Electrochemical Evaluation of Choline Bromide-Based Electrolyte for Hybrid Supercapacitors
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
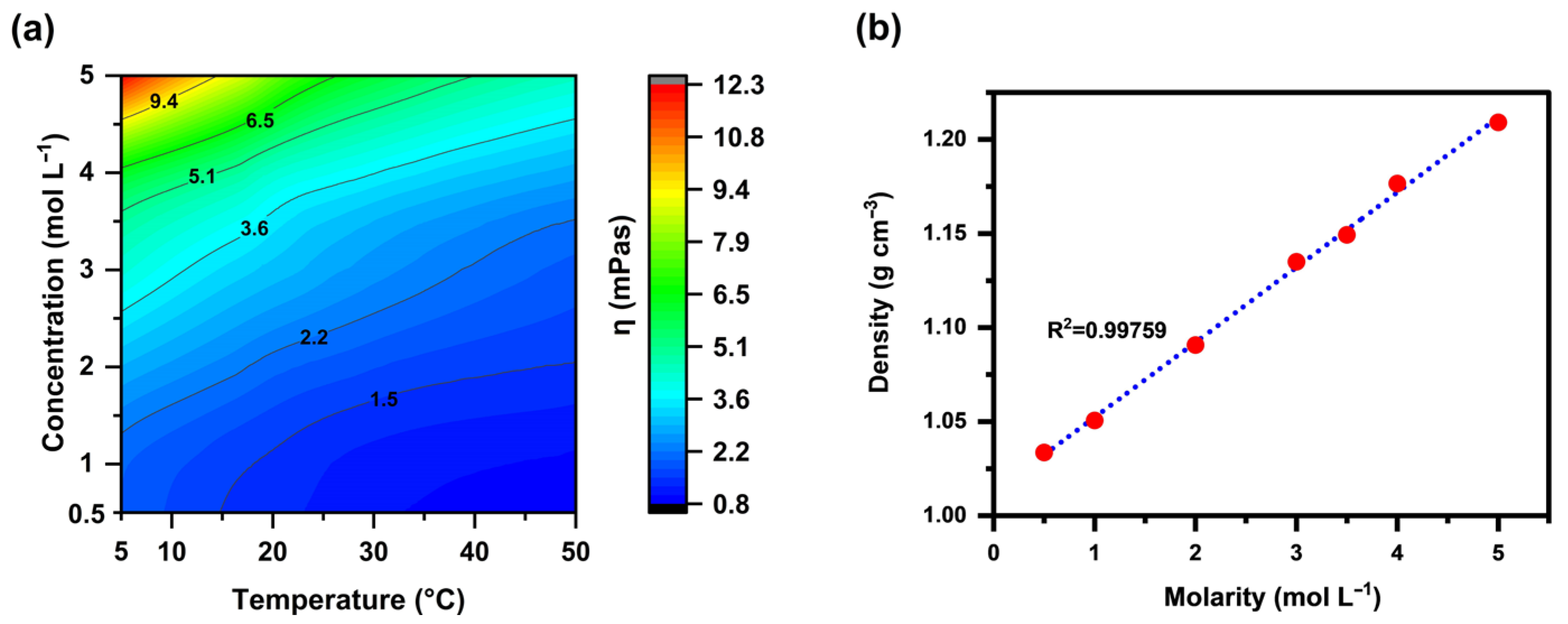
3.1. Physico-Chemical Characterization of ChBr Aqueous Solutions
3.2. Determination of Potential Electrolyte Stability Window
3.3. Electrochemical Investigations of 2-Electrode Cells: CV, GCD, and EIS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, B.K.; Sy, S.; Yu, A.; Zhang, J. Electrochemical Supercapacitors for Energy Storage and Conversion. In Handbook of Clean Energy Systems; Yan, J., Ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 1–25. ISBN 978-1-118-38858-7. [Google Scholar]
- Afif, A.; Rahman, S.M.; Tasfiah Azad, A.; Zaini, J.; Islan, M.A.; Azad, A.K. Advanced Materials and Technologies for Hybrid Supercapacitors for Energy Storage—A Review. J. Energy Storage 2019, 25, 100852. [Google Scholar] [CrossRef]
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest Advances in Supercapacitors: From New Electrode Materials to Novel Device Designs. Chem. Soc. Rev. 2017, 46, 6816–6854. [Google Scholar] [CrossRef] [PubMed]
- Pavlenko, V.; Kalybekkyzy, S.; Knez, D.; Abbas, Q.; Mansurov, Z.; Bakenov, Z.; Ng, A. Revisiting the Carbon Mesopore Contribution towards Improved Performance of Ionic Liquid–Based EDLCs at Sub-Zero Temperatures. Ionics 2022, 28, 893–901. [Google Scholar] [CrossRef]
- Pavlenko, V.V.; Zakharov, A.Y.; Ayaganov, Z.E.; Mansurov, Z.A. Nanoporous Carbon Materials: Modern Production Methods and Applications. Russ. Chem. Rev. 2024, 93, RCR5122. [Google Scholar] [CrossRef]
- Ye, W.; Wang, H.; Ning, J.; Zhong, Y.; Hu, Y. New Types of Hybrid Electrolytes for Supercapacitors. J. Energy Chem. 2021, 57, 219–232. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspectives for Electrochemical Capacitors and Related Devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Yamazaki, S.; Ito, T.; Murakumo, Y.; Naitou, M.; Shimooka, T.; Yamagata, M.; Ishikawa, M. Hybrid Capacitors Utilizing Halogen-Based Redox Reactions at Interface between Carbon Positive Electrode and Aqueous Electrolytes. J. Power Sources 2016, 326, 580–586. [Google Scholar] [CrossRef]
- Shah, S.S.; Aziz, M.A.; Yamani, Z.H. Recent Progress in Carbonaceous and Redox-Active Nanoarchitectures for Hybrid Supercapacitors: Performance Evaluation, Challenges, and Future Prospects. Chem. Rec. 2022, 22, e202200018. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A Review on Recent Advances in Hybrid Supercapacitors: Design, Fabrication and Applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145. [Google Scholar] [CrossRef]
- Chun, S.-E.; Evanko, B.; Wang, X.; Vonlanthen, D.; Ji, X.; Stucky, G.D.; Boettcher, S.W. Design of Aqueous Redox-Enhanced Electrochemical Capacitors with High Specific Energies and Slow Self-Discharge. Nat. Commun. 2015, 6, 7818. [Google Scholar] [CrossRef] [PubMed]
- Eredia, M.; Bellani, S.; Zappia, M.I.; Gabatel, L.; Galli, V.; Bagheri, A.; Beydaghi, H.; Bianca, G.; Conticello, I.; Pellegrini, V.; et al. High-Energy Density Aqueous Supercapacitors: The Role of Electrolyte pH and KI Redox Additive. APL Mater. 2022, 10, 101102. [Google Scholar] [CrossRef]
- Sayed, D.M.; Taha, M.M.; Ghanem, L.G.; El-Deab, M.S.; Allam, N.K. Hybrid Supercapacitors: A Simple Electrochemical Approach to Determine Optimum Potential Window and Charge Balance. J. Power Sources 2020, 480, 229152. [Google Scholar] [CrossRef]
- Abbas, Q.; Fitzek, H.; Pavlenko, V.; Gollas, B. Towards an Optimized Hybrid Electrochemical Capacitor in Iodide Based Aqueous Redox-Electrolyte: Shift of Equilibrium Potential by Electrodes Mass-Balancing. Electrochim. Acta 2020, 337, 135785. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, K.; Chen, Y. Wide Voltage Aqueous Asymmetric Supercapacitors: Advances, Strategies, and Challenges. Adv. Funct. Mater. 2022, 32, 2108107. [Google Scholar] [CrossRef]
- Przygocki, P.; Abbas, Q.; Gorska, B.; Béguin, F. High-Energy Hybrid Electrochemical Capacitor Operating down to −40 °C with Aqueous Redox Electrolyte Based on Choline Salts. J. Power Sources 2019, 427, 283–292. [Google Scholar] [CrossRef]
- Wang, Z.; Béguin, F. Implementation of a Choline Bis(Trifluoromethylsulfonyl)Imide Aqueous Electrolyte for Low Temperature EDLCs Enabled by a Cosolvent. J. Energy Chem. 2022, 70, 84–94. [Google Scholar] [CrossRef]
- Adanuvor, P.K.; White, R.E.; Lorimer, S.E. The Effect of the Tribromide Complex Reaction on the Oxidation/Reduction Current of the Br2/Br− Electrode. J. Electrochem. Soc. 1987, 134, 1450–1454. [Google Scholar] [CrossRef]
- Kaliyaraj Selva Kumar, A.; Miao, R.; Li, D.; Compton, R.G. Do Carbon Nanotubes Catalyse Bromine/Bromide Redox Chemistry? Chem. Sci. 2021, 12, 10878–10882. [Google Scholar] [CrossRef]
- Küttinger, M.; Wlodarczyk, J.K.; Daubner, D.; Fischer, P.; Tübke, J. High Energy Density Electrolytes for H2/Br2 Redox Flow Batteries, Their Polybromide Composition and Influence on Battery Cycling Limits. RSC Adv. 2021, 11, 5218–5229. [Google Scholar] [CrossRef]
- Zhao, Y.; Ding, Y.; Song, J.; Peng, L.; Goodenough, J.B.; Yu, G. A Reversible Br2/Br− Redox Couple in the Aqueous Phase as a High-Performance Catholyte for Alkali-Ion Batteries. Energy Environ. Sci. 2014, 7, 1990–1995. [Google Scholar] [CrossRef]
- Tang, X.; Lui, Y.H.; Chen, B.; Hu, S. Functionalized Carbon Nanotube Based Hybrid Electrochemical Capacitors Using Neutral Bromide Redox-Active Electrolyte for Enhancing Energy Density. J. Power Sources 2017, 352, 118–126. [Google Scholar] [CrossRef]
- Li, C.; Yoshida, Y.; Date, R.; Matsushita, K.; Ishii, Y.; Kawasaki, S. Bromine Aqueous Electrolyte Redox Capacitor Using Carbon Nanotubes. MAT Express 2018, 8, 555–561. [Google Scholar] [CrossRef]
- Petrov, M.M.; Konev, D.V.; Kuznetsov, V.V.; Antipov, A.E.; Glazkov, A.T.; Vorotyntsev, M.A. Electrochemically Driven Evolution of Br-Containing Aqueous Solution Composition. J. Electroanal. Chem. 2019, 836, 125–133. [Google Scholar] [CrossRef]
- Li, Q.; Haque, M.; Kuzmenko, V.; Ramani, N.; Lundgren, P.; Smith, A.D.; Enoksson, P. Redox Enhanced Energy Storage in an Aqueous High-Voltage Electrochemical Capacitor with a Potassium Bromide Electrolyte. J. Power Sources 2017, 348, 219–228. [Google Scholar] [CrossRef]
- Wang, Z.-S.; Sayama, K.; Sugihara, H. Efficient Eosin Y Dye-Sensitized Solar Cell Containing Br−/Br3− Electrolyte. J. Phys. Chem. B 2005, 109, 22449–22455. [Google Scholar] [CrossRef]
- Tang, L.; Lu, W.; Li, X. Electrolytes for Bromine-Based Flow Batteries: Challenges, Strategies, and Prospects. Energy Storage Mater. 2024, 70, 103532. [Google Scholar] [CrossRef]
- Degoulange, D.; Rousse, G.; Grimaud, A. Two-Step Mechanism for Halogen Intercalation in Graphite Enabled by Aqueous Biphasic Systems. ACS Energy Lett. 2023, 8, 4397–4405. [Google Scholar] [CrossRef]
- Akinwolemiwa, B.; Wei, C.; Yang, Q.; Yu, L.; Xia, L.; Hu, D.; Peng, C.; Chen, G.Z. Optimal Utilization of Combined Double Layer and Nernstian Charging of Activated Carbon Electrodes in Aqueous Halide Supercapattery through Capacitance Unequalization. J. Electrochem. Soc. 2018, 165, A4067–A4076. [Google Scholar] [CrossRef]
- Li, P.; Li, C.; Guo, X.; Li, X.; Zhi, C. Metal-Iodine and Metal-Bromine Batteries: A Review. Bull. Chem. Soc. Jpn. 2021, 94, 2036–2042. [Google Scholar] [CrossRef]
- Prehal, C.; Fitzek, H.; Kothleitner, G.; Presser, V.; Gollas, B.; Freunberger, S.A.; Abbas, Q. Persistent and Reversible Solid Iodine Electrodeposition in Nanoporous Carbons. Nat. Commun. 2020, 11, 4838. [Google Scholar] [CrossRef] [PubMed]
- Khayyam Nekouei, R.; Mofarah, S.S.; Maroufi, S.; Tudela, I.; Sahajwalla, V. Determination of the Optimum Potential Window for Super- and Pseudocapacitance Electrodes via in-Depth Electrochemical Impedance Spectroscopy Analysis. J. Energy Storage 2022, 56, 106137. [Google Scholar] [CrossRef]
- Bredar, A.R.C.; Chown, A.L.; Burton, A.R.; Farnum, B.H. Electrochemical Impedance Spectroscopy of Metal Oxide Electrodes for Energy Applications. ACS Appl. Energy Mater. 2020, 3, 66–98. [Google Scholar] [CrossRef]
- Laheäär, A.; Przygocki, P.; Abbas, Q.; Béguin, F. Appropriate Methods for Evaluating the Efficiency and Capacitive Behavior of Different Types of Supercapacitors. Electrochem. Commun. 2015, 60, 21–25. [Google Scholar] [CrossRef]
- Ho, P.C.; Bianchi, H.; Palmer, D.A.; Wood, R.H. Conductivity of Dilute Aqueous Electrolyte Solutions at High Temperatures and Pressures Using a Flow Cell. J. Solut. Chem. 2000, 29, 217–235. [Google Scholar] [CrossRef]
- Ding, M.S.; Von Cresce, A.; Xu, K. Conductivity, Viscosity, and Their Correlation of a Super-Concentrated Aqueous Electrolyte. J. Phys. Chem. C 2017, 121, 2149–2153. [Google Scholar] [CrossRef]
- Mahiuddin, S.; Ismail, K. Study of the Concentration Dependence of the Conductance of Aqueous Electrolytes. J. Phys. Chem. 1984, 88, 1027–1031. [Google Scholar] [CrossRef]
- Li, Y.G.; Lu, J.F. Theory of Electrolyte Solutions; Tsinghua University Press: Beijing, China, 2005; ISBN 978-7-302-09828-7. [Google Scholar]
- Hamann, C.H.; Hamnett, A.; Vielstich, W. Electrochemistry; Wiley: Hoboken, NJ, USA, 2007; ISBN 978-3-527-31069-2. [Google Scholar]
- Marcus, Y.; Hefter, G. Ion Pairing. Chem. Rev. 2006, 106, 4585–4621. [Google Scholar] [CrossRef]
- Fuoss, R.M. Conductance-Concentration Function for the Paired Ion Model. J. Phys. Chem. 1978, 82, 2427–2440. [Google Scholar] [CrossRef]
- Carey, D.M.; Korenowski, G.M. Measurement of the Raman Spectrum of Liquid Water. J. Chem. Phys. 1998, 108, 2669–2675. [Google Scholar] [CrossRef]
- Ahmed, M.; Namboodiri, V.; Singh, A.K.; Mondal, J.A.; Sarkar, S.K. How Ions Affect the Structure of Water: A Combined Raman Spectroscopy and Multivariate Curve Resolution Study. J. Phys. Chem. B 2013, 117, 16479–16485. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Fang, W.; Li, T.; Li, F.; Sun, C.; Li, Z.; Huang, Y.; Men, Z. An Insight into Liquid Water Networks through Hydrogen Bonding Halide Anion: Stimulated Raman Scattering. J. Appl. Phys. 2016, 119, 163104. [Google Scholar] [CrossRef]
- Kitadai, N.; Sawai, T.; Tonoue, R.; Nakashima, S.; Katsura, M.; Fukushi, K. Effects of Ions on the OH Stretching Band of Water as Revealed by ATR-IR Spectroscopy. J. Solut. Chem. 2014, 43, 1055–1077. [Google Scholar] [CrossRef]
- Burikov, S.; Dolenko, T.; Patsaeva, S.; Starokurov, Y.; Yuzhakov, V. Raman and IR Spectroscopy Research on Hydrogen Bonding in Water-Ethanol Systems. Mol. Phys. 2010, 108, 2427–2436. [Google Scholar] [CrossRef]
- Zhuravlev, Y.; Gordienko, K.; Dyagilev, D.; Luzgarev, S.; Ivanova, S.; Prosekov, A. Structural, Electronic, and Vibrational Properties of Choline Halides. Mater. Chem. Phys. 2020, 246, 122787. [Google Scholar] [CrossRef]
- Hickstein, D.D.; Goldfarbmuren, R.; Darrah, J.; Erickson, L.; Johnson, L.A. Rapid, Accurate, and Precise Concentration Measurements of a Methanol–Water Mixture Using Raman Spectroscopy. OSA Contin. 2018, 1, 1097. [Google Scholar] [CrossRef]
- Pichugov, R.; Konev, D.; Speshilov, I.; Abunaeva, L.; Petrov, M.; Vorotyntsev, M.A. Analysis of the Composition of Bromide Anion Oxidation Products in Aqueous Solutions with Different pH via Rotating Ring-Disk Electrode Method. Membranes 2022, 12, 820. [Google Scholar] [CrossRef]
- Galeano, C.; Meier, J.C.; Soorholtz, M.; Bongard, H.; Baldizzone, C.; Mayrhofer, K.J.J.; Schüth, F. Nitrogen-Doped Hollow Carbon Spheres as a Support for Platinum-Based Electrocatalysts. ACS Catal. 2014, 4, 3856–3868. [Google Scholar] [CrossRef]
- Tang, H.; Chen, J.H.; Huang, Z.P.; Wang, D.Z.; Ren, Z.F.; Nie, L.H.; Kuang, Y.F.; Yao, S.Z. High Dispersion and Electrocatalytic Properties of Platinum on Well-Aligned Carbon Nanotube Arrays. Carbon 2004, 42, 191–197. [Google Scholar] [CrossRef]
- Perez, J.; Gonzalez, E.R.; Ticianelli, E.A. Oxygen Electrocatalysis on Thin Porous Coating Rotating Platinum Electrodes. Electrochim. Acta 1998, 44, 1329–1339. [Google Scholar] [CrossRef]
- Fic, K.; Morimoto, S.; Frąckowiak, E.; Ishikawa, M. Redox Activity of Bromides in Carbon-Based Electrochemical Capacitors. Batter. Supercaps 2020, 3, 1080–1090. [Google Scholar] [CrossRef]
- Fic, K.; Meller, M.; Frackowiak, E. Interfacial Redox Phenomena for Enhanced Aqueous Supercapacitors. J. Electrochem. Soc. 2015, 162, A5140–A5147. [Google Scholar] [CrossRef]
- Rufford, T.E.; Hulicova-Jurcakova, D.; Khosla, K.; Zhu, Z.; Lu, G.Q. Microstructure and Electrochemical Double-Layer Capacitance of Carbon Electrodes Prepared by Zinc Chloride Activation of Sugar Cane Bagasse. J. Power Sources 2010, 195, 912–918. [Google Scholar] [CrossRef]
- Shrestha, D. Activated Carbon and Its Hybrid Composites with Manganese (IV) Oxide as Effectual Electrode Materials for High Performance Supercapacitor. Arab. J. Chem. 2022, 15, 103946. [Google Scholar] [CrossRef]
- Alkhalaf, S.; Ranaweera, C.K.; Kahol, P.K.; Siam, K.; Adhikari, H.; Mishra, S.R.; Perez, F.; Gupta, B.K.; Ramasamy, K.; Gupta, R.K. Electrochemical Energy Storage Performance of Electrospun CoMn2O4 Nanofibers. J. Alloys Compd. 2017, 692, 59–66. [Google Scholar] [CrossRef]
- Pillay, B.; Newman, J. The Influence of Side Reactions on the Performance of Electrochemical Double-Layer Capacitors. J. Electrochem. Soc. 1996, 143, 1806–1814. [Google Scholar] [CrossRef]
- Jing, L.; Zhuo, K.; Sun, L.; Zhang, N.; Su, X.; Chen, Y.; Hu, X.; Feng, R.; Wang, J. The Mass-Balancing between Positive and Negative Electrodes for Optimizing Energy Density of Supercapacitors. J. Am. Chem. Soc. 2024, 146, 14369–14385. [Google Scholar] [CrossRef]
- Shaalan, N.M.; Ahmed, F.; Kumar, S.; Ahmad, M.M.; Al-Naim, A.F.; Hamad, D. Electrochemical Performance of Potassium Bromate Active Electrolyte for Laser-Induced KBr-Graphene Supercapacitor Electrodes. Inorganics 2023, 11, 109. [Google Scholar] [CrossRef]
- Maher, M.; Hassan, S.; Shoueir, K.; Yousif, B.; Abo-Elsoud, M.E.A. Activated Carbon Electrode with Promising Specific Capacitance Based on Potassium Bromide Redox Additive Electrolyte for Supercapacitor Application. J. Mater. Res. Technol. 2021, 11, 1232–1244. [Google Scholar] [CrossRef]
- Ede, S.R.; Anantharaj, S.; Kumaran, K.T.; Mishra, S.; Kundu, S. One Step Synthesis of Ni/Ni(OH) 2 Nano Sheets (NSs) and Their Application in Asymmetric Supercapacitors. RSC Adv. 2017, 7, 5898–5911. [Google Scholar] [CrossRef]
- Kubra, K.T.; Javaid, A.; Sharif, R.; Ali, G.; Iqbal, F.; Salman, A.; Shaheen, F.; Butt, A.; Iftikhar, F.J. Facile Synthesis and Electrochemical Study of a Ternary Hybrid PANI/GNP/MnO2 as Supercapacitor Electrode Material. J. Mater. Sci. Mater. Electron. 2020, 31, 12455–12466. [Google Scholar] [CrossRef]
- Giffin, G.A. The Role of Concentration in Electrolyte Solutions for Non-Aqueous Lithium-Based Batteries. Nat. Commun. 2022, 13, 5250. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y.; Meng, J.; Shen, N.; Liu, H.; Guo, C.; Bao, W.; Li, J.; Yao, D.; Yu, F. Boosting the Capacitance of MOF-Derived Carbon-Based Supercapacitors by Redox-Active Bromide Ions. Chem. Eng. J. Adv. 2023, 14, 100484. [Google Scholar] [CrossRef]
- Ayaganov, Z.; Pavlenko, V.; Haque, S.F.B.; Tanybayeva, A.; Ferraris, J.; Zakhidov, A.; Mansurov, Z.; Bakenov, Z.; Ng, A. A Comprehensive Study on Effect of Carbon Nanomaterials as Conductive Additives in EDLCs. J. Energy Storage 2024, 78, 110035. [Google Scholar] [CrossRef]
- Mirzaei-Saatlo, M.; Faraji, S.; Fakhraei, M.; Moradi-Alavian, S.; Asghari, E.; Shekaari, H. Phase Change Ionogel Based Choline Formate as a Green Solid-State Electrolyte with Novel Poly (Aniline-Co-4-Nitroaniline) Electrode Material for Flexible Supercapacitors. J. Energy Storage 2024, 82, 110534. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, D.; She, W.; Wang, J.; Gao, S.; Wang, Y.; Wang, K. Remarkably Improving the Specific Energy of Supercapacitor Based on a Biomass-Derived Interconnected Hierarchical Porous Carbon by Using a Newly-Developed Mixed Alkaline Aqueous Electrolyte with Widened Operation Voltage. J. Power Sources 2021, 492, 229666. [Google Scholar] [CrossRef]
- Xu, L.H.; Wu, D.; Zhong, M.; Wang, G.B.; Chen, X.Y.; Zhang, Z.J. The Construction of a New Deep Eutectic Solvents System Based on Choline Chloride and Butanediol: The Influence of the Hydroxyl Position of Butanediol on the Structure of Deep Eutectic Solvent and Supercapacitor Performance. J. Power Sources 2021, 490, 229365. [Google Scholar] [CrossRef]
- Abbas, Q.; Béguin, F. Sustainable Carbon/Carbon Supercapacitors Operating Down to −40 °C in Aqueous Electrolyte Made with Cholinium Salt. ChemSusChem 2018, 11, 975–984. [Google Scholar] [CrossRef]
- Schrade, S.; Zhao, Z.; Supiyeva, Z.; Chen, X.; Dsoke, S.; Abbas, Q. An Asymmetric MnO2|activated Carbon Supercapacitor with Highly Soluble Choline Nitrate-Based Aqueous Electrolyte for Sub-Zero Temperatures. Electrochim. Acta 2022, 425, 140708. [Google Scholar] [CrossRef]
- Sandhiya, M.; Vivekanand; Suresh Balaji, S.; Sathish, M. Unrevealed Performance of NH4VO3 as a Redox-Additive for Augmenting the Energy Density of a Supercapacitor. J. Phys. Chem. C 2021, 125, 8068–8079. [Google Scholar] [CrossRef]
- Pavlenko, V.V.; Temirkulova, K.M.; Zakharov, A.Y.; Aubakirov, Y.A.; Ayaganov, Z.E. Optimization of the Porous Structure of Carbon Electrodes for Hybrid Supercapacitors with a Redox Electrolyte Based on Potassium Bromide. Eurasian Chem.-Technol. J. 2024, 25, 201–210. [Google Scholar] [CrossRef]
- Abbas, Q.; Babuchowska, P.; Frąckowiak, E.; Béguin, F. Sustainable AC/AC Hybrid Electrochemical Capacitors in Aqueous Electrolyte Approaching the Performance of Organic Systems. J. Power Sources 2016, 326, 652–659. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Z. Porous Carbon Derived from Herbal Plant Waste for Supercapacitor Electrodes with Ultrahigh Specific Capacitance and Excellent Energy Density. Waste Manag. 2020, 106, 250–260. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Chen, J.; Su, H.; Liu, F.; Yang, W. One-Step Synthesis of Hierarchically Porous Carbons for High-Performance Electric Double Layer Supercapacitors. J. Power Sources 2016, 315, 120–126. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, X.; Zhang, H.; Zhang, D.; Ma, Y. A Comparative Study of Activated Carbon-Based Symmetric Supercapacitors in Li2SO4 and KOH Aqueous Electrolytes. J. Solid State Electrochem. 2012, 16, 2597–2603. [Google Scholar] [CrossRef]
- Li, G.; Li, Q.; Ye, J.; Fu, G.; Han, J.; Zhu, Y. Activated Carbon from the Waste Water Purifier for Supercapacitor Application. J. Solid. State Electrochem. 2017, 21, 3169–3177. [Google Scholar] [CrossRef]
- Mohd Hanappi, M.F.Y.; Deraman, M.; Suleman, M.; Mohd Nor, N.S.; Sazali, N.E.S.; Hamdan, E.; Moh Tajuddin, N.S.; Basri, N.H.; Mohd Jasni, M.R.; Othman, M.A.R. Influence of Aqueous KOH and H2SO4 Electrolytes Ionic Parameters on the Performance of Carbon-Based Supercapacitor Electrodes. Funct. Mater. Lett. 2017, 10, 1750013. [Google Scholar] [CrossRef]
- Senthilkumar, S.T.; Selvan, R.K.; Ponpandian, N.; Melo, J.S.; Lee, Y.S. Improved Performance of Electric Double Layer Capacitor Using Redox Additive (VO2+/VO2+) Aqueous Electrolyte. J. Mater. Chem. A 2013, 1, 7913. [Google Scholar] [CrossRef]
- Shang, T.; Xu, Y.; Li, P.; Han, J.; Wu, Z.; Tao, Y.; Yang, Q.-H. A Bio-Derived Sheet-like Porous Carbon with Thin-Layer Pore Walls for Ultrahigh-Power Supercapacitors. Nano Energy 2020, 70, 104531. [Google Scholar] [CrossRef]
- Balaji, S.S.; Karnan, M.; Anandhaganesh, P.; Tauquir, S.M.; Sathish, M. Performance Evaluation of B-Doped Graphene Prepared via Two Different Methods in Symmetric Supercapacitor Using Various Electrolytes. Appl. Surf. Sci. 2019, 491, 560–569. [Google Scholar] [CrossRef]
- Wemme, D.; Petrouleas, V.; Panagiotopoulos, N.; Filippakis, S.E.; Lemmon, R.M. High-resolution solid-state nuclear magnetic resonance and x-ray structure study of choline chloride, bromide, and iodide. J. Phys. Chem. 1983, 87, 999–1003. [Google Scholar] [CrossRef]





| Electrode Materials | SSA, m2 g−1 | Electrolyte | Cell Voltage | Current Density (A g−1) | Energy Density (Wh kg−1) | Power Density (W kg−1) | C (F g−1) | ΔC (%) | CE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Choline-based electrolytes | ||||||||||
| Kansai Coke, Maxsorb MSP-20X | 2306 | 3.5 mol L−1 ChBr | 1.9 | 0.5 | 41 | 226 | 330 | 98% after 10,000 cycles at 2 A g−1 | 97% | This work |
| 5 | 36 | 2297 | 301 | |||||||
| Poly (aniline-co-4-nitroaniline) | - | Choline formate/ 2-hydroxyethyl cellulose = 3/1 | 0.6 | 0.5 | ~30 | 150 | 594 | ~90% after 5000 cycles at 5 A g−1 | ~96% at 5 A g−1 | [68] |
| Interconnected hierarchical porous carbon (IHPC) | 3463 | 0.1 mol L−1 ChOH + 1 mol L−1 KOH | 1.3 | 0.5 | ~20 | 203 | 462 | ~91% after 30,000 cycles at 5 A g−1 | 100% at 5 A g−1 | [69] |
| Activated Carbon | 1900 | ChCl/1, 2-butanediol = 1/4 | 2.0 | 1.0 | ~16 | 1k | 116 | ~87% after 10,000 cycles at 4 A g−1 | - | [70] |
| (−) Kansai Coke, Maxsorb/(+)Kuraray, YP-80F | 1962/1735 | 5 mol kg−1 ChNO3 +0.5 mol kg−1 ChI | 1.5 | 0.1 | ~12 | 3.0 k | 81 per total mass of electrodes | 92% after 20 k cycles at 0.5 A g−1 | ~86% Energy eff. | [17] |
| MgO-templated hierarchical carbon | ~2000 | 5 mol kg−1 choline bis(trifluoromethylsulfonyl)imide in M0.75W0.25 | 1.6 | 0.5 | ~8 | 50 | 128 | 81% after 20,000 cycles at 1 A g−1 | - | [18] |
| DLC Supra 30 from Norit | 1869 | 5 mol kg−1 ChCl | 1.5 | 0.2 | - | - | 126 | ~98% after 10,000 cycles at 1 A g−1 | ~99% | [71] |
| (+)MnO2/CNT|YP80F(−) | - | 5 mol L−1 ChNO3 | 0.3–1.8 | 1.0 | - | - | 38 for cell | ~97% after 10,000 | 90% | [72] |
| Redox active electrolytes | ||||||||||
| Fuzhou Yihuan Carbon Co., Fuzhou, China, YEC-8A | 1898 | 1 mol L−1 KBr | 2.0 | 0.25 | ~33 | - | - | - | ~93% | [30] |
| S-doped graphene | 215 | 0.01 mol L−1 NH4VO3 + 1 mol L−1 H2SO4 | 1.6 | 3.0 | 32 | 2370 | 364 | 85% after 10,000 at 10 A g−1 | 99% at 10 A/g | [73] |
| AC/MgO templated carbon | 2315/1976 | 5 mol L−1 NaNO3 + 0.5 mol L−1 KBr | 1.8 | 0.5 | 27 | - | 239 | 75% after 5000 cycles at 2 A g−1 | 94 | [74] |
| AC | 2180 | 1 mol L−1 Li2SO4 + 0.5 mol L−1 KI | 1.6 | 0.2 | 26 | ~80 | 300 | 91% after floating for 120 h | 71% energy efficiency | [75] |
| Kuraray, YP-80F | 2112 | 1 mol L−1 KBr | 1.9 | 1.0 | 12 | 15 kW kg−1 at 15 A g−1 | 92 | 81% after 10,000 cycles at 15 A g−1 | ~98% | [26] |
| Other common aqueous electrolytes | ||||||||||
| Neutral | ||||||||||
| AC from Salvia miltiorrhiza flowers | 1715 | 1 mol L−1 Na2SO4 | 1.8 | 0.5 | ~22 | 448 | 198 | 91% after 10,000 cycles at 10 A g−1 in 6 M KOH | - | [76] |
| Hierarchically porous carbons | 3003 | 1 mol L−1 Na2SO4 | 1.6 | 0.5 | ~21 | 400 | 240 | 91% after 2000 cycles at 5 A g−1 | 93% | [77] |
| AC, Carbosino Co., Ltd., Shanghai, China | 2500 | 1 mol L−1 Li2SO4 | 1.6 | 0.25 | ~17 | 200 | 190 | 92% after 10,000 cycles at 1 A g−1 | 99% | [78] |
| Acid | ||||||||||
| AC from waste water | 1103 | 1 mol L−1 H2SO4 | 1.0 | 1.0 | ~15 | ~937 | ~123 | ~66% after 6000 cycles at 5 A/g | - | [79] |
| AC | 903 | 1 mol L−1 H2SO4 | 1.0 | 0.5 | 10 | 490 | 545 | - | - | [80] |
| AC from Eichhornia crassipes | 683 | 1 mol L−1 H2SO4 | 0.8 | 1 mA cm−2 | ~9 | ~315 | ~441 | 91% after 4000 cycles at 5 mA cm−2 | ~81% | [81] |
| Alkaline | ||||||||||
| AC | 3577 | 6 mol L−1 KOH | 1.0 | 0.1 | ~10 | ~25 | 330 | 95% after 10,000 at 1 A/g | - | [82] |
| B-doped graphene | 170 | 20% KOH | 1.0 | 1.0 | ~5 | 502 | 286 | 96% after 10,000 cycles at 20 A/g | - | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayaganov, Z.; Malchik, F.; Bakenov, Z.; Mansurov, Z.; Maldybayev, K.; Kurbatov, A.; Ng, A.; Pavlenko, V. Electrochemical Evaluation of Choline Bromide-Based Electrolyte for Hybrid Supercapacitors. Energies 2024, 17, 5580. https://doi.org/10.3390/en17225580
Ayaganov Z, Malchik F, Bakenov Z, Mansurov Z, Maldybayev K, Kurbatov A, Ng A, Pavlenko V. Electrochemical Evaluation of Choline Bromide-Based Electrolyte for Hybrid Supercapacitors. Energies. 2024; 17(22):5580. https://doi.org/10.3390/en17225580
Chicago/Turabian StyleAyaganov, Zhanibek, Fyodor Malchik, Zhumabay Bakenov, Zulkhair Mansurov, Kaiyrgali Maldybayev, Andrey Kurbatov, Annie Ng, and Vladimir Pavlenko. 2024. "Electrochemical Evaluation of Choline Bromide-Based Electrolyte for Hybrid Supercapacitors" Energies 17, no. 22: 5580. https://doi.org/10.3390/en17225580
APA StyleAyaganov, Z., Malchik, F., Bakenov, Z., Mansurov, Z., Maldybayev, K., Kurbatov, A., Ng, A., & Pavlenko, V. (2024). Electrochemical Evaluation of Choline Bromide-Based Electrolyte for Hybrid Supercapacitors. Energies, 17(22), 5580. https://doi.org/10.3390/en17225580







