Abstract
This paper examines the current and prospective greenhouse gas (GHG) emissions of e-fuels produced via electrolysis and Fischer–Tropsch synthesis (FTS) for the years 2021, 2030, and 2050 for use in Germany. The GHG emissions are determined by a scenario approach as a combination of a literature-based top-down and bottom-up approach. Considered process steps are the provision of feedstocks, electrolysis (via solid oxide co-electrolysis; SOEC), synthesis (via Fischer–Tropsch synthesis; FTS), e-crude refining, eventual transport to, and use in Germany. The results indicate that the current GHG emissions for e-fuel production in the exemplary export countries Saudi Arabia and Chile are above those of conventional fuels. Scenarios for the production in Germany lead to current GHG emissions of 2.78–3.47 kgCO2-eq/L e-fuel in 2021 as the reference year and 0.064–0.082 kgCO2-eq/L e-fuel in 2050. With a share of 58–96%, according to the respective scenario, the electrolysis is the main determinant of the GHG emissions in the production process. The use of additional renewable energy during the production process in combination with direct air capture (DAC) are the main leverages to reduce GHG emissions.
1. Introduction
In mid-2021, the German government tightened its climate targets with the goal of greenhouse gas neutrality by 2045 [1]. E-fuels (synthetic fuels derived from power, carbon dioxide and water) will probably make a decisive contribution to achieving these climate targets, particularly in the transport sector as there are certain areas that are difficult or impossible to decarbonize by electrification such as aviation and deep-sea shipping. In these cases, e-fuels can contribute to reducing the use of fossil fuels and thus the level of greenhouse gas (GHG) emissions. Political decision makers in Germany and the European Union already claim e-fuels to be a sustainable alternative to conventional fossil fuels and designate the prospective use of e-fuels in the mobility sector [2]. Due to the high political interest in e-fuels, the question of their environmental impact and their contribution potential to reducing GHG emissions arises.
Therefore, this paper evaluates the GHG emissions of e-fuels used in Germany. While the focus lies on domestic production, for the purpose of comparison, Saudi Arabia and Chile are considered as two exemplary countries to represent possible export regions with existing or planned economic relations. Different production routes exist, using carbon dioxide, water, and electricity as resources. The conversion efficiency from electricity to fuel is currently still low at approximately 44% [3]. Solid oxide fuel cells (SOFCs) as a technology on the verge of commercial use offer great potential for improvement in electrolysis due to their comparatively high theoretical efficiency and the possibility to generate synthesis gas from carbon dioxide and water in a single process step. In [4], the conversion efficiency from electricity to hydrogen, based on the lower heating value, is quantified as 76.8%, compared to a maximum of 70% for alkaline electrolysis and a maximum of 72% for proton exchange membrane (PEM) electrolysis until 2030. For co-electrolysis in solid oxide electrolyzer cells (SOECs), due to the continuous development, a longer time horizon of 2050 instead of 2030 was chosen.
In this paper, an innovative system configuration combining SOECs for synthesis gas production and Fischer–Tropsch synthesis (FTS) for the conversion to crude fuel is studied, leading to the research question: “What are the potential GHG emissions of e-fuels produced via the SOEC and FTS route for use in Germany?”
For the assessment of the environmental impacts, data from the literature can hardly be referred to, since to date, only a few life cycle analyses exist on the production of liquid hydrocarbons from carbon dioxide via the Fischer–Tropsch route [5]. To the authors’ knowledge, no study has previously examined the environmental impacts of precisely the system configuration at hand with the combination of Co-SOEC and FTS, although [6,7,8] have examined its technical performance. Another relevant aspect is the distribution of environmental impacts among the individual process steps in order to identify the levers (other than electrolysis) for increasing overall efficiency. In the existing literature, however, merely the overall process is usually considered. This paper, therefore, aims to break down the evaluated GHG emissions of e-fuels by process step wherever possible.
2. Materials and Methods
As described above, there have been few studies to date on the environmental impact of the production of synthetic fuels from electricity and CO2 via the FTS route. Existing studies also differ in their structure and results. For this reason, a combination of two methods was intentionally chosen to categorize the literature data and validate own calculation results. The methodology used in this work is a combination of literature-based top-down and bottom-up analyses. In a top-down analysis using a comprehensive review of the existing literature, the range and average values of overall GHG emissions were quantified in absolute terms and their distribution to the main process steps was determined in relative figures. In a complementary approach, the bottom-up analysis calculated GHG emissions in absolute terms by multiplying the necessary energy usage, provided that it was known for the respective process step, with the corresponding emission factor. Where data were missing, as was the case with the Fischer–Tropsch synthesis step, the determined relative value from the top-down analysis was used for approximation. The absolute values of the overall GHG emissions resulting from the two different approaches were finally cross-checked.
For solid oxide co-electrolysis as a technology under continuous development and due to the innovative combination with Fischer–Tropsch synthesis, a bottom-up approach was used. Fischer–Tropsch synthesis, as a well-established technology (using fossil fuels as ground stock), on the other hand, has been the subject of numerous studies, of which [9,10] are to be mentioned primarily, though not in combination with SOECs. Here, a literature-based top-down analysis is at an advantage and also served to cross-check the results from the bottom-up approach. As an indicator of the environmental impact along the process chain of e-fuel production and use, the potential GHG emissions, measured in CO2 equivalents (CO2-eq), were assessed. The considered process steps were the provision of feedstocks including direct air capture (DAC), electrolysis (via SOEC), synthesis (via FTS), e-crude refining, and transport and use. A scenario-based approach was applied in order to estimate and compare future emissions. In this context, the emissions (CO2-eq) were determined for the years 2021 (as a reference), 2030, and 2050 for production sites in Germany, Saudi Arabia, and Chile. The latter countries were chosen for reasons of data availability as well as existing trade relations [11,12] and are supposed to be representative of the regions Middle East and South America.
The input for the production of each kilogram of synthesis gas (syngas; a mixture of CO and H2) as feedstock of the FTS, was found to be 4.87 kWh of electricity, 0.77 kg H2O, and 0.7 kg CO2 in 2021 [13]. According to [13], the demand of H2O and CO2 remains constant for prospective syngas production. Owing to productivity increases, the electricity demand is expected to be reduced to 4.26 kWh/kg of syngas by 2050. Based on an interpolation, an electricity demand of 4.66 kWh/kg of syngas can be assumed by 2030. Furthermore, 0.77 kg H2O and 0.7 kg CO2 are required. The GHG emissions per kg e-fuel were determined by multiplying the input demand with the corresponding emission factor (in CO2-eq). Regarding electricity, the current country-specific electricity mix was applied. For estimating future emission factors, the self-imposed targets for the electricity mix of the respective countries were used. Taking into account the upstream chain emissions, this leads to a current (2021) emission factor of 485 gCO2-eq/kWh in Germany (220 gCO2-eq/kWh in 2030; 14 gCO2-eq/kWh in 2050) [14,15]. In Chile, the use of electricity from the public grid (Sistema Eléctrico Nacional) leads to 636 gCO2-eq/kWh [14,16,17]. For 2030, 338 gCO2-eq/kWh and 14 gCO2-eq/kWh for 2050 will be emitted [15,18]. In Saudi Arabia, 676 gCO2-eq/kWh are emitted (338 gCO2-eq/kWh in 2030; 122 gCO2-eq/kWh in 2050) [19,20]. The calculation of the future CO2-eq emissions is based on the share of renewable energy in the grid according to the self-proclaimed targets of the respective country and their current composition of the electricity mix. In Table 1, the described feedstock demand per kg synthesis gas and the CO2-eq/kWh are displayed.

Table 1.
Feedstock demand of synthesis gas and CO2-eq/kWh.
For the provision of H2O as a feedstock, firstly, the local availability of freshwater and saltwater was determined. Secondly, the electricity demand of freshwater (ground- and surface water) treatment and the desalination of salt water was reviewed. The considered technologies for freshwater treatment are 1. conventional pre-treatment (process steps: iron removal, chemical softening, and filtration) in combination with reverse osmosis (R.O.), 2. conventional pre-treatment in combination with ion exchange technology, 3. combined treatment. For the salt water treatment, R.O. as the dominating technology on the market according to [21,22] was chosen. Based on [14], the respective energy demands (in kWh/m3) are 1. 2.9 kWh/m3 in 2021, 1.9 kWh/m3 in 2030, 0.9 kWh/m3 in 2050; 2. 0.7 kWh/m3 in 2021, 0.6 kWh/m3 in 2030, 0.5 kWh/m3 in 2050; 3. 3.3 kWh/m3 in 2021, 2.3 kWh/m3 in 2030, 1.3 kWh/m3 in 2050. R.O. technology has an expected energy demand of 2.35 kWh/m3 up to 2030 and 2 kWh/m3 in 2050 (see Table 2).

Table 2.
Electricity demand for water treatment.
The technologies included in this analysis for the provision of CO2 are 1. DAC, 2. Biomass (organic waste), 3. CO2 capture from cement production. The electricity demand of DAC technologies varies between 1.4 and 2.8 kWh/kg CO2 according to data from the literature [23,24]. In the following, an average of 2.1 kWh/kg CO2 was used. According to [25], 0.46 kWh/kg CO2 is necessary while using a pressure swing absorption process. For the CO2 capture during the cement production by means of Selexol© scrubbing, 0.31 kWh/kg CO2 are required [14]. The Selexol© process involves the utilization of a liquid physical solvent to extract acidic gases from both synthetic and natural gas streams [26]. A total of eight scenarios of the above-mentioned alternatives for the provision of feedstock for the electrolysis was considered (see Appendix A).
To identify the environmental impacts of Fischer–Tropsch synthesis in particular, initially, literature research was carried out, mainly using the metadata service Google Scholar as the biggest academic database and search terms both in English and German. The search terms were then entered into other minor databases, both open-access and commercial, in order to cross-check the search results. Search terms were “Fischer Tropsch synthetic fuels”, “LCA Fischer Tropsch”, “LCA solar fuels”, “LCA wind fuels”, “Fischer Tropsch fuels renewable energy”/“Fischer Tropsch Kraftstoffe erneuerbare Energien”. Based on the abstracts, all relevant articles from the first five pages of the respective search results i.e., those works focusing on Fischer–Tropsch fuels produced from electricity, were then reviewed in detail. Subsequently, the references cited in these papers were included to broaden the literature review. This step was used as an indicator to reveal a too-narrow choice of search terms eventually. Since only one of the eleven studies evaluated in the final loop was included in the literature search as a reference of another study, the choice of search terms can be deemed sufficiently diversified and therefore comprehensive.
In [27], the GHG emissions over the entire life cycle of an aviation fuel and naphtha production plant using solar energy are investigated. Water and carbon dioxide are captured from air and converted to syngas in a thermochemical reactor before being converted to jet fuel via Fischer–Tropsch synthesis. Specific life cycle GHG emissions are calculated to 14.7 gCO2-eq/MJ jet fuel and 17.7 gCO2-eq/MJ naphtha in the solar stand-alone solution design case, with different values due to the different energy densities and emission factors during combustion. Fischer–Tropsch synthesis accounts for 16% of the emissions, i.e., 2.3 gCO2-eq/MJ jet fuel and 2.8 gCO2-eq/MJ naphtha, which are largely composed of the syngas leakage and the combustion of the light hydrocarbons in the cogeneration plant. The plant construction for the FT reactor including follow-up treatment plays a minor role and is not mentioned separately in this analysis. Using solar energy (instead of the CHP plant) also for post-treatment of the FT hydrocarbons leads to a reduction in emission levels.
Also, in [5], a life cycle analysis of GHG emissions from the production of liquid fuels by Fischer–Tropsch synthesis is performed. The investigated system consists of a direct air capture plant (for CO2), coupled with a Fischer–Tropsch synthesis plant, designed for diesel production. Syngas is obtained from the reverse water gas shift reaction using the captured CO2 and water as inputs. In the design case, the required electrical energy is supplied from the power grid with a comparatively very low assumed emission factor of 13 gCO2-eq/kWh, and an oxygen-fueled calciner is considered as an alternative to an electrically powered one. The greenhouse gas emissions over the entire life cycle for Fischer–Tropsch synthesis and electrolysis are calculated in the design case to be 7.3 gCO2-eq/MJ, based on the calorific value of the synthetic diesel fuel and for both types of calciner. There is no direct comparability to the values from [27], because of the combined assessment of electrolysis and synthesis as well as the different product, requiring different process control.
The greenhouse gas emissions as well as the cumulative energy demands of different production routes of liquid hydrocarbons from CO2, water, and electrical energy are also calculated and compared in [28]. In this study, the three carbon dioxide sources, natural gas, biomass, or ambient air, and three electricity sources (natural gas, biomass combustion, or photovoltaic) are combined into nine conceivable production options, five of which are further evaluated. Hydrogen is obtained via alkaline electrolysis and fed to the reverse water gas shift reaction for syngas production. Included in the comparison are the gas-to-liquid and biomass-to-liquid processes as reference technologies. GHG emissions for DAC of CO2 and electricity generation from photovoltaic are calculated to 30 gCO2-eq/MJ of fuel and the cumulative energy demand (CED) (also called ‘primary energy consumption’) is 2.90 MJPrim. per MJ fuel.
In [29], the focus of the study is on the influence of electrolysis and the choice of system boundary. The considered stand-alone solution uses wind or solar energy for electricity generation. Hydrogen is produced by low-temperature electrolysis, without further specification, and syngas is generated via reverse water gas shift reaction, where the CO2 stems from any (non-defined) source. This solution causes greenhouse gas emissions of only 3.8 gCO2-eq/MJ of fuel if the hydrogen is separated and recycled from the exhaust gas of the FT reactor. Without hydrogen recycling, the production route actually represents an emission sink with a value of −8.3 gCO2-eq/MJ of fuel, since the combustion of the hydrogen-containing off-gas generates additional electricity that replaces grid electricity with a higher emission factor. However, the GHG emissions associated with the upstream chains of CO2 input are not taken into account, so these values can in no way be used for a direct comparison. Rather, this production route should be seen as an add-on to other processes serving as a point source for CO2. One example of such an integrated system is a plant for the production of ethanol from corn combined with Fischer–Tropsch synthesis, which provides the carbon dioxide for fuel production. Its life cycle greenhouse gas emissions are 37.6 gCO2-eq/MJ fuel with hydrogen recycling and 35.3 gCO2-eq/MJ fuel without, which is equivalent to savings of about 60% compared to petroleum-based fuels. This order of magnitude is also given in [30]. The importance of the choice of system boundary for the results is emphasized in the study to draw attention to possible implications for allowance trading regulations. The lack of uniform and binding specifications for LCAs on synthetic fuels also makes it difficult to compare results among the different analyzed studies and research groups.
In [13], the individual process steps for the production of synthetic fuels via the Fischer–Tropsch route are investigated and evaluated separately with regard to their sustainability potentials. The system includes hydrogen electrolysis by proton exchange membrane electrolysis cell (PEMEC), DAC, reverse water gas shift reaction and Fischer–Tropsch synthesis for diesel and kerosene production. The combustion of the fuels is not considered in the life cycle analysis. As power sources, the German electricity mix from 2020 and an onshore wind farm for the year 2050 are compared. The greenhouse gas emissions using the 2020 electricity mix are 314.1 gCO2-eq/MJ fuel (assumed calorific value of 44.7 MJ/kg), whereby the share of hydrogen supply is about six times as large as that of the other process steps (supply of CO2 and electricity as well as FT plant construction). The relative shares of catalysts appear almost negligible; they are at best guessed at in the visual representation of the results. In the projection for wind power and technological advances in 2050, the relative importance of the respective shares has shifted significantly; hydrogen supply now accounts for about half of the emissions, while CO2 supply contributes more than 30% and FT plant construction more than 12%. Taking into account the carbon dioxide credit, a negative value of −39.8 gCO2-eq/MJ fuel is calculated overall.
In [14], the production of e-fuels via FT synthesis is structured into technical modules that form different combinations with each other to be systematically compared in terms of environmental impacts. Also in this study, the construction and operation of the synthesis plant itself is found to account for only a small proportion of the GHG emissions in each of the different generic pathways considered. For the pathways using DAC and renewable energy sources, the respective shares in emissions attributed to synthesis range from 9% to 15% at present, prospectively decreasing to between 3% and 4% in 2050. If the synthesis plant is utilized to a theoretical maximum of 8000 operation hours per year, the shares in emissions even decrease to 4–10% at present and to 1–2% in 2050, respectively. The overall greenhouse gas emissions of most pathways remain below the averaged reference value of 89 gCO2-eq/MJ for fossil gasoline and diesel, many even between 8 and 20 gCO2-eq/MJ of fuel. For pathways with CO2 from fossil point sources, however, this is only true as long as the environmental burdens of the upstream chains are attributed to the source (memorandum fossil CO2). Otherwise, their GHG emissions exceed that of the fossil reference.
In [31], the state of the art and the prospects for a future supply of alternative aviation fuels is discussed, including e-fuels. In this context, the results of [32,33], considering the greenhouse gas emissions for production, transport, distribution, and dispensing of e-fuels from renewable electricity, are summarized. No emissions are attributed to the production when renewable energy and direct air capture are used. Transport, distribution, and dispensing cause overall emissions of approximately 1 gCO2-eq per MJ of final fuel. In [32], it is argued that emissions from the construction of power generation facilities and production sites, as well as the combustion process, are often excluded from life cycle analyses.
In [34], the GHG emissions range between a minimum of 32 gCO2-eq/MJ (with electricity input from hydropower) and a maximum of 301 gCO2-eq/MJ (with electricity purchased from the grid) for an assumed plant efficiency of 65%, or between 31.5 gCO2-eq/MJ and 282.1 gCO2-eq/MJ at 70% efficiency, respectively. The combination with wind power results in GHG emissions of about 40 gCO2-eq/MJ, while photovoltaics achieve 50 gCO2-eq/MJ. If the heat required for DAC is provided by waste heat instead of burning natural gas, the GHG emissions drop to a minimum of 8.4 gCO2-eq/MJ for use of hydropower, 11 gCO2-eq per MJ for wind power, 28 gCO2-eq per MJ for photovoltaics, and to a maximum of 259.1 gCO2-eq/MJ for grid electricity. Emissions from the construction of power generation plants and production sites, all located in Germany, are taken into account. The study considers high-temperature electrolysis and DAC in combination with FTS and subsequently upgrading to final fuel.
Considering the latter part, the upgrading of the synthesis product, the majority of the literature sources evaluated do not provide precise information on the refining of e-crude, although either referring to end products such as diesel or explicitly assuming the end product to correspond to diesel [13,14,28,32,33,34]. Only in [27], the energy demand for the post-treatment of the FT product is quantified with the equivalent of 5 kJ electrical and 31 kJ thermal energy per MJ product (LHV), and there, it is provided in-process by the combustion of the light hydrocarbon fractions (C1–C4) in a cogeneration plant, since the direct recycling of the light hydrocarbons into the synthesis process contradicts common practice in existing GtL plants.
A qualitative comparison between the refining of fossil crude oil and e-crude as a product of Fischer–Tropsch synthesis is provided in [35], concluding that the energy requirement for refining the synthetic fuel is lower than in the conventional case as several process steps with relatively high energy demand can be eliminated while others are modified or new ones added. The available data vary by a power of ten and range from 0.036 MJ per MJ product (LHV) in [27] to an upper limit of 0.13 MJ per MJ (LHV) in [36], which again is consistent with data in [37,38], or 0.43 MJ per MJ (LHV) in [35], respectively. Curiously enough, a comparison of the data used in the older studies for the crude oil throughput in U.S. refineries in the mid 1970s and the required energy demand with the more recent studies shows that the values quoted can still be seen as up to date.
The system designs from the selected literature were compared in the next step with regard to their components, process steps, and system boundaries in order to obtain information on the comparability of the results for the environmental impacts. Finally, the extent to which the studies provided information on the distribution of environmental impacts among the individual process steps was examined and the assumptions found were compared. It was assumed that if the process design is sufficiently similar, a comparison with regard to the derived GHG emissions between individual pathways from [14] and other literature sources is permissible. The process design must include direct air capture of CO2, hydrogen from electrolysis, synthesis via the Fischer–Tropsch route, and consideration of plant construction, refining, and combustion of the fuel. The supply pathways 10 to 19 from [14], as well as three other literature sources [13,28,34], met the similarity criteria defined above and consequently were included in the comparison.
In a next step, it was assumed that processes with similar enough designs and GHG emissions in a comparable order of magnitude also have a similar distribution of the respective environmental impacts among the individual process steps. From [14], where a systematic comparison between different generic supply pathways is conducted on the basis of uniform assumptions, an arithmetic mean value for the proportional contributions of the individual process steps to the specific GHG emissions of the fuel was calculated. As the data showed wide variation, an interval was then defined for the Fischer–Tropsch synthesis’s share, which comprised at least 70% of the supply pathways. That interval of the share was, in accordance with the available data, defined for the years 2015, 2030, and 2050 and subsequently used in the bottom-up analysis as a basis for the assumptions on the Fischer–Tropsch synthesis’s contribution to the emissions, where the year 2015 served as a reference for the current status.
In order to determine the GHG emissions of the transport of e-crude, nine transport routes and means were examined. Specifically, the production of e-crude in Chile or Saudi Arabia was considered. For the transport route within Germany, it was assumed that the electrolysis and FTS plants are located in the immediate vicinity of refineries. The entire transport route was divided into two sections. The first section describes the transport of e-crude via ocean-going tankers to Europe. In the second section, the e-crude is further transported within Europe to the refineries via pipelines or barges. An overview of the transport routes can be found in Appendix C.
For the first section of the transport route, a distinction was made between the route from Saudi Arabia to Europe and Chile to Europe. The calculation of the GHG emissions for the first transport section is based on [39], which has worked out the specific GHG emissions for different transport routes based on a sample in the scope of 70,000 oil shipments. The transport route from Saudi Arabia to Europe using the trade route from the Arabian gulf to Europe was estimated at 5126 km. The specific GHG emissions for the route from Chile to Europe were extrapolated using a transport distance of 14,000 km.
The pipeline routes as the second transport section include the Wilhelmshaven–Hamburg (NDO), Wilhelmshaven–Wesseling (NWO), Rotterdam–Wesel (RRP–Wesel), Rotterdam–Wesseling (RRP–Wesseling), and Trieste–Karlsruhe (TAL) connections. Regarding the GHG emissions of the transport of e-fuel, only consumption at pumping stations was taken into account on the basis of the available literature i.e., any differences in altitude along the route were omitted due to the limited availability of consumption data. Furthermore, with regard to pipeline transport, only the German electricity mix was used as a general basis for the calculations of energy consumption. The GHG emissions for pipeline transportation are based on [40], which states that pumping stations along the pipeline route require about 0.5% of the transported energy for their operation. Based on the length of the route, the associated number of pumping stations, the density of the e-crude, and the average amount of crude oil transported each year, the GHG emissions were determined. Since this approximate procedure is prone to a certain inaccuracy in the determined GHG emissions, an overall average value of the transport routes was added to the total GHG emissions of the e-fuel use in Germany to account for the emissions linked to the crude and fuel transport.
3. Results
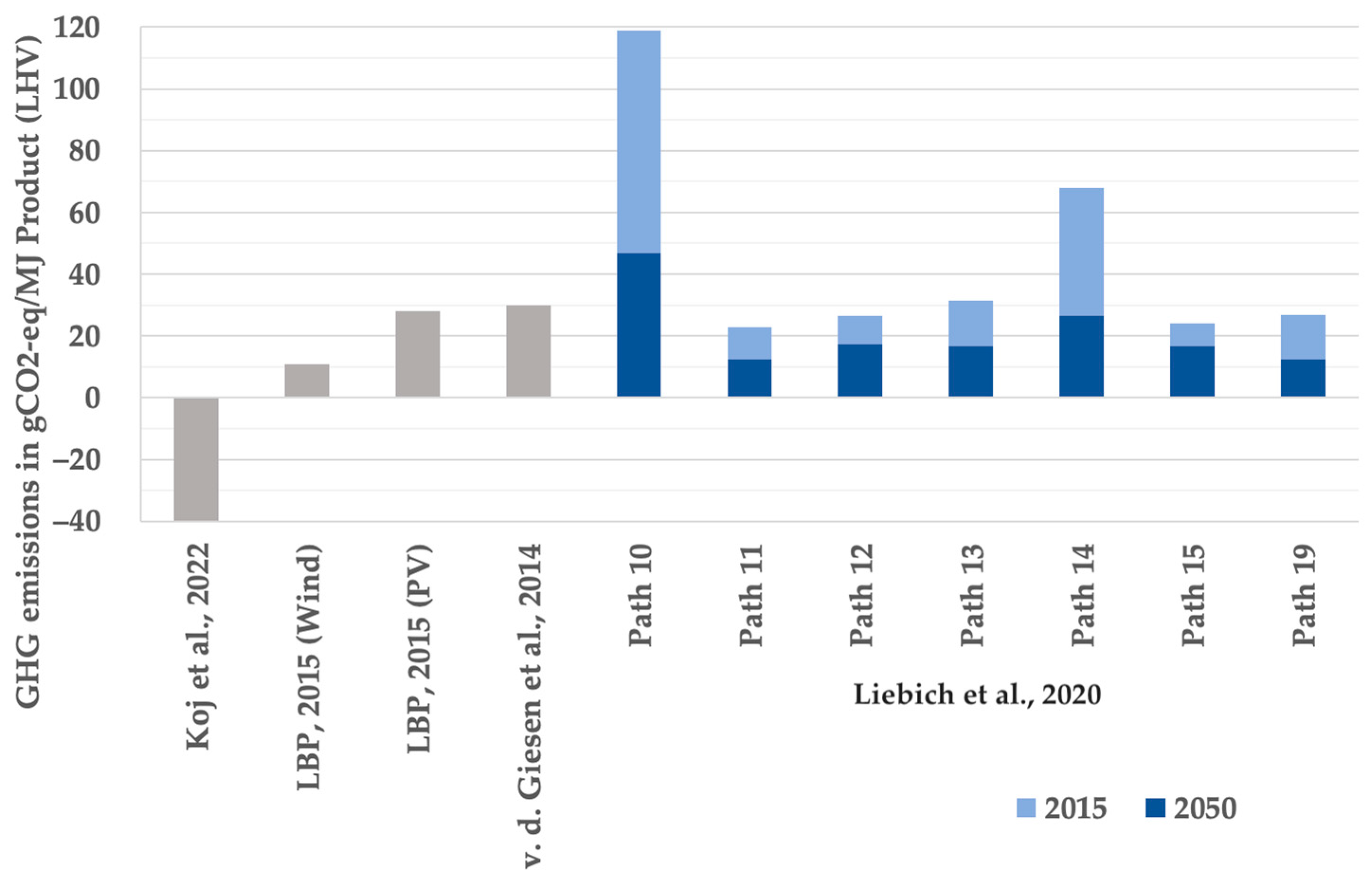
The results found in the literature study were checked for comparability according to the previously explained specification. The calculated GHG emissions over the entire life cycle for supply paths 10 to 19 from [14] as well as for the similar system designs from [13,28,34] were deemed comparable accordingly and are illustrated in Figure 1.
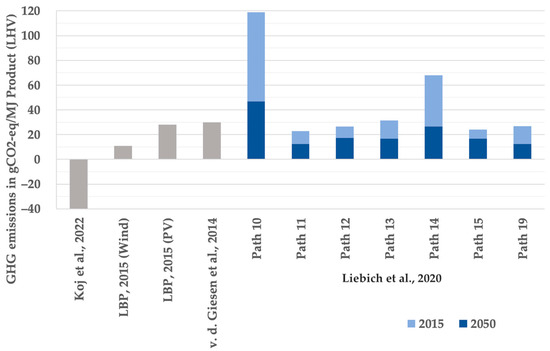
Figure 1.
GHG emissions in gCO2-eq per MJ product (LHV) of Fischer–Tropsch fuels from literature review, pathways 10 to 19 at full load operation of the electricity source [13,14,28,34].

Table 3.
System details for the studies on the horizontal axis label in Figure 1.
From Figure 1, as a first approximation, GHG emissions of 20 to 30 gCO2-eq per MJ product (LHV) of Fischer–Tropsch fuels can be derived. At the same time, the great variety in the results is apparent, ranging from −40 gCO2-eq to 119 gCO2-eq per MJ, the latter even exceeding the refence value for fossil fuels of 89 gCO2-eq per MJ from [14].
One of the aims of this work was to break down the distribution of GHG emissions between the individual process steps wherever possible. However, only source [14] in the literature study provided information on the environmental impacts of the synthesis step. For own calculations in the bottom-up analysis, a relative share of the synthesis in the total GHG emissions was therefore used, which was determined as an arithmetic mean value of a fluctuation interval. For this purpose, the percentage shares of the synthesis plant in the GHG emissions of the electricity-based power-to-liquid pathways from [14] utilizing both DAC as a CO2 source and renewable electricity sources i.e., pathways 10–19, were calculated. In the reference year 2015, the share of synthesis in the GHG emissions for the majority (over 70%) of these pathways is between 9% and 15% for full utilization of the electricity source, with 12% as an arithmetic mean value. The same value is used in the bottom-up analysis for the reference year 2021. By 2030, this share falls below 5%, with an arithmetic mean value of the fluctuation interval of 4%; another 1% of decline can be witnessed up to 2050. The derivation of relative shares from [14] served as an intermediate step in order to ultimately calculate absolute GHG emissions. As it can be assumed that the synthesis step is performed at the same efficiency in the three considered countries, the absolute GHG emissions are independent of the location. Accordingly, the relative shares presented in Table 4 differ between the countries, while for Germany, they correspond to the mean value derived from [14] in the intermediate step.

Table 4.
Relative contribution of the three main e-fuel production steps to CO2-eq emissions.
From the bottom-up approach, it can be seen that freshwater availability is a crucial limiting factor for e-fuel production. The analysis of the AQUASTAT database on water resources of the Food and Agriculture Organization (FAO) of the United Nations shows that in Germany and Chile, further extraction of freshwater would be possible. In contrast, Saudi Arabia faces critical water stress and can therefore not provide further groundwater or surface freshwater. Consequently, the production of e-fuels in Saudi Arabia needs to be based on desalinated saltwater.
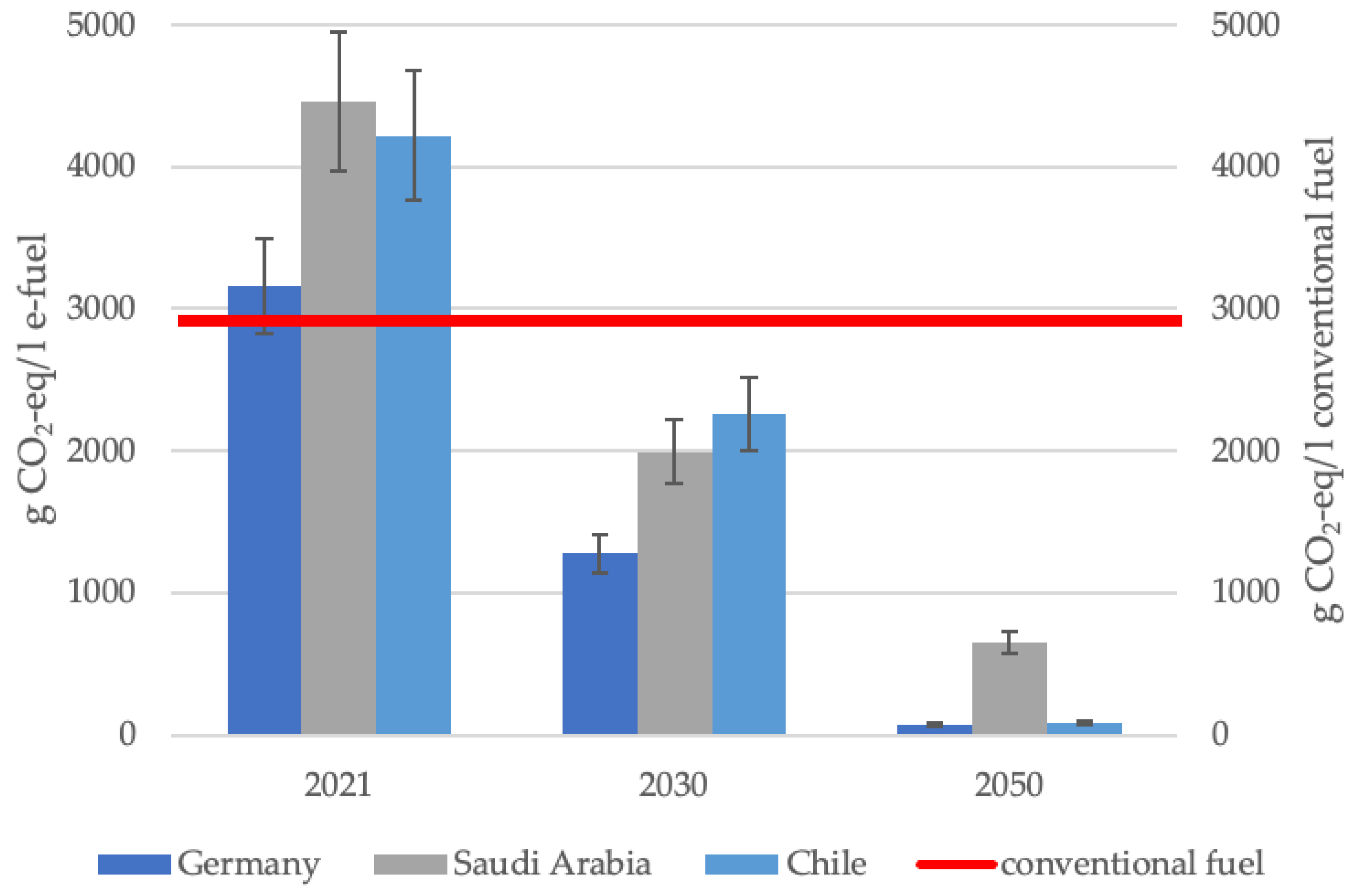
Figure 2 illustrates the CO2-eq emissions of e-fuels while including all considered scenarios for the provision of feedstocks and puts them in comparison with conventional fuel. Taking into account the upstream chain emissions, 2.73 kgCO2-eq/L gasoline and 3.08 kgCO2-eq/L diesel are emitted [41], leading to an average of 2.91 kgCO2-eq/L for conventional fuel. The red line illustrates the CO2-eq of conventional fuel, serving as a benchmark against which the considered scenario of e-fuel consumption in Germany is compared. It delineates the threshold beyond which the CO2-eq of e-fuels surpass the CO2-eq of conventional fuel.
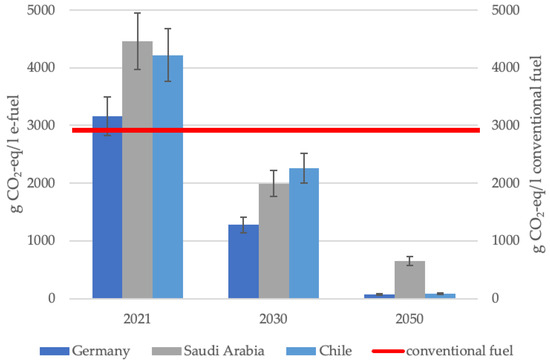
Figure 2.
CO2-eq emissions per liter e-fuel compared with conventional fuel.
Figure 2 contains the average of eight considered scenarios for the respective country and year. Additionally, the variation in scenarios is mapped by the error bars.
The comparison of the specific GHG emissions of e-fuels and conventional fuels shows the following:
- In every scenario for e-fuel production in the reference year 2021, the GHG emissions for production in Saudi Arabia and Chile are still higher than those of conventional fuels.
- For production in Germany, four out of eight scenarios lead to higher GHG emissions in the reference year 2021, while the remaining four scenarios lead to marginal CO2-eq savings already in 2021 (see Appendix B).
- For all countries, the scenarios from 2030 and beyond lead to less CO2-eq/L compared to conventional fuels.
As shown in Table 4, the process step contributing the most to GHG emissions is electrolysis.
The energy mix used for feedstock production is therefore the major factor for GHG emissions during the production of e-fuels. Due to the efforts of the considered countries to increase the share of renewable energies in their energy mix, the specific CO2-eq emissions will decrease over time. Moreover, increased efficiency in the production process will reduce the energy demand for the provision of feedstocks and electrolysis.
4. Discussion
The objective of this article was to answer the following research question: “What are the GHG emissions of e-fuels produced via the SOEC and FTS route for use in Germany?” The scenarios considered in this context show that currently (2021), the emissions for e-fuel production using grid electricity still mostly exceed those of fossil fuels, which is in line with the findings in [13,14,27,30,34]. The exceptions are four scenarios for production in Germany, which show slightly reduced CO2-eq emissions already in 2021. Although the GHG emissions from the use of e-fuels in Germany decrease drastically in the considered scenarios for 2050, it cannot be concluded that e-fuels should be a direct substitute for fossil fuels allowing for a continuation of current consumption patterns. The usage of e-fuels in motorized individual transportation in particular has major disadvantages. Firstly, as shown above, in most scenarios, the current GHG emissions of e-fuels still exceed those of conventional fuels. Secondly, the costs of e-fuels are not yet economically feasible. Current prices are estimated at 3.4 EUR/L and are predicted to reach 2 EUR/L in 2030 [42]. On the contrary, private companies’ claims of reaching final prices of 1.2 EUR/L in 2030 exist [43]. Yet, no scientific studies or real-life experiences can back this claim. Thirdly, the energy conversion efficiency at 10–35% is much below that of direct or battery electric vehicles [44]. Fourthly, the production of e-fuels is at present only available in R&D facilities and first smaller-scale production sites. Norsk e-fuel as the currently largest manufacturer is aiming for a capacity of 50 mio·l/year (corresponding to 40,000 tons/year) after completion of their first plant in 2026 and a supply of over 250 mio·l/year from a total of three plants in 2030 [45]. Therefore, broad application of e-fuels is not yet secured. In conclusion, a broad substitution of fossil fuels by e-fuels is so far neither applicable, economically feasible, nor energy efficient compared to direct electricity usage. Nevertheless, e-fuels offer an important option for defossilization in areas that cannot be electrified and are therefore difficult to decarbonize, and where, in addition, energy carriers with high energy density are needed, as pointed out in a similar way in [28]. These are, in particular, heavy non-road machinery such as rescue vehicles used in natural disaster areas, military vehicles, aviation, and deep-sea shipping. The exclusive usage of e-fuels in niche areas is likewise discussed in [28].
Regarding the determined GHG emissions, this study is also based on the assumption that the considered countries (Germany, Saudi Arabia, Chile) meet their self-defined targets for the expansion of renewable energies. On the basis of historical data, however, this would require a much greater effort on the part of the countries concerned than in the past. If the respective shares of renewable energies in the national grid remain below the targets aspired to, the GHG emissions increase compared to the presented results. Furthermore, in order to effectively reduce the GHG emissions, additional renewable energy is required for e-fuel production as is also emphasized in [34]. The additionality is essential to avoid CO2-eq balance shifts within a country. On the one hand, the redistributed use of renewable energy would lead to a reduction in e-fuel emissions if considered individually. On the other hand, without additional (newly built) renewable energy in the electricity grid, the renewable energy demand allocated to all other consumers would simply be substituted with fossil energy, which would actually lead to an increase in the overall GHG emissions of a country due to the increased overall energy demand. Therefore, the importance of mandatory requirements for the determination of the system boundary against the background of emission allowance trading is also emphasized in [29], supporting our findings.
In the scope of this article, solely the current water availability in the three analyzed countries has been taken into account for the use of ground/surface water. However, according to [46], the future water availability in Germany and Chile might also differ e.g., as a consequence of climate change. Hence, the availability, cost of freshwater and the social acceptance of freshwater use for e-fuel production can lead to future challenges and have to be further evaluated. The alternative use of salt water desalination does not lead to a supply issue. Yet, this energy-intensive process also requires additional renewable energy [14]. Lastly, other potential environmental impacts (such as on marine ecosystems due to the brine disposal) must also be further investigated for desalination processes.
It was assumed that if the system designs of the investigated processes are similar enough, the calculated GHG emissions for the individual supply paths from [14] are comparable with the data from the other literature sources [13,28,34]. This assumption was used to check the consistency of the various literature values and see if the distribution of the environmental impacts among the individual process steps from [14] could be used for orientation. In this next step, it was assumed that similar enough process designs also result in similar shares of the respective process steps in the environmental impacts, and that the average share of synthesis for the supply paths from [14] could therefore be transferred to our bottom-up calculation. As the synthesis step is of minor importance in terms of environmental impact compared to the electrolysis and CO2 capture, this assumption was not considered to lead to a significant miscalculation. Furthermore, the results of our calculation are consistent with the conclusion in [13], according to which the share of electrolysis accounts for about six times the environmental impact of the other process steps. The findings of this study also correspond with other studies that focus on e-fuel production even though deviating production processes have been examined [28,29,47].
One aspect that often is excluded from LCA studies but also deserves some attention is the refining of e-crude. As outlined above, it is difficult to quantify the required energy demand and even more, the associated emissions. However, as soon as e-crude is more than just a blend in existing refineries, a different infrastructure will be required, resulting in emissions not only for operation, but also for the construction of the corresponding plants [35].
Overall, in recent studies, the environmental impacts of high-temperature electrolysis are estimated to still be higher compared to other types of electrolysis [14]. This results from the assumed significantly lower stack lifetime of a technology still under development, which cannot be fully compensated for by the efficiency advantage at this stage. However, it should also be noted that a service life of 10,000 operating hours as assumed in [14] may now no longer correspond to the actual state of the art due to the technical progress. Furthermore, while effects of variation in location, CO2 source, power source for electrolysis, electrolysis type, and transport route are directly compared in [14], the synthesis type remains constant. In order to find the most efficient production route, a further comparison with a system combining high-temperature co-electrolysis and methanol synthesis would therefore be interesting for future work.
In our study, we focused exclusively on the greenhouse gas emissions of e-fuels produced by high-temperature electrolysis in combination with Fischer–Tropsch synthesis. We thereby excluded other aspects, yet not for lack of importance. In particular, the social aspects and other impacts of a global e-fuel production and trade network must be considered separately. Research in this field is absolutely necessary in order to avert negative side effects, especially in the producing countries, and not to drift into a new energy colonialism [48,49,50].
5. Conclusions
Overall, e-fuels can be an environmentally beneficial alternative to conventional fuels. Nevertheless, it is crucial that additional renewable energy be used along the whole production chain, which must be designed as efficiently as possible. Otherwise, as shown in this study, the GHG emissions may even exceed those of conventional fossil fuels. Finally, the application should be prioritized and limited to use cases that are hard to decarbonize. In the ecological dimension, aspects other than a sole reduction in GHG emissions have to be investigated further, such as the disposal of brine stemming from sea water desalination plants or land use due to power generation plants. More research is also needed on other aspects from the social sustainability dimension especially, which have so far tended to be left out of many debates on e-fuels [48,49,50].
Author Contributions
Conceptualization, F.L., B.S. and T.G.; Funding acquisition, T.G.; Investigation, F.L., B.S. and J.P.; Methodology, F.L., B.S. and T.G.; Project administration, T.G.; Supervision, T.G.; Writing—original draft, F.L., B.S. and J.P.; Writing—review and editing, F.L. and B.S. All authors have read and agreed to the published version of the manuscript.
Funding
Based on a resolution of the German parliament, the research leading to this article was funded by the Federal Ministry for Economics and Climate Action; Project Management Jülich, Funding reference number: 03EN5003C, (ESyRE—Efficient Synthesis and Electricity Recovery from E-Fuels). We acknowledge financial support by Wuppertal Institut für Klima, Umwelt, Energie gGmbH within the funding program Open Access Publishing.
Data Availability Statement
Acknowledgments
The good cooperation with the Fraunhofer Institute for Ceramic Technologies and Systems (IKTS) and the Entwicklungs- und Vertriebsgesellschaft Brennstoffzelle (EBZ) mbH in the underlying ESyRE project is gratefully acknowledged. We also thank the University of Stuttgart Institute for Acoustics and Building Physics Department of Life Cycle Engineering for making their study available as an original literature source.
Conflicts of Interest
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Appendix A

Table A1.
Overview of the scenarios considered for the provision of feedstocks for the electrolysis.
Table A1.
Overview of the scenarios considered for the provision of feedstocks for the electrolysis.
| Scenario | Electricity Source | Water Treatment * | CO2 Source |
|---|---|---|---|
| 1 | Grid | Conventional pre-treatment and reverse osmosis | Direct air capture |
| 2 | Grid | Conventional pre-treatment and ion exchange | Biomass (organic waste) |
| 3 | Grid | Combined treatment | Direct air capture |
| 4 | Grid | Reverse osmosis | Direct air capture |
| 5 | Grid | Conventional pre-treatment and reverse osmosis | Biomass (organic waste) |
| 6 | Grid | Conventional pre-treatment and reverse osmosis | Cement production |
| 7 | Grid | Reverse osmosis | Biomass (organic waste) |
| 8 | Grid | Reverse osmosis | Cement Production |
* For Saudi Arabia, the water treatment is restricted to reverse osmosis of salt water.
Appendix B

Table A2.
GHG emissions of the considered scenarios according to country and year.
Table A2.
GHG emissions of the considered scenarios according to country and year.
| Gramm CO2-eq Emissions/L e-Fuel | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2021 | 2030 | 2050 | |||||||
| Scenario | Germany | Saudi Arabia | Chile | Germany | Saudi Arabia | Chile | Germany | Saudi Arabia | Chile |
| 1 | 3476 | 4923 | 4654 | 1403 | 2202 | 2498 | 82 | 726 | 92 |
| 2 | 3475 | 4922 | 4652 | 1403 | 2201 | 2497 | 82 | 726 | 92 |
| 3 | 3476 | 4924 | 4654 | 1403 | 2202 | 2498 | 82 | 726 | 92 |
| 4 | 3476 | 4923 | 4653 | 1403 | 2202 | 2498 | 82 | 726 | 92 |
| 5 | 2902 | 4044 | 3829 | 1195 | 1797 | 2042 | 66 | 582 | 75 |
| 6 | 2789 | 3966 | 3753 | 1116 | 1761 | 2000 | 64 | 569 | 74 |
| 7 | 2902 | 4046 | 3828 | 1195 | 1798 | 2042 | 66 | 582 | 75 |
| 8 | 2789 | 3966 | 3752 | 1116 | 1762 | 2000 | 64 | 569 | 74 |
Appendix C

Table A3.
Overview of the transport routes and means.
Table A3.
Overview of the transport routes and means.
| Route | Transportation | Distance in km | Pumping Stations | |
|---|---|---|---|---|
| 1 | Saudi Arabia–Europe | Ocean tanker | 5126 | - |
| 2 | Chile–Europe | Ocean tanker | 14,000 | - |
| 3 | Wilhelmshaven–Hamburg | Pipeline | 142 | 1 |
| 4 | Wilhelmshaven–Wesseling | Pipeline | 391 | 3 |
| 5 | Europoort–Wesel | Pipeline | 220 | 2 |
| 6 | Europoort–Wesseling | Pipeline | 280 | 2 |
| 7 | Triest–Karlsruhe | Pipeline | 731 | 10 |
| 8 | Europoort–Wesel | Inland vessel | 365 | - |
| 9 | Europoort–Wesseling | Inland vessel | 666 | - |
Appendix D
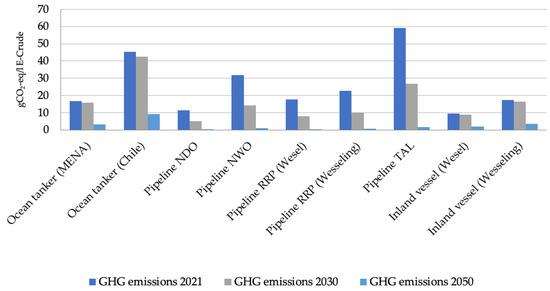
Figure A1.
GHG emissions of the considered transport routes and means at the different points of time analyzed.
Figure A1.
GHG emissions of the considered transport routes and means at the different points of time analyzed.
References
- Bundesregierung. Bundes-Klimaschutzgesetz (KSG); Bundesregierung: Berlin, Germany, 2021; p. 10.
- Bundesregierung. Modernisierungspaket fuer Klimaschutz und Planungsbeschleunigung; Bundesregierung: Berlin, Germany, 2023; p. 10.
- Agora Verkehrswende; Agora Energiewende; Frontier Economics. Die Zukünftigen Kosten Strombasierter Synthetischer Brennstoffe; Agora Verkehrswende: Berlin, Germany; Agora Energiewende: Berlin, Germany; Frontier Economics: Berlin, Germany, 2018; p. 12. [Google Scholar]
- Mathiesen, B.V.; Ridjan, I.; Connolly, D.; Nielsen, M.P.; Vang Hendriksen, P.; Bjerg Mogensen, M.; Højgaard Jensen, S.; Dalgaard Ebbesen, S. Technology Data for High Temperature Solid Oxide Electrolyser Cells, Alkali and PEM Electrolysers; Aalborg University: Aalborg, Danmark, 2013; p. 17. [Google Scholar]
- Liu, C.M.; Sandhu, N.K.; McCoy, S.T.; Bergerson, J.A. A Life Cycle Assessment of Greenhouse Gas Emissions from Direct Air Capture and Fischer-Tropsch Fuel Production. Sustain. Energy Fuels 2020, 4, 3129–3142. [Google Scholar] [CrossRef]
- Pratschner, S.; Hammerschmid, M.; Müller, F.J.; Müller, S.; Winter, F. Simulation of a Pilot Scale Power-to-Liquid Plant Producing Synthetic Fuel and Wax by Combining Fischer–Tropsch Synthesis and SOEC. Energies 2022, 15, 4134. [Google Scholar] [CrossRef]
- Frost, L.; Elangovan, E.; Hartvigsen, J. Production of Synthetic Fuels by High-Temperature Co-Electrolysis of Carbon Dioxide and Steam with Fischer-Tropsch Synthesis. Can. J. Chem. Eng. 2016, 94, 636–641. [Google Scholar] [CrossRef]
- Cinti, G.; Baldinelli, A.; Di Michele, A.; Desideri, U. Integration of Solid Oxide Electrolyzer and Fischer-Tropsch: A Sustainable Pathway for Synthetic Fuel. Appl. Energy 2016, 162, 308–320. [Google Scholar] [CrossRef]
- De Klerk, A. Fischer-Tropsch Refining; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; ISBN 978-3-527-32605-1. [Google Scholar]
- Marano, J.J.; Ciferno, J.P. Life-Cycle Greenhouse-Gas Emissions Inventory for Fischer-Tropsch Fuels; U.S. Department of Energy, National Energy Technology Laboratory: Washington, DC, USA, 2001; p. 186.
- Federal Ministry for Economic Affairs and Climate Action. Available online: https://www.bmwk.de/Redaktion/EN/Artikel/Energy/international-energy-policy-2.html (accessed on 2 May 2023).
- Energy Partnership Chile-Alemania. Available online: https://www.energypartnership.cl/home/ (accessed on 2 May 2023).
- Koj, J.C.; Harzendorf, F.; Zelt, O.; Taubitz, A. Band II—Lebenszyklusorientierte Analysen und Kritikalitätsanalyse von Power-to-X- Optionen; Virtuelles Institut Strom zu Gas und Wärme NRW: Essen, Germany, 2022. [Google Scholar]
- Liebich, A.; Fröhlich, T.; Münter, D.; Fehrenbach, H. Systemvergleich Speicherbarer Energieträger aus Erneuerbaren Energien. 2020. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/479/publikationen/texte_2020_68_anhang_detailanalysen_zum_systemvergleich_speicherbarer_energietraeger_aus_erneuerbaren_energien.pdf (accessed on 6 October 2022).
- Umweltbundesamt. Available online: https://www.umweltbundesamt.de/themen/luft/emissionen-von-luftschadstoffen/spezifische-emissionsfaktoren-fuer-den-deutschen (accessed on 5 December 2022).
- Energía Abierta. Available online: http://energiaabierta.cl/visualizaciones/factor-de-emision-sic-sing/ (accessed on 16 December 2022).
- Palma Behnke, R.; Jiménez Estévez, G.; Alarcón Arias, I. Las Energías Renevables No Convencionales en el Mercado Eléctrico Chileno. 2020, p. 22. Available online: https://energia.gob.cl/sites/default/files/ernc_mercado_electrico_chileno_baja_resolucion.pdf (accessed on 16 December 2022).
- Ministerio de Energía. Available online: https://energia.gob.cl/noticias/nacional/ministro-jobet-anuncia-nueva-meta-las-ernc-representaran-el-40-de-la-matriz-al-2030 (accessed on 17 December 2022).
- British Broadcasting Corporation. Available online: https://www.bbc.com/news/world-middle-east-58955584 (accessed on 11 December 2022).
- Government Saudi Arabia. Available online: https://www.my.gov.sa/wps/portal/snp/aboutksa/environmentalProtection (accessed on 11 December 2022).
- Fraunhofer Institute for Interfacial Engineering and Biotechnology IGB. Available online: https://www.igb.fraunhofer.de/de/forschung/thermische-trennverfahren/projekte/desol.html (accessed on 17 December 2022).
- Mohite, S.; Prasad, E. Water Desalination Equipment Market by Technology (Reverse Osmosis, Multi-Stage Flash (MSF) Distillation, Multi-Effect Distillation (MED), and Others), Source (Seawater, Brachish Water, River Water, and Others), and Application (Municipal, Industiral, and Others): Global Opportunity Analysis and Industry Forecast 2021–2030. 2021. Available online: https://www.alliedmarketresearch.com/water-desalination-equipment-market-A13994 (accessed on 17 December 2022).
- Geoengineering Monitor Geoengineering. Direct Air Capture (DAC). 2021, p. 1. Available online: https://www.boell.de/sites/default/files/2021-01/GM_DAC_de.pdf (accessed on 21 December 2022).
- McQueen, N.; Vaz Gomes, K.; McCormick, C.; Blumanthal, K.; Pisciotta, M.; Wilcox, J. A Review of Direct Air Capture (DAC): Scaling up Commercial Technologies and Innovating for the Future. Prog. Energy 2021, 3, 032001. [Google Scholar] [CrossRef]
- Fröhlich, T.; Blömer, S.; Münter, D.; Brischke, L.-A. CO2-Quellen Für Die PtX-Herstellung in Deutschland—Technologien, Umweltwirkung, Verfügbarkeit. 2019, p. 12. Available online: https://www.ifeu.de/fileadmin/uploads/ifeu_paper_03_2019_CO2-Quellen-f%C3%BCr-PtX.pdf (accessed on 21 December 2022).
- Ghasem, N. Selexol Process DEPG (Selexol Process) Is Applied for Capturing Both CO2 and Hydrogen Sulfide (H2S). In Advances in Carbon Capture; Elsevier: Amsterdam, The Netherlands, 2020; p. 464. [Google Scholar]
- Falter, C.; Batteiger, V.; Sizmann, A. Climate Impact and Economic Feasibility of Solar Thermochemical Jet Fuel Production. Environ. Sci. Technol. 2016, 50, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Van der Giesen, C.; Kleijn, R.; Kramer, G. Energy and Climate Impacts of Producing Synthetic Hydrocarbon Fuels from CO2. Environ. Sci. Technol. 2014, 48, 7111–7121. [Google Scholar] [CrossRef] [PubMed]
- Zang, G.; Sun, P.; Elgowainy, A.; Bafana, A.; Wang, M. Life Cycle Analysis of Electrofuels: Fischer–Tropsch Fuel Production from Hydrogen and Corn Ethanol Byproduct CO2. Environ. Sci. Technol. 2021, 55, 3888–3897. [Google Scholar] [CrossRef] [PubMed]
- Ausfelder, F.; Tran, D.D. Optionen für Ein Nachhaltiges Energiesystem Mit Power-to-X-Technologien. 2022. Available online: https://www.kopernikus-projekte.de/lw_resource/datapool/systemfiles/elements/files/EC7C18F68BCE7C0DE0537E695E86F60F/live/document/221025_DEC_P2X4_V08_Web.pdf (accessed on 17 November 2022).
- Roth, A.; Batteiger, V.; Riegel, F. Power-to-Liquids Potentials and Perspectives for the Future Supply of Renewable Aviation Fuel; German Environment Agency: Berlin, Germany, 2016.
- Hass, H.; Huss, A.; Mass, H. Well-to-Wheels Analysis of Future Automotive Fuels and Powertrains in the European Context—Tank-to-Wheels (TTW) Report; JEC (JRC, EUCAR, Concawe): Luxembourg, 2014. [Google Scholar]
- Eberle, U.; Bölkow, L. FVV Study “Renewables in Transport 2050: Empowering a Sustainable Mobility Future with Zero Emission Fuels from Renewable Electricity”. 2016. Available online: https://www.researchgate.net/publication/292143392_FVV_Study_Renewables_in_Transport_2050_Empowering_a_sustainable_mobility_future_with_zero_emission_fuels_from_renewable_electricity#fullTextFileContent (accessed on 25 January 2023).
- Universität Stuttgart Institut für Akustik und Bauphysik. Available online: https://www.uni-stuttgart.de/universitaet/aktuelles/meldungen/Oekobilanzbericht_fuer_synthetischen_Diesel-Kraftstoff_aus_Wasser_und_CO2_erschienen/ (accessed on 17 May 2023).
- Maitles, P.; De Klerk, A. Greener Fischer-Tropsch Processes for Fuels and Feedstocks, 1st ed.; Maitlis, P.M., de Klerk, A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; ISBN 978-3-527-32945-8. [Google Scholar]
- Haynes, V. Energy Use in Petroleum Refineries; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1976. [CrossRef]
- Affeldt, J.; Kuther, T. Auch Verbrenner Fahren Mit Strom. 2018. Available online: https://www.next-mobility.de/auch-verbrenner-fahren-mit-strom-a-678284/?p=4#:~:text=Laut%20einer%20Anfrage%20des%20Department,Angabe%20durch%20die%20GEMNIS%2DDatenbank (accessed on 7 February 2023).
- Burkert, A. Available online: https://www.springerprofessional.de/elektromobilitaet/dieselmotor/endenergiebezogene-analyse-diesel-versus-elektromobilitaet/16673694 (accessed on 7 February 2022).
- Greene, S.; Jia, H.; Rubio-Domingo, G. Well-to-tank carbon emissions from crude oil maritime transportation. Transp. Res. Part D Transp. Environ. 2020, 88, 102587. [Google Scholar] [CrossRef]
- Seljom, P. Oil and Natural Gas Logistics; Energy Technology System Analysis Programme: Athens, Greece, 2011. [Google Scholar]
- Wietschel, M.; Moll, C.; Oberle, S.; Lux, B.; Timmerberg, S.; Neuling, U.; Kaltschmitt, M.; Ashley-Belbin, N. Klimabilanz, Kosten und Potenziale Verschiedener Kraftstoffarten und Antriebssysteme für PKW und LKW; Fraunhofer ISI: Karlsruhe, Germany, 2019. [Google Scholar]
- Burchardt, J.; Franke, K.; Herhold, P.; Hohaus, M.; Humpert, H.; Päivärinta, J.; Richenhagen, E.; Ritter, D.; Schönberger, S.; Schröder, J.; et al. Klimapfade 2.0—Ein Wirtschaftsprogramm für Klima und Zukunft. 2021, p. 126. Available online: https://bdi.eu/media/publikationen#/publikation/news/klimapfade-2-0-ein-wirtschaftsprogramm-fuer-klima-und-zukunft (accessed on 6 February 2023).
- Bosch. Available online: https://www.bosch.com/stories/denners-view-synthetic-fuels-and-electromobility/ (accessed on 23 May 2023).
- Ueckerdt, F.; Bauer, C.; Dirnaichner, A.; Everall, J.; Sacchi, R.; Luderer, G. Potential and Risks of Hydrogen-Based e-Fuels in Climate Change Mitigation. Nat. Clim. Change 2021, 11, 384–393. [Google Scholar] [CrossRef]
- Norsk E-Fuel. Available online: https://www.norsk-e-fuel.com/projects (accessed on 8 January 2024).
- United Nations. The United Nations World Water Development Report 2022—Groundwater Making the Invisible Visible; United Nations: New York, NY, USA, 2022.
- Sunfire Fuel Cells GmbH. Available online: https://www.sunfire-home.de/files/sunfire/images/content/landingpages/home/Sunfire-Home_Broschu%cc%88re_2021.pdf (accessed on 28 September 2022).
- Müller, F.; Tunn, J.; Kalt, T. Hydrogen Justice. Environ. Res. Lett. 2022, 17, 115006. [Google Scholar] [CrossRef]
- Hanusch, F.; Schad, M. Hydrogen research: Technology first, society second? GAIA 2021, 30, 82–86. [Google Scholar] [CrossRef]
- Claar, S. Kein Ende Des Grünen Kolonialismus: Der Europäische Green Deal Reproduziert Die Abhängigkeiten von Afrika. PROKLA 2021, 51, 141–148. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).