Abstract
Iron-based oxygen carriers (OCs) have received much attention due to their low costs, high mechanical strengths and high-temperature stabilities in the chemical looping gasification (CLG) of biomass, but their chemical reactivity is very ordinary. Converter steel slags (CSSs) are steelmaking wastes and rich in Fe2O3, CaO and MgO, which have good oxidative ability and good stability as well as catalytic effects on biomass gasification. Therefore, the composite OCs prepared by mechanically mixing CSSs with iron-based OCs are expected to be used to increase the hydrogen production in the CLG of biomass. In this study, the catalytic performance of CSS/Fe2O3 composite OCs prepared by mechanically mixing CSSs with iron-based OCs on the gasification of brewers’ spent grains (BSGs) were investigated in a tubular furnace experimental apparatus. The results showed that when the weight ratio of the CSSs in composite OCs was 0.5, the relative volume fraction of hydrogen reached the maximum value of 49.1%, the product gas yield was 0.85 Nm3/kg and the gasification efficiency was 64.05%. It could be found by X-ray diffraction patterns and scanning electron microscope characterizations that the addition of CSSs helped to form MgFe2O4, which are efficient catalysts for H2 production. Owing to the large and widely distributed surface pores of CSSs, mixing them with iron-based OCs was beneficial for catalytic steam reforming to produce hydrogen.
1. Introduction
Brewers’ spent grains (BSGs) are the residues produced after the fermentation of barley during the brewing process of beer. For each ton of beer, about 0.25 tons of BSGs will be produced. In China, the annual output of beer was more than 35 million kiloliters in 2023. In this situation, the annual production of BSGs has surpassed 10 million tons and continues to rise. Untreated BSGs will rapidly deteriorate after 4–7 days and pollute the environment [1]. The accumulation of untreated BSGs poses various environmental risks, including hypoxia and eutrophication of water bodies, as well as the salinization and acidification of soil [2]. BSGs were frequently utilized as animal feed due to their high content of crude protein and micronutrients [3]. However, after being exposed to the air for a certain period, BSGs may become rancid due to fermentation. Long term or excessive feeding of these BSGs can cause poisoning in animals. When dried, BSGs possess a high heating value and can be incinerated for thermal energy utilization [3]. Nevertheless, the high inherent moisture content in BSGs presents challenges to their combustion, including elevated carbon content in the residues, incomplete combustion, and potential NOx emissions due to their higher nitrogen content [4].
Chemical looping gasification (CLG) is realized between an oxidation reactor and a reduction reactor. This process uses highly active oxygen carrier particles for circulation to play a role in carrying oxygen, carrying heat and catalyzing reactions [5]. In the reduction reactor, nitrogen-free hydrogen-rich syngas is produced, while in the oxidation reactor, the reduced oxygen carriers (OCs) are oxidized and regenerated. The CLG process also can use calcium oxide as a carrier of carbon dioxide between two reactors. The capture of the CO2 from the reduction reactor by the CaO particles can increase the rate of the forward water–gas shift reaction, thus resulting in a higher hydrogen yield in the product gas [6]. The utilization of OCs can avoid the use of expensive pure oxygen for excluding the production of nitrogen. Therefore, using CLG technology for the thermochemical conversion of BSGs can achieve high-value utilization of BSGs while disposing of them. In addition, many OCs have a catalytic effect on the thermal decomposition or reforming of tars formed, resulting in the production of other small molecules and the tar reduction in product gas [7]. In a word, the performances of carrier particles, involving redox, capturing CO2 or catalysis, are critical for the production of high-quality syngas.
Currently, oxides of Fe, Ni, Cu, Mn or Co are common alternative oxygen-carrying materials. Among these, iron-based OCs are particularly attractive due to their low cost, high mechanical strength and thermal stability [8,9]. However, iron-based OCs exhibited mediocre reactivity in the gasification reactions of different feedstocks, such as coal, biomass [9,10], biomass char [11], microalgae [12], cellulose [13] and petroleum coke [14,15]. The reactivities of iron-based oxides are not excellent, which may hinder their application and development [16,17]. To enhance the reactivities, different alkaline earth metals (e.g., Ca, Ba, or Mg) can be used to modify iron-based OCs. These modifications improve the oxygen carriers’ reactivities and enhance the diffusivity of lattice oxygen [18,19,20]. Steel slags (SSs) are solid wastes produced in the steelmaking and steel refining processes which damage the environment when improperly managed. The SSs contain metallic elements like Fe, Ca and Mg, and their main crystalline structures belong to perovskite and wüstite. Some studies showed that using SSs as OCs in chemical looping gasification can ensure the high reactivity of OCs [21,22].
Some scholars used iron-based OCs to produce syngas through chemical looping gasification of a certain type of biomass feedstock. For instance, Nguyen et al. [23] explored the efficiency of syngas production in the chemical looping gasification of torrefied woodchips within a pilot-scale bubbling fluidized bed system, utilizing iron ore as an OC. Their findings indicated that the carbon conversion efficiency for iron ore peaked at 91.42% when the oxygen-carrier-to-biomass ratio (OBR) was set at 6, while the content of H2 reached a peak of 42.89% for iron ore at a steam-to-biomass mass ratio (SBR) of 1.4. Their study showed that the increase in the OBR value caused a reduction in H2 production, a downward trend in syngas yield and an upward trend in carbon conversion efficiency. For H2 production in the CLG process, the SBR value is crucial. Nguyen’s study also stated that the content of H2 increased with the steam amount used; higher SBR values also enhanced the syngas yield as well as the product gas yield and process efficiencies, but excess steam may cause a decline in the CLG performance due to heat loss in the gasifier. Huang et al. [24] used iron-based OCs to produce syngas through the chemical looping gasification of rice husks and analyzed the effects of the ratio of oxygen carriers to rice husks (O/C), temperature, residence time and preparation methods of iron-based OCs. Their findings indicated that as the O/C ratio rose from 0.5 to 3.0, there was a gradual increase in gas production, the H2/CO ratio, CO2 yield and carbon conversion efficiency. Conversely, the yields of H2, CO and CH4, as well as the lower heating value (LHV), experienced a gradual decline.
Some studies have also explored the synergistic effects of two different feedstocks in chemical looping co-gasification (CLCG). Luo et al. [25] conducted a CLCG of rice husks with coal in a fixed-bed reactor using iron ore as an oxygen carrier and investigated the influence of parameters such as the ratio of oxygen carriers to rice husks (O/C), reaction temperature, reaction residence time and rice husk blending ratio on the gasification performance. The findings indicated that the CLCG process delivered the optimal performance at an oxygen/carbon ratio of 0.2, a furnace temperature of 900 °C and a steam flow rate of 0.125 g/min. When the blending ratio of rice husks was 50%, the synergistic effect reached the highest value. In another study, Liu et al. [26] carried out a CLCG of pine wood and polyethylene using CaO/Fe2O3 oxygen carriers in a fixed-bed reactor and thermogravimetric analyzer (TGA) and investigated the effects of the mass ratio of Fe2O3 to biomass and temperature, and the mass ratio of steam to biomass, on the syngas yield, H2/CO ratio and cold gas efficiency. It was found that the optimal product distribution was obtained at a gasification temperature of 850 °C, a mass ratio of Fe2O3 to biomass of 0.25, and a mass ratio of steam to biomass of 0.25. The addition of polyethylene increased the syngas and hydrogen yield, and the optimal addition ratio of polyethylene was 75%.
Beyond the feedstocks, attention has been focused on the choice of OCs. Sun et al. [27] proposed chemical looping deoxygenation gasification (CLDG) for the preparation of high-quality syngas with an adjustable H2/CO ratio and the use of CO2. In their study, a thermodynamic simulation was conducted to verify the feasibility of CLDG using Aspen Plus. Biomass was gasified to produce biochar, tar and syngas in the designed deoxygenation reactor. Through two deoxygenation reactions (2CaO + 2Fe + 3H2O = Ca2Fe2O5 + 3H2 and 2CaO + 2Fe + 3CO2 = Ca2Fe2O5 + 3CO), Ca2Fe2O5 was synthesized. The desired H2/CO ratio in the syngas could be obtained by regulating the H2O and CO2 flow rates, while tar cracking was achieved through CaO as catalysis. Wei et al. [28] investigated the CLG of biomass in a fluidized bed reactor using Fe–Ni bimetallic oxides as OCs. Their experimental results indicated that the concentrations of CO, H2 and CH4 in the syngas, along with the carbon conversion rate and gasification efficiency, increased with rising reaction temperatures, while CO2 fractions decreased due to exothermic processes. However, it was observed that as the biomass-to-oxygen-carrier ratio increased, the carbon conversion efficiency declined. This could be due to the limited availability of reactive sites on the oxygen carrier at higher biomass loadings.
Several studies have investigated the role of steel slags in promoting CLG. Zhang et al. [29] conducted an in-depth investigation to evaluate the properties of basic oxygen furnace slags (BOFSs) and electric arc furnace slags (EAFSs) as OCs for the CLG of sewage sludge. Their findings revealed that the surface roughness and wear resistance of BOFSs were higher than those of EAFSs. Additionally, at both 800 °C and 900 °C under identical redox cycles, the oxygen transport capacity of BOFSs exceeded that of EAFSs, resulting in a significantly higher content of combustible components in each cycle. These findings offer a theoretical foundation for using steel slags as OCs in the CLG of solid wastes. Niu et al. [15] used several Ca- and Mg-rich steelmaking wastes from the steelmaking industry as OCs in the CLG of biomass. Their study indicated that the reducibility of Linz–Donawitz converter slags (LD slags) and blast furnace dust (BF dust) was superior to that of hematite, and LD slags presented excellent stability during multiple redox cycles, while BF dust showed poor stability. Furthermore, Ca- and Mg-rich waste exhibited a higher syngas production, which could be attributed to the improved reduction rate of Fe2O3 and gasification rate of biomass by Ca and Mg in steelmaking waste. Pan et al. [30] used phosphogypsum (PG) and steel slags (SSs) to prepare OCs for hydrogen-enriched syngas production through CLG technology. In their study, the reaction mechanism and the impact of operating parameters were analyzed using FactSage 7.1 simulations and then validated through fixed-bed tests. The outcomes demonstrated that the SSs notably boosted the effectiveness of the developed OCs.
In this study, iron-based OCs were first prepared by the co-precipitation method and put into a tubular furnace for the gasification of BSGs. The effects of operation parameters such as the reaction zone temperature T, the OCs-to-BSGs mass ratio (OC/SG) and the steam flow rate Qsteam on the gasification performance were investigated in the presence of the iron-based OCs. The optimal process conditions for BSGs gasification were determined by the product gas yield and gasification efficiency. Subsequently, CSS/Fe2O3 composite OCs were prepared by mechanically mixing converter steel slags with iron-based OCs. The effects of the weight ratio of CSSs in CSS/Fe2O3 composite OCs on the CLG process of BSGs were investigated, and then the optimal weight ratio of CSSs in the composite OCs was determined. Finally, the CSS/Fe2O3 composite OCs were analyzed by X-ray diffraction patterns and characterized by scanning electron microscope images in order to explore their function mechanism on the CLG process.
2. Experimental Section
2.1. Materials
BSGs from Qingdao Brewery in China were used as biomass raw materials for gasification. Before the experiment, they were first naturally air-dried, then pulverized into particles with particle sizes of 100–200 μm and finally put into an electrothermal blowing dry box at 105 °C for 6 h. The proximate analysis and ultimate analysis results of BSGs are shown in Table 1.

Table 1.
Proximate analysis and ultimate analysis results of BSGs.
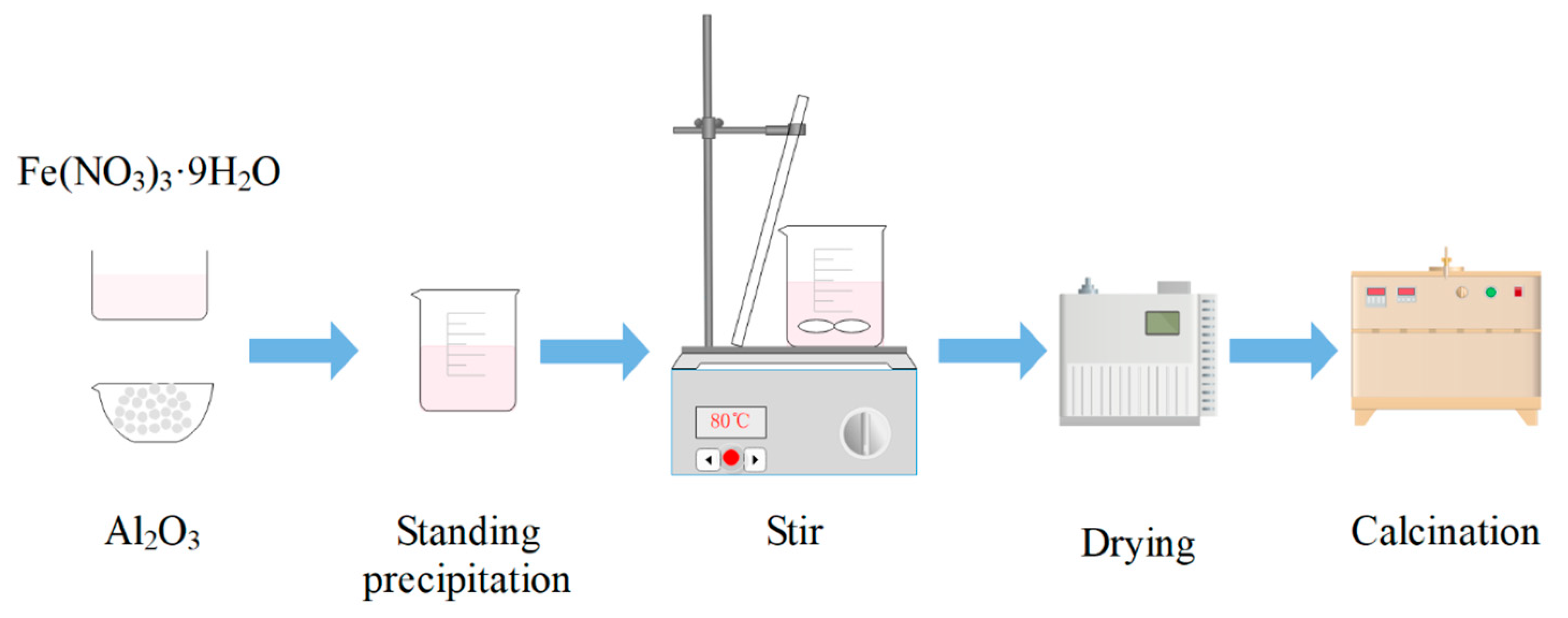
The iron-based OCs used were prepared from Fe(NO3)3·9H2O and α-Al2O3 by co-precipitation method. The masses of Fe(NO3)3·9H2O and α-Al2O3 were calculated based on a molar ratio of Fe2O3 to carrier α-Al2O3 of 6:4. The preparation procedure of iron-based OCs is shown in Figure 1. In a glass beaker, Fe(NO3)3·9H2O and α-Al2O3 were mixed together, and distilled water was added to the beaker and stirred well. The beaker was allowed to stand for 4 h and then placed in a water bath so the mixture could be stirred at 80 °C until no water was separated out. Then, the mixture was put into a blowing dry box under a constant temperature of 160 °C to be dried, and the drying time was 24 h. After this process, the oxygen carriers’ precursors were obtained. Finally, the oxygen carriers’ precursors were calcined at 900 °C in a muffle furnace for 6 h to obtain the samples of iron-based OCs. Oxygen carrier samples were pulverized into particles with particle sizes of 200–300 μm.
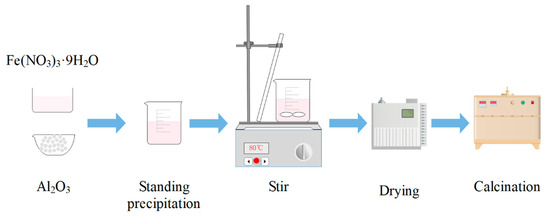
Figure 1.
Preparation procedure of iron-based oxygen carriers.
The CSSs came from a steelmaking plant in Hebei province of China, and were pulverized and then calcined in a muffle furnace at 900 °C for 1 h. The main compositions of the CSSs were analyzed by an X-ray fluorescence spectrometer (XRF). Table 2 lists the main composition of the CSSs before calcination.

Table 2.
Main composition of converter steel slags.
2.2. Experimental Apparatus and Methods
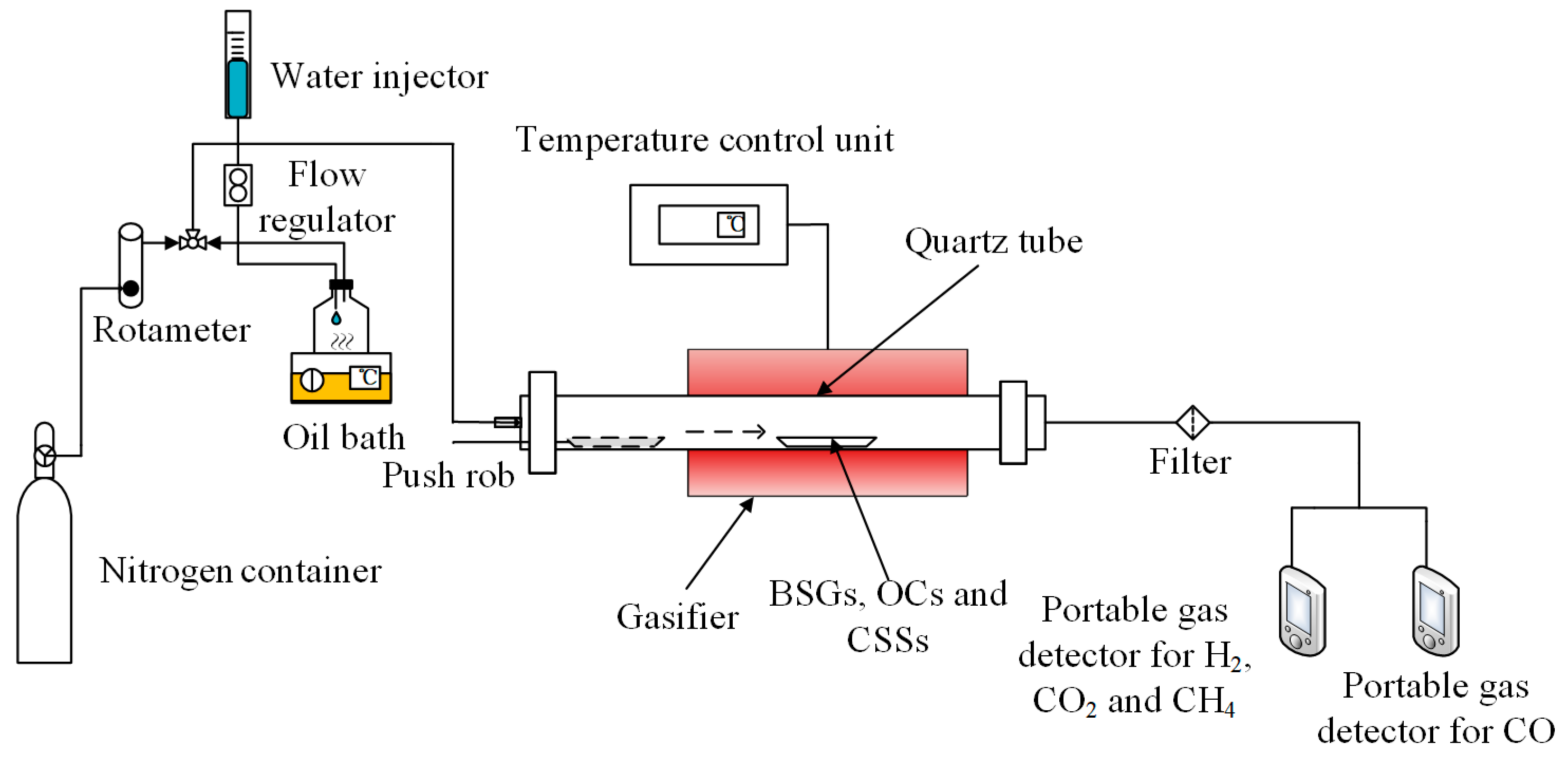
The gasification experiments were carried out in a self-designed experimental apparatus, which mainly consisted of a gas supply section, a steam generator, a tubular furnace and two portable gas detectors (MS400-CO-K and MS400-K) produced by SAACOO Instruments Ltd. in Chongqing of China, as shown in Figure 2. A ceramic tube with an inner diameter of 18 mm and a length of 540 mm was used in the tubular furnace. Distilled water was fed into the steam generator at a fixed flow rate where it was heated to steam in an instant. The generated steam was mixed with nitrogen as the gasification agent, and it was fed into the tubular furnace by nitrogen as carrier gas. The flow rate of nitrogen was controlled by a rotameter.
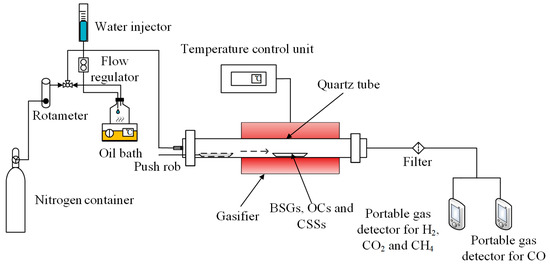
Figure 2.
Schematic illustration of experimental apparatus for gasification of BSGs.
A certain number of OCs or CSSs were homogeneously mixed with 0.25 g of BSGs and placed in the corundum boat. Prior to the reaction, the corundum boat was positioned at the reactor inlet. The reactor was heated to the controlled temperature in air and purged with a mixed airflow of nitrogen and water vapor for 15 min to keep the reactor under a mixed atmosphere. The corundum boat was then rapidly pushed into the center position in the reactor for the CLG. The whole experiment process was carried out under the mixed atmosphere of nitrogen and water vapor. At the end of the reaction, the reactor was cooled to room temperature in a nitrogen atmosphere. The gas produced at the reactor outlet was condensed and dried, with the volume percentages of the four gases—H2, CH4, CO and CO2—monitored in real time using two portable gas detectors (MS400-CO-K and MS400-K).
The phases present in different OCs or CSSs were determined by the X-ray diffraction (XRD) method. The XRD instrument type used in this study was Ultima IV. All samples were scanned at angles ranging from 10° to 80°. Among these, the fresh CSS/Fe2O3 composite OCs and used CSS/Fe2O3 composite OCs were mixed powders, which were scanned at a rate of 2°/min. The fresh iron-based OCs and calcined CSSs were scanned at a rate of 10°/min.
The surface microstructures of different OCs or CSSs were characterized by the scanning electron microscope (SEM) method. The SEM instrument type used in this study was JSM-7800F. The SEM images of different samples were taken at magnifications of 5000× and 10,000×.
The chemical reactivities of different OCs were investigated by temperature-programmed reduction of hydrogen (H2-TPR) tests in a chemisorption analyzer. The H2-TPR instrument type used was Micromeritics AutochemIII2930, and the manufacturer is Micromeritics located in Norcross, GA, USA. The carrier gas used for H2-TPR tests was a mixture of H2 and Ar, with H2 accounting for 10%, and the measured flow rate was 30 mL/min. The heating rate was 10 °C/min.
The cyclic stability of CSS/Fe2O3 composite OCs during the CLG of BSGs was investigated by ten redox cycle experiments at 900 °C. After each CLG experiment, air was introduced into the gasifier and blown for 30 min to ensure complete oxidation of the reduced OCs.
2.3. Data Evaluation
The relative volume fraction Ri of a certain gas component i (CO, H2, CO2 or CH4), relative to the sum of CO, H2, CO2 and CH4, was calculated using Equation (1).
where Ri was dimensionless; Vi represents the volume fraction of gas component i in the measured product gas, %; and indicates the volume fraction of the sum of CO, H2, CO2 and CH4 in the measured product gas, %.
The low calorific value of syngas, QL, kJ/m3, is the most critical parameter for evaluating the quality of gas produced by gasification, calculated using the following formula [23]:
where RCO, RH2 and RCH4 denote the relative volume fractions of CO, H2 and CH4 to the sum of CO, H2, CO2 and CH4, respectively, without dimension.
The total gas production per unit mass of BSGs gasification is defined as
where is the volume flow rate of nitrogen, set to a fixed value according to the experimental needs; t is the time for the reaction to collect the gas; represents the volume fraction of nitrogen in the total product gas; and is the mass of the BSGs.
The gas production Gi for each gas component can be expressed as
where Gi is defined as the volume of each gas component produced per unit mass of solid fuel under standard conditions.
The measured syngas yield Gy can be expressed as
The gasification efficiency η can be calculated by the following equation:
where the gasification efficiency η is defined as the ratio of the lower calorific value of the product gas to the lower calorific value of the feedstocks and QL,B is the lower calorific value of BSGs feedstocks, kJ/kg.
3. Chemical Reactions Involved
This study investigated the effects of the main operating parameters—such as temperature, mass ratio of OCs to BSGs, flow rate of water vapor and mass ratio of OCs to converter steel slags—on the CLG of BSGs. When biomass is heated in a limited supply of oxygen, it is first pyrolyzed or broken down into solid carbon, condensable gases and non-condensable gases. The condensable gases include tar, heavier hydrocarbons and water vapor, while the non-condensable gas mixture comprises lower-molecular-weight gases such as carbon dioxide, carbon monoxide, methane, ethane and ethylene [31]. Solid carbon, condensable gases and lower-molecular-weight non-condensable gases, along with lattice oxygen from the OCs, participate in gasification reactions to produce combustible or synthesis gas. This process involves numerous chemical reactions, which we illustrate using solid carbon and lower-molecular-weight non-condensable gases as raw materials. The related reactions are detailed in Reactions (R1)–(R13). Since the hydrocarbons in the product gas are mainly dominated by methane, the overall trend of hydrocarbons in the gasification process was revealed by analyzing the change rule of methane with respect to the operating parameters.
Oxidations of solid carbon and lower-molecular-weight non-condensable gases involve the following reactions [8]:
The producer gas reaction produces hydrogen and carbon monoxide from carbon and water vapor, as expressed by the following reaction:
The water–gas shift reaction is used for maximization of hydrogen production and is expressed by the following reaction:
The gasification of char in carbon dioxide is known as Boudouard reaction, and is expressed as follows:
The two main purposes of using catalysts in the gasification of BSGs are the removal of tar from the product gas and reduction of the methane content in the product gas. Catalytic gasification was developed in response to the need for tar reforming in which both alkaline earth metal catalysts and iron-based catalysts are effective [32]. As the product gas passes through the catalyst particles, the tar or condensable hydrocarbons can be reformed with steam or carbon dioxide at the catalyst surface to produce additional hydrogen and carbon monoxide. In this way, the catalytic tar reforming reaction yields extra fuel gas, replacing unwanted tar or soot, thereby increasing both the gas yield and the calorific value of the product gas [33].
The steam reforming reaction of tar or condensable hydrocarbons can be expressed simply, as in the following reaction [34]:
The carbon dioxide reforming (or dry reforming) reaction of tar or condensable hydrocarbons can similarly be written as in the following reaction [34]:
Calcined converter steel slags contain alkaline earth metal oxides such as CaO and MgO, which are very effective and inexpensive for the reduction of tar [35]. These materials can serve as catalysts for the thermal conversion of tar when mixed with BSGs. However, alkaline earth metal oxides are less effective for methane conversion, unless they serve as a support, a promoter or a constituent element in specific mineral phases [36].
Catalytic steam reforming or catalytic carbon dioxide reforming of lower-molecular-weight hydrocarbons in the presence of iron-based catalysts can be utilized to remove lower-molecular-weight hydrocarbons such as methane from the product gas [37]. This type of reforming is crucial for syngas production, especially when methane is undesirable and a precise ratio of CO and H2 in the product gas is required. In the presence of the catalysts, methane is converted to CO and H2 by reacting with steam at a high temperature (typically 750–950 °C) [38] or is reformed by carbon dioxide at a temperature range of 700 to 900 °C [39].
The steam reforming of methane can be expressed as follows:
The carbon dioxide reforming of methane offers the unique advantage of reducing two greenhouse gases (CO2 and CH4) in a single reaction. This reaction is highly heat absorbing, and can be expressed as follows:
Methane reforming requires high thermal stability of the catalyst [39], and MgO in CSSs has both a high temperature stability and high surface area [15], making it suitable as a carrier for the catalyst. The addition of alkali metals helps to improve the stability and selectivity of methane-reforming catalysts. Alkali metals can enhance the alkalinity of the catalyst surface, improve the adsorption capacity of reaction gases, increase the dispersion of active constituent Fe and suppress the carbon deposition on the catalyst surface [38,39]. Table 3 presents the main composition of the ashes in BSGs, which contain a high content of inherent potassium and a significant amount of inherent sodium. This composition enhances the auxiliary effects of the potassium and sodium during the gasification process, thereby reducing tar yield and converting lower-molecular-weight hydrocarbons, such as methane, to a certain extent, to CO and H2 in the product gas.

Table 3.
Main compositions of ashes in brewers’ spent grains.
4. Results and Discussion
4.1. Effects of Temperature
The influence of gasification temperature on chemical looping gasification is primarily reflected in its effects on the reaction rate and the extent of reversible reactions. Experiments were carried out at five different gasifier temperatures in the range of 750 °C–950 °C to investigate the effects of the gasifier temperature on the CLG of BSGs. The mass ratio of the OCs to BSGs was set as 1.0, and the flow rate of water vapor was maintained at 0.125 g/min.
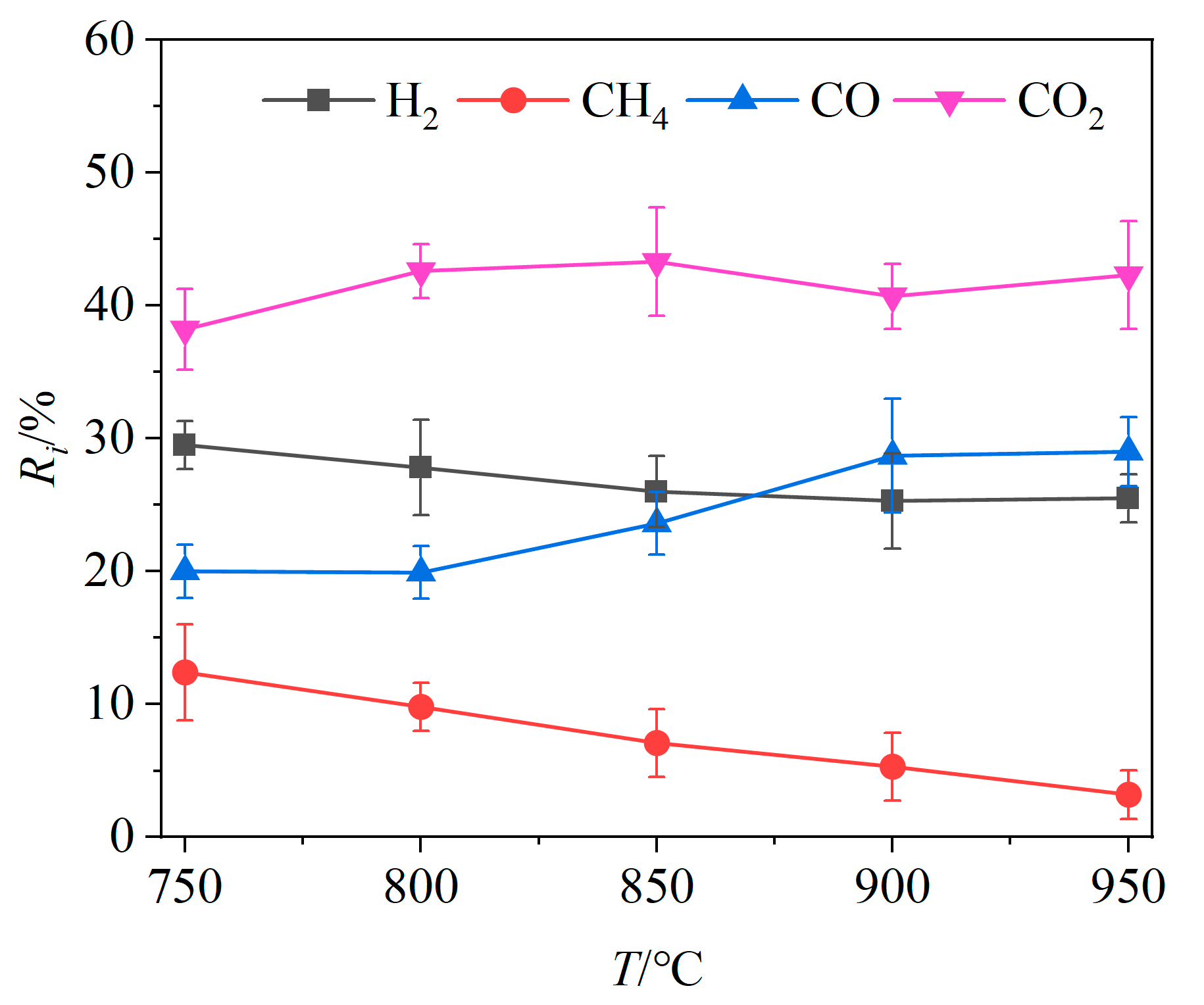
The relative volume fractions Ri of the four main gas components in the product gas at different temperatures are shown in Figure 3. As the gasifier temperature increased, the relative volume fractions of CO and CO2 exhibited an overall increasing trend, increasing, respectively, by 9.08% and 2.46%, while the relative volume fractions of H2 and CH4 declined by 3.96% and 9.19%, respectively. In this temperature interval, the reactivity of Fe2O3 is enhanced at elevated temperatures, promoting oxidation reactions of unburned combustible gases and reducing the relative volume fractions of H2 and CH4. In addition, the reactions between carbon-containing combustibles and OCs (Reactions (R1) and (R2), Reactions (R4)–(R10)), the producer gas reaction (Reaction (R11)) and the Boudouard reaction (Reaction (R13)) are all heat absorbing. Higher temperatures promote these heat-absorbing reactions, leading to an increase in the CO and CO2 content. While the intensification of the partial oxidation (Reactions (R5)–(R9)) also raised the H2 content, the intensification of the H2 oxidation (Reaction (R3)) accelerated the thermal conversion of H2, resulting in an overall decrease in its relative volume fraction as the temperature rises. Therefore, the relative volume fractions of CO2 and CO showed an increasing trend. When the gasifier temperature exceeded 900 °C, the relative volume fractions of CO and CO2 did not change significantly, likely due to the propensity of brewers’ spent grains to undergo coking at excessively high temperatures, which reduces gas production [40].
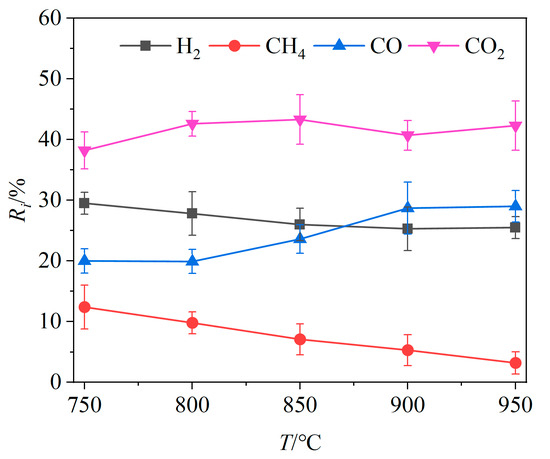
Figure 3.
Effects of gasifier temperature on relative volume fractions of gas components.
Since the relative volume fractions of combustible gases such as H2 and CH4 tend to decrease at higher temperatures, the QL value decreased from 10,729.65 kJ/kg to 8069.22 kJ/kg when the gasifier temperature was increased from 750 °C to 950 °C. This indicates that higher temperatures lead to a reduction in the calorific value of the product gas, which is detrimental for obtaining gaseous products with a higher calorific value.
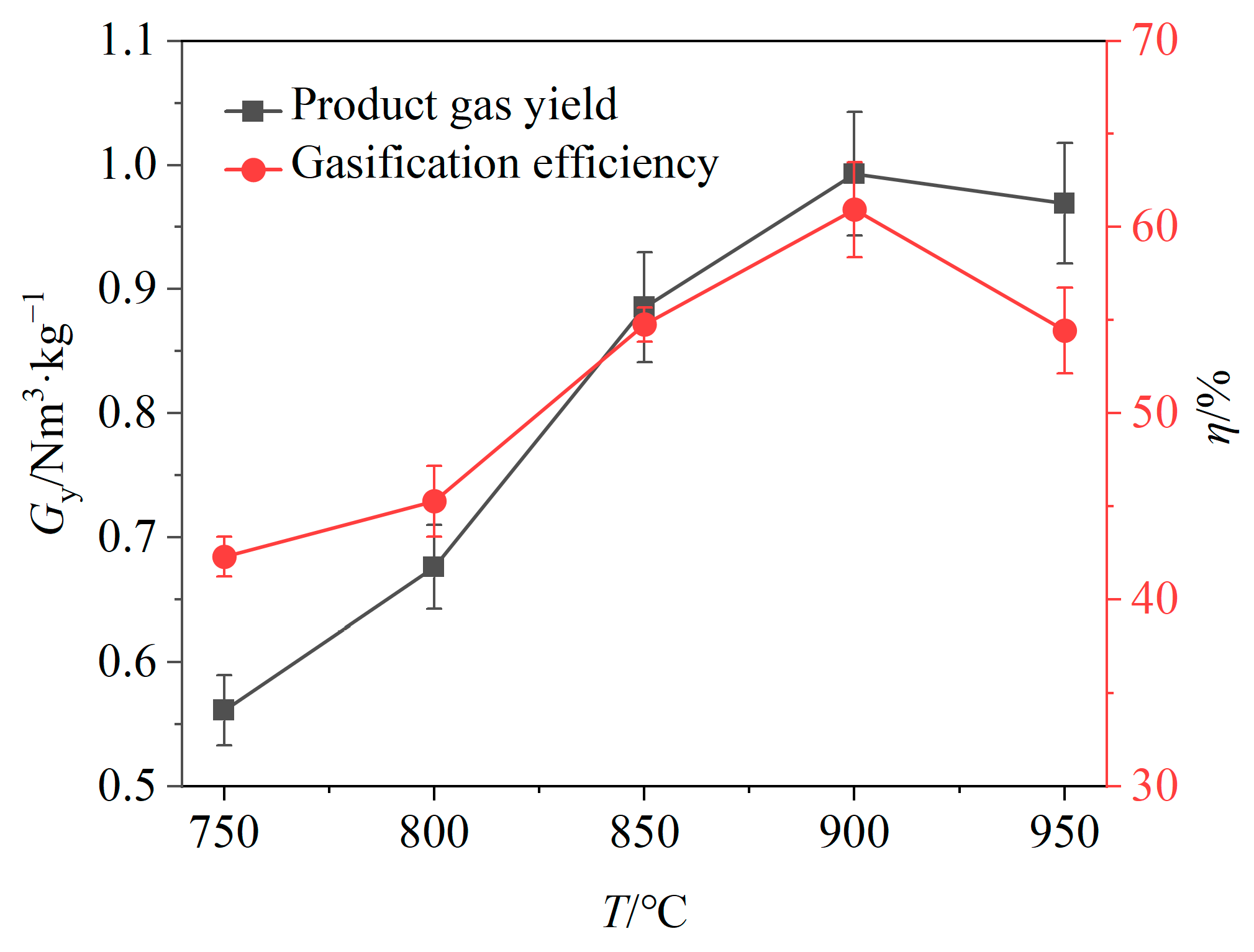
The impact of the gasification temperature on the gas yield and gasification efficiency is depicted in Figure 4. It is evident that both gas yield and gasification efficiency generally increase with a rising temperature. This suggests that high temperatures promote pyrolysis of solid products, gasification of carbon and reactions between the BSGs and OCs, leading to an increase in the relative volume fractions of gaseous products. When the temperature increased from 750 °C to 900 °C, the gas yield and gasification efficiency both exhibited upward trends, increasing by 0.44 Nm3/kg and 18.65%, respectively. However, when the temperature increased from 900 °C to 950 °C, both the gas yield and gasification efficiency declined, decreasing by 0.03 Nm3/kg and 6.50%, respectively.
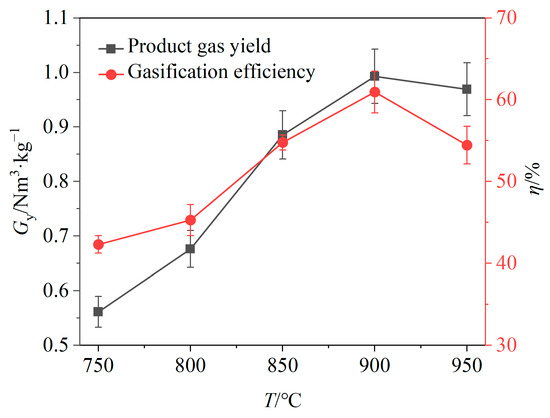
Figure 4.
Effects of temperature on gas yield and gasification efficiency.
The gasifier temperature significantly influences the performance of the chemical looping gasification. An increase in temperature markedly improves the syngas yield and gasification efficiency; however, it is essential to avoid excessively high temperatures because of the low ash melting point of biomass. Higher temperatures may lead to bed sintering and ash deposition on the surface of OCs [25]. In addition, gasification efficiency is an important indicator for evaluating the conversion results of gasification feedstocks. As the gasifier temperature was increased from 900 °C to 950 °C, the gasification efficiency obviously decreased, prompting the selection of 900 °C for subsequent experiments.
4.2. Effects of OC/SG Ratio
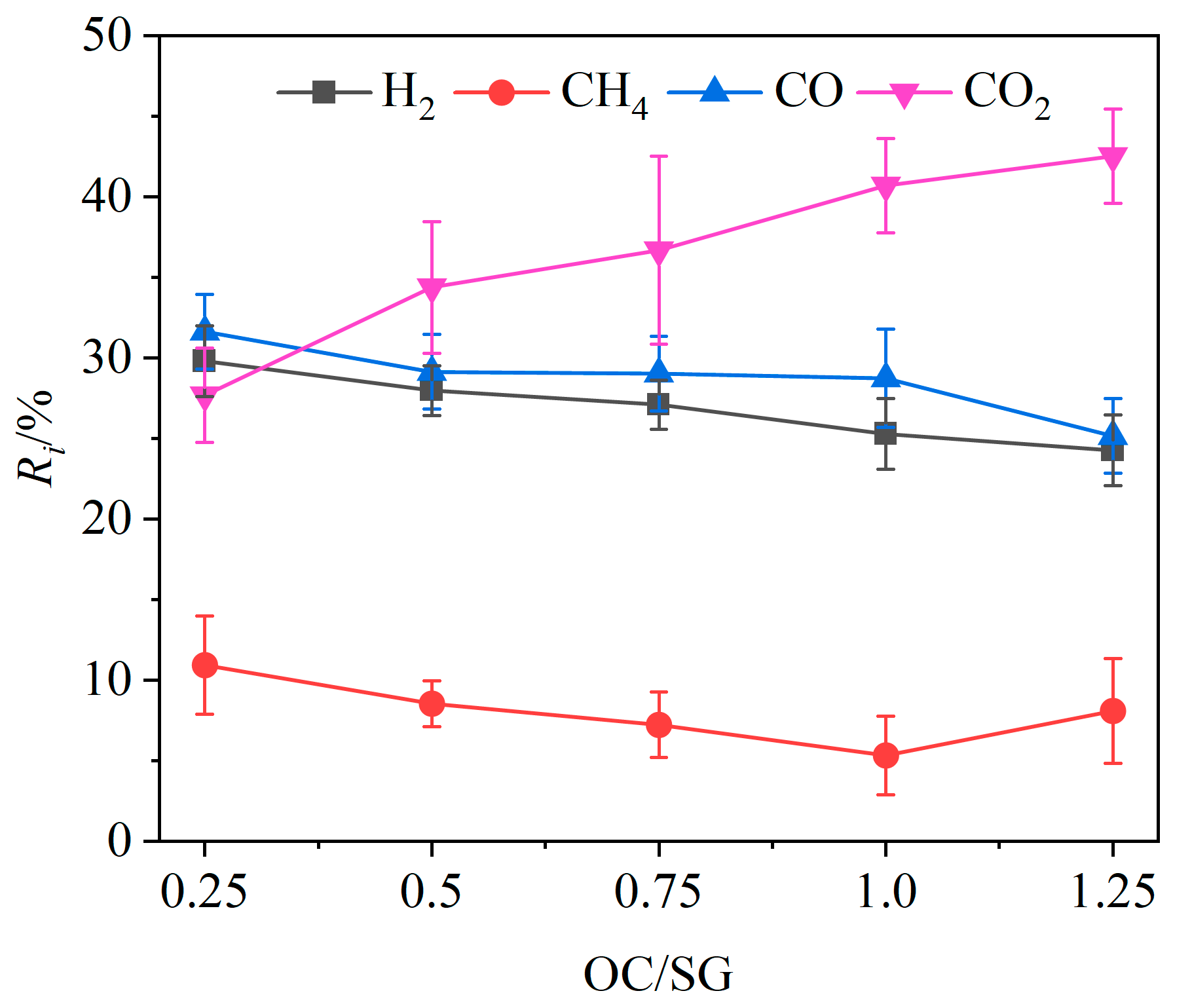
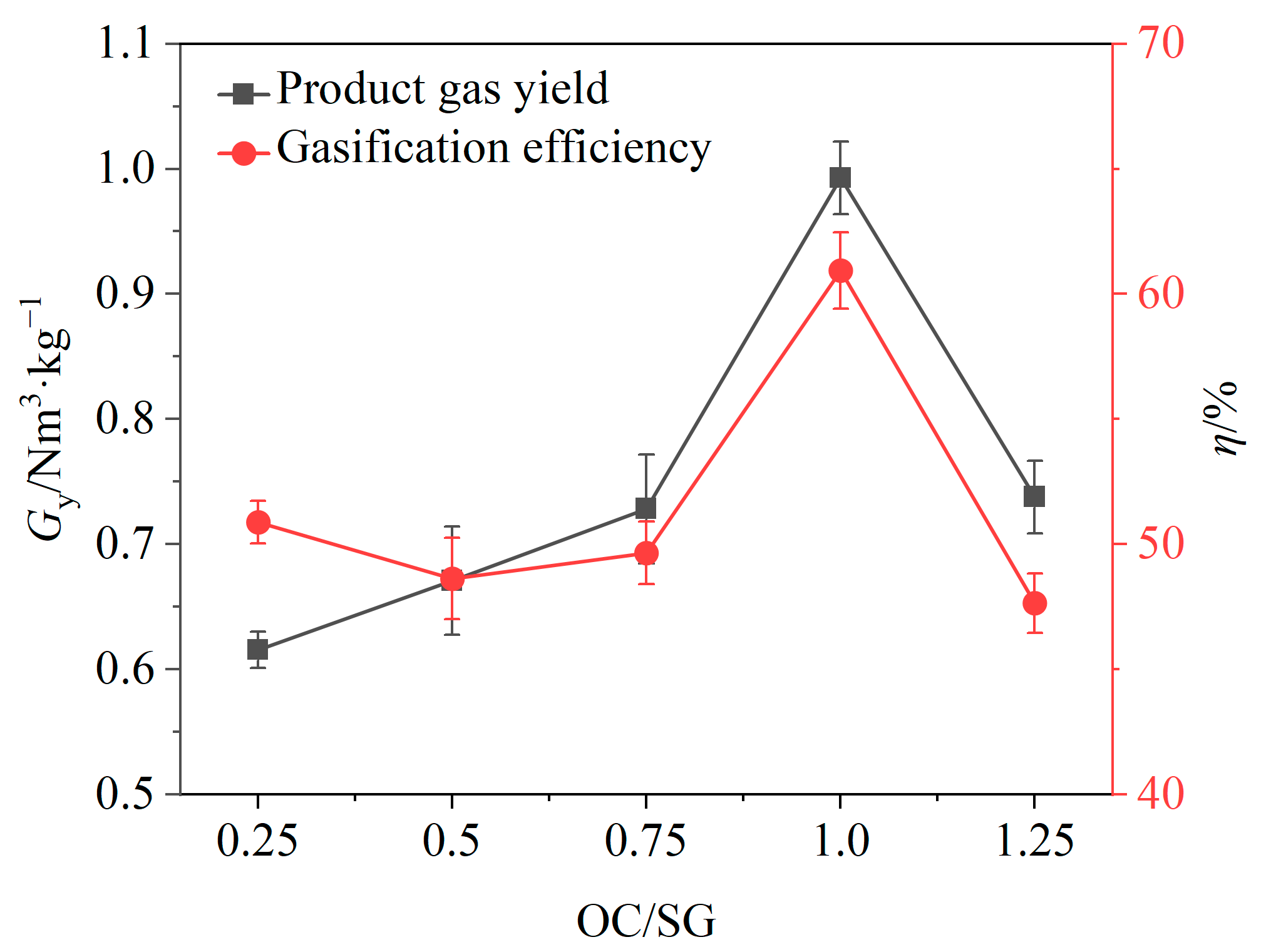
Under the conditions of a gasifier temperature of 900 °C and a steam flow rate of 0.125 g/min, the volume fraction of each component in the product gas, gas yield and gasification efficiency were investigated at OC/SG ratios of 0.25, 0.5, 0.75, 0.1 and 0.125, as shown in Figure 5 and Figure 6.
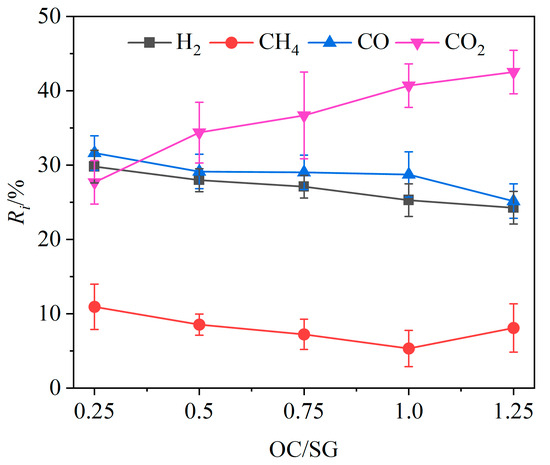
Figure 5.
Effects of OC/SG ratio on relative volume fractions of gas components.
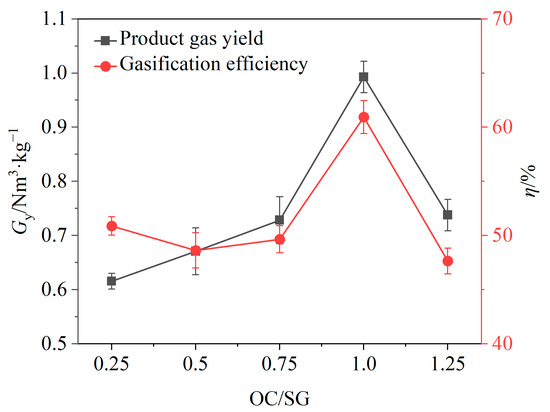
Figure 6.
Effects of OC/SG ratio on gas yield and gasification efficiency.
The variation in the relative volume fraction Ri of different gas components with OC/SG ratios is shown in Figure 5. The relative volume fractions of CO, H2 and CH4 in the product gas exhibited an overall decreasing trend, while the relative volume fraction of CO2 showed a corresponding increase as the OC/SG ratio increased. Specifically, when the OC/SG ratio was raised from 0.25 to 1.25, the relative volume fractions of CO and H2 decreased by 6.48% and 5.53%, respectively, while the relative volume fraction of CO2 increased significantly by 14.85%. The overall variation trend of the relative volume fraction of CH4 was also downward. These change laws can be attributed to the increase in the mass of the OCs with the rising OC/SG ratio, which enhanced the availability of oxygen for the CLG. This increased oxygen supply promoted chemical reactions between the OCs and combustible gases such as H2 and CH4 (Reactions (R3), (R5) and (R6)), leading to greater hydrogen consumption. Moreover, the additional lattice oxygen facilitated oxidation reactions of carbon and CO (Reactions (R1), (R2) and (R4)), resulting in a decrease in the relative volume fraction of CO as carbon-containing species were completely oxidized to CO2. Therefore, as the mass of the OCs increased, the relative volume fraction of CO tended to decrease, while the relative volume fraction of CO2 tended to increase. When the OC/SG ratio increased from 0.25 to 1.25, the value of QL also decreased from 11,716.63 kJ/kg to 9174.46 kJ/kg according to Equation (2).
The effects of the OC/SG ratio on the gasification efficiency η and gas yield Gy are shown in Figure 6. When the OC/SG ratio increased from 0.25 to 1.0, Gy increased from 0.62 Nm3/kg to 1.0 Nm3/kg, and the gasification efficiency increased from 50.87% to 60.94%; when the OC/SG ratio increased from 1.0 to 1.25, there was a substantial decrease in the gas yield and gasification efficiency. It is evident that both the gas yield and gasification efficiency were relatively low when the OC/SG ratio was either below or above 1. When the OC/SG ratio was equal to 1, the gas yield and gasification efficiency both reached the maximum value. The reason may be that when the OC/SG ratio is less than 1, the mass of OCs is relatively low, which cannot provide enough lattice oxygen for the chemical looping gasification of BSGs, and the catalytic effects of OCs are not good, resulting in a low gas production rate [41]. However, if the OC/SG ratio exceeds 1, the excessive mass of the OCs may lead to an abundance of lattice oxygen, causing the chemical looping gasification process to transition into a combustion process, thereby increasing the CO2 content [42]. The experimental results indicated that an OC/SG ratio of 1 was optimal for the chemical looping gasification of BSGs. Consequently, subsequent experiments were conducted with the OC/SG ratio set to 1.
4.3. Effects of Steam Flow Rate
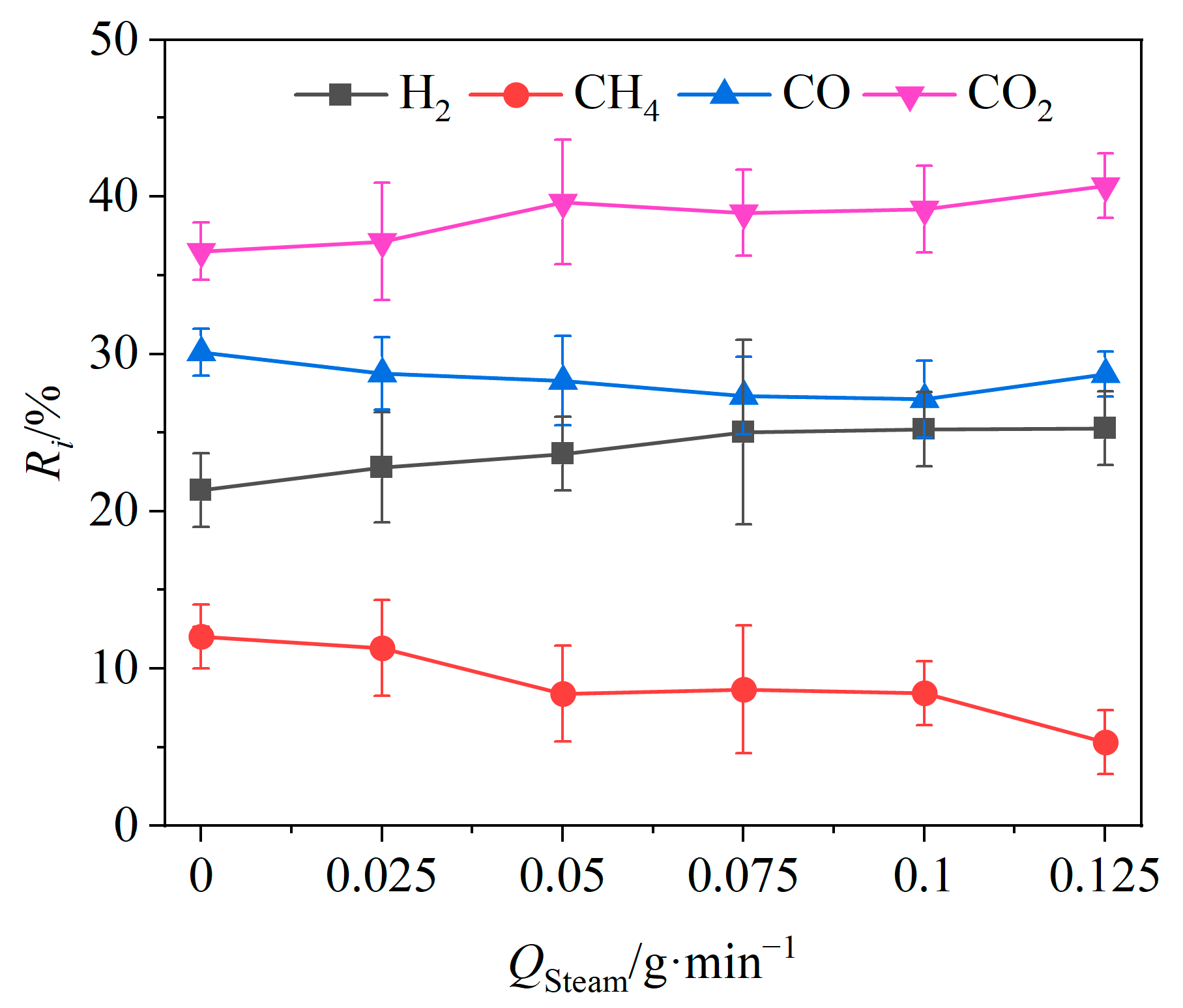
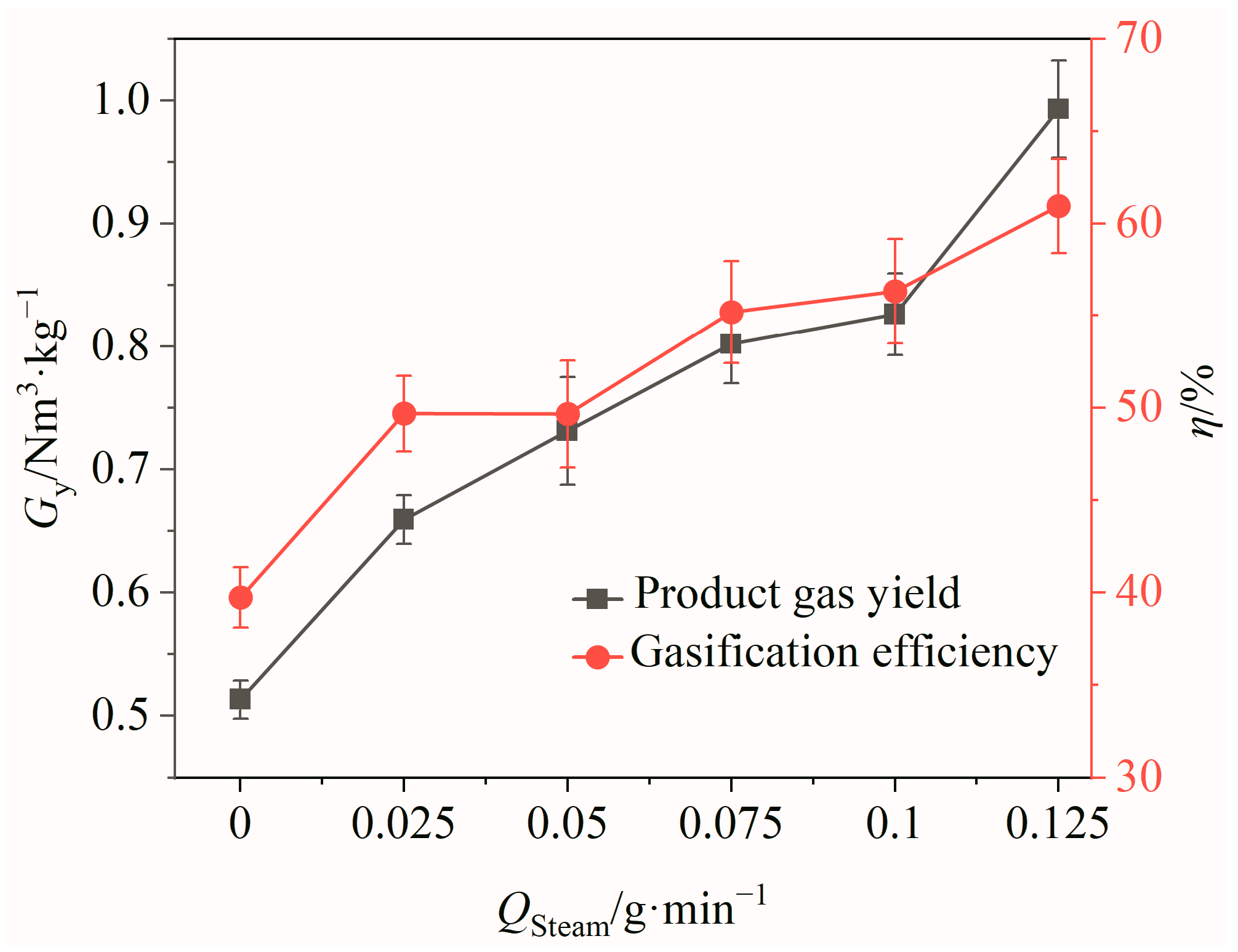
According to the above experimental results, the gasification efficiency reached its peak at 900 °C, when the mass ratio of OCs to BSGs was 1. Therefore, under this condition, the effects of the steam flow rate on the CLG performances of BSGs was investigated. For the experiments, 0.25 g of BSGs was weighed, and controlled steam flow rates of 0, 0.025, 0.05, 0.075, 0.1, and 0.125 g/min were utilized under stable working conditions.
The effects of the vapor flow rate Qsteam on the relative volume fractions of the gas components are shown in Figure 7. When Qsteam was increased from 0 to 0.125 g/min, the relative volume fractions of CO2 and H2 showed an upward trend, increasing by 4.17% and 3.94%. The relative volume fractions of CO and CH4 showed a decreasing trend, decreasing by 1.39% and 6.7%, respectively. Higher steam flow rates promoted the producer gas reaction of carbon (Reaction (R7)) as well as reforming reactions of tar (Reactions (R10) and (R11)) and methane (Reaction (R12)), resulting in an increase in the relative volume fraction of H2 in the product gas. Meanwhile, the transformation of CO to CO2 was expedited by the intensified water–gas shift reaction (Reaction (R8)) due to the increased steam flow. Thus, higher steam flow rates yielded greater amounts of CO2 and H2.
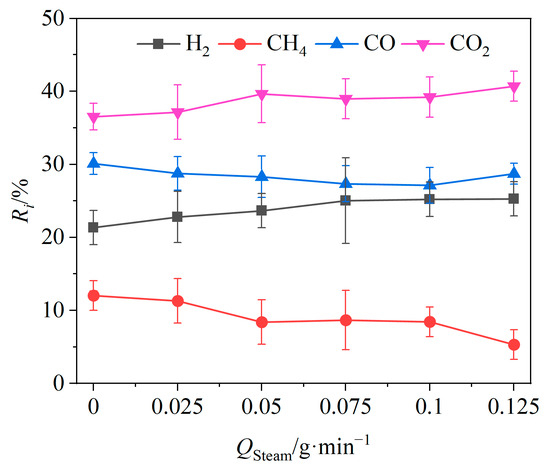
Figure 7.
Effects of steam flow rate on relative volume fractions of gas components.
The effects of the steam flow rate Qsteam on the gas yield and gasification efficiency of the product gas are shown in Figure 8. It can be seen that with the increase in Qsteam, there was an increasing trend for both the gas yield and gasification efficiency. When Qsteam was increased from 0 to 0.125 g/min, the product gas yield and gasification efficiency increased by 0.48 Nm3/kg and 21.23%, respectively. This indicated that the BSG gasification reaction was more effective at higher steam flow rates, leading to a greater conversion of BSGs into gaseous products and an increase in H2 production. However, it is important to note that the steam flow rate cannot be elevated indefinitely, as steam generation requires energy. Considering energy consumption and utilization efficiency, a suitable steam flow rate of 0.125 g/min was ultimately selected for this study.
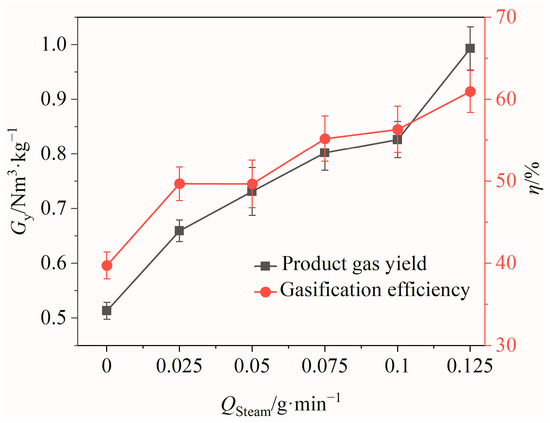
Figure 8.
Effects of steam flow rate on product gas yield and gasification efficiency.
4.4. Effects of the Weight Ratio of CSSs in CSS/Fe2O3 Composite OCs
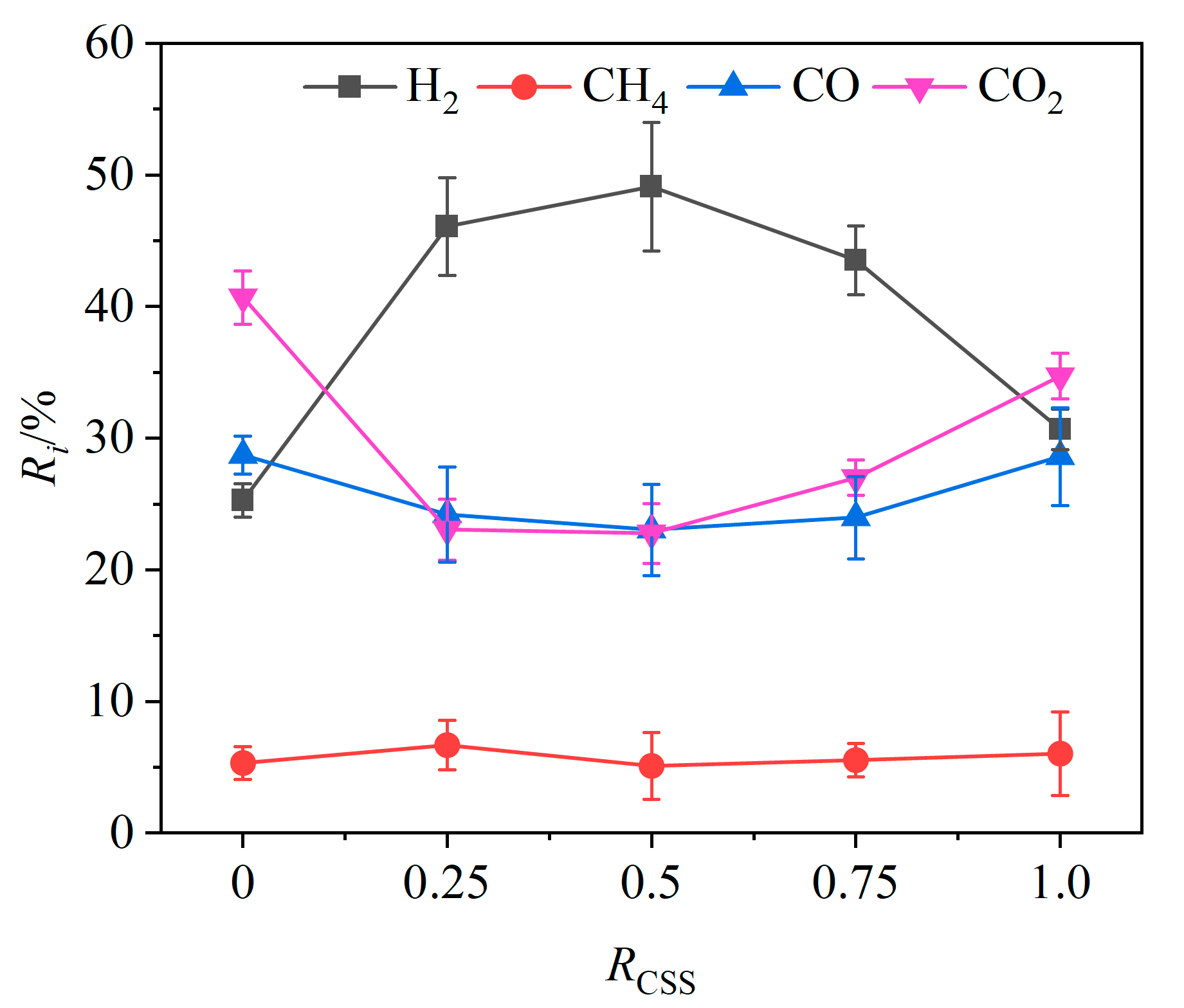
The CSS/Fe2O3 composite OCs were made by mechanically mixing CSSs with iron-based OCs. The effects of the weight ratio RCSS of CSSs in CSS/Fe2O3 composite OCs on the relative volume fractions of gas components are shown in Figure 9. As the RCSS value increased from 0 to 100%, the relative volume fraction of H2 initially increased and then decreased, while the volume fractions of CO and CO2 exhibited the opposite trend. The relative volume fraction of CH4 showed no distinct change rule. When the RCSS value was lower than 0.5, the relative volume fraction of H2 increased with the increasing RCSS value and reached a maximum value of 49.1% at an RCSS value of 0.5, indicating that there exists an optimal weight ratio of CSSs in composite OCs to maximize the relative volume fraction of hydrogen in the product gas. According to the XRF analysis of the CSSs presented in Table 2, the contents of calcium oxide (CaO) and magnesium oxide (MgO) were notably high. Both CaO and MgO not only have the effect of absorbing CO2 but also have the catalytic effect of promoting chemical looping gasification. According to the theory of chemical equilibrium, when CaO and MgO absorb CO2, it will cause the water–gas shift reaction (R8) to proceed in the positive direction, resulting in an increase in the amount of H2 and a decrease in the amount of CO. Furthermore, the abundance of alkaline earth metals such as Ca and Mg in CSSs can act as metal catalysts, enhancing the reforming of tar produced during the chemical looping gasification of BSGs, thereby further increasing hydrogen production [43].
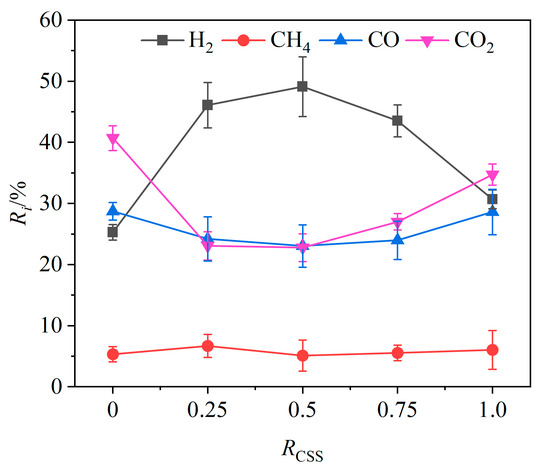
Figure 9.
Effects of weight ratio of CSSs on relative volume fractions of gas components.
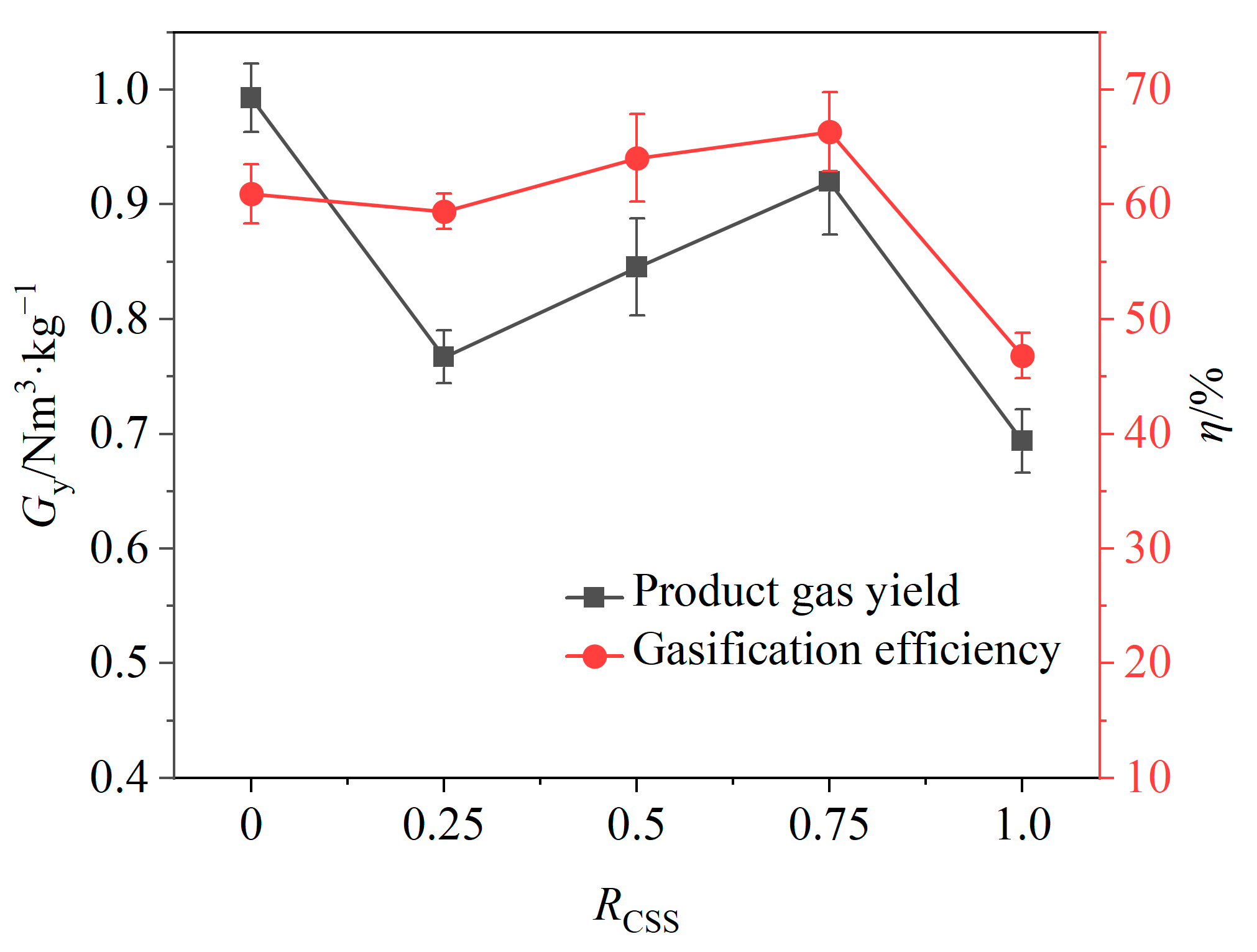
At 0.5 < RCSS < 0.75, gas yield continued to increase with the increasing RCSS values (shown in Figure 10), while the relative volume fraction of H2 began to decrease and the relative volume fractions of CO2 and CO began to increase. This behavior may be attributed to the adequate supply of lattice oxygen within this weight ratio range, where increasing the weight ratio of CSSs facilitates the conversion of tar to CO and H2, resulting in higher gas yields. Additionally, the large and widely distributed surface pores of CSSs enhanced the release of lattice oxygen compared to iron-based OCs, promoting oxidation reactions that generated more CO and CO2, while allowing H2 to be fully oxidized.
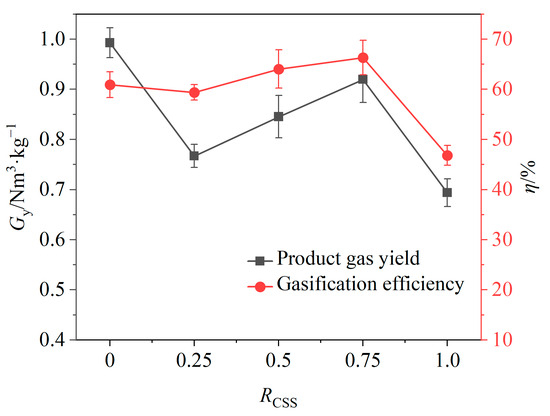
Figure 10.
Effects of weight ratio of CSSs on gas yield and gasification efficiency.
When the RCSS > 0.75, as the RCSS value continued to increase, the gas yield and gasification efficiency began to decline. At this time, the weight ratio of CSSs became too large, leading to insufficient provision of lattice oxygen. While the large and widely distributed surface pores of CSSs facilitated the generation of CO and CO2 through oxidation reactions, it also promoted the oxidation of H2. In addition, excess CSSs may partially cover the iron-based OCs, reducing the contact between the combustible substances and the iron-based OCs [44].
The effects of the weight ratio of CSSs in composite OCs on the product gas yield and gasification efficiency are shown in Figure 10. It can be seen that when the RCSS value was 0.5 and the relative volume fraction of H2 reached the maximum value, the gas yield and the gasification efficiency would increase with the further increase in the RCSS value, and the gasification efficiency reached the maximum value at the RCSS value of 0.75. It shows that the addition of CSSs can improve gasification efficiency. However, in order to meet the need of producing a high percentage of hydrogen, the RCSS value of 0.5 was finally selected as the optimal weight ratio of CSSs in composite OCs.
4.5. Analysis and Characterization of CSS/Fe2O3 Composite OCs
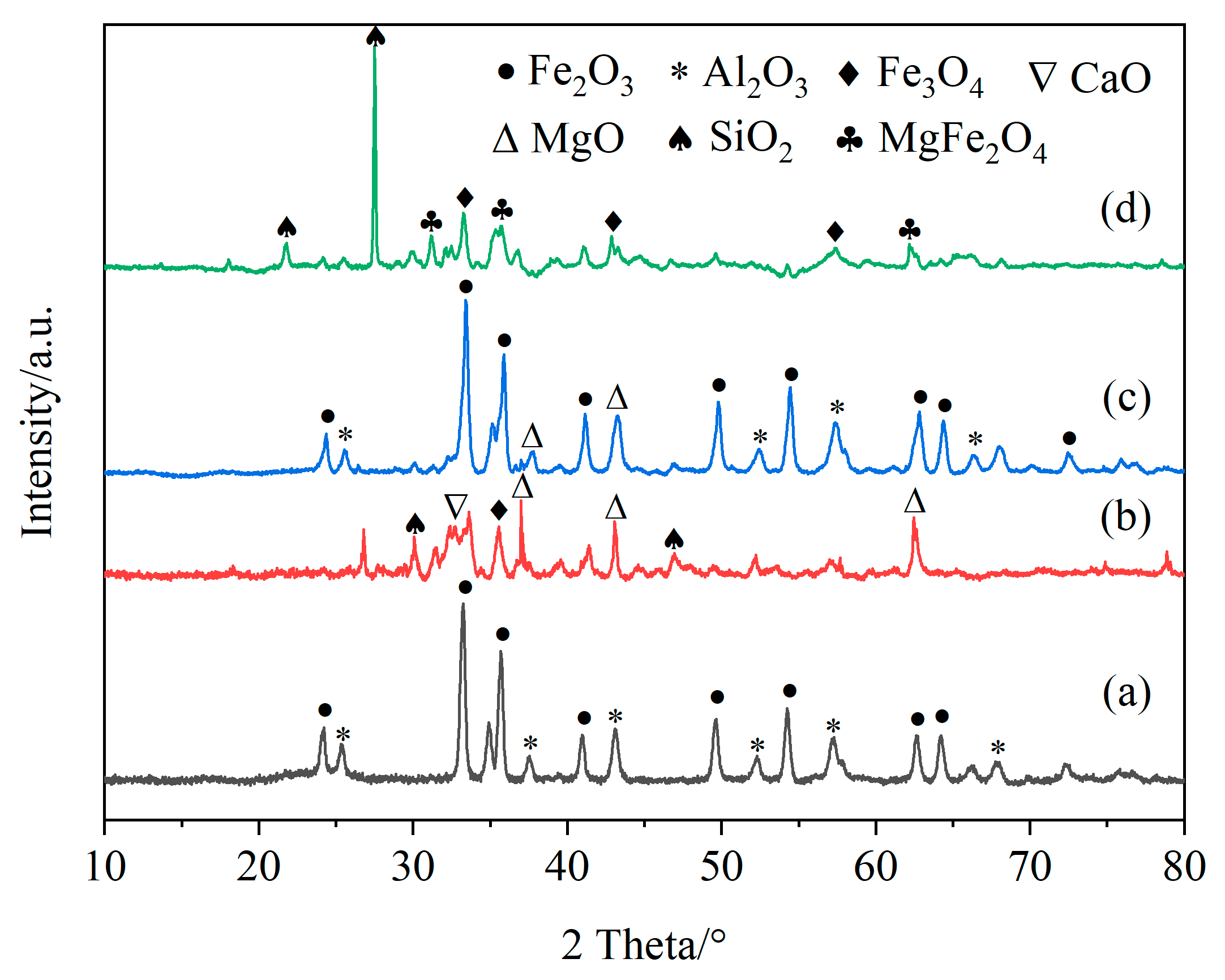
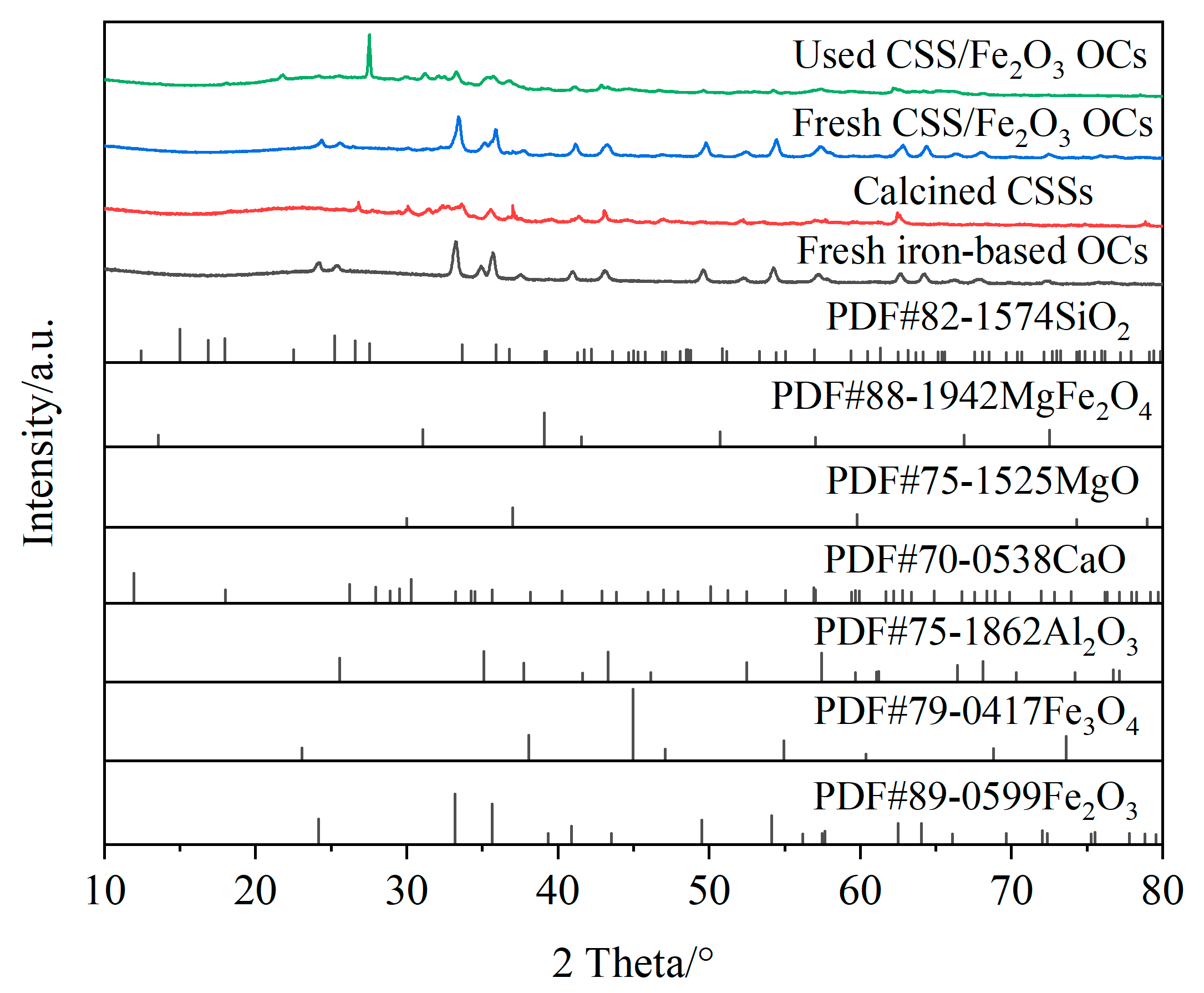
Figure 11 shows the XRD patterns of fresh iron-based OCs, calcined CSSs, fresh CSS/Fe2O3 composite OCs and the CSS/Fe2O3 composite OCs after use in chemical looping gasification, and Figure 12 presents the reference XRD patterns of Fe2O3, Fe3O4, Al2O3, CaO, MgO, MgFe2O4 and SiO2. The phases present in different materials before and after chemical looping gasification can be determined by the XRD method. Figure 11a indicates that the primary component of the fresh iron-based OCs was Fe2O3. Figure 11b demonstrates that the main components of CSSs after calcination were Fe3O4, MgO and CaO, which were suitable for mixing with iron-based OCs to form CSS/Fe2O3 composite OCs.
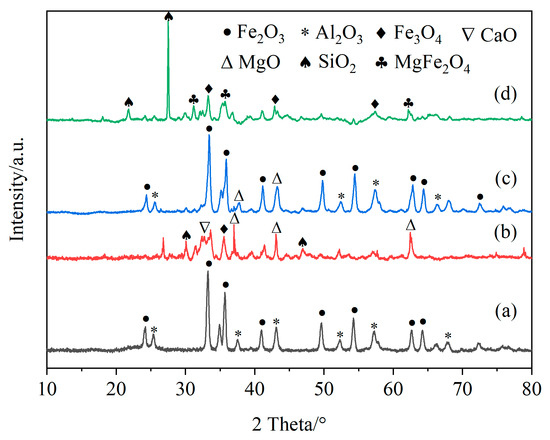
Figure 11.
The XRD patterns of fresh iron-based oxygen carriers (a), calcined CSSs (b), fresh CSS/Fe2O3 oxygen carriers (c) and used CSS/Fe2O3 oxygen carriers (d).
Figure 12.
The reference XRD patterns of Fe2O3, Fe3O4, Al2O3, CaO, MgO, MgFe2O4 and SiO2.
Figure 11c shows the XRD pattern of the fresh unreacted CSS/Fe2O3 composite OCs, which were mainly composed of Fe2O3 and MgO. In Figure 11d, the XRD pattern of the CSS/Fe2O3 composite OCs after being used in chemical looping gasification is depicted. It is observed that Fe2O3 was reduced to Fe3O4, with a certain amount of MgFe2O4 also present. This indicates that the lattice oxygen in the CSS/Fe2O3 composite OCs was released and MgO was converted to MgFe2O4. Notably, FeO and pure Fe were not detected after the gasification, likely due to the preferential reduction of Fe2O3 to Fe3O4 [45].
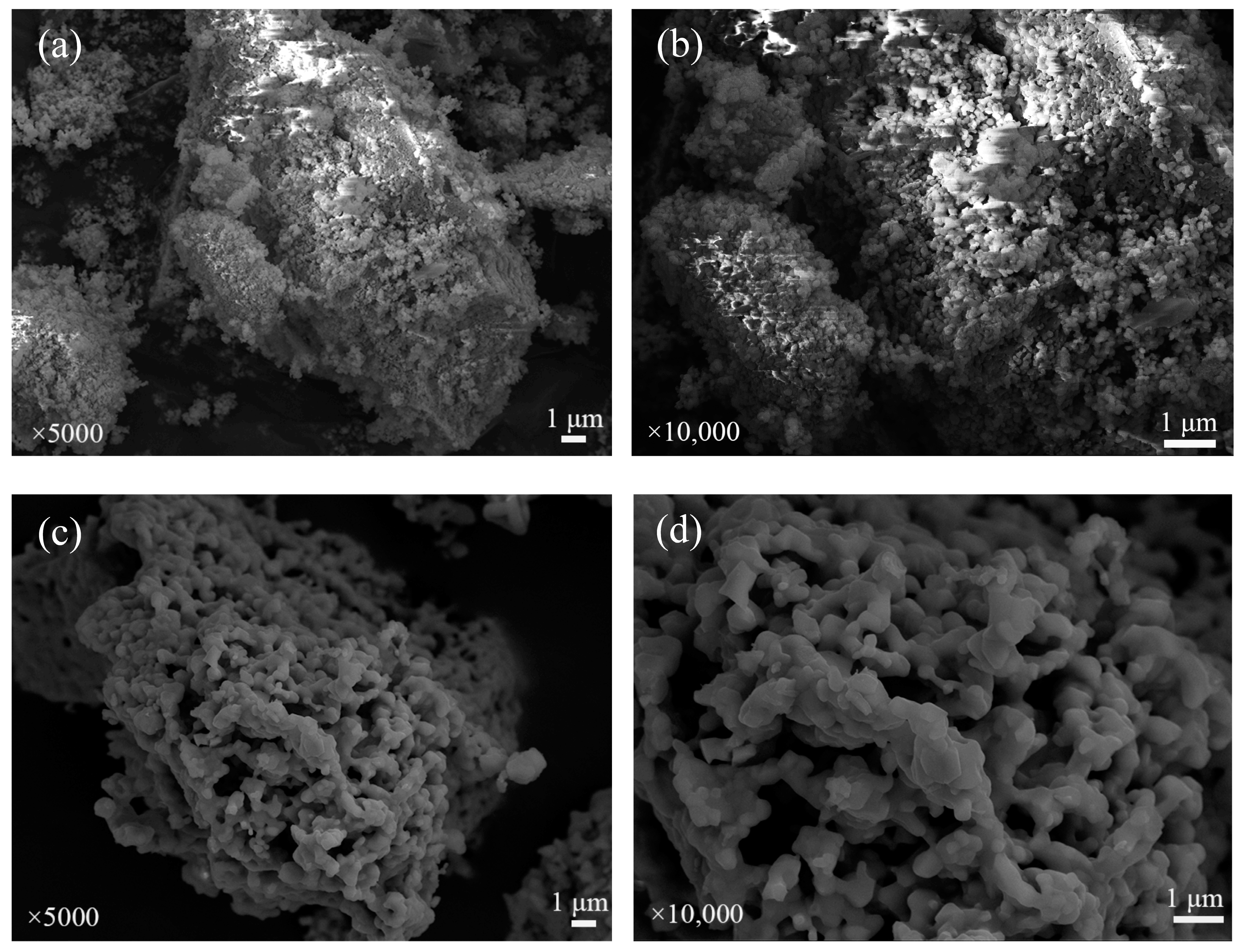
The SEM images of fresh iron-based OCs, calcined CSSs, fresh CSS/Fe2O3 composite OCs and the CSS/Fe2O3 composite OCs after being used in chemical looping gasification are shown sequentially in Figure 13. The surface microstructures of different materials before and after chemical looping gasification can be characterized by the SEM method. Figure 13a,b illustrate that the fresh iron-based oxygen carrier particles were uniformly distributed on the aluminum oxide, featuring a granular and porous surface that facilitated reactions between the OCs and the BSGs. Figure 13c,d show that the surface pores of the CSSs were large and widely distributed, which was beneficial for catalytic steam reforming to produce hydrogen. After mechanically mixing the iron-based OCs with CSSs to make CSS/Fe2O3 composite OCs, as shown in Figure 13e,f, in addition to the fresh iron-based oxygen carrier particles, there were also some calcined CSS particles adhering to the surface of the aluminum oxide. Figure 13g,h show that many obvious pores appeared on the surface of the inert carrier Al2O3 while the composite OCs were being used in chemical looping gasification, which enhanced the specific surface area and created more active sites for gas molecule entry, facilitating the gasification reactions. The XRD pattern of Figure 11d indicates the emergence of several post-reaction diffraction peaks that represent MgFe2O4, along with the formation of some large grains, as illustrated in the SEM image of Figure 13g,h, suggesting that the interactions between MgO and Fe2O3 resulted in the formation of spinel ferrite MgFe2O4, although slight sintering was observed after the gasification. Related studies showed that spinel ferrites are efficient catalysts for H2 production via the steam reforming of pyrolysis volatiles, which exhibit a prolonged service life without compromising H2 selectivity [46]. This stable spinel also served as a support for the active Fe2O3 [15,47]. Spinel ferrites contributed to the chemical looping process, exhibiting good oxygen transferability and wide distributions of metal cations because of their metal synergistic effects in the spine structure [48].
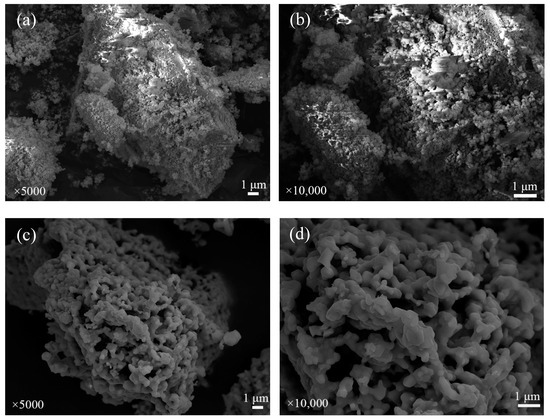

Figure 13.
The SEM images of different OCs. (a) Fresh iron-based OCs (5000×), (b) fresh iron-based OCs (10,000×), (c) calcined CSSs (5000×), (d) calcined CSSs (10,000×), (e) fresh CSS/Fe2O3 oxygen carriers (5000×), (f) fresh CSS/Fe2O3 oxygen carriers (10,000×), (g) used CSS/Fe2O3 oxygen carriers (5000×) and (h) used CSS/Fe2O3 oxygen carriers (10,000×).
4.6. Chemical Reactivity of CSS/Fe2O3 Composite OCs
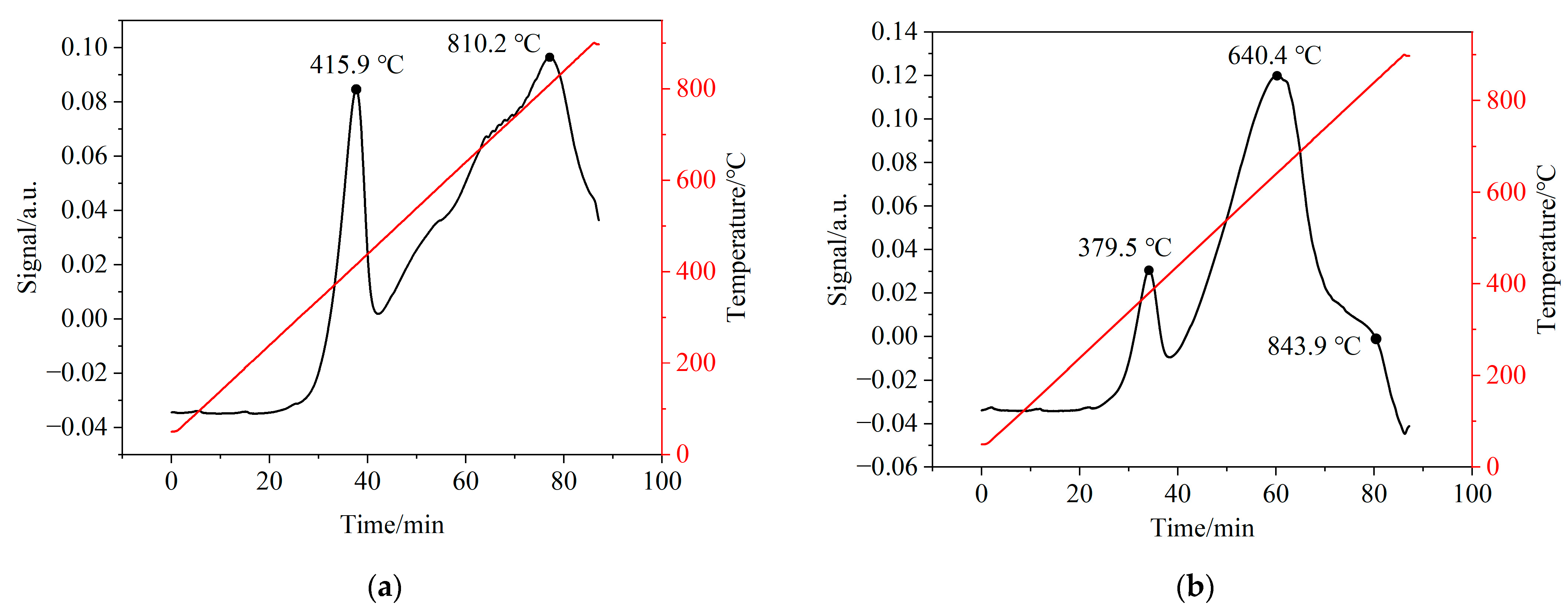
In order to investigate the interaction between CSSs and iron-based OCs, H2-TPR tests were performed to evaluate the chemical reactivity of CSS/Fe2O3 composite OCs. Figure 14 illustrates the H2-TPR patterns of iron-based OCs and CSS/Fe2O3 composite OCs. Figure 14a exhibits two reduction peaks at 415.9 °C and 810.2 °C for iron-based OCs, corresponding to the reduction of Fe2O3 to Fe3O4 and reduction of Fe3O4 to FeO, respectively. Figure 14b exhibits three reduction peaks at 379.5 °C, 640.4 °C and 843.9 °C for CSS/Fe2O3 composite OCs indicating the formation of new reduction centers or intermediate products that were ferrites of alkaline earth metals such as MgFe2O4. Compared to iron-based OCs, the CSS/Fe2O3 composite OCs exhibited a relatively lower temperature for the first reduction peak. This phenomenon was attributed to the catalytic effect of minor constituents such as CaO and MgO present in the CSSs, which significantly facilitated the reduction process of the OCs. The CSS/Fe2O3 composite OCs demonstrated a higher rate of hydrogen consumption, i.e., greater chemical reactivity, compared to the iron-based OCs. This observation might be attributed to the interaction between the alkaline earth metal oxides in CSSs and iron-based OCs.
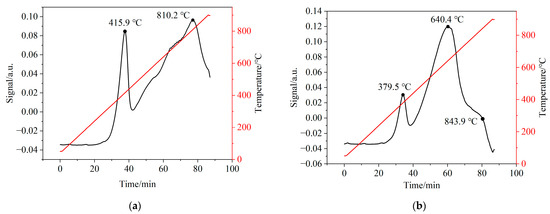
Figure 14.
The H2-TPR patterns of different oxygen carriers. (a) Fresh iron-based OCs, (b) fresh CSS/Fe2O3 composite oxygen carriers.
4.7. Effects of Cycle Number
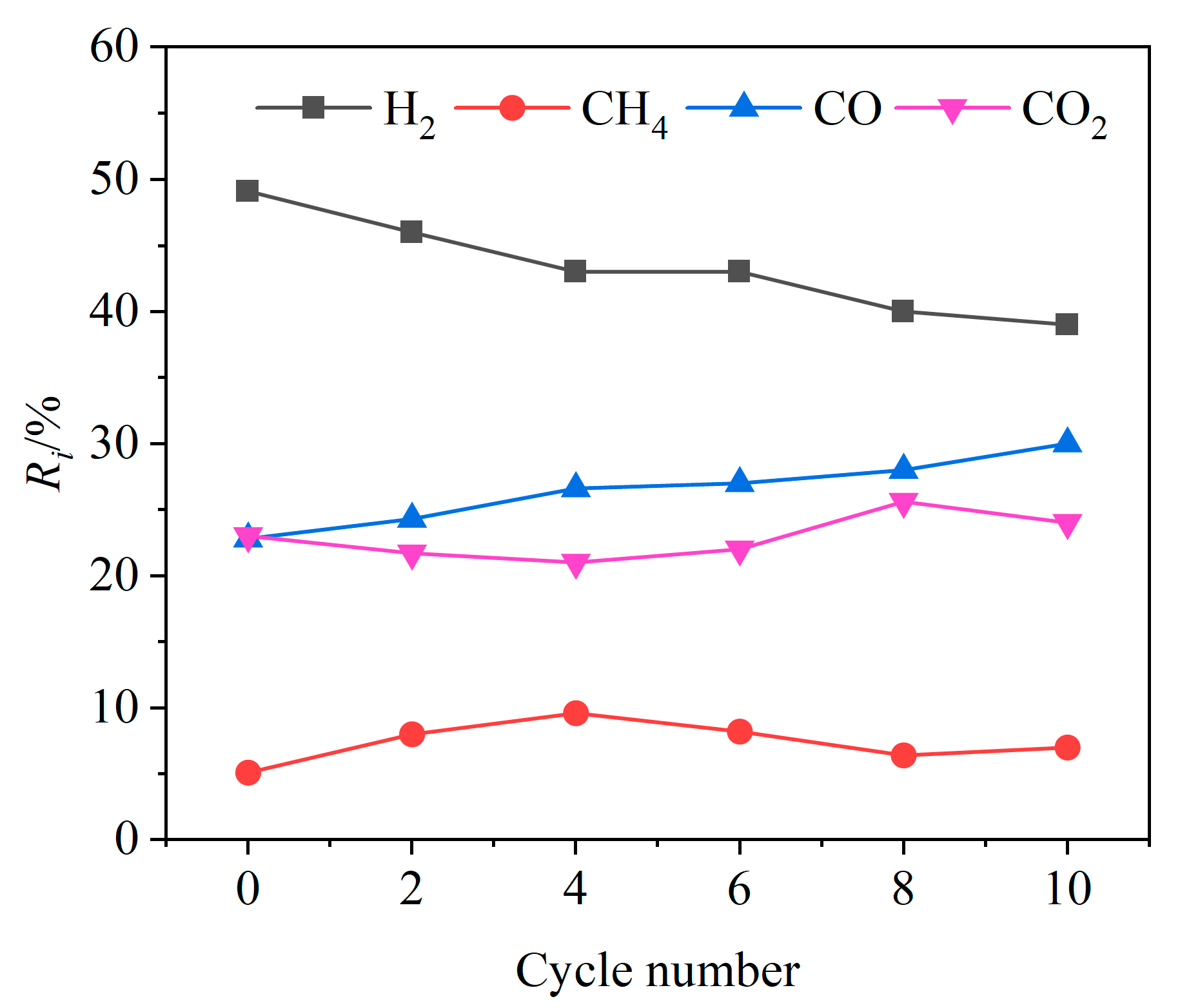
In order to investigate the cyclic stability of CSS/Fe2O3 composite OCs during the CLG of BSGs, ten redox cycle experiments were conducted at 900 °C. As shown in Figure 15, with the increase in the cycle number, the relative volume fraction Ri of H2 in the syngas showed a slow decreasing trend, while the Ri value of CO showed an increasing trend and the Ri values of CO2 and CH4 both showed no obvious variation trends. This was mainly due to the weakening of the oxygen release capacity of the CSS/Fe2O3 composite OCs as the cycle number increased. At lower cycle numbers, the oxides of calcium and magnesium in the CSSs could effectively absorb CO2, which led to a lower value in the relative volume fraction of CO2. After the cycle number exceeded four, the reaction between the oxides of alkaline earth metals and CO2 slightly weakened. This was beneficial for the Boudouard reaction, resulting in an increase in the Ri value of CO with the increase in the cycle number. After the 8th cycle, as the cycle number further increased, the Ri value of H2 in the syngas tended to be relatively stable, with the Ri value of H2 remaining at around 40%. This indicated that the CSS/Fe2O3 composite OCs had a certain cyclic stability.
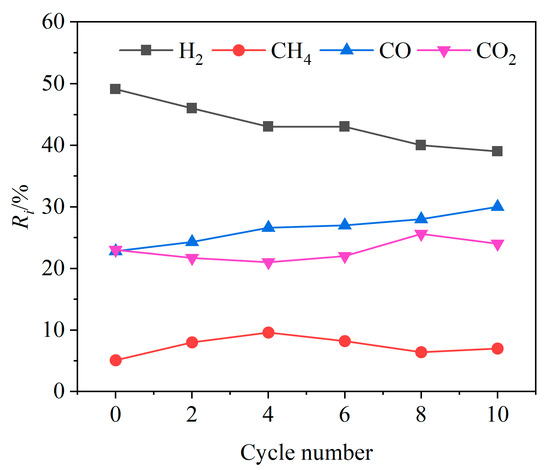
Figure 15.
Relative volume fractions of gas components at different cycle numbers.
4.8. Performance Comparison of CSS/Fe2O3 Composite OCs and Other OCs
After investigating the catalytic performance, catalytic gasification efficiency, chemical reactivity and cyclic stability of CSS/Fe2O3 composite OCs in the CLG for hydrogen production, a comparison was made among the performances of CSS/Fe2O3 composite OCs, iron-based OCs [25] and perovskites OCs [49], as listed in Table 4.

Table 4.
Performance comparison of CSS/Fe2O3 composite OCs, iron-based OCs and perovskites.
5. Conclusions
The effects of the weight ratio of CSSs in CSS/Fe2O3 composite OCs on the CLG process of BSGs were investigated, and the optimal weight ratio of CSSs was determined. The CSS/Fe2O3 composite OCs were analyzed by X-ray diffraction patterns and characterized by scanning electron microscope images in order to explore their function mechanism on the CLG process.
The presence of alkaline earth metals such as Ca and Mg in CSSs can act as metal catalysts, promoting hydrogen production. There exists an optimal weight ratio RCSS of CSSs in CSS/Fe2O3 composite OCs to maximize the relative volume fraction of hydrogen in the product gas. When the RCSS value was 0.5, the relative volume fraction of hydrogen reached the maximum value of 49.1%; furthermore, the product gas yield was 0.85 Nm3/kg and the gasification efficiency was 64.05%.
The fresh unreacted CSS/Fe2O3 composite OCs were mainly composed of Fe2O3 and MgO. After mechanically mixing the iron-based OCs with CSSs, there were some calcined CSS particles adhering to the surface of the aluminum oxide. The surface pores of the CSSs were large and widely distributed, which were beneficial for catalytic steam reforming to produce hydrogen.
From the XRD pattern and SEM image of the CSS/Fe2O3 composite OCs after use in chemical looping gasification, it can be observed that the interactions between MgO in CSSs and Fe2O3 in iron-based OCs resulted in the formation of spinel ferrite MgFe2O4, which is an efficient catalyst for H2 production via steam reforming of pyrolysis volatiles.
The CSS/Fe2O3 composite OCs demonstrated a higher chemical reactivity compared to the iron-based OCs, which was attributed to the interaction between the alkaline earth metal oxides in CSSs and iron-based OCs. As the cycle number increased, the oxygen release capacity of the CSS/Fe2O3 composite OCs weakened, but after the 8th cycle, the Ri value of H2 tended to be relatively stable.
Author Contributions
Conceptualization, H.J.; methodology, H.J.; validation, M.Y.; formal analysis, M.Y.; investigation, M.Y.; resources, M.Y., H.J. and Y.L.; data curation, M.Y.; writing—original draft preparation, M.Y. and H.J.; writing—review and editing, H.J., X.Z., C.W., Y.L. and H.Y.; visualization, M.Y. and H.J.; supervision, H.J., C.W., Y.L. and H.Y.; project administration, H.J.; funding acquisition, H.J., C.W., Y.L. and H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (Grant No. 2023YFB4104301-3), the International Science and Technology Cooperation Program of Qingdao City (Grant No. 24-1-6-ghgg-9-hz), the Huaneng Group Science and Technology Research Project (Grant No. HNKJ23-H71) and the Qingdao Postdoctoral Applied Research Project.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Xie, Z.; Dan, M.; Zhao, G. Recent advances in microbial high-value utilization of brewer’s spent grain. Bioresour. Technol. 2024, 408, 131197. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Li, Z. A coupling strategy for comprehensive utilization of distillers’ grains towards energy recovery and carbon sequestration. Energy Convers. Manag. 2023, 275, 116494. [Google Scholar] [CrossRef]
- Ferreira, S.; Monteiro, E.; Calado, L.; Silva, V.; Brito, P.; Vilarinho, C. Experimental and modeling analysis of brewers’ spent grains gasification in a downdraft reactor. Energies 2019, 12, 4413. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Dieringer, P.; Marx, F.; Ströhle, J.; Epple, B. System hydrodynamics of a 1 MWth dual circulating fluidized bed chemical looping gasifier. Energies 2023, 16, 5630. [Google Scholar] [CrossRef]
- Qiu, Y.; Zeng, D.; Xiao, R. Hydrogen production from biomass-based chemical looping: A critical review and perspectives. Energy Fuels 2024, 38, 13819–13836. [Google Scholar] [CrossRef]
- Ponzio, A.; Kalisz, S.; Blasiak, W. Effect of operating conditions on tar and gas composition in high temperature air/steam gasification (HTAG) of plastic containing waste. Fuel Process. Technol. 2006, 87, 223–233. [Google Scholar] [CrossRef]
- Luo, M.; Yi, Y.; Wang, S. Review of hydrogen production using chemical-looping technology. Renew. Sustain. Energy Rev. 2018, 81, 3186–3214. [Google Scholar] [CrossRef]
- Huang, Z.; He, F.; Zhu, H. Thermodynamic analysis and thermogravimetric investigation on chemical looping gasification of biomass char under different atmospheres with Fe2O3 oxygen carrier. Appl. Energy 2015, 157, 546–553. [Google Scholar] [CrossRef]
- Hu, J.; Li, C.; Zhang, Q. Using chemical looping gasification with Fe2O3/Al2O3 oxygen carrier to produce syngas (H2+CO) from rice straw. Int. J. Hydrog. Energy 2019, 44, 3382–3386. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Y.; Fu, J. Chemical looping gasification of biomass char using iron ore as an oxygen carrier. Int. J. Hydrogen Energy 2016, 41, 17871–17883. [Google Scholar] [CrossRef]
- Hu, Z.; Jiang, E.; Ma, X. The effect of oxygen carrier content and temperature on chemical looping gasification of microalgae for syngas production. J. Energy Inst. 2019, 92, 474–487. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, B.; Wu, S. Chemical looping gasification of lignocellulosic biomass with iron-based oxygen carrier: Products distribution and kinetic analysis on gaseous products from cellulose. Fuel Process. Technol. 2019, 193, 361–371. [Google Scholar] [CrossRef]
- Wang, L.; Feng, X.; Shen, L. Carbon and sulfur conversion of petroleum coke in the chemical looping gasification process. Energy 2019, 179, 1205–1216. [Google Scholar] [CrossRef]
- Niu, X.; Shen, L. Ca- and Mg-rich waste as high active carrier for chemical looping gasification of biomass. Chin. J. Chem. Eng. 2021, 38, 145–154. [Google Scholar] [CrossRef]
- Mayer, F.; Bidwe, A.R.; Schope, A. Comparison of a new micaceous iron oxide and ilmenite as oxygen carrier for Chemical looping combustion with respect to syngas conversion. Appl. Energy 2014, 113, 1863–1868. [Google Scholar] [CrossRef]
- Song, T.; Wu, J.; Zhang, H. Characterization of an Australia hematite oxygen carrier in chemical looping combustion with coal. Int. J. Greenh. Gas Control 2012, 11, 326–336. [Google Scholar] [CrossRef]
- Bao, J.; Li, Z.; Cai, N. Reduction Kinetics of Foreign-Ion-Promoted Ilmenite Using Carbon Monoxide (CO) for Chemical Looping Combustion. Ind. Eng. Chem. Res. 2013, 52, 10646–10655. [Google Scholar] [CrossRef]
- Huang, W.C.; Kuo, Y.L.; Su, Y.M. A facile method for sodium-modified Fe2O3/Al2O3 oxygen carrier by an air atmospheric pressure plasma jet for chemical looping combustion process. Chem. Eng. J. 2017, 316, 15–23. [Google Scholar] [CrossRef]
- Zhong, H.; Er, D.; Wen, L. Theoretical study on influence of CaO and MgO on the reduction of FeO by CO. Appl. Surf. Sci. 2017, 399, 630–637. [Google Scholar] [CrossRef]
- Hildor, F.; Leion, H.; Linderholm, C.J. Steel converter slag as an oxygen carrier for chemical-looping gasification. Fuel Process. Technol. 2020, 210, 106576. [Google Scholar] [CrossRef]
- Di, Z.; Cao, Y.; Yang, F. Studies on steel slag as an oxygen carrier for chemical looping combustion. Fuel 2018, 226, 618–626. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Alobaid, F.; Epple, B. Chemical looping gasification of torrefied woodchips in a bubbling fluidized bed test rig using iron-based oxygen carriers. Renew. Energy 2021, 172, 34–45. [Google Scholar] [CrossRef]
- Huang, X.; Wu, J.; Wang, M.; Ma, X.; Jiang, E.; Hu, Z. Syngas production by chemical looping gasification of rice husk using Fe-based oxygen carrier. J. Energy Inst. 2020, 93, 1261–1270. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, H.; Wang, S. Syngas production by chemical looping co-gasification of rice husk and coal using an iron-based oxygen carrier. Fuel 2022, 309, 122100. [Google Scholar] [CrossRef]
- Liu, Q.; Hu, C.; Peng, B. High H2/CO ratio syngas production from chemical looping co-gasification of biomass and poly-ethylene with CaO/Fe2O3 oxygen carrier. Energy Convers. Manag. 2019, 199, 111951. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, Z.; Toan, S. Chemical looping deoxygenated gasification: An implication for efficient biomass utilization with high-quality syngas modulation and CO2 reduction. Energy Convers. Manag. 2020, 215, 112913. [Google Scholar] [CrossRef]
- Wei, G.; He, F.; Zhao, Z. Performance of Fe–Ni bimetallic oxygen carriers for chemical looping gasification of biomass in a 10 kWth interconnected circulating fluidized bed reactor. Int. J. Hydrogen Energy 2015, 40, 16021–16032. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Zhang, L. Characteristics of steel slag as an oxygen carrier for chemical looping gasification of sewage sludge. Energy 2022, 247, 123534. [Google Scholar] [CrossRef]
- Pan, Q.; Ma, L.; Du, W. Hydrogen-enriched syngas production by lignite chemical looping gasification with composite oxygen carriers of phosphogypsum and steel slag. Energy 2022, 241, 122927. [Google Scholar] [CrossRef]
- Wang, B.; Ma, Z.; Li, S.; Dai, J.; Patrascu, M.; Gao, X. Experiment investigation and multiscale modeling of biomass oxidative fast pyrolysis in a fluidized bed reactor. Chem. Eng. J. 2024, 501, 157546. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, M.; Xu, S.; Feng, Y. Hydrogen and methane mixture from biomass gasification coupled with catalytic tar reforming, methanation and adsorption enhanced reforming. Fuel Process. Technol. 2019, 192, 147–153. [Google Scholar] [CrossRef]
- Hildor, F.; Soleimanisalim, A.H.; Seemann, M.; Mattisson, T.; Leion, H. Tar characteristics generated from a 10 kWth chemical-looping biomass gasifier using steel converter slag as an oxygen carrier. Fuel 2023, 331, 125770. [Google Scholar] [CrossRef]
- Kuttin, K.W.; Leghari, A.; Yu, H.; Xia, Z.; Ding, L.; Yu, G. Carbon dioxide-steam reforming gasification of carbonized bio-mass pellet for high syngas yield and TAR reduction through CFD modeling. Chem. Eng. Sci. 2024, 287, 119716. [Google Scholar] [CrossRef]
- Abdalazeez, A.; Li, T.; Cao, Y.; Wang, W.; Abuelgasim, S.; Liu, C. Syngas production from chemical looping gasification of rice husk-derived biochar over iron-based oxygen carriers modified by different alkaline earth metals. Int. J. Hydrogen Energy 2022, 47, 40881–40894. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Liu, L. Role and mechanism of calcium-based catalysts for methane dry reforming: A review. Fuel 2024, 355, 129329. [Google Scholar] [CrossRef]
- Shah, M.; Mesfer, M.K.A.; Danish, M. Design and optimization of Ni–Fe–La based catalytic system for CO2 utilization for sustainable syngas production via dry reforming of methane. J. Energy Inst. 2023, 110, 101346. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Hu, Y.H. Steam reforming of methane: Current states of catalyst design and process upgrading. Renew. Sustain. Energy Rev. 2021, 149, 111330. [Google Scholar] [CrossRef]
- Nguyen, D.L.T.; Tran, A.V.; Vo, D.N. Methane dry reforming: A catalyst challenge awaits. J. Ind. Eng. Chem. 2024, 140, 169–189. [Google Scholar] [CrossRef]
- Abdalazeez, A.; Wang, W.; Abuelgasim, S. Syngas production from chemical looping reforming of ethanol over iron-based oxygen carriers: Theoretical analysis and experimental investigation. Chin. J. Chem. Eng. 2021, 38, 123–131. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, H.; Huang, Z. Chemical looping gasification coupled with steam reforming of biomass using NiFe2O4: Kinetic analysis of DAEM-TI, thermodynamic simulation of OC redox, and a loop test. Chem. Eng. J. 2020, 395, 125046. [Google Scholar] [CrossRef]
- Shen, X.; Yan, F.; Zhang, Z. Enhanced and environment-friendly chemical looping gasification of crop straw using red mud as a sinter-resistant oxygen carrier. Waste Manag. 2021, 121, 354–364. [Google Scholar] [CrossRef]
- Wu, Y.; Liao, Y.; Liu, G. Syngas production by chemical looping gasification of biomass with steam and CaO additive. Int. J. Hydrogen Energy 2018, 43, 19375–19383. [Google Scholar] [CrossRef]
- Liu, G.; Liao, Y.; Wu, Y. Synthesis gas production from microalgae gasification in the presence of Fe2O3 oxygen carrier and CaO additive. Appl. Energy 2018, 212, 955–965. [Google Scholar] [CrossRef]
- Leion, H.; Jerndal, E.; Steenari, B.M. Solid fuels in chemical-looping combustion using oxide scale and unprocessed iron ore as oxygen carriers. Fuel 2009, 88, 1945–1954. [Google Scholar] [CrossRef]
- Xu, L.; Wang, L.; Li, Y.; Song, Q.; Tian, Z.; Wang, C.; Zhao, M.; Gao, N. A novel high-entropy spinel ferrites (CoNiCuZnMg)Fe2O4 catalyst for H2 production via steam reforming of derived volatiles from polypropylene and waste cooking oil. Chem. Eng. J. 2024, 488, 150767. [Google Scholar] [CrossRef]
- Das, S.; Biswas, A.; Tiwary, C.S.; Paliwal, M. Hydrogen production using chemical looping technology: A review with emphasis on H2 yield of various oxygen carriers. Int. J. Hydrogen Energy 2022, 47, 28322–28352. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, K.; Zhao, Z.; He, F.; Huang, Z.; Wei, G. Identifying the roles of MFe2O4 (M=Cu, Ba, Ni, and Co) in the chemical looping reforming of char, pyrolysis gas and tar resulting from biomass pyrolysis. Int. J. Hydrogen Energy 2019, 44, 4674–4687. [Google Scholar] [CrossRef]
- Chen, J.; Wang, X.; Gai, D.; Wang, J. B-site semi-doped LaFeO3 perovskite oxygen carrier for biomass chemical looping steam gasification to produce hydrogen-rich syngas. Int. J. Hydrogen Energy 2025, 103, 446–455. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).