Experimental Investigation of the Effects of Inorganic Components on the Supercritical Water Gasification of Semi-Coke
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
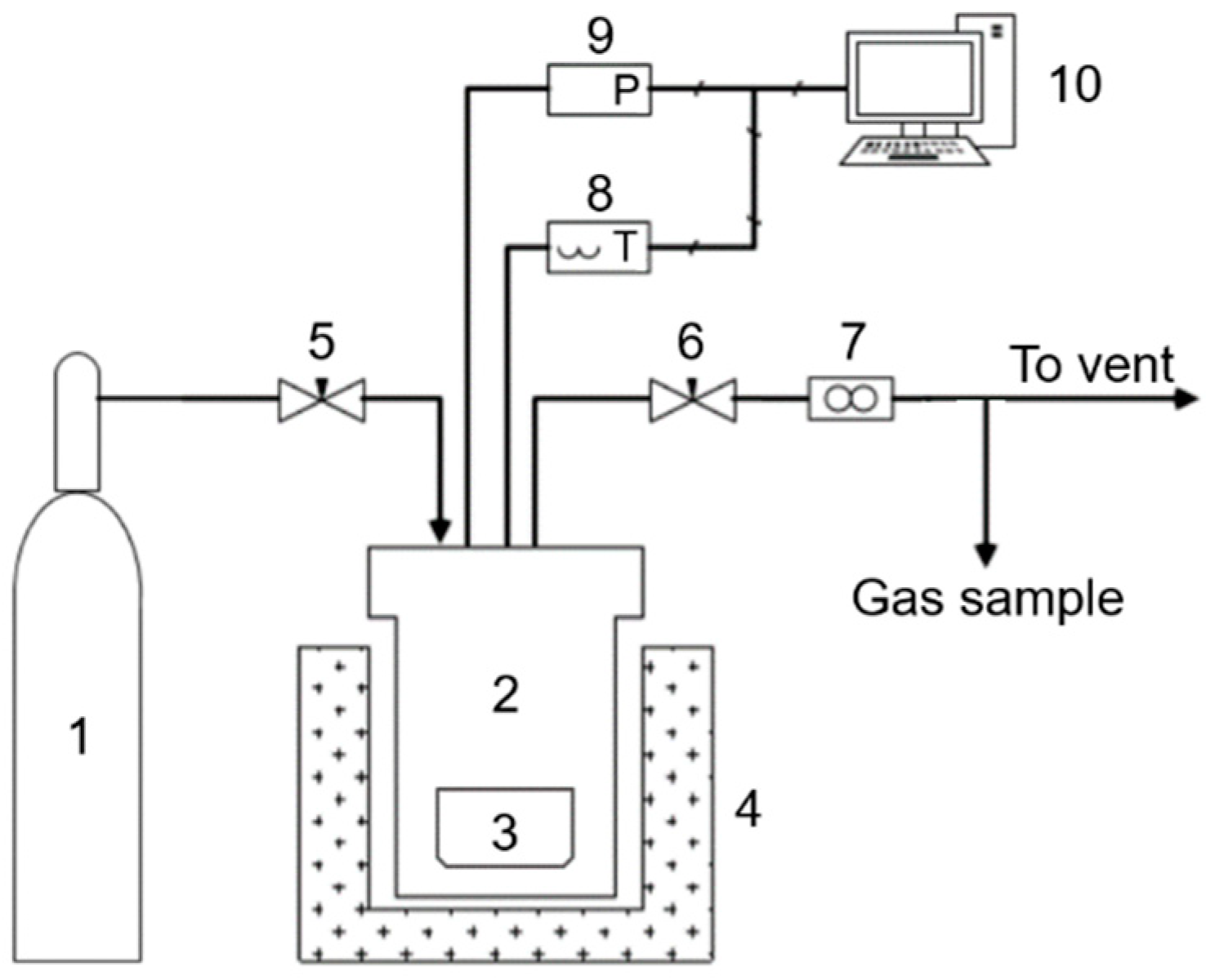
2.2. SCWG Experimental Setup
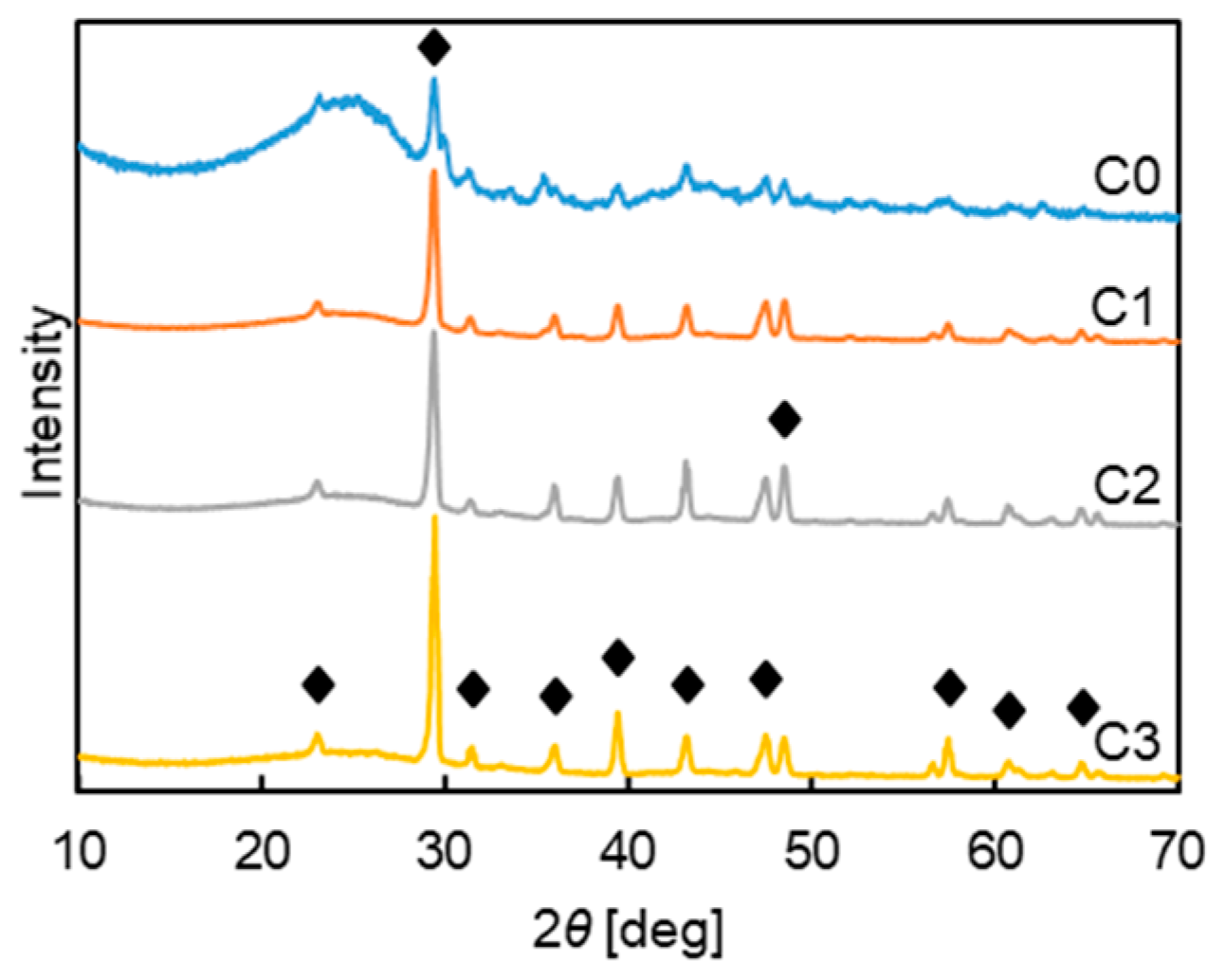
2.3. Characterization Analysis
2.4. Data Analysis
3. Results and Discussion
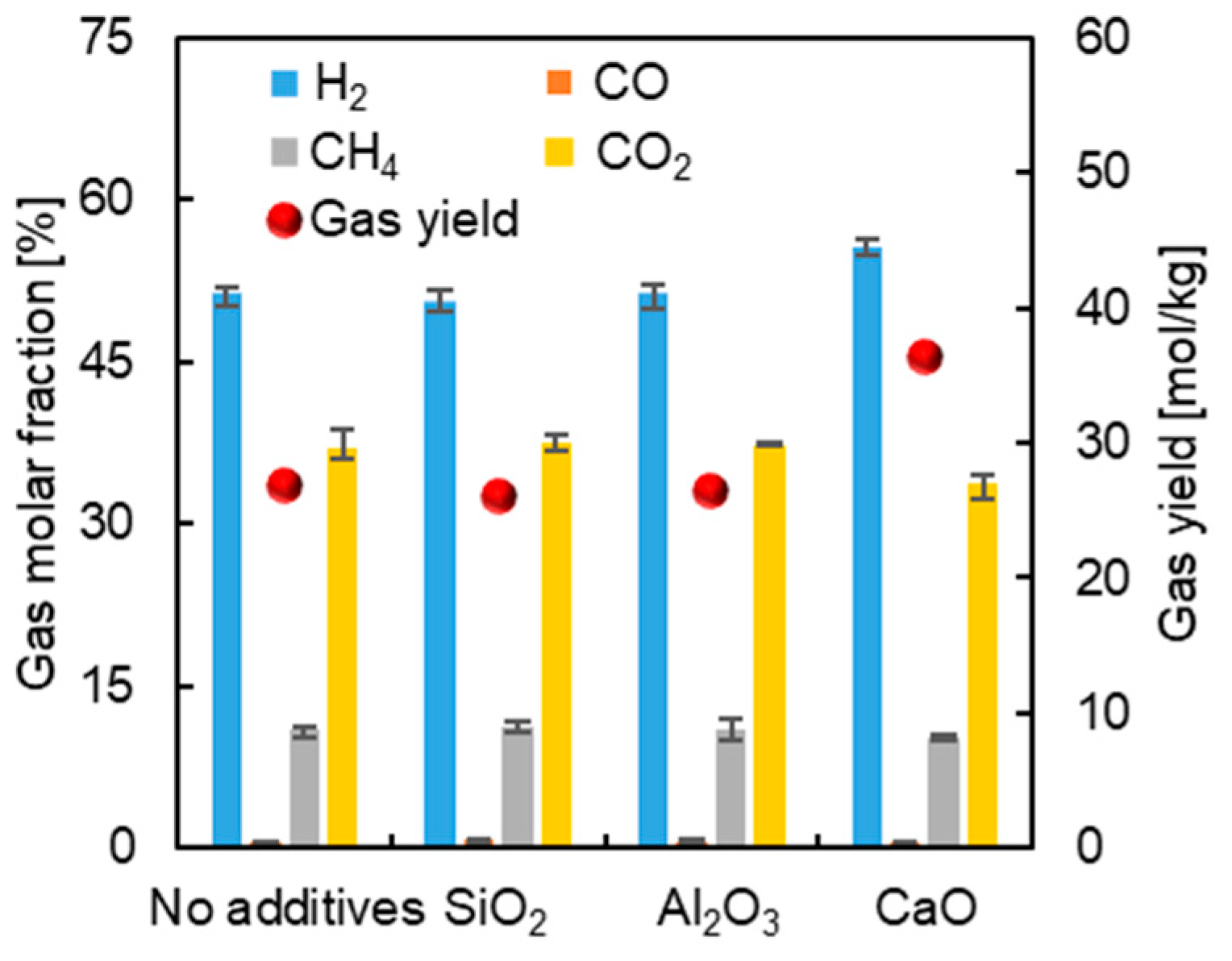
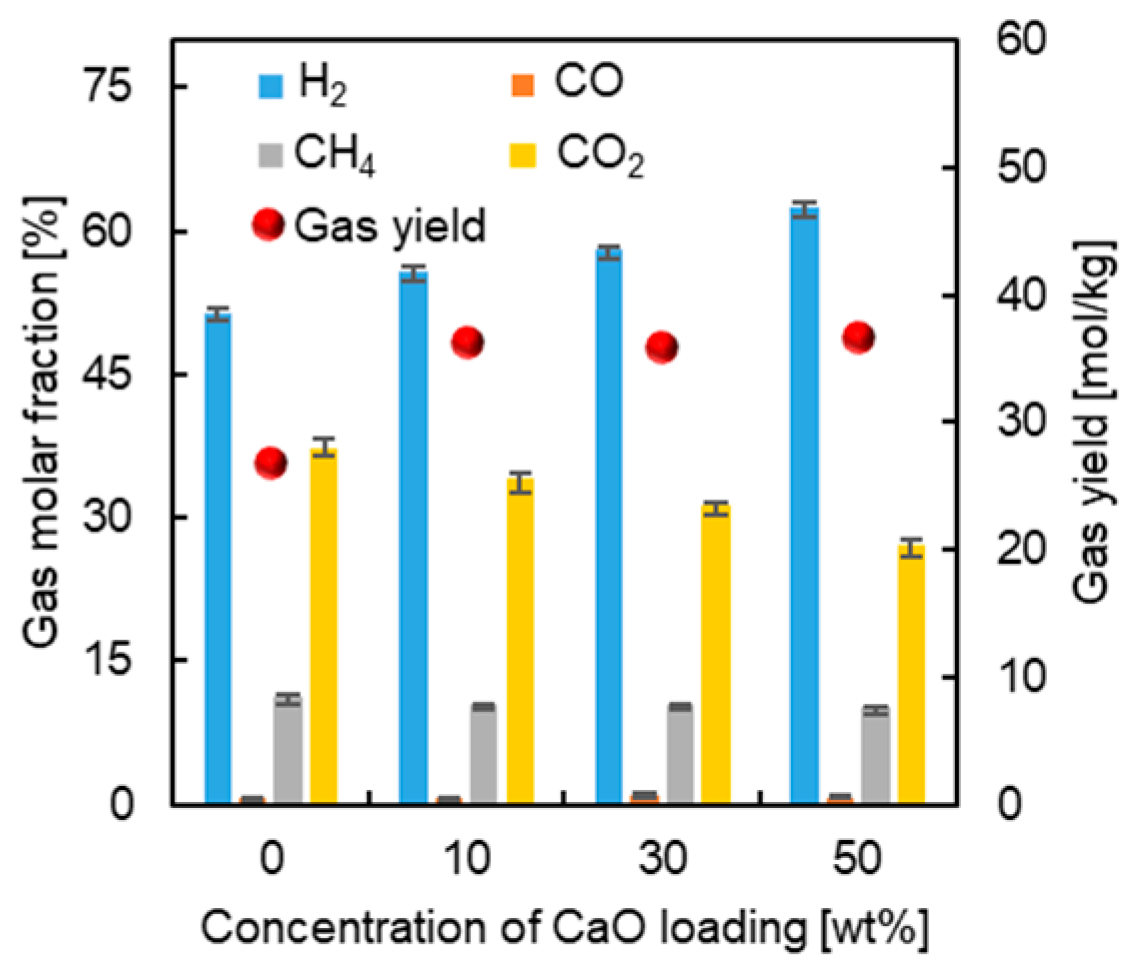
3.1. Effect of Mineral Components without K2CO3
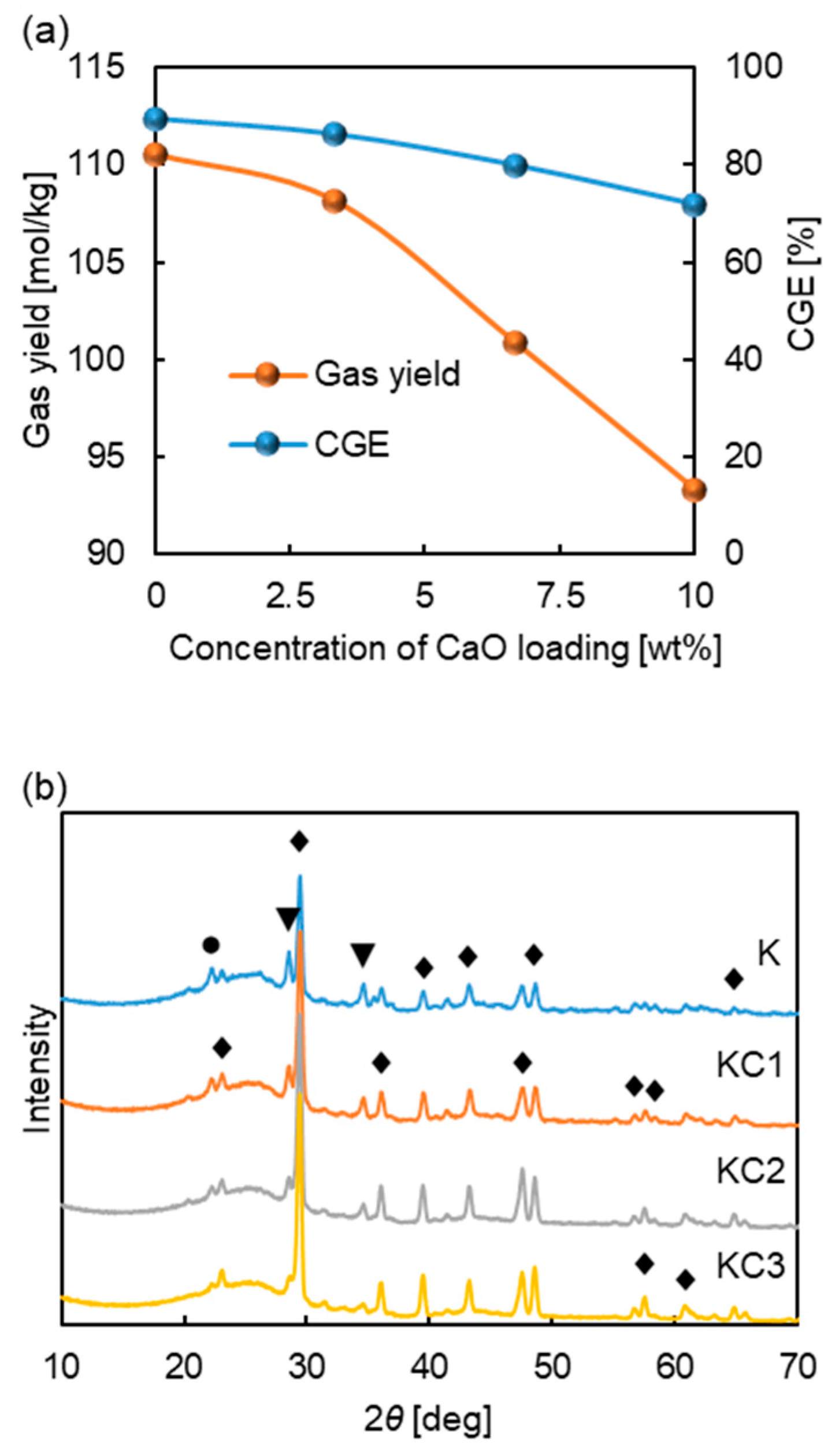
3.2. Effect of Mineral Components with K2CO3
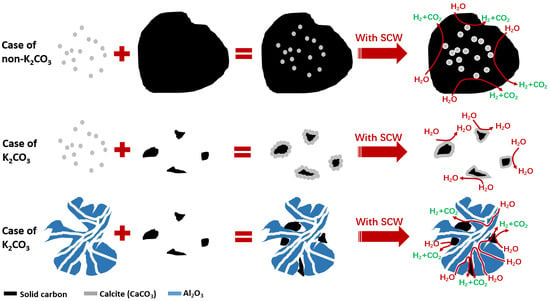
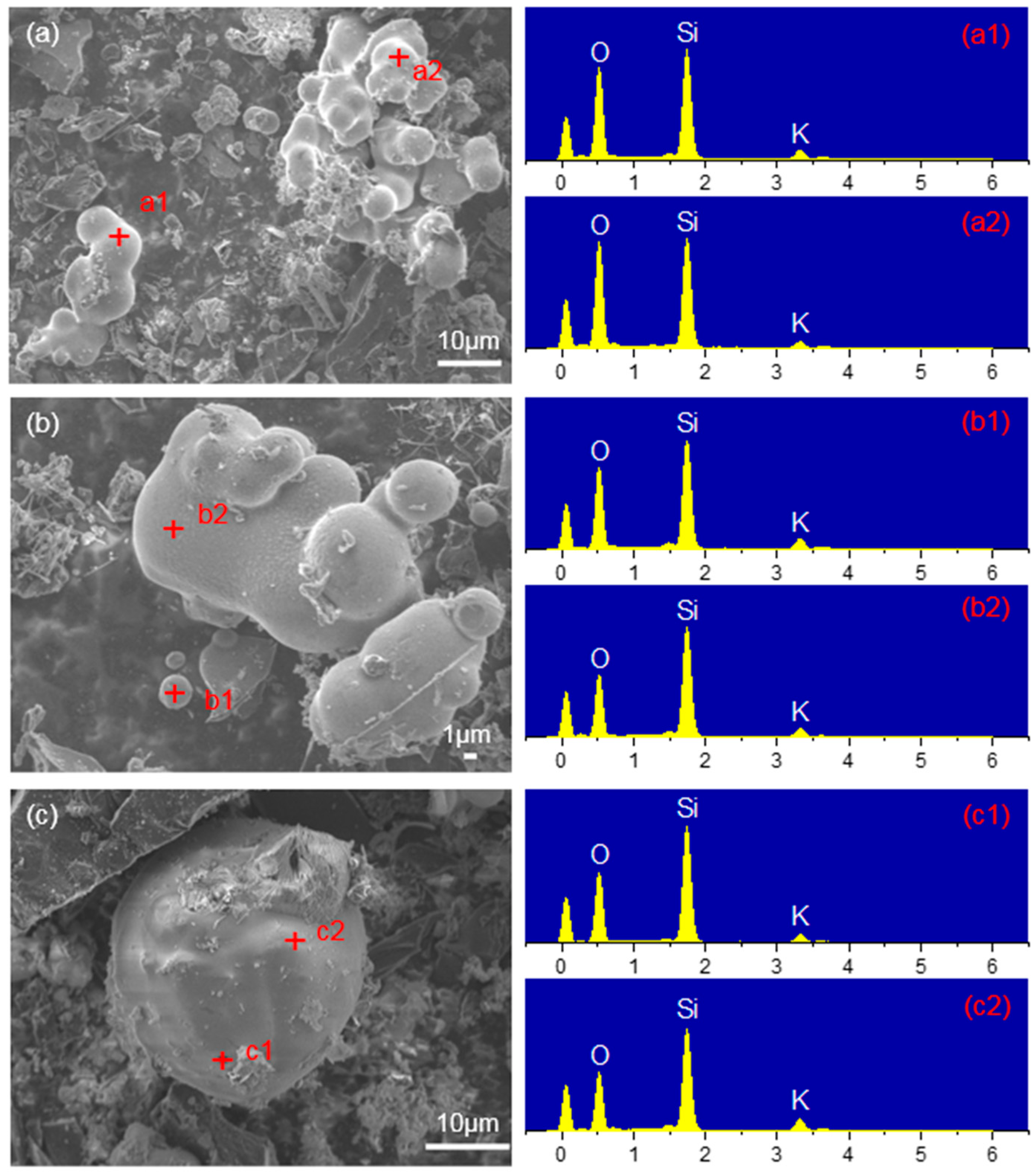
3.2.1. Mechanism Analysis of SiO2 Inhibiting Gasification
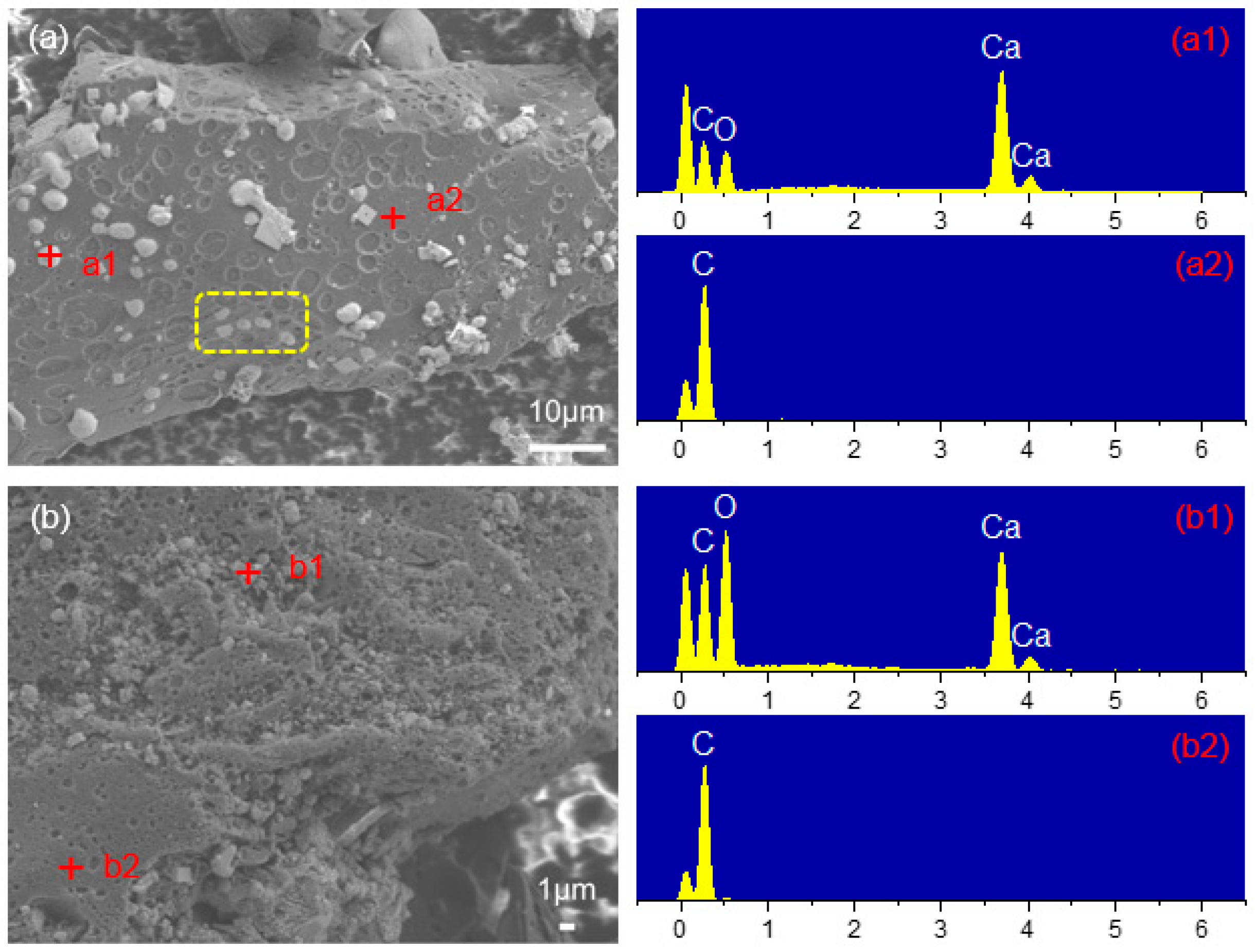
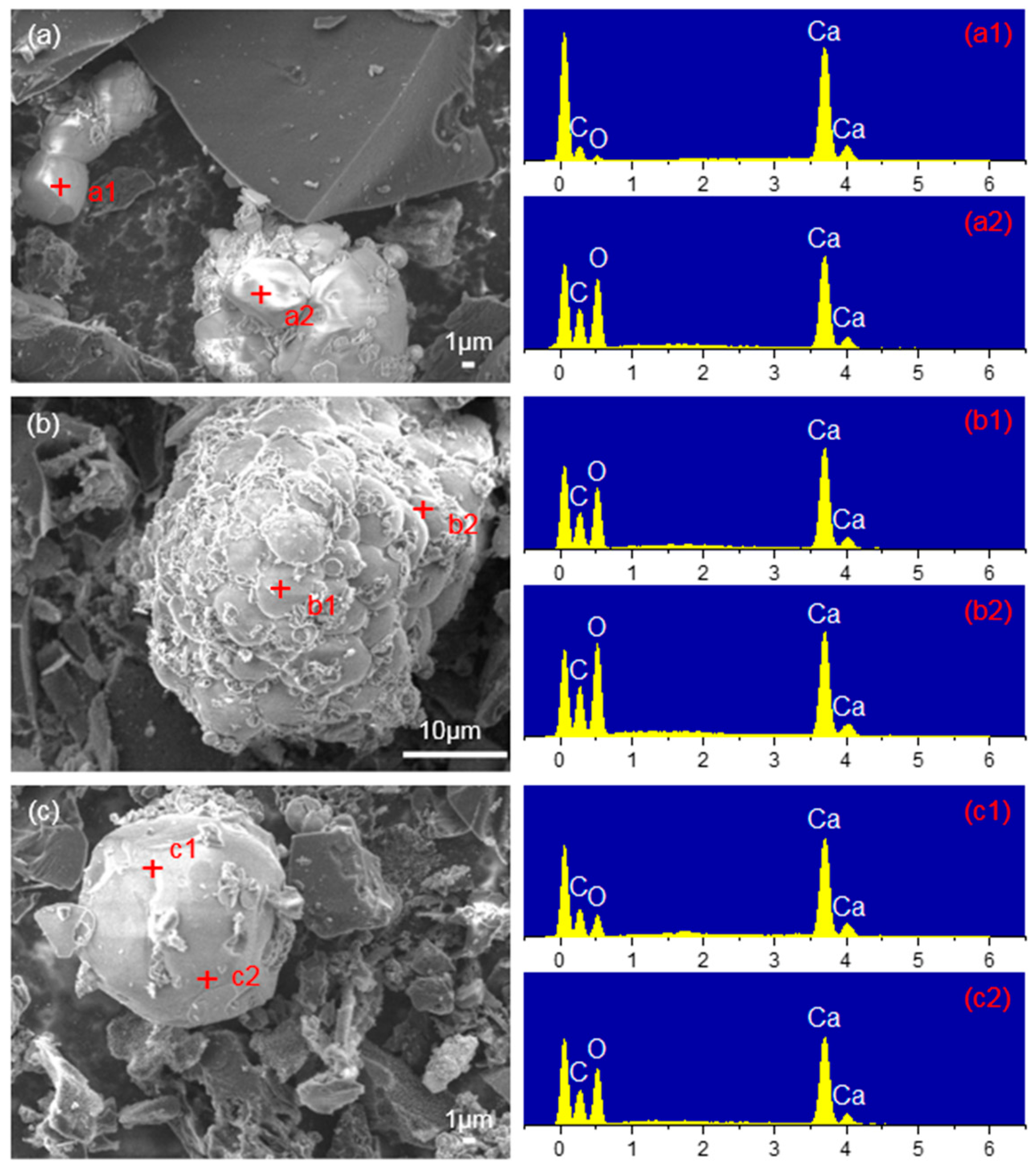
3.2.2. Mechanism Analysis of CaO Inhibiting Gasification
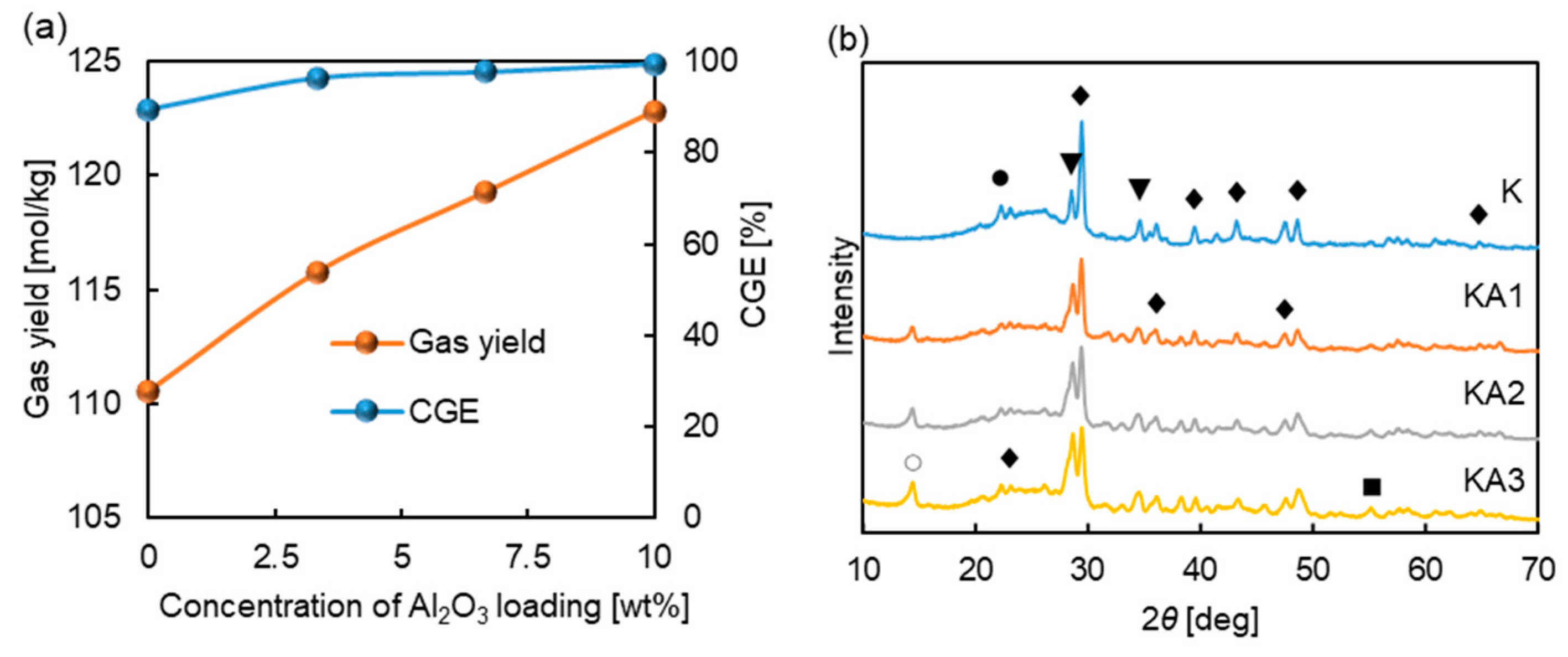
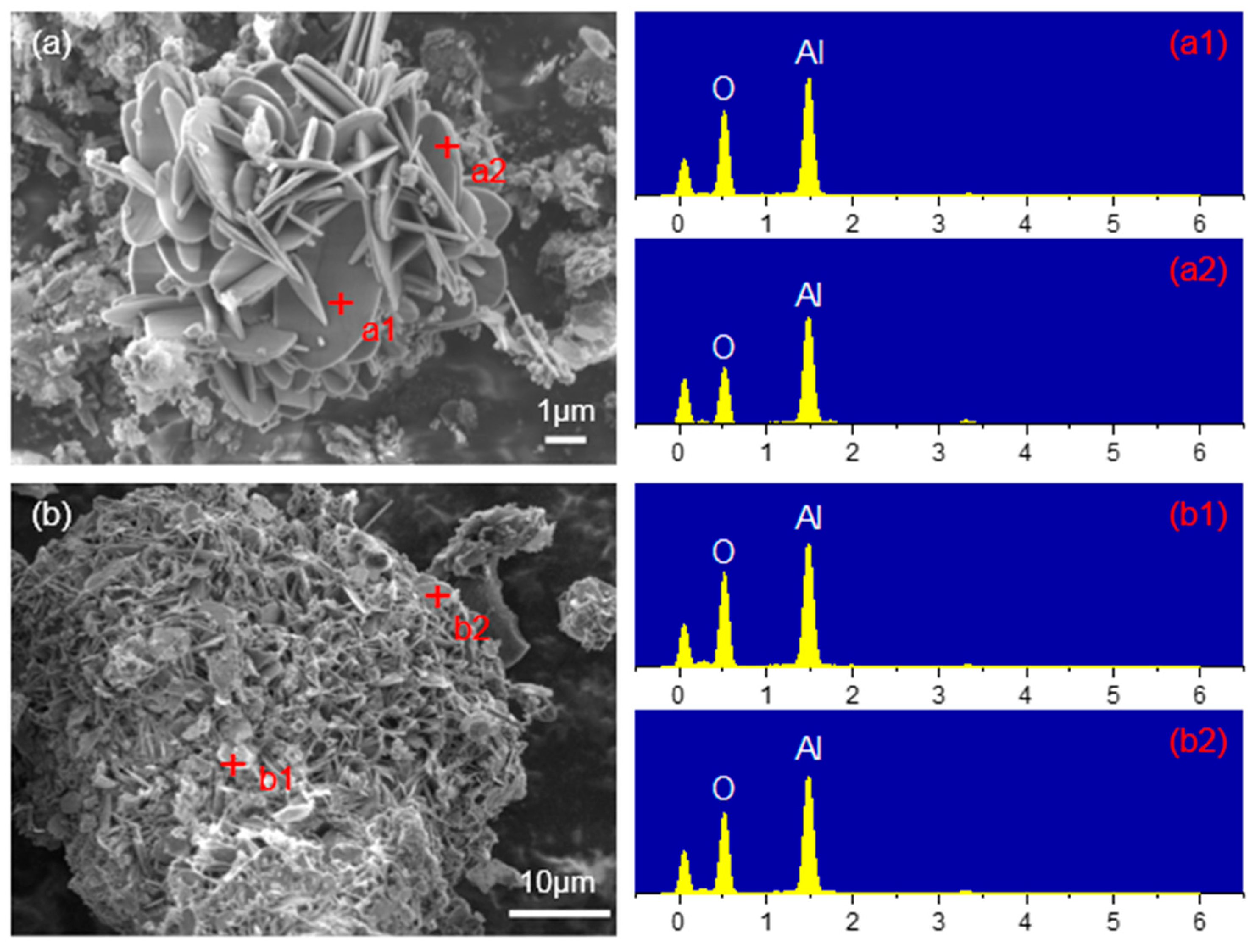
3.2.3. Analysis of the Mechanism of Al2O3 Promoting Gasification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krishnamoorthy, V.; Pisupati, S.V. A Critical Review of Mineral Matter Related Issues during Gasification of Coal in Fixed, Fluidized, and Entrained Flow Gasifiers. Energies 2015, 8, 10430–10463. [Google Scholar] [CrossRef]
- Liu, S.; Qi, C.; Jiang, Z.; Zhang, Y.; Niu, M.; Li, Y.; Dai, S.; Finkelman, R.B. Mineralogy and geochemistry of ash and slag from coal gasification in China: A review. Int. Geol. Rev. 2017, 60, 717–735. [Google Scholar] [CrossRef]
- Cao, X.; Kong, L.; Bai, J.; Ge, Z.; He, C.; Li, H.; Bai, Z.; Li, W. Effect of water vapor on coal ash slag viscosity under gasification condition. Fuel 2019, 237, 18–27. [Google Scholar] [CrossRef]
- Beér, J.M. Combustion technology developments in power generation in response to environmental challenges. Prog. Energy Combust. Sci. 2000, 26, 301–327. [Google Scholar] [CrossRef]
- Chiesa, P.; Consonni, S.; Kreutz, T.; Williams, R. Co-production of hydrogen, electricity and CO2 from coal with commercially readytechnology. PartA—Performance and emissions. Int. J. Hydrogen Energy 2005, 30, 747–767. [Google Scholar] [CrossRef]
- Savage, P.E.; Gopalan, S.; Mizan, T.I.; Martino, C.J.; Brock, E.E. Reactions at Supercritical Conditions: Applications and Fundamentals. AIChE J. 1995, 41, 1723–1778. [Google Scholar] [CrossRef]
- Kalinichev, A.G.; Heinzinger, K. Molecular dynamics of supercritical water—A computer simulation of vibrational spectra with the flexible BJH potential. Geochim. Cosmochim. Acta 1994, 59, 641–650. [Google Scholar] [CrossRef]
- Hoffmann, M.M.; Conradi, M.S. Are There Hydrogen Bonds in Supercritical Water? J. Am. Chem. Soc. 1997, 119, 3811–3817. [Google Scholar] [CrossRef]
- Guo, L.; Ou, Z.; Liu, Y.; Ge, Z.; Jin, H.; Ou, G.; Song, M.; Jiao, Z.; Jing, W. Technological innovations on direct carbon mitigation by ordered energy conversion and full resource utilization. Carbon. Neutrality 2022, 1, 4. [Google Scholar] [CrossRef]
- Khandelwal, K.; Nanda, S.; Boahene, P.; Dalai, A.K. Hydrogen production from supercritical water gasification of canola residues. Int. J. Hydrogen Energy 2024, 49, 1518–1527. [Google Scholar] [CrossRef]
- Ge, Z.; Jin, H.; Guo, L. Hydrogen production by catalytic gasification of coal in supercritical water with alkaline catalysts: Explore the way to complete gasification of coal. Int. J. Hydrogen Energy 2014, 39, 19583–19592. [Google Scholar] [CrossRef]
- Qu, X.; Zhou, X.; Yan, X.; Zhang, R.; Bi, J. Behavior of Alkaline-Metal Catalysts in Supercritical Water Gasification of Lignite. Chem. Eng. Technol. 2018, 41, 1682–1689. [Google Scholar] [CrossRef]
- Mu, R.; Liu, M.; Zhang, P.; Yan, J. System design and thermo-economic analysis of a new coal power generation system based on supercritical water gasification with full CO2 capture. Energy 2023, 285, 129384. [Google Scholar] [CrossRef]
- Wang, S.; Xie, R.; Liu, J.; Zhao, P.; Liu, H.; Wang, X. Numerical Analysis of the Temperature Characteristics of a Coal—Supercritical Water-Fluidized Bed Reactor for Hydrogen Production. Machines 2023, 11, 546. [Google Scholar] [CrossRef]
- Tian, Y.; Feng, H.; Zhang, Y.; Li, Q.; Liu, D. New insight into Allam cycle combined with coal gasification in supercritical water. Energy Convers. Manag. 2023, 292, 117432. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.; Han, W.; Xue, X.; Ma, W.; Jin, H. Efficient coal-based power generation via optimized supercritical water gasification with chemical recuperation. Appl. Therm. Eng. 2024, 238, 122164. [Google Scholar] [CrossRef]
- Mu, R.; Liu, M.; Yan, J. Advanced exergy analysis on supercritical water gasification of coal compared with conventional O2-H2O and chemical looping coal gasification. Fuel Process. Technol. 2023, 245, 107742. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, H.; Xie, X.; Wang, S.; Liu, J.; Wang, X.; Xie, R.; Zuo, S. Efficient Surrogate-Assisted Parameter Analysis for Coal-Supercritical Water Fluidized Bed Reactor with Adaptive Sampling. Machines 2023, 11, 295. [Google Scholar] [CrossRef]
- Ward, C.R. Analysis, origin and significance of mineral matter in coal: An updated review. Int. J. Coal Geol. 2016, 165, 1–27. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, S. Changes in char structure due to inorganic matters during anthracite pyrolysis. J. Anal. Appl. Pyrolysis 2017, 127, 170–175. [Google Scholar] [CrossRef]
- Song, Y.; Feng, W.; Li, N.; Li, Y.; Zhi, K.; Teng, Y.; He, R.; Zhou, H.; Liu, Q. Effects of demineralization on the structure and combustion properties of Shengli lignite. Fuel 2016, 183, 659–667. [Google Scholar] [CrossRef]
- Ma, Z.; Bai, J.; Bai, Z.; Kong, L.; Guo, Z.; Yan, J.; Li, W. Mineral Transformation in Char and Its Effect on Coal Char Gasification Reactivity at High Temperatures, Part 2: Char Gasification. Energy Fuels 2014, 28, 1846–1853. [Google Scholar] [CrossRef]
- Bai, J.; Li, W.; Li, C.-z.; Bai, Z.; Li, B. Influences of minerals transformation on the reactivity of high temperature char gasification. Fuel Process. Technol. 2010, 91, 404–409. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, X.; Gu, J.; Wu, Y.; Gao, J. Interactions between Carbon and Metal Oxides and Their Effects on the Carbon_CO2 Reactivity at High Temperatures. Energy Fuels 2007, 21, 1827–1831. [Google Scholar] [CrossRef]
- Wang, J.; Morishita, K.; Takarada, T. High-Temperature Interactions between Coal Char and Mixtures of calcium Oxide, Quartz, and Kaolinite. Energy Fuels 2001, 15, 1145–1152. [Google Scholar] [CrossRef]
- Kuznetsov, P.N.; Kuznetsova, L.I.; Mikhlin, Y.L. Chemical Composition of Surface Species in Pyrolyzed Brown Coals and Their Evolution during the Steam Gasification Reaction. Energy Fuels 2019, 33, 1892–1900. [Google Scholar] [CrossRef]
- Ban, Y.; Liu, Q.; Zhou, H.; Li, N.; Zhao, B.; Shi, S.; He, R.; Zhi, K. Catalytic effect of representative calcium salts on the steam gasification of a Shengli lignite. Fuel 2019, 255, 115832. [Google Scholar] [CrossRef]
- Cheng, Z.; Jin, H.; Liu, S.; Guo, L.; Xu, J.; Su, D. Hydrogen production by semicoke gasification with a supercritical water fluidized bed reactor. Int. J. Hydrogen Energy 2016, 41, 16055–16063. [Google Scholar] [CrossRef]
- Jin, H.; Chen, Y.; Ge, Z.; Liu, S.; Ren, C.; Guo, L. Hydrogen production by Zhundong coal gasification in supercritical water. Int. J. Hydrogen Energy 2015, 40, 16096–16103. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, L.; Liang, Q.; Xu, J.; Lin, K.; Liu, H. In situ experimental and modeling study on coal char combustion for coarse particle with effect of gasification in air (O2/N2) and O2/CO2 atmospheres. Fuel 2018, 233, 177–187. [Google Scholar] [CrossRef]
- Liu, Y.; Guan, Y.-J.; Zhang, K. Gasification reactivity and morphology of coal chars formed in N2 and CO2 atmospheres. Chem. Pap. 2018, 72, 2045–2054. [Google Scholar] [CrossRef]
- Du, Y.; Wang, C.A.; Xin, H.; Che, D.; Mathews, J.P. Competitive or additive behavior for H2O and CO2 gasification of coal char? Exploration via simplistic atomistic simulation. Carbon 2019, 141, 226–237. [Google Scholar] [CrossRef]
- GB/T30733-2014; Determination of Total Carbon, Hydrogen and Nitrogen Content in Coal-Instrumental Method. Standardization Administration of the P.R.C.: Beijing, China, 2014.
- GB/T30732-2014; Proximate Analysis of Coal-Instrumental Method. Standardization Administration of the P.R.C.: Beijing, China, 2014.
- GB/T37673-2019; Determination of Silicon, Aluminum, Iron, Calcium, Magnesium, Sodium, Potassium, Phosphorus, Titanium, Manganese, Strontium and Barium in Coal Ash—X-ray Fluorescence Spectrometric Method. Administration of the P.R.C.: Beijing, China, 2019.
- Liu, S.; Jin, H.; Wei, W.; Guo, L. Gasification of indole in supercritical water Nitrogen transformation mechanisms and kinetics. Int. J. Hydrogen Energy 2016, 41, 15985–15997. [Google Scholar] [CrossRef]
- Chase, M.W. NIST-JANAF thermochemical tables. J. Phys. Chem. Ref. Data 1998, 9, 703–736. [Google Scholar]
- Su, X.; Guo, L.; Jin, H. Mathematical Modeling for Coal Gasification Kinetics in Supercritical Water. Energy Fuels 2016, 30, 9028–9035. [Google Scholar] [CrossRef]
- Yu, G.; Yu, D.; Liu, F.; Yu, X.; Han, J.; Wu, J.; Xu, M. Different catalytic action of ion-exchanged calcium in steam and CO2 gasification and its effects on the evolution of char structure and reactivity. Fuel 2019, 254, 115609. [Google Scholar] [CrossRef]
- Jiang, M.-Q.; Zhou, R.; Hu, J.; Wang, F.-C.; Wang, J. Calcium-promoted catalytic activity of potassium carbonate for steam gasification of coal char: Influences of calcium species. Fuel 2012, 99, 64–71. [Google Scholar] [CrossRef]
- Arnold, R.A.; Hill, J.M. Effect of calcium and barium on potassium-catalyzed gasification of ash-free carbon black. Fuel 2019, 254, 115647. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Zhu, M.; Zhang, Z.; Zhou, B.; Shen, G.; Zhang, D. A phenomenological investigation into potassium migration and ash sintering characteristics during p.f. combustion of lignites with and without K2CO3 addition. Appl. Therm. Eng. 2019, 148, 64–77. [Google Scholar] [CrossRef]
- Li, J.; Zhu, M.; Zhang, Z.; Zhang, K.; Shen, G.; Zhang, D. The mineralogy, morphology and sintering characteristics of ash deposits on a probe at different temperatures during combustion of blends of Zhundong lignite and a bituminous coal in a drop tube furnace. Fuel Process. Technol. 2016, 149, 176–186. [Google Scholar] [CrossRef]
- Zhang, J.; Li, J.; Mao, Y.; Bi, J.; Zhu, M.; Zhang, Z.; Zhang, L.; Zhang, D. Effect of CaCO3 addition on ash sintering behaviour during K2CO3 catalysed steam gasification of a Chinese lignite. Appl. Therm. Eng. 2017, 111, 503–509. [Google Scholar] [CrossRef]



| Ultimate Analysis [wt%] | Proximate Analysis [wt%, Air-Dry Base] | |||||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O a | Moisture | Ash | Volatiles | Fixed carbon |
| 65.59 | 2.25 | 0.95 | 2.26 | 7.75 | 0.70 | 21.20 | 14.58 | 63.52 |
| Ash composition [wt%] | ||||||||
| CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | Na2O | MgO | TiO2 | Others |
| 32.77 | 22.12 | 22.09 | 11.96 | 6.57 | 1.31 | 0.84 | 0.50 | 1.84 |
| Case | K2CO3 [wt%] | SiO2 [wt%] | Al2O3 [wt%] | CaO [wt%] |
|---|---|---|---|---|
| 1 | 0 | 0 | 0 | 0 |
| 2 | 0 | 10 | 0 | 0 |
| 3 | 0 | 0 | 10 | 0 |
| 4 | 0 | 0 | 0 | 10 |
| 5 | 0 | 0 | 0 | 30 |
| 6 | 0 | 0 | 0 | 50 |
| 7 | 40 | 0 | 0 | 0 |
| 8 | 40 | 10 | 0 | 0 |
| 9 | 40 | 0 | 10 | 0 |
| 10 | 40 | 0 | 0 | 10 |
| 11 | 40 | 3.33 | 0 | 0 |
| 12 | 40 | 6.67 | 0 | 0 |
| 13 | 40 | 0 | 3.33 | 0 |
| 14 | 40 | 0 | 6.67 | 0 |
| 15 | 40 | 0 | 0 | 3.33 |
| 16 | 40 | 0 | 0 | 6.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, P.; Lv, Z.; Sun, C.; Jin, H.; He, L.; Ren, T.; Cheng, Z. Experimental Investigation of the Effects of Inorganic Components on the Supercritical Water Gasification of Semi-Coke. Energies 2024, 17, 1193. https://doi.org/10.3390/en17051193
Sun P, Lv Z, Sun C, Jin H, He L, Ren T, Cheng Z. Experimental Investigation of the Effects of Inorganic Components on the Supercritical Water Gasification of Semi-Coke. Energies. 2024; 17(5):1193. https://doi.org/10.3390/en17051193
Chicago/Turabian StyleSun, Panpan, Zhaobin Lv, Chuanjiang Sun, Hui Jin, Long He, Tong Ren, and Zening Cheng. 2024. "Experimental Investigation of the Effects of Inorganic Components on the Supercritical Water Gasification of Semi-Coke" Energies 17, no. 5: 1193. https://doi.org/10.3390/en17051193
APA StyleSun, P., Lv, Z., Sun, C., Jin, H., He, L., Ren, T., & Cheng, Z. (2024). Experimental Investigation of the Effects of Inorganic Components on the Supercritical Water Gasification of Semi-Coke. Energies, 17(5), 1193. https://doi.org/10.3390/en17051193