Application of Proton Ionic Liquid in the Process of Obtaining Bioethanol from Hemp Stalks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pretreatment with PIL
2.2. Enzymatic Hydrolysis
2.3. Alcohol Fermentation
2.4. Analytical Techniques
- R—original value
- μ—mean of the data
- σ—standard deviation of the data.
- Ce—ethanol concentration (g·L−1)
- V—sample volume (L)
- M—total amount of substrate in the sample (g s.s.)
- C—cellulose and hemicellulose concentration in the material (%)
- 1.1—cellulose to glucose conversion factor
- 0.51—glucose to ethanol conversion factor
3. Results
3.1. Effect of PIL Pretreatment on Composition Analysis
3.2. Fourier Transform Analysis of Infrared Spectra (FTIR)
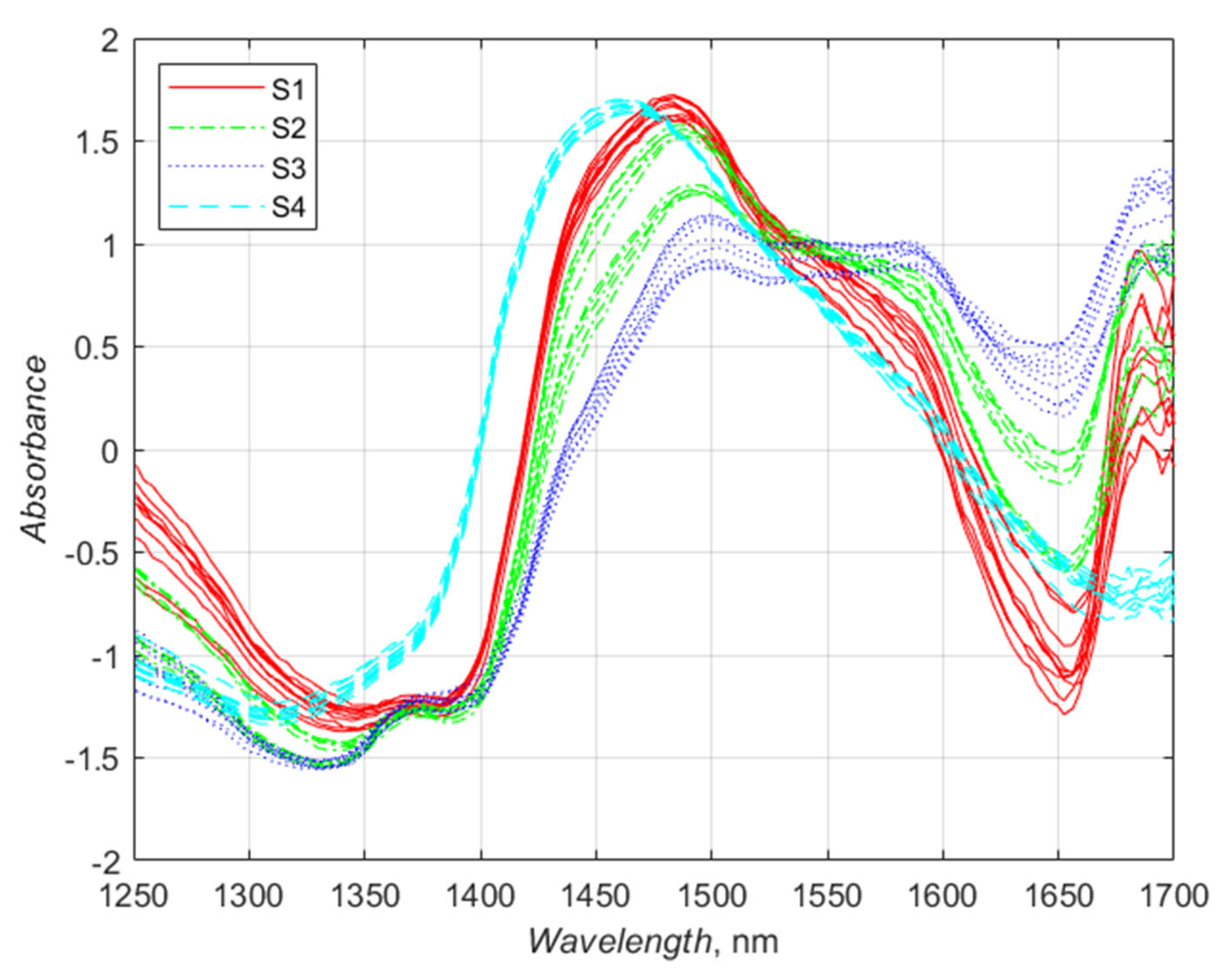
3.3. Analysis of NIR Spectra
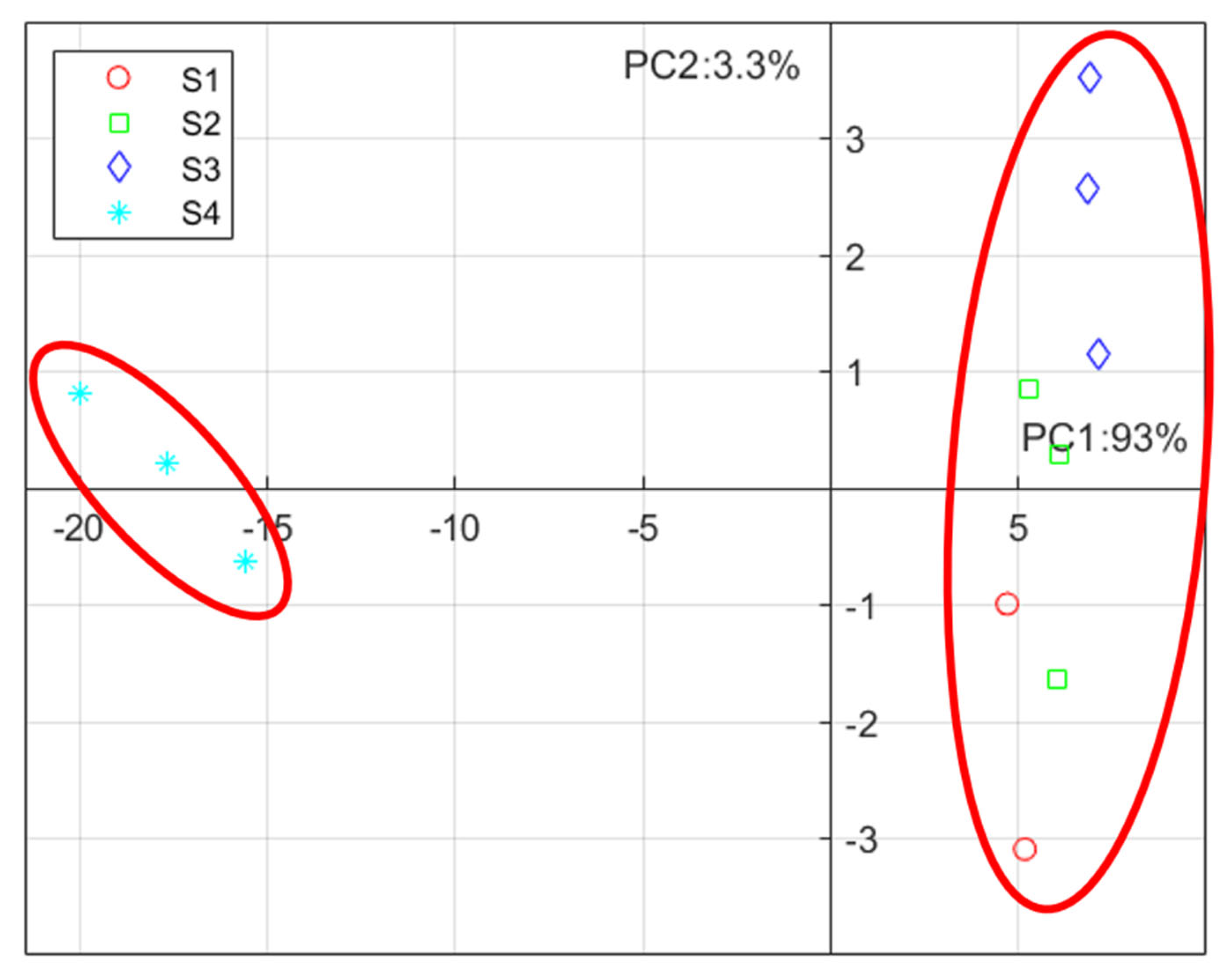
3.4. PCA Analysis of NIR Spectra
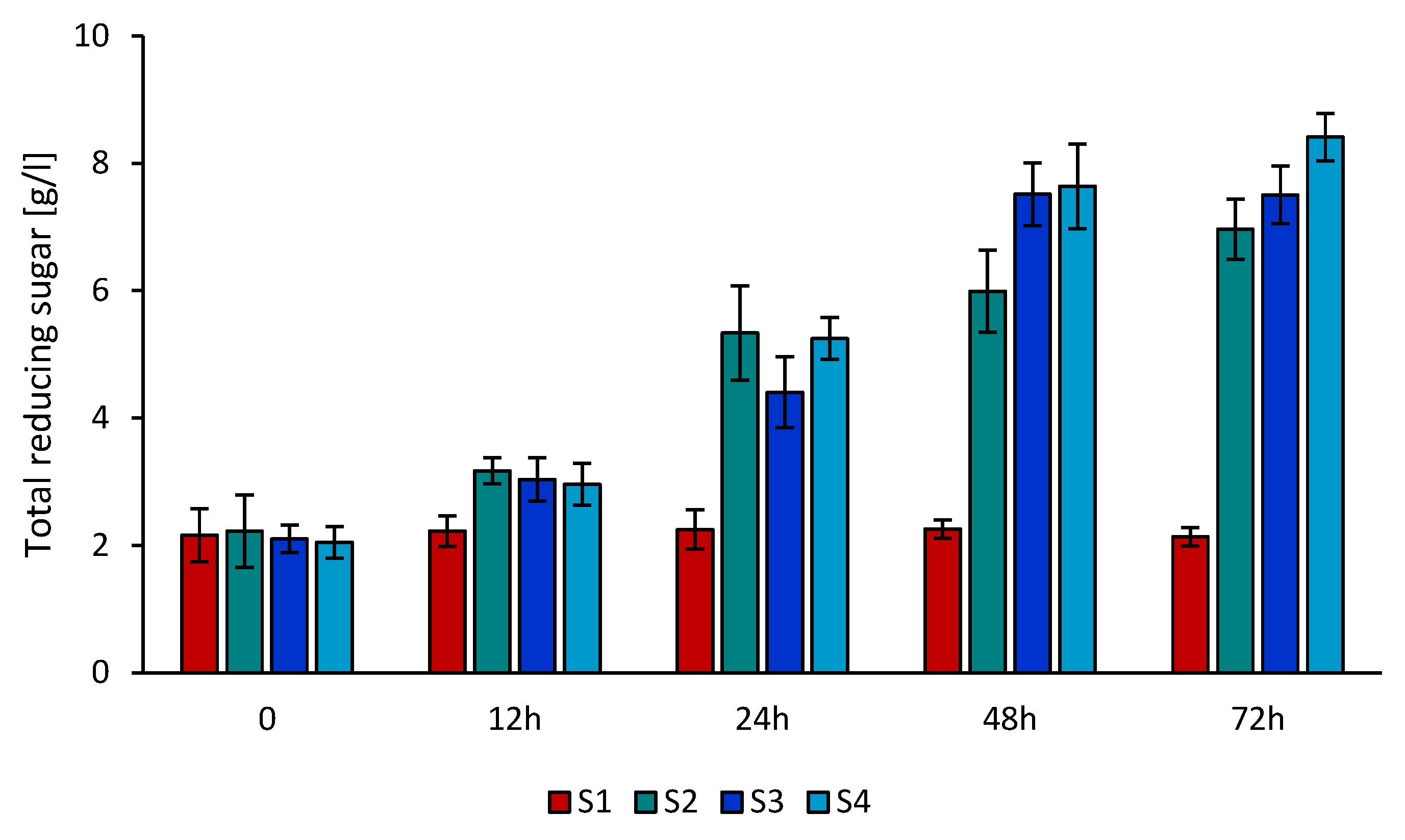
3.5. Effect of Using PIL for Pretreatment of Hemp Stalks on the Enzymatic Hydrolysis and Alcohol Fermentation Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, J.; Xu, Y.; Wang, W.; Griffin, J.; Roozeboom, K.; Wang, D. Bioconversion of Industrial Hemp Biomass for Bioethanol Production: A Review. Fuel 2020, 281, 118725. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, R.; Kumar, V.; Rani, A.; Jain, R. Cannabis sativa: A Plant Suitable for Phytoremediation and Bioenergy Production. In Phytoremediation Potential of Bioenergy Plants; Bauddh, K., Singh, B., Korstad, J., Eds.; Springer: Singapore, 2017; pp. 269–285. ISBN 978-981-10-3083-3. [Google Scholar]
- Kaniewski, R.; Pniewska, I.; Kubacki, A.; Strzelczyk, M.; Chudy, M.; Oleszak, G. Konopie Siewne (Cannabis sativa L.)—Wartościowa Roślina Użytkowa i Lecznicza. PF 2017, 18, 139–144. [Google Scholar] [CrossRef]
- Parvez, A.M.; Lewis, J.D.; Afzal, M.T. Potential of Industrial Hemp (Cannabis sativa L.) for Bioenergy Production in Canada: Status, Challenges and Outlook. Renew. Sustain. Energy Rev. 2021, 141, 110784. [Google Scholar] [CrossRef]
- Rehman, M.S.U.; Rashid, N.; Saif, A.; Mahmood, T.; Han, J.-I. Potential of Bioenergy Production from Industrial Hemp (Cannabis sativa): Pakistan Perspective. Renew. Sustain. Energy Rev. 2013, 18, 154–164. [Google Scholar] [CrossRef]
- Tareen, A.K.; Sultan, I.N.; Songprom, K.; Laemsak, N.; Sirisansaneeyakul, S.; Vanichsriratana, W.; Parakulsuksatid, P. Two-Step Pretreatment of Oil Palm Trunk for Ethanol Production by Thermotolerent Saccharomyces Cerevisiae SC90. Bioresour. Technol. 2021, 320, 124298. [Google Scholar] [CrossRef] [PubMed]
- Atitallah, I.B.; Ntaikou, I.; Antonopoulou, G.; Bradai, C.; Mechichi, T.; Lyberatos, G. Effect of Alkaline/Hydrogen Peroxide Pretreatment on Date Palm Fibers: Induced Chemical and Structural Changes and Assessment of Ethanol Production Capacity via Pichia Anomala and Pichia Stipitis. Biomass Conv. Bioref. 2022, 12, 4473–4489. [Google Scholar] [CrossRef]
- Odorico, F.H.; Morandim-Giannetti, A.D.A.; Lucarini, A.C.; Torres, R.B. Pretreatment of Guinea Grass (Panicum Maximum) with the Ionic Liquid 1-Ethyl-3-Methyl Imidazolium Acetate for Efficient Hydrolysis and Bioethanol Production. Cellulose 2018, 25, 2997–3009. [Google Scholar] [CrossRef]
- Shafiei, M.; Zilouei, H.; Zamani, A.; Taherzadeh, M.J.; Karimi, K. Enhancement of Ethanol Production from Spruce Wood Chips by Ionic Liquid Pretreatment. Appl. Energy 2013, 102, 163–169. [Google Scholar] [CrossRef]
- Reina, L.; Botto, E.; Mantero, C.; Moyna, P.; Menéndez, P. Production of Second Generation Ethanol Using Eucalyptus Dunnii Bark Residues and Ionic Liquid Pretreatment. Biomass Bioenergy 2016, 93, 116–121. [Google Scholar] [CrossRef]
- Pyrchla, K.; Tomczak, A.; Zaniewicz, G.; Pyrchla, J.; Kowalska, P. Analysis of the Dynamic Height Distribution at the Estuary of the Odra River Based on Gravimetric Measurements Acquired with the Use of a Light Survey Boat—A Case Study. Sensors 2020, 20, 6044. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Bao, X.; Zhou, C.; Zhang, L.; Yagoub, A.E.-G.A.; Yang, H.; Ma, H. Ultrasound-Ionic Liquid Enhanced Enzymatic and Acid Hydrolysis of Biomass Cellulose. Ultrason. Sonochem. 2018, 41, 410–418. [Google Scholar] [CrossRef]
- Nakasu, P.Y.S.; Verdía Barbará, P.; Firth, A.E.J.; Hallett, J.P. Pretreatment of Biomass with Protic Ionic Liquids. Trends Chem. 2022, 4, 175–178. [Google Scholar] [CrossRef]
- Haykir, N.I.; Soysal, K.; Yaglikci, S.; Gokce, Y. Assessing the Effect of Protic Ionic Liquid Pretreatment of Pinus radiata from Different Perspectives Including Solvent-Water Ratio. J. Wood Chem. Technol. 2021, 41, 236–248. [Google Scholar] [CrossRef]
- Achinivu, E.C.; Cabrera, M.; Umar, A.; Yang, M.; Baral, N.R.; Scown, C.D.; Simmons, B.A.; Gladden, J.M. In Situ Synthesis of Protic Ionic Liquids for Biomass Pretreatment. ACS Sustain. Chem. Eng. 2022, 10, 12090–12098. [Google Scholar] [CrossRef]
- Ziaei-Rad, Z.; Pazouki, M.; Fooladi, J.; Azin, M.; Gummadi, S.N.; Allahverdi, A. Investigation of a Robust Pretreatment Technique Based on Ultrasound-Assisted, Cost-Effective Ionic Liquid for Enhancing Saccharification and Bioethanol Production from Wheat Straw. Sci. Rep. 2023, 13, 446. [Google Scholar] [CrossRef]
- Mohd Azhar, S.H.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Mohd Faik, A.A.; Rodrigues, K.F. Yeasts in Sustainable Bioethanol Production: A Review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Techaparin, A.; Thanonkeo, P.; Klanrit, P. High-Temperature Ethanol Production Using Thermotolerant Yeast Newly Isolated from Greater Mekong Subregion. Braz. J. Microbiol. 2017, 48, 461–475. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of Cellulase Activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of Lignocellulose: Formation of Inhibitory by-Products and Strategies for Minimizing Their Effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Stuart, B.H. Infrared Spectroscopy: Fundamentals and Applications, 1st ed.; Analytical Techniques in the Sciences; Wiley: Hoboken, NJ, USA, 2004; ISBN 978-0-470-85427-3. [Google Scholar]
- Liu, Z.; Li, L.; Liu, C.; Xu, A. Pretreatment of Corn Straw Using the Alkaline Solution of Ionic Liquids. Bioresour. Technol. 2018, 260, 417–420. [Google Scholar] [CrossRef]
- Chambon, C.L.; Chen, M.; Fennell, P.S.; Hallett, J.P. Efficient Fractionation of Lignin- and Ash-Rich Agricultural Residues Following Treatment With a Low-Cost Protic Ionic Liquid. Front. Chem. 2019, 7, 246. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, J.R.; Gírio, F.M.; Łukasik, R.M. The Effect of the Chemical Character of Ionic Liquids on Biomass Pre-Treatment and Posterior Enzymatic Hydrolysis. Molecules 2019, 24, 808. [Google Scholar] [CrossRef] [PubMed]
- Gräsvik, J.; Winestrand, S.; Normark, M.; Jönsson, L.J.; Mikkola, J.-P. Evaluation of Four Ionic Liquids for Pretreatment of Lignocellulosic Biomass. BMC Biotechnol. 2014, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Rosa, M.F.; Medeiros, E.S.; Malmonge, J.A.; Gregorski, K.S.; Wood, D.F.; Mattoso, L.H.C.; Glenn, G.; Orts, W.J.; Imam, S.H. Cellulose Nanowhiskers from Coconut Husk Fibers: Effect of Preparation Conditions on Their Thermal and Morphological Behavior. Carbohydr. Polym. 2010, 81, 83–92. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the Cell Wall and Cellulose Content of Developing Cotton Fibers Investigated by FTIR Spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.-C.; Popescu, C.-M.; Lisa, G.; Sakata, Y. Evaluation of Morphological and Chemical Aspects of Different Wood Species by Spectroscopy and Thermal Methods. J. Mol. Struct. 2011, 988, 65–72. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- Poletto, M.; Pistor, V.; Zeni, M.; Zattera, A.J. Crystalline Properties and Decomposition Kinetics of Cellulose Fibers in Wood Pulp Obtained by Two Pulping Processes. Polym. Degrad. Stab. 2011, 96, 679–685. [Google Scholar] [CrossRef]
- Tarchoun, A.F.; Trache, D.; Klapötke, T.M.; Derradji, M.; Bessa, W. Ecofriendly Isolation and Characterization of Microcrystalline Cellulose from Giant Reed Using Various Acidic Media. Cellulose 2019, 26, 7635–7651. [Google Scholar] [CrossRef]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and Quantitative Analysis of Lignocellulosic Biomass Using Infrared Techniques: A Mini-Review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef]
- Fackler, K.; Stevanic, J.S.; Ters, T.; Hinterstoisser, B.; Schwanninger, M.; Salmén, L. FT-IR Imaging Microscopy to Localise and Characterise Simultaneous and Selective White-Rot Decay within Spruce Wood Cells. Holzforschung 2011, 65, 411–420. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, A.; Ibrahim, S.A.; Yang, H.; Huang, W. Isolation and Characterization of Microcrystalline Cellulose from Pomelo Peel. Int. J. Biol. Macromol. 2018, 111, 717–721. [Google Scholar] [CrossRef]
- Renugadevi, K.; Devan, P.K.; Chandra Sekhara Reddy, M.; Karthik, P.; Thomas, T. Optimization of Surface Treatment in Calotropis Gigantea (CG)-Fibre Yarn by Simple Techniques and Characterization of CG Fibre Yarn Reinforced Laminate. J. Mater. Res. Technol. 2020, 9, 12187–12200. [Google Scholar] [CrossRef]
- Owolabi, A.F.; Haafiz, M.K.M.; Hossain, M.S.; Hussin, M.H.; Fazita, M.R.N. Influence of Alkaline Hydrogen Peroxide Pre-Hydrolysis on the Isolation of Microcrystalline Cellulose from Oil Palm Fronds. Int. J. Biol. Macromol. 2017, 95, 1228–1234. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Hu, H. Prediction of Hot-Water-Soluble Extractive, Pentosan and Cellulose Content of Various Wood Species Using FT-NIR Spectroscopy. Bioresour. Technol. 2013, 140, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Liu, J.; Liu, S.; Yang, Q. Application of FT-NIR-DR and FT-IR-ATR Spectroscopy to Estimate the Chemical Composition of Bamboo (Neosinocalamus affinis Keng). Holzforschung 2011, 65, 689–696. [Google Scholar] [CrossRef]
- Lande, S.; Van Riel, S.; Høibø, O.A.; Schneider, M.H. Development of Chemometric Models Based on near Infrared Spectroscopy and Thermogravimetric Analysis for Predicting the Treatment Level of Furfurylated Scots Pine. Wood Sci. Technol. 2010, 44, 189–203. [Google Scholar] [CrossRef]
- Correia, J.A.D.C.; Júnior, J.E.M.; Gonçalves, L.R.B.; Rocha, M.V.P. Alkaline Hydrogen Peroxide Pretreatment of Cashew Apple Bagasse for Ethanol Production: Study of Parameters. Bioresour. Technol. 2013, 139, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.V.P.; Rodrigues, T.H.S.; De Macedo, G.R.; Gonçalves, L.R.B. Enzymatic Hydrolysis and Fermentation of Pretreated Cashew Apple Bagasse with Alkali and Diluted Sulfuric Acid for Bioethanol Production. Appl. Biochem. Biotechnol. 2009, 155, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.H.S.; De Barros, E.M.; De Sá Brígido, J.; Da Silva, W.M.; Rocha, M.V.P.; Gonçalves, L.R.B. The Bioconversion of Pretreated Cashew Apple Bagasse into Ethanol by SHF and SSF Processes. Appl. Biochem. Biotechnol. 2016, 178, 1167–1183. [Google Scholar] [CrossRef]
- Reis, C.L.B.; Silva, L.M.A.E.; Rodrigues, T.H.S.; Félix, A.K.N.; Santiago-Aguiar, R.S.D.; Canuto, K.M.; Rocha, M.V.P. Pretreatment of Cashew Apple Bagasse Using Protic Ionic Liquids: Enhanced Enzymatic Hydrolysis. Bioresour. Technol. 2017, 224, 694–701. [Google Scholar] [CrossRef]
- Selig, M.J.; Vinzant, T.B.; Himmel, M.E.; Decker, S.R. The Effect of Lignin Removal by Alkaline Peroxide Pretreatment on the Susceptibility of Corn Stover to Purified Cellulolytic and Xylanolytic Enzymes. Appl. Biochem. Biotechnol. 2009, 155, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Karagöz, P.; Rocha, I.V.; Özkan, M.; Angelidaki, I. Alkaline Peroxide Pretreatment of Rapeseed Straw for Enhancing Bioethanol Production by Same Vessel Saccharification and Co-Fermentation. Bioresour. Technol. 2012, 104, 349–357. [Google Scholar] [CrossRef]
- Das, S.; Bhattacharya, A.; Halder, S.; Ganguly, A.; Gu, S.; Ting, Y.-P.; Chatterjee, P.K. Optimization of Enzymatic Saccharification of Water Hyacinth Biomass for Bio-Ethanol: Comparison between Artificial Neural Network and Response Surface Methodology. Sustain. Mater. Technol. 2015, 3, 17–28. [Google Scholar] [CrossRef]
- Chandel, A.K.; Kapoor, R.K.; Singh, A.; Kuhad, R.C. Detoxification of Sugarcane Bagasse Hydrolysate Improves Ethanol Production by Candida Shehatae NCIM 3501. Bioresour. Technol. 2007, 98, 1947–1950. [Google Scholar] [CrossRef] [PubMed]
- Soares, L.B.; Bonan, C.I.D.G.; Biazi, L.E.; Dionísio, S.R.; Bonatelli, M.L.; Andrade, A.L.D.; Renzano, E.C.; Costa, A.C.; Ienczak, J.L. Investigation of Hemicellulosic Hydrolysate Inhibitor Resistance and Fermentation Strategies to Overcome Inhibition in Non-Saccharomyces Species. Biomass Bioenergy 2020, 137, 105549. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Machado, E.M.S.; Carneiro, L.M.; Teixeira, J.A. Sugars Metabolism and Ethanol Production by Different Yeast Strains from Coffee Industry Wastes Hydrolysates. Appl. Energy 2012, 92, 763–768. [Google Scholar] [CrossRef]
- Sathesh-Prabu, C.; Murugesan, A.G. Potential Utilization of Sorghum Field Waste for Fuel Ethanol Production Employing Pachysolen Tannophilus and Saccharomyces Cerevisiae. Bioresour. Technol. 2011, 102, 2788–2792. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, F.A.; Ruiz, H.A.; Silvino Dos Santos, E.; Teixeira, J.A.; De Macedo, G.R. Bioethanol Production by Saccharomyces Cerevisiae, Pichia Stipitis and Zymomonas Mobilis from Delignified Coconut Fibre Mature and Lignin Extraction According to Biorefinery Concept. Renew. Energy 2016, 94, 353–365. [Google Scholar] [CrossRef]
- Le, T.D.T.; Nguyen Truong, V.P.; Ngo, M.T.T.; Kim, T.H.; Oh, K.K. Pretreatment of Corn Stover Using an Extremely Low-Liquid Ammonia (ELLA) Method for the Effective Utilization of Sugars in Simultaneous Saccharification and Fermentation (SSF) of Ethanol. Fermentation 2021, 7, 191. [Google Scholar] [CrossRef]
- Sipos, B.; Kreuger, E.; Svensson, S.-E.; Réczey, K.; Björnsson, L.; Zacchi, G. Steam Pretreatment of Dry and Ensiled Industrial Hemp for Ethanol Production. Biomass Bioenergy 2010, 34, 1721–1731. [Google Scholar] [CrossRef]
- Gschwend, F.J.V.; Malaret, F.; Shinde, S.; Brandt-Talbot, A.; Hallett, J.P. Rapid Pretreatment of Miscanthus Using the Low-Cost Ionic Liquid Triethylammonium Hydrogen Sulfate at Elevated Temperatures. Green Chem. 2018, 20, 3486–3498. [Google Scholar] [CrossRef]
- Malaret, F.; Gschwend, F.J.V.; Lopes, J.M.; Tu, W.-C.; Hallett, J.P. Eucalyptus Red Grandis Pretreatment with Protic Ionic Liquids: Effect of Severity and Influence of Sub/Super-Critical CO 2 Atmosphere on Pretreatment Performance. RSC Adv. 2020, 10, 16050–16060. [Google Scholar] [CrossRef] [PubMed]
- Nakasu, P.Y.S.; Clarke, C.J.; Rabelo, S.C.; Costa, A.C.; Brandt-Talbot, A.; Hallett, J.P. Interplay of Acid–Base Ratio and Recycling on the Pretreatment Performance of the Protic Ionic Liquid Monoethanolammonium Acetate. ACS Sustain. Chem. Eng. 2020, 8, 7952–7961. [Google Scholar] [CrossRef]


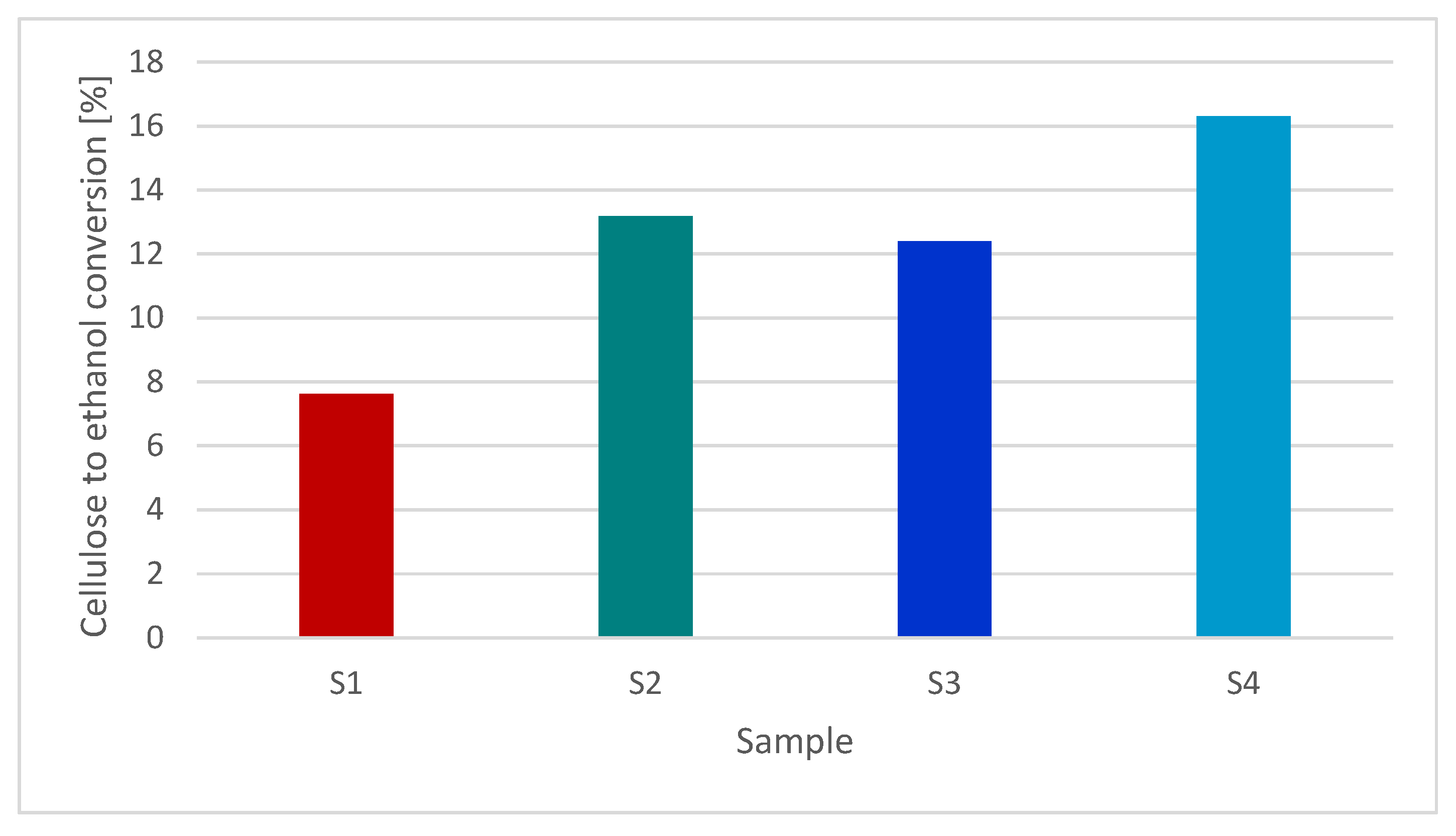
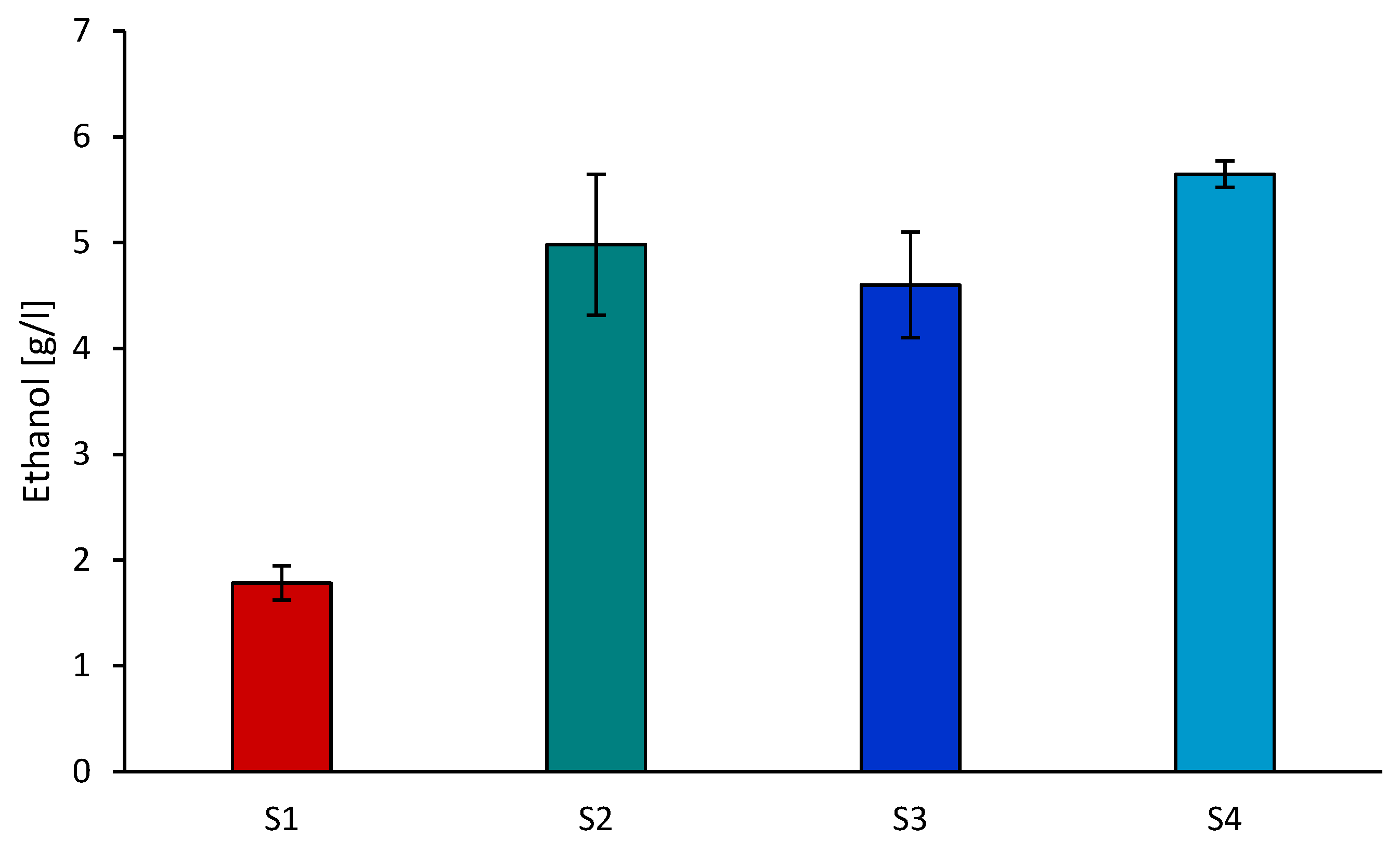
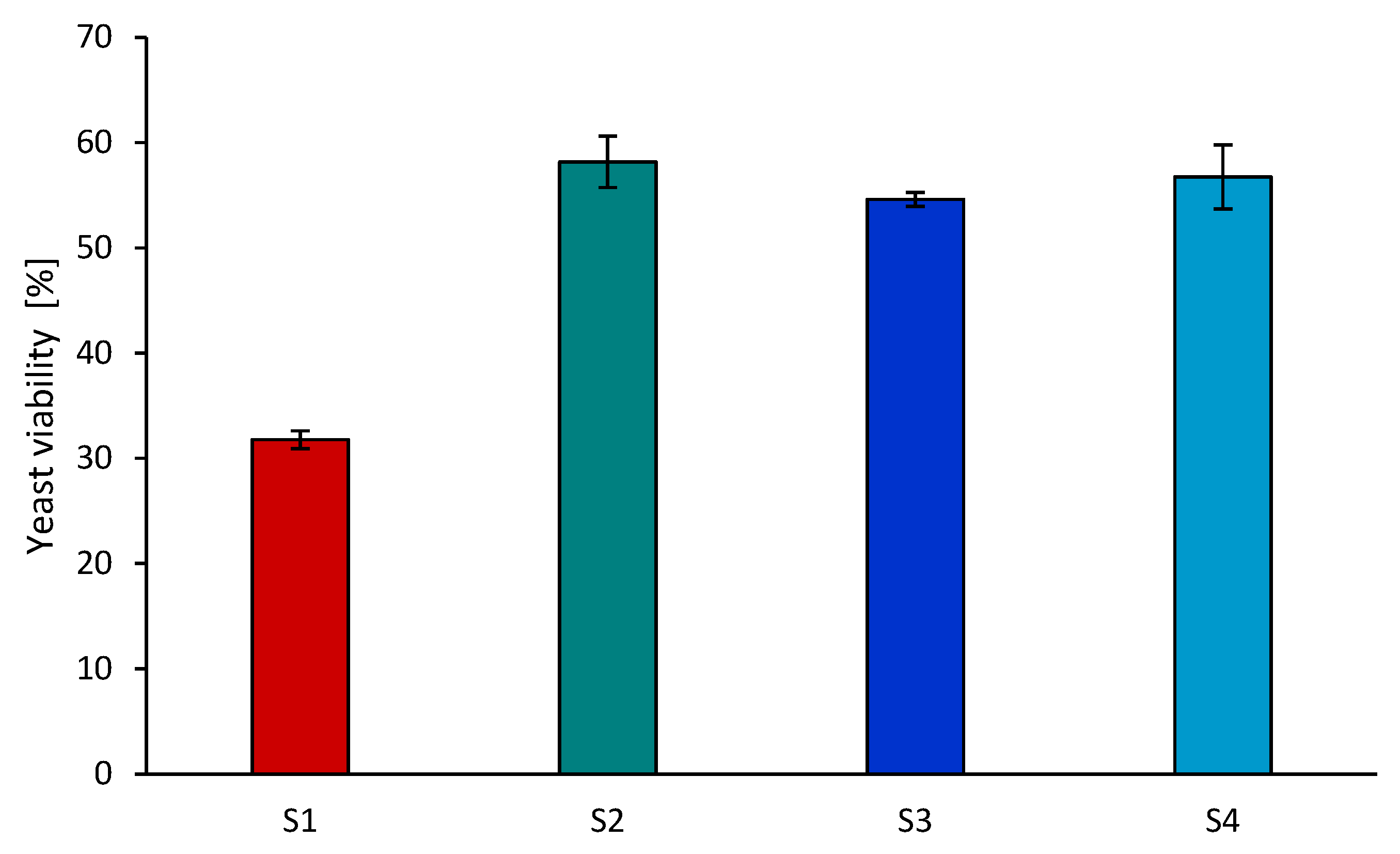
| Sample | Celulose [%] | Lignin [%] | Hemicelulose [%] |
|---|---|---|---|
| S1 | 41.54 | 18.08 | 22.87 |
| S2 | 54.41 | 11.08 | 18.00 |
| S3 | 66.09 | 8.43 | 10.21 |
| S4 | 76.28 | 3.10 | 4.32 |
| Feedstock | Pretreated | Fermentable Sugar Content (g/L) | Ethanol Concentration (g/L) | References |
|---|---|---|---|---|
| Sweet sorghum juice | Squeezing in a juice extractor | 200 | 37.47–72.69 at 37 °C 16.52–53.89 at 40 °C | [18] |
| Spent coffee grounds | Acid hydrolysis | 50 | 11.7 | [49] |
| Sorghum stover | Alkaline treatment | 200 | 56–68 | [50] |
| Coconut fiber mature | Hydrothermal pretreatment catalyzed with sodium hydroxide (HPCSH) | 50 | 10.81–11.65 | [51] |
| Wheat straw | Low cost ionic liquid/ultrasound irradiation IL/US | 100 | 38.9 after 15 min treatment 42.0 after 30 min treatment | [16] |
| Corn stover | Extremely low-liquid ammonia (ELLA) | 27.9 | 14.5 | [52] |
| Industrial hemp stem | Stem with SO2 | 12.7 | Separated fiber 21.3 Whole slurry 18.4 | [53] |
| Industrial hemp silage | Stem with SO2 | 10.8 | Separated fiber 20.3 Whole slurry 15.4 | [53] |
| Industrial hemp | Liquid hot water (LHW) | 21.59 | 10.9 | [1] |
| Industrial hemp | H2SO4 treatment | 28.04 | 13.8 | [1] |
| Industrial hemp | NaOH treatment | 40.08 | 20.3 | [1] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smuga-Kogut, M.; Walendzik, B.; Lewicka-Rataj, K.; Kogut, T.; Bychto, L.; Jachimowicz, P.; Cydzik-Kwiatkowska, A. Application of Proton Ionic Liquid in the Process of Obtaining Bioethanol from Hemp Stalks. Energies 2024, 17, 972. https://doi.org/10.3390/en17040972
Smuga-Kogut M, Walendzik B, Lewicka-Rataj K, Kogut T, Bychto L, Jachimowicz P, Cydzik-Kwiatkowska A. Application of Proton Ionic Liquid in the Process of Obtaining Bioethanol from Hemp Stalks. Energies. 2024; 17(4):972. https://doi.org/10.3390/en17040972
Chicago/Turabian StyleSmuga-Kogut, Małgorzata, Bartosz Walendzik, Katarzyna Lewicka-Rataj, Tomasz Kogut, Leszek Bychto, Piotr Jachimowicz, and Agnieszka Cydzik-Kwiatkowska. 2024. "Application of Proton Ionic Liquid in the Process of Obtaining Bioethanol from Hemp Stalks" Energies 17, no. 4: 972. https://doi.org/10.3390/en17040972
APA StyleSmuga-Kogut, M., Walendzik, B., Lewicka-Rataj, K., Kogut, T., Bychto, L., Jachimowicz, P., & Cydzik-Kwiatkowska, A. (2024). Application of Proton Ionic Liquid in the Process of Obtaining Bioethanol from Hemp Stalks. Energies, 17(4), 972. https://doi.org/10.3390/en17040972







