Silicon/Biomass Carbon Composite as a Low-Cost Anode for Lithium-Ion Batteries
Abstract
1. Introduction
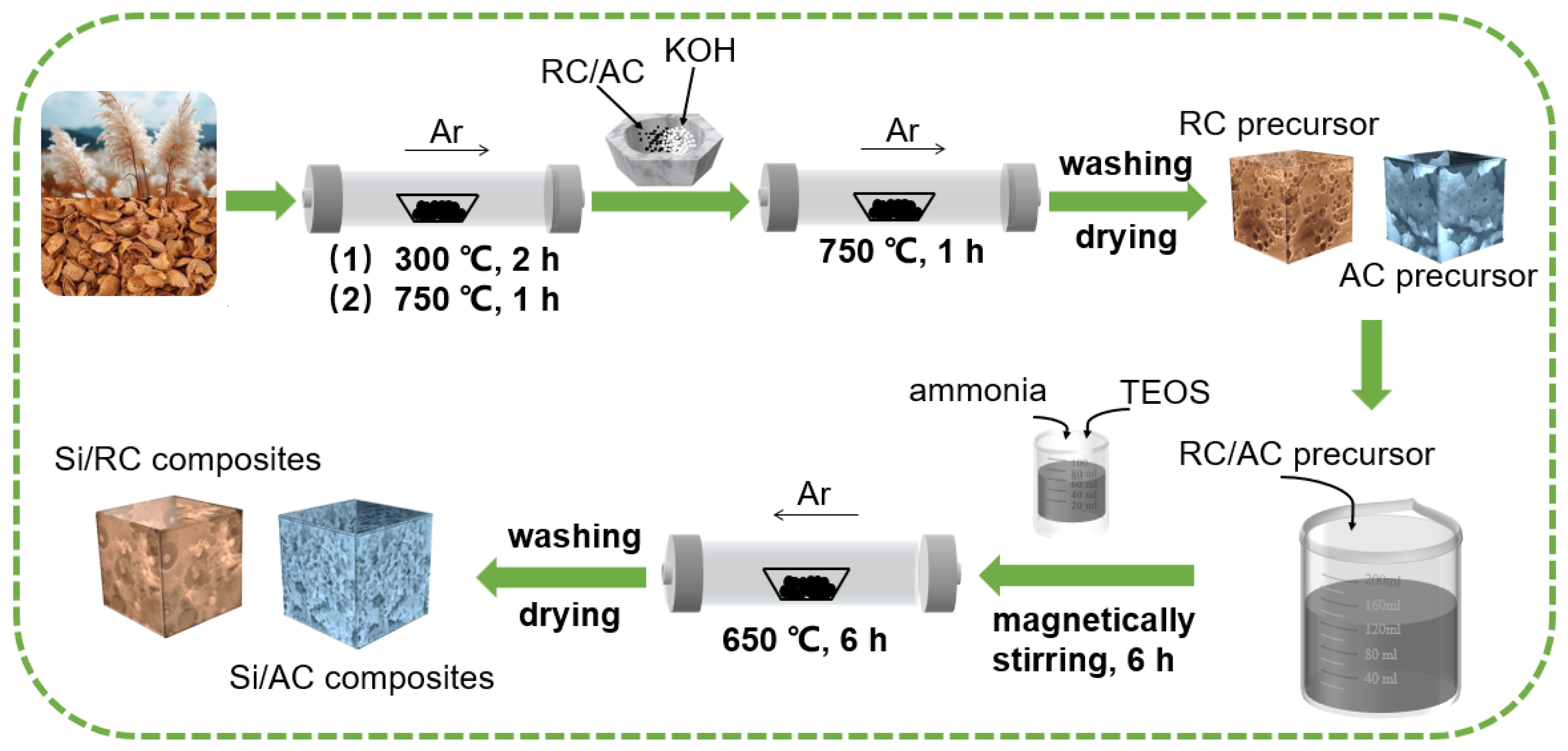
2. Materials and Methods
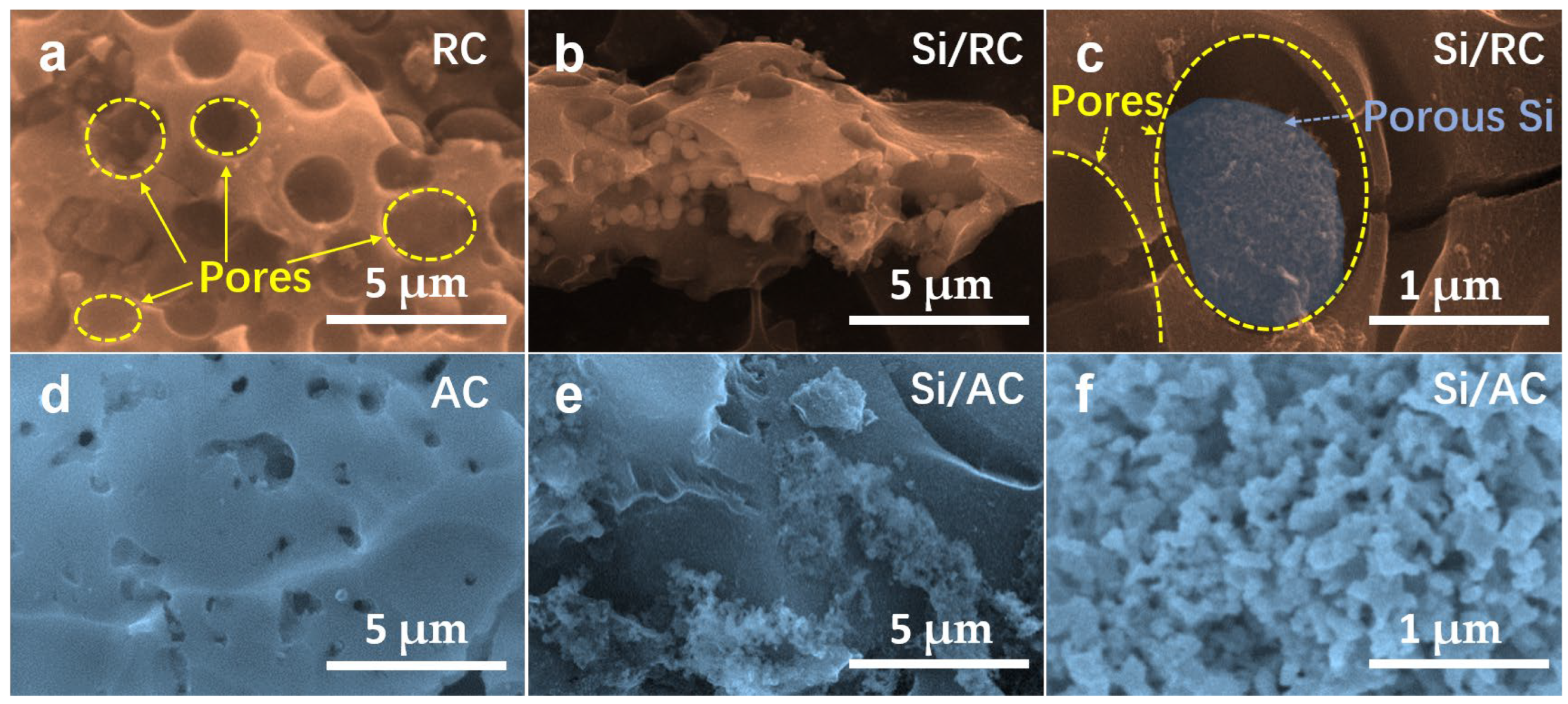
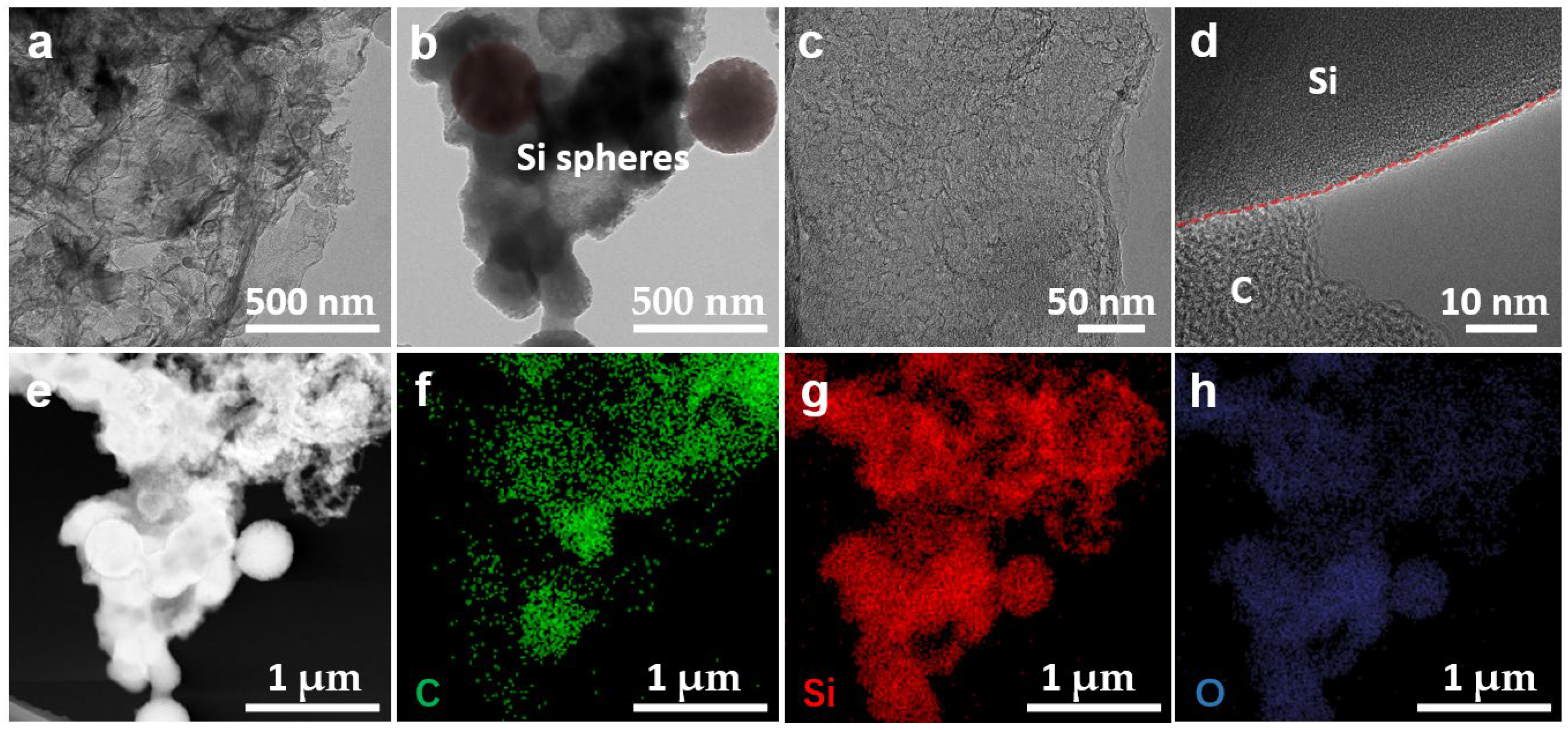
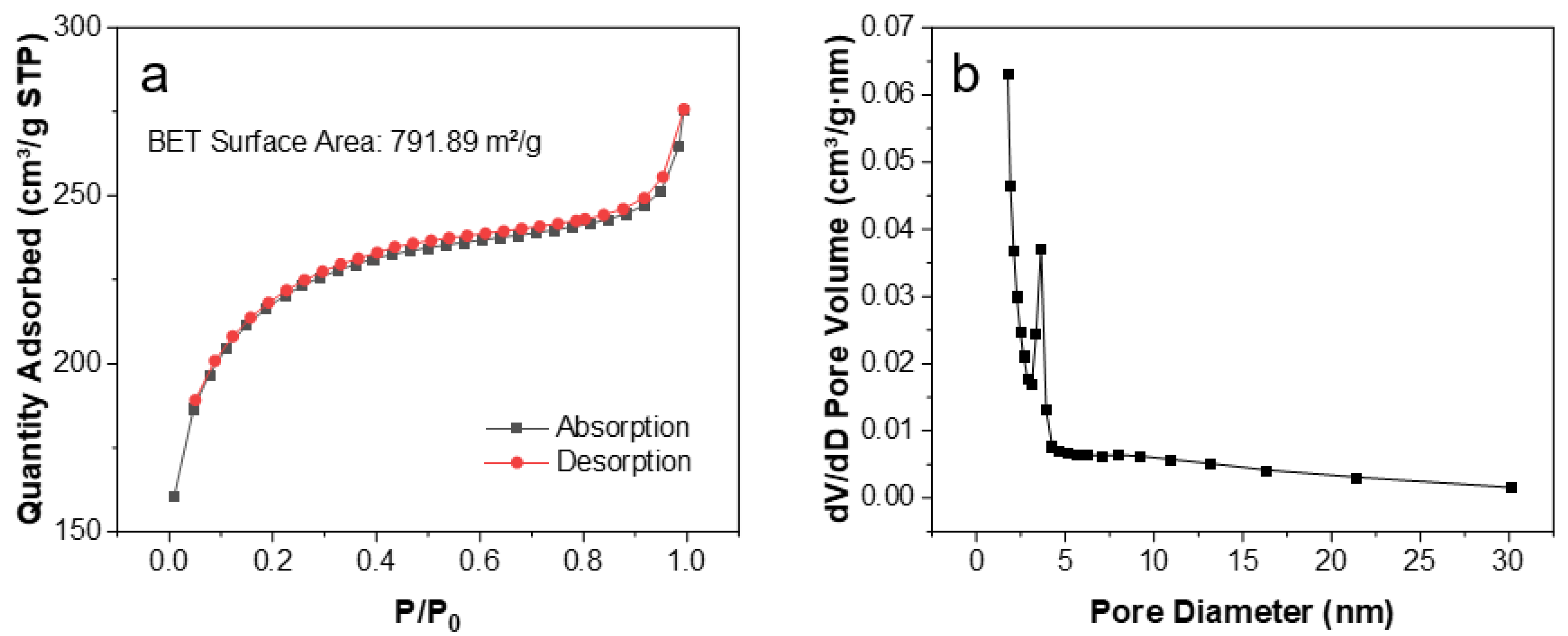
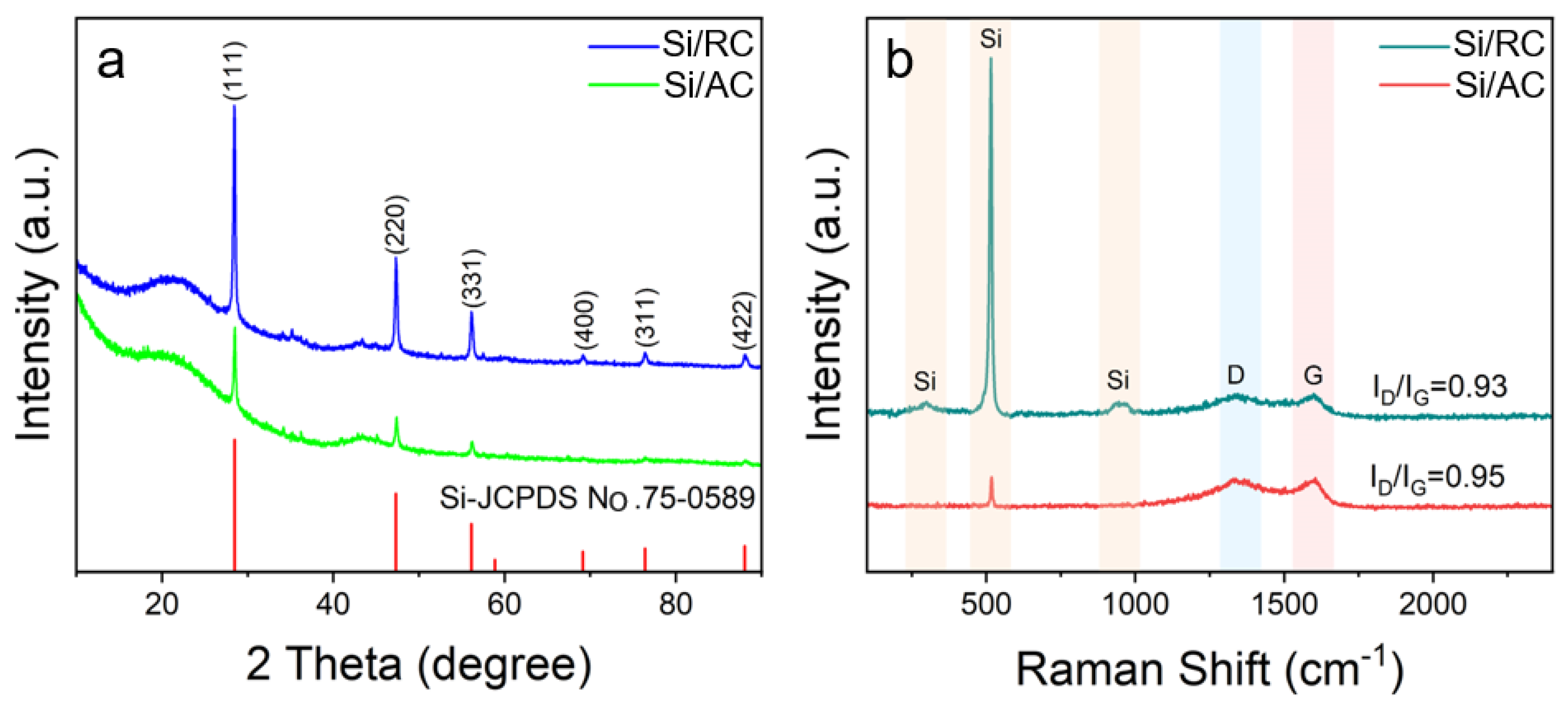
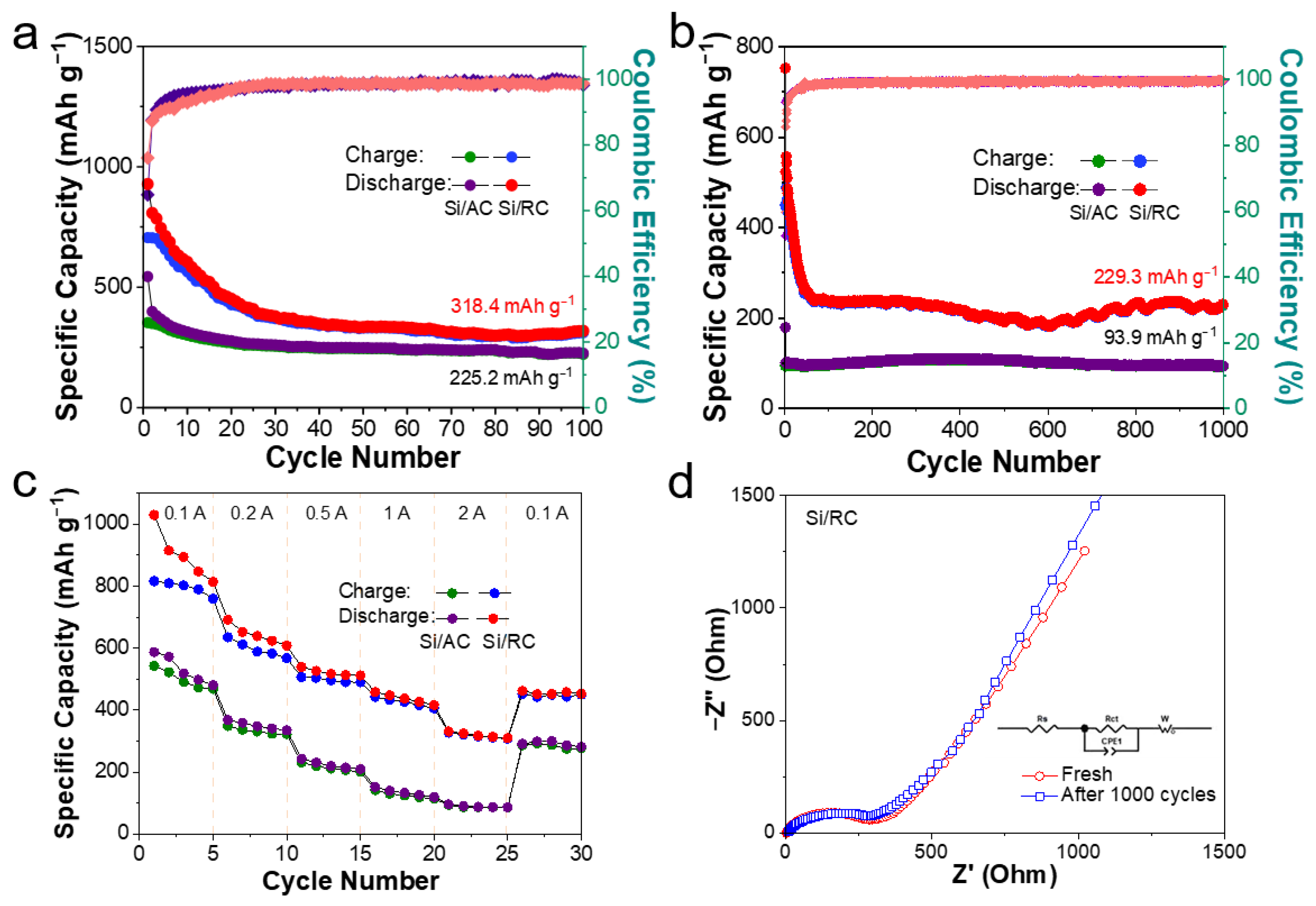
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dong, L.S.; Wang, Z.G.; Mi, C.; Zhao, W.M.; Qin, C.L.; Luo, C.; Wang, Z.F. Defect-rich hierarchical porous spinel MFe2O4 (M = Ni, Co, Fe, Mn) as high-performance anode for lithium ion batteries. Mater. Today Chem. 2024, 35, 101853. [Google Scholar] [CrossRef]
- Wang, Z.F.; Wang, H.Y.; Liu, X.L.; Chen, Y.X.; Zhao, Y.; Zhang, Y.G.; Han, Q.Q.; Qin, C.L.; Bakenov, Z.; Wang, Y.C.; et al. Single Zn atoms anchored on hollow carbon nanofiber network for dendrite-free lithium metal anode of flexible Li-S full cell. Rare Met. 2023, 42, 3705–3717. [Google Scholar] [CrossRef]
- Xu, J.J.; Cai, X.Y.; Cai, S.M.; Shao, Y.X.; Hu, C.; Lu, S.R.; Ding, S.J. High-energy lithium-ion batteries: Recent progress and a promising future in applications. Energy Environ. Mater. 2023, 6, e12450. [Google Scholar] [CrossRef]
- He, J.H.; Meng, J.K.; Huang, Y.H. Challenges and recent progress in fast-charging lithium-ion battery materials. J. Power Sources 2023, 570, 232965. [Google Scholar] [CrossRef]
- Sun, X.; Qin, C.L.; Zhao, B.Y.; Jia, S.F.; Wang, Z.F.; Yang, T.Z.; Liu, X.C.; Pan, L.N.; Zheng, L.L.; Luo, D.; et al. A cation and anion dual-doping strategy in novel Li-rich Mn-based cathode materials for high-performance Li metal batteries. Energy Storage Mater. 2024, 70, 103559. [Google Scholar] [CrossRef]
- Oh, P.; Yun, J.; Park, S.; Nam, G.; Liu, M.; Cho, J. Recent advances and prospects of atomic substitution on layered positive materials for lithium-ion battery. Adv. Energy Mater. 2021, 11, 2003197. [Google Scholar] [CrossRef]
- Dong, L.S.; Luo, C.; Wang, Y.C.; Meng, S.J.; Zhao, W.M.; Qin, C.L.; Wang, Z.F. Prelithiation enhanced initial Coulombic efficiency and cycling stability of spinel (FeCoCrNiMn)3O4 high entropy oxide anode. J. Energy Storage 2024, 98, 113185. [Google Scholar] [CrossRef]
- Wang, Z.F.; Yan, Y.J.; Zhang, Y.G.; Chen, Y.X.; Peng, X.Y.; Wang, X.; Zhao, W.M.; Qin, C.L.; Liu, Q.; Liu, X.J.; et al. Single-atomic Co-B2N2 sites anchored on carbon nanotube arrays promote lithium polysulfide conversion in lithium-sulfur batteries. Carbon Energy 2023, 5, e306. [Google Scholar] [CrossRef]
- Gao, T.; Xu, C.Y.; Li, R.Q.; Zhang, R.; Wang, B.L.; Jiang, X.F.; Hu, M.; Bando, Y.; Kong, D.S.; Dai, P.C.; et al. Biomass-derived carbon paper to sandwich magnetite anode for long-life Li-ion battery. ACS Nano 2019, 13, 11901–11911. [Google Scholar] [CrossRef]
- Thangaraj, B.; Solomon, P.A.; Wongyao, N.; Helal, M.I.; Abdullah, A.; Abedrabbo, S.; Hassan, J. Synthesis of reduced graphene oxide nanosheets from sugarcane dry leaves by two-stage pyrolysis for antibacterial activity. Nano Mater. Sci. 2024, 6, 625–634. [Google Scholar] [CrossRef]
- Wu, H.; Fei, G.; Gao, X.; Guo, X.; Gong, X.; Ma, X.; Wang, Q.; Xu, S. Research progress on preparation and application of polyaniline and its composite materials. China Powder Sci. Technol. 2023, 29, 70–80. [Google Scholar]
- Wang, L.S.; Wang, Y.X.; Sun, W.; Wang, C.W.; Meng, X.S. Preparation of lightweight and high-strength ceramsite from highly doped coal fly ash. Trans. Nonferrous Met. Soc. China 2023, 33, 3885–3898. [Google Scholar] [CrossRef]
- Jin, C.B.; Nai, J.W.; Sheng, O.W.; Yuan, H.D.; Zhang, W.K.; Tao, X.Y.; Lou, X.W. Biomass-based materials for green lithium secondary batteries. Energy Environ. Sci. 2021, 14, 1326–1379. [Google Scholar] [CrossRef]
- Pan, X.Y.; Caikang Wang, C.K.; Li, B.; Ma, M.Z.; Sun, H.; Zhan, G.W.; Wang, K.; Fan, M.M.; Ding, L.F.; Fu, G.T.; et al. Coupling Enteromorpha prolifera-derived N-doped biochar with Cu-Mo2C clusters for selective CO2 hydrogenation to CO. Adv. Powder Mater. 2025, 4, 100259. [Google Scholar] [CrossRef]
- She, S.H.; Sukma, L.P.P.; Peng, M.M.; Shirai, H.; Suzuki, Y.; Kamiya, K.; Qian, E.W. Hierarchically structured macro-mesoporous carbon catalysts for saccharification of cellulose. Green Carbon, 2025; in press. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, F.; Chen, J. Structural design and application of fiber-based electrocatalytic materials. China Powder Sci. Technol. 2024, 30, 161–170. [Google Scholar]
- Yang, X.F.; Chen, X.F.; Qiu, J.Y.; Li, M.; Ming, H.; Zhang, S.T.; Zhang, T.T. Controllable synthesis of silicon/carbon hollow microspheres using renewable sources for high energy lithium-ion battery. J. Solid State Chem. 2021, 296, 121968. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Wang, Y.J.; Meng, Y.; Tan, G.Q.; Guo, Y.; Xiao, D. Porous nitrogen-doped carbon tubes derived from reed catkins as a high-performance anode for lithium ion batteries. RSC Adv. 2016, 6, 98434–98439. [Google Scholar] [CrossRef]
- Liu, J.; Kopold, P.; van Aken, P.A.; Maier, J.; Yu, Y. Energy storage materials from nature through nanotechnology: A sustainable route from reed plants to a silicon anode for lithium-ion batteries. Angew. Chem. Int. Ed. 2015, 54, 9632–9636. [Google Scholar] [CrossRef]
- Zhao, W.M.; Wen, J.J.; Zhao, Y.M.; Wang, Z.F.; Shi, Y.R.; Zhao, Y. Hierarchically porous carbon derived from biomass reed fowers as highly stable Li-ion battery anode. Nanomaterials 2020, 10, 346. [Google Scholar] [CrossRef] [PubMed]
- Demir, E.; Aydin, M.; Arie, A.A.; Demir-Cakan, R. Apricot shell derived hard carbons and their tin oxide composites as anode materials for sodium-ion batteries. J. Alloys Compd. 2019, 788, 1093–1102. [Google Scholar] [CrossRef]
- Hernandez-Rentero, C.; Marangon, V.; Olivares-Marin, M.; Gomez-Serrano, V.; Caballero, A.; Morales, J.; Hassoun, J. Alternative lithium-ion battery using biomass-derived carbons as environmentally sustainable anode. J. Colloid Interface Sci. 2020, 573, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, C.; Qi, H.; Yu, K.F.; Li, X.J. Formation mechanism and characterization of porous biomass carbon for excellent performance lithium-ion batteries. RSC Adv. 2018, 8, 12666–12671. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.Z.; Lin, C.H.; Yang, W.; Meng, Y.; Guo, Y.; Li, M.L.; Xiao, D. Hierarchically porous nitrogen-rich carbon derived from wheat straw as an ultra-high-rate anode for lithium ion batteries. J. Mater. Chem. A 2014, 2, 9684–9690. [Google Scholar] [CrossRef]
- Chen, F.; Yang, J.; Bai, T.; Long, B.; Zhou, X.Y. Facile synthesis of few-layer graphene from biomass waste and its application in lithium ion batteries. J. Electroanal. Chem. 2016, 768, 18–26. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, K.X.; Li, G.D.; Chen, J.S. Hierarchical porous carbon derived from rice straw for lithium ion batteries with high-rate performance. Electrochem. Commun. 2009, 11, 130–133. [Google Scholar] [CrossRef]
- Wang, L.P.; Schnepp, Z.; Titirici, M.M. Rice husk-derived carbon anodes for lithium ion batteries. J. Mater. Chem. A 2013, 1, 5269–5273. [Google Scholar] [CrossRef]
- Lotfabad, E.M.; Ding, J.; Cui, K.; Kohandehghan, A.; Kalisvaart, W.P.; Hazelton, M.; Mitlin, D. High-density sodium and lithium ion battery anodes from banana peels. ACS Nano 2014, 8, 7115–7129. [Google Scholar] [CrossRef]
- Um, J.H.; Ahn, C.Y.; Kim, J.; Jeong, M.; Sung, Y.E.; Cho, Y.H.; Kim, S.S.; Yoon, W.S. From grass to battery anode: Agricultural biomass hemp-derived carbon for lithium storage. RSC Adv. 2018, 8, 32231–32240. [Google Scholar] [CrossRef]
- Liu, X.; Chen, M.; Ma, J.; Liang, J.; Li, C.; Chen, C.; He, H. Advances in the synthesis strategies of carbon-based single atom catalysts and their electrochemical applications. China Powder Sci. Technol. 2024, 30, 35–45. [Google Scholar]
- Li, P.; Zhao, G.Q.; Zheng, X.B.; Xu, X.; Yao, C.H.; Sun, W.P.; Dou, S.X. Recent progress on silicon-based anode materials for practical lithium-ion battery applications. Energy Storage Mater. 2018, 15, 422–446. [Google Scholar] [CrossRef]
- Cheng, Z.L.; Jiang, H.; Zhang, X.L.; Cheng, F.Y.; Wu, M.H.; Zhang, H.J. Fundamental understanding and facing challenges in structural design of porous Si-based anodes for lithium-ion batteries. Adv. Funct. Mater. 2023, 33, 2301109. [Google Scholar] [CrossRef]
- Shi, Q.T.; Zhou, J.H.; Ullah, S.; Yang, X.Q.; Tokarska, K.; Trzebicka, B.; Ta, H.Q.; Rummeli, M.H. A review of recent developments in Si/C composite materials for Li-ion batteries. Energy Storage Mater. 2021, 34, 735–754. [Google Scholar] [CrossRef]
- Roland, A.; Fullenwarth, J.; Ledeuil, J.B.; Martinez, H.; Louvain, N.; Monconduit, L. How carbon coating or continuous carbon pitch matrix influence the silicon electrode/electrolyte interfaces and the performance in Li-ion batteries. Battery Energy 2022, 1, 20210009. [Google Scholar] [CrossRef]
- Dou, F.; Shi, L.Y.; Chen, G.R.; Zhang, D.S. Silicon/carbon composite anode materials for lithium-ion batteries. Electrochem. Energy Rev. 2019, 2, 149–198. [Google Scholar] [CrossRef]
- Liu, Q.; Ji, Y.X.; Yin, X.M.; Li, J.W.; Liu, Y.J.; Hu, X.; Wen, Z.H. Magnesiothermic reduction improved route to high-yield synthesis of interconnected porous Si@C networks anode of lithium ions batteries. Energy Storage Mater. 2022, 46, 384–393. [Google Scholar] [CrossRef]
- Dos Santos-Gomez, L.; Cuesta, N.; Camean, I.; Garcia-Granda, S.; Garcia, A.B.; Arenillas, A. A promising silicon/carbon xerogel composite for high-rate and high-capacity lithium-ion batteries. Electrochim. Acta 2022, 426, 140790. [Google Scholar] [CrossRef]
- Chen, M.X.; Cao, W.Y.; Wang, L.C.; Ma, X.; Han, K. Chessboard-like silicon/graphite anodes with high cycling stability toward practical lithium-ion batteries. ACS Appl. Energy Mater. 2021, 4, 775–783. [Google Scholar] [CrossRef]
- Yang, Z.W.; Qiu, L.; Zhang, M.K.; Zhong, Y.N.; Zhong, B.H.; Song, Y.; Wang, G.K.; Liu, Y.X.; Wu, Z.G.; Guo, X.D. Carbon dioxide solid-phase embedding reaction of silicon-carbon nanoporous composites for lithium-ion batteries. Chem. Eng. J. 2021, 423, 130127. [Google Scholar] [CrossRef]
- Tan, Y.; Jiang, T.T.; Chen, G.Z. Mechanisms and product options of magnesiothermic reduction of silica to silicon for lithium-ion battery applications. Front. Energy Res. 2021, 9, 651386. [Google Scholar] [CrossRef]
- Wang, Z.F.; Zhang, X.M.; Liu, X.L.; Zhang, Y.G.; Zhao, W.M.; Li, Y.Y.; Qin, C.L.; Bakenov, Z. High specific surface area bimodal porous carbon derived from biomass reed flowers for high performance lithium-sulfur batteries. J. Colloid Interface Sci. 2020, 569, 22–33. [Google Scholar] [CrossRef]
- Hui, X.B.; Zhao, R.Z.; Zhang, P.; Li, C.X.; Wang, C.X.; Yin, L.W. Low-temperature reduction strategy synthesized Si/Ti3C2 MXene composite anodes for high-performance Li-ion batteries. Adv. Energy Mater. 2019, 9, 1901065. [Google Scholar] [CrossRef]
- Ahn, J.; Kim, H.S.; Pyo, J.; Lee, J.K.; Yoo, W.C. Variation in crystalline phases: Controlling the selectivity between silicon and silicon carbide via magnesiothermic reduction using silica/carbon composites. Chem. Mater. 2016, 28, 1526–1536. [Google Scholar] [CrossRef]
- Parekh, M.H.; Parikh, V.P.; Kim, P.J.; Misra, S.; Qi, Z.M.; Wang, H.Y.; Pol, V.G. Encapsulation and networking of silicon nanoparticles using amorphous carbon and graphite for high performance Li-ion batteries. Carbon 2019, 148, 36–43. [Google Scholar] [CrossRef]
- Chen, X.N.; Wang, X.H.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Chen, P.Y.; Liu, W.; Wang, H.; Jiang, Y.; Niu, X.B.; Wang, L.P. Semi-ionic C-F bond enabling fluorinated carbons rechargeable as Li-ion batteries cathodes. J. Colloid Interface Sci. 2023, 649, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Chuan, X.Y.; Masse, R.C.; Huang, D.B.; Li, S.; Cao, G.Z. A three layer design with mesoporous silica encapsulated by a carbon core and shell for high energy lithium ion battery anodes. J. Mater. Chem. A 2015, 3, 22739–22749. [Google Scholar] [CrossRef]
- Du, F.H.; Li, B.; Fu, W.; Xiong, Y.J.; Wang, K.X.; Chen, J.S. Surface binding of polypyrrole on porous silicon hollow nanospheres for Li-ion battery anodes with high structure stability. Adv. Mater. 2014, 26, 6145–6150. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.H.; Cao, C.B.; Idrees, F.; Ma, X.L. Hierarchical porous nitrogen-doped carbon nanosheets derived from silk for ultrahigh-capacity battery anodes and supercapacitors. ACS Nano 2015, 9, 2556–2564. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.I.; Kong, K.; Choi, S.; Na, M.Y.; Kim, K.B.; Kim, W.T.; Kim, D. Synthesis of porosity controllable nanoporous silicon with a self-coated nickel layer for lithium-ion batteries. J. Power Sources 2021, 495, 229802. [Google Scholar] [CrossRef]
- Jeong, B.J.; Jiang, F.; Sung, J.Y.; Jung, S.P.; Oh, D.W.; Gnanamuthu, R.; Vediappan, K.; Lee, C.W. Biomass-Derived carbon utilization for electrochemical energy enhancement in lithium-ion batteries. Nanomaterials 2024, 14, 999. [Google Scholar] [CrossRef]
- Huang, A.Q.; Tu, Y.B.; Yu, Q.C. Preparation and electrochemical properties of nitrogen-doped starch hard carbon anode materials for lithium-ion battery. Int. J. Electrochem. Sci. 2024, 19, 100744. [Google Scholar] [CrossRef]
- Luna, F.; Morales, J.; Caballero, A. Biomass porous carbons derived from banana peel waste as sustainable anodes for lithi-um-ion batteries. Materials 2021, 14, 5995. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, S.J.; He, J.F.; Liang, K.; Li, J.B.; Huang, X.B.; Ren, Y.R. Based on N, F, and P co-doping biomass carbon to construct 3D porous carbon coated LiFePO4 for preparing lithium-ion batteries. J. Ind. Eng. Chem. 2024, 137, 376–386. [Google Scholar] [CrossRef]
- Metyouy, K.; Karbak, M.; Ghamouss, F.; Chafik, T. Tuning the morphology of argan shells-based activated carbon towards applications as an anode for lithium-ion battery. Diam. Relat. Mater. 2024, 145, 111152. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, H.; Ma, T.; Deng, H.; Zha, Z. Effect of cotton stalk particle size on the structure of biochar and the perfor-mance of anode for lithium-ion battery. J. Phys. Chem. Solids 2022, 169, 110845. [Google Scholar] [CrossRef]
- Yokokura, T.J.; Rodriguez, J.R.; Pol, V.G. Waste biomass-derived carbon anode for enhanced lithium storage. ACS Omega 2020, 5, 19715–19720. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.; Saravanan, S.; Ahmed, A.T.A.; Inamdar, A.I.; Im, H.; Lee, S.; Kim, D.Y. Spherical activated-carbon nanoparticles derived from biomass green tea wastes for anode material of lithium-ion battery. Mater. Lett. 2019, 240, 189–192. [Google Scholar] [CrossRef]
- Zheng, S.; Luo, Y.; Zhang, K.Y.; Liu, H.G.; Hu, G.B.; Qin, A.M. Nitrogen and phosphorus co-doped mesoporous carbon nanosheets derived from bagasse for lithium-ion batteries. Mater. Lett. 2021, 290, 129459. [Google Scholar] [CrossRef]


| Materials | Source | Current Density (mA/g) | Cycle Number | Capacity (mAh/g) | Ref. |
|---|---|---|---|---|---|
| LiFeO4@C | Potato peel | 0.1C | 100 | 150.4 | [51] |
| N doping carbon | Corn starch | 0.2C | 100 | 273 | [52] |
| Porous carbon | Banana peel | 0.2C | 200 | 272 | [53] |
| LFP@NFPC-2 | Bacterial residues | 1C | 200 | 161.2 | [54] |
| Honeycomb-AC | Argan shells | 50 | 200 | 220 | [55] |
| Activated carbon | Cotton stalks | 100 | 100 | 271.7 | [56] |
| Activated carbon | Avocado seeds | 100 | 100 | 400 | [57] |
| Spherical mesoporous carbon | Waste green tea | 100 | 100 | 498 | [58] |
| Porous carbon | Bagasse | 2000 | 200 | 198.7 | [59] |
| Si/C composites | Reed catkins | 0.2C | 100 | 318.4 | This work |
| 1C | 1000 | 229.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Z.; Xu, Z.; Li, H.; Xiong, H.; Liu, X.; Qin, C.; Wang, Z. Silicon/Biomass Carbon Composite as a Low-Cost Anode for Lithium-Ion Batteries. Energies 2025, 18, 972. https://doi.org/10.3390/en18040972
Meng Z, Xu Z, Li H, Xiong H, Liu X, Qin C, Wang Z. Silicon/Biomass Carbon Composite as a Low-Cost Anode for Lithium-Ion Batteries. Energies. 2025; 18(4):972. https://doi.org/10.3390/en18040972
Chicago/Turabian StyleMeng, Ziying, Ziqing Xu, Heng Li, Hanqing Xiong, Xijun Liu, Chunling Qin, and Zhifeng Wang. 2025. "Silicon/Biomass Carbon Composite as a Low-Cost Anode for Lithium-Ion Batteries" Energies 18, no. 4: 972. https://doi.org/10.3390/en18040972
APA StyleMeng, Z., Xu, Z., Li, H., Xiong, H., Liu, X., Qin, C., & Wang, Z. (2025). Silicon/Biomass Carbon Composite as a Low-Cost Anode for Lithium-Ion Batteries. Energies, 18(4), 972. https://doi.org/10.3390/en18040972








