Research and Application Progress of Aerogel Materials in the Field of Batteries and Supercapacitors
Abstract
:1. Introduction
2. Preparation Methods of Aerogels
2.1. Sol–Gel Method
2.2. Template Method
2.3. Self-Assembly
2.4. Electrospinning
2.5. Three-Dimensional Printing
3. The Application of Aerogels in Batteries and Supercapacitors
3.1. Used as Separators
3.1.1. Heat Insulation Protection
3.1.2. Inhibition of Shuttle Effect
3.1.3. Suppressing Lithium Dendrites
3.2. As Solid-State Electrolytes
3.3. As Electrodes
3.3.1. As the Anode
3.3.2. As the Cathode
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sam, D.K.; Sam, E.K.; Lv, X. Application of biomass-derived nitrogen-doped carbon aerogels in electrocatalysis and supercapacitors. Chem. Electro. Chem. 2020, 7, 3695–3712. [Google Scholar]
- Sarawade, P.B.; Kim, J.K.; Kim, H.K. High specific surface area teos-based aerogels with large pore volume prepared at an ambient pressure. Appl. Surf. Sci. 2007, 254, 574–579. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.Y.; Pan, D.F.; Hou, Y. Robust, Fire-Retardant, and Water-Resistantb Wood/Polyimide Composite Aerogels with a Hierarchical Pore Structure for Thermal Insulation. Gels 2023, 9, 467–479. [Google Scholar] [PubMed]
- Kistler, S.S. Coherent expanded aerogels and jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Lu, G.; Wang, X.D.; Duan, Y.Y. Effects of non-ideal structures and high temperatures on the insulation properties of aerogel-based composite materials. J. Non-Cryst. Solids 2011, 357, 3822–3829. [Google Scholar] [CrossRef]
- Cuce, E.; Cuce, P.M.; Wood, C.J. Toward aerogel based thermal superinsulation in buildings: A comprehensive review. Renew. Sust. Energ. Rev. 2014, 34, 273–299. [Google Scholar] [CrossRef]
- Meador, M.A.; Aleman, C.R.; Hanson, K.; Ramirez, N.; Vivod, S.L.; Wilmoth, N.; McCorkle, L. Polyimide aerogels with amide cross-links: A low cost alternative for mechanically strong polymer aerogels. ACS Appl. Mater. Interfaces 2015, 7, 1240–1249. [Google Scholar] [CrossRef]
- Feng, J.; Wang, X.; Jiang, Y.; Du, D.; Feng, J. Study on Thermal Conductivities of Aromatic Polyimide Aerogels. ACS Appl. Mater. Interfaces 2016, 8, 12992–12996. [Google Scholar] [CrossRef]
- Ji, X.; Zhou, Q.; Qiu, G. Preparation of monolithic silica-based aerogels with high thermal stability by ambient pressure drying. Ceram. Int. 2018, 44, 11923–11931. [Google Scholar] [CrossRef]
- He, S.; Li, Z.; Shi, X. Rapid synthesis of sodium silicate based hydrophobic silica aerogel granules with large surface area. Adv. Powder Technol. 2015, 26, 537–541. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.P.; Gustavsen, A. Aerogel insulation for building applications: A state-of-the-art review. Energ. Build. 2011, 43, 761–769. [Google Scholar] [CrossRef]
- Dorcheh, A.S.; Abbasi, M.H. Silica aerogel; synthesis, properties and characterization. J. Mater. Process. Technol. 2008, 199, 10–26. [Google Scholar] [CrossRef]
- Tokudome, Y.; Nakanishi, K.; Kanamori, K.; Fujita, K.; Akamatsu, H.; Hanada, T. Structural characterization of hierarchically porous alumina aerogel and xerogel monoliths. J. Colloid Interf. Sci. 2009, 338, 506–513. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.H.; Tian, J.; Li, H.D.; Sang, Y.H.; Yu, G.W.; Cai, H.Q.; Liu, H.; Wong, C.P.; Umar, A. Hierarchical porous carbon aerogel derived from bagasse for high performance supercapacitor electrode. Nanoscale 2014, 6, 12120–12129. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, S.; Ren, J.; Jia, Y.A.; Chen, C.M.; Komarneni, S.; Yang, D.J.; Yao, X.D. Electronic Structure Tuning in Ni3FeN/r-GO Aerogel toward Bifunctional Electrocatalyst for Overall Water Splitting. ACS Nano 2018, 12, 245–253. [Google Scholar] [CrossRef]
- Raagulan, K.; Kim, B.M.; Chai, K.Y. Recent Advancement of Electromagnetic Interference (EMI) Shielding of Two Dimensional (2D) MXene and Graphene Aerogel Composites. Nanomaterials 2020, 10, 702. [Google Scholar] [CrossRef]
- Osman, S.H.; Kamarudin, S.K.; Basri, S.; Karim, N.A. Organic aerogel as electro-catalytic support in low-temperature fuel cell. Inter. J. Energ. Res. 2022, 46, 16264–16280. [Google Scholar] [CrossRef]
- Sen, S.; Singh, A.; Bera, C.; Roy, S.; Kailasam, K. Recent developments in biomass derived cellulose aerogel materials for thermal insulation application: A review. Cellulose 2022, 29, 4805–4833. [Google Scholar] [CrossRef]
- El Kadib, A.; Bousmina, M. Chitosan Bio-Based Organic-Inorganic Hybrid Aerogel Microspheres. Chem.-Eur. J. 2012, 18, 8264–8277. [Google Scholar] [CrossRef]
- Gurav, J.L.; Jung, I.K.; Park, H.H.; Kang, E.S.; Nadargi, D.Y. Silica Aerogel: Synthesis and Applications. J. Nanomater. 2010, 2010, 409310. [Google Scholar] [CrossRef]
- Lin, J.M.; Li, G.Z.; Liu, W.; Qiu, R.X.; Wei, H.Y.; Zong, K.; Cai, X.K. A review of recent progress on the silica aerogel monoliths: Synthesis, reinforcement, and applications. J. Mater. Sci. 2021, 56, 10812–10833. [Google Scholar] [CrossRef]
- Lee, K.H.; Arshad, Z.; Dahshan, A.; Alshareef, M.; Alsulami, Q.A.; Bibi, A.; Lee, E.J.; Nawaz, M.; Zubair, U.; Javid, A. Porous Aerogel Structures as Promising Materials for Photocatalysis, Thermal Insulation Textiles, and Technical Applications: A Review. Catalysts 2023, 13, 1286. [Google Scholar] [CrossRef]
- Cao, L.Y.; Wang, C.W.; Huang, Y.X. Structure optimization of graphene aerogel-based composites and applications in batteries and supercapacitors. Chem. Eng. J. 2023, 454, 140094. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Zhou, J.P.; Pei, Z.Y.; Sui, Z.Y.; Liang, Y.; Xu, X.F.; Li, Y.P.; Li, Y.L.; Qiu, J.Y.; Chen, Q. Hierarchical Porous and Three-Dimensional MXene/SiO2 Hybrid Aerogel through a Sol-Gel Approach for Lithium-Sulfur Batteries. Molecules 2022, 27, 7073–7084. [Google Scholar] [CrossRef] [PubMed]
- Parale, V.G.; Kim, Y.; Phadtare, V.D.; Kim, T.; Choi, H.; Patil, U.M.; Park, H.H. In Situ Sol-Gel Assembly of Graphitic Carbonitride Nanosheet-Supported Colloidal Binary Metal Sulfide into Nanosandwich-Like Multifunctional 3D Macroporous Aerogel Catalysts for Asymmetric Supercapacitor and Electrocatalytic Oxygen and Hydrogen Evolution. Inter. J. Energ. Res. 2023, 2023, 6645822–6645842. [Google Scholar] [CrossRef]
- Elsehsah, K.A.A.A.; Noorden, Z.A.; Saman, N.M. Graphene aerogel electrodes: A review of synthesis methods for high-performance supercapacitors. J. Energy Storage 2024, 97, 112788–112814. [Google Scholar] [CrossRef]
- Teo, N.; Gu, Z.P.; Jana, S.C. Polyimide-based aerogel foams, via emulsion-templating. Polymer 2024, 157, 95–102. [Google Scholar] [CrossRef]
- Birdsong, B.K.; Wu, Q.; Hedenqvist, M.S.; Capezza, A.J.; Andersson, R.L.; Svagan, A.J.; Das, O.; Mensah, R.A.; Olsson, R.T. Flexible and fire-retardant silica/cellulose aerogel using bacterial cellulose nanofibrils as template material. Mater. Adv. 2024, 5, 5041–5051. [Google Scholar] [CrossRef]
- Du, Y.X.; Liu, L.B.; Xiang, Y.; Zhang, Q. Enhanced electrochemical capacitance and oil-absorbability of N-doped graphene aerogel by using amino-functionalized silica as template and doping agent. J. Power Sources 2018, 379, 240–248. [Google Scholar] [CrossRef]
- Chang, M.J.; Zhu, W.Y.; Ni, F.R.; Wang, H.; Li, X.; Yang, L.Q.; Li, H.L.; Liu, J. Controllable synthesis of phenolic resin-based carbon aerogel templated by graphene oxide for high-performance supercapacitors. Colloids Surface A 2023, 681, 132736–132743. [Google Scholar] [CrossRef]
- Chen, W.F.; Li, S.R.; Chen, C.H.; Yan, L.F. Self-Assembly and Embedding of Nanoparticles by In Situ Reduced Graphene for Preparation of a 3D Graphene/Nanoparticle Aerogel. Adv. Mater. 2011, 23, 5679. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; He, D.W.; Zhang, Y.N. Hydrothermal Self-assembly of Manganese Dioxide/Manganese Carbonate/Reduced Graphene Oxide Aerogel for Asymmetric Supercapacitors. Electrochim. Acta 2015, 164, 154–162. [Google Scholar] [CrossRef]
- Lamberti, A.; Gigot, A.; Bianco, S.; Fontana, M.; Castellino, M.; Tresso, E.; Pirri, C.F. Self-assembly of graphene aerogel on copper wire for wearable fiber-shaped supercapacitors. Carbon 2016, 105, 649–654. [Google Scholar] [CrossRef]
- Luo, L.; Chen, Z.; Ke, H.Z.; Sha, S.; Cai, G.M.; Li, D.W.; Yang, H.J.; Yang, X.W.; Zhang, R.Q.; Li, J.Q.; et al. Facile synthesis of three-dimensional MgFe2O4/graphene aerogel composites for high lithium storage performance and its application in full cell. Mater. Design 2019, 182, 108043. [Google Scholar] [CrossRef]
- Lai, H.R.; Ma, X.J.; Shang, P.H.; Chen, X.; Wang, Y.Q.; Li, J.Y.; Ge, Z. Building polyaniline: Poly(styrenesulfonate)-carbon multifunctional skeleton on aerogel like substrate for high performance gel-electrolyte-friendly fiber-shaped hollow porous electrode. Mater. Today Chem. 2024, 41, 102293. [Google Scholar] [CrossRef]
- Zhang, L.P.; Feng, G.H.; Li, X.L.; Cui, S.Z.; Ying, S.W.; Feng, X.M.; Mi, L.W.; Chen, W.H. Synergism of surface group transfer and in-situ growth of silica-aerogel induced high-performance modified polyacrylonitrile separator for lithium/sodium-ion batteries. J. Membr. Sci. 2019, 577, 137–144. [Google Scholar] [CrossRef]
- Li, V.C.F.; Mulyadi, A.; Dunn, C.K.; Deng, Y.L.; Qi, H.J. Direct Ink Write 3D Printed Cellulose Nanofiber Aerogel Structures with Highly Deformable, Shape Recoverable, and Functionalizable Properties. ACS Sustain. Chem. Eng. 2018, 6, 2011–2022. [Google Scholar] [CrossRef]
- Yan, J.; Zhi, G.; Kong, D.Z.; Wang, H.; Xu, T.T.; Zang, J.H.; Shen, W.X.; Xu, J.M.; Shi, Y.M.; Dai, S.G.; et al. 3D printed rGO/CNT microlattice aerogel for a dendrite-free sodium metal anode. J. Mater. Chem. A 2020, 8, 19843–19854. [Google Scholar] [CrossRef]
- Xue, Z.C.; Ru, Y.Y.; Wang, Z.Z.; Li, Q.; Yu, M.F.; Li, J.; Sun, H. A 3D-printed freestanding graphene aerogel composite photocathode for high-capacity and long-life photo-assisted Li-O2 batteries. Nanoscale 2023, 15, 14877–14885. [Google Scholar] [CrossRef]
- Yuan, S.J.; Fan, W.; Wang, D.; Zhang, L.S.; Miao, Y.E.; Lai, F.L.; Liu, T.X. 3D printed carbon aerogel microlattices for customizable supercapacitors with high areal capacitance. J. Mater. Chem. A 2021, 9, 423–432. [Google Scholar] [CrossRef]
- Liu, C.; Li, Q.; Xue, Z.C.; Zhang, T.Y.; Sun, H. Cathode material of non-aqueous lithium-oxygen battery based on 3D-printed graphene aerogel. Electron. Comp. Mater. 2022, 41, 908–915. [Google Scholar]
- Liu, X.Y.; Ma, W.L.; Yang, T.Y.; Qiu, Z.R.; Wang, J.B.; Li, Y.H.; Wang, Y.; Huang, Y. Multilevel Heterogeneous Interfaces Enhanced Polarization Loss of 3D Printed Graphene/NiCoO2/Selenides Aerogels for Boosting Electromagnetic Energy Dissipation. ACS Nano 2024, 18, 10184–10195. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z.M. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, M.Y.; Lin, C.E.; Zhu, B.K. Progress in polymeric separators for lithium ion batteries. RSC Adv. 2015, 5, 89848–89860. [Google Scholar] [CrossRef]
- Feng, G.H.; Li, Z.H.; Mi, L.W.; Zheng, J.Y.; Feng, X.M.; Chen, W.H.; Li, Z.H. Separator induced enhanced safety and low polarization for lithium-ion batteries. J. Power Sources 2018, 376, 177–183. [Google Scholar] [CrossRef]
- Kim, G.; Kim, J.; Jeong, J.; Lee, D.; Kim, M.; Lee, S.; Kim, S.; Lee, H.; Han, H. High Temperature Applicable Separator by Using Polyimide Aerogel/Polyethylene Double-Layer Composite Membrane for High-Safety Lithium Ion Battery. Int. J. Electrochem. Sci. 2019, 14, 7133–7148. [Google Scholar] [CrossRef]
- Liu, M.C.; Chen, H.J.; Wu, G.; Wang, X.L.; Wang, Y.H. Multifunctional robust aerogel separator towards high-temperature, large-rate, long-cycle lithium-ion batteries. Chin. Chem. Lett. 2023, 34, 107546. [Google Scholar] [CrossRef]
- Deng, Y.R.; Pan, Y.L.; Zhang, Z.X.; Fu, Y.Y.; Gong, L.L.; Liu, C.Y.; Yang, J.Z.; Zhang, H.P.; Cheng, X.D. Novel Thermotolerant and Flexible Polyimide Aerogel Separator Achieving Advanced Lithium-Ion Batteries. Adv. Funct. Mater. 2021, 32, 2106176–2106185. [Google Scholar] [CrossRef]
- Yuan, H.D.; Chen, X.L.; Zhou, G.M.; Zhang, W.K.; Luo, J.M.; Huang, H.; Gan, Y.P.; Liang, C.; Xia, Y.; Zhang, J.; et al. Efficient Activation of Li2S by Transition Metal Phosphides Nanoparticles for Highly Stable Lithium-Sulfur Batteries. ACS Energy Lett. 2017, 2, 1711–1719. [Google Scholar] [CrossRef]
- Li, S.Q.; Leng, D.; Li, W.Y.; Qie, L.; Dong, Z.H.; Cheng, Z.Q.; Fan, Z.Y. Recent progress in developing Li2S cathodes for Li-S batteries. Energy Storage Mater. 2020, 27, 279–296. [Google Scholar] [CrossRef]
- Meng, Q.H.; Jin, Q.; Wang, X.Y.; Lv, W.B.; Ma, X.Z.; Li, L.; Wu, L.L.; Gao, H.; Zhu, C.C.; Zhang, X.T. 3D Ti3C2Tx aerogel-modified separators for high-performance Li-S batteries. J. Alloys Compd. 2020, 816, 153155. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Gong, L.L.; Pan, Y.L.; Deng, Y.R.; Zhou, Y.; Cheng, X.D.; Zhang, H.P. Coral-like interconnected carbon aerogel modified separator for advanced lithium-sulfur batteries. Electrochim. Acta 2020, 354, 136637–136645. [Google Scholar] [CrossRef]
- Tan, S.Y.; Wu, Y.F.; Kan, S.T.; Bu, M.M.; Liu, Y.; Yang, L.S.; Yang, Y.H.; Liu, H.T. A combination of MnO2-decorated graphene aerogel modified separator and I/N codoped graphene aerogel sulfur host to synergistically promote LieS battery performance. Electrochim. Acta 2020, 348, 136173–136180. [Google Scholar] [CrossRef]
- Liu, Z.E.; Hu, Z.W.; Jiang, X.A.; Zhang, Y.; Wang, X.W.; Zhang, S.G. Multi-functional ZnS quantum Dots/Graphene aerogel modified separator for high performance lithium-sulfur batteries. Electrochim. Acta 2022, 422, 140496–140504. [Google Scholar] [CrossRef]
- Ding, L.Y.; Yue, X.Y.; Zhang, X.H.; Chen, Y.M.; Liu, J.J.; Shi, Z.Q.; Wang, Z.Y.; Yan, X.Z.; Liang, Z. A polyimine aerogel separator with electron cloud design to boost Li-ion transport for stable Li metal batteries. Proc. Natl. Acad. Sci. USA 2023, 120, 2314264120. [Google Scholar] [CrossRef]
- Sheng, L.; Li, Z.L.; Hsueh, C.H.; Liu, L.; Wang, J.L.; Tang, Y.P.; Wang, J.; Xu, H.; He, X.M. Suppression of lithium dendrite by aramid nanofibrous aerogel separator. J. Power Sources 2021, 515, 230608–230614. [Google Scholar] [CrossRef]
- Yin, F.; Jin, Q.; Zhang, X.T.; Wu, L.L. Design of a 3D CNT/Ti3C2Tx aerogel-modified separator for Li-S batteries to eliminate both the shuttle effect and slow redox kinetics of polysulfides. New Carbon Mater. 2022, 37, 724–731. [Google Scholar] [CrossRef]
- Su, L.W.; Zhu, Y.J.; Liu, L.H.; Zhan, X.Y.; Wu, H.; Chen, H.; Shen, C.Q.; Wang, L.B. Highly porous CMC-Li aerogel frameworks supported polymer solid electrolytes for lithium metal batteries. Electrochem. Commun. 2022, 140, 107336–107339. [Google Scholar] [CrossRef]
- Da, X.Y.; Chen, J.; Qin, Y.Y.; Zhao, J.Y.; Jia, X.; Zhao, Y.J.; Deng, X.T.; Li, Y.N.; Gao, N.; Su, Y.Q.; et al. CO2-Assisted Induced Self-Assembled Aramid Nanofiber Aerogel Composite Solid Polymer Electrolyte for All-Solid-State Lithium-Metal Batteries. Adv. Energy Mater. 2024, 14, 2303527–2303539. [Google Scholar] [CrossRef]
- Wang, D.; Li, Z.Y.; Yang, L.; Zhang, J.; Wei, Y.H.; Feng, Q.; Wei, Q.F. Hydrogel electrolyte based on sodium polyacrylate/KOH hydrogel reinforced with bacterial cellulose aerogel for flexible supercapacitors. Chem. Eng. J. 2023, 454, 140090–140099. [Google Scholar] [CrossRef]
- Zhou, X.S.; Lv, P.Y.; Li, M.X.; Xu, J.; Cheng, G.G.; Yuan, N.Y.; Ding, J.N. Graphene Oxide Aerogel Foam Constructed All-Solid Electrolyte Membranes for Lithium Batteries. Langmuir 2022, 38, 32573264. [Google Scholar] [CrossRef] [PubMed]
- Vareda, J.P.; Fonseca, A.C.; Ribeiro, A.C.F.; Pontinha, A.D.R. Silica-Poly(Vinyl Alcohol) Composite Aerogel: A Promising Electrolyte for Solid-State Sodium Batteries. Gels 2024, 10, 293–307. [Google Scholar] [CrossRef]
- Liu, L.; Cai, Y.H.; Zhao, Z.K.; Ma, C.W.; Li, C.L.; Mu, D.B. A succinonitrile-infiltrated silica aerogel synergistically-reinforced hybrid solid electrolyte for durable solid-state lithium metal batteries. Mater. Chem. Front. 2022, 6, 430–439. [Google Scholar] [CrossRef]
- Li, M.R.; Qi, S.G.; Li, S.L.; Du, L. Realizing Scalable Nano-SiO2-Aerogel-Reinforced Composite Polymer Electrolytes with High Ionic Conductivity via Rheology-Tuning UV Polymerization. Molecules 2023, 28, 756–765. [Google Scholar] [CrossRef]
- Nargatti, K.I.; Subhedar, A.R.; Ahankari, S.S.; Grace, A.N.; Dufresne, A.; Subhedar, A.R. Nanocellulose-basedaerogel electrodesfor supercapacitors: A review. Carbohyd. Polym. 2022, 297, 120039. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.L.; Ge, Y.J.; Qing, P.; Jin, Y.K.; Chen, L.B.; Mei, L. Lightweight 3D Lithiophilic Graphene Aerogel Current Collectors for Lithium Metal Anodes. Materials 2024, 17, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Nandy, S.; Fortunato, E.; Martins, R. Green economy and waste management: An inevitable plan for materials science. Prog. Nat. Sci.-Mater. 2022, 32, 1–9. [Google Scholar] [CrossRef]
- Nandy, S.; Goswami, S.; Marques, A.; Gaspar, D.; Grey, P.; Cunha, I.; Nunes, D.; Pimentel, A.; Igreja, R.; Barquinha, P.; et al. Cellulose: A Contribution for the Zero e-Waste Challenge. Adv. Mater. Technol. 2021, 6, 2000994. [Google Scholar] [CrossRef]
- Goswami, S.; Dillip, G.R.; Nandy, S.; Banerjee, A.N.; Pimentel, A.; Joo, S.W.; Martins, R.; Fortunato, E. Biowaste-derived carbon black applied to polyaniline-based high-performance supercapacitor microelectrodes: Sustainable materials for renewable energy applications. Electrochim. Acta 2019, 316, 202–218. [Google Scholar] [CrossRef]
- Pham, C.D.; Tran, K.D.; Truong, T.M.; Le, P.K. Cellulose-derived carbon aerogel from rice straw for high-performance lithium-ion battery anodes. Biomass Convers. Bior. 2024, 1–9. [Google Scholar] [CrossRef]
- Liao, L.X.; Ma, T.; Fang, T.; Zhang, J.Y.; Chai, B.; Zhu, L.; Ding, J.Q.; Kou, H.Z.; Xu, Y.L.; Hou, Y.J.; et al. Carbon aerogel capsulated cobalt oxides with nanomicro-hierarchical architectures as anodes for lithium-ion batteries. New J. Chem. 2024, 48, 9486–9497. [Google Scholar] [CrossRef]
- Ma, Y.; Gu, Y.T.; He, Y.; Wei, L.; Lian, Y.B.; Pan, W.Y.; Li, X.J.; Su, Y.H.; Peng, Y.; Deng, Z.; et al. Fast-charging and dendrite-free lithium metal anode enabled by partial lithiation of graphene aerogel. Nano Res. 2022, 15, 9792–9799. [Google Scholar] [CrossRef]
- Wang, S.G.; Xie, Y.; Hou, Y.L.; Zeng, R.; Qin, T.; Guan, H.B.; Zhao, D.L. Graphene aerogel encapsulated Co3O4 open-ended microcage anode with enhanced performance for lithium-ion batteries. Appl. Surf. Sci. 2022, 605, 154759–154769. [Google Scholar] [CrossRef]
- Li, Z.Y.; Zhao, D.H.; Fu, H.; Wang, A.; Cao, H.M.; Zong, P. Conductive copper ion-reinforced sodium alginate aerogel based free-standing Si anode for Li-ion storage. Solid State Ion. 2024, 409, 116529–116538. [Google Scholar] [CrossRef]
- Carvalho, J.T.; Correia, A.; Cordeiro, N.J.A.; Coelho, J.; Lourenço, S.A.; Fortunato, E.; Martins, R.; Pereira, L. MoS2 decorated carbon fiber yarn hybrids for the development of freestanding flexible supercapacitors. NPJ 2D Mater. Appl. 2024, 8, 20–28. [Google Scholar] [CrossRef]
- Versaci, D.; Canale, I.; Goswami, S.; Amici, J.; Francia, C.; Fortunato, E.; Martins, R.; Pereira, L.; Bodoardo, S. Molybdenum disulfide/polyaniline interlayer for lithium polysulphide trapping in lithium-sulphur batteries. J. Power Sources 2022, 521, 230945. [Google Scholar] [CrossRef]
- Wu, L.Q.; Feng, L.; Mao, X.X.; Niu, J.Y.; Xin, W.H.; Wang, D. Construction of porous MoS2-Mo2C@C aerogel for use as superior lithium-ion battery anode. J. Energy Storage 2023, 70, 108011–108020. [Google Scholar] [CrossRef]
- Wen, W.X.; Xie, Y.; Wang, H.Y.; Zhang, B.H.; Hou, Y.L.; Chen, J.Z.; Zhao, D.L. Graphene aerogel encapsulated Co3O4 microcubes derived from prussian blue analog as integrated anode with enhanced Li-ion storage properties. Ceram. Int. 2023, 49, 11788–11795. [Google Scholar] [CrossRef]
- Cao, M.J.; Feng, Y.; Wang, D.Y.; Gu, X.L.; Yao, J.F. Bacterial cellulose-derived porous carbon aerogel containing FeS as the self-supporting anode for high-efficiency lithium-ion storage. J. Energy Storage 2024, 95, 112645–112652. [Google Scholar] [CrossRef]
- Tian, H.; Xu, Z.Z.; Liu, K.; Wang, D.; Ren, L.L.; Wei, Y.M.; Chen, L.H.; Chen, Y.Y.; Liu, S.H.; Yang, H.X. Heterogeneous bimetallic selenides encapsulated within graphene aerogel as advanced anodes for sodium ion batteries. J. Colloid Interf. Sci. 2024, 670, 152–162. [Google Scholar] [CrossRef]
- Tian, H.; Jia, Y.D.; Wang, Y.T.; Qiao, Y.W.; Ji, P.G.; Zhang, C.W.; Gou, H.Y.; Wang, G.K. Graphene Aerogel Armed 3D Ordered Mesoporous Carbonas Versatile Anode Platforms for Sodium-Ion Storage Devices. Adv. Funct. Mater. 2024, 2406827. [Google Scholar] [CrossRef]
- Wang, Z.X.; Huang, Z.X.; Wang, H.; Li, W.D.; Wang, B.Y.; Xu, J.M.; Xu, T.T.; Zang, J.H.; Kong, D.Z.; Li, X.J.; et al. 3D-Printed Sodiophilic V2CTx/rGO-CNT MXene Microgrid Aerogel for Stable Na Metal Anode with High Areal Capacity. ACS Nano 2022, 16, 9105–9116. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Wang, H.; Li, W.D.; Tian, B.F.; Xu, T.T.; Kong, D.Z.; Huang, S.Z.; Liu, K.K.; Li, X.J.; Yang, H.Y.; et al. A simple and effective host for sodium metal anode: A 3D-printed high pyrrolic-N doped graphene microlattice aerogel. J. Mater. Chem. A 2022, 10, 16842–16852. [Google Scholar] [CrossRef]
- Pan, D.H.; Yang, H.Y.; Liu, Y.Y.; Wang, H.; Xu, T.T.; Kong, D.Z.; Yao, J.J.; Shi, Y.M.; Li, X.J.; Yang, H.Y.; et al. Ultrahigh areal capacity and long cycling stability of sodium metal anodes boosted using a 3D-printed sodiophilic MXene/rGO microlattice aerogel. Nanoscale 2023, 15, 17482–17493. [Google Scholar] [CrossRef]
- Liu, J.J.; Cheng, M.; Liu, Q.Q.; Wang, R.R.; Wei, Y.H.; Ma, W.J.; Hu, J.; Wei, T.; Ling, Y.; Liu, B.; et al. Bimetallic Bi-Sn micro-/nanospheres@cellulose nanocrystal derived carbon aerogel composite anode for high-performance Mg-ion batteries. Compos. Commun. 2023, 39, 101553–101558. [Google Scholar] [CrossRef]
- Cheng, M.; Liu, J.J.; Wang, X.M.; Li, Y.B.; Xia, W.T.; Liu, Q.Q.; Hu, J.; Wei, T.; Ling, Y.; Liu, B.; et al. In-situ synthesis of Bi nanospheres anchored in 3D interconnected cellulose nanocrystal derived carbon aerogel as anode for high-performance Mg-ion batteries. Chem. Eng. J. 2023, 451, 138824–138834. [Google Scholar] [CrossRef]
- Chen, Z.H.; Zhao, J.; Lv, W.J.; Lv, Z.Z.; Li, G.X.; Guo, C.S.; Liu, T.T.; Meng, Z.Y.; Tang, J.G.; Hui, J.S. 3D MXene/PVA Aerogel Enabling a Dendrite-Free Zn Metal Anode. ACS Appl. Mater. Interfaces 2024, 16, 35104–35113. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, G.Y.; Misra, S.; Nelson, J.; Toney, M.F.; Cui, Y. High-Capacity Micrometer-Sized Li2S Particles as Cathode Materials for Advanced Rechargeable Lithium-Ion Batteries. J. Am. Chem. Soc. 2012, 134, 15387–15394. [Google Scholar] [CrossRef]
- Cai, K.P.; Song, M.K.; Cairns, E.J.; Zhang, Y.G. Nanostructured Li2S-C Composites as Cathode Material for High-Energy Lithium/Sulfur Batteries. Nano Lett. 2012, 12, 6474–6479. [Google Scholar] [CrossRef]
- He, J.R.; Chen, Y.F.; Lv, W.G.; Wen, K.C.; Xu, C.; Zhang, W.L.; Li, Y.R.; Qin, W.; He, W.D. From Metal Organic Framework to Li2S@C Co N Nanoporous Architecture: A High Capacity Cathode for Lithium Sulfur Batteries. ACS Nano 2016, 10, 10981–10987. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, J.B.; You, J.J.; Chi, X.Y.; Du, C.Q.; Cheng, S.Q. rGO@S Aerogel Cathode for High Performance Lithium-Sulfur Batteries. Int. J. Electrochem. Sci. 2022, 17, 220251–220261. [Google Scholar] [CrossRef]
- Hu, J.L.; Zhang, L.Z. Highly conductive freestanding sulfur cathode based on low-defect graphene-PEDOT: PSS aerogel for Li-S batteries. Mater. Lett. 2024, 354, 135435–135438. [Google Scholar] [CrossRef]
- Lin, X.Y.; Li, W.Y.; Pan, X.; Wang, S.; Fan, Z.Y. Electrocatalytic and Conductive Vanadium Oxide on Carbonized Bacterial Cellulose Aerogel for the Sulfur Cathode in Li-S Batteries. Batteries 2023, 9, 14–26. [Google Scholar] [CrossRef]
- Shan, X.Y.; Guo, Z.X.; Zhang, C.; Wang, W.Q.; Zhao, L.J. Nickel aerogel @ ultra-thin NiCo-LDH nanosheets integrated freestanding film as a collaborative adsorption and accelerated conversion cathode to improve the rate performance of lithium sulfur batteries. Chem. Eng. J. 2024, 488, 151105–151115. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Duan, H.; Xie, S.Y.; Dai, R.Y.; Rong, J.H.; Kang, F.Y.; Dong, L.B. Aerogel-structured MnO2 cathode assembled by defect-rich ultrathin nanosheets for zinc-ion batteries. Chem. Eng. J. 2024, 441, 136008–136015. [Google Scholar] [CrossRef]
- Xiang, Y.S.; Chen, F.Y.; Tang, B.; Zhou, M.Q.; Li, X.L.; Wang, R.H. Novel Zn0.079V2O5⋅0.53H2O/Graphene aerogel as high-rate and long-life cathode materials of aqueous zinc-ion batteries. J. Colloid Interf. Sci. 2024, 664, 1002–1011. [Google Scholar] [CrossRef]
- Xing, X.H.; Wang, X.; Wang, W.Y.; Yang, C.; Wang, H.Z. Hierarchically porous N-doped carbon nanosheet aerogel cathodes for Zn-ion hybrid supercapacitors with superhigh energy density. J. Energy Storage 2023, 68, 107822–107830. [Google Scholar] [CrossRef]
- Yao, J.J.; Li, F.Z.; Zhou, R.Y.; Guo, C.C.; Liu, X.R.; Zhu, Y.R. Phosphorous-doped carbon nanotube/reduced graphene oxide aerogel cathode enabled by pseudocapacitance for high energy and power zinc-ion hybrid capacitors. Chin. Chem. Lett. 2024, 35, 108354–108360. [Google Scholar] [CrossRef]
- Gültekin, S.Y.; Güler, A.; Kuruahmet, D.; Güngör, H.; Singil, M.M.; Uzun, E.; Akbulut, H.; Güler, M.O. Graphene aerogel-supported Na3V2(PO4)3/C cathodes for sodium-ion batteries. Diam. Relat. Mater. 2023, 139, 110399–110412. [Google Scholar] [CrossRef]
- Zhang, H.W.; Shen, L.X.; Geng, X.D.; Zhang, J.X.; Jiang, Y.; Ma, H.T.; Liu, Q.L.; Yang, K.; Ma, J.L.; Zhu, N. MXene-rGO aerogel assisted Na3.5MnTi(PO4) cathode for high-performance sodium-ion batteries. Chem. Eng. J. 2023, 466, 143132–143140. [Google Scholar] [CrossRef]


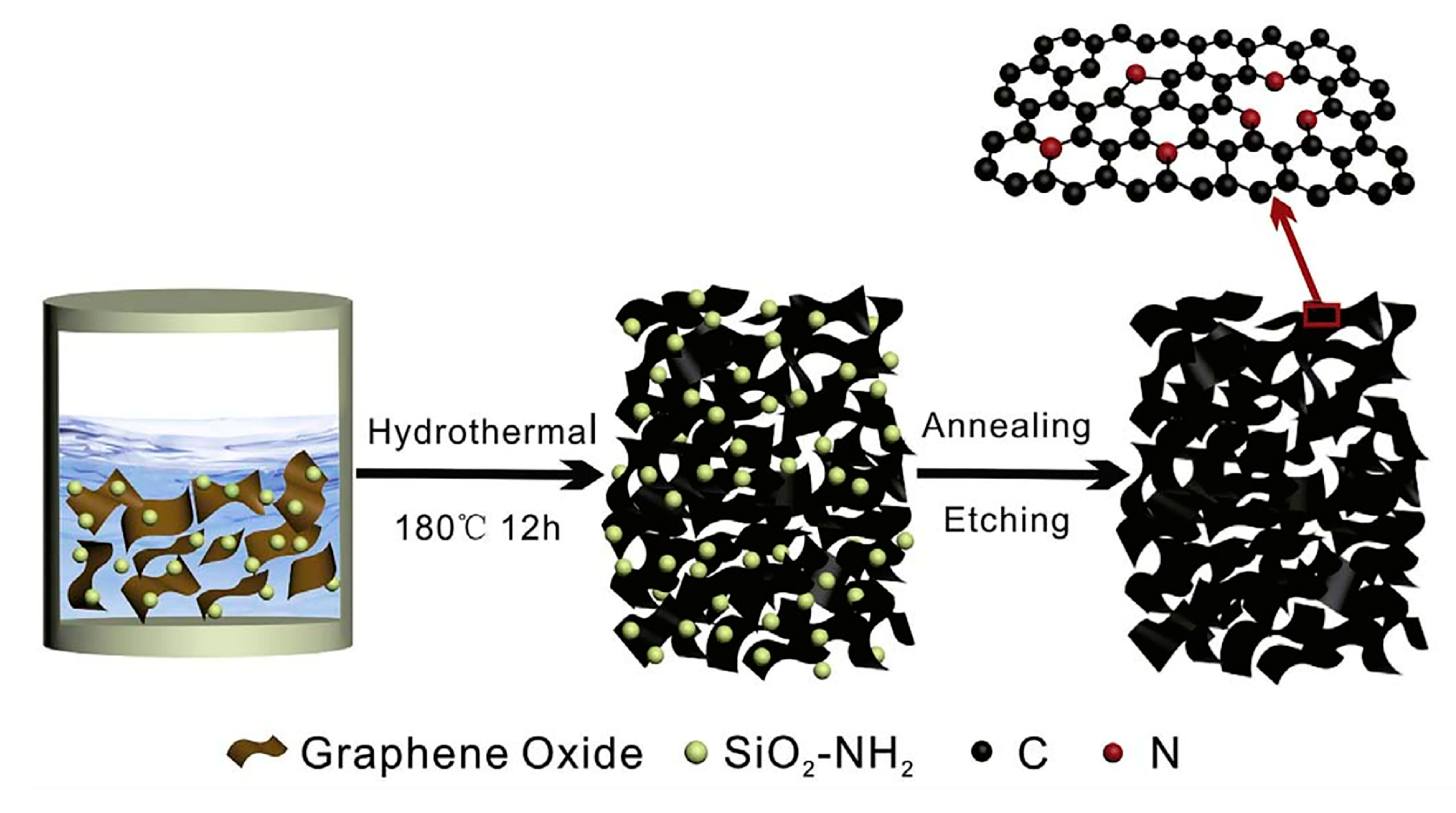
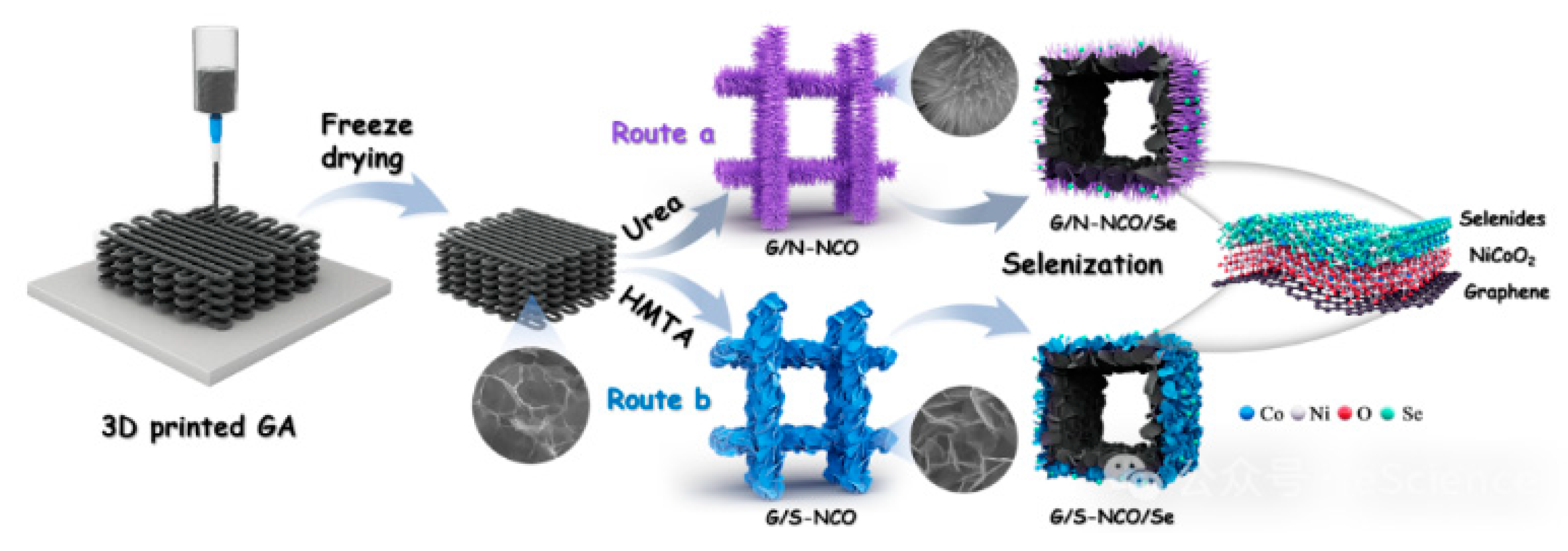
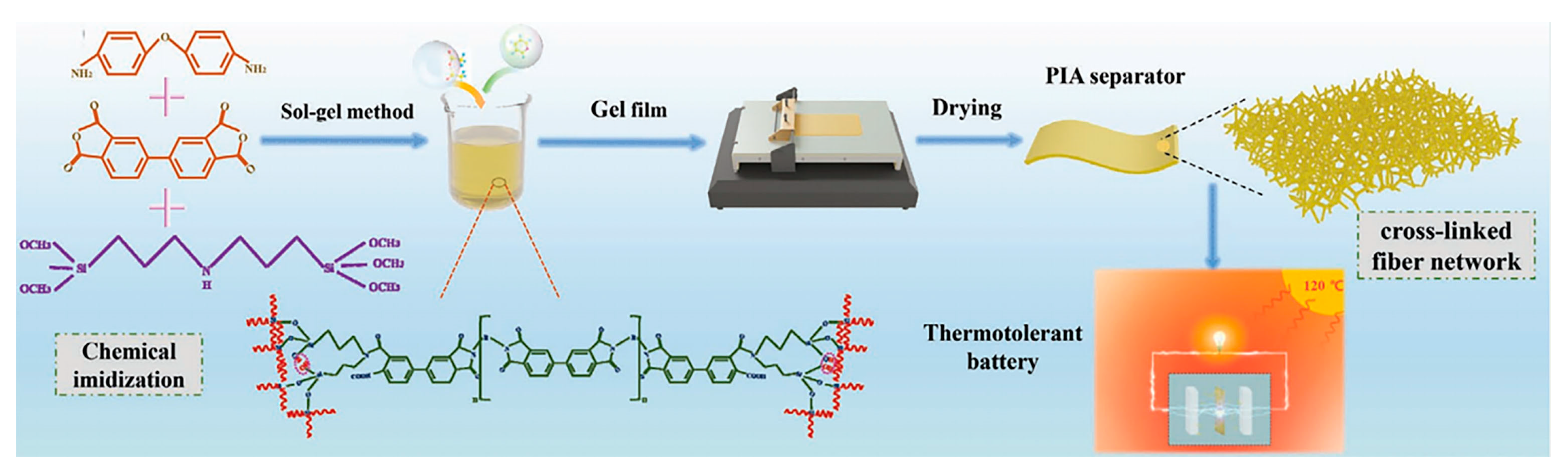

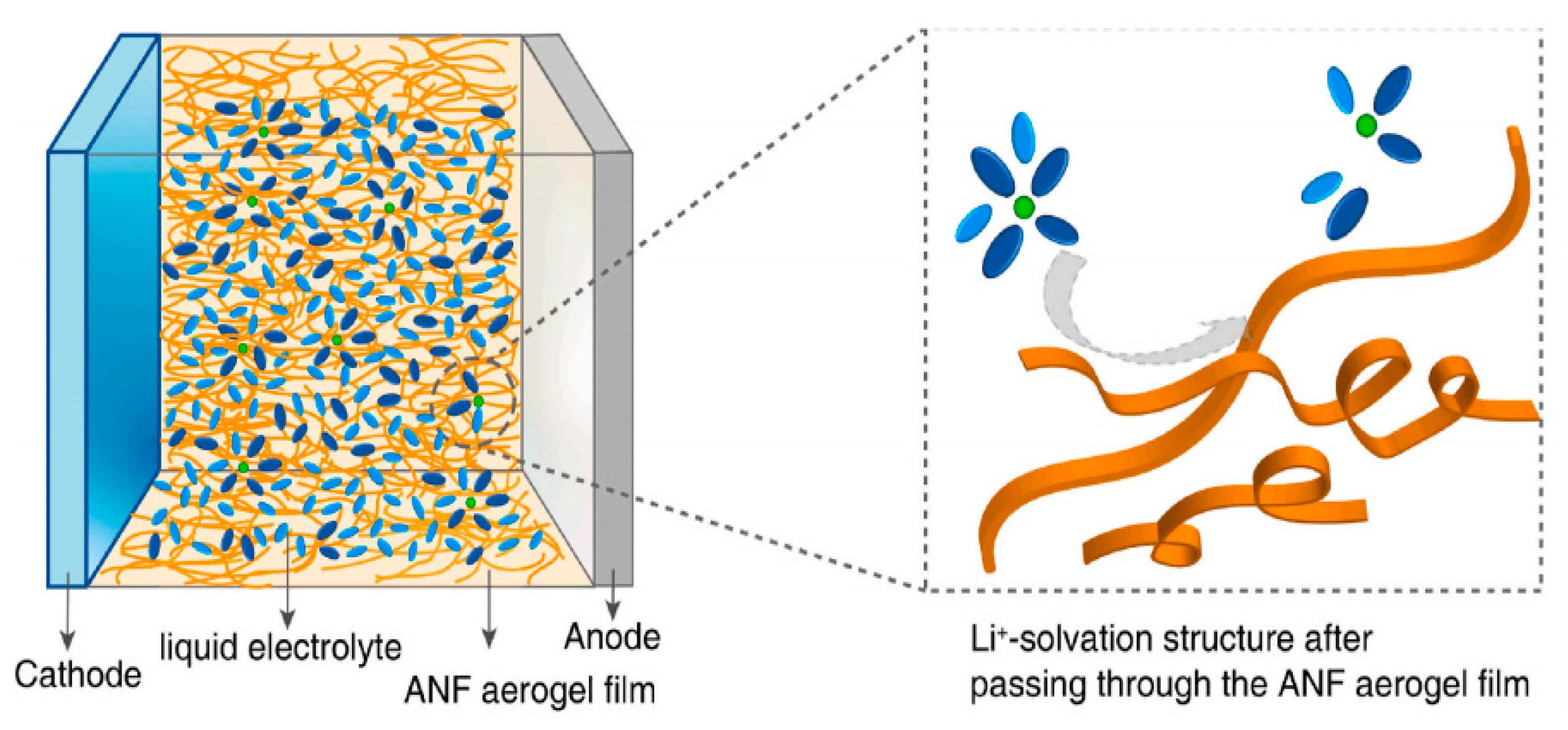
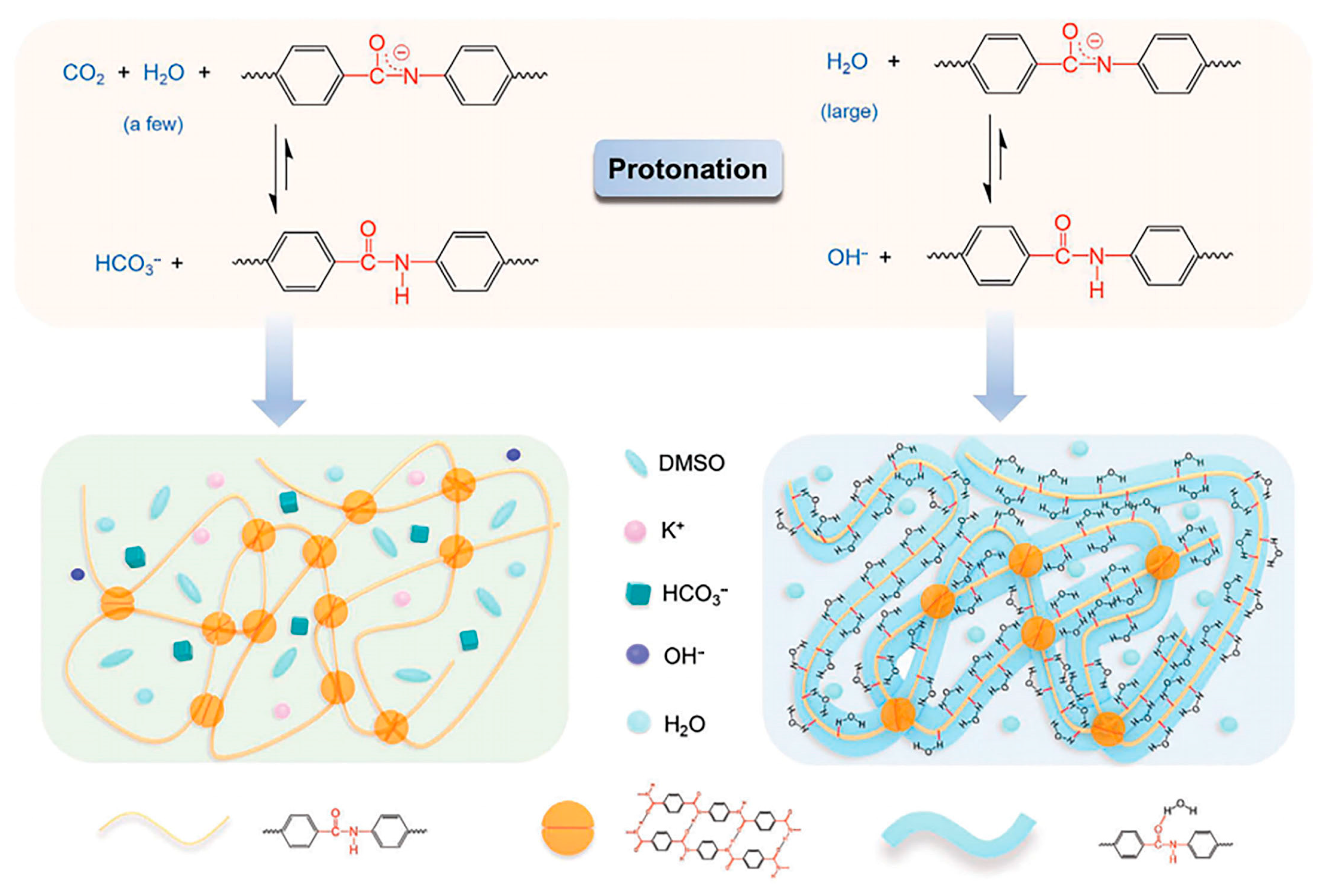
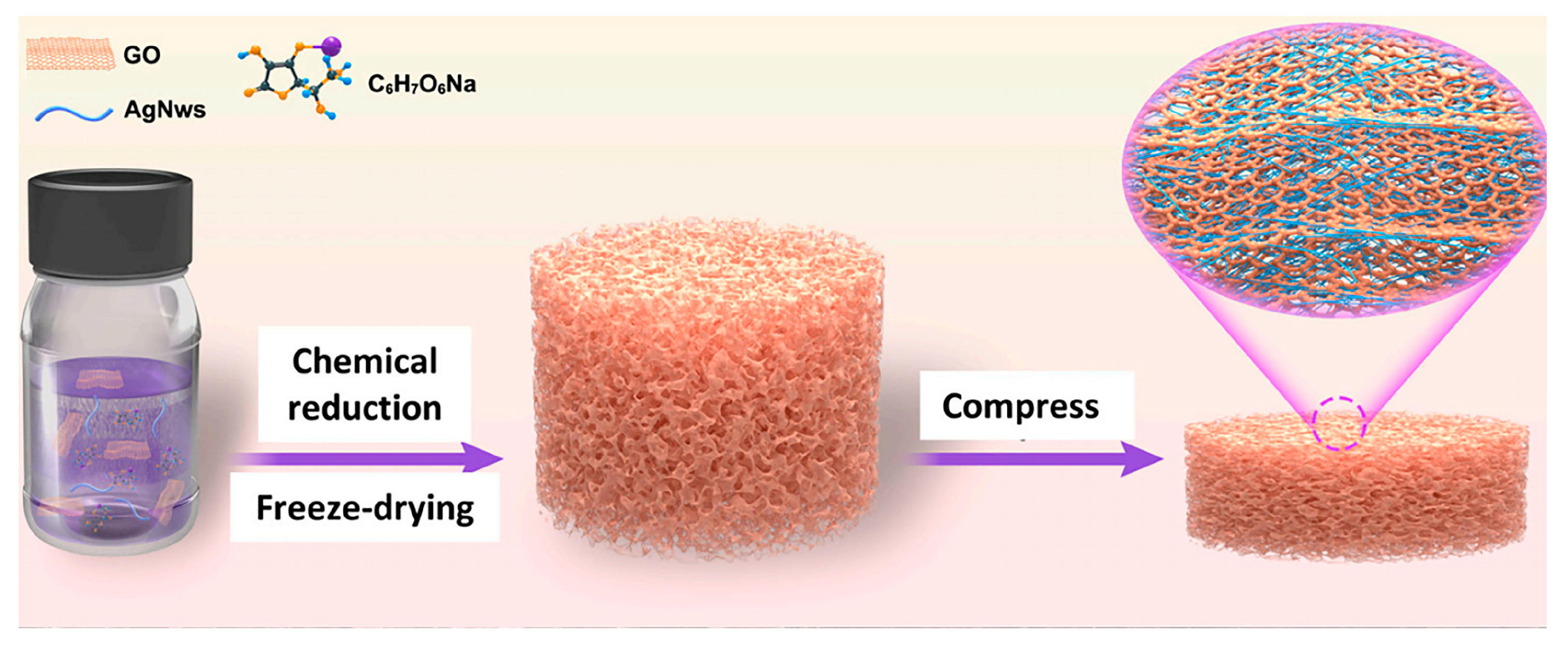
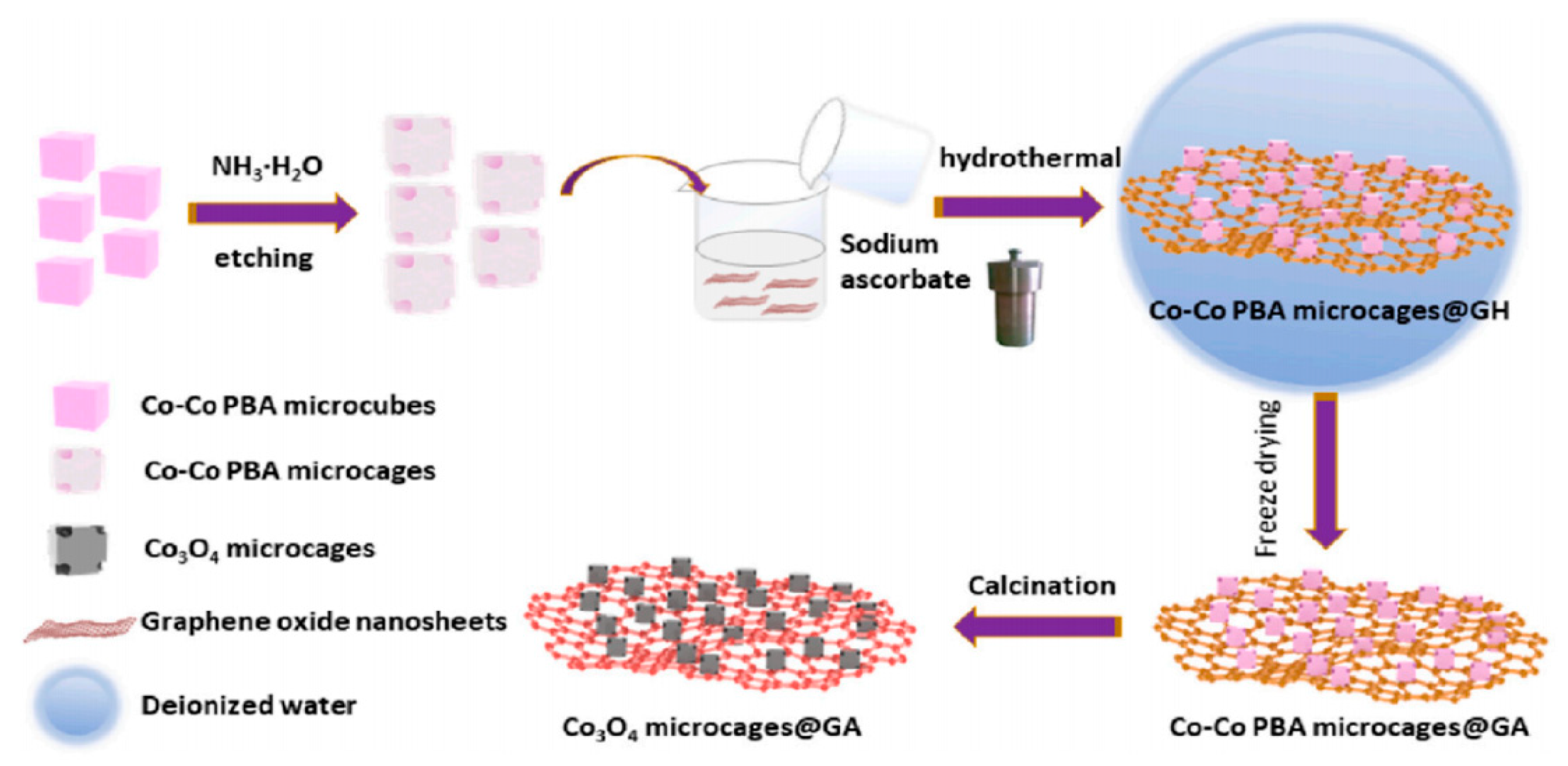
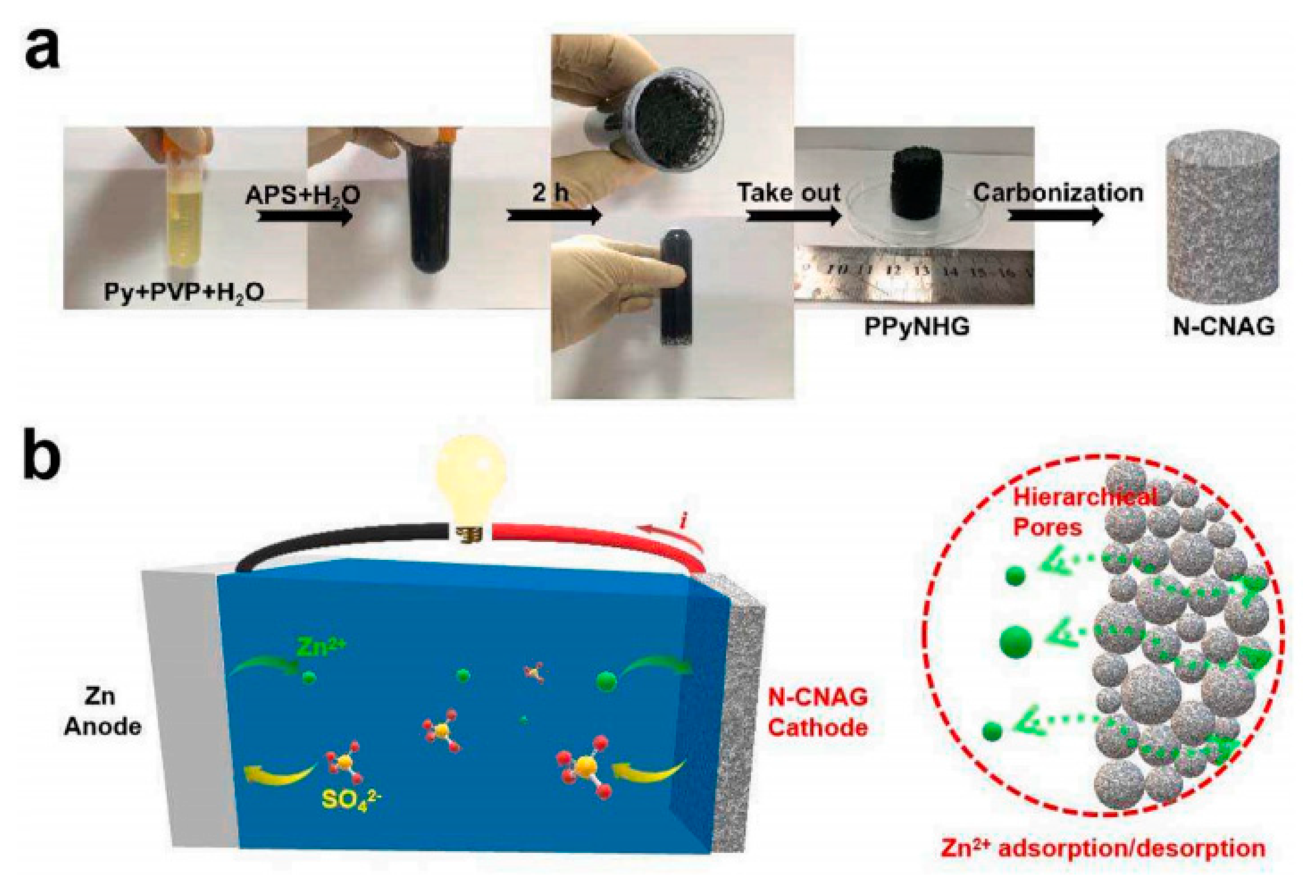
| Separator Material | Capacity | Cycle | Rate | Ref. |
|---|---|---|---|---|
| ANFs | 102 mA h/g | 600 | 5 C | [48] |
| PIA | 118 mA h/g | 1000 | 1 C | [49] |
| Ti3C2Tx Aerogel | 423 mA h/g | 1500 | 1 C | [52] |
| MG@Sep | 902 mA h/g | 200 | 0.2 C | [54] |
| Graphene Aerogel/Polypropylene | 590 mA h/g | 200 | 0.5 C | [55] |
| PIA | 86.1 mA h/g | 200 | 1 C | [56] |
| CNT/Ti3C2Tx | 582.8 mA h/g | 800 | 0.5 C | [58] |
| Aerogel as Electrode Material | Capacity | Cycle | Rate | Ref. |
|---|---|---|---|---|
| Co3O4@NCA-1 | 600.2 mA h/g | 200 | 0.1 A/g | [69] |
| Co3O4 microcages@GA | 1439 mA h/g | 200 | 1 A/g | [71] |
| Si@CNTs | 835.6 mA h/g | 100 | 0.1 A/g | [72] |
| MoS2-Mo2C@C | 375.1 mA h/g | 3000 | 10 m A/g | [73] |
| FeS@C/CBC | 669 mA h/g | 1000 | 1 m A/g | [74] |
| Co3O4@GA | 1234.9 mA h/g | 200 | 1 A/g | [75] |
| MoSe2-Cu1.82Se@GA | 444.8 mA h/g | 1000 | 1 A/g | [76] |
| FeSe2/GA@PMC-2 | 345.1 mA h/g | 1000 | 5 A/g | [77] |
| 3DP-NGA | 85.3 mA h/g | 1000 | 0.1 A/g | [79] |
| Ti3C2Tx/rGO | 85.3 mA h/g | 500 | 0.1 A/g | [80] |
| CNC-CA@Bi-Sn | 187 mA h/g | 500 | 1 A/g | [81] |
| CNC-CA@Bi-NS | 346 mA h/g | 100 | 0.5 A/g | [82] |
| NMWAs@NiCo-LDH/S | 647.1 mA h/g | 700 | 5 A/g | [90] |
| MXene-rGO | 154.98 mA h/g | 5000 | 2 A/g | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Li, Q. Research and Application Progress of Aerogel Materials in the Field of Batteries and Supercapacitors. Energies 2024, 17, 4981. https://doi.org/10.3390/en17194981
Chen J, Li Q. Research and Application Progress of Aerogel Materials in the Field of Batteries and Supercapacitors. Energies. 2024; 17(19):4981. https://doi.org/10.3390/en17194981
Chicago/Turabian StyleChen, Junyong, and Qingyuan Li. 2024. "Research and Application Progress of Aerogel Materials in the Field of Batteries and Supercapacitors" Energies 17, no. 19: 4981. https://doi.org/10.3390/en17194981
APA StyleChen, J., & Li, Q. (2024). Research and Application Progress of Aerogel Materials in the Field of Batteries and Supercapacitors. Energies, 17(19), 4981. https://doi.org/10.3390/en17194981







