Ammonia Combustion: Internal Combustion Engines and Gas Turbines
Abstract
:1. Introduction
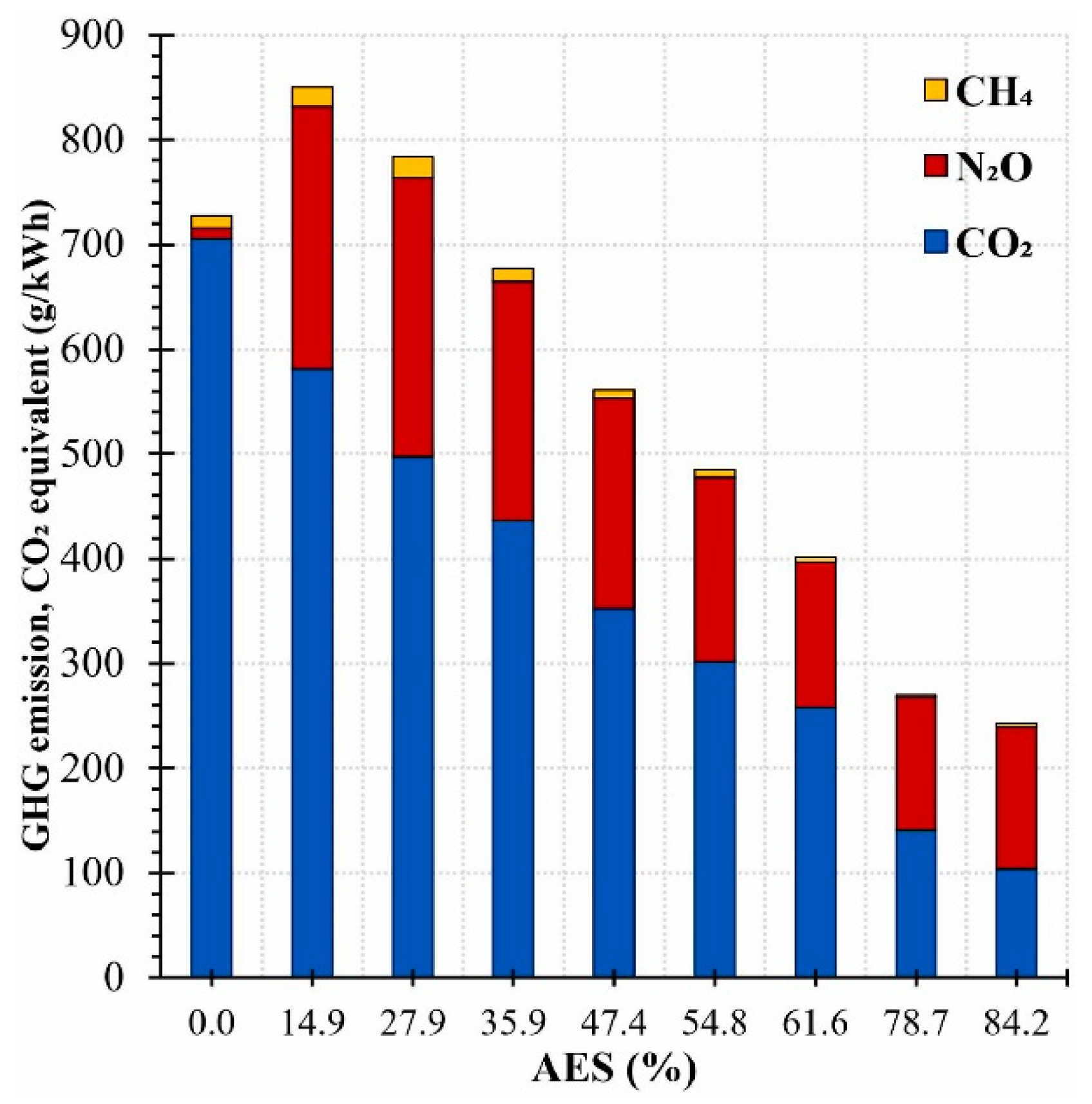
2. Ammonia Combustion in Internal Combustion Engines
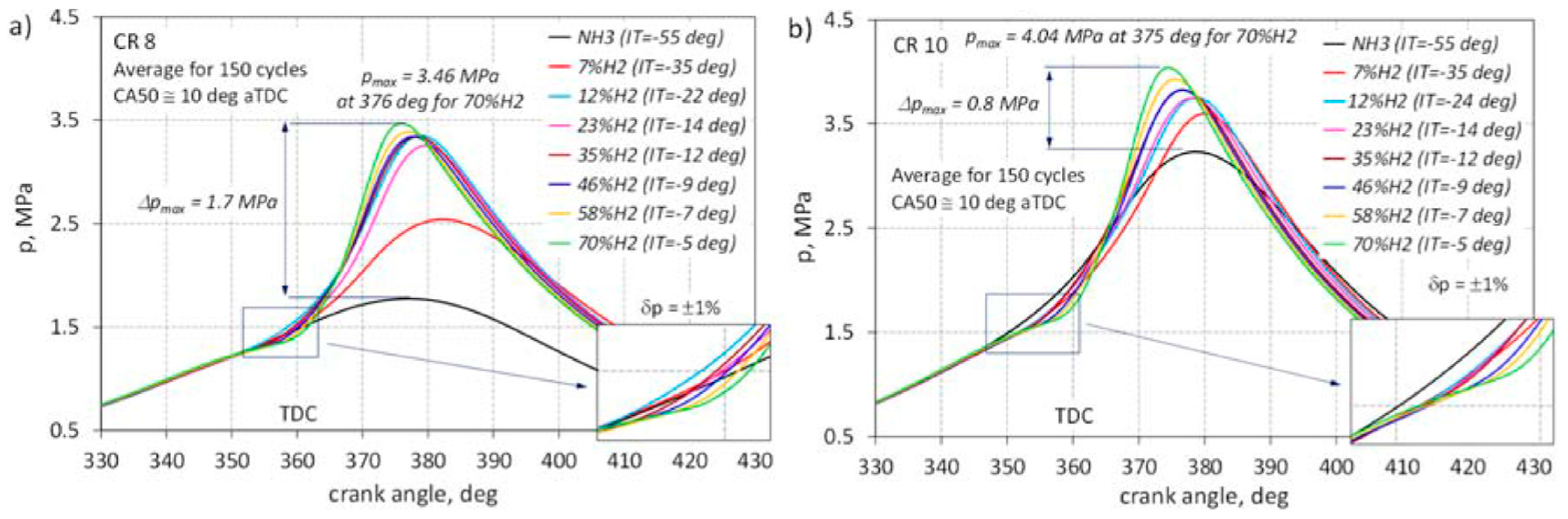
2.1. Ammonia Combustion
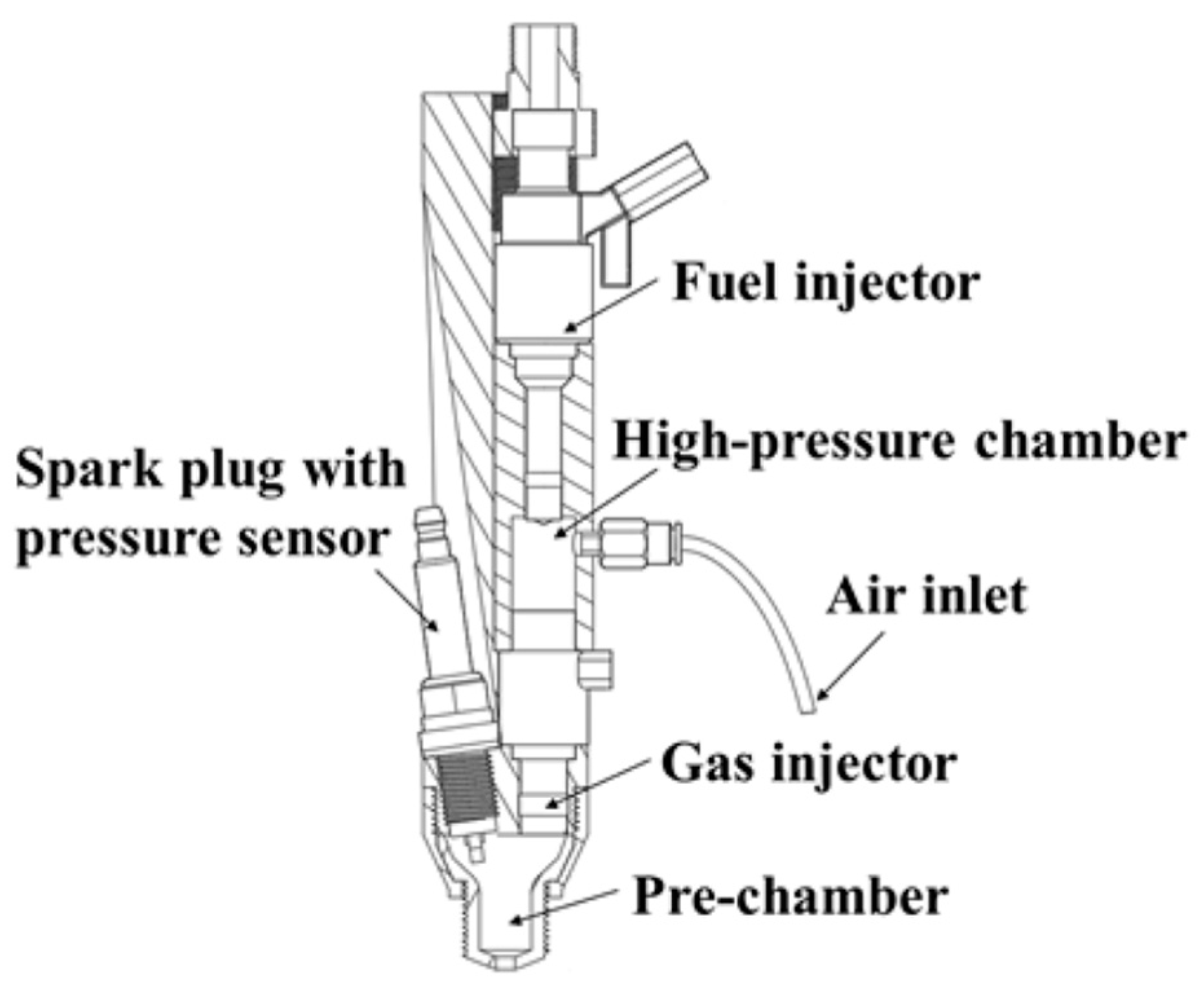
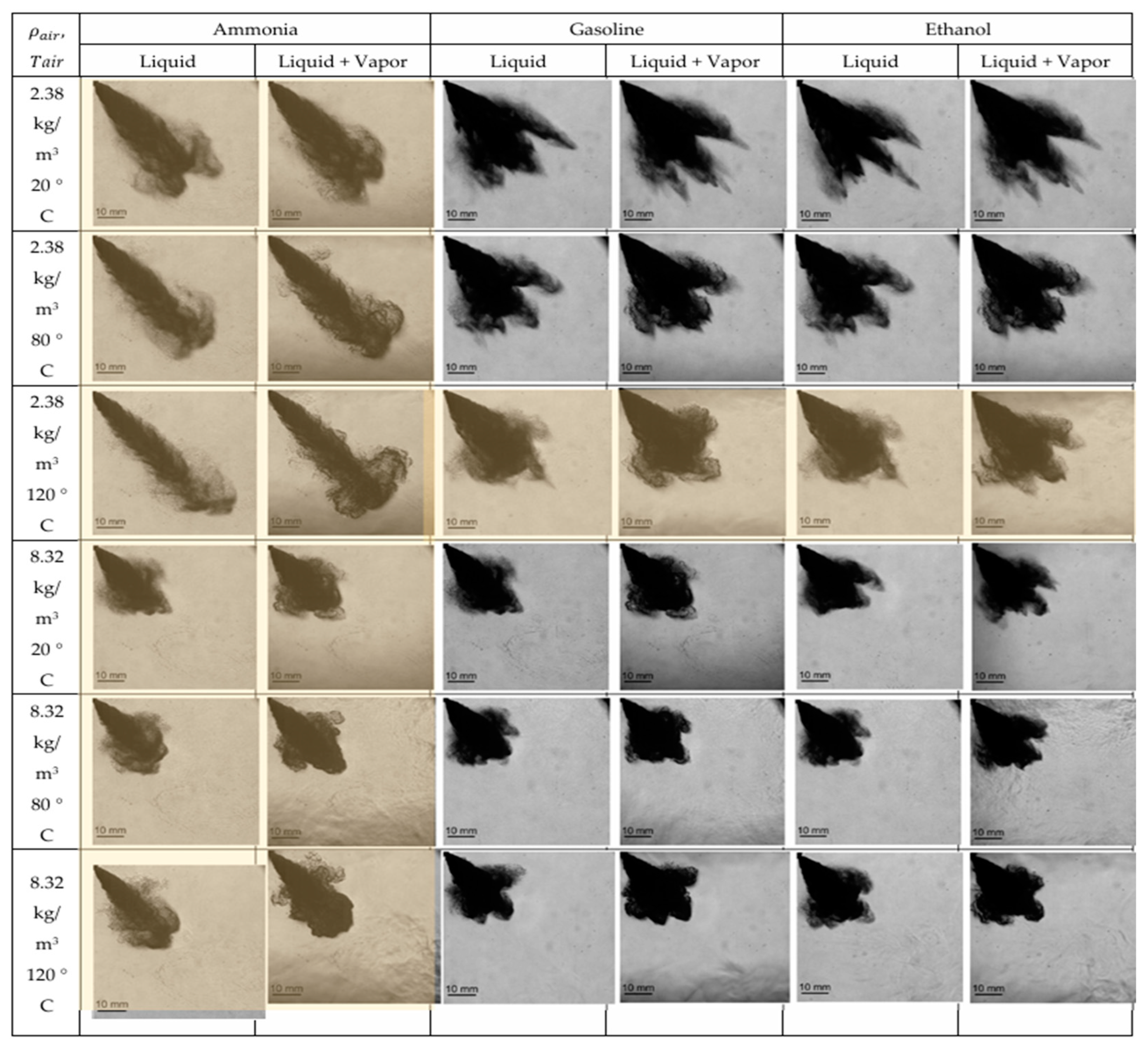
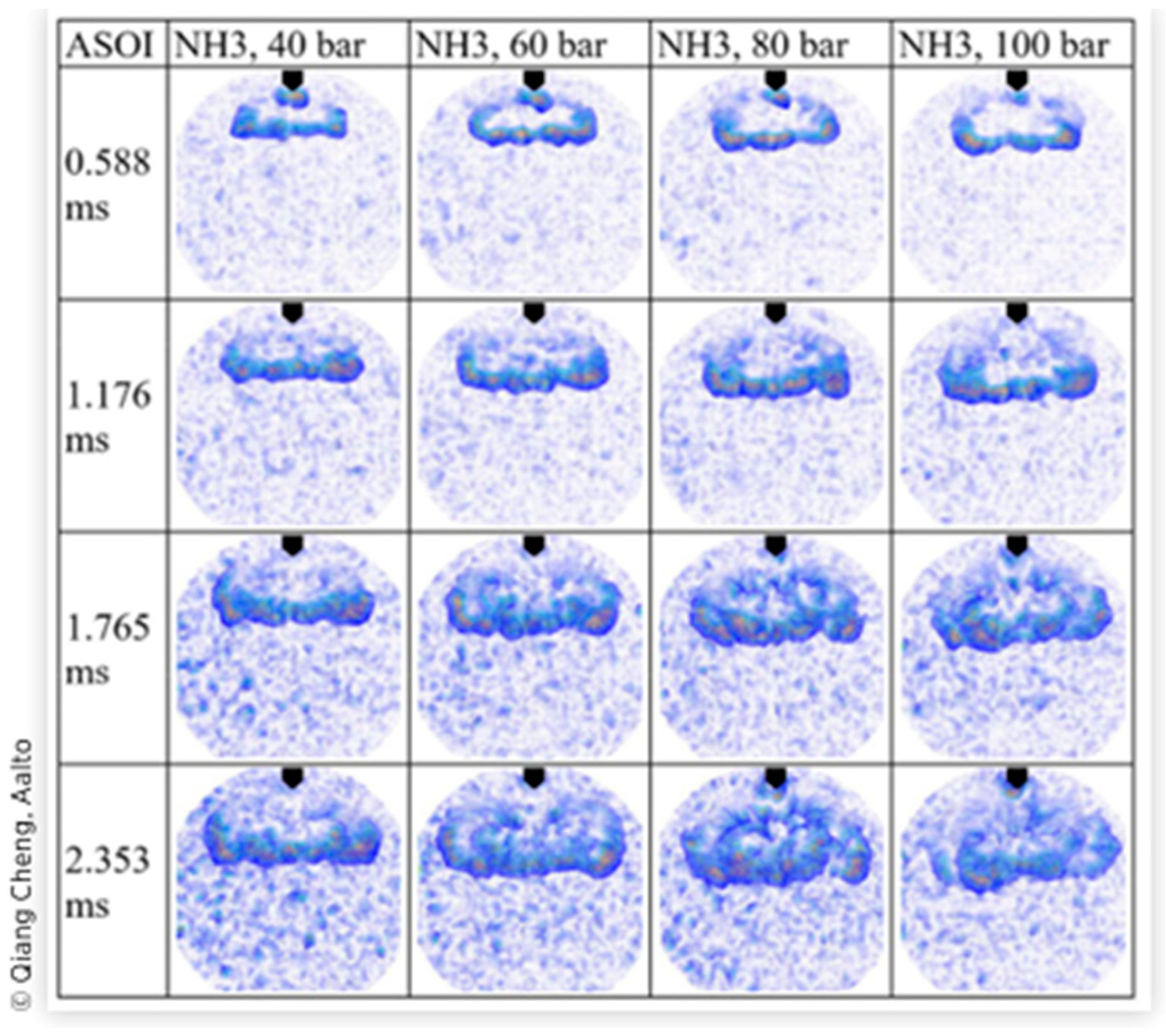
2.2. Direct Liquid Ammonia Injection Spray Characteristics and Effect on Combustion Performance
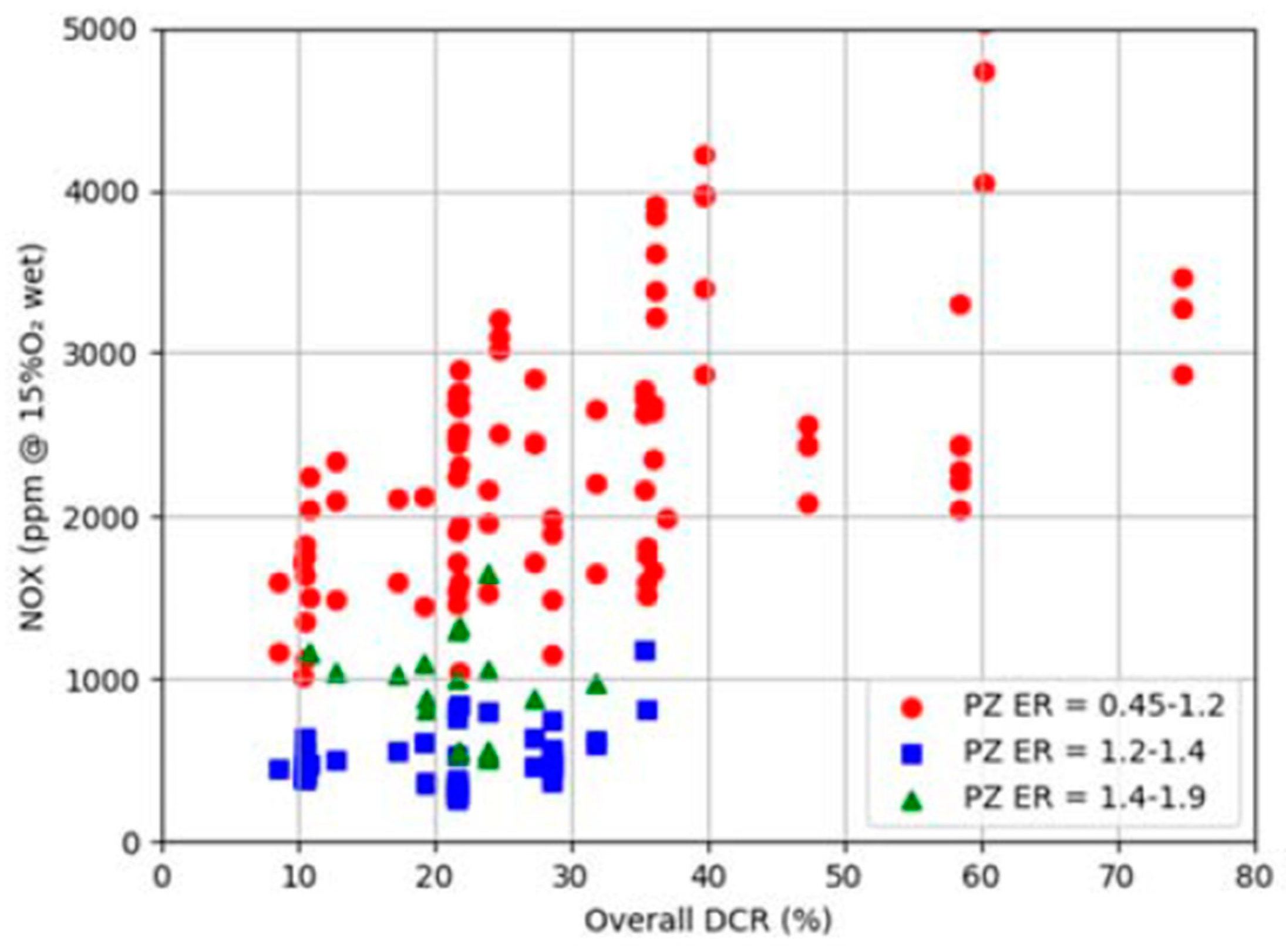
3. Ammonia Combustion in Gas Turbines
4. Conclusions
- Ammonia’s uniqueness highlights its potential in the transition to net zero, owing to its carbon-free combustion, high energy density, and undeniable potential for gas turbine and internal combustion engines. However, it is crucial to recognize ammonia’s inherent challenges and complexity, raising a safety concern and the issue related to public acceptance as a fuel. Therefore, these challenges need to be addressed rigorously.
- For spark ignition engines, achieving ammonia combustion efficiency and reliable ignition will not only demand advanced technologies such as turbulent jet ignition and partially cracked ammonia but also an increased comprehension of the combustion kinetics of ammonia. The gap between laboratory investigations and real-world deployment must be bridged and will require fundamental research coupled with practical application.
- Compression ignition engines offer the potential for ammonia utilization in maritime transportation sectors. Implementing multi-fuel injection strategies and secondary promoter fuels provide ways to control emissions and improve performance. However, with these integrated solutions it is essential to balance the cost and complexity of the system to guarantee its feasibility.
- Gas turbines provide a remarkable, exciting frontier for the combustion of ammonia, innovative combustor design, and ammonia–hydrogen or methane blending that can play a key role in achieving decarbonization; nonetheless, critical challenges like NOx emissions still need to be addressed and demand the precise control of the combustion conditions and a robust after-treatment system.
- Outlook: Ammonia’s journey to becoming a mainstream solution will become a reality only by addressing its limitations thoroughly, which implies developing advancing emission control technologies, scalable safety solutions, and combustion performance efficiency in collaboration with academics, industry, and policymakers.
- Ammonia is far from perfect, like a broad range of fuels; however, its role in achieving net zero emissions is vital. Therefore, a pragmatic approach is necessary to leverage its strengths while addressing its weaknesses, and prioritizing innovation can push ammonia forward in becoming a cornerstone of energy systems in the future.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| ICEs | Internal combustion engines |
| CI | Compression ignition engines |
| SI | Spark ignition engines |
| NH3 | Ammonia |
| LNH3 | Liquid ammonia |
| H2 | Hydrogen |
| CH4 | Methane |
| NOx | Nitrogen oxides |
| N2O | Nitrous oxide |
| CO2 | Carbon dioxide |
| GTs | Gas turbines |
| BSEC | Brake-specific energy consumption |
| BTDC | Before top dead centre |
| HC | Hydrocarbon |
| CO | Carbon monoxide |
| IMEP | Indicated mean effective pressure |
| COV | Coefficient of variation |
| CR | Compression ratio |
| λ | Air ratio |
| GWP | Global warming potential |
| ΔH° | Standard enthalpy change |
| Ru | Ruthenium |
| Ni–Pt | Nickel–platinum |
| AEC | Ammonia electrolyte cell |
| TJI | Prechamber turbulent jet ignition |
| PAC | Plasma-assisted combustion |
| O | Oxygen |
| OH | Hydroxyl radical |
| HNO | Nitroxyl radical |
| SCR | Selective catalytic reduction |
| RCCI | Reactivity-controlled compression ignition |
| ITE | Indicated thermal efficiency |
| GHG | Greenhouse gas |
| PM | Particulate matter |
| HVO | Hydrotreated vegetable oil |
| CEN | Combustion enhancer containing alkyl nitrates |
| CA | Crank angle |
| ATDC | After top dead centre |
| TDC | Top dead centre |
| Pc/Pv | Pressure chamber/ Pressure vapour ratio |
| ϕ | Vapor-to-liquid density ratio |
| GDI | Gasoline direct injection |
| L/D | Aspect ratio (length-to-diameter orifice) |
| Rp | Degree of superheat |
| ER | Equivalent ratio |
| DLE | Dry low emissions |
| MILD | Moderate or intense low-oxygen dilution |
| EGR | Exhaust gas recirculation |
| RQL | Rich-burn, quick-quench, lean-burn |
| DCR | Duty cycle ratio |
| HCN | Hydrogen cyanide |
| DCR | Decomposition rate of ammonia |
References
- UNFCCC. Adoption of the Paris Agreement—Paris Agreement Text English; UNFCCC: Bonn, Germany, 2015. [Google Scholar]
- Do Sacramento, E.; Carvalho, P.C.; De Lima, L.; Veziroglu, T. Feasibility study for the transition towards a hydrogen economy: A case study in Brazil. Energy Policy 2013, 62, 3–9. [Google Scholar] [CrossRef]
- Giddey, S.; Badwal, S.; Kulkarni, A. Review of electrochemical ammonia production technologies and materials. Int. J. Hydrogen Energy 2013, 38, 14576–14594. [Google Scholar] [CrossRef]
- Yapicioglu, A.; Dincer, I. A review on clean ammonia as a potential fuel for power generators. Renew. Sustain. Energy Rev. 2019, 103, 96–108. [Google Scholar] [CrossRef]
- Dimitriou, P.; Javaid, R. A review of ammonia as a compression ignition engine fuel. Int. J. Hydrogen Energy 2020, 45, 7098–7118. [Google Scholar] [CrossRef]
- Mozafari-Varnusfadrani, A. Predictions and Measurements of Spark-Ignition Engine Characteristics Using Ammonia and Other Fuels; Queen Mary University of London: London, UK, 1988. [Google Scholar]
- Duynslaegher, C.; Jeanmart, H.; Vandooren, J. Ammonia combustion at elevated pressure and temperature conditions. Fuel 2010, 89, 3540–3545. [Google Scholar] [CrossRef]
- Vukadinovic, V.; Habisreuther, P.; Zarzalis, N. Experimental study on combustion characteristics of conventional and alternative liquid fuels. J. Eng. Gas Turbines Power 2012, 134, 121504. [Google Scholar] [CrossRef]
- Grannell, S.M.; Assanis, D.N.; Bohac, S.V.; Gillespie, D.E. The fuel mix limits and efficiency of a stoichiometric, ammonia, and gasoline dual fueled spark ignition engine. J. Eng. Gas Turbines Power. 2008, 130, 042802. [Google Scholar] [CrossRef]
- Bicer, Y.; Dincer, I.; Vezina, G.; Raso, F. Impact assessment and environmental evaluation of various ammonia production processes. Environ. Manag. 2017, 59, 842–855. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, B.; Li, Q.; Ma, T.; Hu, S.; Griffy-Brown, C. CO2 emissions and mitigation potential in China’s ammonia industry. Energy Policy 2010, 38, 3701–3709. [Google Scholar] [CrossRef]
- Andres, R.J.; Boden, T.A.; Bréon, F.-M.; Ciais, P.; Davis, S.; Erickson, D.; Gregg, J.S.; Jacobson, A.; Marland, G.; Miller, J. A synthesis of carbon dioxide emissions from fossil-fuel combustion. Biogeosciences 2012, 9, 1845–1871. [Google Scholar] [CrossRef]
- Andres, R.J.; Gregg, J.S.; Losey, L.; Marland, G.; Boden, T.A. Monthly, global emissions of carbon dioxide from fossil fuel consumption. Tellus B Chem. Phys. Meteorol. 2011, 63, 309–327. [Google Scholar] [CrossRef]
- Zamfirescu, C.; Dincer, I. Ammonia as a green fuel and hydrogen source for vehicular applications. Fuel Process. Technol. 2009, 90, 729–737. [Google Scholar] [CrossRef]
- Mørch, C.S.; Bjerre, A.; Gøttrup, M.P.; Sorenson, S.C.; Schramm, J. Ammonia/hydrogen mixtures in an SI-engine: Engine performance and analysis of a proposed fuel system. Fuel 2011, 90, 854–864. [Google Scholar] [CrossRef]
- Duynslaegher, C.; Contino, F.; Vandooren, J.; Jeanmart, H. Modeling of ammonia combustion at low pressure. Combust. Flame 2012, 159, 2799–2805. [Google Scholar] [CrossRef]
- Sahoo, B.; Sahoo, N.; Saha, U. Effect of engine parameters and type of gaseous fuel on the performance of dual-fuel gas diesel engines—A critical review. Renew. Sustain. Energy Rev. 2009, 13, 1151–1184. [Google Scholar] [CrossRef]
- Zhou, L.; Zhong, L.; Liu, Z.; Wei, H. Toward highly-efficient combustion of ammonia–hydrogen engine: Prechamber turbulent jet ignition. Fuel 2023, 352, 129009. [Google Scholar] [CrossRef]
- Cai, T.; Zhao, D.; Gutmark, E. Overview of fundamental kinetic mechanisms and emission mitigation in ammonia combustion. Chem. Eng. J. 2023, 458, 141391. [Google Scholar] [CrossRef]
- Riviera. WinGD on Track to Deliver Ammonia Engines in 2025. Available online: https://www.rivieramm.com/news-content-hub/news-content-hub/wingd-on-track-to-deliver-ammonia-engines-in-2025-76985 (accessed on 10 October 2024).
- Lindstrand, N. MAN Energy Solutions Is Developing a Fuel-Flexible, Two-Stroke Ammonia Engine as a Key Technology in the Maritime Energy Transition. MAN. 2023. Available online: https://www.man-es.com/discover/two-stroke-ammonia-engine (accessed on 15 October 2024).
- Alder, I. Wärtsilä Continues to Set the Pace for Marine Decarbonisation with Launch of World-First 4-Stroke Engine-Based Ammonia Solution. Wärtsilä Corporation. 2023. Available online: https://www.wartsila.com/media/news/15-11-2023-wartsila-continues-to-set-the-pace-for-marine-decarbonisation-with-launch-of-world-first-4-stroke-engine-based-ammonia-solution-3357985 (accessed on 17 October 2024).
- Line, N. Maritime Consortium Successfully Completes Ammonia Co-Firing Test Using Cutting-Edge Ammonia-Fueled Engine. 2023. Available online: https://www.nyk.com/english/news/2023/20230516_01.html (accessed on 20 October 2024).
- Rimmele, C. MAHLE Powertrain Drives the Development of Ammonia Combustion for Heavy Duty Engines. 2023. Available online: https://www.mahle-powertrain.com/en/news-and-press/press-releases/mahle-powertrain-drives-the-development-of-ammonia-combustion-for-heavy-duty-engines-91713#:~:text=MAHLE%20Powertrain%20drives%20the%20development%20of%20ammonia%20combustion%20for%20heavy%20duty%20engines (accessed on 25 October 2024).
- Hydrofuel. Engine Conversions. 2023. Available online: https://www.mahle-powertrain.com/en/news-and-press/press-releases/mahle-powertrain-drives-the-development-of-ammonia-combustion-for-heavy-duty-engines-91713 (accessed on 26 October 2024).
- Kable, G. GAC and Toyota Develop Ammonia Engine for 90% CO2 Reduction. Four-Cylinder Engine Is the First Ammonia-Fuelled Engine for Passenger Cars; Could Be a Hydrogen Alternative. 2023. Available online: https://www.autocar.co.uk/car-news/new-cars/gac-and-toyota-develop-ammonia-engine-90-co2-reduction (accessed on 30 October 2024).
- Ma, F.; Guo, L.; Li, Z.; Zeng, X.; Zheng, Z.; Li, W.; Zhao, F.; Yu, W. A review of current advances in ammonia combustion from the fundamentals to applications in internal combustion engines. Energies 2023, 16, 6304. [Google Scholar] [CrossRef]
- Tornatore, C.; Marchitto, L.; Sabia, P.; De Joannon, M. Ammonia as green fuel in internal combustion engines: State-of-the-art and future perspectives. Front. Mech. Eng. 2022, 8, 944201. [Google Scholar] [CrossRef]
- Jespersen, M.C.; Rasmussen, T.Ø.H.; Ivarsson, A. Widening the operation limits of a SI engine running on neat ammonia. Fuel 2024, 358, 130159. [Google Scholar] [CrossRef]
- Ryu, K.; Zacharakis-Jutz, G.E.; Kong, S.-C. Effects of gaseous ammonia direct injection on performance characteristics of a spark-ignition engine. Appl. Energy 2014, 116, 206–215. [Google Scholar] [CrossRef]
- Grannell, S.M.; Assanis, D.N.; Gillespie, D.E.; Bohac, S.V. Exhaust emissions from a stoichiometric, ammonia and gasoline dual fueled spark ignition engine. In Proceedings of the Internal Combustion Engine Division Spring Technical Conference, Milwaukee, WI, USA, 3–6 May 2009; pp. 135–141. [Google Scholar]
- Kurien, C.; Varma, P.S.; Mittal, M. Effect of ammonia energy fractions on combustion stability and engine characteristics of gaseous (ammonia/methane) fuelled spark ignition engine. Int. J. Hydrogen Energy 2023, 48, 1391–1400. [Google Scholar] [CrossRef]
- Mounaïm-Rousselle, C.; Bréquigny, P.; Dumand, C.; Houillé, S. Operating limits for ammonia fuel spark-ignition engine. Energies 2021, 14, 4141. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Contino, F.; Mounaïm-Rousselle, C. Experimental study on ammonia/hydrogen/air combustion in spark ignition engine conditions. Fuel 2020, 269, 117448. [Google Scholar] [CrossRef]
- Mounaïm-Rousselle, C.; Brequigny, P. Ammonia as fuel for low-carbon spark-ignition engines of tomorrow’s passenger cars. Front. Mech. Eng. 2020, 6, 70. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, W.; Liu, S.; Wang, W.; Peng, Y.; Wang, Z. A review on ammonia-hydrogen fueled internal combustion engines. ETransportation 2023, 18, 100288. [Google Scholar] [CrossRef]
- Pyrc, M.; Gruca, M.; Tutak, W.; Jamrozik, A. Assessment of the co-combustion process of ammonia with hydrogen in a research VCR piston engine. Int. J. Hydrogen Energy 2023, 48, 2821–2834. [Google Scholar] [CrossRef]
- Westlye, F.R.; Ivarsson, A.; Schramm, J. Experimental investigation of nitrogen based emissions from an ammonia fueled SI-engine. Fuel 2013, 111, 239–247. [Google Scholar] [CrossRef]
- Dinesh, M.; Pandey, J.K.; Kumar, G. Study of performance, combustion, and NOx emission behavior of an SI engine fuelled with ammonia/hydrogen blends at various compression ratio. Int. J. Hydrogen Energy 2022, 47, 25391–25403. [Google Scholar] [CrossRef]
- Ryu, K.; Zacharakis-Jutz, G.E.; Kong, S.-C. Performance enhancement of ammonia-fueled engine by using dissociation catalyst for hydrogen generation. Int. J. Hydrogen Energy 2014, 39, 2390–2398. [Google Scholar] [CrossRef]
- Ezzat, M.; Dincer, I. Development and assessment of a new hybrid vehicle with ammonia and hydrogen. Appl. Energy 2018, 219, 226–239. [Google Scholar] [CrossRef]
- Koike, M.; Suzuoki, T.; Takeuchi, T.; Homma, T.; Hariu, S.; Takeuchi, Y. Cold-start performance of an ammonia-fueled spark ignition engine with an on-board fuel reformer. Int. J. Hydrogen Energy 2021, 46, 25689–25698. [Google Scholar] [CrossRef]
- Kim, J.; Um, D.; Kwon, O. Hydrogen production from burning and reforming of ammonia in a microreforming system. Energy Convers. Manag. 2012, 56, 184–191. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, D.; Sun, D.; Semlitsch, B. Electrical power, energy efficiency, NO and CO emissions investigations of an ammonia/methane-fueled micro-thermal photovoltaic system with a reduced chemical reaction mechanism. Energy 2024, 305, 132248. [Google Scholar] [CrossRef]
- Meng, X.; Zhao, C.; Cui, Z.; Zhang, X.; Zhang, M.; Tian, J.; Long, W.; Bi, M. Understanding of combustion characteristics and NO generation process with pure ammonia in the pre-chamber jet-induced ignition system. Fuel 2023, 331, 125743. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, J.; Cui, Z.; Xiong, S.; Yin, S.; Wang, Q.; Long, W. Visualization study on the effects of pre-chamber jet ignition and methane addition on the combustion characteristics of ammonia/air mixtures. Fuel 2023, 338, 127204. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, L.; Wei, H. Experimental investigation on the performance of pure ammonia engine based on reactivity controlled turbulent jet ignition. Fuel 2023, 335, 127116. [Google Scholar] [CrossRef]
- Uddeen, K.; Tang, Q.; Shi, H.; Magnotti, G.; Turner, J. A novel multiple spark ignition strategy to achieve pure ammonia combustion in an optical spark-ignition engine. Fuel 2023, 349, 128741. [Google Scholar] [CrossRef]
- Guo, B.; Ichiyanagi, M.; Kajiki, K.; Aratake, N.; Zheng, Q.; Kodaka, M.; Suzuki, T. Combustion analysis of ammonia fueled high compression ratio si engine with glow plug and sub-chamber-effects of ammonia content under condition of co-combustion with gasoline/ammonia/air. Int. J. Automot. Eng. 2022, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, Y.; van’t Veer, K.; Gorbanev, Y.; Neyts, E.C.; Schneider, W.F.; Bogaerts, A. Plasma catalysis for ammonia synthesis: A microkinetic modeling study on the contributions of Eley–Rideal reactions. ACS Sustain. Chem. Eng. 2021, 9, 13151–13163. [Google Scholar] [CrossRef]
- Faingold, G.; Lefkowitz, J.K. A numerical investigation of NH3/O2/He ignition limits in a non-thermal plasma. Proc. Combust. Inst. 2021, 38, 6661–6669. [Google Scholar] [CrossRef]
- Chiong, M.-C.; Chong, C.T.; Ng, J.-H.; Mashruk, S.; Chong, W.W.F.; Samiran, N.A.; Mong, G.R.; Valera-Medina, A. Advancements of combustion technologies in the ammonia-fuelled engines. Energy Convers. Manag. 2021, 244, 114460. [Google Scholar] [CrossRef]
- Niki, Y.; Yoo, D.-H.; Hirata, K.; Sekiguchi, H. Effects of ammonia gas mixed into intake air on combustion and emissions characteristics in diesel engine. In Proceedings of the Internal Combustion Engine Division Fall Technical Conference, Greenville, SC, USA, 9–12 October 2016. [Google Scholar]
- Niki, Y.; Nitta, Y.; Sekiguchi, H.; Hirata, K. Emission and combustion characteristics of diesel engine fumigated with ammonia. In Proceedings of the Internal Combustion Engine Division Fall Technical Conference, San Diego, CA, USA, 4–7 November 2018. [Google Scholar]
- Jin, S.; Wu, B.; Zi, Z.; Yang, P.; Shi, T.; Zhang, J. Effects of fuel injection strategy and ammonia energy ratio on combustion and emissions of ammonia-diesel dual-fuel engine. Fuel 2023, 341, 127668. [Google Scholar] [CrossRef]
- Førby, N.; Thomsen, T.B.; Cordtz, R.F.; Bræstrup, F.; Schramm, J. Ignition and combustion study of premixed ammonia using GDI pilot injection in CI engine. Fuel 2023, 331, 125768. [Google Scholar] [CrossRef]
- Xu, L.; Xu, S.; Bai, X.-S.; Repo, J.A.; Hautala, S.; Hyvönen, J. Performance and emission characteristics of an ammonia/diesel dual-fuel marine engine. Renew. Sustain. Energy Rev. 2023, 185, 113631. [Google Scholar] [CrossRef]
- Pei, Y.; Wang, D.; Jin, S.; Gu, Y.; Wu, C.; Wu, B. A quantitative study on the combustion and emission characteristics of an Ammonia-Diesel Dual-fuel (ADDF) engine. Fuel Process. Technol. 2023, 250, 107906. [Google Scholar] [CrossRef]
- Mi, S.; Wu, H.; Pei, X.; Liu, C.; Zheng, L.; Zhao, W.; Qian, Y.; Lu, X. Potential of ammonia energy fraction and diesel pilot-injection strategy on improving combustion and emission performance in an ammonia-diesel dual fuel engine. Fuel 2023, 343, 127889. [Google Scholar] [CrossRef]
- Nadimi, E.; Przybyła, G.; Lewandowski, M.T.; Adamczyk, W. Effects of ammonia on combustion, emissions, and performance of the ammonia/diesel dual-fuel compression ignition engine. J. Energy Inst. 2023, 107, 101158. [Google Scholar] [CrossRef]
- Dupuy, A.; Brequigny, P.; Schmid, A.; Frapolli, N.; Mounaïm-Rousselle, C. Experimental study of RCCI engine-Ammonia combustion with diesel pilot injection. J. Ammon. Energy 2023, 1. [Google Scholar] [CrossRef]
- Samson, R.; Morin, A.-G.; Foucher, F. Effects of the Combustion Enhancer Containing Alkyl Nitrate (CEN) to Dodecane and HVO as Pilot Fuels on a Compression Ignition Engine Operating in Dual-Fuel with Ammonia; SAE Technical Paper; SAE International: Warrendale, PA, USA, 2023. [Google Scholar]
- Zhang, Z.; Long, W.; Dong, P.; Tian, H.; Tian, J.; Li, B.; Wang, Y. Performance characteristics of a two-stroke low speed engine applying ammonia/diesel dual direct injection strategy. Fuel 2023, 332, 126086. [Google Scholar] [CrossRef]
- Bjørgen, K.O.P.; Emberson, D.R.; Løvås, T. Combustion of liquid ammonia and diesel in a compression ignition engine operated in high-pressure dual fuel mode. Fuel 2024, 360, 130269. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Niki, Y.; Takasaki, K.; Kobayashi, H.; Miyanagi, A. NH3 combustion using three-layer stratified fuel injection for a large two-stroke marine engine: Experimental verification of the concept. Appl. Energy Combust. Sci. 2022, 10, 100071. [Google Scholar] [CrossRef]
- Scharl, V.; Sattelmayer, T. Spectroscopic investigation of diesel-piloted ammonia spray combustion. Fuel 2024, 358, 130201. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, W.; Zhang, R.; Wei, H. Comparative study on combustion and flame characteristics of NH3 dual-fuel engine under CI vs SI combustion modes. Fuel 2024, 359, 130525. [Google Scholar] [CrossRef]
- Hyun Ho Park. An Experimental Study on High-Pressure Spray Characteristics of Ammonia by Using Schlieren & Mie-Scattering Method. 2018. Available online: https://hdl.handle.net/10371/141371 (accessed on 12 October 2024).
- Okafor, E.C.; Yamashita, H.; Hayakawa, A.; Somarathne, K.K.A.; Kudo, T.; Tsujimura, T.; Uchida, M.; Ito, S.; Kobayashi, H. Flame stability and emissions characteristics of liquid ammonia spray co-fired with methane in a single stage swirl combustor. Fuel 2021, 287, 119433. [Google Scholar] [CrossRef]
- Pelé, R.; Mounaïm-Rousselle, C.; Bréquigny, P.; Hespel, C.; Bellettre, J. First study on ammonia spray characteristics with a current GDI engine injector. Fuels 2021, 2, 253–271. [Google Scholar] [CrossRef]
- Fan, Y.; Ohtomo, M.; Kasuga, S.; Iki, N.; Kurata, O.; Tsujimura, T.; Furutani, H. Characteristics of ammonia spray injected by pressure-swirl atomizers. In Proceedings of the International Conference on Liquid Atomization and Spray Systems (ICLASS), Edinburgh, UK, 22–26 July 2018. [Google Scholar]
- Cheng, Q.; Ojanen, K.; Diao, Y.; Kaario, O.; Larmi, M. Dynamics of the ammonia spray using high-speed schlieren imaging. SAE Tech. Pap. 2022, 2022. [Google Scholar] [CrossRef]
- Sher, E.; Bar-Kohany, T.; Rashkovan, A. Flash-boiling atomization. Prog. Energy Combust. Sci. 2008, 34, 417–439. [Google Scholar] [CrossRef]
- Colson, S.; Yamashita, H.; Oku, K.; Somarathne, K.D.K.A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Study on the effect of injection temperature and nozzle geometry on the flashing transition of liquid ammonia spray. Fuel 2023, 348, 128612. [Google Scholar] [CrossRef]
- Aresi, L. General Electric: GE and IHI Sign Agreement to Develop Ammonia Fuels Roadmap Across Asia; GE Gas Power: Tokyo, Japan; GE (NYSE: GE) and IHI Corporation (IHI) GE Gas Power: Tokyo, Japan, 2021; Available online: https://www.marketscreener.com/quote/stock/GE-AEROSPACE-4823/news/General-Electric-GE-and-IHI-Sign-Agreement-to-Develop-Ammonia-Fuels-Roadmap-across-Asia-35664503/ (accessed on 12 October 2024).
- Skjervold, V. DECAMMP—Decomposed Ammonia for Carbon-Free Power Generation. 2023. Available online: https://www.sintef.no/en/projects/2023/decammp-decomposed-ammonia-for-carbon-free-power-generation/ (accessed on 13 April 2023).
- Kurata, O.; Iki, N.; Matsunuma, T.; Inoue, T.; Tsujimura, T.; Furutani, H.; Kobayashi, H.; Hayakawa, A. Performances and emission characteristics of NH3–air and NH3CH4–air combustion gas-turbine power generations. Proc. Combust. Inst. 2017, 36, 3351–3359. [Google Scholar] [CrossRef]
- Hayakawa, A.; Arakawa, Y.; Mimoto, R.; Somarathne, K.K.A.; Kudo, T.; Kobayashi, H. Experimental investigation of stabilization and emission characteristics of ammonia/air premixed flames in a swirl combustor. Int. J. Hydrogen Energy 2017, 42, 14010–14018. [Google Scholar] [CrossRef]
- Kurata, O.; Iki, N.; Inoue, T.; Matsunuma, T.; Tsujimura, T.; Furutani, H.; Kawano, M.; Arai, K.; Okafor, E.C.; Hayakawa, A. Development of a wide range-operable, rich-lean low-NOx combustor for NH3 fuel gas-turbine power generation. Proc. Combust. Inst. 2019, 37, 4587–4595. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Pugh, D.; Marsh, P.; Bulat, G.; Bowen, P. Preliminary study on lean premixed combustion of ammonia-hydrogen for swirling gas turbine combustors. Int. J. Hydrogen Energy 2017, 42, 24495–24503. [Google Scholar] [CrossRef]
- Okafor, E.C.; Somarathne, K.K.A.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Kobayashi, H. Towards the development of an efficient low-NOx ammonia combustor for a micro gas turbine. Proc. Combust. Inst. 2019, 37, 4597–4606. [Google Scholar] [CrossRef]
- Sorrentino, G.; Sabia, P.; Bozza, P.; Ragucci, R.; de Joannon, M. Low-NOx conversion of pure ammonia in a cyclonic burner under locally diluted and preheated conditions. Appl. Energy 2019, 254, 113676. [Google Scholar] [CrossRef]
- Božo, M.G.; Vigueras-Zuniga, M.; Buffi, M.; Seljak, T.; Valera-Medina, A. Fuel rich ammonia-hydrogen injection for humidified gas turbines. Appl. Energy 2019, 251, 113334. [Google Scholar] [CrossRef]
- Božo, M.G.; Mashruk, S.; Zitouni, S.; Valera-Medina, A. Humidified ammonia/hydrogen RQL combustion in a trigeneration gas turbine cycle. Energy Convers. Manag. 2021, 227, 113625. [Google Scholar] [CrossRef]
- Rocha, R.C.; Costa, M.; Bai, X.-S. Combustion and emission characteristics of ammonia under conditions relevant to modern gas turbines. Combust. Sci. Technol. 2021, 193, 2514–2533. [Google Scholar] [CrossRef]
- Ditaranto, M.; Saanum, I.; Larfeldt, J. Experimental study on high pressure combustion of decomposed ammonia: How can ammonia be best used in a gas turbine? In Turbo Expo: Power for Land, Sea, and Air; American Society of Mechanical Engineers: New York, NY, USA, 2021. [Google Scholar] [CrossRef]
- Somarathne, K.; Yamashita, H.; Colson, S.; Okafor, E.; Hayakawa, A.; Kudo, T.; Kobayashi, H. Liquid ammonia spray combustion and emission characteristics with gaseous hydrogen/air co-firing. In Proceedings of the 13th Asia-Pacific Conference on Combustion, Abu Dhabi, United Arab Emirates, 5–9 December 2021. [Google Scholar]
- Okafor, E.C.; Kurata, O.; Yamashita, H.; Inoue, T.; Tsujimura, T.; Iki, N.; Hayakawa, A.; Ito, S.; Uchida, M.; Kobayashi, H. Liquid ammonia spray combustion in two-stage micro gas turbine combustors at 0.25 MPa; Relevance of combustion enhancement to flame stability and NOx control. Appl. Energy Combust. Sci. 2021, 7, 100038. [Google Scholar] [CrossRef]
- Somarathne, K.D.K.A.; Yamashita, H.; Colson, S.; Oku, K.; Honda, K.; Okafor, E.C.; Hayakawa, A.; Kudo, T.; Kobayashi, H. Towards the development of liquid ammonia/air spray combustion in a gas turbine-like combustor at moderately high pressure. Appl. Energy Combust. Sci. 2023, 16, 100215. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Marsh, R.; Runyon, J.; Pugh, D.; Beasley, P.; Hughes, T.; Bowen, P. Ammonia–methane combustion in tangential swirl burners for gas turbine power generation. Appl. Energy 2017, 185, 1362–1371. [Google Scholar] [CrossRef]
- Okafor, E.C.; Somarathne, K.K.A.; Ratthanan, R.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Tsujimura, T.; Furutani, H.; Kobayashi, H. Control of NOx and other emissions in micro gas turbine combustors fuelled with mixtures of methane and ammonia. Combust. Flame 2020, 211, 406–416. [Google Scholar] [CrossRef]
- Kenanoğlu, R.; Baltacioğlu, E. An experimental investigation on hydroxy (HHO) enriched ammonia as alternative fuel in gas turbine. Int. J. Hydrogen Energy 2021, 46, 29638–29648. [Google Scholar] [CrossRef]
- Ávila, C.D.; Cardona, S.; Abdullah, M.; Younes, M.; Jamal, A.; Guiberti, T.F.; Roberts, W.L. Experimental assessment of the performance of a commercial micro gas turbine fueled by ammonia-methane blends. Appl. Energy Combust. Sci. 2023, 13, 100104. [Google Scholar] [CrossRef]




| Fuel | Formula | Storage Temp. [°C] | Storage Pressure [kPa] | Density [kg/m3] | Lower Heating Value [MJ/kg] | Air/Fuel Ratio by Weight | Specific Energy [MJ/kg] | Auto-Ignition Temperature [°C] |
|---|---|---|---|---|---|---|---|---|
| Ammonia | NH3 | 25 | 1030 | 600 | 18.8 | 6.05 | 2.64 | 651 |
| Hydrogen (gas) | H2 | 25 | 24,821 | 17.5 | 120 | 34.32 | 3.40 | 571 |
| Hydrogen (liquid) | H2 | −253 | 102 | 71 | 120 | 34.32 | 3.40 | 571 |
| Diesel | C12H23 | 25 | 101.3 | 850 | 45 | 14.32 | 2.77 | 254 |
| Gasoline | C7H17 | 25 | 101.3 | 700 | 42.5 | 15.29 | 2.58 | 370 |
| Ethanol | C2H5OH | 25 | 101.3 | 790 | 27 | 8.95 | 2.70 | 423 |
| Methanol | CH3OH | 25 | 101.3 | 780 | 19.5 | 6.44 | 2.69 | 464 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eyisse, E.F.; Nadimi, E.; Wu, D. Ammonia Combustion: Internal Combustion Engines and Gas Turbines. Energies 2025, 18, 29. https://doi.org/10.3390/en18010029
Eyisse EF, Nadimi E, Wu D. Ammonia Combustion: Internal Combustion Engines and Gas Turbines. Energies. 2025; 18(1):29. https://doi.org/10.3390/en18010029
Chicago/Turabian StyleEyisse, Edith Flora, Ebrahim Nadimi, and Dawei Wu. 2025. "Ammonia Combustion: Internal Combustion Engines and Gas Turbines" Energies 18, no. 1: 29. https://doi.org/10.3390/en18010029
APA StyleEyisse, E. F., Nadimi, E., & Wu, D. (2025). Ammonia Combustion: Internal Combustion Engines and Gas Turbines. Energies, 18(1), 29. https://doi.org/10.3390/en18010029








