Synthesis of Guanidine and Its Deposition on Bacterial Cellulose as Green Heterogeneous Catalyst for Transesterification to Methyl Esters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Guanidine Synthesis
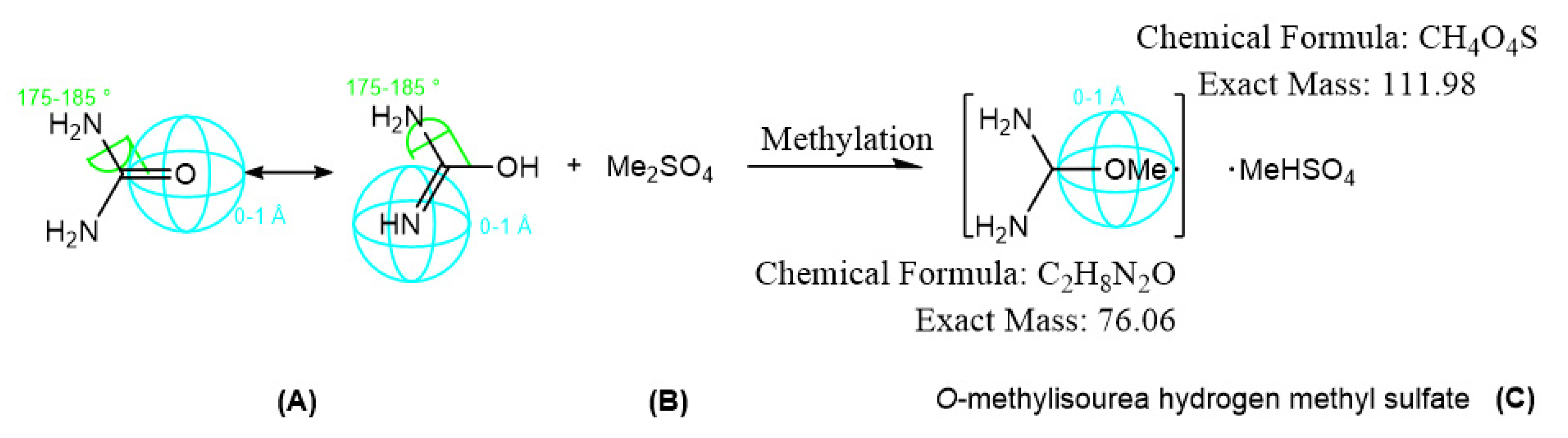
2.2.1. Synthesis of O-methylisourea Hydrogen Methyl Sulfate Precursor
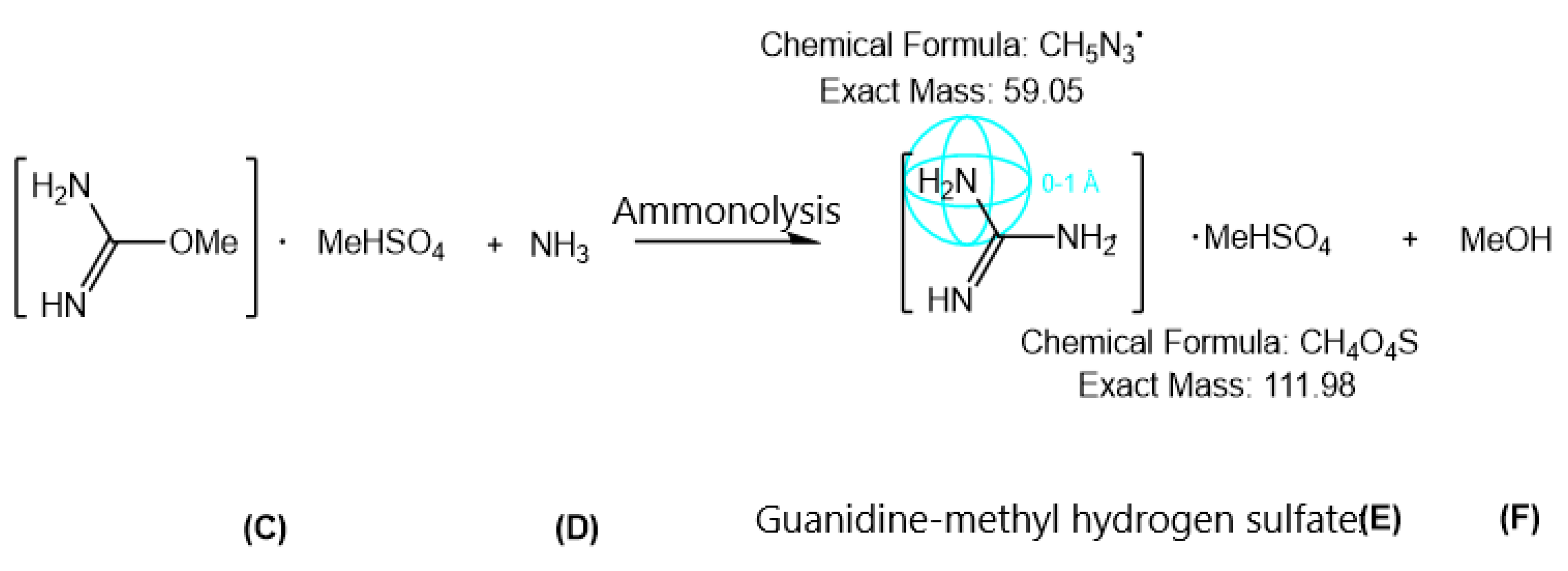
2.2.2. Synthesis of Guanidine-Methyl Hydrogen Sulfate Precursor
2.2.3. Synthesis of Alcoholic Guanidine
2.3. Catalytic Support Preparation
2.4. Heterogeneous Catalyst Synthesis
2.4.1. Treatment with Potassium Hydroxide
2.4.2. Treatment of BCKOH with Guanidine
2.5. Catalyst Analysis
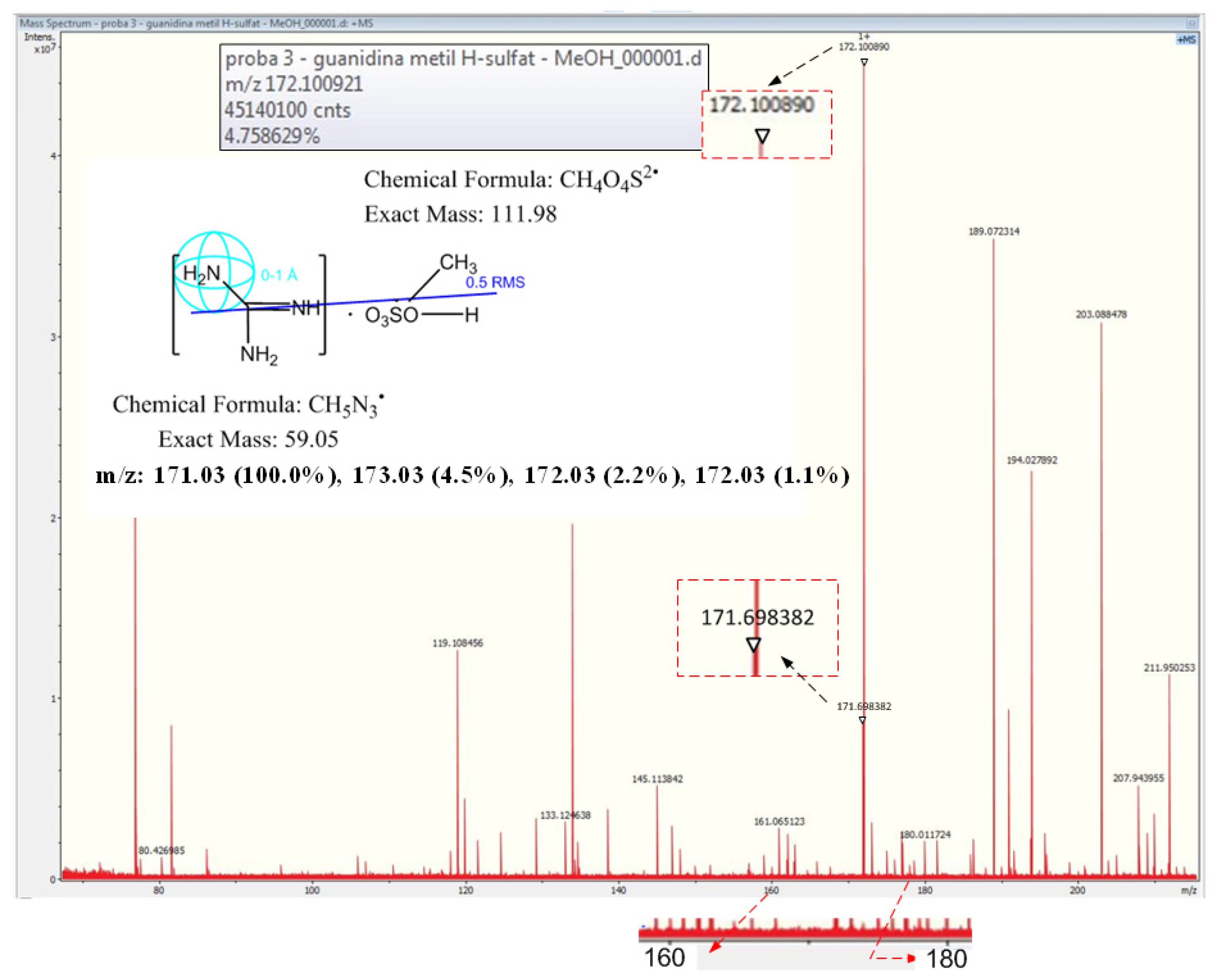
2.5.1. High-Resolution Mass Spectrometry Analysis for Guanidine
2.5.2. Fourier Transform Infrared Spectroscopy for Guanidine
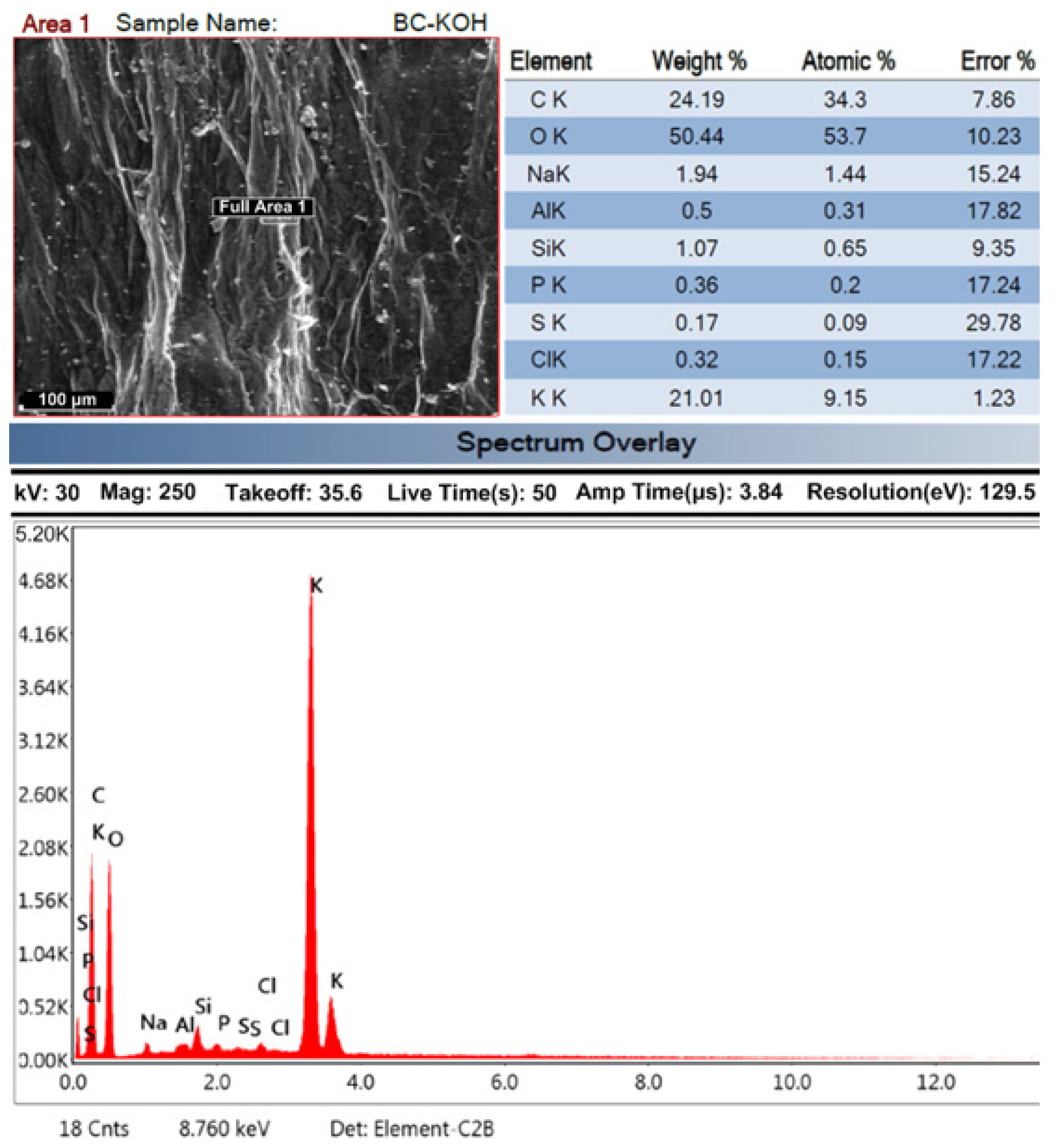
2.5.3. SEM Analysis for Heterogeneous Catalyst
2.6. Catalyst Testing
3. Results and Discussion
3.1. Analysis of Guanidine Synthesis Products
3.1.1. Fourier Transform Infrared Spectroscopy for Guanidine
3.1.2. High-Resolution Mass Spectrometry for Guanidine
3.2. Heterogeneous Catalyst Analysis
SEM Analysis
3.3. Catalyst Testing in Transesterification
- (1)
- (2)
- Considering the orthogonality of the data matrix, calculate the β coefficients in Equation (1) with Equations (3)–(7).
- (3)
- From the matrix containing experimental data, extract the matrix containing data referring to experiments characterizing the reproducibility measurements and compute their variance (Equations (8) and (9)) and the standard deviation of β coefficients (Equation (10)).
- (4)
- Establish the number of freedom degrees characterizing the experiences in the center of the experimental plan (Equation (11)), choose the confidence interval (Equation (12)) for β coefficients, and calculate the theoretical value of the Student variable (tνα), by solving Equation (13).
- (5)
- Evaluate the Student variable value associated to each β coefficient (Equations (14)–(18)).
- (6)
- Verify the significance of each β coefficient by comparing its computed Student variable value with tνα, as follows:
- (7)
- Build the statistical model with the significant β coefficients and prove with the Fischer test that the model is adequate.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ameen, M.; Ahmad, M.; Zafar, M.; Munir, M.; Mujtaba, M.M.; Sultana, S.; Rozina; El-Khatib, S.E.; Soudagar, M.E.M.; Kalam, M.A. Prospects of Catalysis for Process Sustainability of Eco-Green Biodiesel Synthesis via Transesterification: A State-Of-The-Art Review. Sustainability 2022, 14, 7032. [Google Scholar] [CrossRef]
- Balakrishnan, N.K.; Teoh, Y.H.; How, H.G.; Le, T.D.; Nguyen, H.T. An Experimental Investigation on the Characteristics of a Compression Ignition Engine Fuelled by Diesel-Palm Biodiesel–Ethanol/Propanol Based Ternary Blends. Energies 2023, 16, 1003. [Google Scholar] [CrossRef]
- Sonthalia, A.; Kumar, N. Performance Improvement and Emission Reduction Potential of Blends of Hydrotreated Used Cooking Oil, Biodiesel and Diesel in a Compression Ignition Engine. Energies 2023, 16, 7431. [Google Scholar] [CrossRef]
- Karlsson Potter, H.; Yacout, D.M.M.; Henryson, K. Climate Assessment of Vegetable Oil and Biodiesel from Camelina Grown as an Intermediate Crop in Cereal-Based Crop Rotations in Cold Climate Regions. Sustainability 2023, 15, 12574. [Google Scholar] [CrossRef]
- Jabeen, M.; Munir, M.; Abbas, M.M.; Ahmad, M.; Waseem, A.; Saeed, M.; Kalam, M.A.; Zafar, M.; Sultana, S.; Mohamed, A.; et al. Sustainable Production of Biodiesel from Novel and Non-Edible Ailanthus altissima (Mill.) Seed Oil from Green and Recyclable Potassium Hydroxide Activated Ailanthus Cake and Cadmium Sulfide Catalyst. Sustainability 2022, 14, 10962. [Google Scholar] [CrossRef]
- De Feo, G.; Ferrara, C.; Giordano, L.; Ossèo, L.S. Assessment of Three Recycling Pathways for Waste Cooking Oil as Feedstock in the Production of Biodiesel, Biolubricant, and Biosurfactant: A Multi-Criteria Decision Analysis Approach. Recycling 2023, 8, 64. [Google Scholar] [CrossRef]
- Wirawan, S.S.; Solikhah, M.D.; Setiapraja, H.; Sugiyono, A. Biodiesel implementation in Indonesia: Experiences and future perspectives. Renew. Sustain. Energy Rev. 2024, 189, 113911. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration (EIA). Petroleum and Other Liquids. Available online: https://www.eia.gov/dnav/pet/TblDefs/pet_cons_821use_tbldef2.asp (accessed on 10 December 2023).
- Gogoaşă, C.I.; Răducanu, C.E.; Petraş, L.E.; Cioroiu Tîrpan, D.R.; Vasilievici, G.; Mîrţ, A.L.; Dobre, T.; Pârvulescu, O.C. Bacterial Cellulose and Biodegradable Superbase for Heterogeneous Transesterification to Alkyl Esters. Catalysts 2023, 13, 1431. [Google Scholar] [CrossRef]
- Argaw Shiferaw, K.; Mathews, J.M.; Yu, E.; Choi, E.-Y.; Tarte, N.H. Sodium Methoxide/Zeolite-Supported Catalyst for Transesterification of Soybean Waste Cooking Oil for Biodiesel Production. Inorganics 2023, 11, 163. [Google Scholar] [CrossRef]
- Marinković, M.; Waisi, H.; Blagojević, S.; Zarubica, A.; Ljupković, R.; Krstić, A.; Janković, B. The effect of process parameters and catalyst support preparation methods on the catalytic efficiency in transesterification of sunflower oil over heterogeneous KI/Al2O3-based catalysts for biodiesel production. Fuel 2022, 315, 123246. [Google Scholar] [CrossRef]
- Alharbi, W.; Alharbi, K.H.; Roselin, L.S.; Savidha, R.; Selvin, R. Nanosized Silica-Supported 12-Tungstophosphoric Acid: A Highly Active and Stable Catalyst for the Alkylation of p-Cresol with tert-Butanol. Catalysts 2023, 13, 1432. [Google Scholar] [CrossRef]
- Ishikawa, T. Superbases for Organic Synthesis: Guanidines, Amidines, Phosphazenes and Related Organocatalysts; John Wiley and Sons: New York, NY, USA, 2009; ISBN 978-0-470-51800-7. [Google Scholar]
- Alder, R.W.; Bowman, P.S.; Steele, W.R.S.; Winterman, D.R. The remarkable basicity of 1,8-bi(dimethylamino)naphthalene. Chem. Commun. 1968, 13, 723–724. [Google Scholar] [CrossRef]
- Schwesinger, R. Extremely strong, non-ionic bases: Syntheses and applications. Chimia 1985, 39, 69–272. [Google Scholar]
- Verkade, J.G.; Kisanga, P.B. Proazaphosphatranes: A synthesis methodology trip from their discovery to vitamin A. Tetrahedron 2003, 59, 7819–7858. [Google Scholar] [CrossRef]
- Caubere, P. Unimetal super bases. Chem. Rev. 1993, 93, 2317–2334. [Google Scholar] [CrossRef]
- Haflinger, G.; Kuske, F.K.H. The Chemistry of Amidines and Imidates; Patai, S., Rapport, Z., Eds.; John Wiley and Sons: Chichester, UK, 1991; Volume 2, pp. 1–100. [Google Scholar]
- Avram, M. Organic Chemistry; Academica R.S.R.: Bucharest, Romania, 1983; Volume 1. [Google Scholar]
- Rojas, O.J. Cellulose Chemistry and Properties: Fibers, Nanocelluloses and Advanced Materials; Advances in Polymer Science; Springer: Cham, Switzerland, 2016; p. 271. ISBN 978-3-319-26013-6. [Google Scholar]
- Dobre, T.; Stoica, A.; Pârvulescu, O.C.; Stroescu, M.; Iavorschi, G. Factors influence on bacterial cellulose growth in static reactors. Rev. Chim. 2008, 59, 591–594. [Google Scholar] [CrossRef]
- Rainer, J.; Luiz, F.F. Production and application of microbial cellulose. Polym. Degrad. Stab. 1998, 59, 101–106. [Google Scholar]
- Vandamme, E.J.; De Baets, S.; Vanbaelen, A.; Jori, K.; De Wulf, P. Improved production of bacterial cellulose and its application potential. Polym. Degrad. Stab. 1998, 59, 93–99. [Google Scholar] [CrossRef]
- Ge, J.; Yin, Y. Responsive photonic crystals. Angew. Chem. Int. 2011, 50, 1492–1522. [Google Scholar] [CrossRef]
- Tatsumi, M.; Teramoto, Y.; Nishio, Y. Polymer composite reinforced by locking-in liquid-crystalline assembly of cellulose nanocrystallites. Biomacromolecules 2012, 13, 1584–1591. [Google Scholar] [CrossRef]
- Kelly, J.A.; Yu, M.; Hamad, W.Y.; MacLachlan, M.J. Large crack-free freestanding films with chiral nematic structures. Adv. Opt. Mater. 2013, 1, 295–299. [Google Scholar] [CrossRef]
- Ben-Moshe, M.; Alexeev, V.L.; Asher, S.A. Fast responsive crystalline colloidal array photonic crystal glucose sensors. Anal. Chem. 2006, 78, 5149–5157. [Google Scholar] [CrossRef]
- Boscornea, C. Biochemistry Course, I Master, Faculty of Chemical Engineering and Biotechnologies; National University of Science and Technology Politehnica Bucharest: Bucharest, Romania, 2013. [Google Scholar]
- Parvulescu, O.C.; Isopencu, G.; Busuioc, C.; Raducanu, C.; Mocanu, A.; Deleanu, I.; Stoica-Guzun, A. Chapter 17—Antimicrobial bacterial cellulose composites as textile materials. In Antimicrobial Textiles from Natural Resources; Mondal, I.H., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 513–556. [Google Scholar]
- Raducanu, C.E. New Solutions Regarding Catalytic Transesterification Integration with Separation in Biodiesel Technology. Ph.D. Thesis, National University of Science and Technology Politehnica Bucharest, Bucharest, Romania, 2020. [Google Scholar]
- Roberts, E.; Griffiths, J. The Synthesis of Guanidine from Urea; Waltham Abbey: Essex, UK, 1949; p. 41. [Google Scholar]
- Stoica Guzun, A.; Stroescu, M.; Jinga, S.I.; Voicu, G.; Grumezescu, A.M.; Holban, A.M. Plackett–Burman experimental design for bacterial cellulose–silica composites synthesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 280–288. [Google Scholar] [CrossRef]
- Stoica-Guzun, A.; Jecu, L.; Gheorghe, A.; Raut, I.; Stroescu, M.; Ghiurea, M.; Danila, M.; Jipa, I.; Fruth, V. Biodegradation of Poly(vinyl alcohol) and Bacterial Cellulose Composites by Aspergillus niger. J. Polym. Environ. 2011, 19, 1969–1979. [Google Scholar] [CrossRef]
- Păvăloiu, R.D.; Stroescu, M.; Pârvulescu, O.C.; Dobre, T. Composite hydrogels of bacterial cellulose-carboxymethyl cellulose for drug release. Rev. Chim. 2014, 65, 948–951. [Google Scholar]
- Hestrin, S.; Schramm, M. Synthesis of cellulose by Acetobacter xylinum. II. Preparation of freeze-dried cells capable of polymerizing glucose to cellulose. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Dobre, T.G.; Sanchez Marcano, J.G. Statistic Modeling. Chemical Engineering: Modeling, Simulation and Similitude; Wiley: Weinheim, Germany, 2007. [Google Scholar]











| Exp | z1 | z2 | z3 | z4 |
|---|---|---|---|---|
| 1 | 5 | 2 | 6 | 75 |
| 2 | 5 | 2 | 4 | 75 |
| 3 | 5 | 4 | 6 | 75 |
| 4 | 5 | 4 | 4 | 75 |
| 5 | 10 | 2 | 6 | 75 |
| 6 | 10 | 2 | 4 | 75 |
| 7 | 10 | 4 | 6 | 75 |
| 8 | 10 | 4 | 4 | 75 |
| 9 | 5 | 2 | 6 | 105 |
| 10 | 5 | 2 | 4 | 105 |
| 11 | 5 | 4 | 6 | 105 |
| 12 | 5 | 4 | 4 | 105 |
| 13 | 10 | 2 | 6 | 105 |
| 14 | 10 | 2 | 4 | 105 |
| 15 | 10 | 4 | 6 | 105 |
| 16 | 10 | 4 | 4 | 105 |
| 17 | 7.5 | 3 | 5 | 90 |
| 18 | 7.5 | 3 | 5 | 90 |
| 19 | 7.5 | 3 | 5 | 90 |
| 20 | 7.5 | 3 | 5 | 90 |
| Exp | x1 | x2 | x3 | x4 | ||
|---|---|---|---|---|---|---|
| 1 | −1 | −1 | 1 | −1 | 0.9478 | 0.7112 |
| 2 | −1 | −1 | −1 | −1 | 0.9514 | 0.6938 |
| 3 | −1 | 1 | 1 | −1 | 0.9172 | 0.8013 |
| 4 | −1 | 1 | −1 | −1 | 0.9356 | 0.7461 |
| 5 | 1 | −1 | 1 | −1 | 0.9411 | 0.8333 |
| 6 | 1 | −1 | −1 | −1 | 0.9363 | 0.8915 |
| 7 | 1 | 1 | 1 | −1 | 0.9347 | 0.8624 |
| 8 | 1 | 1 | −1 | −1 | 0.9332 | 0.8527 |
| 9 | −1 | −1 | 1 | 1 | 0.9521 | 0.9108 |
| 10 | −1 | −1 | −1 | 1 | 0.9575 | 0.8817 |
| 11 | −1 | 1 | 1 | 1 | 0.9405 | 0.9302 |
| 12 | −1 | 1 | −1 | 1 | 0.9334 | 0.8963 |
| 13 | 1 | −1 | 1 | 1 | 0.9421 | 0.9835 |
| 14 | 1 | −1 | −1 | 1 | 0.9453 | 0.8381 |
| 15 | 1 | 1 | 1 | 1 | 0.9447 | 0.9738 |
| 16 | 1 | 1 | −1 | 1 | 0.9441 | 0.9399 |
| 17 | 0 | 0 | 0 | 0 | 0.9441 | 0.8915 |
| 18 | 0 | 0 | 0 | 0 | 0.9294 | 0.8769 |
| 19 | 0 | 0 | 0 | 0 | 0.9219 | 0.8672 |
| 20 | 0 | 0 | 0 | 0 | 0.9382 | 0.8782 |
| Equation | x1 | x2 | x3 | x4 | Max. Yield |
|---|---|---|---|---|---|
| Equation (22) | 1.290 | 0 | 0.1 | 0 | 0.946 |
| Equation (23) | 1.510 | 2.920 | 1.480 | 0.362 | 0.964 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Răducanu, C.E.; Dobre, T.; Mihăiescu, D.E.; Moroşan, A.; Jidveian, R.; Cioroiu Tîrpan, D.R.; Vasiliu, A.D.; Gogoaşă, C.I.; Pârvulescu, O.C.; Trică, B. Synthesis of Guanidine and Its Deposition on Bacterial Cellulose as Green Heterogeneous Catalyst for Transesterification to Methyl Esters. Energies 2024, 17, 1344. https://doi.org/10.3390/en17061344
Răducanu CE, Dobre T, Mihăiescu DE, Moroşan A, Jidveian R, Cioroiu Tîrpan DR, Vasiliu AD, Gogoaşă CI, Pârvulescu OC, Trică B. Synthesis of Guanidine and Its Deposition on Bacterial Cellulose as Green Heterogeneous Catalyst for Transesterification to Methyl Esters. Energies. 2024; 17(6):1344. https://doi.org/10.3390/en17061344
Chicago/Turabian StyleRăducanu, Cristian Eugen, Tănase Dobre, Dan Eduard Mihăiescu, Alina Moroşan, Roxana Jidveian, Doinița Roxana Cioroiu Tîrpan, Alexandru Dan Vasiliu, Cristina Ionela Gogoaşă, Oana Cristina Pârvulescu, and Bogdan Trică. 2024. "Synthesis of Guanidine and Its Deposition on Bacterial Cellulose as Green Heterogeneous Catalyst for Transesterification to Methyl Esters" Energies 17, no. 6: 1344. https://doi.org/10.3390/en17061344
APA StyleRăducanu, C. E., Dobre, T., Mihăiescu, D. E., Moroşan, A., Jidveian, R., Cioroiu Tîrpan, D. R., Vasiliu, A. D., Gogoaşă, C. I., Pârvulescu, O. C., & Trică, B. (2024). Synthesis of Guanidine and Its Deposition on Bacterial Cellulose as Green Heterogeneous Catalyst for Transesterification to Methyl Esters. Energies, 17(6), 1344. https://doi.org/10.3390/en17061344









