Abstract
Lipases are essential in many industrial processes. Although microbial lipases are widely used, plant lipases remain more accessible and abundant, particularly in germinated kernels. This study aims to evaluate the catalytic potential of lipase extract powder of germinated rubber kernels in transesterification reaction. Germinated rubber kernels, lipase extract powder of germinated rubber kernels, and crude oils of palm (PKO), Jatropha curcas (JCO), and rubber (RSO) were characterized. The presence of lipase in the plant extract powder was evidenced by FT-IR and SEM-EDX analyses and hydrolysis reaction. Biodiesel was produced from crude rubber oil. The results showed that germinated rubber kernels have high moisture (33.48%), protein (15.75%), and fat (50.11%) contents. The optimum hydrolytic activities of lipase on PKO, JCO, and RSO were 25.67 U/mL, 26.67 U/mL, and 31 U/mL, respectively, at pH 5. Lipase extract concentration, temperature, and storage time influenced the lipase hydrolytic activity. The optimum biodiesel yield (29.63%) was obtained at 30 °C. The addition of co-solvents (water and n-hexane) to the reaction mixture increased yields from 20.47% (without co-solvent) to 31.06% and 21.85%, respectively. These insights show that germinated rubber seeds are rich in oil and contain lipase with good hydrolytic and catalytic activity.
1. Introduction
The valorization of natural resources through sustainable and environmentally friendly processes represents a significant step towards a greener and more sustainable future. The rubber plant (Hevea brasiliensis), belonging to the family Euphorbiaceae, is the tallest latex-bearing plant cultivated and represents the primary source of natural rubber [1]. It is cultivated in tropical regions such as Côte d’Ivoire. The tree consists of leaves, seeds, and the trunk (bark, latex, and wood). The rubber seed is an underutilized by-product, with only a small proportion of it typically employed for seeding purposes [2]. As Africa’s leading rubber producer, Côte d’Ivoire produces 75,000–100,000 tonnes of rubber seed per year. Nevertheless, only 10–15% of the seeds are employed in the production of seedlings, with the majority remaining in the field as agricultural waste, either germinating naturally or rotting [3,4]. The rubber seed is currently of great interest to industrialists, as it represents a promising avenue for promoting the rubber tree as a sustainable alternative resource for biodiesel production. The rubber seed contains a lipid-rich kernel in excess of 50%. Tambunan et al. [5] have demonstrated that the kernel of the rubber seed contains a considerable quantity of oil, representing between 40 and 50% of the seed’s total mass. This makes the rubber seed an attractive source of raw material for the production of biodiesel. Furthermore, the rubber kernel is also a potential source of enzyme (lipase) [6], which enhances the versatility of rubber seed utilization.
Lipases, or triacylglycerol acyl hydrolases (EC 3.1.1.3), are carboxylic ester hydrolases present in all living microbes, animals, and plants. They play an important role in lipid biochemistry [7,8]. Microbial lipases account for over 60% of commercial enzymes, animal 18%, plant 11%, and algal 3% [9]. Lipases are employed in a multitude of applications, including detergents, food, pharmaceuticals, cosmetics, agrochemicals, biotechnologies, and biofuel production [9,10]. The primary physiological function of lipases is the hydrolysis of triglycerides into diglycerides, monoglycerides, fatty acids, and glycerol [8], as shown in the work of Ahmad et al. [11] by studying the hydrolytic activity of lipase from endosperm of rubber seeds (i.e., the ungerminated kernels). However, studies have shown that it is advantageous to use germinated seeds rather than ungerminated seeds. This is because germination increases the biocatalytic activity of lipase. The lipases present in seeds play a crucial role in the growth and development of the embryo, providing it with the energy required for growth and expansion. Consequently, lipases are present in higher concentrations during the germination of seeds [12]. For example, Kouteu [13] demonstrated that the hydrolytic activity of lipase from germinated Jatropha curcas seeds was significantly higher than that of lipase from ungerminated Jatropha curcas seeds. Furthermore, lipases are capable of catalyzing esterification, interesterification, and transesterification reactions in aqueous or non-aqueous media, a property that contributes to their versatility [10,14]. The versatility of the reactions that lipases can catalyze and the diversity of their catalytic properties mean that they have an important role to play in industrial applications [15].
The industrial production of biodiesel by transesterification is generally carried out by chemical catalysis, but enzymatic catalysis is of great interest because lipases have considerable biotechnological potential [16]. In fact, the advantages of lipases have led researchers to pay particular attention to them as biocatalysts. The most studied are microbial lipases. An example of microbial lipase is Candida rugosa, which is used to catalyze the transesterification of vegetable oils [17]. However, these are very expensive. Consequently, plant lipases, which are widely available, easily accessible, and easily extracted from plant organs; can be used under mild reaction conditions; and facilitate glycerol recovery, could be used as a substitute for microbial lipases [18,19]. Plant lipases also have the advantage of being used directly in reactions with or without prior purification [20,21] and are easy to handle, avoiding the need for complex separation processes [22].
Several studies have been carried out on the use of plant lipase as a biocatalyst in biodiesel production [13,23]. However, to our knowledge, transesterification catalyzed by lipase from rubber kernels has not yet been studied. In this work, we are focusing specifically on lipase from germinated rubber kernels and the total use of the kernel (the oil and the lipase powder). Indeed, in order to add value to unused rubber seeds in plantations, the aim of this work is to evaluate the catalytic potential of plant extract powder of germinated rubber kernels containing lipase in the transesterification reaction of crude rubber oil for biodiesel production. The germinated rubber kernel, lipase extract powder of germinated rubber kernel, and crude oils of palm kernel (PKO), Jatropha curcas (JCO), and rubber seed (RSO) were characterized. The presence of lipase in the plant extract powder was evidenced by FT-IR and SEM-EDX analyses and the hydrolysis reaction of PKO, JCO, and RSO. Biodiesel was produced from crude rubber oil.
2. Materials and Methods
2.1. Materials and Chemicals
Lipase extract powder was obtained from germinated rubber seeds. Crude oils of palm kernel, Jatropha curcas, and rubber seeds were extracted. UV-Vis spectrophotometer (JENWAY 6305, Cole-Parmer Ltd., Beacon Road Stone, UK), pH meter (HANNA HI2209, Woonsocket, RI, USA), Fourier-transform infrared spectroscopy (FT-IR, Nicolet 6700 Thermo-Scientific, Madison, WI, USA), scanning electron microscope (SEM, Thermo Scientific Phenom ProX, Eindhoven, The Netherlands), X-ray spectroscopy (EDX, Thermo Fisher Scientific, Eindhoven, The Netherlands), Thin layer chromatography (TLC, MACHEREY-NAGEL GmbH & Co KG, Germany), gas chromatography with flame ionization detector (GC-FID, Varian 450-GC, USA), sodium di-hydrogen phosphate/di-sodium hydrogen phosphate (99%), bovine serum albumin (99%), n-hexane (99%), ethanol (99%), acetone (99%), Arabic gum, and phenolphthalein (99%) from Sigma-Aldrich were used in the experiments. The experiments were carried out in triplicate, and the results were processed by statistical analysis.
2.2. Pre-Treatment and Characterization of Germinated Rubber Seeds
Both germinated and ungerminated rubber seeds were collected in bags in a rubber plantation during the seed drop period of August and September 2021 at Alépé (Côte d’Ivoire). After harvesting, the germinated seeds were separated from ungerminated seeds. One part of the germinated seeds was used for fresh kernel characterization, while the other part was stored in a refrigerator at 4 °C for lipase extract powder preparation. The pre-treatment carried out prior to characterization consisted of seed manual dehulling and kernel grinding using a mixer (Nasco, BL-J2001AK-CB, Côte d’Ivoire). Parameters such as moisture, dry matter, ash, protein, and fat were determined using standard methods [24].
2.2.1. Moisture
A crystallizer container was dried in the oven (Memmert GmbH + Co.KG, Germany) for 30 min, then taken out and left to cool in the desiccator for 15 min until it returned to room temperature, after which it was weighed. Next, 2 g of the sample was added to the container and placed in the oven at a temperature of 105 ± 2 °C for 15 h. After this time, the container and its contents were transferred to the desiccator; once cooled, the container and sample were weighed. The sample was considered dry when a constant mass was obtained.
Moisture was calculated using the Formula (1):
: the mass of the empty container in grams;
: the mass of the container with the sample before drying in grams;
: the mass of the container with the sample after drying to constant weight in grams.
2.2.2. Dry Matter
Dry matter was determined from the moisture (2).
2.2.3. Ash
Ash on dry matter was determined by the muffle furnace calcination method. Next, 3 g of the sample was placed in crucibles weighed to vacuum beforehand and calcined in a muffle furnace (Nabertherm, Cole-Parmer, Germany) at 550 °C for 6 h. The difference in mass before and after calcination is used to obtain the ash content, which is expressed as a percentage of dry matter.
Ash was calculated using Formula (3):
: the mass of the empty crucible in grams;
: the mass of the crucible with the sample in grams;
: the mass of the container with the ash in grams.
2.2.4. Fat
Fat content was determined using the Soxhlet extraction method. In an extraction cartridge weighed to vacuum beforehand, 10 g of sample was introduced and sealed with hydrophilic cotton. Next, 300 mL of n-hexane was introduced into a 500 mL flask, also weighed to vacuum beforehand. After setting up the circuit (refrigerator–Soxhlet–flask), the system was refluxed for 6 h. At the end of the set time, the extraction cartridge was removed and placed in a stream of air to remove most of the residual solvent. The hexane still retained in the Soxhlet was transferred to the flask and distilled at 45 °C. Once the separation of the two components (solvent/fat) was complete, the flask containing the fat was placed in an oven at 105 ± 2 °C for 30 min, then left to cool to room temperature in a desiccator before being weighed.
The fat content was calculated using Formula (4):
: the mass of the test sample in grams;
: the mass of the empty flask in grams;
: the mass of the flask containing the fat after drying in grams.
2.3. Extraction and Physicochemical Properties of Crude Oils
Palm kernel, Jatropha curcas, and rubber seeds were de-hulled by hand and sun-dried for 7 days. The crude vegetable oils were obtained by pressing the dried kernels using an automatic OUKANING press at 115 °C under 20 bars. The oils obtained were allowed to settle for 24 h and then filtered to obtain a crude vegetable oil free from impurities.
The physicochemical properties of these crude oils were determined: color according to ASTM D1209 [25], acidity according to ISO 660 [26], kinematic viscosity at 40 °C according to ASTM D445 [27], density at 31 °C according to NF 6883 [28], calorific value according to [29], and water content according to NF T60–201 [30].
2.3.1. Acid Value
Dissolve 0.4 g of oil in 10 mL of a mixture of diethyl ether and ethanol (1/1, v/v) in an Erlenmeyer flask. After adding 10 mL of alcoholic potash, the mixture was stirred. The excess alcoholic potash was determined in the presence of phenolphthalein using a hydrochloric acid solution of normality N until the color was removed. A blank test was carried out under the same conditions, and the acid value was calculated using Formula (5):
and : respective volumes of hydrochloric acid solution poured into the test sample and the blank test in mL;
: normality of the hydrochloric acid solution in meq.g.mL−1;
: mass of test sample in grams;
282: molar mass of oleic acid in g.mol−1.
2.3.2. Kinematic Viscosity
It was carried out at 40 °C using a viscometer (FUNGILAB EAGLE, Barcelona, Spain). They allow simple but accurate measurements of the kinematic viscosity of translucent Newtonian fluids by measuring the mean time required for the substance to fall. The viscometers are coupled to a thermostatic bath, allowing the temperature of the sample to be regulated during viscosity measurements.
Kinematic viscosity was calculated using Formula (6):
: kinematic viscosity in mm2.s−1;
c: constant in mm2.s−2;
: average time for the substance to fall in s.
2.3.3. Density
The density or specific mass (NF 6883) represents the mass of the unit volume at a temperature. Next, 1 mL of dry oil and water are weighed. The masses determined for the test sample and distilled water are used to determine the density.
It was determined at 31 °C according to Formula (7):
are the respective densities of the oil and distilled water in g.mL−1.
2.3.4. Calorific Value
The calorific value was calculated using Formula (8).
and are the respective iodine and saponification values.
2.4. Plant Lipase Extraction
Extraction of plant lipase was carried out according to Okunwaye et al. [31], with some modifications.
2.4.1. Lipase Extract Powder Preparation
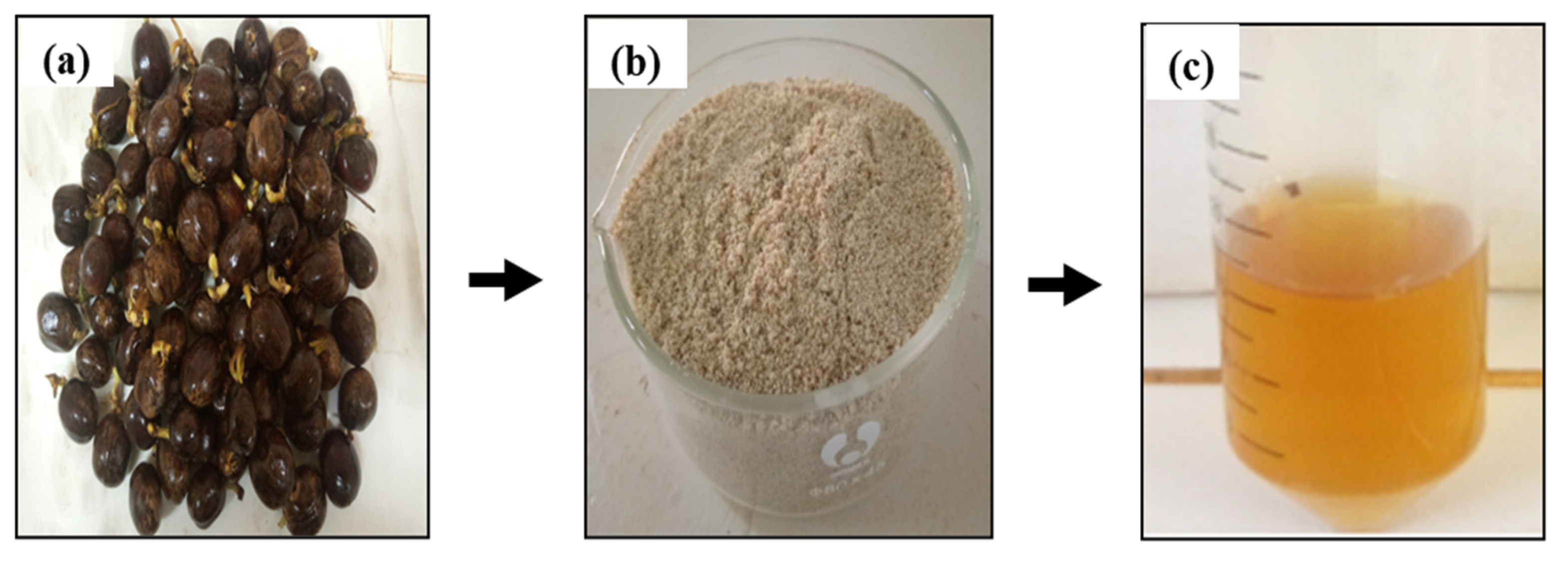
An amount of 100 g of germinated rubber seeds (Figure 1a) was de-hulled in order to obtain the kernel. The kernels were ground using a Nasco mixer (BL-J2001AK-CB, Côte d’Ivoire). Subsequently, the crushed kernel was subjected to drying at 30 °C for 24 h in an air oven, with the objective of preventing thermal degradation. The delipidation of the dried, crushed material was conducted with cold n-hexane at a ratio of 1:3 (w/v) for 24 h under agitation (400 rpm), with the objective of removing fat. Subsequently, the supernatant was filtered under vacuum using a Buchner funnel and washed with n-hexane. The excess hexane in the delipidated crushed material was completely evaporated at room temperature in a fume hood for 6 h. The delipidated crushed material was then folded and sieved through a 400 µm (Figure 1b). The lipase extract powder obtained was packed in hermetically sealed plastic boxes and stored in a refrigerator at 4 °C until required. It will be used as a biocatalyst in transesterification reactions.
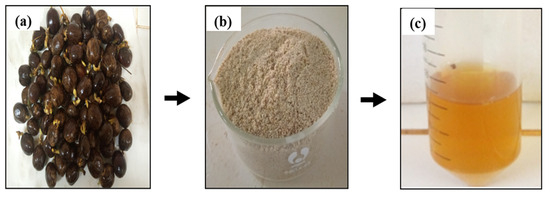
Figure 1.
(a) Germinated rubber seeds; (b) lipase extract powder; (c) lipase solution.
2.4.2. Lipase Solution Preparation
Lipase solution preparation was conducted by incubating 1 g of lipase extract powder in 20 mL of sodium phosphate buffer solution (0.2 M, pH 5) for 30 min. Following this, the mixture was subjected to centrifugation at 2000 rpm for 15 min. The resulting supernatant was then collected and stored at 4 °C until use (Figure 1c).
2.5. Protein Content in Lipase Solution
Protein concentration was determined using the Bradford method [32], with bovine serum albumin (BSA) employed as the standard. A stock solution of bovine serum albumin was prepared in distilled water at a concentration of 1 mg/mL. Subsequently, a series of concentrations of standards, commencing with the stock solution, was prepared by cascade dilution (1/2). One hundred microliters (100 µL) of each standard and sample solution was transferred into clean, dry vial tubes. To this solution, 3 mL of Bradford reagent was added and vortexed. The tubes were then incubated at room temperature for a period of 15 min prior to analysis. Subsequently, the absorbance of the BSA and samples was determined by means of a UV-vis spectrophotometer at a wavelength of 595 nm.
The amount of protein was determined using the formula from the BSA calibration curve (9):
y and x represent the absorbance value and the protein content (mg/mL), respectively. R2 is the correlation coefficient.
y = 0.4149x − 0.0454; R2 = 0.996
2.6. Evidence of Lipase by Hydrolysis Reaction
The presence of lipase in the plant extract powder was confirmed by the hydrolysis reaction. The lipase solution was used to study the hydrolytic activity of lipase from germinated rubber kernel on crude oils of palm kernel (PKO), Jatropha curcas (JCO), and rubber seed (RSO). The hydrolytic activity of the lipase was quantified using the methodology proposed Gadge et al. [33], with certain modifications. An emulsion was prepared by combining 0.5 g of crude vegetable oil, 2 mL of Arabic gum (3%, w/v), and 5 mL of sodium phosphate buffer (0.2 M). The reaction was initiated by the addition of 1 mL of the enzyme solution to the emulsified substrate. The reaction mixture was incubated for a period of 10 min at 350 rpm, after which the reaction was terminated by the addition of 10 mL of a solution of acetone and ethanol in a 1:1 ratio. The free fatty acid released in each emulsified sample was titrated with sodium hydroxide (0.1 M) until the reaction reached its endpoint, with 1% phenolphthalein serving as the color indicator. A control test was conducted for each crude oil employed.
Hydrolytic activity was calculated according to Formula (10):
V1NaOH is the volume of NaOH from the blank assay (mL); V2NaOH is the volume of NaOH from the assay with the lipase solution (mL); CNaOH is the concentration of NaOH (g/mol); X is the amount of lipase solution taken (mL); and t is the reaction time (min).
U is defined as the amount of lipase required to release 1 µmol of fatty acid equivalent per minute under the test conditions.
2.6.1. Effect of pH on Lipase Hydrolytic Activity
The effect of pH on the hydrolytic activity of plant-derived rubber lipase was investigated by incubating plant extract samples in a sodium phosphate buffer (0.2 M) at varying pH values (ranging from 5 to 9) at room temperature for 30 min. The hydrolytic activity of the lipase was evaluated and quantified in accordance with the methodology and formula outlined in Section 2.5 [33].
2.6.2. Effect of Extract Concentration on Lipase Hydrolytic Activity
The effect of extract concentration on the hydrolytic activity of plant-derived rubber lipase was investigated by preparing samples of lipase solution in a sodium phosphate buffer (0.2 M, pH 5) at varying concentrations (12.5, 25, 50, 100, and 200 mg/mL). The hydrolytic activity of lipase was then evaluated and calculated in accordance with the methodology outlined in Section 2.5 [33].
2.6.3. Determination of Lipase Solution Thermal Stability
The lipase solution samples were prepared in 0.2 M sodium phosphate buffer (pH 5) and incubated at temperatures between 20 and 70 °C, with 10 °C increments, for one hour (1 h). After incubation, the lipase solutions were cooled to room temperature [34]. The hydrolytic activity of lipase was then tested and calculated in accordance with the procedure and formula given in Section 2.5 [33].
2.6.4. Determination of Lipase Storage Stability
The storage stability of the lipase was investigated by storing it at 4 °C for 30 days. At 5-day intervals, 1 mL samples of the lipase solution were taken to determine its hydrolytic activity [34,35]. The hydrolytic activity of lipase was tested and calculated according to the procedure and formula given in Section 2.5 [33].
2.7. Enzymatic Transesterification Reaction
The catalytic activity of lipase was investigated through the transesterification reaction. The reaction was conducted using a lipase extract powder derived from germinated rubber kernels. This extract was used on rubber kernels’ crude oil. The enzymatic transesterification was conducted in accordance with the methodology proposed by Shamsudin et al. [36], with minor modifications. The reactions were conducted in 50 mL flasks containing a total of four grams of crude oil, combined with alcohol in a molar ratio. Ethanol was added in successive fractions (three times) over a 12 h interval. Subsequently, the crude plant powder extract was added to the mixture (12.5%, w/w) and incubated under constant magnetic stirring at 300 rpm for a 48 h reaction period. Afterwards, the mixture was subjected to centrifugation at 2000 rpm for 30 min. Subsequently, the supernatant obtained by centrifugation was subjected to gas chromatography with flame ionization detection (GC-FID, Varian 450-GC) for the determination of fatty acid ethyl esters.
2.7.1. Evaluation of Effect of Temperature on the Catalytic Activity of Lipase
In order to evaluate the effect of temperature on lipase catalytic activity, transesterification reactions were conducted at temperatures of 30 °C, 40 °C, 50 °C, and 60 °C for a period of 48 h. The catalytic activity of lipase was then determined and calculated in accordance with the methodology outlined in Section 2.7 and Section 2.10.
2.7.2. Aqueous Transesterification Reaction
In order to evaluate the effect of water on the catalytic activity of the lipase present in the crude extract, different quantities of water were added to the reaction medium (25 µL, 50 µL, 75 µL, and 100 µL). The reaction was conducted at 37 °C for 48 h. The catalytic activity of the lipase was determined and calculated in accordance with the methodology outlined in Section 2.7 and Section 2.10.
2.7.3. Co-Solvent Addition in Transesterification Reaction
The transesterification reactions were conducted initially without the addition of a co-solvent, followed by the introduction of water (100 µL) and n-hexane (100 µL). The reactions were conducted at 37 °C for 48 h. Following this period, the catalytic activity of lipase was determined and calculated in accordance with the methodology outlined in Section 2.7 and Section 2.10.
2.8. FT-IR Analysis
The samples were analyzed using Fourier transform infrared spectroscopy (FT-IR, Nicolet 6700, Thermo-Scientific, Madison, USA), equipped with an attenuated total reflectance (ATR) accessory. A pressure applicator with a rotary knob was employed to compress the samples and enhance contact with the ATR diamond crystal. Infrared radiation from the spectrometer, with a fixed angle of incidence of 45 °C, was reflected through the crystal and penetrated the sample via an evanescent wave. The infrared spectra were processed using the OMNIC Professional 7 software package, which is integrated into the control computer, in the range of 4000 cm−1 to 400 cm−1 [37].
2.9. SEM-EDX Analysis
The morphology and microstructures of the samples were analyzed using a scanning electron microscope (SEM, Thermo Scientific Phenom ProX, Eindhoven, The Netherlands) equipped with an EDX analyzer at FOV: 537 µm, Mode: 15 kV—Image, Detector: BSD Full [38].
2.10. Analysis of Ethyl Ester Formation by Gas Chromatography with Flame Ionization Detector
The standard NF EN 14103 method was used to quantify the ethyl esters (or biodiesel yield) formed at the end of the reactions. A mass (100 mg) of sample was weighed into a flask (10 mL). Then, 5 mL of a solution consisting of ethyl heptadecanoate (the standard) and n-hexane (99%), prepared at a concentration (10 mg/mL), was added to the flask. The n-hexane was run through the GC-FID first, followed by the biodiesel samples obtained from the crude oil of rubber seeds.
Fatty acid ethyl ester content (%r) was determined by the following Formula (11):
: the total peak area of the fatty acid ethyl ester;
: the area of the ethyl heptadecanoate peak;
: the concentration of the ethyl heptadecanoate solution in mg/mL;
: the volume of the ethyl heptadecanoate solution in mL;
: the mass of the sample taken in mg.
3. Results and Discussion
3.1. Characteristics of Germinated Rubber Kernels
The characteristics of the germinated rubber kernels are presented in Table 1. The obtained results show that the harvested germinated rubber kernels contained relatively high moisture content (33.48%). Most of the moisture in rubber seeds is stored in the kernel, and this content is between 29 and 32% [39]. However, it can be very high (50%) or very low (1.5%) [40]. According to Sugebo et al. [41], the moisture content of rubber kernels may be due to the harvesting season, the environment in which the tree was grown, and/or varietal factors. The content obtained in this study could be explained by the harvesting season (rainy season) and the method used to preserve the seeds, which would have favored an increase in the water content of the kernels. The ash content on dry matter is 3.36%. This result is consistent with previous studies that reported that the ash content on dry matter of rubber kernels would be in the range of 3 to 5% [40,42]. However, some works have shown lower contents in the range of 0.24% [40,43]. The protein content obtained was 15.75%. This shows that germinated rubber kernels constitute a good source of protein. According to Koné et al. and Oluodo et al. [3,40], rubber kernels, with a protein content ranging from 11 to 35%, are considered a protein supplement in livestock feed. The fat content obtained was 50.11%. This result agrees with several works that reported 48.78% [39] and 49.3% [42]. Oil is one of the most abundant components of rubber seed. Nowadays, this oil is receiving a lot of attention, mainly as an alternative feedstock in biodiesel production [4].

Table 1.
Composition (%) of germinated rubber kernels.
3.2. Physicochemical Properties of Crude Oils Extracted
The physicochemical properties of the crude oils from palm kernel (PKO), Jatropha curcas (JCO), and rubber seed (RSO) used in this study are presented in Table 2. The studied parameters are very important and must be considered, as they can be limiting factors for the direct use of vegetable oil in a diesel engine [44]. Vegetable oils are the most widely used energy source in the world and are the main feedstock for biodiesel production [45]. The results obtained show that oils have different colors and are liquid at room temperature (31 °C): golden yellow for JCO, light yellow for PKO, and brown for RSO. This observed difference in color could be related to the extraction process of the oils or to the presence of natural pigments in the kernels that affect the color of the oil.

Table 2.
Physicochemical properties of JCO, PKO, and RSO.
The acid values obtained for JCO, PKO, and RSO were high: 18.70 mg KOH.g−1, 25.05 mg KOH.g−1, and 41.51 mg KOH.g−1, respectively. The high acidity of the oils observed indicates that the oils have a high content of free fatty acids. An increase in this could be detrimental to the quality of the oil. This property is an indicator of oil deterioration [46,47]. This high level of free fatty acids could be explained by the conditions under which the seeds are harvested, post-harvest, storage of the seeds after drying, or extraction of the oils. Poor storage of the kernels could have a negative impact on the quality of the oil [48]. However, methods such as the use of low-acid materials and enzymatic deacidification are recommended to reduce the acidity of vegetable oils [48,49]. In this study, the reduction of the free fatty acid content in PKO, JCO, and RSO oils was not considered, because one of the advantages of using lipases as biocatalysts in the transesterification reaction is that the acidity of the oil does not influence the lipase activity. The kinematic viscosities of JCO, PKO, and RSO obtained are 34.67 cSt, 29.49 cSt, and 31.93 cSt at 40 °C, respectively. As these viscosities are very high, they do not favor atomization or spraying during fuel injection. According to Reksowardojo et al. [50], increasing the injection pressure leads to incomplete combustion and is likely to foul the injector noses, cylinders, and pistons. This also leads to clogging of the engine feed components [51]. To overcome these problems, some experimenters have proposed the use of preheating chambers. Other possible methods to reduce the viscosity of these oils and improve their properties as biofuels are transesterification or oil–diesel blending [44]. All the oils have a density within the range of the Ivorian diesel specification (min 0.820; max 0.880): 0.84 for JCO, 0.87 for PKO, and 0.84 for RSO [52]. The calorific values of the oils are higher than 35 MJ.kg−1: 39.58 MJ.kg−1 for JCO, 38.08 MJ.kg−1 for PKO, and 39.90 MJ.kg−1 for RSO. This indicates that these crude oils are therefore fuels capable of producing energy to power a diesel engine [44]. The absence of water in the oils could be explained by the method used to extract it. The values obtained for the physicochemical properties are within the range of results reported in the literature [46,53].
3.3. Lipase Extract Powder Characterization
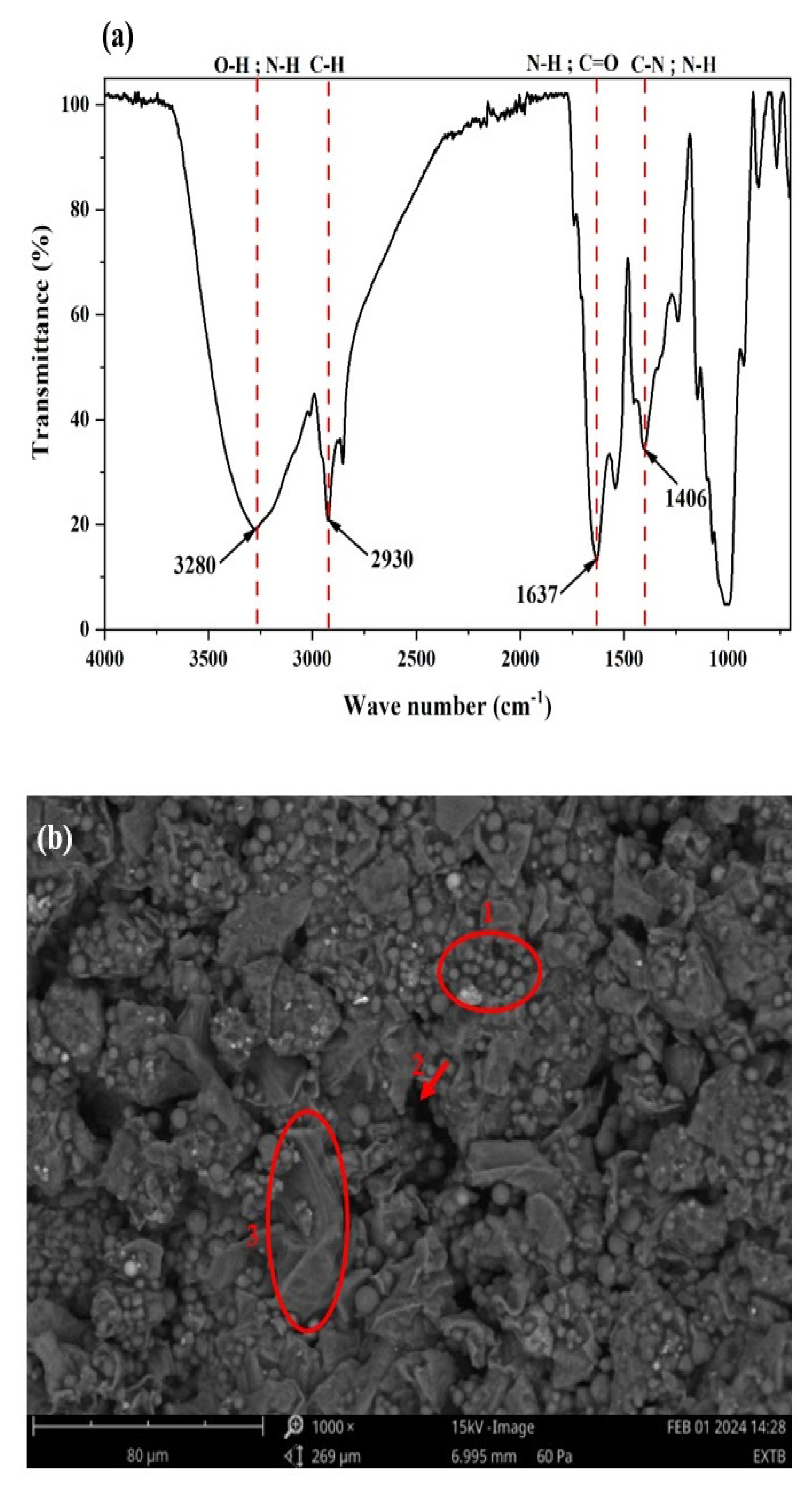
Figure 2 shows the FT-IR spectrum obtained between 4000 and 400 cm−1 (a), the SEM micrograph (b), and the EDX analysis obtained by SEM (c) of lipase extract powder of germinated rubber.
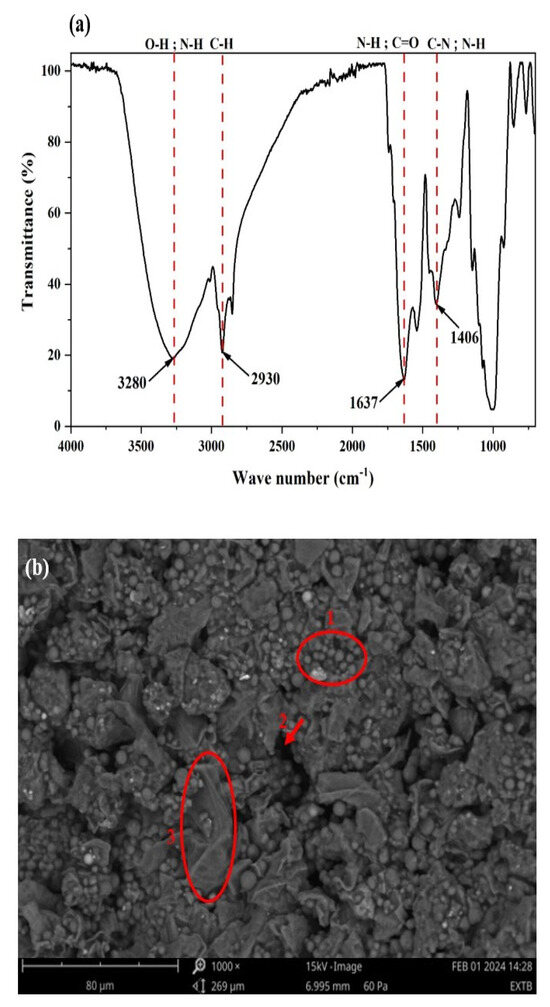
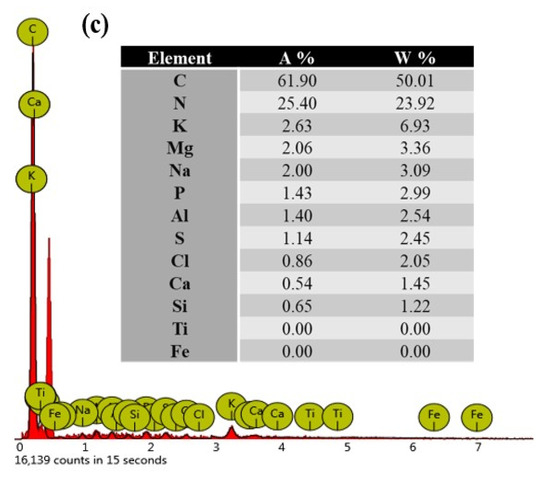
Figure 2.
(a) FT–IR spectrum (4000–400 cm−1); (b) SEM micrograph; (c) EDX analysis obtained by SEM of the lipase extract powder.
Analysis of the spectrum allowed characteristic peaks to be assigned to the functional groups present on the surface of the lipase extract powder (Figure 2a). The characteristic bands of the extract are the following: a band at 3273 cm−1 attributed to the stretching of the O-H and N-H functional groups; a broad band between 2924 cm−1 and 2854 cm−1 attributed to the stretching of the C-H group; a band at 1632 cm−1 corresponding to the stretching of the O-H groups whose vibration overlaps the stretching of the C=O group; and a band at 1401 cm−1 attributed to the stretching of the C-N, N-H, and -CH2 functional groups [38].
Figure 2b shows the morphology of germinated rubber lipase extract powder. It shows different morphologies on the surface of the lipase powder. This irregularity of morphology on the surface could indicate the presence of enzyme in the powder. The photograph shows spherical aggregates (1), irregularly shaped macropores (2), and smooth sheets (3).
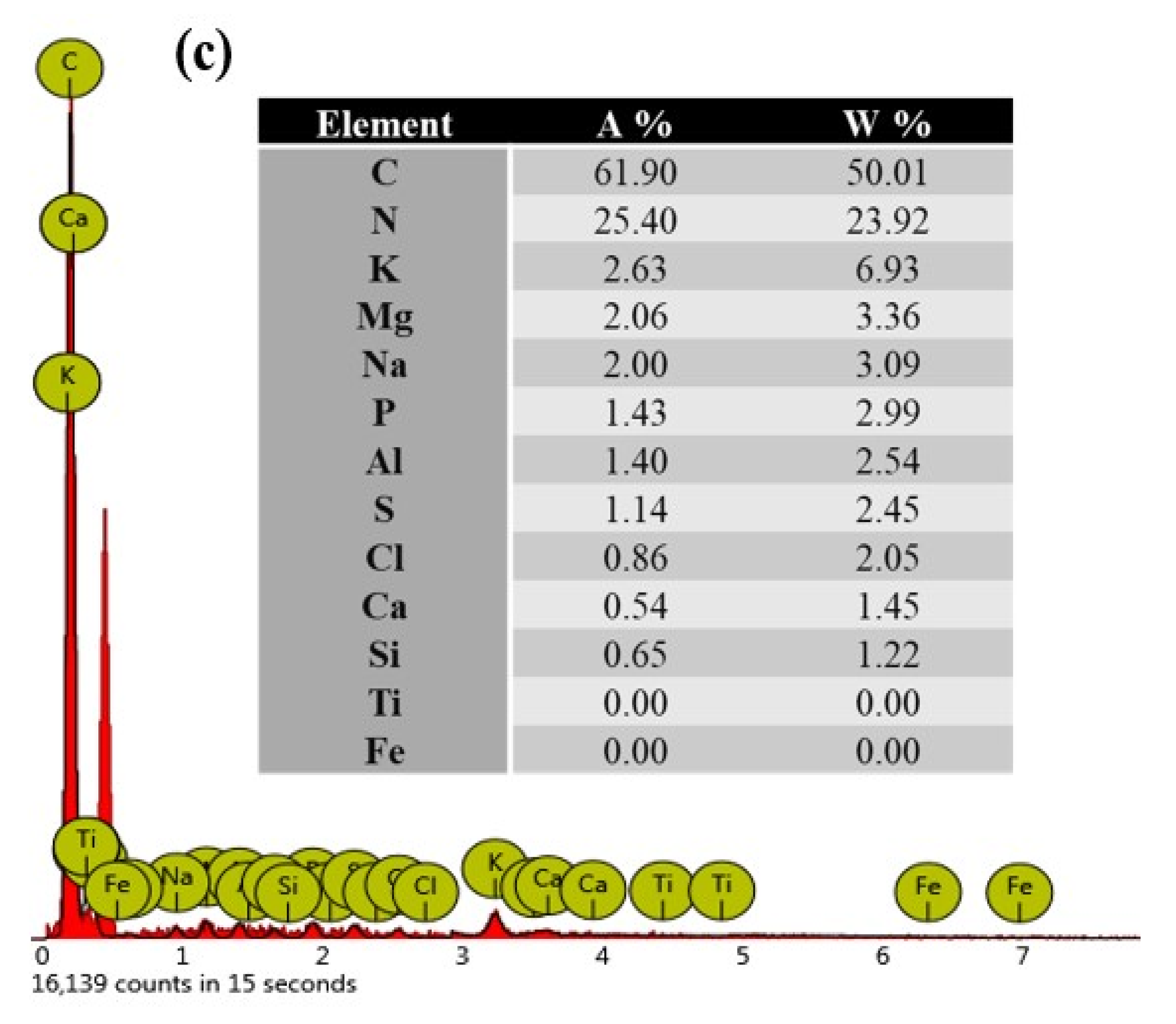
The EDX results shown in Figure 2c revealed the chemical composition of the lipase extract powder to be 50.01% carbon, 23.92% nitrogen, 6.93% potassium, 3.36% magnesium, 3.09% sodium, 2.99% phosphorus, 2.54% aluminum, 2.45% sulfur, 2.05% chlorine, 1.45% calcium, and 1.22% silicon.
3.4. Quantification of Protein in Solution
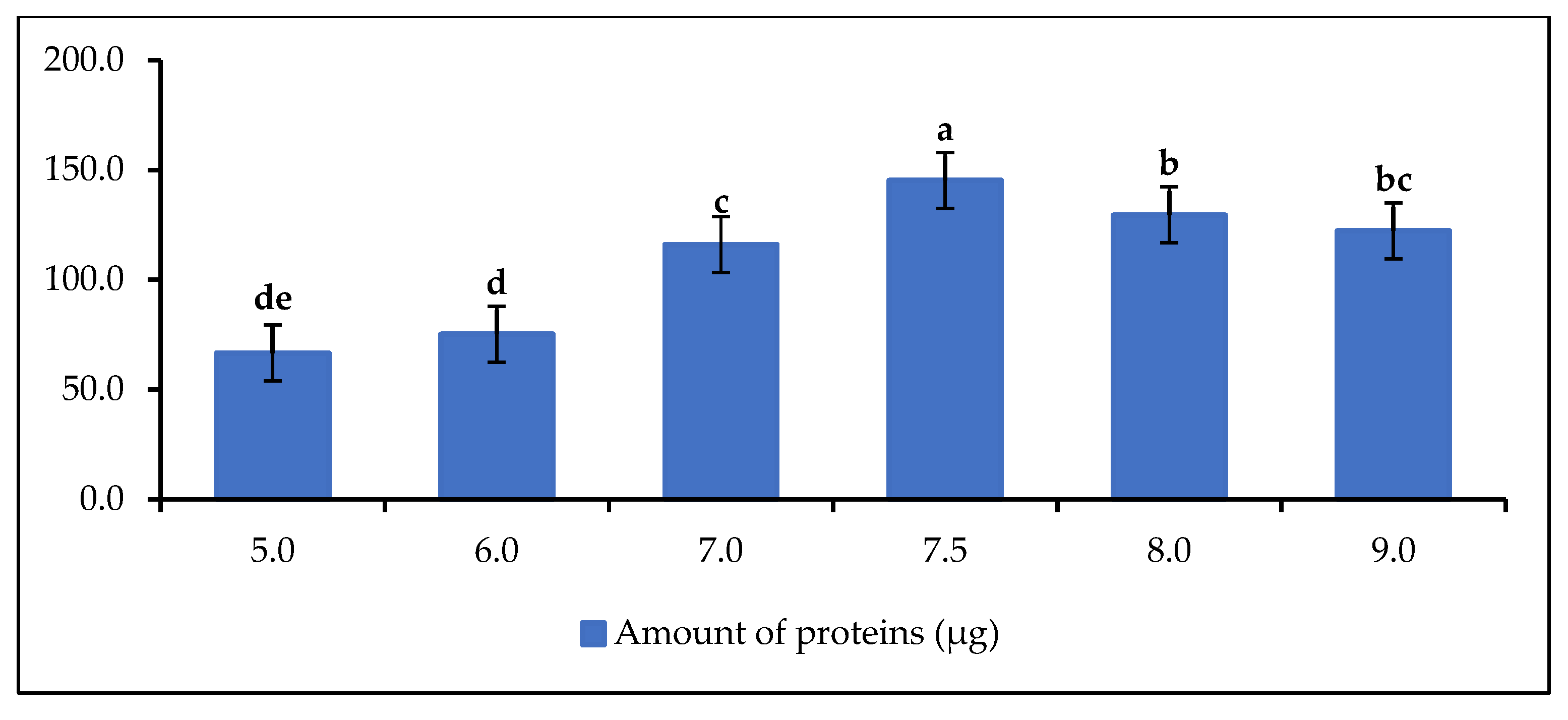
The influence of the pH of the buffer solution on the protein (lipase) extraction capacity was investigated in Figure 3.
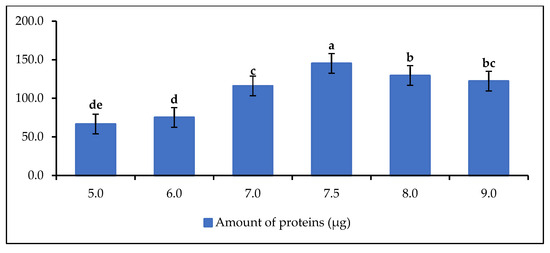
Figure 3.
Amount of protein as a function of pH buffer solution at C = 50 mg/mL. Letters show statistical significance between mean.
The results show that the protein content is related to the pH of the extraction solution. The amount of protein increases from pH 5 to reach the optimum at pH 7.5 and then decreases, but not significantly. Therefore, the optimum extraction pH in this study is 7.5. This result confirms the results of Mortazavi and Aghaei, who immobilized Candida rugosa lipase by adsorption on Na-sepiolite with a monolayer surfactant (MSEP) at the optimum pH of 7.5 [54]. Taghizadeh et al. [55] performed protein assay and immobilization of porcine pancreatic lipase (PPL) at pH 7.5. The highest protein levels were found in the range of pH 7.5 to pH 9. This shows that the phosphate buffer solution has a good lipase recovery capacity in this pH range or demonstrates the good lipase extraction capacity of the buffer solution in an alkaline environment [56]. As mentioned above, the extracts of delipidated rubber seeds are known to be a good source of protein. Due to this good protein content, rubber seeds are commonly used in animal feed [57,58].
3.5. Lipase Hydrolytic Activity
3.5.1. Effect of Buffer pH on Lipase Hydrolytic Activity
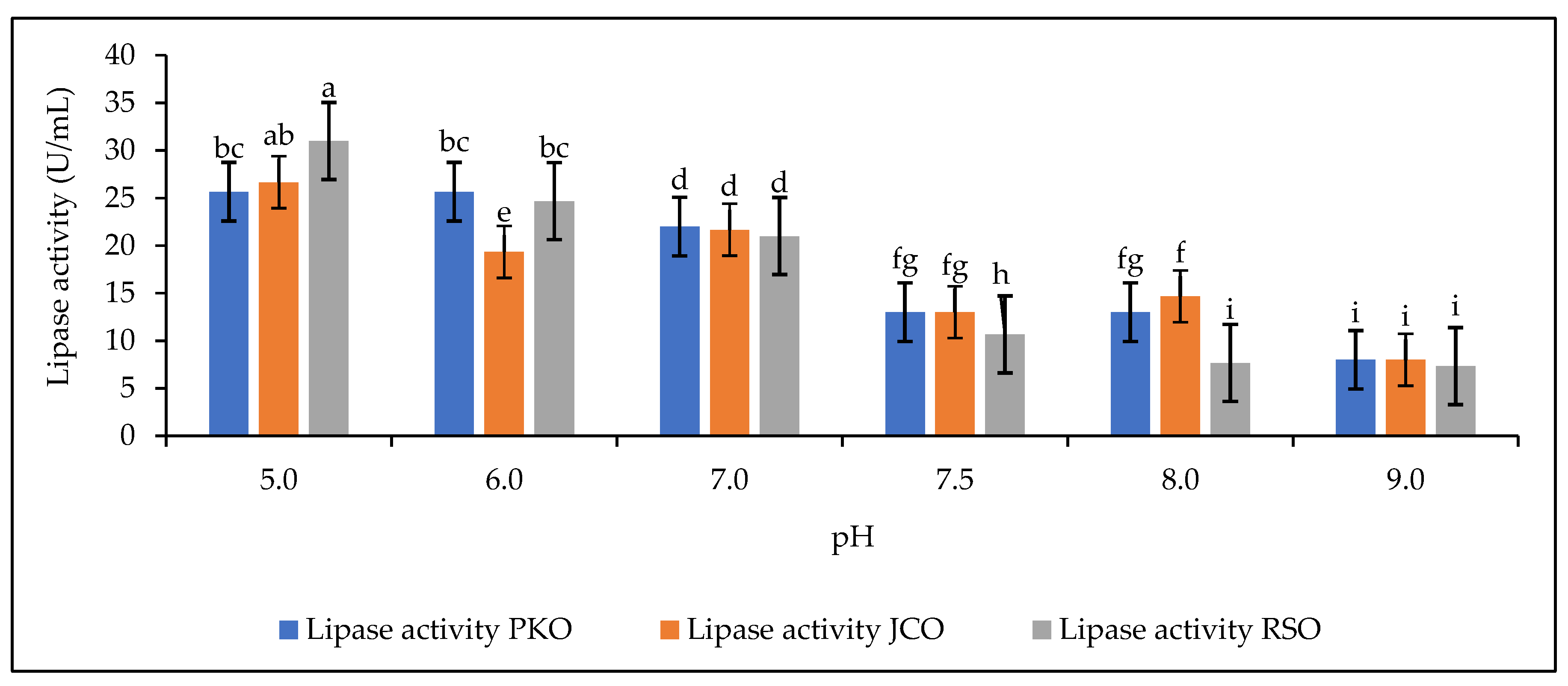
The effect of buffer pH on lipase hydrolytic activity is shown in Figure 4.
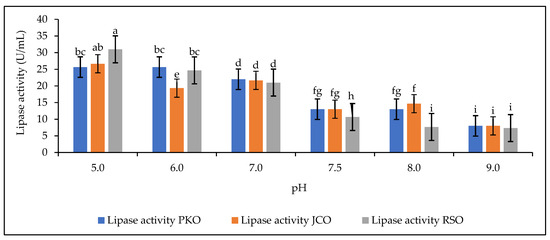
Figure 4.
Lipase activity as a function of buffer pH. Letters show statistical significance between mean.
The results showed a variation in lipase activity for each oil used (PKO, JCO, and RSO) over the range of pH 5 to pH 9. This is because soluble or free lipase is sensitive to changes in pH [59]. It was found that the hydrolytic activity of lipase decreased as the pH of the buffer solution tended towards alkalinity. A drastic drop in the performance of lipase activity during hydrolysis was observed from pH 7.5 to 9, while the above results showed a higher protein content in the solution at this pH range (Figure 3). This decrease in lipase hydrolytic activity could be due to the presence of an emulsifying compound in the reaction mixture (Arabic gum), which could have an inhibitory effect on the active site [60]. The addition of Arabic gum to the reaction mixture is used to facilitate the emulsion of the oil–lipase solution [61]. Arabic gum can be used alone in the reaction mixture or in combination with a second emulsifier, as in the work of Hoppe and Theimer, who used Arabic gum and deoxycholate together [19,62]. For each plant oil used, the optimal pH range is from pH 5 to pH 8. According to Robinson, the optimal pH range for enzyme activity is from pH 6 to pH 8, but some may be more active below or above this range [60]. In our study, the highest hydrolytic activities were observed at pH 5 for PKO, JCO, and RSO: 25.67 U/mL, 26.67 U/mL, and 31 U/mL, respectively. Thus, our study shows that pH 5 is the optimum for good hydrolytic activity of lipases in solution. This result does not confirm that obtained by Weerasooriya and Kumarasinghe [63], who worked on rubber kernel lipases, specifically on the isolation of alkaline lipase from rubber seeds, and obtained 8 as the optimum pH. Fractionation to use only alkaline lipase in the supernatant may explain this difference in results. Various environmental factors can affect the rate of reactions catalyzed by enzymes by causing reversible or irreversible changes in the protein structure. Enzymes are sensitive to pH; changes in pH can alter the ionization of the functional groups of the enzyme active site and substrate. This affects the binding of the substrate to the active site [60].
3.5.2. Lipase Hydrolytic Activity According Extract Concentration
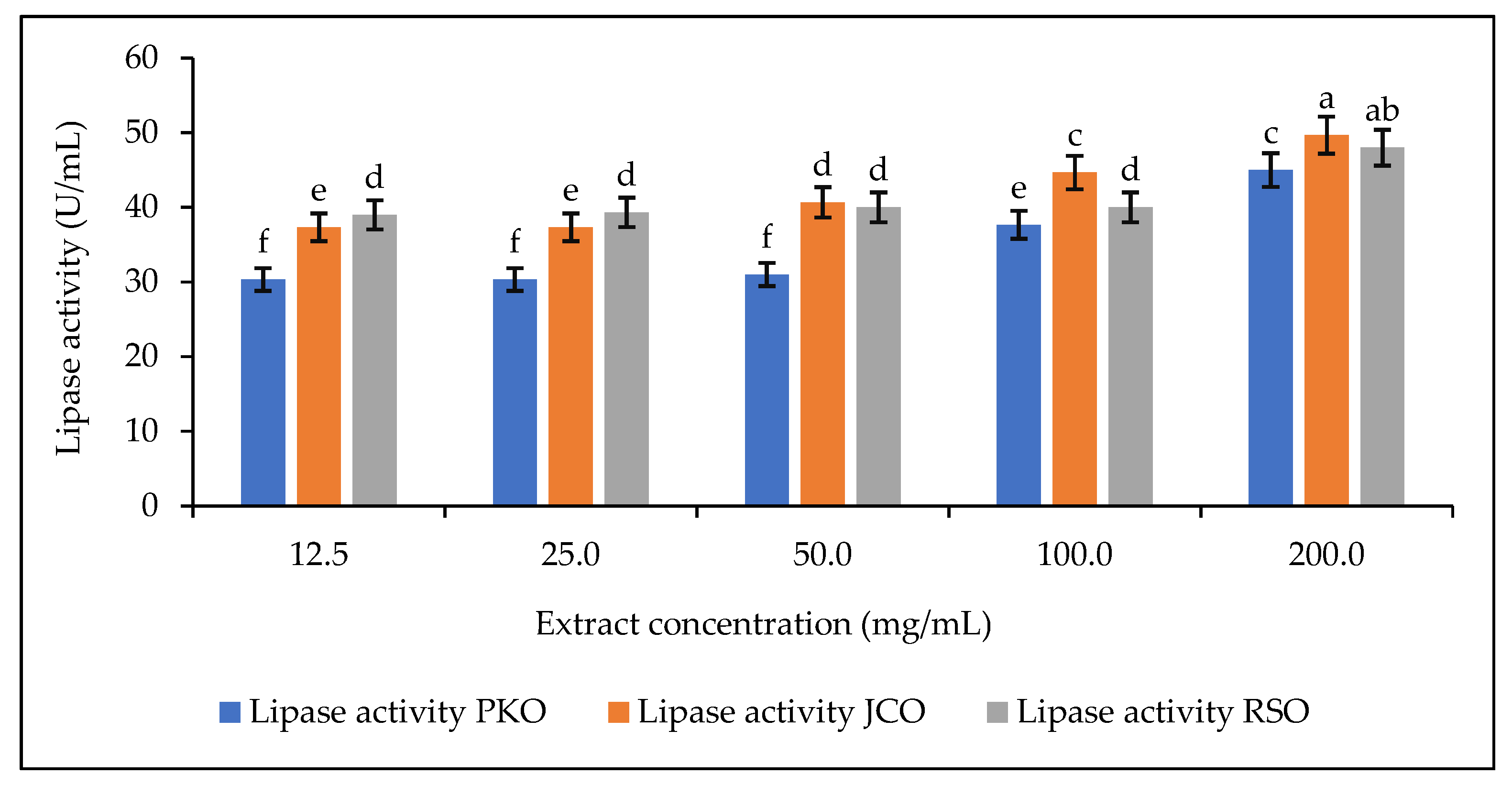
After determining the required pH of the sodium phosphate buffer solution for optimal rubber lipase hydrolytic activity, a concentration range for the preparation of the lipase solution was defined after preliminary experiments to study its effect on the lipase hydrolytic activity. The hydrolytic activity of rubber kernel lipase in solution was determined at different extract concentrations using phosphate buffer (12.5 mg/mL to 200 mg/mL) at pH 5. The results are shown in Figure 5.
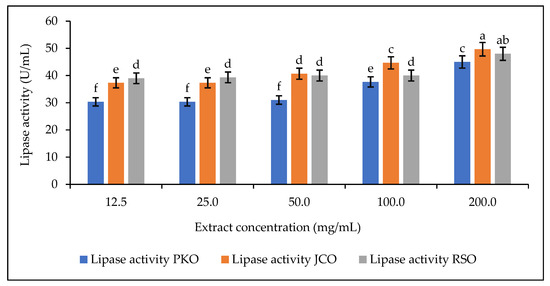
Figure 5.
Lipase activity as a function of extract concentration. Letters show statistical significance between mean.
For each of the oils used, it was observed that the hydrolytic activity of the lipase increased in proportion to the increase in the concentration of the extract, i.e., from 12.5 to 200 mg/mL. This can be explained by the increase in the amount of lipase in the solutions. Increasing the concentration of lipase causes an increase in its hydrolytic activity and even an increase in the speed of the reaction. This observation is due to the availability of active lipase sites in the reaction mixture. The hydrolytic activity of the lipase decreases or stops when there are no more active lipase sites available [64]. In this part of the study, which focuses on the hydrolytic activity of rubber lipase, the aim is to observe good lipase activity while reducing its use. Therefore, the concentration defined for the preparation of the lipase solution for the rest of the experiments is 50 mg/mL.
3.5.3. Effect of Temperature on Lipase Hydrolytic Activity
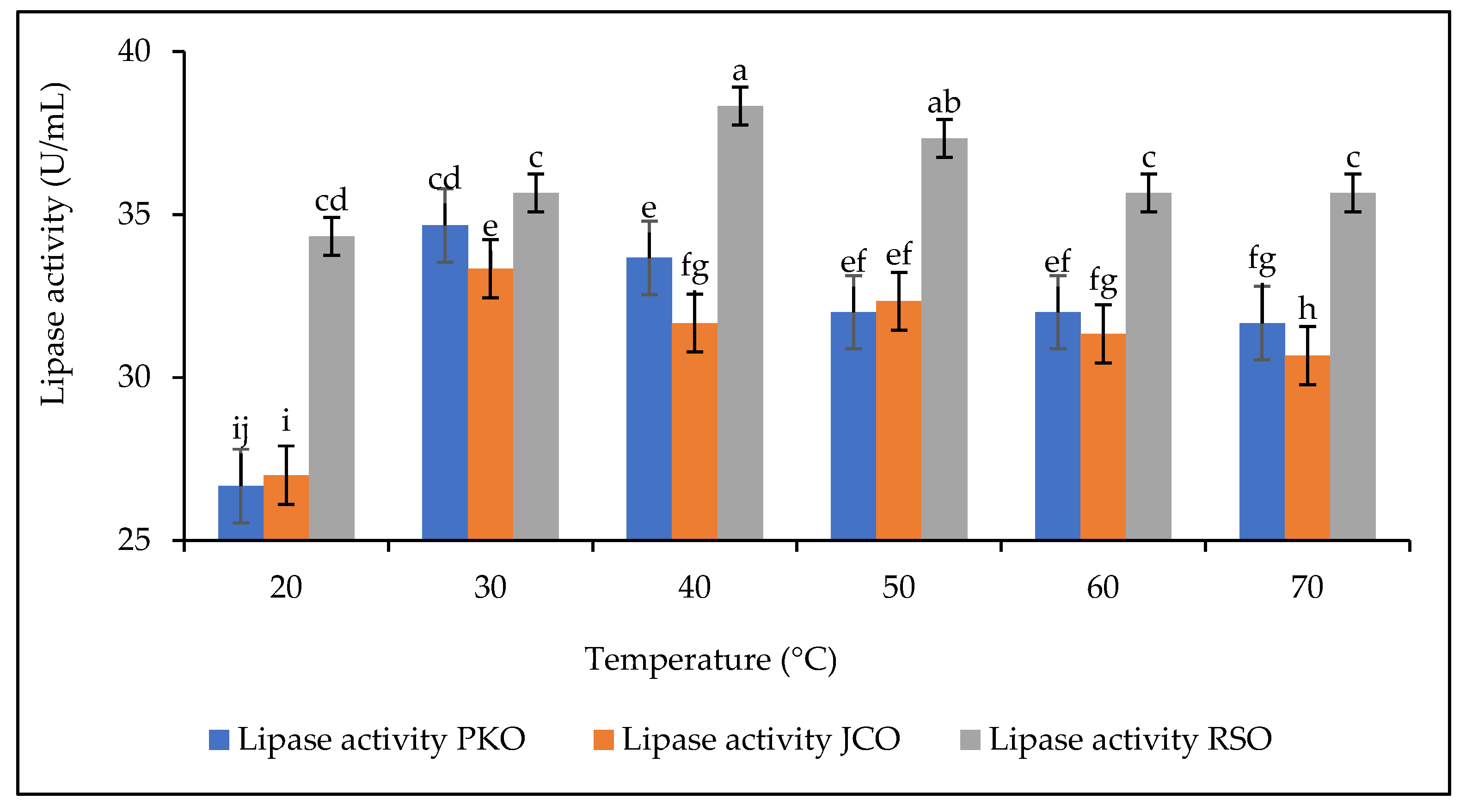
The thermal stability of lipases is one of the most important properties to be studied. The effect of temperature on the hydrolytic activity of lipase was studied in the temperature range of 30–70 °C (Figure 6). The experiments were carried out at a constant extract concentration of 50 mg/mL at pH 5.
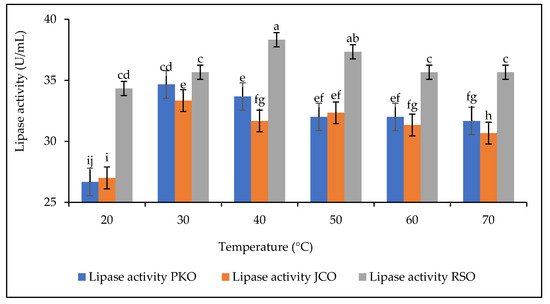
Figure 6.
Lipase stability as a function of temperature. Letters show statistical significance between mean.
The results showed that for all oils there was an increase in the hydrolytic activity of lipases up to the optimum temperature. The optimum hydrolytic activity of lipase on PKO and JCO was 34.67 U/mL and 33.33 U/mL, respectively, corresponding to the optimum temperature of 30 °C. In contrast, the optimum hydrolytic activity of lipase on RSO was 38.33 U/mL, corresponding to an optimum temperature of 40 °C. The lipase from germinated rubber seeds was found to have good thermal stability between 30 °C and 40 °C. The results also showed that lipase is sensitive to temperature increase. Above the optimum temperature, the hydrolytic activity of lipase decreases. This decrease in hydrolytic activity can be explained by changes in the physical and chemical properties of lipase due to its progressive structural denaturation [34,65].
3.5.4. Effect of Storage Time on Lipase Hydrolytic Activity
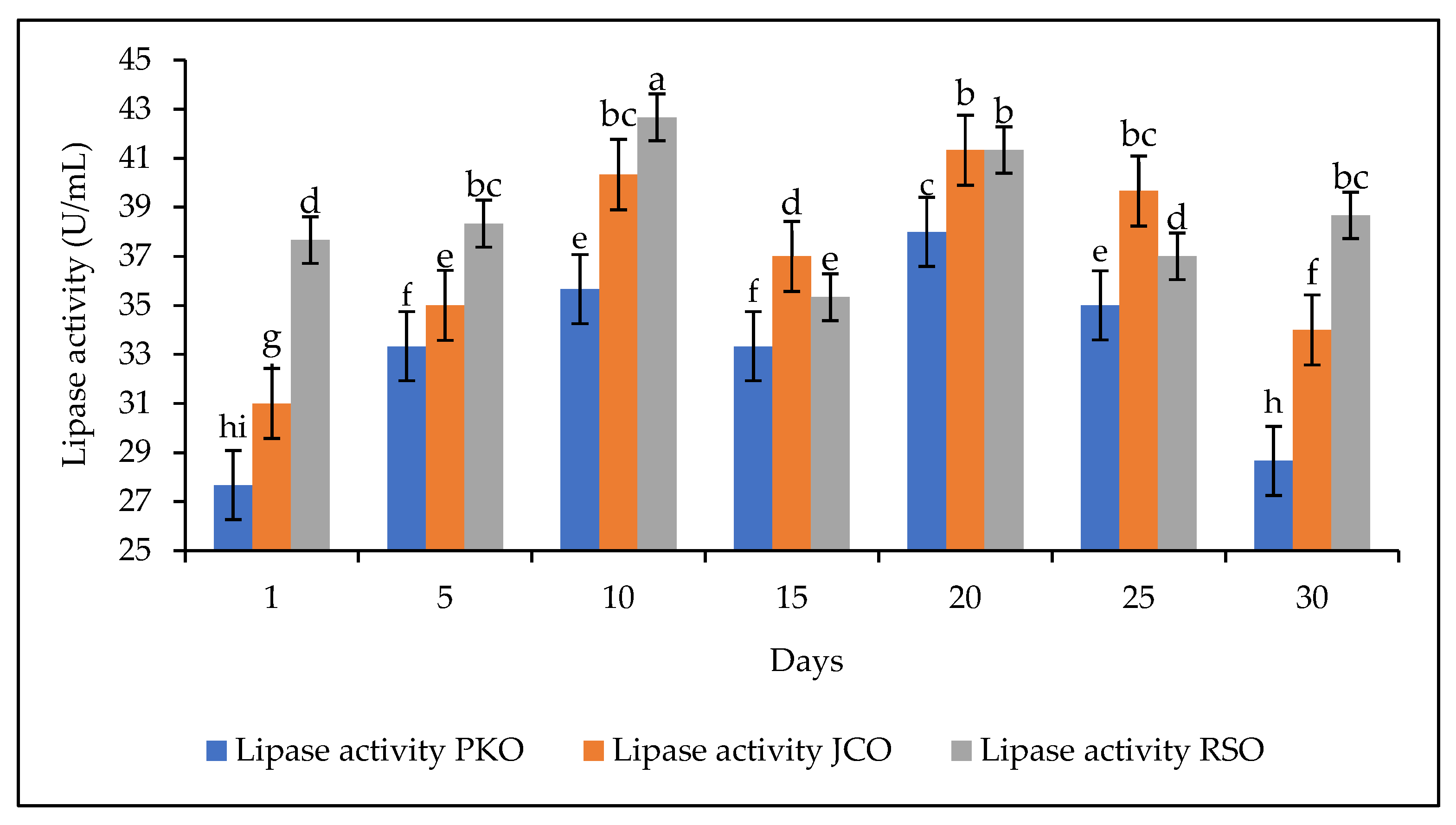
The storage stability of the lipase is also an important parameter to study, especially if an industrial application of the lipase is envisaged. The lipase storage stability study was performed by storing the lipase solution at 4 °C for 30 days. The experiments were carried out at a constant extract concentration of 50 mg/mL at pH 5. A decrease in the hydrolytic activity of the lipase was observed on the fifteenth day for each of the oils used. This was followed by an increase in activity on day 20 and a further decrease on day 30 for PKO and JCO. On day 30, RSO showed an increase in hydrolytic activity (Figure 7).
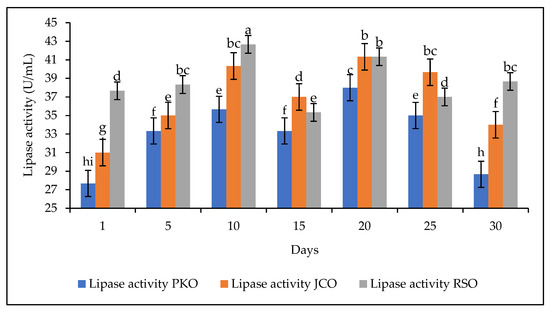
Figure 7.
Storage stability of lipase at 4 °C for 30 days. Letters show statistical significance between mean.
These observations demonstrate the unstable nature of lipase in solution. This confirms observations from other studies. Free lipases are less stable than immobilized lipases [34,66]. Immobilization is a method used to improve the stability of the enzyme so that it can be used repeatedly [67]. Studies have shown that free lipases are less stable in storage than immobilized lipases. Work by Mohammadi et al. showed that immobilized lipases SiO2-lipase and SiO2-lipase entrapped in a PVA/Alg (poly(vinyl alcohol)/alginate) hydrogel retained 76.5% and 43.4% of their initial activity, respectively, during 30 days of storage at 4 °C, while free lipase retained 13.7% of its initial activity under the same conditions [34].
3.6. Lipase Catalytic Activity on RSO
Hydrolysis tests, along with SEM-EDX and FTIR analyses, revealed the presence of lipase in the plant extract powder. Since the aim of the study was to add value to rubber seeds germinated in unused plantations, the rubber extract powder was used directly as a biocatalyst in the transesterification of crude rubber oil (RSO). The catalytic activity of the lipase was also investigated.
3.6.1. Composition of Interest Product Obtained After Transesterification Reaction
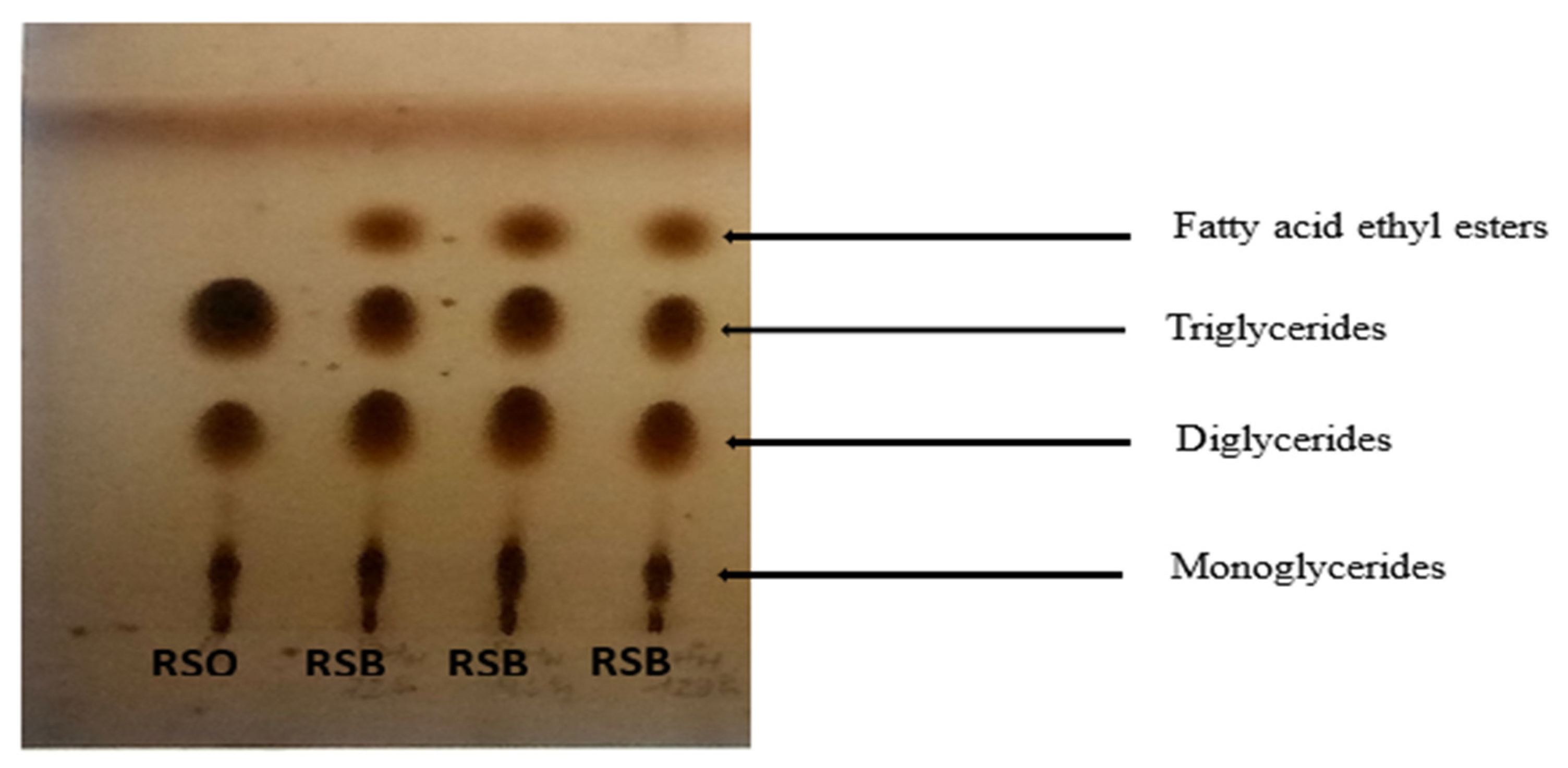
The identification of the compounds in the products obtained from the reactions was carried out on TLC plates. The plates showed the presence of fatty acid ethyl esters, triglycerides, diglycerides, and monoglycerides in the reactions carried out (Figure 8). Indeed, the transesterification of triglycerides with alcohol in the presence of a catalyst gives fatty acid alkyl esters as the main product and glycerol as the secondary product. The first step is the conversion of triglycerides into diglycerides, followed by the conversion of diglycerides into monoglycerides and monoglycerides into glycerol. This produces one molecule of alkyl ester from each glyceride at each stage [68].
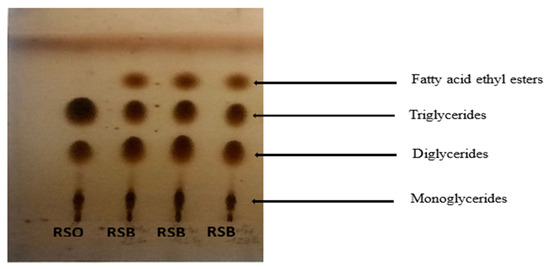
Figure 8.
Evidence of the presence of fatty acid ethyl esters in the products obtained with RSO after the transesterification reaction.
3.6.2. Effect of Temperature on Lipase Catalytic Activity
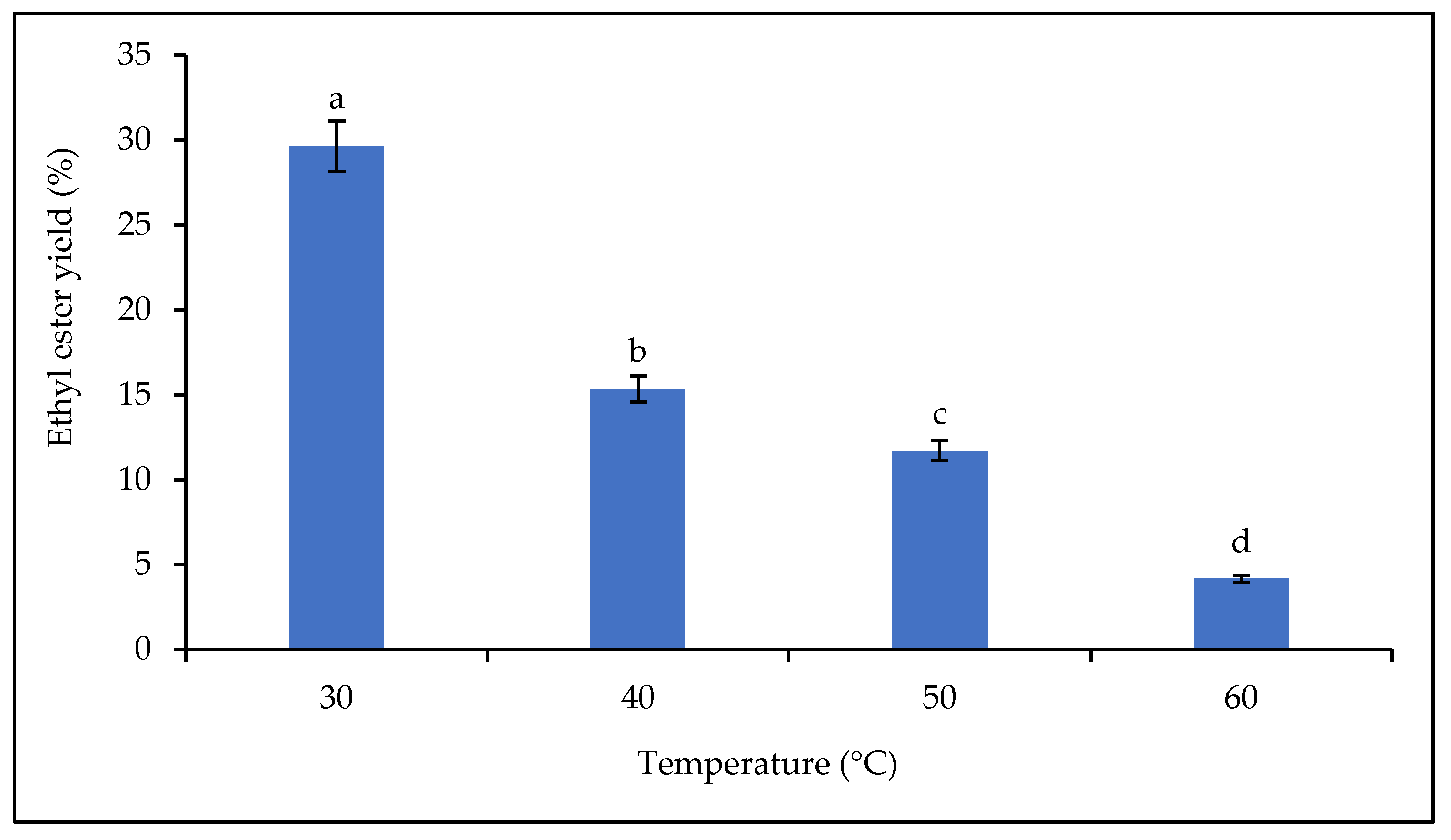
The effect of temperature on lipase catalytic activity was carried out over the temperature range of 30–60 °C, and the yields of fatty acid alkyl esters are shown in Figure 9. The results show that the yield decreased with increasing temperature. The highest biodiesel yield was obtained at 30 °C with 29.63%. Thereafter, a decrease in yield was observed from 40 °C to 60 °C (from 15.35% to 4.16%). The production of biodiesel by enzymatic catalysis requires mild conditions and is less energy-intensive than the chemical method [69]. Temperature has a clear effect on the catalytic activity of plant lipases. In general, a decrease in the yield of fatty acid alkyl esters from equilibrium synthesis is observed when the reaction temperature is higher than the physiological activity temperature of the lipase [20].
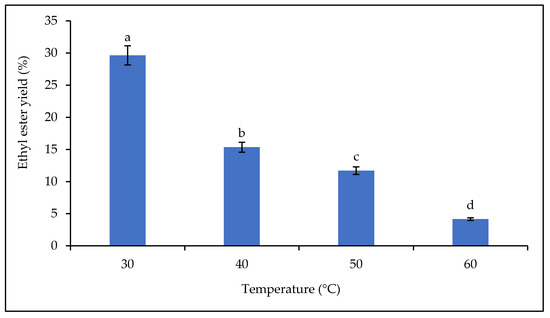
Figure 9.
Effect of temperature on the ethyl ester yield. Letters show statistical significance between mean.
Studies have shown that the desirable temperature range for enzymatic reactions is between 30 and 40 °C. Increasing the temperature above 40 °C could denature the enzyme (mainly plant lipases), which would reduce the rate of conversion of triglycerides to fatty acid alkyl esters. A temperature above 40 °C could destroy the structure/conformation of the plant lipase and lead to its deactivation [70,71]. This loss of active conformation is associated with the breaking of hydrogen bonds that control protein folding [20].
3.6.3. Effect of Water on Lipase Catalytic Activity
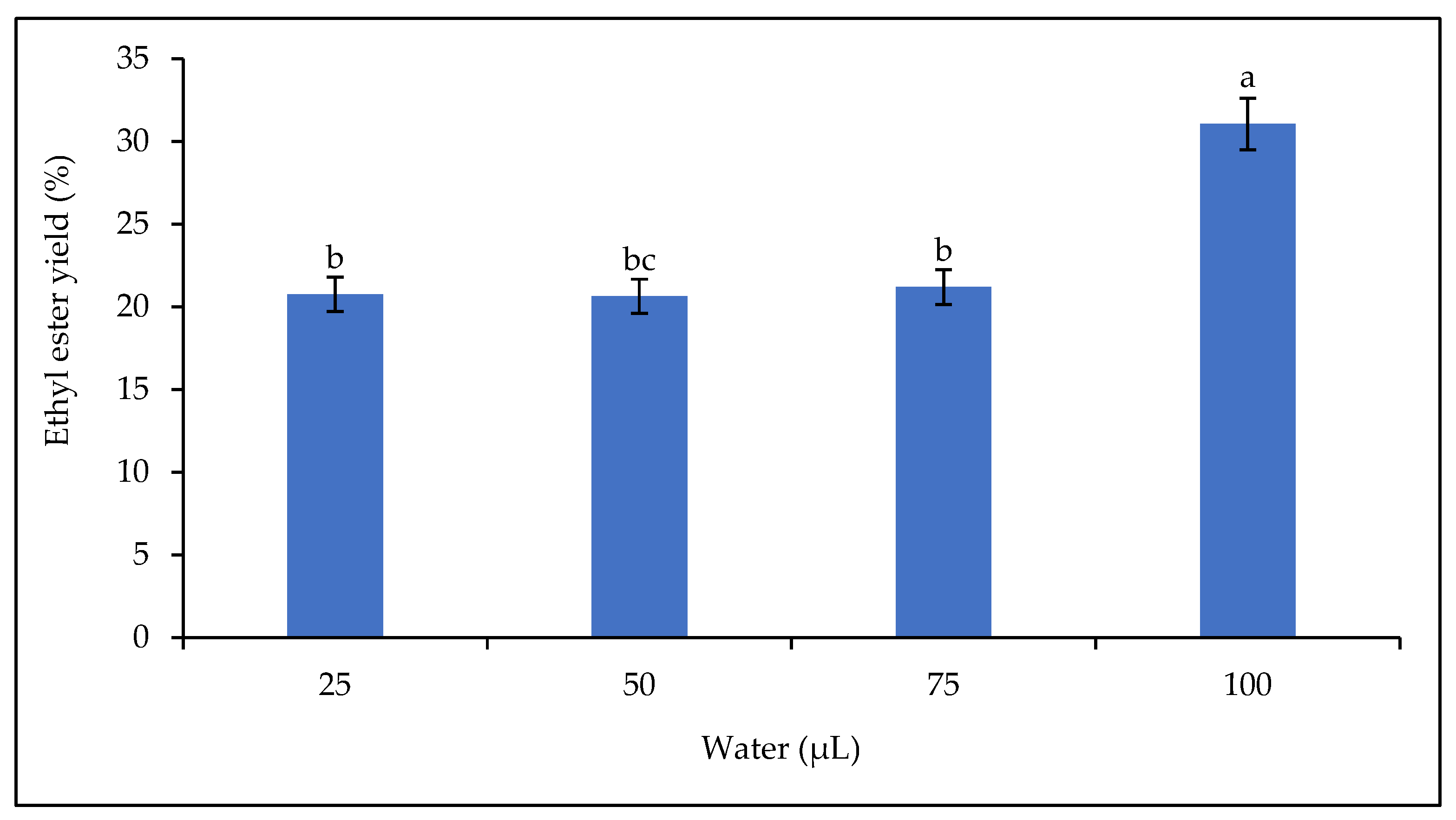
Figure 10 shows that the biodiesel yield increased with the amount of water in the reaction medium (from 25 µL to 100 µL), with an optimum yield of 31.06% at 100 µL.
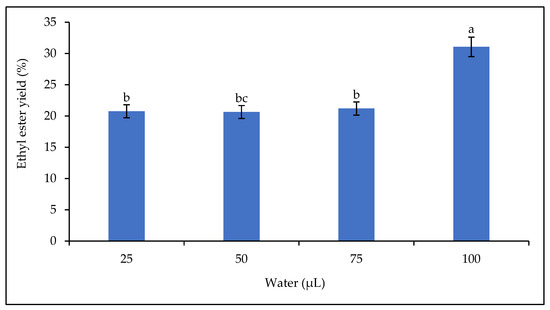
Figure 10.
Effect of water on the ethyl ester yield. Letters show statistical significance between mean.
The presence of water in the reaction mixture is an essential parameter in enzymatic catalysis [72]. The presence of water in the reaction mixture affects the catalytic activity and stability of lipase. This effect could be explained by a change in the conformation of the lipase [16,73] or by the composition of the reaction mixture, shifting the equilibrium of the transesterification reaction in favor of undesired hydrolysis of the substrate [20]. Adequate water content is necessary to maintain high enzymatic activity when organic solvents are used [74]. The optimum water content for good lipase activity is 2–5% [74]. In this study, the optimum yield was obtained at 3.18% water. The results in Figure 10 show that increasing the amount of water in the reaction mixture increases the catalytic activity of the lipase as the yield increases up to the highest water content. This observation agrees with some results in the literature, but not with others. Lipases are characterized by their specific properties. Therefore, the effect of water in the reaction mixture on the rate of conversion of triglycerides to alkyl esters could be related to the substrate used but also to the origin of the lipase. According to Guo et al. [71], high water content is detrimental to the lipase. It would reduce the contact between the lipase and the oil, thereby reducing the efficiency of the lipase. Some research has shown that a high water content in the reaction mixture reduces the catalytic activity of the lipase, for example, the addition of more than 5% water in the transesterification reaction of soybean oil with immobilized R. oryzae and Novozym 435 lipases [74].
3.6.4. Effect of Co-Solvent Addition on Lipase Catalytic Activity
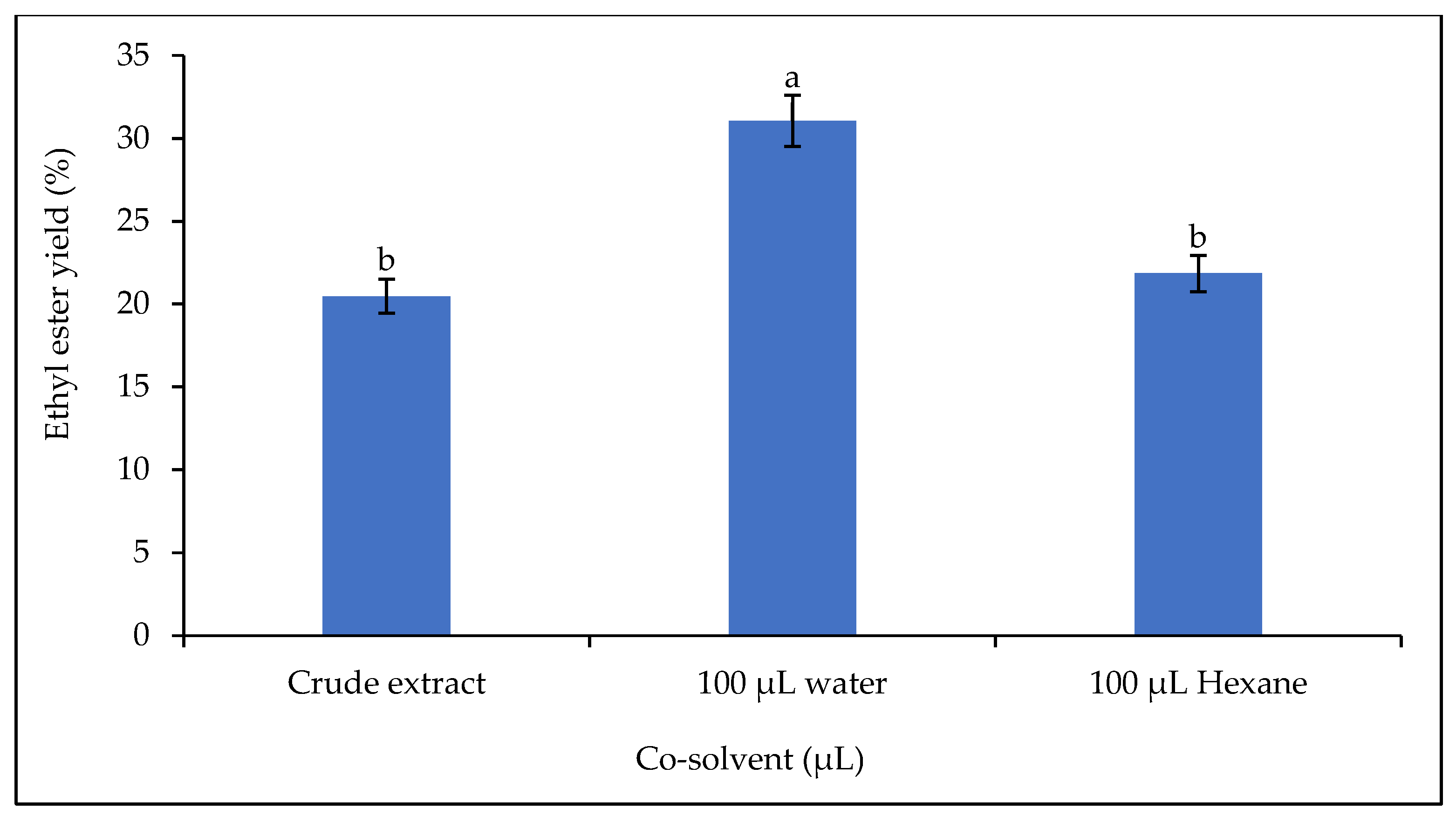
In this work, the influence of 3% w/w polar (water) and non-polar (n-hexane) solvents on the catalytic activity of the lipase was investigated (Figure 11). Incubation of water and n-hexane in the reaction mixture increased the yield by 51.73% when water was added and by 6.74% when n-hexane was added.
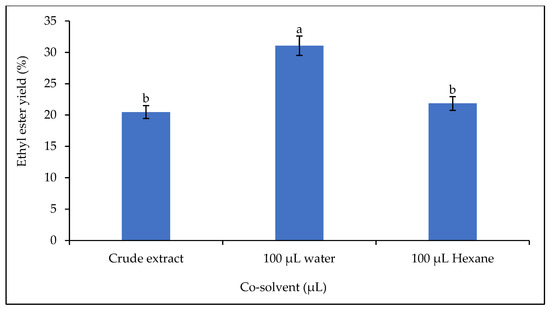
Figure 11.
Effect of co-solvent on the ethyl ester yield. Letters show statistical significance between mean.
Immiscibility between the alcohol and the vegetable oil sometimes makes it difficult for the lipase to act during the transesterification reaction. This immiscibility can lead to the formation of interfaces that are resistant to mass transfer. The addition of an organic solvent to the reaction mixture has been found to be a remedy [75]. The co-solvent is also necessary to improve the rate of the transesterification reaction. The addition of a co-solvent is sometimes used to solve the problem of a slow reaction as the co-solvent facilitates a single-phase reaction [76,77]. It accelerates the reaction by homogenizing it into a single phase, while the catalyst accelerates the reaction by reducing the activation energy [53]. However, a high content of organic solvent in the reaction mixture could be problematic, as it could denature the lipases and therefore reduce lipase activity or even inactivate them completely [75]. The two solvents used contributed to the increase in ethyl ester yield, but water had a greater effect than n-hexane. This finding could be explained by the fact that lipase has an affinity for the water in the reaction mixture. The primary function of lipases is hydrolysis, so the presence of water in the reaction mixture would have changed the conformation of the lipase and made it more stable.
4. Conclusions
This study aimed to valorize germinated rubber seeds for biodiesel production by using the lipase they contain as a biocatalyst and the oil as a feedstock for the transesterification reaction. To this end, the presence of lipase in the raw plant extract was first demonstrated in the hydrolysis reaction. Biodiesel was then produced using oil and lipase extract powder from the germinated rubber seed. Germinated rubber seeds are a good source of protein (15.75%) and rich in fat (50.11%). Protein extraction is maximal in the basic environment of the extraction buffer solution (pH 7.5–pH 9). Temperature, extract concentration, and storage time affect the hydrolytic activity of lipase. However, it showed good hydrolytic activity on the three oils used. The optimum test conditions were observed at pH 5. Lipase also shows catalytic activity in both non-aqueous and aqueous media. The use of co-solvents in the reaction mixture had a positive effect on the rate of conversion of triglycerides to fatty acid alkyl esters (yield). The optimum yield was 29.63% at 30 °C. The addition of water and n-hexane to the reaction mixture increased the yield from 20.47% (without co-solvent) to 31.06% and 21.85%, respectively. Lipase from germinated rubber seeds has good hydrolytic and catalytic activity. However, in order to use the lipase continuously in the transesterification reaction and increase the yield, it would be desirable to isolate the lipase and fix it on a carrier. It could be used as an additive in the production of several interesting products (detergents, paper) or for bioenergy applications.
Author Contributions
Conceptualization, M.J.C.A.; Methodology, M.J.C.A.; Software, K.E.K. and A.A.; Validation, M.J.C.A., K.E.K., A.A., W.K.I.O. and K.B.Y.; Formal Analysis, K.E.K.; Investigation, K.B.Y.; Resources, W.K.I.O. and K.B.Y.; Data Curation, K.E.K. and A.A.; Writing—Original Draft Preparation, M.J.C.A. and K.E.K.; Writing—Review and Editing, M.J.C.A., K.E.K., A.A., W.K.I.O. and K.B.Y.; Supervision, W.K.I.O. and K.B.Y.; Project Administration, K.B.Y.; Funding Acquisition, K.B.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the African Center of Excellence for the Valorization of Waste into high added value Product (CEA-VALOPRO), grant number 1679 01 T, and the Intra-Africa Program of the European Union, grant number 624193.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the World Bank (WB), the French Development Agency (AFD), the African Center of Excellence for the Valorization of Waste into high added value Product (CEA-VALOPRO) of Institut National Polytechnique Félix Houphouët-Boigny, Laboratoire des Energies Renouvelables et Efficacité Energétique (LabEREE) de l’Institut International d’Ingénierie de l’Eau et de l’Environnement (2IE), and the Intra-Africa mobility program of the European Union (RésIng project) for the arrangements put in place to carry out this study.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
References
- Cheng, L.; Jiang, H.; Xie, G.; Wang, J.; Peng, W.; Zhou, L.; An, F. Photosynthesis and Latex Burst Characteristics in Different Varieties of Rubber Trees (Hevea Brasiliensis) under Chilling Stress, Combing Bark Tensile Property and Chemical Component Analysis. Forests 2024, 15, 1408. [Google Scholar] [CrossRef]
- Lestari, A.; Yerizam, M.; Hasan, A. Characterization of Rubber Seed (Hevea Brasiliensis) as Raw Material for The Production of Biofuel. J. Appl. Agric. Sci. Technol. 2023, 7, 217–224. [Google Scholar] [CrossRef]
- Koné, G.A.; Good, M.; Kouba, M. Performance of Guinea Fowl Fed Hevea Seed Meal or Cashew Nut Meal as a Partial Substitute for Soya Bean Meal. Animal 2020, 14, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Widyarani; Coulen, S.C.W.; Sanders, J.P.M.; Bruins, M.E. Valorisation of Proteins from Rubber Tree. Waste Biomass Valorization 2017, 8, 1027–1041. [Google Scholar] [CrossRef]
- Tambunan, B.; Ambarita, H.; Sitorus, T.; Sebayang, A.; Masudie, A. An Overview of Physicochemical Properties and Engine Performance Using Rubber Seed Biodiesel–Plastic Pyrolysis Oil Blends in Diesel Engines. Automot. Exp. 2024, 6, 551–583. [Google Scholar] [CrossRef]
- Hussain, M.; Khan, I.; Jiang, B.; Zheng, L.; Pan, Y.; Hu, J.; Ashraf, A.; Salah, A.; Salahuddin, A.; Al-Ansi, W.; et al. Lipases: Sources, Immobilization Techniques, and Applications. Int. J. Environ. Agric. Biotechnol. 2023, 8, 094–121. [Google Scholar] [CrossRef]
- Godoy, C.A.; Pardo-Tamayo, J.S.; Barbosa, O. Microbial Lipases and Their Potential in the Production of Pharmaceutical Building Blocks. Int. J. Mol. Sci. 2022, 23, 9933. [Google Scholar] [CrossRef]
- Liu, X.; Kokare, C. Chapter 17–Microbial Enzymes of Use in Industry. In Biotechnology of Microbial Enzymes (Second Edition); Brahmachari, G., Ed.; Academic Press: New York, NY, USA, 2023; Volume 11, pp. 405–444. ISBN 978-0-443-19059-9. [Google Scholar]
- Okino-Delgado, C.H.; Pereira, M.S.; Prado, D.Z.D.; Fleuri, L.F. Evaluation of the Influence of Chemical and Physical Factors on Mixtures of Fungal and Plant Lipases. An. Acad. Bras. DeCiências 2022, 94, e20201268. [Google Scholar] [CrossRef]
- Gonawan, F.N.; Romli, M.M.; Zuhan, M.K.N.M.; Jaya, M.A.T. Immobilization of Candida Rugosa Lipase on the Glutaraldehyde-Activated Chitosan Beads. J. Chem. Eng. Ind. Biotechnol. 2022, 8, 33–41. [Google Scholar] [CrossRef]
- Ahmad, N.F.B.; Veny, H.; Che Mat, S.Z. Investigation on Enzymatic Activity of Rubber Seed as Source of Plant Lipase. Malays. J. Chem. Eng. Technol. 2020, 3, 45–50. [Google Scholar] [CrossRef]
- Al-Haidari, A.; Khudhair, S.; Alsaadawi, I. Extraction and Purification of Lipases Enzyme from Germinating Seeds of Four Crops. Iraqi J. Sci. 2020, 61, 2182–2188. [Google Scholar] [CrossRef]
- Kouteu, N.P. Mise En Œuvre Des Lipases Végétales Issues Des Graines Dans La Catalyse Enzymatique d’esters Éthyliques d’huiles Végétales Pour La Production de Biodiesel. Ph.D. Thesis, Université de Montpellier, SupAgro, Montpellier, France, 2017. [Google Scholar]
- Aghaei, H.; Ghavi, M.; Hashemkhani, G.; Keshavarz, M. Utilization of Two Modified Layered Doubled Hydroxides as Supports for Immobilization of Candida Rugosa Lipase. Int. J. Biol. Macromol. 2020, 162, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Alloue, W.A.M.; Aguedo, M.; Destain, J.; Ghalfi, H.; Blecker, C.; Wathelet, J.-P.; Thonart, P. Les lipases immobilisées et leurs applications. Biotechnol. Agron. Soc. Env. 2008, 12, 57–68. [Google Scholar]
- Barros, M.; Fleuri, L.F.; Macedo, G.A. Seed Lipases: Sources, Applications and Properties–a Review. Braz. J. Chem. Eng. 2010, 27, 15–29. [Google Scholar] [CrossRef]
- Xie, W.; Huang, M. Fabrication of Immobilized Candida Rugosa Lipase on Magnetic Fe3O4-Poly(Glycidyl Methacrylate-Co-Methacrylic Acid) Composite as an Efficient and Recyclable Biocatalyst for Enzymatic Production of Biodiesel. Renew. Energy 2020, 158, 474–486. [Google Scholar] [CrossRef]
- Moussavou, M.R.W.; Brunschwig, C.; Baréa, B.; Villeneuve, P.; Blin, J. Assessing the Enzyme Activity of Different Plant Extracts of Biomasses from Sub-Saharan Africa for Ethyl Biodiesel Production. Energy Fuels 2016, 30, 2356–2364. [Google Scholar] [CrossRef]
- Nanssou, K.P.A.; Baréa, B.; Barouh, N.; Blin, J.; Villeneuve, P. Lipase Activity of Tropical Oilseed Plants for Ethyl Biodiesel Synthesis and Their Typo- and Regioselectivity. J. Agric. Food Chem. 2016, 64, 8838–8847. [Google Scholar] [CrossRef]
- Moussavou, M.R.W.; Brunschwig, C.; Baréa, B.; Villeneuve, P.; Blin, J. Are Plant Lipases a Promising Alternative to Catalyze Transesterification for Biodiesel Production? Prog. Energy Combust. Sci. 2013, 39, 441–456. [Google Scholar] [CrossRef]
- Seth, S.; Chakravorty, D.; Dubey, V.K.; Patra, S. An Insight into Plant Lipase Research–Challenges Encountered. Protein Expr. Purif. 2014, 95, 13–21. [Google Scholar] [CrossRef]
- Istyami, A.; Sari, M.; Gultom, C.; Prakoso, T.; Soerawidjaja, T. Exploration of Novel Lipase from Plant Seeds and Plant Latexes. Indones. J. Chem. Res. 2024, 12, 1–8. [Google Scholar] [CrossRef]
- Moussavou, M.R.W. Synthèse Enzymatique D’esters Ethyliques D’huiles Végétales pour la Production de Biodiesel à L’aide de Lipases Végétales Issues de la Biomasse Africaine. Ph.D. Thesis, Université de Montpellier, SupAgro, Montpellier, France, 2014. [Google Scholar]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 18th ed.; AOAC: Baltimore, MD, USA, 2005. [Google Scholar]
- ASTM D1209; Test Method for Color of Clear Liquids (Platinum-Cobalt Scale). American Society for Testing and Materials ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]
- ISO 660; Animal and Vegetable Fats and Oils: Determination of Acid Value and Acidity. ISO: Geneva, Switzerland, 2020.
- ASTM D445; Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (Incl. Calculation of Dynamic Viscosity). American Society for Testing and Materials ASTM International: West Conshohocken, PA, USA, 2006.
- NF 6883; Animal and Vegetable Fats and Oils Determination of Conventional Mass per Volume (Litre Weight in Air). ISO: Geneva, Switzerland, 2017.
- Batel, W.; Graef, M.; Mejer, G.J.; Moller, R.; Schoedder, F.; Batel, W.; Graef, M.; Mejer, G.J.; Moller, R.; Schoedder, F. Vegetable Oils as Sources of Fuel and Energy. Grund. Landtech 1980, 30, 40–51. [Google Scholar]
- NF T60-201; Animal and Vegetable Fats and Oils: Determination of Moisture and Volatile Matter Content. ISO: Geneva, Switzerland, 2016.
- Okunwaye, T.; Obibuzor, J.U.; Okogbenin, E.A. Purification and Biochemical Properties of Lipase from Raphia Palm Fruit Mesocarp. Afr. J. Biochem. Res. 2015, 9, 73–80. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Gadge, P.P.; Madhikar, S.D.; Yewle, J.N.; Jadhav, U.U.; Chougale, A.D.; Zambare, V.P.; Padul, M.V. Biochemical Studies of Lipase from Germinating Oil Seeds (Glycine Max). Am. J. Biochem. Biotechnol. 2011, 7, 141–145. [Google Scholar] [CrossRef]
- Mohammadi, N.S.; Khiabani, M.S.; Ghanbarzadeh, B.; Mokarram, R.R. Improvement of Lipase Biochemical Properties via a Two-Step Immobilization Method: Adsorption onto Silicon Dioxide Nanoparticles and Entrapment in a Polyvinyl Alcohol/Alginate Hydrogel. J. Biotechnol. 2020, 323, 189–202. [Google Scholar] [CrossRef]
- Paichid, N.; Yunu, T.; Klomklao, S.; Prasertsan, P.; Sangkharak, K. Enhanced Synthesis of Fatty-Acid Methyl Ester Using Oil from Palm Oil Mill Effluents and Immobilized Palm Lipase. J. Am. Oil Chem. Soc. 2018, 95, 1373–1384. [Google Scholar] [CrossRef]
- Shamsudin, M.I.; Tan, L.S.; Tsuji, T.; Kiew, P.L. Production and Characterization of Biodiesel from Canola Oil through Enzymatic Transesterification. J. Phys. Conf. Ser. 2022, 2259, 012023. [Google Scholar] [CrossRef]
- Khan, M.F.; Kundu, D.; Hazra, C.; Patra, S. A Strategic Approach of Enzyme Engineering by Attribute Ranking and Enzyme Immobilization on Zinc Oxide Nanoparticles to Attain Thermostability in Mesophilic Bacillus Subtilis Lipase for Detergent Formulation. Int. J. Biol. Macromol. 2019, 136, 66–82. [Google Scholar] [CrossRef]
- Ameri, A.; Forootanfar, H.; Behnam, B.; Shakibaie, M.; Ameri, A.; Daneshpajooh, M.; Najafi, A.; Amirheidari, B. Optimization of Immobilization of Pseudomonas Cepacia Lipase on Multiwalled Carbon Nanotubes Functionalized with Glycyrrhizin and Tween 80. 3 Biotech 2021, 11, 260. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, W.; Cao, H. Biorefinery Methods for Extraction of Oil and Protein from Rubber Seed. Bioresour. Bioprocess. 2021, 8, 45. [Google Scholar] [CrossRef]
- Oluodo, L.A.; Huda, N.; Komilus, C.F. Potential Utilization of Rubber Seed Meal as Feed and Food. Int. J. Eng. 2018, 7, 64–71. [Google Scholar] [CrossRef]
- Sugebo, B.; Demrew, Z.; Feleke, S.; Molla, M. Evaluation and Characterization of Rubber Seed Oil for Biodiesel Production. In Biomass Conversion and Biorefinery; Springer: Berlin/Heidelberg, Germany, 2021; pp. 1–11. [Google Scholar] [CrossRef]
- Udo, M.D.; Ekpo, U.; Ahamefule, F.O. Effects of Processing on the Nutrient Composition of Rubber Seed Meal. J. Saudi Soc. Agric. Sci. 2018, 17, 297–301. [Google Scholar] [CrossRef]
- Sharma, B.B.; Saha, R.K.; Saha, H. Effets de l’alimentation Avec de La Farine de Graines de Caoutchouc Détoxifiée Sur Les Performances de Croissance et Les Indices Hématologiques Des Alevins de Labeo Rohita (Hamilton). Anim. Feed Sci. Technol. 2014, 193, 84–92. [Google Scholar] [CrossRef]
- Kouassi, K.E.; Abolle, A.A.; Yao, K.B.; Boa, D.; Adouby, K.; Drogui, P.; Tyagi, R.D. Optimization of Rubber Seed Oil Transesterification to Biodiesel Using Experimental Designs and Artificial Neural Networks. Green Sustain. Chem. 2017, 8, 39–61. [Google Scholar] [CrossRef]
- Brahma, S.; Nath, B.; Basumatary, B.; Das, B.; Saikia, P.; Patir, K.; Basumatary, S. Biodiesel Production from Mixed Oils: A Sustainable Approach towards Industrial Biofuel Production. Chem. Eng. J. Adv. 2022, 10, 100284. [Google Scholar] [CrossRef]
- Halim, A.H.A.; Zamberi, M.M.; Husin, M.H.M.; Haminudin, N.F.; Idral, F.; Ghani, S.A. Physicochemical Properties of Jatropha Curcas Oil as a Potential Feedstock for Biodiesel Production. Proc. Mech. Eng. Res. Day 2022, 283–284. [Google Scholar]
- Novidzro, K.M.; Wokpor, K.; Fagla, B.A.; Koudouvo, K.; Dotse, K.; Osseyi, E.; Koumaglo, K.H. Etude de quelques paramètres physicochimiques et analyse des éléments minéraux, des pigments chlorophylliens et caroténoïdes de l’huile de graines de Griffonia simplicifolia. Int. J. Biol. Chem. Sci. 2019, 13, 2360. [Google Scholar] [CrossRef]
- Domonhedo, H.; Cros, D.; Nodichao, L.; Billotte, N.; Ahanhanzo, C. Enjeux et amélioration de la réduction de l’acidité dans les fruits mûrs du palmier à huile, Elaeis guineensis Jacq. (synthèse bibliographique). Biotechnol. Agron. Société Environ. 2018, 22, 54–66. [Google Scholar] [CrossRef]
- Ducret, A.; Pina, M.; Montet, D.; Graille, J. Désacidification enzymatique des huiles hyperacides. Oléagineux 1989, 44, 603–607. [Google Scholar]
- Reksowardojo, I.; Bui, H.N.; Sok, R.; Kilgour, A.J.; Brodjonegoro, T.P.; Soerawidjaja, T.H. The Effect of Biodiesel Fuel Rubber (Hevea Brasiliensis) Seed Oil on a Direct Injection (DI) Diesel Engine. Asean Eng. J. 2011, 1, 65–81. [Google Scholar] [CrossRef]
- Jain, S.; Sharma, M.P. Stability of Biodiesel and Its Blends: A Review. Renew. Sustain. Energy Rev. 2010, 14, 667–678. [Google Scholar] [CrossRef]
- Kouassi, E.K.; Abolle, A.; Yao, B.; Boa, D. Essais de Transestérifications comparées par méthanolyse et éthanolyse de l’huile de palme: Mesure de la densité et de la viscosité en relation avec la structure moléculaire. Int. J. Innov. Appl. Stud. 2015, 12, 918–930. [Google Scholar]
- Paul, A.K.; Borugadda, V.B.; Reshad, A.S.; Bhalerao, M.S.; Tiwari, P.; Goud, V.V. Comparative Study of Physicochemical and Rheological Property of Waste Cooking Oil, Castor Oil, Rubber Seed Oil, Their Methyl Esters and Blends with Mineral Diesel Fuel. Mater. Sci. Energy Technol. 2021, 4, 148–155. [Google Scholar] [CrossRef]
- Mortazavi, S.; Aghaei, H. Make Proper Surfaces for Immobilization of Enzymes: Immobilization of Lipase and α-Amylase on Modified Na-Sepiolite. Int. J. Biol. Macromol. 2020, 164, 1–12. [Google Scholar] [CrossRef]
- Taghizadeh, T.; Ameri, A.; Talebian-Kiakalaieh, A.; Mojtabavi, S.; Ameri, A.; Forootanfar, H.; Tarighi, S.; Faramarzi, M.A. Lipase@zeolitic Imidazolate Framework ZIF-90: A Highly Stable and Recyclable Biocatalyst for the Synthesis of Fruity Banana Flavour. Int. J. Biol. Macromol. 2021, 166, 1301–1311. [Google Scholar] [CrossRef]
- Eze, O.F.; Chatzifragkou, A.; Charalampopoulos, D. Properties of Protein Isolates Extracted by Ultrasonication from Soybean Residue (Okara). Food Chem. 2022, 368, 130837. [Google Scholar] [CrossRef]
- Onoji, S.E.; Iyuke, S.E.; Igbafe, A.I.; Daramola, M.O. Hevea Brasiliensis (Rubber Seed) Oil: Modeling and Optimization of Extraction Process Parameters Using Response Surface Methodology and Artificial Neural Network Techniques. Biofuels 2019, 10, 677–691. [Google Scholar] [CrossRef]
- Takase, M.; Zhao, T.; Zhang, M.; Chen, Y.; Liu, H.; Yang, L.; Wu, X. An Expatiate Review of Neem, Jatropha, Rubber and Karanja as Multipurpose Non-Edible Biodiesel Resources and Comparison of Their Fuel, Engine and Emission Properties. Renew. Sustain. Energy Rev. 2015, 43, 495–520. [Google Scholar] [CrossRef]
- Hasan, N.; Yie, T.; Zain, N.; Suhaimi, M. Immobilization of Candida Rugosa Lipase in PVA-Alginate-Sulfate Beads for Waste Cooking Oil Treatment. J. Teknol. 2015, 74, 221–228. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: Principles and Biotechnological Applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef]
- Santos, K.C.; Cassimiro, D.M.J.; Avelar, M.H.M.; Hirata, D.B.; de Castro, H.F.; Fernández-Lafuente, R.; Mendes, A.A. Characterization of the Catalytic Properties of Lipases from Plant Seeds for the Production of Concentrated Fatty Acids from Different Vegetable Oils. Ind. Crops Prod. 2013, 49, 462–470. [Google Scholar] [CrossRef]
- Hoppe, A.; Theimer, R.R. Rapeseed Lipase—PH Dependent Specificity for Native Lipid Body Autolysis and Lipolysis of Artifical Oil Droplets. J. Plant Physiol. 1997, 151, 390–398. [Google Scholar] [CrossRef]
- Weerasooriya, M.K.B.; Kumarasinghe, A.A.N. Isolation of Alkaline Lipase from Rubber Seed—Partial Purification, Characterization and Its Potential Applications as a Detergent Additive. Indian J. Chem. Technol. 2012, 19, 244–249. [Google Scholar]
- Yao, L.W.; Ahmed Khan, F.S.; Mubarak, N.M.; Karri, R.R.; Khalid, M.; Walvekar, R.; Abdullah, E.C.; Mazari, S.A.; Ahmad, A.; Dehghani, M.H. Insight into Immobilization Efficiency of Lipase Enzyme as a Biocatalyst on the Graphene Oxide for Adsorption of Azo Dyes from Industrial Wastewater Effluent. J. Mol. Liq. 2022, 354, 118849. [Google Scholar] [CrossRef]
- Tizchang, S.; Khiabani, M.S.; Mokarram, R.R.; Hamishehkar, H.; Bahramifar, N.; Hoon, P.J.; Pyo, S.H. Bacterial Cellulose Nano Crystal as Hydrocolloid Matrix in Immobilized β-Galactosidase onto Silicon Dioxide Nanoparticles. LWT 2020, 123, 109091. [Google Scholar] [CrossRef]
- Ali, Z.; Tian, L.; Zhao, P.; Zhang, B.; Ali, N.; Khan, M.; Zhang, Q. Immobilization of Lipase on Mesoporous Silica Nanoparticles with Hierarchical Fibrous Pore. J. Mol. Catal. B Enzym. 2016, 134, 129–135. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Kim, S.M.; Mahmoud, S.; Younesi, H.; Nader, B.; Ji, H.P.; Sang-Hyun, P. Lipase-immobilized chitosan-crosslinked magnetic nanoparticle as a biocatalyst for ring opening esterification of itaconic anhydride. Biochem. Eng. J. 2019, 143, 141–150. [Google Scholar] [CrossRef]
- Aarthy, M.; Saravanan, P.; Gowthaman, M.K.; Rose, C.; Kamini, N.R. Enzymatic Transesterification for Production of Biodiesel Using Yeast Lipases: An Overview. Chem. Eng. Res. Des. 2014, 92, 1591–1601. [Google Scholar] [CrossRef]
- Arumugam, A.; Thulasidharan, D.; Jegadeesan, G.B. Process Optimization of Biodiesel Production from Hevea Brasiliensis Oil Using Lipase Immobilized on Spherical Silica Aerogel. Renew. Energy 2018, 116, 755–761. [Google Scholar] [CrossRef]
- Badgujar, V.C.; Badgujar, K.C.; Yeole, P.M.; Bhanage, B.M. Enhanced Biocatalytic Activity of Immobilized Steapsin Lipase in Supercritical Carbon Dioxide for Production of Biodiesel Using Waste Cooking Oil. Bioprocess Biosyst. Eng. 2019, 42, 47–61. [Google Scholar] [CrossRef]
- Guo, J.; Sun, S.; Liu, J. Conversion Des Déchets d’huile de Palme de Friture En Biodiesel En Utilisant La Lipase A Libre de Candida Antarctica Comme Nouveau Catalyseur. Fuel 2020, 267, 117323. [Google Scholar] [CrossRef]
- Green, K.D.; Nakajima, M. Evaluation of Immobilized Modified Lipase: Aqueous Preparation and Reaction Studies in n-Hexane. J. Am. Oil Chem. Soc. 1998, 75, 1519–1526. [Google Scholar] [CrossRef]
- Palocci, C.; Soro, S.; Cernia, E.; Fiorillo, F.; Belsito, C.M.A.; Monacelli, B.; Delle Monache, G.; Pasqua, G. Isoenzymes Lipolytiques Du Latex d’Euphorbia. Plant Sci. 2003, 165, 577–582. [Google Scholar] [CrossRef]
- Binhayeeding, N.; Klomklao, S.; Prasertsan, P.; Sangkharak, K. Improvement of Biodiesel Production Using Waste Cooking Oil and Applying Single and Mixed Immobilised Lipases on Polyhydroxyalkanoate. Renew. Energy 2020, 162, 1819–1827. [Google Scholar] [CrossRef]
- Quayson, E.; Amoah, J.; Rachmadona, N.; Hama, S.; Yoshida, A.; Kondo, A.; Ogino, C. Biodiesel-Mediated Biodiesel Production: A Recombinant Fusarium Heterosporum Lipase-Catalyzed Transesterification of Crude Plant Oils. Fuel Process. Technol. 2020, 199, 106278. [Google Scholar] [CrossRef]
- Mujtaba, M.A.; Haeng, M.; Masjuki, H.H.; Kalam, M.A.; Farooq, M.; Manzoore, E.M.S.; Gul, M.; Afzal, A.; Waqar, A.; Asad, R.; et al. Effect of primary and secondary alcohols as oxygenated additives on the performance and emission characteristics of diesel engine. Energy Rep. 2021, 7, 1116–1124. [Google Scholar] [CrossRef]
- Baweja, S.; Trehan, A.; Kumar, R. Combustion, Performance, and Emission Analysis of a CI Engine Fueled with Mustard Oil Biodiesel Blended in Diesel Fuel. Fuel 2021, 292, 120346. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).