. Prod. 2021, 171, 113936. [Google Scholar] [CrossRef]
- Wang, X.; Morrison, W.; Du, Z.; Wan, Y.; Lin, X.; Chen, P.; Ruan, R. Biomass temperature profile development and its implications under the microwave-assisted pyrolysis condition. Appl. Energy 2012, 99, 386–392. [Google Scholar] [CrossRef]
- Konstantinavičienė, J. Assessment of Potential of Forest Wood Biomass in Terms of Sustainable Development. Sustain. 2023, 15, 13871. [Google Scholar] [CrossRef]
- Gonçalves, M.; Freire, F.; Garcia, R. Material flow analysis of forest biomass in Portugal to support a circular bioeconomy. Resour. Conserv. Recycl. 2021, 169. [Google Scholar] [CrossRef]
- Chen, X.; Che, Q.; Li, S.; Liu, Z.; Yang, H.; Chen, Y.; Wang, X.; Shao, J.; Chen, H. Recent developments in lignocellulosic biomass catalytic fast pyrolysis: Strategies for the optimization of bio-oil quality and yield. Fuel Process. Technol. 2019, 196, 106180. [Google Scholar] [CrossRef]
- Salema, A.A.; Ani, F.N. Pyrolysis of oil palm empty fruit bunch biomass pellets using multimode microwave irradiation. Bioresour. Technol. 2012, 125, 102–107. [Google Scholar] [CrossRef]
- Foong, S.Y.; Liew, R.K.; Yang, Y.; Cheng, Y.W.; Yek, P.N.Y.; Wan Mahari, W.A.; Lee, X.Y.; Han, C.S.; Vo, D.V.N.; Van Le, Q.; et al. Valorization of biomass waste to engineered activated biochar by microwave pyrolysis: Progress, challenges, and future directions. Chem. Eng. J. 2020, 389, 124401. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Papari, S.; Hawboldt, K. A review on the pyrolysis of woody biomass to bio-oil: Focus on kinetic models. Renew. Sustain. Energy Rev. 2015, 52, 1580–1595. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Ellis, N.; Kim, C.S.; Bi, X. Microwave-assisted catalytic biomass pyrolysis: Effects of catalyst mixtures. Appl. Catal. B Environ. 2019, 253, 226–234. [Google Scholar] [CrossRef]
- Fan, J.; Shuttleworth, P.S.; Gronnow, M.; Breeden, S.W.; Clark, J.H.; Macquarrie, D.J.; Budarin, V.L. Influence of Density on Microwave Pyrolysis of Cellulose. ACS Sustain. Chem. Eng. 2018, 6, 2916–2920. [Google Scholar] [CrossRef]
- Ge, S.; Yek, P.N.Y.; Cheng, Y.W.; Xia, C.; Wan Mahari, W.A.; Liew, R.K.; Peng, W.; Yuan, T.Q.; Tabatabaei, M.; Aghbashlo, M.; et al. Progress in microwave pyrolysis conversion of agricultural waste to value-added biofuels: A batch to continuous approach. Renew. Sustain. Energy Rev. 2021, 135, 110148. [Google Scholar] [CrossRef]
- Tabakaev, R.; Kalinich, I.; Dimitryuk, I.; Asilbekov, A.; Astafev, A.; Ibraeva, K.; Shanenkov, I.; Mostovshchikov, A.; Chumerin, P. Experimental study of microwave processing of pine nut shells into a high-calorie gas: Main results and physicochemical features. J. Anal. Appl. Pyrolysis 2023, 176, 106264. [Google Scholar] [CrossRef]
- Li, J.; Dai, J.; Liu, G.; Zhang, H.; Gao, Z.; Fu, J.; He, Y.; Huang, Y. Biochar from microwave pyrolysis of biomass: A review. Biomass Bioenergy 2016, 94, 228–244. [Google Scholar] [CrossRef]
- Akbari, M.; Kumar, A. The development of data-intensive techno-economic models for the comparison of renewable natural gas production from six different biomass feedstocks for the decarbonization of energy demand sectors. Fuel 2024, 358, 130107. [Google Scholar] [CrossRef]
- Patel, P.; Vaezi, M.; Billal, M.M.; Kumar, A. Development of data-intensive techno-economic models for the assessment of a biomass, waste heat, and MSW integrated waste-to-electricity facility. Resour. Conserv. Recycl. Adv. 2023, 20, 200188. [Google Scholar] [CrossRef]
- Tisserant, A.; Hu, X.; Liu, Q.; Xie, Z.; Zhao, W.; Cherubini, F. Biochar and Its Potential to Deliver Negative Emissions and Better Soil Quality in Europe. Earth’s Futur. 2023, 11, e2022EF003246. [Google Scholar] [CrossRef]
- França, L.C.d.J.; e Silva, C.S.J.; Mucida, D.P.; da Costa, J.S.; Gomide, L.R. Towards renewable energy projects under sustainable watersheds principles for forest biomass supply. Biomass Bioenergy 2023, 176, 106916. [Google Scholar] [CrossRef]
- Brunel, C.; Farnet Da Silva, A.M.; Lerch, T.Z.; Gros, R. Influence of tree residue retention in Mediterranean forest on soil microbial communities responses to frequent warming and drying events. Eur. J. Soil Biol. 2023, 118, 103541. [Google Scholar] [CrossRef]
- Freer-Smith, P.; Bailey-Bale, J.H.; Donnison, C.L.; Taylor, G. The good, the bad, and the future: Systematic review identifies best use of biomass to meet air quality and climate policies in California. GCB Bioenergy 2023, 15, 1312–1328. [Google Scholar] [CrossRef]
- Natural Resources Canada. Is Forest Bioenergy Good for the Environment? Natural Resources Canada: Ottawa, ON, Canada, 2010. [Google Scholar]
- Serra, R.; Niknia, I.; Paré, D.; Titus, B.; Gagnon, B.; Laganière, J. From conventional to renewable natural gas: Can we expect GHG savings in the near term? Biomass Bioenergy 2019, 131, 105396. [Google Scholar] [CrossRef]
- Jordan, M.; Meisel, K.; Dotzauer, M.; Schröder, J.; Cyffka, K.F.; Dögnitz, N.; Schmid, C.; Lenz, V.; Naumann, K.; Daniel-Gromke, J.; et al. The controversial role of energy crops in the future German energy system: The trade offs of a phase-out and allocation priorities of the remaining biomass residues. Energy Rep. 2023, 10, 3848–3858. [Google Scholar] [CrossRef]
- Rijal, P.; Bras, P.; Garrido, S.; Matias, J.; Pimentel, C.; Carvalho, H. Residual Forestry Biomass Supply Chain: A Mapping Approach. Int. J. Ind. Eng. Manag. 2023, 14, 244–256. [Google Scholar] [CrossRef]
- Negi, H.; Suyal, D.C.; Soni, R.; Giri, K.; Goel, R. Indian Scenario of Biomass Availability and Its Bioenergy-Conversion Potential. Energies 2023, 16, 5805. [Google Scholar] [CrossRef]
- Gan, X.; Guo, B.; Ma, Z.; Fang, M.; Yan, Y.; Liu, W. The Effect of Forest Growth Rate on Climate Change Impacts of Logging Residue Utilization. Atmosphere 2023, 14, 1270. [Google Scholar] [CrossRef]
- Watanabe, M.D.B.; Hu, X.; Ballal, V.; Cavalett, O.; Cherubini, F. Climate change mitigation potentials of on grid-connected Power-to-X fuels and advanced biofuels for the European maritime transport. Energy Convers. Manag. X 2023, 20, 100418. [Google Scholar] [CrossRef]
- Hu, J.; Jåstad, E.O.; Bolkesjø, T.F.; Rørstad, P.K. Impact of large-scale Bio-CCS deployment on forest biomass competition and forest industry production. Biomass Bioenergy 2023, 175, 106896. [Google Scholar] [CrossRef]
- Ibitoye, S.E.; Mahamood, R.M.; Jen, T.C.; Loha, C.; Akinlabi, E.T. An overview of biomass solid fuels: Biomass sources, processing methods, and morphological and microstructural properties. J. Bioresour. Bioprod. 2023, 8, 333–360. [Google Scholar] [CrossRef]
- Rehan, M.; Amir Raza, M.; Ghani Abro, A.; M Aman, M.; Mohammad Ibrahim Ismail, I.; Sattar Nizami, A.; Imtiaz Rashid, M.; Summan, A.; Shahzad, K.; Ali, N. A sustainable use of biomass for electrical energy harvesting using distributed generation systems. Energy 2023, 278, 128036. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Shetti, N.P.; Reddy, K.R.; Nadagouda, M.N.; Badawi, M.; Bonilla-Petriciolet, A.; Aminabhavi, T.M. Valorization of biowastes for clean energy production, environmental depollution and soil fertility. J. Environ. Manag. 2023, 332, 117410. [Google Scholar] [CrossRef]
- Zhou, C.H.; Xia, X.; Lin, C.X.; Tong, D.S.; Beltramini, J. Beltramini Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 2011, 40, 5588–5617. [Google Scholar] [CrossRef]
- Wang, F.; Ouyang, D.; Zhou, Z.; Page, S.J.; Liu, D.; Zhao, X. Lignocellulosic biomass as sustainable feedstock and materials for power generation and energy storage. J. Energy Chem. 2021, 57, 247–280. [Google Scholar] [CrossRef]
- European Union. Infographics on Biomass Sources and Uses in the EU-27; European Commission: 2022. Available online: https://knowledge4policy.ec.europa.eu/publication/infographics-biomass-sources-uses-eu-27-2017-data_en (accessed on 25 September 2024).
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo de Medeiros, G.; do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Morgan, H.M.; Bu, Q.; Liang, J.; Liu, Y.; Mao, H.; Shi, A.; Lei, H.; Ruan, R. A review of catalytic microwave pyrolysis of lignocellulosic biomass for value-added fuel and chemicals. Bioresour. Technol. 2017, 230, 112–121. [Google Scholar] [CrossRef]
- Al Shra’Ah, A.; Helleur, R. Microwave pyrolysis of cellulose at low temperature. J. Anal. Appl. Pyrolysis 2014, 105, 91–99. [Google Scholar] [CrossRef]
- Wang, N.; Tahmasebi, A.; Yu, J.; Xu, J.; Huang, F.; Mamaeva, A. A Comparative study of microwave-induced pyrolysis of lignocellulosic and algal biomass. Bioresour. Technol. 2015, 190, 89–96. [Google Scholar] [CrossRef] [PubMed]
- European Commission Forests. Available online: https://international-partnerships.ec.europa.eu/publications/forest-partnerships-factsheets_en (accessed on 20 August 2024).
- Chiang, L.E.; Castro, F.A.; Molina, F.A. Socioeconomic and environmental benefits of substituting firewood with charcoal briquettes produced from biomass residues in the Forestry Belt in Chile. Energy Sustain. Dev. 2023, 77, 101341. [Google Scholar] [CrossRef]
- Wang, R.; Cai, W.; Yu, L.; Li, W.; Zhu, L.; Cao, B.; Li, J.; Shen, J.; Zhang, S.; Nie, Y.; et al. A high spatial resolution dataset of China’s biomass resource potential. Sci. Data 2023, 10, 384. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.P.; Teixeira, S.; Teixeira, J.C. Characterization of the physicochemical and thermal properties of different forest residues. Biomass Bioenergy 2023, 175, 106870. [Google Scholar] [CrossRef]
- Węgiel, A.; Jakubowski, J.; Molińska-Glura, M.; Polowy, K.; Węgiel, J.; Gornowicz, R. Effect of logging residue removal and mechanical site preparation on productivity of the subsequent Scots pine (Pinus sylvestris L.) stands. Ann. For. Sci. 2023, 80, 5. [Google Scholar] [CrossRef]
- Arpia, A.A.; Chen, W.H.; Lam, S.S.; Rousset, P.; de Luna, M.D.G. Sustainable biofuel and bioenergy production from biomass waste residues using microwave-assisted heating: A comprehensive review. Chem. Eng. J. 2021, 403, 126233. [Google Scholar] [CrossRef]
- Motasemi, F.; Afzal, M.T. A review on the microwave-assisted pyrolysis technique. Renew. Sustain. Energy Rev. 2013, 28, 317–330. [Google Scholar] [CrossRef]
- Hadiya, V.; Popat, K.; Vyas, S.; Varjani, S.; Vithanage, M.; Kumar Gupta, V.; Núñez Delgado, A.; Zhou, Y.; Loke Show, P.; Bilal, M.; et al. Biochar production with amelioration of microwave-assisted pyrolysis: Current scenario, drawbacks and perspectives. Bioresour. Technol. 2022, 355, 127303. [Google Scholar] [CrossRef]
- Velvizhi, G.; Jacqueline, P.J.; Shetti, N.P.; Latha, K.; Mohanakrishna, G.; Aminabhavi, T.M. Emerging trends and advances in valorization of lignocellulosic biomass to biofuels. J. Environ. Manag. 2023, 345, 118527. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Tejasvi, R. A review on role of process parameters on pyrolysis of biomass and plastics: Present scope and future opportunities in conventional and microwave-assisted pyrolysis technologies. Process Saf. Environ. Prot. 2022, 162, 435–462. [Google Scholar] [CrossRef]
- Ronsse, F.; Nachenius, R.W.; Prins, W. Carbonization of Biomass. Elsevier B.V.: Amsterdam, The Netherlands, 2015; ISBN 9780444632906. [Google Scholar]
- Huang, Y.F.; Chiueh, P.T.; Lo, S.L. A review on microwave pyrolysis of lignocellulosic biomass. Sustain. Environ. Res. 2016, 26, 103–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, W.T. 5—Hydrothermal liquefaction of protein-containing feedstocks. In Direct Thermochemical Liquefaction for Energy Applications; Rosendahl, L., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 127–168. ISBN 9780081010297. [Google Scholar]
- Guo, H.; Chang, Y.; Lee, D.J.; Tien Thanh, N.; Mostapha, M.; Lam, M.K.; Ishak, S.; Kanna Dasan, Y.; Lim, J.W.; Tan, I.S.; et al. Cellulosic biomass fermentation for biofuel production: Review of artificial intelligence approaches. Renew. Sustain. Energy Rev. 2024, 98, 95–123. [Google Scholar] [CrossRef]
- Guo, H.; Chang, Y.; Lee, D.J. Enzymatic saccharification of lignocellulosic biorefinery: Research focuses. Bioresour. Technol. 2018, 252, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Stickel, J.J.; Elander, R.T.; Mcmillan, J.D.; Brunecky, R. Chapter 4 Enzymatic Hydrolysis of Lignocellulosic Biomass. In Bioprocessing of Renewable Resources to Commodity Bioproducts; Virendra, S., Bisaria, A.K., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2014; pp. 77–103. [Google Scholar]
- Tien Thanh, N.; Mostapha, M.; Lam, M.K.; Ishak, S.; Kanna Dasan, Y.; Lim, J.W.; Tan, I.S.; Lau, S.Y.; Chin, B.L.F.; Hadibarata, T. Fundamental understanding of in-situ transesterification of microalgae biomass to biodiesel: A critical review. Energy Convers. Manag. 2022, 270, 116212. [Google Scholar] [CrossRef]
- Santos, F.; Eichler, P.; de Queiroz, J.H.; Gomes, F. (Eds.) Chapter 11—Production of second-generation ethanol from sugarcane. In Sugarcane Biorefinery, Technology and Perspectives; Academic Press: Cambridge, MA, USA, 2020; pp. 195–228. ISBN 9780128142363. [Google Scholar]
- Prado, J.M.; Lachos-Perez, D.; Forster-Carneiro, T.; Rostagno, M.A. Sub- and supercritical water hydrolysis of agricultural and food industry residues for the production of fermentable sugars: A review. Food Bioprod. Process. 2016, 98, 95–123. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Conversion of Biomass to Biofuels and Life Cycle Assessment: A Review; Springer International Publishing: Cham, Switzerland, 2021; Volume 19, ISBN 0123456789. [Google Scholar]
- García-Velásquez, C.A.; Cardona, C.A. Comparison of the biochemical and thermochemical routes for bioenergy production: A techno-economic (TEA), energetic and environmental assessment. Energy 2019, 172, 232–242. [Google Scholar] [CrossRef]
- Liu, T.; Miao, P.; Shi, Y.; Tang, K.H.D.; Yap, P.S. Recent advances, current issues and future prospects of bioenergy production: A review. Sci. Total Environ. 2022, 810, 152181. [Google Scholar] [CrossRef]
- Syed, N.R.; Zhang, B.; Mwenya, S.; Aldeen, A.S. A Systematic Review on Biomass Treatment Using Microwave-Assisted Pyrolysis under PRISMA Guidelines. Molecules 2023, 28, 5551. [Google Scholar] [CrossRef]
- Andooz, A.; Eqbalpour, M.; Kowsari, E.; Ramakrishna, S.; Ansari Cheshmeh, Z. A comprehensive review on pyrolysis from the circular economy point of view and its environmental and social effects. J. Clean. Prod. 2023, 388, 136021. [Google Scholar] [CrossRef]
- Roy, P.; Dias, G. Prospects for pyrolysis technologies in the bioenergy sector: A review. Renew. Sustain. Energy Rev. 2017, 77, 59–69. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Vuppaladadiyam, S.S.V.; Awasthi, A.; Sahoo, A.; Rehman, S.; Pant, K.K.; Murugavelh, S.; Huang, Q.; Anthony, E.; Fennel, P.; et al. Biomass pyrolysis: A review on recent advancements and green hydrogen production. Bioresour. Technol. 2022, 364, 128087. [Google Scholar] [CrossRef]
- Oh, S.; Lee, J.; Lam, S.S.; Kwon, E.E.; Ha, J.M.; Tsang, D.C.W.; Ok, Y.S.; Chen, W.H.; Park, Y.K. Fast hydropyrolysis of biomass Conversion: A comparative review. Bioresour. Technol. 2021, 342, 126067. [Google Scholar] [CrossRef]
- Pahnila, M.; Koskela, A.; Sulasalmi, P.; Fabritius, T.; Roy, P.; Dias, G.; Andooz, A.; Eqbalpour, M.; Kowsari, E.; Ramakrishna, S.; et al. A Review of Pyrolysis Technologies and the Effect of Process Parameters on Biocarbon Properties. Renew. Sustain. Energy Rev. 2023, 388, 6936. [Google Scholar] [CrossRef]
- Resende, F.L.P. Recent advances on fast hydropyrolysis of biomass. Catal. Today 2016, 269, 148–155. [Google Scholar] [CrossRef]
- Vignesh, N.S.; Soosai, M.R.; Chia, W.Y.; Wahid, S.N.; Varalakshmi, P.; Moorthy, I.M.G.; Ashokkumar, B.; Arumugasamy, S.K.; Selvarajoo, A.; Chew, K.W. Microwave-assisted pyrolysis for carbon catalyst, nanomaterials and biofuel production. Fuel 2022, 313, 123023. [Google Scholar] [CrossRef]
- Fricler, V.Y.; Nyashina, G.S.; Vershinina, K.Y.; Vinogrodskiy, K.V.; Shvets, A.S.; Strizhak, P.A. Microwave pyrolysis of agricultural waste: Influence of catalysts, absorbers, particle size and blending components. J. Anal. Appl. Pyrolysis 2023, 171, 105962. [Google Scholar] [CrossRef]
- Abdelsayed, V.; Ellison, C.; Trubetskaya, A.; Smith, M.; Shekhawat, D. Effect of Microwave and Thermal Co-pyrolysis of Low Rank Coal and Pine Wood on Product Distributions and Char Structure. Energy Fuels 2019, 33, 7069–7082. [Google Scholar] [CrossRef]
- Li, X.; Li, K.; Geng, C.; El Mashad, H.; Li, H.; Yin, W. Biochar from microwave pyrolysis of artemisia slengensis: Characterization and methylene blue adsorption capacity. Appl. Sci. 2019, 9, 1813. [Google Scholar] [CrossRef]
- Su, G.; Ong, H.C.; Cheah, M.Y.; Chen, W.H.; Lam, S.S.; Huang, Y. Microwave-assisted pyrolysis technology for bioenergy recovery: Mechanism, performance, and prospect. Fuel 2022, 326, 124983. [Google Scholar] [CrossRef]
- Ao, W.; Fu, J.; Mao, X.; Kang, Q.; Ran, C.; Liu, Y.; Zhang, H.; Gao, Z.; Li, J.; Liu, G.; et al. Microwave assisted preparation of activated carbon from biomass: A review. Renew. Sustain. Energy Rev. 2018, 92, 958–979. [Google Scholar] [CrossRef]
- Yadav, S.P.S.; Bhandari, S.; Bhatta, D.; Poudel, A.; Bhattarai, S.; Yadav, P.; Ghimire, N.; Paudel, P.; Shrestha, J.; Oli, B. Biochar application: A sustainable approach to improve soil health. J. Agric. Food Res. 2023, 11, 100498. [Google Scholar]
- Zhou, R.; Lei, H.; Julson, J.L. Effects of reaction temperature, time and particle size on switchgrass microwave pyrolysis and reaction kinetics. Int. J. Agric. Biol. Eng. 2013, 6, 53–61. [Google Scholar] [CrossRef]
- Zheng, A.; Xia, S.; Cao, F.; Liu, S.; Yang, X.; Zhao, Z.; Tian, Y.; Li, H. Directional valorization of eucalyptus waste into value-added chemicals by a novel two-staged controllable pyrolysis process. Chem. Eng. J. 2021, 404, 127045. [Google Scholar] [CrossRef]
- Luo, H.; Bao, L.; Kong, L.; Sun, Y. Low temperature microwave-assisted pyrolysis of wood sawdust for phenolic rich compounds: Kinetics and dielectric properties analysis. Bioresour. Technol. 2017, 238, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Karthäuser, J.; Biziks, V.; Frauendorf, H.; Hoffmann, L.; Raskop, S.; Roggatz, D.; Militz, H. Substituting phenol in phenol–formaldehyde resins for wood modification by phenolic cleavage products from vacuum low-temperature microwave-assisted pyrolysis of softwood kraft lignin. Cellulose 2023, 30, 7277–7293. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Wang, L.; Yadavalli, G.; Wei, Y.; Zhang, X.; Zhu, L.; Liu, Y. Biofuel production from catalytic microwave pyrolysis of Douglas fir pellets over ferrum-modified activated carbon catalyst. J. Anal. Appl. Pyrolysis 2015, 112, 74–79. [Google Scholar] [CrossRef]
- Reddy, B.R.; Sridevi, V.; Kumar, T.H.; Rao, C.S.; Palla, V.C.S.; Suriapparao, D.V.; Undi, G.S. Synthesis of renewable carbon biorefinery products from susceptor enhanced microwave-assisted pyrolysis of agro-residual waste: A review. Process Saf. Environ. Prot. 2022, 164, 354–372. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Liu, S.; Peng, P.; Min, M.; Cheng, Y.; Anderson, E.; Zhou, N.; Fan, L.; Liu, C.; et al. Effects of feedstock characteristics on microwave-assisted pyrolysis—A review. Bioresour. Technol. 2017, 230, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lin, L.; Ju, T.; Meng, F.; Han, S.; Chen, K.; Jiang, J. Microwave-assisted pyrolysis of solid waste for production of high-value liquid oil, syngas, and carbon solids: A review. Renew. Sustain. Energy Rev. 2024, 189, 113979. [Google Scholar] [CrossRef]
- Sharma, A.K.; Ghodke, P.K.; Goyal, N.; Bobde, P.; Kwon, E.E.; Lin, K.Y.A.; Chen, W.H. A critical review on biochar production from pine wastes, upgradation techniques, environmental sustainability, and challenges. Bioresour. Technol. 2023, 387, 129632. [Google Scholar] [CrossRef] [PubMed]
- Lapshinov, B.A. Temperature Measurement Methods in Microwave Heating Technologies. Meas. Tech. 2021, 64, 453–462. [Google Scholar] [CrossRef]
- Chemat, F.; Esveld, E. Microwave super-heated boiling of organic liquids: Oigin, effect and application. Chem. Eng. Technol. 2001, 24, 735–744. [Google Scholar] [CrossRef]
- Feng, H.; Yin, Y.; Tang, J. Microwave Drying of Food and Agricultural Materials: Basics and Heat and Mass Transfer Modeling. Food Eng. Rev. 2012, 4, 89–106. [Google Scholar] [CrossRef]
- Anwar, J.; Shafique, U.; Zaman, W.U.; Rehman, R.; Salman, M.; Dar, A.; Anzano, J.M.; Ashraf, U.; Ashraf, S. Microwave chemistry: Effect of ions on dielectric heating in microwave ovens. Arab. J. Chem. 2015, 8, 100–104. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Tanneru, H.K.; Reddy, B.R. A review on the role of susceptors in the recovery of valuable renewable carbon products from microwave-assisted pyrolysis of lignocellulosic and algal biomasses: Prospects and challenges. Environ. Res. 2022, 215, 114378. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, H.S.; Raghavan, V.G.S.; Santos, T.; Hennetier, L.; Costa, V.A.F.; Costa, L.C.; Liu, H.P.; Chen, T.P.; Li, Y.; Song, Z.Y.; et al. Temperature rise characteristics of ZhunDong coal during microwave pyrolysis. J. Manuf. Process. 2016, 148, 92–100. [Google Scholar] [CrossRef]
- Ramaswamy, H.S.; Rauber, J.M.; Raghavan, G.V.; Van De Voort, F.R. Evaluation of shielded thermocouples for measuring temperature of foods in a microwave oven. J. Food Sci. Technol. 1998, 35, 325–329. [Google Scholar]
- Santos, T.; Hennetier, L.; Costa, V.A.F.; Costa, L.C. Microwave versus conventional porcelain firing: Temperature measurement. J. Manuf. Process. 2019, 41, 92–100. [Google Scholar] [CrossRef]
- Siddique, I.J.; Salema, A.A. Unraveling the metallic thermocouple effects during microwave heating of biomass. Energy 2023, 267, 126529. [Google Scholar] [CrossRef]
- Wada, D.; Sugiyama, J.I.; Zushi, H.; Murayama, H. An optical fiber sensing technique for temperature distribution measurements in microwave heating. Meas. Sci. Technol. 2015, 26, 085105. [Google Scholar] [CrossRef]
- Lapshinov, B.A.; Mamontov, A.V. Application of Spectral Pyrometry Under Conditions of Intense Microwave Electromagnetic Fields. Meas. Tech. 2020, 63, 741–746. [Google Scholar] [CrossRef]
- Valverde, C.; Rodríguez-García, M.M.; Rojas, E.; Bayón, R. State of the art of the fundamental aspects in the concept of microwave-assisted heating systems. Int. Commun. Heat Mass Transf. 2024, 156, 107594. [Google Scholar] [CrossRef]
- Gulyaev, I.P.; Dolmatov, A.V. Spectral-brightness pyrometry: Radiometric measurements of non-uniform temperature distributions. Int. J. Heat Mass Transf. 2018, 116, 1016–1025. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Tahir, F.; Pradhan, S.; Al-Ansari, T.; McKay, G. Life cycle assessment of biofuel production from waste date stones using conventional and microwave pyrolysis. Energy Convers. Manag. X 2024, 21, 100510. [Google Scholar] [CrossRef]
- Seow, Y.X.; Tan, Y.H.; Kansedo, J.; Tan, I.S.; Chin, B.L.F.; Mubarak, N.M.; Bin Mohiddin, M.N.; Yek, P.N.Y.; Chan, Y.S.; Abdullah, M.O. Pyrolysis assessment of palm kernel shell waste valorization to sulfonated magnetic biochar from techno-economic and energy perspectives. Discov. Appl. Sci. 2024, 6, 398. [Google Scholar] [CrossRef]
- Muniyappan, D.; Lima, G.R.; Pereira, A.O.; Gopi, R.; Ramanathan, A. Multivariate combined optimization strategy and comparative life-cycle assessment of biomass and plastic residues via microwave co-pyrolysis approach towards a sustainable synthesis of renewable hydrocarbon fuel. J. Environ. Chem. Eng. 2023, 11, 111436. [Google Scholar] [CrossRef]
- Fodah, A.E.M.; Abdelwahab, T.A.M. Process optimization and technoeconomic environmental assessment of biofuel produced by solar powered microwave pyrolysis. Sci. Rep. 2022, 12, 12572. [Google Scholar] [CrossRef]
- Foong, S.Y.; Chan, Y.H.; Yek, P.N.Y.; Lock, S.S.M.; Chin, B.L.F.; Yiin, C.L.; Lan, J.C.W.; Lam, S.S. Microwave-assisted pyrolysis in biomass and waste valorisation: Insights into the life-cycle assessment (LCA) and techno-economic analysis (TEA). Chem. Eng. J. 2024, 491, 151942. [Google Scholar] [CrossRef]
- Ren, X.; Shanb Ghazani, M.; Zhu, H.; Ao, W.; Zhang, H.; Moreside, E.; Zhu, J.; Yang, P.; Zhong, N.; Bi, X. Challenges and opportunities in microwave-assisted catalytic pyrolysis of biomass: A review. Appl. Energy 2022, 315, 118970. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Liu, S.; Fan, L.; Zhou, N.; Min, M.; Cheng, Y.; Peng, P.; Anderson, E.; Wang, Y.; et al. Microwave-Assisted Pyrolysis of Biomass for Bio-Oil Production. In Pyrolysis; InTech: Houston, TX, USA, 2017. [Google Scholar]
- Fernandes, A.; Cruz-Lopes, L.; Esteves, B.; Evtuguin, D.V. Microwaves and Ultrasound as Emerging Techniques for Lignocellulosic Materials. Materials 2023, 16, 7351. [Google Scholar] [CrossRef]
- Ravikumar, C.; Senthil Kumar, P.; Subhashni, S.K.; Tejaswini, P.V.; Varshini, V. Microwave assisted fast pyrolysis of corn cob, corn stover, saw dust and rice straw: Experimental investigation on bio-oil yield and high heating values. Sustain. Mater. Technol. 2017, 11, 19–27. [Google Scholar] [CrossRef]
- Thue, P.S.; Adebayo, M.A.; Lima, E.C.; Sieliechi, J.M.; Machado, F.M.; Dotto, G.L.; Vaghetti, J.C.P.; Dias, S.L.P. Preparation, characterization and application of microwave-assisted activated carbons from wood chips for removal of phenol from aqueous solution. J. Mol. Liq. 2016, 223, 1067–1080. [Google Scholar] [CrossRef]
- Giorcelli, M.; Das, O.; Sas, G.; Försth, M.; Bartoli, M. A review of bio-oil production through microwave-Assisted pyrolysis. Processes 2021, 9, 561. [Google Scholar] [CrossRef]
- Yang, Y.; Shahbeik, H.; Shafizadeh, A.; Masoudnia, N.; Rafiee, S.; Zhang, Y.; Pan, J.; Tabatabaei, M.; Aghbashlo, M. Biomass microwave pyrolysis characterization by machine learning for sustainable rural biorefineries. Renew. Energy 2022, 201, 70–86. [Google Scholar] [CrossRef]
- Pritam, K.; Puppala, H.; Palla, S.; Suriapparao, D.V.; Basak, T. A two-step hybrid multi-criteria approach to analyze the significance of parameters affecting microwave-assisted pyrolysis. Process Saf. Environ. Prot. 2023, 171, 975–985. [Google Scholar] [CrossRef]
- Zheng, A.; Zhao, K.; Sun, J.; Jiang, L.; Zhao, Z.; Huang, Z.; Wei, G.; He, F.; Li, H. Effect of microwave-assisted organosolv fractionation on the chemical structure and decoupling pyrolysis behaviors of waste biomass. J. Anal. Appl. Pyrolysis 2018, 131, 120–127. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Luo, K.; Shao, J.; Yang, H. The influence of microwave drying on biomass pyrolysis. Energy and Fuels 2008, 22, 67–74. [Google Scholar] [CrossRef]
- Liang, J.; Xu, X.; Yu, Z.; Chen, L.; Liao, Y.; Ma, X. Effects of microwave pretreatment on catalytic fast pyrolysis of pine sawdust. Bioresour. Technol. 2019, 293, 122080. [Google Scholar] [CrossRef]
- Karthäuser, J.; Biziks, V.; Frauendorf, H.; Mai, C.; Militz, H. Vacuum Low-Temperature Microwave-Assisted Pyrolysis of Technical Lignins. Polymers 2022, 14, 3383. [Google Scholar] [CrossRef]
- Venegas-Vásconez, D.; Arteaga-Pérez, L.E.; Aguayo, M.G.; Romero-Carrillo, R.; Guerrero, V.H.; Tipanluisa-Sarchi, L.; Alejandro-Martín, S. Analytical Pyrolysis of Pinus radiata and Eucalyptus globulus: Effects of Microwave Pretreatment on Pyrolytic Vapours Composition. Polymers 2023, 15, 3790. [Google Scholar] [CrossRef]
- Yang, X.; Cui, C.; Zheng, A.; Zhao, Z.; Wang, C.; Xia, S.; Huang, Z.; Wei, G.; Li, H. Ultrasonic and microwave assisted organosolv pretreatment of pine wood for producing pyrolytic sugars and phenols. Ind. Crop. Prod. 2020, 157, 112921. [Google Scholar] [CrossRef]
- Duan, X.; Srinivasakannan, C.; Peng, J.; Zhang, L.; Zhang, Z. Comparison of activated carbon prepared from Jatropha hull by conventional heating and microwave heating. Biomass Bioenergy 2011, 35, 3920–3926. [Google Scholar] [CrossRef]
- Mašek, O.; Budarin, V.; Gronnow, M.; Crombie, K.; Brownsort, P.; Fitzpatrick, E.; Hurst, P. Microwave and slow pyrolysis biochar—Comparison of physical and functional properties. J. Anal. Appl. Pyrolysis 2013, 100, 41–48. [Google Scholar] [CrossRef]
- Merckel, R.D. Fast and Microwave-Induced Pyrolysis Bio-Oil from Eucalyptus Grandis: Possibilities for Upgrading. Master’s Thesis, University of Pretoria (South Africa), Pretoria, South Africa, 2014. [Google Scholar]
- Du, J.; Liu, P.; Zuo-Hua, L.; Da-Gui, S.; Chang-Yuan, T. Fast pyrolysis of biomass for bio-oil with ionic liquid and microwave irradiation. J. Fuel Chem. Technol. 2010, 38, 554–559. [Google Scholar] [CrossRef]
- Wan, Y.; Chen, P.; Zhang, B.; Yang, C.; Liu, Y.; Lin, X.; Ruan, R. Microwave-assisted pyrolysis of biomass: Catalysts to improve product selectivity. J. Anal. Appl. Pyrolysis 2009, 86, 161–167. [Google Scholar] [CrossRef]
- Bartoli, M.; Rosi, L.; Giovannelli, A.; Frediani, P.; Frediani, M. Bio-oil from residues of short rotation coppice of poplar using a microwave assisted pyrolysis. J. Anal. Appl. Pyrolysis 2016, 119, 224–232. [Google Scholar] [CrossRef]
- Lo, S.L.; Huang, Y.F.; Chiueh, P.T.; Kuan, W.H. Microwave Pyrolysis of Lignocellulosic Biomass. Energy Procedia 2017, 105, 41–46. [Google Scholar] [CrossRef]
- Huang, Y.F.; Chiueh, P.T.; Kuan, W.H.; Lo, S.L. Effects of lignocellulosic composition and microwave power level on the gaseous product of microwave pyrolysis. Energy 2015, 89, 974–981. [Google Scholar] [CrossRef]
- Shi, K.; Yan, J.; Menéndez, J.A.; Luo, X.; Yang, G.; Chen, Y.; Lester, E.; Wu, T. Production of H2-Rich Syngas From Lignocellulosic Biomass Using Microwave-Assisted Pyrolysis Coupled With Activated Carbon Enabled Reforming. Front. Chem. 2020, 8, 3. [Google Scholar] [CrossRef]
- Wauts, J. Catalytic Microwave Pyrolysis to Produce Upgraded Bio-Oil. Master’s Thesis, University of Pretoria (South Africa), Pretoria, South Africa, 2016; pp. 1–111. [Google Scholar]
- Gronnow, M.J.; Budarin, V.L.; Mašek, O.; Crombie, K.N.; Brownsort, P.A.; Shuttleworth, P.S.; Hurst, P.R.; Clark, J.H. Torrefaction/biochar production by microwave and conventional slow pyrolysis—Comparison of energy properties. GCB Bioenergy 2013, 5, 144–152. [Google Scholar] [CrossRef]
- Huang, Y.F.; Chiueh, P.T.; Kuan, W.H.; Lo, S.L. Product distribution and heating performance of lignocellulosic biomass pyrolysis using microwave heating. Energy Procedia 2018, 152, 910–915. [Google Scholar] [CrossRef]
- Dong, Q.; Xiong, Y. Kinetics study on conventional and microwave pyrolysis of moso bamboo. Bioresour. Technol. 2014, 171, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.T.; Sanz, A.B.; Nozal, L.; Castro, F.; Alonso, R.; Aguirre, J.L.; González, S.D.; Matía, M.P.; Novella, J.L.; Peinado, M.; et al. Microwave-assisted pyrolysis of Mediterranean forest biomass waste: Bioproduct characterization. J. Anal. Appl. Pyrolysis 2017, 127, 278–285. [Google Scholar] [CrossRef]
- Parvez, A.M.; Afzal, M.T.; Jiang, P.; Wu, T. Microwave-assisted biomass pyrolysis polygeneration process using a scaled-up reactor: Product characterization, thermodynamic assessment and bio-hydrogen production. Biomass Bioenergy 2020, 139, 105651. [Google Scholar] [CrossRef]
- Nhuchhen, D.R.; Afzal, M.T.; Dreise, T.; Salema, A.A. Characteristics of biochar and bio-oil produced from wood pellets pyrolysis using a bench scale fixed bed, microwave reactor. Biomass Bioenergy 2018, 119, 293–303. [Google Scholar] [CrossRef]
- Sellamuthu, S.; Chowdhury, Z.Z.; Khalid, K.; Shibly, S.M.; Rahman, M.M.; Rana, M.; Badruddin, I.A.; Khaleed, H.M.T.; Kamangar, S.; Johan, M.R.B.; et al. Mathematical Modelling and Optimization for Facile Synthesis of Structured Activated Carbon (ACs) from Adansonia kilima (Baobab) Wood Chips Integrating Microwave-Assisted Pyrolysis for the Elimination of Lead (II) Cations from Wastewater Effluents. Molecules 2023, 28, 6640. [Google Scholar] [CrossRef]
- Zhao, Z.; Jiang, Z.; Lin, L.; Qiu, R.; Yan, K. Synthesis of alkoxyphenols-rich bio-oil by microwave-assisted catalytic pyrolysis of wood over MoS2 catalyst. Renew. Energy 2023, 219, 119491. [Google Scholar] [CrossRef]
- Khelfa, A.; Rodrigues, F.A.; Koubaa, M.; Vorobiev, E. Microwave-assisted pyrolysis of pine wood sawdust mixed with activated carbon for bio-oil and bio-char production. Processes 2020, 8, 1437. [Google Scholar] [CrossRef]
- Wu, C.; Budarin, V.L.; Gronnow, M.J.; De Bruyn, M.; Onwudili, J.A.; Clark, J.H.; Williams, P.T. Conventional and microwave-assisted pyrolysis of biomass under different heating rates. J. Anal. Appl. Pyrolysis 2014, 107, 276–283. [Google Scholar] [CrossRef]
- Lin, Y.C.; Wu, T.Y.; Jhang, S.R.; Yang, P.M.; Hsiao, Y.H. Hydrogen production from banyan leaves using an atmospheric-pressure microwave plasma reactor. Bioresour. Technol. 2014, 161, 304–309. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, S.; Wang, B.; Shi, C.; Nie, Y. Microwave –assisted pyrolysis aspen wood for production of valuable products under different temperatures. Arab. J. Chem. 2023, 16, 105187. [Google Scholar] [CrossRef]
- Wang, C.; Lei, H.; Zhao, Y.; Qian, M.; Kong, X.; Mateo, W.; Zou, R.; Ruan, R. Integrated harvest of phenolic monomers and hydrogen through catalytic pyrolysis of biomass over nanocellulose derived biochar catalyst. Bioresour. Technol. 2021, 320, 124352. [Google Scholar] [CrossRef] [PubMed]
- Huo, E.; Duan, D.; Lei, H.; Liu, C.; Zhang, Y.; Wu, J.; Zhao, Y.; Huang, Z.; Qian, M.; Zhang, Q.; et al. Phenols production form Douglas fir catalytic pyrolysis with MgO and biomass-derived activated carbon catalysts. Energy 2020, 199, 117459. [Google Scholar] [CrossRef]
- Wallace, C.A.; Afzal, M.T.; Saha, G.C. Effect of feedstock and microwave pyrolysis temperature on physio-chemical and nano-scale mechanical properties of biochar. Bioresour. Bioprocess. 2019, 6, 33. [Google Scholar] [CrossRef]
- Dutta, B.; Gariepy, Y.; Raghavan, G.S.V. Effects of process parameters and selective heating on microwave pyrolysis of lignocellulosic biomass for biochar production. Can. Biosyst. Eng. Genie Biosyst. 2016, 57, 323–332. [Google Scholar] [CrossRef]
- Zhou, C.; Deng, Z.; Zhang, Y.; Li, X.; Liu, Y.; Fu, J.; Chen, L.; Yuan, Y.; Jin, Y.; Dai, J.; et al. Pyrolysis of typical solid wastes in a continuously operated microwave-assisted auger pyrolyser: Char characterization, analysis and energy balance. J. Clean. Prod. 2022, 373, 133818. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J. A comparative investigation into the formation behaviors of char, liquids and gases during pyrolysis of pinewood and lignocellulosic components. Bioresour. Technol. 2014, 170, 262–269. [Google Scholar] [CrossRef]
- Wang, X.H.; Chen, H.P.; Ding, X.J.; Yang, H.P.; Zhang, S.H.; Shen, Y.Q. Properties of gas and char from microwave pyrolysis of pine sawdust. BioResources 2009, 4, 946–959. [Google Scholar] [CrossRef]
- Nzediegwu, C.; Arshad, M.; Ulah, A.; Naeth, M.A.; Chang, S.X. Fuel, thermal and surface properties of microwave-pyrolyzed biochars depend on feedstock type and pyrolysis temperature. Bioresour. Technol. 2021, 320, 124282. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Ren, S.; Wang, L.; Holladay, J.; Zhang, Q.; Tang, J.; Ruan, R. Phenol and phenolics from lignocellulosic biomass by catalytic microwave pyrolysis. Bioresour. Technol. 2011, 102, 7004–7007. [Google Scholar] [CrossRef]
- Bu, Q. Catalytic Microwave Pyrolysis of Biomass for Renewable Phenols and Fuels. Ph.D. Thesis, Washington State University, Pullman, WA, USA, 2013. [Google Scholar]
- Miura, M.; Kaga, H.; Sakurai, A.; Kakuchi, T.; Takahashi, K. Rapid pyrolysis of wood block by microwave heating. J. Anal. Appl. Pyrolysis 2004, 71, 187–199. [Google Scholar] [CrossRef]
- Ren, S.; Lei, H.; Wang, L.; Bu, Q.; Chen, S.; Wu, J.; Julson, J.; Ruan, R. Biofuel production and kinetics analysis for microwave pyrolysis of Douglas fir sawdust pellet. J. Anal. Appl. Pyrolysis 2012, 94, 163–169. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Ren, S.; Wang, L.; Zhang, Q.; Tang, J.; Ruan, R. Production of phenols and biofuels by catalytic microwave pyrolysis of lignocellulosic biomass. Bioresour. Technol. 2012, 108, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lei, H.; Zhang, Y.; Qian, K.; Villota, E.; Qian, M.; Yadavalli, G.; Sun, H. Production of renewable alkyl-phenols from catalytic pyrolysis of Douglas fir sawdust over biomass-derived activated carbons. Appl. Energy 2018, 220, 426–436. [Google Scholar] [CrossRef]
- Moen, J.; Yang, C.; Zhang, B.; Lei, H.; Hennessy, K.; Wan, Y.; Liu, Y.; Le, Z.; Chen, P.; Ruan, R. Catalytic microwave assisted pyrolysis of aspen. Int. J. Agric. Biol. Eng. 2009, 2, 70–75. [Google Scholar] [CrossRef]
- Yang, X.; Cui, C.; Zheng, A.; Zhao, Z.Z.; Wang, C.C.C.; Xia, S.; Huang, Z.Z.; Wei, G.; Li, H.H.; Karthäuser, J.; et al. Pyrolysis of oil palm empty fruit bunch biomass pellets using multimode microwave irradiation. J. Anal. Appl. Pyrolysis 2023, 15, 175–179. [Google Scholar]
- Suriapparao, D.V.; Vinu, R. Biomass waste conversion into value-added products via microwave-assisted Co-Pyrolysis platform. Renew. Energy 2021, 170, 400–409. [Google Scholar] [CrossRef]
- Vorhauer-Huget, N.; Seidenbecher, J.; Bhaskaran, S.; Schenkel, F.; Briest, L.; Gopalkrishna, S.; Barowski, J.; Dernbecher, A.; Hilfert, L.; Rolfes, I.; et al. Dielectric and physico-chemical behavior of single thermally thick wood blocks under microwave assisted pyrolysis. Particuology 2024, 86, 291–303. [Google Scholar] [CrossRef]
- Klinger, J.L.; Westover, T.L.; Emerson, R.M.; Williams, C.L.; Hernandez, S.; Monson, G.D.; Ryan, J.C. Effect of biomass type, heating rate, and sample size on microwave-enhanced fast pyrolysis product yields and qualities. Appl. Energy 2018, 228, 535–545. [Google Scholar] [CrossRef]
- Mamaeva, A.; Tahmasebi, A.; Tian, L.; Yu, J. Microwave-assisted catalytic pyrolysis of lignocellulosic biomass for production of phenolic-rich bio-oil. Bioresour. Technol. 2016, 211, 382–389. [Google Scholar] [CrossRef]
- Shang, H.; Lu, R.R.; Shang, L.; Zhang, W.H. Effect of additives on the microwave-assisted pyrolysis of sawdust. Fuel Process. Technol. 2015, 131, 167–174. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Wang, L.; Wei, Y.; Zhu, L.; Liu, Y.; Liang, J.; Tang, J. Renewable phenols production by catalytic microwave pyrolysis of Douglas fir sawdust pellets with activated carbon catalysts. Bioresour. Technol. 2013, 142, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yu, Z.; Xu, H.; Wan, K.; Liao, Y.; Ma, X. Microwave-assisted co-pyrolysis of Chlorella vulgaris and wood sawdust using different additives. Bioresour. Technol. 2019, 273, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Yan, J.; Luo, X.; Lester, E.; Wu, T. Microwave-assisted pyrolysis of bamboo coupled with reforming by activated carbon for the production of hydrogen-rich syngas. Energy Procedia 2017, 142, 1640–1646. [Google Scholar] [CrossRef]
- Makepa, D.C.; Chihobo, C.H.; Ruziwa, W.R.; Musademba, D. Microwave-assisted pyrolysis of pine sawdust: Process modelling, performance optimization and economic evaluation for bioenergy recovery. Heliyon 2023, 9, e14688. [Google Scholar] [CrossRef]
- Zhou, N.; Zhou, J.; Dai, L.; Guo, F.; Wang, Y.; Li, H.; Deng, W.; Lei, H.; Chen, P.; Liu, Y.; et al. Syngas production from biomass pyrolysis in a continuous microwave assisted pyrolysis system. Bioresour. Technol. 2020, 314, 123756. [Google Scholar] [CrossRef] [PubMed]
- Parvez, A.M.; Wu, T.; Afzal, M.T.; Mareta, S.; He, T.; Zhai, M. Conventional and microwave-assisted pyrolysis of gumwood: A comparison study using thermodynamic evaluation and hydrogen production. Fuel Process. Technol. 2019, 184, 1–11. [Google Scholar] [CrossRef]
- Dong, Y.; Tian, B.; Guo, F.; Du, S.; Zhan, Y.; Zhou, H.; Qian, L. Application of low-cost Fe-based catalysts in the microwave-assisted pyrolysis of macroalgae and lignocellulosic biomass for the upgradation of bio-oil. Fuel 2021, 300, 120944. [Google Scholar] [CrossRef]
- Chen, M.; Wang, J.; Zhang, M.; Chen, M.; Zhu, X.; Min, F.; Tan, Z. Catalytic effects of eight inorganic additives on pyrolysis of pine wood sawdust by microwave heating. J. Anal. Appl. Pyrolysis 2008, 82, 145–150. [Google Scholar] [CrossRef]
- Borges, F.C.; Du, Z.; Xie, Q.; Trierweiler, J.O.; Cheng, Y.; Wan, Y.; Liu, Y.; Zhu, R.; Lin, X.; Chen, P.; et al. Fast microwave assisted pyrolysis of biomass using microwave absorbent. Bioresour. Technol. 2014, 156, 267–274. [Google Scholar] [CrossRef]
- Ellison, C.R.; Hoff, R.; Mărculescu, C.; Boldor, D. Investigation of microwave-assisted pyrolysis of biomass with char in a rectangular waveguide applicator with built-in phase-shifting. Appl. Energy 2020, 259, 114217. [Google Scholar] [CrossRef]
- Undri, A.; Abou-Zaid, M.; Briens, C.; Berruti, F.; Rosi, L.; Bartoli, M.; Frediani, M.; Frediani, P. Bio-oil from pyrolysis of wood pellets using a microwave multimode oven and different microwave absorbers. Fuel 2015, 153, 464–482. [Google Scholar] [CrossRef]
- Lestinsky, P.; Grycova, B.; Pryszcz, A.; Martaus, A.; Matejova, L. Hydrogen production from microwave catalytic pyrolysis of spruce sawdust. J. Anal. Appl. Pyrolysis 2017, 124, 175–179. [Google Scholar] [CrossRef]
- Li, L.; Cao, K.; Cai, D.; Zhang, Z.; Zhao, Z.; Yu, M.; Zhang, L.; Zhang, Q.; Zou, G.; Wang, C. Influences of iron additives on microwave-assisted pyrolysis of woody biomass and microwave-induced discharge with spherical bio-char. Energy 2023, 276, 127549. [Google Scholar] [CrossRef]
- Guo, H.; Qin, X.; Cheng, S.; Xing, B.; Jiang, D.; Meng, W.; Xia, H. Production of high-quality pyrolysis product by microwave–assisted catalytic pyrolysis of wood waste and application of biochar. Arab. J. Chem. 2023, 16, 104961. [Google Scholar] [CrossRef]
- Li, M.; Yu, Z.; Bin, Y.; Huang, Z.; He, H.; Liao, Y.; Zheng, A.; Ma, X. Microwave-assisted pyrolysis of eucalyptus wood with MoO3 and different nitrogen sources for coproducing nitrogen-rich bio-oil and char. J. Anal. Appl. Pyrolysis 2022, 167, 105666. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; He, Y.; An, X.; Fan, D.; Wu, Z. Microwave-assisted catalytic pyrolysis of apple wood to produce biochar: Co-pyrolysis behavior, pyrolysis kinetics analysis and evaluation of microbial carriers. Bioresour. Technol. 2021, 320, 124345. [Google Scholar] [CrossRef]
- Li, L.; Tan, Y.; Sun, J.; Zhang, Y.; Zhang, L.; Deng, Y.; Cai, D.; Song, Z.; Zou, G.; Bai, Y. Characteristics and kinetic analysis of pyrolysis of forestry waste promoted by microwave-metal interaction. Energy 2021, 232, 121095. [Google Scholar] [CrossRef]
- Gao, Q.; Budarin, V.L.; Cieplik, M.; Gronnow, M.; Jansson, S. PCDDs, PCDFs and PCNs in products of microwave-assisted pyrolysis of woody biomass—Distribution among solid, Liquid and gaseous phases and effects of material composition. Chemosphere 2016, 145, 193–199. [Google Scholar] [CrossRef]
- McKeown, M.S.; Trabelsi, S.; Tollner, E.W. Effects of temperature and material on sensing moisture content of pelleted biomass through dielectric properties. Biosyst. Eng. 2016, 149, 1–10. [Google Scholar] [CrossRef]
- Wang, C.; Ouyang, S.; Shen, Z.; Cai, B.; Zhao, C.; Peng, H.; Zhang, Y. Research on the determination method of biomass dielectric properties based on mixing rules. Biomass Convers. Biorefinery 2024, 2–11. [Google Scholar] [CrossRef]
- Ellison, C.; McKeown, M.S.; Trabelsi, S.; Boldor, D. Dielectric properties of biomass/biochar mixtures at microwave frequencies. Energies 2017, 10, 502. [Google Scholar] [CrossRef]
- Motasemi, F.; Salema, A.A.; Afzal, M.T. Dielectric characterization of corn stover for microwave processing technology. Fuel Process. Technol. 2015, 131, 370–375. [Google Scholar] [CrossRef]
- Fan, X.; Li, B.; Zi, W.; Kang, M.; Wu, H.; Bian, J.; Sun, M.Y. Microwave dielectric characterization and loss mechanism of biowaste during pyrolysis. Energy Convers. Manag. 2024, 301, 118075. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).