Abstract
Jerusalem artichoke (Helianthus tuberosus L.) is a plant with considerable potential for energy generation due to its rapid growth, high biomass yield, and resistance to environmental stresses. The aim of this study was to determine the influence of the nitrogen fertilization strategy on the yield and energy balance in the production technology of Jerusalem artichoke (JA) in a perennial cropping system. The article presents the results of a three-year experiment which was conducted in Poland to determine the effect of different N rates (0, 50, 75, and 100 kg ha−1) supplied with mineral fertilizers and liquid digestate on the energy balance in the production of JA aerial biomass. The experiment had a randomized block design with three replications. The demand for energy in JA cultivation reached 16.2–26.3 (year 1) and 2.9–14.6 GJ ha−1 (years 2 and 3). Energy inputs in the cultivation technology were reduced by 17–19% (year 1) and 35–47% (years 2 and 3) when mineral fertilizers were replaced with digestate. Jerusalem artichoke yields were lowest in the technology without fertilization (12.5 Mg ha−1 DM). Dry matter yield increased significantly (by 43–55%) after the application of 75 kg N ha−1, regardless of fertilizer type. The energy output of biomass peaked (230.1 GJ ha−1) in response to a mineral fertilizer rate of 75 kg N ha−1. In turn, the highest energy gain (218.5 GJ ha−1) was noted after the application of digestate at a rate equivalent to 75 kg N ha–1. The energy efficiency ratio was highest in the technology without fertilization (20.1) and after the application of digestate at a rate equivalent to 75 kg N ha−1 (19.7). Regardless of the factors that limit agricultural production, the energy balance of JA biomass production was most favorable when JA was fertilized with digestate at a rate equivalent to 75 kg N ha−1. The results of this study may pave the way for future research on novel agronomic strategies for sustainable bioenergy production, including nutrient recycling.
1. Introduction
The use of fossil fuels in energy generation is regarded as the main cause of global climate change. Most efforts to minimize the adverse changes of climate change focus on the search for alternative sources of renewable energy [1,2,3,4]. Various sources of renewable energy are utilized in different regions of the world, depending on geographic location, climate, and the availability of natural resources. Countries with high solar energy potential may invest in solar farms, whereas wind farms may be preferred in countries with high wind energy capacity. Biomass is a highly versatile source of renewable energy [5,6]. Due to its widespread availability, biomass is an attractive energy source in many regions of the world with diverse climates. As a result, biomass can significantly contribute to the global energy transition and the implementation of sustainable and eco-friendly power generation systems [7]. Biomass has a number of additional advantages, in particular when it is produced as a dedicated energy crop: (i) it contributes to the management of marginal land and land protection against degradation through the use of best agronomic practices, and (ii) unlike wind and solar energy which fluctuate on a seasonal basis, biomass can be converted into electricity and heat throughout the year, thus stabilizing energy generation [8]. Biomass derived from lignocellulosic crops [8,9,10,11] that contain cellulose, hemicellulose (polysaccharides), and lignin (an aromatic polymer that acts as a binding agent for cellulose and hemicellulose) [12] is the most widely available source of renewable energy in the world. Lignocellulosic biomass is the third most important energy source after crude oil and coal [13]. It is one of the most sustainable sources of organic carbon (Corg) with net zero emissions of CO2 [12]. In addition, most lignocellulosic crops, including dedicated energy crops, do not compete with food crops [14].
Maize (Zea mays L.) is the most popular lignocellulosic crop for bioenergy generation in Europe [15]. Maize is characterized by high biomass yields [16,17], high biochemical methane potential, and high biogas potential [18]. In Europe, the highest number of agricultural biogas plants (10,600) are operated in Germany [19]. Germany is the global leader in terms of installed electrical capacity of biogas plants (7500 MWel) (59% and 41% of European and global electrical biogas capacity, respectively) [20]. Maize accounts for 73% of biomass feedstocks in German biogas plants [21]. In 2012, regulatory limits were imposed on the proportions of various feedstocks in biogas plants, and the so-called “maize cap” was introduced to promote the use of alternative substrates [22]. Maize is usually grown in the proximity of biogas plants to guarantee regular feedstock supplies. When grown as an energy crop, maize decreases the biodiversity of agricultural ecosystems and competes with food crops for arable land [23]. In Europe, maize is grown in high-input production technologies characterized by high fertilizer consumption, intensive tillage, and relatively high use of pesticides. High-input production technologies exert a negative impact on the environment, in particular when maize is grown in large-scale monocultures [8,24,25].
The biomass of Jerusalem artichoke (JA, Helianthus tuberosus L.) is an alternative substrate for renewable energy production [26,27,28,29,30,31]. Jerusalem artichoke has numerous advantages, including (i) low environmental and agronomic requirements [29,32,33,34]; (ii) tolerance to drought and soil salinity; and (iii) low fertilizer requirements [32,33,34]. Jerusalem artichoke can be grown as an annual plant (where tubers and aerial biomass are harvested each year) or as a perennial plant (where only aerial biomass is harvested annually) [28,29,35,36]. Both tubers and aerial biomass constitute valuable substrates for biogas and bioethanol production [26,29,37,38]. The yield potential of JA tubers and aerial biomass has been estimated at 70–100 Mg ha−1 fresh matter (FM) [39,40] and 24–80 Mg ha−1 dry matter (DM) [29,31,40,41,42,43,44]. The energy inputs (EIs) in the cultivation technology of JA range from 12–28 GJ ha−1 (in a perennial cropping system, where only aerial biomass is harvested annually) [30,31] to 32–45 GJ ha−1 (in an annual cropping system, where both tubers and aerial biomass are harvested each year) [45]. In perennial cropping systems, mineral fertilization is the most energy-intensive operation that accounts for 32–53% (in the year of plantation establishment) to 60–87% of total EI (successive years) [30,31]. Mineral fertilizers have a high share of total EI in crop production technologies mainly because fertilizer manufacture is an energy-intensive process [31]. In addition, intensive mineral fertilization, even at rates that are optimal for a given crop species under local conditions, has a negative impact on the ecosystem and the environment [46,47]. Organic fertilizers derived from agricultural (liquid and solid manure, slurry) and industrial production (municipal sewage sludge, digestate from agricultural biogas plants) offer an alternative to mineral fertilizers. Recycled fertilizers can decrease EI in crop production technologies [48,49] and eliminate/minimize the negative environmental impact of mineral fertilizers [47] without compromising agricultural productivity [50].
Digestate, a by-product from the anaerobic digestion of lignocellulosic biomass, is a popular organic fertilizer [16,51,52,53,54]. Biogas produced during anaerobic digestion is used for power generation, whereas digestate can be applied as organic fertilizer in agricultural production [55,56,57]. Digestate is a rich source of nitrogen (N), phosphorus, potassium, sulfur, micronutrients, and organic matter [58,59,60,61,62]. Digestate decreases the use of mineral fertilizers and mined resources (phosphate rock and natural gas) that are used in the production of mineral fertilizers [63]. Digestate contains mineral nutrients that are readily available to plants and significantly accelerate crop growth [64]. Digestate is also abundant in organic matter which increases the content of Corg in soil [65,66], initiates microbial processes [67,68], and stimulates the activity of soil enzymes [69]. As a result, digestate regulates the rate at which nutrients are released from organic compounds into the soil [64,65]. Digestate also improves soil structure [70] and soil’s ability to retain nutrients and water [46,52]. In the European Union (EU), around 180 Tg of digestate (a mixture of liquid manure and energy crops) is produced each year, mostly (67%) in agricultural farms [71]. Digestate is produced continuously in biogas plants, and it has to be adequately managed [72,73]. Prolonged storage of digestate is expensive and requires dedicated technical equipment [74]. Direct application in the field is the most popular and the cheapest method of digestate management [75,76]. However, effective groundwater protection measures are required when digestate is used as agricultural fertilizer. Soil nutrient levels have to be monitored, and legal standards concerning maximum nutrient loading rates have to be observed [77]. Directly after application, digestate should be incorporated into the topsoil layer to maximize productivity and protect the environment [78]. Adequate management of digestate in agricultural production is a direct and effective method of implementing the zero-waste concept as one of the pillars of a circular economy [71].
Research studies analyzing the effect of various methods of mineral fertilizer and digestate application on the yield of silage maize biomass, perennial grasslands, and winter triticale (×Triticosecale Wittm. ex A. Camus) intercropped with red clover (Trifolium pratense L.) demonstrated that digestate (solid and liquid fractions) induced a similar increase in biomass yields to mineral fertilizers [79]. Complete substitution of mineral fertilizers with digestate was possible in the intercropping system, and the greatest increase in biomass yields was noted when mineral fertilizers were combined with digestate, which reduced mineral fertilizer input by up to 66% [79]. In a study by Stolarski et al. [80], JA biomass yields increased when digestate was applied at a rate equivalent to 170 kg N ha−1. At a lower fertilizer rate (85 kg N ha−1), digestate induced a smaller increase in yields than mineral fertilizers [80]. In the work of Lee et al. [53], digestate applied at a rate equivalent to 150 kg N ha−1 increased biomass yields in switchgrass (Panicum virgatum L.) by 14%. At lower N rates (50 or 100 kg ha−1), digestate was less effective than mineral fertilizers. In turn, Nabel et al. [52] reported a correlation between fertilizer type, soil, and the biomass yield of Virginia fanpetals [Sida hermaphrodita (L.) Rusby]. In nutrient-poor soils (with a high content of the particle size fraction >2 mm and a low content of Corg), the biomass yield of Virginia fanpetals was 30% higher after the application of digestate than mineral fertilizers. However, mineral fertilizers induced a greater increase in biomass yields in nutrient-rich soils (with a high content of particle size fractions <2 mm). According to Dubis et al. [54], digestate and mineral fertilizers led to a comparable increase in the yields of Amur silvergrass [Miscanthus sacchariflorus (Maxim.) Hack.]. These observations indicate that digestate has a positive influence on the yields of energy crops. However, digestate’s impact on the energy balance in the cultivation technology of energy crops remains insufficiently investigated [49,54,81]. There is some evidence to indicate that recycled fertilizers (sewage sludge and solid digestate) improved the energy balance in the production of Amur silvergrass. Organic fertilizers decreased EI by 34–40% (sewage sludge) and 41–48% (digestate) [54]. In the work of Dubis et al. [54], the energy output (EO) was highest when M. sacchariflorus was supplied with 100 kg N ha−1, regardless of fertilizer type (mineral fertilizer vs. digestate or sewage sludge). In turn, energy gain (EG) peaked in response to digestate applied at a rate equivalent to 160 kg N ha−1. The energy efficiency ratio (EER) was also higher when M. sacchariflorus was fertilized with digestate or sewage sludge than mineral fertilizers [54]. In the cultivation of M. × giganteus, EI decreased by 32–34% (without affecting biomass yields) and the EER increased by 43−52% when mineral fertilizers were replaced with sewage sludge [82]. In the production technology of H. tuberosus, EI decreased by 27–32% and the EER increased by 44–48% when mineral fertilizers were replaced with sewage sludge [30].
The use of digestate as fertilizer in the production of non-food crops is a sustainable approach to waste management [81]. This biofertilizer can also increase yields in the production of JA biomass. At the same time, biomass produced with the use of digestate constitutes a valuable substrate or co-substrate for agricultural biogas plants; therefore, it is an important source of biogas and digestate in a circular economy. The production technology of JA biomass has to be intensified to ensure that EO exceeds EI. This is an important consideration because biomass conversion to energy will be environmentally and economically justified only if more energy is generated than input into the system. The influence of digestate fertilization on biomass yields and energy balance in the production of JA biomass has not been examined extensively to date. Therefore, the aim of this study was to determine the effect of N supplied with mineral fertilizers (ammonium nitrate, 34% N) and organic fertilizers (liquid digestate, 0.49–0.54% N) on biomass yields and energy balance (energy inputs, energy output, energy gain, and the energy efficiency ratio) in the cultivation technology of JA in a perennial cropping system (where only aerial biomass was harvested each year).
2. Materials and Methods
2.1. Field Experiment
The experiment was conducted in 2021–2023 at the Agricultural Experiment Station (AES) in Bałdy (Tomaszkowo village; 53°42′44′′ N, 20°25′54′′ E; elevation: 130 m above sea level; North-East Poland) which is part of the University of Warmia and Mazury in Olsztyn. The experimental variables were the N rate and two types of fertilizers: (i) control (without fertilization), (ii) 50 kg N ha−1 supplied with mineral fertilizers, (iii) 50 kg N ha−1 supplied with digestate, (iv) 75 kg N ha−1 supplied with mineral fertilizers, (v) 75 kg N ha−1 supplied with digestate, (vi) 100 kg N ha−1 supplied with mineral fertilizers, and (vii) 100 kg N ha−1 supplied with digestate. The experiment had a randomized block design with three replications. The experiment was established on Haplic Luvisol originating from boulder clay [83]. The plot area was 15 m2 (6.0 × 2.5 m). Oat (Avena sativa L.) was the preceding crop. Before the experiment, soil samples (5 samples per plot) were collected at a depth of 0–20 cm. The experiment was established on slightly acidic soil (pH 5.8) with organic carbon (Corg) and total nitrogen (Nt) content of 8.1 and 1.06 g kg−1, respectively. The content of available nutrients was determined at 206 mg P2O5 kg−1, 165 mg K2O kg−1, 45 mg Mg kg−1; 10 mg kg−1, 0.3 mg B kg−1; 93 mg Mn kg−1; 1 mg Cu kg−1, 7 mg Zn kg−1, and 910 mg Fe kg−1. The chemical composition of the soil was analyzed in the Chemical–Agricultural Station in Olsztyn according to the procedure described by Bardsley and Lancaster [84] and Houba et al. [85].
Nitrogen was applied as ammonium nitrate (34% N). In treatments with mineral fertilization, 65 kg P2O5 ha−1 (enriched superphosphate, 40% P2O5) and 140 K2O ha−1 (potash salt, 50% K2O) were applied in addition to N. Liquid digestate from a mesophilic digestion process was obtained from an agricultural biogas plant operated by EKODAMIR sp. z o.o., sp. k. in Jarnołtowo (situated 80 km from the experimental site). Digestate had alkaline pH (pH 7.8–8.9), DM content of 47 g kg−1, and the following chemical composition: 4.9–5.4 g Nt kg−1 DM, 1.2–1.4 g P kg−1, 3.4–3.9 g K kg−1, 1.2–1.3 g Ca kg−1, 0.2 g Mg kg−1 0.02–0.03 mg Cd kg−1, 1.1 mg Ni kg−1, 0.34–0.76 mg Pb kg−1, and 0.67 mg Cr kg−1. Digestate rates (ii, iv, and vi) were calculated in each year of the experiment based on the content of Nt and DM (Table S1). The chemical composition of digestate was determined at the Aquanet Laboratorium sp. z o. o. in Poznań (Central-East Poland). In the first year of the experiment (plantation establishment), mineral fertilizers and digestate were applied before tuber planting. In years 2 and 3, fertilizers were applied before the beginning of the spring growing season. In all years of the study, only aerial biomass was harvested, and JA tubers were left in the soil for three years. The production process in the year of plantation establishment (year 1) and productive years (years 2 and 3) is presented in Table 1.

Table 1.
Production process of Jerusalem artichoke.
2.2. Energy Input Analysis
The EIs associated with the cultivation technology of JA were determined based on diesel fuel consumption, the performance of tractors and agricultural machines, and human labor which were measured directly in the experimental fields in AES in Bałcyny (Table S2). The weight and operating time of tractors and agricultural machines were determined based on the work of Muzalewski [86,87]. The following factors were included in the calculation of EI in the production technology of JA: transport of agricultural equipment and materials from the farm to the experimental field, biomass transport from the experimental field to the farm (1 km), and digestate transport from the biogas plant to the experimental field (80 km). The energy equivalents for calculating EI are presented in Table 2. Total EIs were calculated by summing up the EI associated with diesel oil consumption (energy carriers), labor, tractors and agricultural machines (fixed assets), JA tubers for planting, fertilizers, and pesticides (agricultural materials) according to the formula proposed by Bogucka and Jankowski [31].

Table 2.
Energy equivalents of inputs associated with the production of Jerusalem artichoke.
2.3. Biomass Processing
The fresh matter yield (FMY) of JA was calculated directly after harvest, and the results were expressed per hectare. The moisture content of the harvested biomass was determined by drying 1 kg samples from each plot at a temperature of 105 °C in a Binder FD 53 ventilated oven (Binder GmbH, Tuttlingen, Germany) until constant weight. The results were used to calculate the DM content and dry matter yield (DMY) of JA biomass according to the formulas presented by Bogucka and Jankowski [31].
2.4. Energy Output Analysis
The higher heating value (HHV) of JA biomass was determined by adiabatic combustion in a C 6000 calorimeter (IKA-Werke GmbH & CO. KG, Staufen, Germany). The lower heating value (LHV) of JA biomass was calculated based on the formula presented by Kopetz et al. [89]. The EO of JA biomass was calculated as the product of FMY and LHV [31].
2.5. Energy Gain and the Energy Efficiency Ratio
The EG and EER were calculated to determine the energy balance in the cultivation technology of JA. Energy gain was calculated as the difference between EO and EI [31]. The EER was determined as the ratio of the volume of energy accumulated in JA biomass (EO) to the energy invested in the biomass production technology (EI) [31].
2.6. Statistical Analysis
The values of FMY, DMY, DM, LHV, EO, EG, and EER were processed by ANOVA with the use of a randomized block design model in Statistica v. 13.3 [90]. The F-values in ANOVA are presented in Table 3. Treatment means were compared based on studentized range distribution in Tukey’s Honest Significant Difference (HSD) test.

Table 3.
F-test statistics in ANOVA.
3. Results
3.1. Weather Conditions
Weather conditions varied across years during the three-year experiment (Table 4). In the first and second growing seasons (April-September), the mean daily temperature was 0.4 °C and 0.8 °C lower, respectively, than the long-term average (14.5 °C). In the third growing season, the mean daily temperature approximated the long-term average. The first growing season (2021) was characterized by very high precipitation. Total rainfall exceeded the long-term average by 46% (533 vs. 366 mm), and May, July, and August were particularly wet months. In the remaining growing seasons (2022 and 2023), precipitation was 12% and 25% below the long-term average, respectively. The third growing season was particularly dry with very low rainfall between April and July (36%, 72%, 42%, and 54% below the long-term average in April, May, June, and July, respectively) (Table 4).

Table 4.
Weather conditions during the experiment (2021–2023) and the long-term average (1981–2010) in Tomaszkowo village (AES in Bałdy). Data were acquired with the use of the PM Ecology automatic weather station (PM Ecology sp. z o. o, Gdynia, Poland).
3.2. Energy Inputs
The EIs associated with the cultivation technology of JA in a perennial cropping system (where only aerial biomass was harvested each year) were differentiated by the N rate, type of fertilizer, and year (Table 5). In year 1 (plantation establishment), EIs in the JA biomass production technology without fertilization were determined at 16.2 GJ ha−1. The N rates of 50, 75, and 100 kg N ha−1 supplied with mineral fertilizers increased EI in biomass production by 39%, 51%, and 63%, respectively. In treatments fertilized with digestate, the EI was lower by 17–19% (i.e., by 3.8–5.1 GJ ha−1). In years 2 and 3, no tillage or planting operations were performed, and EIs were lower by 45–56% (mineral fertilization), 55–72% (digestate), and 76–82% (no fertilization) relative to the year of plantation establishment. In years 2 and 3, EI in the JA biomass production technology without fertilization reached 3.8 and 2.9 GJ ha−1, respectively. In these years, EIs were highest in cultivation technologies with mineral fertilizers (9.9–14.6 GJ ha−1 y−1). The application of digestate decreased EI in the JA cultivation technology by 35–47% (Table 5) due to the lower energy value of digestate relative to mineral fertilizers (6.2–10.1 vs. 3.1–4.1 GJ ha−1) (Table 6). The EI related to digestate transport and application (labor, tractors and machines, and diesel fuel) were only 0.4 and 0.9 GJ ha−1 higher than the EI associated with mineral fertilizer rates of 50 and 100 kg N ha−1, respectively. The EI associated with digestate transport and application did not exert a negative impact on the EO of JA biomass because digestate has a lower energy value (by 3.1–6.0 GJ ha−1) than mineral fertilizers (Table 6).

Table 5.
Energy inputs in the production technology of Jerusalem artichoke biomass, per agricultural operation (2021, 2022, 2023).

Table 6.
Energy inputs in the production technology of Jerusalem artichoke biomass, per energy flux (2021, 2022, 2023).
The EIs associated with each agronomic operation are presented in Table 5. In year 1, tuber planting was the most energy-intensive operation (50% of total EI) in the JA biomass production technology without fertilization. In years 2 and 3, the most energy-intensive operations were biomass harvest and transport (63% and 51% of total EI, respectively) and weed control (33% and 44% of total EI, respectively). Nitrogen fertilization not only increased the demand for energy in the JA cultivation technology, but also modified the structure of EI. In year 1, tuber planting (31–36% of total EI) and fertilization (28–39%) were the main EI in production technologies with mineral fertilization. In production technologies involving digestate, the most energy-intensive operations were tuber planting (38–43% of total EI), biomass harvest and transport (21–24%), and fertilization (14–24%). In years 2 and 3, N fertilization was the main EI regardless of fertilizer type. In these years, N fertilization accounted for 57–78% (mineral fertilizers) and 33–63% (digestate) of total EI (Table 5).
An analysis of the EI by energy fluxes revealed considerable differences between years (year 1 vs. years 2 and 3) and fertilizer types (mineral fertilizers vs. digestate) (Table 6). In year 1, the highest EIs were associated with materials (44–65%) and energy carriers (24–40%). The share of agricultural materials in total EI increased, whereas the share of energy carriers fuel decreased with a rise in the N rate (50, 75, and 100 kg ha−1), regardless of fertilizer type. In years 2 and 3, fertilizer type (mineral vs. organic) induced the greatest differences in the structure of EI. In production technologies without fertilization and in technologies where digestate was applied as a source of nutrients, energy carriers were the main EI (67–69% and 39–49% of total EI, respectively). In production technologies with mineral fertilization, energy consumption was determined mainly by mineral fertilizers that accounted for 56–77% of total EI (Table 6).
3.3. Biomass Yield
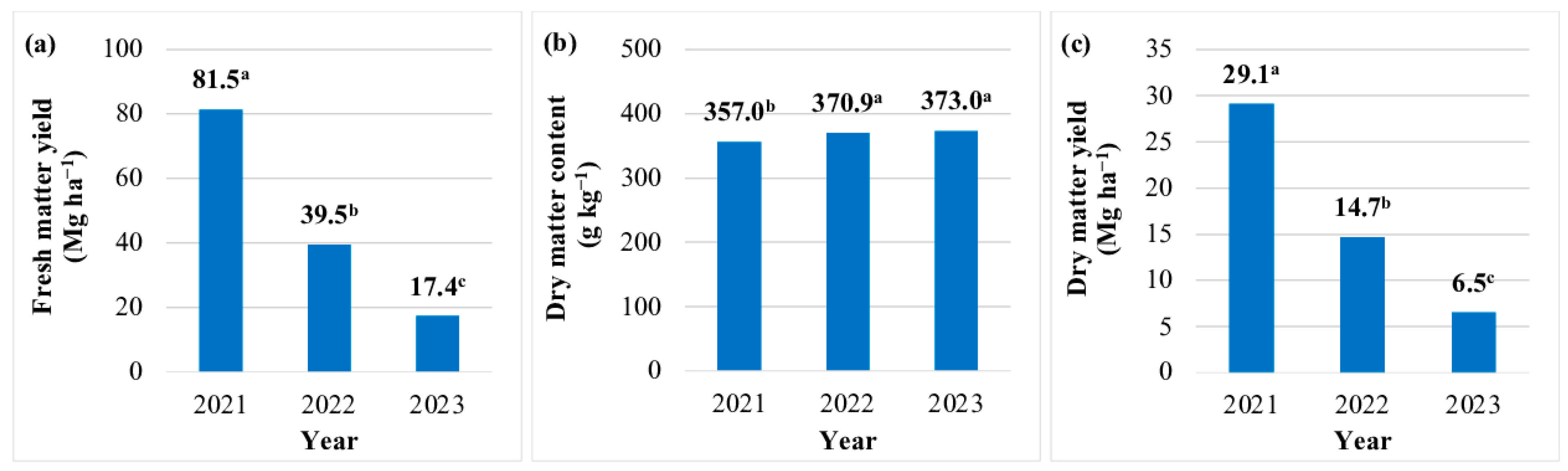
The FMY of JA aerial biomass was highest (81.5 Mg ha−1) in year 1. In years 2 and 3, the FMY was 52% and 79% lower, respectively (Figure 1a). The FMY of JA aerial biomass was lowest in the production technology without fertilization 34.3 Mg ha−1) (Table 7). The FMY increased by 32–40% in response to the N rate of 50 kg N ha−1, regardless of fertilizer type. An increase in the N rate to 75 kg N ha−1 was justified only when JA was fertilized with digestate (FMY increased by 18%). A further increase in the N rate (100 kg N ha−1) did not lead to a significant increase in FMY, irrespective of fertilizer type (Table 7). The DM content of JA biomass differed across each year. This parameter was lowest in year 1 (357.0 g kg−1), and it increased by 4% in years 2 and 3 (Figure 1b). The DM content of JA biomass was not significantly affected by the N rate or fertilizer type (Table 3 and Table 7).
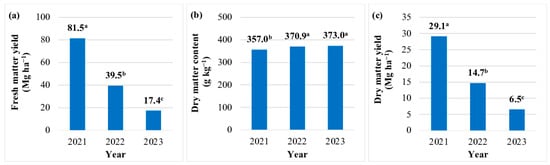
Figure 1.
Yield and moisture content of Jerusalem artichoke biomass in each year of the study (average values for the fertilization strategy). Means with the same letter do not differ significantly at p ≤ 0.05 in Tukey’s HSD test.

Table 7.
Yield and moisture content of Jerusalem artichoke biomass (average for 2021–2023).
The DMY of JA biomass was highest (29.1 Mg ha−1) in the first year of the experiment. In years 2 and 3, the DMY of aerial biomass was lower by 14.4 and 22.6 Mg ha−1 (by 50% and 78%), respectively (Figure 1c). This parameter was lowest (12.5 Mg ha−1 DM) in the JA biomass production technology without fertilization. The N rate of 50 kg ha−1 increased DMY by 30–40%, regardless of fertilizer type. The N rate of 75 kg ha−1 increased DMY (by 11–19%) only after the application of digestate. The highest N rate (100 kg ha−1) did not induce a significant increase in DMY, irrespective of fertilizer type (Table 7).
3.4. Energy Output
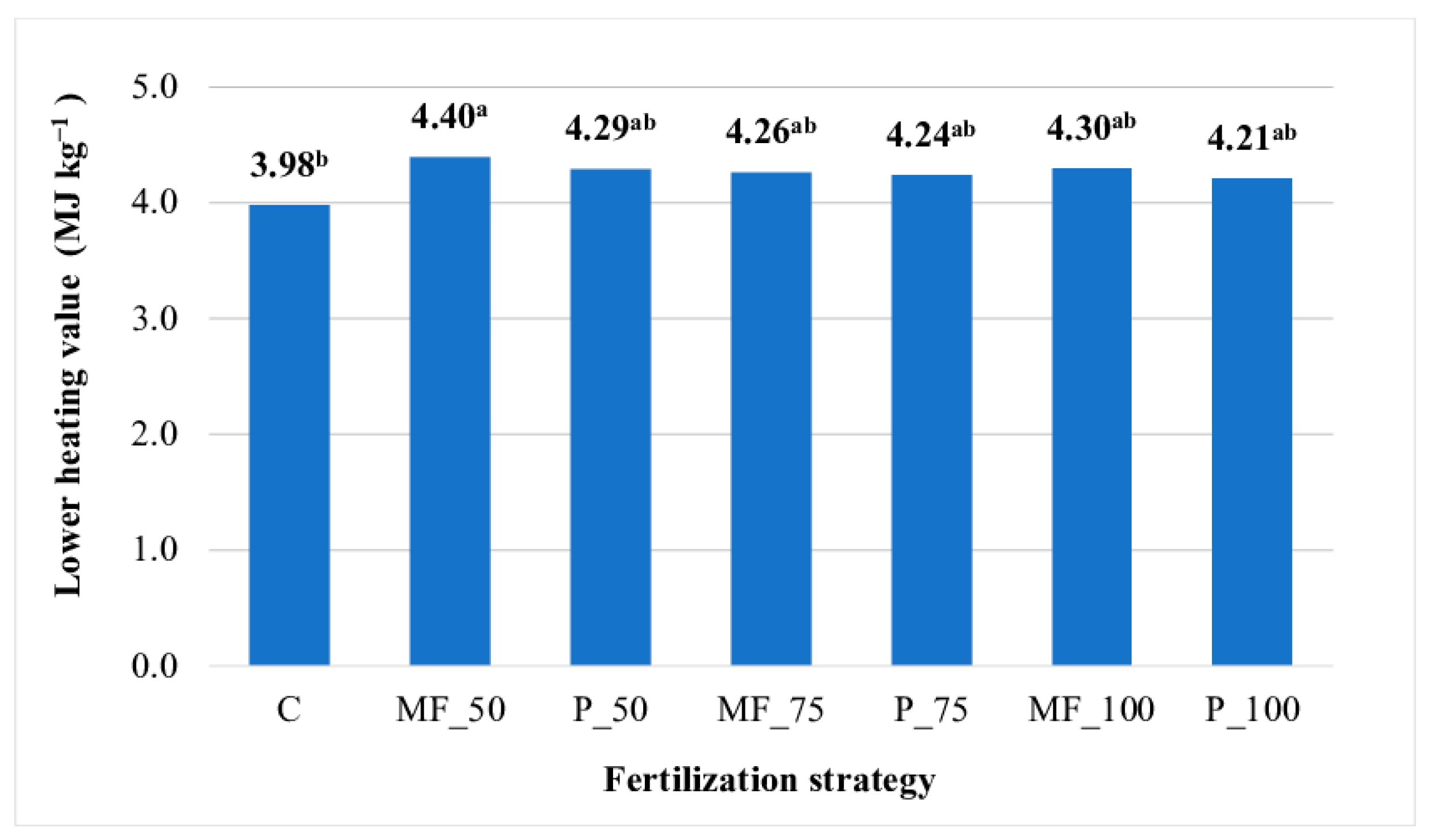
The LHV of JA aerial biomass did not differ significantly across the experimental years (years 1–3). This parameter was lowest in the JA biomass production technology without fertilization (3.98 MJ kg−1). A significant increase in the LHV of biomass (by 11%) was observed after the application of 50 kg N ha–1 in mineral form (Figure 2).
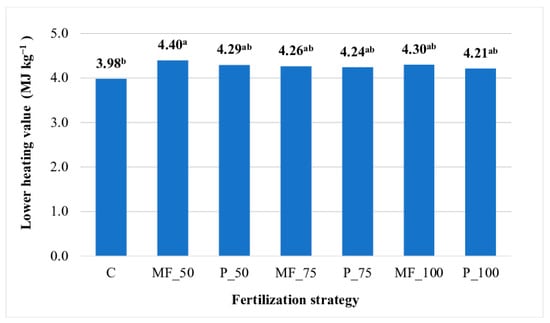
Figure 2.
Lower heating value (MJ kg−1) of Jerusalem artichoke biomass (average for 2021−2023). Means with the same letter do not differ significantly at p ≤ 0.05 in Tukey’s HSD test. C—unfertilized control; MF_50—mineral fertilizers (50 kg N ha−1); P_50—digestate (50 kg N ha−1); MF_75—mineral fertilizers (75 kg N ha−1); P_75—digestate (75 kg N ha−1); MF_100—mineral fertilizers (100 kg N ha−1); P_50—digestate (100 kg N ha−1).
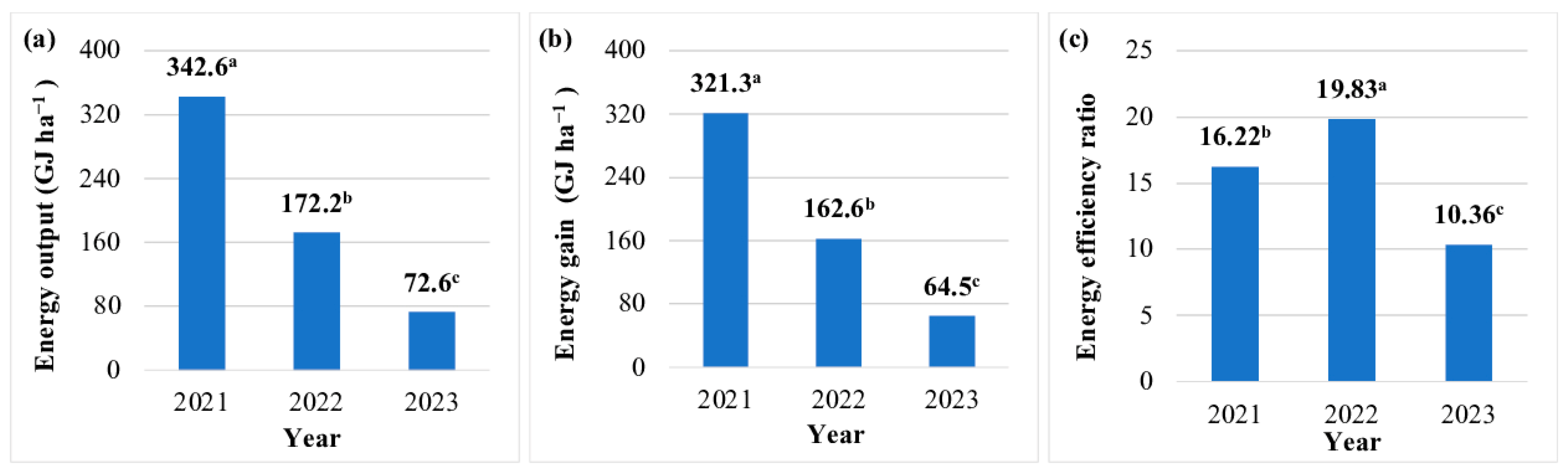
The EO of JA biomass was highest in the year of plantation establishment (342.6 GJ ha−1), and it decreased by 50% in year 2 and by 79% in year 3 (Figure 3a). The EO was lowest in the JA biomass production technology without fertilization (134.8 GJ ha−1) (Table 8). Nitrogen fertilization increased the EO by 42–55% (50 kg ha−1), 56–71% (75 kg ha−1), and 46–47% (100 kg ha−1). The EO of aerial biomass was highest (230.1 GJ ha–1) in the production technology where mineral N was supplied at 75 kg ha−1 (Table 8).
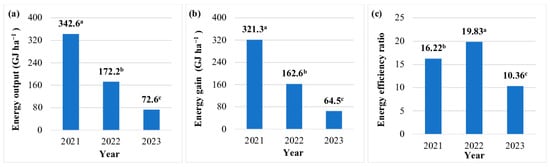
Figure 3.
Energy balance in the production technology of Jerusalem artichoke biomass in each year of the study (average values for the fertilization strategy). Means with the same letter do not differ significantly at p ≤ 0.05 in Tukey’s HSD test.

Table 8.
Energy balance in the production technology of Jerusalem artichoke biomass (average for 2021–2023).
3.5. Energy Gain and the Energy Efficiency Ratio
The EG in JA cultivation was highest in the year of plantation establishment (321.3 GJ ha−1). This parameter was 49% and 80% lower in years 2 and 3, respectively (Figure 3b). During the entire three-year experiment, the average EG was lowest (127.2 GJ ha−1) in the JA biomass production technology without fertilization and highest (218.5 GJ ha−1) in the production technology where digestate was applied at a rate equivalent to 75 kg N ha−1. An increase in the N rate to 100 kg ha−1 decreased EG by 16–18%, regardless of fertilizer type (Table 8).
The EER of the examined JA cultivation technologies was very high (6.5 to 29.1) in all years and fertilization regimes (Figure 3b). The EER peaked (29.1) in year 1, and it was two times and 4.5 times lower in years 2 and 3, respectively. The EER was highest in the JA biomass production technology without fertilization (20.1) and in the technology where digestate was applied at a rate equivalent to 75 kg N ha−1 (19.7). The remaining N rates led to a significant decrease in the EER. It should be noted that mineral fertilizers induced a greater decrease in the EER than digestate (33–49% vs. 12–27%). During the entire three-year experiment, the average EER was 21% higher in the biomass production technologies where JA was fertilized with digestate than in technologies involving mineral fertilizers (14.6 vs. 12.1) (Table 8).
4. Discussion
4.1. Energy Inputs
Energy crops should be characterized by high biomass yields, high EO, and relatively low EI in the production process [82]. These parameters can be improved by modifying the energy intensity of the cultivation technology [91,92,93,94]. In the work of Fang et al. [45], the EI associated with JA cultivation in North-Eastern and Eastern China reached 34.7 and 44.7 GJ ha−1, respectively. According to Stolarski et al. [81], the EI in the cultivation technology of JA in North-Eastern Poland ranged from 10.8–19.8 to 32.3–59.1 GJ ha−1. In the present study, EI ranged from 16.2 (no fertilization) to 18.7–22.5 and 21.2–26.3 GJ ha−1 (50 and 100 kg N ha−1, respectively) in the year of plantation establishment. In years 2 and 3, EI decreased by 45–56% (mineral fertilizers), 55–72% (digestate), and 76–82% (no fertilization). In the work of Jankowski et al. [30] and Bogucka and Jankowski [31], the demand for energy also decreased in the second and third year of biomass production (by 35–47% relative to the year of plantation establishment). In the perennial cropping system, EIs were lower due to the absence of planting and tillage operations in successive years of the experiment. Perennial cropping promotes soil organic matter accumulation, carbon sequestration, and the diversity of soil-dwelling microorganisms. It also reduces the demand for fossil fuels and decreases greenhouse gas emissions.
In the biomass production of perennial crops, the demand for energy is influenced mainly by agricultural materials (root cuttings, rhizomes, and tubers) and mineral fertilizers in the first year of cultivation. In perennial cropping systems, mineral fertilization is the most energy-intensive agricultural operation in successive years of cultivation [30,31,49,82]; present study, Table 6. In modern cultivation technologies, the demand for energy can be reduced through the use of alternative fertilizers [95], including digestate [16,51,54], digested biogas crops and slurry [96], municipal sewage sludge [30,49,54,82], and meat and bone meal [48]. Energy inputs were reduced by 27–34% when mineral fertilizers were replaced with sewage sludge in the cultivation of JA [30], cup plant (Silphium perfoliatum L.) [49], and giant miscanthus [Miscanthus × giganteus (Greef et Deuter)] [82]. In the current experiment, the replacement of mineral fertilizers with digestate decreased the demand for energy in JA production by 17–19% (year 1) to 55–72% (years 2 and 3). In the cultivation technology of Amur silvergrass, EIs were also reduced (by 41–48%) when digestate was used instead of mineral fertilizers [54].
The present study demonstrated that digestate is an alternative fertilizer that significantly decreases the demand for mineral fertilizers in the production of JA biomass. Mineral fertilizers are among the most energy-intensive inputs in farming, mainly because their production requires considerable amounts of energy (non-renewable resources, including fossil fuels). The results of this experiment indicate that EI associated with JA production in a perennial cropping system can be reduced by up to 55–72% when mineral fertilizers are replaced with digestate. This decrease can significantly reduce energy consumption in large-scale farms. The replacement of mineral fertilizers with recycled fertilizers not only lowers EI in the production process, but also decreases agricultural producers’ dependence on external energy suppliers. As a result, the production process will be less influenced by fluctuations in the prices of mineral fertilizers and unstable supplies, thus providing farmers with greater autonomy in managing the production process. Biomass producers who use recycled fertilizers are less vulnerable to changes in the energy market.
4.2. Biomass Yield
The biomass yield of JA is influenced mainly by the availability of water [97,98,99]. Drought, in particular during flowering and tuber formation, decreases the accumulation of DM [100] and biomass yields in JA by 20% [97,101] to 25% [102]. In the present study, the FMY and DMY of JA aerial biomass were highest in the wet and warm year of 2021 (the first growing season). In turn, biomass yields were lowest in the third growing season which was characterized by prolonged drought (April-July). The biomass yields of JA were also affected by weather conditions in the work of Jankowski et al. [31], Piskier [35], Sawicka [43], and Skiba [103].
Jerusalem artichoke yields are strongly determined by climate [28,29,31,34,40,43,102,104,105,106,107]. The yield potential of this crop species is lowest (<15 Mg DM ha−1) in the subarctic climate (Dfc—Köppen climate classification) of North America, Northern Europe, Eastern Europe, and North Asia [28,108,109,110]. Aerial biomass yields can reach 30 Mg ha−1 DM in the humid continental climate of Central Europe (Dfb) and the cold semi-arid climate (BSk) of the Middle East ([31,34,40,43,107,111,112]; present study, Figure 1c). In Poland, the aerial biomass yield of JA ranges from 6–28 Mg ha−1 DM on nutrient-poor soils [43,113] to 35–50 Mg ha−1 DM on soils that are more abundant in nutrients and water [43,101]. The present experiment was established on slightly acidic Haplic Luvisol originating from boulder clay, and the DMY of JA aerial biomass was determined at 6.5 to 29.1 Mg ha−1. In studies conducted by Stolarski et al. [81] and Jankowski et al. [30] in North-Eastern Poland, the DMY of JA aerial biomass reached 5.9–13.5 and 5.1–16.4 Mg ha−1, respectively. The highest JA yields (up to 40 Mg ha−1 DM) are noted in the Mediterranean climate (Cs) of Western and Southern Europe and in the cold desert climate (BWk) of East Asia [98,102,104,105,106,114,115,116,117].
Nitrogen fertilization is essential for the growth and development of JA plants. The optimal N rate in JA cultivation ranges from 52 to 190 kg ha−1 [29,34,102,104,117]. In Northern China, aerial biomass yields increased in response to 50–75 kg N ha−1, whereas maximum tuber yields were achieved already after the application of 25 kg N ha−1 [118]. In another study conducted in China, the optimal N rate in JA production was 20–50 kg ha−1 [117]. In the United Kingdom, the N rate of 56 kg ha−1 induced the greatest increase in JA biomass yields [119]. In Spain, JA biomass yields peaked in response to 100 kg N ha−1 [99]. In Central Germany, N fertilization increased biomass yields up to the rate of 120 kg ha−1. In this study, the DMY of JA aerial biomass was highest (19.4 Mg ha−1) after the application of 75 kg N ha−1. An increase in the N rate to 100 kg ha−1 decreased DMY by 14%. However, in some studies, N fertilization exerted no yield-forming effects in JA cultivation [28,104]. Research studies conducted in various parts of the world (China, United Kingdom, Spain, Germany) have shown that the optimal N rates are determined by local agroecological conditions [28,29,34,99,102,104,117,118,119]; present study, Table 7. The above implies that N fertilization strategies should be adapted to soil conditions and climate in a given region. Further research is needed to develop optimal N rates for JA biomass production in different climate zones with the aim of promoting sustainable management of agricultural resources, reducing eutrophication of surface waters, and decreasing greenhouse gas emissions.
The biomass yields of JA can be improved not only through the application of mineral fertilizers containing N, but also through the use of organic fertilizers, including digestate [80,120]. Fertilization is regarded as the cheapest method of digestate management [120,121], which promotes the recovery and recycling of organic and mineral compounds [58]. Research has shown that digestate has a positive impact on crop yields [59,122,123]. In a pot experiment conducted by Szymańska et al. [124], digestates obtained from typical agricultural substrates and wastes from the agri-food industry significantly increased maize biomass yields. In comparison with the unfertilized control treatment, maize biomass yields were more than three times higher in treatments fertilized with agricultural digestates and more than two times higher in treatments supplied with agri-food waste digestates. According to Siebielec et al. [125], digestate enhanced maize and winter wheat (Triticum aestivum L.) yields, but decreased winter rapeseed (Brassica napus L.) yields. Digestate exerted similar or greater yield-forming effects than mineral fertilizers in the work of Dubis et al. [54], Sapp et al. [126], Barłóg et al. [127,128], and Jamison et al. [129]. In the current study, digestate was more productive than mineral fertilizers only when applied at a rate equivalent to 75 kg N ha−1. Lower (50 kg N ha−1) and higher (100 kg N ha−1) equivalent rates of digestate exerted a similar effect on biomass yields to mineral fertilizers.
In some studies, digestate had no effect on crop yields [130], whereas other researchers reported on the negative effects of digestate fertilizers [131,132,133]. According to Alburquerque et al. [134], some digestates contain heavy metals (mainly zinc and copper) at concentrations that are toxic to plants. Digestates with a high content of potassium, sodium, and chlorine may also exert phytotoxic effects. Seed germination and plant growth may be inhibited in soils that are abundant in these elements [135,136]. According to Tigini et al. [136], McLachlan et al. [137], and Teglia et al. [138], the phytotoxic effects of digestate can also be attributed to a high concentration of -N which inhibits germination. In turn, Sogn et al. [139] observed that the fertilizing value of digestate increases with a rise in -N levels. Digestate did not exert phytotoxic effects in the present study. The highest N rate (100 kg ha−1) decreased aerial biomass yields in JA plants that were fertilized with digestate as well as in plants that were supplied with mineral fertilizers.
The study demonstrated that the use of digestate as fertilizer promotes nutrient recycling, reduces waste, and contributes to the sustainable management of non-renewable resources. This fertilization strategy minimizes waste storage and its adverse impact on the environment. Digestate decreases the demand for mineral fertilizers, thus reducing greenhouse gas emissions and minimizing the chemical pollution of soils and groundwater. However, the presence of heavy metals and high concentrations of potassium, sodium, and chlorine in digestates can contribute to soil salinity and phytotoxicity. Therefore, the chemical composition of digestate should be monitored to reduce these risks. The results of this experiment indicate that digestate can be as effective or, in some cases, more effective than mineral fertilizers. Digestate offers a cheaper alternative to mineral fertilizers, thus decreasing costs in agricultural production. The use of digestate in the farming sector also contributes to effective waste management.
4.3. Energy Output
In energy crops, the EO of agricultural phytomass is determined mainly by the energy intensity of the production process [26,91,92,93,94]. An increase in EI associated with cultivation technology generally increases the EO of biomass [82,91,92,93,94,140,141]. In a study by Epie et al. [28], the EO of JA aerial biomass ranged from 182 to 269 GJ ha−1. Ivanova et al. [142] estimated the EO of JA aerial biomass at 495 GJ ha−1. In Poland, the EO of JA aerial biomass ranges from 60 to 321 GJ ha−1 ([30,31,35]; present study, Figure 3a). In this study, the EO of JA aerial biomass peaked (230.1 GJ ha−1) in response to 75 kg N ha−1 in mineral form. An equivalent N rate supplied with digestate decreased EO by 8%. Seleiman et al. [143] reported that sewage sludge increased the EO of maize and hemp (Cannabis sativa L.) by 5–6% and 20%, respectively. Dubis et al. [82] demonstrated that the EO of giant miscanthus biomass peaked after the application of 160 kg N ha−1, regardless of fertilizer type (mineral fertilizers vs. sewage sludge). In a study by Dubis et al. [82], the greatest increase in the EO of Amur silvergrass was noted in response to 100 kg N ha−1 in mineral form or 160 kg N ha−1 supplied with sewage sludge and digestate. Jankowski et al. [30] found that the EO of JA aerial biomass was influenced by the type of N fertilizer and plantation age. In year 1, EO was higher in treatments fertilized with sewage sludge. In year 2, higher EO was achieved in treatments supplied with mineral fertilizers. In year 3, the EO of biomass was not differentiated by the type of fertilizer.
Considerable variations in the EO of JA biomass supplied with different types and rates of N fertilizers (mineral vs. organic) indicate that this macronutrient should be rationally managed. Large-scale use of recycled fertilizers not only contributes to a circular economy, but also promotes the sustainable production of energy crops. The results of research studies examining the influence of various fertilization strategies on the yield of energy crops will enable farmers to adopt agricultural practices to plant requirements, thus increasing the energy efficiency of the entire biomass production system. The prices of mineral fertilizers have risen significantly in recent years, which is why recycled fertilizers can generate substantial economic benefits for agricultural producers. Digestates offer an attractive option for farmers due to lower fertilization costs and comparable or even higher energy value of the obtained biomass. The current study revealed that the EO of JA biomass is also strongly influenced by local agroecological conditions. The above implies that agricultural producers should adapt their fertilization strategies to local requirements by selecting the most appropriate type (mineral vs. organic) and rate of N fertilizer.
4.4. Energy Gain and the Energy Efficiency Ratio
An increase in the EI associated with the production technology of energy crops generally increases the EG (by increasing DMY and EO) and decreases the EER (the increase in EI exceeds the increase in EO) [30,82]. The EG in the production technology of JA aerial biomass ranges from 41–88 to 168–346 GJ ha−1 ([30,31,45,81]; present study, Figure 3b). In the current experiment, EG was lowest (127 GJ ha−1) in the JA biomass production technology without fertilization and highest (218 GJ ha−1) when digestate was applied at a rate equivalent to 75 kg N ha−1. An increase in the N rate to 100 kg ha−1 decreased EG by 16–18%, regardless of fertilizer type. In the work of Jankowski et al. [30], EG peaked in the cultivation technology where JA was fertilized with sewage sludge at a rate equivalent to 100 kg N ha−1. Organic fertilizers also improved EG in the cultivation of giant miscanthus (160 kg N ha−1 supplied with sewage sludge] and Amur silvergrass (160 kg N ha−1 supplied with digestate] [54,92].
In Europe, the EER in the production technology of JA biomass ranges from 7–18 ([30,35,81]; present study, Figure 3c) to 30 [31]. An increase in EI can decrease the EER of the JA biomass production technology by 32% to 80% [30,31,81]. According to Jankowski et al. [30] and Stolarski et al. [81], production technologies without fertilization are characterized by the highest EER values. In JA production, fertilization decreases the EER by 51–71% (85–100 kg N ha−1) and 47–79% (160–170 kg N ha−1). The EER is affected not only by the N rate, but also by the type of fertilizer [30,35,81]. In the work of Piskier [35], the EER was 31–41% higher in JA cultivation technologies involving mineral fertilizers than mineral-organic fertilizers (50:50) or organic fertilizers. Stolarski et al. [81] reported that the use of dry digestate and torrefied digestate decreased the EER in the JA biomass production technology by 46–55% and 49–62%, respectively, relative to production technologies involving mineral fertilizers. In the present study, the EER was highest in the JA biomass production technology without fertilization (20.1) and in the technology where liquid digestate was applied at a rate equivalent to 75 kg N ha−1 (19.7). Mineral fertilizers decreased the EER by 33–37% (50–70 kg N ha−1) and 49% (100 kg N ha−1). Jankowski et al. [30] also found that the EER was nearly two times higher when JA was supplied with organic fertilizers (sewage sludge) than mineral fertilizers. The replacement of mineral fertilizers with sewage sludge or digestate also exerted a positive impact on the EER in the production of giant miscanthus and Amur silvergrass [54,82].
The study demonstrated that digestate induced a greater increase in EG and the EER in the production technology of JA biomass than mineral fertilizers. This suggests that mineral fertilizers can be replaced with digestate when JA biomass is produced in a perennial cropping system, thus reducing the carbon footprint associated with biomass production and transport. Digestate can also decrease fertilization costs, which is a very important consideration in the face of the recent increase in the prices of mineral fertilizers. The transition to organic fertilizers (in particular recycled fertilizers) can generate economic benefits, especially for small-area farms, where capital is the main factor that limits agricultural production. Regardless of farm size (scale of production), the application of digestate as fertilizer constitutes an attractive management strategy by improving the energy balance in the production of energy crops. When JA biomass is produced in a perennial cropping system, the replacement of mineral fertilizers with digestate has positive implications for the environment and agricultural practice by promoting sustainable development and reducing fertilizers’ contribution to climate change.
5. Conclusions
In the year of plantation establishment, the demand for energy in the JA biomass production technology without fertilization reached 16.2 GJ ha−1. The application of mineral fertilizers at 50, 75, and 100 kg N ha−1 increased EI by 39%, 51%, and 63%, respectively. Energy inputs were 45–82% lower in productive years (years 2 and 3). The replacement of mineral fertilizers with digestate decreased EI by 17–19% (year 1) and 35–47% (years 2 and 3). Higher EIs associated with digestate transport and application (by 0.7 GJ ha−1) were compensated by the lower energy value of digestate relative to mineral fertilizers (difference of 4.2–6.4 GJ ha−1). Biomass yields peaked (19.4 Mg ha−1 DM) in the production technology where digestate was applied at a rate equivalent to 75 kg N ha−1. The EO of JA biomass was highest in year 1 (342.6 GJ ha−1), and it was 50% lower in year 2 and 79% lower in year 3. The EO of JA aerial biomass was lowest (134.8 GJ ha−1) in the production technology without fertilization. Nitrogen increased the EO of JA aerial biomass by 42–71%. Jerusalem artichoke biomass was characterized by the highest EO (230.1 GJ ha−1) in the production technology where N was applied at 75 kg ha–1 in mineral form. In turn, EG peaked (218.5 GJ ha−1) in response to digestate applied at a rate equivalent to 75 kg N ha−1. The EER was highest in the JA biomass production technology without fertilization (20.1) and in the technology where digestate was applied at a rate equivalent to 75 kg N ha−1 (19.7). The remaining N rates significantly decreased the EER of the production technology. Mineral fertilizers induced a greater decrease in the EER than organic fertilizers (33–49% vs. 12–27%). Production technologies involving digestate were characterized by higher EER (by 21% on average) than technologies involving mineral fertilizers (14.6 vs. 12.1). The energy balance (EG and EER) in the production of JA aerial biomass was most favorable when JA was fertilized with digestate at a rate equivalent to 75 kg N ha−1, regardless of farm size and factors that limit agricultural production (land resources in small-area farms and capital in large-area farms). The results of this study may pave the way for future research on novel agronomic strategies for sustainable bioenergy production, including nutrient recycling. In the future, a long-term study should also be undertaken to assess the impact of digestate fertilizers on JA biomass yields, the chemical and biological properties of soil, and greenhouse gas emissions under different agroecological conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en17205202/s1, Table S1. Nitrogen-equivalent rates of digestate; Table S2. Parameters, performance, and fuel consumption of agricultural machines in the production of Jerusalem artichoke (2021–2023).
Author Contributions
Conceptualization, K.J.J. and B.D.; methodology, K.J.J. and B.D.; software, K.J.J. and B.D.; validation, K.J.J.; formal analysis, K.J.J. and B.D.; investigation, K.J.J. and B.D.; resources, K.J.J. and B.D.; data curation, K.J.J. and B.D.; writing—original draft preparation, K.J.J. and B.D.; writing—review and editing, K.J.J. and B.D.; visualization, K.J.J. and B.D.; supervision, K.J.J.; project administration, K.J.J.; funding acquisition, K.J.J. All authors have read and agreed to the published version of the manuscript.
Funding
The results presented in this paper were obtained as part of a comprehensive study of the Faculty of Agriculture and Forestry University of Warmia and Mazury in Olsztyn (grant No. 30.610.013–110). The research was funded by the Minister of Science under the Regional Initiative of Excellence program.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors are grateful to Die Topinambur Manufaktur in Heimenkirch (Bavaria, Germany) for supplying Jerusalem artichoke tubers for the study. We would also like to thank the staff of the AES in Bałcyny for technical support during the experiment.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fatma, S.; Hameed, A.; Noman, M.; Ahmed, T.; Shahid, M.; Tariq, M.; Sohail, I.; Tabassum, R. Lignocellulosic biomass: A sustainable bioenergy source for the future. Protein Pept. Lett. 2018, 25, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.; Nanda, S.; Acharya, B.; Dalai, A.K. A review of thermochemical conversion of waste biomass to biofuels. Energies 2022, 15, 6352. [Google Scholar] [CrossRef]
- Silwadi, M.; Mousa, H.; AL-Hajji, B.Y.; A-LWahaibi, S.S.; AL-Harrasi, Z.Z. Enhancing biogas production by anaerobic digestion of animal manure. Int. J. Green Energy 2022, 20, 257–264. [Google Scholar] [CrossRef]
- Azni, M.A.; Khalid, R.M.; Hasran, U.A.; Kamarudin, S.K. Review of the effects of fossil fuels and the need for a hydrogen fuel cell policy in Malaysia. Sustainability 2023, 15, 4033. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Warmiński, K.; Krzyżaniak, M.; Olba-Zięty, E.; Akincza, M. Bioenergy technologies and biomass potential vary in Northern European countries. Renew. Sustain. Energy Rev. 2020, 133, 110238. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Ahmed, N.A.; Ogunkunle, O. Optimization of biogas yield from lignocellulosic materials with different pretreatment methods: A review. Biotechnol. Biofuels 2021, 14, 159. [Google Scholar] [CrossRef]
- Von Cossel, M.; Pereira, L.A.; Lewandowski, I. Deciphering substrate-specific methane yields of perennial herbaceous wild plant species. Agronomy 2021, 11, 451. [Google Scholar] [CrossRef]
- Lewandowski, I.; Bahrs, E.; Dahmen, N.; Hirth, T.; Rausch, T.; Weidtmann, A. Biobased value chains for a growing bioeconomy. GCB Bioenergy 2019, 11, 4–8. [Google Scholar] [CrossRef]
- Von Cossel, M.; Amarysti, C.; Wilhelm, H.; Priya, N.; Winkler, B.; Hoerner, L. The replacement of maize (Zea mays L.) by cup plant (Silphium perfoliatum L.) as biogas substrate and its implications for the energy and material flows of a large biogas plant. Biofuels Bioprod. Biorefining 2020, 14, 152–179. [Google Scholar] [CrossRef]
- Von Cossel, M.; Mangold, A.; Iqbal, Y.; Hartung, J.; Lewandowski, I.; Kiesel, A. How to generate yield in the first year—A three-year experiment on miscanthus (Miscanthus × giganteus (Greef et Deuter)) establishment under maize (Zea mays L.). Agronomy 2019, 9, 237. [Google Scholar] [CrossRef]
- Manyi-Loh, C.E.; Lues, R. Anaerobic digestion of lignocellulosic biomass: Substrate characteristics (challenge) and innovation. Fermentation 2023, 9, 755. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Sanjukta, S.; Bhatia, L.; Saha, K.; Mudgil, D.; Shadangi, K.P.; Srivastava, R.K.; Pattnaik, B.; Arya, R.K. Utilization of agricultural waste biomass and recycling towards circular bioeconomy. Environ. Sci. Pollut. Res. 2023, 30, 8526–8539. [Google Scholar] [CrossRef]
- Haberzettl, J.; Hilgert, P.; von Cossel, M. A Critical review on lignocellulosic biomass yield modeling and the bioenergy potential from marginal land. Agronomy 2021, 11, 2397. [Google Scholar] [CrossRef]
- Kamperidou, V.; Terzopoulou, P. Anaerobic digestion of lignocellulosic waste materials. Sustainability 2021, 13, 12810. [Google Scholar] [CrossRef]
- Gissén, C.; Prade, T.; Kreuger, E.; Nges, I.A.; Rosenqvist, H.; Svensson, S.E.; Lantz, M.; Mattsson, J.E.; Börjesson, P.; Björnsson, L. Comparing energy crops for biogas production—Yields, energy input and costs in cultivation using digestate and mineral fertilization. Biomass Bioenergy 2014, 64, 199–210. [Google Scholar] [CrossRef]
- Purdy, S.J.; Maddison, A.L.; Nunn, C.P.; Winters, A.; Timms-Taravella, E.; Jones, C.M.; Clifton-Brown, J.C.; Donnison, I.S.; Gallagher, J.A. Could Miscanthus replace maize as the preferred substrate for anaerobic digestion in the United Kingdom? Future breeding strategies. GCB Bioenergy 2017, 9, 1122–1139. [Google Scholar] [CrossRef]
- Kiesel, A.; Lewandowski, I. Miscanthus as biogas substrate—Cutting tolerance and potential for anaerobic digestion. GCB Bioenergy 2017, 9, 153–167. [Google Scholar] [CrossRef]
- Gustafsson, M.; Ammenberg, J. IEA Bionergy Task 37—A Perspective on the State of the Biogas Industry from Selected Member Countries. 2022. Available online: https://www.ieabioenergy.com/blog/publications/a-perspective-on-the-state-of-the-biogas-industry-from-selected-member-countries-of-iea-bioenergy-task-37/ (accessed on 5 April 2024).
- Brémond, U.; Bertrandias, A.; Steyer, J.P.; Bernet, N.; Carrere, H. A vision of European biogas sector development towards 2030: Trends and challenges. J. Clean. Prod. 2021, 287, 125065. [Google Scholar] [CrossRef]
- FNR. Anbau und Verwendung Nachwachsender Rohstoffe in Deutschland. 2022. Available online: https://www.fnr.de/ftp/pdf/berichte/22004416.pdf (accessed on 5 April 2024).
- Yang, X.; Liu, Y.; Thrän, D.; Bezama, A.; Wang, M. Effects of the German Renewable Energy Sources Act and environmental, social and economic factors on biogas plant adoption and agricultural land use change. Energy Sustain. Soc. 2021, 11, 6. [Google Scholar] [CrossRef]
- Jurgutis, L.; Šlepetienė, A.; Amalevičiūtė-Volungė, K.; Volungevičius, J.; Šlepetys, J. The effect of digestate fertilisation on grass biogas yield and soil properties in field-biomass-biogas-field renewable energy production approach in Lithuania. Biomass Bioenergy 2021, 153, 106211. [Google Scholar] [CrossRef]
- Mayer, F.; Gerin, P.A.; Noo, A.; Lemaigre, S.; Stilmant, D.; Schmit, T.; Leclech, N.; Ruelle, L.; Gennen, J.; von Francken-Welz, H.; et al. Assessment of energy crops alternative to maize for biogas production in the greater region. Bioresour. Technol. 2014, 166, 358–367. [Google Scholar] [CrossRef] [PubMed]
- Vogel, E.; Deumlich, D.; Kaupenjohann, M. Bioenergy maize and soil erosion—Risk assessment and erosion control concepts. Geoderma 2016, 261, 80–92. [Google Scholar] [CrossRef]
- Matías, J.; Encinar, J.M.; González, J.; González, J.F. Optimisation of ethanol fermentation of Jerusalem artichoke tuber juice using simple technology for a decentralised and sustainable ethanol production. Energy Sustain. Dev. 2015, 25, 34–39. [Google Scholar] [CrossRef]
- Piskier, T. A method of estimation of the caloric value of the biomass. Part I. Biomass energy potential. J. Mech. Energy Eng. 2017, 1, 189–194. [Google Scholar]
- Epie, K.E.; Santanen, A.; Mäkelä, P.S.A.; Stoddard, F.L. Fertilizer and intercropped legumes as nitrogen source for Jerusalem artichoke (Helianthus tuberosus L.) tops for bioenergy. Agric. Food Sci. 2018, 27, 199–205. [Google Scholar] [CrossRef]
- Rossini, F.; Provenzano, M.E.; Kuzmanović, L.; Ruggeri, R. Jerusalem artichoke (Helianthus tuberosus L.): A versatile and sustainable crop for renewable energy production in Europe. Agronomy 2019, 9, 528. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Dubis, B.; Kozak, M. Sewage sludge and the energy balance of Jerusalem artichoke production. A six-year field experiment in Poland. Energy 2021, 276, 127478. [Google Scholar] [CrossRef]
- Bogucka, B.; Jankowski, K.J. The effect of harvest strategy on the energy potential of Jerusalem artichoke. Ind. Crops Prod. 2022, 177, 114473. [Google Scholar] [CrossRef]
- Long, X.H.; Zhao, J.; Liu, Z.P.; Rengel, Z.; Liu, L.; Shao, H.B.; Tao, Y. Applying geostatistics to determine the soil quality improvement by Jerusalem artichoke in coastal saline zone. Ecol. Eng. 2014, 70, 319–326. [Google Scholar] [CrossRef]
- Yang, L.; He, Q.S.; Corscadden, K.; Udenigwe, C.C. The prospects of Jerusalem artichoke in functional food ingredients and bioenergy production. Biotechnol. Rep. 2015, 5, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Bogucka, B.; Pszczółkowska, A.; Okorski, A.; Jankowski, K. The effects of potassium fertilization and irrigation on the yield and health status of Jerusalem artichoke (Helianthus tuberosus L.). Agronomy 2021, 11, 234. [Google Scholar] [CrossRef]
- Piskier, T. Energy potential of Jerusalem artichoke. Probl. Inżynierii Rol. 2009, 1, 133–136. (In Polish) [Google Scholar]
- Krochmal-Marczak, B.; Sawicka, B.; Barbaś, P. Utility meaning of Jerusalem Artichoke. In Jerusalem Artichoke Food Science and Technology: Helianthus tuberosus; Sawicka, B., Krochmal-Marczak, B., Eds.; Springer: Singapore, 2022; pp. 91–138. [Google Scholar]
- Kim, S.; Kim, C.H. Evaluation of whole Jerusalem artichoke (Helianthus tuberosus L.) for consolidated bioprocessing ethanol production. Renew. Energy 2014, 65, 83–91. [Google Scholar] [CrossRef]
- Qiu, Y.; Lei, P.; Zhang, Y.; Sha, Y.; Zhan, Y.; Xu, Z.; Li, S.; Xu, H.; Ouyang, P. Recent advances in bio-based multi-products of agricultural Jerusalem artichoke resources. Biotechnol. Biofuels 2018, 11, 151. [Google Scholar] [CrossRef]
- Sawicka, B.; Skiba, D. The influence of diversified mineral fertilization on potassium, phosphorus, and magnesium content in Helianthus tuberosus L. tubers. Pol. J. Environ. Stud. 2007, 16, 231–234. [Google Scholar]
- Izsáki, Z.; Kádi, G.N. Biomass accumulation and nutrient uptake of Jerusalem artichoke (Helianthus tuberosus L.). Am. J. Plant Sci. 2013, 4, 1629–1640. [Google Scholar] [CrossRef]
- Kays, S.J.; Nottingham, S.F. Biology and Chemistry of Jerusalem Artichoke: Helianthus tuberosus L.; CRC Press: Boca Raton, FL, USA; Taylor and Francis Group: London, UK, 2008; p. 478. [Google Scholar]
- Liu, Z.X.; Han, L.P.; Steinberger, Y.; Xie, G.H. Genetic variation and yield performance of Jerusalem artichoke germplasm collected in China. Agric. Sci. China 2011, 10, 668–678. [Google Scholar] [CrossRef]
- Sawicka, B. Jerusalem artichoke (Helianthus tuberosus L.). Biology, Culture, the Importance of Utility; Publishing House of the University of Life Sciences: Lublin, Poland, 2016; p. 241. (In Polish) [Google Scholar]
- Dybek, B.; Anders, D.; Hołaj-Krzak, J.T.; Hałasa, Ł.; Maj, G.; Kapłan, M.; Klimek, K.; Filipczak, G.; Wałowski, G. Assessment of the prospects of Polish non-food energy agriculture in the context of a renewable energy source. Energies 2023, 16, 3315. [Google Scholar] [CrossRef]
- Fang, Y.R.; Liu, J.A.; Steinberger, Y.; Xie, G.H. Energy use efficiency and economic feasibility of Jerusalem artichoke production on arid and coastal saline lands. Ind. Crops Prod. 2018, 117, 131–139. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Galic, M.; Mesic, M.; Zgorelec, Z. Influence of organic and mineral fertilization on soil greenhouse gas emissions. A review. Agric. Conspec. Sci. 2019, 85, 1–8. [Google Scholar]
- Jankowski, K.J.; Nogalska, A. Meat and bone meal and the energy balance of winter oilseed rape—A case study in north-eastern Poland. Energies 2022, 15, 3853. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Kołodziej, B.; Dubis, B.; Sugier, D.; Antonkiewicz, J.; Szatkowski, A. The effect of sewage sludge on the energy balance of cup plant biomass production. A six-year field experiment in Poland. Energy 2023, 276, 127478. [Google Scholar] [CrossRef]
- Adegbeye, M.J.; Reddy, P.R.K.; Obaisi, A.I.; Elghandour, M.M.M.Y.; Oyebamiji, K.J.; Salem, A.Z.M.; Morakinyo-Fasipe, O.T.; Cipriano-Salazar, M.; Camacho-Díaz, L.M. Sustainable agriculture options for production, greenhouse gasses and pollution alleviation, and nutrient recycling in emerging and transitional nations—An overview. J. Clean Prod. 2020, 242, 118319. [Google Scholar] [CrossRef]
- Barbosa, D.B.P.; Nabel, M.; Jablonowski, N.D. Biogas-digestate as nutrient source for biomass production of Sida hermaphrodita, Zea mays L. and Medicago sativa L. Energy Procedia 2014, 59, 120–126. [Google Scholar] [CrossRef]
- Nabel, M.; Schrey, S.D.; Poorter, H.; Koller, R.; Jablonowski, N.D. Effects of digestate fertilization on Sida hermaphrodita: Boosting biomass yields on marginal soils by increasing soil fertility. Biomass Bioenergy 2017, 107, 207–213. [Google Scholar] [CrossRef]
- Lee, M.S.; Urgun-Demirtas, M.; Shen, Y.; Zumpf, C.; Anderson, E.K.; Rayburn, A.L.; Lee, D.K. Effect of digestate and digestate supplemented with biochar on switchgrass growth and chemical composition. Biomass Bioenergy 2021, 144, 105928. [Google Scholar] [CrossRef]
- Dubis, B.; Szatkowski, A.; Jankowski, K.J. Sewage sludge, digestate, and mineral fertilizer application affects the yield and energy balance of Amur silvergrass. Ind. Crops Prod. 2022, 175, 114235. [Google Scholar] [CrossRef]
- Slepetiene, A.; Volungevicius, J.; Jurgutis, L.; Liaudanskiene, I.; Amaleviciute-Volunge, K.; Slepetys, J.; Ceseviciene, J. The potential of digestate as a biofertilizer in eroded soils of Lithuania. Waste Manag. 2020, 102, 441–451. [Google Scholar] [CrossRef]
- Ramirez, J.; McCabe, B.; Jensen, P.D.; Speight, R.; Harrison, M.; van den Berg, L.; O’Hara, I. Wastes to profit: A circular economy approach to value-addition in livestock industries. Anim. Prod. Sci. 2021, 61, 541–550. [Google Scholar] [CrossRef]
- Kovačić, Đ.; Lončarić, Z.; Jović, J.; Samac, D.; Popović, B.; Tišma, M. Digestate management and processing practices: A review. Appl. Sci. 2022, 128, 9216. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, C.; Campoy, M.; Carrasc, L.; Nájera, I.; Baixauli, C.; Caravaca, F.; Roldán, A.; Cegarra, J.; Bernal, M.P. Agricultural use of digestate for horticultural crop production and improvement of soil properties. Eur. J. Agron. 2012, 42, 119–128. [Google Scholar] [CrossRef]
- Šimon, T.; Kunzová, E.; Friedlová, M. The effect of digestate, cattle slurry, and mineral fertilization on the winter wheat yield and soil quality parameters. Plant Soil Environ. 2015, 61, 522–527. [Google Scholar] [CrossRef]
- Riva, C.; Orzi, V.; Carozzi, M.; Acutis, M.; Boccasile, G.; Lonati, S.; Tambone, F.; D’Imporzano, G.; Adani, F. Short-term experiments in using digestate products as substitutes for mineral (N) fertilizer: Agronomic performance, odours, and ammonia emission impacts. Sci. Total Environ. 2016, 547, 206–214. [Google Scholar] [CrossRef]
- Przygocka-Cyna, K.; Grzebisz, W. Biogas digestate—Benefits and risks for soil fertility and crop quality—An evaluation of grain maize response. Open Chem. 2018, 16, 258–271. [Google Scholar] [CrossRef]
- Brtnicky, M.; Kintl, A.; Holatko, J.; Hammerschmiedt, T.; Mustafa, A.; Kucerik, J.; Vitez, T.; Prichystalova, J.; Baltazar, T.; Elbl, J. Effect of digestates derived from the fermentation of maize-legume intercropped culture and maize monoculture application on soil properties and plant biomass production. Chem. Biol. Technol. Agric. 2022, 9, 43. [Google Scholar] [CrossRef]
- Evangelisti, S.; Lettieri, P.; Borello, D.; Clift, R. Life cycle assessment of energy from waste via anaerobic digestion: A UK case study. Waste Manag. 2014, 34, 226–237. [Google Scholar] [CrossRef]
- Abubaker, J.; Risberg, K.; Pell, M. Biogas residues as fertilisers—Effect on wheat growth and soil microbial activities. Appl. Energy 2012, 99, 126–134. [Google Scholar] [CrossRef]
- Odlare, M.; Pell, M.; Svensson, K. Changes in soil chemical and microbiological properties during 4 years of application of various organic residues. Waste Manag. 2008, 28, 1246–1253. [Google Scholar] [CrossRef]
- Odlare, M.; Arthurson, V.; Pell, M.; Svensson, K.; Nehrenheim, E.; Abubaker, J. Land application of organic waste—Effects on the soil ecosystem. Appl. Energy 2011, 88, 2210–2218. [Google Scholar] [CrossRef]
- Hupfauf, S.; Bachmann, S.; Juárez, M.F.D.; Insam, H.; Eichler-Löbermann, B. Biogas digestates affect crop P uptake and soil microbial community composition. Sci. Total Environ. 2016, 542 Pt B, 1144–1154. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Romeo, F.; Mallamaci, C.; Muscolo, A. Digestate application on two different soils: Agricultural benefit and risk. Waste Biomass Valoriz. 2021, 12, 4341–4353. [Google Scholar] [CrossRef]
- Galvez, A.; Sinicco, T.; Cayuela, M.L.; Mingorance, M.D.; Fornasier, F.; Mondini, C. Short term effects of bioenergy by-products on soil C and N dynamics, nutrient availability and biochemical properties. Agric. Ecosyst. Environ. 2012, 160, 3–14. [Google Scholar] [CrossRef]
- Różyło, K.; Oleszczuk, P.; Jośko, I.; Kraska, P.; Kiecińska-Poppe, E.; Andruszczak, S. An ecotoxicological evaluation of soil fertilized with biogas residues or mining waste. Environ. Sci. Pollut. Res. 2015, 22, 7833–7842. [Google Scholar] [CrossRef]
- Di Maria, F.; Sisani, F.; El-Hoz, M.; Mersky, R.L. How collection efficiency and legal constraints on digestate management can affect the effectiveness of anaerobic digestion of bio-waste: An analysis of the Italian context in a life cycle perspective. Sci. Total Environ. 2020, 726, 138555. [Google Scholar] [CrossRef]
- Deng, L.; Liu, Y.; Wang, W. Utilization of digestate. In Biogas Technology; Deng, L., Liu, Y., Wang, W., Eds.; Springer Nature: Singapore, 2020; pp. 319–363. [Google Scholar]
- Egene, C.E.; Sigurnjak, I.; Regelink, I.C.; Schoumans, O.F.; Adani, F.; Michels, E.; Sleutel, S.; Tack, F.M.G.; Meers, E. Solid fraction of separated digestate as soil improver: Implications for soil fertility and carbon sequestration. J. Soils Sediments 2021, 121, 678–688. [Google Scholar] [CrossRef]
- Plana, P.V.; Noche, B. A review of the current digestate distribution models: Storage and transport. In WIT Transactions on Ecology and the Environment; Brebbia, C.A., Itoh, H., Eds.; WIT Press: Southampton, UK, 2016; p. 346. [Google Scholar]
- Baştabak, B.; Koçar, G. A review of the biogas digestate in agricultural framework. J. Mater. Cycles Waste. Manag. 2020, 22, 1318–1327. [Google Scholar] [CrossRef]
- Doyeni, M.O.; Stulpinaite, U.; Baksinskaite, A.; Suproniene, S.; Tilvikiene, V. The effectiveness of digestate use for fertilization in an agricultural cropping system. Plants 2021, 10, 1734. [Google Scholar] [CrossRef]
- Fuchs, W.; Drosg, B. Assessment of the state of the art of technologies for the processing of digestate residue from anaerobic digesters. Water Sci. Technol. 2013, 67, 1984–1993. [Google Scholar] [CrossRef]
- Möller, K. Effects of anaerobic digestion on soil carbon and nitrogen turnover, N emissions, and soil biological activity. A review. Agron. Sustain. Dev. 2015, 35, 1021–1041. [Google Scholar] [CrossRef]
- Ehmann, A.; Thumm, U.; Lewandowski, I. Fertilizing potential of separated biogas digestates in annual and perennial biomass production systems. Front. Sustain. Food. Syst. 2018, 2, 12. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Krzyżaniak, M.; Warminski, K.; Tworkowski, J.; Szczukowski, S. Perennial herbaceous crops as a feedstock for energy and industrial purposes: Organic and mineral fertilizers versus biomass yield and efficient nitrogen utilization. Ind. Crops Prod. 2017, 107, 244–259. [Google Scholar] [CrossRef]
- Stolarski, M.J.; Krzyżaniak, M.; Warmiński, K.; Tworkowski, J.; Szczukowski, S.; Olba-Zięty, E.; Gołaszewski, J. Energy efficiency of perennial herbaceous crops production depending on the type of digestate and mineral fertilizers. Energy 2017, 134, 50–60. [Google Scholar] [CrossRef]
- Dubis, B.; Jankowski, K.J.; Załuski, D.; Sokólski, M. The effect of sewage sludge fertilization on the biomass yield of giant miscanthus and the energy balance of the production process. Energy 2020, 206, 118189. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2022. FAO: Rome, Italy, 2022. Available online: https://eurasian-soil-portal.info/wp-content/uploads/2022/07/wrb_fourth_edition_2022-3.pdf (accessed on 10 April 2024).
- Bardsley, C.E.; Lancaster, J.D. Determination of reserve sulfur and soluble sulfates in soils. Soil Sci. Soc. Am. J. 1960, 24, 265–268. [Google Scholar] [CrossRef]
- Houba, V.J.G.; van der Lee, J.J.; Novozamsky, I. Part 5A: Soil analysis procedures, other procedures. In Soil and Plant Analysis; Department of Soil Science and Plant Nutrition, Agricultural University: Wageningen, The Netherlands, 1995. [Google Scholar]
- Muzalewski, A. Operating Costs of Agricultural Machines, No. 24; IBMER: Warszawa, Poland, 2009; p. 52. (In Polish) [Google Scholar]
- Muzalewski, A. Operating Costs of Agricultural Machines, No. 25; Institute of Technology and Life Sciences Publishing House: Falenty–Warszawa, Poland, 2010; p. 56. (In Polish) [Google Scholar]
- Wójcicki, Z. Equipment, Materials and Energy Inputs in Growth-Oriented Farms; IBMER: Warszawa, Poland, 2000. (In Polish) [Google Scholar]
- Kopetz, H.; Jossart, J.; Ragossnig, H.; Metschina, C. European Biomass Statistics; European Biomass Association (AEBIOM): Brussels, Belgium, 2007. [Google Scholar]
- TIBCO Software, Inc. STATISTICA (Data Analysis Software System); TIBCO Software, Inc.: Palo Alto, CA, USA, 2017. [Google Scholar]
- Budzyński, W.; Szempliński, W.; Parzonka, A.; Sałek, T. Agricultural productivity, energy efficiency and costs associated with growing selected energy crops for biogas production. In Production and Processing of Agricultural and Aquatic Biomass for Biogas Plants and Gasification Units; Gołaszewski, J., Ed.; Publishing House of the University Warmia and Mazury in Olsztyn: Olsztyn, Poland, 2014; pp. 11–282. (In Polish) [Google Scholar]
- Jankowski, K.J.; Dubis, B.; Sokólski, M.M.; Załuski, D.; Bórawski, P.; Szempliński, W. Biomass yield and energy balance of Virginia fanpetals in different production technologies in north-eastern Poland. Energy 2019, 185, 612–623. [Google Scholar] [CrossRef]
- Dubis, B.; Jankowski, K.J.; Sokolski, M.; Załuski, D.; Borawski, P.; Szempliński, W. Biomass yield and energy balance of fodder galega in different production technologies: An 11-year field experiment in a large-area farm in Poland. Renew. Energy 2020, 154, 813–825. [Google Scholar] [CrossRef]
- Jankowski, K.J.; Sokólski, M.M.; Dubis, B.; Załuski, D.; Szempliński, W. Sweet sorghum—Biomass production and energy balance at different levels of agricultural inputs. A six-year field experiment in north-eastern Poland. Eur. J. Agron. 2020, 119, 126119. [Google Scholar] [CrossRef]
- Kyttä, V.; Helenius, J.; Tuomisto, H.L. Carbon footprint and energy use of recycled fertilizers in arable farming. J. Clean. Prod. 2021, 287, 125063. [Google Scholar] [CrossRef]
- Plöchl, M.; Heiermann, M.; Linke, B.; Schelle, H. Biogas crops—Part II: Balance of greenhouse gas emissions and energy from using field crops for anaerobic digestion. Agric. Eng. Int. 2009, 11, 1–11. [Google Scholar]
- Denoroy, P. The crop physiology of Helianthus tuberosus L.: A model orientated view. Biomass Bioenergy 1996, 11, 11–32. [Google Scholar] [CrossRef]
- Baldini, M.; Danuso, F.; Monti, A.; Amaducci, M.T.; Stevanato, P.; De Mastro, G. Chicory and Jerusalem artichoke productivity in different areas of Italy, in relation to water availability and time of harvest. Ital. J. Agron. 2006, 1, 291–308. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Sousa, L.; Cabanas, J.E.; Arrobas, M. Tuber yield and leaf mineral composition of Jerusalem artichoke (Helianthus tuberosus L.) grown under different cropping practices. Span. J. Agric. Res. 2007, 5, 545–553. [Google Scholar] [CrossRef]
- Monti, A.; Amaducci, M.T.; Venturi, G. Growth response, leaf gas exchange and fructans accumulation of Jerusalem artichoke (Helianthus tuberosus L.) as affected by different water regimes. Eur. J. Agron. 2005, 23, 136–145. [Google Scholar] [CrossRef]
- Sawicka, B.; Skiba, D.; Kiełtyka-Dadasiewicz, A.; Danilčenko, H. Jerusalem artichoke (Helianthus tuberosus L.) as energy raw material. In Proceedings of the 9th International Scientific Conference Rural Development, Kovno, Lithuania, 26–28 September 2019; pp. 336–342. [Google Scholar]
- Liu, Z.X.; Spiertz, J.H.J.; Sha, J.; Xue, S.; Xie, G.H. Growth and yield performance of Jerusalem artichoke clones in a semiarid region of China. Agron. J. 2012, 104, 1538–1546. [Google Scholar] [CrossRef]
- Skiba, D. Variability in Yield and Quality of Selected Features of Several Cultivars of Helianthus tuberosus L. under Different Fertilization. Ph.D. Thesis, University of Life Sciences in Lublin, Lublin, Poland, 2014; p. 234. (In Polish). [Google Scholar]
- Matías, J.; González, J.; Cabanillas, J.; Royano, L. Influence of NPK fertilisation and harvest date on agronomic performance of Jerusalem artichoke crop in the Guadiana Basin (Southwestern Spain). Ind. Crops Prod. 2013, 48, 191–197. [Google Scholar] [CrossRef]
- Liu, Z.X.; Steinberger, Y.; Chen, X.; Wang, J.S.; Xie, G.H. Chemical composition and potential ethanol yield of Jerusalem artichoke in a semi-arid region of China. Ital. J. Agron. 2015, 10, 603. [Google Scholar] [CrossRef]
- Long, X.H.; Shao, H.B.; Liu, L.; Liu, L.P.; Liu, Z.U. Jerusalem artichoke: A sustainable biomass feedstock for biorefinery. Renew. Sustain. Energy Rev. 2016, 54, 1382–1388. [Google Scholar] [CrossRef]
- Farzinmehr, S.; Rezaei, J.H.; Fazaeli, H. Effect of harvesting frequency and maturity stage of Jerusalem artichoke forage on yield, chemical composition and in vitro fermentation of the tubers and forage. Span. J. Agric. Res. 2020, 18, e0602. [Google Scholar] [CrossRef]
- Labergh, C.; Sackston, W.E. Adaptability and diseases of Jerusalem artichoke (Helianthus tuberosus) in Quebec. Can. J. Plant. Sci. 1987, 67, 349–353. [Google Scholar] [CrossRef]
- Slepetys, J.; Kadziuliene, Z.; Sarunaite, L.; Tilvikiene, V.; Kryzeviciene, A. Biomass potential of plants grown for bioenergy production. In Growing and Processing Technologies of Energy Crops, Proceedings of the International Scientific Conference Renewable Energy and Energy Efficiency, Jelgava, Latvia, 28–30 May 2012; Rivža, P., Rivža, S., Eds.; Latvia University of Agriculture: Jelgava, Latvia, 2012; pp. 66–72. [Google Scholar]
- Chupina, M.P.; Stepanov, A.F. Assessment of photosynthetic productivity of new perennial forage crops in forest-steppe conditions of Western Siberia. IOP Conf. Ser. Earth Environ. Sci. 2021, 624, 012121. [Google Scholar] [CrossRef]
- Cepl, J.; Kasal, P.; Souckova, H.; Svobodova, A.; Bucher, P. Non-food production of Jerusalem artichoke (Helianthus tuberosus) and possibilities of its energetic utilization. In Actual Tasks on Agricultural Engineering, Proceedings of the 40th International Symposium on Agricultural Engineering, Opatija, Croatia, 21–24 February 2012; University of Zagreb Faculty of Agriculture: Zagreb, Croatia, 2012; pp. 517–526. [Google Scholar]
- Kaszás, L.; Alshaal, T.; El-Ramady, H.; Kovács, Z.; Koroknai, J.; Elhawat, N.; Nagy, É.; Cziáky, Z.; Fári, M.; Domokos-Szabolcsy, É. Identification of bioactive phytochemicals in leaf protein concentrate of Jerusalem artichoke (Helianthus tuberosus L.). Plants 2020, 9, 889. [Google Scholar] [CrossRef] [PubMed]
- Faber, A.; Stasiak, M.; Kuś, J. Preliminary evaluation of productivity of the selected energy crops. Prog. Plant Protect. 2007, 47, 339–346. (In Polish) [Google Scholar]
- Baldini, M.; Danuso, F.; Turi, M.; Vannozzi, G.P. Evaluation of new clones of Jerusalem artichoke (Helianthus tuberosus L.) for inulin and sugar yield from stalks and tubers. Ind. Crops Prod. 2004, 19, 25–40. [Google Scholar] [CrossRef]
- Englert, H.; Lewandowski, I.; Böhmel, C.; Vetter, A.; Hartmann, H. Angebaute Biomasse. In Energie aus Biomasse; Kaltschmitt, M., Hartmann, H., Hofbauer, H., Eds.; Springer: Berlin-Heidelberg, Germany, 2009; pp. 75–134. (In German) [Google Scholar]
- Liebhard, P.; Zeitlhofer, C.; Kaul, H.P.; Amon, T. Methanbildungsvermögen und Biogasqualität bei der Vergärung von Topinamburkraut. In Topinambur-eine Pflanze mit vielen Verwendungsmöglichkeiten; Landwirtschaftliches Technologiezentrum Augustenberg (LTZ): Karlsruhe, Germany, 2009; pp. 2.11–2.17. Available online: https://www.topinambur-verein.de/Download/Wissenswertes/Workshop-Tagungsband.pdf (accessed on 5 April 2024). (In German)
- Kai, G.; Tie-Xia, X.; Qi-Bing, W. Nitrogen fertilization, irrigation, and harvest times affect biomass and energy value of Helianthus tuberosus L. J. Plant Nutr. 2016, 39, 1906–1914. [Google Scholar] [CrossRef]
- Gao, K.; Zhu, T.; Han, G. Water and nitrogen interactively increased the biomass production of Jerusalem artichoke (Helianthus tuberosus L.) in semi-arid area. Afr. J. Biotechnol. 2011, 10, 6466–6472. [Google Scholar]
- Mays, D.A.; Buchanan, W.; Bradford, B.N.; Giordano, P.M. Fuel production potential of several agricultural crops. In Advances in New Crops, Proceedings of the First National Symposium “New Crops: Research, Development, Economics”, Indianapolis, IN, USA, 23–26 October 1990; Janick, J., Simon, J.E., Eds.; Timber Press: Portland, OR, USA, 1990; pp. 260–263. [Google Scholar]
- Solé-Bundó, M.; Cucina, M.; Folch, M.; Tàpias, J.; Gigliotti, G.; Garfi, M.; Ferrer, I. Assessing the agricultural reuse of the digestate from microalgae anaerobic digestion and co-digestion with sewage sludge. Sci. Total Environ. 2017, 586, 1–9. [Google Scholar] [CrossRef]
- Romero-Güiza, M.S.; Mata-Alvarez, J.; Chimenos Rivera, J.M.; Garcia, S.A. Nutrient recovery technologies for anaerobic digestion systems: An overview. Rev. Ion 2016, 29, 7–26. [Google Scholar] [CrossRef]
- Cavalli, D.; Cabassi, G.; Borrelli, L.; Geromel, G.; Degano, L.; Gallina, P.M. Nitrogen fertilizer replacement value of undigested liquid cattle manure and digestates. Eur. J. Agron. 2016, 73, 34–41. [Google Scholar] [CrossRef]
- Lošák, T.; Hlušek, J.; Válka, T.; Elbl, J.; Vitěz, T.; Běliková, H.; von Bennewitz, E. The effect of fertilization with digestate on kohlrabi yields and quality. Plant Soil Environ. 2016, 62, 274–278. [Google Scholar] [CrossRef]
- Szymańska, M.; Szara, E.; Sosulski, T.; Stępień, W.; Pilarski, K.; Pilarska, A.A. Chemical properties and fertilizer value of ten different anaerobic digestates. Fresenius Environ. Bull. 2018, 27, 3425–3432. [Google Scholar]
- Siebielec, G.; Siebielec, S.; Lipski, D. Long-term impact of sewage sludge, digestate, and mineral fertilizers on plant yield and soil biological activity. J. Clean. Prod. 2018, 178, 372–379. [Google Scholar] [CrossRef]
- Sapp, M.; Harrison, M.; Hany, U.; Charlton, A.; Thwaites, R. Comparing the effect of digestate and chemical fertiliser on soil bacteria. Appl. Soil. Ecol. 2015, 86, 1–9. [Google Scholar] [CrossRef]
- Barłóg, P.; Hlisnikovský, L.; Kunzová, E. Effect of digestate on soil organic carbon and plant-available nutrient content compared to cattle slurry and mineral fertilization. Agronomy 2020, 10, 379. [Google Scholar] [CrossRef]
- Barłóg, P.; Hlisnikovský, L.; Kunzová, E. Yield, content and nutrient uptake by winter wheat and spring barley in response to applications of digestate, cattle slurry and NPK mineral fertilizers. Arch. Agron. Soil Sci. 2020, 66, 1481–1496. [Google Scholar] [CrossRef]
- Jamison, J.; Khanal, S.K.; Nguyen, N.H.; Deenik, J.L. Assessing the effects of digestates and combinations of digestates and fertilizer on yield and nutrient use of Brassica juncea (Kai Choy). Agronomy 2021, 11, 509. [Google Scholar] [CrossRef]
- Båth, B.; Elfstrand, S. Use of red clover-based green manure in leek cultivation. Biol. Agric. Hortic. 2008, 25, 269–286. [Google Scholar] [CrossRef]
- Kolář, L.; Kužel, S.; Peterka, J.; Štindl, P.; Plát, V. Agrochemical value of organic matter of fermenter wastes in biogas production. Plant Soil Environ. 2008, 54, 321–328. [Google Scholar] [CrossRef]
- Kupper, L.; Bucheli, T.D.; Brandi, R.C.; Ortelli, D.; Edder, P. Dissipation of pesticides during composting and anaerobic digestion of source-separated organic waste at full-scale plants. Bioresour. Technol. 2008, 99, 7988–7994. [Google Scholar] [CrossRef]
- Kolář, L.; Kužel, S.; Peterka, J.; Borová-Batt, J. Agrochemical value of the liquid phase of wastes from fermenters during biogas production. Plant Soil Environ. 2010, 56, 23–27. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, C.; Ferrer-Costa, A.; Carrasco, L.; Cegarra, J.; Abad, M.; Bernal, M.P. Assessment of the fertiliser potential of digestate from farm and agroindustrial residues. Biomass Bioenergy 2012, 40, 181–189. [Google Scholar] [CrossRef]
- Pivato, A.; Vanin, S.; Raga, R.; Lavagnolo, M.C.; Barausse, A.; Rieple, A.; Laurent, A.; Cossu, R. Use of digestate from decentralized on-farm biogas plant as fertilizer in soil: An ecotoxicological study for future indicators in risk and life cycle assessment. Waste Manag. 2016, 49, 378–389. [Google Scholar] [CrossRef] [PubMed]
- Tigini, V.; Franchino, M.; Bona, F.; Varese, G.C. Is digestate safe? A study on its ecotoxicity and environmental risk on pig manure. Sci. Total Environ. 2016, 551–552, 127–132. [Google Scholar] [CrossRef] [PubMed]
- McLachlan, K.L.; Chong, C.; Voroney, R.P.; Liu, H.W.; Holbein, B.E. Assessing the potential phytotoxicity of digestates during processing of municipal solid waste by anaerobic digestion: Comparison to aerobic compost. Acta Hortic. 2004, 638, 225–230. [Google Scholar] [CrossRef]
- Teglia, C.; Tremier, A.; Martel, J.L. Characterization of solid digestate: Part II, assessment of the quality and suitability for composting of six digested products. Waste Biomass Valoriz. 2011, 2, 113–126. [Google Scholar] [CrossRef]
- Sogn, T.A.; Dragicevic, I.; Linjordet, R.; Krogstad, T.; Eijsink, V.G.H.; Eich-Greatorex, S. Recycling of biogas digestates in plant production: NPK fertilizer value and risk of leaching. Int. J. Recyc. Org. Waste Agric. 2018, 7, 49–58. [Google Scholar] [CrossRef]
- Amaducci, S.; Colauzzi, M.; Battini, F.; Fracasso, A.; Perego, A. Effect of irrigation and nitrogen fertilization on the production of biogas from maize and sorghum in a water limited environment. Eur. J. Agron. 2016, 76, 54–65. [Google Scholar] [CrossRef]
- Garofalo, P.; D’Andrea, L.; Vonella, A.V.; Rinaldi, M.; Palumbo, A.D. Sweet sorghum in a bioethanol supply chain: Effects of different soil and nitrogen management on energy performances and greenhouse gas emissions. Ital. J. Agrometeorol. 2016, 21, 15–24. [Google Scholar]
- Ivanova, T.; Muntean, A.; Titei, V.; Havrland, B.; Kolarikova, M. Energy crops utilization as an alternative agricultural production. Agron. Res. 2015, 13, 311–317. [Google Scholar]
- Seleiman, M.F.; Santanen, A.; Jaakkola, S.; Ekholm, P.; Hartikainen, H.; Stoddard, F.L.; Mäkelä, P.S.A. Biomass yield and quality of bioenergy crops grown with synthetic and organic fertilizers. Biomass Bioenergy 2013, 59, 477–485. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).