Study on the Properties of Compound Surfactants with PO Groups
Abstract
1. Introduction
2. Experimental
2.1. Instruments and Materials
2.2. Methods
2.2.1. Salt Solubility Resistance
2.2.2. Determination of Compound Proportion
2.2.3. Determination of Interfacial Tension
2.2.4. Determination of Emulsion Stability
2.2.5. Experimental Test of Phase Behavior
3. Results and Discussion
3.1. Determination of Salt Solubility and Compound System
3.2. Effect of the Number of PO Groups on the Interfacial Tension of the Composite System
3.3. Effect of Emulsification Stability
3.4. Study on the Phase Ability in the Formation of C12PO10S/APS Complex System
3.4.1. Effects of Different Oil–Water Ratios and NaCl Concentrations on Winsor Ⅲ Microemulsion
3.4.2. Evaluation of the Stability of the Winsor Ⅲ Microemulsion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pu, W.F.; Du, D.J.; Tang, Y.L.; Wang, S. Synthesis of an Alkyl Polyoxyethylene Ether Sulfonate Surfactant and Its Application in Surfactant Flooding. J. Surfactants Deterg. 2018, 21, 687–697. [Google Scholar] [CrossRef]
- Riswati, S.S.; Bae, W.; Park, C.; Permadi, A.K.; Novriansyah, A. Nonionic Surfactant to Enhance the Performances of Alkaline-Surfactant-Polymer Flooding with a Low Salinity Constraint. Appl. Sci. 2020, 10, 3752. [Google Scholar] [CrossRef]
- Muhammad, S.K.; Ibnelwaleed, A.H.; Abdullah, S.S. Review on Surfactant Flooding: Phase Behavior, Retention, IFT, and Field Applications. Energy Fuels 2017, 31, 7701–7720. [Google Scholar]
- Nilanjan, P.; Neha, S.; Divya, L.K.V.; Ajay, M. Interfacial behaviour, wettability alteration and emulsification characteristics of a novel surfactant: Implications for enhanced oil recovery. Chem. Eng. Sci. 2018, 187, 200–212. [Google Scholar]
- Braccalenti, E.; Del Gaudio, L.; Belloni, A.; Albonico, P.; Radaelli, E.; Bartosek, M. Enhancing Oil Recovery with Nanoemulsion Flooding. In Proceedings of the Offshore Mediterranean Conference and Exhibition, Ravenna, Italy, 29–31 March 2017. [Google Scholar]
- Aljabri, N.M.; Shi, N.; Cavazos, A. Nanoemulsion: An emerging technology for oilfield applications between limitations and potentials. J. Pet. Sci. Eng. 2022, 208, 109306. [Google Scholar] [CrossRef]
- Zhao, X.Z.G.; Ling, Y.G.; Guang, Z.L.; Huo, X.C.; Quan, S.L.; Dong, F.; Yu, J. Micellar solubilization of petroleum fractions by heavy alkylbenzene sulfonate surfactant. J. Mol. Liq. 2021, 329, 115519. [Google Scholar] [CrossRef]
- Kesarwani, H.; Saxena, A.; Mandal, A.; Sharma, S. Anionic/nonionic surfactant mixture for enhanced oil recovery through the investigation of adsorption, interfacial, rheological, and rock wetting characteristics. Energy Fuels 2021, 35, 3065–3078. [Google Scholar] [CrossRef]
- Pal, N.; Vajpayee, M.; Mandal, A. Cationic/nonionic mixed surfactants as enhanced oil recovery fluids: Influence of mixed micellization and polymer association on interfacial, rheological, and rock-wetting characteristics. Energy Fuels 2019, 33, 6048–6059. [Google Scholar] [CrossRef]
- Liu, R.; Liu, R.F.; Shi, J.P.; Chu, Y.J.; Du, D.J. Interfacial Properties and Efficient Imbibition Mechanism of Anionic–Nonionic Surfactants in Shale Porous Media. Energy Fuels 2023, 37, 11955–11968. [Google Scholar] [CrossRef]
- Souayeh, M.; Al-Maamari, R.S.; Karimi, M.; Aoudia, M. Wettability alteration and oil recovery by surfactant assisted low salinity water in carbonate rock: The impact of nonionic/anionic surfactants. J. Pet. Sci. Eng. 2021, 197, 108108. [Google Scholar] [CrossRef]
- Chen, J.; Hu, X.; Fang, Y. Cooperative effects of polypropylene oxide spacers and alkyl chains on dynamic amphipathicity of extended surfactants. J. Mol. Liq. 2020, 311, 113276. [Google Scholar] [CrossRef]
- Liu, X.C.; Zhao, Y.X.; Li, Q.X.; Niu, J.P. Surface tension, interfacial tension and emulsification of sodium dodecyl sulfate extended surfactant. Colloid. Surf. A 2016, 494, 201–208. [Google Scholar] [CrossRef]
- Wang, Z.S.; Zhou, Z.H.; Han, L.; Chen, X.; He, H.J.; Zhang, Q.; Xu, Z.C.; Gong, Q.T.; Ma, G.Y.; Zhang, L. The mechanism for lowering interfacial tension by extended surfactant containing ethylene oxide and propylene oxide groups. J. Mol. Liq. 2022, 359, 119364. [Google Scholar] [CrossRef]
- He, H.J.; Xiao, H.; Cao, X.L.; Yuan, F.Q.; Jiang, X.D.; Zhang, L. A helical shape of polyoxypropylene chain for extended surfactant molecule at the water/oil interface: Theoretical and experimental study. Fuel 2022, 312, 122835. [Google Scholar] [CrossRef]
- Feng, J.J.; Yan, Z.H.; Song, J.M.; He, J.C.; Zhao, G.; Fan, H.M. Study on the structure-activity relationship between the molecular structure of sulfate gemini surfactant and surface activity, thermodynamic properties and foam properties. Chem. Eng. Sci. 2021, 245, 116857. [Google Scholar] [CrossRef]
- Long, G.; Yan, L.; Song, S.H. Dynamic interfacial tensions of alkyl alcohol polyoxypropylene-oxyehtylene ether sulfonate solutions. J. Petrol. Sci. Eng. 2016, 141, 9–15. [Google Scholar]
- Zhang, M.J.; He, Z.Q.; Mao, C.Y.; Fang, Y. Structure-activity relationship of Nonylphenol Polyether Sulfate/φ-sulfonate Surfactants. J. Jiangnan Univ. 2012, 11, 216–220. [Google Scholar]
- Luan, H.; Zhou, Z.; Xu, C. Study on the Synergistic Effects between Petroleum Sulfonate and a Nonionic-Anionic Surfactant for Enhanced Oil Recovery. Energies 2022, 15, 1177. [Google Scholar] [CrossRef]
- Wang, K.L.; Zhang, W.; Zhuang, Y.T.; Li, G.; Su, Y. Effect of Alkali and Surfactant dosage on Emulsion Stability of weak Alkali Ternary system. J. Northeast Pet. Univ. 2020, 44, 48–55. [Google Scholar]
- Hammond, C.E.; Acosta, E.J. On the characteristic curvature of alkyl-polypropylene oxide sulfate extended surfactants. J. Surfactants Deterg. 2012, 15, 157–165. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Zhang, L.; Cao, X.L.; Song, X.W.; Jin, Z.Q.; Zhang, L.; Zhao, S. Effect of electrolytes on interfacial tensions of alkyl ether carboxylate solutions. Energy Fuels 2013, 27, 3122–3129. [Google Scholar] [CrossRef]
- Velasquez, J.; Scorzza, C.; Vejar, F. Effect of temperature and other variables on the optimum formulation of anionic extended surfactant-alkane-brine systems. J. Surfactants Deterg. 2010, 13, 69–73. [Google Scholar] [CrossRef]
- Forgiarini, M.A.; Scorzza, C.; Velasquez, J. Influence of the mixed propoxy/ethoxy spacer arrangement order and of the ionic head group nature on the adsorption and aggregation of extended surfactants. J. Surfactants Deterg. 2010, 13, 451–458. [Google Scholar] [CrossRef]
- Zhang, X.J.; Zhou, Z.H.; Han, L. Mechanism responsible for the reduction of interfacial tension by extended surfactants. Colloid. Surf. A 2022, 634, 128013. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.; Liu, H.E.; Ning, T.F.; Yu, Y.F.; Liu, Y.R. Effect of cosurfactant and oil-water ratio on phase behavior of microemulsion. Fine Chem. Ind. 2020, 37, 1645–1652. [Google Scholar]
- Zhao, S.; Zhou, Z.H.; Shang, G.Y.N. Effect of bivalent cations on the interfacial tensions of extended anionic surfactant solutions. J. Mol. Liq. 2022, 349, 118162. [Google Scholar] [CrossRef]
- Meng, D.L.; Cong, L.; Jian, Y. Effect of number of oxypropylene on dynamic interfacial tensions of extended surfactants. Colloid. Surf. A 2019, 570, 429–437. [Google Scholar]
- Mengual, O.; Meunier, G.; Cayre, I. TURBISCAN MA 2000: Multiple scattering measurement for concentrated emulsion and suspension instability analysis. Talanta 1999, 50, 445–456. [Google Scholar] [CrossRef]
- Yan, S.; Deac, A.; Geoff, G.Z.Z. Assessing Physical Stability of Colloidal Dispersions Using a Turbiscan Optical Analyzer. Mol. Pharmaceut. 2019, 16, 877–885. [Google Scholar]
- Kang, W.; Xu, B.; Wang, Y.; Yuan, L.; Shan, X.; An, F.; Liu, J. Stability mechanism of W/O crude oil emulsion stabilized by polymer and surfactant. Colloid. Surf. A 2011, 384, 555–560. [Google Scholar] [CrossRef]
- Chen, J.; Hu, X.Y.; Fang, Y.; Liu, H.H.; Xia, Y.M. Comparative study of conventional/ethoxylated/exte-nded n-alkylsulfate surfactants. Langmuir 2019, 35, 3116–3125. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, D.; Hu, J. Effect of Surfactant Molecular Structure on Emulsion Stability Investigated by Interfacial Dilatational Rheology. Polymers 2021, 13, 1127. [Google Scholar] [CrossRef]
- Winsor, P.A. Solvent Properties of Amphiphilic Compounds; Butterworths Scientific Publications: London, UK, 1954. [Google Scholar]
- Huh, C. Interfacial tensions and solubilizing ability of a microemulsion phase that coexists with oil and brine. J. Colloid. Interf. Sci. 1979, 71, 408–426. [Google Scholar] [CrossRef]
- Ting, L.Q.; Cong, W.W.; Qing, Q.Y. Performance evaluation of microemulsion of naphthenic petroleum sulfonate complex system. Oil Gas Surf. Eng. 2023, 42, 12–17. [Google Scholar]
- Zhi, W.Z.; Wei, D.; Zhong, L. Properties of microemulsion formed by alkylaryl sulfonates with different chain lengths. Chem. J. Chin. Univ. 2012, 33, 395–399. [Google Scholar]
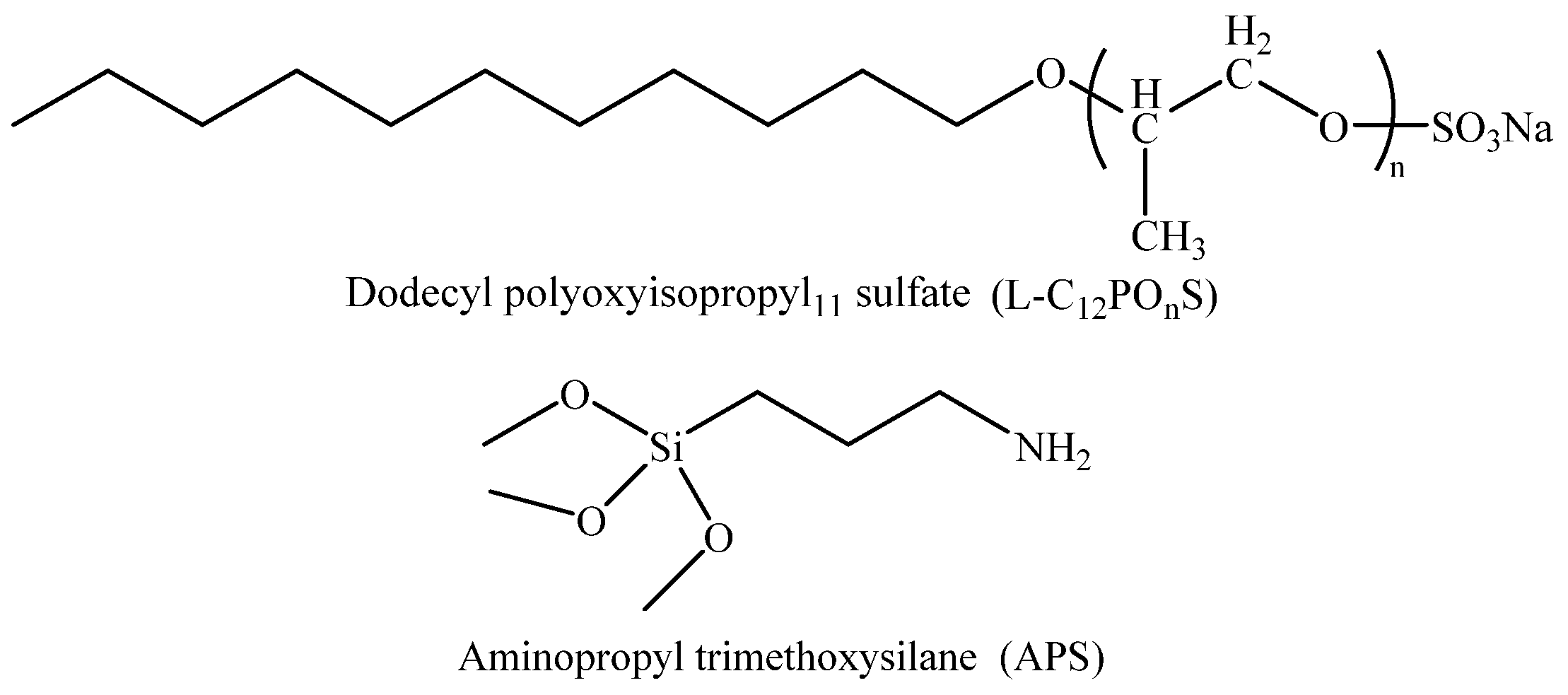




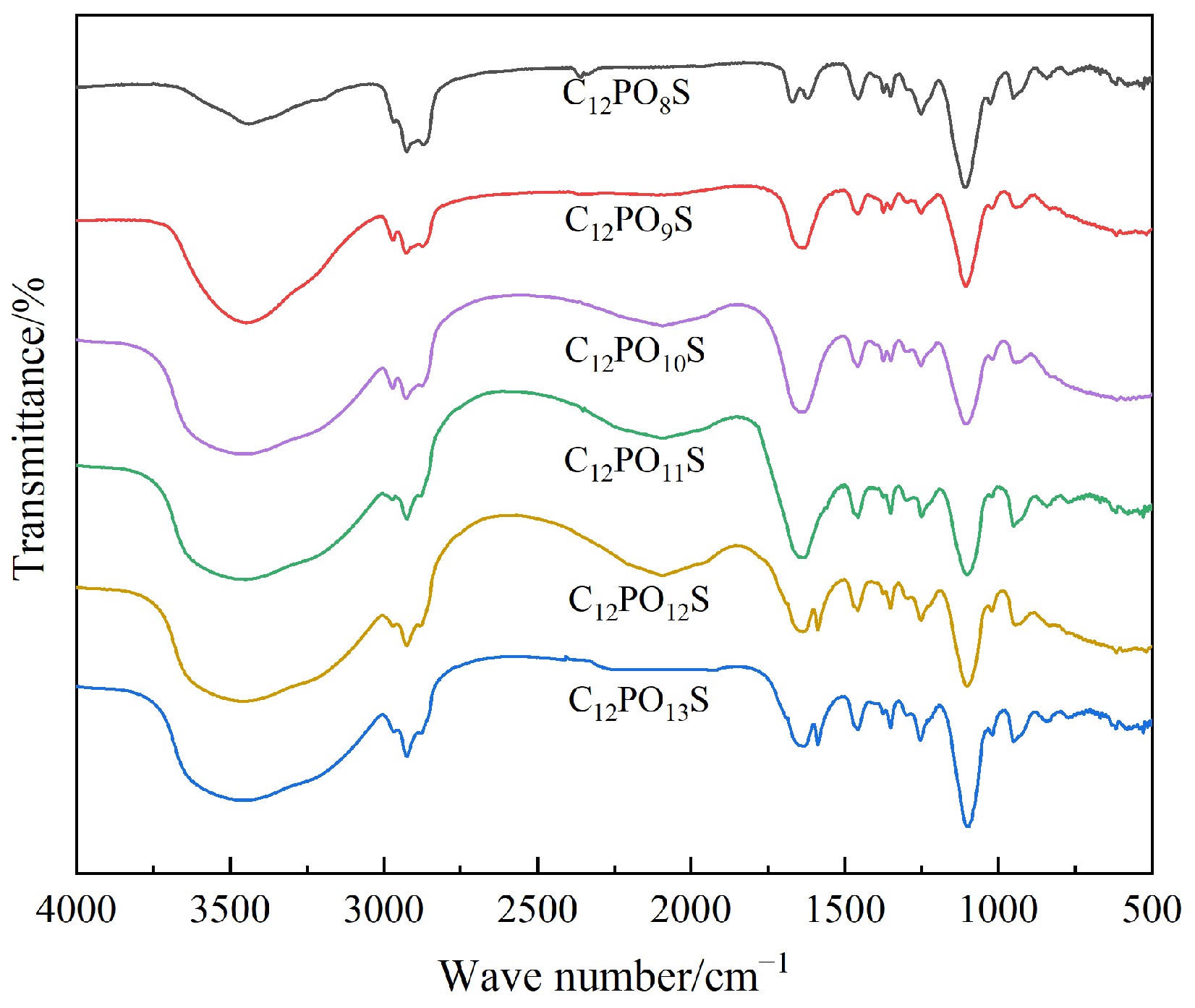
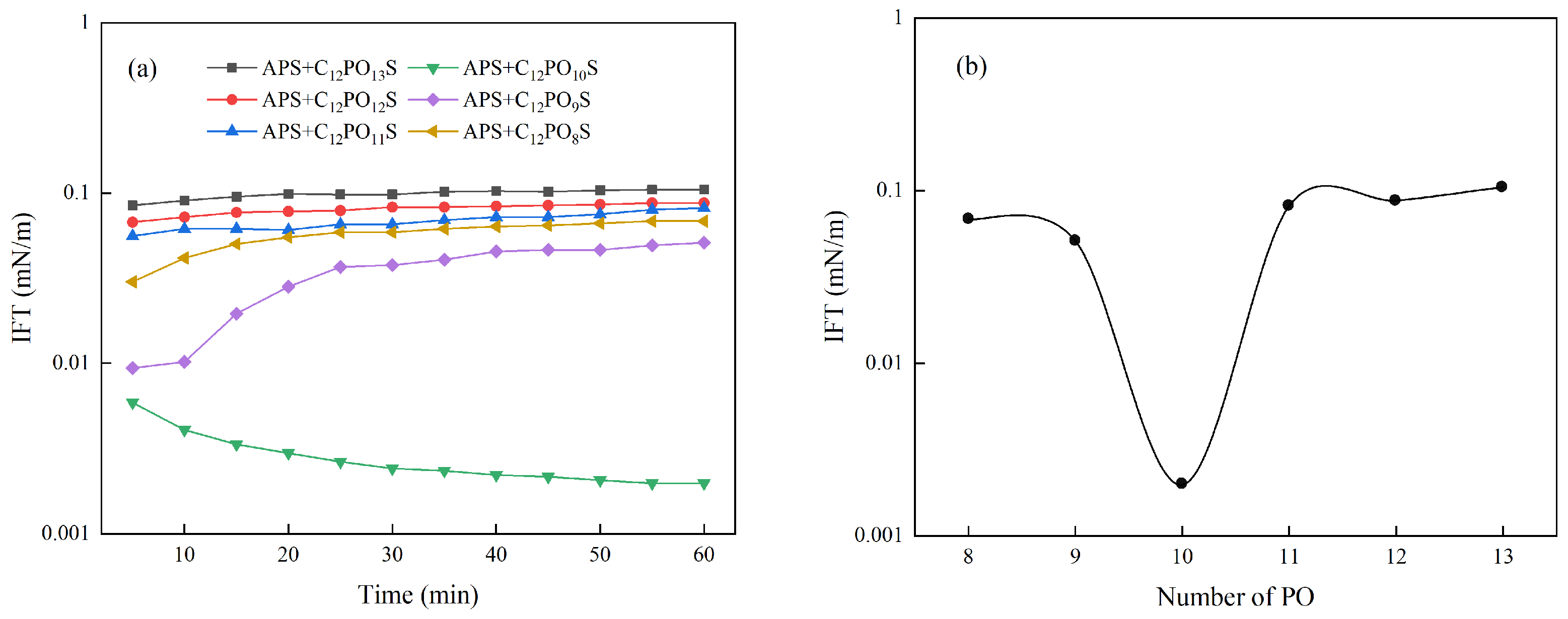
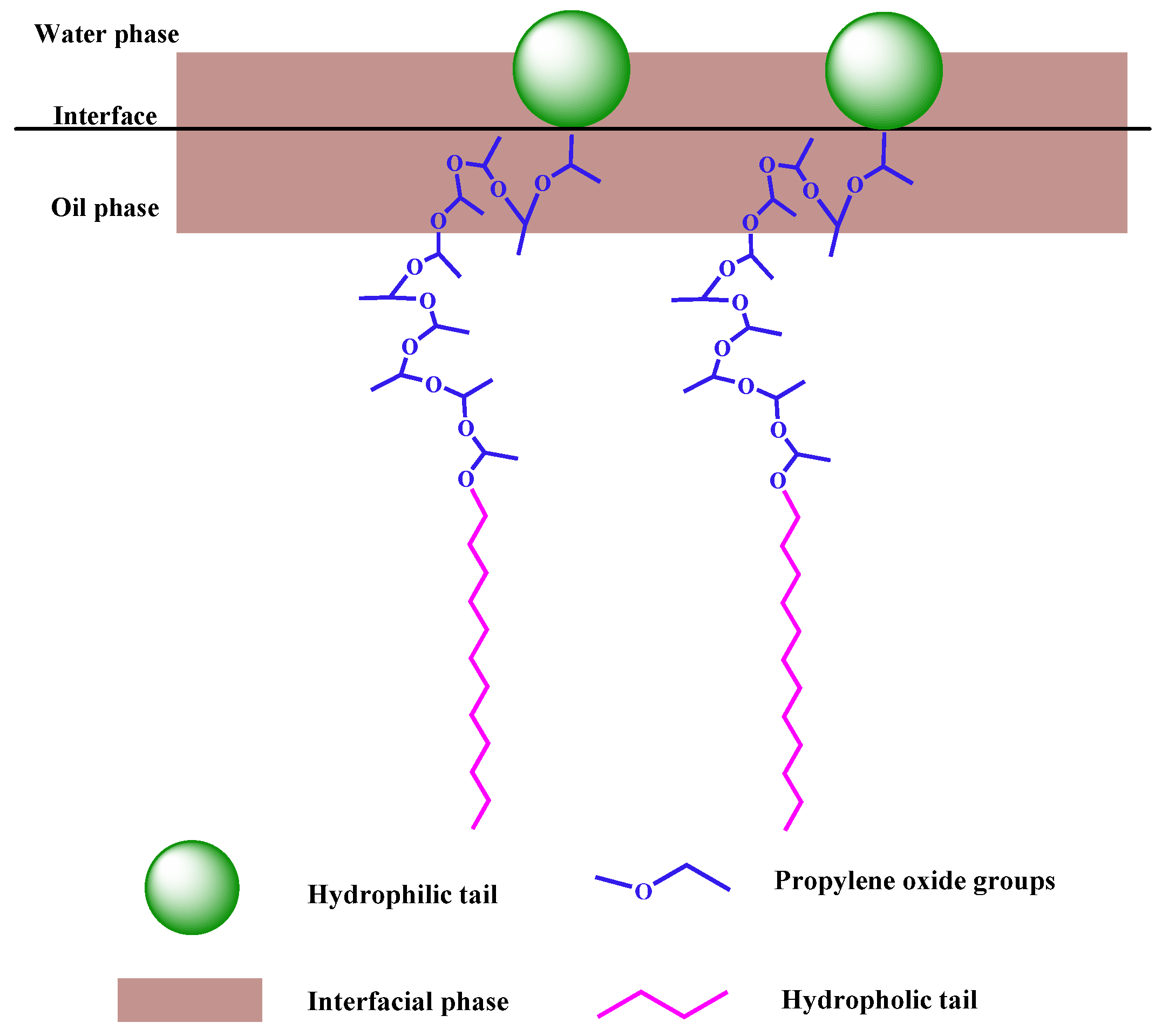


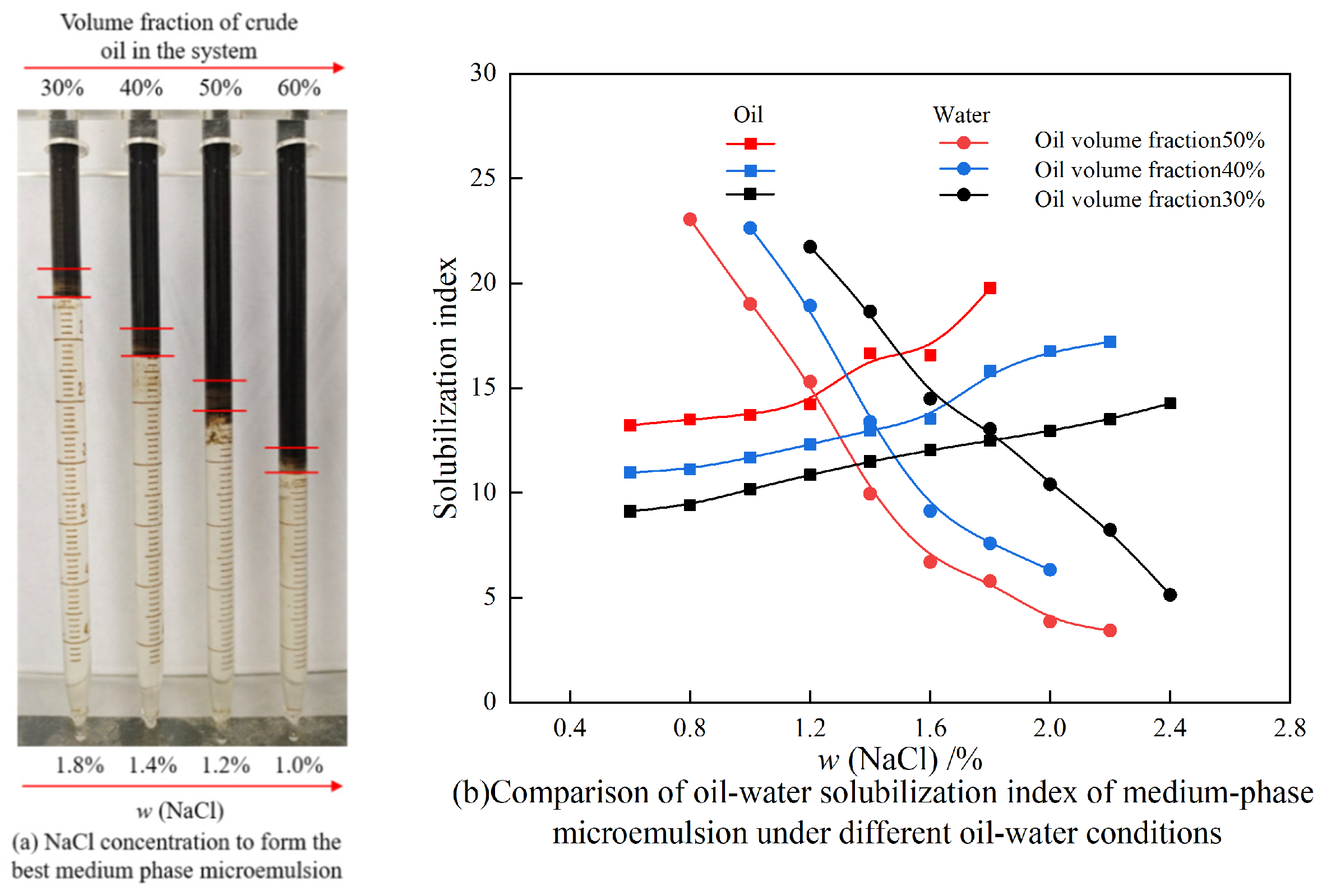

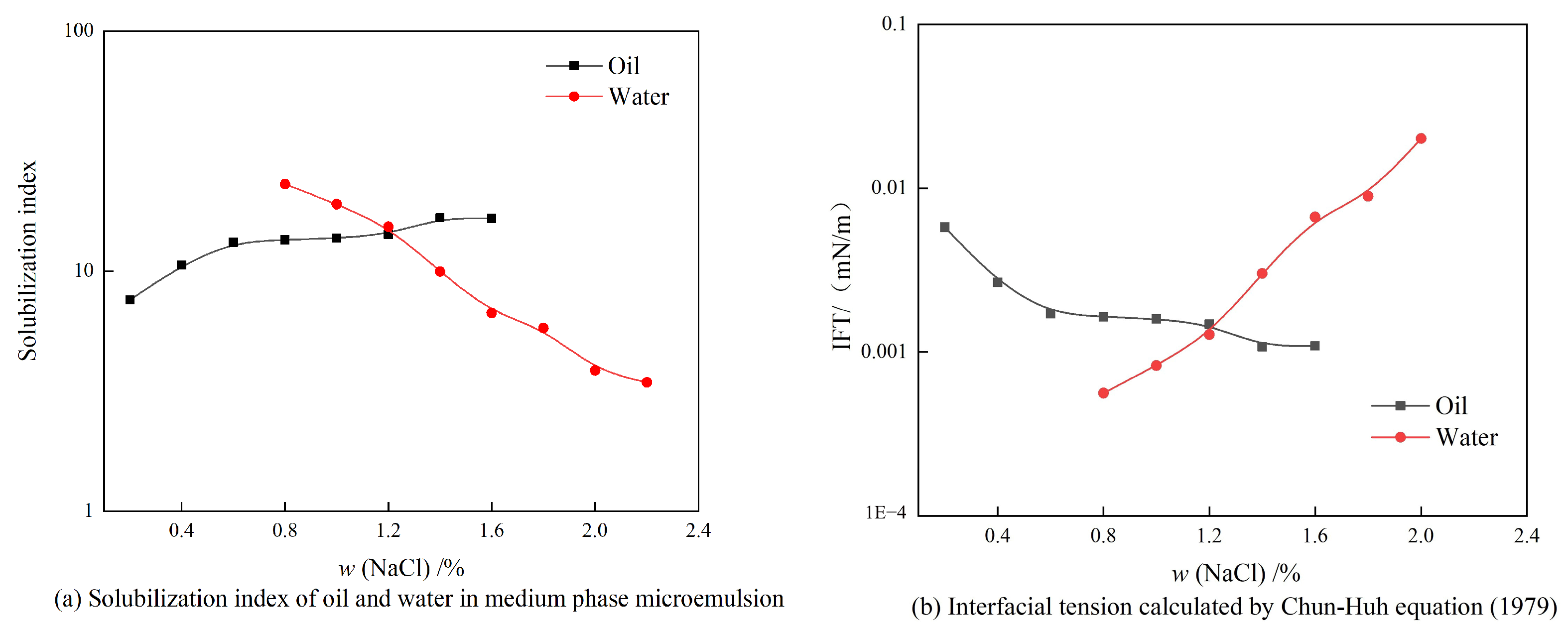
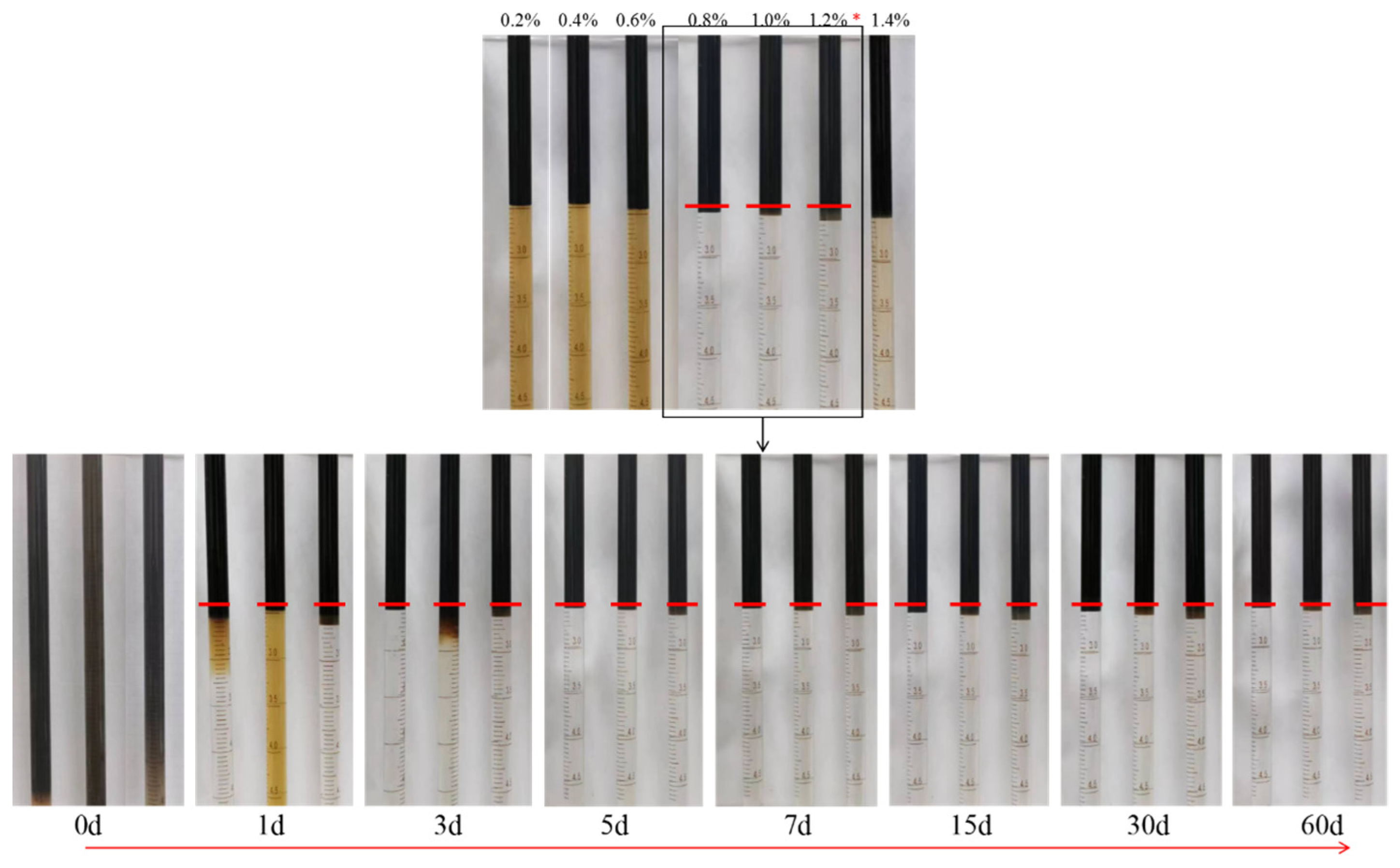
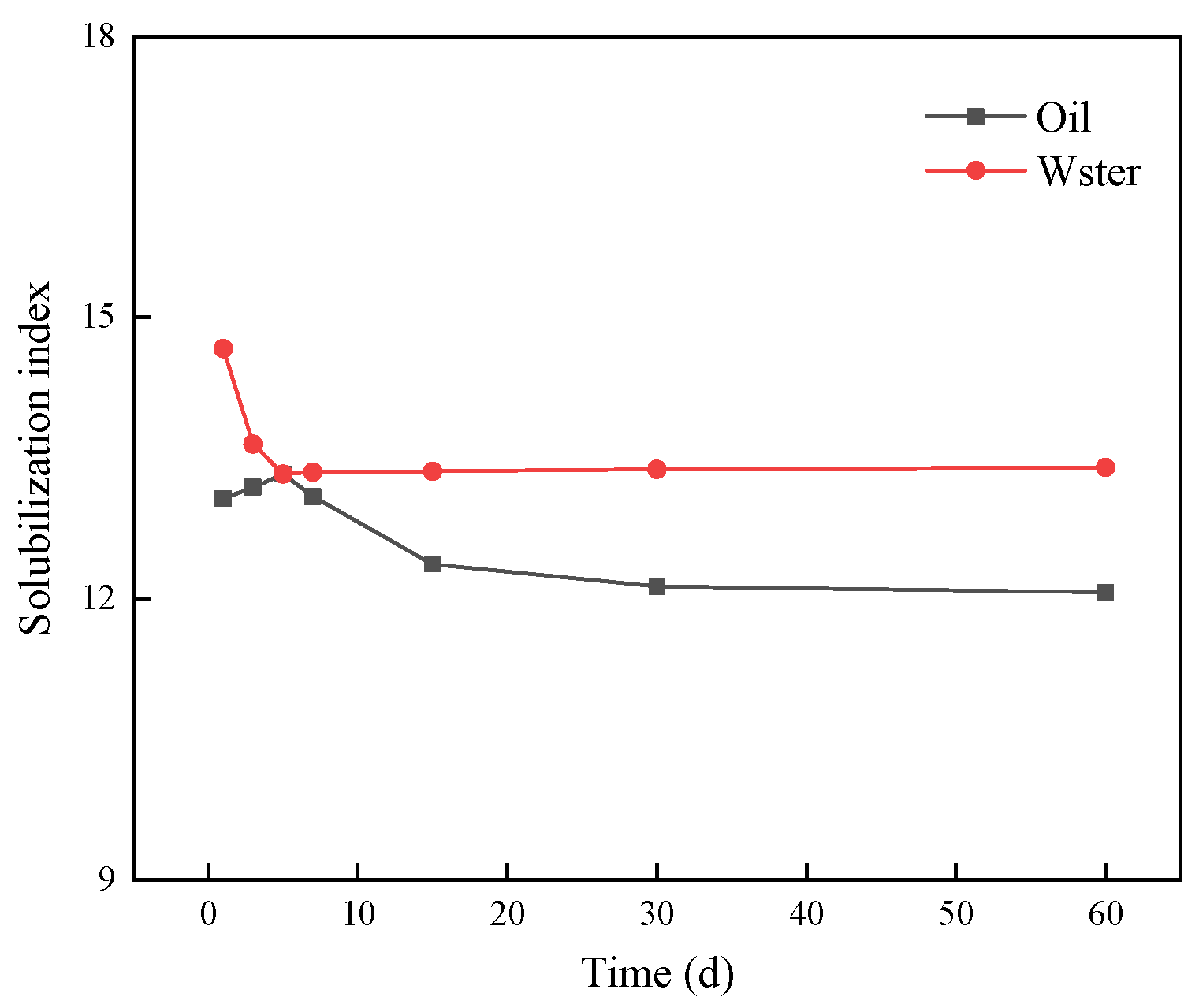
| Ionic Species | K++Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | CO32− | Total |
|---|---|---|---|---|---|---|---|---|
| Salinity/(mg/L) | 1826.39 | 15.03 | 10.34 | 1007.03 | 14.41 | 2297.4 | 421.82 | 5592.53 |
| Total Mineralization Value (mg/L) | Na+ Concentration (mg/L) |
|---|---|
| 2000 | 787.13 |
| 4000 | 1574.26 |
| 6000 | 2361.4 |
| 8000 | 3148.53 |
| 10,000 | 3935.66 |
| 12,000 | 4722.79 |
| 14,000 | 5509.92 |
| 16,000 | 6297.06 |
| 18,000 | 7084.19 |
| 20,000 | 7871.32 |
| NaCl/(mg/L) | LBAS | APS | Compound System of LBAS and APS |
|---|---|---|---|
| 0 | C | C | C |
| 2000 | T | C | C |
| 4000 | T | C | C |
| 6000 | P | C | C |
| 8000 | Y | C | C |
| 10,000 | Y | C | C |
| 12,000 | Y | C | P |
| 14,000 | Y | C | P |
| 16,000 | Y | C | Y |
| 18,000 | Y | C | Y |
| 20,000 | Y | C | Y |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Wang, X.; Hu, F.; Wang, X.; Yang, Z.; Zhu, X.; Li, G.; Wang, K. Study on the Properties of Compound Surfactants with PO Groups. Energies 2024, 17, 513. https://doi.org/10.3390/en17020513
Wang B, Wang X, Hu F, Wang X, Yang Z, Zhu X, Li G, Wang K. Study on the Properties of Compound Surfactants with PO Groups. Energies. 2024; 17(2):513. https://doi.org/10.3390/en17020513
Chicago/Turabian StyleWang, Biao, Xue Wang, Futang Hu, Xiangyu Wang, Zhongji Yang, Xiuyu Zhu, Gen Li, and Keliang Wang. 2024. "Study on the Properties of Compound Surfactants with PO Groups" Energies 17, no. 2: 513. https://doi.org/10.3390/en17020513
APA StyleWang, B., Wang, X., Hu, F., Wang, X., Yang, Z., Zhu, X., Li, G., & Wang, K. (2024). Study on the Properties of Compound Surfactants with PO Groups. Energies, 17(2), 513. https://doi.org/10.3390/en17020513





