Low-Frequency Electrical Heating for In Situ Conversion of Shale Oil: Modeling Thermal Dynamics and Decomposition
Abstract
1. Introduction
2. Model Construction
2.1. Numerical Methods
- In this model, the phases are defined as follows: the aqueous phase consists mostly of liquid water; the gas phase includes both steam and CH4; and the solid phase is composed of kerogen and coke. Moreover, liquid hydrocarbons—heavy oil and light oil—are modeled as distinct fluids due to the pronounced variations in their viscosities and densities, which necessitate their separate treatment.
- To simplify the pyrolysis of shale oil and to compute the thermophysical and transport properties, this model proposes that heavy oil, light oil, and natural gas consist solely of C22H46, C11H24, and CH4, respectively.
- The flow of each fluid phase obeys Darcy’s law.
| Description | Equation |
|---|---|
| Darcy’s law (flow velocity of fluids) | |
| The mass conservation equation | |
| The modified version of Stone’s relative permeability method [38] | |
| Heat transfer (heat convection and conduction) | |
| Heat transfer (heat convection and conduction) |
2.2. Verifications
2.3. Model Settings
3. Results
3.1. Evolution of Temperature
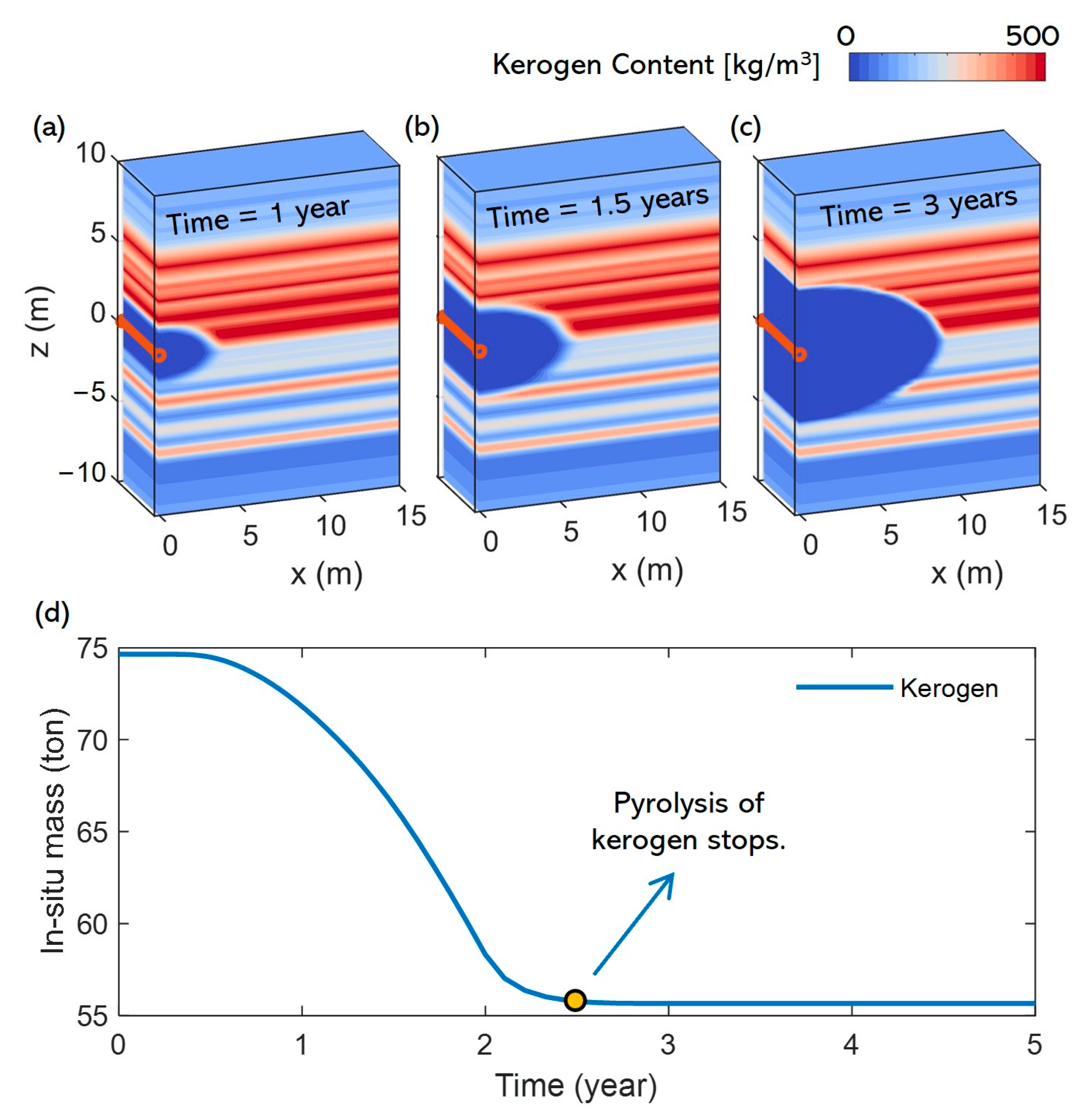
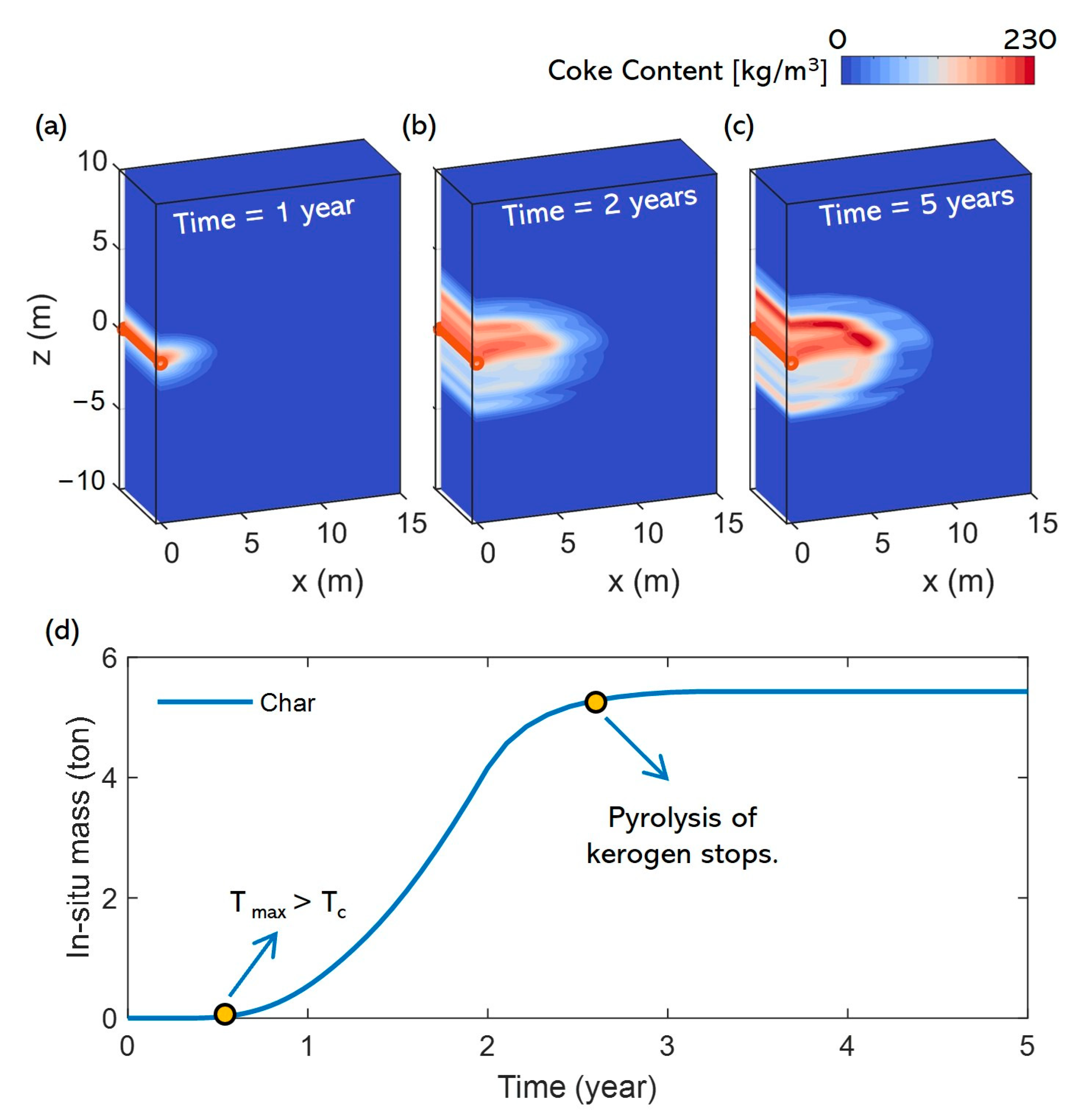
3.2. Pyrolysis Characteristics
- (a)
- In the early stage of heating, the maximum temperature in the reservoir reaches the pyrolysis temperature of kerogen but not that of heavy oil. During this stage, heavy oil primarily receives input from the pyrolysis of kerogen and does not undergo pyrolysis itself. However, heating and production occur simultaneously, and some heavy oil may be extracted through the horizontal well during this phase. Due to the high viscosity of heavy oil and its relatively low concentration near the well, the production rate is low. As a result of these combined factors, the total content of heavy oil in the reservoir remains relatively stable during this stage.
- (b)
- Since the difference in pyrolysis temperatures between heavy oil and kerogen is not significant, the first stage is relatively short (about 0.7 years). During the subsequent heating process, the temperature near the wellbore reaches the level required for heavy oil pyrolysis. At this point, heavy oil and kerogen undergo pyrolysis simultaneously. The heavy oil near the wellbore is almost completely pyrolyzed, and the remaining heavy oil is displaced to the outer areas of the wellbore by the light oil and other substances produced. Near the wellbore, there is almost no heavy oil during this stage, and the heavy oil in the reservoir no longer decreases due to production but is primarily consumed through pyrolysis. However, since the continuous heating causes more kerogen to undergo pyrolysis, the supply of heavy oil still exceeds the amount consumed by pyrolysis. As a result, the total content of heavy oil in the reservoir continues to increase, forming a high-saturation heavy oil layer outside the horizontal well. This stage persists until the end of the heating process.
- (c)
- After heating ends and the light oil and other substances within the heavy oil layer have been extracted through the horizontal well, heavy oil moves toward the wellbore due to pressure differences and concentration gradients, subsequently occupying the pores near the production well and being produced. At this stage, both the pyrolysis of heavy oil and kerogen in the reservoir cease, making the production of heavy oil the only factor leading to the decline in its content within the reservoir.
3.3. Production Characteristics
3.4. Sensitivity Analysis
4. Conclusions
- The use of low-frequency electric heating to heat the reservoir allows for the extraction of shale oil through a single well, thereby improving production efficiency. Additionally, this method does not require high reservoir permeability, making it suitable for tight shale oil reservoirs with low permeability.
- In the process of in situ shale oil conversion, the production of heavy oil is divided into two stages because of pyrolysis and displacement by light oil and other substances, and this production will continue for an extended period.
- The sensitivity analysis of heating strategies and reservoir properties revealed that higher heating power can reduce the proportion of heavy oil in the products, allowing further pyrolysis into light oil and other substances. Additionally, reservoirs with higher porosity are conducive to oil and gas production. Conducting in situ shale oil conversion in formations with better thermal conductivity may improve heating efficiency but also increase the proportion of heavy oil in the products.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hu, S.; Zhao, W.; Hou, L.; Yang, Z.; Zhu, R.; Wu, S.; Bai, B.; Jin, X. Development potential and technical strategy of continental shale oil in China. Shiyou Kantan Yu Kaifa/Pet. Explor. Dev. 2020, 47, 819–828. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, R.; Hu, S.; Hou, L.; Wu, S. Accumulation contribution differences between lacustrine organic-rich shales and mudstones and their significance in shale oil evaluation. Pet. Explor. Dev. 2020, 47, 1160–1171. [Google Scholar] [CrossRef]
- Crawford, P.M.; Biglarbigi, K.; Dammer, A.R.; Knaus, E. Advances in world oil shale production technologies. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 21–24 September 2008; pp. 4101–4111. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, G.; Tang, W.; Wang, D.; Wang, K.; Liu, J.; Du, D. A review of commercial development of continental shale oil in China. Energy Geosci. 2022, 3, 282–289. [Google Scholar] [CrossRef]
- Crawford, P.M.; Killen, J.C. New challenges and directions in oil shale development technologies. In Oil Shale: A Solution to the Liquid Fuel Dilemma; Ogunsola, O.I., Hartstein, A.M., Ogunsola, O., Eds.; American Chemical Society: Washington, DC, USA, 2010; Chapter 2; Volume 1032, pp. 21–60. [Google Scholar] [CrossRef]
- Fowler, T.D.; Vinegar, H.J. Oil shale ICP—Colorado field pilots. In Proceedings of the SPE Western Regional Meeting 2009, San Jose, CA, USA, 24–26 March 2009; pp. 270–284. [Google Scholar] [CrossRef]
- Fan, Y.; Durlofsky, L.J.; Tchelepi, H.A. Numerical simulation of the in-situ upgrading of oil shale. SPE J. 2010, 15, 368–381. [Google Scholar] [CrossRef]
- Huang, H.; Yu, H.; Xu, W.; Lyu, C.; Micheal, M.; Xu, H.; Liu, H.; Wu, H. A coupled thermo-hydro-mechanical-chemical model for production performance of oil shale reservoirs during in-situ conversion process. Energy 2023, 268, 126700. [Google Scholar] [CrossRef]
- Ryan, R.C.; Fowler, T.D.; Beer, G.L.; Nair, V. Shell’s in situ conversion process-from laboratory to field pilots. ACS Symp. Ser. 2010, 1032, 161–183. [Google Scholar] [CrossRef]
- Lee, K.; Moridis, G.J.; Ehlig-Economides, C.A. A Comprehensive simulation model of kerogen pyrolysis for the in-situ upgrading of oil shales. SPE J. 2016, 21, 1612–1630. [Google Scholar] [CrossRef]
- Lee, K.; Moridis, G.; Ehlig-Economides, C. In situ upgrading of oil shale by Steamfrac in multistage transverse fractured horizontal well system. Energy Sources A Recovery Util. Environ. Eff. 2016, 38, 3034–3041. [Google Scholar] [CrossRef]
- Riveros, G.V.; Barrios, H. Steam injection experiences in heavy and extra-heavy oil fields, Venezuela. In Proceedings of the SPE International Heavy Oil Conference and Exhibition 2011, Kuwait City, Kuwait, 12–14 December 2011; pp. 265–279. [Google Scholar] [CrossRef]
- Yang, D.; Zhao, Y.; Kang, Z. Numerical simulation of in situ exploitation of oil shale by injecting high-temperature steam. Oil Shale 2019, 36, 483–500. [Google Scholar] [CrossRef]
- Wang, L.; Yang, D.; Zhao, J.; Zhao, Y.; Kang, Z. Changes in oil shale characteristics during simulated in-situ pyrolysis in superheated steam. Oil Shale 2018, 35, 230–241. [Google Scholar] [CrossRef]
- Ifticene, M.A.; Yuan, C.; Al-Muntaser, A.A.; Onishchenko, Y.V.; Emelianov, D.A.; Varfolomeev, M.A. Behavior and kinetics of the conversion/combustion of oil shale and its components under air condition. Fuel 2022, 324, 124597. [Google Scholar] [CrossRef]
- Khakimova, L.; Bondarenko, T.; Cheremisin, A.; Myasnikov, A.; Varfolomeev, M. High pressure air injection kinetic model for Bazhenov Shale Formation based on a set of oxidation studies. J. Pet. Sci. Eng. 2019, 172, 1120–1132. [Google Scholar] [CrossRef]
- Gao, Y.; Wan, T.; Dong, Y.; Li, Y. Numerical and experimental investigation of production performance of in-situ conversion of shale oil by air injection. Energy Rep. 2022, 8, 1099–1112. [Google Scholar] [CrossRef]
- Martins, M.F.; Salvador, S.; Thovert, J.F.; Debenest, G. Co-current combustion of oil shale—Part 1: Characterization of the solid and gaseous products. Fuel 2010, 89, 144–151. [Google Scholar] [CrossRef]
- Pei, S.; Wang, Y.; Zhang, L.; Huang, L.; Cui, G.; Zhang, P.; Ren, S. An innovative nitrogen injection assisted in-situ conversion process for oil shale recovery: Mechanism and reservoir simulation study. J. Pet. Sci. Eng. 2018, 171, 507–515. [Google Scholar] [CrossRef]
- Brandt, A.R.; Boak, J.; Burnham, A.K. Carbon dioxide emissions from oil shale derived liquid fuels. ACS Symp. Ser. 2010, 1032, 219–248. [Google Scholar] [CrossRef]
- Zhang, Z.B.; Montilla, M.J.B.; Li, S.D.; Li, X.; Xing, J.P.; Hu, Y.Z. Numerical evaluations on the fluid production in the in-situ conversion of continental shale oil reservoirs. Pet. Sci. 2024, 21, 2485–2501. [Google Scholar] [CrossRef]
- Vakhin, A.V.; Khelkhal, M.A.; Tajik, A.; Gafurov, M.R.; Morozov, O.G.; Nasybullin, A.R.; Karandashov, S.A.; Ponomarev, A.A.; Krapivnitskaia, T.O.; Glyavin, M.Y.; et al. The role of nanodispersed catalysts in microwave application during the development of unconventional hydrocarbon reserves: A review of potential applications. Processes 2021, 9, 420. [Google Scholar] [CrossRef]
- Vermeulen, F.E.; Chute, F.S.; Cervenan, M.R. Physical Modelling of the Electromagnetic Heating of Oil Sand and Other Earth-Type and Biological Materials. Can. Electr. Eng. J. 1979, 4, 19–28. [Google Scholar] [CrossRef]
- Pan, Y.; Xiao, L.; Chen, C.; Yang, S. Development of Radio Frequency Heating Technology for Shale Oil Extraction. Appl. Sci. 2012, 2, 66–69. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Leung, C.; Gao, F. A fully coupled numerical model for microwave heating enhanced shale gas recovery. Energies 2018, 11, 1608. [Google Scholar] [CrossRef]
- Vermeulen, F.E.; Chute, F.S. Electromagnetic Techniques in the In-Situ Recovery of Heavy Oils. J. Microw. Power 1983, 18, 15–29. [Google Scholar] [CrossRef]
- McGee, B.C.; Vermeulen, F.E. The Mechanisms of Electrical Heating for the Recovery of Bitumen from Oil Sands. J. Can. Pet. Technol. 2007, 46. [Google Scholar] [CrossRef]
- McGee, B.C. Electro-thermal pilot in the Athabasca oil sands: Theory versus performance. World Oil 2008, 229, 47–54. [Google Scholar] [CrossRef]
- Zhao, E.; Hou, J.; Ji, Y.; Liu, Y.; Bai, Y. Energy recovery behavior of low-frequency electric heating assisted depressurization in Class 1 hydrate deposits. Fuel 2022, 309, 122185. [Google Scholar] [CrossRef]
- Zhao, E.; Hou, J.; Du, Q.; Liu, Y.; Ji, Y.; Bai, Y. Numerical modeling of gas production from methane hydrate deposits using low-frequency electrical heating assisted depressurization method. Fuel 2021, 290, 120075. [Google Scholar] [CrossRef]
- Wall, E.T. Interaction of Microwave Energy with Fuel Precursors. J. Microw. Power 1983, 18, 31–36. [Google Scholar] [CrossRef]
- Vermeulen, F.E.; McGee, B. In situ electromagnetic heating for hydrocarbon recovery and environmental remediation. J. Can. Pet. Technol. 2000, 39, 24–28. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, Z.; Li, S.; Li, X.; Lu, C. Numerical Evaluation of Gas Hydrate Production Performance of the Depressurization and Backfilling with In-Situ Supplemental Heat Method. ACS Omega 2021, 6, 12275–12286. [Google Scholar] [CrossRef]
- Briceño Montilla, M.J.; Li, S.; Zhang, Z.; Li, X.; Sun, Y.; Ma, S. Theoretical Analysis of the Effect of Electrical Heat in Situ Injection on the Kerogen Decomposition for the Development of Shale Oil Deposits. Energies 2023, 16, 5007. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Li, S.; He, J.; Li, X.; Xu, T.; Lu, C.; Qin, X. Optimization of the natural gas hydrate hot water injection production method: Insights from numerical and phase equilibrium analysis. Appl. Energy 2024, 361, 122963. [Google Scholar] [CrossRef]
- Braun, R.; Burnham, A. Chemical Reaction Model for Oil and Gas Generation from Type I and Type II Kerogen; Lawrence Livermore National Lab.: Livermore, CA, USA, 1993. [Google Scholar] [CrossRef]
- Lee, K. Rigorous simulation model of kerogen pyrolysis for the in situ upgrading of oil shales. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, December 2014. Available online: https://hdl.handle.net/1969.1/153864 (accessed on 1 September 2024).
- Stone, H.L. Probability Model for Estimating Three-Phase Relative Permeability. JPT J. Pet. Technol. 1970, 22, 214–218. [Google Scholar] [CrossRef]
- Pei, S.; Huang, L.; Zhang, L.; Ren, S. Experimental Study on thermal cracking reactions of ultra-heavy oils during air injection assisted in-situ upgrading process. J. Pet. Sci. Eng. 2020, 195, 107850. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, S.; Hou, L. Connotation and Strategic Role of In-situ Conversion Processing of Shale Oil Underground in the Onshore China. Shiyou Kantan Yu Kaifa/Pet. Explor. Dev. 2018, 454, 537–545. [Google Scholar] [CrossRef]
- Lei, G.; Li, Z.; Yao, C.; Ma, X.; Wang, D. Numerical simulation on oil shale in-situ upgrading by steam injection. In Proceedings of the IET Conference Publications, Kuala Lumpur, Malaysia, 14–15 November 2016. [Google Scholar] [CrossRef]
- Engineering Toolbox. Available online: https://www.engineeringtoolbox.com/classification-coal-d_164.html (accessed on 4 September 2023).
- Zhao, B.; Li, Y.; Huang, W.; Wang, X.; Chen, C. Experimental study on mechanical properties of shale rock and its strength criterion. Arab. J. Geosci. 2021, 14, 264. [Google Scholar] [CrossRef]
- Younglove, B.A.; Ely, J.F. Thermophysical Properties of Fluids II: Methane, Ethane, Propane, Isobutene, and Normal Butane. J. Phys. Chem. Ref. Data 1987, 16, 577–798. [Google Scholar] [CrossRef]
- Wagner, W.; Kretzschmar, H.J. International Steam Tables, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Yaws, C.L. Yaws’ Handbook of Thermodynamic and Physical Properties of Chemical Compounds; Knovel: Beaumont, TX, USA, 2003; Available online: www.knovel.com/knovel2/Toc.jsp (accessed on 18 May 2022).
- Berkovich, A.J.; Levy, J.H.; Schmidt, S.J.; Young, B.R. Heat capacities and enthalpies for some Australian oil shales from non-isothermal modulated DSC. Thermochim. Acta 2000, 357, 41–45. [Google Scholar] [CrossRef]
- Jin, J.; Liu, J.; Jiang, W.; Cheng, W.; Zhang, X.J.E. Evolution of the anisotropic thermal conductivity of oil shale with temperature and its relationship with anisotropic pore structure evolution. Energies 2022, 15, 8021. [Google Scholar] [CrossRef]

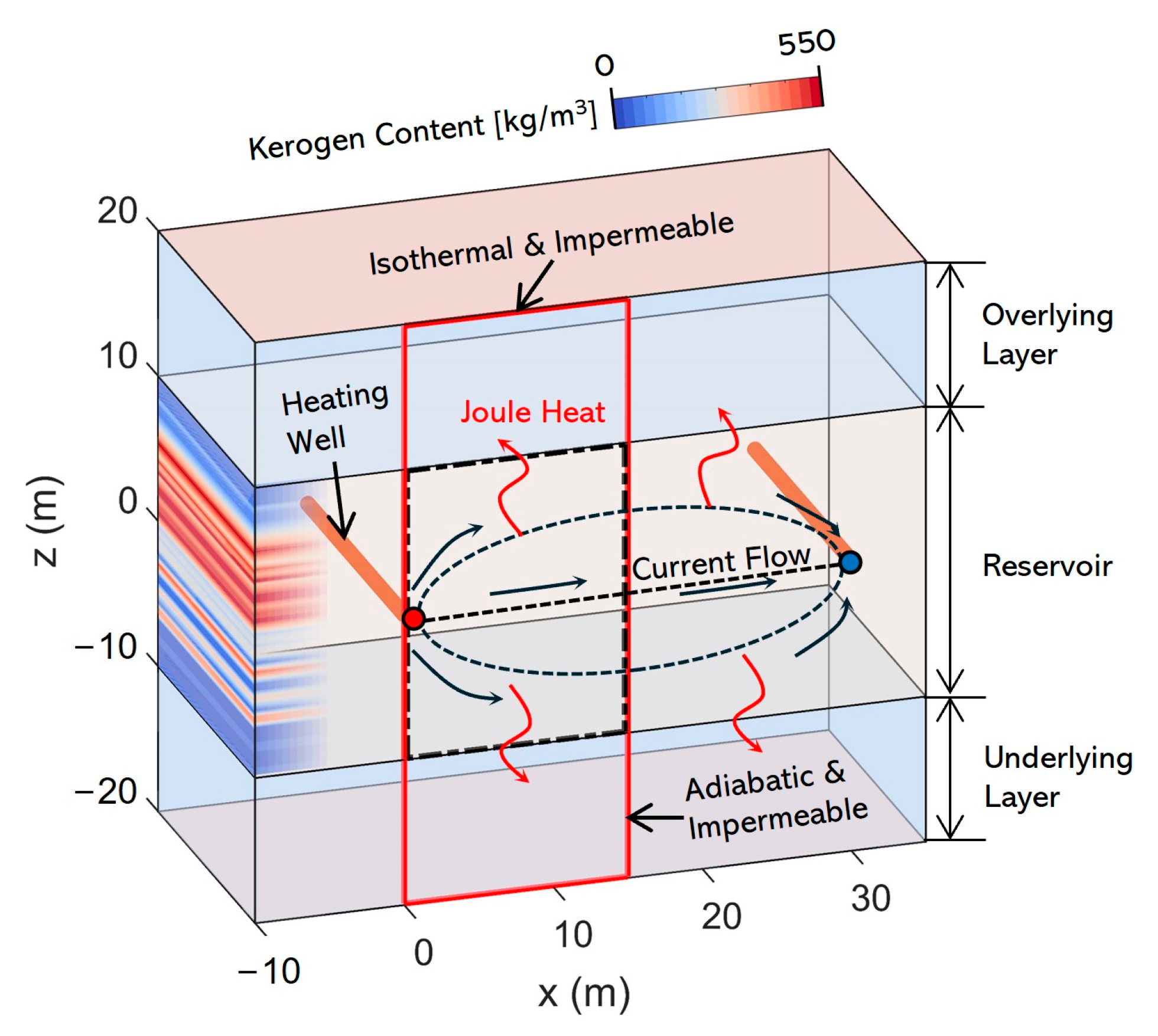
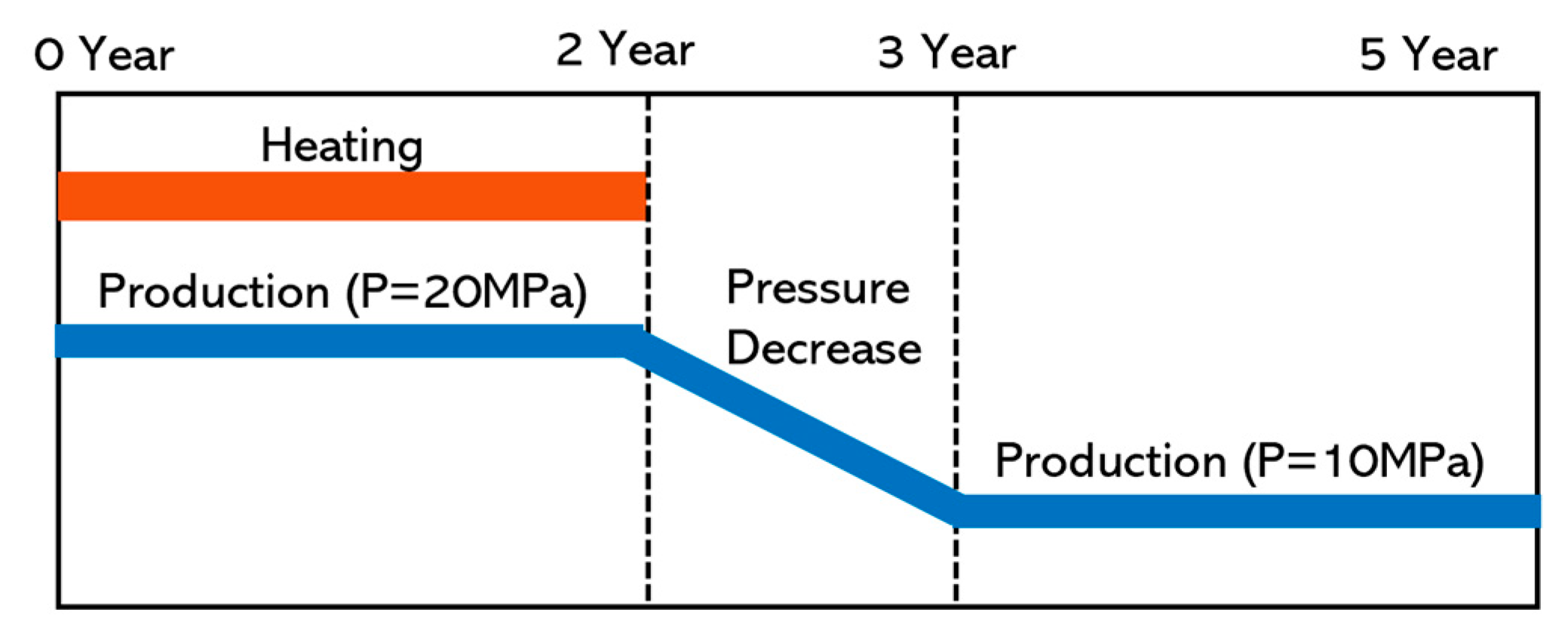






| Parameters | Values |
|---|---|
| Fluid/Solid Properties | |
| Density of solids and liquids | , , [41], and [42,43] |
| Density/viscosity of methane | Functions of pressure and temperature [44] |
| Density/viscosity of steam | Functions of pressure and temperature [45] |
| Oil viscosity | Functions of temperature [46] |
| Rock density | 2500 kg/m3 |
| Rock heat capacity | 2000 J/kg·K [47] |
| Reservoir properties | |
| Permeability | Horizontal: 10 mD, vertical: 2 mD |
| Heat conductivity | Horizontal: 2.0 W·m−1·K−1, vertical: 0.5 W·m−1·K−1 [48] |
| Initial conditions | |
| Temperature | 350 K |
| Pressure | 20 MPa |
| Porosity | Variation with depth [40] |
| Fluid saturations | Variation with depth [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Xie, Z.; Montilla, M.J.B.; Li, S.; Li, X. Low-Frequency Electrical Heating for In Situ Conversion of Shale Oil: Modeling Thermal Dynamics and Decomposition. Energies 2024, 17, 5401. https://doi.org/10.3390/en17215401
Zhang Z, Xie Z, Montilla MJB, Li S, Li X. Low-Frequency Electrical Heating for In Situ Conversion of Shale Oil: Modeling Thermal Dynamics and Decomposition. Energies. 2024; 17(21):5401. https://doi.org/10.3390/en17215401
Chicago/Turabian StyleZhang, Zhaobin, Zhuoran Xie, Maryelin Josefina Briceño Montilla, Shouding Li, and Xiao Li. 2024. "Low-Frequency Electrical Heating for In Situ Conversion of Shale Oil: Modeling Thermal Dynamics and Decomposition" Energies 17, no. 21: 5401. https://doi.org/10.3390/en17215401
APA StyleZhang, Z., Xie, Z., Montilla, M. J. B., Li, S., & Li, X. (2024). Low-Frequency Electrical Heating for In Situ Conversion of Shale Oil: Modeling Thermal Dynamics and Decomposition. Energies, 17(21), 5401. https://doi.org/10.3390/en17215401







