Abstract
The results of testing the ignition properties of fuels in the form of blends of diesel oil with pyrolysis oil produced from tires, used as an additive at concentrations of 0, 5, 7, 10, 15, and 20% m/m, are presented in this paper. The experiment included the preparation of distillation curves and the determination of the flash points, derived cetane number, and calculated cetane ratios. The results are related to the limits indicated in selected standards and regulations on requirements for marine- and land-based compression ignition engine fuels. The obtained results show the suitability of pyrolysis oil and the possibility of its use as an additive to fossil fuels, which fits in with the requirements of the policies currently being developed for reducing the use of fossil fuels and building a circular economy.
1. Introduction
Current environmental challenges aimed at reducing climate change, alongside the problem of depleted fossil fuel resources, are prompting scientists and engineers to look for alternative energy sources. Pyrolytic oil (also known as post-pyrolysis or post-recycling oil) is recycled oil (RF) produced from used tires. RF and biodiesel, derived from vegetable oils, are promising solutions. The use of such fuels fits in with the environmental requirements for and the building of a closed-loop economy. Fuels refined with vegetable or post-recycling additives can, therefore, potentially be used as replacements or additives for diesel oil. A process such as pyrolysis allows for waste to be converted into fuels with functional properties that meet technological and environmental requirements.
Pyrolysis oil is the product of a pyrolysis process in which waste materials, such as used tires, undergo high-temperature decomposition under anaerobic conditions. This product contains a mixture of hydrocarbon compounds with a wide range of boiling points, which influences its varied physicochemical properties. As shown in a study [1], the density of pyrolysis oil is similar to diesel fuel (about 930 kg/m3), and the calorific value is about 42 MJ/kg, which places it, with these two parameters in mind, between gasoline and diesel fuel. However, the relatively high content of sulfur and solid pollutants, including ash, as well as the low value of the cetane number, are a significant barrier to its direct use in internal combustion engines. Biodiesel is a renewable fuel with properties similar to those of traditional diesel. Incorporating biodiesel into pyrolytic oil blends can improve the lubricating properties of the fuel and reduce emissions compared with conventional petroleum diesel. Such additives can reduce the content of pollutants such as NOx (common notation for nitrogen oxides NO and NO2) and PM (particulate matter) in exhaust [2]. Pyrolysis oil from tires, when blended with biodiesel, makes it a potential alternative fuel [3,4]. The results presented in refs. [5,6] indicate that the combustion process can be optimized by using biogas as a booster fuel.
Studies indicate that the use of additives, such as biodiesel, can effectively modify combustion characteristics and improve engine performance [7]. Mixtures of pyrolytic oil and biodiesel show synergistic effects that favorably influence their use as alternative fuels. In a previous study [8], it was shown that blends with up to 30% pyrolytic oil are stable and do not require modifications to engine design. Hybrid blends such as TP10KB20 (10% pyrolysis oil, 20% biodiesel, and 70% diesel) have a higher heating value and better lubricity properties compared with conventional diesel, which reduces engine wear [9]. The addition of a 10% mixture of fatty acid methyl esters (FAMEs) from rapeseed and sunflower oil significantly reduced the coefficient of friction and mechanical wear, indicating the beneficial effect of FAME additives on lubricating film durability and ignition properties [10].
Various purification methods are used, such as oxidative desulfurization and vacuum distillation [11], to reduce the content of sulfur and other impurities in pyrolytic oil. In a previous paper [12], the effectiveness of extraction techniques using methanol and adsorption on silica gel was presented, which reduced sulfur by more than 60%. The purified pyrolytic oil can then be used as a fuel component in transportation applications. The use of alternative fuels, such as pyrolysis oil and biodiesel, contributes to reducing environmental impact. The process of pyrolyzing used tires reduces landfill waste and the greenhouse gas emissions associated with burning them in the open atmosphere [13]. In addition, blending pyrolysis oil with biodiesel can reduce NOx, CO (carbon oxide), and HC (hydrocarbons) emissions, which has been confirmed in experimental studies [14].
Pyrolysis oil and biodiesel, thus, have the potential to revolutionize the alternative fuel market as bridge fuels before the introduction of low-carbon and carbon-free fuels, a goal currently being pursued by industrialized countries. Their production allows for the use of waste and renewable resources, which makes them attractive from the point of view of the economy [15,16]. The challenge remains to optimize pyrolysis processes further and develop technologies for the purification and modification of fuels to meet the standards required by the transportation industry [17].
Pyrolytic oil offers a number of environmental and technological advantages. Its widespread use, however, requires further research into the properties of fuel blends, purification processes, and the impact on engine performance and durability. In this article, the authors present the results of a study of the ignition properties of fuels prepared as blends of diesel and pyrolytic oil. The authors performed an experiment to determine the effect of the addition of pyrolysis oil to diesel fuel on the ignition temperature, volatility of the fuel, and its propensity to spontaneous combustion.
The flash point of a substance, according to EN IEC 60079-10-1:2021-09 [18], is the lowest temperature of a liquid at which, under standard conditions, the liquid produces vapor in sufficient quantity to form a combustible mixture with air [19]. This is the lowest temperature of the analytical sample, corrected for an atmospheric pressure of 101.3 kPa, at which the application of an ignition source will momentarily ignite the vapor above the surface of the test liquid [20].
Auto-ignition delay is defined as the time between the atomization of a flammable substance and the start of the combustion process after auto-ignition has occurred [21]. The indicator used in evaluating the self-ignition properties of diesel oils (fuels for compression ignition engines) is the cetane number (CN). Since the determination of CN requires the use of a special CFR (Cooperative Fuel Research) test engine, for example, according to ASTM D613-23 [22], which is a very expensive solution, a number of substitute indicators are used in practice, such as the use of analyzers to determine the derived cetane number (DCN) and the use of computed cetane indices (CCIs).
The experiment is designed to evaluate the effect of pyrolysis oil as a diesel additive and the limiting of the content of pyrolysis oil in the diesel blend to meet the requirements of selected standards in the context of the ignition properties of the tested fuel blends. The authors refer to the compliance of the tested fuel blends with the requirements of the Ministry of Economy of Poland and the ISO 8217 standard [23] characterizing marine fuels. The authors also discovered the ignition temperature values, the distillation curves, and the derived cetane numbers and then calculate the cetane indices for the fuel blends tested. Based on the obtained results, conclusions are drawn regarding the acceptable content of pyrolysis oil as a diesel oil additive in the context of the correct conditions for the realization of the combustion process in compression ignition internal combustion engines fueled with fuel blends of the mentioned type.
Our goal is to conduct as comprehensive an analysis as possible of pyrolytic oil as an additive to diesel fuel. Due to the need to investigate various properties of pyrolytic oil and its mixtures with diesel fuel, including ignition properties, impact on engine component wear, rheological properties, and the effect on the composition of exhaust gases emitted by the engine, it was necessary to divide the entire issue into a series of partial tasks. In this article, we present the results of research on the suitability of pyrolytic oil as an additive to diesel fuel, focusing on the ignition properties of the mixture and the compliance of the mixtures with the relevant standards in this area.
2. Materials and Methods
In this experiment, the ignition properties of fuels obtained as a mixture of diesel oil without FAME additives (D100 oil) with recycled fuel (RF) oil obtained from tire pyrolysis were evaluated. Pure base oils for making blends and blends of D100 with RF were tested at known mass percentages of RF in the blend equal to 5% m/m, 7% m/m, 10% m/m, 15% m/m, and 20% m/m. Oils with the characteristics shown in Table 1 were used to make the blends. Appendix A lists the dominant components of pyrolysis oil identified by mass spectrometry.

Table 1.
Characteristics of base fuels for making blends.
In the experiment, the flashpoint of the tested fuels in the closed cup was determined using the Pensky–Martens method in accordance with PN-EN ISO 2719:2016-08+A1:2021-06 [27] with the Flashpoint Pensky–Martens Semi-Automatic apparatus (Walter Herzog GmbH, Lauda-Königshofen, Germany). This apparatus was also used to determine the initial boiling point of the fuels tested. It is the lowest temperature at which the appearance of gas bubbles throughout the entire volume of the tested liquid is observed, resulting from the boiling of the lightest fractions of the fuel [31,32].
There are a number of standards and indicators describing fuel volatility [33], including those based on the temperatures at which specific amounts of fuel are distilled during distillation. These often correspond to shares equal to 10%, 50%, and 90% by volume of the fuel. One such indicator is the temperature volatility index (TVI), which was used in the experiment. The TVI is determined according to the following formula [34,35]:
where t10, t50, and t90 (°C) are recovery temperatures at which 10%, 50%, and 90% by volume of the fuel are distilled.
This is a dimensionless indicator describing the evaporability of a given fuel, and the smaller it is, the greater the volume of the fuel that evaporates at lower temperatures.
The spontaneous ignition capacities of the tested fuels were determined using DCN and CCI. These indicators are interpreted analogously to CN. It is assumed that, for marine fuels, indices above 45 correspond to very good ignition properties, while those below 25 characterize very poor self-ignition properties [36]. The DCN value was determined using the Herzog Cetane ID 510 instrument analyzer (PAC L.P., Houston, TX, USA) in accordance with ASTM D7668 (2017) [37]. The CCI, on the other hand, was calculated based on the density and temperature or temperatures extracted from the distillation curve of the fuel in question. Distillation curves were performed in accordance with PN EN ISO 3405:2019-05 [38] using the OptiDistTM apparatus (Walter Herzog GmbH, Lauda-Königshofen, Germany). The CCI can be calculated using two- and four-variable equations [39]. The two-parameter relationship for calculating the calculated cetane index is included in the ASTM D976 standard and has the following form [40]:
where ρ15 (g/cm3) is the density at 15 °C and t50 (°C) is the receiving temperature during distillation of 50% of the fuel volume.
The four-parameter relationship, on the other hand, is outlined in ASTM D4737 [41] and includes two procedures depending on the type of fuel classified according to the ASTM D975 specification [42]. Procedure B for fuels included in grade no. 2-D S15 and procedure A for other fuels. The equation for procedure A is of the following form [43]:
where ρ15 (g/cm3) is the density at 15 °C and t10, t50, and t90 (°C) are the receiving temperatures during the distillation of 10%, 50%, and 90% of the fuel volume, respectively.
In contrast, the four-parameter equation for procedure B is described by the following formula:
The fuels tested do not correspond to grade no. 2-D S15, for which the maximum temperature value of t90 is 282 °C. Thus, the experiment used the relations (3) and (4).
3. Results and Discussion
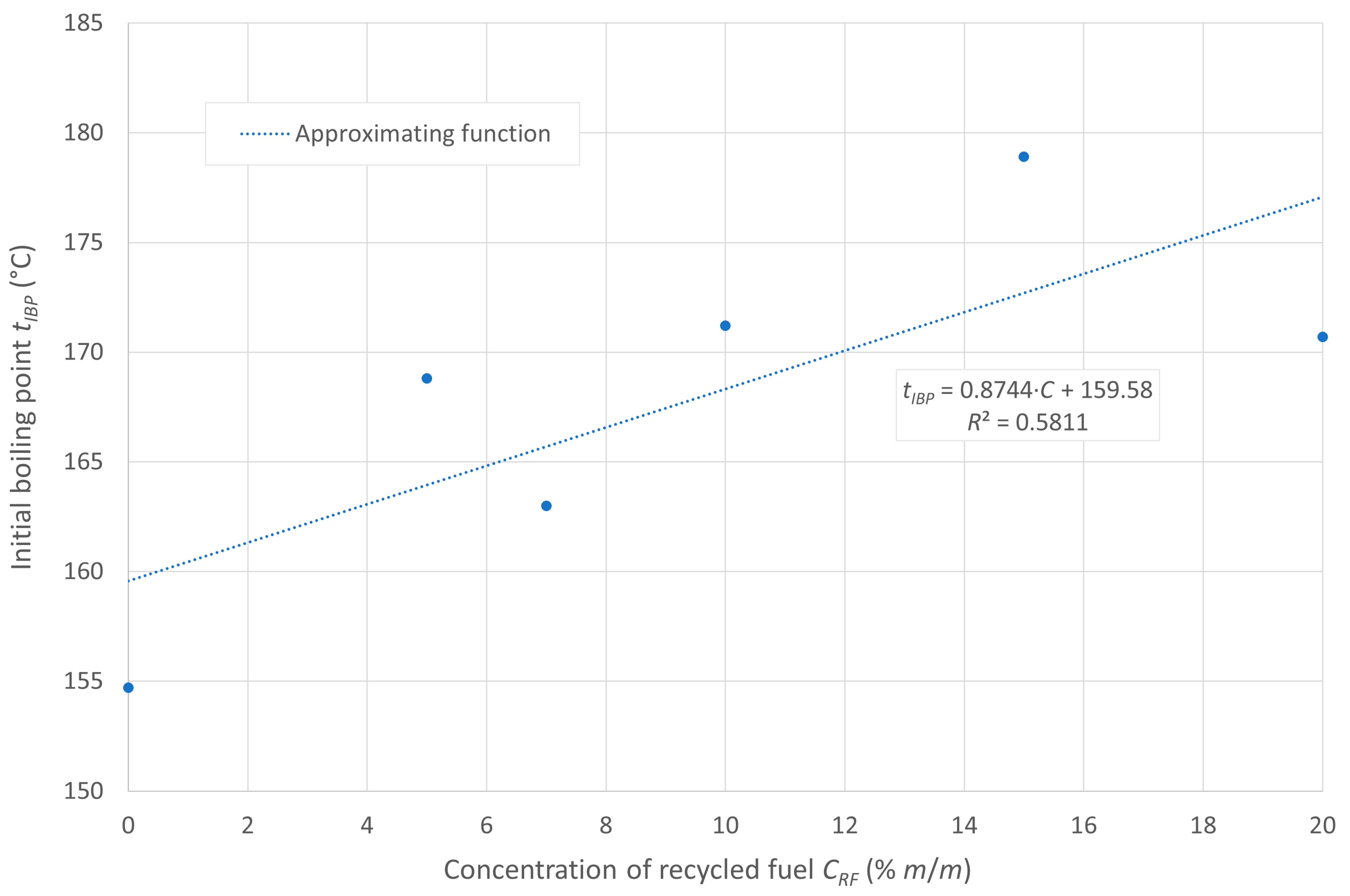
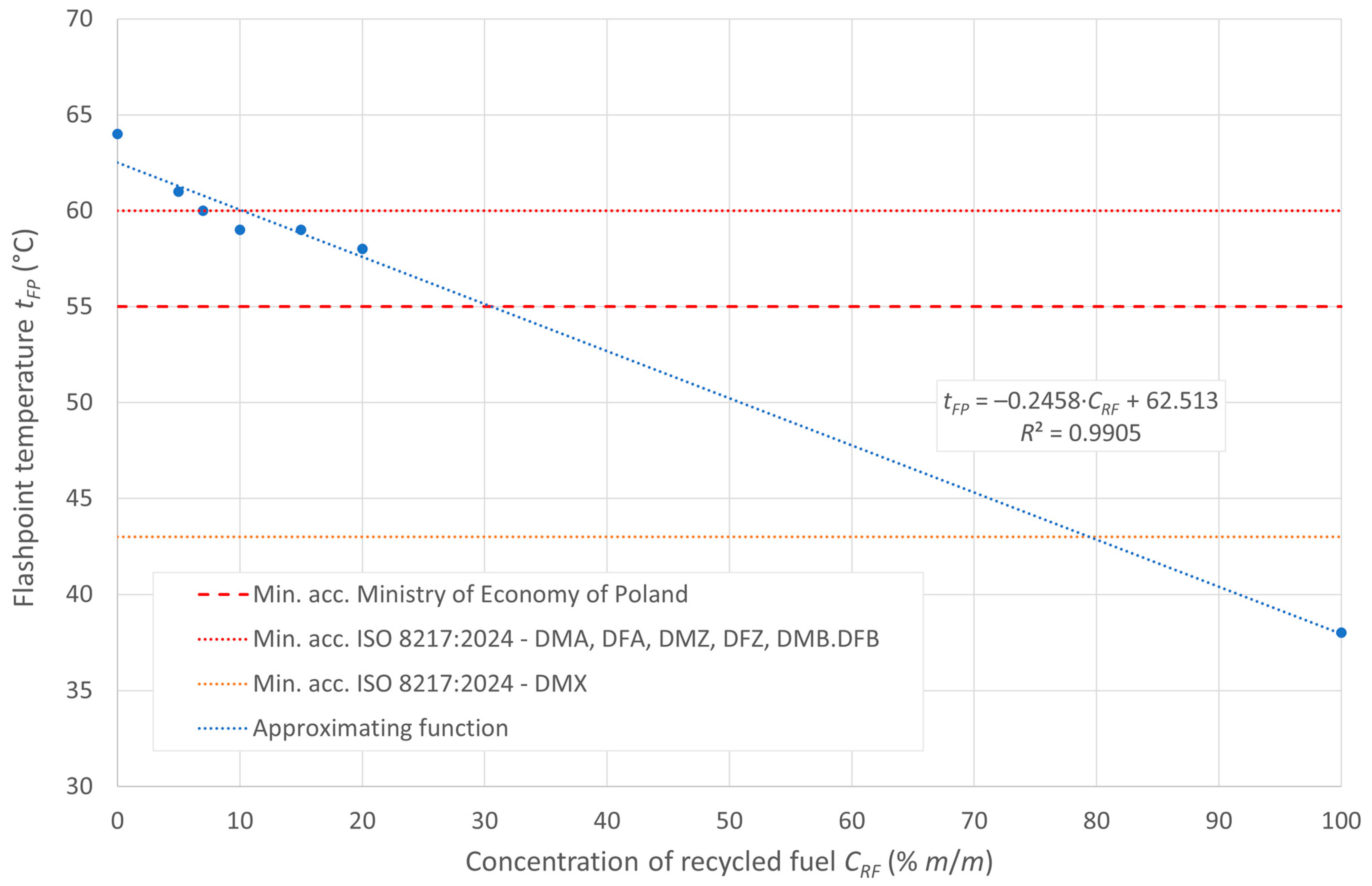
The flash point of the tested fuels decreases with increasing concentrations of recycled oil in the diesel blend, as recycled oil has a lower flash point than diesel D100. In the case analyzed, the ignition temperature is inversely proportional to the boiling onset temperature, which is due to the higher amount of heavy hydrocarbons in the composition of the post-recycling fuel. The boiling onset temperature is shown in Figure 1, while the flash point is displayed in Figure 2.
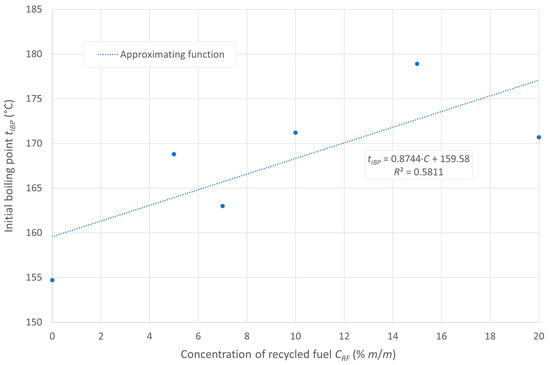
Figure 1.
Initial boiling point of the tested fuels.
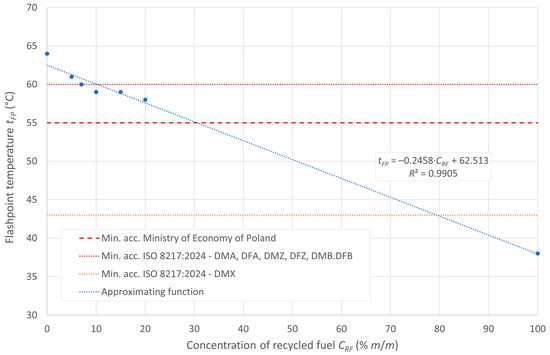
Figure 2.
Flash point of the tested fuels.
Under the initial assumption of the existence of linear relationships between these temperatures and the content of post-recycled fuel in the blends, the aforementioned relationships can be represented by the approximating lines, as shown in the figures. The linear models in the cases presented show a relatively good fit to the empirical data.
According to ISO 8217:2024 [23], the minimum flashpoint value is defined as 43 °C for DMX distillation grade fuels and 60 °C for other non-FAME marine distillation fuels, namely DMA, DMZ, and DMB. The tested blends meet the required limits for distillate fuels in the concentration range for recycled oil concentrations < 5% m/m. Moreover, for the grade fuel DMX, the standard is met by all the blends in the range of the tested concentrations of the recycled oil blended with diesel oil, which has up to 20% recycled oil content in the blend. In turn, the document “Regulation of the Minister of Economy of 9 October 2015, on quality requirements for liquid fuels” [44] specifies for internal combustion engine fuels a minimum permissible flashpoint of 55 °C, as shown in the figure with a red dashed line. All the tested mixtures meet the requirements of this document.
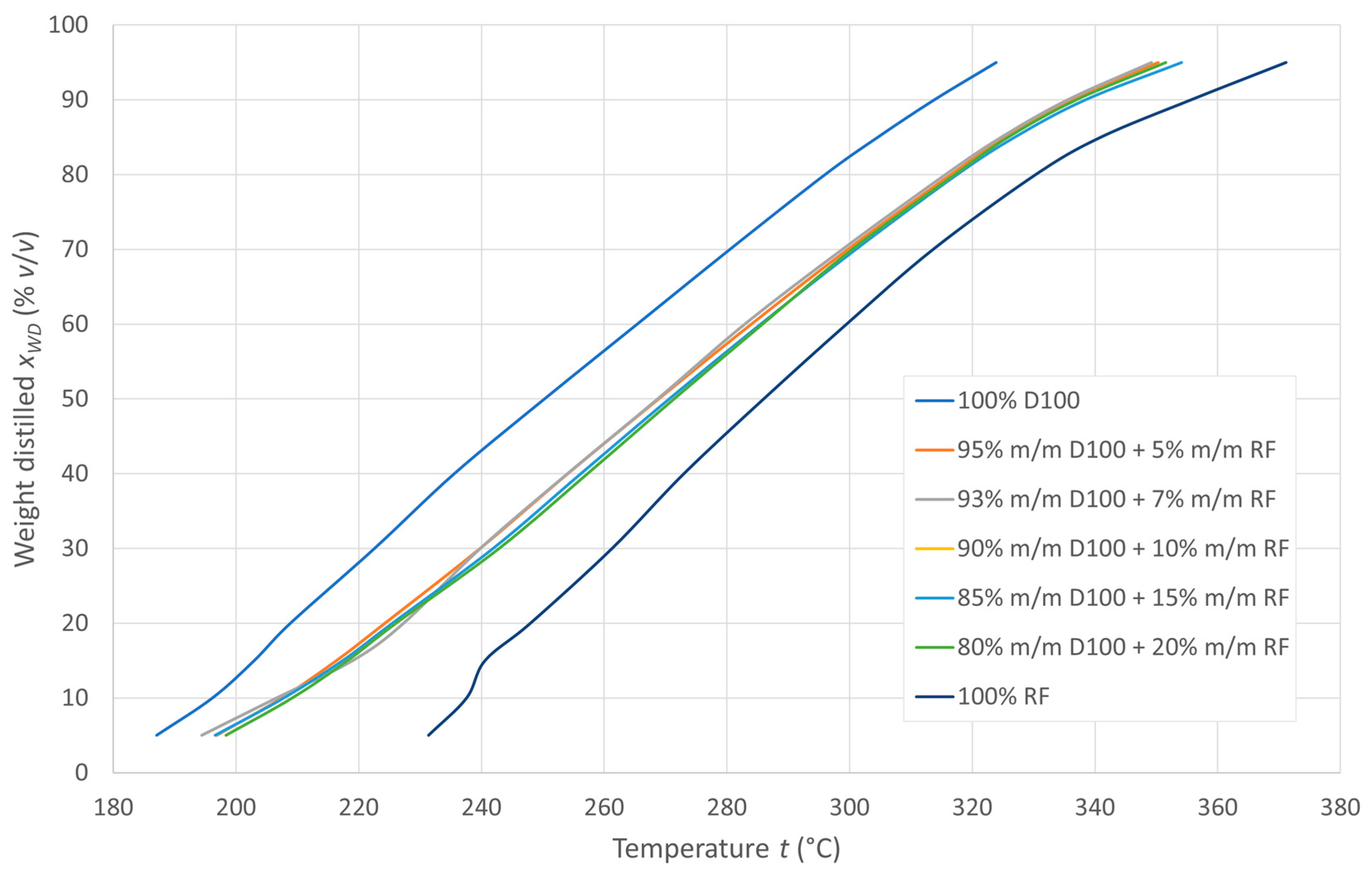
Distillation curves were prepared over the entire range of distillation temperatures for the fuel blends tested, as shown in Figure 3. All diesel-recycled oil mixtures in the concentration range of 5–20% m/m recycled oil in the mixture show similar evaporation characteristics. Receipt temperatures of 10, 50, and 90% v/v of the tested fuels and their blends were used to determine the calculated CCI ignition rates.
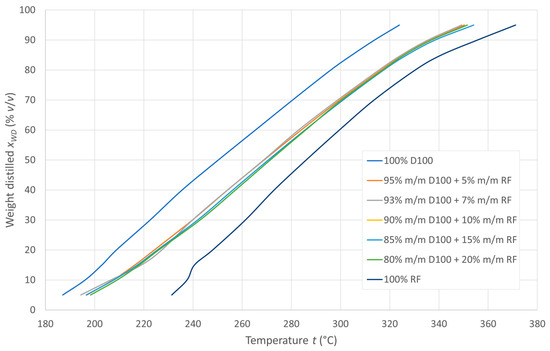
Figure 3.
Distillation curves of the tested fuels.
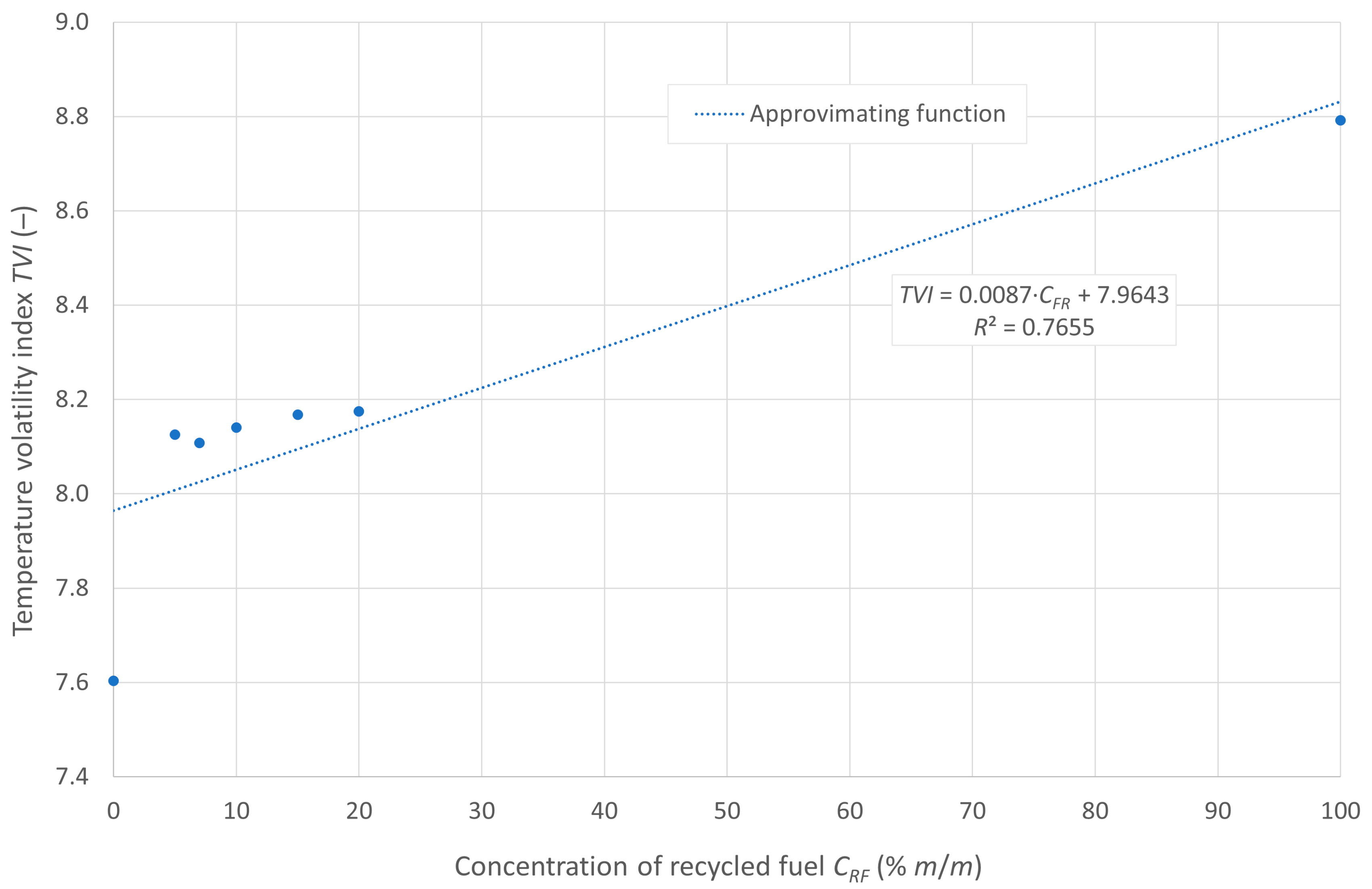
The variability of the temperature volatility index (TVI) as a function of the pyrolytic oil content in the mixture with diesel fuel is shown in Figure 4.
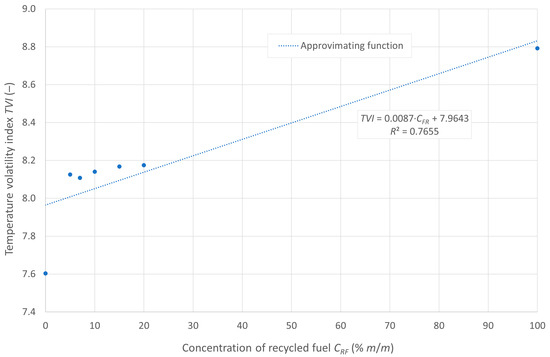
Figure 4.
Temperature volatility index of the tested fuels.
The curve shows good alignment with the empirical data, as evidenced by the coefficient of determination. The lowest TVI value is observed for pure diesel fuel. The tested fuel mixtures exhibit a similar TVI value of approximately 8.1, while pure pyrolytic oil demonstrates the lowest evaporability, with a TVI of about 8.8.
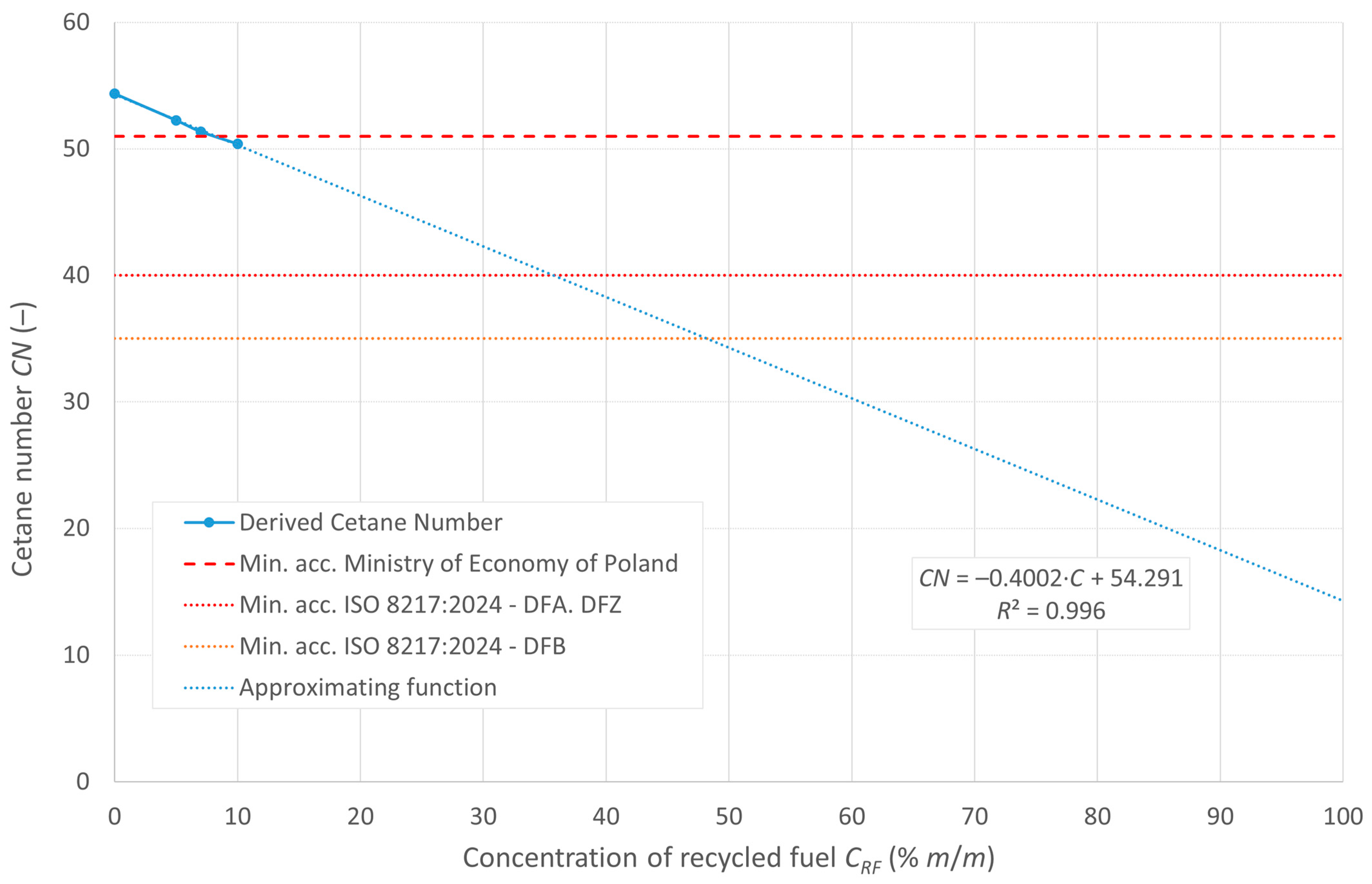
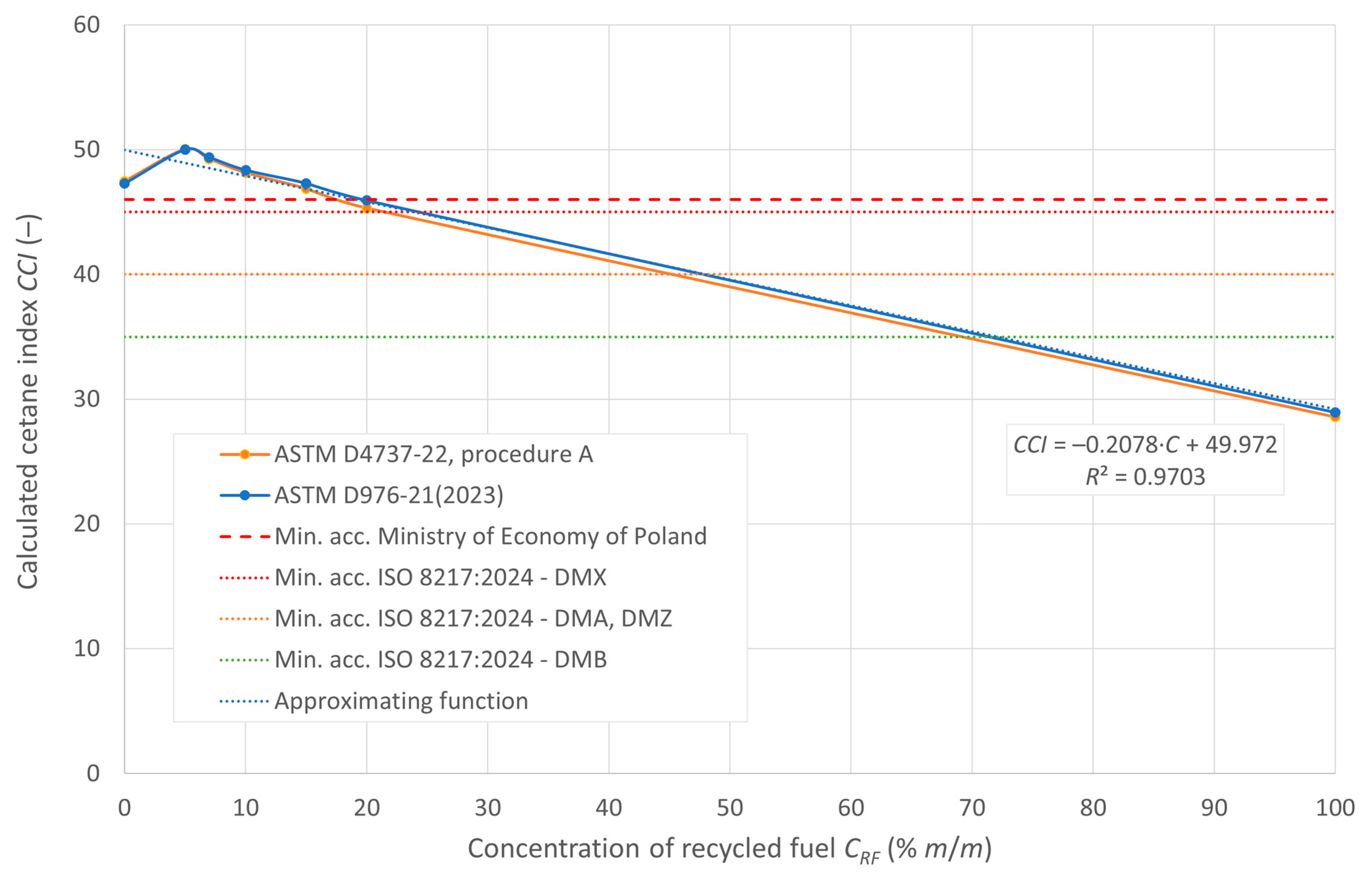
The cetane number, denoted as DCN, and the calculated CCI ignition rates are shown in Figure 5 and Figure 6, respectively. The figures also show the minimum limits set by the relevant regulations.
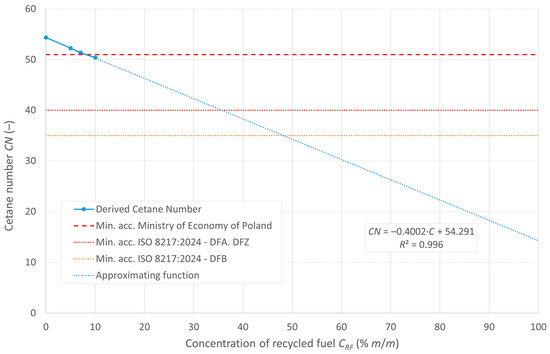
Figure 5.
Cetane number of the tested fuels.
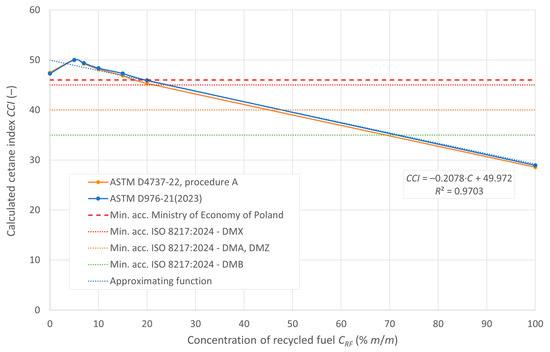
Figure 6.
Calculated cetane index of the tested fuels.
DCN, due to the limitations of the measuring apparatus and the excessive ignition delay, was measured for concentrations of up to 10% recycled oil in the diesel mixture. The trend line extended beyond the measured range indicates that the potential DCN values for concentrations of pyrolytic oil in the mixture above 20% are beyond the limit of the analyzer’s measurement range. All the indicators decrease with increasing recycled oil content in the mixture. According to the guidelines of the RMG of the Minister of Economy of Poland, the minimum value of the cetane number for diesel oils is 51, so tested fuel blends with RF content of up to 7% meet this requirement.
The ISO 8217:2024 standard [23] specifies the allowable cetane number values for DFA, DFZ, and DFB fuel categories. All the tested blends meet the requirements for these fuel categories.
According to the RMG guidelines of the Minister of Economy of Poland, the minimum permissible value of the cetane index is 46. For the fuels tested using both calculation methods indicated in ASTM standards, i.e., the two- and four-parameter equation, the blends meet the cetane index requirements for recycled oil content in the blend of 15% m/m and less. However, for distillate fuels classified in ISO 8217:2024 that do not contain FAME, the minimum cetane index value is 45 for DMX fuel, 40 for DMA and DMZ fuel, and 35 for DMB fuel. All the fuel mixtures tested in the experiment meet these requirements.
4. Conclusions
This study showed that the ignition temperature of the fuels decreases as the recycled oil (RF) content in the diesel blend increases. This is due to the lower ignition temperature of RF compared with diesel (D100). The minimum required flash point, according to ISO 8217:2024, is 43 °C for DMX distillation fuels and 60 °C for DMA, DMZ, and DMB fuels. Mixtures containing less than 5% m/m RF meet the requirements for distillation fuels. For DMX-grade fuels, standards are met throughout the range tested (up to 20% m/m RF). All the tested blends also meet the Polish requirement for a minimum flash point of 55 °C.
The distillation curves of the tested mixtures show similar evaporation characteristics in the 5–20% m/v RF concentration range. Starting temperatures of 10, 50, and 90% v/v fuels were used to determine CCI ignition rates that, similar to the cetane number of DCN, decrease with increasing RF content. DCN, measured for concentrations up to 10% RO, indicates that blends of up to 7% RF meet the minimum required cetane number value (i.e., 51) according to Polish guidelines. The minimum cetane numbers/cetane indices for all the tested mixtures are within these standards.
The research presented is an introduction to further research on engine performance and exhaust gas emission. These topics are the subjects of our current research, and we intend to present the results of the impact of adding pyrolytic oil from tires in selected concentrations to diesel fuel on the composition of exhaust gases from the test engine in the future.
Author Contributions
Conceptualization, L.C., M.S., P.B. (Piotr Brożek) and R.P.; methodology, L.C., M.S., P.B. (Piotr Brożek) and R.P.; software, L.C., M.S., T.P., P.B. (Piotr Brożek) and R.P.; validation, L.C., M.S., T.P., P.B. (Piotr Brożek) and R.P.; formal analysis, L.C., M.S., T.P., P.B. (Piotr Brożek) and R.P.; investigation, L.C., M.S., T.P., R.P. and P.B. (Paweł Borowski); resources, L.C., M.S., P.B., T.P., P.B. (Piotr Brożek), T.P. and P.B. (Paweł Borowski); data curation, L.C., M.S., T.P., P.B. (Piotr Brożek) and R.P.; writing—original draft preparation, L.C., M.S., T.P., P.B. (Piotr Brożek) and R.P.; writing—review and editing, L.C., M.S., T.P. and P.B. (Piotr Brożek); visualization, L.C., M.S., T.P., P.B. (Piotr Brożek), P.B. (Paweł Borowski) and R.P.; supervision, L.C.; project administration, L.C.; funding acquisition, L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly funded by the Ministry of Science and Higher Education (MEiSW) of Poland, grant number 1/S/KSO/24.
Data Availability Statement
All data are available in this paper.
Acknowledgments
The authors would like to thank the following people for their technical support in conducting the research presented in this article: Magdalena Szmukała and Barbara Żurańska (commissioned laboratory measurements).
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the study’s design, collection, analysis, interpretation of data, writing the manuscript, or deciding to publish the results.
Appendix A. Pyrolysis Oil Data

Table A1.
Dominant components in pyrolysis oil.
Table A1.
Dominant components in pyrolysis oil.
| Retention Time RT (min) | Name of the Component | Chemical Abstracts Service Registry Number (CAS) | Similarity Index SI (–) |
|---|---|---|---|
| 4.1 | Cyclobutane, (1-methylethylidene)- | 1528-22-9 | 92 |
| 4.3 | Toluene | 108-88-3 | 97 |
| 5.7 | Ethylbenzene | 100-41-4 | 96 |
| 5.8 | o-Xylene | 95-47-6 | 96 |
| 6.1 | Benzene, 1,3-dimethyl- | 108-38-3 | 84 |
| 6.5 | Benzene, (1-methylethyl)- | 98-82-8 | 88 |
| 7.0 | Benzene, 1-ethyl-4-methyl- | 622-96-8 | 87 |
| 7.3 | Tricyclo[3.1.0.0(2,4)]hex-3-ene-3-carbonitrile | 103495-51-8 | 70 |
| 7.4 | Benzene, 1,2,4-trimethyl- | 95-63-6 | 85 |
| 7.8 | D-Limonene | 5989-27-5 | 93 |
| 8.5 | Benzene, (2-methyl-1-propenyl)- | 768-49-0 | 84 |
| 9.2 | Benzene, 1-methyl-2-(2-propenyl)- | 1587-04-8 | 88 |
| 9.6 | 1H-Indene, 2,3-dihydro-4,7-dimethyl- | 6682-71-9 | 76 |
| 10.8 | Naphthalene, 1-methyl- | 90-12-0 | 87 |
| 11.2 | 2,4,4,6,6,8,8-Heptamethyl-2-nonene | 39761-73-4 | 75 |
| 11.5 | 1H-Indene, 1,1,3-trimethyl- | 2177-45-9 | 83 |
| 11.6 | Tetradecane | 629-59-4 | 66 |
| 12.0 | Naphthalene, 1,6-dimethyl- | 575-43-9 | 92 |
| 12.9 | Naphthalene, 1,6,7-trimethyl- | 2245-38-7 | 96 |
| 14.0 | Heneicosane | 629-94-7 | 91 |
| 15.5 | Hexadecanenitrile | 629-79-8 | 93 |
| 16.9 | Octadecanenitrile | 638-65-3 | 93 |
References
- Williams, P.T. Pyrolysis of waste tyres: A review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Cho, H.M.; Xu, C. Effect of Biodiesel Blended Fuel on the Performance and Emission Characteristics of Diesel Engines—A Review. Int. J. Appl. Mech. Eng. 2022, 27, 215–231. [Google Scholar] [CrossRef]
- Arya, S.; Sharma, A.; Rawat, M.; Agrawal, A. Tyre pyrolysis oil as an alternative fuel: A review. Mater. Today Proc. 2020, 28, 2481–2484. [Google Scholar] [CrossRef]
- Kumaravel, S.T.; Murugesan, A.; Kumaravel, A. Tyre pyrolysis oil as an alternative fuel for diesel engines—A review. Renew. Sustain. Energy Rev. 2016, 60, 1678–1685. [Google Scholar] [CrossRef]
- Tudu, K.; Murugan, S.; Patel, S.K. Effect of tyre derived oil-diesel blend on the combustion and emissions characteristics in a compression ignition engine with internal jet piston geometry. Fuel 2016, 184, 89–99. [Google Scholar] [CrossRef]
- Karagöz, M.; Polat, F.; Sarıdemir, S.; Yeşilyurt, M.K.; Ağbulut, Ü. An experimental assessment on dual fuel engine behavior powered by waste tire-derived pyrolysis oil–biogas blends. Fuel Process. Technol. 2022, 229, 107177. [Google Scholar] [CrossRef]
- Murugan, S.; Ramaswamy, M.C.; Nagarajan, G. The use of tyre pyrolysis oil in diesel engines. Waste Manag. 2008, 28, 2743–2749. [Google Scholar] [CrossRef] [PubMed]
- Umeki, E.R.; de Oliveira, C.F.; Torres, R.B.; dos Santos, R.G. Physico-chemistry properties of fuel blends composed of diesel and tire pyrolysis oil. Fuel 2016, 185, 236–242. [Google Scholar] [CrossRef]
- Auti, S.M.; Rathod, W.S. Effect of hybrid blends of raw tyre pyrolysis oil, karanja biodiesel and diesel fuel on single cylinder four stokes diesel engine. Energy Rep. 2021, 7, 2214–2220. [Google Scholar] [CrossRef]
- Sułek, M.W.; Kulczycki, A.; Małysa, A. Ocena Smarności Mieszanin Estrów Metylowych Kwasów Tłuszczowych Otrzymywanych z Olejów Roślinnych w Oleju Napędowym. Tribologia 2009, 4, 189–197. [Google Scholar]
- Wądrzyk, M.; Janus, R.; Rządzik, B.; Lewandowski, M.; Budzyń, S. Pyrolysis Oil from Scrap Tires as a Source of Fuel Components: Manufacturing, Fractionation, and Characterization. Energy Fuels 2020, 34, 5917–5928. [Google Scholar] [CrossRef]
- Al-Lal, A.-M.; Bolonio, D.; Llamas, A.; Lapuerta, M.; Canoira, L. Desulfurization of pyrolysis fuels obtained from waste: Lube oils, tires and plastics. Fuel 2015, 150, 208–216. [Google Scholar] [CrossRef]
- Zerin, N.H.; Rasul, M.G.; Jahirul, M.I.; Sayem, A.S.M. End-of-life tyre conversion to energy: A review on pyrolysis and activated carbon production processes and their challenges. Sci. Total Environ. 2023, 905, 166981. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Yadav, S.; Gomey, A.K.; Choubey, A.K.; Kumar, R. Assessment of performance and emission characteristics of CI engine using tyre pyrolysis oil and biodiesel blends by nano additives: An experimental study. J. Energy Inst. 2024, 117, 101825. [Google Scholar] [CrossRef]
- Han, W.; Han, D.; Chen, H. Pyrolysis of Waste Tires: A Review. Polymers 2023, 15, 1604. [Google Scholar] [CrossRef] [PubMed]
- Pyshyev, S.; Lypko, Y.; Chervinskyy, T.; Fedevych, O.; Kułażyński, M.; Pstrowska, K. Application of tyre derived pyrolysis oil as a fuel component. S. Afr. J. Chem. Eng. 2023, 43, 342–347. [Google Scholar] [CrossRef]
- Yaqoob, H.; Teoh, Y.H.; Jamil, M.A.; Gulzar, M. Potential of tire pyrolysis oil as an alternate fuel for diesel engines: A review. J. Energy Inst. 2021, 96, 205–221. [Google Scholar] [CrossRef]
- PN-EN IEC 60079-10-1:2021-09; Explosive Atmospheres-Part 10-1: Classification of Areas-Explosive Gas Atmospheres. PKN: Warszawa, Poland, 2021.
- Pang, W.-Q.; Yetter, R.A.; DeLuca, L.T.; Zarko, V.; Gany, A.; Zhang, X.-H. Boron-based composite energetic materials (B-CEMs): Preparation, combustion and applications. Prog. Energy Combust. Sci. 2022, 93, 101038. [Google Scholar] [CrossRef]
- CNBOP-PIB-BW03P; Metody Badania Temperatury Zapłonu Substancji Ciekłych Niebezpiecznych Pożarowo-Wytyczne. CNBOP-PIB: Józefów, Poland, 2016.
- Chybowski, L. Diagnozowanie Silników Okrętowych z Zapłonem Samoczynnym w Oparciu o Analizę Procesów Wtrysku i Spalania Paliwa; Maritime University of Szczecin Press: Szczecin, Poland, 2019. [Google Scholar]
- ASTM D613-23; Standard Test Method for Cetane Number of Diesel Fuel Oil. ASTM: West Conshohocken, PA, USA, 2023.
- ISO 8217:2024; Petroleum Products—Fuels (Class F)—Specifications of Marine Fuels. 7th ed. ISO: Geneva, Switzerland, 2024.
- PN-EN ISO 12185:2002; Ropa Naftowa i Przetwory Naftowe-Oznaczanie Gęstości-Metoda Oscylacyjna z U-rurką. PKN: Warszawa, Poland, 2002.
- PN-EN ISO 3104:2021-03; Petroleum Products—Transparent and Opaque Liquids-Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity. PKN: Warszawa, Poland, 2021.
- PN-C-04062:2018-05; Przetwory Naftowe-Oznaczanie Ciepła Spalania Paliw Ciekłych w Bombie Kalorymetrycznej i Obliczanie Wartości Opałowej z Zastosowaniem Wzorów Empirycznych. PKN: Warszawa, Poland, 2018.
- ISO 2719:2016; Determination of Flash Point—Pensky-Martens Closed Cup Method. 4th ed. ISO: Geneva, Switzerland, 2016.
- PN-EN 12937: 2005; Przetwory Naftowe-Oznaczanie Wody-Miareczkowanie Kulometryczne Metodą Karla Fischera. PKN: Warszawa, Poland, 2005.
- ISO 8754:2003; Petroleum Products—Determination of Sulfur Content—Energy-Dispersive X-Ray Fluorescence Spectrometry. ISO: Geneva, Switzerland, 2003.
- ASTM D6595-17; Standard Test Method for Determination of Wear Metals and Contaminants in Used Lubricating Oils or Used Hydraulic Fluids by Rotating Disc Electrode Atomic Emission Spectrometry. ASTM: West Conshohocken, PA, USA, 2022.
- Chybowski, L. The Initial Boiling Point of Lubricating Oil as an Indicator for the Assessment of the Possible Contamination of Lubricating Oil with Diesel Oil. Energies 2022, 15, 7927. [Google Scholar] [CrossRef]
- Klyus, O.; Szczepanek, M.; Kidacki, G.; Krause, P.; Olszowski, S.; Chybowski, L. The Effect of Internal Combustion Engine Nozzle Needle Profile on Fuel Atomization Quality. Energies 2024, 17, 266. [Google Scholar] [CrossRef]
- Yanowitz, J.; McCormick, R.L. Review: Fuel Volatility Standards and Spark-Ignition Vehicle Driveability. SAE Int. J. Fuels Lubr. 2016, 9, 408–429. [Google Scholar] [CrossRef]
- Sokal, W. Wskaźniki Charakterystyczne Paliw Ciekłych. Available online: https://slideplayer.pl/slide/59984/ (accessed on 28 August 2022).
- Urbański, P. Paliwa i Smary; Fundacja Rozwoju Wyższej Szkoły Morskiej w Gdyni: Gdynia, Poland, 1999. [Google Scholar]
- Yanowitz, J.; Ratcliff, M.; McCormick, R.; Taylor, J.; Murphy, M. Compendium of Experimental Cetane Numbers; National Renewable Energy Laboratory: Golden, BC, Canada, 2014. [Google Scholar]
- ASTM D 7668-17; Standard Test Method for Determination of Derived Cetane Number (DCN) of Diesel Fuel OilsIgnition Delay and Combustion Delay Using a Constant Volume Combustion Chamber Method. ASTM: West Conshohocken, PA, USA, 2017.
- PN-EN ISO 3405:2019-05; Petroleum Products and Similar Products of Natural and Synthetic Origin-Determination of the Fractional Composition by Distillation at Atmospheric Pressure. PKN: Warszawa, Poland, 2019.
- Ramadan, O.; Menard, L.; Gardiner, D.; Wilcox, A.; Webster, G. Performance Evaluation of the Ignition Quality Testers Equipped with TALM Precision Package (TALM-IQTTM) Participating in the ASTM NEG Cetane Number Fuel Exchange Program; SAE Technical Paper; SAE: Warrendale, PA, USA, 2017. [Google Scholar] [CrossRef]
- ASTM D976-21; Standard Test Method for Calculated Cetane Index of Distillate Fuels. ASTM: West Conshohocken, PA, USA, 2023.
- ASTM D4737-22; Standard Test Method for Calculated Cetane Index by Four Variable Equation. ASTM: West Conshohocken, PA, USA, 2023.
- ASTM D975-21; Standard Specification for Diesel Fuel. ASTM: West Conshohocken, PA, USA, 2022.
- ASTM D4294-21; Standard Test Method for Sulfur in Petroleum and Petroleum Products by Energy Dispersive X-Ray Fluorescence Spectrometry. ASTM: West Conshohocken, PA, USA, 2021.
- Minister Gospodarki RP. Rozporządzenie Ministra Gospodarki z dnia 9 Października 2015 r. w Sprawie Wymagań Jakościowych dla Paliw Ciekłych; Ministerstwo Gospodarki RP: Warszawa, Poland, 2015.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).