Ozonation and Changes in Biodegradable Organic Substances in Drinking Water Treatment: The Future of Green Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
3.1. The Impact of the Concentration and Contact Time of O3 with Water on the Content of Selected Forms of Organic Carbon
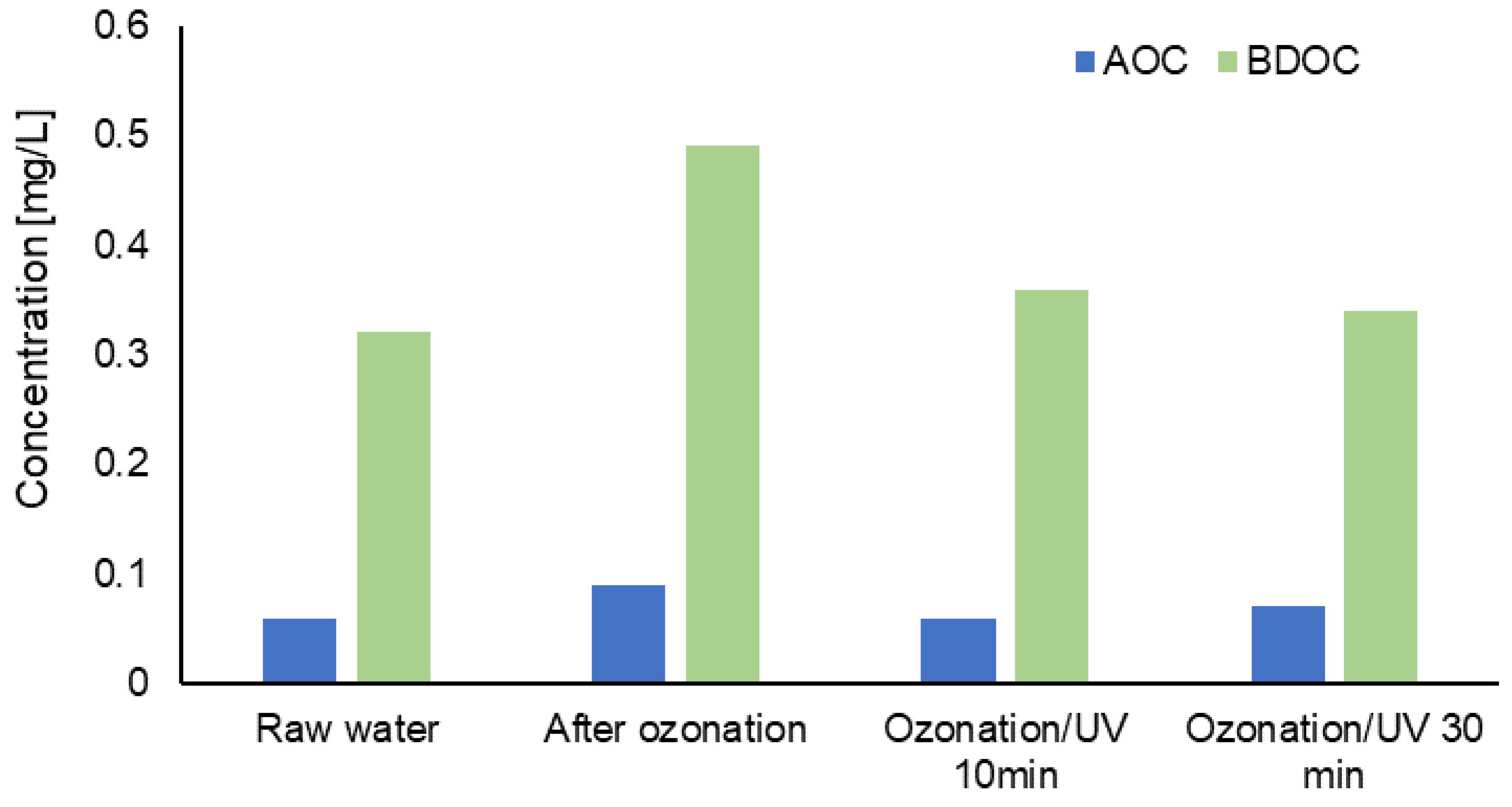
3.2. Evaluation of Changes in the Content of Selected Forms of Organic Carbon in Water after the Process of Ozonation Assisted by UV Radiation
4. Discussion of the Results
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prest, E.I.; Hammes, F.; van Loosdrecht, M.C.M.; Vrouwenvelder, J.S. Biological stability of drinking water: Controlling factors, methods, and challenges. Front. Microbiol. 2016, 7, 45. [Google Scholar] [CrossRef]
- Liviac, D.; Creus, A.; Marcos, R. Genotoxic evaluation of the non-halogenated disinfection by-products nitrosodimethylamine and nitrosodiethylamine. J. Hazard. Mater. 2011, 185, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, A.D.; Lekkas, T.D.; Golfinopoulos, S.K. Kinetics of the formation and decomposition of chlorination by-products in surface waters. Chem. Eng. J. 2004, 100, 139–148. [Google Scholar] [CrossRef]
- Liao, Y.; Ji, W.; Wang, Z.; Tian, Y.; Peng, J.; Li, W.; Pan, Y.; Li, A. Effects of alternative disinfection methods on the characteristics of effluent organic matter and the formation of disinfection byproducts. Environ. Pollut. 2024, 340, 122796. [Google Scholar] [CrossRef] [PubMed]
- Zacariah, L.; Sanchez-Rosario, H.S.; Klima, A.; Lide, T.; Schu, K.A. TOC/Conductivity: Surrogate measurements potentially guiding greater utilization of treated produced water. Energies 2023, 16, 206. [Google Scholar] [CrossRef]
- Escobar, I.C.; Randall, A. Assimilable organic carbon (AOC) and biodegradable dissolved organic carbon (BDOC): Complementary measurements. Water Res. 2001, 35, 4444–4454. [Google Scholar] [CrossRef] [PubMed]
- Wanga, Q.; Taoa, T.; Xina, K. The Relationship between Water biostability and initial bacterial growth variations to different organic carbon concentrations. Procedia Eng. 2014, 89, 160–167. [Google Scholar] [CrossRef][Green Version]
- Chen, W.T.; Chien, C.C.; Ho, W.S.; Ou, J.H.; Chen, S.C.; Kao, C.M. Effects of treatment processes on AOC removal and changes of bacterial diversity in a water treatment plant. J. Environ. Manag. 2022, 311, 114853. [Google Scholar] [CrossRef] [PubMed]
- Wolska, M. Biological stability of water in water distribution systems. The effect of water treatment trials. Environ. Prot. Eng. 2015, 41, 147–157. [Google Scholar] [CrossRef]
- Kilb, B.; Lange, B.; Schaule, G.; Flemming, H.C.; Wingender, J. Contamination of drinking water by coliforms from biofilms grown on rubber-coated valves. Int. J. Hyg. Environ. Health 2003, 206, 563–573. [Google Scholar] [CrossRef]
- Huck, P.M. Measurement of Biodegradable Organic Matter and Bacterial Growth Potential in Drinking Water. J. AWWA 1990, 82, 78–86. Available online: https://www.jstor.org/stable/41292977 (accessed on 21 December 2023).
- Van der Kooij, D. Assimilable organic carbon as an indicator of bacterial regrowth. J. Am. Water Works Assoc. 1982, 84, 57–65. [Google Scholar] [CrossRef]
- Van der Kooij, D. Biological stability: A multidimensional quality aspect of treated water. J. Water Air Soil Pollut. 2000, 123, 25–34. [Google Scholar] [CrossRef]
- Eichler, S.; Christen, R.; Höltje, C.; Westphal, P.; Bötel, J.; Brettar, I.; Mehling, A.; Höfle, M. Composition and dynamics of bacterial communities of a drinking water supply system as assessed by RNA- and DNA-based 16S rRNA gene fingerprinting. Appl. Environ. Microbiol. 2006, 72, 1858–1872. [Google Scholar] [CrossRef]
- Lautenschlager, K.; Boon, N.; Wang, Y.; Egli, T.; Hammes, F. Overnight stagnation of drinking water in household taps induces microbialgrowth and changes in community composition. Water Res. 2010, 44, 4868–4877. [Google Scholar] [CrossRef]
- Wilson, C.; Lukowicz, R.; Merchant, S.; Valquier-Flynn, H.; Caballero, J.; Sandoval, J.; Okuom, M.; Huber, C.; Brooks, T.D.; Wilson, E.; et al. Quantitative and Qualitative Assessment Methods for Biofilm Growth: A Mini-review. Res. Rev. J. Eng. Technol. 2017, 6. Available online: http://www.rroij.com/open-access/quantitative-and-qualitative-assessment-methods-for-biofilm-growth-a-minireview-.pdf (accessed on 24 October 2017).
- Bhuyan, B.; Yadav, M.; Giri, S.J.; Begum, S.; Das, S.; Phukan, A.; Priyadarshani, P.; Sarkar, S.; Jayswal, A.; Kabyashree, K.; et al. Microliter spotting and micro-colonyobservation: A rapid and simple approach for counting bacterial colony forming units. J. Microbiol. Methods. 2023, 207, 106707. [Google Scholar] [CrossRef]
- He, H.; Li, T.; He, C.; Chen, J.; Chu, H.; Dong, B. Removal of natural organic matter in full-scale conventional and advanced water treatment plants: Assimilable organic carbon and its precursors. Chem. Eng. J. Adv. 2021, 8, 100183. [Google Scholar] [CrossRef]
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption (Recast) (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/eli/dir/2020/2184/oj (accessed on 23 December 2020).
- Simon, M.; Joshi, H. A review on green technologies for the rejuvenation of polluted surface water bodies: Field-scale feasibility, challenges, and future perspectives. J. Environ. Chem. Eng. 2021, 9, 105763. [Google Scholar] [CrossRef]
- Rolbiecki, S.; Rolbiecki, R.; Kuśmierek-Tomaszewska, R.; Żarski, J.; Jagosz, B.; Kasperska-Wołowicz, W.; Sadan, H.; Łangowski, A. Influence of forecast climate changes on water needs of Jerusalem Artichoke Grown in the Kuyavia Region in Poland. Energies 2023, 16, 533. [Google Scholar] [CrossRef]
- Eaton, A.D.; Clesceri, L.S.; Rice, E.W.; Greenberg, A.E. (Eds.) Standard Methods for the Examination of Water and Wastewater, 21st ed.; Centennial Edition, Procedure 9215, 9217, 9050, 9060; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Rosińska, A.; Rakocz, K. The influence UV/chlorination process on changes of biodegradable fraction in water. J. Clean. Prod. 2021, 278, 123947. [Google Scholar] [CrossRef]
- PN-EN ISO 6222:2002; Water Quality-Enumeration of Culturable Microorganisms-Colony Count by Inoculation in a Nutrient Agar Culture Medium. Polish Committee for Standardization: Warsaw, Poland, 2013.
- Świderska-Broz, M.; Wolska, M. Efficiency of surface water treatment processes at removing biodegradable organic substances. Environ. Protect. 2011, 33, 77–80. [Google Scholar]
- Li, W.-T.; Cao, M.-J.; Young, T.; Ruffino, B.; Dodd, M.; Li, A.-M.; Korshin, G. Application of UV absorbance and fluorescence indicators to assess the formation of biodegradable dissolved organic carbon and bromate during ozonation. Water Res. 2017, 111, 154–162. [Google Scholar] [CrossRef]
- Świetlik, J.; Raczyk-Stanisławiak, U.; Nawrocki, J. The influence of disinfection on aquatic biodegradable organic carbon formation. Water Res. 2009, 43, 463–473. [Google Scholar] [CrossRef]
- Raczyk-Stanisławiak, U.; Świetlik, J.; Dąbrowska, A.; Nawrocki, J. Biodegradability of organic by-products after natural organic matter oxidation with ClO2—Case study. Water Res. 2004, 38, 1044–1054. [Google Scholar] [CrossRef]
- Jyoti, K.K.; Pandit, A.B. Ozone and cavitation for water disinfection. Biochem. Eng. J. 2004, 18, 9–19. [Google Scholar] [CrossRef]
- Pick, F.C.; Fish, K.E.; Boxall, J.B. Assimilable organic carbon cycling within drinking water distribution systems. Water Res. 2021, 198, 117147. [Google Scholar] [CrossRef]
- Ren, X.; Chen, H. Effect of residual chlorine on the interaction between bacterial growth and assimilable organic carbon and biodegradable organic carbon in reclaimed water. Sci. Total Environ. 2021, 752, 141223. [Google Scholar] [CrossRef]
- Dukan, S.; Levi, Y.; Piriou, P. Dynamic modeling of bacterial growth in drinking water networks. Water Res. 1996, 30, 1991–2002. [Google Scholar] [CrossRef]
- Thayanukul, P.; Kirisu, F.; Kasuga, I.; Furumai, H. Evaluation of microbial regrowth potential by assimilable organic carbon in various reclaimed water and distribution systems. Water Res. 2013, 47, 225–232. [Google Scholar] [CrossRef]
- Hammes, F.; Salhi, E.; Köster, O.; Kaiser, H.-P.; Egli, T.; Von Gunten, U. Mechanistic and kinetic evaluation of organic disinfection by-product and assimilable organic carbon (AOC) formation during the ozonation of drinking water. Water Res. 2006, 40, 2275–2286. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.C.; Welte, B.; Montie, A. Removal of biodegradable dissolved organic carbon in a water treatment plant. Wat. Res. 1992, 26, 1673–1680. [Google Scholar] [CrossRef]
- Polańska, M.; Huysman, K.; Van Keer, C. Investigation of assimilable organic carbon (AOC) in flemish drinking water. Water Res. 2005, 39, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Yavich, A.A.; Lee, K.H.; Chen, K.C.; Pape, L.; Masten, S.J. Evaluation of biodegradability of NOM after ozonation. Water Res. 2004, 38, 2839–2846. [Google Scholar] [CrossRef]
- Klymenko, N.A.; Kozyatnyk, I.P.; Savchyna, I.A. Removing of fulvic acids by ozonation and biological active carbon filtration. Water Res. 2010, 44, 5316–5322. [Google Scholar] [CrossRef]
- Weinrich, L.A.; Giraldo, E.; LeChevallier, M.W. Development and application of a escence-based test for assimilable organic carbon in reclaimed waters. Appl. Environ. Microbiol. 2009, 75, 7385–7390. [Google Scholar] [CrossRef]
- Ghernaout, D.; Ghernaout, B.; Naceur, M.W. Embodying the chemical water treatment in the green chemistry—A review. Desalination 2011, 271, 1–10. [Google Scholar] [CrossRef]

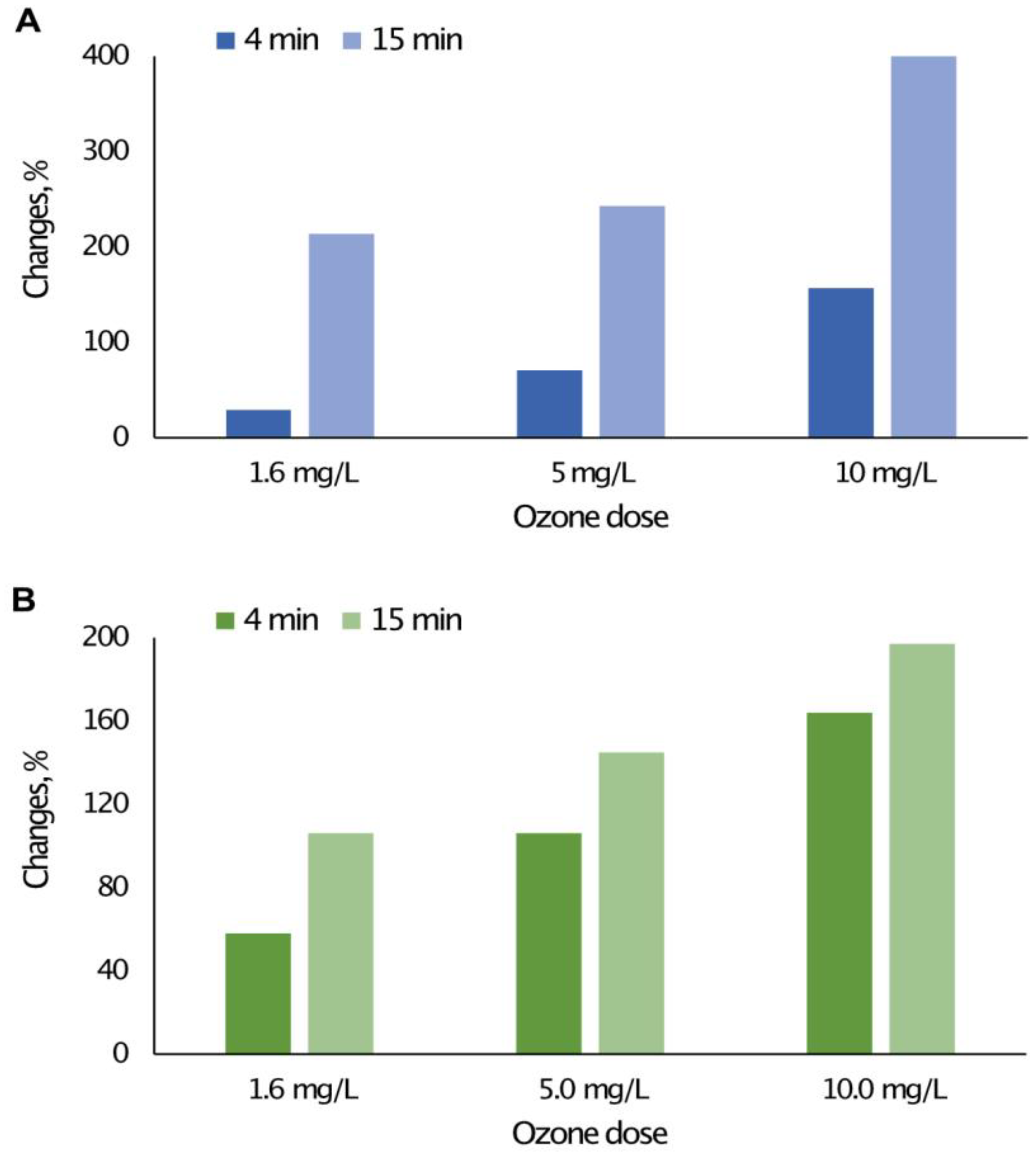
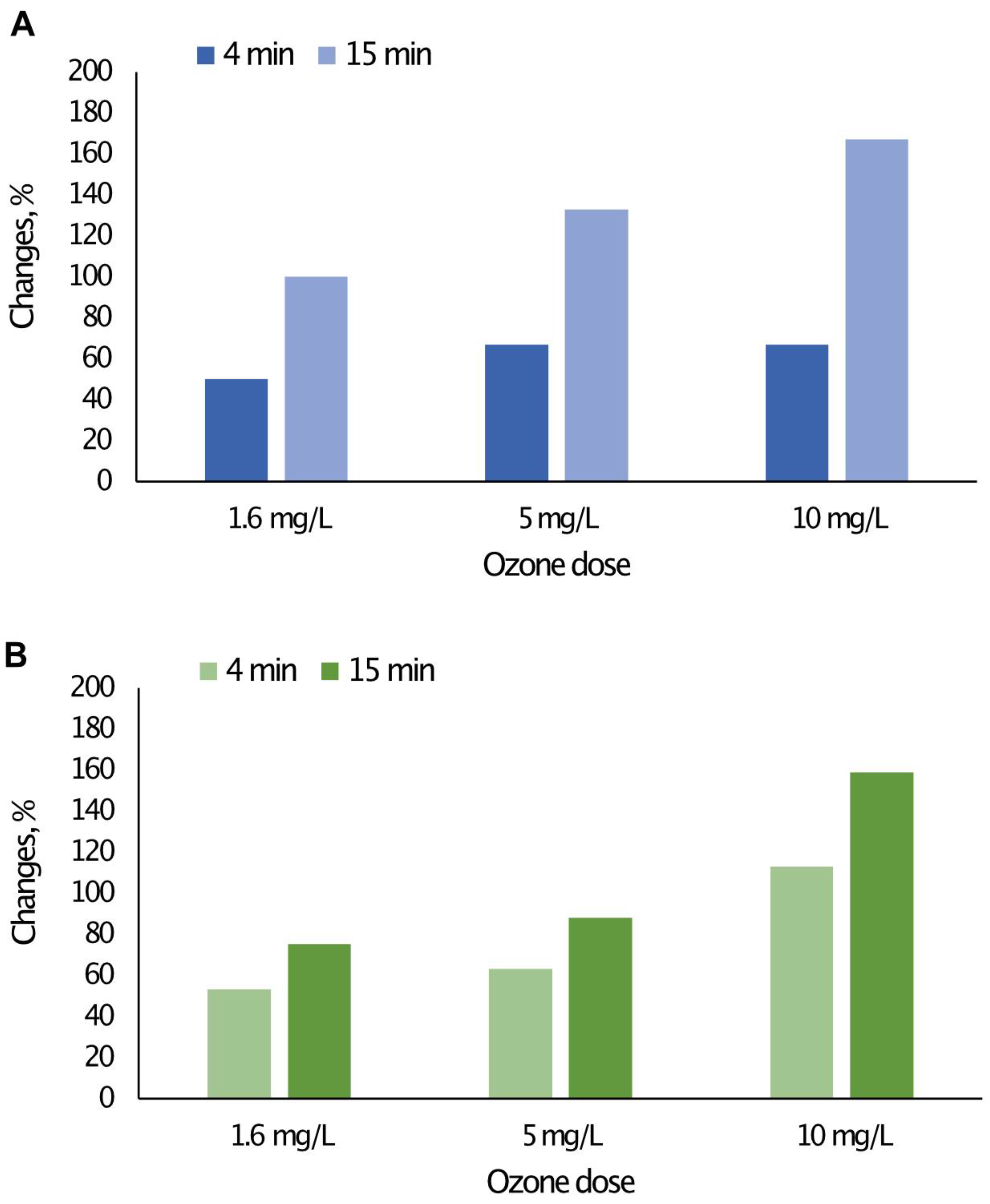
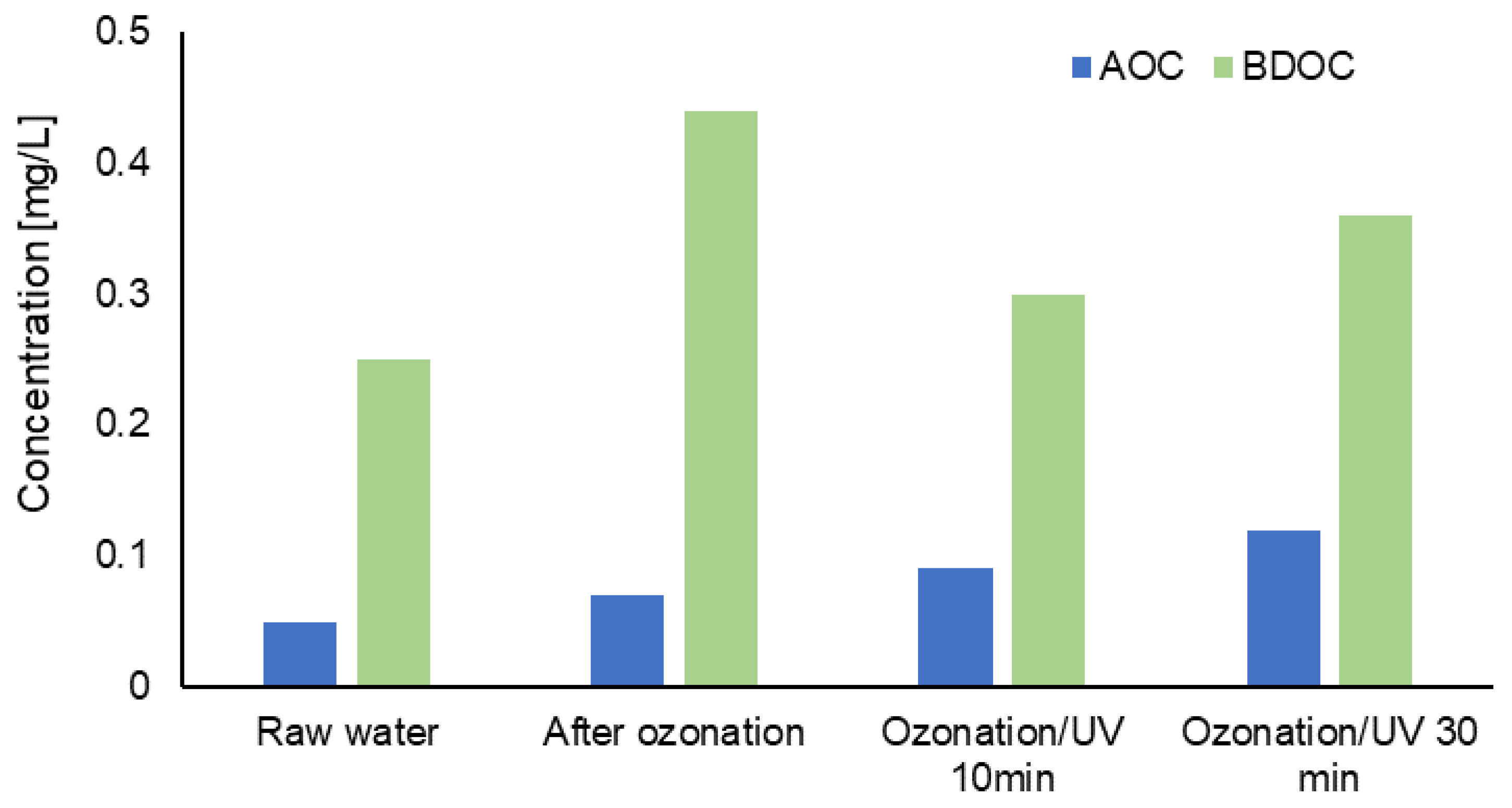
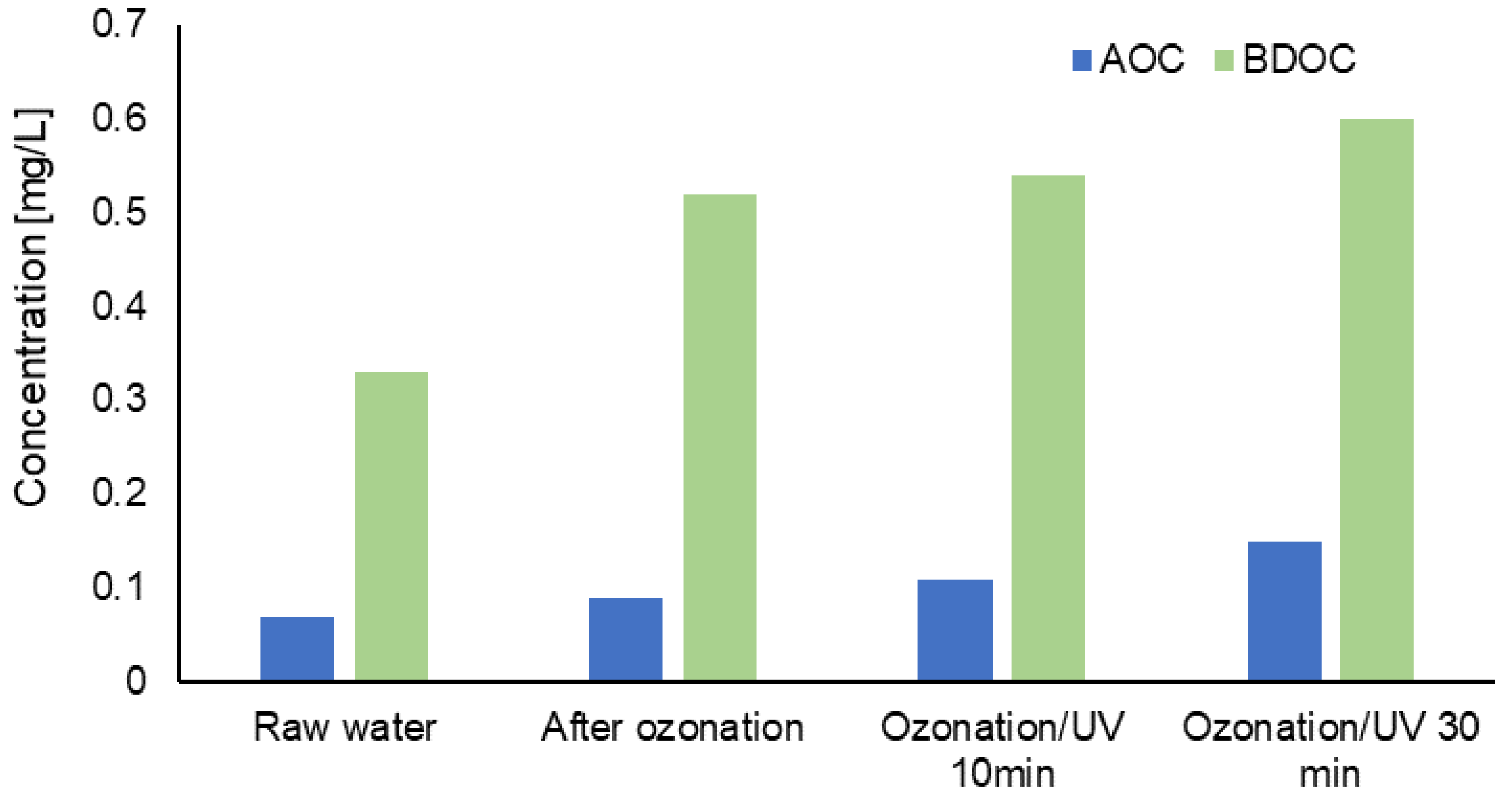
| Water Intake | Type of Disinfectant | Contact Time/Flow Rate |
|---|---|---|
| W | O3 concentration
| 9 min |
| M | O3 disinfection O3 concentration in the contact chamber 0.30 mgO3/L ±0.05,
| 17.5–27.5 min |
| L | Disinfection with the solution NaClO Disinfectant concentration 0.15 mgNaOCl/L | flow velocity in the contact chamber 15 m3/h. |
| R | Disinfection with the solution NaClO Disinfectant concentration 0.16–0.17 mgNaOCl/L | flow velocity in the contact chamber 25 m3/h. |
| Organic Carbon Fraction | Raw Water | O3 Dose | Ozonation/UV | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1.6 mg/L | 5.0 mg/L | 10.0 mg/L | |||||||
| 4 min | 15 min | 4 min | 15 min | 4 min | 15 min | 10 min | 30 min | ||
| TOC | 9.80 | 9.30 | 9.10 | 9.20 | 9.00 | 9.20 | 8.80 | 9.6 | 9.3 |
| DOC | 9.20 | 9.00 | 8.80 | 8.90 | 8.60 | 8.60 | 8.30 | 9.0 | 8.7 |
| AOC | 0.05 | 0.07 | 0.18 | 0.09 | 0.18 | 0.12 | 0.33 | 0.09 | 0.45 |
| BDOC | 0.25 | 0.44 | 0.56 | 0.50 | 0.56 | 0.62 | 0.75 | 0.30 | 0.36 |
| Organic Carbon Fraction | Raw Water | O3 Dose | Ozonation/UV | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1.6 mg/L | 5.0 mg/L | 10.0 mg/L | |||||||
| 4 min | 15 min | 4 min | 15 min | 4 min | 15 min | 10 min | 15 min | ||
| TOC | 13.05 | 11.50 | 11.10 | 10.86 | 10.65 | 9.84 | 9.79 | 11.44 | 11.26 |
| DOC | 11.65 | 10.42 | 9.85 | 9.65 | 9.55 | 10.02 | 9.88 | 10.98 | 10.77 |
| AOC | 0.07 | 0.09 | 0.22 | 0.12 | 0.24 | 0.18 | 0.35 | 0.11 | 0.15 |
| BDOC | 0.33 | 0.52 | 0.68 | 0.68 | 0.81 | 0.87 | 0.98 | 0.54 | 0.60 |
| Organic Carbon Fraction | Raw Water | O3 Doze | Ozonation/UV | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1.6 mg/L | 5.0 mg/L | 10.0 mg/L | |||||||
| 4 min | 15 min | 4 min | 15 min | 4 min | 15 min | 10 min | 30 min | ||
| TOC | 13.02 | 12.20 | 12.00 | 12.12 | 11.98 | 11.85 | 11.56 | 11.45 | 11.23 |
| DOC | 10.68 | 10.00 | 9.85 | 9.85 | 9.83 | 9.82 | 9.78 | 10.34 | 9.96 |
| AOC | 0.060 | 0.090 | 0.120 | 0.100 | 0.140 | 0.100 | 0.160 | 0.06 | 0.07 |
| BDOC | 0.32 | 0.49 | 0.56 | 0.52 | 0.60 | 0.68 | 0.83 | 0.36 | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosińska, A.; Rakocz, K. Ozonation and Changes in Biodegradable Organic Substances in Drinking Water Treatment: The Future of Green Technology. Energies 2024, 17, 530. https://doi.org/10.3390/en17020530
Rosińska A, Rakocz K. Ozonation and Changes in Biodegradable Organic Substances in Drinking Water Treatment: The Future of Green Technology. Energies. 2024; 17(2):530. https://doi.org/10.3390/en17020530
Chicago/Turabian StyleRosińska, Agata, and Klaudia Rakocz. 2024. "Ozonation and Changes in Biodegradable Organic Substances in Drinking Water Treatment: The Future of Green Technology" Energies 17, no. 2: 530. https://doi.org/10.3390/en17020530
APA StyleRosińska, A., & Rakocz, K. (2024). Ozonation and Changes in Biodegradable Organic Substances in Drinking Water Treatment: The Future of Green Technology. Energies, 17(2), 530. https://doi.org/10.3390/en17020530










