Investigation of Cotton Stalk-Derived Hydrothermal Bio-Oil: Effects of Mineral Acid/Base and Oxide Additions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
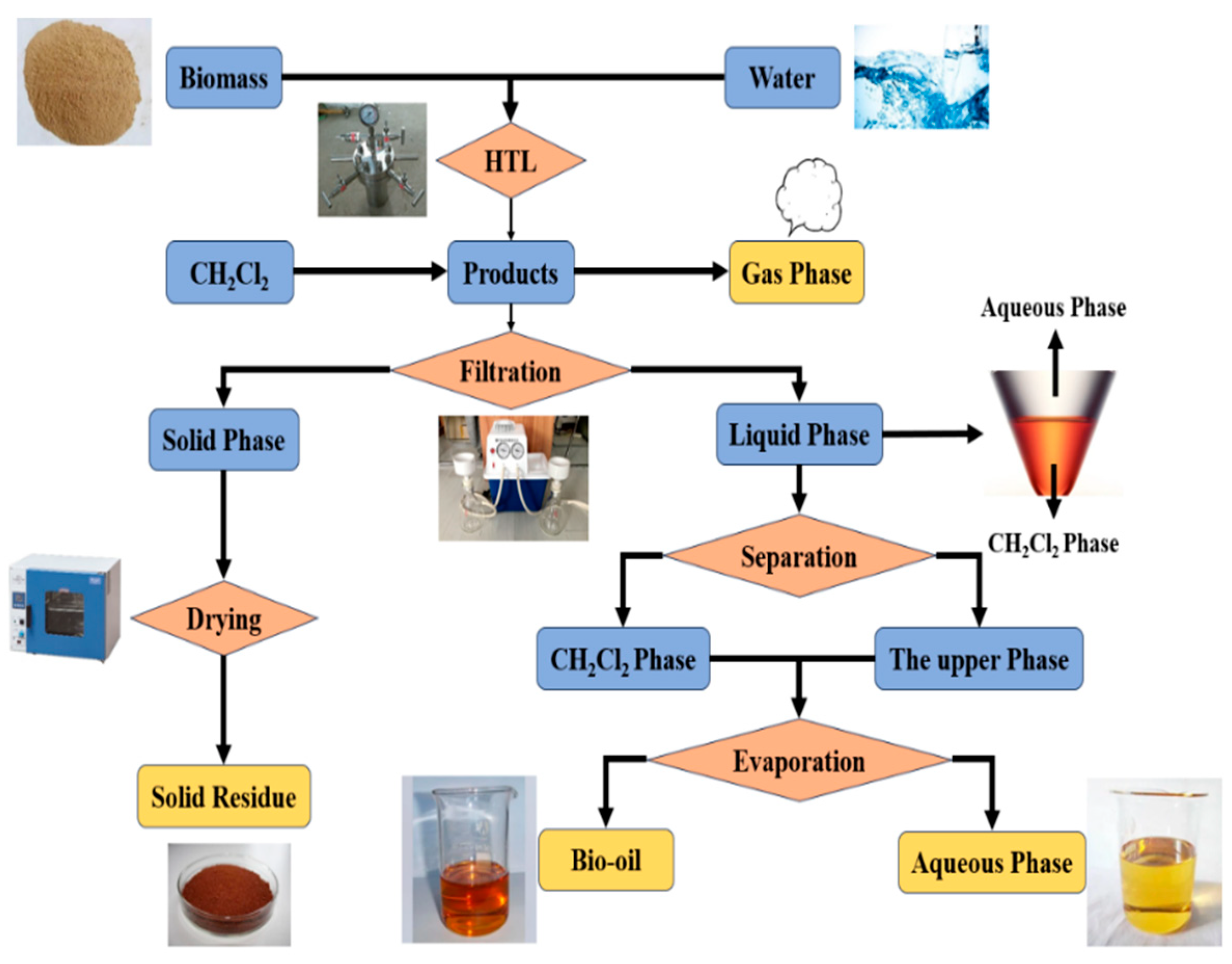
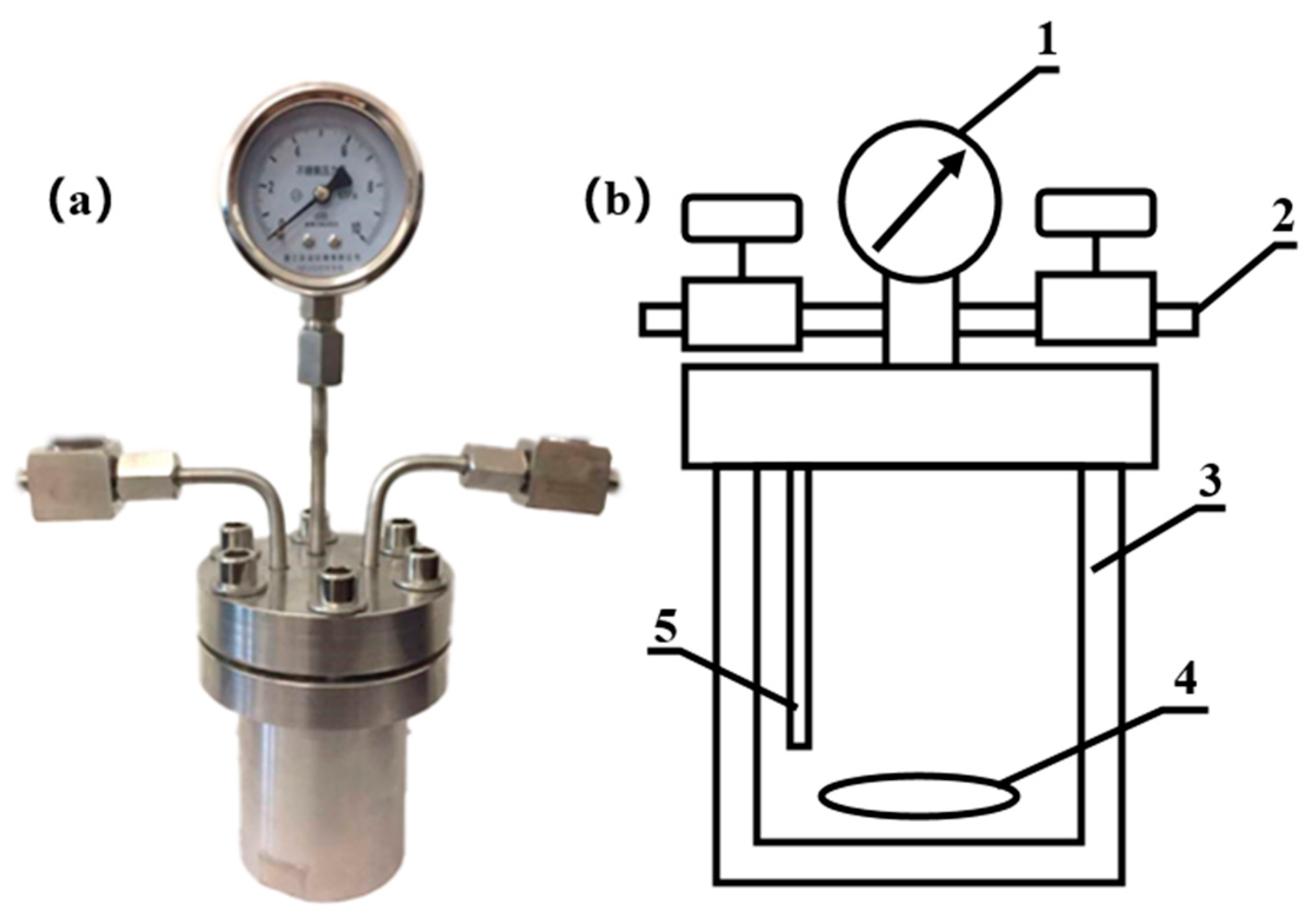
2.2. Hydrothermal Liquefaction Technology (HTL) Process and Product Separation Method
2.3. Materials and Product Analysis
3. Results
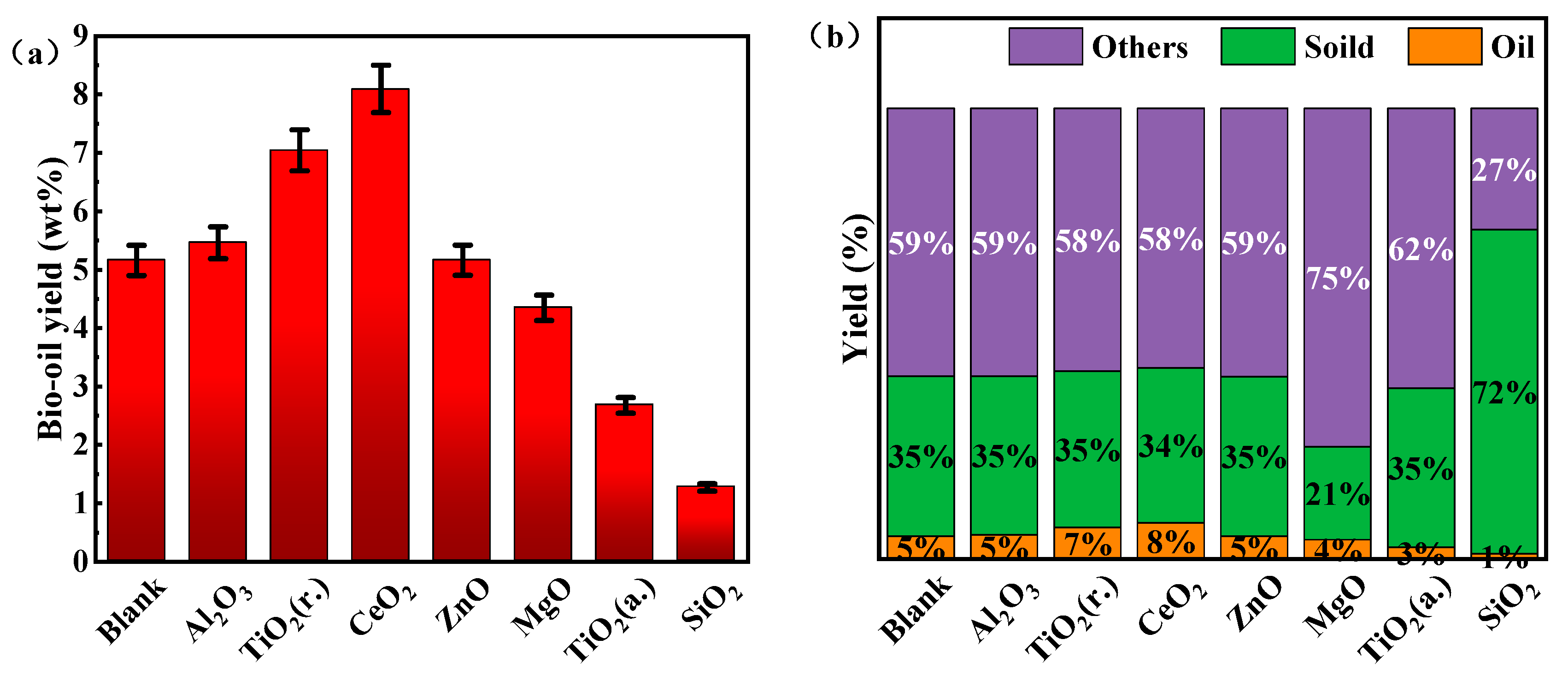
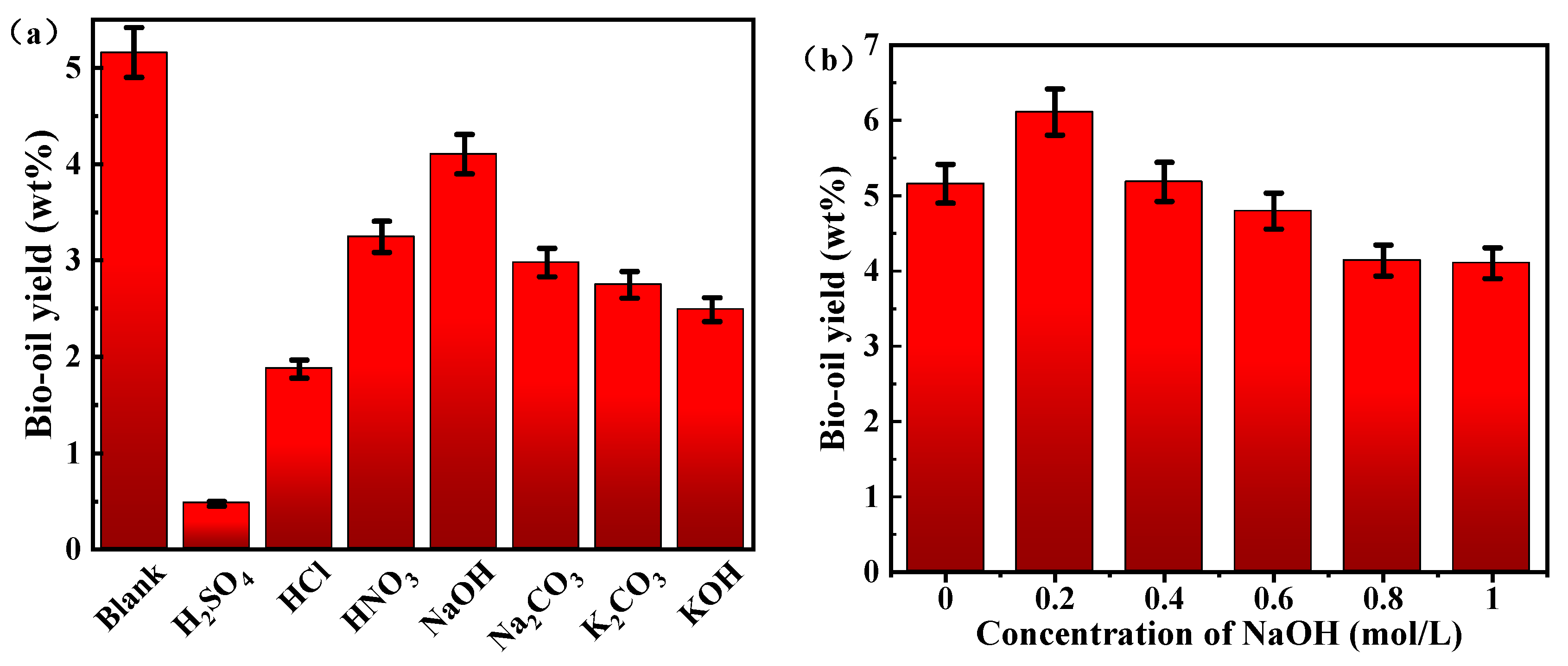
3.1. Effect of Catalytic on Products Distribution from HTL
3.2. CS Bio-Oil Characterization
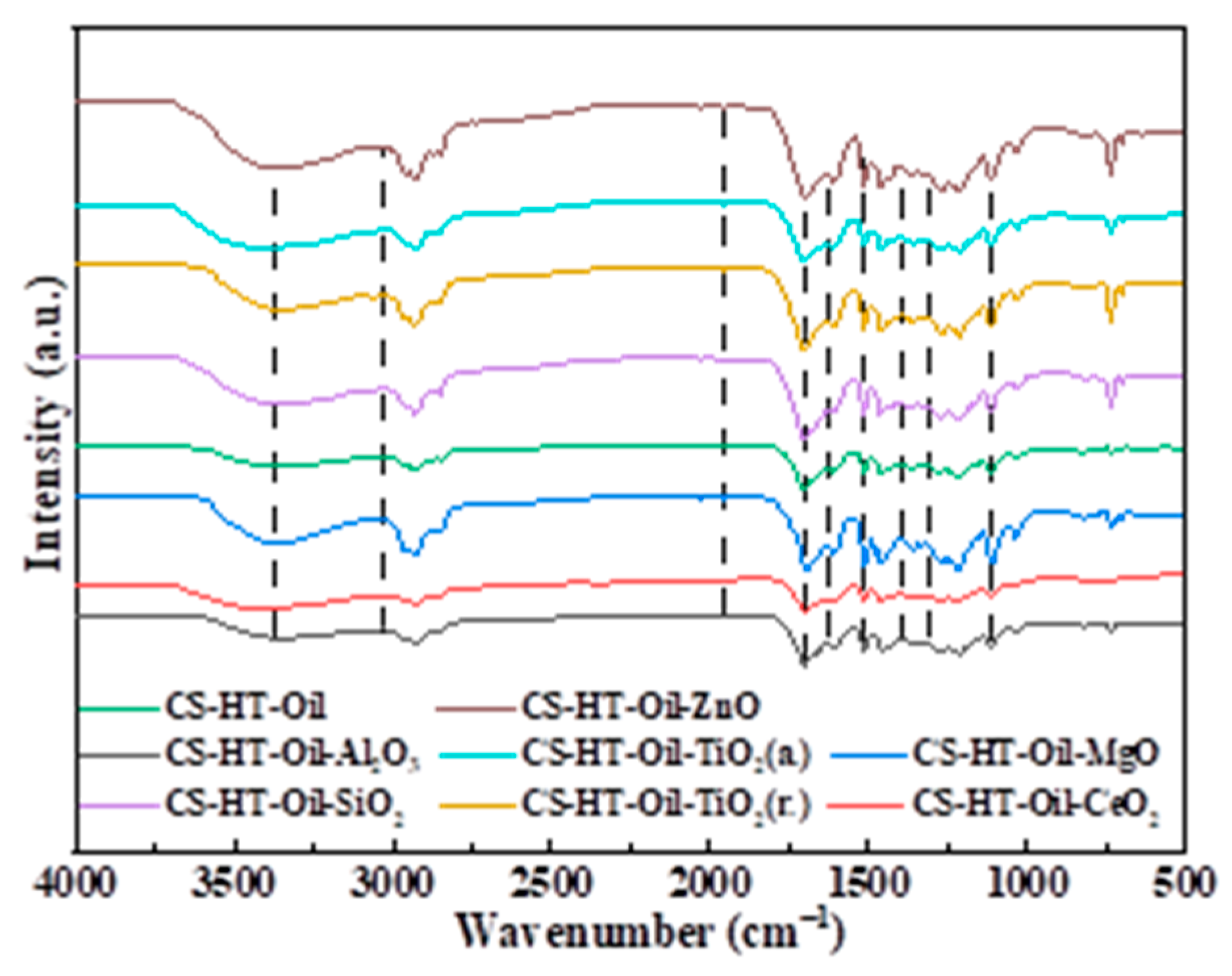
3.2.1. FTIR
3.2.2. Elemental Analysis
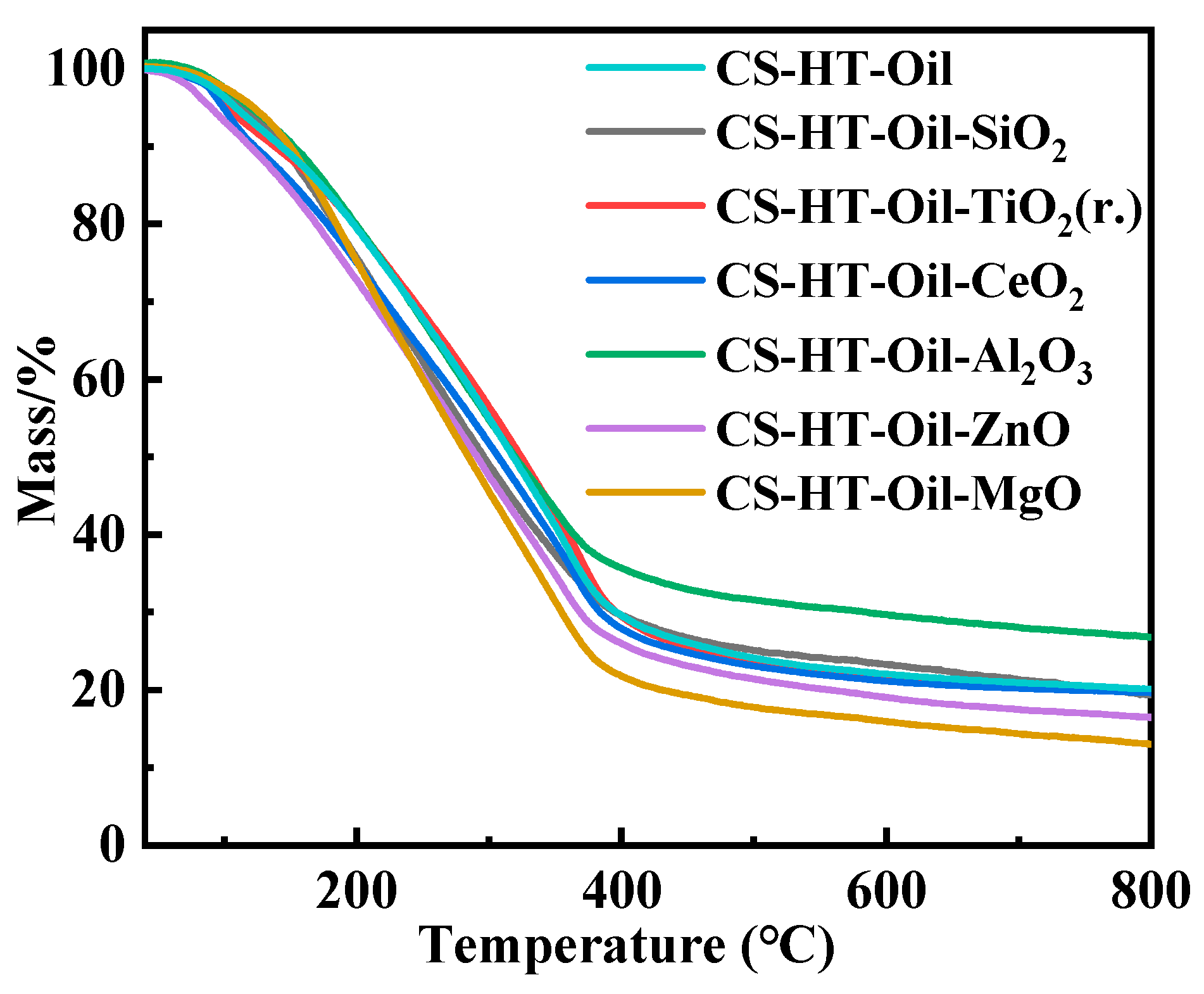
3.2.3. TGA
3.2.4. GC-MS
3.3. Catalyst Characterization
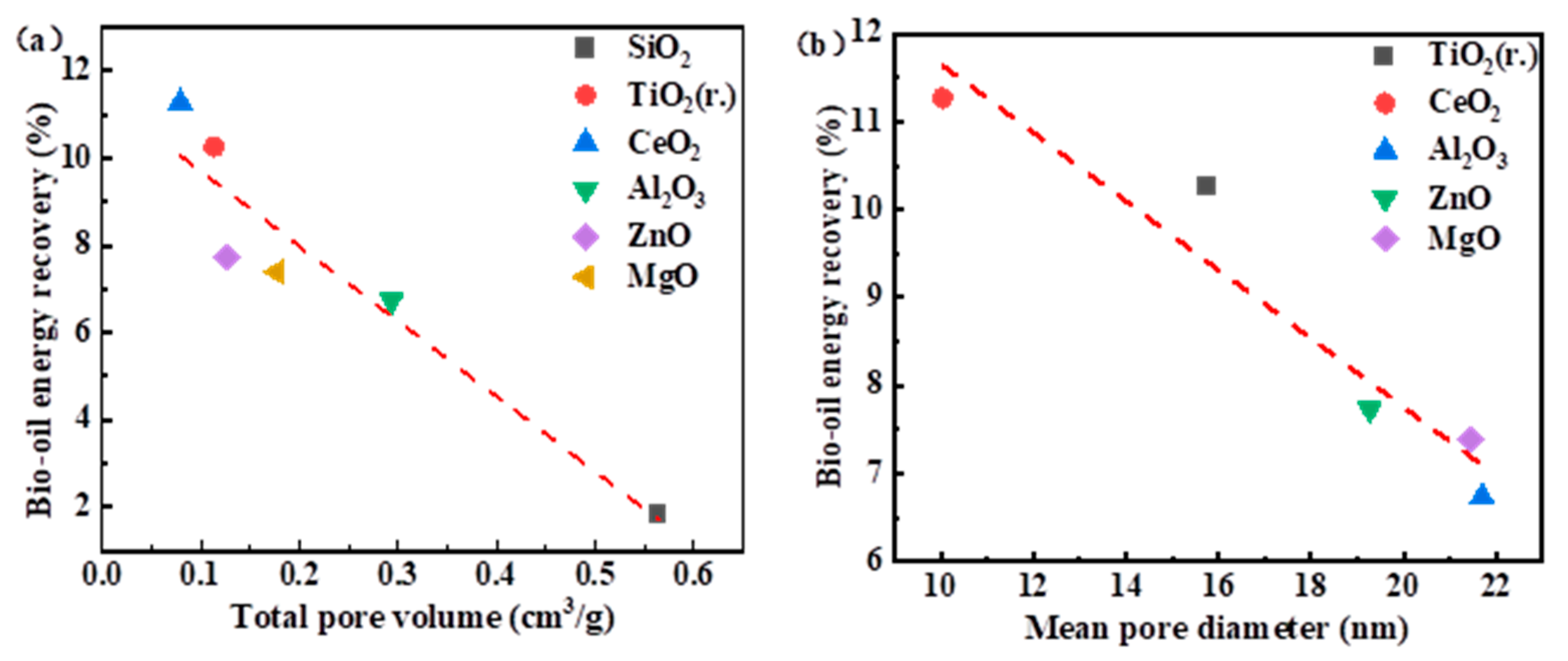
3.3.1. BET
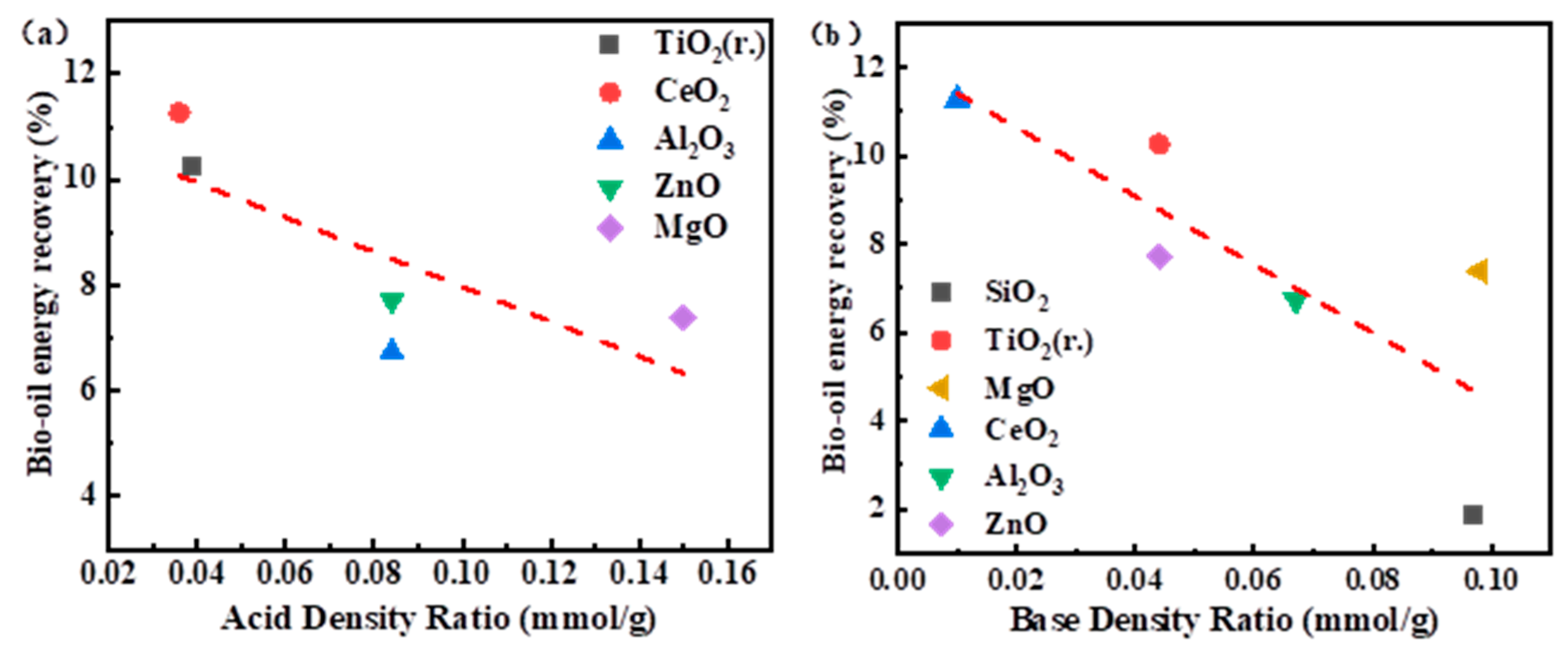
3.3.2. TPD
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Usman, M.; Cheng, S.; Boonyubol, S.; Cross, J.S. From Biomass to Biocrude: Innovations in Hydrothermal Liquefaction and Upgrading. Energy Convers. Manag. 2024, 302, 118093. [Google Scholar] [CrossRef]
- Shahbeik, H.; Panahi, H.K.S.; Dehhaghi, M.; Guillemin, G.J.; Fallahi, A.; Hosseinzadeh-Bandbafha, H.; Amiri, H.; Rehan, M.; Raikwar, D.; Latine, H.; et al. Biomass to Biofuels Using Hydrothermal Liquefaction: A Comprehensive Review. Renew. Sustain. Energy Rev. 2024, 189, 113976. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, F.; Djandja, J.O.; Zhang, S.-L.; Xu, Y.-P.; Duan, P.-G. Hydrothermal Liquefaction of Crop Straws: Effect of Feedstock Composition. Fuel 2020, 265, 116946. [Google Scholar] [CrossRef]
- Shao, Y.; Huo, W.; Ye, R.; Liu, Y.; Ajmal, M.; Lu, W. Hydrothermal Humification of Lignocellulosic Components: Who Is Doing What? Chem. Eng. J. 2023, 457, 141180. [Google Scholar] [CrossRef]
- Roy, P.; Jahromi, H.; Rahman, T.; Baltrusaitis, J.; Hassan, E.B.; Torbert, A.; Adhikari, S. Hydrotreatment of Pyrolysis Bio-Oil with Non-Edible Carinata Oil and Poultry Fat for Producing Transportation Fuels. Fuel Process. Technol. 2023, 245, 107753. [Google Scholar] [CrossRef]
- Leng, L.; Zhou, J.; Li, T.; Vlaskin, M.; Zhan, H.; Peng, H.; Huang, H.; Li, H. Nitrogen Heterocycles in Bio-Oil Produced from Hydrothermal Liquefaction of Biomass: A Review. Fuel 2023, 335, 126995. [Google Scholar] [CrossRef]
- Deng, G.; Tang, X.; Ma, X.; Ling, S.; Fei, F.; Mao, Q.; Li, J. Effects of Different Catalytic Liquefaction of Bio-Oil on Hydrothermal Upgrading of Heavy Oil: A Comprehensive Analysis of Composition, Desulfurization and Hydrogenation. J. Anal. Appl. Pyrolysis 2024, 179, 106455. [Google Scholar] [CrossRef]
- Paiva Pinheiro Pires, A.; Garcia-Perez, M.; Olarte, M.V.; Kew, W.; Schmidt, A.; Zemaitis, K.; Denson, M.; Terrell, E.; McDonald, A.; Han, Y. Comparison of the Chemical Composition of Liquids from the Pyrolysis and Hydrothermal Liquefaction of Lignocellulosic Materials. Energy Fuels 2023, 37, 7221–7236. [Google Scholar] [CrossRef]
- Kurzyna-Szklarek, M.; Cybulska, J.; Zdunek, A. Analysis of the Chemical Composition of Natural Carbohydrates–An Overview of Methods. Food Chem. 2022, 394, 133466. [Google Scholar] [CrossRef]
- Elhenawy, Y.; Fouad, K.; Bassyouni, M.; Al-Qabandi, O.A.; Majozi, T. Yield and Energy Outputs Analysis of Sawdust Biomass Pyrolysis. Energy Convers. Manag. X 2024, 22, 100583. [Google Scholar] [CrossRef]
- Wang, H.; Han, X.; Zeng, Y.; Xu, C.C. Development of a Global Kinetic Model Based on Chemical Compositions of Lignocellulosic Biomass for Predicting Product Yields from Hydrothermal Liquefaction. Renew. Energy 2023, 215, 118956. [Google Scholar] [CrossRef]
- Cheng, F.; Tompsett, G.A.; Murphy, C.M.; Maag, A.R.; Carabillo, N.; Bailey, M.; Hemingway, J.J.; Romo, C.I.; Paulsen, A.D.; Yelvington, P.E.; et al. Synergistic Effects of Inexpensive Mixed Metal Oxides for Catalytic Hydrothermal Liquefaction of Food Wastes. ACS Sustain. Chem. Eng. 2020, 8, 6877–6886. [Google Scholar] [CrossRef]
- Hirano, Y.; Miyata, Y.; Taniguchi, M.; Funakoshi, N.; Yamazaki, Y.; Ogino, C.; Kita, Y. Fe-Assisted Hydrothermal Liquefaction of Cellulose: Effects of Hydrogenation Catalyst Addition on Properties of Water-Soluble Fraction. J. Anal. Appl. Pyrolysis 2020, 145, 104719. [Google Scholar] [CrossRef]
- Aturagaba, G.; Egesa, D.; Mubiru, E.; Tebandeke, E. Catalytic Hydrothermal Liquefaction of Water Hyacinth Using Fe3O4/NiO Nanocomposite: Optimization of Reaction Conditions by Response Surface Methodology. J. Sustain. Bioenergy Syst. 2023, 13, 73–98. [Google Scholar] [CrossRef]
- Prasannamedha, G.; Kumar, P.S.; Shankar, V. Facile Route for Synthesis of Fe0/Fe3C/γ-Fe2O3 Carbon Composite Using Hydrothermal Carbonization of Sugarcane Bagasse and Its Use as Effective Adsorbent for Sulfamethoxazole Removal. Chemosphere 2022, 289, 133214. [Google Scholar] [CrossRef]
- Grams, J.; Jankowska, A.; Goscianska, J. Advances in Design of Heterogeneous Catalysts for Pyrolysis of Lignocellulosic Biomass and Bio-Oil Upgrading. Microporous Mesoporous Mater. 2023, 362, 112761. [Google Scholar] [CrossRef]
- Biswas, B.; Kumar, A.; Fernandes, A.C.; Saini, K.; Negi, S.; Muraleedharan, U.D.; Bhaskar, T. Solid Base Catalytic Hydrothermal Liquefaction of Macroalgae: Effects of Process Parameter on Product Yield and Characterization. Bioresour. Technol. 2020, 307, 123232. [Google Scholar] [CrossRef]
- Liu, J.; Xiu, P.; Zhu, Y.; Wang, K.; Tong, S.; Ma, Y.; Guo, H.; Gu, X. Catalytic Hydrogenolysis of Lignin over Ni/CeO2-Al2O3 Catalysts with Formic Acid as Hydrogen Source. Catal. Commun. 2024, 187, 106868. [Google Scholar] [CrossRef]
- Shangdiar, S.; Cheng, P.-C.; Chen, S.-C.; Amesho, K.T.T.; Ponnusamy, V.K.; Lin, Y.-C. Enhancing Sugar Yield for Bioconversion of Rice Straw: Optimization of Microwave-Assisted Pretreatment Using Dilute Acid Hydrolysis. Environ. Technol. Innov. 2023, 32, 103313. [Google Scholar] [CrossRef]
- Elhenawy, Y.; Fouad, K.; Mansi, A.; Bassyouni, M.; Gadalla, M.; Ashour, F.; Majozi, T. Experimental Analysis and Numerical Simulation of Biomass Pyrolysis. J. Therm. Anal. Calorim. 2024, 1–15. [Google Scholar] [CrossRef]
- Gao, J.; Zeng, J.; Zhu, S.; Ma, H.; Yao, R.; Zhao, Y.; He, Z. In-Situ Catalytic Bio-Oil Production from Hydrothermal Liquefaction of Cu-Impregnated Water Hyacinth: Screening of Reaction Parameters. J. Energy Inst. 2023, 109, 101308. [Google Scholar] [CrossRef]
- Prakash, S.; Radha; Sharma, K.; Dhumal, S.; Senapathy, M.; Deshmukh, V.P.; Kumar, S.; Madhu; Anitha, T.; Balamurugan, V.; et al. Unlocking the Potential of Cotton Stalk as a Renewable Source of Cellulose: A Review on Advancements and Emerging Applications. Int. J. Biol. Macromol. 2024, 261, 129456. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, Y.; Xu, D.; Guan, Q. A Review on Recent Advances in Clean Microalgal Bio-Oil Production via Catalytic Hydrothermal Deoxygenation. J. Clean. Prod. 2022, 366, 132978. [Google Scholar] [CrossRef]
- Yan, M.; Liu, Y.; Wen, X.; Yang, Y.; Cui, J.; Chen, F.; Hantoko, D. Effect of Operating Conditions on Hydrothermal Liquefaction of Kitchen Waste with Ethanol-Water as a Co-Solvent for Bio-Oil Production. Renew. Energy 2023, 215, 118949. [Google Scholar] [CrossRef]
- Forero, J.A.J.; Tran, T.H.T.; Tana, T.; Baker, A.; Beltramini, J.; Doherty, W.O.S.; Moghaddam, L. Hydrothermal Liquefaction of Sugarcane Bagasse to Bio-Oils: Effect of Liquefaction Solvents on Bio-Oil Stability. Fuel 2022, 312, 122793. [Google Scholar] [CrossRef]
- Du, J.; Gao, L.; Yang, Y.; Chen, G.; Guo, S.; Omran, M.; Chen, J.; Ruan, R. Study on Thermochemical Characteristics Properties and Pyrolysis Kinetics of the Mixtures of Waste Corn Stalk and Pyrolusite. Bioresour. Technol. 2021, 324, 124660. [Google Scholar] [CrossRef]
- He, Y.; Zhao, Y.; Chai, M.; Zhou, Z.; Sarker, M.; Li, C.; Liu, R.; Cai, J.; Liu, X. Comparative Study of Fast Pyrolysis, Hydropyrolysis and Catalytic Hydropyrolysis of Poplar Sawdust and Rice Husk in a Modified Py-GC/MS Microreactor System: Insights into Product Distribution, Quantum Description and Reaction Mechanism. Renew. Sustain. Energy Rev. 2020, 119, 109604. [Google Scholar] [CrossRef]
- Kumar, M.; Mishra, P.K.; Upadhyay, S.N. Pyrolysis of Saccharum Munja: Optimization of Process Parameters Using Response Surface Methodology (RSM) and Evaluation of Kinetic Parameters. Bioresour. Technol. Rep. 2019, 8, 100332. [Google Scholar] [CrossRef]
- Nzediegwu, C.; Arshad, M.; Ulah, A.; Naeth, M.A.; Chang, S.X. Fuel, Thermal and Surface Properties of Microwave-Pyrolyzed Biochars Depend on Feedstock Type and Pyrolysis Temperature. Bioresour. Technol. 2021, 320, 124282. [Google Scholar] [CrossRef]
- Chen, G.; Hu, M.; Du, G.; Tian, S.; He, Z.; Liu, B.; Ma, W. Hydrothermal Liquefaction of Sewage Sludge by Microwave Pretreatment. Energy Fuels 2020, 34, 1145–1152. [Google Scholar] [CrossRef]
- Yan, L.; Wang, Y.; Li, J.; Zhang, Y.; Ma, L.; Fu, F.; Chen, B.; Liu, H. Hydrothermal Liquefaction of Ulva Prolifera Macroalgae and the Influence of Base Catalysts on Products. Bioresour. Technol. 2019, 292, 121286. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wang, S.; Cao, B.; Hu, Y.; Abomohra, A.E.-F.; Wang, Q.; Qian, L.; Liu, L.; Liu, X.; He, Z.; et al. Optimization of Hydrothermal Co-Liquefaction of Seaweeds with Lignocellulosic Biomass: Merging 2nd and 3rd Generation Feedstocks for Enhanced Bio-Oil Production. Energy 2019, 173, 413–422. [Google Scholar] [CrossRef]
- Isa, Y.M.; Ganda, E.T. Bio-Oil as a Potential Source of Petroleum Range Fuels. Renew. Sustain. Energy Rev. 2018, 81, 69–75. [Google Scholar] [CrossRef]
- De Caprariis, B.; Bavasso, I.; Bracciale, M.P.; Damizia, M.; De Filippis, P.; Scarsella, M. Enhanced Bio-Crude Yield and Quality by Reductive Hydrothermal Liquefaction of Oak Wood Biomass: Effect of Iron Addition. J. Anal. Appl. Pyrolysis 2019, 139, 123–130. [Google Scholar] [CrossRef]
- Wu, X.-F.; Zhou, Q.; Li, M.-F.; Li, S.-X.; Bian, J.; Peng, F. Conversion of Poplar into Bio-Oil via Subcritical Hydrothermal Liquefaction: Structure and Antioxidant Capacity. Bioresour. Technol. 2018, 270, 216–222. [Google Scholar] [CrossRef]
- Moazezi, M.R.; Bayat, H.; Tavakoli, O.; Hallajisani, A. Hydrothermal Liquefaction of Chlorella Vulgaris and Catalytic Upgrading of Product: Effect of Process Parameter on Bio-Oil Yield and Thermodynamics Modeling. Fuel 2022, 318, 123595. [Google Scholar] [CrossRef]
- Rana, M.; Ghosh, S.; Nshizirungu, T.; Park, J.-H. Catalytic Depolymerization of Kraft Lignin to High Yield Alkylated-Phenols over CoMo/SBA-15 Catalyst in Supercritical Ethanol. RSC Adv. 2023, 13, 30022–30039. [Google Scholar] [CrossRef]
- Ding, Y.-J.; Zhao, C.-X.; Liu, Z.-C. Catalytic Hydrothermal Liquefaction of Rice Straw for Production of Monomers Phenol over Metal Supported Mesoporous Catalyst. Bioresour. Technol. 2019, 294, 122097. [Google Scholar] [CrossRef]
- Wang, Y.; Akbarzadeh, A.; Chong, L.; Du, J.; Tahir, N.; Awasthi, M.K. Catalytic Pyrolysis of Lignocellulosic Biomass for Bio-Oil Production: A Review. Chemosphere 2022, 297, 134181. [Google Scholar] [CrossRef]


| C (wt%) | N (wt%) | H (wt%) | S (wt%) | O 1 (wt%) | H/C | O/C | HHV (MJ/kg) | Energy Recovery (%) | |
|---|---|---|---|---|---|---|---|---|---|
| CS | 45.96 | 1.02 | 5.97 | 0.09 | 43.5 | 1.56 | 0.71 | 16.30 | - |
| CS-HT-Oil | 61.52 | 0.62 | 5.734 | 0.262 | 31.864 | 1.12 | 0.39 | 23.32 | 7.38 |
| CS-HT-Oil-SiO2 | 61.00 | 0.69 | 6.086 | 0.342 | 31.882 | 1.2 | 0.39 | 23.65 | 1.85 |
| CS-HT-Oil-TiO2(r.) | 61.46 | 0.76 | 5.978 | 0.445 | 31.357 | 1.17 | 0.38 | 23.76 | 10.26 |
| CS-HT-Oil-CeO2 | 60.25 | 1.00 | 5.905 | 0.513 | 32.332 | 1.18 | 0.40 | 23.07 | 11.27 |
| CS-HT-Oil-Al2O3 | 59.41 | 0.75 | 5.822 | 1.319 | 32.699 | 1.18 | 0.41 | 22.68 | 6.73 |
| CS-HT-Oil-ZnO | 62.48 | 0.8 | 6.085 | 0.327 | 30.308 | 1.17 | 0.36 | 24.43 | 7.73 |
| CS-HT-Oil-MgO | 67.31 | 1.51 | 6.441 | 0.659 | 24.08 | 1.15 | 0.27 | 27.71 | 7.39 |
| Entry | Compound | SiO2 | TiO2 | CeO2 | Al2O3 | ZnO | MgO | Blank |
|---|---|---|---|---|---|---|---|---|
| 1 | N-ethyl formamide | 19.83 | 8.43 | 27.62 | 9.42 | 13.47 | - | - |
| 2 | 5-Nitro-6-chloro-2,4(1H,3H)-Pyrimidinedione | - | 10.12 | - | 5.74 | - | - | - |
| 3 | Acetaldehyde | - | - | 36.21 | 41.55 | 27.58 | ||
| 4 | 1,1-Dimethylcyclopropane | - | - | 19.91 | 34.07 | - | - | |
| 5 | 2,4-Dichloro-5-oxo2-hexenedioic acid | - | - | 8.23 | 19.91 | 19.93 | - | - |
| 6 | 2-Ethylacridine | - | - | - | - | - | 1.75 | 11.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Wang, J.; Ming, H.; Hu, H.; Dou, X.; Xiao, Y.; Cheng, L.; Hu, Z. Investigation of Cotton Stalk-Derived Hydrothermal Bio-Oil: Effects of Mineral Acid/Base and Oxide Additions. Energies 2024, 17, 4854. https://doi.org/10.3390/en17194854
Zhang L, Wang J, Ming H, Hu H, Dou X, Xiao Y, Cheng L, Hu Z. Investigation of Cotton Stalk-Derived Hydrothermal Bio-Oil: Effects of Mineral Acid/Base and Oxide Additions. Energies. 2024; 17(19):4854. https://doi.org/10.3390/en17194854
Chicago/Turabian StyleZhang, Libo, Jianing Wang, Hui Ming, Hanjun Hu, Xintong Dou, Yepeng Xiao, Lihua Cheng, and Zhun Hu. 2024. "Investigation of Cotton Stalk-Derived Hydrothermal Bio-Oil: Effects of Mineral Acid/Base and Oxide Additions" Energies 17, no. 19: 4854. https://doi.org/10.3390/en17194854
APA StyleZhang, L., Wang, J., Ming, H., Hu, H., Dou, X., Xiao, Y., Cheng, L., & Hu, Z. (2024). Investigation of Cotton Stalk-Derived Hydrothermal Bio-Oil: Effects of Mineral Acid/Base and Oxide Additions. Energies, 17(19), 4854. https://doi.org/10.3390/en17194854





