Advanced Fuel Based on Semi-Coke and Cedarwood: Kinetic Characteristics and Synergetic Effects
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Combustion Characteristics
2.3. Estimation Methods of Synergy
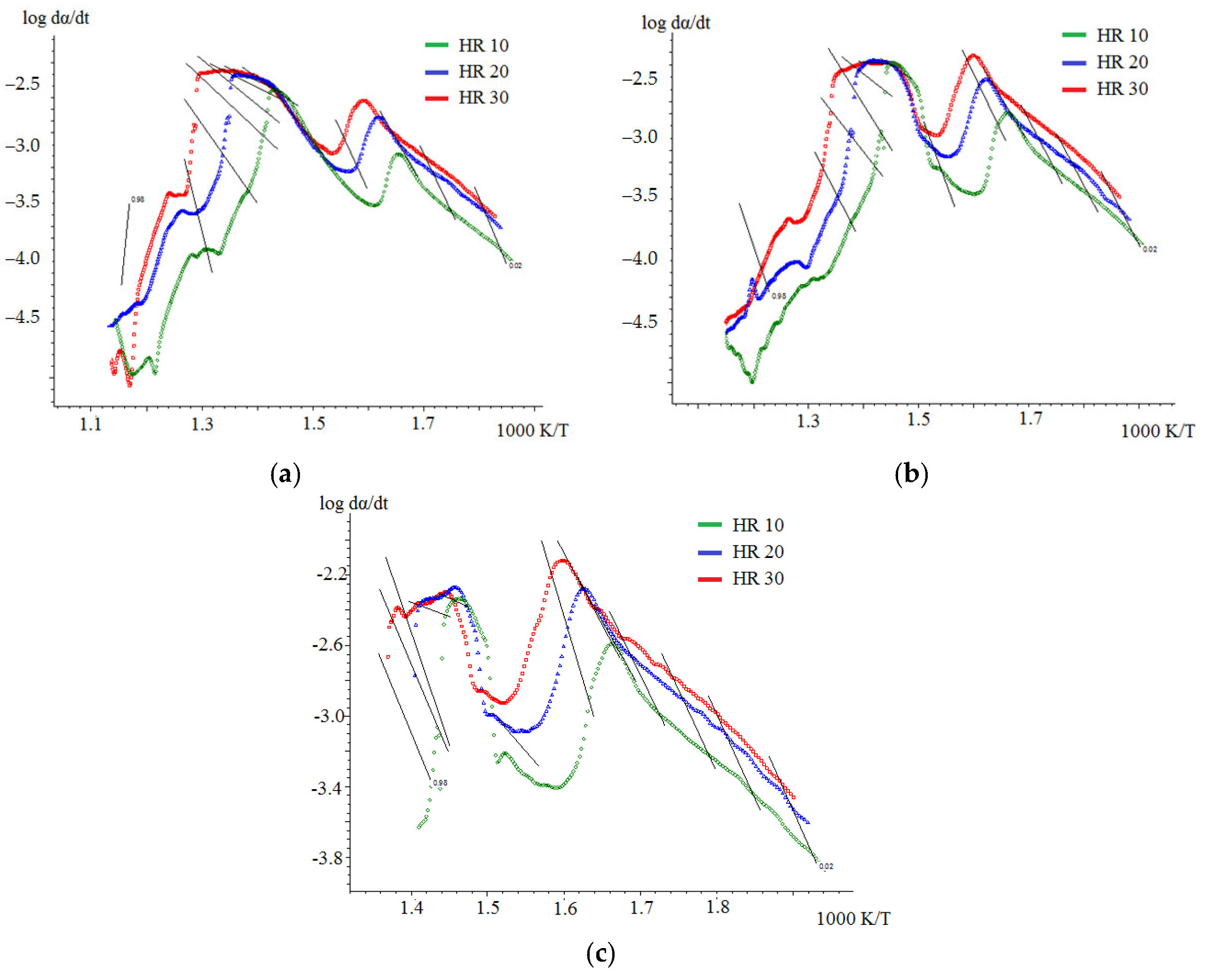
2.4. Kinetic Analysis
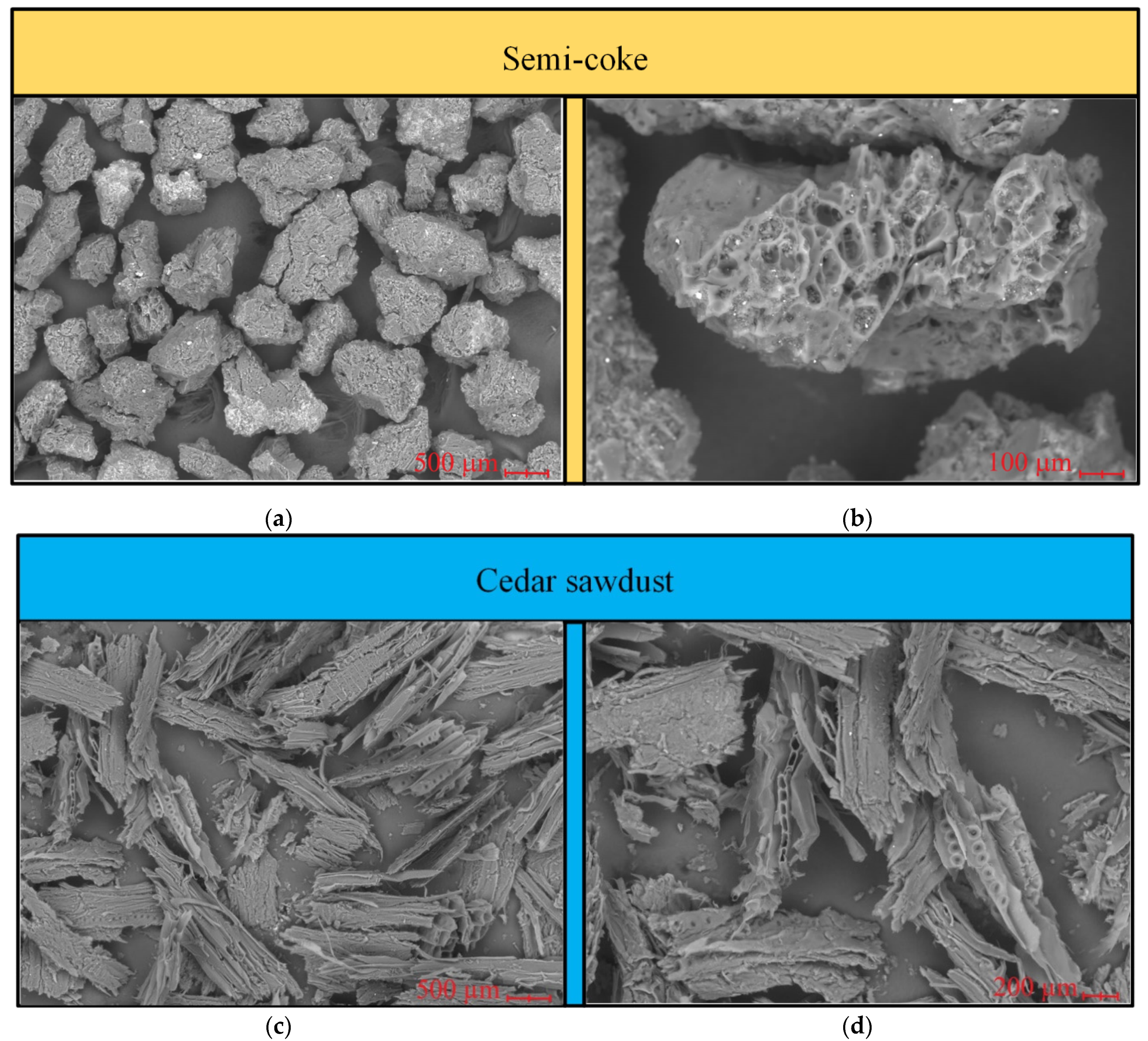
2.5. SEM
2.6. Ash Analysis
3. Results
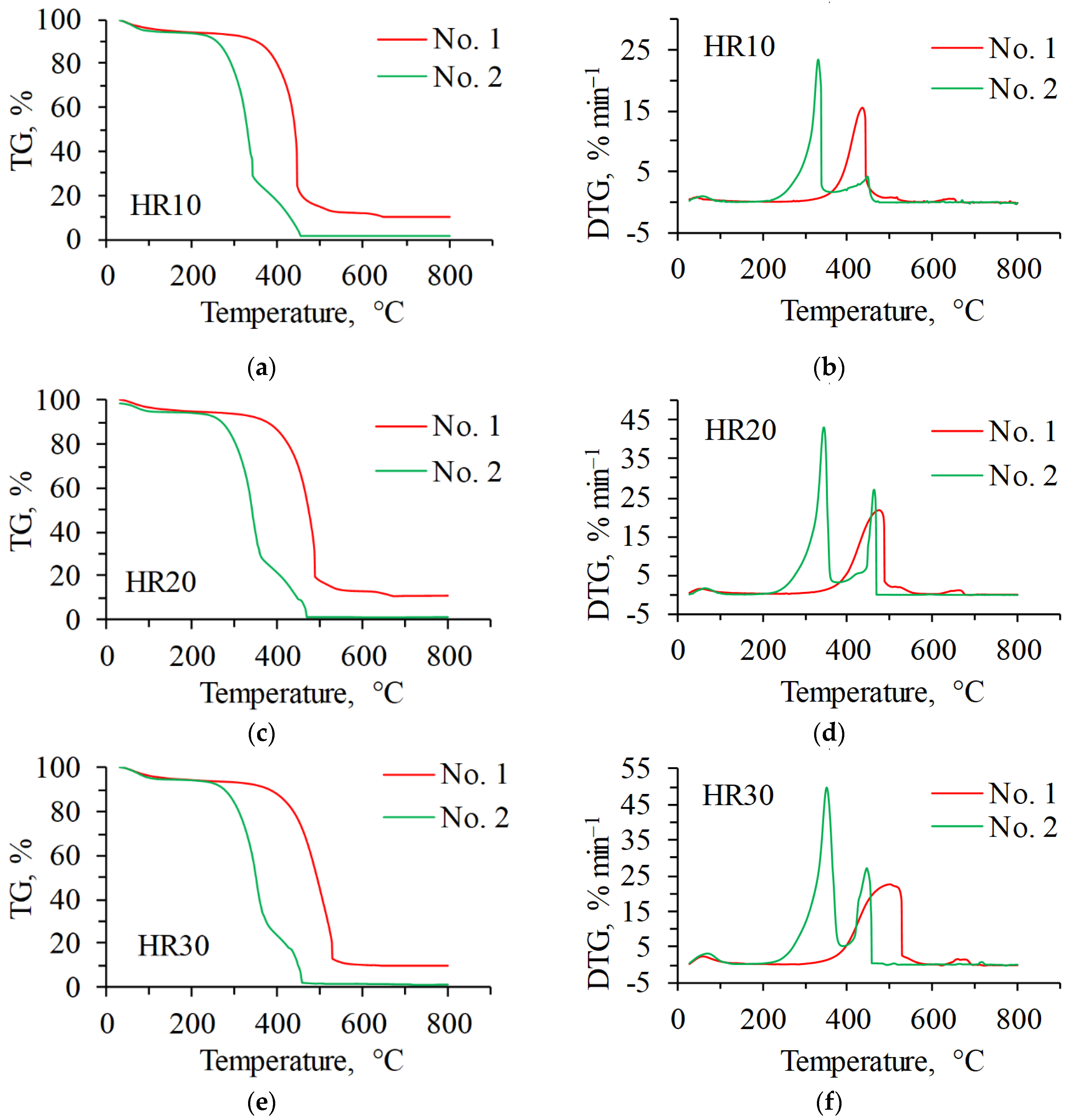
3.1. Individual Combustion
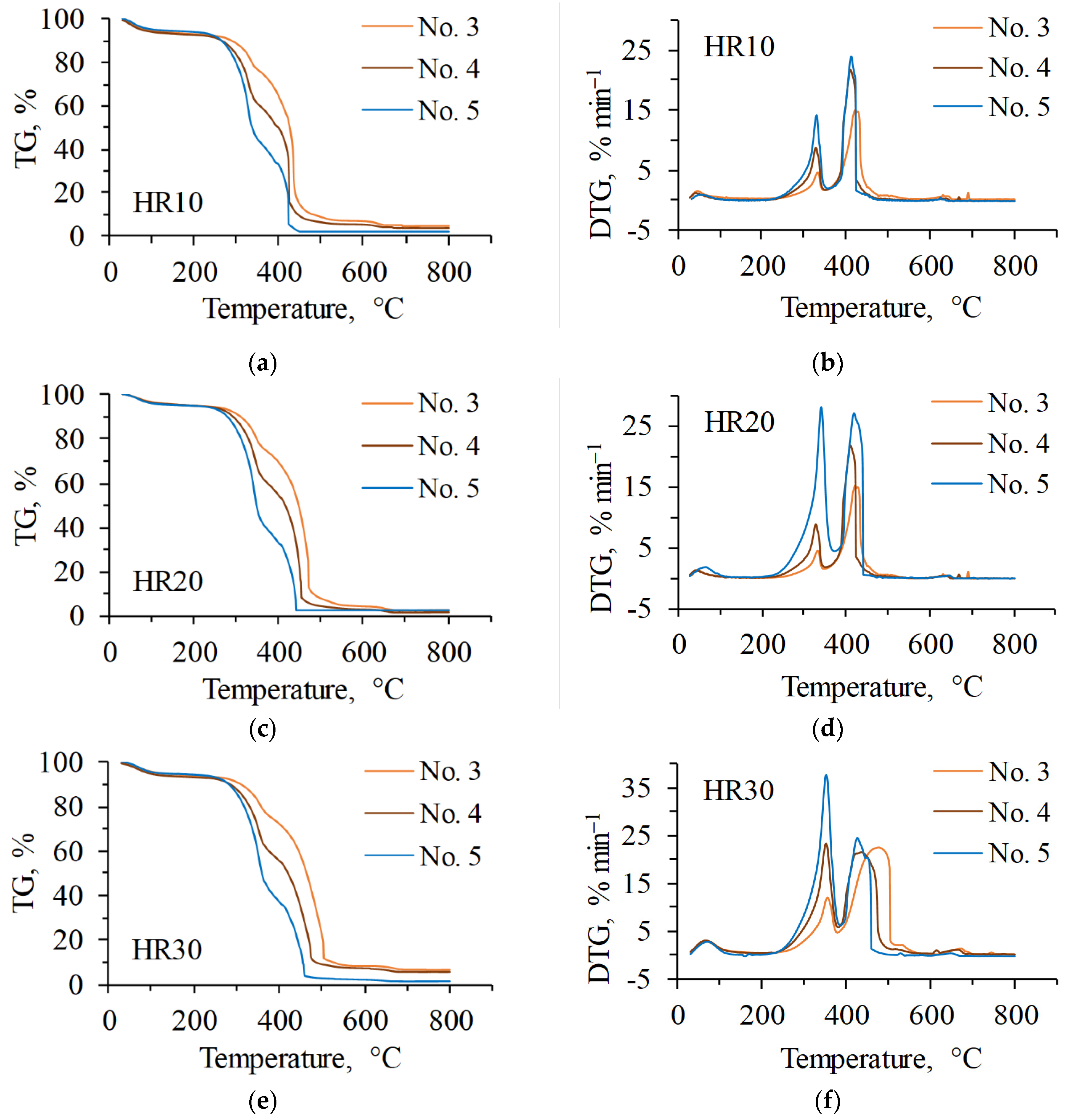
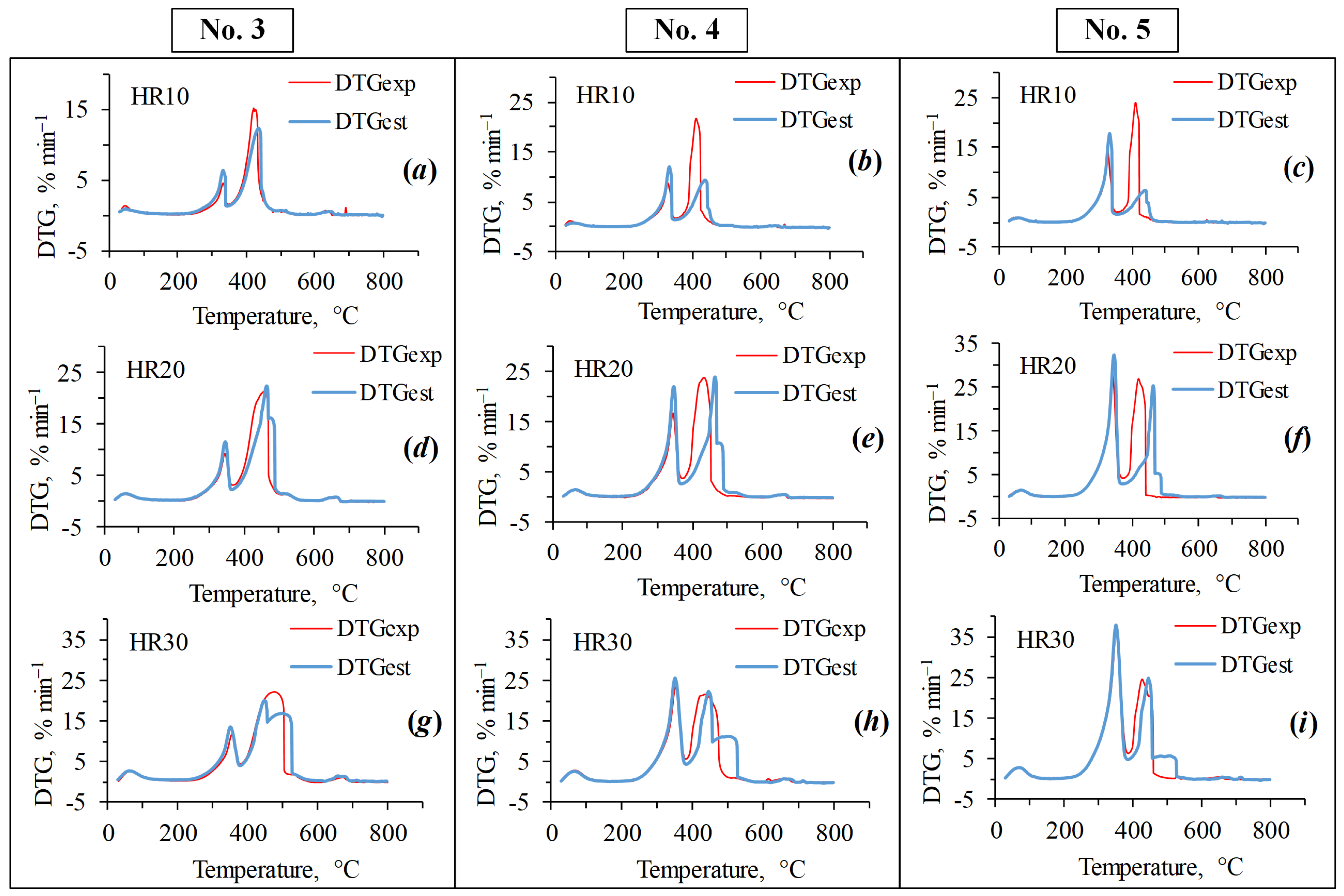
3.2. Co-Combustion Characteristics of Blends
3.3. Interaction between Blend Components—Synergistic Effects
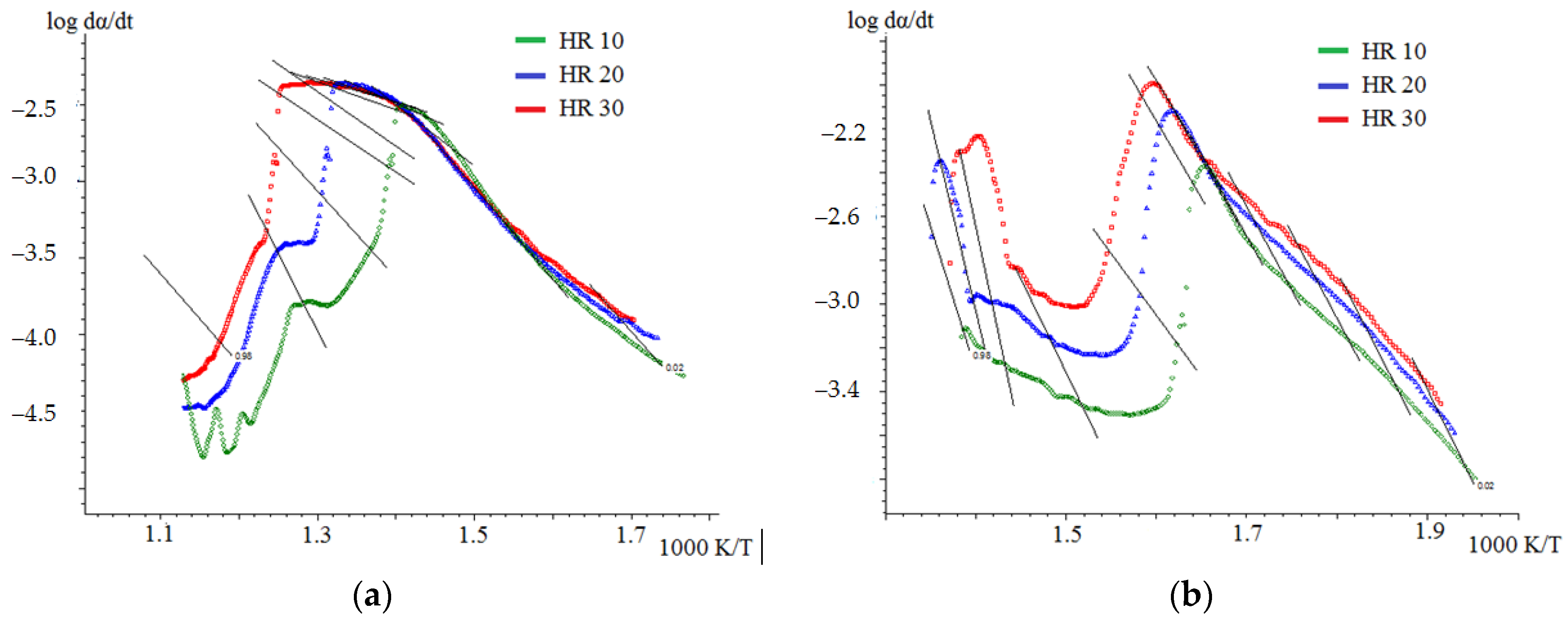
3.4. Kinetic Analysis
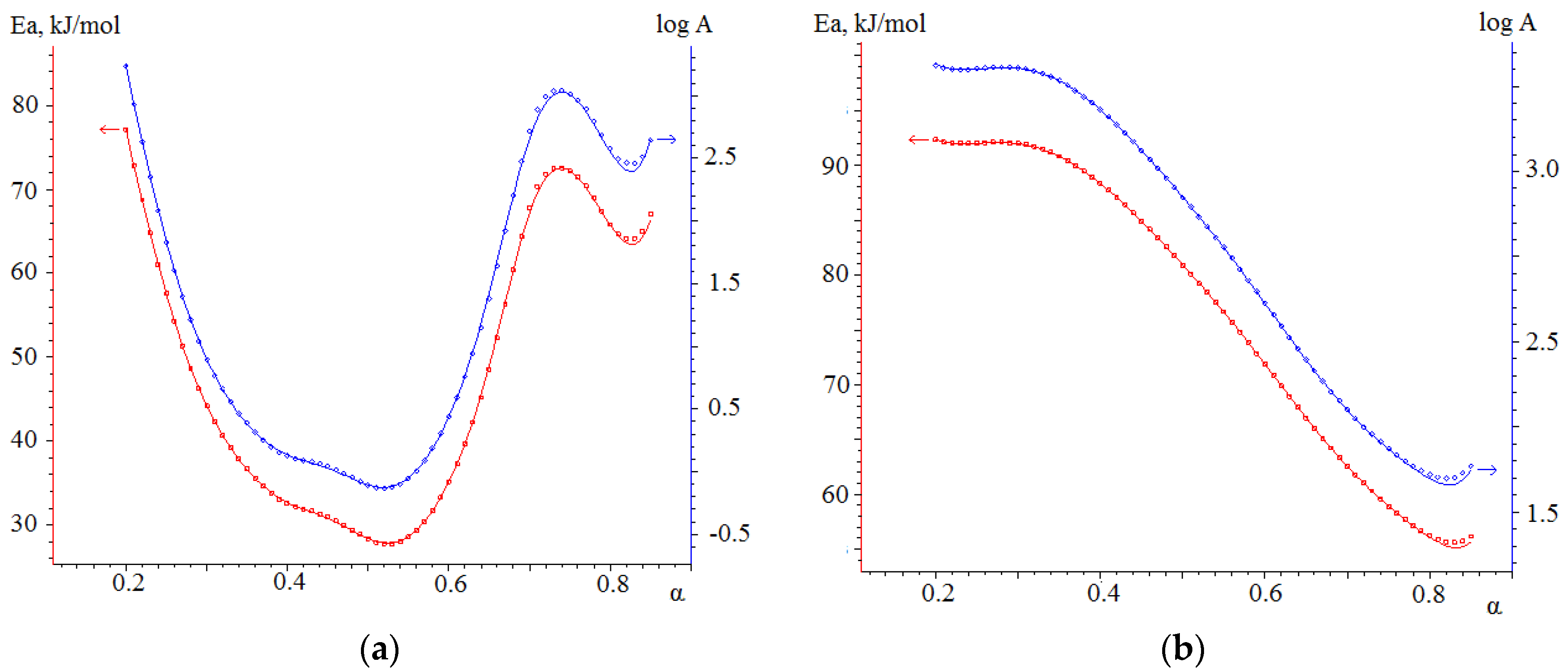
3.4.1. Kinetic Analysis of Individual Fuels
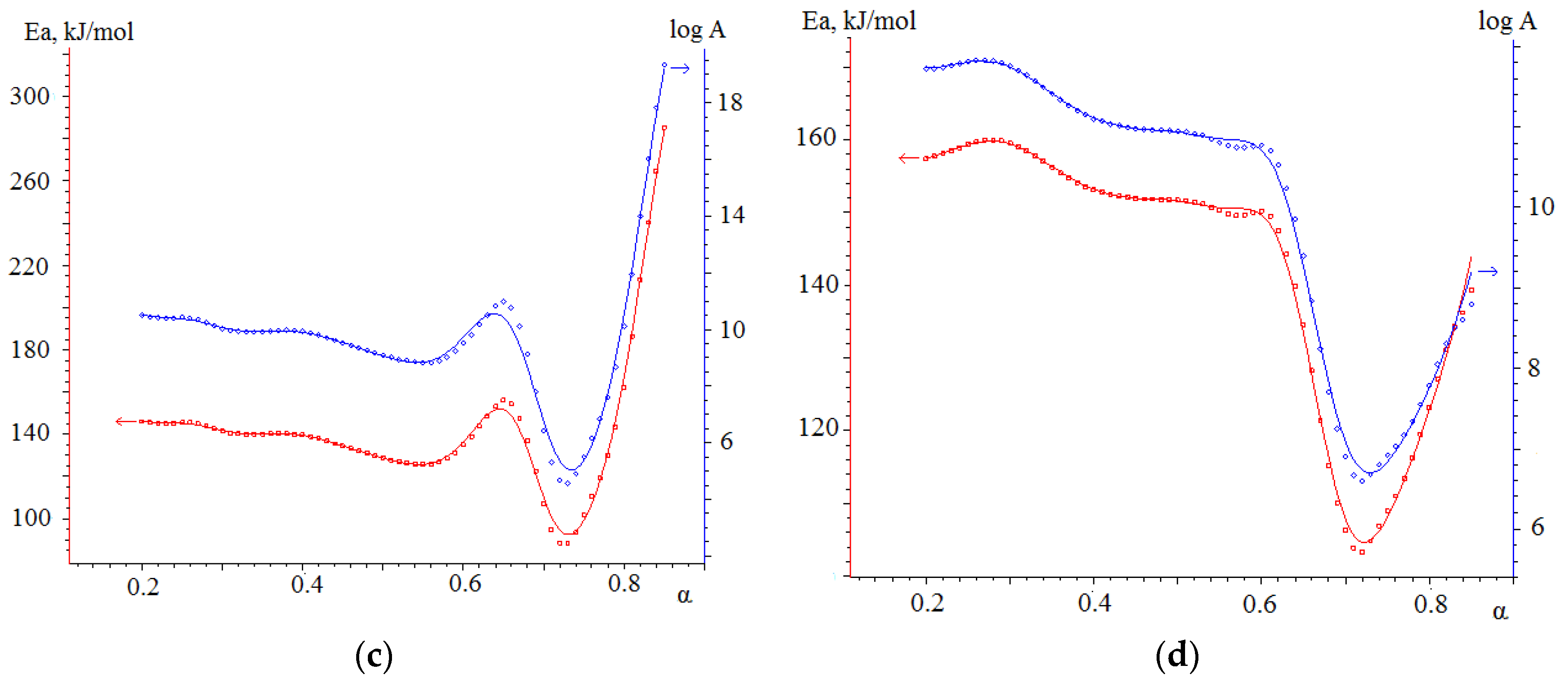
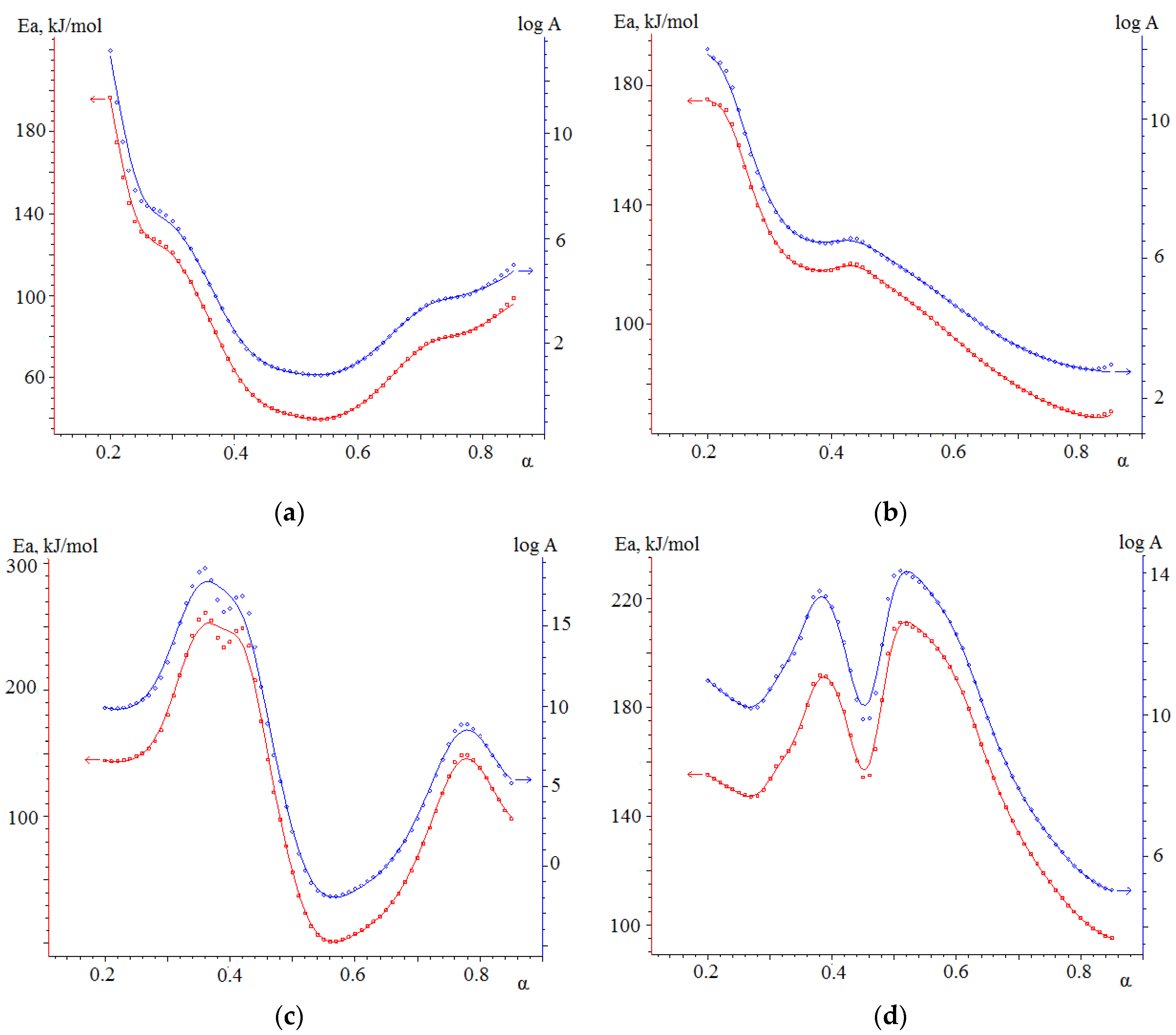
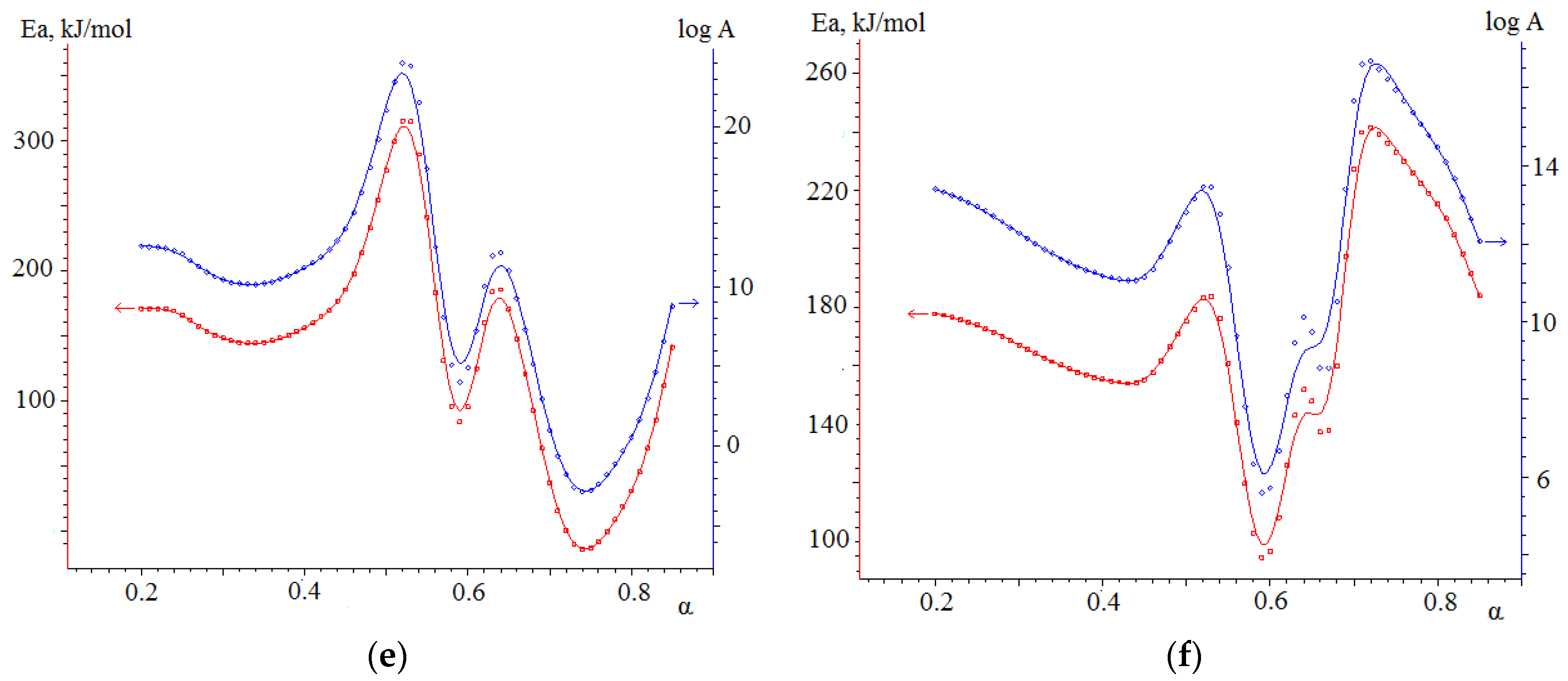
3.4.2. Kinetic Analysis of Co-Combustion
4. Conclusions
- (1)
- The obtained semi-coke has a low volatile matter content of 8.4%, which may have an adverse effect on its combustion in the boiler furnace, and a high calorific value of 32.1 MJ/kg. Adding biomass with high volatile matter content (80.2%) to semi-coke will increase the volatile matter content of the mixture, which will have a positive effect on the combustion of the mixtures.
- (2)
- According to the results of the thermal analysis, the main combustion characteristics were established for the investigated fuels and mixtures. The ignition temperature of semi-coke is 38% higher than that of biomass, the burnout temperature is 45% higher, and the combustion index is five times lower than that of biomass. At least 50% of biomass should be added to semi-coke; there is a 7% increase in combustion index compared to semi-coke; further increase in biomass content in the mixture will increase the combustion index, which will have a positive effect on the combustion of the mixture.
- (3)
- Both positive and negative synergetic interactions between mixture components are observed during the combustion of mixtures. Synergetic effects reducing the maximum heating rate at all heating rates are mainly observed in the combustion of biomass volatiles. Synergetic effects positively influencing the combustion process were observed in the combustion of biomass coke residue and semi-coke. There is no dependence between the heating rate of the studied fuels and the synergetic effects occurring between the components of the mixtures influencing the combustion process.
- (4)
- The results obtained in the determination of kinetic parameters show higher activation energy values for biomass combustion (113.5 kJ/mol) compared to semi-coke (76.5 kJ/mol). The kinetic analysis found that the combustion of mixtures overlaps different stages of combustion processes of individual fuels, resulting in lower activation energy values for such joint processes. Thus, the mixture based on 75% semi-coke and 25% cedar sawdust has the lowest activation energy value, high values of the pre-exponential multiplier, sufficient amount of volatile components, and, in our case, is a composition with a large synergetic effect.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gür, T.M. Carbon Dioxide Emissions, Capture, Storage and Utilization: Review of Materials Processes and Technologies. Prog. Energy Combust. Sci. 2022, 89, 100965. [Google Scholar] [CrossRef]
- Overland, I.; Loginova, J. The Russian coal industry in an uncertain world: Finally pivoting to Asia? Energy Res. Soc. Sci. 2023, 102, 103150. [Google Scholar] [CrossRef]
- Baxter, L. Biomass-Coal Cofiring: An Overview of Technical Issues. Green Energy Technol. 2011, 28, 43–73. [Google Scholar] [CrossRef]
- Variny, M.; Varga, A.; Rimár, M.; Janošovský, J.; Kizek, J.; Lukáč, L.; Jablonský, G.; Mierka, O. Advances in biomass co-combustion with fossil fuels in the European context: A review. Processes 2021, 9, 100. [Google Scholar] [CrossRef]
- Pazheri, F.R.; Othman, M.F.; Malik, N.H. A review on global renewable electricity scenario. Renew. Sust. Energ. Rev. 2014, 31, 835–845. [Google Scholar] [CrossRef]
- Sahu, S.G.; Chakraborty, N.; Sarkar, P. Coal-biomass co-combustion: An overview. Renew. Sust. Energ. Rev. 2014, 39, 575–586. [Google Scholar] [CrossRef]
- Al-Mansour, F.; Zuwala, J. An evaluation of biomass co-firing in Europe. Biomass Bioenergy 2010, 34, 620–629. [Google Scholar] [CrossRef]
- Ding, G.; He, B.; Yao, H.; Cao, Y.; Su, L.; Duan, Z. Co-combustion behaviors of municipal solid waste and low-rank coal semi-coke in air or oxygen/carbon dioxide atmospheres. J. Therm. Anal. Calorim. 2021, 143, 619–635. [Google Scholar] [CrossRef]
- Wang, P.; Wang, C.; Wang, C.; Du, Y.; Che, D. Experimental investigation on co-combustion characteristics of semi-coke and coal: Insight into synergy and blending method. Process Saf. Environ. Prot. 2023, 175, 290–302. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, K.; Ni, J.; Wu, J.; Ji, P.; Zeng, W.; Li, J.; Zhao, B.; Kou, M. Numerical simulation of co-combustion characteristics of semicoke and coke breeze in an ironmaking blast furnace. Fuel 2023, 335, 127113. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, W.; Liang, S.; Pan, X. Study on the co-combustion behavior of semi-coke and typical biomass: Combustion, NO emission and ash characteristics analysis. Fuel 2024, 358, 130068. [Google Scholar] [CrossRef]
- Liu, H.-P.; Liang, W.-X.; Qin, H.; Wang, Q. Synergy in co-combustion of oil shale semi-coke with torrefied cornstalk. Appl. Therm. Eng. 2016, 109, 653–662. [Google Scholar] [CrossRef]
- Qi, H.; Sun, R.; Peng, J.; Meng, X.; Cao, Z.; Wang, Z.; Ren, X.; Yuan, M.; Zhang, L.; Ding, S. Experimental investigation on the ignition and combustion characteristics of pyrolyzed char and bituminous coal blends. Fuel 2020, 281, 118732. [Google Scholar] [CrossRef]
- Qin, H.; Wang, W.; Liu, H.; Zhang, L.; Wang, Q.; Shi, C.; Yao, K. Thermal behavior research for co-combustion of furfural residue and oil shale semi-coke. Appl. Therm. Eng. 2017, 120, 19–25. [Google Scholar] [CrossRef]
- Zheng, S.; Hu, Y.; Wang, Z.; Cheng, X. Experimental investigation on ignition and burnout characteristics of semi-coke and bituminous coal blends. J. Energy Inst. 2020, 93, 1373–1381. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, X.; Wang, C.; Zhao, N.; Wang, P.; Che, D. Experimental investigation on combustion and NO formation characteristics of semi-coke and bituminous coal blends. Fuel 2019, 247, 87–96. [Google Scholar] [CrossRef]
- Hu, L.; Zhang, Y.; Chen, D.; Fang, J.; Zhang, M.; Wu, Y.; Zhang, H.; Li, Z.; Lyu, J. Experimental study on the combustion and NOx emission characteristics of a bituminous coal blended with semi-coke. Appl. Therm. Eng. 2019, 160, 113993. [Google Scholar] [CrossRef]
- Zhao, R.; Qin, J.; Chen, T.; Wu, J. TG-FTIR study on co-combustion of bituminous coal semicoke and lignite. J. Therm. Anal. Calorim. 2022, 147, 1849–1858. [Google Scholar] [CrossRef]
- ISO 11722:1999; Solid Mineral Fuels—Hard Coal—Determination of Moisture in the General Analysis Test Sample by Drying in Nitrogen. International Organization for Standardization: Geneva, Switzerland, 1999.
- ISO 1171:2010; Solid Mineral Fuel—Determination of Ash. International Organization for Standardization: Geneva, Switzerland, 2010.
- ISO 562:2010; Hard Coal and Coke—Determination of Volatile Matter. International Organization for Standardization: Geneva, Switzerland, 2010.
- ISO 1928:2009; Solid Mineral Fuels—Determination of Gross Calorific Value by the Bomb Calorimetric Method and Calculation of Net Calorific Value. International Organization for Standardization: Geneva, Switzerland, 2009.
- ASTM D5373-14e1; Standard Test Methods for Determination of Carbon, Hydrogen and Nitrogen in Analysis Samples of Coal and Carbon in Analysis Samples of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2014.
- Oladejo, J.M.; Adegbite, S.; Pang, C.H.; Liu, H.; Parvez, A.M.; Wu, T. A novel index for the study of synergistic effects during the co-processing of coal and biomass. Appl. Energy. 2017, 188, 215–225. [Google Scholar] [CrossRef]
- Moon, C.; Sung, Y.; Ahn, S.; Kim, T.; Choi, G.; Kim, D. Effect of blending ratio on combustion performance in blends of biomass and coals of different ranks. Exp. Therm. Fluid Sci. 2013, 47, 232–240. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Hoekman, S.K.; Srinivasan, M.P.; Balasubramanian, R. Thermogravimetric investigation of hydrochar-lignite co-combustion. Bioresour. Technol. 2012, 123, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Zhuikov, A.V.; Glushkov, D.O.; Kuznetsov, P.N.; Grishina, I.I.; Samoilo, A.S. Ignition of two-component and three-component fuel mixtures based on brown coal and char under slow heating conditions. J. Therm. Anal. Calorim. 2022, 147, 11965–11976. [Google Scholar] [CrossRef]
- Wang, C.; Wang, F.; Yang, Q.; Liang, R. Thermogravimetric studies of the behavior of wheat straw with added coal during combustion. Biomass Bioenergy 2009, 33, 50–56. [Google Scholar] [CrossRef]
- Raza, M.; Abu-Jdayil, B.; Al-Marzouqi, A.H.; Inayat, A. Inayat Kinetic and thermodynamic analyses of date palm surface fibers pyrolysis using Coats-Redfern method. Renew. Energy 2022, 183, 67–77. [Google Scholar] [CrossRef]
- Isaac, K.; Bada, S.O. The co-combustion performance and reaction kinetics of refuse derived fuels with South African high ash coal. Heliyon 2020, 6, e03309. [Google Scholar] [CrossRef]
- Yuan, Y.; Zuo, H.; Wang, J.; Gao, Y.; Xue, Q.; Wang, J. Co-combustion behavior, kinetic and ash melting characteristics analysis of clean coal and biomass pellet. Fuel 2022, 324, 124727. [Google Scholar] [CrossRef]
- Armakan, S.; Civan, M.; Yurdakul, S. Determining co-combustion characteristics, kinetics and synergy behaviors of raw and torrefied forms of two distinct types of biomass and their blends with lignite. J. Therm. Anal. Calorim. 2022, 147, 12855–12869. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Japan 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A quick, direct method for the determination of activation energy from thermogravimetric data. J. Polym. Sci. Part B Polym. Lett. 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Doyle, C.D. Estimating isothermal life from thermogravimetric data. J. Appl. Polym. Sci. 1962, 6, 639–642. [Google Scholar] [CrossRef]
- Glushkov, D.O.; Zhuikov, A.V.; Nurpeiis, A.E.; Paushkina, K.K.; Kuznechenkova, D.A. Ignition behavior of a mixture of brown coal and biomass during the movement of fine particles in a hot air flow. Fuel 2024, 363, 131010. [Google Scholar] [CrossRef]
- Kuznetsov, P.N.; Kuznetsova, L.I.; Bimer, J.; Salbut, P.; Gruber, R.; Brodzki, D. Quantitative relation between the macromolecular characteristics of brown coal and its reactivity in conversion with tetralin. Fuel 1997, 76, 189–193. [Google Scholar] [CrossRef]
| No. | Proximate Analysis/% | Ultimate Analysis/% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| MCa | Ad | VCdaf | HHV | Cdaf | Hdaf | Ndaf | Sdaf | Odaf | |
| % | % | % | MJ/kg | % | % | % | % | % | |
| 1 | 0.8 | 9.1 | 8.4 | 32.1 | 91.2 | 2.4 | 2.2 | 0.2 | 4.0 |
| 2 | 6.4 | 0.2 | 80.2 | 19.2 | 51.3 | 5.4 | 0.3 | – | 43.0 |
| Oxides, % | No.1—Semi-Coke | No.2—Cedar Sawdust |
|---|---|---|
| SiO2 | 50.5 | 10.5 |
| Al2O3 | 10.0 | 1.3 |
| Fe2O3 | 13.7 | 1.1 |
| CaO | 23.0 | 55.1 |
| MgO | 4.5 | 4.0 |
| TiO2 | 0.1 | 0.1 |
| Na2O | 0.2 | 0.6 |
| K2O | 0.3 | 19.2 |
| SO3 | 6.0 | 2.3 |
| P2O5 | 0.04 | 3.1 |
| ZnO | 0.05 | 0.9 |
| Cl | 0.01 | 0.3 |
| MnO | 0.05 | 1.5 |
| Samples | HR, °C min−1 | Temperature Interval Stage II, °C | Stage II, min | Temperature Interval Stage III, °C | Stage III, min | Burning, min |
|---|---|---|---|---|---|---|
| No. 1 | 10 | 295–405 | 9.5 | 406–653 | 26.5 | 36.0 |
| 20 | 310–435 | 5.1 | 436–672 | 13.1 | 18.2 | |
| 30 | 290–440 | 4.2 | 441–690 | 9.2 | 13.4 | |
| No. 2 | 10 | 201–299 | 9.4 | 300–461 | 16.7 | 26.1 |
| 20 | 202–311 | 5.2 | 312–469 | 8.2 | 13.4 | |
| 30 | 202–318 | 3.8 | 319–458 | 4.7 | 8.5 | |
| No. 3 | 10 | 240–349 | 21.2 | 350–644 | 30.0 | 51.2 |
| 20 | 230–401 | 8.0 | 402–669 | 14.0 | 22.0 | |
| 30 | 240–406 | 5.1 | 407–689 | 9.9 | 15.0 | |
| No. 4 | 10 | 233–301 | 6.6 | 302–669 | 37.2 | 43.8 |
| 20 | 220–313 | 4.6 | 314–665 | 17.7 | 22.3 | |
| 30 | 229–318 | 2.9 | 319–675 | 12.0 | 14.9 | |
| No. 5 | 10 | 216–301 | 8.0 | 302–625 | 33.1 | 41.1 |
| 20 | 204–311 | 5.4 | 312–456 | 7.4 | 12.8 | |
| 30 | 206–316 | 3.5 | 317–489 | 5.9 | 9.4 |
| Samples | HR, °C min−1 | RDTG, % min–1 | Ti, °C | TDTG, °C | Tb, °C | S × 10–7, min–2 °C–3 |
|---|---|---|---|---|---|---|
| No. 1 | 10 | 295–405 | 9.5 | 406–653 | 26.5 | 36.0 |
| 20 | 310–435 | 5.1 | 436–672 | 13.1 | 18.2 | |
| 30 | 290–440 | 4.2 | 441–690 | 9.2 | 13.4 | |
| No. 2 | 10 | 201–299 | 9.4 | 300–461 | 16.7 | 26.1 |
| 20 | 202–311 | 5.2 | 312–469 | 8.2 | 13.4 | |
| 30 | 202–318 | 3.8 | 319–458 | 4.7 | 8.5 | |
| No. 3 | 10 | 240–349 | 21.2 | 350–644 | 30.0 | 51.2 |
| 20 | 230–401 | 8.0 | 402–669 | 14.0 | 22.0 | |
| 30 | 240–406 | 5.1 | 407–689 | 9.9 | 15.0 | |
| No. 4 | 10 | 233–301 | 6.6 | 302–669 | 37.2 | 43.8 |
| 20 | 220–313 | 4.6 | 314–665 | 17.7 | 22.3 | |
| 30 | 229–318 | 2.9 | 319–675 | 12.0 | 14.9 | |
| No. 5 | 10 | 216–301 | 8.0 | 302–625 | 33.1 | 41.1 |
| 20 | 204–311 | 5.4 | 312–456 | 7.4 | 12.8 | |
| 30 | 206–316 | 3.5 | 317–489 | 5.9 | 9.4 |
| Blends | Stage II | Stage III | |||
|---|---|---|---|---|---|
| DTGexp, % min−1 | DTGest, % min−1 | DTGexp, % min−1 | DTGest, % min−1 | ||
| Heating rate 10 °C min−1 | No. 3 | 4.5 | 6.4 | 15.1 | 12.4 |
| No. 4 | 8.8 | 12.1 | 21.7 | 9.4 | |
| No. 5 | 14.3 | 17.7 | 24.1 | 6.4 | |
| Heating rate 20 °C min−1 | No. 3 | 9.4 | 11.6 | 21.5 | 22.5 |
| No. 4 | 16.8 | 22.0 | 23.7 | 24.0 | |
| No. 5 | 28.1 | 32.5 | 27.2 | 25.4 | |
| Heating rate 30 °C min−1 | No. 3 | 11.8 | 13.6 | 22.3 | 20.2 |
| No. 4 | 23.4 | 25.7 | 21.5 | 22.4 | |
| No. 5 | 37.7 | 37.8 | 24.6 | 24.8 | |
| Friedman Model | OFW Model | Friedman Model | OFW Model | |
|---|---|---|---|---|
| Ea, kJ/mol | Ea, kJ/mol | Ea, kJ/mol | Ea, kJ/mol | |
| Stage II | n/a | n/a | 140.1 | 155.1 |
| Stage III | n/a | n/a | 113.5 | 120.9 |
| average | 63.6 | 76.5 | n/a | n/a |
| Sample | Transformation Degree | Friedman Model Ea, kJ/mol | OFW Model Ea, kJ/mol |
|---|---|---|---|
| No. 3 | α = 0–0.3 | 141.7 | 157.0 |
| α = 0.3–0.7 | 58.8 | 107.8 | |
| α = 0.7–1 | 87.1 | 77.9 | |
| No. 4 | α = 0–0.4 | 176.1 | 159.0 |
| α = 0.4–0.7 | 67.4 | 179.4 | |
| α = 0.7–1 | 120.0 | 114.0 | |
| No. 5 | α = 0–0.5 | 166.6 | 165.0 |
| α =0.5–0.7 | 164.5 | 143.2 | |
| α = 0.7–1 | 104.5 | 158.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuikov, A.; Irtyugo, L.; Samoilo, A.; Zhuikova, Y.; Grishina, I.; Pyanykh, T.; Chicherin, S. Advanced Fuel Based on Semi-Coke and Cedarwood: Kinetic Characteristics and Synergetic Effects. Energies 2024, 17, 4963. https://doi.org/10.3390/en17194963
Zhuikov A, Irtyugo L, Samoilo A, Zhuikova Y, Grishina I, Pyanykh T, Chicherin S. Advanced Fuel Based on Semi-Coke and Cedarwood: Kinetic Characteristics and Synergetic Effects. Energies. 2024; 17(19):4963. https://doi.org/10.3390/en17194963
Chicago/Turabian StyleZhuikov, Andrey, Lily Irtyugo, Alexander Samoilo, Yana Zhuikova, Irina Grishina, Tatyana Pyanykh, and Stanislav Chicherin. 2024. "Advanced Fuel Based on Semi-Coke and Cedarwood: Kinetic Characteristics and Synergetic Effects" Energies 17, no. 19: 4963. https://doi.org/10.3390/en17194963
APA StyleZhuikov, A., Irtyugo, L., Samoilo, A., Zhuikova, Y., Grishina, I., Pyanykh, T., & Chicherin, S. (2024). Advanced Fuel Based on Semi-Coke and Cedarwood: Kinetic Characteristics and Synergetic Effects. Energies, 17(19), 4963. https://doi.org/10.3390/en17194963








