Characteristics of Biodiesel Produced from Crude Palm Oil through Non-Alcohol Synthesis Route Using Dimethyl Carbonate and Immobilized Eco-Enzyme Catalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
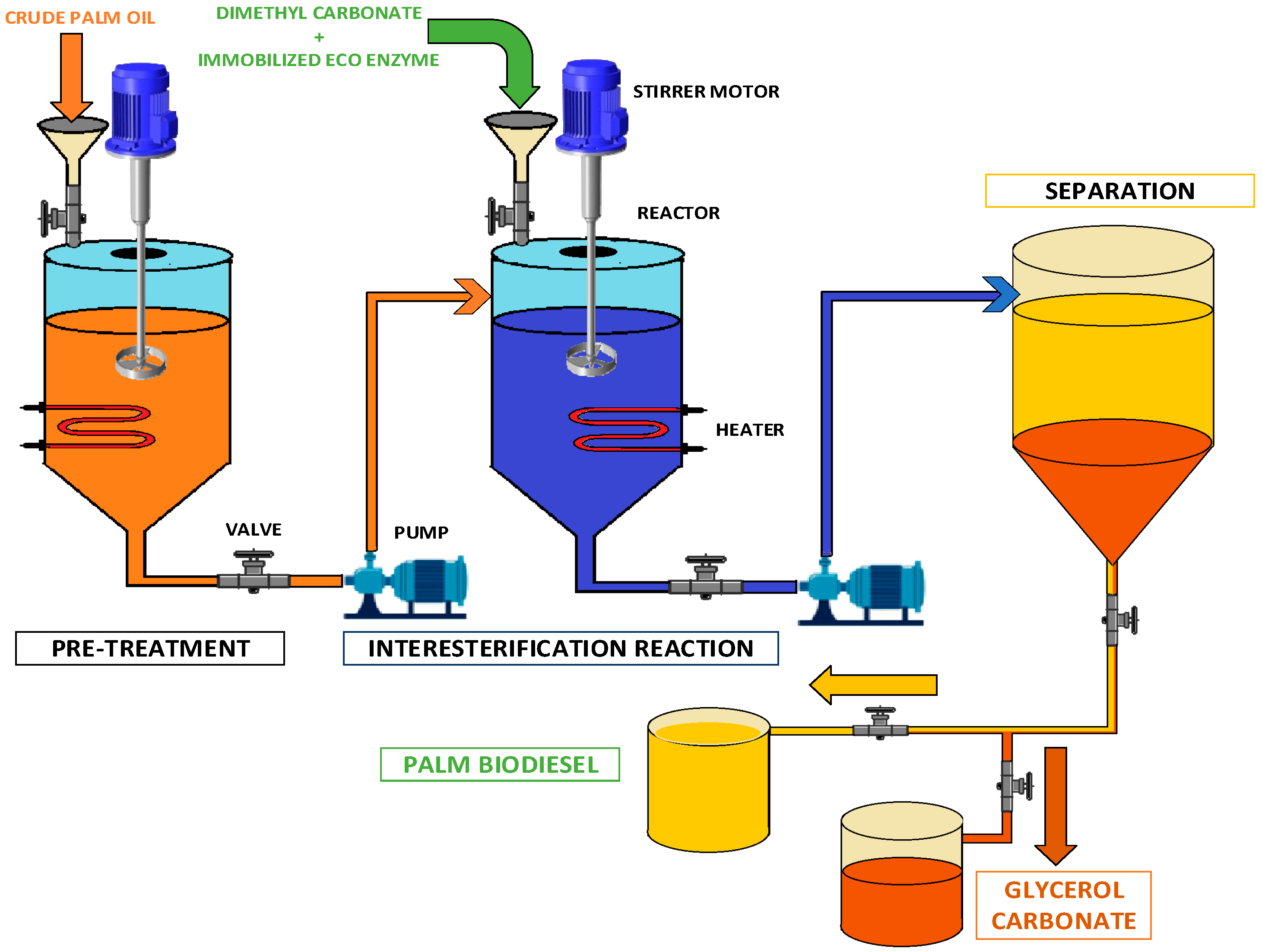
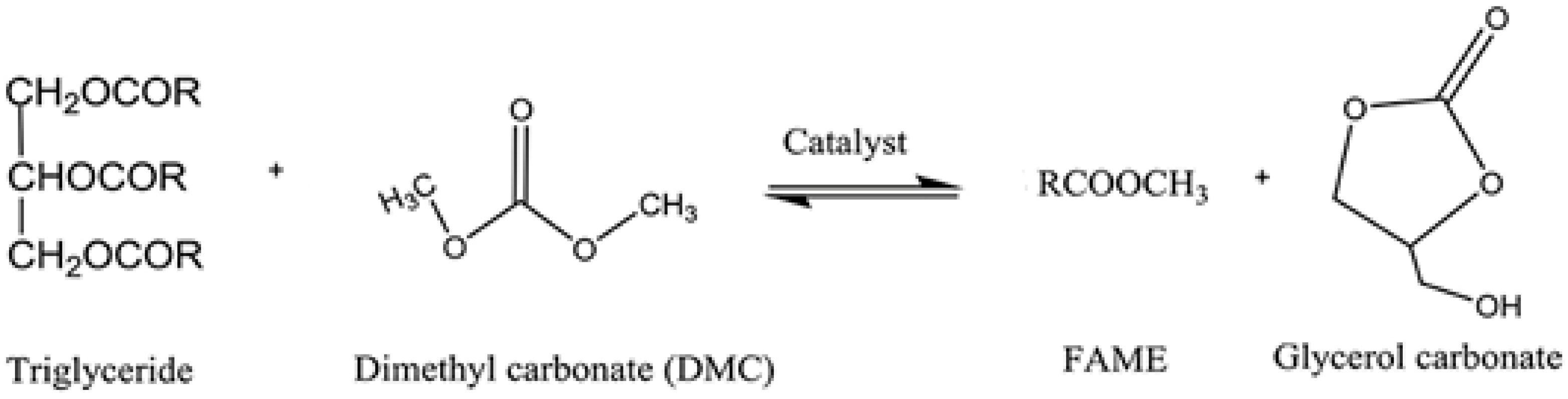
2.3. Production Methods and Interesterification of Biodiesel
3. Results and Discussion
3.1. Conversion Yield of Biodiesel Interesterification
3.2. Characterization of Yield from Biodiesel Interesterification
3.2.1. Density Measurement
3.2.2. Measurement of Biodiesel Viscosity
3.2.3. Flash-Point Test
3.2.4. Calorific Value Test
3.2.5. Cetane Number Test
3.3. Analysis of Gas Chromatography–Mass Spectroscopy
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CPO | crude palm oil |
| DMC | dimethyl carbonate |
| FFA | free fatty acid |
| FAME | fatty acid methyl ester |
| HHV | high heating value |
| LHV | low heating value |
| CO | coconut oil |
| SO | soybean oil |
| SVO | straight vegetable oil |
| CFO | catfish oil |
| ASTM | American Society for Testing and Materials |
| EN | European Norm/European Standard |
| GC-MS | gas chromatography–mass spectrometry |
| Nomenclature | |
| CO | carbon monoxide |
| CO2 | carbon dioxide |
| NOx | nitrogen oxide |
| KOH | potassium hydroxide |
| NaOH | sodium hydroxide |
| MgAl | magnesium aluminide |
| K | potassium |
| HCl | hydrogen chloride |
| H2SO4 | sulfuric acid |
| Na | sodium |
References
- Tnopo, S.J.; Kolo, S.M.D.; Presson, J. Synthesis of Methyl Ester from Feun Kase (Thevetia Peruviana) Seed Oil with Varying Molar Concentration of NaOH Catalyst. J. Chem. Sci. Appl. 2023, 1, 29–35. [Google Scholar]
- Purba, P.P. Effect of Catalyst Amount and Reaction Time on Oxidation and Acid Values in Biodiesel from Cooking Oil Waste. Bachelor’s Thesis, Medan Area University Repository, Medan, Indonesia, 2023; pp. 6–18. Available online: https://repositori.uma.ac.id/jspui/handle/123456789/20113 (accessed on 2 December 2023).
- Azhari, I. The Effect of Catalysts on the Characteristics of Biodiesel from Cooking Oil Waste. Thesis Book. Medan Area University Repository, Medan, Indonesia, 2022; pp. 1–31. Available online: https://repositori.uma.ac.id/jspui/handle/123456789/20114 (accessed on 2 December 2023).
- Kosuru, S.M.Y.; Delhiwala, Y.; Koorla, P.B.; Mekala, M. A review on the biodiesel production: Selection of catalyst, Pre-treatment, Post treatment methods. Green Technol. Sustain. 2024, 2, 100061. [Google Scholar] [CrossRef]
- Budiman, A.A.; Samik, S. Article Review: Review Article: Biodiesel Production from Used Cooking Oil using the Transesterification Method Using a Catalyst. UNESA J. Chem. 2023, 12, 36–38. [Google Scholar]
- Darmawan, A.P.; Zamhari, M.; Junaidi, R. Utilization of Natural Zeolite as a Catalyst for Biodiesel Synthesis. Innov. J. Soc. Sci. Res. 2023, 3, 5703–5714. [Google Scholar]
- Tujjohra, F.; Alam, M.S.; Rahman, M.M.; Rahman, M.M. An Eco-Friendly Approach of Biodiesel Production from Tannery Fleshing Wastes by Crude Neutral Protease Enzyme. Clean. Eng. Technol. 2023, 14, 100638. [Google Scholar] [CrossRef]
- Razzaq, L.; Mujtaba, M.; Shahbaz, M.; Nawaz, S.; Khan, H.M.; Hussain, A.; Ishtiaq, U.; Kalam, M.; Soudagar, M.E.M.; Ismail, K.A.; et al. Effect of Biodiesel-Dimethyl Carbonate Blends on Engine Performance, Combustion and Emission Characteristics. Alexandria Eng. J. 2022, 61, 5111–5121. [Google Scholar] [CrossRef]
- Dewi, S.K.; Yuliati, L.; Widodo, A.S. Characterization of Biodiesel Transesterification Products Using Centrifugation Speed Variations in the Fame Separation Process. J. Mech. Eng. Technol. 2023, 14, 83–95. [Google Scholar] [CrossRef]
- Sumarna, D.; Mulawarman, U. Study of Red Palm Oil Processing Methods. Proceeding Natl. Chem. Conf. 2019, 2014, 1–5. [Google Scholar]
- Sarip, M.S.M.; Morad, N.A.; Aziz, M.K.T.A.; Saparin, N.; Nawi, M.A.H.M. Composition of Crude Palm Oil Extracted Using Hot Compressed Water Extraction. J. Oleo Sci. 2023, 72, 33–38. [Google Scholar] [CrossRef]
- Felletti, S.; Spedicato, M.; Bozza, D.; De Luca, C.; Presini, F.; Giovannini, P.P.; Carraro, M.; Macis, M.; Cavazzini, A.; Catani, M.; et al. Dimethyl Carbonate as a Green Alternative to Acetonitrile in Reversed-Phase Liquid Chromatography. Part I: Separation of Small Molecules. J. Chromatogr. A 2023, 1712, 464530. [Google Scholar] [CrossRef]
- Yang, M.; Li, H.; He, L. Synthesis of Dimethyl Carbonate via Transesterification of Ethylene Carbonate and Methanol using Recyclable Li/NaY Zeolite. Asian J. Org. Chem. 2022, 11, e202200224. [Google Scholar] [CrossRef]
- Bunsaksit, C.; Methaapanon, R.; Bumroongsakulsawat, P.; Bumroongsri, P.; Kim-Lohsoontorn, P. Exploring the Possibility of Carbon Neutral Dimethyl Carbonate Production through Urea Alcoholysis Processes. J. Clean. Prod. 2024, 434, 139586. [Google Scholar] [CrossRef]
- Aydoğdu, S.; Kapucu, N. A Review on The Achievement of Enzymatic Glycerol Carbonate Production. Turkish J. Chem. 2022, 46, 1376–1396. [Google Scholar] [CrossRef]
- Benny, N.; Shams, R.; Dash, K.K.; Pandey, V.K.; Bashir, O. Recent Trends in Utilization of Citrus Fruits in Production of Eco-Enzyme. J. Agric. Food Res. 2023, 13, 100657. [Google Scholar] [CrossRef]
- Kalita, P.; Basumatary, B.; Saikia, P.; Das, B.; Basumatary, S. Biodiesel as Renewable Biofuel Produced via Enzyme-Based Catalyzed Transesterification. Energy Nexus 2022, 6, 100087. [Google Scholar] [CrossRef]
- Kusumaningtyas, R.D.; Purnamasari, I.; Mahmudati, R.; Prasetiawan, H. Interesterification Reaction of Vegetable Oil and Alkyl Acetate as Alternative Route for Glycerol-Free Biodiesel Synthesis. In Biofuels and Bioenergy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 435–452. [Google Scholar] [CrossRef]
- Esan, A.O.; Olalere, O.A.; Gan, C.-Y.; Smith, S.M.; Ganesan, S. Synthesis of Biodiesel from Waste Palm Fatty Acid Distillate (PFAD) and Dimethyl Carbonate (DMC) Via Taguchi Optimisation Method. Biomass Bioenergy 2021, 154, 106262. [Google Scholar] [CrossRef]
- Rashid, K.T.; Mansour, K.; Abid, M.F.; Ali, S.M.; Abed, K.N. Synthesis of Dimethyl Carbonate for Enhancement of Gasoline Performance. J. King Saud Univ.-Eng. Sci. 2019, 31, 171–177. [Google Scholar] [CrossRef]
- Setiadi; Hamid; Nawfal, F. Synthesis of Biodiesel from Palm Oil with Dimethyl Carbonate and Methanol as Reagent Variation Using KOH and Enzyme Catalyst. IOP Conf. Ser. Earth Environ. Sci. 2018, 105, 012103. [Google Scholar] [CrossRef]
- EN 590; Biodiesel Standard Guidelines. European Committee for Standarization: Brussel, Belgia, 2022; pp. 3–15. Available online: https://cdn.standards.iteh.ai/samples/70815/1d6a0c7cc9524b9794579f46ae3aa744/SIST-EN-590-2022.pdf (accessed on 15 December 2023).
- Nugraha, R.P. Advancement of Utilizing Dimethyl Carbonate and Diethyl Carbonate as Eco-Friendly Additives in Gasoline. Dissertation Book. Sepuluh Nopember Institute of Technology, Surabaya, Indonesia, 2019; pp. 16–58. Available online: https://repository.its.ac.id/62463/1/02211660010007-Dissertation.pdf (accessed on 2 December 2023).
- Bardier, F.B. Green Chemistry. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 77–82. [Google Scholar] [CrossRef]
- Worldwide Fuel Charter Committee. Biodiesel Guidelines. March 2009; pp. 1–14. Available online: http://www.acea.be/uploads/publications/20090423_B100_Guideline.pdf (accessed on 2 December 2023).
- Merck Chemicals and Life Sciences. Product Identifier: Dimethyl Carbonate. Available online: www.Sigmaaldrich.com (accessed on 7 December 2023).
- ASTM D1298; Standard Test Method for Density, Relative Density (Specific Gravity), or API Gravity of Crude Petroleum and Liquid Petroleum Products by Hydrometer Method. ASTM International: West Conshohocken, PA, USA, 2003; Volume 9.1, p. 221. [CrossRef]
- ASTM D445; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (the Calculation of Dynamic Viscosity). ASTM International: West Conshohocken, PA, USA, 2017; Volume Part 71: S, pp. 126–133. [CrossRef]
- Koehler Instrument Company. Textbook of Koehler General Test Equipment and Method; 85 Corporate Drive Holtsville: New York, NY, USA, 2022; pp. 46–47. [Google Scholar]
- ASTM D2699; Standard Test Method for Research Octane Number of Spark-Ignition Engine Fuel 1. American Society for Testing and Materials: West Conshohocken, PA, USA, 2023; pp. 1–48. Available online: http://www.ansi.org (accessed on 12 December 2023).
- ASTM D93; Standard Test Methods for Flash Point by Pensky-Martens Closed Cup Tester. ASTM International: West Conshohocken, PA, USA, 2017; p. 80112.
- ASTM D5769; American Society for Testing and Materials. Annual Book of ASTM D5769 QC Standards. ASTM International: New Haven, CT, USA, 2003; pp. 1–8. Available online: https://www.astm.org/d5769-22.html (accessed on 12 December 2023).
- Anwar, F.; Tariq, M.; Nisar, J.; Ali, G.; Kanwal, H. Optimization of Biodiesel Yield from Non-Food Karanja Seed Oil: Characterization and Assessment of Fuel Properties. Sustain. Chem. Environ. 2023, 3, 100035. [Google Scholar] [CrossRef]
- Wang, W.; Liu, H.; Li, F.; Wang, H.; Ma, X.; Li, J.; Zhou, L.; Xiao, Q. Effects of Unsaturated Fatty Acid Methyl Esters on The Oxidation Stability of Biodiesel Determined by Gas Chromatography-Mass Spectrometry and Information Entropy Methods. Renew. Energy 2021, 175, 880–886. [Google Scholar] [CrossRef]
- Burmana, A.D.; Tambun, R.; Haryanto, B.; Sarah, M.; Alexander, V. Recycling Heterogeneous Catalyst Waste in Biodiesel Production Using Methanol and Hydrochloric Acid: A Case Study on The Washing Effect with Lauric Acid as Raw Material. Case Stud. Chem. Environ. Eng. 2023, 8, 100510. [Google Scholar] [CrossRef]
- Makareviciene, V.; Sendzikiene, E.; Gumbyte, M. Application of Simultaneous Oil Extraction and Transesterification in Biodiesel Fuel Synthesis: A review. Energies 2020, 13, 2204. [Google Scholar] [CrossRef]
- Sinaga, S.V.; Haryanto, A.; Triyono, S. Effect of Temperature and Reaction Time on Making Biodiesel from Used Cooking Oil. J. Tek. Pertan. Lampung 2014, 3, 27–34. Available online: http://www.youtube.com (accessed on 15 December 2023).
- Simões, S.S.; Ribeiro, J.S.; Celante, D.; Brondani, L.N.; Castilhos, F. Heterogeneous Catalyst Screening for Fatty Acid Methyl Esters Production Through Interesterification Reaction. Renew. Energy 2020, 146, 719–726. [Google Scholar] [CrossRef]
- Kampars, V.; Abelniece, Z.; Lazdovica, K.; Kampare, R. Interesterification of Rapeseed Oil with Methyl Acetate in The Presence of Potassium Tert-Butoxide Solution in Tetrahydrofuran. Renew. Energy 2020, 158, 668–674. [Google Scholar] [CrossRef]
- Christopher, L.P.; Kumar, H.; Zambare, V.P. Enzymatic biodiesel: Challenges and Opportunities. Appl. Energy 2014, 119, 497–520. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Khilji, I.A.; Sirohi, R.; Pandey, A.; Baskar, G.; Satyavolu, J. Maximizing the Value of Biodiesel Industry Waste: Exploring Recover, Recycle, and Reuse for Sustainable Environment. Environ. Technol. Innov. 2023, 32, 103447. [Google Scholar] [CrossRef]
- Dhawan, M.S.; Barton, S.C.; Yadav, G.D. Interesterification of Triglycerides with Methyl Acetate for The Co-Production Biodiesel and Triacetin Using Hydrotalcite as A Heterogenous Base Catalyst. Catal. Today 2021, 375, 101–111. [Google Scholar] [CrossRef]
- Dharmawan, A.; Fauzi, A.; Putri, E.; Pacheco, P.; Dermawan, A.; Nuva, N.; Amalia, R.; Sudaryanti, D. Bioenergy Policy: The Biodiesel Sustainability Dilemma in Indonesia. Int. J. Sustain. Dev. Plan. 2020, 15, 537–546. [Google Scholar] [CrossRef]
- Rozina; Ahmad, M.; Elnaggar, A.Y.; Teong, L.K.; Sultana, S.; Zafar, M.; Munir, M.; Hussein, E.E.; Abidin, S.Z.U. Sustainable and Eco-Friendly Synthesis of Biodiesel from Novel and Non-Edible Seed Oil of Monotheca buxifolia Using Green Nano-Catalyst of Calcium Oxide. Energy Convers. Manag. X 2022, 13, 100142. [Google Scholar] [CrossRef]
- ASTM D6571; Standard Test Method for Determination of Compression Resistance and Recovery Properties of Highloft Nonwoven Fabric Using Static Force Loading. American Society for Testing and Materials: West Conshohocken, PA, USA, 2010; pp. 1–6. Available online: https://www.astm.org/d6571-01.html (accessed on 15 December 2023).
- EN 14214; The Requirements and Test Methods for FAME Biodiesel. European Committee for Standarization: Brussel, Belgia, 2019; pp. 1–11. Available online: https://cdn.standards.iteh.ai/samples/68664/f2c4423443d244afa68d894b03089fe1/SIST-EN-14214-2012-A2-2019.pdf (accessed on 15 December 2023).
- China GB/T 20828–2007; Biodiesel Blend Stock (BD 100) for Diesel Engine Fuels. National Standards of People’s Republic of China. National Petroleum Products and Lubricants Standardization Technical Committee: Beijing, China, 2015; pp. 1–9. Available online: https://www.chinesestandard.net/PDF/English.aspx/GBT20828-2007 (accessed on 15 December 2023).
- Visioli, L.J.; Nunes, A.L.B.; Wancura, J.H.C.; Enzweiler, H.; Vernier, L.J.; de Castilhos, F. Batch and continuous γ-alumina-catalyzed FAME production from soybean oil deodorizer distillate by interesterification. Fuel 2023, 351, 128954. [Google Scholar] [CrossRef]
- Agu, C.M.; Ani, K.A.; Abiazieije, P.O.; Omeje, J.A.; Ekuma, J.C.; Umelo, U.E.; Omukwu, O.H.; Nwankwo, E.D.; Chinedu, M.P. Biodiesel production from waste cat fish oil using heterogeneous catalyst from cat fish born: A viable waste management approach, and ANN modeling of biodiesel yield. Waste Manag. Bull. 2023, 1, 172–181. [Google Scholar] [CrossRef]
- Wati, I.; Pertiwi, D.S. The Effect of Time, Temperature and Transesterification Reaction Ratio on Biodiesel Quality of Kesambi Seed Oil (Schleichera Oleosa Lour). Migasian 2023, 7, 31–44. [Google Scholar] [CrossRef]
- Russo, D.; Portarapillo, M.; Di Benedetto, A. Flash Point of Biodiesel/Glycerol/Alcohol Mixtures for Safe Processing and Storage. J. Loss Prev. Process Ind. 2023, 83, 105077. [Google Scholar] [CrossRef]
- Prak, D.L.; Hamilton, M.; Banados, R.; Cowart, J. Density, Viscosity, Speed of Sound, Flash Point, Bulk Modulus, and Surface Tension of Mixtures of Military Jet Fuel JP-5 and Biodiesels Dataset. Data Br. 2022, 41, 107849. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, D.C.D.; Carareto, N.D.D.; Neto, A.M.B.; Gerbaud, V.; da Costa, M.C. Flash Point Prediction with UNIFAC Type Models of Ethylic Biodiesel and Binary/Ternary Mixtures of FAEEs. Fuel 2020, 281, 118717. [Google Scholar] [CrossRef]
- Barbarey, M.S.; Seleman, M.M.E.-S.; El Kheshen, A.A.; Zawrah, M.F. Utilization of Ladle Furnace Slag for Fabrication of Geopolymer: Its Application as Catalyst for Biodiesel Production. Constr. Build. Mater. 2024, 411, 134226. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, W.; Bao, H.; Li, F.; Zhou, L. Biodiesel-Diesel Blend Optimized via Leave-One Cross-Validation Based on Kinematic Viscosity, Calorific Value, and Flash Point. Ind. Crops Prod. 2023, 191, 115914. [Google Scholar] [CrossRef]
- Pawar, S.; Hole, J.; Bankar, M.; Khan, S.; Wankhade, S. Use of Fatty Acid Chemical Composition for Predicting Higher Calorific Value of Biodiesel. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Aqilah, S.; My, O.; Saifuddin, N. Non-Catalytic Microwave Assisted Transesterification of Palm Oil with Dimethyl Carbonate. J. Mech. Eng. Technol. 2020, 12, 37–52. [Google Scholar]
- Ghiasi, M.M.; Mohammadzadeh, O.; Zendehboudi, S. Reliable Connectionist Tools to Determine Biodiesel Cetane Number Based on Fatty Acids Methyl Esters Content. Energy Convers. Manag. 2022, 264, 115601. [Google Scholar] [CrossRef]
- Kumbhar, V.; Pandey, A.; Sonawane, C.R.; El-Shafay, A.S.; Panchal, H.; Chamkha, A.J. Statistical Analysis on Prediction of Biodiesel Properties from Its Fatty Acid Composition. Case Stud. Therm. Eng. 2022, 30, 101775. [Google Scholar] [CrossRef]
- Dzakiroh, A.; Rahmadina, N.; Syarif, A.; Rusnadi, I. The Use of Deep Eutectic Solvent in Reducing Used Cooking Oil FFA and the Effect of Stirring Speed and Time. Energy Engineering Study Program, Department of Chemical Engineering. Bachelor’s Thesis, Politeknik Negeri Sriwijaya, Kota Palembang, Indonesia, 2023; pp. 125–129. [Google Scholar]
- Setyawardhani, D.A.; Distantina, S.; Henfiana, H.; Dewi, A.S. Improving the Quality of Biodiesel from Saturated Fatty Acids of Rubber Seed Oil using the Hydrolysis Process. Ekuilibrium 2021, 9, 47–50. Available online: https://jurnal.uns.ac.id/ekuilibrium/article/view/49534 (accessed on 5 January 2024).
- Saifuddin, S.; Shalihah, N.; Nahar, N.; Nageubri, R. Synthesis and Characterization of Biodiesel Production from Wolffia Using Homogeneous KOH Catalyst by In Situ Transesterification. Devot. J. Community Serv. 2023, 4, 778–787. [Google Scholar] [CrossRef]
- de Carvalho Rochade, W.F.; Sheen, D.A. Determination of Physicochemical Properties of Petroleum Derivatives and Biodiesel Using GC/MS and Chemometric Methods with Uncertainty Estimation. Fuel 2019, 243, 413–422. [Google Scholar] [CrossRef]

| Parameters | Unit | Value |
|---|---|---|
| Density at 150 °C | kg/m3 | 850–900 |
| Viscosity at 400 °C | mm2/s (cSt) | 3.5–5.0 |
| Flash Point | °C | 120 |
| Cetane Number | min | 51 |
| Calorific Value (Gross Specific Energy) | MJ/kg | Max 45.4 |
| Sulfur Content | mg/kg | 10.0 |
| Carbon Residue | % (m/m) | 0.30 |
| Sulfated Ash Content | % (m/m) | 0.01 |
| Water Content | mg/kg | 200 |
| Total Contamination | mg/kg | 24 |
| Oxidative Stability | g/m3 | 25 |
| Acid Value | mg KOH/g | 0.50 |
| Iodine Value | g I/100 g | 130 |
| Linolenic Acid Content | % (m/m) | 0.20 |
| Monoglyceride Content | % (m/m) | 0.80 |
| Diglyceride Content | % (m/m) | 0.20 |
| Free Glycerin | % (m/m) | 0.02 |
| Total Glycerin | % (m/m) | 0.25 |
| Alkali Metals (Na + K) | mg/kg | 5.0 |
| Run | DMC Ratio (Mol) | Reaction Time (Minute) | Temperature (°C) | Catalyst Ratio (%) | Yield (%) | Density (g/mL) | Viscosity (mm2/s) |
|---|---|---|---|---|---|---|---|
| 1 | 2.5 | 45 | 47.5 | 3 | 68.82 | 0.87 | 5.06 |
| 2 | 2 | 60 | 50 | 4 | 66.35 | 0.87 | 5.47 |
| 3 | 2.5 | 75 | 47.5 | 5 | 71.63 | 0.87 | 4.63 |
| 4 | 1.5 | 75 | 47.5 | 5 | 63.76 | 0.88 | 6.34 |
| 5 | 1.5 | 75 | 47.5 | 3 | 62.68 | 0.88 | 6.80 |
| 6 | 2 | 60 | 55 | 6 | 66.89 | 0.87 | 5.38 |
| 7 | 2 | 60 | 55 | 2 | 64.55 | 0.87 | 5.99 |
| 8 | 2.5 | 45 | 47.5 | 5 | 70.41 | 0.87 | 4.63 |
| 9 | 1.5 | 45 | 62.5 | 3 | 62.87 | 0.88 | 6.80 |
| 10 | 1.5 | 75 | 62.5 | 3 | 63.23 | 0.88 | 6.75 |
| 11 | 2 | 90 | 55 | 4 | 65.83 | 0.87 | 5.47 |
| 12 | 2 | 60 | 55 | 4 | 66.35 | 0.87 | 5.44 |
| 13 | 3 | 60 | 55 | 4 | 73.65 | 0.86 | 3.73 |
| 14 | 2 | 60 | 55 | 4 | 65.83 | 0.87 | 5.48 |
| 15 | 2 | 60 | 55 | 4 | 65.48 | 0.87 | 5.48 |
| 16 | 1.5 | 45 | 62.5 | 5 | 63.14 | 0.88 | 6.34 |
| 17 | 2 | 60 | 55 | 4 | 66.22 | 0.87 | 5.47 |
| 18 | 1.5 | 75 | 62.5 | 5 | 63.58 | 0.88 | 6.34 |
| 19 | 2 | 60 | 55 | 4 | 65.48 | 0.87 | 5.48 |
| 20 | 2 | 60 | 55 | 4 | 66.61 | 0.87 | 5.48 |
| 21 | 1.5 | 45 | 47.5 | 5 | 62.87 | 0.88 | 6.34 |
| 22 | 1 | 60 | 55 | 4 | 58.83 | 0.93 | 6.92 |
| 23 | 2.5 | 45 | 62.5 | 3 | 69.28 | 0.87 | 5.06 |
| 24 | 2.5 | 75 | 62.5 | 5 | 72.19 | 0.87 | 4.63 |
| 25 | 1.5 | 45 | 47.5 | 3 | 62.14 | 0.88 | 6.80 |
| 26 | 2 | 60 | 70 | 4 | 67.21 | 0.87 | 5.47 |
| 27 | 2.5 | 75 | 47.5 | 3 | 68.46 | 0.87 | 5.06 |
| 28 | 2 | 30 | 55 | 4 | 66.69 | 0.87 | 5.47 |
| 29 | 2.5 | 45 | 62.5 | 5 | 71.71 | 0.87 | 4.63 |
| 30 | 2.5 | 75 | 62.5 | 3 | 68.95 | 0.87 | 5.06 |
| European Biodiesel Standard (EN 590) | 0.850–0.900 g/mL | 3.5–5.0 mm2/s (cSt) | |||||
| Run | DMC Ratio (Mol) | Reaction Time (Minute) | Temperature (°C) | Catalyst Ratio (%) | Flash Point (°C) | Calorific Value (MJ/kg) | Cetane Number (CN) |
|---|---|---|---|---|---|---|---|
| 4 | 1.5 | 75 | 47.5 | 5 | 103 | 30.1 | 70.6 |
| 5 | 1.5 | 75 | 47.5 | 3 | 100 | 29.3 | 76.7 |
| 6 | 2 | 60 | 55 | 6 | 108 | 27.9 | 79.3 |
| 7 | 2 | 60 | 55 | 2 | 110 | 28.1 | 80.5 |
| 12 | 2 | 60 | 55 | 4 | 105 | 29.7 | 82.9 |
| 8 | 2.5 | 45 | 47.5 | 5 | 106 | 28.1 | 83.3 |
| 23 | 2.5 | 45 | 62.5 | 3 | 109 | 30.1 | 84.3 |
| 24 | 2.5 | 75 | 62.5 | 5 | 110 | 30.7 | 84.6 |
| 13 | 3 | 60 | 55 | 4 | 113 | 34.4 | 86.1 |
| European Biodiesel Standard (EN 590) | Min. 120 °C | Max. 45.4 | Min. 51 | ||||
| Peak | Retention Time (Minutes) | Area (mg/g) | Compound Name |
|---|---|---|---|
| 1 | 13.014 | 41.45 | Hexadecanoic acid, ethyl ester (CAS) ethyl |
| 2 | 16.195 | 33.36 | Oleic acid |
| 3 | 16.460 | 16.13 | Ethyl oleate |
| 4 | 22.063 | 2.19 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl) |
| 5 | 24.792 | 2.93 | Oleoyl chloride |
| 6 | 26.546 | 3.95 | Squalene |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balfas, R.N.; Muhammad Syam, A.; Muhammad, M.; Setiawan, A.; Fithra, H. Characteristics of Biodiesel Produced from Crude Palm Oil through Non-Alcohol Synthesis Route Using Dimethyl Carbonate and Immobilized Eco-Enzyme Catalyst. Energies 2024, 17, 1551. https://doi.org/10.3390/en17071551
Balfas RN, Muhammad Syam A, Muhammad M, Setiawan A, Fithra H. Characteristics of Biodiesel Produced from Crude Palm Oil through Non-Alcohol Synthesis Route Using Dimethyl Carbonate and Immobilized Eco-Enzyme Catalyst. Energies. 2024; 17(7):1551. https://doi.org/10.3390/en17071551
Chicago/Turabian StyleBalfas, Reza Nageubri, Azhari Muhammad Syam, Muhammad Muhammad, Adi Setiawan, and Herman Fithra. 2024. "Characteristics of Biodiesel Produced from Crude Palm Oil through Non-Alcohol Synthesis Route Using Dimethyl Carbonate and Immobilized Eco-Enzyme Catalyst" Energies 17, no. 7: 1551. https://doi.org/10.3390/en17071551
APA StyleBalfas, R. N., Muhammad Syam, A., Muhammad, M., Setiawan, A., & Fithra, H. (2024). Characteristics of Biodiesel Produced from Crude Palm Oil through Non-Alcohol Synthesis Route Using Dimethyl Carbonate and Immobilized Eco-Enzyme Catalyst. Energies, 17(7), 1551. https://doi.org/10.3390/en17071551







