Sustainable Valorization of CO2 through Nuclear Power-to-X Pathways
Abstract
:1. Introduction
2. Chemical Pathways to Valorize CO2
2.1. Synthesis Gas (Syngas)
2.1.1. Thermocatalytic Pathway
2.1.2. Chemical Looping Pathway
| Hemicycle 1 (Reduction step) | Hemicycle 2 (Oxidation step) | |
| (R11) | (R12) | |
| Thermal Redox | Me + Oxidant → MeO + Reduced-oxidant | MeO + Heat → Me + ½O2 |
| Chemical Redox | Me + Oxidant → MeO + Reduced-oxidant | MeO + Reductant → Me + Oxidant |
| Carbonation | MeO + (CO2)sol → MeCO3 | MeCO3 + Heat → MeO + Pure CO2 |
2.1.3. Electrochemical Pathway
2.1.4. Radiation-Induced Pathways
2.2. Methanol
2.2.1. Thermocatalytic Pathways
2.2.2. Electrochemical Pathways
2.2.3. Economic and Environmental Assessments
2.3. Urea
2.4. Polymers
2.4.1. Thermocatalytic Pathways
| Path 1 | (R27) + (R28) | Phosgene, coproducing HCl | |
| Path 2 | (R29) | 2NO + 2CH3OH → 2CH3ONO + H2 | via methyl nitrite, coproducing CO and NO |
| (R30) | 2CH3ONO + CO → CH3OCO2CH3 + 2NO | ||
| Path 3 | (R26) | Methanol carbonylation | |
| Path 4 | (R31) | CO(NH2)2 + CH3OH → CH3OCONH2 + NH3 | Urea pathway, coproducing NH3 |
| (R32) | CH3OCONH2 + CH3OH → CH3OCO2CH3 + NH3 | ||
| Path 5 | (R33) | (CH2)2O + CO2 → (CH2)2OCO2 | Ethylene oxide (EO) pathway, coproducing ethylene glycol (EG) |
| (R34) | (CH2)2OCO2 + 2CH3OH → CH3OCO2CH3 + (CH2)2(OH)2 | ||
| Path 6 | (R24) | Methanol carboxylation |
2.4.2. Radiation-Induced Pathways
2.5. Carbonates and Bicarbonates
3. Integration to Nuclear Power
4. Future Potentialities
5. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
Abbreviations and Acronyms
| Adam & Eva | A&E | integrated energy systems | IES |
| 2-amino-2-methyl-1-propanol | AMP | intermediate heat exchanger | IHX |
| Carbon Recycling International | CRI | hydrogen evolution reaction | HER |
| Chemical looping | CL | life cycle assessment | LCA |
| chemical process | CP | metal | Me |
| dimethyl carbonate | DMC | metal oxide | MeO |
| dimethyl ether | DME | methane dry reforming | MDR |
| Emergy Accounting | EMA | metric tons of heavy metal | MTHM |
| Emissions-to-Liquids | EtL | nuclear energy | NE |
| Ethylene glycol | EG | nuclear hydrogen | NH |
| Ethylene oxide | EO | nuclear power plants | NPP |
| faradaic efficiency | FE | oxidative coupling of methane | OCM |
| formaldehyde-to-urea | F/U | power to gas | PtG |
| fuel cell | FC | power to liquids | PtL |
| functional unit | FU | power to value | PtV |
| gas diffusion electrodes | GDE | power to any energy-carrier | PtX |
| Gas Technology Institute | GTI | renewable natural gas | RNG |
| global warming impact | GWI | spent nuclear fuel | SNF |
References
- Chen, X.; McElroy, M.B.; Wu, Q.; Shu, Y.; Xue, Y. Transition towards higher penetration of renewables: An overview of interlinked technical, environmental and socio-economic challenges. J. Mod. Power Syst. Clean Energy 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Milko, J.; Allen, T.; Fitzpatrick, R. Keeping up with the Advanced Nuclear Industry; Third Way: Washington, DC, USA, 2018; 3p, Available online: https://www.thirdway.org/graphic/keeping-up-with-the-advanced-nuclear-industry (accessed on 8 December 2018).
- Fütterer, M.A.; Fu, L.; Sink, C.; de Groot, S.; Pouchon, M.; Kim, Y.W.; Carré, F.; Tachibana, Y. Status of the very high temperature reactor system. Prog. Nucl. Energy 2014, 77, 266–281. [Google Scholar] [CrossRef]
- US Department of Energy; Nuclear Energy Research Advisory Committe; Generation IV International Forum. A Technology Roadmap for Generation IV Nuclear Energy Systems; GIF-002-00 Report; USA Department of Energy: Washington, DC, USA, 2002; 97p.
- Fumizawa, M.; Kosuge, Y.; Horiuchi, H. Thermal evaluation for a ultra high temperature reactor with pebble type fuels. In Proceedings of the 17th International Conference on Nuclear Engineering, ICONE17, Brussels, Belgium, 12–16 July 2009; Volume 1, pp. 865–872. [Google Scholar] [CrossRef]
- Hashimoto, K.; Yamasaki, M.; Fujimura, K.; Matsui, T.; Izumiya, K.; Komori, M.; El-Moneim, A.A.; Akiyama, E.; Habazaki, H.; Kumagai, N.; et al. Global CO2 recycling—Novel materials and prospect for prevention of global warming and abundant energy supply. Mater. Sci. Eng. A 1999, 267, 200–206. [Google Scholar] [CrossRef]
- Buchholz, O.S.; Van Der Ham, A.G.J.; Veneman, R.; Brilman, D.W.F.; Kersten, S.R.A. Power-to-Gas: Storing surplus electrical energy a design study. In Proceedings of the 12th International Conference on Greenhouse Gas Control Technologies, GHGT 2014; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 63, pp. 7993–8009. [Google Scholar] [CrossRef]
- Hashimoto, K.; Akiyama, E.; Habazaki, H.; Kawashima, A.; Shimamura, K.; Komori, M.; Kumagai, N. Global CO2 Recycling. Zair.-Kankyo 1996, 45, 614–620. [Google Scholar] [CrossRef]
- Habazaki, H.; Yamasaki, M.; Kawashima, A.; Hashimoto, K. Methanation of carbon dioxide on Ni/(Zr-Sm)O(x) catalysts. Appl. Organomet. Chem. 2000, 14, 803–808. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kato, Z.; Kumagai, N.; Izumiya, K. Key materials and systems for the use of renewable energy in the form of methane. In Proceedings of the 28th International Conference on Ocean, Offshore and Arctic Engineering, OMAE2009, Honolulu, HI, USA, 31 May–5 June 2009; Volume 4, pp. 1003–1011. [Google Scholar] [CrossRef]
- Kato, Z.; Izumiya, K.; Kumagai, N.; Hashimoto, K. Energy-saving seawater electrolysis for hydrogen production. J. Solid State Electrochem. 2009, 13, 219–224. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kumagai, N.; Izumiya, K.; Takano, H.; Kato, Z. The production of renewable energy in the form of methane using electrolytic hydrogen generation. Energy Sustain. Soc. 2014, 4, 17. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Gomberg, H.J.; Gordus, A.A. Hydrogen for synthetic fuels via nuclear energy. J. Fusion Energy 1982, 2, 319–339. [Google Scholar] [CrossRef]
- Harth, R.E.; Boltendahl, U. The chemical heat pipe EVA and ADAM. Interdiscip. Sci. Rev. 1981, 6, 221–228. [Google Scholar] [CrossRef]
- Orhan, M.F.; Dincer, I.; Rosen, M.A.; Kanoglu, M. Integrated hydrogen production options based on renewable and nuclear energy sources. Renew. Sustain. Energy Rev. 2012, 16, 6059–6082. [Google Scholar] [CrossRef]
- El-Emam, R.S.; Khamis, I. Advances in nuclear hydrogen production: Results from an IAEA international collaborative research project. Int. J. Hydrogen Energy 2019, 44, 19080–19088. [Google Scholar] [CrossRef]
- Sink, C.J., Jr.; Taylor, A. An overview of the Nuclear hydrogen initiative. In Proceedings of the Topical Conference on Hydrogen 2006, Held at the 2006 AIChE Spring National Meeting, Orlando, FL, USA, 23–27 April 2006; pp. 40–45. [Google Scholar]
- Sakaba, N.; Ohashi, H.; Sato, H.; Hara, T.; Kato, R.; Kunitomi, K. Hydrogen production by high-temperature gas-cooled reactor; conceptual design of advanced process heat exchangers of the HTTR-is hydrogen production system. Trans. At. Energy Soc. Jpn. 2008, 7, 242–256. [Google Scholar] [CrossRef]
- Naterer, G.F.; Dincer, I.; Zamfirescu, C. Hydrogen Production from Nuclear Energy; Springer: London, UK, 2013; Volume 9781447149385, pp. 1–492. [Google Scholar] [CrossRef]
- Elder, R.; Allen, R. Nuclear heat for hydrogen production: Coupling a very high/high temperature reactor to a hydrogen production plant. Prog. Nucl. Energy 2009, 51, 500–525. [Google Scholar] [CrossRef]
- Revankar, S.T. Nuclear hydrogen production. In Storage and HYBRIDIZATION of Nuclear Energy: Techno-Economic Integration of Renewable and Nuclear Energy; Elsevier: Amsterdam, The Netherlands, 2018; pp. 49–117. [Google Scholar] [CrossRef]
- Yan, X.L.; Konishi, S.; Hori, M.; Hino, R. Nuclear hydrogen production: An overview. In Nuclear Hydrogen Production Handbook; CRC Press: Boca Raton, FL, USA, 2016; pp. 47–82. [Google Scholar]
- Petrescu, F.I.T.; Petrescu, R.V.V. Nuclear hydrogen structure and dimensions. Int. J. Hydrogen Energy 2019, 44, 10833–10837. [Google Scholar] [CrossRef]
- O’Brien, J.E. Review of the potential of nuclear hydrogen for addressing energy security and climate change. Nucl. Technol. 2012, 178, 55–65. [Google Scholar] [CrossRef]
- Khan, S.U.D. Using next generation nuclear power reactors for development of a techno-economic model for hydrogen production. Int. J. Energy Res. 2019, 43, 6827–6839. [Google Scholar] [CrossRef]
- Höhlein, B.; Menzer, R.; Range, J. High temperature methanation in the long-distance nuclear energy transport system. Appl. Catal. 1981, 1, 125–139. [Google Scholar] [CrossRef]
- Ramirez-Corredores, M.M.; Gadikota, G.; Huang, E.E.; Gaffney, A.M. Radiation-induced chemistry of carbon dioxide: A pathway to close the carbon loop for a circular economy. Front. Energy Res. 2020, 8, 17. [Google Scholar] [CrossRef]
- Ramirez-Corredores, M.M.; Rollins, H.W.; Morco, R.P.; Zarzana, C.A.; Diaz, L.A. Radiation-induced dry reforming: A negative emission process. J. Clean. Prod. 2023, 429, 139539. [Google Scholar] [CrossRef]
- Ramirez-Corredores, M.M.; Diaz, L.A.; Gaffney, A.M.; Zarzana, C.A. Identification of opportunities for integrating chemical processes for carbon (dioxide) utilization to nuclear power plants. Renew. Sustain. Energy Rev. 2021, 150, 111450. [Google Scholar] [CrossRef]
- Luderer, G.; Vrontisi, Z.; Bertram, C.; Edelenbosch, O.Y.; Pietzcker, R.C.; Rogelj, J.; De Boer, H.S.; Drouet, L.; Emmerling, J.; Fricko, O.; et al. Residual fossil CO2 emissions in 1.5–2 °C pathways. Nat. Clim. Change 2018, 8, 626–633. [Google Scholar] [CrossRef]
- Rissman, J.; Bataille, C.; Masanet, E.; Aden, N.; Morrow, W.R.; Zhou, N.; Elliott, N.; Dell, R.; Heeren, N.; Huckestein, B.; et al. Technologies and policies to decarbonize global industry: Review and assessment of mitigation drivers through 2070. Appl. Energy 2020, 266, 114848. [Google Scholar] [CrossRef]
- Global Carbon Project. Global Carbon Atlas: CO2 Emissions. Available online: http://www.globalcarbonatlas.org/en/CO2-emissions (accessed on 15 February 2024).
- Crippa, M.; Guizzardi, D.; Schaaf, E.; Monforti-Ferrario, F.; Quadrelli, R.; Risquez Martin, A.; Rossi, S.; Vignati, E.; Muntean, M.; Brandao De Melo, J.; et al. GHG Emissions of All World Countries—2023; Publications Office of the European Union, European Commission Joint Research Centre: Luxembourg, 2023; 268p. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A. Utilisation of CO2 as a chemical feedstock: Opportunities and challenges. Dalton Trans. 2007, 28, 2975–2992. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Kumagai, N.; Izumiya, K.; Kato, Z. Carbon dioxide, the feedstock for using renewable energy. In Proceedings of the European Materials Research Society (E-MRS) 2010 Fall Meeting, Warsaw, Poland, 13–17 September 2010. [Google Scholar]
- Dibenedetto, A.; Angelini, A.; Stufano, P. Use of carbon dioxide as feedstock for chemicals and fuels: Homogeneous and heterogeneous catalysis. J. Chem. Technol. Biotechnol. 2014, 89, 334–353. [Google Scholar] [CrossRef]
- Grignard, B.; Gennen, S.; Jérôme, C.; Kleij, A.W.; Detrembleur, C. Advances in the use of CO2 as a renewable feedstock for the synthesis of polymers. Chem. Soc. Rev. 2019, 48, 4466–4514. [Google Scholar] [CrossRef]
- Müller, L.J.; Kätelhön, A.; Bringezu, S.; McCoy, S.; Suh, S.; Edwards, R.; Sick, V.; Kaiser, S.; Cuéllar-Franca, R.; El Khamlichi, A.; et al. The carbon footprint of the carbon feedstock CO2. Energy Environ. Sci. 2020, 13, 2979–2992. [Google Scholar] [CrossRef]
- Global CCS Institute. The Global Status of CCS 2023; Global CCS Institute: Melbourne, Australia, 2023; 98p, Available online: https://status23.globalccsinstitute.com/ (accessed on 15 January 2024).
- Budinis, S.; Fajardy, M.; Greenfield, C. Carbon Capture, Utilisation and Storage. Available online: https://www.iea.org/energy-system/carbon-capture-utilisation-and-storage (accessed on 15 June 2024).
- Allen, M.; Babiker, M.; Chen, Y.; de Coninck, H.; Connors, S.; van Diemen, R.; Dube, O.P.; Ebi, K.L.; Engelbrecht, F.; Ferrat, M.; et al. Global Warming of 1.5 °C; Special Report; Intergovernmental Panel on Climate Change (IPCC); Cambridge University Press: Cambridge, UK, 2018; 540p, Available online: https://www.ipcc.ch/sr15/ (accessed on 20 September 2021).
- Fernández, A.; Spencer, T.; McGlade, C.; Remme, U.; Brent, W.; D’Ambrosio, D. Net Zero Roadmap: A Global Pathway to Keep the 1.5 °C Goal in Reach; International Energy Agency: online, 2023; 226p, Available online: https://www.iea.org/reports/net-zero-roadmap-a-global-pathway-to-keep-the-15-0c-goal-in-reach (accessed on 2 November 2023).
- Riahi, K.; Schaeffer, R.; Arango, J.; Calvin, K.; Guivarch, C.; Hasegawa, Y.; Jiang, K.; Kriegler, E.; Matthews, R.; Peters, G.P.; et al. Mitigation pathways compatible with Long-term goals. In Climate Change 2022: Mitigation of Climate Change; Shukla, P.R., Skea, J., Slade, R., Eds.; Intergovernmental Panel on Climate Change (IPCC): Cambridge, UK; New York, NY, USA, 2023; pp. 295–408. Available online: https://www.ipcc.ch/report/ar6/wg3/downloads/ (accessed on 9 April 2024).
- Ramirez-Corredores, M.M.; Goldwasser, M.R.; Falabella de Sousa Aguiar, E. Decarbonization. In Decarbonization as a Route towards Sustainable Circularity; Springer International Publishing: Cham, Switzerland, 2023; pp. 15–101. [Google Scholar] [CrossRef]
- Ramirez-Corredores, M.M.; Goldwasser, M.R.; Falabella de Sousa Aguiar, E. Sustainable Circularity. In Decarbonization as a Route towards Sustainable Circularity; Springer International Publishing: Cham, Switzerland, 2023; pp. 103–125. [Google Scholar] [CrossRef]
- General Union Environment Action Programme to 2020 ‘Living Well, within the Limits of Our Planet’, in 1386/2013/EU. The 7th Environment Action Programme: European Union. 2013. 30p. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32013D1386 (accessed on 24 June 2022).
- National Research Council. Carbon Management: Implications for R&D in the Chemical Sciences and Technology; The National Academies Press: Washington, DC, USA, 2001; 236p. [CrossRef]
- Mahajan, D.; Song, C.; Scaroni, A.W. Catalytic Reduction of CO2 into Liquid Fuels: Simulating Reactions under Geologic Formation Conditions. In CO2 Conversion and Utilization; American Chemical Society: Washington, DC, USA, 2002; Volume 809, pp. 166–180. [Google Scholar] [CrossRef]
- Song, C.; Gaffney, A.F.; Fujimoto, K. CO2 Conversion and Utilization; ACS symposium series; Wiley: Washington, DC, USA, 2002; Volume 809, 427p. [Google Scholar]
- Aresta, M. Carbon Dioxide as Chemical Feedstock; Wiley-VCH: Washington, DC, USA, 2010; 394p. [Google Scholar] [CrossRef]
- Fink, J.K. Reactive Polymers Fundamentals and Applications: A Concise Guide to Industrial Polymers, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 1–535. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Green Carbon Dioxide: Advances in CO2 Utilization; Wiley Blackwell: Hoboken, NJ, USA, 2014; Volume 9781118590881, pp. 1–303. [Google Scholar]
- Muradov, N. Liberating energy from carbon: Introduction to decarbonization. In Lecture Notes in Energy; Wiley Blackwell: Hoboken, NJ, USA, 2014; Volume 22, 450p. [Google Scholar]
- Nakagawa, Y.; Honda, M.; Tomishige, K. Direct synthesis of organic carbonates from CO2 and alcohols using heterogeneous oxide catalysts. In Green Carbon Dioxide: Advances in CO2 Utilization; Wiley Blackwell: Hoboken, NJ, USA, 2014; Volume 9781118590881, pp. 119–148. [Google Scholar] [CrossRef]
- Fennell, P.; Anthony, B. Calcium and Chemical Looping Technology for Power Generation and Carbon Dioxide (CO2) Capture; Woodhead Publishing: Cambridge, UK, 2015; 446p. [Google Scholar]
- Aresta, M.; Dibenedetto, A.; Quaranta, E. Reaction Mechanisms in Carbon Dioxide Conversion; Springer: Berlin, Germany, 2016; 423p. [Google Scholar] [CrossRef]
- Kiss, A.A.; Pragt, J.J.; Vos, H.J.; Bargeman, G.; de Groot, M.T. Enhanced process for methanol production by CO2 Hydrogenation. In Computer Aided Chemical Engineering; Kravanja, Z., Bogataj, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 38, pp. 985–990. [Google Scholar] [CrossRef]
- Chiang, P.-C.; Pan, S.-Y. Carbon Dioxide Mineralization and Utilization; Springer: Berlin/Heidelberg, Germany, 2017; 456p. [Google Scholar]
- Karamé, I.; Shaya, J.; Srour, H. Carbon Dioxide Chemistry, Capture and Oil Recovery; InTech Open: Online publication, 2018; 253p. [Google Scholar]
- Aresta, M.; Karimi, I.; Kawi, S. An Economy Based on Carbon Dioxide and Water, Potential of Large Scale Carbon Dioxide Utilization; Springer: Cham, Switzerland, 2019; 436p. [Google Scholar] [CrossRef]
- Klitkou, A.; Fevolden, M.; Capasso, M. From Waste to Value: Valorisation Pathways for Organic Waste Streams in Circular Bioeconomies; Taylor and Francis: Abingdon, UK, 2019; pp. 1–306. [Google Scholar] [CrossRef]
- North, M.; Styring, P. Carbon Dioxide Utilization; De Gruyter: Berlin, Germany, 2019; Volume 1 Fundamentals. [Google Scholar] [CrossRef]
- National Academies of Sciences-Engineering-Medicine. Gaseous Carbon Waste Streams Utilization: Status and Research Needs; The National Academies Press: Washington, DC, USA, 2019; 256p. [CrossRef]
- National Academies of Sciences-Engineering-Medicine. Gaseous carbon waste resources. In Gaseous Carbon Waste Streams Utilization: Status and Research Needs; The National Academies Press: Washington, DC, USA, 2019; pp. 27–38. [Google Scholar] [CrossRef]
- Rafiee, A.; Khalilpour, K.R.; Milani, D. CO2 Conversion and utilization pathways. In Polygeneration with Polystorage for Chemical and Energy Hubs; Khalilpour, K.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 213–245. [Google Scholar] [CrossRef]
- National Academies of Sciences-Engineering-Medicine. Accelerating Decarbonization of the U.S. Energy System; The National Academies Press: Washington, DC, USA, 2021; 268p. [CrossRef]
- Ramirez-Corredores, M.M.; Goldwasser, M.R.; Falabella de Sousa Aguiar, E. Decarbonization as a Route Towards Sustainable Circularity, 1st ed.; Springer: Cham, Switzerland, 2023; Volume XXIX, 153p. [Google Scholar] [CrossRef]
- Aresta, M. Carbon dioxide reduction and uses as a chemical feedstock. In Activation of Small Molecules: Organometallic and Bioinorganic Perspectives; John Wiley and Sons: Hoboken, NJ, USA, 2006; pp. 1–41. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A. Artificial carbon sinks: Utilization of carbon dioxide for the synthesis of chemicals and technological applications. In Greenhouse Gas Sinks; CABI Publishing: Oxfordshire, UK, 2007; pp. 98–114. [Google Scholar]
- Olah, G.A.; Goeppert, A.; Prakash, G.K.S. Beyond Oil and Gas: The Methanol Economy, 2nd ed.; Wiley-VCH: Washington, DC, USA, 2009; 334p. [Google Scholar] [CrossRef]
- Aresta, M. Carbon dioxide: Utilization options to reduce its accumulation in the atmosphere. In Carbon Dioxide as Chemical Feedstock; Wiley-VCH: Washington, DC, USA, 2010; pp. 1–13. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A. Industrial utilization of carbon dioxide (CO2). In Developments and Innovation in Carbon Dioxide (CO2) Capture and Storage Technology; Elsevier Ltd.: Amsterdam, The Netherlands, 2010; Volume 2, pp. 377–410. [Google Scholar] [CrossRef]
- Suib, S.L. New and Future Developments in Catalysis. Activation of Carbon Dioxide; Elsevier B.V.: Amsterdam, The Netherlands, 2013; Volume 7, 644p. [Google Scholar] [CrossRef]
- Shi, F.; Morreale, B. Novel Materials for Carbon Dioxide Mitigation Technology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 207–229. [Google Scholar]
- Styring, P.; Quadrelli, E.A.; Armstrong, K. Carbon Dioxide UtiIisation: Closing the Carbon Cycle; Elsevier: Amsterdam, The Netherlands, 2015; 336p. [Google Scholar]
- Aresta, M. The carbon dioxide problem. In An Economy Based on Carbon Dioxide and Water, Potential of Large Scale Carbon Dioxide Utilization; Aresta, M., Karimi, I., Kawi, S., Eds.; Springer: Cham, Switzerland, 2019; pp. v–xi. [Google Scholar]
- Mazzotti, M.; Abanades, J.C.; Allam, R.; Lackner, K.S.; Meunier, F.; Rubin, E.; Sanchez, J.C.; Yogo, K.; Zevenhoven, R. Mineral carbonation and industrial uses of carbon dioxide. In Special Report on Carbon Dioxide Capture and Storage; Metz, B., Davidson, O., de Coninck, H., Loos, M., Meyer, L., Eds.; Intergovernmental Panel on Climate Change (IPCC); Cambridge University Press: Cambridge, UK, 2005; pp. 319–338. [Google Scholar]
- Aresta, M.; Dibenedetto, A. Carbon dioxide: A valuable source of carbon for chemicals, fuels and materials. In Catalytic Process Development for Renewable Materials; Wiley-VCH: Washington, DC, USA, 2013; pp. 355–385. [Google Scholar] [CrossRef]
- Centi, G.; Perathoner, S. Catalytic transformation of CO2 to fuels and chemicals, with reference to biorefineries. In The Role of Catalysis for the Sustainable Production of Bio-Fuels and Bio-Chemicals; Triantafyllidis, K., Lappas, A., Stöcker, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 529–555. [Google Scholar] [CrossRef]
- Hall, P.J.; Wilson, I.A.G.; Rennie, A. CO2-derived fuels for energy storage. In Carbon Dioxide UtiIisation: Closing the Carbon Cycle; Styring, P., Quadrelli, E.A., Armstrong, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 33–44. [Google Scholar]
- Langanke, J.; Wolf, A.; Peters, M. Polymers from CO2—An industrial perspective. In Carbon Dioxide Utilisation; Styring, P., Quadrelli, E.A., Armstrong, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 59–71. [Google Scholar] [CrossRef]
- Aresta, M.; Nocito, F. Large scale utilization of carbon dioxide: From its reaction with energy rich chemicals to (Co)-processing with water to afford energy rich products. Opportunities and barriers. In An Economy Based on Carbon Dioxide and Water, Potential of Large Scale Carbon Dioxide Utilization; Aresta, M., Karimi, I., Kawi, S., Eds.; Springer: Cham, Switzerland, 2019; pp. 2–34. [Google Scholar]
- Leonzio, G. State-of-the-art overview of CO2 conversions. In Carbon Dioxide Utilization to Sustainable Energy and Fuels; Inamuddin, Boddula, R., Ahamed, M.I., Khan, A., Eds.; Springer Nature: Berlin/Heidelberg, Germany, 2022; pp. 335–353. [Google Scholar] [CrossRef]
- Ushikoshi, K.; Moria, K.; Watanabe, T.; Takeuchi, M.; Saito, M. A 50 kg/day class test plant for methanol synthesis from CO2 and H2. In Studies in Surface Science and Catalysis; Inui, T., Anpo, M., Izui, K., Yanagida, S., Yamaguchi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 1998; Volume 114, pp. 357–362. [Google Scholar] [CrossRef]
- Song, C. CO2 Conversion and utilization: An overview. In CO2 Conversion and Utilization; Song, C., Gaffney, A.F., Fujimoto, K., Eds.; ACS Symposium Series: Washington, DC, USA, 2002; Volume 809, pp. 2–30. [Google Scholar]
- Huijgen, W.J.J.; Comans, R.N.J. Carbon Dioxide Sequestration by Mineral Carbonation: Literature Review; ECN-C-03-016; Energy Research Centre of The Netherlands: Petten, The Netherlands, 2003; 53p. [Google Scholar]
- Gattrell, M.; Gupta, N.; Co, A. A review of the aqueous electrochemical reduction of CO2 to hydrocarbons at copper. J. Electroanal. Chem. 2006, 594, 1–19. [Google Scholar] [CrossRef]
- Ma, J.; Sun, N.; Zhang, X.; Zhao, N.; Xiao, F.; Wei, W.; Sun, Y. A short review of catalysis for CO2 conversion. Catal. Today 2009, 148, 221–231. [Google Scholar] [CrossRef]
- Budzianowski, W.M. Mass-recirculating systems in CO2 capture technologies: A review. Recent Pat. Eng. 2010, 4, 15–43. [Google Scholar] [CrossRef]
- Mikkelsen, M.; Jørgensen, M.; Krebs, F.C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 2010, 3, 43–81. [Google Scholar] [CrossRef]
- Fan, M.S.; Abdullah, A.Z.; Bhatia, S. Catalytic technology for carbon dioxide reforming of methane to synthesis gas. ChemCatChem 2009, 1, 192–208. [Google Scholar] [CrossRef]
- Seemann, M.; Thunman, H. Methane synthesis. In Substitute Natural Gas from Waste; Materazzi, M., Foscolo, P.U., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 221–243. [Google Scholar] [CrossRef]
- Chauvy, R.; De Weireld, G. CO2 Utilization Technologies in Europe: A Short Review. Energy Technol. 2020, 8, 2000627. [Google Scholar] [CrossRef]
- Siddique, M.H.; Maqbool, F.; Shahzad, T.; Waseem, M.; Rasul, I.; Hayat, S.; Afzal, M.; Faisal, M.; Muzammil, S. Recent advances in carbon dioxide utilization as renewable energy. In Green Sustainable Process for Chemical and Environmental Engineering and Science: Carbon Dioxide Capture and Utilization; Elsevier: Amsterdam, The Netherlands, 2023; pp. 197–210. [Google Scholar] [CrossRef]
- Ramirez-Corredores, M.M. A carbon dioxide refinery: The core of a sustainable carbon-based circular economy. Highlights Sustinability 2024, 3, 205–239. [Google Scholar] [CrossRef]
- Dziejarski, B.; Krzyżyńska, R.; Andersson, K. Current status of carbon capture, utilization, and storage technologies in the global economy: A survey of technical assessment. Fuel 2023, 342, 127776. [Google Scholar] [CrossRef]
- Makaryan, I.A.; Sedov, I.V. Progress of Commercial Technologies for Producing Syngas and Hydrogen from Hydrocarbon Gases. Russ. J. Appl. Chem. 2023, 96, 619–642. [Google Scholar] [CrossRef]
- BP. Statistical Review of World Energy; BP: London, UK, 2020; 68p, Available online: https://www.bp.com/en/global/corporate/energy-economics/statistical-review-of-world-energy.html (accessed on 15 November 2021).
- Ginsburg, J.M.; Piña, J.; El Solh, T.; De Lasa, H.I. Coke formation over a nickel catalyst under methane dry reforming conditions: Thermodynamic and kinetic models. Ind. Eng. Chem. Res. 2005, 44, 4846–4854. [Google Scholar] [CrossRef]
- Nikoo, M.K.; Amin, N.A.S. Thermodynamic analysis of carbon dioxide reforming of methane in view of solid carbon formation. Fuel Process. Technol. 2011, 92, 678–691. [Google Scholar] [CrossRef]
- Burkart, M.D.; Hazari, N.; Tway, C.L.; Zeitler, E.L. Opportunities and Challenges for Catalysis in Carbon Dioxide Utilization. ACS Catal. 2019, 9, 7937–7956. [Google Scholar] [CrossRef]
- Jensen, C.; Duyar, M.S. Thermodynamic Analysis of Dry Reforming of Methane for Valorization of Landfill Gas and Natural Gas. Energy Technol. 2021, 9, 2100106. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Zhang, Z.; Hong, X.; Liu, Y. Oxidative reformings of methane to syngas with steam and CO2 catalyzed by metallic Ni based monolithic catalysts. Catal. Commun. 2008, 9, 1040–1044. [Google Scholar] [CrossRef]
- Gao, J.; Hou, Z.; Liu, X.; Zeng, Y.; Luo, M.; Zheng, X. Methane autothermal reforming with CO2 and O2 to synthesis gas at the boundary between Ni and ZrO2. Int. J. Hydrogen Energy 2009, 34, 3734–3742. [Google Scholar] [CrossRef]
- Múnera, J.F.; Carrara, C.; Cornaglia, L.M.; Lombardo, E.A. Combined oxidation and reforming of methane to produce pure H2 in a membrane reactor. Chem. Eng. J. 2010, 161, 204–211. [Google Scholar] [CrossRef]
- Hunt, J.; Ferrari, A.; Lita, A.; Crosswhite, M.; Ashley, B.; Stiegman, A.E. Microwave-Specific Enhancement of the Carbon–Carbon Dioxide (Boudouard) Reaction. J. Phys. Chem. C 2013, 117, 26871–26880. [Google Scholar] [CrossRef]
- Bell, A.T. The impact of nanoscience on heterogeneous catalysis. Science 2003, 299, 1688–1691. [Google Scholar] [CrossRef]
- Rivas, I.; Alvarez, J.; Pietri, E.; Pérez-Zurita, M.J.; Goldwasser, M.R. Perovskite-type oxides in methane dry reforming: Effect of their incorporation into a mesoporous SBA-15 silica-host. Catal. Today 2010, 149, 388–393. [Google Scholar] [CrossRef]
- Kim, D.K.; Stöwe, K.; Müller, F.; Maier, W.F. Mechanistic study of the unusual catalytic properties of a new NiCe mixed oxide for the CO2 reforming of methane. J. Catal. 2007, 247, 101–111. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Liu, H.-M.; Xu, B.-Q. Durable Ni/MgO catalysts for CO2 reforming of methane: Activity and metal–support interaction. J. Mol. Catal. A Chem. 2009, 299, 44–52. [Google Scholar] [CrossRef]
- Liu, D.; Cheo, W.N.E.; Lim, Y.W.Y.; Borgna, A.; Lau, R.; Yang, Y. A comparative study on catalyst deactivation of nickel and cobalt incorporated MCM-41 catalysts modified by platinum in methane reforming with carbon dioxide. Catal. Today 2010, 154, 229–236. [Google Scholar] [CrossRef]
- Mortensen, P.M.; Dybkjær, I. Industrial scale experience on steam reforming of CO2-rich gas. Appl. Catal. A Gen. 2015, 495, 141–151. [Google Scholar] [CrossRef]
- Shang, Z.; Li, S.; Li, L.; Liu, G.; Liang, X. Highly active and stable alumina supported nickel nanoparticle catalysts for dry reforming of methane. Appl. Catal. B Environ. 2017, 201, 302–309. [Google Scholar] [CrossRef]
- Shang, Z.; Li, S.; Wang, Q.; Gu, X.; Liang, X. Nano-engineered nickel catalysts supported on 4-channel A-Al2O3 hollow fibers for dry reforming of methane. AIChE J. 2018, 64, 2625–2631. [Google Scholar] [CrossRef]
- Jin, B.; Shang, Z.; Li, S.; Jiang, Y.B.; Gu, X.; Liang, X. Reforming of methane with carbon dioxide over cerium oxide promoted nickel nanoparticles deposited on 4-channel hollow fibers by atomic layer deposition. Catal. Sci. Technol. 2020, 10, 3212–3222. [Google Scholar] [CrossRef]
- Jin, B.; Li, S.; Liang, X. Enhanced activity and stability of MgO-promoted Ni/Al2O3 catalyst for dry reforming of methane: Role of MgO. Fuel 2021, 284, 119082. [Google Scholar] [CrossRef]
- Jin, B.; Li, S.; Liang, X. High-performance catalytic four-channel hollow fibers with highly dispersed nickel nanoparticles prepared by atomic layer deposition for dry reforming of methane. Ind. Eng. Chem. Res. 2022, 61, 10377–10386. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, A.; Li, X.; Zhang, S.; Zhu, A.; Ma, Y.; Au, C. Ni-modified Mo2C catalysts for methane dry reforming. Appl. Catal. A Gen. 2012, 431–432, 164–170. [Google Scholar] [CrossRef]
- Nagaraja, B.M.; Bulushev, D.A.; Beloshapkin, S.; Chansai, S.; Ross, J.R.H. Potassium-doped Ni-MgO-ZrO2 catalysts for dry reforming of methane to synthesis gas. Top Catal. 2013, 56, 1686–1694. [Google Scholar] [CrossRef]
- Elsayed, N.H.; Roberts, N.R.M.; Joseph, B.; Kuhn, J.N. Low temperature dry reforming of methane over Pt-Ni-Mg/ceria-zirconia catalysts. Appl. Catal. B Environ. 2015, 179, 213–219. [Google Scholar] [CrossRef]
- Rezaei, R.; Moradi, G.; Sharifnia, S. Dry Reforming of Methane over Ni-Cu/Al2O3 Catalyst Coatings in a Microchannel Reactor: Modeling and Optimization Using Design of Experiments. Energy Fuels 2019, 33, 6689–6706. [Google Scholar] [CrossRef]
- Dang, C.; Luo, J.; Yang, W.; Li, H.; Cai, W. Low-temperature catalytic dry reforming of methane over Pd promoted Ni–CaO–ca12al14o33 Multifunctional Catalyst. Ind. Eng. Chem. Res. 2021, 60, 18361–18372. [Google Scholar] [CrossRef]
- Yadav, P.K.; Patrikar, K.; Mondal, A.; Sharma, S. Ni/Co in and on CeO2: A comparative study on the dry reforming reaction. Sustain. Energy Fuels 2023, 7, 3853–3870. [Google Scholar] [CrossRef]
- Jin, B.; Li, S.; Liu, Y.; Liang, X. Engineering metal-oxide interface by depositing ZrO2 overcoating on Ni/Al2O3 for dry reforming of methane. Chem. Eng. J. 2022, 436, 135195. [Google Scholar] [CrossRef]
- Rosen, B.A.; Gileadi, E.; Eliaz, N. Electrodeposited Re-promoted Ni foams as a catalyst for the dry reforming of methane. Catal. Commun. 2016, 76, 23–28. [Google Scholar] [CrossRef]
- Zubenko, D.; Singh, S.; Rosen, B.A. Exsolution of Re-alloy catalysts with enhanced stability for methane dry reforming. Appl. Catal. B Environ. 2017, 209, 711–719. [Google Scholar] [CrossRef]
- Meng, J.; Pan, W.; Gu, T.; Bu, C.; Zhang, J.; Wang, X.; Liu, C.; Xie, H.; Piao, G. One-pot synthesis of a highly active and stable ni-embedded hydroxyapatite catalyst for syngas production via dry reforming of methane. Energy Fuels 2021, 35, 19568–19580. [Google Scholar] [CrossRef]
- Huang, L.; Li, D.; Tian, D.; Jiang, L.; Li, Z.; Wang, H.; Li, K. Optimization of Ni-based catalysts for dry reforming of methane via alloy design: A review. Energy Fuels 2022, 36, 5102–5151. [Google Scholar] [CrossRef]
- Praserthdam, S.; Somdee, S.; Rittiruam, M.; Balbuena, P.B. Computational study of the evolution of ni-based catalysts during the dry reforming of methane. Energy Fuels 2020, 34, 4855–4864. [Google Scholar] [CrossRef]
- Wang, C.; Sun, N.; Zhao, N.; Wei, W.; Sun, Y.; Sun, C.; Liu, H.; Snape, C.E. Coking and deactivation of a mesoporous Ni-CaO-ZrO2 catalyst in dry reforming of methane: A study under different feeding compositions. Fuel 2015, 143, 527–535. [Google Scholar] [CrossRef]
- Osaki, T.; Horiuchi, T.; Suzuki, K.; Mori, T. Suppression of carbon deposition in CO2-reforming of methane on metal sulfide catalysts. Catal. Lett. 1995, 35, 39–43. [Google Scholar] [CrossRef]
- Arora, S.; Prasad, R. An overview on dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Mondal, K.; Sasmal, S.; Badgandi, S.; Chowdhury, D.R.; Nair, V. Dry reforming of methane to syngas: A potential alternative process for value added chemicals—A techno-economic perspective. Environ. Sci. Pollut. Res. 2016, 23, 22267–22273. [Google Scholar] [CrossRef] [PubMed]
- Gallego, J.; Batiot-Dupeyrat, C.; Barrault, J.; Mondragón, F. Severe deactivation of a LaNiO3 perovskite-type catalyst precursor with H2S during methane dry reforming. Energy Fuels 2009, 23, 4883–4886. [Google Scholar] [CrossRef]
- Sokolov, S.; Kondratenko, E.V.; Pohl, M.M.; Rodemerck, U. Effect of calcination conditions on time on-stream performance of Ni/La2O3-ZrO2 in low-temperature dry reforming of methane. Int. J. Hydrogen Energy 2013, 38, 16121–16132. [Google Scholar] [CrossRef]
- Wang, C.; Sun, N.; Zhao, N.; Wei, W.; Zhang, J.; Zhao, T.; Sun, Y.; Sun, C.; Liu, H.; Snape, C.E. The properties of individual carbon residuals and their influence on the deactivation of Ni-CaO-ZrO2 catalysts in CH4 dry reforming. ChemCatChem 2014, 6, 640–648. [Google Scholar] [CrossRef]
- Pawar, V.; Ray, D.; Subrahmanyam, C.; Janardhanan, V.M. Study of short-term catalyst deactivation due to carbon deposition during biogas dry reforming on supported Ni catalyst. Energy Fuels 2015, 29, 8047–8052. [Google Scholar] [CrossRef]
- Zhang, R.-j.; Xia, G.-f.; Li, M.-f.; Wu, Y.; Nie, H.; Li, D.-d. Effect of support on the performance of Ni-based catalyst in methane dry reforming. J. Fuel Chem. Technol. 2015, 43, 1359–1365. [Google Scholar] [CrossRef]
- Lou, Y.; Steib, M.; Zhang, Q.; Tiefenbacher, K.; Horváth, A.; Jentys, A.; Liu, Y.; Lercher, J.A. Design of stable Ni/ZrO2 catalysts for dry reforming of methane. J. Catal. 2017, 356, 147–156. [Google Scholar] [CrossRef]
- Li, R.; Xu, W.; Deng, J.; Zhou, J. Coke-resistant Ni-Co/ZrO2-CaO-based microwave catalyst for highly effective dry reforming of methane by microwave catalysis. Ind. Eng. Chem. Res. 2021, 60, 17458–17468. [Google Scholar] [CrossRef]
- Wang, Y.B.; He, L.; Zhou, B.C.; Tang, F.; Fan, J.; Wang, D.Q.; Lu, A.H.; Li, W.C. Hydroxyapatite nanorods rich in [Ca-O-P] sites stabilized Ni species for methane dry reforming. Ind. Eng. Chem. Res. 2021, 60, 15064–15073. [Google Scholar] [CrossRef]
- Cherbański, R.; Kotkowski, T.; Molga, E. Thermogravimetric analysis of coking during dry reforming of methane. Int. J. Hydrogen Energy 2023, 48, 7346–7360. [Google Scholar] [CrossRef]
- Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837. [Google Scholar] [CrossRef]
- Aziz, M.A.A.; Setiabudi, H.D.; Teh, L.P.; Annuar, N.H.R.; Jalil, A.A. A review of heterogeneous catalysts for syngas production via dry reforming. J. Taiwan Inst. Chem. Eng. 2019, 101, 139–158. [Google Scholar] [CrossRef]
- de Medeiros, F.G.M.; Lopes, F.W.B.; Rego de Vasconcelos, B. Prospects and technical challenges in hydrogen production through dry reforming of methane. Catalysts 2022, 12, 363. [Google Scholar] [CrossRef]
- Du, C.; Lu, P.; Tsubaki, N. Efficient and new production methods of chemicals and liquid fuels by carbon monoxide hydrogenation. ACS Omega 2020, 5, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Bjørgen, M.; Joensen, F.; Spangsberg Holm, M.; Olsbye, U.; Lillerud, K.P.; Svelle, S. Methanol to gasoline over zeolite H-ZSM-5: Improved catalyst performance by treatment with NaOH. Appl. Catal. A Gen. 2008, 345, 43–50. [Google Scholar] [CrossRef]
- Godini, H.R.; Xiao, S.; Jašo, S.; Stünkel, S.; Salerno, D.; Son, N.X.; Song, S.; Wozny, G. Techno-economic analysis of integrating the methane oxidative coupling and methane reforming processes. Fuel Process. Technol. 2013, 106, 684–694. [Google Scholar] [CrossRef]
- Kim, S.; Ryi, S.K.; Lim, H. Techno-economic analysis (TEA) for CO2 reforming of methane in a membrane reactor for simultaneous CO2 utilization and ultra-pure H2 production. Int. J. Hydrogen Energy 2018, 43, 5881–5893. [Google Scholar] [CrossRef]
- Sharifian, R.; van der Wal, H.C.; Wagterveld, R.M.; Vermaas, D.A. Fouling management in oceanic carbon capture via in-situ electrochemical bipolar membrane electrodialysis. Chem. Eng. J. 2023, 458, 141407. [Google Scholar] [CrossRef]
- Udengaard, N.R. Sulfur passivated reforming process lowers syngas H2/CO ratio. Oil Gas J. 1992, 90, 62–67. [Google Scholar]
- Teuner, S.C.; Neumann, P.; Von Linde, F. CO through CO2 reforming—The Calcor standard and Calcor economy processes. Oil Gas Eur. Mag. 2001, 27, 44–46. [Google Scholar]
- Kurz, G.; Teuner, S. Calcor process for CO production. Erdoel Und Kohle Erdgas Petrochem. 1990, 43, 171–172. [Google Scholar]
- Teuner, S.C.; Neumann, P.; Von Linde, F. CO through CO2 reforming: The Calcor standard and Calcor economy processes. Erdoel Erdgas Kohle 2001, 117, 580–582. [Google Scholar]
- GTI. Dry Reforming of Methane to Produce Syngas and Reduce CO2 Emissions. Available online: https://www.gti.energy/dry-reforming-of-methane-to-produce-syngas-and-reduce-CO2-emissions/ (accessed on 3 June 2024).
- Bartesch, T.; Wawrzinek, K.; Behrens, A.; Peschel, A. DRYREF & SYNSPIRE innovation for HyCO applications. In Proceedings of the Global Syngas Technologies Conference, Virtual, 20–21, 27–28 October 2020; Available online: https://globalsyngas.org/wp-content/conference-presentations/2020/2020-w1-d2-m2-DRYREF-Global-Syngas-Conference.pdf (accessed on 4 July 2024).
- Linde Engineering. Smaller Carbon Footprint. Higher Process Efficiency. Available online: https://www.engineering.linde.com/dryref (accessed on 16 March 2024).
- Rostrup-Nielsen, J.R. Sulfur-passivated nickel catalysts for carbon-free steam reforming of methane. J. Catal. 1984, 85, 31–43. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.R. Promotion by poisoning. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1991; Volume 68, pp. 85–101. [Google Scholar] [CrossRef]
- Dibben, H.C.; Olesen, P.; Rostrup-Nielsen, J.R.; Tøttrup, P.B.; Volengaard, N.R. Make low H2/CO syngas using sulfur passivated reforming. Hydrocarb. Process 1986, 65, 71–74. [Google Scholar]
- Mustafaev, I.I.; Gurbanov, M.A.; Hajiev, H.M. Gasification of different types of carbon in steam and carbon dioxide in the presence of γ-radiation. Carbon 1988, 26, 125–128. [Google Scholar] [CrossRef]
- Steib, M. Dry Reforming of Methane—Understanding Metal Support Interactions and Evaluation of Regeneration Protocols. Ph.D. Thesis, Technische Universität München, Munich, Germany, 2017. [Google Scholar]
- York, A.P.E.; Xiao, T.C.; Green, M.L.H.; Claridge, J.B. Methane oxyforming for synthesis gas production. Catal. Rev.-Sci. Eng. 2007, 49, 511–560. [Google Scholar] [CrossRef]
- Shah, Y.T.; Gardner, T.H. Dry reforming of hydrocarbon feedstocks. Catal. Rev.-Sci. Eng. 2014, 56, 476–536. [Google Scholar] [CrossRef]
- Green Car Congress. Linde Pilot Testing Dry Reforming Process to Generate Syngas from CO2 and Methane for Production of Fuels and Chemicals. Available online: https://www.greencarcongress.com/2015/10/20151016-lpr.html (accessed on 8 March 2022).
- Schwab, E.; Milanov, A.; Schunk, S.A.; Behrens, A.; Schödel, N. Dry reforming and reverse water gas shift: Alternatives for syngas production? Chem.-Ing.-Tech. 2015, 87, 347–353. [Google Scholar] [CrossRef]
- Er-Rbib, H.; Bouallou, C.; Werkoff, F. Production of synthetic gasoline and diesel fuel from dry reforming of methane. In Proceedings of the 19th World Hydrogen Energy Conference, WHEC 2012, Toronto, ON, Canada, 3–7 June 2019; pp. 156–165. [Google Scholar] [CrossRef]
- Engineering India. Linde Technologies That Do More with Less. Available online: https://www.linde-engineering.in/en/about_linde_engineering/success-stories/technologies-more-with-less.html? (accessed on 5 June 2024).
- Boretti, A.; Nayfeh, J.; Al-Maaitah, A. Hydrogen production by solar thermochemical water-splitting cycle via a beam down concentrator. Front. Energy Res. 2021, 9, 5. [Google Scholar] [CrossRef]
- Buelens, L.C.; Poelman, H.; Marin, G.B.; Galvita, V.V. 110th anniversary: Carbon dioxide and chemical looping: Current research trends. Ind. Eng. Chem. Res. 2019, 58, 16235–16257. [Google Scholar] [CrossRef]
- Fang, H.; Haibin, L.; Zengli, Z. Advancements in development of chemical-looping combustion: A review. Int. J. Chem. Eng. 2009, 2009, 710515. [Google Scholar] [CrossRef]
- Moghtaderi, B. Review of the recent chemical looping process developments for novel energy and fuel applications. Proc. Energy Fuels 2012, 26, 15–40. [Google Scholar] [CrossRef]
- Barros do Nascimento, R.A.; Pimenta de Macedo, H.; Melo, D.M.A.; Santiago, R.C.; Rodrigues de Araújo, T.; Medeiros, R.L.B.A.; Adánez, J. Structure and reactivity of brazilian iron ores as low-cost oxygen carriers for chemical looping combustion. Ind. Eng. Chem. Res. 2022, 61, 2469–2482. [Google Scholar] [CrossRef]
- Zhu, M.; Song, Y.; Chen, S.; Li, M.; Zhang, L.; Xiang, W. Chemical looping dry reforming of methane with hydrogen generation on Fe2O3/Al2O3 oxygen carrier. Chem. Eng. J. 2019, 368, 812–823. [Google Scholar] [CrossRef]
- Guerrero-Caballero, J.; Kane, T.; Haidar, N.; Jalowiecki-Duhamel, L.; Löfberg, A. Ni, Co, Fe supported on Ceria and Zr doped Ceria as oxygen carriers for chemical looping dry reforming of methane. Catal. Today 2019, 333, 251–258. [Google Scholar] [CrossRef]
- Chein, R.Y.; Hsu, W.H. Thermodynamic analysis of syngas production via chemical looping dry reforming of methane. Energy 2019, 180, 535–547. [Google Scholar] [CrossRef]
- Löfberg, A.; Kane, T.; Guerrero-Caballero, J.; Jalowiecki-Duhamel, L. Chemical looping dry reforming of methane: Toward shale-gas and biogas valorization. Chem. Eng. Process. Process Intensif. 2017, 122, 523–529. [Google Scholar] [CrossRef]
- Li, D.; Xu, R.; Gu, Z.; Zhu, X.; Qing, S.; Li, K. Chemical-looping conversion of methane: A review. Energy Technol. 2020, 8, 1900925. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, H.S.; Lee, M.; Lee, J.W. Ni-Fe-Al mixed oxide for combined dry reforming and decomposition of methane with CO2 utilization. Catal. Today 2021, 368, 86–95. [Google Scholar] [CrossRef]
- Sastre, D.; Galván, C.Á.; Pizarro, P.; Coronado, J.M. Enhanced performance of CH4 dry reforming over La0.9Sr0.1FeO3/YSZ under chemical looping conditions. Fuel 2022, 309, 122122. [Google Scholar] [CrossRef]
- Petrakopoulou, F.; Tsatsaronis, G.; Boyano, A.; Morosuk, T. Exergoeconomic and exergoenvironmental evaluation of power plants including CO2 capture. Chem. Eng. Res. Des. 2011, 89, 1461–1469. [Google Scholar] [CrossRef]
- Petrakopoulou, F.; Tsatsaronis, G. Can carbon dioxide capture and storage from power plants reduce the environmental impact of electricity generation? Energy Fuels 2014, 28, 5327–5338. [Google Scholar] [CrossRef]
- Petrescu, L.; Cormos, C.C. Environmental assessment of IGCC power plants with pre-combustion CO2 capture by chemical & calcium looping methods. J. Clean. Prod. 2017, 158, 233–244. [Google Scholar] [CrossRef]
- Bareschino, P.; Mancusi, E.; Urciuolo, M.; Paulillo, A.; Chirone, R.; Pepe, F. Life cycle assessment and feasibility analysis of a combined chemical looping combustion and power-to-methane system for CO2 capture and utilization. Renew. Sustain. Energy Rev. 2020, 130, 109962. [Google Scholar] [CrossRef]
- Chirone, R.; Paulillo, A.; Coppola, A.; Scala, F. Carbon capture and utilization via calcium looping, sorption enhanced methanation and green hydrogen: A techno-economic analysis and life cycle assessment study. Fuel 2022, 328, 125255. [Google Scholar] [CrossRef]
- Peres, C.B.; Resende, P.M.R.; Nunes, L.J.R.; Morais, L.C.D. Advances in carbon capture and use (CCU) technologies: A comprehensive review and CO2 mitigation potential analysis. Clean. Technol. 2022, 4, 1193–1207. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Zhang, Y.; Wu, Q.; Xin, L.; Zhou, Y.; Yin, K.; Wang, Y.; Li, X.; Cui, P. A novel power, DME, and ammonia polygeneration system using Aspen plus based on the integration of biomass gasification and syngas chemical looping. Energy Convers. Manag. 2024, 299, 117808. [Google Scholar] [CrossRef]
- Xiang, D.; Li, P.; Yuan, X.; Cao, H.; Liu, L.; Liu, Y. Energy consumption and greenhouse gas emissions of shale gas chemical looping reforming process integrated with coal gasification for methanol production. Appl. Therm. Eng. 2021, 193, 116990. [Google Scholar] [CrossRef]
- Baker, R.; Alizadeh Sahraei, O.; Dal-Cin, M.M.; Bensebaa, F. A technology development matrix for carbon capture: Technology status and R&D gap assessment. Front. Energy Res. 2022, 10, 908658. [Google Scholar] [CrossRef]
- Martínez-Ramón, N.; Romay, M.; Iribarren, D.; Dufour, J. Life-cycle assessment of hydrogen produced through chemical looping dry reforming of biogas. Int. J. Hydrogen Energy 2024, 78, 373–381. [Google Scholar] [CrossRef]
- Virla, L.D.; de Oliveira, C.F.; Camacho, F.G.; Kaur, M.; Mahinpey, N. Chemical looping reforming (CLR) for syngas production. In Advances in Synthesis Gas. Methods, Technologies and Applications; Syngas Production and Preparation; Elsevier: Amsterdam, The Netherlands, 2022; Volume 1, pp. 149–177. [Google Scholar] [CrossRef]
- Hosokawa, Y.; Kajiya, S.; Ohshima, A.; Ishida, N.; Washio, M.; Usuki, A. CO2 conversion by high-dose rate electron beam irradiation: One-step, metal-free and simultaneous production of H2, CO, CH4, c2h6 and organic acids from an acid-decomposed CaCO3/additive EtOH mixture. Green Chem. 2019, 21, 3091–3098. [Google Scholar] [CrossRef]
- Meyer, W.; La-Belle, D.W.; Rassoul, S.A. The effects of Co-60 gamma radiation on the rmal decomposition of carbonate compounds. Int. J. Appl. Radiat. Isot. 1973, 24, 315–318. [Google Scholar] [CrossRef]
- Álvarez, A.; Borges, M.; Corral-Pérez, J.J.; Olcina, J.G.; Hu, L.; Cornu, D.; Huang, R.; Stoian, D.; Urakawa, A. CO2 Activation over catalytic surfaces. ChemPhysChem 2017, 18, 3135–3141. [Google Scholar] [CrossRef]
- Dioxide Materials. CO2 Electrolyzers. Available online: https://dioxidematerials.com/technology/CO2-electrolysis/ (accessed on 25 January 2024).
- OCO Chem. Carbon FluX Electrolyzer™. Available online: https://ocochem.com/product/ (accessed on 25 January 2024).
- Twelve. Opus™ System. Available online: https://www.twelve.co/post/how-the-opus-system-by-twelve-turns-CO2-into-products (accessed on 25 January 2024).
- Chen, C.; Khosrowabadi Kotyk, J.F.; Sheehan, S.W. Progress toward commercial application of electrochemical carbon dioxide reduction. Chem 2018, 4, 2571–2586. [Google Scholar] [CrossRef]
- Küngas, R. Review—Electrochemical CO2 reduction for CO production: Comparison of low- and high-temperature electrolysis technologies. J. Electrochem. Soc. 2020, 167, 044508. [Google Scholar] [CrossRef]
- O’Brien, J.E.; McKellar, M.G.; Harvego, E.A.; Stoots, C.M. High-temperature electrolysis for large-scale hydrogen and syngas production from nuclear energy—Summary of system simulation and economic analyses. Int. J. Hydrogen Energy 2010, 35, 4808–4819. [Google Scholar] [CrossRef]
- Hartvigsen, J.; Elangovan, S.; O’Brien, J.; Stoots, C.; Herring, J. Operation of high temperature steam electrolyzer module. ECS Trans. 2007, 7, 357–363. [Google Scholar] [CrossRef]
- Delacourt, C.; Ridgway, P.L.; Kerr, J.B.; Newman, J. Design of an electrochemical cell making syngas (CO+ H2) from CO2 and H2O reduction at room temperature. J. Electrochem. Soc. 2008, 155, B42–B49. [Google Scholar] [CrossRef]
- McKellar, M.G.; O’Brien, J.E.; Stoots, C.M.; Hawkes, G.L. Process model for the production of syngas via high temperature co-electrolysis. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, IMECE 2007, Seattle, WA, USA, 11–15 November 2007; pp. 691–699. [Google Scholar] [CrossRef]
- McKellar, M.G.; Hawkes, G.L.; O’Brien, J.E. The production of syngas via high temperature electrolysis and biomass gasification. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, IMECE 2008, Boston, MA, USA, 31 October–6 November 2008; pp. 229–235. [Google Scholar] [CrossRef]
- O’Brien, J.E.; McKellar, M.G.; Stoots, C.M.; Herring, J.S.; Hawkes, G.L. Parametric study of large-scale production of syngas via high-temperature co-electrolysis. Int. J. Hydrogen Energy 2009, 34, 4216–4226. [Google Scholar] [CrossRef]
- Stoots, C.; O’Brien, J.; Hartvigsen, J. Results of recent high temperature coelectrolysis studies at the Idaho National Laboratory. Int. J. Hydrogen Energy 2009, 34, 4208–4215. [Google Scholar] [CrossRef]
- Stoots, C.M.; O’Brien, J.E.; Herring, J.S.; Hartvigsen, J.J. Syngas production via high-temperature coelectrolysis of steam and carbon dioxide. J. Fuel Cell Sci. Technol. 2009, 6, 0110141–01101412. [Google Scholar] [CrossRef]
- Fu, Q.; Mabilat, C.; Zahid, M.; Brisse, A.; Gautier, L. Syngas production via high-temperature steam/CO2 co-electrolysis: An economic assessment. Energy Environ. Sci. 2010, 3, 1382–1397. [Google Scholar] [CrossRef]
- Stoots, C.M.; O’Brien, J.E.; Condie, K.G.; Hartvigsen, J.J. High-temperature electrolysis for large-scale hydrogen production from nuclear energy—Experimental investigations. Int. J. Hydrogen Energy 2010, 35, 4861–4870. [Google Scholar] [CrossRef]
- Redissi, Y.; Bouallou, C. Valorization of carbon dioxide by co-electrolysis of CO2/H2O at high temperature for syngas production. In Proceedings of the 11th International Conference on Greenhouse Gas Control Technologies, GHGT 2012, Kyoto, Japan, 18–22 November 2012; pp. 6667–6678. [Google Scholar] [CrossRef]
- Lister, T.E.; Dufek, E.J. Chlor-syngas: Coupling of electrochemical technologies for production of commodity chemicals. Energy Fuels 2013, 27, 4244–4249. [Google Scholar] [CrossRef]
- Foit, S.R.; Vinke, I.C.; de Haart, L.G.J.; Eichel, R.-A. Power-to-Syngas: An Enabling Technology for the Transition of the Energy System? Angew. Chem. Int. Ed. 2017, 56, 5402–5411. [Google Scholar] [CrossRef]
- Hernández, S.; Amin Farkhondehfal, M.; Sastre, F.; Makkee, M.; Saracco, G.; Russo, N. Syngas production from electrochemical reduction of CO2: Current status and prospective implementation. Green Chem. 2017, 19, 2326–2346. [Google Scholar] [CrossRef]
- Ripatti, D.S.; Veltman, T.R.; Kanan, M.W. Carbon monoxide gas diffusion electrolysis that produces concentrated C2 products with high single-pass conversion. Joule 2019, 3, 240–256. [Google Scholar] [CrossRef]
- Leonard, M.E.; Clarke, L.E.; Forner-Cuenca, A.; Brown, S.M.; Brushett, F.R. Investigating electrode flooding in a flowing electrolyte, gas-fed carbon dioxide electrolyzer. ChemSusChem 2020, 13, 400–411. [Google Scholar] [CrossRef]
- Weekes, D.M.; Salvatore, D.A.; Reyes, A.; Huang, A.; Berlinguette, C.P. Electrolytic CO2 reduction in a flow cell. Acc. Chem. Res. 2018, 51, 910–918. [Google Scholar] [CrossRef]
- Higgins, D.; Hahn, C.; Xiang, C.; Jaramillo, T.F.; Weber, A.Z. Gas-diffusion electrodes for carbon dioxide reduction: A new paradigm. ACS Energy Lett. 2019, 4, 317–324. [Google Scholar] [CrossRef]
- Durrani, J. Can Catalysis Save Us from Our CO2 Problem? Available online: https://www.chemistryworld.com/news/can-catalysis-save-us-from-our-CO2-problem/3010555.article (accessed on 24 June 2022).
- Zhang, Y.; Guo, S.-X.; Zhang, X.; Bond, A.M.; Zhang, J. Mechanistic understanding of the electrocatalytic CO2 reduction reaction—New developments based on advanced instrumental techniques. Nano Today 2020, 31, 100835. [Google Scholar] [CrossRef]
- Lim, R.J.; Xie, M.; Sk, M.A.; Lee, J.-M.; Fisher, A.; Wang, X.; Lim, K.H. A review on the electrochemical reduction of CO2 in fuel cells, metal electrodes and molecular catalysts. Catal. Today 2014, 233, 169–180. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Y.; Ma, L.; Zhu, G.; Wang, Y.; Xue, X.; Chen, R.; Yang, S.; Jin, Z. Progress and Perspective of Electrocatalytic CO2 Reduction for Renewable Carbonaceous Fuels and Chemicals. Adv. Sci. 2018, 5, 1700275. [Google Scholar] [CrossRef] [PubMed]
- Küngas, R.; Blennow, P.; Heiredal-Clausen, T.; Holt, T.; Rass-Hansen, J.; Primdahl, S. Systematic lifetime testing of stacks in CO2 electrolysis. ECS Trans. 2017, 78, 2895–2905. [Google Scholar] [CrossRef]
- Artz, J.; Müller, T.E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable conversion of carbon dioxide: An integrated review of catalysis and life cycle assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef]
- Li, T.; Lees, E.W.; Goldman, M.; Salvatore, D.A.; Weekes, D.M.; Berlinguette, C.P. Electrolytic conversion of bicarbonate into co in a flow cell. Joule 2019, 3, 1487–1497. [Google Scholar] [CrossRef]
- Li, L.; Yang, J.; Li, L.; Huang, Y.; Zhao, J. Electrolytic reduction of CO2 in KHCO3 and alkanolamine solutions with layered double hydroxides intercalated with gold or copper. Electrochim. Acta 2022, 402, 139523. [Google Scholar] [CrossRef]
- Zhang, Z.; Lees, E.W.; Ren, S.; Mowbray, B.A.W.; Huang, A.; Berlinguette, C.P. Conversion of Reactive Carbon Solutions into CO at Low Voltage and High Carbon Efficiency. ACS Cent. Sci. 2022, 8, 749–755. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Nakazato, R.; Matsumoto, K.; Kakesu, M.; Rosero-Navarro, N.C.; Miura, A.; Tadanaga, K. Electrocatalytic property of Zn-Al layered double hydroxides for CO2 electrochemical reduction. J. Asian Ceram. Soc. 2023, 11, 406–411. [Google Scholar] [CrossRef]
- Yue, P.; Kang, Z.; Fu, Q.; Li, J.; Zhang, L.; Zhu, X.; Liao, Q. Life cycle and economic analysis of chemicals production via electrolytic (bi)carbonate and gaseous CO2 conversion. Appl. Energy 2021, 304, 117768. [Google Scholar] [CrossRef]
- Zhu, W.; Michalsky, R.; Metin, Ö.; Lv, H.; Guo, S.; Wright, C.J.; Sun, X.; Peterson, A.A.; Sun, S. Monodisperse au nanoparticles for selective electrocatalytic reduction of CO2 to CO. J. Am. Chem. Soc. 2013, 135, 16833–16836. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.; Reske, R.; Zeng, Z.; Zhao, Z.-J.; Greeley, J.; Strasser, P.; Cuenya, B.R. Exceptional size-dependent activity enhancement in the electroreduction of CO2 over Au nanoparticles. J. Am. Chem. Soc. 2014, 136, 16473–16476. [Google Scholar] [CrossRef] [PubMed]
- Ham, Y.S.; Choe, S.; Kim, M.J.; Lim, T.; Kim, S.K.; Kim, J.J. Electrodeposited Ag catalysts for the electrochemical reduction of CO2 to CO. Appl. Catal. B Environ. 2017, 208, 35–43. [Google Scholar] [CrossRef]
- Lees, E.W.; Goldman, M.; Fink, A.G.; Dvorak, D.J.; Salvatore, D.A.; Zhang, Z.; Loo, N.W.X.; Berlinguette, C.P. Electrodes Designed for Converting Bicarbonate into CO. ACS Energy Lett. 2020, 5, 2165–2173. [Google Scholar] [CrossRef]
- Verma, S.; Lu, X.; Ma, S.; Masel, R.I.; Kenis, P.J.A. The effect of electrolyte composition on the electroreduction of CO2 to CO on Ag based gas diffusion electrodes. Phys. Chem. Chem. Phys. 2016, 18, 7075–7084. [Google Scholar] [CrossRef]
- Larrea, C.; Torres, D.; Avilés-Moreno, J.R.; Ocón, P. Multi-parameter study of CO2 electrochemical reduction from concentrated bicarbonate feed. J. CO2 Util. 2022, 57, 101878. [Google Scholar] [CrossRef]
- Pimlott, D.J.; Jewlal, A.; Kim, Y.; Berlinguette, C.P. Oxygen-resistant CO2 reduction enabled by electrolysis of liquid feedstocks. J. Am. Chem. Soc. 2023, 145, 25933–25937. [Google Scholar] [CrossRef]
- Pimlott, D.J.D.; Jewlal, A.; Mowbray, B.A.W.; Berlinguette, C.P. Impurity-Resistant CO2 Reduction Using Reactive Carbon Solutions. ACS Energy Lett. 2023, 8, 1779–1784. [Google Scholar] [CrossRef]
- Kim, Y.; Lees, E.W.; Berlinguette, C.P. Permeability matters when reducing CO2 in an electrochemical flow cell. ACS Energy Lett. 2022, 7, 2382–2387. [Google Scholar] [CrossRef]
- Diaz, L.A.; Gao, N.; Adhikari, B.; Lister, T.E.; Dufek, E.J.; Wilson, A.D. Electrochemical production of syngas from CO2 captured in switchable polarity solvents. Green Chem. 2018, 20, 620–626. [Google Scholar] [CrossRef]
- Gao, N.; Quiroz Arita, C.; Diaz, L.A.; Lister, T.E. Intensified co-electrolysis process for syngas production from captured CO2. J. CO2 Util. 2020, 43, 101365. [Google Scholar] [CrossRef]
- Lee, G.; Li, Y.C.; Kim, J.Y.; Peng, T.; Nam, D.H.; Sedighian Rasouli, A.; Li, F.; Luo, M.; Ip, A.H.; Joo, Y.C.; et al. Electrochemical upgrade of CO2 from amine capture solution. Nat. Energy 2021, 6, 46–53. [Google Scholar] [CrossRef]
- Pérez-Gallent, E.; Vankani, C.; Sánchez-Martínez, C.; Anastasopol, A.; Goetheer, E. Integrating CO2 Capture with Electrochemical Conversion Using Amine-Based Capture Solvents as Electrolytes. Ind. Eng. Chem. Res. 2021, 60, 4269–4278. [Google Scholar] [CrossRef]
- Ahmad, N.; Wang, X.; Sun, P.; Chen, Y.; Rehman, F.; Xu, J.; Xu, X. Electrochemical CO2 reduction to CO facilitated by MDEA-based deep eutectic solvent in aqueous solution. Renew. Energy 2021, 177, 23–33. [Google Scholar] [CrossRef]
- Wang, M.; Herzog, H.J.; Hatton, T.A. CO2 Capture Using Electrochemically Mediated Amine Regeneration. Ind. Eng. Chem. Res. 2020, 59, 7087–7096. [Google Scholar] [CrossRef]
- O’rear, D.J. Process for Conversion of Lpg and CH4 to syNgas and Higher Valued Products. Patent No. US6774148, 10 August 2004. Also published as GB0426607, US20030236312. [Google Scholar]
- Johnston, D.A. Carbon Dioxide Absorbed from Air and Hydrogen from Electrolysis of Water, for Production of Carbon Monoxide, Alcohols, Fischer-Tropsch Hydrocarbons & Fuels. Patent No. GB2448685, 29 October 2008. [Google Scholar]
- Olah, G.A.; Prakash, G.K.S. Electrolysis of Carbon Dioxide in Aqueous Media to Carbon Monoxide and Hydrogen for Production of Methanol. Patent No. US7704369, 27 April 2010. Also published as CN101743343, AU2008276180, CA2690980, EP2167706, ES2659978, JP2010533784, JP5144755, KR20100036317, US2009014336, WO2009012154. [Google Scholar]
- Olah, G.A.; Prakash, G.K.S. Electrolysis of Carbon Dioxide in Aqueous Media to Carbon Monoxide and Hydrogen for Production of Methanol. Patent No. US8138380, 20 March 2012. Also published as US2010193370. [Google Scholar]
- Sivasankar, N.; Cole, E.B.; Teamey, K. Electrochemical Production of Synthesis Gas from Carbon Dioxide. Patent No. US8721866, 13 May 2014. Also published as AU2011282783, BR112013002217, CA2805852, CN103119204, EP2598670, JP2013532774, KR20140012018, US8721866, US10119196, US2011114504, US2014238871, WO2012015921. [Google Scholar]
- Masel, R.I.; Salehi-Khojin, A. Electrocatalytic Process for Carbon Dioxide Conversion. Patent No. US9012345, 21 April 2015. Also published as US2013157174. [Google Scholar]
- Masel, R.I.; Qingmei, C.H.E.N.; Liu, Z.; Kutz, R. Electrochemical Device for Converting Carbon Dioxide to a Reaction Product. Patent No. US9481939, 1 November 2016. Also published as US2016108530. [Google Scholar]
- Kuhl, K.P.; Cave, E.R.; Leonard, G. Reactor with Advanced Architecture for the Electrochemical Reaction of CO2, CO and Other Chemical Compounds. Patent No. US2017321333, 9 November 2017. Also published as WO2017192787. [Google Scholar]
- Masel, R.I.; Salehi-Khojin, A.; Kutz, R. Electrocatalytic Process for Carbon Dioxide Conversion. Patent No. US9815021, 14 September 2017. Also published as US2017259206, WO2017176600. [Google Scholar]
- Masel, R.I.; Salehi-Khojin, A. Electrocatalytic Process for Carbon Dioxide Conversion. Patent No. US9555367, 31 January 2017. Also published as US2015209722. [Google Scholar]
- Krishnamurthy, K.; Gärtner, L.-E.; Rupieper, A.; Bostick, D. Process and Apparatus for Manufacturing Carbon Monoxide. Patent No. WO2018228723, 20 December 2018. [Google Scholar]
- Masel, R.I. Devices for Electrocatalytic Conversion of Carbon Dioxide. Patent No. US10173169, 26 April 2018. Also published as US2018111083. [Google Scholar]
- Rüger, D. Synthesis Gas Production from CO2 and H2O in a CO-Electrolysis. Patent No. WO2018228643, 20 December 2018. [Google Scholar]
- Sivasankar, N.; Cole, E.B.; Teamey, K. Electrochemical Production of Synthesis Gas from Carbon Dioxide. Patent No. US10119196, 6 November 2018. Also published as AU2011282783, BR112013002217, CA2805852, CN103119204, EP2598670, JP2013532774, KR20140012018, US8721866, US2011114504, US2014238871, WO2012015921. [Google Scholar]
- Lister, T.E.; Dufek, E.J.; Wilson, A.D.; Diaz Aldana, L.A.; Adhikari, B.; Gao, N. Methods and Systems for the Electrochemical Reduction of Carbon Dioxide Using Switchable Polarity Materials. Patent No. WO2019070526, 11 April 2019. [Google Scholar]
- Masel, R.I. Electrocatalytic Process for Carbon Dioxide Conversion. Patent No. US2019211463, 11 July 2019. [Google Scholar]
- Kuhl, K.P.; Cave, E.R.; Leonard, G. Reactor with Advanced Architecture for the Electrochemical Reaction of CO2, CO and Other Chemical Compounds. Patent No. US10822709, 3 November 2020. Also published as CA3022807, CA3022812, CA3124239, CN109643813, CN109643816, CN115198294, CN116231017, DK3453064, DK3453065, EP3453064, EP3828315, EP3954807, ES2879900, ES2885066, HUE055019, HUE056900, JP2019515142, JP2019520474, JP2021059788, JP6784776, JP6816165, JP7194368, PL3453064, PL3453065, PT3453064, PT3453065, US10648091, US11124886, US11680327, US2017321333, US2017321334, US2020354843, US2021164116, US2022010437, WO2017192787, WO2017192788. [Google Scholar]
- Kuhl, K.P.; Cave, E.R.; Leonard, G. Reactor with Advanced Architecture for the Electrochemical Reaction of CO2, CO and Other Chemical Compounds. Patent No. US11124886, 21 September 2021. Also published as CA3022807, CA3022812, CA3124239, CN109643813, CN109643816, CN115198294, CN116231017, DK3453064, DK3453065, EP3453064, EP3828315, EP3954807, ES2879900, ES2885066, HUE055019, HUE056900, JP2019515142, JP2019520474, JP2021059788, JP6784776, JP6816165, JP7194368, PL3453064, PL3453065, PT3453064, PT3453065, US10648091, US10822709, US11680327, US2017321333, US2017321334, US2020354843, US2021164116, US2022010437, WO2017192787, WO2017192788. [Google Scholar]
- Lister, T.E.; Dufek, E.J.; Wilson, A.D.; Diaz Aldana, L.A.; Adhikari, B.; Gao, N. Methods and Systems for the Electrochemical Reduction of Carbon Dioxide Using Switchable Polarity Materials. Patent No. US10975477, 13 April 2021. Also published as US2020255958, WO2019070526. [Google Scholar]
- Omersa, K. Carbon Dioxide Conversion Using Combined Fuel Cell and Electrolysis Cell. Patent No. US2021313608, 7 October 2021. [Google Scholar]
- Fontecave, M.; Mougel, V.; Tran, N.H.; Wakerley, D.; Lamaison, S. Method for Converting Carbon Dioxide (CO2) into Syngas by an Electrolysis Reaction. Patent No. US2022064804, 3 March 2022. Also published as CA3123971, DK3670701, EP3670700, EP3670701, ES2929181, JP2022515169, US2022056602, WO2020127772, WO2020127821. [Google Scholar]
- Rüger, D. Synthesis Gas Production from CO2 and H2O in a CO-Electrolysis. Patent No. US11214488, 4 January 2022. Also published as CA3067061, DK3472370, EP3415661, EP3472370, US2020095124, WO2018228643. [Google Scholar]
- Zhang, C.; Pan, L.; Guo, H.; Xu, X.; Wang, J. System for Preparing Synthesis Gas by Reducing Electrolytic Urea-Carbon Dioxide. Patent No. CN218115613, 23 December 2022. [Google Scholar]
- Kuhl, K.P.; Cave, E.R.; Leonard, G. Reactor with Advanced Architecture for the Electrochemical Reaction of CO2, CO and Other Chemical Compounds. Patent No. US11680327, 20 June 2023. Also published as CA3022807, CA3022812, CA3124239, CN109643813, CN109643816, CN115198294, CN116231017, DK3453064, DK3453065, EP3453064, EP3828315, EP3954807, ES2879900, ES2885066, HUE055019, HUE056900, JP2019515142, JP2019520474, JP2021059788, JP6784776, JP6816165, JP7194368, PL3453064, PL3453065, PT3453064, PT3453065, US10648091, US10822709, US11124886, US2017321333, US2017321334, US2020354843, US2021164116, US2022010437, WO2017192787, WO2017192788. [Google Scholar]
- Schreiber, A.; Peschel, A.; Hentschel, B.; Zapp, P. Life cycle assessment of power-to-syngas: Comparing high temperature co-electrolysis and steam methane reforming. Front. Energy Res. 2020, 8, 533850. [Google Scholar] [CrossRef]
- Harteck, P.; Dondes, S. Decomposition of carbon dioxide by ionizing radiation. Part I. J. Chem. Phys. 1955, 23, 902–908. [Google Scholar] [CrossRef]
- Hirschfelder, J.O.; Taylor, H.S. The alpha-particle reactions in carbon monoxide, oxygen and carbon dioxide systems. J. Chem. Phys. 1938, 6, 783–790. [Google Scholar] [CrossRef]
- Lind, S.C.; Bardwell, D.C. The chemical action of gaseous ions produced by alpha particles. VI. Reactions of the oxides of carbon. J. Am. Chem. Soc. 1925, 47, 2675–2697. [Google Scholar] [CrossRef]
- Kalashnikov, N.A.; Kalinichenko, B.S.; Shvetsov, I.K. Influence of reagent phase state, form and dose rate irradiation on efficiency of radiolytic decomposition of water and carbon dioxide. At. Energiya 1992, 72, 47–53. [Google Scholar]
- Avdonina, E.N. Hot hydrogen atoms reactions and the mechanisms of liquid normal alkanes radiolysis. Radiat. Eff. 1977, 31, 241–248. [Google Scholar] [CrossRef]
- Huerta Parajon, M.; Rajesh, P.; Mu, T.; Pimblott, S.M.; LaVerne, J. H atom yields in the radiolysis of water. Radiat. Phys. Chem. 2008, 77, 1203–1207. [Google Scholar] [CrossRef]
- Schofield, J.; Reiff, S.C.; Pimblott, S.M.; LaVerne, J.A. Radiolytic hydrogen generation at silicon carbide-water interfaces. J. Nucl. Mater. 2016, 469, 43–50. [Google Scholar] [CrossRef]
- Laverne, J.A.; Pimblott, S.M. New mechanism for H2 formation in water. J. Phys. Chem. A 2000, 104, 9820–9822. [Google Scholar] [CrossRef]
- Pimblott, S.M.; La Verne, J.A. Molecular product formation in the electron radiolysis of water. Radiat. Res. 1992, 129, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, C.; Liu, W.; Zhang, D. Factors influencing hydrogen yield in water radiolysis and implications for hydrocarbon generation: A review. Arab. J. Geosci. 2018, 11, 542. [Google Scholar] [CrossRef]
- Southworth, J.S.; Pimblott, S.M.; Orr, R.M.; Koehler, S.P.K. A novel method for measuring the radiolysis yields of water adsorbed on ZrO2 nanoparticles. Radiat. Phys. Chem. 2020, 174, 108924. [Google Scholar] [CrossRef]
- Watanabe, D.; Yoshida, T.; Allen, C.; Tanabe, T. Enhancement of gamma-ray radiolysis of carbon dioxide with the assistance of solid materials. J. Radioanal. Nucl. Chem. 2007, 272, 461–465. [Google Scholar] [CrossRef]
- Ikezoe, Y.; Sato, S.; Shimizu, S.; Matsuoka, S. Effect of water on the radiolysis of carbon dioxide. Radiat. Phys. Chem. 1981, 17, 69–70. [Google Scholar] [CrossRef]
- Ryazantsev, S.V.; Feldman, V.I. Matrix-isolation studies on the radiation-induced chemistry in H2O/CO2 systems: Reactions of oxygen atoms and formation of HOCO radical. J. Phys. Chem. A 2015, 119, 2578–2586. [Google Scholar] [CrossRef]
- Wu, X.Z.; Hatashita, M.; Enokido, Y.; Kakihana, H. Reduction of carbon dioxide in γ ray irradiated carbon dioxide: Water system containing cu2+ and so32. Chem. Lett. 2000, 5, 572–573. [Google Scholar] [CrossRef]
- Kalashnikov, N.A.; Kalinichenko, B.S.; Shvetsov, I.K. Effect of the reagent phase and the radiation power and type on the radiolysis efficiency of water and carbon dioxide. Sov. At. Energy 1992, 72, 43–48. [Google Scholar] [CrossRef]
- Kalashnikov, N.A.; Kalinichenko, B.S.; Shvetsov, I.K. Effect of the temperature, pressure, and inorganic gas impurities on the radiolysis efficiency of water and carbon dioxide. Sov. At. Energy 1992, 72, 49–53. [Google Scholar] [CrossRef]
- Kalinichenko, B.S.; Kalashnikov, N.A.; Kulazhko, V.G.; Shvetsov, I.K. Method of Producing Carbon Monoxide. Patent No. SU1426943, 30 September 1988. [Google Scholar]
- Kalinichenko, B.S.; Kalashnikov, N.A.; Kulazhko, V.G.; Shvetsov, I.K. Method of Producing Carbon Monoxide. Patent No. SU1490074, 30 June 1989. [Google Scholar]
- Kalinichenko, B.S.; Kalashnikov, N.A.; Kulazhko, V.G.; Shvetsov, I.K. Method of Producing Hydrogen by Radiolysis of Water Vapor. Patent No. RU1824375, 30 June 1993. [Google Scholar]
- Ikezoe, Y.; Sato, S. Radiation chemical reactions in carbon dioxide-propane system formation of carbon monoxide by fission fragments. J. Nucl. Sci. Technol. 1976, 13, 503–507. [Google Scholar] [CrossRef]
- Ikezoe, Y.; Sato, S. Radiation chemical reactions in carbon dioxide–propane system. II. Reoxidation of carbon monoxide at low propane concentration. Bull. Chem. Soc. Jpn. 1978, 51, 33–36. [Google Scholar] [CrossRef]
- Dyer, A.; Moorse, G.E. The radiolysis of simple gas mixtures-I. Rates of production and destruction of methane in mixtures with carbon dioxide as a major constituent. Radiat. Phys. Chem. 1982, 20, 315–321. [Google Scholar] [CrossRef]
- Lind, S.C.; Bardwell, D.C. The chemical action of gaseous ions produced by alpha particles. IX. Saturated hydrocarbons. J. Am. Chem. Soc. 1926, 48, 2335–2351. [Google Scholar] [CrossRef]
- Honig, R.E.; Sheppard, C.W. An experimental comparison of the chemical effects of deuterons and of alpha particles on methane and n-butane. J. Phys. Chem. 1946, 50, 119–143. [Google Scholar] [CrossRef]
- Camps, J.A.; Turnbull, D.M. Synthetic Gas Production for Methanol—Current and Future Trends. Hydrog. Prod. Mark. 1980, 116, 123–146. [Google Scholar]
- Sehested, J. Industrial and scientific directions of methanol catalyst development. J. Catal. 2019, 371, 368–375. [Google Scholar] [CrossRef]
- Herman, R.G.; Klier, K.; Simmons, G.W.; Finn, B.P.; Bulko, J.B.; Kobylinski, T.P. Catalytic synthesis of methanol from COH2. I. Phase composition, electronic properties, and activities of the Cu/ZnO/M2O3 catalysts. J. Catal. 1979, 56, 407–429. [Google Scholar] [CrossRef]
- Mehta, S.; Simmons, G.W.; Klier, K.; Herman, R.G. Catalytic synthesis of methanol from COH2. II. Electron microscopy (TEM, STEM, microdiffraction, and energy dispersive analysis) of the Cu ZnO and Cu/ZnO/Cr2O3 catalysts. J. Catal. 1979, 57, 339–360. [Google Scholar] [CrossRef]
- Herman, R.G.; Simmons, G.W.; Klier, K. Catalytic synthesis of methanol from CO/H2 III. the role of alumina and ceria in the cu/zno system. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1981; Volume 7, pp. 475–489. [Google Scholar] [CrossRef]
- Klier, K.; Chatikavanij, V.; Herman, R.G.; Simmons, G.W. Catalytic synthesis of methanol from COH2. IV. The effects of carbon dioxide. J. Catal. 1982, 74, 343–360. [Google Scholar] [CrossRef]
- Bozzano, G.; Manenti, F. Efficient methanol synthesis: Perspectives, technologies and optimization strategies. Prog. Energy Combust. Sci. 2016, 56, 71–105. [Google Scholar] [CrossRef]
- Ali, K.A.; Abdullah, A.Z.; Mohamed, A.R. Recent development in catalytic technologies for methanol synthesis from renewable sources: A critical review. Renew. Sustain. Energy Rev. 2015, 44, 508–518. [Google Scholar] [CrossRef]
- Jadhav, S.G.; Vaidya, P.D.; Bhanage, B.M.; Joshi, J.B. Catalytic carbon dioxide hydrogenation to methanol: A review of recent studies. Chem. Eng. Res. Des. 2014, 92, 2557–2567. [Google Scholar] [CrossRef]
- Ramos, F.S.; Farias, A.M.D.D.; Borges, L.E.P.; Monteiro, J.L.; Fraga, M.A.; Sousa-Aguiar, E.F.; Appel, L.G. Role of dehydration catalyst acid properties on one-step DME synthesis over physical mixtures. Gas-Liq. Technol. Pap. Present. 12th Braz. Congr. Catal. 2005, 101, 39–44. [Google Scholar] [CrossRef]
- Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to olefins (MTO): From fundamentals to commercialization. ACS Catal. 2015, 5, 1922–1938. [Google Scholar] [CrossRef]
- Ye, M.; Li, H.; Zhao, Y.; Zhang, T.; Liu, Z. MTO Processes Development: The Key of Mesoscale Studies. In Mesoscale Modeling in Chemical Engineering Part II, 2015; Li, J., Marin, G.B., Eds.; Academic Press Inc.: Cambridge, MA, USA, 2015; Volume 47, pp. 279–335. [Google Scholar] [CrossRef]
- Gogate, M.R. Methanol-to-olefins process technology: Current status and future prospects. Pet. Sci. Technol. 2019, 37, 559–565. [Google Scholar] [CrossRef]
- Carbon Recycling International (CRI); HS Orka. George Olah CO2 to Renewable Methanol Plant. Available online: https://www.chemicals-technology.com/projects/george-olah-renewable-methanol-plant-iceland/ (accessed on 17 August 2024).
- Carbon Recycling International (CRI). Emission to Liquid (ETL) Technology. Available online: https://www.carbonrecycling.is/george-olah (accessed on 17 August 2023).
- Carbon Recycling International (CRI). First large-Scale CO2 to Methanol Plant Inaugurated. Available online: https://carbonrecycling.com/about/news/first-large-scale-CO2-to-methanol-plant-inaugurated (accessed on 1 May 2024).
- Goehna, H.; Koenig, P. Producing methanol from CO2. CHEMTECH 1994, 24, 36–39. [Google Scholar]
- Konig, P.; Gohna, H. Process of Producing Methanol. Patent No. US5631302, 20 May 1997. [Google Scholar]
- Konig, P.; Gohna, H. Process of Producing Methanol. Patent No. US5827901, 27 October 1998. [Google Scholar]
- Müller, D.; Bormann, A. Process and Plant for Producing Methanol. Patent No. US8471076, 25 June 2013. Also published as ATE528274, BRPI0816071, CN101790501, DE102007040707, EG25675, EP2181083, MX2010002206, MY183147, US2011065966, WO2009030353. [Google Scholar]
- Kopetsch, H. Process and Plant for Producing Methanol. Patent No. US8629190, 14 January 2014. Also published as CN102171170, CN105732313, DE102008049622, EP2328854, US2011178187, WO2010037441. [Google Scholar]
- Brehm, L.; Göhna, H.; König, P. Process and Reactor System for Synthesis of Methanol with Cycle Gas and Purge Gas Recycling. Patent No. EP3016924, 11 May 2016. Also published as WO2014173452. [Google Scholar]
- Rodrigues, M.T.; Zonetti, P.C.; Alves, O.C.; Soua-Aguiar, E.F.; Borges, L.P.; Appel, L.E.G. RWGS reaction employing Ni/Mg(Al,Ni)O—The role of the O vacancies. Appl. Catal. A Gen. 2017, 543, 98–103. [Google Scholar] [CrossRef]
- Uhm, S.J.; Han, S.H.; Song, S.M.; Joo, O.S. Process for the Production of Methanol from Waste Gas. Patent No. KR0138587, 1 May 1998. Also published as WO9606064, JPH09510734, KR960007519. [Google Scholar]
- Joo, O.-S.; Jung, K.-D.; Moon, I.; Rozovskii, A.Y.; Lin, G.I.; Han, S.-H.; Uhm, S.-J. Carbon dioxide hydrogenation to form methanol via a reverse-water-gas-shift reaction (the camere process). Ind. Eng. Chem. Res. 1999, 38, 1808–1812. [Google Scholar] [CrossRef]
- Joo, O.S.; Jung, K.D.; Jung, Y. CAMERE process for methanol synthesis from CO2 hydrogenation. In Studies in Surface Science and Catalysis; Elsevier Inc.: Amsterdam, The Netherlands, 2004; Volume 153, pp. 67–72. [Google Scholar]
- Anicic, B.; Trop, P.; Goricanec, D. Comparison between two methods of methanol production from carbon dioxide. Energy 2014, 77, 279–289. [Google Scholar] [CrossRef]
- Toro, C.; Sciubba, E. Sabatier based power-to-gas system: Heat exchange network design and thermoeconomic analysis. Appl. Energy 2018, 229, 1181–1190. [Google Scholar] [CrossRef]
- Vogt, C.; Monai, M.; Kramer, G.J.; Weckhuysen, B.M. The renaissance of the Sabatier reaction and its applications on Earth and in space. Nat. Catal. 2019, 2, 188–197. [Google Scholar] [CrossRef]
- Rihko-Struckmann, L.K.; Peschel, A.; Hanke-Rauschenbach, R.; Sundmacher, K. Assessment of methanol synthesis utilizing exhaust CO2 for chemical storage of electrical energy. Proc. Ind. Eng. Chem. Res. 2010, 49, 11073–11078. [Google Scholar] [CrossRef]
- Liang, X.L.; Xie, J.R.; Liu, Z.M. A Novel Pd-decorated Carbon Nanotubes-promoted Pd-ZnO Catalyst for CO2 Hydrogenation to Methanol. Catal. Lett. 2015, 145, 1138–1147. [Google Scholar] [CrossRef]
- Kiatphuengporn, S.; Donphai, W.; Jantaratana, P.; Yigit, N.; Föttinger, K.; Rupprechter, G.; Chareonpanich, M. Cleaner production of methanol from carbon dioxide over copper and iron supported MCM-41 catalysts using innovative integrated magnetic field-packed bed reactor. J. Clean. Prod. 2017, 142, 1222–1233. [Google Scholar] [CrossRef]
- Wang, J.; Li, G.; Li, Z.; Tang, C.; Feng, Z.; An, H.; Liu, H.; Liu, T.; Li, C. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol. Sci. Adv. 2017, 3, e1701290. [Google Scholar] [CrossRef]
- Kar, S.; Kothandaraman, J.; Goeppert, A.; Prakash, G.K.S. Advances in catalytic homogeneous hydrogenation of carbon dioxide to methanol. J. CO2 Util. 2018, 23, 212–218. [Google Scholar] [CrossRef]
- Duyar, M.S.; Tsai, C.; Snider, J.L.; Singh, J.A.; Gallo, A.; Yoo, J.S.; Medford, A.J.; Abild-Pedersen, F.; Studt, F.; Kibsgaard, J.; et al. A Highly Active Molybdenum Phosphide Catalyst for Methanol Synthesis from CO and CO2. Angew. Chem.-Int. Ed. 2018, 57, 15045–15050. [Google Scholar] [CrossRef] [PubMed]
- Pacholik, G.; Enzlberger, L.; Benzer, A.; Rameshan, R.; Latschka, M.; Rameshan, C.; Föttinger, K. In situ XPS studies of MoS2-based CO2 hydrogenation catalysts. J. Phys. D Appl. Phys. 2021, 54, 324002. [Google Scholar] [CrossRef]
- Schrenk, F.; Lindenthal, L.; Pacholik, G.; Navratil, T.; Berger, T.M.; Drexler, H.; Rameshan, R.; Ruh, T.; Föttinger, K.; Rameshan, C. Perovskite-Type Oxide Catalysts in CO2 Utilization: A Principal Study of Novel Cu-Doped Perovskites for Methanol Synthesis. Compounds 2022, 2, 378–387. [Google Scholar] [CrossRef]
- Alves, G.A.S.; Pacholik, G.; Pollitt, S.; Wagner, T.; Rameshan, R.; Rameshan, C.; Föttinger, K. Mn-promoted MoS2 catalysts for CO2 hydrogenation: Enhanced methanol selectivity due to MoS2/MnOx interfaces. Catal. Sci. Technol. 2024, 14, 1138–1147. [Google Scholar] [CrossRef]
- Xu, D.; Cheng, B.; Wang, W.; Jiang, C.; Yu, J. Ag2cro4/g-c3n4/graphene oxide ternary nanocomposite Z-scheme photocatalyst with enhanced CO2 reduction activity. Appl. Catal. B Environ. 2018, 231, 368–380. [Google Scholar] [CrossRef]
- Schlager, S.; Dumitru, L.M.; Haberbauer, M.; Fuchsbauer, A.; Neugebauer, H.; Hiemetsberger, D.; Wagner, A.; Portenkirchner, E.; Sariciftci, N.S. Electrochemical reduction of carbon dioxide to methanol by direct injection of electrons into immobilized enzymes on a modified electrode. ChemSusChem 2016, 9, 631–635. [Google Scholar] [CrossRef]
- Schlager, S.; Dibenedetto, A.; Aresta, M.; Apaydin, D.H.; Dumitru, L.M.; Neugebauer, H.; Sariciftci, N.S. Biocatalytic and bioelectrocatalytic approaches for the reduction of carbon dioxide using enzymes. Energy Technol. 2017, 5, 812–821. [Google Scholar] [CrossRef]
- Bansode, A.; Urakawa, A. Towards full one-pass conversion of carbon dioxide to methanol and methanol-derived products. J. Catal. 2014, 309, 66–70. [Google Scholar] [CrossRef]
- Ganesh, I. Conversion of carbon dioxide into methanol—A potential liquid fuel: Fundamental challenges and opportunities (a review). Renew. Sustain. Energy Rev. 2014, 31, 221–257. [Google Scholar] [CrossRef]
- Kommoß, B.; Klemenz, S.; Schmitt, F.; Hocke, E.; Vogel, K.; Drochner, A.; Albert, B.; Etzold, B.; Vogel, H.G. Heterogeneously Catalyzed Hydrogenation of Supercritical CO2 to Methanol. Chem. Eng. Technol. 2017, 40, 1907–1915. [Google Scholar] [CrossRef]
- Álvarez, A.; Bansode, A.; Urakawa, A.; Bavykina, A.V.; Wezendonk, T.A.; Makkee, M.; Gascon, J.; Kapteijn, F. Challenges in the Greener Production of Formates/Formic Acid, Methanol, and DME by Heterogeneously Catalyzed CO2 Hydrogenation Processes. Chem. Rev. 2017, 117, 9804–9838. [Google Scholar] [CrossRef] [PubMed]
- Szima, S.; Cormos, C.-C. Improving methanol synthesis from carbon-free H2 and captured CO2: A techno-economic and environmental evaluation. J. CO2 Util. 2018, 24, 555–563. [Google Scholar] [CrossRef]
- Al-Saydeh, S.A.; Zaidi, S.J. Carbon dioxide conversion to methanol: Opportunities and fundamental challenges. In Carbon Dioxide Chemistry, Capture and Oil Recovery; Iyad, K., Janah, S., Hassan, S., Eds.; IntechOpen: Rijeka, Croatia, 2018; pp. 42–61. [Google Scholar] [CrossRef]
- Marlin, D.S.; Sarron, E.; Sigurbjörnsson, Ó. Process advantages of direct CO2 to methanol synthesis. Front. Chem. 2018, 6, 446. [Google Scholar] [CrossRef] [PubMed]
- Bayomie, O.S.; Bouallou, C. Energy and conversion investigations of different process configurations for hydrogenation of CO2 into methanol from industrial flue gases. Chem. Eng. Trans. 2018, 70, 1345–1350. [Google Scholar] [CrossRef]
- Meunier, N.; Chauvy, R.; Mouhoubi, S.; Thomas, D.; De Weireld, G. Alternative production of methanol from industrial CO2. Renew. Energy 2020, 146, 1192–1203. [Google Scholar] [CrossRef]
- Din, I.U.; Usman, M.; Khan, S.; Helal, A.; Alotaibi, M.A.; Alharthi, A.I.; Centi, G. Prospects for a green methanol thermo-catalytic process from CO2 by using MOFs based materials: A mini-review. J. CO2 Util. 2021, 43, 101361. [Google Scholar] [CrossRef]
- Pacholik, G.; Föttinger, K. Process for Preparing Methanol. Patent No. US2023365481, 16 November 2023. [Google Scholar]
- Hamedi, H.; Brinkmann, T.; Shishatskiy, S. Membrane-assisted methanol synthesis processes and the required permselectivity. Membranes 2021, 11, 596. [Google Scholar] [CrossRef]
- Milani, D.; Khalilpour, R.; Zahedi, G.; Abbas, A. A model-based analysis of CO2 utilization in methanol synthesis plant. J. CO2 Util. 2015, 10, 12–22. [Google Scholar] [CrossRef]
- Abdelaziz, O.Y.; Hosny, W.M.; Gadalla, M.A.; Ashour, F.H.; Ashour, I.A.; Hulteberg, C.P. Novel process technologies for conversion of carbon dioxide from industrial flue gas streams into methanol. J. CO2 Util. 2017, 21, 52–63. [Google Scholar] [CrossRef]
- Simonov, A. Electrolysis Breakthrough Could Solve the Hydrogen Conundrum. 2019. Available online: https://phys.org/news/2019-09-electrolysis-breakthrough-hydrogen-conundrum.html (accessed on 22 January 2022).
- Haldor Topsoe. Electrify Methanol Production for a Sustainable Business. 2019. Available online: https://info.topsoe.com/emethanol (accessed on 5 December 2021).
- Masel, R.I.; Ni, Z.R.; Chen, Q.; Rosen, B.A. Hydrogenation of Formic Acid to Formaldehyde. Patent No. US9193593, 24 November 2015. Also published as AU2014218628, CN105339336, CN107557086, EP2958882, US2014239231, WO2014130962. [Google Scholar]
- Masel, R.I.; Rosen, B.A.; Zhu, W. Devices and Processes for Carbon Dioxide Conversion into Useful Fuels and Chemicals. Patent No. US9181625, 10 November 2015. Also published as CN104822861, EP2898120, US2014093799, WO2014047661, WO201404766. [Google Scholar]
- Masel, R.I. Process for the Sustainable Production of Acrylic Acid. Patent No. US9790161, 17 October 2017. Also published as US2019135726 (9 May 2019), US2018057439 (1 March 2018), US20160207866 (21 July 2016), AU2017200539. [Google Scholar]
- Masel, R.I. System and Process for the Production of Renewable Fuels and Chemicals. Patent No. EP3504359, 3 July 2019. Also published as CN109642332, KR20190043156, WO2018044720. [Google Scholar]
- Kaczur, J.J.; Yang, H.; Sajjad, S.D.; Masel, R.I. Method and System for Electrochemical Production of Formic Acid from Carbon Dioxide. Patent No. US2019010620, 1 October 2019. [Google Scholar]
- Olah, G.A.; Prakash, G.K.S. Efficient and Selective Conversion of Carbon Dioxide to Methanol, Dimethyl Ether and Derived Products. Patent No. US7605293, 20 October 2009. Also published as US20060235091. [Google Scholar]
- Kuhl, K.P.; Hatsukade, T.; Cave, E.R.; Abram, D.N.; Kibsgaard, J.; Jaramillo, T.F. Electrocatalytic conversion of carbon dioxide to methane and methanol on transition metal surfaces. J. Am. Chem. Soc. 2014, 136, 14107–14113. [Google Scholar] [CrossRef]
- Wiranarongkorn, K.; Eamsiri, K.; Chen, Y.S.; Arpornwichanop, A. A comprehensive review of electrochemical reduction of CO2 to methanol: Technical and design aspects. J. CO2 Util. 2023, 71, 102477. [Google Scholar] [CrossRef]
- Ampelli, C.; Genovese, C.; Perathoner, S.; Centi, G.; Errahali, M.; Gatti, G.; Marchese, L. An electrochemical reactor for the CO2 reduction in gas phase by using conductive polymer based electrocatalysts. Chem. Eng. Trans. 2014, 41, 13–18. [Google Scholar] [CrossRef]
- ElMekawy, A.; Hegab, H.M.; Mohanakrishna, G.; Elbaz, A.F.; Bulut, M.; Pant, D. Technological advances in CO2 conversion electro-biorefinery: A step toward commercialization. Bioresour. Technol. 2016, 215, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Masel, R.I.; Liu, Z.; Rosen, B.A. Electrochemical Device Comprising Novel Catalyst Mixtures. Patent No. US9957624, 1 May 2018. Also published as US20170022618. [Google Scholar]
- Hatsukade, T.; Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. Insights into the electrocatalytic reduction of CO2 on metallic silver surfaces. Phys. Chem. Chem. Phys. 2014, 16, 13814–13819. [Google Scholar] [CrossRef]
- Díez-Ramírez, J.; Sánchez, P.; Valverde, J.L.; Dorado, F. Electrochemical promotion and characterization of PdZn alloy catalysts with K and Na ionic conductors for pure gaseous CO2 hydrogenation. J. CO2 Util. 2016, 16, 375–383. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Olah, G.A.; Goeppert, A. Beyond oil and gas: The methanol economy. In Proceedings of the Electrochemistry and Climate Change—219th ECS Meeting, Montreal, QC, Canada, 1–6 May 2011; pp. 31–40. [Google Scholar] [CrossRef]
- Liu, W.C.; Baek, J.; Somorjai, G.A. The Methanol Economy: Methane and Carbon Dioxide Conversion. Top. Catal. 2018, 61, 530–541. [Google Scholar] [CrossRef]
- Goeppert, A.; Czaun, M.; Jones, J.P.; Surya Prakash, G.K.; Olah, G.A. Recycling of carbon dioxide to methanol and derived products-closing the loop. Chem. Soc. Rev. 2014, 43, 7995–8048. [Google Scholar] [CrossRef]
- Shulenberger, A.M.; Jonsson, F.R.; Ingolfsson, O.; Tran, K.-C. Process for Producing Liquid Fuel from Carbon Dioxide and Water. Patent No. US8198338, 12 June 2012. Also published as US2012201717, US8506910, WO2007108014. [Google Scholar]
- Shulenberger, A.M.; Jonsson, F.R.; Ingolfsson, O.; Tran, K.-C. Process and System for Producing Liquid Fuel from Carbon Dioxide and Water. Patent No. US8506910, 13 August 2013. Also published as US2012201717, US8198338, WO2007108014. [Google Scholar]
- Zaromb, S. Apparatus and Methods for Carbon Dioxide Capture and Conversion. Patent No. US8413420, 9 April 2013. [Google Scholar]
- Sigurbjornsson, O.F.; Singh, S.; Eythorsson, D. System and Process to Capture Industrial Emissions and Recycle for the Production of Chemicals. Patent No. WO2014087433, 12 June 2014. [Google Scholar]
- Simmons, W.W.; White, S.P.; Perkins, C. Various Methods and Apparatuses for Multi-Stage Synthesis Gas Generation. Patent No. US9663363, 30 May 2017. Also published as WO2013158343, US2012181483. [Google Scholar]
- Bowker, M.; Hayward, J. Catalyst Suitable for Methanol Synthesis. Patent No. WO2018138512, 2 August 2018. [Google Scholar]
- Li, X.; Xu, Y.; Ru, J.; Yang, K.; Zhang, T. Comprehensive Treatment Device for Carbon Dioxide Generated by Waste Incineration. Patent No. CN218154221, 27 December 2022. [Google Scholar]
- Wix, C.; Stummann, T.D. Conversion of Carbon Dioxide and Water to Synthesis Gas for Producing Methanol and Hydrocarbon Products. Patent No. CA3203055, 30 June 2022. Also published as AU2021405257 CN116601335 TW202241835 WO2022136374. [Google Scholar]
- Al-Rowaili, F.N.; Khaled, M.; Jamal, A.; Onaizi, S.A.; Zahid, U. Electrochemical Reduction of Carbon Dioxide. Patent No. US11512400, 29 November 2022. Also published as US2022186385 WO2022125831. [Google Scholar]
- Hou, C.; Lv, H.; Ding, L.A.; Zhang, X. Peak Regulation Power Generation System and Method Coupled with Carbon Dioxide Capture and Utilization. Patent No. CN114899884, 12 August 2022. [Google Scholar]
- Roustaei, A.H.; Djemai, A. System for Production, Conversion and Reproduction of Gas-Liquid-Gas Hydrogen Cycle with Absorption of Carbon Dioxide, Comprises Water Electrolyzer Device with Electrolysis Chamber, Electrodes and Electrolyte, and Gas Transformation Device. Patent No. FR2939450, 11 June 2010. [Google Scholar]
- Hoppe, W.; Bringezu, S.; Wachter, N. Economic assessment of CO2-based methane, methanol and polyoxymethylene production. J. CO2 Util. 2018, 27, 170–178. [Google Scholar] [CrossRef]
- Ghasemzadeh, K.; Sadati Tilebon, S.M.; Nasirinezhad, M.; Basile, A. Economic Assessment of Methanol Production. In Methanol: Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 613–632. [Google Scholar] [CrossRef]
- Nguyen, T.B.H.; Leonzio, G.; Zondervan, E. Supply chain optimization framework for CO2 capture, utilization, and storage in Germany. Phys. Sci. Rev. 2023, 8, 1685–1711. [Google Scholar] [CrossRef]
- Pratschner, S.; Skopec, P.; Hrdlicka, J.; Winter, F. Power-to-green methanol via CO2 hydrogenation—A concept study including oxyfuel fluidized bed combustion of biomass. Energies 2021, 14, 4638. [Google Scholar] [CrossRef]
- Gaikwad, A.; Maga, D.; Schlüter, S. Comparative Lifecycle Assessment of Methanol and Urea Produced from Steel Mill Gases. Chem.-Ing.-Tech. 2022, 94, 1476–1488. [Google Scholar] [CrossRef]
- Meessen, J. Urea synthesis. Chem. Ing. Tech. 2014, 86, 2180–2189. [Google Scholar] [CrossRef]
- Ding, J.; Ye, R.; Fu, Y.; He, Y.; Wu, Y.; Zhang, Y.; Zhong, Q.; Kung, H.H.; Fan, M. Direct synthesis of urea from carbon dioxide and ammonia. Nat. Commun. 2023, 14, 4586. [Google Scholar] [CrossRef] [PubMed]
- Berghout, N.; McCulloch, S. Putting CO2 to Use; IEA: Paris, France, 2019; 86p, Available online: https://www.iea.org/reports/putting-CO2-to-use (accessed on 18 April 2022).
- Cheng, K.; Cheng, W. Tripolycyanamide Whole Circulation Production Process and Device. Patent No. CN108558783, 21 September 2018. [Google Scholar]
- Lee, J.M. Process for Manufacturing Melamine from Urea. Patent No. US5384404, 24 January 1995. [Google Scholar]
- Li, D. One-Step Method Used for Production of Melamine by Circulating Ammonia Gas as Carrying Gas. Patent No. CN103601693, 2 June 2012. [Google Scholar]
- Mennen, J.H. Integrated Production of Urea and Melamine. Patent No. US9981924, 29 May 2018. [Google Scholar]
- Sioli, G. Process for the Production of High Purity Melamine from Urea. Patent No. US9045439, 2 June 2012. [Google Scholar]
- Brueske, S.; Kramer, C.; Fisher, A. Chemical Bandwidth Study; US Department of Energy, Office of Energy Efficiency & Renewable Energy: Washington, DC, USA, 2015. Available online: https://www.energy.gov/eere/iedo/articles/bandwidth-study-us-chemical-manufacturing (accessed on 21 May 2020).
- Hosono, H.; Kitano, M. Advances in materials and applications of inorganic electrides. Chem. Rev. 2021, 121, 3121–3185. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, V.; Garagounis, I.; Vourros, A.; Vasileiou, E.; Stoukides, M. An electrochemical haber-bosch process. Joule 2020, 4, 142–158. [Google Scholar] [CrossRef]
- Liu, H.; Wei, L.; Liu, F.; Pei, Z.; Shi, J.; Wang, Z.J.; He, D.; Chen, Y. Homogeneous, heterogeneous, and biological catalysts for electrochemical N2 reduction toward NH3 under ambient conditions. ACS Catal. 2019, 9, 5245–5267. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Yu, H.; Xu, Y.; Xue, H.; Li, X.; Wang, H.; Wang, L. Ambient Electrochemical Synthesis of Ammonia from Nitrogen and Water Catalyzed by Flower-Like Gold Microstructures. ChemSusChem 2018, 11, 3480–3485. [Google Scholar] [CrossRef]
- Shipman, M.A.; Symes, M.D. Recent progress towards the electrosynthesis of ammonia from sustainable resources. Catal. Today 2017, 286, 57–68. [Google Scholar] [CrossRef]
- Kyriakou, V.; Garagounis, I.; Vasileiou, E.; Vourros, A.; Stoukides, M. Progress in the Electrochemical Synthesis of Ammonia. Catal. Today 2017, 286, 2–13. [Google Scholar] [CrossRef]
- Topsoe and First Ammonia Launch Zero Emission Ammonia Production with the World’s Largest Reservation of Electrolyzer Capacity. Available online: https://www.topsoe.com/press-releases/topsoe-and-first-ammonia?utm_medium=email&_hsmi=225986923&_hsenc=p2ANqtz-9hzpaCDUilA4TCFqDA1kUOcPkVabbU%E2%80%A6 (accessed on 16 September 2022).
- Knudsen, J.N. Green Ammonia: Brighten Two Horizons at Once. Available online: https://www.topsoe.com/processes/green-ammonia (accessed on 3 December 2023).
- Boyce, J.; Sacchi, R.; Goetheer, E.; Steubing, B. A prospective life cycle assessment of global ammonia decarbonisation scenarios. Heliyon 2024, 10, e27547. [Google Scholar] [CrossRef]
- Shi, L.; Liu, L.; Yang, B.; Sheng, G.; Xu, T. Evaluation of industrial urea energy consumption (EC) based on life cycle assessment (LCA). Sustainability 2020, 12, 3793. [Google Scholar] [CrossRef]
- Shirmohammadi, R.; Aslani, A.; Ghasempour, R.; Romeo, L.M.; Petrakopoulou, F. Exergoenvironmental analysis and thermoeconomic optimization of an industrial post-combustion CO2 capture and utilization installation. J. CO2 Util. 2022, 59, 101927. [Google Scholar] [CrossRef]
- Yang, M.; Rosentrater, K.A. Life Cycle Assessment of Urea-Formaldehyde Adhesive and Phenol-Formaldehyde Adhesives. Environ. Process. 2020, 7, 553–561. [Google Scholar] [CrossRef]
- Litskas, V.D. Environmental Impact Assessment for Animal Waste, Organic and Synthetic Fertilizers. Nitrogen 2023, 4, 16–25. [Google Scholar] [CrossRef]
- Pulselli, F.M.; Patrizi, N.; Focardi, S. Calculation of the unit emergy value of water in an Italian watershed. Ecol. Model. 2011, 222, 2929–2938. [Google Scholar] [CrossRef]
- Lyu, Y.; Raugei, M.; Zhang, X.; Mellino, S.; Ulgiati, S. Environmental cost and impacts of chemicals used in agriculture: An integration of emergy and Life Cycle Assessment. Renew. Sustain. Energy Rev. 2021, 151, 111604. [Google Scholar] [CrossRef]
- Rivas-Aybar, D.; John, M.; Biswas, W. Environmental Life Cycle Assessment of a Novel Hemp-Based Building Material. Materials 2023, 16, 7208. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, Z.; Hu, Y.; Schmidhalter, U.; Zhang, W.; Ruan, S.; Chen, X. Integrated assessment of agronomic, environmental and ecosystem economic benefits of blending use of controlled-release and common urea in wheat production. J. Clean. Prod. 2021, 287, 125572. [Google Scholar] [CrossRef]
- Masjedi, S.K.; Kazemi, A.; Moeinnadini, M.; Khaki, E.; Olsen, S.I. Urea production: An absolute environmental sustainability assessment. Sci. Total Environ. 2024, 908, 168225. [Google Scholar] [CrossRef]
- Marvel, C.S.; Elliott, J.R.; Boettner, F.E.; Yuska, H. The Structure of Urea-Formaldehyde Resins1. J. Am. Chem. Soc. 1946, 68, 1681–1686. [Google Scholar] [CrossRef]
- Nguon, O.; Lagugné-Labarthet, F.; Brandys, F.A.; Li, J.; Gillies, E.R. Microencapsulation by in situ polymerization of amino resins. Polym. Rev 2017, 58, 326–375. [Google Scholar] [CrossRef]
- Pizzi, A. Urea-formaldehyde adhesives. In Advanced Wood Adhesives Technology; CRC Press: Boca Raton, FL, USA, 1994; pp. 19–66. [Google Scholar]
- Rammon, R.M.; Johns, W.E.; Magnuson, J.; Dunker, A.K. The Chemical Structure of UF Resins. J. Adhes. 1986, 19, 115–135. [Google Scholar] [CrossRef]
- Antov, P.; Savov, V.; Krišt’ák, L.; Réh, R.; Mantanis, G.I. Eco-friendly, high-density fiberboards bonded with urea-formaldehyde and ammonium lignosulfonate. Polymers 2021, 13, 220. [Google Scholar] [CrossRef] [PubMed]
- Ormondroyd, G.A. Adhesives for wood composites. In Wood Composites; Ansell, M.P., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 47–66. [Google Scholar] [CrossRef]
- Rowell, R.M. Handbook of Wood Chemistry and Wood Composites, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar] [CrossRef]
- Meyer, B. Urea-Formaldehyde Resins; Addison-Wesley Publishing Co.: London, UK, 1979; 142p. [Google Scholar]
- Myers, G.E.; Koutsky, J.A. Formaldehyde Liberation and Cure Behavior of Urea-Formaldehyde Resins. Holzforschung 1990, 44, 117–126. [Google Scholar] [CrossRef]
- Myers, G.E. New technologies and materials for bonding wood products. Adhes. Age 1988, 31, 31–36. [Google Scholar]
- Myers, G.E.; Koutsky, J.A. Procedure for measuring formaldehyde liberation from formaldehyde-based resins. For. Prod. J. 1987, 37, 56–60. [Google Scholar]
- Myers, G.E. Effect of ventilation rate and board loading on formaldehyde concentration: A critical review of the literature. For. Prod. J. 1984, 34, 59–68. [Google Scholar]
- Myers, G.E. How mole ratio of UF resin affects formaldehyde emission and other properties: A literature critique. For. Prod. J. 1984, 34, 35–41. [Google Scholar]
- Myers, G.E. Formaldehyde emission from particleboard and plywood paneling: Measurement, mechanism, and product standards. For. Prod. J. 1983, 33, 27–37. [Google Scholar]
- Myers, G.E.; Nagaoka, M. Emission of formaldehyde by particleboard: Effect of ventilation rate and loading on air-contamination levels. For. Prod. J. 1981, 31, 39–44. [Google Scholar]
- River, B.H.; Ebewele, R.O.; Myers, G.E. Failure mechanisms in wood joints bonded with urea-formaldehyde adhesives. Holz Als Roh- Und Werkst. 1994, 52, 179–184. [Google Scholar] [CrossRef]
- Ebewele, R.O.; River, B.H.; Myers, G.E. Behavior of amine-modified urea–formaldehyde-bonded wood joints at low formaldehyde/urea molar ratios. J. Appl. Polym. Sci. 1994, 52, 689–700. [Google Scholar] [CrossRef]
- Ebewele, R.O.; River, B.H.; Myers, G.E. Polyamine-modified urea–formaldehyde-bonded wood joints. III. Fracture toughness and cyclic stress and hydrolysis resistance. J. Appl. Polym. Sci. 1993, 49, 229–245. [Google Scholar] [CrossRef]
- Ebewele, R.O.; Myers, G.E.; River, B.H.; Koutsky, J.A. Polyamine-modified urea-formaldehyde resins. I. Synthesis, structure, and properties. J. Appl. Polym. Sci. 1991, 42, 2997–3012. [Google Scholar] [CrossRef]
- Ganesh, I. Conversion of carbon dioxide into several potential chemical commodities following different pathways—A review. Mater. Sci. Forum 2013, 764, 1–82. [Google Scholar] [CrossRef]
- Chan, F.L.; Altinkaya, G.; Fung, N.; Tanksale, A. Low temperature hydrogenation of carbon dioxide into formaldehyde in liquid media. Catal. Today 2018, 309, 242–247. [Google Scholar] [CrossRef]
- Roffael, E. Significance of wood extractives for wood bonding. Appl. Microbiol. Biotechnol. 2016, 100, 1589–1596. [Google Scholar] [CrossRef]
- Dessbesell, L.; Yuan, Z.; Leitch, M.; Paleologou, M.; Pulkki, R.; Xu, C.C. Capacity design of a kraft lignin biorefinery for production of biophenol via a proprietary low-temperature/low-pressure lignin depolymerization process. ACS Sustain. Chem. Eng. 2018, 6, 9293–9303. [Google Scholar] [CrossRef]
- Starace, A.K.; Black, B.A.; Lee, D.D.; Palmiotti, E.C.; Orton, K.A.; Michener, W.E.; Ten Dam, J.; Watson, M.J.; Beckham, G.T.; Magrini, K.A.; et al. Characterization and Catalytic Upgrading of Aqueous Stream Carbon from Catalytic Fast Pyrolysis of Biomass. ACS Sustain. Chem. Eng. 2017, 5, 11761–11769. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Wu, Y.; He, Z.; Wan, H. “Greener” adhesives composed of urea-formaldehyde resin and cottonseed meal for wood-based composites. J. Clean. Prod. 2018, 187, 361–371. [Google Scholar] [CrossRef]
- Zhan, B.; Zhang, L.; Deng, Y.; Fan, M.; Yan, L. Sustainable adhesives for ultra-composites from biomass powder. Chem. Eng. J. 2024, 485, 149984. [Google Scholar] [CrossRef]
- Al-gheethi, A.A.; Memon, Z.A.; Balasbaneh, A.T.; Al-kutti, W.A.; Mokhtar, N.; Othman, N.; Juki, M.I.; Noman, E.A.; Algaifi, H.A. Critical Analysis for Life Cycle Assessment of Bio-Cementitious Materials Production and Sustainable Solutions. Sustainability 2022, 14, 1920. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, J.Y.; Park, S.B.; Han, S.T.; Kang, E.C. Comparative study of the storage stability between a melamine-urea- formaldehyde and a urea-formaldehyde resin. For. Prod. J. 2012, 62, 146–149. [Google Scholar] [CrossRef]
- Priha, E. Formaldehyde release from resin-containing wood board dusts: Evaluation of methods to determine formaldehyde. Appl. Occup. Environ. Hyg. 1996, 11, 465–470. [Google Scholar] [CrossRef]
- Park, B.D.; Lee, S.M.; Roh, J.K. Effects of formaldehyde/urea mole ratio and melamine content on the hydrolytic stability of cured urea-melamine-formaldehyde resin. Eur. J. Wood Wood Prod. 2009, 67, 121–123. [Google Scholar] [CrossRef]
- Park, B.D.; Lee, S.M. Hydrolytic stability of cured urea-melamine-formaldehyde resins depending on hydrolysis conditions and hardener types. J. Korean Wood Sci. Technol. 2015, 43, 672–681. [Google Scholar] [CrossRef]
- Jeong, B.; Park, B.D.; Causin, V. Influence of synthesis method and melamine content of urea-melamine-formaldehyde resins to their features in cohesion, interphase, and adhesion performance. J. Ind. Eng. Chem. 2019, 79, 87–96. [Google Scholar] [CrossRef]
- Suchý, P.; Straková, E.; Herzig, I.; Staňa, J.; Kalusová, R.; Pospíchalová, M. Toxicological risk of melamine and cyanuric acid in food and feed. Interdiscip. Toxicol. 2009, 2, 55–59. [Google Scholar] [CrossRef]
- Bann, B.; Miller, S.A. Melamine And Derivatives Of Melamine. Chem. Rev. 1958, 58, 131–172. [Google Scholar] [CrossRef]
- Braun, D.; Ritzert, H.J. Properties and processing behaviour of urea-melamine-formaldehyde resins. Kunststoffe Ger. Plast. 1987, 77, 29–31. [Google Scholar]
- No, B.Y.; Kim, M.G. Evaluation of melamine-modified urea-formaldehyde resins as particleboard binders. J. Appl. Polym. Sci. 2007, 106, 4148–4156. [Google Scholar] [CrossRef]
- Shi, J.; Ye, S. Effects of addition ammonia modified urea-melamine-formaldehyde resin on the adhesions and formaldehyde emission in plywood. Proc. Adv. Mater. Res. 2010, 113–116, 1226–1229. [Google Scholar] [CrossRef]
- Ranjbaran, S.; Nazerian, M.; Kermanian, H.; Koosha, M.; Garmaroody, E.R. High strength papers impregnated with urea/melamine formaldehyde resin/nanosilica nanocomposite coatings: The effects of paper type, blend ratio and nano-content. Mater. Today Commun. 2020, 25, 101300. [Google Scholar] [CrossRef]
- Li, Z.; Chen, H.; Xu, Q.; Li, X.; Ma, H.; Yuan, Q. Styrene and BPO poly (urea-melamine-formaldehyde) microcapsules prepared via in situ polymerization to promote the self-healing properties of epoxy composites. J. Coat. Technol. Res. 2022, 19, 1837–1850. [Google Scholar] [CrossRef]
- Sokolova, E.G.; Varankina, G.S.; Rusakov, D.S. Urea–melamine formaldehyde resin for the manufacture of water-resistant plywood. Polym. Sci.-Ser. D 2022, 15, 557–561. [Google Scholar] [CrossRef]
- Silva, D.A.L.; Lahr, F.A.R.; Varanda, L.D.; Christoforo, A.L.; Ometto, A.R. Environmental performance assessment of the melamine-urea-formaldehyde (MUF) resin manufacture: A case study in Brazil. J. Clean. Prod. 2015, 96, 299–307. [Google Scholar] [CrossRef]
- Yılmaz, E. Environmental impact assessment of melamine coated medium density fiberboard (MDF-LAM) production and cumulative energy demand: A case study in Türkiye. Case Stud. Constr. Mater. 2024, 20, e02733. [Google Scholar] [CrossRef]
- Lao, W.L.; Ma, L.P.; Wang, C.; Liu, C.W.; Li, Y. Environmental impacts evaluation and promotion measures of wood-based composite doors. J. Build. Eng. 2023, 80, 108164. [Google Scholar] [CrossRef]
- Tang, Y.; Yuan, Z.; Gong, Y.; Yin, M.; Yang, X.; Chen, D.; Yi, J.; Lei, L.; Liu, C.; Li, X.; et al. System and Process for Melamine Production by Gas-Phase Quenching Method of Energy Efficient and Cost Saving Type. Patent No. US9114371, 25 August 2015. [Google Scholar]
- Solmi, M.V.; Schmitz, M.; Leitner, W. CO2 as a Building Block for the Catalytic Synthesis of Carboxylic Acids. In Studies in Surface Science and Catalysis; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 178, pp. 105–124. [Google Scholar] [CrossRef]
- Hölscher, M.; Gürtler, C.; Keim, W.; Müller, T.E.; Peters, M.; Leitner, W. Carbon dioxide as a carbon resource—Recent trends and perspectives. Z. Fur Naturforschung-Sect. B J. Chem. Sci. 2012, 67, 961–975. [Google Scholar] [CrossRef]
- Scott, M.; Westhues, C.G.; Kaiser, T.; Baums, J.C.; Jupke, A.; Franciò, G.; Leitner, W. Methylformate from CO2: An integrated process combining catalytic hydrogenation and reactive distillation. Green Chem. 2019, 21, 6307–6317. [Google Scholar] [CrossRef]
- Schmitz, M.; Solmi, M.V.; Leitner, W. Catalytic processes combining CO2 and alkenes into value-added chemicals: Synthesis of cyclic carbonates, lactones, carboxylic acids, esters, aldehydes, alcohols, and amines. Top. Organomet. Chem. 2019, 63, 17–38. [Google Scholar] [CrossRef]
- Peters, M.; Köhler, B.; Kuckshinrichs, W.; Leitner, W.; Markewitz, P.; Müller, T.E. Chemical technologies for exploiting and recycling carbon dioxide into the value chain. ChemSusChem 2011, 4, 1216–1240. [Google Scholar] [CrossRef] [PubMed]
- Ostapowicz, T.G.; Schmitz, M.; Krystof, M.; Klankermayer, J.; Leitner, W. Carbon dioxide as a C1 building block for the formation of carboxylic acids by formal catalytic hydrocarboxylation. Angew. Chem.-Int. Ed. 2013, 52, 12119–12123. [Google Scholar] [CrossRef] [PubMed]
- Leitner, W.; Franciò, G.; Scott, M.; Westhues, C.; Langanke, J.; Lansing, M.; Hussong, C.; Erdkamp, E. Carbon2Polymer—Chemical Utilization of CO2 in the Production of Isocyanates. Chem.-Ing.-Tech. 2018, 90, 1504–1512. [Google Scholar] [CrossRef]
- Suh, H.S.; Ha, J.Y.; Yoon, J.H.; Ha, C.-S.; Suh, H.; Kim, I. Polyester polyol synthesis by alternating copolymerization of propylene oxide with cyclic acid anhydrides by using double metal cyanide catalyst. React. Funct. Polym. 2010, 70, 288–293. [Google Scholar] [CrossRef]
- Ballivet-Tkatchenko, D.; Dos Santos, J.H.Z.; Philippot, K.; Vasireddy, S. Carbon dioxide conversion to dimethyl carbonate: The effect of silica as support for SnO2 and ZrO2 catalysts. C. R. Chim. 2011, 14, 780–785. [Google Scholar] [CrossRef]
- Kizlink, J.; Pastucha, I. Preparation of dimethyl carbonate from methanol and carbon dioxide in the presence of Sn(IV) and Ti(IV) alkoxides and metal acetates. Collect. Czech. Chem. Commun. 1995, 60, 687–692. [Google Scholar] [CrossRef]
- Sun, W.; Zheng, L.; Wang, Y.; Li, D.; Liu, Z.; Wu, L.; Fang, T.; Wu, J. Study of thermodynamics and experiment on direct synthesis of dimethyl carbonate from carbon dioxide and methanol over yttrium oxide. Ind. Eng. Chem. Res. 2020, 59, 4281–4290. [Google Scholar] [CrossRef]
- Hu, X.; Cheng, H.; Kang, X.; Chen, L.; Yuan, X.; Qi, Z. Analysis of direct synthesis of dimethyl carbonate from methanol and CO2 intensified by in-situ hydration-assisted reactive distillation with side reactor. Chem. Eng. Process.-Process Intensif. 2018, 129, 109–117. [Google Scholar] [CrossRef]
- Guo, C.; Liu, X.; Wang, F.; Cao, Y.; Zheng, S.; He, G. Economic Analysis and Life Cycle Environmental Assessment of Imidazolium-Based Ionic Liquids for Separation of the Methanol/Dimethyl Carbonate Azeotrope. ACS Sustain. Chem. Eng. 2023, 11, 10482–10495. [Google Scholar] [CrossRef]
- Garcia-Herrero, I.; Cuéllar-Franca, R.M.; Enríquez-Gutiérrez, V.M.; Alvarez-Guerra, M.; Irabien, A.; Azapagic, A. Environmental Assessment of Dimethyl Carbonate Production: Comparison of a Novel Electrosynthesis Route Utilizing CO2 with a Commercial Oxidative Carbonylation Process. ACS Sustain. Chem. Eng. 2016, 4, 2088–2097. [Google Scholar] [CrossRef]
- Zhao, S.Y.; Wang, S.P.; Zhao, Y.J.; Ma, X.B. An in situ infrared study of dimethyl carbonate synthesis from carbon dioxide and methanol over well-shaped CeO2. Chin. Chem. Lett. 2017, 28, 65–69. [Google Scholar] [CrossRef]
- Tomishige, K.; Sakaihori, T.; Ikeda, Y.; Fujimoto, K. A novel method of direct synthesis of dimethyl carbonate from methanol and carbon dioxide catalyzed by zirconia. Catal. Lett. 1999, 58, 225–229. [Google Scholar] [CrossRef]
- Stoian, D.C.; Taboada, E.; Llorca, J.; Molins, E.; Medina, F.; Segarra, A.M. Boosted CO2 reaction with methanol to yield dimethyl carbonate over Mg–Al hydrotalcite-silica lyogels. Chem. Commun. 2013, 49, 5489–5491. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Dibenedetto, A.; Pastore, C.; Pápai, I.; Schubert, G. Reaction mechanism of the direct carboxylation of methanol to dimethylcarbonate: Experimental and theoretical studies. Top. Catal. 2006, 40, 71–81. [Google Scholar] [CrossRef]
- Tundo, P.; Selva, M. The chemistry of dimethyl carbonate. Acc. Chem. Res. 2002, 35, 706–716. [Google Scholar] [CrossRef]
- Tundo, P.; Perosa, A. Green organic syntheses: Organic carbonates as methylating agents. Chem. Rec. 2002, 2, 13–23. [Google Scholar] [CrossRef]
- Lui, Y.W.; Chan, B.; Lui, M.Y. Methylation with Dimethyl Carbonate/Dimethyl Sulfide Mixtures: An Integrated Process without Addition of Acid/Base and Formation of Residual Salts. ChemSusChem 2022, 15, e202102538. [Google Scholar] [CrossRef]
- Delledonne, D.; Rivetti, F.; Romano, U. Developments in the production and application of dimethylcarbonate. Appl. Catal. A Gen. 2001, 221, 241–251. [Google Scholar] [CrossRef]
- Astruc, L.; Miranda, J.; Donis, I.R.; Vialle, C.; Sablayrolles, C. Dimethyl Carbonate Production by Urea Transesterification, Process Simulation and Environmental Assessment. In Computer Aided Chemical Engineering; Elsevier B.V.: Amsterdam, The Netherlands, 2020; Volume 48, pp. 97–102. [Google Scholar] [CrossRef]
- Huang, S.; Yan, B.; Wang, S.; Ma, X. Recent advances in dialkyl carbonates synthesis and applications. Chem. Soc. Rev. 2015, 44, 3079–3116. [Google Scholar] [CrossRef]
- Monteiro, J.G.M.S.; De Queiroz Fernandes Araújo, O.; De Medeiros, J.L. Sustainability metrics for eco-technologies assessment, part I: Preliminary screening. Clean Technol. Environ. Policy 2009, 11, 209–214. [Google Scholar] [CrossRef]
- Monteiro, J.G.M.S.; de Queiroz Fernandes Araújo, O.; de Medeiros, J.L. Sustainability metrics for eco-technologies assessment, Part II. Life cycle analysis. Clean Technol. Environ. Policy 2009, 11, 459–472. [Google Scholar] [CrossRef]
- Lee, Y.G.; Lee, H.U.; Lee, J.M.; Kim, N.Y.; Jeong, D.H. Design of Dimethyl Carbonate (DMC) Synthesis Process Using CO2, Techno-economic Analysis, and Life Cycle Assessment. Korean J. Chem. Eng. 2024, 41, 117–133. [Google Scholar] [CrossRef]
- Aresta, M.; Quaranta, E.; Dibenedetto, A.; Tommasi, I.; Marciniec, B. CO2-catalysed carbamation of aminofunctional silanes. Appl. Organomet. Chem. 2000, 14, 871–873. [Google Scholar] [CrossRef]
- Tundo, P.; McElroy, C.R.; Aricò, F. Synthesis of carbamates from amines and dialkyl carbonates: Influence of leaving and entering groups. Synlett 2010, 2010, 1567–1571. [Google Scholar] [CrossRef]
- Sun, J.; Aly, K.I.; Kuckling, D. A novel one-pot process for the preparation of linear and hyperbranched polycarbonates of various diols and triols using dimethyl carbonate. RSC Adv. 2017, 7, 12550–12560. [Google Scholar] [CrossRef]
- Liu, T.; Wang, G.; Yang, X. Controlled synthesis of aliphatic polycarbonate diols using dimethyl carbonate and various diols. J. Chin. Chem. Soc. 2022, 69, 995–1001. [Google Scholar] [CrossRef]
- Tundo, P.; Aricò, F.; McElroy, C.R. The Greening of Chemistry. In The Chemical Element: Chemistry’s Contribution to Our Global Future, 1st ed.; Wiley-VCH: Hoboken, NJ, USA, 2011; pp. 189–233. [Google Scholar] [CrossRef]
- Dernehl, C.U. Health hazards associated with polyurethane foams. J. Occup. Med. 1966, 8, 59–62. [Google Scholar]
- González, S.G.; Bravo, A.R.; Carpintero, V.C.; Cuenca-Romero, L.A. Towards an ecological transition in the construction sector through the production of new eco-efficient products. Proc. E3S Web Conf. 2023, 379, 04004. [Google Scholar] [CrossRef]
- Rodrigo-Bravo, A.; Alameda Cuenca-Romero, L.; Calderón, V.; Rodríguez, Á.; Gutiérrez-González, S. Comparative Life Cycle Assessment (LCA) between standard gypsum ceiling tile and polyurethane gypsum ceiling tile. Energy Build. 2022, 259, 111867. [Google Scholar] [CrossRef]
- Rey-Álvarez, B.; Silvestre, J.; García-Martínez, A.; Sánchez-Montañés, B. A comparative approach to evaluate the toxicity of building materials through life cycle assessment. Sci. Total Environ. 2024, 912, 168897. [Google Scholar] [CrossRef] [PubMed]
- Llantoy, N.; Chàfer, M.; Cabeza, L.F. A comparative life cycle assessment (LCA) of different insulation materials for buildings in the continental Mediterranean climate. Energy Build. 2020, 225, 110323. [Google Scholar] [CrossRef]
- Klug, V.; Schöggl, J.P.; Dallinger, D.; Hiebler, K.; Stueckler, C.; Steiner, A.; Arzt, A.; Kappe, C.O.; Baumgartner, R.J. Comparative Life Cycle Assessment of Different Production Processes for Waterborne Polyurethane Dispersions. ACS Sustain. Chem. Eng. 2021, 9, 8980–8989. [Google Scholar] [CrossRef]
- Mohamed, N.A.; El-Dash, K.M.; Attia, T.M.; Abdel-Monem, M. Analysis of alternative building envelope solutions to improve energy efficiency. In Proceedings of the 2023 2nd International Conference on Smart Cities 4.0, Smart Cities 4.0 2023, Cairo, Egypt, 22–24 October 2023; pp. 138–145. [Google Scholar] [CrossRef]
- Liang, S.; Liu, H.; Jiang, T.; Song, J.; Yang, G.; Han, B. Highly efficient synthesis of cyclic carbonates from CO2 and epoxides over cellulose/KI. Chem. Commun. 2011, 47, 2131–2133. [Google Scholar] [CrossRef]
- Nodehi, M.; Aguayo, F.; Madey, N.; Zhou, L. A Comparative Review of Polymer, Bacterial-based, and Alkali-Activated (also Geopolymer) Binders: Production, Mechanical, Durability, and Environmental impacts (life cycle assessment (LCA)). Constr. Build. Mater. 2024, 422, 135816. [Google Scholar] [CrossRef]
- Fridrihsone, A.; Romagnoli, F.; Kirsanovs, V.; Cabulis, U. Life Cycle Assessment of vegetable oil based polyols for polyurethane production. J. Clean. Prod. 2020, 266, 121403. [Google Scholar] [CrossRef]
- Bachmann, M.; Kätelhön, A.; Winter, B.; Meys, R.; Müller, L.J.; Bardow, A. Renewable carbon feedstock for polymers: Environmental benefits from synergistic use of biomass and CO2. Faraday Discuss. 2021, 230, 227–246. [Google Scholar] [CrossRef]
- Polo Fonseca, L.; Duval, A.; Luna, E.; Ximenis, M.; De Meester, S.; Avérous, L.; Sardon, H. Reducing the carbon footprint of polyurethanes by chemical and biological depolymerization: Fact or fiction? Curr. Opin. Green Sustain. Chem. 2023, 41, 100802. [Google Scholar] [CrossRef]
- Recupido, F.; Lama, G.C.; Ammendola, M.; Bossa, F.D.L.; Minigher, A.; Campaner, P.; Morena, A.G.; Tzanov, T.; Ornelas, M.; Barros, A.; et al. Rigid composite bio-based polyurethane foams: From synthesis to LCA analysis. Polymer 2023, 267, 125674. [Google Scholar] [CrossRef]
- Silva, R.; Barros-Timmons, A.; Quinteiro, P. Life cycle assessment of fossil- and bio-based polyurethane foams: A review. J. Clean. Prod. 2023, 430, 139697. [Google Scholar] [CrossRef]
- Wiatrowski, M.; Tan, E.C.D.; Davis, R. TEA and LCA of bio-based polyurethanes. In Rethinking Polyester Polyurethanes: Algae Based Renewable, Sustainable, Biodegradable and Recyclable Materials; Elsevier: Amsterdam, The Netherlands, 2023; pp. 153–176. [Google Scholar] [CrossRef]
- Zhou, C.H.; Beltramini, J.N.; Lu, G.Q. Chemoselective catalytic conversion of glycerol as a biorenewable source to valuable commodity chemicals. Chem. Soc. Rev. 2008, 37, 527–549. [Google Scholar] [CrossRef] [PubMed]
- Pagliaro, M.; Rossi, M. The Future of Glycerol: New Usages for a Versatile Raw Material; RSC Publishing: Cambridge, UK, 2008; 127p. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, X.; Shen, Y. Commodity Chemicals Derived from Glycerol, an Important Biorefinery Feedstock. Chem. Rev. 2008, 110, 1807. [Google Scholar] [CrossRef] [PubMed]
- Ozorio, L.P.; Pianzolli, R.; Da Cruz MacHado, L.; Miranda, J.L.; Turci, C.C.; Guerra, A.C.O.; Souza-Aguiar, E.F.; Mota, C.J.A. Metal-impregnated zeolite Y as efficient catalyst for the direct carbonation of glycerol with CO2. Appl. Catal. A Gen. 2015, 504, 187–191. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Pastore, C.; Aresta, M. Direct carboxylation of alcohols to organic carbonates: Comparison of the Group 5 element alkoxides catalytic activity. An insight into the reaction mechanism and its key steps. Catal. Today 2006, 115, 88–94. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Nocito, F.; Pastore, C. A study on the carboxylation of glycerol to glycerol carbonate with carbon dioxide: The role of the catalyst, solvent and reaction conditions. J. Mol. Catal. A Chem. 2006, 257, 149–153. [Google Scholar] [CrossRef]
- Hammond, C.; Lopez-Sanchez, J.A.; Hasbi Ab Rahim, M.; Dimitratos, N.; Jenkins, R.L.; Carley, A.F.; He, Q.; Kiely, C.J.; Knight, D.W.; Hutchings, G.J. Synthesis of glycerol carbonate from glycerol and urea with gold-based catalysts. Dalton Trans. 2011, 40, 3927–3937. [Google Scholar] [CrossRef]
- Yoo, J.W.; Mouloungui, Z. Catalytic carbonylation of glycerin by urea in the presence of zinc mesoporous system for the synthesis of glycerol carbonate. In Studies in Surface Science and Catalysis; Elsevier Inc.: Amsterdam, The Netherlands, 2003; Volume 146, pp. 757–760. [Google Scholar] [CrossRef]
- Wang, L.; Ma, Y.; Wang, Y.; Liu, S.; Deng, Y. Efficient synthesis of glycerol carbonate from glycerol and urea with lanthanum oxide as a solid base catalyst. Catal. Commun. 2011, 12, 1458–1462. [Google Scholar] [CrossRef]
- Christy, S.; Noschese, A.; Lomelí-Rodriguez, M.; Greeves, N.; Lopez-Sanchez, J.A. Recent progress in the synthesis and applications of glycerol carbonate. Curr. Opin. Green Sustain. Chem. 2018, 14, 99–107. [Google Scholar] [CrossRef]
- Ahamad, A.; Singh, P.; Tiwary, D. Plastic and Microplastic in the Environment: Management and Health Risks; Wiley: Hoboken, NJ, USA, 2022; pp. 1–299. [Google Scholar] [CrossRef]
- Kumar, A.; Thakur, V.K.; Nezhad, H.Y.; Lee, K.S. Prospects of sustainable polymers. Sci. Rep. 2024, 14, 9430. [Google Scholar] [CrossRef]
- Kosloski-Oh, S.C.; Fieser, M.E. From source to disposal: Cracking the code for sustainable polymers. One Earth 2023, 6, 587–590. [Google Scholar] [CrossRef]
- Zeng, X.; Chen, Q.; Zhao, C.; Xie, S.; Xie, H.; Huang, C. Eugenol-derived Organic Liquids as an In-Situ CO2 Capturing and Conversion System for Eugenol-based Polycarbonate Synthesis. Chem.-Asian J. 2022, 17, e202200503. [Google Scholar] [CrossRef] [PubMed]
- Torres-Giner, S. Sustainable Polymer Technologies for a Circular Economy. Appl. Sci. 2023, 13, 5864. [Google Scholar] [CrossRef]
- Sadeghi, K.; Jeon, Y.; Seo, J. Roadmap to the sustainable synthesis of polymers: From the perspective of CO2 upcycling. Prog. Mater. Sci. 2023, 135, 101103. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Wu, F.; Mincheva, R.; Hakkarainen, M.; Raquez, J.M.; Mielewski, D.F.; Narayan, R.; Netravali, A.N.; Misra, M. Sustainable polymers. Nat. Rev. Methods Primers 2022, 2, 46. [Google Scholar] [CrossRef]
- Okolie, O.; Kumar, A.; Edwards, C.; Lawton, L.A.; Oke, A.; McDonald, S.; Thakur, V.K.; Njuguna, J. Bio-Based Sustainable Polymers and Materials: From Processing to Biodegradation. J. Compos. Sci. 2023, 7, 213. [Google Scholar] [CrossRef]
- Alper, E.; Yuksel Orhan, O. CO2 utilization: Developments in conversion processes. Petroleum 2017, 3, 109–126. [Google Scholar] [CrossRef]
- Elmas, S.; Subhani, M.A.; Leitner, W.; Müller, T.E. Anion effect controlling the selectivity in the zinc-catalysed copolymerisation of CO2 and cyclohexene oxide. Beilstein J. Org. Chem. 2015, 11, 42–49. [Google Scholar] [CrossRef]
- Leitner, W.; Müller, T.E.; Gürtler, C. How can we put the climate killer CO2 to good use? In Annual Report: Science for a Better Life; Bayer: Leverkusen, Germany, 2010; pp. 32–37. [Google Scholar]
- Gütlbauer, F.; Getoff, N. Radiation chemical carboxylation of hydroxycompounds. Int. J. Appl. Radiat. Isot. 1965, 16, 673–680. [Google Scholar] [CrossRef]
- Krapfenbauer, K.F.; Getoff, N. Radiation-and photo-induced formation of salicylic acid from phenol and CO2 in aqueous solution. World Resour. Rev. 1997, 9, 421–433. [Google Scholar]
- Getoff, N. Radiation chemistry and the environment. Radiat. Phys. Chem. 1999, 54, 377–384. [Google Scholar] [CrossRef]
- Getoff, N.; Solar, S.; Quint, R.M. One-electron oxidation of mitomycin C and its corresponding peroxyl radicals. A steady-state and pulse radiolysis study. Radiat. Phys. Chem. 1997, 50, 575–583. [Google Scholar] [CrossRef]
- Getoff, N.; Solar, S.; Richter, U.B.; Haenel, M.W. Pulse radiolysis of pyrene in aprotic polar organic solvents: Simultaneous formation of pyrene radical cations and radical anions. Radiat. Phys. Chem. 2003, 66, 207–214. [Google Scholar] [CrossRef]
- Getoff, N.; Schenck, G.O. On the formation of amino acids by gamma-ray-induced carboxylation of amines in aqueous solutions. Radiat. Res. 1967, 31, 486–505. [Google Scholar] [CrossRef] [PubMed]
- Fjodorov, V.V.; Getoff, N. Radiation induced carboxylation of methanol under elevated CO2-pressure. Radiat. Phys. Chem. 1983, 22, 841–848. [Google Scholar] [CrossRef]
- Getoff, N.; Gütlbauer, F.; Schenck, G.O. Radiation-chemical carboxylation of formic acid and methanol in aqueous solution. Int. J. Appl. Radiat. Isot. 1966, 17, 341–349. [Google Scholar] [CrossRef]
- Caputo, G.; Galia, A.; Scrò, F.; Spadaro, G.; Filardo, G. Gamma radiation induced polymerization of vinyl monomers in dense CO2. Radiat. Phys. Chem. 2002, 63, 45–51. [Google Scholar] [CrossRef]
- Hayashi, K. Radiation and photo-induced ionic polymerization. Polym. J. 1980, 12, 571–581. [Google Scholar] [CrossRef]
- Bartoníček, B. Gamma radiolysis of solid succinic acid. Int. J. Radiat. Phys. Chem. 1973, 5, 361–369. [Google Scholar] [CrossRef]
- Wang, W.; Chang, Z.; Wang, M.; Zhang, Z. Effect of carboxyl on vulcanization and mechanical properties of carboxylated acrylic rubber prepared by 60Co-γ-ray-induced polymerization. J. Appl. Polym. Sci. 2006, 102, 5587–5594. [Google Scholar] [CrossRef]
- Dispenza, C.; Filardo, G.; Silvestri, G.; Spadaro, G. Carboxylation of linear low density polyethylene through gamma irradiation in presence of supercritical carbon dioxide. Grafted groups analysis via derivatization procedures. Colloid Polym. Sci. 1997, 275, 390–395. [Google Scholar] [CrossRef]
- Spadaro, G.; De Gregorio, R.; Galia, A.; Valenza, A.; Filardo, G. Gamma radiation induced maleation of polypropylene using supercritical CO2: Preliminary results. Polymer 2000, 41, 3491–3494. [Google Scholar] [CrossRef]
- Clean Energy Technologies. Canada’s CO2 Capture & Storage Technology Roadmap; CANMET Energy Technology Centre: Devon, AL, Canada, 2006; 89p, Available online: https://natural-resources.canada.ca/our-natural-resources/energy-sources-distribution/carbon-management/canadas-co2-capture-and-storage-technology-roadmap/4327 (accessed on 29 June 2022).
- Zhang, Y. Progress in carbon dioxide sequestration by mineral carbonation. CIESC J. 2007, 58, 1. [Google Scholar]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M.M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [PubMed]
- Delgado Torróntegui, M. Assessing the Mineral Carbonation Science and Technology; Swiss Federal Institute of Technology, ETH: Zurich, Switzerland, 2010. [Google Scholar]
- Romanov, V.; Soong, Y.; Carney, C.; Rush, G.E.; Nielsen, B.; O’Connor, W. Mineralization of Carbon Dioxide: A Literature Review. ChemBioEng Rev. 2015, 2, 231–256. [Google Scholar] [CrossRef]
- Aminu, M.D.; Nabavi, S.A.; Rochelle, C.A.; Manovic, V. A review of developments in carbon dioxide storage. Appl. Energy 2017, 208, 1389–1419. [Google Scholar] [CrossRef]
- Wang, B.; Pan, Z.; Cheng, H.; Zhang, Z.; Cheng, F. A review of carbon dioxide sequestration by mineral carbonation of industrial byproduct gypsum. J. Clean. Prod. 2021, 302, 126930. [Google Scholar] [CrossRef]
- Bocin Dumitriu, A.; Perez Fortes, M.D.M.; Tzimas, E.; Sveen, T. Carbon Capture and Utilisation Workshop: Background and Proceedings; JRC Publications: Luxembourg, 2013; 77p, Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC86324 (accessed on 22 July 2021).
- Romão, I.S.S. Production of Magnesium Carbonates from Serpentinites for CO2 Mineral Sequestration-Optimisation towards Industrial Application. Ph.D. Thesis, Abo Akademi University, Turku, Findland, 2015. [Google Scholar]
- Romão, I.S.; Gando-Ferreira, L.M.; Da Silva, M.M.V.G.; Zevenhoven, R. CO2 sequestration with serpentinite and metaperidotite from Northeast Portugal. Miner. Eng. 2016, 94, 104–114. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Fagerlund, J.; Nduagu, E.; Romão, I.; Jie, B.; Highfield, J. Carbon storage by mineralisation (CSM): Serpentinite rock carbonation via Mg(OH)2 reaction intermediate without CO2 pre-separation. In Proceedings of the 11th International Conference on Greenhouse Gas Control Technologies, GHGT 2012, Kyoto, Japan, 18–22 November 2012; pp. 5945–5954. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Ruijg, G.J.; Comans, R.N.J.; Witkamp, G.J. Energy consumption and net CO2 sequestration of aqueous mineral carbonation. Ind. Eng. Chem. Res. 2006, 45, 9184–9194. [Google Scholar] [CrossRef]
- Santos, R.M.; Verbeeck, W.; Knops, P.; Rijnsburger, K.; Pontikes, Y.; Van Gerven, T. Integrated mineral carbonation reactor technology for sustainable carbon dioxide sequestration: ‘CO2 Energy Reactor’. In Proceedings of the 11th International Conference on Greenhouse Gas Control Technologies, GHGT 2012, Kyoto, Japan, 18–22 November 2012; pp. 5884–5891. [Google Scholar] [CrossRef]
- Lackner, K.S.; Wendt, C.H.; Butt, D.P.; Joyce Jr, E.L.; Sharp, D.H. Carbon dioxide disposal in carbonate minerals. Energy 1995, 20, 1153–1170. [Google Scholar] [CrossRef]
- Chen, Z.Y.; O’Connor, W.K.; Gerdemann, S.J. Chemistry of aqueous mineral carbonation for carbon sequestration and explanation of experimental results. Environ. Prog. 2006, 25, 161–166. [Google Scholar] [CrossRef]
- O’Connor, W.K.; Dahlin, D.C.; Rush, G.E.; Gerdemann, S.J.; Penner, L.R. Energy and economic evaluation of ex situ aqueous mineral carbonation. In Greenhouse Gas Control Technologies; Elsevier Ltd.: Amsterdam, The Netherlands, 2005; pp. 2011–2015. [Google Scholar] [CrossRef]
- Gerdemann, S.J.; O’Connor, W.K.; Dahlin, D.C.; Penner, L.R.; Rush, H. Ex situ aqueous mineral carbonation. Environ. Sci. Technol. 2007, 41, 2587–2593. [Google Scholar] [CrossRef] [PubMed]
- Benhelal, E.; Rashid, M.I.; Rayson, M.S.; Brent, G.F.; Oliver, T.; Stockenhuber, M.; Kennedy, E.M. Direct aqueous carbonation of heat activated serpentine: Discovery of undesirable side reactions reducing process efficiency. Appl. Energy 2019, 242, 1369–1382. [Google Scholar] [CrossRef]
- Fernandez, A.; West, K. Technology Roadmap—Low-Carbon Transition in the Cement Industry; International Energy Agency: Paris, France, 2018; 62p. [Google Scholar]
- Olajire, A.A. A review of mineral carbonation technology in sequestration of CO2. J. Pet. Sci. Eng. 2013, 109, 364–392. [Google Scholar] [CrossRef]
- Zevenhoven, R.; Romão, I.; Slotte, M. Mineralisation of CO2 using serpentinite rock—Towards industrial application. In Proceedings of the IEAGHG/IETS Iron & Steel Industry CCUS & Process Integration Workshop, Tokyo, Japan, 5–7 November 2013; 26p. Available online: https://ieaghg.org/docs/General_Docs/Iron%20and%20Steel%202%220Secured%220presentations/203_1520%1520Mikko%1520Helle.pdf (accessed on 13 July 2024).
- Doucet, F.J. Scoping Study on CO2 Mineralization Technologies; CGS-2011-007 Report; CGS: Pretoria, South Africa, 2001; 88p, Available online: https://www.academia.edu/4061042/Scoping_study_on_CO2_mineralization_technologies (accessed on 13 July 2024).
- Hemmati, A.; Shayegan, J.; Bu, J.; Yeo, T.Y.; Sharratt, P. Process optimization for mineral carbonation in aqueous phase. Int. J. Miner. Process. 2014, 130, 20–27. [Google Scholar] [CrossRef]
- Priestnall, M. Making Money from Mineralisation of CO2. Carbon Capture J. 3 February 2013. 3p. Available online: https://www.carboncapturejournal.com/news/making-money-from-mineralisation-of-co2/3251.aspx?Category=a#:~:text=Mineral%20carbonation%20brings%20opportunities%20particularly%20to%20the,wider%20industrial%20and%20power%20generation%20CCS%20markets.d (accessed on 29 June 2022).
- Zevenhoven, R. Metals production, CO2 mineralization and LCA. Metals 2020, 10, 16. [Google Scholar] [CrossRef]
- Priestnall, M. Method and System of Sequestrating Carbon Dioxide. Patent No. GB2515995, 14 January 2015. [Google Scholar]
- Priestnall, M. Method and System of Activation of Mineral Silicate Minerals. Patent No. US9963351, 8 May 2018. Also published as CN106573197, DK3129125, EP3129125, ES2824676, GB2516141, PL3129125, US2017029284, WO2015154887. [Google Scholar]
- Priestnall, M. Can Mineral Carbonation Be Used for Industrial Carbon Dioxide Sequestration? Environmental Chemistry Group Buletin. 2014. 3p. Available online: https://www.envchemgroup.com/can-mineral-carbonation-be-used-for-industrial-co2-sequestration.html (accessed on 29 June 2022).
- Priestnall, M. Decarbonising flue gas using CO2 mineralisation—Project experience on ships. In Proceedings of the Keeping the Momentum; Geological Society: London, UK, 2014; 9p. [Google Scholar]
- Priestnall, M. CO2 mineralisation via fuel cells. How to make CCS profitable. In Proceedings of the Finding Petroleum CCS Forum; Geological Society: London, UK, 2010; 14p; Available online: http://www.findingpetroleum.com/files/event15/ccc.pdf (accessed on 29 June 2022).
- Evans, M. Programme and Business Plan. In Direct Air CO2 Capture & Mineralisation; Cambridge Carbon Capture: Cambridge, UK, 2022; Chapter 4; pp. 14–18. Available online: https://assets.publishing.service.gov.uk/media/6281f0cd8fa8f5561cd9b1b7/cambridge-carbon-cam202029.pdf (accessed on 29 June 2022).
- Fernandez Pales, A.; Remme, U.; Berly, T.; McCulloch, S.; Stanley, T.; Vass, T.; Teter, J.; Abergel, T. Exploring Clean Energy Pathways: The Role of CO2 Storage; International Energy Agency (IEA): Paris, France, 2019; 105p. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Li, Y.; Pan, X.; Poon, C.-S.; Xie, Z. Influence of carbonated recycled concrete aggregate on properties of cement mortar. Constr. Build. Mater. 2015, 98, 1–7. [Google Scholar] [CrossRef]
- Alberici, S.; Noothout, P.; Mir, G.U.R.; Stork, M.; Wiersma, F.; Mac Dowell, N.; Shah, N.; Fennell, P. Assessing the Potential of CO2 Utilisation in the UK; Gardiner, A., Ed.; Final Report SISUK17099; 2017; 138p. Available online: https://assets.publishing.service.gov.uk/media/5cc9bd9c40f0b64c1e187664/SISUK17099AssessingCO2_utilisationUK_ReportFinal_260517v2__1_.pdf (accessed on 25 May 2024).
- O’Connor, W.K.; Dahlin, D.C.; Rush, G.E.; Dahlin, C.L.; Collins, W.K. Carbon dioxide sequestration by direct mineral carbonation: Process mineralogy of feed and products. Miner. Metall. Process. 2002, 19, 95–101. [Google Scholar] [CrossRef]
- O’Connor, W.K.; Dahlin, D.C.; Rush, G.E.; Gedermann, S.J.; Penner, L.R.; Nilsen, D.N. Aqueous Mineral Carbonation; DOE/ARC-TR-04-002; National Energy Technology Laboratory: Albany, OR, USA, 2005; 463p.
- O’Connor, W.K.; Dahlin, D.C.; Turner, P.C.; Walters, R.P. Carbon dioxide sequestration by ex-situ mineral carbonation. Technology 1999, 7, 115–123. [Google Scholar]
- Teir, S.; Eloneva, S.; Revitzer, H.; Zevenhoven, R.; Salminen, J.; Fogelholm, C.-J.; Poylio, E. Method of Producing Calcium Carbonate from Waste and by-Products. Patent No. US8603428, 10 December 2013. [Google Scholar]
- Batuecas, E.; Liendo, F.; Tommasi, T.; Bensaid, S.; Deorsola, F.A.; Fino, D. Recycling CO2 from flue gas for CaCO3 nanoparticles production as cement filler: A Life Cycle Assessment. J. CO2 Util. 2021, 45, 101446. [Google Scholar] [CrossRef]
- Khoo, Z.Y.; Ho, E.H.Z.; Li, Y.; Yeo, Z.; Low, J.S.C.; Bu, J.; Chia, L.S.O. Life cycle assessment of a CO2 mineralisation technology for carbon capture and utilisation in Singapore. J. CO2 Util. 2021, 44, 101378. [Google Scholar] [CrossRef]
- Zhuang, Q.; Clements, B.; Li, Y. From ammonium bicarbonate fertilizer production process to power plant CO2 capture. Int. J. Greenh. Gas Control 2012, 10, 56–63. [Google Scholar] [CrossRef]
- Dowsett, M.R.; Lewis, C.M.; North, M.; Parkin, A. Exploring the scope of capacitance-assisted electrochemical carbon dioxide capture. Dalton Trans. 2018, 47, 10447–10452. [Google Scholar] [CrossRef] [PubMed]
- Lamb, K.J.; Dowsett, M.R.; Chatzipanagis, K.; Scullion, Z.W.; Kröger, R.; Lee, J.D.; Aguiar, P.M.; North, M.; Parkin, A. Capacitance-Assisted Sustainable Electrochemical Carbon Dioxide Mineralisation. ChemSusChem 2018, 11, 137–148. [Google Scholar] [CrossRef]
- Balucan, R.D.; Dlugogorski, B.Z.; Kennedy, E.M.; Belova, I.V.; Murch, G.E. Energy cost of heat activating serpentinites for CO2 storage by mineralisation. Int. J. Greenh. Gas Control 2013, 17, 225–239. [Google Scholar] [CrossRef]
- Britten, R. System for Halting the Increase in Atmospheric Carbon Dioxide and Method of Operation Thereof. Patent No. US2011171107, 14 July 2011. Abandoned. [Google Scholar]
- Jones, J.D.; St Angelo, D. Removing Carbon Dioxide from Waste Streams through Co-Generation of Carbonate and/or Bicarbonate Minerals. Patent No. US9205375, 8 December 2015. Also published as AT543562, AU2008302064, BRPI0817116, CA2930096, CA2699572, CN101970084, EP2205341, ES2386060, GEP20135757, HK1143107, JP2010540214, JP2013066887, JP2015186801, JP6110103, JP6145129, KR101485854, KR20100101069, PL2205341, RU2010115481, RU2012144036, RU2477168, RU2531827, US2009127127, WO2009039445, ZA201001932. [Google Scholar]
- Sano, K.; Saito, H.; Miyamoto, H.; Imada, T. Carbon Dioxide Fixation Method and Carbon Dioxide Fixation System. Patent No. US2022297058, 22 September 2022. Also published as JP2022145080. [Google Scholar]
- Sano, K.; Saito, H.; Miyamoto, H.; Suzuki, A. Carbon Dioxide Fixation System and Method Using Seawater Electrolysis. Patent No. US2023051291, 16 February 2023. Also published as JP2023025798. [Google Scholar]
- Idir, R.; Cyr, M.; Tagnit-Hamou, A. Use of waste glass in cement-based materials. Environ. Ingénierie Développement (Déchets Sci. Et Tech.) 2015, 57, 7. [Google Scholar] [CrossRef]
- Glavind, M. Sustainability of cement, concrete and cement replacement materials in construction. In Sustainability of Construction Materials; Elsevier Ltd.: Amsterdam, The Netherlands, 2009; pp. 120–147. [Google Scholar] [CrossRef]
- Riley, B.J.; McFarlane, J.; DelCul, G.D.; Vienna, J.D.; Contescu, C.I.; Forsberg, C.W. Molten salt reactor waste and effluent management strategies: A review. Nucl. Eng. Des. 2019, 345, 94–109. [Google Scholar] [CrossRef]
- Was, G.S.; Petti, D.; Ukai, S.; Zinkle, S. Materials for future nuclear energy systems. J. Nucl. Mater. 2019, 527, 151837. [Google Scholar] [CrossRef]
- Mignacca, B.; Locatelli, G. Economics and finance of Molten Salt Reactors. Prog. Nucl. Energy 2020, 129, 103503. [Google Scholar] [CrossRef]
- Roper, R.; Harkema, M.; Sabharwall, P.; Riddle, C.; Chisholm, B.; Day, B.; Marotta, P. Molten salt for advanced energy applications: A review. Ann. Nucl. Energy 2022, 169, 108924. [Google Scholar] [CrossRef]
- Wu, J.; Chen, J.; Cai, X.; Zou, C.; Yu, C.; Cui, Y.; Zhang, A.; Zhao, H. A Review of Molten Salt Reactor Multi-Physics Coupling Models and Development Prospects. Energies 2022, 15, 8296. [Google Scholar] [CrossRef]
- Rehm, T.E. Advanced nuclear energy: The safest and most renewable clean energy. Curr. Opin. Chem. Eng. 2023, 39, 100878. [Google Scholar] [CrossRef]
- Wilson, J.F.; Srinivasan, S.S.; Moore, B.M.; Henderson, L.; Iii, S.E.; Sharma, P.C. Hydrogen Production Using Nuclear Energy; NP-T-4.2; International Atomic Energy Agency: Vienna, Austria, 2013; 400p. [Google Scholar]
- Kugeler, K.; Schulten, R.; Kugeler, M.; Niessen, H.F.; Roeth-Kamat, M.; Hohn, H.; Woike, O.; Germer, J.H. The Pebble-Bed High-Temperature Reactor as a Source of Nuclear Process Heat. Julich, Germany. 1974, Volume 4. 84p. Available online: http://inis.iaea.org/search/search.aspx?orig_q=RN:07275833 (accessed on 8 December 2018).
- Singh, J.; Niessen, H.F.; Harth, R.; Fedders, H.; Reutler, H.; Panknin, W.; Müller, W.D.; Harms, H.G. The nuclear heated steam reformer—Design and semitechnical operating experiences. Nucl. Eng. Des. 1984, 78, 179–194. [Google Scholar] [CrossRef]
- Niessen, H.F.; Bhattacharyya, A.T.; Busch, M.; Hesse, K.; Zentis, A. Erprobung und Versuchsergebnisse des PNP-Teströhrenspaltofens in der EVA II-Anlage [Test and Results of the Test Tubes, Chemical Heat Pipe PNP in Eva II-Anlage]; Juel-2231/FZJ-2014-04914; Kernforschungsanlage Jülich GmbH; Zentralbiliothek Verlag: Jülich, Germany, 1988. [Google Scholar]
- Harth, R.; Jansing, W.; Teubner, H. Experience gained from the EVA II and KVK operation. Nucl. Eng. Des. 1990, 121, 173–182. [Google Scholar] [CrossRef]
- Kugeler, K.; Kugeler, M.; Niessen, H.F.; Hammelmann, K.H. Steam reformers heated by helium from high temperature reactors. Nucl. Eng. Des. 1975, 34, 129–145. [Google Scholar] [CrossRef]
- Fedders, H.; Harth, R.; Höhlein, B. Experiments for combining nuclear heat with the methane steam-reforming process. Nucl. Eng. Des. 1975, 34, 119–127. [Google Scholar] [CrossRef]
- Harth, R.; Jansing, W.; Mauersberger, R.; Teubner, H. Status of High-Temperature Reactor Component Development for Process Heat Generation. Atomkernenerg. Kerntech. 1985, 47, 176–183. [Google Scholar]
- Dumm, K.; Theymann, W.; Der Decken, C.B.V. Design and qualification of components for high-temperature reactors. Nucl. Eng. Des. 1990, 121, 193–198. [Google Scholar] [CrossRef]
- Höhlein, B.; Niessen, H.; Range, J.; Schiebahn, H.J.R.; Vorwerk, M. Methane from synthesis gas and operation of high-temperature methanation. Nucl. Eng. Des. 1984, 78, 241–250. [Google Scholar] [CrossRef]
- Kugeler, K.; Niessen, H.F.; Röth-Kamat, M.; Böcker, D.; Rüter, B.; Theis, K.A. Transport of nuclear heat by means of chemical energy (nuclear long-distance energy). Nucl. Eng. Des. 1975, 34, 65–72. [Google Scholar] [CrossRef]
- Fedders, H.; Höhlein, B. Operating a pilot plant circuit for energy transport with hydrogen-rich gas. Int. J. Hydrogen Energy 1982, 7, 793–800. [Google Scholar] [CrossRef]
- Boltendahl, U.; Harth, R. Wärmetransport auf kaltem Wege [Transport of Heat Energy using Cold Gases]. Nukleare Fernenergie, Projekt ADAM und EVA. Bild Der Wiss. 1980, 17, 44–55. [Google Scholar]
- Kesten, A.S.; Boedeker, L.R.; Couch, H.T. A self-driven chemical heat pipe. J. Heat Recovery Syst. 1983, 3, 311–319. [Google Scholar] [CrossRef]
- Nuernberg, H.W.; Wolff, G. Energy Conversion Method. Patent No. US3558047, 26 January 1971. [Google Scholar]
- Kugeler, K.; Kugeler, M.; Niessen, H.F.; Hohn, H. Considerations on high temperature reactors for process heat applications. Nucl. Eng. Des. 1975, 34, 15–32. [Google Scholar] [CrossRef]
- Inagaki, Y.; Hino, R.; Hada, K.; Haga, K.; Nishihara, T.; Takeda, T.; Shiozawa, S. Out-of-pile demonstration test program of HTTR hydrogen production system by steam reforming of natural gas. In Proceedings of the 5th International Conference on Nuclear Engineering, ICONE5, Nice, France, 26–30 May 1997; p. 172. [Google Scholar]
- Takeda, T.; Iwatsuki, J. Counter-permeation of deuterium and hydrogen through INCONEL 600®. Nucl. Technol. 2004, 146, 83–95. [Google Scholar] [CrossRef]
- Hishida, M.; Nekoya, S.; Takizuka, T.; Emori, K.; Ogawa, M.; Ouchi, M.; Okamoto, Y.; Sanokawa, K.; Nakano, T.; Hagiwara, T.; et al. Construction and Performance Tests of Hydrogen Secondary Cooling System, (I) Facility and Performance of Components. Nippon Genshiryoku Gakkaishi/J. At. Energy Soc. Jpn. 1980, 22, 181–188. [Google Scholar] [CrossRef]
- Hishida, M.; Takizuka, T.; Ogawa, M.; Nekoya, S.; Emori, K.; Ouchi, M.; Sanokawa, K. Construction and Performance Tests of Hydrogen Secondary Cooling System, (II) Results of Performance Tests. Nippon Genshiryoku Gakkaishi/J. At. Energy Soc. Jpn. 1980, 22, 326–334. [Google Scholar] [CrossRef]
- Tochio, D.; Shimizu, A.; Hamamoto, S.; Sakaba, N. Helium leak and chemical impurities control technology in HTTR. J. Nucl. Sci. Technol. 2014, 51, 1407–1412. [Google Scholar] [CrossRef]
- Nishihara, T.; Sakaki, A.; Inagaki, Y.; Takami, K. Development of high temperature isolation valve for the HTTR hydrogen production system. Trans. At. Energy Soc. Jpn. 2004, 3, 381–387. [Google Scholar] [CrossRef]
- Inaba, Y.; Nishihara, T.; Nitta, Y. Analytical study on fire and explosion accidents assumed in HTGR hydrogen production system. Nucl. Technol. 2004, 146, 49–57. [Google Scholar] [CrossRef]
- Inaba, Y.; Nishihara, T.; Groethe, M.A.; Nitta, Y. Study on explosion characteristics of natural gas and methane in semi-open space for the HTTR hydrogen production system. Nucl. Eng. Des. 2004, 232, 111–119. [Google Scholar] [CrossRef]
- Verfondern, K.; Nishihara, T. Safety aspects of the combined HTTR/steam reforming complex for nuclear hyrogen production. Prog. Nucl. Energy 2005, 47, 527–534. [Google Scholar] [CrossRef]
- Inaba, Y.; Ohashi, H.; Nishihara, T.; Sato, H.; Inagaki, Y.; Takeda, T.; Hayashi, K.; Takada, S. Study on control characteristics for HTTR hydrogen production system with mock-up test facility System controllability test for fluctuation of chemical reaction. Nucl. Eng. Des. 2005, 235, 111–121. [Google Scholar] [CrossRef]
- Hada, K.; Nishihara, T.; Shibata, T.; Shiozawa, S. Design of a steam reforming system to be connected to the HTTR. In Proceedings of the Third JAERI Symposium on HTGR Technologies, Oarai, Japan, 15–16 February 1996; pp. 229–240. [Google Scholar]
- Nishihara, T.; Shimizu, A.; Inagaki, Y.; Tanihira, M. Flow Scheme and Controllability of the HTTR Hydrogen Production System. Trans. At. Energy Soc. Jpn. 2003, 2, 517–524. [Google Scholar] [CrossRef]
- Tanaka, T.; Baba, O.; Shiozawa, S.; Okubo, M.; Tobioka, T. Present status of HTTR construction and its testing program. In Proceedings of the JAERI seminar on HTGR technologies, Tokai, Japan, 7–8 November 1994. 26p. [Google Scholar]
- Ohashi, H.; Inaba, Y.; Nishihara, T.; Inagaki, Y.; Takeda, T.; Hayashi, K.; Katanishi, S.; Takada, S.; Ogawa, M.; Shiozawa, S. Performance test results of mock-up test facility of httr hydrogen production system. J. Nucl. Sci. Technol. 2004, 41, 385–392. [Google Scholar] [CrossRef]
- Ohashi, H.; Inaba, Y.; Nishihara, T.; Takeda, T.; Hayashi, K.; Takada, S.; Inagaki, Y. Development of control technology for HTTR hydrogen production system with mock-up test facility. System controllability test for loss of chemical reaction. Nucl. Eng. Des. 2006, 236, 1396–1410. [Google Scholar] [CrossRef]
- Inagaki, Y.; Ohashi, H.; Inaba, Y.; Sato, H.; Nishihara, T.; Takeda, T.; Hayashi, K.; Ogawa, M. Research and development on system integration technology for connection of hydrogen production systems to an HTGR. Nucl. Technol. 2007, 157, 111–119. [Google Scholar] [CrossRef]
- Onuki, K.; Inagaki, Y.; Hino, R.; Tachibana, Y. Research and development on nuclear hydrogen production using HTGR at JAERI. Prog. Nucl. Energy 2005, 47, 496–503. [Google Scholar] [CrossRef]
- Karasawa, H.; Shiina, K.; Sasahira, A.; Hoshino, K. Storage and hydrogen production usage of recycling material in the flexible fuel cycle initiative. In Proceedings of the International Conference on Nuclear Energy Systems for Future Generation and Global Sustainability, Tsukuba, Japan, 9–13 October 2005. 6p. [Google Scholar]
- Icerman, L. Open-loop chemical heat pipes. Energy 1979, 4, 1187–1188. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Park, K.T.; Lee, W.; Lim, H.; Kwon, Y.; Kang, S. Current achievements and the future direction of electrochemical CO2 reduction: A short review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 769–815. [Google Scholar] [CrossRef]
- Burdyny, T.; Smith, W.A. CO2 reduction on gas-diffusion electrodes and why catalytic performance must be assessed at commercially-relevant conditions. Energy Environ. Sci. 2019, 12, 1442–1453. [Google Scholar] [CrossRef]
- Jensen, S.H.; Larsen, P.H.; Mogensen, M. Hydrogen and synthetic fuel production from renewable energy sources. Int. J. Hydrogen Energy 2007, 32, 3253–3257. [Google Scholar] [CrossRef]
- Fujiwara, S.; Kasai, S.; Yamauchi, H.; Yamada, K.; Makino, S.; Matsunaga, K.; Yoshino, M.; Kameda, T.; Ogawa, T.; Momma, S.; et al. Hydrogen production by high temperature electrolysis with nuclear reactor. Prog. Nucl. Energy 2008, 50, 422–426. [Google Scholar] [CrossRef]
- Kasai, S.; Fujiwara, S.; Yamada, K.; Ogawa, T.; Matsunaga, K.; Yoshino, M.; Hoashp, E.; Makino, S. Nuclear hydrogen production by high-temperature electrolysis. Trans. At. Energy Soc. Jpn. 2009, 8, 122–141. [Google Scholar] [CrossRef]
- Chang, J. Status of nuclear hydrogen production technology development project in Korea. In Proceedings of the International Conference on Non-Electric Applications of Nuclear Power: Seawater Desalination, Hydrogen Production and Other Industrial Applications, Oarai, Japan, 16–19 April 2007; pp. 130–132. [Google Scholar]
- International Atomic Energy Agency. Non-electric applications of nuclear power: Seawater desalination, hydrogen production and other industrial applications. In Proceedings of the International Conference on Non-Electric Applications of Nuclear Power, Oarai, Japan, 16–19 April 2007. [Google Scholar]
- Bents, D.J.; Scullin, V.J.; Chang, B.J.; Johnson, D.W.; Garcia, C.P.; Jakupca, I.J. Hydrogen-oxygen PEM regenerative fuel cell development at Nasa Glenn Research Center. Fuel Cells Bull. 2006, 2006, 12–14. [Google Scholar] [CrossRef]
- Sohal, M.S.; O’Brien, J.E.; Stoots, C.M.; Sharma, V.I.; Yildiz, B.; Virkar, A. Degradation issues in solid oxide cells during high temperature electrolysis. J. Fuel Cell Sci. Technol. 2012, 9, 011017. [Google Scholar] [CrossRef]
- Zhang, X.; O’Brien, J.E.; O’Brien, R.C.; Housley, G.K. Durability evaluation of reversible solid oxide cells. J. Power Sources 2013, 242, 566–574. [Google Scholar] [CrossRef]
- O’Brien, J.E.; O’Brien, R.C.; Zhang, X.; Tao, G.G.; Butler, B.J. Long-term performance of solid oxide stacks with electrode-supported cells operating in the steam electrolysis mode. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, IMECE 2011, Denver, CO, USA, 11–17 November 2011; pp. 495–503. [Google Scholar]
- Harvego, E.A.; O’Brien, J.E.; McKellar, M.G. System evaluation and life-cycle cost analysis of a commercial scale high-temperature electrolysis hydrogen production plant. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, IMECE 2012, Houston, TX, USA, 9–15 November 2012; pp. 875–884. [Google Scholar] [CrossRef]
- Schmeda-Lopez, D.; McConnaughy, T.B.; McFarland, E.W. Radiation enhanced chemical production: Improving the value proposition of nuclear power. Energy 2018, 162, 491–504. [Google Scholar] [CrossRef]
- Livingston, P. Radiation Reduction of Carbon Dioxide: A New Chemical Industry? ChemRxiv 2020, 1, 14. [Google Scholar] [CrossRef]
- LaTourrette, T.; Light, T.; Knopman, D.; Bartis, J.T. Managing Spent Nuclear Fuel. Strategy Alternatives and Policy Implications. RAND Corporation Monograph Series 2010. 95p. Available online: https://www.rand.org/content/dam/rand/pubs/monographs/2010/RAND_MG970.pdf (accessed on 23 January 2023).
- Mccullum, R. Integrated Used Fuel Management: Industry Perspectives; Rod McCullum, Nuclear Energy Institute, Nuclear Waste Technical Review Board: Las Vegas, NV, USA, 2009; 15p. Available online: https://www.nwtrb.gov/docs/default-source/meetings/2009/june/mccullum.pdf?sfvrsn=5 (accessed on 23 January 2023).
- Office of Civilian Radioactive Waste Management. The Report to the President and the Congress by the Secretary of Energy on the Need for a Second Repository; DOE/RW-0595; U.S. Department of Energy: Las Vegas, NV, USA, 10 December 2008; 15p.
- Chu, M.S.Y. Spent nuclear fuel recycling practices, technologies and impact on geological repository systems. In Geological Repository Systems for Safe Disposal of Spent Nuclear Fuels and Radioactive Waste; Elsevier Inc.: Amsterdam, The Netherlands, 2010; pp. 29–42. [Google Scholar] [CrossRef]
- Paviet, P.; Simpson, M.F. Nuclear fuel recycling. In Nuclear Engineering Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 387–469. [Google Scholar] [CrossRef]
- Buckner, M.R.; Burchill, W.E. The Case for Nuclear Fuel Recycling. Nuclear News. February 2016, pp. 42–48. Available online: https://www.ans.org/pubs/magazines/download/article-1011/ (accessed on 23 January 2023).
- Perkins, C.; Jovanovic, Z.; Laska, T.E. Various Methods and Apparatuses for a Radiant-Heat Driven Chemical Reactor. Patent No. US8814961, 26 August 2014. Also published as US20120241677. [Google Scholar]
- Simmons, W.W.; Perkins, C.; Jovanovic, Z.; Hilton, C.; Popp, P.; Schramm, B.J.; Turner, J.T. Various Methods and Apparatuses for Ultra-High Heat Flux Chemical Reactor. Patent No. US9011560, 21 April 2015. Also published as US2012145965, CN102985195, WO2011155962, CA2799827, AU2010355257. [Google Scholar]
- Wickham, A.J.; Best, J.V.; Wood, C.J. Recent advances in the ories of carbon dioxide radiolysis and radiolytic graphite corrosion. Radiat. Phys. Chem. 1977, 10, 107–117. [Google Scholar] [CrossRef]
- Ramirez-Corredores, M.M. Sustainable production of CO2-derived materials. NPJ Mater. Sustain. 2024, in press.

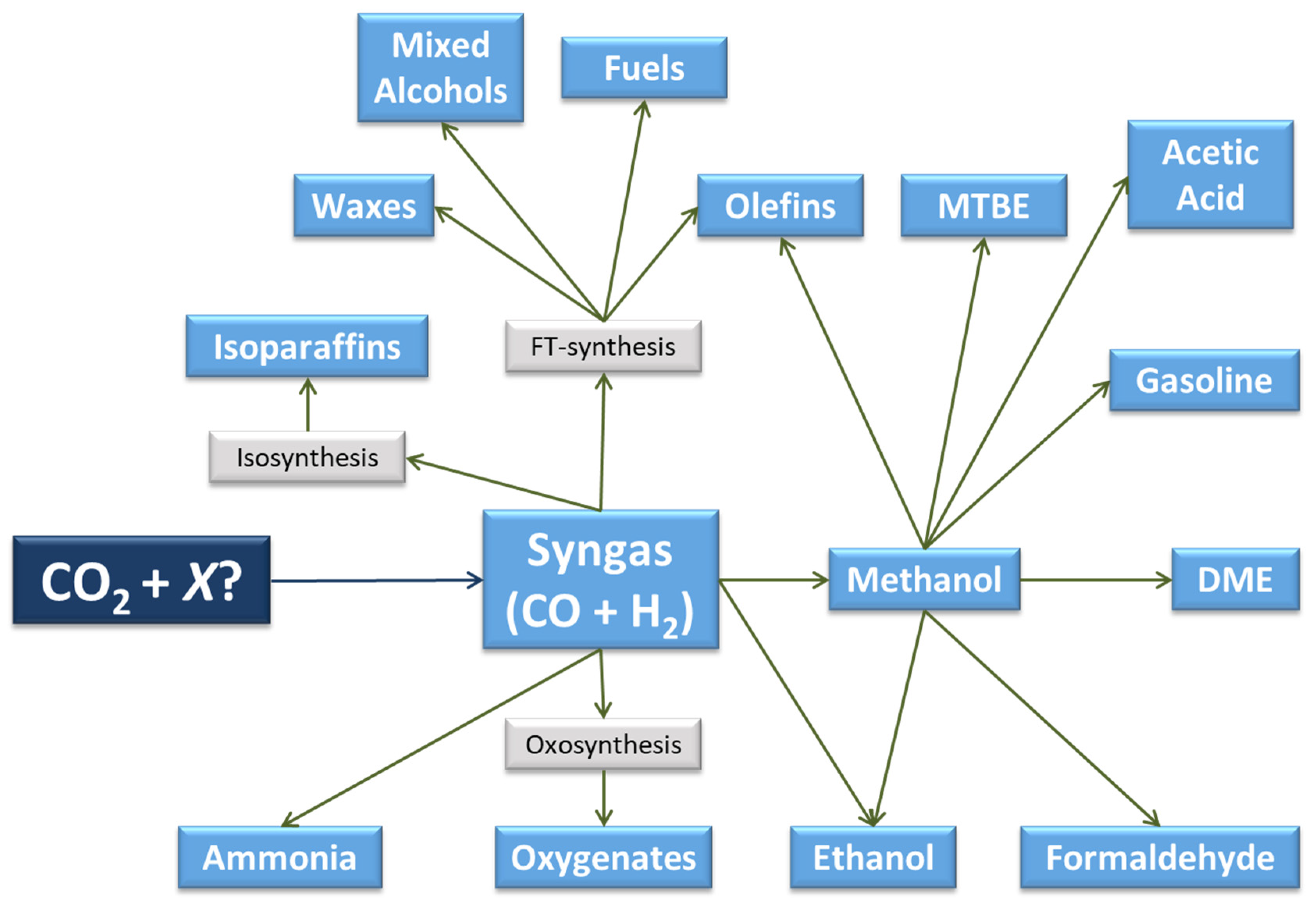
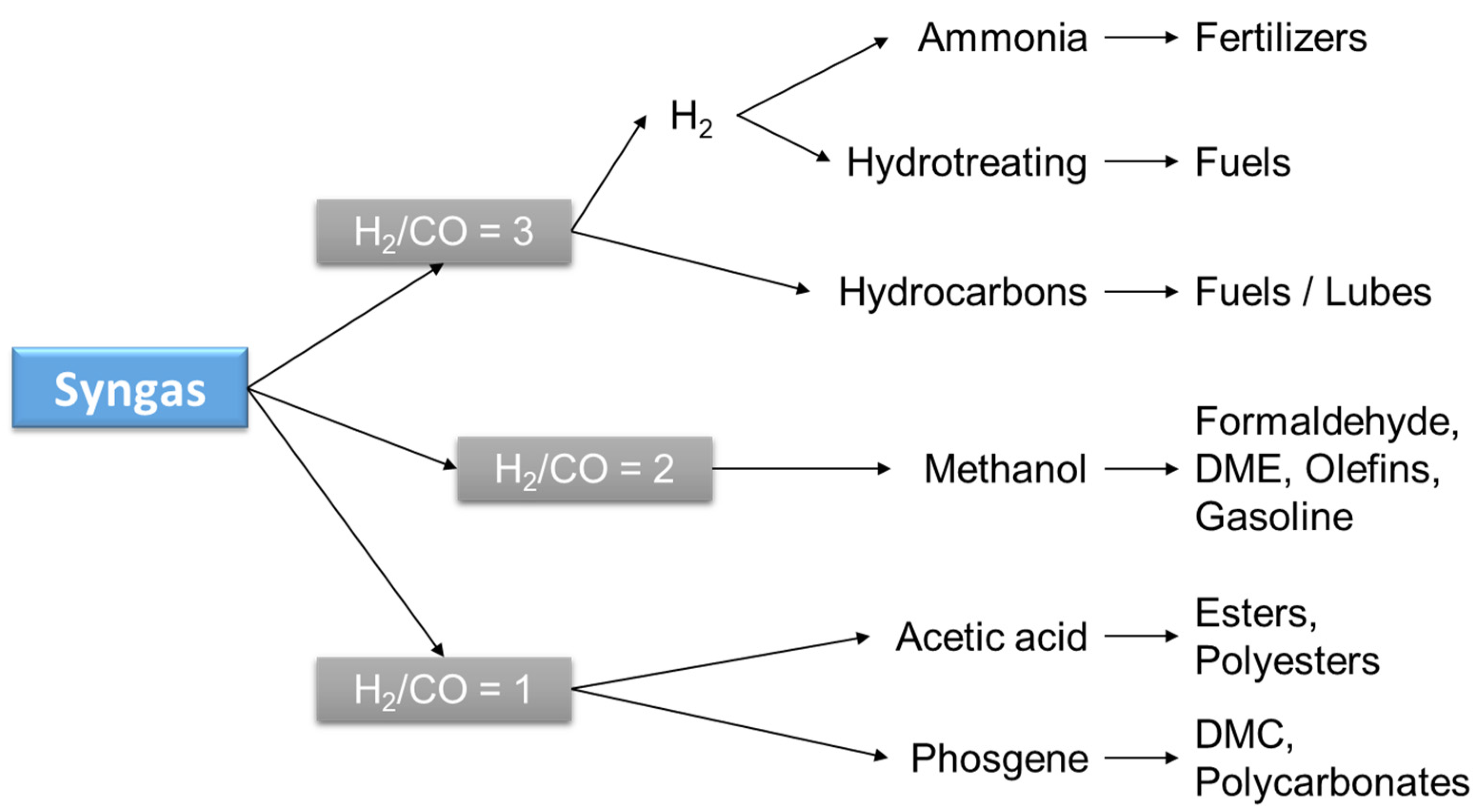
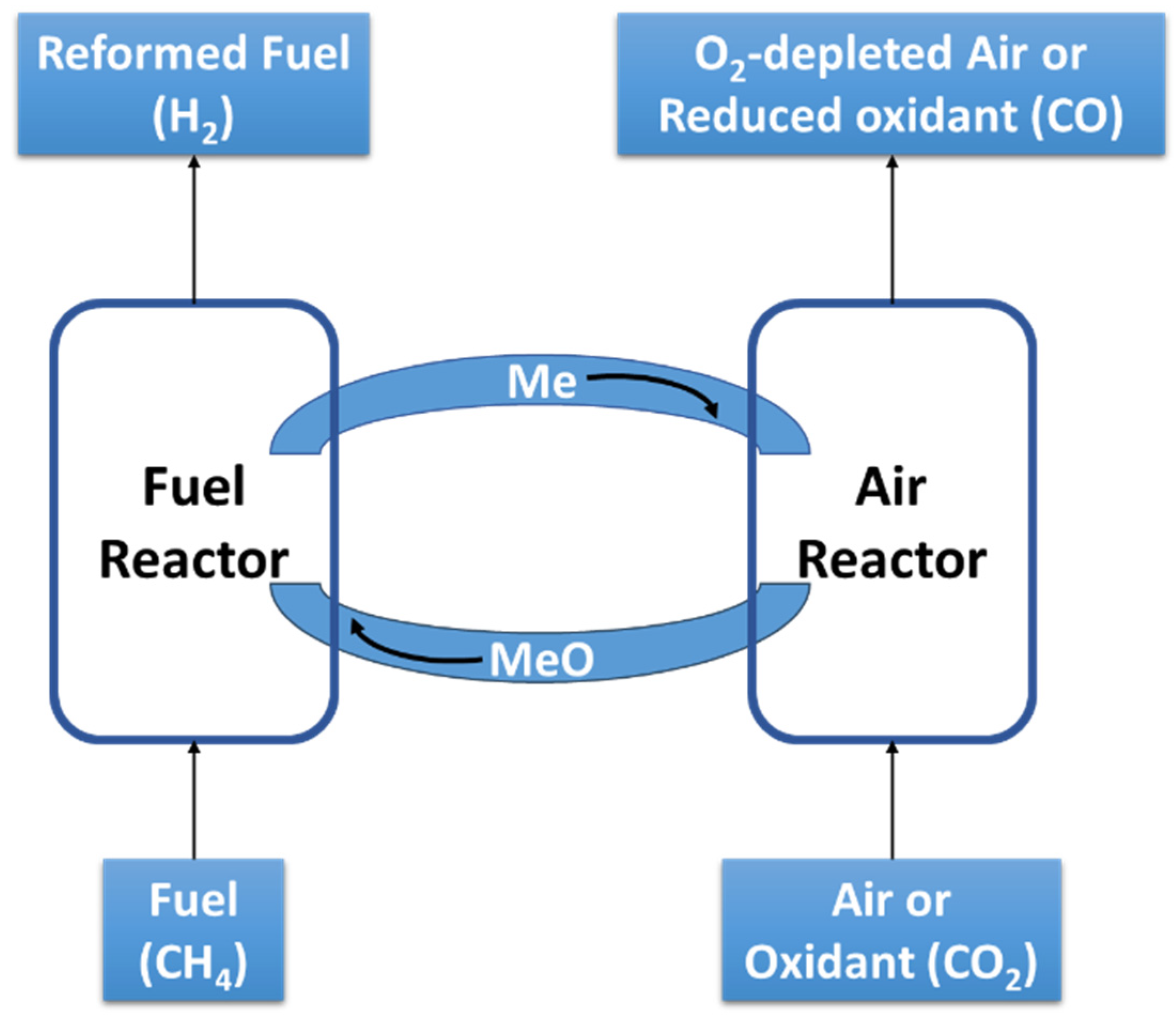
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez-Corredores, M.M. Sustainable Valorization of CO2 through Nuclear Power-to-X Pathways. Energies 2024, 17, 4977. https://doi.org/10.3390/en17194977
Ramirez-Corredores MM. Sustainable Valorization of CO2 through Nuclear Power-to-X Pathways. Energies. 2024; 17(19):4977. https://doi.org/10.3390/en17194977
Chicago/Turabian StyleRamirez-Corredores, Maria Magdalena. 2024. "Sustainable Valorization of CO2 through Nuclear Power-to-X Pathways" Energies 17, no. 19: 4977. https://doi.org/10.3390/en17194977
APA StyleRamirez-Corredores, M. M. (2024). Sustainable Valorization of CO2 through Nuclear Power-to-X Pathways. Energies, 17(19), 4977. https://doi.org/10.3390/en17194977





