Study on the Performance and Emissions of Triple Blends of Diesel/Waste Plastic Oil/Vegetable Oil in a Diesel Engine: Advancing Eco-Friendly Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Plastic Oil/SVOs Double Blends, and Diesel/Plastic Oil/SVOs Triple Blends
2.2. Characterization of the Physical–Chemical Properties of the Biofuel Mixtures
2.3. Performance and Exhaust Emissions of a Diesel Engine Fueled with the PO/SVO Double Blends and Diesel/PO/SVOs Triple Blends
3. Results and Discussion
3.1. Physicochemical Properties of PO/SVO Double Blends, and D/PO/SVO Triple Blends
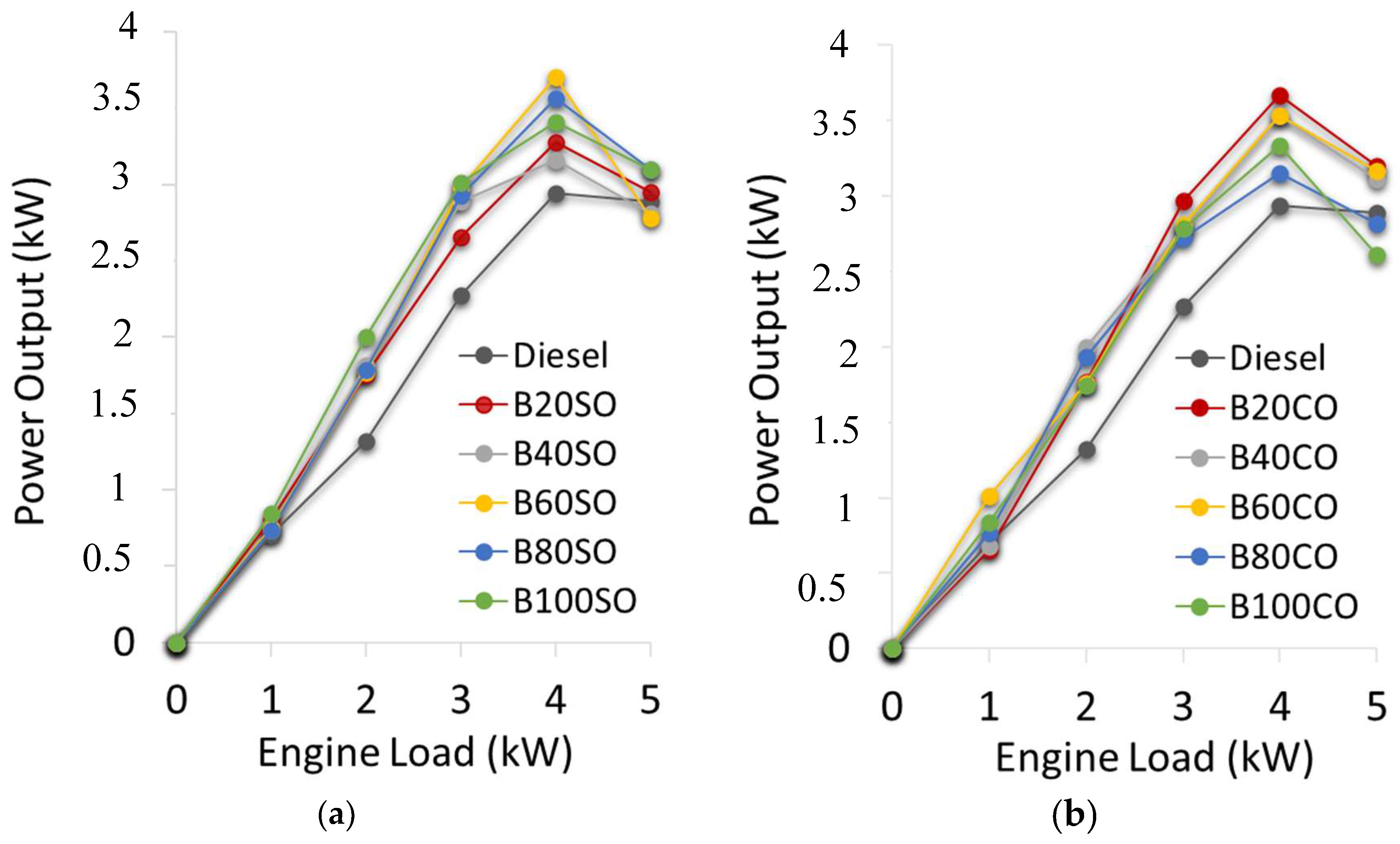
3.2. Mechanical Performance of a Diesel Engine, Working as Electric Generator, Fed with Different Biofuel Blends
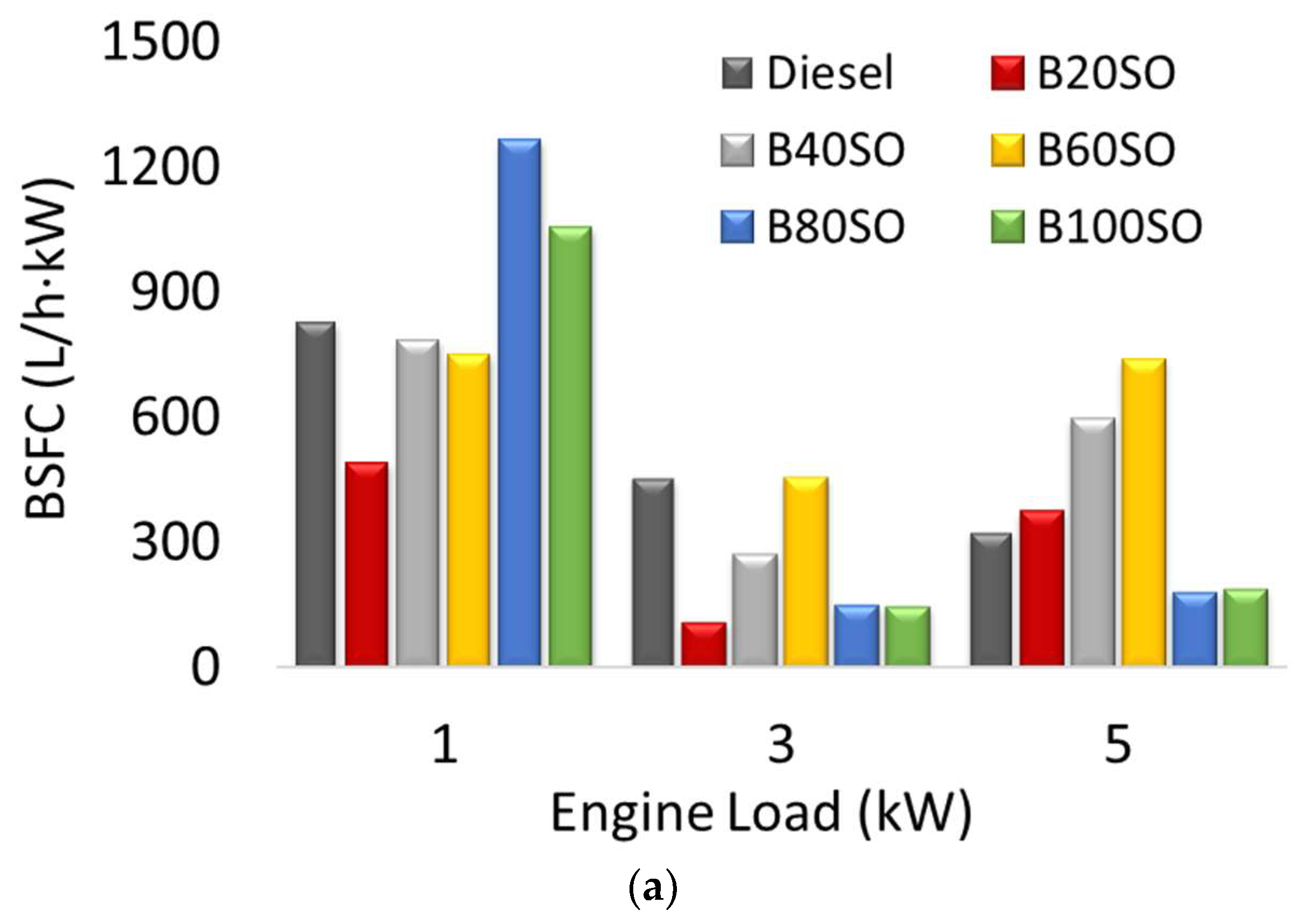
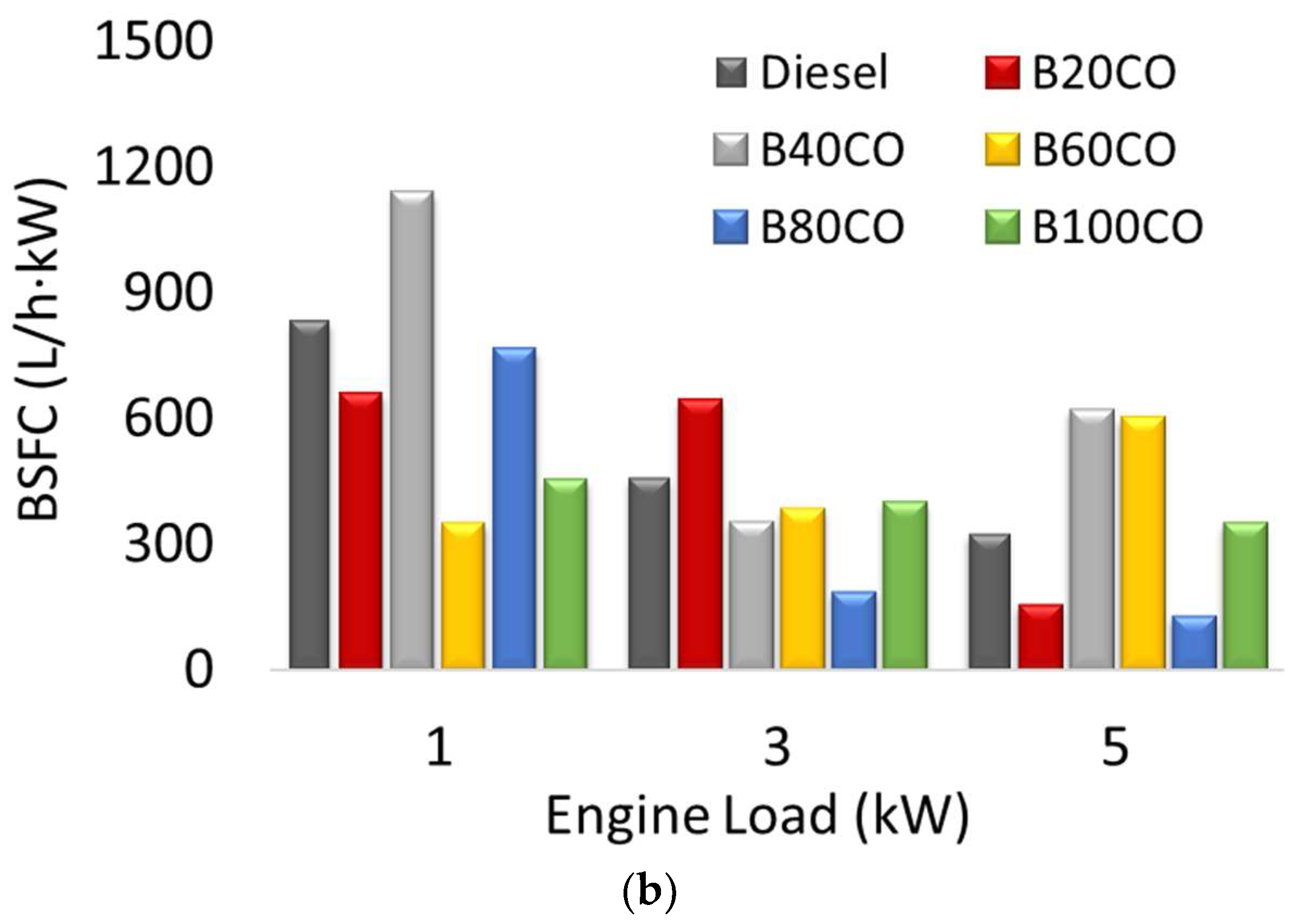
3.3. Brake-Specific Fuel Consumption (BSFC)
3.4. Exhaust Emissions from Diesel Engine
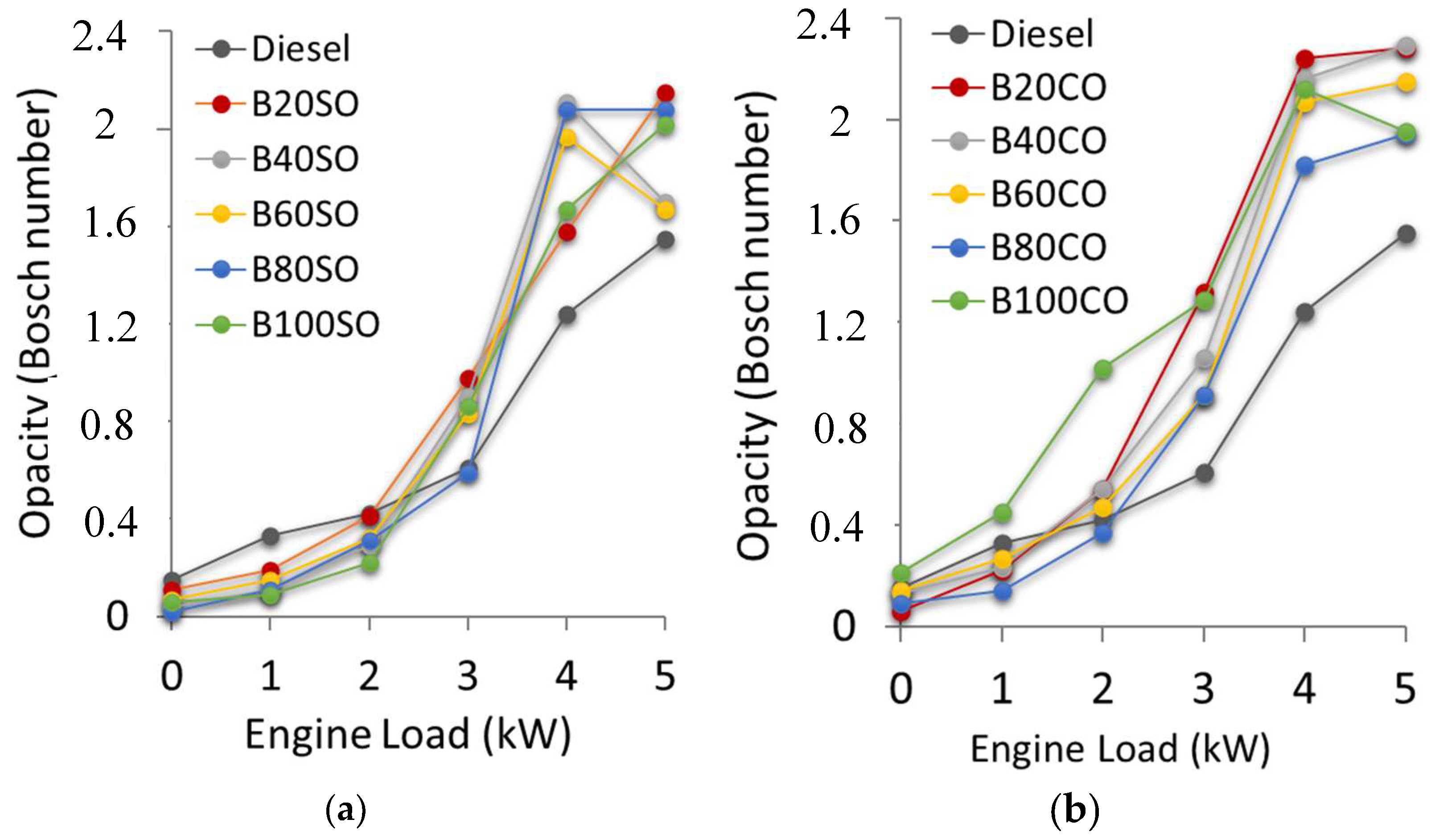
3.4.1. Soot Emissions
3.4.2. Carbon Monoxide (CO) Emissions
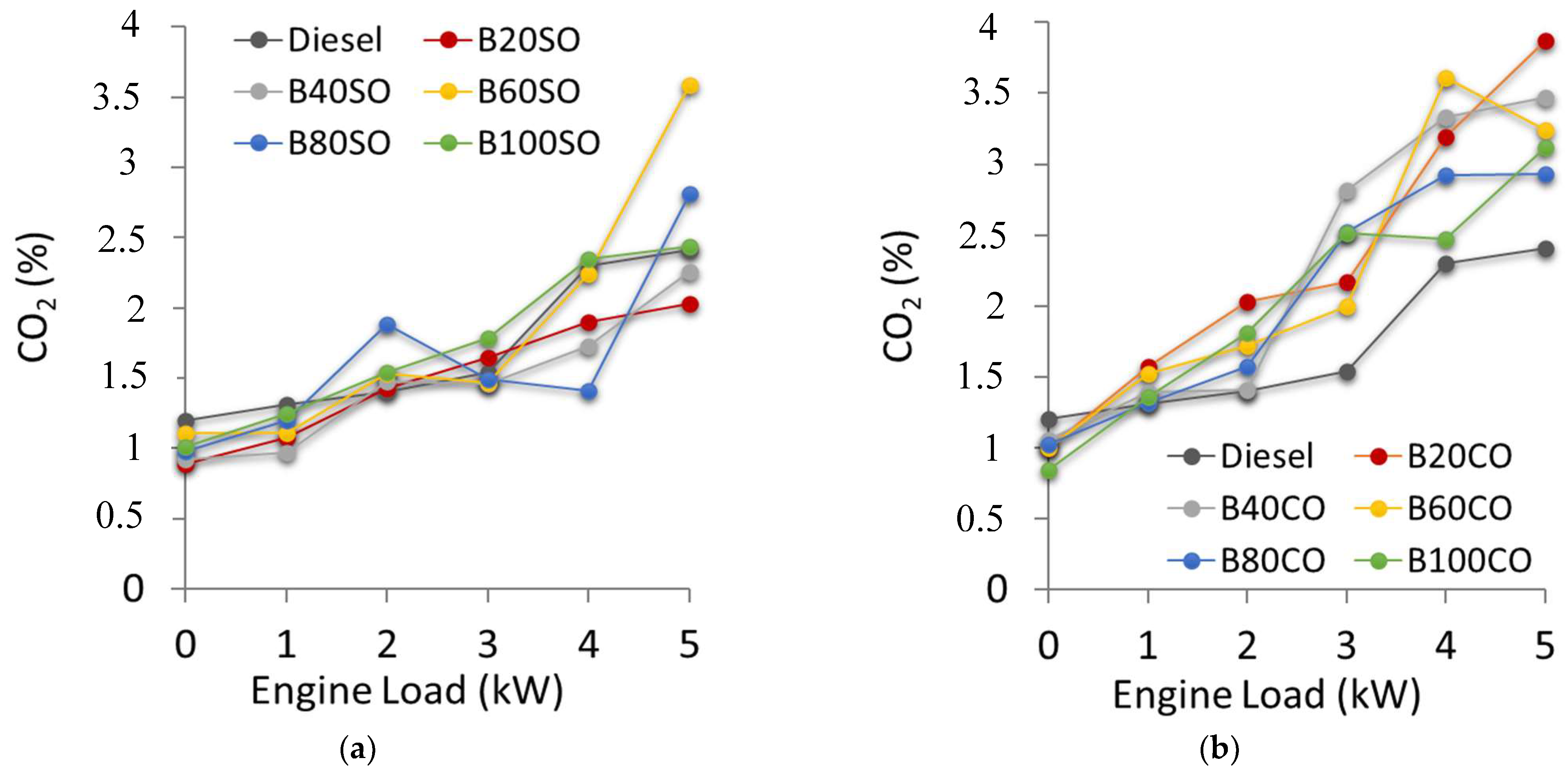
3.4.3. Carbon Dioxide (CO2) Emissions
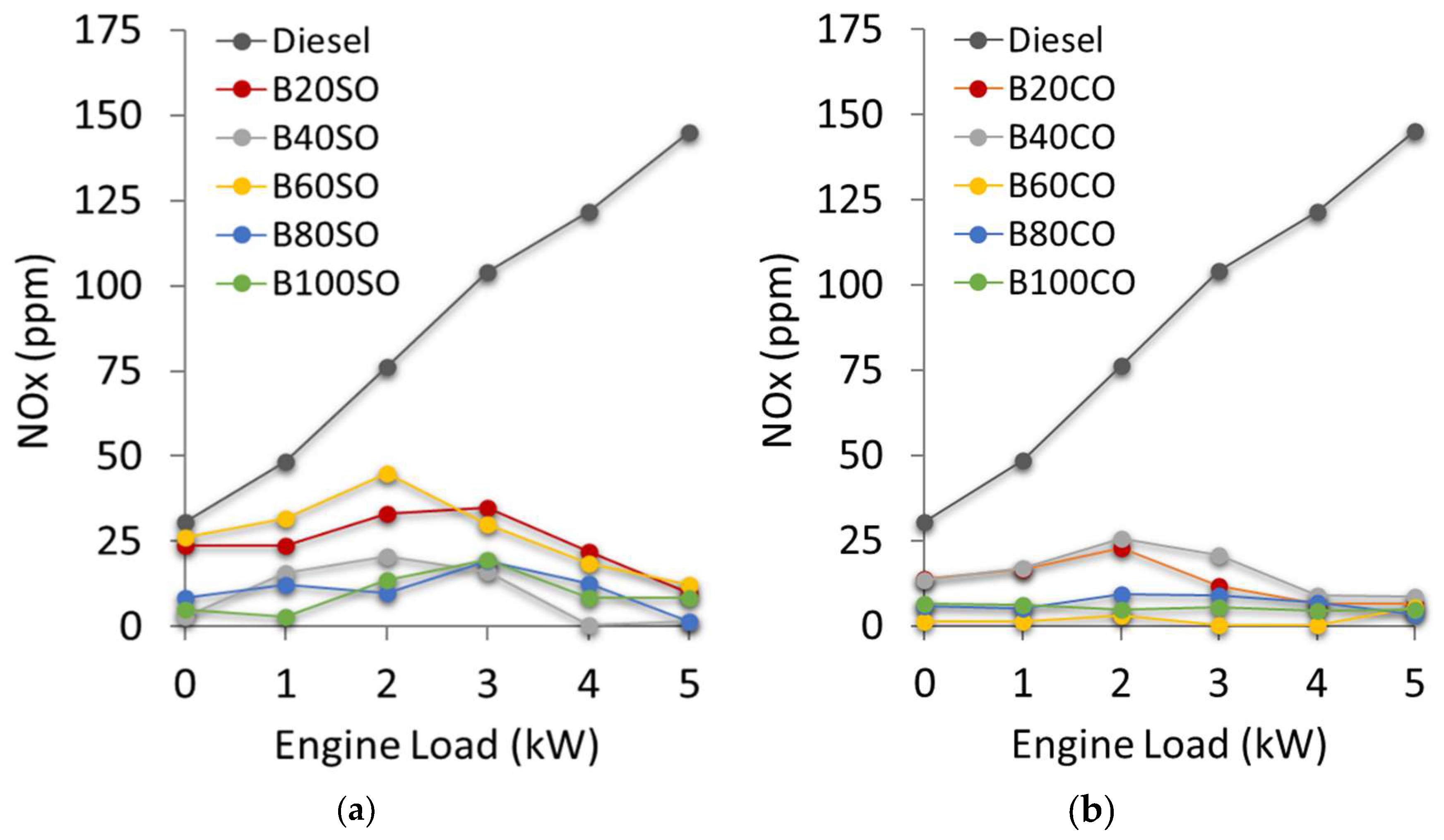
3.4.4. Nitrogen Oxides (NOx) Emissions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASTM | American Society for Testing and Materials |
| B0 | 100% diesel |
| B20CO | 80% diesel + 20% plastic oil/castor oil blend |
| B40CO | 60% diesel + 40% plastic oil/castor oil blend |
| B60CO | 40% diesel + 60% plastic oil/castor oil blend |
| B80CO | 20% diesel + 80% plastic oil/castor oil blend |
| B100CO | 100% plastic oil/castor oil blend |
| B20SO | 80% diesel + 20% plastic oil/sunflower oil blend |
| B40SO | 60% diesel + 40% plastic oil/sunflower oil blend |
| B60SO | 40% diesel + 60% plastic oil/sunflower oil blend |
| B80SO | 20% diesel + 80% plastic oil/sunflower oil blend |
| B100SO | 100% plastic oil/sunflower oil blend |
| BSFC | Brake-specific fuel consumption |
| C.I. | Compression ignition |
| CN | Cetane number |
| CO | Castor oil |
| CP | Cloud point |
| cSt | Centistokes |
| CV | Calorific value |
| D | Diesel |
| ISO | International Standards Organization |
| LVS | Low-viscosity solvent |
| PP | Pour point. |
| Rpm | Revolutions per minute |
| SO | Sunflower oil |
| SVO | Straight vegetable oil |
| VO | Vegetable oil |
| W | Watts |
| Symbols | |
| C | Calibration constant (mm2/s)/s |
| T | Flow time (s) |
| υ | Viscosity (centistokes) |
References
- Lebreton1, L.; Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 2019, 5, 6. [Google Scholar] [CrossRef]
- Borrelle, S.B.; Ringma, J.; Law, K.L.; Monnahan, C.C.; Lebreton, L.; McGivern, A.; Murphy, E.; Jambeck, J.; Leonard, G.H.; Hilleary, M.A.; et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science 2020, 369, 1515–1518. [Google Scholar] [CrossRef] [PubMed]
- Amankwa, M.O.; Tetteh, E.K.; Mohale, G.T.; Dagba, G.; Opoku, P. The production of valuable products and fuel from plastic waste in Africa. Discov. Sustain. 2021, 2, 31. [Google Scholar] [CrossRef]
- Zorpas, A.A.; Navarro-Pedreño, J.; Jeguirim, M.; Dimitriou, G.; Almendro-Candel, M.B.; Argirusis, C.; Vardopoulos, I.; Loizia, P.; Chatziparaskeva, G.; Papamichael, I. Crisis in leadership vs waste management. Euro-Mediterr. J. Environ. Integr. 2021, 6, 80. [Google Scholar] [CrossRef]
- Law, K.L.; Starr, N.; Siegler, T.R.; Jambeck, J.R.; Mallos, N.J.; Leonard, G.H. The United States’ contribution of plastic waste to land and ocean. Sci. Adv. 2020, 6, eabd0288. [Google Scholar] [CrossRef] [PubMed]
- Meijer, L.J.J.; van Emmerik, T.; van der Ent, R.; Schmidt, C.; Lebreton, L. More than 1000 rivers account for 80% of global riverine plastic emissions into the ocean. Sci. Adv. 2021, 7, eaaz5803. [Google Scholar] [CrossRef]
- Nyika, J.; Dinka, M. Recycling plastic waste materials for building and construction Materials: A minireview. Mater. Today Proc. 2022, 62, 3257–3262. [Google Scholar] [CrossRef]
- Li, N.; Liu, H.; Cheng, Z.; Yan, B.; Chen, G.; Wang, S. Conversion of plastic waste into fuels: A critical review. J. Hazard. Mater. 2022, 424, 127460. [Google Scholar] [CrossRef]
- Al-dalain, R.; Celebi, D. Planning a mixed fleet of electric and conventional vehicles for urban freight with routing and replacement considerations. Sustain. Cities Soc. 2021, 73, 103105. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mohanty, K. Co-pyrolysis of waste biomass and waste plastics (polystyrene and waste nitrile gloves) into renewable fuel and value-added chemicals. Carbon Resour. Convers. 2020, 3, 145–155. [Google Scholar] [CrossRef]
- Dobó, Z.; Kecsmár, G.; Nagy, G.; Koós, T.; Muránszky, G.; Ayari, M. Characterization of Gasoline-like Transportation Fuels Obtained by Distillation of Pyrolysis Oils from Plastic Waste Mixtures. Energy Fuels 2021, 35, 2347–2356. [Google Scholar] [CrossRef]
- Fahim, I.; Mohsen, O.; ElKayaly, D. Production of Fuel from Plastic Waste: A Feasible Business. Polymers 2021, 13, 915. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R.K.; Sarojini, J.; Rajak, U.; Verma, T.N.; Ağbulut, Ü. Alternative fuel production from waste plastics and their usability in light duty diesel engine: Combustion, energy, and environmental analysis. Energy 2023, 265, 126140. [Google Scholar] [CrossRef]
- EN 590:2022; Automotive Fuels-Diesel-Requirements and Test Methods. IEC: Geneva, Switzerland, 2022.
- Estevez, R.; Aguado-Deblas, L.; Bautista, F.M.; Luna, D.; Luna, C.; Calero, J.; Posadillo, A.; Romero, A.A. Biodiesel at the Crossroads: A Critical Review. Catalysts 2019, 9, 1033. [Google Scholar] [CrossRef]
- Mizik, T.; Gyarmati, G. Three Pillars of Advanced Biofuels’ Sustainability. Fuels 2022, 3, 607–626. [Google Scholar] [CrossRef]
- Estevez, R.; Aguado-Deblas, L.; López-Tenllado, F.J.; Luna, C.; Calero, J.; Romero, A.A.; Bautista, F.M.; Luna, D. Biodiesel is dead: Long life to advanced biofuels. A 2 comprehensive critical review. Energies 2022, 15, 3173. [Google Scholar] [CrossRef]
- Long, F.; Liu, W.; Jiang, X.; Zhai, Q.; Cao, X.; Jiang, J.; Xu, J. State-of-the-art technologies for biofuel production from triglycerides: A review. Renew. Sustain. Energy Rev. 2021, 148, 111269. [Google Scholar] [CrossRef]
- Pfleger, B.F.; Takors, R. Recent progress in the synthesis of advanced biofuel and bioproducts. Curr. Opin. Biotechnol. 2023, 80, 102913. [Google Scholar] [CrossRef]
- Hongloi, N.; Prapainainar, P.; Prapainainar, C. Review of green diesel production from fatty acid deoxygenation over Ni-based catalysts. Mol. Catal. 2022, 523, 111696. [Google Scholar] [CrossRef]
- Mat, S.C.; Idroas, M.Y.; Teoh, Y.H.; Hamid, M.F. An Investigation of Viscosities, Calorific Values and Densities of Binary Biofuel Blends. MATEC Web Conf. 2017, 135, 00004. [Google Scholar] [CrossRef][Green Version]
- Vallinayagam, R.; Vedharaj, S.; Yang, W.; Roberts, W.L.; Dibble, R.W. Feasibility of using less viscous and lower cetane (LVLC) fuels in a diesel engine: A review. Renew. Sustain. Energy Rev. 2015, 51, 1166–1190. [Google Scholar] [CrossRef]
- Mat, S.C.; Idroas, M.Y.; Hamid, M.F.; Zainal, Z.A. Performance and emissions of straight vegetable oils and its blends as a fuel in diesel engine: A review. Renew. Sustain. Energy Rev. 2018, 82, 808–823. [Google Scholar] [CrossRef]
- Mat, S.C.; Idroas, M.Y.; Teoh, Y.H.; Hamid, M.F. Physicochemical, Performance, Combustion and Emission Characteristics of Melaleuca Cajuputi Oil-Refined Palm Oil Hybrid Biofuel Blend. Energies 2018, 11, 3146. [Google Scholar] [CrossRef]
- Mat, S.C.; Idroas, M.Y.; Teoh, Y.H.; Hamid, M.F. Assessment of basic properties and thermal analysis of hybrid biofuel blend. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 2073–2082. [Google Scholar] [CrossRef]
- Mat, S.C.; Idroas, M.Y.; Teoh, Y.H.; Hamid, M.F. Optimization of viscosity and density of refined palm Oil-Melaleuca Cajuputi oil binary blends using mixture design method. Renew. Energy 2019, 133, 393–400. [Google Scholar] [CrossRef]
- Prakash, T.; Geo, V.E.; Martin, L.J.; Nagalingam, B. Evaluation of pine oil blending to improve the combustion of high viscous (castor oil) biofuel compared to castor oil biodiesel in a CI engine. Heat Mass Transf. 2019, 55, 1491–1501. [Google Scholar] [CrossRef]
- Kasiraman, G.; Nagalingam, B.; Balakrishnan, M. Performance, emission and combustion improvements in a direct injection diesel engine using cashew nutshell oil as fuel with camphor oil blending. Energy 2012, 47, 116–124. [Google Scholar] [CrossRef]
- Martin, M.L.J.; Geo, V.E.; Singh, D.K.J.; Nagalingam, B. A comparative analysis of different methods to improve the performance of cotton seed oil fueled diesel engine. Fuel 2012, 102, 372–378. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; Herfatmanesh, M.R.; Geo, V.E.; Peng, Z. Experimental investigation into the effect of magnetic fuel reforming on diesel combustion and emissions running on wheat germ and pine oil. Fuel Process. Technol. 2019, 186, 116–124. [Google Scholar] [CrossRef]
- Coughlin, B.; Hoxie, A. Combustion characteristics of ternary fuel Blends: Pentanol, butanol and vegetable oil. Fuel 2017, 196, 488–496. [Google Scholar] [CrossRef]
- Lujaji, F.; Kristóf, L.; Bereczky, A.; Mbarawa, M. Experimental investigation of fuel properties, engine performance, combustion and emissions of blends containing croton oil, butanol, and diesel on a CI engine. Fuel 2011, 90, 505–510. [Google Scholar] [CrossRef]
- Atmanlı, A.; Ileri, E.; Yüksel, B. Experimental investigation of engine performance and exhaust emissions of a diesel engine fueled with diesel–n-butanol–vegetable oil blends. Energy Convers. Manag. 2014, 81, 312–321. [Google Scholar] [CrossRef]
- Atmanli, A.; Ileri, E.; Yuksel, B.; Yilmaz, N. Extensive analyses of diesel–vegetable oil–n-butanol ternary blends in a diesel engine. Appl. Energy 2015, 145, 155–162. [Google Scholar] [CrossRef]
- Atmanlı, A.; Yüksel, B.; İleri, E. Experimental investigation of the effect of diesel–cotton oil–n-butanol ternary blends on phase stability, engine performance and exhaust emission parameters in a diesel engine. Fuel 2013, 109, 503–511. [Google Scholar] [CrossRef]
- Atmanlı, A.; Yüksel, B.; İleri, E.; Karaoglan, D. Response surface methodology-based optimization of diesel–n-butanol–cotton oil ternary blend ratios to improve engine performance and exhaust emission characteristics. Energy Convers. Manag. 2015, 90, 383–394. [Google Scholar] [CrossRef]
- Atmanlı, A.; İleri, E.; Yüksel, B. Effects of higher ratios of n-butanol addition to diesel–vegetable oil blends on performance and exhaust emissions of a diesel engine. J. Energy Inst. 2015, 88, 209–220. [Google Scholar] [CrossRef]
- Atmanli, A.; Ileri, E.; Yilmaz, N. Optimization of diesel–butanol–vegetable oil blend ratios based on engine operating parameters. Energy 2016, 96, 569–580. [Google Scholar] [CrossRef]
- Krishnamoorthy, V.; Dhanasekaran, R.; Rana, D.; Saravanan, S.; Kumar, B.R. A comparative assessment of ternary blends of three bio-alcohols with waste cooking oil and diesel for optimum emissions and performance in a CI engine using response surface methodology. Energy Convers. Manag. 2018, 156, 337–357. [Google Scholar] [CrossRef]
- Eiadtrong, S.; Maliwan, K.; Theppaya, T.; Kattiyawan, T.; Prateepchaikul, G.; Leevijit, T. An investigation to utilize ternary diesel-palm fatty acid distillate-10 wt% n-butanol blends as simply novel diesel substitutes. Fuel 2021, 289, 119965. [Google Scholar] [CrossRef]
- Ileri, E.; Atmanli, A.; Yilmaz, N. Comparative analyses of n-butanol-rapeseed oil-diesel blend with biodiesel, diesel and biodiesel-diesel fuels in a turbocharged direct injection diesel engine. J. Energy Inst. 2016, 89, 586–593. [Google Scholar] [CrossRef]
- EL-Seesy, A.I.; He, Z.; Hassan, H.; Balasubramanian, D. Improvement of combustion and emission characteristics of a diesel engine working with diesel/jojoba oil blends and butanol additive. Fuel 2020, 279, 118433. [Google Scholar] [CrossRef]
- Saleh, H.E. Performance and emissions characteristics of direct injection diesel engine fueled by diesel-jojoba oil-butanol blends with hydrogen peroxide. Fuel 2021, 285, 119048. [Google Scholar] [CrossRef]
- Vinod Babu, M.; Madhu Murthy, K.; Amba Prasad Rao, G. Butanol and pentanol: The promising biofuels for CI engines—A review. Renew. Sustain. Energy Rev. 2017, 78, 1068–1088. [Google Scholar] [CrossRef]
- Kumar, B.R.; Saravanan, S. Use of higher alcohol biofuels in diesel engines: A review. Renew. Sustain. Energy Rev. 2016, 60, 84–115. [Google Scholar] [CrossRef]
- Prakash, T.; Geo, V.E.; Martin, L.J.; Nagalingam, B. Effect of ternary blends of bioethanol, diesel and castor oil on performance, emission and combustion in a CI engine. Renew. Energy 2018, 122, 301–309. [Google Scholar] [CrossRef]
- Yilmaz, N.; Atmanli, A.; Vigil, F.M. Quaternary blends of diesel, biodiesel, higher alcohols and vegetable oil in a compression ignition engine. Fuel 2018, 212, 462–469. [Google Scholar] [CrossRef]
- Swaminathan, S.; Subramanian, T.; Martin, L.J.; Beddhannan, N. Emission profiling of CI engine fueled with neem and wintergreen oil blend with hexanol and octanol manifold injection. Environ. Sci. Pollut. Res. 2020, 27, 17505–17515. [Google Scholar] [CrossRef]
- Mat, S.C.; Idroas, M.Y.; Teoh, Y.H.; Hamid, M.F.; Sharudin, H.; Pahmi, M.A.A.H. Optimization of ternary blends among refined palm oil-hexanol melaleuca cajuputi oil and engine emissions analysis of the blends. Renew. Energy 2022, 196, 451–461. [Google Scholar] [CrossRef]
- Kumar, A.; Rajan, K.; Naraynan, M.R.; Kumar, K.R.S. Performance and Emission Characteristics of a Di Diesel Engine Fueled with Cashew Nut Shell Oil (CNSO)-Diesel Blends with Diethyl Ether as Additive. Appl. Mech. Mater. 2015, 787, 746–750. [Google Scholar] [CrossRef]
- Kumar, A.; Rajan, K.; Kumar, K.R.S.; Maiyappan, K.; Rasheed, U.T. Green fuel utilization for diesel engine, combustion and emission analysis fuelled with CNSO diesel blends with Diethyl ether as additive. Front. Automob. Mech. Eng. IOP Conf. Ser. Mater. Sci. Eng. 2017, 197, 012013. [Google Scholar] [CrossRef]
- Krishnamoorthi, M.; Malayalamurthi, R. Experimental investigation on the availability, performance, combustion and emission distinctiveness of bael oil/diesel/diethyl ether blends powered in a variable compression ratio diesel engine. Heat Mass Transf. 2018, 54, 2023–2044. [Google Scholar] [CrossRef]
- Krishnamoorthi, M.; Malayalamurthi, R. Availability analysis, performance, combustion and emission behavior of bael oil-diesel-diethyl ether blends in a variable compression ratio diesel engine. Renew. Energy 2018, 119, 235–252. [Google Scholar] [CrossRef]
- Krishnamoorthi, M.; Malayalamurthi, R. The influence of charge air temperature and exhaust gas recirculation on the availability analysis, performance and emission behavior of diesel-bael oil-diethyl ether blend operated diesel engine. J. Mech. Sci. Technol. 2018, 32, 1835–1847. [Google Scholar] [CrossRef]
- Krishnamoorthi, M.; Malayalamurthi, R. Experimental investigation on performance, emission behavior and exergy analysis of a variable compression ratio engine fueled with diesel-aegle marmelos oil-diethyl ether blends. Energy 2017, 128, 312–328. [Google Scholar] [CrossRef]
- Krishnamoorthi, M.; Malayalamurthi, R. Combined Effect of Compression Ratio, Injection Pressure, and Injection Timing on Performance and Emission of a DI Compression Ignition Engine Fueled with Diesel–Aegle Marmelos Oil–Diethyl Ether Blends Using Response Surface Methodology. Energy Fuels 2017, 31, 11362–11376. [Google Scholar] [CrossRef]
- Krishna, R.; Bandewar, A.G.; Dongare, V.K. Experimental Investigations of Blending Diethyl Ether in Karanja Vegetable Oil using A Multi-Cylinder Diesel Engine. Int. J. Res. Innov. Technol. 2014, 1, 70–73. [Google Scholar]
- Aguado-Deblas, L.; Hidalgo-Carrillo, J.; Bautista, F.; Luna, D.; Luna, C.; Calero, J.; Posadillo, A.; Romero, A.; Estevez, R. Diethyl Ether as an Oxygenated Additive for Fossil Diesel/Vegetable Oil Blends: Evaluation of Performance and Emission Quality of Triple Blends on a Diesel Engine. Energies 2020, 13, 1542. [Google Scholar] [CrossRef]
- Aguado-Deblas, L.; Estevez, R.; Hidalgo-Carrillo, J.; Bautista, F.M.; Luna, C.; Calero, J.; Posadillo, A.; Romero, A.A.; Luna, D. Outlook for Direct Use of Sunflower and Castor Oils as Biofuels in Compression Ignition Diesel Engines, Being Part of Diesel/Ethyl Acetate/Straight Vegetable Oil Triple Blends. Energies 2020, 13, 4836. [Google Scholar] [CrossRef]
- Aguado-Deblas, L.; Hidalgo-Carrillo, J.; Bautista, F.M.; Luna, C.; Calero, J.; Alejandro Posadillo, A.; Romero, A.A.; Luna, D.; Estevez, R. Biofuels from Diethyl Carbonate and Vegetable Oils for Use in Triple Blends with Diesel Fuel: Effect on Performance and Smoke Emissions of a Diesel Engine. Energies 2020, 13, 6584. [Google Scholar] [CrossRef]
- Aguado-Deblas, L.; Hidalgo-Carrillo, J.; Bautista, F.M.; Luna, C.; Calero, J.; Alejandro Posadillo, A.; Romero, A.A.; Luna, D.; Estevez, R. Evaluation of Dimethyl Carbonate as Alternative Biofuel. Performance and Smoke Emissions of a Diesel Engine Fueled with Diesel/Dimethyl Carbonate/Straight Vegetable Oil Triple Blends. Sustainability 2021, 13, 1749. [Google Scholar] [CrossRef]
- Aguado-Deblas, L.; Hidalgo-Carrillo, J.; Bautista, F.M.; Luna, D.; Luna, C.; Calero, J.; Alejandro Posadillo, A.; Romero, A.A.; Estevez, R. Acetone Prospect as an Additive to Allow the Use of Castor and Sunflower Oils as Drop-In Biofuels in Diesel/Acetone/Vegetable Oil Triple Blends for Application in Diesel Engines. Molecules 2020, 25, 2935. [Google Scholar] [CrossRef]
- Aguado-Deblas, L.; López-Tenllado, F.J.; Luna, D.; Bautista, F.M.; Romero, A.A.; Estevez, R. Advanced Biofuels from ABE (Acetone/Butanol/Ethanol) and Vegetable Oils (Castor or Sunflower Oil) for Using in Triple Blends with Diesel: Evaluation on a Diesel Engine. Materials 2022, 15, 6493. [Google Scholar] [CrossRef]
- Veza, I.; Said, M.F.M.; Latiff, Z.A. Recent advances in butanol production by acetone-butanol-ethanol (ABE) fermentation. Biomass Bioenergy 2021, 144, 105919. [Google Scholar] [CrossRef]
- Veza, I.; Said, M.F.M.; Latiff, Z.A. Progress of acetone-butanol-ethanol (ABE) as biofuel in gasoline and diesel engine: A review. Fuel Process. Technol. 2019, 196, 106179. [Google Scholar] [CrossRef]
- Algayyim, S.J.M.; Wandel, A.P.; Yusaf, T.; Hamawand, I. Production and application of ABE as a biofuel. Renew. Sustain. Energy Rev. 2018, 82, 1195–1214. [Google Scholar] [CrossRef]
- Lia, Y.; Tanga, W.; Chena, Y.; Liua, J.; Lee, C.F. Potential of acetone-butanol-ethanol (ABE) as a biofuel. Fuel 2019, 242, 673–686. [Google Scholar] [CrossRef]
- Atmanli, A. Effects of a cetane improver on fuel properties and engine characteristics of a diesel engine fueled with the blends of diesel, hazelnut oil and higher carbon alcohol. Fuel 2016, 172, 209–217. [Google Scholar] [CrossRef]
- Paneerselvam, P.; Venkadesan, G.; Panithasan, M.S.; Alaganathan, G.; Wierzbicki, S.; Mikulski, M. Evaluating the Influence of Cetane Improver Additives on the Outcomes of a Diesel Engine Characteristics Fueled with Peppermint Oil Diesel Blend. Energies 2021, 14, 2786. [Google Scholar] [CrossRef]
- Estevez, R.; Aguado-Deblas, L.; Posadillo, A.; Hurtado, B.; Bautista, F.; Hidalgo, J.M.; Luna, C.; Calero, J.; Romero, A.; Luna, D. Performance and Emission Quality Assessment in a Diesel Engine of Straight Castor and Sunflower Vegetable Oils, in Diesel/Gasoline/Oil Triple Blends. Energies 2019, 12, 2181. [Google Scholar] [CrossRef]
- Mani, M.; Subash, C.; Nagarajan, G. Performance, emission and combustion characteristics of a DI diesel engine using waste plastic oil. Appl. Therm. Eng. 2009, 29, 2738–2744. [Google Scholar] [CrossRef]
- Pakiya Pradeep, A.; Gowthaman, S. Combustion and emission characteristics of diesel engine fuelled with waste plastic oil—A review. Int. J. Ambient. Energy 2022, 43, 1269–1287. [Google Scholar] [CrossRef]
- Pawar Harshal, R.; Lawankar Shailendra, M. Waste plastic Pyrolysis oil Alternative Fuel for CI Engine—A Review. Res. J. Eng. Sci. 2013, 2, 26–30. [Google Scholar]
- Bridjesh, P.; Geetha, N.K. Effect of Diethyl Carbonate as Additive to Waste Plastic Oil on Performance and Emission of a Diesel Engine. Orient. J. Chem. 2020, 36, 189–194. [Google Scholar] [CrossRef]
- Khan, M.Z.H.; Sultana, M.; Al-Mamun, M.R.; Hasan, M.R. Pyrolytic Waste Plastic Oil and Its Diesel Blend: Fuel Characterization. J. Environ. Public Health 2016, 2016, 7869080. [Google Scholar] [CrossRef] [PubMed]
- Kaimal, V.K.; Vijayabalan, P. An investigation on the effects of using DEE additive in a DI diesel engine fueled with waste plastic oil. Fuel 2016, 180, 90–96. [Google Scholar] [CrossRef]
- Abnisa, F. Enhanced Liquid Fuel Production from Pyrolysis of Plastic Waste Mixtures Using a Natural Mineral Catalyst. Energies 2023, 16, 1224. [Google Scholar] [CrossRef]
- Ramavathu, J.; Kota, T.R. Combustion performance and emission characteristics on HCCI engine of waste plastic pyrolysis oil biodiesel blends with external PFI and vaporizer. Int. J. Sustain. Eng. 2020, 13, 204–217. [Google Scholar] [CrossRef]
- Hoang, A.T.; Murugesan, P.; Elumalai, P.V.; Balasubramanian, D.; Parida, S.; Jayabal, C.P.; Nachippan, M.; Kalam, M.A.; Truong, T.H.; Cao, D.N.; et al. Strategic combination of waste plastic/tire pyrolysis oil with biodiesel for natural gas-enriched HCCI engine: Experimental analysis and machine learning model. Energy 2023, 280, 128233. [Google Scholar] [CrossRef]
- Awang, M.S.N.; Zulkifli, N.W.M.; Abbas, M.M.; Zulkifli, M.S.A.; Kalam, M.A.; Yusoff, M.N.A.M.; Ahmad, M.H.; Daud, W.M.A.W. Effect of plastic pyrolytic oil and waste cooking biodiesel on tribological properties of palm biodiesel–diesel fuel blends. Ind. Lubr. Tribol. 2022, 74, 932–942. [Google Scholar] [CrossRef]
- Kaewbuddee, C.; Sukjit, E.; Srisertpol, J.; Maithomklang, S.; Wathakit, K.; Klinkaew, N.; Liplap, P.; Arjharn, W. Evaluation of Waste Plastic Oil-Biodiesel Blends as Alternative Fuels for Diesel Engines. Energies 2020, 13, 2823. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, S.; Sarma, S.J.; Brar, S.K. A comprehensive review of castor oil-derived renewable and sustainable industrial products. Environ. Prog. Sustain. Energy 2023, 42, e14008. [Google Scholar] [CrossRef]
- Mahari, W.A.W.; Chong, C.T.; Cheng, C.K.; Lee, C.L. Production of value-added liquid fuel via microwave co-pyrolysis of used frying oil and plastic waste. Energy 2018, 162, 309–317. [Google Scholar] [CrossRef]
- Kizza, R.; Banadda, N.; Seay, J. Qualitative and energy recovery potential analysis: Plastic-derived fuel oil versus conventional diesel oil. Clean Technol. Environ. Policy 2022, 24, 789–800. [Google Scholar] [CrossRef]
- ASTM D2270; Standard Practice for Calculating Viscosity Index from Kinematic Viscosity at 40 °C and 100 °C. ASTM License Agreement: West Conshohocken, PA, USA, 2016.
- UNE-EN ISO 3675:1999; Crude Petroleum and Liquid Petroleum Products. Laboratory Determination of Density. Hydrometer Method. ISO: Geneva, Switzerland, 1999.
- EN 23015; Petroleum Products-Determination of Cloud Point. ISO: Geneva, Switzerland, 1994.
- ASTM D2500; Standard Test Method for Cloud Point of Petroleum Products and Liquid Fuels. ASTM: West Conshohocken, PA, USA, 2023.
- ISO 3016; Petroleum and Related Products from Natural or Synthetic Sources. Determination of Pour Point. ISO: Geneva, Switzerland, 2019.
- ASTM D97; Standard Test Method for Pour Point of Petroleum Products. ASTM: West Conshohocken, PA, USA, 2023.
- ASTM D2156; Standard Test Method for Smoke Density in Flue Gases from Burning Distillate Fuels. ASTM License Agreement: West Conshohocken, PA, USA, 2018.
- Venugopal, T.; Ramesh, A. Effective utilisation of butanol along with gasoline in a spark ignition engine through a dual injection system. Appl. Therm. Eng. 2013, 59, 550–558. [Google Scholar] [CrossRef]
- Salamanca, M.; Mondragón, F.; Agudelo, J.R.; Benjumea, P.; Santamaría, A. Variations in the chemical composition and morphology of soot induced by the unsaturation degree of biodiesel and a biodiesel blend. Combust. Flame 2012, 159, 1100–1108. [Google Scholar] [CrossRef]


| Property | Diesel | Sunflower Oil | Castor Oil | Plastic Oil |
|---|---|---|---|---|
| Density at 15 °C (kg/m3) | 820 | 920 | 962 | 1045 |
| Kinematic viscosity at 40 °C (cSt) 1 | 3.20 ± 0.03 | 37.80 ± 0.46 | 226.20 ± 0.55 | 2.47 ± 0.01 |
| Calorific value (MJ/kg) | 35.1 | 34.3 | 35.5 | 42.0 |
| Flash point (°C) | 66 | 220 | 228 | 15 |
| Auto-ignition temperature (°C) | 250 | 316 | 448 | 261 |
| Cetane number | 50 | 37 | 40 | 60–71 |
| Plastic Oil (% by Volume) | PO/SO (cSt, Centistokes) | PO/CO (cSt, Centistokes) |
|---|---|---|
| 0 | 37.8 ± 0.46 | 226.2 ± 0.55 |
| 10 | 28.12 ± 0.05 | 150.61 ± 0.54 |
| 30 | 14.15 ± 0.05 | 62.24 ± 0.09 |
| 60 | 6.74 ± 0.04 | 8.79 ± 0.25 |
| 80 | 4.29 ± 0.01 | 5.94 ± 0.05 |
| 90 | 3.12 ± 0.02 | 4.20 ± 0.03 |
| 100 | 2.47 ± 0.01 | 2.47 ± 0.01 |
| Fuel Blend | D/PO/SO | Kinematic Viscosity (cSt) | Density (kg/m3) | Cloud Point (°C) | Pour Point (°C) |
|---|---|---|---|---|---|
| B0 | 100/0/0 | 3.20 ± 0.03 | 820.84 ± 0.02 | −6.0 ± 1.0 | −16.0 ± 1.2 |
| B20SO | 80/16/4 | 3.23 ± 0.03 | 829.85 ± 0.02 | −4.8 ± 1.5 | −8.1 ± 0.7 |
| B40SO | 60/32/8 | 3.40 ± 0.01 | 840.82 ± 0.03 | −4.4 ± 0.7 | −8.8 ± 1.2 |
| B60SO | 40/48/12 | 3.70 ± 0.04 | 898.95 ± 0.06 | −3.9 ± 0.9 | −6.4 ± 1.0 |
| B80SO | 20/64/16 | 4.03 ± 0.01 | 926.87 ± 0.03 | −3.3 ± 1.0 | −8.6 ± 0.3 |
| B100SO | 0/80/20 | 4.29 ± 0.01 | 951.67 ± 0.03 | −1.8 ± 0.8 | −9.5 ± 0.9 |
| Fuel Blend | D/PO/CO | Kinematic Viscosity (cSt) | Density (kg/m3) | Cloud Point (°C) | Pour Point (°C) |
|---|---|---|---|---|---|
| B0 | 100/0/0 | 3.20 ± 0.03 | 820.84 ± 0.02 | −6.0 ± 1.0 | −16.0 ± 1.0 |
| B20CO | 80/18/2 | 3.21 ± 0.04 | 831.67 ± 0.02 | −4.1 ± 1.5 | −9.5 ± 1.2 |
| B40CO | 60/36/4 | 3.23 ± 0.03 | 842.55 ± 0.04 | −3.4 ± 0.9 | −9.8 ± 1.4 |
| B60CO | 40/54/6 | 3.45 ± 0.03 | 853.83 ± 0.03 | −4.8 ± 1.4 | −8.3 ± 0.9 |
| B80CO | 20/72/8 | 3.72 ± 0.03 | 860.00 ± 0.01 | −6.2 ± 0.9 | −8.9 ± 1.0 |
| B100CO | 0/90/10 | 4.20 ± 0.03 | 881.67 ± 0.04 | −3.6 ± 1.1 | −8.5 ± 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Estevez, R.; Aguado-Deblas, L.; López-Tenllado, F.J.; Bautista, F.M.; Romero, A.A.; Luna, D. Study on the Performance and Emissions of Triple Blends of Diesel/Waste Plastic Oil/Vegetable Oil in a Diesel Engine: Advancing Eco-Friendly Solutions. Energies 2024, 17, 1322. https://doi.org/10.3390/en17061322
Estevez R, Aguado-Deblas L, López-Tenllado FJ, Bautista FM, Romero AA, Luna D. Study on the Performance and Emissions of Triple Blends of Diesel/Waste Plastic Oil/Vegetable Oil in a Diesel Engine: Advancing Eco-Friendly Solutions. Energies. 2024; 17(6):1322. https://doi.org/10.3390/en17061322
Chicago/Turabian StyleEstevez, Rafael, Laura Aguado-Deblas, Francisco J. López-Tenllado, Felipa M. Bautista, Antonio A. Romero, and Diego Luna. 2024. "Study on the Performance and Emissions of Triple Blends of Diesel/Waste Plastic Oil/Vegetable Oil in a Diesel Engine: Advancing Eco-Friendly Solutions" Energies 17, no. 6: 1322. https://doi.org/10.3390/en17061322
APA StyleEstevez, R., Aguado-Deblas, L., López-Tenllado, F. J., Bautista, F. M., Romero, A. A., & Luna, D. (2024). Study on the Performance and Emissions of Triple Blends of Diesel/Waste Plastic Oil/Vegetable Oil in a Diesel Engine: Advancing Eco-Friendly Solutions. Energies, 17(6), 1322. https://doi.org/10.3390/en17061322









