A Review on Catalytic Hydrolysis of Ammonia Borane for Hydrogen Production
Abstract
1. Introduction
2. Exploration of the Catalytic Mechanism, Testing and Evaluation Methods
2.1. The Catalytic Mechanism of AB Hydrolysis
- (a)
- Nucleophilic substitution mechanism
- (b)
- Oxidative addition and reductive elimination
- (c)
- Bimolecular activation
2.2. Testing and Evaluation Methods of Catalyst Performance
2.2.1. Performance Test of Dehydrogenation in Laboratory
2.2.2. Standardized Description of Catalytic Performance Evaluation Methods
- (a)
- Hydrogen Generation Rate (HGR)
- (b)
- Turnover Frequency (TOF)
- (c)
- Apparent Activation Energy (Ea)
- (d)
- Stability
3. Strategies for the Optimization and Control of Catalytic Performance
3.1. Strategies for Performance Optimization Based on Number and Dispersion of Active Centers
3.1.1. Optimization of Active Center by Size Effect
3.1.2. Optimization of Active Center by Morphology
- (a)
- Unsupported transition metal oxides
- (b)
- Catalysts with special support morphology
3.1.3. Optimization of Active Center by Modification of Support

3.1.4. Optimization of Active Center by Stabilizer
3.2. Strategies for Performance Optimization Based on Enhancing Adsorption and Activation of Reactants
3.2.1. Optimization of Adsorption and Activation of Reactants by Bimetallic Alloys

3.2.2. Optimization of Adsorption and Activation of Reactants by Polymetallic Alloys
3.2.3. Optimization of Adsorption and Activation of Reactants by Metal–Support Interaction
- (a)
- Oxide Supports
- (b)
- Carbon Supports
- (c)
- Other Supports

3.3. Strategies for Performance Optimization Based on Hydrogen Desorption
3.4. Strategies for Controlling Catalytic Performance

| Catalyst | TOF (min−1)/ (mL·min−1·gCat−1) | Temperature (K) | nmetal/nAB | Ea (kJ/mol) | Particle Size (nm) | Preparation Method | Durability | Reference |
|---|---|---|---|---|---|---|---|---|
| Rh/C-300A-350H | 3308 | 298 | — | 35 | 1.65 | Low-temperature oxidative thermal redispersion strategy | 40.4%/5 | [30] |
| Ru/VO-Co3O4 | 2114 | 298 | — | 58.8 | 2.8 | — | 92.5%/6 | [31] |
| Pd/alk-Ti3C2 | 230.6 | 298 | — | 21.2 | 4.9 | Direct reduction method | 40%/5 | [32] |
| Rh/C | 1246 | 298 | — | 40.9 | — | In situ reduction | 61.2%/8 | [37] |
| Ru/HPCM | 440 | 303 | — | 43 | 1.41 | Iron citrate pyrolysis | 50%/8 | [38] |
| Co/FeCeO2-0.6 | 92.8 | 298 | — | — | 10 | — | — | [47] |
| Cu0.6Co0.4O@CN | 57.5 | 298 | — | 38.4 | 7 | — | — | [48] |
| Ru–MgO/HBC | 784 | 298 | — | 50.09 | 1.7 | Wet impregnation method | 89.9%/10 | [49] |
| 1.5Co1.5Ni/α-MoC | 321.1 | 298 | — | — | — | Impregnation method | — | [51] |
| Ni0.7Co1.3P/GO | 153.9 | 298 | 0.026 | 43.2 | 5 | Two-step strategy | 95.2%/7 | [52] |
| CuMoO4−CoMoO4 | 104.7 | 298 | — | 38.4 | 2–3 μm | Template-free approach | — | [53] |
| Ni/FeNiOx-25 | 72.3 | 303 | — | 39.18 | 1–2 | — | 100%/6 | [54] |
| Pt/CNTs-O-HT | 567 | 303 | 0.0047 | — | 1.3 | — | — | [56] |
| Co/CTF-1 | 42.3 | 298 | 0.05 | 42.7 | 7.3 | — | — | [57] |
| Ni2Pt@ZIF-8 | 600 | 293 | — | 23.3 | 2 | Co-reduction method | — | [58] |
| Rh1/VO2 | — | — | — | 38.7 | — | — | — | [59] |
| CuFeCo@MIL-101 | 23.2 | 298 | 0.073 | 37.1 | 2.6 | Impregnation–reduction method | 60%/7 | [60] |
| RuMoP@MOF-199 | 753.6 | 298 | — | 46.9 | 2.1 | Liquid impregnation method | 79%/5 | [61] |
| Ni1.2Fe0.8@CN-G | 23.25 | 298 | — | 38.24 | 4 | Pyrolysis | — | [62] |
| Co3B-CoP/h-BN | 37 | 303 | — | 51.8 | — | — | — | [63] |
| RuPt-Ti/Ti3C2 | 1293 | 293 | — | 28.6 | 1.89 | Impregnation–reduction method | — | [64] |
| NiCoP/OPC-300 | 68.03 | 298 | 0.042 | 38.9 | 1.2 | One-step chemical reduction method | 85%/5 | [66] |
| Co-Co3O4/CDS | 6816 mLH2·min−1·gCo−1 | 298 | — | — | — | Hydrothermal process | — | [67] |
| Rh/OPNC | 433 | 298 | — | 26.4 | 2.88 | Air-mediated pyrolysis method | 62%/5 | [68] |
| Ru1Ni1.9/NCS | 824 | 298 | — | 26.5 | 2.3 | Impregnation–reduction method | 67%/5 | [69] |
| Pt/CNT-5W | 710 | 303 | — | 27.8 | 1.4 | Two-step method | 45%/5 | [70] |
| Pd0.1Cu0.9/T-PC | 279 | 298 | — | 57 | 2.9 | In situ reduction | 44.4%/10 | [71] |
| Rh/PCNs | 513.2 | 298 | — | 46.5 | 2.3 | Simple pyrolysis | 66%/6 | [77] |
| Ni−MoOx/(P)NCS | 85.7 | 298 | — | 29.6 | 3 | Phosphate-mediated method | 54%/5 | [88] |
| Ni/CTFPh | 14.3 | 303 | — | 34.8 | 2.2 | Metal vapor synthesis | — | [89] |
| Co/CoNx-CNT-33-800T | 7833 mLH2 gCo−1min−1 | 313 | 0.0654 | 46.17 | — | — | 75%/40 | [90] |
| 5Pt/G1600-O3-60 | 618.9 | 298 | — | — | — | Atomic layer deposition | 88%/6 | [78] |
| MoO3-doped MnCo2O4 (0.10) | 26.4 | 298 | — | 34.24 | — | In situ synthesis | — | [79] |
| Ni0.5Cu0.5Co2O4 nanoplatelets | 80.2 | 298 | — | 28.4 | 200 | — | — | [91] |
| Ni0.5Cu0.5Co2O4 microspheres | 65.1 | 298 | — | 29.5 | 40 | — | — | [91] |
| Ni0.5Cu0.5Co2O4 nanoparticles | 45.5 | 298 | — | 43.2 | 50 | — | — | [91] |
| Pt-Ni | 302.3 | — | — | 42.1 | — | — | — | [92] |
| Plate-like Cu2O–CoO | 34.1 | 298 | — | — | Thickness of 40 nm | — | — | [93] |
| Rh/h-NCNWs | 1234 | 298 | — | 36.94 | — | — | 60%/5 | [94] |
| Cu0.6Ni0.4Co2O4 | 119.5 | 298 | — | 33.91 | — | — | 70%/8 | [95] |
| Cu0.5Ni0.5Co2O4 (Mo = 0.10) | 195.25 | 298 | — | — | — | — | — | [96] |
| CoNiP/GO | 134.6 | 298 | — | 44.12 | — | — | 84.6%/5 | [97] |
| Co–Fe–B@g-C3N4/NF | 14,005 mLH2·min−1·gCat−1 | 298 | — | 45 | — | Chemical deposition method | — | [98] |
| Co-Mo-B/NF | 6027.1 mLH2·min−1·gCat−1 | 298 | — | 43.6 | 65 | Electroless plating method | — | [99] |
| CuO/Co3O4@C-4 | 18.8 | 298 | — | 18.5 | — | Hydrothermal method | — | [100] |
| Ru/B-U-TiO2 | 1287 | 298 | — | 37.96 | — | — | 65%/8 | [101] |
| Co0.7Cu0.3@NHPC-800 | — | 303 | — | 26.2 | — | — | — | [103] |
| Co@N-C-700 | 5.6 | 298 | 31 | 9 | One-step thermolysis | 97.2%/10 | [104] | |
| Ru/NPC | 813 | 298 | — | 24.95 | — | In situ reduction | 67.3%/5 | [105] |
| Rh/NPC | 473.5 | 298 | 0.003 | 40.2 | 6.03 | Pyrolysis method | 54.0%/8 | [106] |
| Ru/Ti3C2−xNx | 1334 | 298 | — | — | 1.54 | Microwave heating polyol method | — | [107] |
| Co0.5Ru0.5/CosNC | 1068 | 298 | — | 18.96 | — | — | 100%/10 | [80] |
| Ru/OCB | 602 | 298 | — | 34.3 | 2.0 | Microwave-assisted solid-state strategy | 52%/5 | [108] |
| Pt/MXene-O3 | 265 | 303 | — | 69 | 0.6 | — | — | [109] |
| Ru/ONC | 556 | 298 | — | 34.3 | 1.69 | Gas-phase oxidation strategy | — | [81] |
| Ru@PC-5–700 | 405.9 | 303 | — | — | 1.3 | — | 58.3%/7 | [110] |
| Ru0.50Ni0.50@WSC | 251 | 303 | — | 45.3 | — | Facile adsorption–NaBH4 reduction method | — | [111] |
| CoP–CoO/NCDs | 89.56 | 298 | — | 41 | 58.82 | — | — | [82] |
| Ru/BNC | 1854 | 298 | — | 26.31 | 1.56 | — | — | [112] |
| Rh/N-U-TiO2 | 721 | 298 | — | 20.05 | 3.28 | — | 62%/5 | [113] |
| Cu/Cu0.76Co2.24O4-VO | 28.46 | 298 | — | 24.36 | — | — | — | [114] |
| Rh0.75Co0.25/Ni@ Ni-N-C | 223.8 | 303 | — | 28.63 | 3.69 | Maceration reduction method | — | [115] |
| Rh/C-SC | 336 | 298 | — | 37.1 | 4.1 | — | 50%/5 | [83] |
| PtPd3 | 4034 mL·min−1·gCat−1 | 298 | — | 14.56 | 10 | — | 70.8%/5 | [116] |
| CF-BT-Ru | 322 | 308 | — | 32.41 | 2.6 | — | — | [84] |
| Ru/3DNPC-500 | 584 | 298 | — | 31 | 1.32 | High-temperature pyrolysis | 50%/7 | [121] |
| Pd/IPCN | 122.8 | 298 | — | 29.1 | 2.17 | — | — | [122] |
| P2-Cu-Co3O4@CNF | 35.6 | 303 | — | 29.86 | — | Nanoconfinement method and a facile ion-doping approach | — | [123] |
| Cu0.5Ni0.5/h-BN | 6.33 | 303 | — | 23.02 | — | Adsorption–chemical reduction | — | [125] |
| CPFC-MS@NiAl-LDH@RhxNi1−x | 13 | 298 | — | 40.3 | — | — | — | [127] |
| Pt-Co/GQDs | 520 | 303 | — | 45.3 | 13 | — | — | [129] |
| CoRu0.5/CQDs | 814.7 | 298 | — | 39.29 | 4.25 | One-step hydrothermal | — | [130] |
| AuNi@ZIF-8 | 40 | 298 | — | 37.4 | — | — | — | [131] |
| SCo0.43Cu0.57 | 5.68 | 298 | 31.06 | 18.23 | Acid etching method | 71.8%/5 | [132] | |
| Ni–Zn/SiO2 | 4.3 | 298 | 0.025 | — | 8.5 | — | — | [133] |
| Ru0.8Ni0.2/g-C3N4-rGO | 905 | 303 | 0.0016 | 27.2 | 1.4 | Adsorption–chemical reduction | 55%/6 | [135] |
| Ru0.25Pd0.75@g-pC | 214.49 | 303 | — | 47.3 | 1.5 | One-pot calcination method | 62%/18 | [136] |
| Ru0.075Co0.925/NPC | 754 | 298 | — | 30.5 | 26.68 | Maceration reduction method | 56%/5 | [137] |
| NiMn-decorated CNFs | 58.2 | 303 | — | 38.9 | 60 | — | — | [138] |
| Ru0.6Co0.4/P25 | 443.7 | 298 | — | 43.9 | 25 | — | — | [141] |
| CoCu-NC-5 | 8.12 | 298 | — | 34.25 | 7.95 | — | — | [142] |
| Fe-CoP@C | 183.5 | 298 | — | 30.6 | — | — | — | [143] |
| Ru2Fe1/N–C | 424 | 298 | — | 33.7 | 3 | Impregnation–co-reduction | 26%/5 | [144] |
| Pt0.1%Co3%/TiO2 | 1530 | 298 | 0.0008 | 63.8 | 1.3 | Step-by-step reduction method | 100%/5 | [145] |
| Pt76Au12Co12 | 450 | 298 | — | 18.47 | — | Sequential Digestive reduction | 56%/5 | [146] |
| Cu0.4Co0.6 Pt0.0075O/RGO | 854 | — | — | 39.8 | 2.89 | — | 80%/8 | [147] |
| Pt1.5/CoCu0.4-NC | 1636.82 | 298 | — | 41.78 | 9.31 | Liquid-phase reduction method | — | [148] |
| Ni0.3Pd0.7Mo0.2 NPs | 252.7 | 298 | — | 52.3 | 5.93 | In situ reduction method | 15%/5 | [149] |
| np-RuNiFeCo | 148.2 | 298 | — | 25.3 | 4 | — | — | [150] |
| Rh0.8Ru0.2Ni0.25@MMT-S | 2961 | 298 | — | 29.7 | 2 | Impregnation method | — | [151] |
| Cu0.8Ni0.1Co0.1 @MIL-101 | 72.1 | 298 | 0.0027 | 29.1 | 2.8 | Solvent evaporation method | 73%/8 | [152] |
| Pt/Co3O4 NCs | 721 | 298 | — | 31.3 | 1.2 | — | 86.6%/10 | [154] |
| Co-CoOx/TiO2@N-C | 5905 mL·min−1·gCo−1 | 298 | — | 38.5 | — | Sol–gel method | 85%/5 | [155] |
| Pd0/Co3O4 | 3048 | 298 | — | 62 | — | Impregnation/reduction method | 100%/10 | [156] |
| Vo–Co–Sn5:2 | 17.6 | 298 | — | 45.95 | 5–15 | Co-precipitation–calcination method | 82.6%/14 | [157] |
| 1.5-PdTVO | 240 | 298 | — | 34.6 | 2 | — | — | [158] |
| A20-Pd5 | 17.4 | — | — | 41 | 20 | Solid-state approach | — | [159] |
| Ru/Co2.28Cu0.72O4/C7.5 | 2020 | 298 | — | 26.3 | 1.4 | — | — | [160] |
| Vco-Co3O4 | 934 | 298 | — | 32.65 | 45.9 | — | — | [161] |
| 1.5-RTVO-4 | 1370 | 298 | — | 46.3 | 1.9 | — | — | [162] |
| RuPd-TiO2-VO | 2750 | 298 | — | 32 | 2 | — | 90%/10 | [163] |
| Co-CN-O-100 | 11,410 mL·min−1·gCat−1 | 313 | — | 39.41 | — | — | 80%/5 | [164] |
| CoCu1Mo3-NC-O-15 | 24.44 | 298 | — | 28.44 | — | — | — | [165] |
| Ru0/SiO2-Fe3O4 | 127 | 298 | 0.0079 | 54 | 3.75 | Maceration reduction method | 100%5 | [166] |
| Ni0/CoFe2O4 | 38.3 | 298 | — | 62.7 | — | Two-step impregnation–reduction method | 38%/10 | [167] |
| Pt/MoO3−x-500 | 2268.6 | 298 | — | 13.97 | 1.8 | — | — | [169] |
| Pd0.75@Ag0.25/SiO2 | 109.99 | 303 | — | 42.26 | 10 | Seed-mediated stepwise reduction | 83.2%/5 | [170] |
| Rh0/CoFe2O4 | 720 | 298 | 0.00048 | 66 | 2.18 | Two-step impregnation–reduction method | 100%/5 | [172] |
| Rh/CoFe2O4-SB-H2 | 1894 | 298 | — | 59.3 | — | Impregnation–reduction method | 75%/10 | [173] |
| Ru/PC | 744 | 298 | — | 39.11 | 1.5 | Salt template-assisted in situ construction | — | [174] |
| Co–Mo2C/NC | 18,876 mLH2·min−1·gCo−1 | 298 | — | 49.8 | 4.71 | One-step method | 77.6%3 | [175] |
| Pd1Rh4/Ti3C2 | 338 | 298 | — | 33.8 | 4 | Microwave-assisted reduction method | — | [176] |
| Ru@Co-NC | 568 | 298 | — | 24.2 | 2.25 | Sodium chloride template method | 59.8%/5 | [177] |
| Cu0.9Ni0.1/Ti3C2Tx | 2429 h−1 | 323 | — | 41.61 | 1.84 | Wet-chemical co-reduction method | 95.5%/5 | [178] |
| Ni-MoOx/(P)NC | 85.7 | 298 | — | 29.6 | 3 | Phosphate-mediated method | 54%/5 | [88] |
| Ru/TASC-NaOH | 582 | 303 | — | 43 | 1.6 | — | — | [179] |
| Rh/MXene | 288.4 | 298 | — | 54.2 | 2.55 | Wet impregnation method | 99%/7 | [180] |
| CuCo/PDA-Ti3C2 | 71.8 | 293 | — | 45.89 | 1.8 | Surficial alkaline functional strategy | — | [181] |
| Ni/Ti3C2Tx−4 | 161 | 298 | 0.074 | 59.3 | 3.07 | — | — | [182] |
| Ru/Ti2.5V0.5C2 | 1072 | 298 | — | 43 | — | — | — | [183] |
| Rh0.8Ru0.2/ SP-ZSM-5-100 | 1006 | 298 | 0.001 | 56.5 | 0.7 | Incipient wetness impregnation method | — | [184] |
| Ru/S-1@C(RSC-2) | 892 | 298 | — | 36.8 | 3 | In situ reduction method. | — | [185] |
| Cu0.5@Co0.5-MOF/5 | 129.8 | 298 | — | 26.5 | 5.5 | Hydrothermal method | 95%/5 | [186] |
| PtCo2@COF | 486 | 293 | — | 34.5 | 2 | — | — | [187] |
| Cu0.8Ni0.2@ZrO2/NC/RGO | 40.9 | 303 | — | 33.24 | 2.5 | — | — | [188] |
| NiCoP/CoP | 30.3 | 298 | — | 25.89 | — | Three-step hydrothermal–oxidation–phosphorization | — | [189] |
| CuCo(O)@CN | 12.4 | 298 | — | 33.8 | 30 | Thermal reduction | 64.7%/5 | [190] |
| Co2P/(0.59-Cu3P)-NC | 729.6 | 298 | — | 63.5 | — | — | — | [191] |
| Pd1Ru2NPs@Alkyne-PVA gel | 247.93 | 298 | — | 33.02 | 2 | In situ reduction | — | [192] |
| Ru1Ni4/APTS-rGO | 1559 | 298 | — | 37.2 | 2 | One-step in situ co-reduction | 49.9%/5 | [193] |
| Pt2Ox | 2800 | — | — | — | 0.96 | Bottom-up approach | 100%/5 | [194] |
| CoP/Co2Ps | 64.1 | 298 | — | 38.8 | — | Salt-induced phase transformation | 100%/10 | [196] |
| CoO0.5P0.5@CS | 37 | 298 | — | 41 | 7.87 | — | — | [197] |
| MS@Pt@EuW10@PNIPAM | 51.1 | 298 | — | 74.89 | — | — | — | [201] |
| NiNPs/ZIF-8 | 85.7 | 298 | — | 28 | 2.7 | Deposition–precipitation (DP) method | — | [202] |
| Ru1Co9/TiO2 | 1408 | 298 | — | 33.25 | — | Co-precipitation and reduction | — | [203] |
| Pd/CGP-GO-Fe3O4 | 36.5 | 303 | — | 27.4 | — | Co-precipitation method | — | [204] |
| Rh0-Co3O4 | 1800 | 298 | 0.00024 | 61.7 | — | Two-step impregnation–reduction method | 100%/5 | [205] |
| CuNi/Co3O4 | 30.5 | 298 | — | 41.8 | 100 in diameter | Impregnation–reduction method | 50%/5 | [206] |
| NiCoP/NF | — | 298 | — | 58.95 | — | Low-temperature induced phosphating method | — | [207] |
| Ru-Ni-NF | 539.6 | 298 | — | 36.4 | — | Spontaneous redox reaction | — | [208] |
| CFNP@CF foam | 12.5 | 303 | — | 23.4 | 20 | Electrodeposition | 99.5%/3 | [209] |
| CoP NA/Ti | 6500 mLH2·min−1·g−1 | — | — | 41 | — | — | 100%/20 | [210] |
| Co–Mo–B/CC | 3916.1 | 298 | — | 25.2 | — | Electroless plating | 55.9%/5 | [211] |
| Rh@GQDs | 469 | 303 | 0.002 | 54.85 | 2.3 | — | — | [213] |
| Co/HNTA | — | 298 | — | 10.8 | 3.07 | — | — | [214] |
| Pt/ZIF-67 | 687 | 303 | — | 45.43 | 2.15 | — | — | [215] |
| Ru/MoAl1−xB | 494 | 303 | — | 39.2 | 2.4 | — | — | [216] |
4. By-Product Analysis and Regeneration Strategies in AB Hydrolysis
4.1. Identification of By-Products of Hydrolysis Reaction
4.2. The Regeneration Strategies of Ammonia Borane

5. Conclusions and Outlook
5.1. Conclusions
5.2. Outlook
Supplementary Materials
Funding
Conflicts of Interest
Correction Statement
References
- Tschan, M.J.-L.; Brulé, E.; Haquette, P.; Thomas, C.M. Synthesis of Biodegradable Polymers from Renewable Resources. Polym. Chem. 2012, 3, 836–851. [Google Scholar] [CrossRef]
- Goeppert, A.; Czaun, M.; Prakash, G.K.S.; Olah, G.A. Air as the Renewable Carbon Source of the Future: An Overview of CO2 Capture from the Atmosphere. Energy Environ. Sci. 2012, 5, 7833–7853. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Li, M.; Bai, Y.; Zhang, C.; Song, Y.; Jiang, S.; Grouset, D.; Zhang, M. Review on the Research of Hydrogen Storage System Fast Refueling in Fuel Cell Vehicle. Int. J. Hydrogen Energy 2019, 44, 10677–10693. [Google Scholar] [CrossRef]
- Huo, J.; Zhang, K.; Wei, H.; Fu, L.; Zhao, C.; He, C.; Hu, X. A Review on Hydrogen Production from Ammonia Borane: Experimental and Theoretical Studies. Chin. Chem. Lett. 2023, 34, 108280. [Google Scholar] [CrossRef]
- Niermann, M.; Beckendorff, A.; Kaltschmitt, M.; Bonhoff, K. Liquid Organic Hydrogen Carrier (LOHC)—Assessment Based on Chemical and Economic Properties. Int. J. Hydrogen Energy 2019, 44, 6631–6654. [Google Scholar] [CrossRef]
- Rusman, N.A.A.; Dahari, M. A Review on the Current Progress of Metal Hydrides Material for Solid-State Hydrogen Storage Applications. Int. J. Hydrogen Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Xu, Q.; Yu, J. Nanopore-Supported Metal Nanocatalysts for Efficient Hydrogen Generation from Liquid-Phase Chemical Hydrogen Storage Materials. Adv. Mater. 2020, 32, 2001818. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Du, H.; Lu, Z. Hydrolysis of Ammonia Borane for Hydrogen Production. Chem. Prog. 2020, 32, 1930–1951. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Z.; Chen, W.; Wu, S.; Li, G.; Chou, S. Non-Noble Metal-Based Catalysts Applied to Hydrogen Evolution from Hydrolysis of Boron Hydrides. Small Struct. 2021, 2, 2000135. [Google Scholar] [CrossRef]
- Shore, S.G.; Parry, R.W. The crystalline compound ammonia-borane,1 H3NBH3. J. Am. Chem. Soc. 1955, 77, 6084–6085. [Google Scholar] [CrossRef]
- Zadick, A.; Dubau, L.; Artyushkova, K.; Serov, A.; Atanassov, P.; Chatenet, M. Nickel-Based Electrocatalysts for Ammonia Borane Oxidation: Enabling Materials for Carbon-Free-Fuel Direct Liquid Alkaline Fuel Cell Technology. Nano Energy 2017, 37, 248–259. [Google Scholar] [CrossRef]
- Akbayrak, S.; Özkar, S. Ammonia Borane as Hydrogen Storage Materials. Int. J. Hydrogen Energy 2018, 43, 18592–18606. [Google Scholar] [CrossRef]
- Demirci, U.B. Ammonia Borane, a Material with Exceptional Properties for Chemical Hydrogen Storage. Int. J. Hydrogen Energy 2017, 42, 9978–10013. [Google Scholar] [CrossRef]
- Guan, S.; Liu, Y.; Zhang, H.; Shen, R.; Wen, H.; Kang, N.; Zhou, J.; Liu, B.; Fan, Y.; Jiang, J.; et al. Recent Advances and Perspectives on Supported Catalysts for Heterogeneous Hydrogen Production from Ammonia Borane. Adv. Sci. 2023, 10, 2300726. [Google Scholar] [CrossRef]
- Ma, J.L.; Cao, H.Y.; Zhang, X.X.; Chen, D. Preparation, Structure and Thermolysis Characteristics of Ammonia Borane. Key Eng. Mater. 2016, 680, 529–533. [Google Scholar] [CrossRef]
- Ergüven, H.; Figen, A.K.; Pişkin, S. Ammonia Borane–Boron Composites for Hydrogen Release: Thermolysis Kinetics. Energy Sources Part Recovery Util. Environ. Eff. 2017, 39, 613–617. [Google Scholar] [CrossRef]
- Li, H.; Yao, Z.; Wang, X.; Zhu, Y.; Chen, Y. Review on Hydrogen Production from Catalytic Ammonia Borane Methanolysis: Advances and Perspectives. Energy Fuels 2022, 36, 11745–11759. [Google Scholar] [CrossRef]
- Yüksel Alpaydın, C.; Gülbay, S.K.; Ozgur Colpan, C. A Review on the Catalysts Used for Hydrogen Production from Ammonia Borane. Int. J. Hydrogen Energy 2020, 45, 3414–3434. [Google Scholar] [CrossRef]
- Kang, N.; Wang, C.; Astruc, D. Hydrogen Evolution upon Ammonia Borane Solvolysis: Comparison between the Hydrolysis and Methanolysis Reactions. Chemistry 2023, 5, 886–899. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.; Du, X.; Sun, S.; Yu, X.; Zhang, X.; Lu, Z.; Li, L.; Yang, X. Hydrogen Production from Ammonia Borane Hydrolysis Catalyzed by Non-Noble Metal-Based Materials: A Review. J. Mater. Sci. 2021, 56, 2856–2878. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Akins, D.L.; Lee, J.W. Calorimetric and Microscopic Studies on the Noncatalytic Hydrothermolysis of Ammonia Borane. Ind. Eng. Chem. Res. 2011, 50, 10407–10413. [Google Scholar] [CrossRef]
- Basu, S.; Abiad, M.G.; Zheng, Y.; Campanella, O.H.; Varma, A. Transport Characteristics of Dehydrogenated Ammonia Borane and Sodium Borohydride Spent Fuels. Int. J. Hydrogen Energy 2010, 35, 2063–2072. [Google Scholar] [CrossRef]
- Hwang, H.T.; Al-Kukhun, A.; Varma, A. Hydrogen for Vehicle Applications from Hydrothermolysis of Ammonia Borane: Hydrogen Yield, Thermal Characteristics, and Ammonia Formation. Ind. Eng. Chem. Res. 2010, 49, 10994–11000. [Google Scholar] [CrossRef]
- Al-Kukhun, A.; Hwang, H.T.; Varma, A. A Comparison of Ammonia Borane Dehydrogenation Methods for Proton-Exchange-Membrane Fuel Cell Vehicles: Hydrogen Yield and Ammonia Formation and Its Removal. Ind. Eng. Chem. Res. 2011, 50, 8824–8835. [Google Scholar] [CrossRef]
- Diwan, M.; Hwang, H.T.; Al-Kukhun, A.; Varma, A. Hydrogen Generation from Noncatalytic Hydrothermolysis of Ammonia Borane for Vehicle Applications. AIChE J. 2011, 57, 259–264. [Google Scholar] [CrossRef]
- Chandra, M.; Xu, Q. A High-Performance Hydrogen Generation System: Transition Metal-Catalyzed Dissociation and Hydrolysis of Ammonia–Borane. J. Power Sources 2006, 156, 190–194. [Google Scholar] [CrossRef]
- Navlani-García, M.; Salinas-Torres, D.; Cazorla-Amorós, D. Hydrolytic Dehydrogenation of Ammonia Borane Attained by Ru-Based Catalysts: An Auspicious Option to Produce Hydrogen from a Solid Hydrogen Carrier Molecule. Energies 2021, 14, 2199. [Google Scholar] [CrossRef]
- Akbayrak, S.; Özkar, S. Magnetically Isolable Pt0/Co3O4 Nanocatalysts: Outstanding Catalytic Activity and High Reusability in Hydrolytic Dehydrogenation of Ammonia Borane. ACS Appl. Mater. Interfaces 2021, 13, 34341–34348. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, Y.; Guo, A.; Mao, M.; Wang, Y.; Long, Y.; Fan, G. Universal Low-Temperature Oxidative Thermal Redispersion Strategy for Green and Sustainable Fabrication of Oxygen-Rich Carbons Anchored Metal Nanoparticles for Hydrogen Evolution Reactions. Chem. Eng. J. 2022, 433, 133648. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Peng, Y.; Ma, X.; Fan, G. Oxygen Vacancies and Morphology Engineered Co3O4 Anchored Ru Nanoparticles as Efficient Catalysts for Ammonia Borane Hydrolysis. Int. J. Hydrogen Energy 2022, 47, 7793–7801. [Google Scholar] [CrossRef]
- Yao, F.; Guan, S.; Bian, L.; Fan, Y.; Liu, X.; Zhang, H.; Li, B.; Liu, B. Ensemble-Exciting Effect in Pd/Alk-Ti3C2 on the Activity for Efficient Hydrogen Production. ACS Sustain. Chem. Eng. 2021, 9, 12332–12340. [Google Scholar] [CrossRef]
- Lai, S.-W.; Lin, H.-L.; Lin, Y.-P.; Yu, T.L. Hydrolysis of Ammonia–Borane Catalyzed by an Iron–Nickel Alloy on an SBA-15 Support. Int. J. Hydrogen Energy 2013, 38, 4636–4647. [Google Scholar] [CrossRef]
- He, T.; Xiong, Z.; Wu, G.; Chu, H.; Wu, C.; Zhang, T.; Chen, P. Nanosized Co- and Ni-Catalyzed Ammonia Borane for Hydrogen Storage. Chem. Mater. 2009, 21, 2315–2318. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, L.; Chen, Y.; Shen, G.; Wang, L.; Zhang, X.; Zou, J.-J. Mo-Doped Ni-Based Catalyst for Remarkably Enhancing Catalytic Hydrogen Evolution of Hydrogen-Storage Materials. Int. J. Hydrogen Energy 2020, 45, 15560–15570. [Google Scholar] [CrossRef]
- Zahmakıran, M.; Durap, F.; Özkar, S. Zeolite Confined Copper(0) Nanoclusters as Cost-Effective and Reusable Catalyst in Hydrogen Generation from the Hydrolysis of Ammonia-Borane. Int. J. Hydrogen Energy 2010, 35, 187–197. [Google Scholar] [CrossRef]
- Hu, M.; Ming, M.; Xu, C.; Wang, Y.; Zhang, Y.; Gao, D.; Bi, J.; Fan, G. Towards High-Efficiency Hydrogen Production through Insitu Formation of Well-Dispersed Rhodium Nanoclusters. Chemsuschem 2018, 11, 3253–3258. [Google Scholar] [CrossRef] [PubMed]
- Zhong, F.; Wang, Q.; Xu, C.; Yang, Y.; Wang, Y.; Zhang, Y.; Gao, D.; Bi, J.; Fan, G. Ultrafine and Highly Dispersed Ru Nanoparticles Supported on Nitrogen-Doped Carbon Nanosheets: Efficient Catalysts for Ammonia Borane Hydrolysis. Appl. Surf. Sci. 2018, 455, 326–332. [Google Scholar] [CrossRef]
- Lu, R.; Hu, M.; Xu, C.; Wang, Y.; Zhang, Y.; Xu, B.; Gao, D.; Bi, J.; Fan, G. Hydrogen Evolution from Hydrolysis of Ammonia Borane Catalyzed by Rh/g-C3N4 under Mild Conditions. Int. J. Hydrogen Energy 2018, 43, 7038–7045. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Liu, J.; Wang, S.; Song, Y.; Zhang, J. Design Strategies for Shape-Controlled Nanocatalysts for Efficient Dehydrogenation of Ammonia Borane: A Review. J. Alloys Compd. 2023, 961, 171001. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, Y.; Liu, Q.; Zhou, M.; Mi, G.; Du, X. Hierarchically Alloyed Pd–Cu Microarchitecture with Tunable Shapes: Morphological Engineering, and Catalysis for Hydrogen Evolution Reaction of Ammonia Borane. Int. J. Hydrogen Energy 2019, 44, 30226–30236. [Google Scholar] [CrossRef]
- Metin, Ö.; Özkar, S. Hydrogen Generation from the Hydrolysis of Ammonia-Borane and Sodium Borohydride Using Water-Soluble Polymer-Stabilized Cobalt(0) Nanoclusters Catalyst. Energy Fuels 2009, 23, 3517–3526. [Google Scholar] [CrossRef]
- Mao, S.; Wang, Z.; Luo, Q.; Lu, B.; Wang, Y. Geometric and Electronic Effects in Hydrogenation Reactions. ACS Catal. 2023, 13, 974–1019. [Google Scholar] [CrossRef]
- Shang, N.-Z.; Feng, C.; Gao, S.-T.; Wang, C. Ag/Pd Nanoparticles Supported on Amine-Functionalized Metal–Organic Framework for Catalytic Hydrolysis of Ammonia Borane. Int. J. Hydrogen Energy 2016, 41, 944–950. [Google Scholar] [CrossRef]
- Yang, X.; Li, Q.; Li, L.; Lin, J.; Yang, X.; Yu, C.; Liu, Z.; Fang, Y.; Huang, Y.; Tang, C. CuCo Binary Metal Nanoparticles Supported on Boron Nitride Nanofibers as Highly Efficient Catalysts for Hydrogen Generation from Hydrolysis of Ammonia Borane. J. Power Sources 2019, 431, 135–143. [Google Scholar] [CrossRef]
- Tao, L.; Wang, Y.; Zou, Y.; Zhang, N.; Zhang, Y.; Wu, Y.; Wang, Y.; Chen, R.; Wang, S. Charge Transfer Modulated Activity of Carbon-Based Electrocatalysts. Adv. Energy Mater. 2020, 10, 1901227. [Google Scholar] [CrossRef]
- Song, J.; Wu, F.; Lu, Y.; Zhang, X. F-Doped CeO2 Supported Co-Based Nanoparticles for Enhanced Photocatalytic H2 Evolution from Ammonia Borane. Int. J. Hydrogen Energy 2023, 48, 13202–13212. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, S.; Shen, R.; Peng, Z.; Liu, B.; Li, J.; Zhang, Z.; Li, B. A Catalytic Copper/Cobalt Oxide Interface for Efficient Hydrogen Generation. ENERGY Environ. Mater. 2023, 6, e12279. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Z.; Li, J.; Gang, H.; Mei, D.; Yin, D.; Deng, R.; Zhu, Y.; Li, X.; Wang, N.; et al. Engineering a Hollow Bowl-like Porous Carbon-Confined Ru–MgO Hetero-Structured Nanopair as a High-Performance Catalyst for Ammonia Borane Hydrolysis. Mater. Horiz. 2024, 11, 2032–2040. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhang, Q.; Huo, J.; Fu, L. An Efficient Single Atom Catalysts Os/P3C Sheet for Ammonia Borane Dehydrogenation. Chin. Chem. Lett. 2022, 33, 3281–3286. [Google Scholar] [CrossRef]
- Ge, Y.Z.; Qin, X.T.; Li, A.; Deng, Y.C.; Lin, L.L.; Zhang, M.T.; Yu, Q.L.; Li, S.W.; Peng, M.; Xu, Y.; et al. Maximizing the Synergistic Effect of CoNi Catalyst on α-MoC for Robust Hydrogen Production. J. Am. Chem. Soc. 2021, 143, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.-C.; Li, Q.; Wang, C.-J.; Peng, C.-Y.; Chen, Q.-Q.; Ye, H.-F.; Fu, W.-F.; Che, C.-M.; López, N.; Chen, Y. Ternary Ni–Co–P Nanoparticles as Noble-Metal-Free Catalysts to Boost the Hydrolytic Dehydrogenation of Ammonia-Borane. Energy Environ. Sci. 2017, 10, 1770–1776. [Google Scholar] [CrossRef]
- Feng, Y.; Shao, Y.; Chen, X.; Zhang, Y.; Liu, Q.; He, M.; Li, H. Sea-Urchin-like Hollow CuMoO4 –CoMoO4 Hybrid Microspheres, a Noble-Metal-like Robust Catalyst for the Fast Hydrogen Production from Ammonia Borane. ACS Appl. Energy Mater. 2021, 4, 633–642. [Google Scholar] [CrossRef]
- Guan, S.; An, L.; Chen, Y.; Liu, X.; Shi, J.; Sun, Y.; Fan, Y.; Liu, B. Enhancing Effect of Fe2+ Doping of Ni/NiO Nanocomposite Films on Catalytic Hydrogen Generation. ACS Appl. Mater. Interfaces 2021, 13, 42909–42916. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Chandra, M. Catalytic Activities of Non-Noble Metals for Hydrogen Generation from Aqueous Ammonia–Borane at Room Temperature. J. Power Sources 2006, 163, 364–370. [Google Scholar] [CrossRef]
- Chen, W.; Ji, J.; Duan, X.; Qian, G.; Li, P.; Zhou, X.; Chen, D.; Yuan, W. Unique Reactivity in Pt/CNT Catalyzed Hydrolytic Dehydrogenation of Ammonia Borane. Chem. Commun. 2014, 50, 2142. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; He, T.; Liu, L.; Chen, W.; Zhang, M.; Wu, G.; Chen, P. Covalent Triazine Framework Supported Non-Noble Metal Nanoparticles with Superior Activity for Catalytic Hydrolysis of Ammonia Borane: From Mechanistic Study to Catalyst Design. Chem. Sci. 2017, 8, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, C.; Wang, Q.; Martinez-Villacorta, A.M.; Escobar, A.; Chong, H.; Wang, X.; Moya, S.; Salmon, L.; Fouquet, E.; et al. Highly Selective and Sharp Volcano-Type Synergistic Ni2Pt@ZIF-8-Catalyzed Hydrogen Evolution from Ammonia Borane Hydrolysis. J. Am. Chem. Soc. 2018, 140, 10034–10042. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, H.; Zhang, W.; Zhao, X.; Qiu, J.; Li, A.; Zheng, X.; Hu, Z.; Si, R.; Zeng, J. Supported Rhodium Catalysts for Ammonia-Borane Hydrolysis: Dependence of the Catalytic Activity on the Highest Occupied State of the Single Rhodium Atoms. Angew. Chem. Int. Ed. 2017, 56, 4712–4718. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S. Low-Cost CuFeCo@MIL-101 as an Efficient Catalyst for Catalytic Hydrolysis of Ammonia Borane. Int. J. Hydrogen Energy 2020, 45, 10433–10441. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Z.; Chen, C.; Kuang, F. Constructing MOF-199 Anchored RuMoP Nanoparticles as a High-Performance Catalyst for Boosting the Hydrolysis of AB. Int. J. Hydrogen Energy 2023, 48, 14670–14680. [Google Scholar] [CrossRef]
- Cui, C.; Liu, Y.; Mehdi, S.; Wen, H.; Zhou, B.; Li, J.; Li, B. Enhancing Effect of Fe-Doping on the Activity of Nano Ni Catalyst towards Hydrogen Evolution from NH3BH3. Appl. Catal. B Environ. 2020, 265, 118612. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Wei, H.; Wang, C.; Liu, T.; Wu, X.; Ashraf, S.; Mehdi, S.; Guan, S.; Fan, Y.; et al. Atomic-Bridge Structure in B-Co-P Dual-Active Sites on Boron Nitride Nanosheets for Catalytic Hydrogen Generation. Appl. Catal. B Environ. 2022, 314, 121495. [Google Scholar] [CrossRef]
- Guan, S.; Yuan, Z.; Zhao, S.; Zhuang, Z.; Zhang, H.; Shen, R.; Fan, Y.; Li, B.; Wang, D.; Liu, B. Efficient Hydrogen Generation from Ammonia Borane Hydrolysis on a Tandem Ruthenium-Platinum-Titanium Catalyst. Angew. Chem. Int. Ed. 2024, 63, e202408193. [Google Scholar] [CrossRef] [PubMed]
- Veeraraghavan Ramachandran, P.; Kulkarni, A.S. Nucleophilic Displacement of Ammonia from Ammonia Borane for the Preparation of Alkylamine-, Pyridine- and Phosphine-Boranes. RSC Adv. 2014, 4, 26207. [Google Scholar] [CrossRef]
- Qu, X.; Jiang, R.; Li, Q.; Zeng, F.; Zheng, X.; Xu, Z.; Chen, C.; Peng, J. The Hydrolysis of Ammonia Borane Catalyzed by NiCoP/OPC-300 Nanocatalysts: High Selectivity and Efficiency, and Mechanism. Green Chem. 2019, 21, 850–860. [Google Scholar] [CrossRef]
- Wu, H.; Wu, M.; Wang, B.; Yong, X.; Liu, Y.; Li, B.; Liu, B.; Lu, S. Interface Electron Collaborative Migration of Co–Co3O4/Carbon Dots: Boosting the Hydrolytic Dehydrogenation of Ammonia Borane. J. Energy Chem. 2020, 48, 43–53. [Google Scholar] [CrossRef]
- Peng, Y.; He, Y.; Wang, Y.; Long, Y.; Fan, G. Sustainable One-Pot Construction of Oxygen-Rich Nitrogen-Doped Carbon Nanosheets Stabilized Ultrafine Rh Nanoparticles for Efficient Ammonia Borane Hydrolysis. J. Colloid Interface Sci. 2021, 594, 131–140. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Peng, Y.; Wang, Y.; Long, Y.; Fan, G. Air-Engaged Fabrication of Nitrogen-Doped Carbon Skeleton as an Excellent Platform for Ultrafine Well-Dispersed RuNi Alloy Nanoparticles toward Efficient Hydrolysis of Ammonia Borane. FUEL 2021, 297, 120750. [Google Scholar] [CrossRef]
- Chen, W.; Fu, W.; Qian, G.; Zhang, B.; Chen, D.; Duan, X.; Zhou, X. Synergistic Pt-WO3 Dual Active Sites to Boost Hydrogen Production from Ammonia Borane. iScience 2020, 23, 100922. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Yuan, H.; Wen, H.; Zhang, H.; Ashraf, S.; Guan, S.; Liu, T.; Mehdi, S.; Shen, R.; et al. Coupling Atom Ensemble and Electron Transfer in PdCu for Superior Catalytic Kinetics in Hydrogen Generation. Nano Res. 2023, 16, 9012–9021. [Google Scholar] [CrossRef]
- Huo, J.; Wei, H.; Fu, L.; Zhao, C.; He, C. Highly Active Fe36Co44 Bimetallic Nanoclusters Catalysts for Hydrolysis of Ammonia Borane: The First-Principles Study. Chin. Chem. Lett. 2023, 34, 107261. [Google Scholar] [CrossRef]
- Mao, D.; Zhang, J.; Wu, Y.; Qin, H.; Zheng, Y.; Li, L.-C. The Electronic Structures of Non-Metal (N, S) Doped Cobalt Phosphide Catalysts and the Catalytic Mechanism for the Hydrogen Evolution Reaction of Ammonia Borane: A Theoretical Study. New J. Chem. 2023, 47, 1724–1730. [Google Scholar] [CrossRef]
- Akbayrak, S.; Özkar, S. Ruthenium(0) nanoparticles supported on xonotlite nanowire: A long-lived catalyst for hydrolytic dehydrogenation of ammonia-borane. Dalton Transactions 2014, 43, 1797–1805. [Google Scholar] [CrossRef]
- Zhou, J.; Meng, X.; Yan, J.; Liu, X. Co/MoS2 nanocomposite catalyzed H2 evolution upon dimethylamine-borane hydrolysis and in situ tandem reaction. Inorg. Chem. Commun. 2021, 130, 108691. [Google Scholar] [CrossRef]
- Guo, K.; Li, H.; Yu, Z. Size-Dependent Catalytic Activity of Monodispersed Nickel Nanoparticles for the Hydrolytic Dehydrogenation of Ammonia Borane. ACS Appl. Mater. Interfaces 2018, 10, 517–525. [Google Scholar] [CrossRef]
- Uzundurukan, A.; Devrim, Y. Carbon nanotube-graphene hybrid supported platinum as an effective catalyst for hydrogen generation from hydrolysis of ammonia borane. Int. J. Hydrogen Energy 2019, 44, 26773–26782. [Google Scholar] [CrossRef]
- Yang, J.; Fu, W.; Chen, C.; Chen, W.; Huang, W.; Yang, R.; Kong, Q.; Zhang, B.; Zhao, J.; Chen, C.; et al. Atomic Design and Fine-Tuning of Subnanometric Pt Catalysts to Tame Hydrogen Generation. ACS Catal. 2021, 11, 4146–4156. [Google Scholar] [CrossRef]
- Wang, X.; Liu, D.; Song, S.; Zhang, H.J. Pt@CeO2 Multicore@Shell Self-Assembled Nanospheres: Clean Synthesis, Structure Optimization, and Catalytic Applications. J. Am. Chem. Soc. 2013, 135, 15864–15872. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, K.; Wang, K.; Wang, M.; Liu, Y.; Jiang, J.; Liu, T.; Liang, E.; Li, B. Out-of-Plane CoRu Nanoalloy Axially Coupling CosNC for Electron Enrichment to Boost Hydrogen Production. Appl. Catal. B Environ. 2022, 318, 121890. [Google Scholar] [CrossRef]
- Guo, A.; Peng, Y.; Mao, M.; Wang, Y.; Long, Y.; Li, Q.; Fan, G. Surface Property and Spatial Confinement Engineering for Achieving Ru Nanoclusters on O/N-Doped Hollow Carbon towards Enhanced Hydrogen Production. Fuel 2021, 306, 121722. [Google Scholar] [CrossRef]
- Wu, H.; Cheng, Y.; Wang, B.; Wang, Y.; Wu, M.; Li, W.; Liu, B.; Lu, S. Carbon Dots-Confined CoP-CoO Nanoheterostructure with Strong Interfacial Synergy Triggered the Robust Hydrogen Evolution from Ammonia Borane. J. Energy Chem. 2021, 57, 198–205. [Google Scholar] [CrossRef]
- Chen, J.; Hu, M.; Ming, M.; Xu, C.; Wang, Y.; Zhang, Y.; Wu, J.; Gao, D.; Bi, J.; Fan, G. Carbon-Supported Small Rh Nanoparticles Prepared with Sodium Citrate: Toward High Catalytic Activity for Hydrogen Evolution from Ammonia Borane Hydrolysis. Int. J. Hydrogen Energy 2018, 43, 2718–2725. [Google Scholar] [CrossRef]
- Fu, L.; Cai, L. Ru Nanoparticles Loaded on Tannin Immobilized Collagen Fibers for Catalytic Hydrolysis of Ammonia Borane. Int. J. Hydrogen Energy 2021, 46, 10749–10762. [Google Scholar] [CrossRef]
- Shen, Y.; Bo, X.K.; Tian, Z.F.; Wang, Y.Z.; Guo, X.K.; Xie, M.J.; Gao, F.; Lin, M.; Guo, X.F.; Ding, W.P. Fabrication of Highly Dispersed/Active Ultrafine Pd Nanoparticle Supported Catalysts: A Facile Solvent-Free In Situ Dispersion/Reduction Method. Green Chem. 2017, 19, 2646–2652. [Google Scholar] [CrossRef]
- Chen, G.; Wang, R.; Zhao, W.; Kang, B.; Gao, D.; Li, C.; Lee, J.Y. Effect of Ru Crystal Phase on the Catalytic Activity of Hydrolytic Dehydrogenation of Ammonia Borane. J. Power Sources 2018, 396, 148–154. [Google Scholar] [CrossRef]
- Ju, Y.; Cho, T.; Lee, K.; Kim, J.; Yoon, C.W.; Kim, J. Intrinsic Size-Dependent Activity of Pt Nanoparticles without Masking by Heterogeneous Oxidation States of Pt for Hydrolytic Dehydrogenation of NH3BH3. Int. J. Energy Res. 2022, 46, 9771–9781. [Google Scholar] [CrossRef]
- Liu, W.; Yao, L.; Sun, X.; Wang, W.; Feng, G.; Yao, Q.; Zhang, L.; Lu, Z. Ultrafine Ni-MoOx Nanoparticles Anchored on Nitrogen-Doped Carbon Nanosheets: A Highly Efficient Noble-Metal-Free Catalyst for Ammonia Borane Hydrolysis. ChemSusChem 2024, 17, e202400415. [Google Scholar] [CrossRef]
- Punzi, E.; Nguyen, X.T.; Pitzalis, E.; Mandoli, A.; Onor, M.; Marelli, M.; Poggini, L.; Tuci, G.; Giambastiani, G.; Evangelisti, C. Ultrasmall Nickel Nanoparticles on a Covalent Triazine Framework for Ammonia Borane Hydrolysis and Transfer Hydrogenation of Nitroaromatics. ACS Appl. Nano Mater. 2024, 7, 6916–6926. [Google Scholar] [CrossRef]
- Poon, P.-C.; Wang, Y.; Li, W.; Suen, D.W.-S.; Lam, W.W.Y.; Yap, D.Z.J.; Mehdi, B.L.; Qi, J.; Lu, X.-Y.; Wong, E.Y.C.; et al. Synergistic Effect of Co Catalysts with Atomically Dispersed CoNx Active Sites on Ammonia Borane Hydrolysis for Hydrogen Generation. J. Mater. Chem. A 2022, 10, 5580–5592. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, J.; Ye, H.; Li, L.; Wang, H.; Li, X.; Zhang, X.; Li, H. Ni0.5Cu0.5Co2O4 Nanocomposites, Morphology, Controlled Synthesis, and Catalytic Performance in the Hydrolysis of Ammonia Borane for Hydrogen Production. Nanomaterials 2019, 9, 1334. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Pei, Q.J.; Yu, Y.; Jing, Z.J.; Wang, J.T.; He, T. Syntheses of Pt-Ni Hollow Nanoalloy for Hydrogen Generation from Catalytic Hydrolysis of Ammonia Borane. ChemCatChem 2020, 12, 4257–4261. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, H.; Chen, X.; Lv, F.; Li, Y.; Zhu, Y.; Xu, C.; Zhang, X.; Liu, H.-R.; Li, H. Simple Synthesis of Cu2O–CoO Nanoplates with Enhanced Catalytic Activity for Hydrogen Production from Ammonia Borane Hydrolysis. Int. J. Hydrogen Energy 2020, 45, 17164–17173. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, Y.; Liu, S.; Wu, J.; Fan, G.; Yu, X. Architecture Engineering toward Highly Active Rh Integrated Porous Carbon with Diverse Flexible Channels for Hydrogen Evolution. New J. Chem. 2023, 47, 16228–16234. [Google Scholar] [CrossRef]
- Lu, D.; Liao, J.; Zhong, S.; Leng, Y.; Ji, S.; Wang, H.; Wang, R.; Li, H. Cu0.6Ni0.4Co2O4 Nanowires, a Novel Noble-Metal-Free Catalyst with Ultrahigh Catalytic Activity towards the Hydrolysis of Ammonia Borane for Hydrogen Production. Int. J. Hydrogen Energy 2018, 43, 5541–5550. [Google Scholar] [CrossRef]
- Lu, D.; Liao, J.; Leng, Y.; Zhong, S.; He, J.; Wang, H.; Wang, R.; Li, H. Mo-Doped Cu0.5Ni0.5Co2O4 Nanowires, a Strong Substitute for Noble-Metal-Based Catalysts towards the Hydrolysis of Ammonia Borane for Hydrogen Production. Catal. Commun. 2018, 114, 89–92. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, K.; Yuan, G.; Kang, Z.; Zhong, J. Highly Efficient CoNiP Nanoboxes on Graphene Oxide for the Hydrolysis of Ammonia Borane. Chem. Eng. J. 2022, 428, 131219. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.-X.; Ren, J.; Zhang, D.; Xu, F.-Y.; Zhang, K.; Cao, Z.-Q.; Sun, Q.-J.; Li, G.-D.; Wu, S.-W.; et al. Hydrogen Production from Hydrolysis of NaBH4 Solution over Co–Fe–B@g-C3N4/NF Thin Film Catalyst. Rare Met. 2024, 43, 2648–2659. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, K.; Wang, D.; Meng, W.; Qi, N.; Cao, Z.; Zhang, K.; Chen, H.; Li, G. Highly Efficient Hydrogen Evolution from the Hydrolysis of Ammonia Borane Solution with the Co–Mo–B/NF Nanocatalyst. Renew. Energy 2020, 154, 453–460. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Feng, Y.; Wang, H.; Liao, J.; Ren, J.; Zhou, W.; He, M.; Li, H. Rattle-Structured CuO/Co3O4@C Microspheres, a Potent Bifunctional Catalyst for Hydrogen Production from Ammonia Borane Hydrolysis and Methanolysis. Appl. Surf. Sci. 2023, 636, 157840. [Google Scholar] [CrossRef]
- Yuan, M.; Guo, A.; Chen, Y.; Wang, X.; Fan, G.; Yu, X. Three Birds, One-Stone Strategy for Fabrication of Hierarchically Arrayed Ru/B-U-TiO2 Nanoribbon Assemblies toward Efficient Hydrogen Evolution. Appl. Surf. Sci. 2023, 641, 158552. [Google Scholar] [CrossRef]
- Yousef, A.; Brooks, R.M.; El-Halwany, M.M.; EL-Newehy, M.H.; Al-Deyab, S.S.; Barakat, N.A.M. Cu0/S-Doped TiO2 Nanoparticles-Decorated Carbon Nanofibers as Novel and Efficient Photocatalyst for Hydrogen Generation from Ammonia Borane. Ceram. Int. 2016, 42, 1507–1512. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Z.; Ma, C.; Han, X.; Dong, X.; Dong, Z.; Zhang, X. N-Doped Hierarchical Porous Carbon Embedded Synergistic Bimetallic CoCu NPs with Unparalleled Catalytic Performance. ChemCatChem 2019, 11, 2415–2422. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Cheng, F.; Tao, Z.; Chen, J. Cobalt Nanoparticles Embedded in Porous N-Doped Carbon as Long-Life Catalysts for Hydrolysis of Ammonia Borane. Catal. Sci. Technol. 2016, 6, 3443–3448. [Google Scholar] [CrossRef]
- Chu, H.; Li, N.; Qiu, S.; Zou, Y.; Xiang, C.; Xu, F.; Sun, L. Ruthenium Supported on Nitrogen-Doped Porous Carbon for Catalytic Hydrogen Generation from NH3BH3 Hydrolysis. Int. J. Hydrogen Energy 2019, 44, 1774–1781. [Google Scholar] [CrossRef]
- Zhong, F.; Wang, Q.; Xu, C.; Wang, Y.; Xu, B.; Zhang, Y.; Fan, G. Catalytically Active Rhodium Nanoparticles Stabilized by Nitrogen Doped Carbon for the Hydrolysis of Ammonia Borane. Int. J. Hydrogen Energy 2018, 43, 22273–22280. [Google Scholar] [CrossRef]
- Liang, L.; Bian, L.; Fan, Y.; Guan, S.; Liu, X.; Sun, Q.; Liu, B. Nitrogen Doping Excited Ru and Ti3C2−xNx Support for Hydrogen Generation from Ammonia Borane. Fuel 2023, 339, 127445. [Google Scholar] [CrossRef]
- Sun, T.; Wang, Y.; Long, Y.; Li, Q.; Fan, G. Ultrafast, Dry Microwave-Assisted Surface Property Modulations to Boost Carbon Stabilized Ru Nanocatalyst for Catalytic Hydrogen Evolution. Fuel 2022, 309, 122203. [Google Scholar] [CrossRef]
- Slot, T.K.; Yue, F.; Xu, H.; Ramos-Fernandez, E.; Sepulveda-Escribano, A.; Sofer, Z.; Rothenberg, G.; Shiju, N.R. Surface Oxidation of Ti(3)C(2)T(x)Enhances the Catalytic Activity of Supported Platinum Nanoparticles in Ammonia Borane Hydrolysis. 2d Mater. 2021, 8, 015001. [Google Scholar] [CrossRef]
- Jiang, R.; Meng, J.; Yang, S.; Peng, Z.; Liu, P.; Zheng, X. Ru Nanoclusters Confined in N, O-Codoped Porous Carbon as Robust Catalysts for Hydrolytic Dehydrogenation of NH3BH3. Appl. Surf. Sci. 2022, 606, 154795. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, S.; Liu, P.; Zhu, Q.; Zheng, X. Nickel-Promoted Ruthenium Nanocatalysts for Controllable Hydrogen Production from NH3BH3 Hydrolysis. Appl. Surf. Sci. 2025, 688, 162345. [Google Scholar] [CrossRef]
- Song, S.; Wu, S.; He, Y.; Zhang, Y.; Fan, G.; Long, Y.; Song, S. Boron/Nitrogen-Trapping and Regulative Electronic States around Ru Nanoparticles towards Bifunctional Hydrogen Production. J. Colloid Interface Sci. 2024, 672, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Yuan, M.; Fan, G.; Long, Y. Enhanced Hydrogen Generation from Ammonia Borane Hydrolysis over Nitrogen-Modified Urchin-like TiO2-Anchored Rh Nanoparticles: Active Site and Water Dissociation Regulating. Fuel 2024, 376, 132748. [Google Scholar] [CrossRef]
- Wang, C.; Ren, Y.; Zhao, J.; Sun, S.; Du, X.; Wang, M.; Ma, G.; Yu, H.; Li, L.; Yu, X.; et al. Oxygen Vacancy-Attired Dual-Active-Sites Cu/Cu0.76Co2.24O4 Drives Electron Transfer for Efficient Ammonia Borane Dehydrogenation. Appl. Catal. B Environ. 2022, 314, 121494. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Liu, L.-C.; Zhang, C.-X.; Zhu, H.-L.; Zheng, Y.-Q. The Doped Co on Rh/Ni@Ni–N–C That Weakened the Catalytic Performance for Ammonia Borane Hydrolysis. Int. J. Hydrogen Energy 2023, 48, 2640–2651. [Google Scholar] [CrossRef]
- Wang, J.; Hui, B.; Jia, T.; Chen, X.; Yu, X.; Li, L.; Zhang, X.; Lu, Z.; Yang, X. PVP-Adjusted Crystal Surfaces of PtPd Nanoparticles for Enhancing the Catalytic Hydrolysis of Ammonia Borane. ACS Appl. Nano Mater. 2024, 7, 9490–9498. [Google Scholar] [CrossRef]
- Umegaki, T.; Imai, H.; Xu, Q.; Kojima, Y. In-situ synthesis of porous silica-ruthenium composite catalyst for hydrolysis of ammonia borane. J. Porous Mater. 2024, 31, 2043–2052. [Google Scholar] [CrossRef]
- Umegaki, T.; Uchida, T.; Imai, H.; Xu, Q.; Kojima, Y. Fabrication of an In-Situ Synthesized Porous Silica-Ruthenium-Nickel Composite Catalyst for Hydrolysis of Ammonia Borane. ChemistrySelect 2024, 9, e202401433. [Google Scholar] [CrossRef]
- Ozay, H.; Tercan, M.; Ozay, O. Utilization of Superabsorbent Xanthan Films Cross-Linked with Ru0 Nanoparticles for Hydrogen Generation from Ammonia Borane. Int. J. Hydrogen Energy 2024, 61, 367–376. [Google Scholar] [CrossRef]
- Chen, X.; Wu, G.; Chen, J.; Chen, X.; Xie, Z.; Wang, X. Synthesis of “Clean” and Well-Dispersive Pd Nanoparticles with Excellent Electrocatalytic Property on Graphene Oxide. J. Am. Chem. Soc. 2011, 133, 3693–3695. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, C.; Chen, Q.; Ming, M.; Wang, Y.; Sun, T.; Zhang, Y.; Gao, D.; Bi, J.; Fan, G. Nitrogen-Doped Carbon-Stabilized Ru Nanoclusters as Excellent Catalysts for Hydrogen Production. ACS Sustain. Chem. Eng. 2019, 7, 1178–1184. [Google Scholar] [CrossRef]
- Mao, M.; Chen, Q.; Wu, J.; Fan, G. Anchoring and Space-Confinement Effects to Synthesize Ultrasmall Pd Nanoparticles for Efficient Ammonia Borane Hydrolysis. Int. J. Hydrogen Energy 2020, 45, 27244–27253. [Google Scholar] [CrossRef]
- Yuan, C.; Xu, T.; Guo, M.; Zhang, T.; Yu, X. Cation/Anion-Doping Induced Electronic Structure Regulation Strategy to Boost the Catalytic Hydrogen Evolution from Ammonia Borane Hydrolysis. Appl. Catal. B Environ. 2023, 321, 122044. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Y.; Liao, Q.; Zhang, W.; Huang, Z.; Chen, X.; Shao, Y.; Dong, H.; Liu, Q.; Li, H. Modulation the Electronic Structure of Hollow Structured CuO-NiCo2O4 Nanosphere for Enhanced Catalytic Activity towards Methanolysis of Ammonia Borane. Fuel 2023, 332, 126045. [Google Scholar] [CrossRef]
- Liu, K.; Yang, S.; Chen, Y.; Zhang, W.; Liu, P.; Zheng, X. Enhanced Catalytic Behavior of H-BN Supported CuNi Bimetallic Catalysts in Hydrolytic Dehydrogenation of NH3BH3. Int. J. Hydrogen Energy 2022, 47, 33741–33753. [Google Scholar] [CrossRef]
- Yan, J.-M.; Zhang, X.-B.; Han, S.; Shioyama, H.; Xu, Q. Magnetically Recyclable Fe-Ni Alloy Catalyzed Dehydrogenation of Ammonia Borane in Aqueous Solution under Ambient Atmosphere. J. Power Sources 2009, 194, 478–481. [Google Scholar] [CrossRef]
- Abbas, Y.; Zuhra, Z.; Majeed, S.; Khan, M.S.; Basharat, M.; Tyagi, D.; Ali, S.; Wu, Z.; Liu, Z. Calcined polycyclotriphosphazene@NiAl-LDH@RhxNi1-x: A Novel Hierarchically Oriented Composition Tunable Catalyst for Green and Sustainable Hydrogen Generation. J. Environ. Chem. Eng. 2022, 10, 107645. [Google Scholar] [CrossRef]
- Furukawa, S.; Komatsu, T. Intermetallic Compounds: Promising Inorganic Materials for Well-Structured and Electronically Modified Reaction Environments for Efficient Catalysis. ACS Catal. 2017, 7, 735–765. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, F.; Xiao, T.; Jiang, L.; Chen, W.; Tan, X. Effective Hydrolysis of NH3BH3 for Hydrogen Evolution by the Novel Graphene Quantum Dots Loaded Bimetallic Nanoparticles (Pt-Co/GQDs). Environ. Prog. Sustain. Energy 2023, 42, e14161. [Google Scholar] [CrossRef]
- Li, W.; Zhao, Y.; Liu, Y.; Sun, M.; Waterhouse, G.I.N.; Huang, B.; Zhang, K.; Zhang, T.; Lu, S. Exploiting Ru-Induced Lattice Strain in CoRu Nanoalloys for Robust Bifunctional Hydrogen Production. Angew. Chem. Int. Ed. 2021, 60, 3290–3298. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Wei, X.; Shen, R.; Li, B.; Cal, E.G.; Moya, S.; Salmon, L.; Wang, C.; Coy, E.; Berlande, M.; et al. Fast Au-Ni@ZIF-8-Catalyzed Ammonia Borane Hydrolysis Boosted by Dramatic Volcano-Type Synergy and Plasmonic Acceleration. Appl. Catal. B Environ. 2023, 320, 121957. [Google Scholar] [CrossRef]
- Wang, C.Y.; Li, L.L.; Yu, X.F.; Lu, Z.M.; Zhang, X.H.; Wang, X.X.; Yang, X.J.; Zhao, J.L. Regulation of d-Band Electrons to Enhance the Activity of Co-Based Non-Noble Bimetal Catalysts for Hydrolysis of Ammonia Borane. ACS Sustain. Chem. Eng. 2020, 8, 8256–8266. [Google Scholar] [CrossRef]
- Furukawa, S.; Nishimura, G.; Takayama, T.; Komatsu, T. Highly Active Ni- and Co-Based Bimetallic Catalysts for Hydrogen Production From Ammonia-Borane. Front. Chem. 2019, 7, 138. [Google Scholar] [CrossRef]
- Akdim, O.; Demirci, U.B.; Miele, P. A Bottom-up Approach to Prepare Cobalt-Based Bimetallic Supported Catalysts for Hydrolysis of Ammonia Borane. Int. J. Hydrogen Energy 2013, 38, 5627–5637. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Q.; Meng, J.; Yang, Y.; Peng, Z.; Zheng, X. Enhanced Catalytic Performance of RuNi Alloy Nanoclusters toward Hydrolytic Dehydrogenation of NH3BH3. Appl. Surf. Sci. 2022, 605, 154709. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, R.; Liu, S.; Zheng, G.; Liu, P.; Zheng, X. Desirable Performance and Mechanism of RuPd Nanoalloys in Catalyzing Hydrolytic Dehydrogenation of NH3BH3. J. Alloys Compd. 2024, 983, 173932. [Google Scholar] [CrossRef]
- Li, G.; Wei, N.; Wang, Y. Active Clusters Ensemble Effect of Bimetallic RuCo Alloys for Efficient Hydrogen Production from Ammonia Borane. Appl. Surf. Sci. 2023, 610, 155459. [Google Scholar] [CrossRef]
- Abutaleb, A.; Zouli, N.; El-Halwany, M.M.; Ubaidullah, M.; Yousef, A. Graphitic Nanofibers Supported NiMn Bimetallic Nanoalloys as Catalysts for H2 Generation from Ammonia Borane. Int. J. Hydrogen Energy 2021, 46, 35248–35260. [Google Scholar] [CrossRef]
- Wang, H.; Gu, X.-K.; Zheng, X.; Pan, H.; Zhu, J.; Chen, S.; Cao, L.; Li, W.-X.; Lu, J. Disentangling the Size-Dependent Geometric and Electronic Effects of Palladium Nanocatalysts beyond Selectivity. Sci. Adv. 2019, 5, eaat6413. [Google Scholar] [CrossRef]
- Wang, J.; Tian, M.; Ma, H.; Yu, X.; Li, L.; Zhang, X.; Lu, Z.; Yang, X. Study on the Influence Factors of Pt-Based Catalyst on Dehydrogen Performance of Ammonia Borane Hydrolysis-Which Is More Important, Geometric Effects or Electronic Effects? Fuel 2024, 358, 130167. [Google Scholar] [CrossRef]
- Wen, H.; Shen, R.; Liu, Y.; Huang, X.; Liu, S.; Peng, Z.; Wu, X.; Guo, X.; Liang, E.; Yuan, H.; et al. Insights into Boosting Catalytic Hydrogen Evolution over Co Doping Ru Nanoparticles. Fuel 2023, 351, 128950. [Google Scholar] [CrossRef]
- Song, Y.; Gao, C.; Liu, J.; Liu, Z. Fabrication of Multiatomic Structure of Cu-CoO/Co Interface for Efficient Hydrogen Generation from Ammonia Borane Hydrolysis. Int. J. Hydrogen Energy 2023, 48, 26162–26172. [Google Scholar] [CrossRef]
- Xu, W.; Li, W.; Liu, M.; Guo, X.; Wen, H.; Li, B. P-Bridged Fe-X-Co Coupled Sites in Hollow Carbon Spheres for Efficient Hydrogen Generation. J. Colloid Interface Sci. 2024, 660, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Huang, X.; Wen, H.; Shen, R.; Liu, Y.; Guo, X.; Li, B. Ru-Fe Nanoalloys Supported on N-Doped Carbon as Efficient Catalysts for Hydrogen Generation from Ammonia Borane. Sustain. Energy Fuels 2020, 4, 3677–3686. [Google Scholar] [CrossRef]
- Meng, Y.; Sun, Q.; Zhang, T.; Zhang, J.; Dong, Z.; Ma, Y.; Wu, Z.; Wang, H.; Bao, X.; Sun, Q.; et al. Cobalt-Promoted Noble-Metal Catalysts for Efficient Hydrogen Generation from Ammonia Borane Hydrolysis. J. Am. Chem. Soc. 2023, 145, 5486–5495. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.-L.; Zhang, D.-F.; Yang, Z.; Chen, T.-W.; Zhai, J. PtAuCo Trimetallic Nanoalloys as Highly Efficient Catalysts toward Dehydrogenation of Ammonia Borane. ACS Sustain. Chem. Eng. 2020, 8, 3734–3742. [Google Scholar] [CrossRef]
- Xu, J.; Feng, K.; Chen, Y.; Zhong, J. Ternary Metallic CuxCo1−xPtyO/RGO Catalyst with Internal Synergistic Effect for Efficient Hydrolysis of Ammonia-Borane. Appl. Surf. Sci. 2021, 537, 147823. [Google Scholar] [CrossRef]
- Li, M.; Liu, J.; Zhang, W.; Zhao, Y.; Wang, J.; Liu, F.; Li, J.; Guo, X.; Li, X. Fabrication of Nano Pt–Co–Cu Sites in the Heterostructured Catalysts for Hydrogen Generation. ACS Appl. Nano Mater. 2024, 7, 22061–22070. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Y.; Zhang, W.; Peng, X.; Tang, Q.; Ding, Q.; Deng, X.; Zhang, H. NiPdMo Nanoparticles Reduced by Cs[Closo-B6H7] as High-Performance Catalysts for Hydrogen Generation from Hydrolysis of Ammonia Borane. Int. J. Hydrogen Energy 2024, 60, 451–457. [Google Scholar] [CrossRef]
- Jin, S.; Li, Y.; Yang, Y.; Zhang, W. Structured RuNiFeCo Multicomponent Alloys with Exceptional Catalytic Activity for Ammonia Borane Hydrolytic Dehydrogenation. Mater. Today Nano 2024, 26, 100485. [Google Scholar] [CrossRef]
- Li, J.; Feng, Y.; Li, X.; Zhang, T.; Liu, X.; Wang, N.; Sun, Q. Sub-2 Nm Ternary Metallic Alloy Encapsulated within Montmorillonite Interlayers for Efficient Hydrogen Generation from Ammonia Borane Hydrolysis. ACS Catal. 2024, 14, 14665–14677. [Google Scholar] [CrossRef]
- Liang, Z.; Xiao, X.; Yu, X.; Huang, X.; Jiang, Y.; Fan, X.; Chen, L. Non-Noble Trimetallic Cu-Ni-Co Nanoparticles Supported on Metal-Organic Frameworks as Highly Efficient Catalysts for Hydrolysis of Ammonia Borane. J. Alloys Compd. 2018, 741, 501–508. [Google Scholar] [CrossRef]
- Pan, C.-J.; Tsai, M.-C.; Su, W.-N.; Rick, J.; Akalework, N.G.; Agegnehu, A.K.; Cheng, S.-Y.; Hwang, B.-J. Tuning/Exploiting Strong Metal-Support Interaction (SMSI) in Heterogeneous Catalysis. J. Taiwan Inst. Chem. Eng. 2017, 74, 154–186. [Google Scholar] [CrossRef]
- Li, M.; Zhang, S.; Zhao, J.; Wang, H. Maximizing Metal-Support Interactions in Pt/Co3O4 Nanocages to Simultaneously Boost Hydrogen Production Activity and Durability. ACS Appl. Mater. Interfaces 2021, 13, 57362–57371. [Google Scholar] [CrossRef]
- Yang, G.; Guan, S.; Mehdi, S.; Fan, Y.; Liu, B.; Li, B. Co-CoOx Supported onto TiO2 Coated with Carbon as a Catalyst for Efficient and Stable Hydrogen Generation from Ammonia Borane. Green Energy Environ. 2021, 6, 236–243. [Google Scholar] [CrossRef]
- Akbayrak, S.; Özkar, S. Palladium Nanoparticles Supported on Cobalt(II,III) Oxide Nanocatalyst: High Reusability and Outstanding Catalytic Activity in Hydrolytic Dehydrogenation of Ammonia Borane. J. Colloid Interface Sci. 2022, 626, 752–758. [Google Scholar] [CrossRef]
- Wang, H.-Z.; Shao, Y.-X.; Feng, Y.-F.; Tan, Y.-J.; Liao, Q.-Y.; Chen, X.-D.; Zhang, X.-F.; Guo, Z.-H.; Li, H. Heterostructured Co3O4–SnO2 Composites Containing Oxygen Vacancy with High Activity and Recyclability toward NH3BH3 Dehydrogenation. Rare Met. 2023, 42, 3013–3023. [Google Scholar] [CrossRef]
- Shen, R.; Liu, Y.; Wen, H.; Wu, X.; Peng, Z.; Mehdi, S.; Liu, T.; Zhang, H.; Guan, S.; Liang, E.; et al. Engineering Vacancy-Atom Ensembles to Boost Catalytic Activity toward Hydrogen Evolution. ENERGY Environ. Mater. 2023, 6, e12292. [Google Scholar] [CrossRef]
- Patra, D.; Garg, R.; Gautam, U.K.; Gopalan, B. Mitigation of Polyborate Precipitation on Pd/Fe2O3 Sites during Ammonia Borane Hydrolysis: An Alternate Insight into the Role of Oxygen Vacancies. Int. J. Hydrogen Energy 2023, 48, 28333–28342. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, H.; Li, Y.; Qin, L.; Hu, Z.; Sheng, M.; Gan, C.; Huang, Y. Cube CoCu-ZIF Derived Ru/Co2.28Cu0.72O4/C7.5 for Superb H2 Production: Morphology and Steam Oxidation Induced Charge Transfer. J. Alloys Compd. 2024, 981, 173729. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, C.; Yang, S.; Luo, Y.; Ai, L.; Jiang, J. Co-Vacancy Rich Co3O4 Catalyst Enables Efficient Hydrogen Generation from the Hydrolysis of Ammonia Borane. Inorg. Chem. Commun. 2022, 146, 110178. [Google Scholar] [CrossRef]
- Shen, R.; Liu, Y.; Wen, H.; Liu, T.; Peng, Z.; Wu, X.; Ge, X.; Mehdi, S.; Cao, H.; Liang, E.; et al. Engineering VO-Ti Ensemble to Boost the Activity of Ru towards Water Dissociation for Catalytic Hydrogen Generation. Appl. Catal. B Environ. 2022, 306, 121100. [Google Scholar] [CrossRef]
- Shen, R.; Liu, Y.; Liu, S.; Guan, S.; Zhang, H.; Mehdi, S.; Ashraf, S.; Xiao, T.-H.; Liang, E.; Jiang, J.; et al. Oxygen Vacancy Promoting Artificial Atom (RuPd) by d-Orbital Coupling for Efficient Water Dissociation. Nano Res. 2024, 17, 7045–7052. [Google Scholar] [CrossRef]
- Guan, S.; An, L.; Ashraf, S.; Zhang, L.; Liu, B.; Fan, Y.; Li, B. Oxygen Vacancy Excites Co3O4 Nanocrystals Embedded into Carbon Nitride for Accelerated Hydrogen Generation. Appl. Catal. B Environ. 2020, 269, 118775. [Google Scholar] [CrossRef]
- Liu, J.; Li, M.; Zhang, W.; Li, X.; Zhao, Y.; Zhu, C.; Li, S. Controllable Preparation of Co-Based Catalysts Doped with Cu and Mo for Boosting Hydrogen Evolution. J. Alloys Compd. 2024, 1003, 175629. [Google Scholar] [CrossRef]
- Taşçı, E.; Akbayrak, S.; Özkar, S. Ruthenium(0) Nanoparticles Supported on Silica Coated Fe3O4 as Magnetically Separable Catalysts for Hydrolytic Dehydrogenation of Ammonia Borane. Int. J. Hydrogen Energy 2018, 43, 15124–15134. [Google Scholar] [CrossRef]
- Manna, J.; Akbayrak, S.; Özkar, S. Nickel(0) Nanoparticles Supported on Bare or Coated Cobalt Ferrite as Highly Active, Magnetically Isolable and Reusable Catalyst for Hydrolytic Dehydrogenation of Ammonia Borane. J. Colloid Interface Sci. 2017, 508, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Gil-San-Millan, R.; Grau-Atienza, A.; Johnson, D.T.; Rico-Francés, S.; Serrano, E.; Linares, N.; García-Martínez, J. Improving Hydrogen Production from the Hydrolysis of Ammonia Borane by Using Multifunctional Catalysts. Int. J. Hydrogen Energy 2018, 43, 17100–17111. [Google Scholar] [CrossRef]
- Zhou, S.; Yang, Y.; Yin, P.; Ren, Z.; Wang, L.; Wei, M. Metal-Support Synergistic Catalysis in Pt/MoO3-x Nanorods toward Ammonia Borane Hydrolysis with Efficient Hydrogen Generation. ACS Appl. Mater. Interfaces 2022, 14, 5275–5286. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, G.; Zhang, J.; Mao, B.; Wei, X.; Song, K. Porous Silica Supported Ag Core-Pd Shell Composite: Seed-Mediated Stepwise Reduction and Tunable Dispersion for Boosting Catalytic Hydrogen Evolution. Fuel 2024, 355, 129473. [Google Scholar] [CrossRef]
- Komova, O.V.; Simagina, V.I.; Pochtar, A.A.; Bulavchenko, O.A.; Ishchenko, A.V.; Odegova, G.V.; Gorlova, A.M.; Ozerova, A.M.; Lipatnikova, I.L.; Tayban, E.S.; et al. Catalytic Behavior of Iron-Containing Cubic Spinel in the Hydrolysis and Hydrothermolysis of Ammonia Borane. Materials 2021, 14, 5422. [Google Scholar] [CrossRef]
- Tonbul, Y.; Akbayrak, S.; Özkar, S. Magnetically Separable Rhodium Nanoparticles as Catalysts for Releasing Hydrogen from the Hydrolysis of Ammonia Borane. J. Colloid Interface Sci. 2019, 553, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, A.; Yang, Y.; Fan, G. Oxygen Vacancy-Engaged Interfacial Charge Transfer Modulation for Upgrade Rh-Catalyzed Hydrogen and Oxygen Productions. Int. J. Hydrogen Energy 2023, 48, 23540–23549. [Google Scholar] [CrossRef]
- Ding, R.; Chen, Q.; Luo, Q.; Zhou, L.; Wang, Y.; Zhang, Y.; Fan, G. Salt Template-Assisted in Situ Construction of Ru Nanoclusters and Porous Carbon: Excellent Catalysts toward Hydrogen Evolution, Ammonia- Borane Hydrolysis, and 4-Nitrophenol Reduction. Green Chem. 2020, 22, 835–842. [Google Scholar] [CrossRef]
- Wu, H.; Liu, L.; Liu, X.; Bian, L.; Chen, Y.; Fan, Y.; Liu, B. In Situ Construction of Co–Mo2C on N-Doped Carbon for Efficient Hydrogen Evolution from Ammonia Borane Hydrolysis. Int. J. Hydrogen Energy 2025, 100, 330–340. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Q.; Bian, L.; Peng, Q.; Han, S.; Liu, B.; Fan, Y. Alloy-Exciting Effect of Palladium-Rhodium on MXene for Enhanced Hydrogen Generation. Int. J. Hydrogen Energy 2024, 49, 1226–1235. [Google Scholar] [CrossRef]
- Ye, M.; Wu, J.; Fan, G. Hierarchical Porous Cobalt/Carbon Hybrid Anchored Ru-Catalyzed Ammonia-Borane Hydrolysis for Efficient H2 Release. Fuel 2022, 321, 123982. [Google Scholar] [CrossRef]
- Yao, S.; Xu, L.; Qin, H.; Ding, X.; Zhao, S.; Ma, Y.; Cui, M.; Lv, Q.; Han, J.; Song, F. Two-Dimensional Titanium Carbide-Supported Ultrafine Non-Noble Bimetallic Nanocatalysts for Remarkable Hydrolytic Evolution from Ammonia Borane. New J. Chem. 2024, 43, 18437–18442. [Google Scholar] [CrossRef]
- Slot, T.K.; Oulego, P.; Sofer, Z.; Bai, Y.; Rothenberg, G.; Raveendran Shiju, N. Ruthenium on Alkali-Exfoliated Ti3(Al0.8Sn0.2)C2 MAX Phase Catalyses Reduction of 4-Nitroaniline with Ammonia Borane. ChemCatChem 2021, 13, 3470–3478. [Google Scholar] [CrossRef]
- Karataş, Y.; Çetin, T.; Akkuş, İ.N.; Akinay, Y.; Gülcan, M. Rh(0) Nanoparticles Impregnated on Two-Dimensional Transition Metal Carbides, MXene, as an Effective Nanocatalyst for Ammonia-Borane Hydrolysis. Int. J. Energy Res. 2022, 46, 11411–11423. [Google Scholar] [CrossRef]
- Qin, H.; Tang, S.; Xu, L.; Li, A.; Lv, Q.; Dong, J.; Liu, L.; Ding, X.; Pan, X.; Yang, X.; et al. Alkaline titanium carbide (MXene) engineering ultrafine non-noble nanocatalysts toward remarkably boosting hydrogen evolution from ammonia borane hydrolysis. J. Alloys Compd. 2025, 1010, 177644. [Google Scholar] [CrossRef]
- Mo, B.; Li, S.; Wen, H.; Zhang, H.; Zhang, H.; Wu, J.; Li, B.; Hou, H. Functional Group Regulated Ni/Ti3C2Tx (Tx = F, −OH) Holding Bimolecular Activation Tunnel for Enhanced Ammonia Borane Hydrolysis. ACS Appl. Mater. Interfaces 2022, 14, 16320–16329. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Liang, L.; Fan, Y.; Liu, X.; Liang, F.; Peng, Q.; Han, S.; Liu, L.; Liu, B. V-Doped Activated Ru/Ti2.5V0.5C2 Dual-Active Center Accelerate Hydrogen Production from Ammonia Borane. J. Colloid Interface Sci. 2024, 671, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Sun, Q.; Zhang, T.; Mayoral, A.; Li, L.; Zhou, X.; Xu, J.; Zhang, P.; Yu, J. Impregnating Subnanometer Metallic Nanocatalysts into Self-Pillared Zeolite Nanosheets. J. Am. Chem. Soc. 2021, 143, 6905–6914. [Google Scholar] [CrossRef]
- Wei, Y.-W.; Yang, G.; Xu, X.-X.; Liu, Y.-Y.; Kang, N.-X.; Li, B.-J.; Wang, Y.-Z.; Zhao, Y.-X. Ultrafine Ru Nanoparticles Anchored on Core–Shell Structured Zeolite-Carbon for Efficient Catalysis of Hydrogen Generation. Rare Met. 2023, 42, 2324–2334. [Google Scholar] [CrossRef]
- Xu, W.; Li, W.; Wen, H.; Ding, J.; Liu, Y.; Li, W.; Li, B. Metal/Metal-Organic Framework Interfacial Ensemble-Induced Dual Site Catalysis towards Hydrogen Generation. Appl. Catal. B Environ. 2021, 286, 119946. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Y.; Wang, H.; Zhang, S. Donor-Acceptor Covalent Organic Frameworks-Confined Ultrafine Bimetallic Pt-Based Nanoclusters for Enhanced Photocatalytic H2 Generation. Nano Res. 2024, 17, 5835–5844. [Google Scholar] [CrossRef]
- Tang, S.; Xu, L.; Ding, X.; Lv, Q.; Qin, H.; Li, A.; Yang, X.; Han, J.; Song, F. Electronic Engineering Induced Ultrafine Non-Noble Nanoparticles for High-Performance Hydrogen Evolution from Ammonia Borane Hydrolysis. Fuel 2025, 381, 133424. [Google Scholar] [CrossRef]
- Chen, X.D.; Luo, X.L.; Zhang, X.F.; Wang, H.Z.; Li, Y.C.; Ye, L.F.; Zheng, J.H.; Li, H. Regulation of Electronic Structures of the Urchin-Like NiCoP/CoP Nanocatalysts for Fast Hydrogen Evolution. Chem. A Eur. J. 2024, 30, e202304266. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, X.; Zhang, X.; Wang, Z.; Yu, R. A MOF-Derived CuCo(O)@ Carbon–Nitrogen Framework as an Efficient Synergistic Catalyst for the Hydrolysis of Ammonia Borane. Inorg. Chem. Front. 2020, 7, 2043–2049. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, L.; Gao, X.; Zhao, Y. Constructing Ultrafine Monodispersed Co2P/(0.59-Cu3P) on Cu Doped CoZn-ZIF Derived Porous N-Doped Carbon for Highly Efficient Dehydrogenation of Ammonia Borane. Nano Res. 2023, 16, 6687–6700. [Google Scholar] [CrossRef]
- Yang, X.; Wei, J.; Wang, Q.; Shuai, M.; Yue, G.; Li, P.; Huang, D.; Astruc, D.; Zhao, P. Pd–Ru Nanocatalysts Derived from a Pd-Induced Aerogel for Dramatic Boosting of Hydrogen Release. Nanoscale 2020, 12, 2345–2349. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Xia, H.; Yang, W.; Liu, M.; Liu, X.; Xiang, J.; Qayum, A.; Hu, L.; Duan, L.; Gao, W.; et al. Remarkably Boosting Ru-Ni Nanocatalysis via Surface/Interface Regulation for Efficient Hydrolytic Dehydrogenation of Ammonia Borane. Fuel 2022, 307, 121800. [Google Scholar] [CrossRef]
- Yan, H.; Lin, Y.; Wu, H.; Zhang, W.; Sun, Z.; Cheng, H.; Liu, W.; Wang, C.; Li, J.; Huang, X.; et al. Bottom-up Precise Synthesis of Stable Platinum Dimers on Graphene. Nat. Commun. 2017, 8, 1070. [Google Scholar] [CrossRef]
- Li, H.J.; Yan, Y.F.; Feng, S.; Chen, Y.R.; Li, L.X.; Zhang, L.; Yang, Z.Q. Hydrogen release mechanism and performance of ammonia borane catalyzed by transition metal catalysts Cu-Co/MgO(100). Int. J. Energy Res. 2018, 43, 921–930. [Google Scholar] [CrossRef]
- Wan, C.; Liu, X.; Wang, J.; Chen, F.; Cheng, D.-G. Heterostructuring 2D Co2P Nanosheets with 0D CoP via a Salt-Assisted Strategy for Boosting Hydrogen Evolution from Ammonia Borane Hydrolysis. Nano Res. 2023, 16, 6260–6269. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, K.; Ashraf, S.; Fan, Y.; Guan, S.; Wu, X.; Liu, Y.; Liu, B.; Li, B. Polar O–Co–P Surface for Bimolecular Activation in Catalytic Hydrogen Generation. Energy Environ. Mater. 2023, 6, e12273. [Google Scholar] [CrossRef]
- Bhunya, S.; Malakar, T.; Ganguly, G.; Paul, A. Combining Protons and Hydrides by Homogeneous Catalysis for Controlling the Release of Hydrogen from Ammonia–Borane: Present Status and Challenges. ACS Catal. 2016, 6, 7907–7934. [Google Scholar] [CrossRef]
- Lu, Z.; Schweighauser, L.; Hausmann, H.; Wegner, H.A. Metal-Free Ammonia-Borane Dehydrogenation Catalyzed by a Bis(Borane) Lewis Acid. Angew. Chem. Int. Ed. 2015, 54, 15556–15559. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Wang, J.; Xu, Y.; Xu, R.; Li, W. Thermo-Controllable Dehydrogenation of Ammonia Borane by Luminescent and Thermo-Responsive Catalysts Based on SiO2@Pt@PABI-Tb@PNIPAM. Appl. Catal. Gen. 2020, 594, 117463. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, J.; Xu, R.; Li, W. Smart Nanocatalyst for Ammonia-Borane Hydrolysis: Thermo-Controlled Hydrogen Generation. Int. J. Hydrogen Energy 2021, 46, 14322–14330. [Google Scholar] [CrossRef]
- Wang, C.; Tuninetti, J.; Wang, Z.; Zhang, C.; Salmon, L.; Moya, S.; Ruiz, J.; Astruc, D. Hydrolysis of Ammonia-Borane over Ni/ZIF-8 Nanocatalyst: High Efficiency, Mechanism and Controlled Hydrogen Release. J. Am. Chem. Soc. 2017, 139, 11610–11615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, J.; Yang, L.; Li, R.; Zhang, F.; Dong, H. Efficient Hydrogen Production from Ammonia Borane Hydrolysis Catalyzed by TiO2-Supported RuCo Catalysts. Int. J. Hydrogen Energy 2021, 46, 3964–3973. [Google Scholar] [CrossRef]
- Jia, H.; Liu, S.; Zheng, G.-P.; Zheng, X.-C.; Wang, X.-Y.; Liu, P. Collagen-Graphene Oxide Magnetic Hybrids Anchoring Pd(0) Catalysts for Efficient H2 Generation from Ammonia Borane. Int. J. Hydrogen Energy 2019, 44, 27022–27029. [Google Scholar] [CrossRef]
- Akbayrak, S.; Tonbul, Y.; Özkar, S. Magnetically Separable Rh0/Co3O4 Nanocatalyst Provides over a Million Turnovers in Hydrogen Release from Ammonia Borane. ACS Sustain. Chem. Eng. 2020, 8, 4216–4224. [Google Scholar] [CrossRef]
- Zhou, J.; Feng, X.; Zhao, Y.; Cui, R.; Wang, D.; Zhang, B. Noble-Metal-Free CuNi/Co3O4 Hybrid Nanosheets as Efficient and Magnetically Recyclable Catalysts for Hydrolysis of Ammonia Borane. J. Alloys Compd. 2022, 923, 166345. [Google Scholar] [CrossRef]
- Asim, M.; Zhang, S.; Wang, Y.; Maryam, B.; Sajid, M.; Shi, C.; Pan, L.; Zhang, X.; Zou, J.-J. Self-Supporting NiCoP for Hydrogen Generation via Hydrolysis of Ammonia Borane. Fuel 2022, 318, 123544. [Google Scholar] [CrossRef]
- Luo, Y.; Tian, Y.; Yang, S.; Jiang, J.; Liu, A.; Gao, H.; Ai, L. Coupling Ultralow-Content Ruthenium with Nickel Hydroxide via Corrosion Engineering for Highly Efficient Hydrogen Generation from Ammonia Borane. Int. J. Hydrogen Energy 2022, 47, 35184–35194. [Google Scholar] [CrossRef]
- Xu, F.; Su, Y.; Cao, Y.; Wu, J.; Guo, W.; Sun, J.; Zheng, X.; Zheng, G. Achieving an Enhancement in Hydrolytic Dehydrogenation of Ammonia Borane Using Nano-Flower CoFeNiP Alloy Catalysts Regulated from Amorphous Nanoparticle Electroplating on Cu Foams. Int. J. Hydrogen Energy 2024, 93, 100–107. [Google Scholar] [CrossRef]
- Cui, L.; Xu, Y.H.; Liu, J.Q. Monolithically integrated CoP nanowire array: An on/off switch for effective on-demand hydrogen generation via hydrolysis of NaBH4 and NH3BH3. Nano Res. 2017, 10, 595–604. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, K.; Zhang, D.; Li, G.; Meng, W.; Wang, D.; Cao, Z.; Zhang, K.; Wu, S. Co–Mo–B Nanoparticles Supported on Carbon Cloth as Effective Catalysts for the Hydrolysis of Ammonia Borane. Int. J. Hydrogen Energy 2020, 45, 14418–14427. [Google Scholar] [CrossRef]
- Zhang, H.; Gu, X.; Song, J. Co, Ni-Based Nanoparticles Supported on Graphitic Carbon Nitride Nanosheets as Catalysts for Hydrogen Generation from the Hydrolysis of Ammonia Borane under Broad-Spectrum Light Irradiation. Int. J. Hydrogen Energy 2020, 45, 21273–21286. [Google Scholar] [CrossRef]
- Chen, W.; Lv, G.; Fu, J.; Ren, H.; Shen, J.; Cao, J.; Liu, X. Demonstration of Controlled Hydrogen Release Using Rh@GQDs during Hydrolysis of NH3BH3. ACS Appl. Mater. Interfaces 2021, 13, 50017–50026. [Google Scholar] [CrossRef]
- Mi, A.; Guo, L.; Yan, Y.; Wang, D.; Shang, H.; Zhao, Y.; Zhang, B. A Solid-System Strategy for Controlled Hydrolytic Release of Hydrogen by Encapsulation of Ammonia Borane in Cobalt Decorated Halloysite Aerogel. ACS Sustain. Chem. Eng. 2024, 12, 5716–5725. [Google Scholar] [CrossRef]
- Rong, Q.; Huang, W.; Xu, F.; Wang, Y.; Wang, C.; Liu, X. “On-off”Controlled H2 Evolution and O2 Evolution upon NH3BH3 Hydrolysis resp·H2O2 Decomposition on Pt/ZIF-67. Fuel 2023, 341, 127721. [Google Scholar] [CrossRef]
- Zhang, C.; Zuo, W.; Ai, L.; Tu, S.; Jiang, J. Two-Dimensional Molybdenum Boride Coordinating with Ruthenium Nanoparticles to Boost Hydrogen Generation from Hydrolytic Dehydrogenation of Ammonia Borane. J. Colloid Interface Sci. 2024, 669, 794–803. [Google Scholar] [CrossRef] [PubMed]
- Kamegawa, T.; Nakaue, T. Complete Hydrogen Release from Aqueous Ammonia-Borane over a Platinum-Loaded Titanium Dioxide Photocatalyst. Chem. Commun. 2015, 51, 16802–16805. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yao, Z.; Zhu, Y.; Wang, X. The Effect of Solvent Properties on the Hydrogen Producing Performance of Catalytic Liquid Phase Ammonia Borane. Fuel 2022, 325, 124849. [Google Scholar] [CrossRef]
- Majumder, D.; Koley, S.; Barik, A.; Ruz, P.; Banerjee, S.; Viswanadh, B.; Barooah, N.; Tripathi, V.S.; Sudarsan, V.; Kumar, A.; et al. Dual Catalytic Activity of a Cucurbit [7]Uril-Functionalized Metal Alloy Nanocomposite for Sustained Hydrogen Generation: Hydrolysis of Ammonia Borane and Electrocatalysts for the Hydrogen Evolution Reaction. Nanoscale 2024, 16, 10801–10811. [Google Scholar] [CrossRef]
- Shingole, M.; Banerjee, S.; Ruz, P.; Kolay, S.; Kumar, A.; Sudarsan, V. Catalytic Hydrogen Generation through Ammonia Borane Hydrolysis Using Metal–Organic Framework via O–H Bond Activation. Energy Fuels 2024, 38, 8968–8978. [Google Scholar] [CrossRef]
- Nakagawa, T.; Uesato, H.; Burrell, A.K.; Ichikawa, T.; Miyaoka, H.; Davis, B.L.; Kojima, Y. Surface-Controlled Conversion of Ammonia Borane from Boron Nitride. Energies 2020, 13, 5569. [Google Scholar] [CrossRef]
- Moussa, G.; Moury, R.; Demirci, U.B.; Miele, P. Borates in Hydrolysis of Ammonia Borane. Int. J. Hydrogen Energy 2013, 38, 7888–7895. [Google Scholar] [CrossRef]
- Liu, C.-H.; Wu, Y.-C.; Chou, C.-C.; Chen, B.-H.; Hsueh, C.-L.; Ku, J.-R.; Tsau, F. Hydrogen Generated from Hydrolysis of Ammonia Borane Using Cobalt and Ruthenium Based Catalysts. Int. J. Hydrogen Energy 2012, 37, 2950–2959. [Google Scholar] [CrossRef]
- Chen, W.; Li, D.; Wang, Z.; Qian, G.; Sui, Z.; Duan, X.; Zhou, X.; Yeboah, I.; Chen, D. Reaction Mechanism and Kinetics for Hydrolytic Dehydrogenation of Ammonia Borane on a Pt/CNT Catalyst. AIChE J. 2017, 63, 60–65. [Google Scholar] [CrossRef]
- Rachiero, G.P.; Demirci, U.B.; Miele, P. Bimetallic RuCo and RuCu Catalysts Supported on γ-Al2O3. A Comparative Study of Their Activity in Hydrolysis of Ammonia-Borane. Int. J. Hydrogen Energy 2011, 36, 7051–7065. [Google Scholar] [CrossRef]
- Demirci, U.B. Ammonia Borane: An Extensively Studied, Though Not Yet Implemented, Hydrogen Carrier. Energies 2020, 13, 3071. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Gagare, P.D. Preparation of Ammonia Borane in High Yield and Purity, Methanolysis, and Regeneration. Inorg. Chem. 2007, 46, 7810–7817. [Google Scholar] [CrossRef] [PubMed]
- Valero-Pedraza, M.-J.; Alligier, D.; Petit, E.; Cot, D.; Granier, D.; Adil, K.; Yot, P.G.; Demirci, U.B. Diammonium Tetraborate Dihydrate as Hydrolytic By-Product of Ammonia Borane in Aqueous Alkaline Conditions. Int. J. Hydrogen Energy 2020, 45, 9927–9935. [Google Scholar] [CrossRef]
- Hausdorf, S. A Procedure for the Regeneration of Ammonia Borane from BNH-Waste Products. Int. J. Hydrogen Energy 2008, 33, 608–614. [Google Scholar] [CrossRef]
- Reller, C.; Mertens, F.O.R.L. A Self-Contained Regeneration Scheme for Spent Ammonia Borane Based on the Catalytic Hydrodechlorination of BCl3. Angew. Chem. Int. Ed. 2012, 51, 11731–11735. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Raju, B.C.; Gagare, P.D. One-Pot Synthesis of Ammonia–Borane and Trialkylamine–Boranes from Trimethyl Borate. Org. Lett. 2012, 14, 6119–6121. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.V.; Kulkarni, A.S. Water-Promoted, Safe and Scalable Preparation of Ammonia Borane. Int. J. Hydrogen Energy 2017, 42, 1451–1455. [Google Scholar] [CrossRef]
- Ramachandran, P.V.; Kulkarni, A.S. The Role of Ammonia in Promoting Ammonia Borane Synthesis. Dalton Trans. 2016, 45, 16433–16440. [Google Scholar] [CrossRef]
- Davis, B.L.; Rekken, B.D.; Michalczyk, R.; Garner, E.B., III; Dixon, D.A.; Kalviri, H.; Baker, R.T.; Thorn, D.L. Lewis Base Assisted B–H Bond Redistribution in Borazine and Polyborazylene. Chem. Commun. 2013, 49, 9095. [Google Scholar] [CrossRef]
- Nagyházi, M.; Turczel, G.; Anastas, P.T.; Tuba, R. Highly Efficient Ammonia Borane Hydrolytic Dehydrogenation in Neat Water Using Phase-Labeled CAAC-Ru Catalysts. ACS Sustain. Chem. Eng. 2020, 8, 16097–16103. [Google Scholar] [CrossRef]
- Diwan, M.; Diakov, V.; Shafirovich, E.; Varma, A. Noncatalytic Hydrothermolysis of Ammonia Borane. Int. J. Hydrogen Energy 2008, 33, 1135–1141. [Google Scholar] [CrossRef]
- Hwang, H.T.; Al-Kukhun, A.; Varma, A. High and Rapid Hydrogen Release from Thermolysis of Ammonia Borane near PEM Fuel Cell Operating Temperatures: Effect of Quartz Wool. Int. J. Hydrogen Energy 2012, 37, 6764–6770. [Google Scholar] [CrossRef]
- Komova, O.V.; Kayl, N.L.; Odegova, G.V.; Netskina, O.V.; Simagina, V.I. Destabilization of NH3BH3 by Water during Hydrothermolysis as a Key Factor in the High Hydrogen Evolution Rates. Int. J. Hydrogen Energy 2016, 41, 17484–17495. [Google Scholar] [CrossRef]
- Pei, P.; Cannon, M.; Quan, G.; Kjeang, E. Effective Hydrogen Release from Ammonia Borane and Sodium Borohydride Mixture through Homopolar Based Dehydrocoupling Driven by Intermolecular Interaction and Restrained Water Supply. J. Mater. Chem. A 2020, 8, 19050–19056. [Google Scholar] [CrossRef]
- Hwang, H.T.; Varma, A. Effect of Boric Acid on Thermal Dehydrogenation of Ammonia Borane: Mechanistic Studies. Int. J. Hydrogen Energy 2013, 38, 1925–1931. [Google Scholar] [CrossRef]
- Gorlova, A.M. Fast Hydrogen Generation from Solid NH3BH3 under Moderate Heating and Supplying a Limited Quantity of CoCl2 or NiCl2 Solution. Renew. Energy 2018, 121, 722–729. [Google Scholar] [CrossRef]
- Komova, O.V.; Odegova, G.V.; Gorlova, A.M.; Bulavchenko, O.A.; Pochtar, A.A.; Netskina, O.V.; Simagina, V.I. Copper–iron mixed oxide catalyst precursors prepared by glycine-nitrate combustion method for ammonia borane dehydrogenation processes. Int. J. Hydrogen Energy 2019, 44, 24277–24291. [Google Scholar] [CrossRef]
- Gorlova, A.M.; Komova, O.V.; Netskina, O.V.; Bulavchenko, O.A.; Lipatnikova, I.L.; Simagina, V.I. Hydrogen for Fuel Cells: Effect of Copper and Iron Oxides on the Catalytic Hydrolysis and Hydrothermolysis of Ammonia Borane. Russ. J. Electrochem. 2020, 56, 170–173. [Google Scholar] [CrossRef]
- Coşkuner, Ö.; Kantürk Figen, A. Hydro-Catalytic Treatment of Organoamine Boranes for Efficient Thermal Dehydrogenation for Hydrogen Production. Int. J. Hydrogen Energy 2021, 46, 35641–35652. [Google Scholar] [CrossRef]
- Simagina, V.I.; Komova, O.V.; Ozerova, A.M.; Netskina, O.V.; Odegova, G.V.; Kayl, N.L.; Filippov, T.N. TiO2-Based Photocatalysts for Controllable Hydrogen Evolution from Ammonia Borane. Catal. Today 2021, 379, 149–158. [Google Scholar] [CrossRef]
- Wolstenholme, D.J.; Traboulsee, K.T.; Hua, Y.; Calhoun, L.A.; McGrady, G.S. Thermal Desorption of Hydrogen from Ammonia Borane: Unexpected Role of Homopolar B-H•••H-B Interactions. Chem. Commun. 2012, 48, 2597. [Google Scholar] [CrossRef]
- Hua, T.Q.; Ahluwalia, R.K. Off-Board Regeneration of Ammonia Borane for Use as a Hydrogen Carrier for Automotive Fuel Cells. Int. J. Hydrogen Energy 2012, 37, 14382–14392. [Google Scholar] [CrossRef]
- Summerscales, O.T.; Gordon, J.C. Regeneration of Ammonia Borane from Spent Fuel Materials. Dalton Trans. 2013, 42, 10075. [Google Scholar] [CrossRef]
- Sutton, A.D.; Burrell, A.K.; Dixon, D.A.; Garner III, E.B.; Gordon, J.C.; Nakagawa, T.; Ott, K.C.; Robinson, J.P.; Vasiliu, M. Sutton et al. Regeneration of Ammonia Borane Spent Fuel by Direct Reaction with Hydrazine and Liquid Ammonia. Science 2011, 331, 1426–1429. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.D.; Davis, B.L.; Bhattacharyya, K.X.; Ellis, B.D.; Gordon, J.C.; Power, P.P. Recycle of Tin Thiolate Compounds Relevant to Ammonia–Boraneregeneration. Chem. Commun. 2010, 46, 148–149. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-L.; Tripathi, A.K.; Chan, H.; Chen, S.-T.; Chang, J.-T.; Nakagawa, T.; Wang, C.-Y. Recyclable Dehydrogenation/Regeneration of Ammonia Borane Nanoconfined in Amino-Functionalized ZIF-8 with 3-Amino-1,2,4-Triazole. ACS Sustainable Chem. Eng. 2023, 11, 6143–6152. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, L.; Chen, X.; Yu, X. Reductive Dechlorination of BCl3 for Efficient Ammonia Borane Regeneration. Dalton Trans. 2015, 44, 753–757. [Google Scholar] [CrossRef] [PubMed]

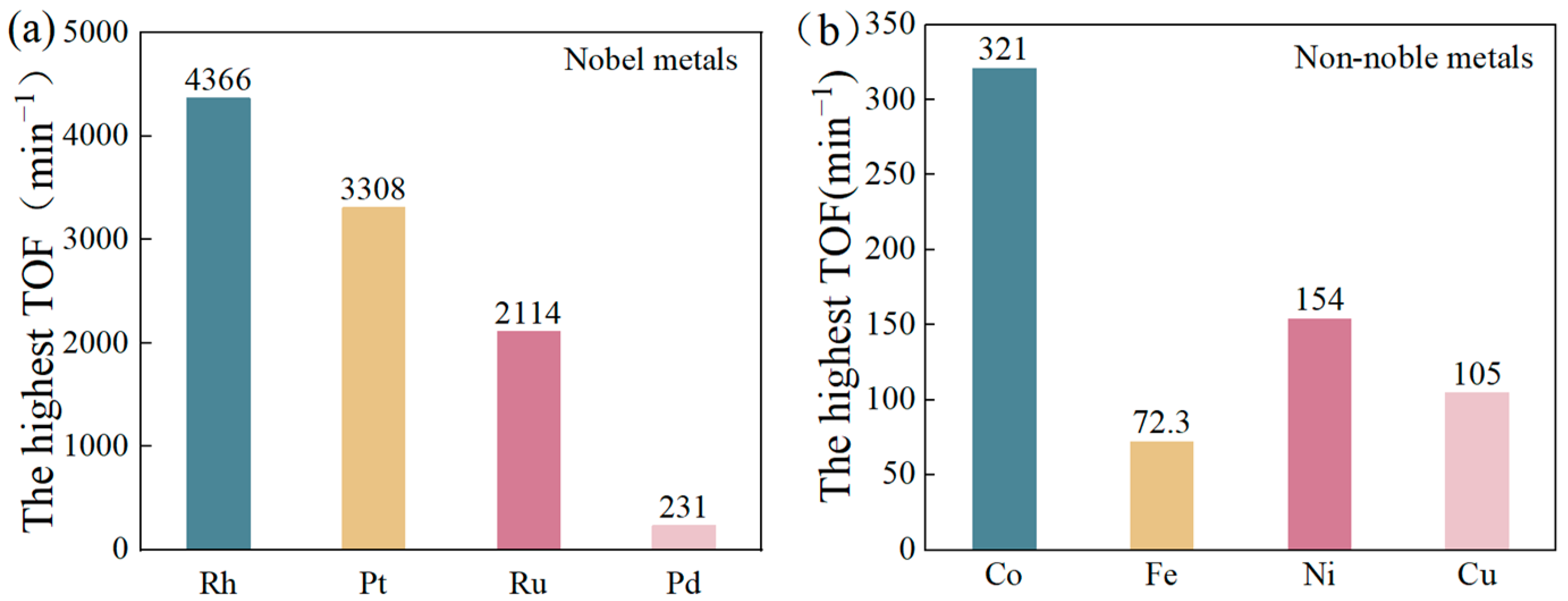




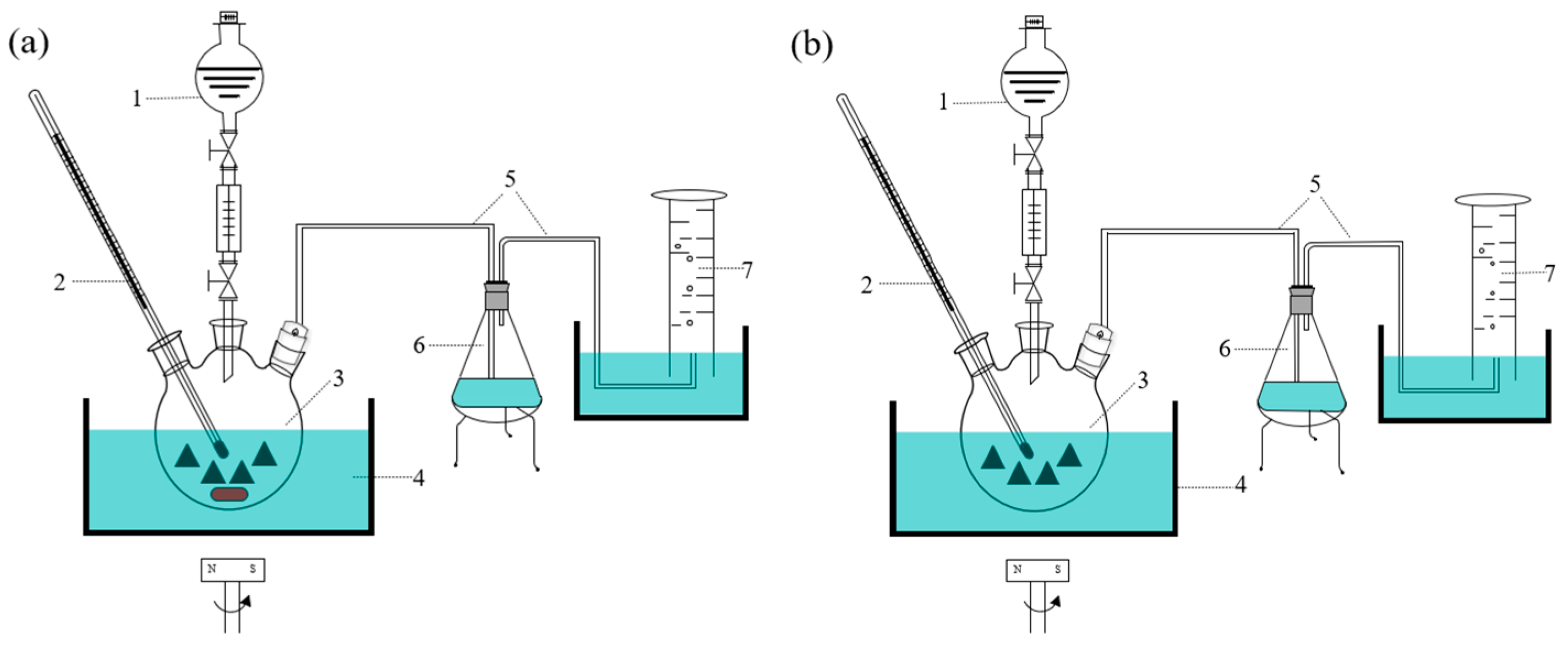




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q.; Ran, W.; Bao, W.; Li, Y. A Review on Catalytic Hydrolysis of Ammonia Borane for Hydrogen Production. Energies 2025, 18, 1105. https://doi.org/10.3390/en18051105
Liu Q, Ran W, Bao W, Li Y. A Review on Catalytic Hydrolysis of Ammonia Borane for Hydrogen Production. Energies. 2025; 18(5):1105. https://doi.org/10.3390/en18051105
Chicago/Turabian StyleLiu, Qingqing, Weizhao Ran, Wenfei Bao, and Yuzhong Li. 2025. "A Review on Catalytic Hydrolysis of Ammonia Borane for Hydrogen Production" Energies 18, no. 5: 1105. https://doi.org/10.3390/en18051105
APA StyleLiu, Q., Ran, W., Bao, W., & Li, Y. (2025). A Review on Catalytic Hydrolysis of Ammonia Borane for Hydrogen Production. Energies, 18(5), 1105. https://doi.org/10.3390/en18051105






