The Gasification of Marine and Coastal Resources for Syngas Production: A Review
Abstract
1. Introduction
1.1. Shores: High Density People Areas
1.2. Energy Consumption
1.3. Energy Transition
1.4. Development of Biomass as a Renewable Energy Source
1.5. Gasification in Short: History, Process, Reactors, and Prospectives
- 1.
- Autothermal: The heat is produced in the gasification reactor via a partial oxidation of the fuel, usually using as air as the oxidant, but also oxygen-enriched air or pure oxygen. Using air implies the presence of nitrogen in the syngas, which reduces its heating value and the concentration of “valuable” compounds such as hydrogen. However, this configuration is the easiest and historical way to operate, since it uses the gasified fuel and only one reactor.
- 2.
- Allothermal: Heat is produced in a separate reactor and supplied to the gasification reactor. This configuration allows the use of other oxidants such as steam or CO2, which only react in endothermic reactions. This leads to a syngas without nitrogen as the inert gas, resulting in higher concentrations of valuable compounds, and therefore, in a higher heating value of the syngas. However, this requires an additional energy source, which could lower the overall energy balance of the gasification process.
- The dry gasification process has a low-moisture fuel and gas atmosphere around the fuel particles in the reactors. It can also be separated into two categories [12,14,19,20]:
- ○
- Fixed bed: The fuel creates a bed which is relatively fixed (moves very slowly) compared to the air going in the reactor and the syngas going out. This implies that suitable fuels for the fixed bed must be able to produce a solid residue (char), creating the bed. In addition, this leads to high requirements for the fuel such as low ash content, high ash melting point, low moisture, … The temperature in such reactors shows a variation along the reactor height, with a peak temperature up to 1300–1400 °C in the partial oxidation area. Fixed-bed reactors cover a wide range of sizes from kW to 5 MW. This category can be decomposed into two types:
- ▪
- Downdraft: Fuel and air are flowing in the same direction in the reactor. This configuration produces syngas with the lowest tar content (<0.1 g/Nm3) among all gasification technologies.
- ▪
- Updraft: Fuel and air are flowing in opposite directions in the reactor. This type leads to a high tar content in the syngas due to opposite directions of air and fuel.
- ○
- Fluidized bed: The fuel is mixed with an inert material which creates a bed. Air is flowing from the bottom at such speed that it creates the fluidization of the inert bed and fuel. The main drawback is the limited operating temperature (900–950 °C) to avoid inert bed particles melting and agglomerating, changing their fluidization properties. This results in lower fuel conversion and higher tar content than downdraft fixed-bed gasifiers. However, fluidized-bed reactors are usually considered as fuel flexible, since they require sufficient grinding to obtain particles that can be fluidized in the same range as the inert bed. Due to the complexity of the fluidization, the lowest fluidized bed size is around 10 MW (for economic reasons) but can cover up to 100 MW.
- Wet gasification processes are rather new, with the main difference being that the medium in the reactor is not gaseous (air and syngas) but liquid, namely water [19,21,22]. In these processes, the reactor is filled with water and fuel at a low concentration (typically 1 to 8% w/w) [21]. The reactor is closed, meaning a discontinuous operation, and temperature and pressure are increased to typical values around 200–500 °C and 80 to 300 bar, respectively [22]. This leads to either a sub- or supercritical state for the water. With these conditions, the fuel decomposes into a solid (char), liquids (aqueous and hydrophobic phases), and gas. After a defined reaction time, the reactor is cooled and gasification products are liberated (gas) and have to be extracted from the water. Since water is the main medium, wet gasification processes seem to be a promising way to valorize wet biomass such as marine resources, sewage sludge, liquid manure, … These wet processes have not been well-developed so far, and are mainly used at the lab scale, which shows a great interest for these technologies.
1.6. Marine Resources as Fuels in Gasification
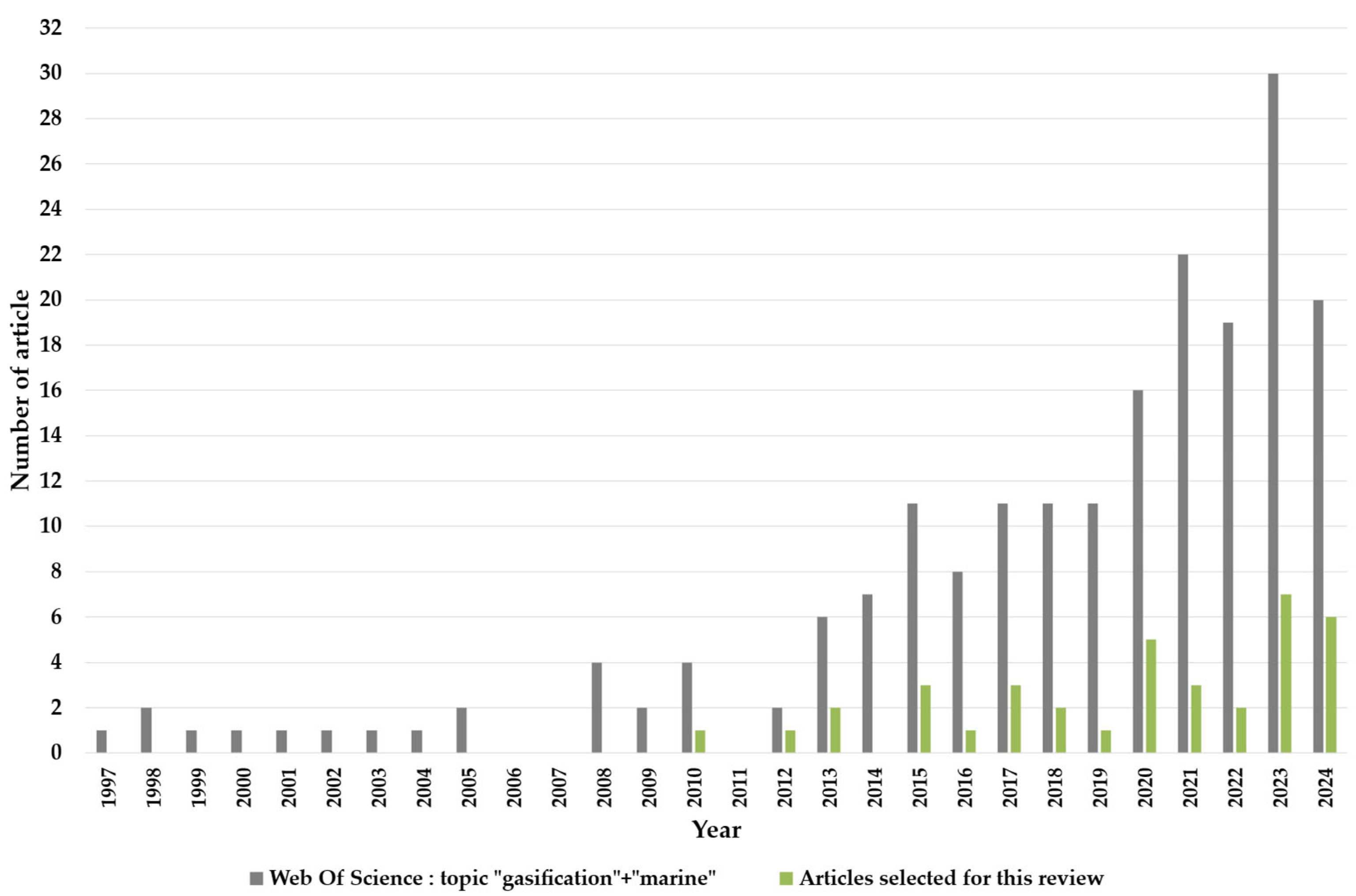
1.7. The Scientific Literature on Marine Resource Gasification
2. Marine Resources
2.1. Resources from the Ocean
2.1.1. Algae
2.1.2. Driftwood
2.1.3. Plastics
2.1.4. Debris
- -
- Recovered sand by sieving, reaching 44,100 t/y.
- -
- About 2470 t/y of sand still remaining in the small-sized (<0.1 m) and medium-sized (0.1–0.3 m) particle fractions.
- -
- Shells and stones representing 2290 t/y and 1490 t/y, respectively.
- -
- The organic fraction, including driftwood in medium-sized particles and organic and driftwood in small-sized particles, reaching almost 5500 t/y.
- -
- Anthropic litter collected during cleaning operation reaching about 870 t/y.
- By hand on the shores at low tides or in shallow waters and using small cutting tools like sickles.
- Using boats to access deeper water areas and using manual rockweed cutting tools or rakes to cut, extract, and collect macroalgae.
- Highly mechanized boats with a rolling grate collecting floating algae and separating water or the so-called “suction harvester” boats, which submerge a pipe sucking the water and tearing algae off of the bottom.
2.2. Resources from the Land
2.2.1. Wood, Crop Residue, and Waste
2.2.2. Invasive Species
3. Gasification of Marine Resources in the Current Literature
3.1. Marine and Coastal Resources Considered as Fuel for Gasification
- -
- 23 resources originated from the sea.
- -
- 6 resources from the land (coastal area).
- -
- 6 classified as other, particularly coal and plastic wastes.
3.2. Gasification Technologies Considered in the Literature
- -
- 13 hydrothermal gasification reactors (in sub- or supercritical conditions).
- -
- 7 fixed-bed reactors.
- -
- 2 thermogravimetric analysis (TGA) reactors.
- -
- 1 free-fall tubular vertical reactor.
- -
- 1 simulation.
- -
- 1 fluidized-bed–spouted-bed reactor.
- (1)
- Subcritical water gasification, or hydrothermal gasification.
- (2)
- Supercritical water gasification.
- (3)
- Hydrothermal liquefaction followed by supercritical gasification of the obtained bio-oil + char.
| Gasification Technology | Quantity kg or kg/h | Time | Temperature—°C | Ref. |
|---|---|---|---|---|
| Supercritical water gasification | 6 × 10−5 kg | 20 min | 400 to 440 | [61] |
| Supercritical water gasification, with or without NaOH and Ni-Al2O3 36 MPa | 1 × 10−3 kg | 30 min | 500 °C | [57] |
| Hydrothermal gasification 79–442 bar | 1 × 10−3 kg | 1 h | 300 to 600 °C | [70] |
| Hydrothermal liquefaction then supercritical water gasification Supercritical gasification: 30–50 MPa | 6 × 10−2 kg | Liquefaction 50 min heating + 60 min reaction Gasification 60 min heating + 120 min reaction | 350 °C liquefaction 600 °C gasification | [58] |
| Supercritical water gasification in the presence of catalysts | 0.7 to 5 × 10−3 kg | 15 to 45 min | 355 to 405 °C | [73] |
| Hydrothermal gasification with catalyst Ru/C | 0.12 × 10−3 kg | 75 min | 410 °C | [67] |
| Supercritical hydrothermal liquefaction and gasification 350 bar | 4 × 10−3 kg | 60 min | 200 to 500 °C | [66] |
| Supercritical water gasification 230 bar | 0.07 to 0.4 × 10−3 kg | 2 to 60 min | 500 to 800 °C | [76] |
| Supercritical water gasification 230 bar | 0.07 to 0.4 × 10−3 kg | 2 to 60 min | 500 to 800 °C | [78] |
| Sub-critical water gasification 8 MPa | 5 to 25 × 10−3 kg | 30 to 90 min | 300 to 400 °C | [59] |
| Catalytic hydrothermal conversion near supercritical water conditions 200 bar | 5 × 10−3 kg | 60 min heating + 60 min reaction | 350 °C | [80] |
| Hydrothermal gasification 240–250 bar | 3 to 7 × 10−3 kg | 7 min to 120 min stand-alone test | 410–550 °C in replicated tests | [62] |
| Supercritical water gasification 210 to 290 bar | n.d. | 2 to 60 min | 500 to 800 °C | [77] |
| Gasification Technology | Quantity kg or kg/h | Time | Temperature—°C | Ref. |
|---|---|---|---|---|
| Double fixed-bed reactor Gasifying agent: CO2 + H2O (20%) in He | 1 × 10−4 kg | 80 min | 400 to 700 °C (100 °C step) | [65] |
| Fixed-bed reactor Steam gasification: argon + steam | 1 × 10−3 kg | 2 h | 650 to 750 °C | [72] |
| Tubular horizontal fixed-bed reactor | 1 × 10−3 kg | 20 min reaction | 700 to 950 °C | [74] |
| Tubular horizontal fixed-bed reactor Steam gasification (50% w/w steam) | 1 × 10−3 kg | 10 min to 6 h | 900 °C | [63] |
| Downdraft fixed bed—“Femto gasifier” Air blown (ventilator before reactor) | 1 × 10+1 kg/h | 137 min | 600 to 900 °C on the grate | [68] |
| Downdraft fixed bed | 2 × 10+1 kg/h | n.d. | n.d. | [40] |
| Tubular horizontal reactor Presence of catalysts (dolomite) Gasifying agent: O2 + H2O | n.d. | n.d. | 850 °C | [71] |
| TGA | 1 × 10−5 kg | 80 min | 900 °C | [69] |
| TGA | 1 × 10−4 kg | 135 min | 700 °C | [81] |
| Free-fall vertical reactor Steam gasification of biochar | 1 × 10−3 kg | 120 min | 750 to 900 °C | [60] |
| Aspen simulation Combined gasifier + SOFC (solid Oxide fuel cell) | 3.6 × 100 kg/h | (Equilibrium simulation) | 750 °C | [56] |
| Fluidized bed co-gasification in spouted-bed reactor | 1 × 100 kg/h | 1.5 to 4 h | 850 °C | [64] |
3.3. Syngas Composition, as Gasification Performance, in the Literature on Marine Resource Gasification
3.3.1. Hydrothermal Processes
| Gasification Type | Fuel | H2 % v/v-dry | CO % v/v-dry | CO2 % v/v-dry | CH4 % v/v-dry | CxHy % v/v-dry | Syngas LHV/HHV MJ/Nm3 | Ref. |
|---|---|---|---|---|---|---|---|---|
| Supercritical water gasification | Ulva Intestinalis | 2 to 4 mmol/g | 1.75 to 1.5 mmol/g | 6 to 11 mmol/g | 3.5 to 2.5 mmol/g | C2H6 1 to 0.5 mmol/g | n.d. | [61] |
| Supercritical water gasification | Chlorella Vulgaris | 18.3 | 5.28 | 45 | 17.1 | 14.3 (C2-C4) | 22.8 | [57] |
| Spirulina Platensis | 21.1 | 4.26 | 36.2 | 21.2 | 16.9 (C2-C4) | 27.9 | ||
| Saccharina Latissima | 24.8 | 4.23 | 50.2 | 12.0 | 8.74 (C2-C4) | 17.3 | ||
| Hydrothermal gasification | Posidonia oceanica | 300 °C: 0 600 °C: 50 | 300 °C: 5–8 600 °C: 0 | 300 °C: 95 600 °C: 25 | 300 °C: 0 600 °C: 25 | C2H6 + C3H8 + C4H10 300 °C: 0 600 °C: 0 Peak at 550 °C: 10 | n.d. | [70] |
| Hydrothermal liquefaction then supercritical water gasification | Nannochloropsis oceanica | 27.77 | 9.76 | 15.26 | 37.35 | C2H6 9.76 | n.d. | [58] |
| Auxenochlorella pyrenoidosa | 30.11 | 0.46 | 26.46 | 32.40 | C2H6 9.89 | n.d. | ||
| Arthrospira platensis | 27.83 | 0.27 | 33.91 | 35.11 | C2H6 2.69 | n.d. | ||
| Schizochytrium limacinum | 34.48 | 0.63 | 17.30 | 36.20 | C2H6 11.22 | n.d. | ||
| Ulva prolifera | 28.17 | 0.00 | 28.30 | 35.95 | C2H6 7.24 | n.d. | ||
| Saccharina japonica | 31.37 | 6.65 | 25.90 | 29.35 | C2H6 6.06 | n.d. | ||
| Zostera marina | 33.72 | 0.27 | 33.81 | 26.68 | C2H6 5.62 | n.d. | ||
| Gracilaria eucheumoides harvey | 21.05 | 12.40 | 38.35 | 25.73 | C2H6 2.57 | n.d. | ||
| Supercritical water gasification with catalysts | Chlorella PTCC 6010 | 1.1–20.9 | 7.7–34.2 | 57.1–84.3 | 0.5–7.7 | n.d. | n.d. | [73] |
| Optimum: 405 °C, 45 min, 1.4% w/w | 20.3 | 8.6 | 64.0 | 7.1 | n.d. | n.d. | ||
| Hydrothermal gasification with catalyst | Nannochloropsis sp. | 35–40 | 0–5 | 43–50 | 9–18 | C2Hx 0–6 | n.d. | [67] |
| Supercritical hydrothermal liquefaction with gasification | Nannochloropsis sp. | 0.39 to 19.3 | 0.2 to 0.4 (only 450 and 500 °C) | 97 to 36 | 0.05 to 32.6 | C2H6 0.64 to 11.4 (300 to 500 °C) | n.d. | [66] |
| Supercritical water gasification of polycarbonate Parameters: 10 min/23 MPa 5% w/w/700 °C | Effect of temperature 500 to 800 °C | 5–30 | 2–15 | 35–70 | 20–35 | C2H4 + C2H6 0–5 | n.d. | [76] |
| Effect of time 2–60 min | 10–30 | 2–20 | 35–40 | 30–35 | C2H4 + C2H6 0–2 | n.d. | ||
| Effect of feedstock concentration 5–25% w/w | 17–25 | 5–7 | 30–35 | 35–45 | C2H4 + C2H6 0–1 | n.d. | ||
| Supercritical water gasification of polypropylene Parameters: 10 min/23 MPa 5% w/w/700 °C | Effect of temperature 500 to 800 °C | 5–37 | 2–5 | 5–15 | 45–70 | C2H4 + C2H6 0–30 | n.d. | [78] |
| Effect of time 2–60 min | 10–30 | 2–5 | 5–10 | 60–75 | C2H4 + C2H6 0–8 | n.d. | ||
| Effect of feedstock concentration 5–25% w/w | 12–18 | 1–4 | 2–5 | 75–85 | C2H4 + C2H6 0–1 | n.d. | ||
| Sub-critical water gasification 400 °C 90 min 8 MPa | Ulva Lactuca 1% w/w | 8 | n.d. | 82 | 10 | n.d. | n.d. | [59] |
| Ulva Lactuca 5% w/w | 2.5 | n.d. | 95 | 2.5 | n.d. | n.d. | ||
| Catalytic hydrothermal gasification | Marine boat wrap | 26 g/kg fuel | n.d. | n.d. | n.d. | n.d. | n.d. | [80] |
| Hydrothermal gasification | Ulva Armoricana Ulva Rotundana | 3–16 | <2 | 40–60 | 3–18 | n.d. | n.d. | [62] |
| Supercritical water gasification of polyethylene terephthalate Parameters: 10 min/23 MPa 5% w/w/700 °C | Effect of temperature 500 to 800 °C | 5–25 | 5–25 | 45–55 | 18–22 | C2H4 + C2H6 0–2 | n.d. | [77] |
| Effect of time 2–60 min | 8–20 | 5–20 | 50–55 | ~20 | C2H4 + C2H6 0–1 | n.d. |
- An increase from 0 to 19% v/v for H2.
- A decrease from 97 to 36% v/v for CO2.
- An increase from 0 to 32% v/v for CH4.
3.3.2. Dry Gasification Processes
| Gasification Type | Fuel | H2 % v/v-dry | CO % v/v-dry | CO2 % v/v-dry | CH4 % v/v-dry | CxHy % v/v-dry | Syngas LHV/HHV MJ/Nm3 | Ref. |
|---|---|---|---|---|---|---|---|---|
| Fixed-bed tube reactor Composition in mmol/gC | Zostera Marina | 22.5 | 2.5 | 12 | 1 | n.d. | n.d. | [72] |
| Torrefied—200 °C | 20 | 2.5 | 10 | 2 | n.d. | n.d. | ||
| Torrefied—250 °C | 18 | 2.5 | 8 | 2 | n.d. | n.d. | ||
| Torrefied—300 °C | 17 | 2 | 7 | 2 | n.d. | n.d. | ||
| Tubular horizontal fixed-bed reactor | Chlorella Vulgaris | 20 to 30 % of measured gas | 22 to 25 % of measured gas | 27 to 37 % of measured gas | 17 to 19 % of measured gas | n.d. | 11 to 13 MJ/m3 | [74] |
| Downdraft fixed bed | Driftwood | 11.0 | 17.2 | 12.3 | 1.6 | n.d. | HHV: 4.2 MJ/m3 | [68] |
| Horizontal tube fixed bed | Posidonia Oceanica | 8 to 15 | 9 to 23 | 12 to 21 | 3 to 7 | C2H4: 1–3 C2H6: 0–1 | n.d. | [71] |
| TGA Composition in g/g-sample(daf) | Rhizophora mucronata | 0.03 | 0.32 | 0.30 | 0.06 | n.d. | n.d. | [69] |
| Bruguiera cylindrica | 0.03 | 0.30 | 0.28 | 0.06 | n.d. | n.d. | ||
| Avicennia marina | 0.04 | 0.32 | 0.44 | 0.06 | n.d. | n.d. | ||
| Eucalyptus | 0.03 | 0.30 | 0.24 | 0.06 | n.d. | n.d. | ||
| Japanese cedar | 0.03 | 0.40 | 0.24 | 0.06 | n.d. | n.d. | ||
| Japanese cypress | 0.03 | 0.44 | 0.22 | 0.06 | n.d. | n.d. | ||
| Free-fall vertical reactor Steam gasification of biochar Temperature 750 °C to 900 °C Composition in mL/g | Corn Stalk (char) | 32–52 | 35–25 | 22–20 | 8–2 | 1–0 | n.d. | [60] |
| Common reed (char) | 38–55 | 32–12 | 30–20 | 10–2 | 1–0 | n.d. | ||
| Sargassum Horneri (char) | 40–58 | 42–8 | 10–30 | 12–2 | <1 | n.d. | ||
| Fluidized-bed co-gasification spouted-bed reactor | 100% coal (* % of carbon in the gas) | 12–20 | 25–40 * | 50–62 * | 3.5–6 * | n.d. | n.d. | [64] |
| 100% algae | Aborted test due to abnormal compartment of the gasification | |||||||
| 10% algae 90% coal | 6–9 | 11–13 | 10–13 | 1 | n.d. | n.d. | ||
4. Limitations of the Current Literature
4.1. Limitations of Syngas and Mass–Energy Balance Analysis
4.2. Operating Conditions Not Representative of Real Applications
4.3. Operating Conditions of the Dry Gasification: A Temperature Minimum
4.4. Limitations of Analysis of By-Products of Gasification: Char, Tar, and Pollutants (H2S, NH3, and HCN)
- Char/ashes: Solid residue containing minerals and potentially enriched in heavy metals. Often, the treatment is only landfilling, sometimes with “hazardous waste” status.
- Tars which can cause clogging of the moving pieces of engines.
- H2S which produces toxic atmospheric pollutants (SO2) and poison catalysts (Fischer–Tropsch).
- NH3 which produces atmospheric pollutants (NOx).
- HCN which produces toxic atmospheric pollutants (NOx).
4.5. Supercritical Gasification: Precipitation of Inorganics Causing Clogging
5. Presentation of Perspectives for Further Studies
5.1. Marine Resources Are Too Wet for Dry Gasification: Ways to Improve Fuel Quality or Process Flexibility
5.1.1. Integration in a Biorefinery Process
5.1.2. Consider Co-Gasification with “Dry” Land Biomass, Including Invasive Land Biomass
5.1.3. Oxy-Gasification
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). Technical Summary. In The Ocean and Cryosphere in a Changing Climate; Cambridge University Press: Cambridge, UK, 2022; pp. 39–70. [Google Scholar] [CrossRef]
- Reimann, L.; Vafeidis, A.T.; Honsel, L.E. Population development as a driver of coastal risk: Current trends and future pathways. In Cambridge Prisms: Coastal Futures; Cambridge University Press: Cambridge, UK, 2023; Volume 1. [Google Scholar] [CrossRef]
- Nicholls, R.J.; Wong, P.P.; Burkett, V.R.; Codignotto, J.O.; Hay, J.E.; McLean, R.F.; Ragoonaden, S.; Woodroffe, C.D. Coastal Systems and Low-Lying Areas Climate Change 2007: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M.L., Canziani, O.F., Palutikof, J.P., van der Linden, P.J., Hanson, C.E., Eds.; Cambridge University Press: Cambridge, UK, 2007; Available online: https://www.ipcc.ch/site/assets/uploads/2018/02/ar4-wg2-chapter6-1.pdf (accessed on 16 September 2024).
- Intergovernmental Panel on Climate Change (IPCC). Annexes. In The Ocean and Cryosphere in a Changing Climate; Cambridge University Press: Cambridge, UK, 2022; pp. 131–202. [Google Scholar] [CrossRef]
- BP. BP Energy Outlook, 2024 ed.; BP: London, UK, 2024. [Google Scholar]
- Global Direct Primary Energy Consumption—Energy Institute—Statistical Review of World Energy (2024), Smil (2017)—With Major Processing by Our World In Data. Available online: https://ourworldindata.org/grapher/global-primary-energy (accessed on 27 November 2024).
- Eight glacial cycles from an Antarctic ice core. Nature 2004, 429, 623–628. [CrossRef]
- Ministry of Public Works Meteoroligical Services and Transport of Fidji (MPWMST). Republic of Fiji—National Energy Policy 2023–2030. Available online: https://islands.irena.org/Publications#regional-reports (accessed on 13 July 2024).
- Chum, H.; Faaij, A.; Moreira, J. Bioenergy. In IPCC Special Report on Renewable Energy Sources and Climate Change Mitigation; IPCC: Geneva, Switzerland, 2011. [Google Scholar]
- Monteiro, E.; Ferreira, S. Some Perspectives for the Gasification Process in the Energy Transition World Scenario. Energies 2023, 16, 5543. [Google Scholar] [CrossRef]
- Magnier, M. L’éclairage au Gaz ou Traité Élémentaire et Pratique à L’usage des Ingénieurs, Directeurs et Contre-Maîtres D’usines à Gaz, Manuels Roret, Paris; Librairie Encyclopédique de Roret: Paris, France, 1849. [Google Scholar]
- Solar Energy Research Institute—SERI. A Survey of Biomass Gasification Volume II-Principles of Gasification; Solar Energy Research Institute: Golden, CO, USA, 1979. [Google Scholar]
- Guettel, R.; Kunz, U.; Turek, T. Reactors for Fischer-Tropsch Synthesis. Chem. Eng. Technol. 2008, 31, 746–754. [Google Scholar] [CrossRef]
- FAO. Wood Gas as Engine Fuel. Food and Agriculture Organization of the United Nations. 1986. Available online: https://www.fao.org/4/t0512e/t0512e00.htm (accessed on 15 October 2024).
- Khlifi, S.; Pozzobon, V.; Lajili, M. A Comprehensive Review of Syngas Production, Fuel Properties, and Operational Parameters for Biomass Conversion. Energies 2024, 17, 3646. [Google Scholar] [CrossRef]
- Susastriawan, A.; Saptoadi, H. Purnomo Small-scale downdraft gasifiers for biomass gasification: A review. Renew. Sustain. Energy Rev. 2017, 76, 989–1003. [Google Scholar] [CrossRef]
- International Energy Agency (IEA). Global Hydrogen Review 2024. November 2024. Available online: www.iea.org (accessed on 13 July 2024).
- Situmorang, Y.A.; Zhao, Z.; Yoshida, A.; Abudula, A.; Guan, G. Small-scale biomass gasification systems for power generation (<200 kW class): A review. Renew. Sustain. Energy Rev. 2020, 117, 109486. [Google Scholar] [CrossRef]
- Shahbaz, M.; Al-Ansari, T.; Aslam, M.; Khan, Z.; Inayat, A.; Athar, M.; Naqvi, S.R.; Ahmed, M.A.; McKay, G. A state of the art review on biomass processing and conversion technologies to produce hydrogen and its recovery via membrane separation. Int. J. Hydrogen Energy 2020, 45, 15166–15195. [Google Scholar] [CrossRef]
- Kumar, A.; Jones, D.D.; Hanna, M.A. Thermochemical Biomass Gasification: A Review of the Current Status of the Technology. Energies 2009, 2, 556–581. [Google Scholar] [CrossRef]
- Farobie, O.; Matsumura, Y.; Syaftika, N.; Amrullah, A.; Hartulistiyoso, E.; Bayu, A.; Moheimani, N.R.; Karnjanakom, S.; Saefurahman, G. Recent advancement on hydrogen production from macroalgae via supercritical water gasification. Bioresour. Technol. Rep. 2021, 16, 100844. [Google Scholar] [CrossRef]
- Basu, P.; Mettanant, V. Biomass Gasification in Supercritical Water—A Review. Int. J. Chem. React. Eng. 2009, 7. [Google Scholar] [CrossRef]
- International Energy Agency. Net Zero by 2050—A Roadmap for the Global Energy Sector. 2021. Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 13 July 2024).
- Clarke, L.; Wei, Y.M.; de la Vega Navarro, A.; Garg, A.; Hahmann, A.N.; Khennas, S.; Azevedo, I.M.; Löschel, A.; Singh, A.K.; Steg, L.; et al. Energy Systems. In IPCC, 2022: Climate Change 2022—Mitigation of Climate Change; Cambridge University Press: Cambridge, UK, 2023; Chapter 6; pp. 613–746. [Google Scholar] [CrossRef]
- Boku, J.H. IEA Bioenergy—Gasification—A Key Technology in the Energy Transition and for the Circular Economy—Workshop Report. 2022. Available online: https://www.unido.org/ (accessed on 13 July 2024).
- Bressan, M.; Campagnoli, E.; Ferro, C.G.; Giaretto, V. A Mass Balance-Based Method for the Anaerobic Digestion of Rice Straw. Energies 2023, 16, 4334. [Google Scholar] [CrossRef]
- Louis, J.; Ballu, S.; Rossi, N.; Lasbleiz, M.; Perrot, T.; Daniel, C.; Cellier, L.; Hénaff, F.; Richier, S. Multi-year renewal of green tides: 18 years of algal mat monitoring (2003–2020) on French coastline (Brittany region). Mar. Pollut. Bull. 2023, 193, 115173. [Google Scholar] [CrossRef]
- ANSES. Green Algae, Risks to Surrounding Populations, Walkers and Workers; ANSES: Maisons-Alfort, France, 2013. [Google Scholar]
- Kolf-Clauw, M.; Chiron, J.; Arnich, N.; Berny, P.; Deserts, R.D.D.; Dunoyer, C.; Guillotin, J.; Laval, A.; Morvan, H.; Yamada, O.; et al. Acute poisoning of wild boars by H2S: Wildlife as sentinels for environmental hazard. Toxicol. Lett. 2012, 211, S92–S93. [Google Scholar] [CrossRef]
- Mishra, K.; Siwal, S.S.; Saini, A.K.; Thakur, V.K. Recent update on gasification and pyrolysis processes of lignocellulosic and algal biomass for hydrogen production. Fuel 2023, 332, 126169. [Google Scholar] [CrossRef]
- Alvarado-Flores, J.J.; Alcaraz-Vera, J.V.; Ávalos-Rodríguez, M.L.; Guzmán-Mejía, E.; Rutiaga-Quiñones, J.G.; Pintor-Ibarra, L.F.; Guevara-Martínez, S.J. Thermochemical Production of Hydrogen from Biomass: Pyrolysis and Gasification. Energies 2024, 17, 537. [Google Scholar] [CrossRef]
- Bhateria, R.; Dhaka, R. Algae as biofuel. Biofuels 2014, 5, 607–631. [Google Scholar] [CrossRef]
- Rony, Z.I.; Rasul, M.; Jahirul, M.; Mofijur, M. Harnessing marine biomass for sustainable fuel production through pyrolysis to support United Nations’ Sustainable Development Goals. Fuel 2024, 358, 130099. [Google Scholar] [CrossRef]
- United Nations Environment Programme. Seaweed Farming—Assessment on the Potential of Sustainable Upscaling for Climate, Communities and the Planet; United Nations Environment Programme: Nairobi, Kenya, 2023. [Google Scholar]
- Rowbotham, J.; Dyer, P.; Greenwell, H.; Theodorou, M. Thermochemical processing of macroalgae: A late bloomer in the development of third-generation biofuels? Biofuels 2012, 3, 441–461. [Google Scholar] [CrossRef]
- European Union, Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives. 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008L0098 (accessed on 15 October 2024).
- Pardilhó, S.; Cotas, J.; Pereira, L.; Oliveira, M.B.; Dias, J.M. Marine macroalgae in a circular economy context: A comprehensive analysis focused on residual biomass. Biotechnol. Adv. 2022, 60, 107987. [Google Scholar] [CrossRef]
- Murphy, E.; Nistor, I.; Cornett, A.; Wilson, J.; Pilechi, A. Fate and transport of coastal driftwood: A critical review. Mar. Pollut. Bull. 2021, 170, 112649. [Google Scholar] [CrossRef]
- Qatarneh, A.F.; Dupont, C.; Ruiz-Villanueva, V.; Perez, D.d.S.; Ashour, R.M.; Piégay, H.; Franca, M.J. Evaluating river driftwood as a feedstock for biochar production. Waste Manag. 2021, 134, 197–205. [Google Scholar] [CrossRef]
- Bartocci, P.; Barbanera, M.; D’amico, M.; Laranci, P.; Cavalaglio, G.; Gelosia, M.; Ingles, D.; Bidini, G.; Buratti, C.; Cotana, F.; et al. Thermal degradation of driftwood: Determination of the concentration of sodium, calcium, magnesium, chlorine and sulfur containing compounds. Waste Manag. 2017, 60, 151–157. [Google Scholar] [CrossRef]
- Tsai, W.-T.; Tsai, Y.-L.; Liu, S.-C. Utilization of driftwood as an energy source and its environmental and economic benefit analysis in Taiwan. BioResource 2011, 6, 4781–4789. [Google Scholar] [CrossRef]
- IUCN. Issues Brief: Plastic Pollution 2024. May 2024. Available online: https://iucn.org/sites/default/files/2024-05/plastic-pollution-issues-brief-may-2024-update.pdf (accessed on 16 October 2024).
- IUCN. Issues Brief: Plastic Pollution 2021. November 2021. Available online: https://iucn.org/sites/default/files/2022-04/marine_plastic_pollution_issues_brief_nov21.pdf (accessed on 16 October 2024).
- Pham, C.K.; Estevez, S.G.; Pereira, J.M.; Herrera, L.; Rodríguez, Y.; Domínguez-Hernández, C.; Villanova-Solano, C.; Hernández-Sánchez, C.; Díaz-Peña, F.J.; Hernández-Borges, J. Three-dimensional evaluation of beaches of oceanic islands as reservoirs of plastic particles in the open ocean. Sci. Total. Environ. 2023, 900, 165798. [Google Scholar] [CrossRef]
- Mallick, K.; Sahu, A.; Dubey, N.K.; Das, A.P. Harvesting marine plastic pollutants-derived renewable energy: A comprehensive review on applied energy and sustainable approach. J. Environ. Manag. 2023, 348, 119371. [Google Scholar] [CrossRef]
- Vonk, G.; Piriou, B.; Dos Santos, P.F.; Wolbert, D.; Vaïtilingom, G. Comparative analysis of wood and solid recovered fuels gasification in a downdraft fixed bed reactor. Waste Manag. 2019, 85, 106–120. [Google Scholar] [CrossRef]
- Ramos, A.; Monteiro, E.; Silva, V.; Rouboa, A. Co-gasification and recent developments on waste-to-energy conversion: A review. Renew. Sustain. Energy Rev. 2018, 81, 380–398. [Google Scholar] [CrossRef]
- Mariyam, S.; Shahbaz, M.; Al-Ansari, T.; Mackey, H.R.; McKay, G. A critical review on co-gasification and co-pyrolysis for gas production. Renew. Sustain. Energy Rev. 2022, 161, 112349. [Google Scholar] [CrossRef]
- Greggio, N.; Carlini, C.; Contin, A.; Soldano, M.; Marazza, D. Exploitable fish waste and stranded beach debris in the Emilia-Romagna Region (Italy). Waste Manag. 2018, 78, 566–575. [Google Scholar] [CrossRef]
- Rudovica, V.; Rotter, A.; Gaudêncio, S.P.; Novoveská, L.; Akgül, F.; Akslen-Hoel, L.K.; Alexandrino, D.A.M.; Anne, O.; Arbidans, L.; Atanassova, M.R.; et al. Valorization of Marine Waste: Use of Industrial By-Products and Beach Wrack Towards the Production of High Added-Value Products. Front. Mar. Sci. 2021, 8, 723333. [Google Scholar] [CrossRef]
- Mac Monagail, M.; Cornish, L.; Morrison, L.; Araújo, R.; Critchley, A.T. Sustainable harvesting of wild seaweed resources. Eur. J. Phycol. 2017, 52, 371–390. [Google Scholar] [CrossRef]
- Rawat, Y.S.; Singh, G.S.; Tekleyohannes, A.T. Optimizing the Benefits of Invasive Alien Plants Biomass in South Africa. Sustainability 2024, 16, 1876. [Google Scholar] [CrossRef]
- Pušić, M.; Ljubojević, M.; Prvulović, D.; Kolarov, R.; Tomić, M.; Simikić, M.; Vejnović, S.; Narandžić, T. Bioenergy and Biopesticides Production in Serbia—Could Invasive Alien Species Contribute to Sustainability? Processes 2024, 12, 407. [Google Scholar] [CrossRef]
- Correia, R.; Quintela, J.C.; Duarte, M.P.; Gonçalves, M. Insights for the Valorization of Biomass from Portuguese Invasive Acacia spp. in a Biorefinery Perspective. Forests 2020, 11, 1342. [Google Scholar] [CrossRef]
- Bandara, W.; Ranasinghe, O.; Perera, P.; Vlosky, R.; Kizha, A.R. Potential to use invasive plants in biomass energy production: A case study Prosopis juliflora in coastal wetlands of Sri Lanka. Trees For. People 2022, 10, 100330. [Google Scholar] [CrossRef]
- Faheem, H.H.; Britt, B.; Rocha, M.; Zhou, S.-H.; Li, C.; Cai, W.; Fan, L. Sensitivity analysis and process optimization for biomass processing in an integrated gasifier-solid oxide fuel cell system. Fuel 2024, 356, 129529. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Lea-Langton, A.R.; Ross, A.B.; Williams, P.T. Catalytic hydrothermal gasification of algae for hydrogen production: Composition of reaction products and potential for nutrient recycling. Bioresour. Technol. 2013, 127, 72–80. [Google Scholar] [CrossRef]
- Duan, P.-G.; Yang, S.-K.; Xu, Y.-P.; Wang, F.; Zhao, D.; Weng, Y.-J.; Shi, X.-L. Integration of hydrothermal liquefaction and supercritical water gasification for improvement of energy recovery from algal biomass. Energy 2018, 155, 734–745. [Google Scholar] [CrossRef]
- Farobie, O.; Syaftika, N.; Masfuri, I.; Rini, T.P.; Es, D.P.L.; Bayu, A.; Amrullah, A.; Hartulistiyoso, E.; Moheimani, N.R.; Karnjanakom, S.; et al. Green algae to green fuels: Syngas and hydrochar production from Ulva lactuca via sub-critical water gasification. Algal Res. 2022, 67, 102834. [Google Scholar] [CrossRef]
- Li, J.; Qiao, Y.; Chen, X.; Zong, P.; Qin, S.; Wu, Y.; Wang, S.; Zhang, H.; Tian, Y. Steam gasification of land, coastal zone and marine biomass by thermal gravimetric analyzer and a free-fall tubular gasifier: Biochars reactivity and hydrogen-rich syngas production. Bioresour. Technol. 2019, 289, 121495. [Google Scholar] [CrossRef]
- Norouzi, O.; Safari, F.; Jafarian, S.; Tavasoli, A.; Karimi, A. Hydrothermal gasification performance of Enteromorpha intestinalis as an algal biomass for hydrogen-rich gas production using Ru promoted Fe–Ni/γ-Al 2 O 3 nanocatalysts. Energy Convers. Manag. 2017, 141, 63–71. [Google Scholar] [CrossRef]
- Graz, Y.; Bostyn, S.; Richard, T.; Bocanegra, P.E.; de Bilbao, E.; Poirier, J.; Gokalp, I. Hydrothermal conversion of Ulva macro algae in supercritical water. J. Supercrit. Fluids 2016, 107, 182–188. [Google Scholar] [CrossRef]
- He, Z.; Saw, W.L.; Lane, D.J.; van Eyk, P.J.; de Nys, R.; Nathan, G.J.; Ashman, P.J. The ash-quartz sand interaction behaviours during steam gasification or combustion of a freshwater and a marine species of macroalgae. Fuel 2019, 263, 116621. [Google Scholar] [CrossRef]
- Alghurabie, I.K.; Hasan, B.O.; Jackson, B.; Kosminski, A.; Ashman, P.J. Fluidized bed gasification of Kingston coal and marine microalgae in a spouted bed reactor. Chem. Eng. Res. Des. 2013, 91, 1614–1624. [Google Scholar] [CrossRef]
- Díaz-Rey, M.; Cortés-Reyes, M.; Herrera, C.; Larrubia, M.; Amadeo, N.; Laborde, M.; Alemany, L. Hydrogen-rich gas production from algae-biomass by low temperature catalytic gasification. Catal. Today 2015, 257, 177–184. [Google Scholar] [CrossRef]
- Brown, T.M.; Duan, P.; Savage, P.E. Hydrothermal Liquefaction and Gasification of Nannochloropsis sp. Energy Fuels 2010, 24, 3639–3646. [Google Scholar] [CrossRef]
- Guan, Q.; Wei, C.; Savage, P.E. Hydrothermal Gasification of Nannochloropsis sp. with Ru/C. Energy Fuels 2012, 26, 4575–4582. [Google Scholar] [CrossRef]
- Puglia, M.; Morselli, N.; Ottani, F.; Pedrazzi, S.; Tartarini, P.; Allesina, G. A preliminary evaluation of different residual biomass potential for energy conversion in a micro-scale downdraft gasifier. Sustain. Energy Technol. Assess. 2023, 57, 103224. [Google Scholar] [CrossRef]
- Sakurai, Y.; Kobayashi, J.; Sakai, Y.; Naruse, I. Pyrolysis and steam gasification properties of mangroves. Chemosphere 2023, 345, 140388. [Google Scholar] [CrossRef]
- Deniz, I.; Vardar-Sukan, F.; Yüksel, M.; Saglam, M.; Ballice, L.; Yesil-Celiktas, O. Hydrogen production from marine biomass by hydrothermal gasification. Energy Convers. Manag. 2015, 96, 124–130. [Google Scholar] [CrossRef]
- Conesa, J.A.; Domene, A. Gasification and pyrolysis of Posidonia oceanica in the presence of dolomite. J. Anal. Appl. Pyrolysis 2015, 113, 680–689. [Google Scholar] [CrossRef]
- Zahra, A.C.A.; Anniwaer, A.; Okura, H.; Chaerusani, V.; Zhang, P.; Rizkiana, J.; Kurnia, I.; Abudula, A.; Guan, G. Addition of torrefied algal biomass to improve land-based biomass gasification for hydrogen-rich gas production. Algal Res. 2023, 74, 103236. [Google Scholar] [CrossRef]
- Samiee-Zafarghandi, R.; Karimi-Sabet, J.; Abdoli, M.A.; Karbassi, A. Supercritical water gasification of microalga Chlorella PTCC 6010 for hydrogen production: Box-Behnken optimization and evaluating catalytic effect of MnO2/SiO2 and NiO/SiO2. Renew. Energy 2018, 126, 189–201. [Google Scholar] [CrossRef]
- Raheem, A.; Dupont, V.; Channa, A.Q.; Zhao, X.; Vuppaladadiyam, A.K.; Taufiq-Yap, Y.-H.; Zhao, M.; Harun, R. Parametric Characterization of Air Gasification of Chlorella vulgaris Biomass. Energy Fuels 2017, 31, 2959–2969. [Google Scholar] [CrossRef]
- Valizadeh, S.; Khani, Y.; Farooq, A.; Kumar, G.; Show, P.L.; Chen, W.-H.; Lee, S.H.; Park, Y.-K. Microalgae gasification over Ni loaded perovskites for enhanced biohydrogen generation. Bioresour. Technol. 2023, 372, 128638. [Google Scholar] [CrossRef]
- Bai, B.; Liu, Y.; Meng, X.; Liu, C.; Zhang, H.; Zhang, W.; Jin, H. Experimental investigation on gasification characteristics of polycarbonate (PC) microplastics in supercritical water. J. Energy Inst. 2020, 93, 624–633. [Google Scholar] [CrossRef]
- Bai, B.; Liu, Y.; Zhang, H.; Zhou, F.; Han, X.; Wang, Q.; Jin, H. Experimental investigation on gasification characteristics of polyethylene terephthalate (PET) microplastics in supercritical water. Fuel 2020, 262, 116630. [Google Scholar] [CrossRef]
- Bai, B.; Wang, W.; Jin, H. Experimental study on gasification performance of polypropylene (PP) plastics in supercritical water. Energy 2020, 191, 116527. [Google Scholar] [CrossRef]
- Subagyono, R.R.D.J.N.; Masdalifa, W.; Aminah, S.; Nugroho, R.A.; Mollah, M.; Allo, V.L.; Gunawan, R. Kinetic Study of Copyrolysis of the Green Microalgae Botryococcus braunii and Victorian Brown Coal by Thermogravimetric Analysis. ACS Omega 2021, 6, 32032–32042. [Google Scholar] [CrossRef]
- Galiwango, E.; Beaulne, M.; Butler, J.; Ma, W.; Austin, K.; Lotfi, S. Catalytic hydrothermal conversion of end-of-life plastic waste in near supercritical water. Int. J. Hydrog. Energy 2024, 87, 1389–1403. [Google Scholar] [CrossRef]
- Hee, J.; Schlögel, K.; Lechthaler, S.; Plaster, J.; Bitter, K.; Blank, L.M.; Quicker, P. Comparative analysis of the behaviour of marine litter in thermochemical waste treatment processes. Processes 2021, 9, 13. [Google Scholar] [CrossRef]
- Venselaar, J. Design Rules for Down Draft Wood Gasifiers a Short Review; Institut Teknologi: Bandung, Indonesia, 1982. [Google Scholar]
- Narayanan, M. Promising biorefinery products from marine macro and microalgal biomass: A review. Renew. Sustain. Energy Rev. 2024, 190, 114081. [Google Scholar] [CrossRef]
- Javed, M.U.; Mukhtar, H.; Hayat, M.T.; Rashid, U.; Mumtaz, M.W.; Ngamcharussrivichai, C. Sustainable processing of algal biomass for a comprehensive biorefinery. J. Biotechnol. 2022, 352, 47–58. [Google Scholar] [CrossRef] [PubMed]
- ANSES. ANSES 2013—Green Algae, Risks to Surrounding Populations, Walkers and Workers. Available online: https://www.anses.fr/en/content/green-algae-risks-surrounding-populations-walkers-and-workers (accessed on 15 October 2024).
- Rouanet, A.; Jeanmart, H. Improvement of syngas quality from two-stage downdraft wood gasification through steam and oxygen injection. Biomass Bioenergy 2024, 180, 106998. [Google Scholar] [CrossRef]

| Fuel/Resource | Type of Resource | Refs. |
|---|---|---|
| Marine Algae (Aspen Plus simulation) | Algae | [56] |
| Saccharina Latissima | Macroalgae | [57] |
| Ulva prolifera | Macroalgae | [58] |
| Saccharina japonica | Macroalgae | [58] |
| Gracilaria eucheumoides harvey | Macroalgae | [58] |
| Ulva Lactuca | Macroalgae | [59] |
| Sargassum Horneri | Macroalgae | [60] |
| Ulva Intestinalis | Macroalgae | [61] |
| Ulva Armoricana | Macroalgae | [62] |
| Ulva Rotundana | Macroalgae | [62] |
| Derbesia tenuissima | Macroalgae | [63] |
| Tetraselmis suecica | Microalgae | [64] |
| Scenedesmus almeriensis | Microalgae | [65] |
| Nannochloropsis sp. | Microalgae | [58,66,67] |
| Auxenochlorella pyrenoidosa | Microalgae | [58] |
| Schizochytrium limacinum | Microalgae | [58] |
| Spirulina Platensis (also, Arthrospira platensis) | Cyanobacteria | [57,58] |
| Driftwood | Wood collected in water | [40,68] |
| Rhizophora mucronata | Mangrove | [69] |
| Bruguiera cylindrica | Mangrove | [69] |
| Avicennia marina | Mangrove | [69] |
| Posidonia Oceanica | Plant (underwater) | [70,71] |
| Zostera Marina | Plant (underwater) | [58,72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vonk, G.; Boy, V.; Lanoisellé, J.-L.; Lendormi, T. The Gasification of Marine and Coastal Resources for Syngas Production: A Review. Energies 2025, 18, 616. https://doi.org/10.3390/en18030616
Vonk G, Boy V, Lanoisellé J-L, Lendormi T. The Gasification of Marine and Coastal Resources for Syngas Production: A Review. Energies. 2025; 18(3):616. https://doi.org/10.3390/en18030616
Chicago/Turabian StyleVonk, Gwendal, Virginie Boy, Jean-Louis Lanoisellé, and Thomas Lendormi. 2025. "The Gasification of Marine and Coastal Resources for Syngas Production: A Review" Energies 18, no. 3: 616. https://doi.org/10.3390/en18030616
APA StyleVonk, G., Boy, V., Lanoisellé, J.-L., & Lendormi, T. (2025). The Gasification of Marine and Coastal Resources for Syngas Production: A Review. Energies, 18(3), 616. https://doi.org/10.3390/en18030616









