The Influence of the Addition of Cement and Zeolite on the Increase in the Efficiency of Sewage Sludge Dewatering in the Pressure Filtration Process †
Abstract
:1. Introduction
2. Materials and Methods
- The doses of mineral substances, i.e., cement, zeolite, and polyelectrolyte;
- The pressure filtration process parameters—variable pressure.
- –
- Polyelectrolyte—Kemira Superfloc® (Kemira, Helsinki, Finland) polyelectrolytes from the C series were used for chemical preparation of sewage sludge: weakly cationic C-494. The gel works by exchanging a charge between the polyelectrolyte chain and the suspension. As a result of this action, the suspension loses stability and becomes capable of coagulation or the formation of flocs. In order to improve the properties and ability to release water, polyelectrolyte was added to the tested sludge at a dose of 2.5 mg/g d.m., regardless of the type of sludge.
- –
- Cement—Portland type, a combination of ground cement clinker and gypsum. Cement clinker is obtained by firing a mixture of ground raw materials containing limestone and aluminosilicates at 1450 °C. The chemical composition of clinker includes allite, tricalcium silicate (50–65% by weight of clinker), belite, dicalcium silicate (about 20% by weight of clinker), brownmillerite, a compound of calcium oxide, aluminum oxide and iron (III) oxide (about 10% by weight of clinker), tricalcium aluminate (about 10% by weight of clinker), and other compounds of aluminum, calcium, and magnesium. Gypsum or a mixture of gypsum and anhydrite, a setting time regulator, and up to 5% of other ingredients (limestone, slag, and pozzolan) were added to the clinker produced from the above mixture.
- –
- Zeolite 13X—synthetic adsorber type 13X. The unit cell of X-type zeolite contains 192 tetrahedra (Si,Al)O4. The basic element of the structure are cuboctahedrons composed of 14 SiO4 tetrahedra and 10 AlO4 tetrahedra. Zeolite 13X has a pore diameter of approximately 1.3 μm and a ball size of 1.3 to 2.3 mm. The tests used doses of 0.8 and 1.6 g/L of sewage sludge.
- r—specific resistance of the sludge under pressure p,
- r0—constant representing the resistivity of the incompressible sludge cake,
- p—filtration pressure,
- s—compressibility coefficient.
- S—compressibility coefficient,
- r1—specific resistance under pressure p1,
- r2—specific resistance under pressure p2.

3. Results
4. Discussion
5. Final Conclusions
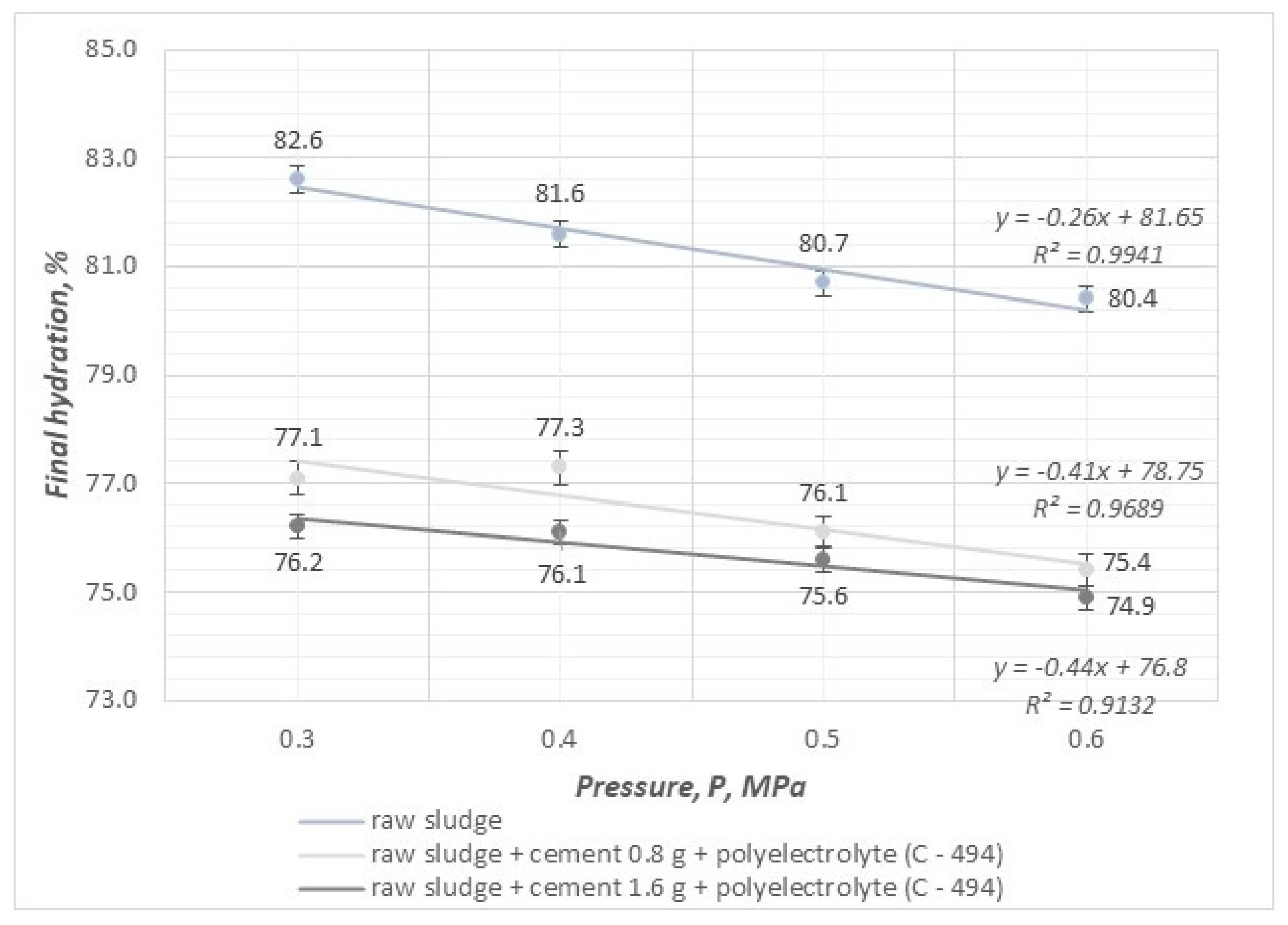
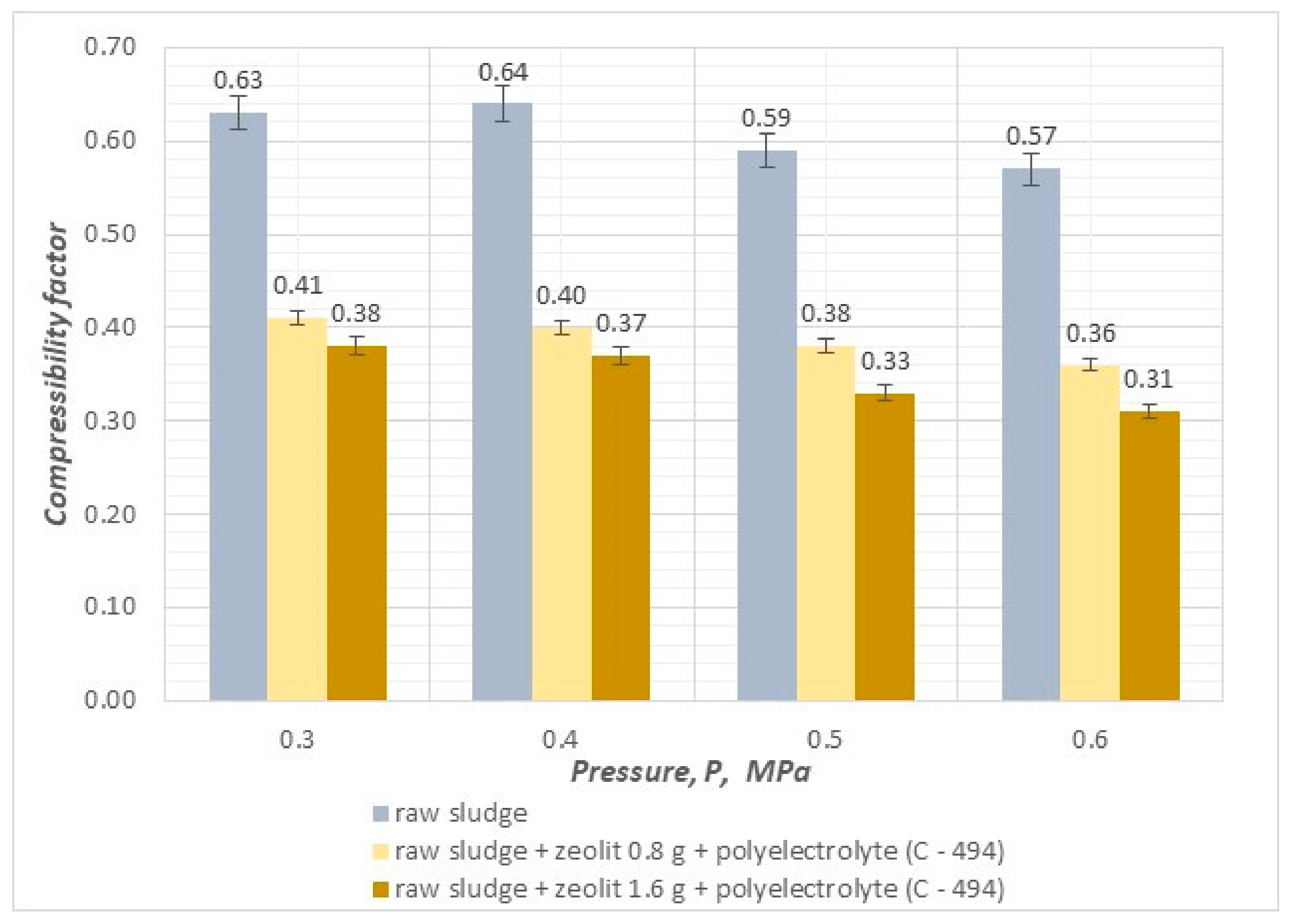
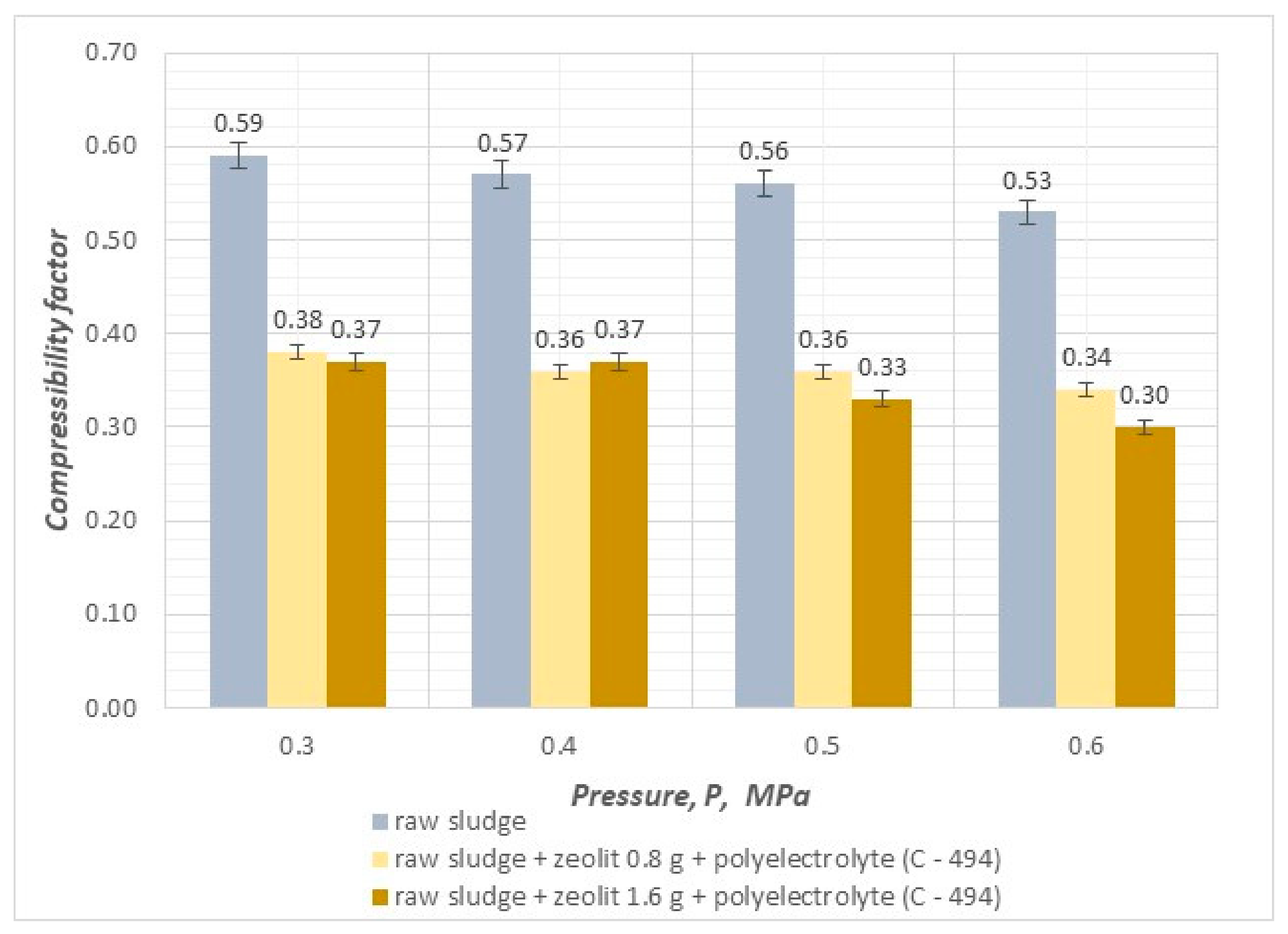
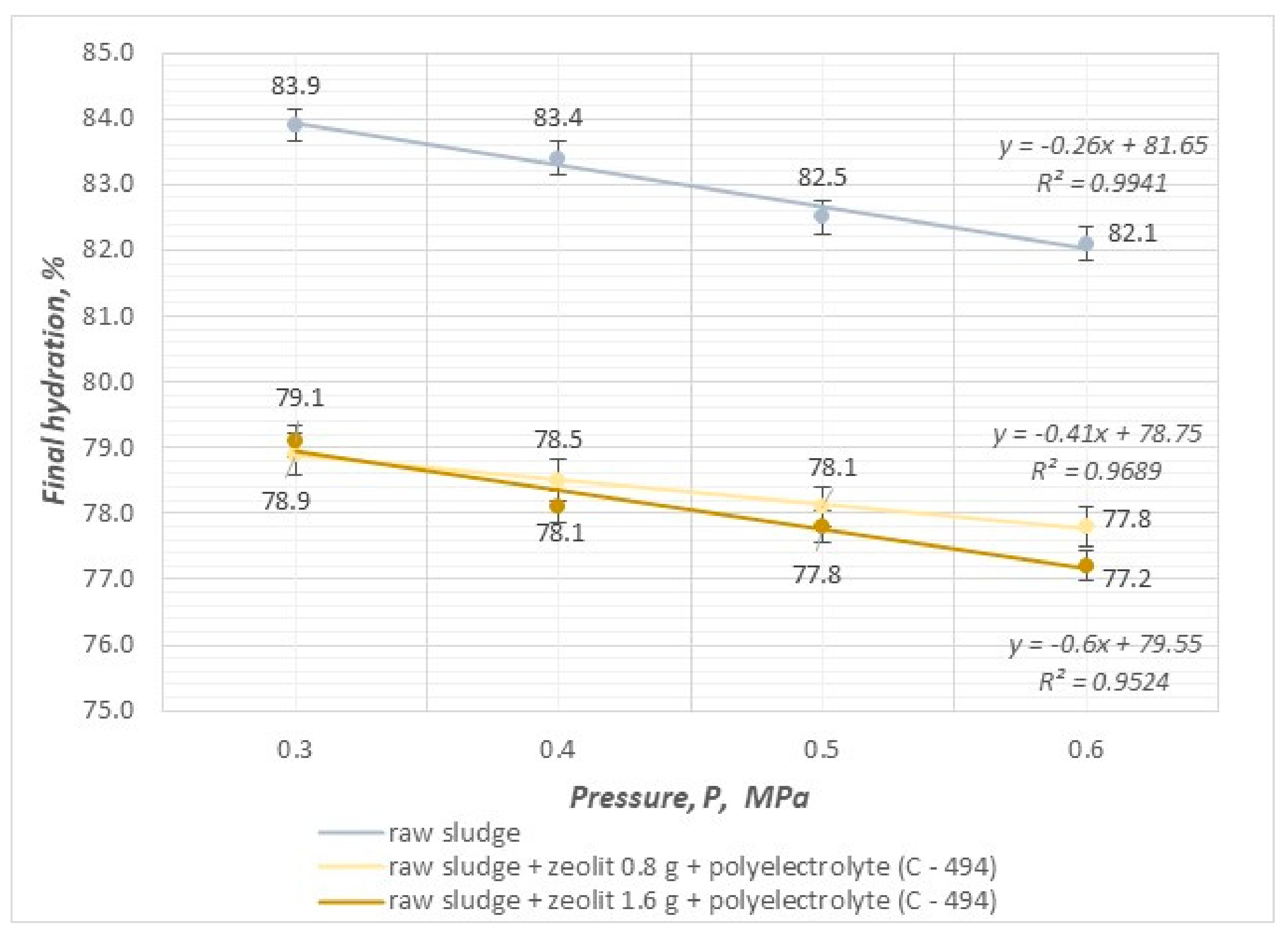
- The compressibility coefficient, taking values in the range of 0.25–0.30, results in a significant increase in efficiency and velocity and affects the reduction in final hydration in the pressure filtration process.
- The value of the sediment compressibility coefficient decreases as the pressure of the filtration process and the dose of mineral substances increase.
- By conducting the pressure filtration process using combined methods of sludge conditioning with an organic reagent (polyelectrolyte) and mineral substances (cement, zeolite), it is possible to achieve a reduction in the compressibility coefficient of sludge, which results in an increase in the efficiency and speed of the process and, at the same time, makes it possible to dewater sludge to a value of 74.9%.
- The use of the highest filtration process pressure of 0.6 MPa leads to the best results in terms of final hydration, efficiency, and filtration speed.
- The addition of zeolites and polyelectrolyte resulted in a decrease in total organic carbon content as the applied pressure increased.
Funding
Data Availability Statement
Conflicts of Interest
References
- Mahmoud, A.; Olivier, J.; Vaxelaire, J.; Hoadley, A.F. Electro-dewatering of wastewater sludge: Influence of the operating conditions and their interactions effects. Water Res. 2011, 45, 2795–2810. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Thapa, K.B.; Hoadley, A.F. Application of filtration aids for improving sludge dewatering properties—A review. Chem. Eng. J. 2011, 171, 373–384. [Google Scholar] [CrossRef]
- Cao, B.; Zhang, W.; Du, Y.; Wang, R.; Usher, S.P.; Scales, P.J.; Wang, D. Compartmentalization of extracellular polymeric substances (EPS) solubilization and cake microstructure in relation to wastewater sludge dewatering behavior assisted by horizontal electric field: Effect of operating conditions. Water Res. 2018, 130, 363–375. [Google Scholar] [CrossRef]
- Cao, B.; Zhang, W.; Wang, Q.; Huang, Y.; Meng, C.; Wang, D. Wastewater sludge dewaterability enhancement using hydroxyl aluminum conditioning: Role of aluminum speciation. Water Res. 2016, 105, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, R.; Zhang, W.; Wu, H.; Wang, D. Carbon-based materials reinforced waste activated sludge electro-dewatering for synchronous fuel treatment. Water Res. 2019, 149, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, Q.; Wang, D.; Li, X.; Zhong, Y.; Li, X.; Deng, Y.; Wang, L.; Yi, K.; Zeng, G. Enhanced dewaterability of waste activated sludge by Fe(II)-activated peroxymonosulfate oxidation. Bioresour. Technol. 2016, 206, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Liu, X.; Ding, A.; Ngo, H.H.; Zhang, R.; Nan, J.; Ma, J.; Li, G. Advanced oxidation processes (AOPs)-based sludge conditioning for enhanced sludge dewatering and micropollutants removal: A critical review. J. Water Process. Eng. 2022, 45, 102468. [Google Scholar] [CrossRef]
- Kamizela, T.; Kowalczyk, M. Impact of Conditioning Substances and Filtration Pressure on Dewatering Efficiency of Sewage Sludge. Energies 2021, 14, 361. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Kamizela, T. Artificial Neural Networks in Modeling of Dewaterability of Sewage Sludge. Energies 2021, 14, 1552. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, W.; Xiao, Z.; Zhou, X.; Zeng, Q. +Efficient dewatering and heavy-metal removal in municipal sewage using oxidants. Chem. Eng. J. 2020, 388, 124298. [Google Scholar] [CrossRef]
- Shen, J.; Zhou, X.; Zhou, L.; Huang, Z.; Huang, S. Effect of sludge conditioning by microbial fuel cell on electro-dewatering performance. J. Water Process. Eng. 2023, 55, 104137. [Google Scholar] [CrossRef]
- Cai, M.; Qian, Z.; Xiong, X.; Dong, C.; Song, Z.; Shi, Y.; Wei, Z.; Jin, M. Cationic polyacrylamide (CPAM) enhanced pressurized vertical electro-osmotic dewatering of activated sludge. Sci. Total Environ. 2022, 818, 151787. [Google Scholar] [CrossRef] [PubMed]
- Saveyn, H.; Pauwels, G.; Timmerman, R.; Van der Meeren, P. Effect of polyelectrolyte conditioning on the enhanced dewatering of activated sludge by application of an electric field during the expression phase. Water Res. 2005, 39, 3012–3020. [Google Scholar] [CrossRef] [PubMed]
- Barber, W. Thermal hydrolysis for sewage treatment: A critical review. Water Res. 2016, 104, 53–71. [Google Scholar] [CrossRef] [PubMed]
- Neyens, E.; Baeyens, J. A review of thermal sludge pre-treatment processes to improve dewaterability. J. Hazard. Mater. 2003, 98, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, H.R.; Devatha, C.P. Review on solid-liquid separation using various conditioning methods. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1166, 012002. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Kamizela, T. Increase in efficiency of separating pollution from sewage sludge through the pressure filtration process. Desalination Water Treat. 2023, 301, 181–189. [Google Scholar] [CrossRef]
- La Heij, E.; Kerkhof, P.; Herwijn, A.; Coumans, W. Fundamental aspects of sludge filtration and expression. Water Res. 1996, 30, 697–703. [Google Scholar] [CrossRef]
- Zhao, Y.; Bache, D. Integrated effects of applied pressure, time, and polymer doses on alum sludge dewatering behaviour. Waste Manag. 2002, 22, 813–819. [Google Scholar] [CrossRef]
- Wu, R.; Lee, D.; Wang, C.; Chen, J.; Tan, R. Novel cake characteristics of waste-activated sludge. Water Res. 2001, 35, 1358–1362. [Google Scholar] [CrossRef]
- Lee, C.; Liu, J. Enhanced sludge dewatering by dual polyelectrolytes conditioning. Water Res. 2000, 34, 4430–4436. [Google Scholar] [CrossRef]








| Unit | Primary Sludge | Mixed Sludge | |
|---|---|---|---|
| Initial hydration | % | 97.1–98.4 ± 0.19 | 97.5–98.1 ± 0.15 |
| Dry mass of sludge | g/L | 29.0–16.0 ± 0.2 | 25.0–19.0 ± 0.18 |
| The content of organic substances | g/L | 18.5–10.2 ± 0.16 | 16.7–13.2 ± 0.18 |
| The content of mineral substances | g/L | 10.5–5.8 ± 0.13 | 8.3–5.8 ± 0.10 |
| pH | - | 6.8–7.1 ± 0.10 | 6.9–7.0 ± 0.10 |
| CST (capillary suction time) | s | 112–128 ± 8.0 | 206–238 ± 19.0 |
| Primary Sludge | Mixed Sludge | ||||||
|---|---|---|---|---|---|---|---|
| Conditioning Method | Pressure MPa | Efficiency kg/m2h | Speed cm3/s | Specific Resistance to Filtration, m/kg × 1013 | Efficiency kg/m2h | Speed cm3/s | Specific Resistance to Filtration, m/kg × 1013 |
| raw sludge | 0.3 | 2.24 ± 0.015 | 0.14 ± 0.009 | 4.24 ± 0.06 | 1.51 ± 0.015 | 0.14 ± 0.009 | 5.59 ± 0.06 |
| 0.4 | 2.31 ± 0.010 | 0.16 ± 0.007 | 4.12 ± 0.08 | 1.87 ± 0.010 | 0.15 ± 0.007 | 5.48 ± 0.08 | |
| 0.5 | 3.01 ± 0.017 | 0.20 ± 0.003 | 4.67 ± 0.08 | 2.12 ± 0.017 | 0.11 ± 0.003 | 5.06 ± 0.08 | |
| 0.6 | 3.71 ± 0.014 | 0.27 ± 0.010 | 4.91 ± 0.04 | 2.56 ± 0.014 | 0.19 ± 0.010 | 5.51 ± 0.04 | |
| raw sludge + cement 0.8 g + polyelectrolyte (C-494) | 0.3 | 8.97 ± 0.033 | 0.38 ± 0.017 | 2.02 ± 0.02 | 7.32 ± 0.022 | 0.34 ± 0.014 | 2.12 ± 0.02 |
| 0.4 | 9.54 ± 0.039 | 0.40 ± 0.019 | 2.96 ± 0.04 | 8.48 ± 0.020 | 0.39 ± 0.016 | 2.34 ± 0.03 | |
| 0.5 | 9.12 ± 0.033 | 0.41 ± 0.019 | 2.54 ± 0.04 | 7.45 ± 0.026 | 0.31 ± 0.015 | 2.68 ± 0.03 | |
| 0.6 | 9.79 ± 0.039 | 0.43 ± 0.021 | 2.59 ± 0.03 | 9.02 ± 0.023 | 0.47 ± 0.019 | 2.94 ± 0.03 | |
| raw sludge + cement 1.6 g + polyelectrolyte (C-494) | 0.3 | 9.17 ± 0.034 | 0.41 ± 0.019 | 2.15 ± 0.04 | 7.48 ± 0.032 | 0.43 ± 0.017 | 3.16 ± 0.05 |
| 0.4 | 9.59 ± 0.039 | 0.49 ± 0.023 | 3.63 ± 0.06 | 8.86 ± 0.033 | 0.41 ± 0.019 | 3.28 ± 0.05 | |
| 0.5 | 11.23 ± 0.043 | 0.45 ± 0.022 | 2.41 ± 0.05 | 9.41 ± 0.035 | 0.44 ± 0.021 | 3.69 ± 0.06 | |
| 0.6 | 11.94 ± 0.046 | 0.53 ± 0.025 | 3.02 ± 0.06 | 9.31 ± 0.037 | 0.49 ± 0.023 | 3.56 ± 0.07 | |
| Primary Sludge | Mixed Sludge | ||||||
|---|---|---|---|---|---|---|---|
| Conditioning Method | Pressure MPa | Efficiency kg/m2h | Speed cm3/s | Specific Resistance to Filtration, m/kg × 1013 | Efficiency kg/m2h | Speed cm3/s | Specific Resistance to Filtration, m/kg × 1013 |
| raw sludge | 0.3 | 2.24 ± 0.015 | 0.14 ± 0.009 | 4.24 ± 0.06 | 1.51 ± 0.015 | 0.14 ± 0.009 | 5.59 ± 0.06 |
| 0.4 | 2.31 ± 0.010 | 0.16 ± 0.007 | 4.12 ± 0.08 | 1.87 ± 0.010 | 0.15 ± 0.007 | 5.48 ± 0.08 | |
| 0.5 | 3.01 ± 0.017 | 0.20 ± 0.003 | 4.67 ± 0.08 | 2.12 ± 0.017 | 0.11 ± 0.003 | 5.06 ± 0.08 | |
| 0.6 | 3.71 ± 0.014 | 0.27 ± 0.010 | 4.91 ± 0.04 | 2.56 ± 0.014 | 0.19 ± 0.010 | 5.51 ± 0.04 | |
| raw sludge + zeolite 0.8 g + polyelectrolyte (C-494) | 0.3 | 6.52 ± 0.031 | 0.38 ± 0.018 | 2.04 ± 0.02 | 5.78 ± 0.017 | 0.32 ± 0.013 | 2.34 ± 0.02 |
| 0.4 | 7.16 ± 0.033 | 0.34 ± 0.016 | 2.24 ± 0.03 | 7.02 ± 0.020 | 0.32 ± 0.015 | 2.06 ± 0.03 | |
| 0.5 | 7.34 ± 0.033 | 0.33 ± 0.019 | 2.36 ± 0.04 | 7.61 ± 0.021 | 0.33 ± 0.014 | 1.89 ± 0.02 | |
| 0.6 | 8.04 ± 0.034 | 0.32 ± 0.017 | 2.19 ± 0.03 | 7.98 ± 0.030 | 0.38 ± 0.017 | 2.08 ± 0.03 | |
| raw sludge + zeolite 1.6 g + polyelectrolyte (C-494) | 0.3 | 7.98 ± 0.034 | 0.34 ± 0.019 | 2.24 ± 0.03 | 7.02 ± 0.027 | 0.32 ± 0.016 | 2.01 ± 0.02 |
| 0.4 | 8.29 ± 0.035 | 0.34 ± 0.020 | 2.04 ± 0.02 | 7.48 ± 0.025 | 0.34 ± 0.016 | 2.24 ± 0.03 | |
| 0.5 | 8.87 ± 0.035 | 0.42 ± 0.022 | 2.19 ± 0.03 | 8.61 ± 0.034 | 0.40 ± 0.020 | 2.94 ± 0.04 | |
| 0.6 | 9.15 ± 0.036 | 0.41 ± 0.024 | 2.67 ± 0.04 | 8.39 ± 0.038 | 0.44 ± 0.026 | 2.76 ± 0.03 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczyk, M. The Influence of the Addition of Cement and Zeolite on the Increase in the Efficiency of Sewage Sludge Dewatering in the Pressure Filtration Process. Energies 2024, 17, 685. https://doi.org/10.3390/en17030685
Kowalczyk M. The Influence of the Addition of Cement and Zeolite on the Increase in the Efficiency of Sewage Sludge Dewatering in the Pressure Filtration Process. Energies. 2024; 17(3):685. https://doi.org/10.3390/en17030685
Chicago/Turabian StyleKowalczyk, Mariusz. 2024. "The Influence of the Addition of Cement and Zeolite on the Increase in the Efficiency of Sewage Sludge Dewatering in the Pressure Filtration Process" Energies 17, no. 3: 685. https://doi.org/10.3390/en17030685
APA StyleKowalczyk, M. (2024). The Influence of the Addition of Cement and Zeolite on the Increase in the Efficiency of Sewage Sludge Dewatering in the Pressure Filtration Process. Energies, 17(3), 685. https://doi.org/10.3390/en17030685







