Investigation of the Behavior of Hydrocarbons during Crude Oil Fouling by High-Resolution Electrospray Ionization Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Simulation of Fouling under Laboratory Conditions
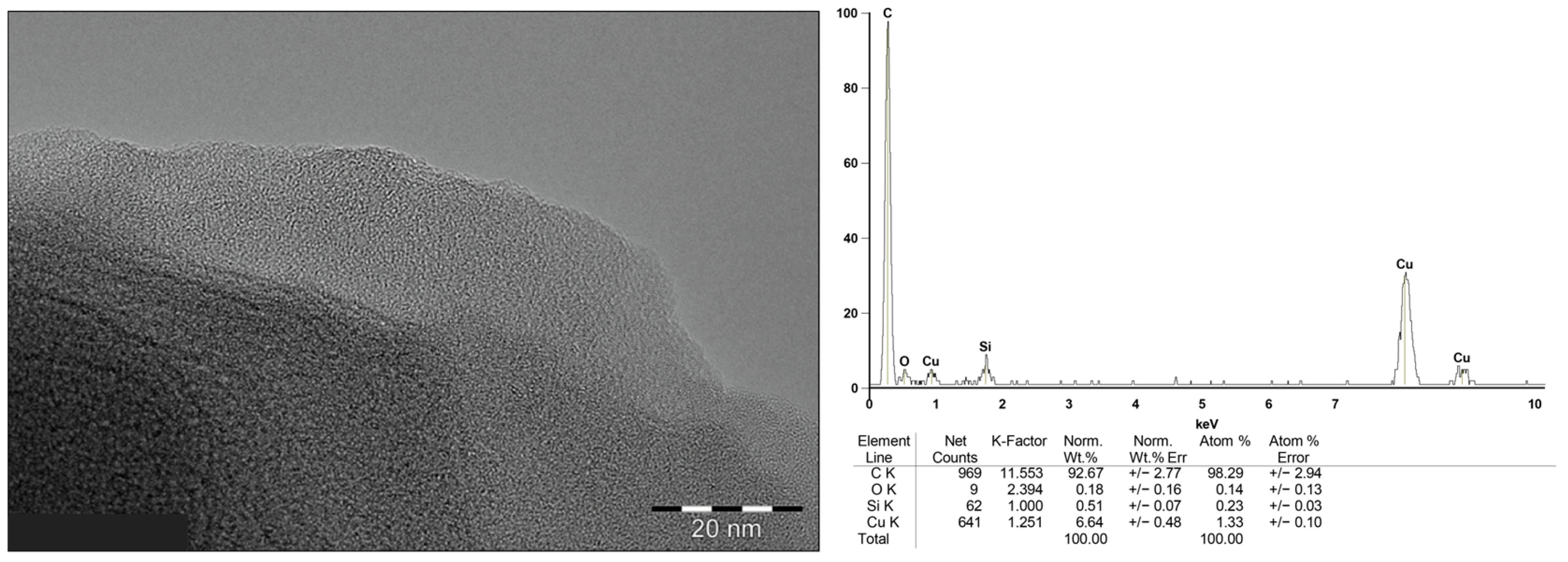
2.2. Solid Deposit Analysis
2.3. Mass Spectrometric Studies and Data Analysis
3. Results and Discussion
3.1. Fouling in a Light Crude Oil Fraction
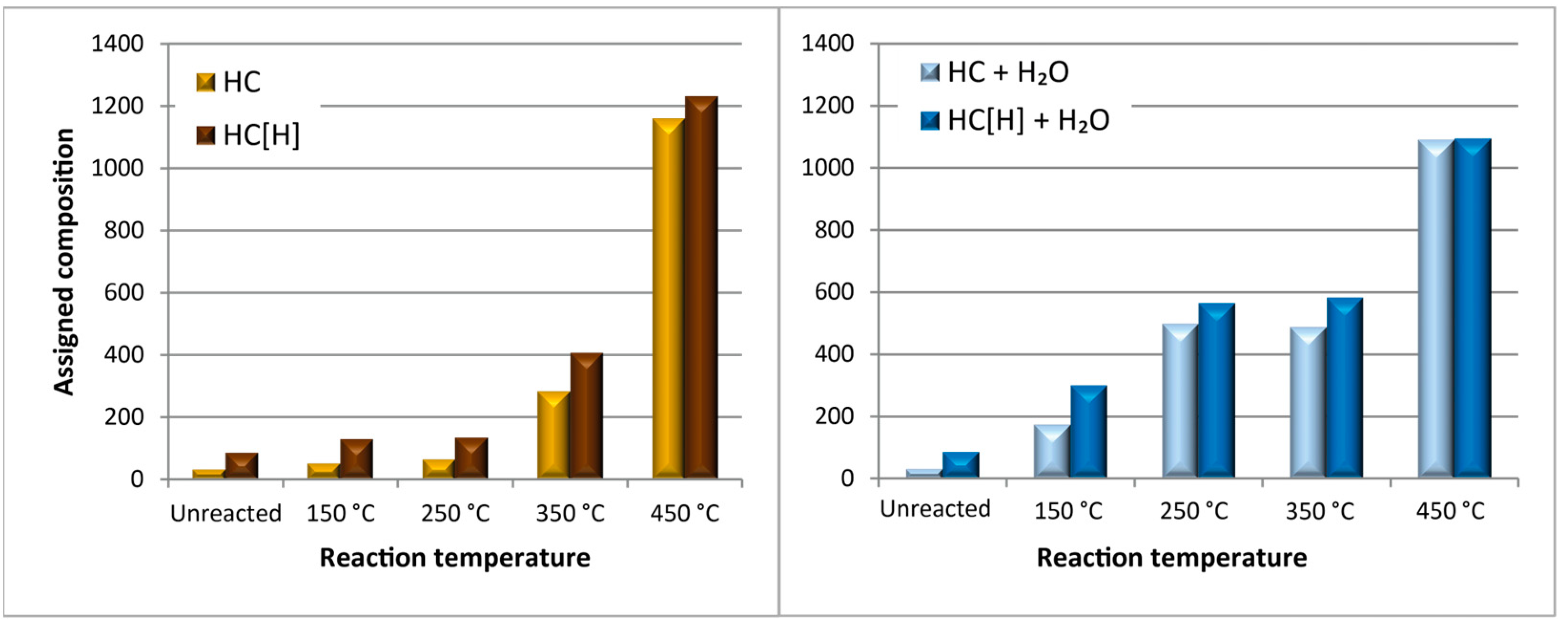
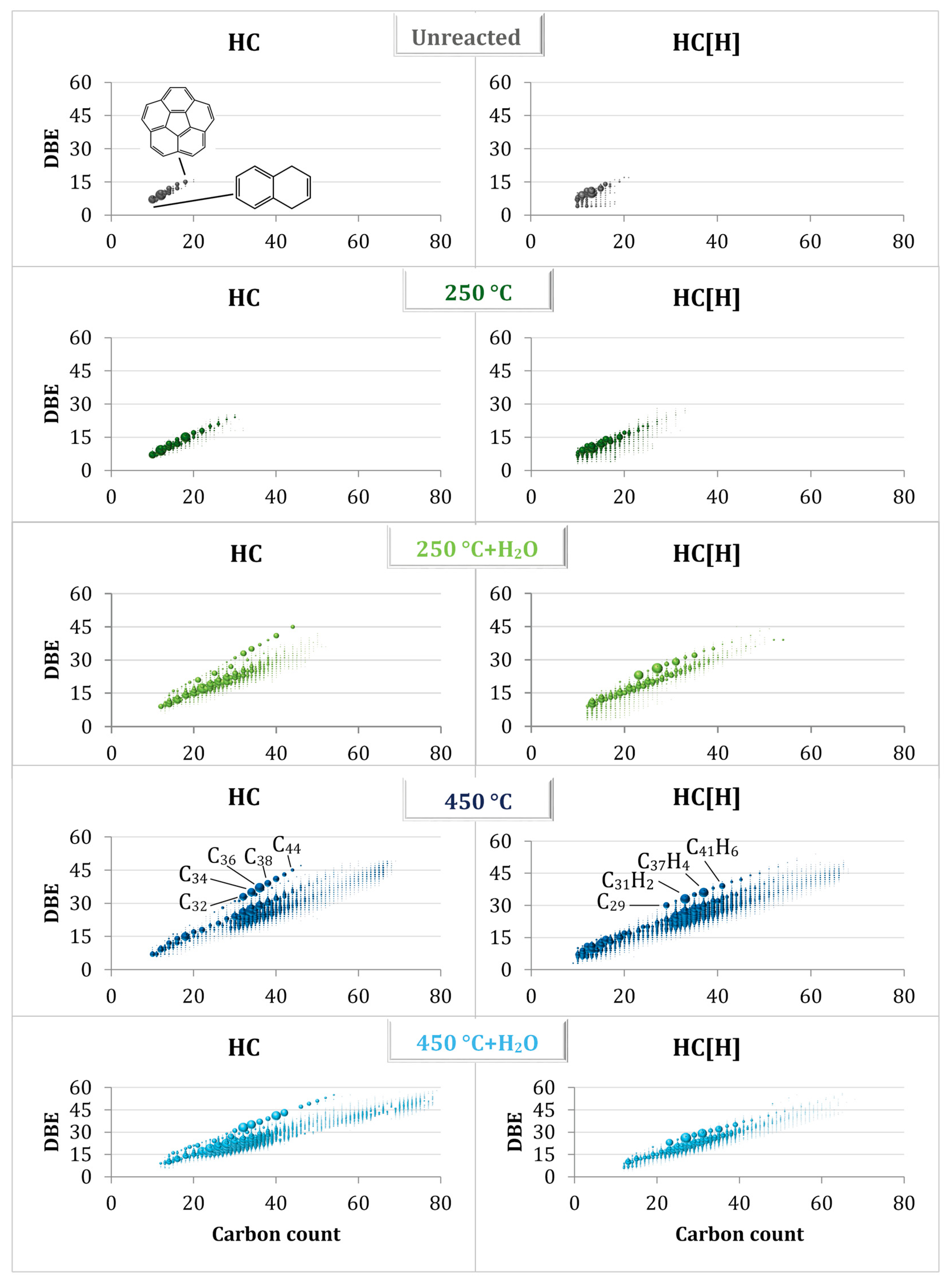
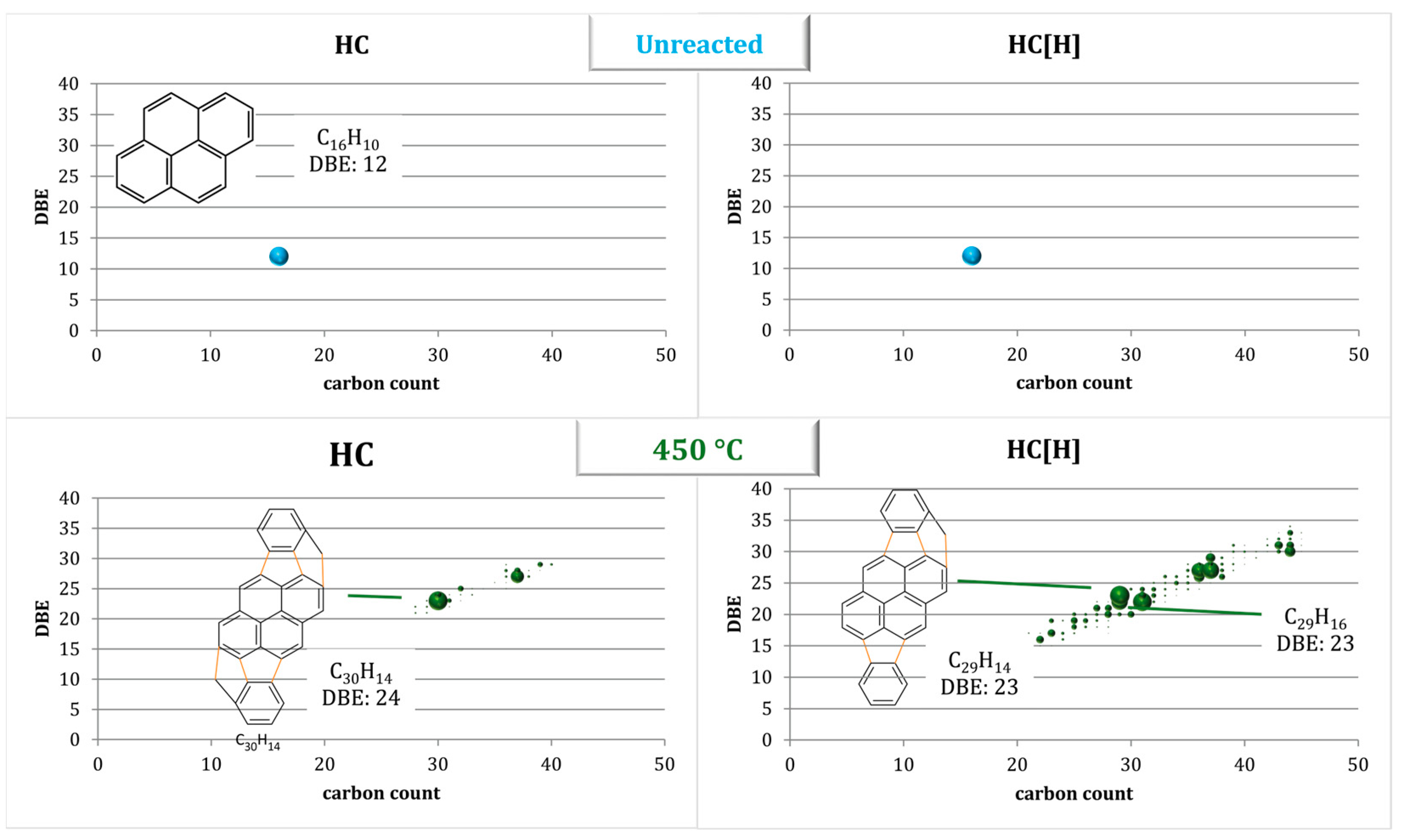
3.2. Studying the Mechanism of Fouling Using a Model Compound
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deshannavar, U.; Rafeen, S.; Marappa Gounder, R.D.S. Crude Oil Fouling: A Review. J. Appl. Sci. 2010, 10, 3167–3174. [Google Scholar] [CrossRef]
- Fan, Z.; Rahimi, P.; McGee, R.; Wen, Q.; Alem, T. Investigation of Fouling Mechanisms of a Light Crude Oil Using an Alcor Hot Liquid Process Simulator. Energy Fuels 2010, 24, 6110–6118. [Google Scholar] [CrossRef]
- Macchietto, S.; Hewitt, G.F.; Coletti, F.; Crittenden, B.D.; Dugwell, D.R.; Galindo, A.; Jackson, G.; Kandiyoti, R.; Kazarian, S.G.; Luckham, P.F.; et al. Fouling in Crude Oil Preheat Trains: A Systematic Solution to an Old Problem. Heat Transf. Eng. 2011, 32, 197–215. [Google Scholar] [CrossRef]
- Müller-Steinhagen, H.; Malayeri, M.R.; Watkinson, A.P. Fouling of Heat Exchangers-New Approaches to Solve an Old Problem. Heat Transf. Eng. 2005, 26, 1–4. [Google Scholar] [CrossRef]
- Wiehe, I. Petroleum fouling: Causes, tools and mitigation methods. In Proceedings of the 9th Topical Conference on Refinery Processing, Orlando, FL, USA, 23–27 April 2006. [Google Scholar]
- Jia, C. Breakthrough and significance of unconventional oil and gas to classical petroleum geology theory. Petrol. Explor. Dev. 2017, 44, 1–10. [Google Scholar] [CrossRef]
- Miller, R.G.; Sorrell, S.R. The future of oil supply. Philos. Trans. R. Soc. A 2014, 372, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Prado, G.; Rao, Y.; de Klerk, A. Nitrogen Removal from Oil: A Review. Energy Fuels 2017, 31, 14–36. [Google Scholar] [CrossRef]
- Watkinson, A.P. Deposition from Crude Oils in Heat Exchangers. Heat Transf. Eng. 2007, 28, 177–184. [Google Scholar] [CrossRef]
- Diaz-Bejarano, E.; Coletti, F.; Macchietto, S. Modeling and Prediction of Shell-Side Fouling in Shell-and-Tube Heat Exchangers. Heat Transf. Eng. 2018, 40, 845–861. [Google Scholar] [CrossRef]
- Saleh, Z.S.; Sheikholeslami, R.; Watkinson, A.P. Fouling Characteristics of a Light Australian Crude Oil. Heat Transf. Eng. 2005, 26, 15–22. [Google Scholar] [CrossRef]
- Beens, J.; Blomberg, J.; Schoenmakers, P.J. Proper Tuning of Comprehensive Two-Dimensional Gas Chromatography (GCxGC) to Optimize the separation of Complex Oil Fractions. J. High Resolut. Chromatogr. 2000, 23, 182–188. [Google Scholar] [CrossRef]
- Epstein, N. Thinking about Heat Transfer Fouling: A 5 × 5 Matrix. Heat Transf. Eng. 1983, 4, 43–56. [Google Scholar] [CrossRef]
- Watkinson, A.P.; Navaneetha-Sundaram, B.; Posarac, D. Fouling of a Sweet Crude Oil under Inert and Oxygenated Conditions. Energy Fuels 2000, 14, 64–69. [Google Scholar] [CrossRef]
- Harris, J.S.; Lane, M.R.; Smith, A.D. Investigating the Impact of Boiling Conditions on the Fouling of a Crude Oil. Heat Transf. Eng. 2017, 38, 703–711. [Google Scholar] [CrossRef]
- Ishiyama, E.M.; Paterson, W.R.; Wilson, D.I. The Effect of Fouling on Heat Transfer, Pressure Drop, and Throughput in Refinery Preheat Trains: Optimization of Cleaning Schedules. Heat Transf. Eng. 2009, 30, 805–814. [Google Scholar] [CrossRef]
- Ramasamy, M.; Deshannavar, U.B. Effect of Bulk Temperature and Heating Regime on Crude Oil Fouling: An Analysis. Adv. Mater. Res. 2014, 917, 189–198. [Google Scholar] [CrossRef]
- Ali, M.; Xing, T.Y.; Alem, T.; Chen, J.W. Investigating the role of aliphatic olefins in fouling of thermally cracked bitumen by testing fouling tendency of model aliphatic olefin compounds in light fractions. Fuel 2024, 360, 130518. [Google Scholar] [CrossRef]
- Hurt, M.; Ovalles, C.; Murray, D.; Rahimi, P. Investigation of the Chemical Composition of the Organic Material Associated with Inorganic Solids Found in Heat Exchanger Deposits: Possible Fouling Precursor in Refinery Operation. Energy Fuels 2023, 37, 14674–14687. [Google Scholar] [CrossRef]
- Lababidi, S.; Schrader, W. Online normal-phase high-performance liquid chromatography/Fourier transform ion cyclotron resonance mass spectrometry: Effects of different ionization methods on the characterization of highly complex crude oil mixtures. Rapid Commun. Mass Spectrom. 2014, 28, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Andersson, J.T.; Schrader, W. Characterization of supercomplex crude oil mixtures: What is really in there? Angew. Chem. 2009, 48, 1788–1791. [Google Scholar] [CrossRef]
- Pinto, D.A.M.; Camargo, S.M.C.; Parra, M.O.; Laverde, D.; Vergara, S.G.; Pinzon, C.B. Formation of fouling deposits on a carbon steel surface from Colombian heavy crude oil under preheating conditions. J. Phys. Conf. Ser. 2016, 687, 012016. [Google Scholar] [CrossRef]
- Venditti, S.; Berrueco, C.; Alvarez, P.; Morgan, T.; Millan-Agorio, M.; Herod, A.A.; Kandiyoti, R. Developing characterisation methods for foulants deposited in refinery heat exchangers. In Proceedings of the Conference: Heat Exchanger Fouling and Cleaning Eurotherm Conference, Schladming, Austria, 14–19 June 2009. [Google Scholar]
- Young, A.; Venditti, S.; Berrueco, C.; Yang, M.; Waters, A.; Davies, H.; Hill, S.; Millan, M.; Crittenden, B. Characterization of Crude Oils and Their Fouling Deposits Using a Batch Stirred Cell System. Heat Transf. Eng. 2011, 32, 216–227. [Google Scholar] [CrossRef]
- Ryder, A.G. Analysis of Crude Petroleum Oils Using Fluorescence Spectroscopy. In Reviews in Fluorescence 2005; Geddes, C.D., Lakowicz, J.R., Eds.; Springer US: Boston, MA, USA, 2005; pp. 169–198. [Google Scholar]
- Aske, N.; Kallevik, H.; Sjöblom, J. Determination of Saturate, Aromatic, Resin, and Asphaltenic (SARA) Components in Crude Oils by Means of Infrared and Near-Infrared Spectroscopy. Energy Fuels 2001, 15, 1304–1312. [Google Scholar] [CrossRef]
- Bennett, C.A.; Appleyard, S.; Gough, M.; Hohmann, R.P.; Joshi, H.M.; King, D.C.; Lam, T.Y.; Rudy, T.M.; Stomierowski, S.E. Industry-Recommended Procedures for Experimental Crude Oil Preheat Fouling Research. Heat Transf. Eng. 2006, 27, 28–35. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, G.; Chang, P.; Wang, X.; Lin, J. Fractal characteristics for coal chemical structure: Principle, methodology and implication. Chaos Solitons Fractals 2023, 173, 113699. [Google Scholar] [CrossRef]
- Wiehe, I.A.; Kennedy, R.J. The Oil Compatibility Model and Crude Oil Incompatibility. Energy Fuels 2000, 14, 56–59. [Google Scholar] [CrossRef]
- García, M.d.C. Crude Oil Wax Crystallization. The Effect of Heavy n-Paraffins and Flocculated Asphaltenes. Energy Fuels 2000, 14, 1043–1048. [Google Scholar] [CrossRef]
- Molina, V.D.; Ariza León, E.; Chaves-Guerrero, A. Understanding the Effect of Chemical Structure of Asphaltenes on Wax Crystallization of Crude Oils from Colorado Oil Field. Energy Fuels 2017, 31, 8997–9005. [Google Scholar] [CrossRef]
- Olsen, J.V.; de Godoy, L.M.; Li, G.; Macek, B.; Mortensen, P.; Pesch, R.; Makarov, A.; Lange, O.; Horning, S.; Mann, M. Parts per million mass accuracy on an Orbitrap mass spectrometer via lock mass injection into a C-trap. Mol. Cell. Proteom. 2005, 4, 2010–2021. [Google Scholar] [CrossRef]
- Panda, S.K.; Andersson, J.T.; Schrader, W. Mass-spectrometric analysis of complex volatile and nonvolatile crude oil components: A challenge. Anal. Bioanal. Chem. 2007, 389, 1329–1339. [Google Scholar] [CrossRef]
- Kondyli, A.; Schrader, W. Understanding “Fouling” in Extremely Complex Petroleum Mixtures. ACS Appl. Energy Mater. 2020, 3, 7251–7256. [Google Scholar] [CrossRef]
- Kondyli, A.; Schrader, W. Study of Crude Oil Fouling from Sulfur-Containing Compounds Using High-Resolution Mass Spectrometry. Energy Fuels 2021, 35, 13022–13029. [Google Scholar] [CrossRef]
- Panda, S.K.; Alawani, N.A.; Lajami, A.R.; Al-Qunaysi, T.A.; Muller, H. Characterization of aromatic hydrocarbons and sulfur heterocycles in Saudi Arabian heavy crude oil by gel permeation chromatography and ultrahigh resolution mass spectrometry. Fuel 2019, 235, 1420–1426. [Google Scholar] [CrossRef]
- Liu, P.; Shi, Q.; Chung, K.; Zhang, Y.; Pan, N.; Zhao, S.; Xu, C. Molecular Characterization of Sulfur Compounds in Venezuela Crude Oil and Its SARA Fractions by Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuels 2010, 24, 5089–5096. [Google Scholar] [CrossRef]
- Wu, Z.; Rodgers, R.P.; Marshall, A.G.; Strohm, J.J.; Song, C. Comparative Compositional Analysis of Untreated and Hydrotreated Oil by Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Energy Fuels 2005, 19, 1072–1077. [Google Scholar] [CrossRef]
- Santos, J.M.; Vetere, A.; Wisniewski, A.; Eberlin, M.N.; Schrader, W. Modified SARA Method to Unravel the Complexity of Resin Fraction(s) in Crude Oil. Energy Fuels 2020, 34, 16006–16013. [Google Scholar] [CrossRef]
- Giraldo-Davila, D.; Chacon-Patino, M.L.; Orrego-Ruiz, J.A.; Blanco-Tirado, C.; Combariza, M.Y. Improving compositional space accessibility in (+) APPI FT-ICR mass spectrometric analysis of crude oils by extrography and column chromatography fractionation. Fuel 2016, 185, 45–58. [Google Scholar] [CrossRef]
- Jones, H.E.; Palacio Lozano, D.C.; Huener, C.; Thomas, M.J.; Aaserud, D.J.; DeMuth, J.C.; Robin, M.P.; Barrow, M.P. Influence of Biodiesel on Base Oil Oxidation as Measured by FTICR Mass Spectrometry. Energy Fuels 2021, 35, 11896–11908. [Google Scholar] [CrossRef]
- Konermann, L.; Ahadi, E.; Rodriguez, A.D.; Vahidi, S. Unraveling the Mechanism of Electrospray Ionization. Anal. Chem. 2013, 85, 2–9. [Google Scholar] [CrossRef]
- Haack, A.; Polaczek, C.; Tsolakis, M.; Thinius, M.; Kersten, H.; Benter, T. Charge Retention/Charge Depletion in ESI-MS: Theoretical Rationale. J. Am. Soc. Mass Spectrom. 2020, 31, 785–795. [Google Scholar] [CrossRef]
- Thinius, M.; Polaczek, C.; Langner, M.; Bräkling, S.; Haack, A.; Kersten, H.; Benter, T. Charge Retention/Charge Depletion in ESI-MS: Experimental Evidence. J. Am. Soc. Mass Spectrom. 2020, 31, 773–784. [Google Scholar] [CrossRef]
- Markert, C.; Thinius, M.; Lehmann, L.; Heintz, C.; Stappert, F.; Wissdorf, W.; Kersten, H.; Benter, T.; Schneider, B.B.; Covey, T.R. Observation of charged droplets from electrospray ionization (ESI) plumes in API mass spectrometers. Anal. Bioanal. Chem. 2021, 413, 5587–5600. [Google Scholar] [CrossRef]
- Schäfer, M.; Drayß, M.; Springer, A.; Zacharias, P.; Meerholz, K. Radical Cations in Electrospray Mass Spectrometry: Formation of Open-Shell Species, Examination of the Fragmentation Behaviour in ESI-MSn and Reaction Mechanism Studies by Detection of Transient Radical Cations. Eur. J. Org. Chem. 2007, 2007, 5162–5174. [Google Scholar] [CrossRef]
- Vetere, A.; Schrader, W. Mass Spectrometric Coverage of Complex Mixtures: Exploring the Carbon Space of Crude Oil. ChemistrySelect 2017, 2, 849–853. [Google Scholar] [CrossRef]
- Watkinson, A.P. Chemical reaction fouling of organic fluids. Chem. Eng. Technol. 1992, 15, 82–90. [Google Scholar] [CrossRef]
- Albright, L.F.; McConnell, C.F.; Welther, K. Types of Coke Formed During the Pyrolysis of Light Hydrocarbons. In Thermal Hydrocarbon Chemistry; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1979; Volume 183, pp. 175–191. [Google Scholar]
- Schorr Wiener, M.; Valdez Salas, B. Corrosion Problems and Solutions in Oil Refining and Petrochemical Industry. Corros. Eng. Sci. Technol. 2018, 53, 80. [Google Scholar] [CrossRef]
- Bhowmik, P.; Emam Hossain, M.D.; Ahmed Shamim, J. Corrosion and Its Control in Crude Oil Refining Process. In Proceedings of the 6th International Mechanical Engineering Conference & 14th Annual Paper Meet, Dhaka, Bangladesh, 28 September 2012. [Google Scholar]
- Farmani, Z.; Vetere, A.; Poidevin, C.; Auer, A.A.; Schrader, W. Studying natural Buckyballs and Buckybowls in fossil materials. Angew. Chem. Int. Ed. 2020, 59, 15008–15013. [Google Scholar] [CrossRef] [PubMed]
- Farmani, Z.; Vetere, A.; Pfänder, N.; Lehmann, C.W.; Schrader, W. Naturally Occurring Allotropes of Carbon. Anal. Chem. 2024, 96, 2968–2974. [Google Scholar] [CrossRef]
- Kharlamov, A.; Kharlamova, G.; Bondarenko, M.; Fomenko, V. Joint Synthesis of Small Carbon Molecules (C3-C11), Quasi-Fullerenes (C40, C48, C52) and their Hydrides. Chem. Eng. Sci. 2013, 1, 32–40. [Google Scholar] [CrossRef]
- Goroff, N.S. Mechanism of Fullerene Formation. Acc. Chem. Res. 1996, 29, 77–83. [Google Scholar] [CrossRef]
- Berné, O.; Montillaud, J.; Joblin, C. Top-down formation of fullerenes in the interstellar medium. Astron. Astrophsics 2015, 577, A133. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondyli, A.; Schrader, W. Investigation of the Behavior of Hydrocarbons during Crude Oil Fouling by High-Resolution Electrospray Ionization Mass Spectrometry. Energies 2024, 17, 1299. https://doi.org/10.3390/en17061299
Kondyli A, Schrader W. Investigation of the Behavior of Hydrocarbons during Crude Oil Fouling by High-Resolution Electrospray Ionization Mass Spectrometry. Energies. 2024; 17(6):1299. https://doi.org/10.3390/en17061299
Chicago/Turabian StyleKondyli, Aikaterini, and Wolfgang Schrader. 2024. "Investigation of the Behavior of Hydrocarbons during Crude Oil Fouling by High-Resolution Electrospray Ionization Mass Spectrometry" Energies 17, no. 6: 1299. https://doi.org/10.3390/en17061299





