Carbon Capture and Storage in Depleted Oil and Gas Reservoirs: The Viewpoint of Wellbore Injectivity
Abstract
:1. Introduction
2. Factors Affecting the Injectivity of CO2
2.1. Innate Reservoir Properties
2.1.1. Permeability
2.1.2. Porosity
2.1.3. Pressure
2.1.4. Well Configuration
2.1.5. Heterogeneity Level of the Storage Medium
2.2. Capillary Trapping
2.3. Residual Oil/Condensate Saturation
2.4. Fluid Properties
2.4.1. Viscosity and Density of Injected CO2
2.4.2. Injected CO2 Purity
2.4.3. Injectant Temperature
| Reference | Study Remarks |
|---|---|
| Jin, Pekot, Smith, Salako, Peterson, Bosshart, Hamling, Mibeck, Hurley and Beddoe [37] | CO2 saturated Mead-Strawn stock-tank oil at 135° F showed that the density of oil increases when CO2 is dissolved in the oil [90]. The gas storage rate in the Bell Creek oil field is linked to the injection rate, decreasing as the injection stabilizes. |
| Kazemzadeh, et al. [91] | The minimum miscible pressure (MMP) is the ideal pressure for cost-effective injection in oil recovery. |
| Barrufet, Bacquet and Falcone [59] | The duration of a project on a gas condensate fluid from the Cusiana field located in the northeast of Bogota, Colombia, in the Lianos basin is determined by injectivity, injection rates, and the number of wells; injection rates do not affect the eventual storage capacity. |
| Tawiah, Duer, Bryant, Larter, O’Brien and Dong [18] | Injection rates in reservoir rocks near the wellbore are influenced by injection pressures, fluid saturation, and fluid mobility. |
2.5. Mineral Dissolution/Precipitation
2.6. Salt Precipitation
2.6.1. Effects of CO2 Flow Rate
2.6.2. Effects of Brine Salinity
2.6.3. Effects of Pore Size
2.6.4. Effects of Particle Size
2.6.5. Effects of Water Saturation
2.6.6. Effects of Temperature
2.7. Asphaltene Precipitation
2.7.1. Effects of Permeability
2.7.2. Effects of Pore Size Distribution
2.7.3. Effects of Temperature
2.7.4. Effects of CO2 Concentration
2.7.5. Effects of Porosity
2.7.6. Effects of Pressure
2.7.7. Effects of Viscosity
2.7.8. Effects of Flow Rate
2.8. Fine Mobilization
2.8.1. Effects of Permeability
2.8.2. Particle Concentration
2.8.3. Injection Rate
2.8.4. Particle Size
2.9. Clay Swelling
2.9.1. Effects of Pressure
2.9.2. Effects on Strain
2.9.3. Effects of Temperature
2.10. Hydrate Formation
3. CO2 Injectivity in Field Cases
3.1. Niagaran Pinnacle Reef Oil Field
3.2. Netherlands Fields
3.3. Malaysia
3.4. Goldeneye
3.5. Cranfield
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iddphonce, R.; Wang, J.; Zhao, L. Review of CO2 injection techniques for enhanced shale gas recovery: Prospect and challenges. J. Nat. Gas Sci. Eng. 2020, 77, 103240. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, J.; Rahman, S.S.; Zhou, N.; Suo, Y. Predicting permeability changes with injecting CO2 in coal seams during CO2 geological sequestration: A comparative study among six SVM-based hybrid models. Sci. Total Environ. 2020, 705, 135941. [Google Scholar] [CrossRef]
- Park, Y.-C.; Kim, S.; Lee, J.H.; Shinn, Y.J. Effect of reducing irreducible water saturation in a near-well region on CO2 injectivity and storage capacity. Int. J. Greenh. Gas Control 2019, 86, 134–145. [Google Scholar] [CrossRef]
- Hoteit, H.; Fahs, M.; Soltanian, M.R. Assessment of CO2 injectivity during sequestration in depleted gas reservoirs. Geosciences 2019, 9, 199. [Google Scholar] [CrossRef]
- Sokama-Neuyam, Y.A.; Ursin, J.R. Experimental and theoretical investigations of CO2 injectivity. AGH Drill. Oil Gas 2016, 33, 245–258. [Google Scholar] [CrossRef]
- Abba, M.K. Enhanced gas recovery by CO2 injection: Influence of monovalent and divalent brines and their concentrations on CO2 dispersion in porous media. J. Nat. Gas Sci. Eng. 2020, 84, 103643. [Google Scholar] [CrossRef]
- Ginting, P. Effect of Colloidal Transport on CO2 Injectivity; University of Stavanger: Stavanger, Norway, 2016. [Google Scholar]
- Wang, X.; Alvarado, V.; Swoboda-Colberg, N.; Kaszuba, J.P. Reactivity of dolomite in water-saturated supercritical carbon dioxide: Significance for carbon capture and storage and for enhanced oil and gas recovery. Energy Convers. Manag. 2013, 65, 564–573. [Google Scholar] [CrossRef]
- Torsæter, M.; Cerasi, P. Geological and geomechanical factors impacting loss of near-well permeability during CO2 injection. Int. J. Greenh. Gas Control 2018, 76, 193–199. [Google Scholar] [CrossRef]
- Peysson, Y.; André, L.; Azaroual, M. Well injectivity during CO2 storage operations in deep saline aquifers—Part 1: Experimental investigation of drying effects, salt precipitation and capillary forces. Int. J. Greenh. Gas Control 2014, 22, 291–300. [Google Scholar] [CrossRef]
- Abba, M.K.; Al-Otaibi, A.; Abbas, A.J.; Nasr, G.G.; Burby, M. Influence of permeability and injection orientation variations on dispersion coefficient during enhanced gas recovery by CO2 injection. Energies 2019, 12, 2328. [Google Scholar] [CrossRef]
- Raza, A.; Gholami, R.; Rezaee, R.; Rasouli, V.; Rabiei, M. Significant aspects of carbon capture and storage—A review. Petroleum 2019, 5, 335–340. [Google Scholar] [CrossRef]
- Al-Hasami, A.; Ren, S.; Tohidi, B. CO2 Injection for Enhanced Gas Recovery and Geo-Storage: Reservoir Simulation and Economics. In Proceedings of the SPE Europec/EAGE Annual Conference, Madrid, Spain, 13–16 June 2005. [Google Scholar]
- Kasahara, J.; Tsuruga, K. An Innovative Method for the 4D Monitor of Storage in CCS(Carbon Dioxide Capture and Storage) and Oil and Gas Reservoirs and Aqufers. In Proceedings of the 2nd Joint BCSR-JCCP Environmental Symposium, Bahrain, 8–10 February 2010. [Google Scholar]
- Valle, L.; Rodríguez, R.; Grima, C.; Martínez, C. Effects of supercritical CO2 injection on sandstone wettability and capillary trapping. Int. J. Greenh. Gas Control 2018, 78, 341–348. [Google Scholar] [CrossRef]
- Min, Y.; Kim, D.; Jun, Y.-S. Effects of Na+ and K+ exchange in interlayers on biotite dissolution under high-temperature and high-CO2-pressure conditions. Environ. Sci. Technol. 2018, 52, 13638–13646. [Google Scholar] [CrossRef]
- De Jong, S.; Spiers, C.; Busch, A. Development of swelling strain in smectite clays through exposure to carbon dioxide. Int. J. Greenh. Gas Control 2014, 24, 149–161. [Google Scholar] [CrossRef]
- Tawiah, P.; Duer, J.; Bryant, S.L.; Larter, S.; O’Brien, S.; Dong, M. CO2 injectivity behaviour under non-isothermal conditions—Field observations and assessments from the Quest CCS operation. Int. J. Greenh. Gas Control 2020, 92, 102843. [Google Scholar] [CrossRef]
- Jung, H.; Espinoza, D.N.; Hosseini, S.A. Wellbore injectivity response to step-rate CO2 injection: Coupled thermo-poro-elastic analysis in a vertically heterogeneous formation. Int. J. Greenh. Gas Control 2020, 102, 103156. [Google Scholar] [CrossRef]
- Ren, B.; Ren, S.; Zhang, L.; Chen, G.; Zhang, H. Monitoring on CO2 migration in a tight oil reservoir during CCS-EOR in Jilin Oilfield China. Energy 2016, 98, 108–121. [Google Scholar] [CrossRef]
- Roy, P.; Morris, J.P.; Walsh, S.D.; Iyer, J.; Carroll, S. Effect of thermal stress on wellbore integrity during CO2 injection. Int. J. Greenh. Gas Control 2018, 77, 14–26. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Q. CO2 breakthrough pressure and permeability for unsaturated low-permeability sandstone of the Ordos Basin. J. Hydrol. 2017, 550, 331–342. [Google Scholar] [CrossRef]
- Ramírez, A.; Hagedoorn, S.; Kramers, L.; Wildenborg, T.; Hendriks, C. Screening CO2 storage options in the Netherlands. Int. J. Greenh. Gas Control 2010, 4, 367–380. [Google Scholar] [CrossRef]
- Rohmer, J.; Pluymakers, A.; Renard, F. Mechano-chemical interactions in sedimentary rocks in the context of CO2 storage: Weak acid, weak effects? Earth Sci. Rev. 2016, 157, 86–110. [Google Scholar] [CrossRef]
- Mackay, E.J. 3—Modelling the Injectivity, Migration and Trapping of CO2 in Carbon Capture and Storage (CCS). In Geological Storage of Carbon Dioxide (CO2); Gluyas, J., Mathias, S., Eds.; Woodhead Publishing: Sawston, UK, 2013; pp. 45–70.e. [Google Scholar]
- Al-Khdheeawi, E.A.; Vialle, S.; Barifcani, A.; Sarmadivaleh, M.; Iglauer, S. Influence of injection well configuration and rock wettability on CO2 plume behaviour and CO2 trapping capacity in heterogeneous reservoirs. J. Nat. Gas Sci. Eng. 2017, 43, 190–206. [Google Scholar] [CrossRef]
- Raza, A.; Rezaee, R.; Gholami, R.; Rasouli, V.; Bing, C.H.; Nagarajan, R.; Hamid, M.A. Injectivity and quantification of capillary trapping for CO2 storage: A review of influencing parameters. J. Nat. Gas Sci. Eng. 2015, 26, 510–517. [Google Scholar] [CrossRef]
- Khurshid, I.; Choe, J. Analysis of asphaltene deposition, carbonate precipitation, and their cementation in depleted reservoirs during CO2 injection. Greenh. Gases Sci. Technol. 2015, 5, 657–667. [Google Scholar] [CrossRef]
- Kalra, S.; Wu, X. CO2 Injection for Enhanced Gas Recovery. In Proceedings of the SPE Western North American and Rocky Mountain Joint Meeting, Denver, CO, USA, 17–18 April 2014. [Google Scholar]
- Gao, S.; Wang, Y.; Jia, L.; Wang, H.; Yuan, J.; Wang, X. CO2–H2O–coal interaction of CO2 storage in coal beds. Int. J. Min. Sci. Technol. 2013, 23, 525–529. [Google Scholar] [CrossRef]
- Santibanez-Borda, E.; Govindan, R.; Elahi, N.; Korre, A.; Durucan, S. Maximising the dynamic CO2 storage capacity through the optimisation of CO2 injection and brine production rates. Int. J. Greenh. Gas Control 2019, 80, 76–95. [Google Scholar] [CrossRef]
- Borda, E.S.; Govindan, R.; Elahi, N.; Korre, A.; Durucan, S. The Development of a Dynamic CO2 Injection Strategy for the Depleted Forties and Nelson Oilfields Using Regression-based Multi-objective Programming. Energy Procedia 2017, 114, 3335–3342. [Google Scholar] [CrossRef]
- Raza, A.; Gholami, R.; Rezaee, R.; Bing, C.H.; Nagarajan, R.; Hamid, M.A. Well selection in depleted oil and gas fields for a safe CO2 storage practice: A case study from Malaysia. Petroleum 2017, 3, 167–177. [Google Scholar] [CrossRef]
- Soltanian, M.R.; Amooie, M.A.; Cole, D.R.; Graham, D.E.; Hosseini, S.A.; Hovorka, S.; Pfiffner, S.M.; Phelps, T.J.; Moortgat, J. Simulating the Cranfield geological carbon sequestration project with high-resolution static models and an accurate equation of state. Int. J. Greenh. Gas Control 2016, 54, 282–296. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, Z.; Park, H.; Shi, J.Q.; Kiyama, T.; Lei, X.; Sun, Y.; Liang, Y. Tracking CO2 plumes in clay-rich rock by distributed fiber optic strain sensing (DFOSS): A laboratory demonstration. Water Resour. Res. 2019, 55, 856–867. [Google Scholar] [CrossRef]
- Jeon, P.R.; Lee, C.-H. Effect of surfactants on CO2 solubility and reaction in CO2-brine-clay mineral systems during CO2-enhanced fossil fuel recovery. Chem. Eng. J. 2020, 382, 123014. [Google Scholar] [CrossRef]
- Jin, L.; Pekot, L.J.; Smith, S.A.; Salako, O.; Peterson, K.J.; Bosshart, N.W.; Hamling, J.A.; Mibeck, B.A.; Hurley, J.P.; Beddoe, C.J. Effects of gas relative permeability hysteresis and solubility on associated CO2 storage performance. Int. J. Greenh. Gas Control 2018, 75, 140–150. [Google Scholar] [CrossRef]
- Valbuena, E.; Barrufet, M. A generalized partial molar volume algorithm provides fast estimates of CO2 storage capacity in depleted oil and gas reservoirs. Fluid Phase Equilibria 2013, 359, 45–53. [Google Scholar] [CrossRef]
- Chauhan, D.S.; Quraishi, M.; Qurashi, A. Recent trends in environmentally sustainable Sweet corrosion inhibitors. J. Mol. Liq. 2021, 326, 115117. [Google Scholar] [CrossRef]
- Usman, B.J.; Ali, S.A. Carbon dioxide corrosion inhibitors: A review. Arab. J. Sci. Eng. 2018, 43, 1–22. [Google Scholar] [CrossRef]
- Perez, T.E. Corrosion in the oil and gas industry: An increasing challenge for materials. Jom 2013, 65, 1033–1042. [Google Scholar] [CrossRef]
- Nejad, A.M. A review of contributing parameters in corrosion of oil and gas wells. Anti Corros. Methods Mater. 2018, 65, 73–78. [Google Scholar] [CrossRef]
- Florez, J.J.A.; Ferrari, J.V. Fluid flow effects on CO2 corrosion: A review of applications of rotating cage methodology. Anti-Corros. Methods Mater. 2019, 66, 507–519. [Google Scholar] [CrossRef]
- Yang, H.-M. Role of organic and eco-friendly inhibitors on the corrosion mitigation of steel in acidic environments—A state-of-art review. Molecules 2021, 26, 3473. [Google Scholar] [CrossRef]
- Liu, Q.; Mao, L.; Zhou, S. Effects of chloride content on CO2 corrosion of carbon steel in simulated oil and gas well environments. Corros. Sci. 2014, 84, 165–171. [Google Scholar] [CrossRef]
- Askari, M.; Aliofkhazraei, M.; Jafari, R.; Hamghalam, P.; Hajizadeh, A. Downhole corrosion inhibitors for oil and gas production—A review. Appl. Surf. Sci. Adv. 2021, 6, 100128. [Google Scholar] [CrossRef]
- Chamkalani, A.; Nareh’ei, M.A.; Chamkalani, R.; Zargari, M.H.; Dehestani-Ardakani, M.R.; Farzam, M. Soft computing method for prediction of CO2 corrosion in flow lines based on neural network approach. Chem. Eng. Commun. 2013, 200, 731–747. [Google Scholar] [CrossRef]
- Hamza, A.; Hussein, I.A.; Al-Marri, M.J.; Mahmoud, M.; Shawabkeh, R.; Aparicio, S. CO2 enhanced gas recovery and sequestration in depleted gas reservoirs: A review. J. Pet. Sci. Eng. 2021, 196, 107685. [Google Scholar] [CrossRef]
- Cui, G.; Yang, L.; Fang, J.; Qiu, Z.; Wang, Y.; Ren, S. Geochemical reactions and their influence on petrophysical properties of ultra-low permeability oil reservoirs during water and CO2 flooding. J. Pet. Sci. Eng. 2021, 203, 108672. [Google Scholar] [CrossRef]
- Mat Razali, N.Z.; Mustapha, K.A.; Kashim, M.Z.; Misnan, M.S.; Md Shah, S.S.; Abu Bakar, Z.A. Critical rate analysis for CO2 injection in depleted gas field, Sarawak Basin, offshore East Malaysia. Carbon Manag. 2022, 13, 294–309. [Google Scholar] [CrossRef]
- Mahmoud, M.; Hussein, I.; Carchini, G.; Shawabkeh, R.; Eliebid, M.; Al-Marri, M.J. Effect of rock mineralogy on Hot-CO2 injection for enhanced gas recovery. J. Nat. Gas Sci. Eng. 2019, 72, 103030. [Google Scholar] [CrossRef]
- Lei, H.; Pingping, S.; Ying, J.; Jigen, Y.; Shi, L.; Aifang, B. Prediction of asphaltene precipitation during CO2 injection. Pet. Explor. Dev. 2010, 37, 349–353. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, H.; Yan, Y.; Liu, X. Adsorption mechanism of CO2/CH4 in kaolinite clay: Insight from molecular simulation. Energy Fuels 2019, 33, 6542–6551. [Google Scholar] [CrossRef]
- Li, X.; Chi, P.; Guo, X.; Sun, Q. CO2-induced asphaltene deposition and wettability alteration on a pore interior surface. Fuel 2019, 254, 115595. [Google Scholar] [CrossRef]
- Fakher, S.; Imqam, A. Asphaltene precipitation and deposition during CO2 injection in nano shale pore structure and its impact on oil recovery. Fuel 2019, 237, 1029–1039. [Google Scholar] [CrossRef]
- Zanganeh, P.; Dashti, H.; Ayatollahi, S. Visual investigation and modeling of asphaltene precipitation and deposition during CO2 miscible injection into oil reservoirs. Fuel 2015, 160, 132–139. [Google Scholar] [CrossRef]
- Baban, A.; Hosseini, M.; Keshavarz, A.; Ali, M.; Hoteit, H.; Amin, R.; Iglauer, S. Robust NMR Examination of the Three-Phase Flow Dynamics of Carbon Geosequestration Combined with Enhanced Oil Recovery in Carbonate Formations. Energy Fuels 2024, 38, 2167–2176. [Google Scholar] [CrossRef]
- Hamza, A.; Hussein, I.A.; Al-Marri, M.J.; Mahmoud, M.; Shawabkeh, R. Impact of clays on CO2 adsorption and enhanced gas recovery in sandstone reservoirs. Int. J. Greenh. Gas Control 2021, 106, 103286. [Google Scholar] [CrossRef]
- Barrufet, M.A.; Bacquet, A.; Falcone, G. Analysis of the storage capacity for CO2 sequestration of a depleted gas condensate reservoir and a saline aquifer. J. Can. Pet. Technol. 2010, 49, 23–31. [Google Scholar] [CrossRef]
- Sokama-Neuyam, Y.A.; Ginting, P.U.R.; Timilsina, B.; Ursin, J.R. The impact of fines mobilization on CO2 injectivity: An experimental study. Int. J. Greenh. Gas Control 2017, 65, 195–202. [Google Scholar] [CrossRef]
- Jeddizahed, J.; Rostami, B. Experimental investigation of injectivity alteration due to salt precipitation during CO2 sequestration in saline aquifers. Adv. Water Resour. 2016, 96, 23–33. [Google Scholar] [CrossRef]
- Li, Z.; Dong, M.; Li, S.; Huang, S. CO2 sequestration in depleted oil and gas reservoirs—Caprock characterization and storage capacity. Energy Convers. Manag. 2006, 47, 1372–1382. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, R.; Kang, X. Modeling the effect of water vaporization and salt precipitation on reservoir properties due to carbon dioxide sequestration in a depleted gas reservoir. Petroleum 2018, 4, 385–397. [Google Scholar] [CrossRef]
- Hosseini, S.A.; Lashgari, H.; Choi, J.W.; Nicot, J.-P.; Lu, J.; Hovorka, S.D. Static and dynamic reservoir modeling for geological CO2 sequestration at Cranfield, Mississippi, USA. Int. J. Greenh. Gas Control 2013, 18, 449–462. [Google Scholar] [CrossRef]
- Goudarzi, A.; Meckel, T.A.; Hosseini, S.A.; Treviño, R.H. Statistical analysis of historic hydrocarbon production data from Gulf of Mexico oil and gas fields and application to dynamic capacity assessment in CO2 storage. Int. J. Greenh. Gas Control 2019, 80, 96–102. [Google Scholar] [CrossRef]
- Ambrose, W.; Lakshminarasimhan, S.; Holtz, M.; Núñez-López, V.; Hovorka, S.D.; Duncan, I. Geologic factors controlling CO2 storage capacity and permanence: Case studies based on experience with heterogeneity in oil and gas reservoirs applied to CO2 storage. Environ. Geol. 2008, 54, 1619. [Google Scholar] [CrossRef]
- Raza, A.; Rezaee, R.; Gholami, R.; Bing, C.H.; Nagarajan, R.; Hamid, M.A. A screening criterion for selection of suitable CO2 storage sites. J. Nat. Gas Sci. Eng. 2016, 28, 317–327. [Google Scholar] [CrossRef]
- Shirbazo, A.; Taghavinejad, A.; Bagheri, S. CO2 Capture and Storage Performance Simulation in Depleted Shale Gas Reservoirs as Sustainable Carbon Resources. J. Constr. Mater. 2021. [Google Scholar] [CrossRef]
- Gan, M.; Nguyen, M.C.; Zhang, L.; Wei, N.; Li, J.; Lei, H.; Wang, Y.; Li, X.; Stauffer, P.H. Impact of reservoir parameters and wellbore permeability uncertainties on CO2 and brine leakage potential at the Shenhua CO2 Storage Site, China. Int. J. Greenh. Gas Control 2021, 111, 103443. [Google Scholar] [CrossRef]
- Akono, A.T.; Druhan, J.L.; Dávila, G.; Tsotsis, T.; Jessen, K.; Fuchs, S.; Crandall, D.; Shi, Z.; Dalton, L.; Tkach, M.K. A review of geochemical-mechanical impacts in geological carbon storage reservoirs. Greenh. Gases Sci. Technol. 2019, 9, 474–504. [Google Scholar] [CrossRef]
- Yu, W.; Lashgari, H.R.; Wu, K.; Sepehrnoori, K. CO2 injection for enhanced oil recovery in Bakken tight oil reservoirs. Fuel 2015, 159, 354–363. [Google Scholar] [CrossRef]
- Kelley, M.; Abbaszadeh, M.; Mishra, S.; Mawalkar, S.; Place, M.; Gupta, N.; Pardini, R. Reservoir characterization from pressure monitoring during CO2 injection into a depleted pinnacle reef-MRCSP commercial-scale CCS demonstration project. Energy Procedia 2014, 63, 4937–4964. [Google Scholar] [CrossRef]
- Islam, M.R.; Chakma, A. Storage and utilization of CO2 in petroleum reservoirs—A simulation study. Energy Convers. Manag. 1993, 34, 1205–1212. [Google Scholar] [CrossRef]
- Streit, J.E.; Hillis, R.R. Building Geomechanical Models for the Safe Underground Storage of Carbon Dioxide in Porous Rock. In Proceedings of the Greenhouse Gas Control Technologies—6th International Conference, Kyoto, Japan, 1–4 October 2002; pp. 495–500. [Google Scholar]
- Recasens, M.; Garcia, S.; Mackay, E.; Delgado, J.; Maroto-Valer, M.M. Experimental study of wellbore integrity for CO2 geological storage. Energy Procedia 2017, 114, 5249–5255. [Google Scholar] [CrossRef]
- Kaldi, J.; Daniel, R.; Tenthorey, E.; Michael, K.; Schacht, U.; Nicol, A.; Underschultz, J.; Backe, G. Containment of CO2 in CCS: Role of Caprocks and Faults. Energy Procedia 2013, 37, 5403–5410. [Google Scholar] [CrossRef]
- Raza, A.; Gholami, R.; Rezaee, R.; Rasouli, V.; Bhatti, A.A.; Bing, C.H. Suitability of depleted gas reservoirs for geological CO2 storage: A simulation study. Greenh. Gases Sci. Technol. 2018, 8, 876–897. [Google Scholar] [CrossRef]
- Karine, S.; Gérald, H.; Joël, B.; Vincent, T. SCA2003-14: Residual Gas Saturation of Sample Originally at Residual Water Saturation in Heterogeneous Sandstone Reservoirs. Available online: https://www.jgmaas.com/SCA/2003/SCA2003-14.pdf (accessed on 21 September 2003).
- Raza, A.; Gholami, R.; Rezaee, R.; Bing, C.H.; Nagarajan, R.; Hamid, M.A. CO2 storage in depleted gas reservoirs: A study on the effect of residual gas saturation. Petroleum 2018, 4, 95–107. [Google Scholar] [CrossRef]
- Saeedi, A.; Rezaee, R. Effect of residual natural gas saturation on multiphase flow behaviour during CO2 geo-sequestration in depleted natural gas reservoirs. J. Pet. Sci. Eng. 2012, 82, 17–26. [Google Scholar] [CrossRef]
- Cao, M.; Gu, Y. Oil recovery mechanisms and asphaltene precipitation phenomenon in immiscible and miscible CO2 flooding processes. Fuel 2013, 109, 157–166. [Google Scholar] [CrossRef]
- Tan, Y.; Nookuea, W.; Li, H.; Thorin, E.; Yan, J. Property impacts on Carbon Capture and Storage (CCS) processes: A review. Energy Convers. Manag. 2016, 118, 204–222. [Google Scholar] [CrossRef]
- Nicot, J.-P.; Solano, S.; Lu, J.; Mickler, P.; Romanak, K.; Yang, C.; Zhang, X. Potential Subsurface Impacts of CO2 Stream Impurities on Geologic Carbon Storage. Energy Procedia 2013, 37, 4552–4559. [Google Scholar] [CrossRef]
- Rother, G.; Ilton, E.S.; Wallacher, D.; Hauβ, T.; Schaef, H.T.; Qafoku, O.; Rosso, K.M.; Felmy, A.R.; Krukowski, E.G.; Stack, A.G. CO2 sorption to subsingle hydration layer montmorillonite clay studied by excess sorption and neutron diffraction measurements. Environ. Sci. Technol. 2013, 47, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, C.M.; Benson, S.M. CO2 Injection for Enhanced Gas Production and Carbon Sequestration. In Proceedings of the SPE International Petroleum Conference and Exhibition in Mexico, Villahermosa, Mexico, 10–12 February 2002. [Google Scholar]
- Vu, H.P.; Black, J.R.; Haese, R.R. The geochemical effects of O2 and SO2 as CO2 impurities on fluid-rock reactions in a CO2 storage reservoir. Int. J. Greenh. Gas Control 2018, 68, 86–98. [Google Scholar] [CrossRef]
- Wang, J.; Ryan, D.; Anthony, E.J.; Wildgust, N.; Aiken, T. Effects of impurities on CO2 transport, injection and storage. Energy Procedia 2011, 4, 3071–3078. [Google Scholar] [CrossRef]
- Ziabakhsh-Ganji, Z.; Kooi, H. Sensitivity of Joule-Thomson cooling to impure CO2 injection in depleted gas reservoirs. Appl. Energy 2014, 113, 434–451. [Google Scholar] [CrossRef]
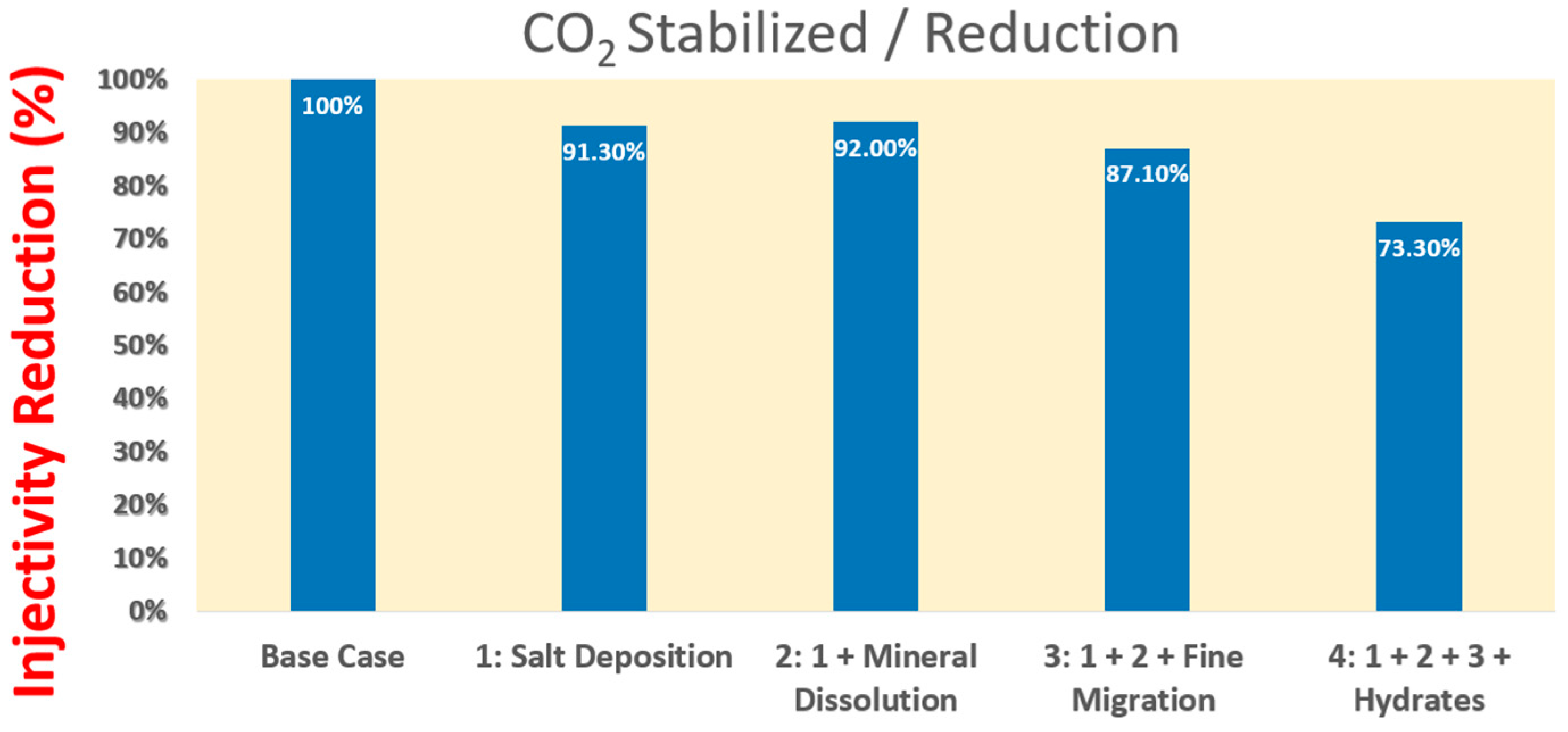
- Machado, M.V.B.; Delshad, M.; Sepehrnoori, K. Injectivity assessment for CCS field-scale projects with considerations of salt deposition, mineral dissolution, fines migration, hydrate formation, and non-Darcy flow. Fuel 2023, 353, 129148. [Google Scholar] [CrossRef]
- Holm, L.; Josendal, V. Mechanisms of oil displacement by carbon dioxide. J. Pet. Technol. 1974, 26, 1427–1438. [Google Scholar] [CrossRef]
- Kazemzadeh, Y.; Parsaei, R.; Riazi, M. Experimental study of asphaltene precipitation prediction during gas injection to oil reservoirs by interfacial tension measurement. Colloids Surf. A Physicochem. Eng. Asp. 2015, 466, 138–146. [Google Scholar] [CrossRef]
- Gaus, I.; Audigane, P.; André, L.; Lions, J.; Jacquemet, N.; Durst, P.; Czernichowski-Lauriol, I.; Azaroual, M. Geochemical and solute transport modelling for CO2 storage, what to expect from it? Int. J. Greenh. Gas Control 2008, 2, 605–625. [Google Scholar] [CrossRef]
- Bacci, G.; Korre, A.; Durucan, S. An experimental and numerical investigation into the impact of dissolution/precipitation mechanisms on CO2 injectivity in the wellbore and far field regions. Int. J. Greenh. Gas Control 2011, 5, 579–588. [Google Scholar] [CrossRef]
- Pudlo, D.; Henkel, S.; Reitenbach, V.; Albrecht, D.; Enzmann, F.; Heister, K.; Pronk, G.; Ganzer, L.; Gaupp, R. The chemical dissolution and physical migration of minerals induced during CO2 laboratory experiments: Their relevance for reservoir quality. Environ. Earth Sci. 2015, 73, 7029–7042. [Google Scholar] [CrossRef]
- Belgodere, C.; Sterpenich, J.; Pironon, J.; Randi, A.; Birat, J.-P. Experimental Study of CO2 Injection in the Triassic Sandstones of Lorraine (Eastern France)–Investigation of Injection Well Injectivity Impairment by Mineral Precipitations. In Proceedings of the Le Studium Conference. Geochemical Reactivity in CO2 Geological Storage Sites, Orleans, France, 25–26 February 2014; pp. 25–26. [Google Scholar]
- Pudlo, D.; Henkel, S.; Enzmann, F.; Heister, K.; Werner, L.; Ganzer, L.; Reitenbach, V.; Albrecht, D.; Gaupp, R. The Relevance of Mineral Mobilization and Dissolution on the Reservoir Quality of Sandstones in CO2 Storage Sites. Energy Procedia 2014, 59, 390–396. [Google Scholar] [CrossRef]
- Wellman, T.P.; Grigg, R.B.; McPherson, B.J.; Svec, R.K.; Lichtner, P.C. Evaluation of CO2-Brine-Reservoir Rock Interaction with Laboratory Flow Tests and Reactive Transport Modeling. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 5–7 February 2003. [Google Scholar]
- Al-Yaseri, A.; Zhang, Y.; Ghasemiziarani, M.; Sarmadivaleh, M.; Lebedev, M.; Roshan, H.; Iglauer, S. Permeability Evolution in Sandstone Due to CO2 Injection. Energy Fuels 2017, 31, 12390–12398. [Google Scholar] [CrossRef]
- Sokama-Neuyam, Y.A.; Ursin, J.R. CO2 Well Injectivity: Effect of Viscous Forces on Precipitated Minerals. In Proceedings of the International Petroleum Technology Conference, Doha, Qatar, 6–9 December 2015. [Google Scholar]
- Miri, R.; Hellevang, H. Salt precipitation during CO2 storage—A review. Int. J. Greenh. Gas Control 2016, 51, 136–147. [Google Scholar] [CrossRef]
- Giorgis, T.; Carpita, M.; Battistelli, A. 2D modeling of salt precipitation during the injection of dry CO2 in a depleted gas reservoir. Energy Convers. Manag. 2007, 48, 1816–1826. [Google Scholar] [CrossRef]
- Yusof, M.A.M.; Mohamed, M.A.; Akhir, N.A.M.; Ibrahim, M.A.; Mardhatillah, M.K. Combined Impact of Salt Precipitation and Fines Migration on CO2 Injectivity Impairment. Int. J. Greenh. Gas Control 2021, 110, 103422. [Google Scholar] [CrossRef]
- Bacci, G.; Korre, A.; Durucan, S. Experimental investigation into salt precipitation during CO2 injection in saline aquifers. Energy Procedia 2011, 4, 4450–4456. [Google Scholar] [CrossRef]
- Ashoori, S.; Balavi, A. An investigation of asphaltene precipitation during natural production and the CO2 injection process. Pet. Sci. Technol. 2014, 32, 1283–1290. [Google Scholar] [CrossRef]
- Hemmati-Sarapardeh, A.; Ahmadi, M.; Ameli, F.; Dabir, B.; Mohammadi, A.H.; Husein, M.M. Modeling asphaltene precipitation during natural depletion of reservoirs and evaluating screening criteria for stability of crude oils. J. Pet. Sci. Eng. 2019, 181, 106127. [Google Scholar] [CrossRef]
- Cruz, A.A.; Amaral, M.; Santos, D.; Palma, A.; Franceschi, E.; Borges, G.R.; Coutinho, J.A.P.; Palácio, J.; Dariva, C. CO2 influence on asphaltene precipitation. J. Supercrit. Fluids 2019, 143, 24–31. [Google Scholar] [CrossRef]
- Kalantari-Dahaghi, A.; Gholami, V.; Moghadasi, J.; Abdi, R. Formation damage through asphaltene precipitation resulting from CO2 gas injection in Iranian carbonate reservoirs. SPE Prod. Oper. 2008, 23, 210–214. [Google Scholar] [CrossRef]
- Shedid, S.A. Influences of asphaltene precipitation on capillary pressure and pore size distribution of carbonate reservoirs. Pet. Sci. Technol. 2001, 19, 503–519. [Google Scholar] [CrossRef]
- Nasrabadi, H.; Moortgat, J.; Firoozabadi, A. New three-phase multicomponent compositional model for asphaltene precipitation during CO2 injection using CPA-EOS. Energy Fuels 2016, 30, 3306–3319. [Google Scholar] [CrossRef]
- Mohammed, S.; Gadikota, G. The influence of CO2 on the structure of confined asphaltenes in calcite nanopores. Fuel 2019, 236, 769–777. [Google Scholar] [CrossRef]
- Papadimitriou, N.; Romanos, G.; Charalambopoulou, G.C.; Kainourgiakis, M.; Katsaros, F.; Stubos, A. Experimental investigation of asphaltene deposition mechanism during oil flow in core samples. J. Pet. Sci. Eng. 2007, 57, 281–293. [Google Scholar] [CrossRef]
- Wang, C.; Li, T.; Gao, H.; Zhao, J.; Gao, Y. Quantitative study on the blockage degree of pores due to asphaltene precipitation in low-permeability reservoirs with NMR technique. J. Pet. Sci. Eng. 2018, 163, 703–711. [Google Scholar] [CrossRef]
- Shedid, S.A.; Zekri, A.Y. Formation damage caused by simultaneous sulfur and asphaltene deposition. SPE Prod. Oper. 2006, 21, 58–64. [Google Scholar] [CrossRef]
- Saeedi Dehaghani, A.; Shadman, M.; Ahmadi, S.; Assaf, M. Modeling the onset point of asphaltene precipitation using the solubility parameter in CO2 injection. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 45, 1–7. [Google Scholar]
- Srivastava, R.; Huang, S.; Dong, M. Asphaltene deposition during CO2 flooding. SPE Prod. Facil. 1999, 14, 235–245. [Google Scholar] [CrossRef]
- Nielsen, B.B.; Svrcek, W.Y.; Mehrotra, A.K. Effects of Temperature and Pressure on Asphaltene Particle Size Distributions in Crude Oils Diluted with n-Pentane. Ind. Eng. Chem. Res. 1994, 33, 1324–1330. [Google Scholar] [CrossRef]
- Jafari Behbahani, T.; Ghotbi, C.; Taghikhani, V.; Shahrabadi, A. Investigation on Asphaltene Deposition Mechanisms during CO2 Flooding Processes in Porous Media: A Novel Experimental Study and a Modified Model Based on Multilayer Theory for Asphaltene Adsorption. Energy Fuels 2012, 26, 5080–5091. [Google Scholar] [CrossRef]
- Rosenbrand, E.; Kjøller, C.; Riis, J.F.; Kets, F.; Fabricius, I.L. Different effects of temperature and salinity on permeability reduction by fines migration in Berea sandstone. Geothermics 2015, 53, 225–235. [Google Scholar] [CrossRef]
- Cihan, A.; Petrusak, R.; Bhuvankar, P.; Alumbaugh, D.; Trautz, R.; Birkholzer, J.T. Permeability Decline by Clay Fines Migration around a Low-Salinity Fluid Injection Well. Groundwater 2022, 60, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Sbai, M.A.; Azaroual, M. Numerical modeling of formation damage by two-phase particulate transport processes during CO2 injection in deep heterogeneous porous media. Adv. Water Resour. 2011, 34, 62–82. [Google Scholar] [CrossRef]
- Xie, Q.; Saeedi, A.; Delle Piane, C.; Esteban, L.; Brady, P.V. Fines migration during CO2 injection: Experimental results interpreted using surface forces. Int. J. Greenh. Gas Control 2017, 65, 32–39. [Google Scholar] [CrossRef]
- Takahashi, S.; Kovscek, A.R. Wettability estimation of low-permeability, siliceous shale using surface forces. J. Pet. Sci. Eng. 2010, 75, 33–43. [Google Scholar] [CrossRef]
- Wang, Y.; Bedrikovetsky, P.; Yin, H.; Othman, F.; Zeinijahromi, A.; Le-Hussain, F. Analytical model for fines migration due to mineral dissolution during CO2 injection. J. Nat. Gas Sci. Eng. 2022, 100, 104472. [Google Scholar] [CrossRef]
- Zhang, M.; de Jong, S.; Spiers, C.J.; Busch, A.; Wentinck, H.M. Swelling stress development in confined smectite clays through exposure to CO2. Int. J. Greenh. Gas Control 2018, 74, 49–61. [Google Scholar] [CrossRef]
- Giesting, P.; Guggenheim, S.; Van Groos, A.F.K.; Busch, A. Interaction of carbon dioxide with Na-exchanged montmorillonite at pressures to 640 bars: Implications for CO2 sequestration. Int. J. Greenh. Gas Control 2012, 8, 73–81. [Google Scholar] [CrossRef]
- Narayanan Nair, A.K.; Cui, R.; Sun, S. Overview of the Adsorption and Transport Properties of Water, Ions, Carbon Dioxide, and Methane in Swelling Clays. ACS Earth Space Chem. 2021, 5, 2599–2611. [Google Scholar] [CrossRef]
- Pham, H.; Nguyen, Q.P. Effect of silica nanoparticles on clay swelling and aqueous stability of nanoparticle dispersions. J. Nanoparticle Res. 2014, 16, 1–11. [Google Scholar] [CrossRef]
- Bibi, I.; Icenhower, J.; Niazi, N.K.; Naz, T.; Shahid, M.; Bashir, S. Clay minerals: Structure, chemistry, and significance in contaminated environments and geological CO2 sequestration. Environ. Mater. Waste 2016, 543–567. [Google Scholar] [CrossRef]
- Anderson, R.; Ratcliffe, I.; Greenwell, H.; Williams, P.; Cliffe, S.; Coveney, P. Clay swelling—A challenge in the oilfield. Earth-Sci. Rev. 2010, 98, 201–216. [Google Scholar] [CrossRef]
- Loganathan, N.; Bowers, G.M.; Yazaydin, A.O.; Schaef, H.T.; Loring, J.S.; Kalinichev, A.G.; Kirkpatrick, R.J. Clay swelling in dry supercritical carbon dioxide: Effects of interlayer cations on the structure, dynamics, and energetics of CO2 intercalation probed by XRD, NMR, and GCMD simulations. J. Phys. Chem. C 2018, 122, 4391–4402. [Google Scholar] [CrossRef]
- Fatah, A.; Mahmud, H.B.; Bennour, Z.; Hossain, M.; Gholami, R. Effect of supercritical CO2 treatment on physical properties and functional groups of shales. Fuel 2021, 303, 121310. [Google Scholar] [CrossRef]
- Favero, V.; Laloui, L. Impact of CO2 injection on the hydro-mechanical behaviour of a clay-rich caprock. Int. J. Greenh. Gas Control 2018, 71, 133–141. [Google Scholar] [CrossRef]
- Pang, J.; Liang, Y.; Masuda, Y.; Matsuoka, T.; Zhang, Y.; Xue, Z. Swelling phenomena of the nonswelling Clay induced by CO2 and Water cooperative adsorption in janus-surface micropores. Environ. Sci. Technol. 2020, 54, 5767–5773. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Misra, S. A review of experimental research on Enhanced Coal Bed Methane (ECBM) recovery via CO2 sequestration. Earth Sci. Rev. 2018, 179, 392–410. [Google Scholar] [CrossRef]
- Miller, J.; Sullivan, C.; Larsen, G.; Kelley, M.; Rike, W.; Gerst, J.; Gupta, N.; Paul, D.; Pardini, R.; Modroo, A. Alternative conceptual geologic models for CO2 injection in a Niagaran pinnacle reef oil field, Northern Michigan, USA. Energy Procedia 2014, 63, 3685–3701. [Google Scholar] [CrossRef]
- Ganesh, P.R.; Mishra, S.; Mawalkar, S.; Gupta, N.; Pardini, R. Assessment of CO2 injectivity and storage capacity in a depleted pinnacle reef oil field in northern Michigan. Energy Procedia 2014, 63, 2969–2976. [Google Scholar] [CrossRef]
- Barnes, D.; Harrison, B.; Grammer, G.M.; Asmus, J. CO2/EOR and geological carbon storage resource potential in the Niagaran Pinnacle Reef Trend, lower Michigan, USA. Energy Procedia 2013, 37, 6786–6799. [Google Scholar] [CrossRef]
- Mishra, S.; Haagsma, A.; Valluri, M.; Gupta, N. Assessment of CO2-enhanced oil recovery and associated geologic storage potential in the Michigan Northern Pinnacle Reef Trend. Greenh. Gases Sci. Technol. 2020, 10, 32–49. [Google Scholar] [CrossRef]
- Orlic, B. Geomechanical effects of CO2 storage in depleted gas reservoirs in the Netherlands: Inferences from feasibility studies and comparison with aquifer storage. J. Rock Mech. Geotech. Eng. 2016, 8, 846–859. [Google Scholar] [CrossRef]
- Gupta, N.; Kelley, M.; Place, M.; Conner, A.; Mawalkar, S.; Mishra, S.; Sminchak, J. Integrated Monitoring Volume: A Summary of Monitoring Studies Conducted in Niagaran Carbonate Pinnacle Reefs During Enhanced Oil Recovery with CO2; Battelle Memorial Inst.: Columbus, OH, USA, 2020. [Google Scholar]
- Sazali, Y.; Sazali, W.; Ibrahim, J.; Dindi, M.; Graham, G.; Gödeke, S. Investigation of high temperature, high pressure, scaling and dissolution effects for carbon capture and storage at a high CO2 content carbonate gas field offshore Malaysia. J. Pet. Sci. Eng. 2019, 174, 599–606. [Google Scholar] [CrossRef]
- Brownsort, P.; Scott, V.; Sim, G.; Haszeldine, S. Carbon Dioxide Transport Plans for Carbon Capture and Storage in the North Sea Region. Available online: https://era.ed.ac.uk/bitstream/handle/1842/16481/wp-2015-02.pdf?sequence=1&isAllowed=y (accessed on 20 July 2015).
- Dale, A.W.; Sommer, S.; Lichtschlag, A.; Koopmans, D.; Haeckel, M.; Kossel, E.; Deusner, C.; Linke, P.; Scholten, J.; Wallmann, K. Defining a biogeochemical baseline for sediments at Carbon Capture and Storage (CCS) sites: An example from the North Sea (Goldeneye). Int. J. Greenh. Gas Control 2021, 106, 103265. [Google Scholar] [CrossRef]
- Esposito, M.; Martinez-Cabanas, M.; Connelly, D.P.; Jasinski, D.; Linke, P.; Schmidt, M.; Achterberg, E.P. Water column baseline assessment for offshore Carbon Dioxide Capture and Storage (CCS) sites: Analysis of field data from the Goldeneye storage complex area. Int. J. Greenh. Gas Control 2021, 109, 103344. [Google Scholar] [CrossRef]
- Tucker, O.; Holley, M.; Metcalfe, R.; Hurst, S. Containment risk management for CO2 storage in a depleted gas field, UK North Sea. Energy Procedia 2013, 37, 4804–4817. [Google Scholar] [CrossRef]
- Hannis, S.; Lu, J.; Chadwick, A.; Hovorka, S.; Kirk, K.; Romanak, K.; Pearce, J. CO2 storage in depleted or depleting oil and gas fields: What can we learn from existing projects? Energy Procedia 2017, 114, 5680–5690. [Google Scholar] [CrossRef]
- Núñez-López, V.; Gil-Egui, R.; Hosseini, S.A. Environmental and operational performance of CO2-EOR as a CCUS technology: A Cranfield example with dynamic LCA considerations. Energies 2019, 12, 448. [Google Scholar] [CrossRef]
- Choi, J.-W.; Nicot, J.-P.; Hosseini, S.A.; Clift, S.J.; Hovorka, S.D. CO2 recycling accounting and EOR operation scheduling to assist in storage capacity assessment at a US gulf coast depleted reservoir. Int. J. Greenh. Gas Control 2013, 18, 474–484. [Google Scholar] [CrossRef]
- Kim, S.; Hosseini, S.A. Above-zone pressure monitoring and geomechanical analyses for a field-scale CO2 injection project in Cranfield, MS. Greenh. Gases Sci. Technol. 2014, 4, 81–98. [Google Scholar] [CrossRef]

| Storage Option | Relative Capacity | Relative Cost | Storage Integrity | Technical Feasibility |
|---|---|---|---|---|
| Active Oil Well (EOR) | Small | Very Low | Good | High |
| Coal Beds | Unknown | Low | Unknown | Unknown |
| Depleted oil/gas wells | Moderate | Low | Good | High |
| Deep Aquifers | Large | Unknown | Unknown | Unknown |
| Mined caverns/salt domes | Large | Very High | Good | High |
| Formation | Hydrocarbon Field | |
|---|---|---|
| Lower Cretaceous Group | Vlieland Sandstone Fm | 1 |
| Lower Germanic Trias Group | Lower Buntsandstein Fm | 0.4 |
| Niedersachsen Group | Friese front Fm (sandstone members) | 0.4 |
| Upper Rotliegend Group | Zechstein Fm (carbonate members) Slochteren Fm (sandstone members) | 0.2 1 |
| Limburg Group | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heidarabad, R.G.; Shin, K. Carbon Capture and Storage in Depleted Oil and Gas Reservoirs: The Viewpoint of Wellbore Injectivity. Energies 2024, 17, 1201. https://doi.org/10.3390/en17051201
Heidarabad RG, Shin K. Carbon Capture and Storage in Depleted Oil and Gas Reservoirs: The Viewpoint of Wellbore Injectivity. Energies. 2024; 17(5):1201. https://doi.org/10.3390/en17051201
Chicago/Turabian StyleHeidarabad, Reyhaneh Ghorbani, and Kyuchul Shin. 2024. "Carbon Capture and Storage in Depleted Oil and Gas Reservoirs: The Viewpoint of Wellbore Injectivity" Energies 17, no. 5: 1201. https://doi.org/10.3390/en17051201
APA StyleHeidarabad, R. G., & Shin, K. (2024). Carbon Capture and Storage in Depleted Oil and Gas Reservoirs: The Viewpoint of Wellbore Injectivity. Energies, 17(5), 1201. https://doi.org/10.3390/en17051201







