Investigation on Graphitization, Surface Functional Groups, and Oxidation Behavior of Soot Particulate Along Exhaust Pipe of Gasoline Direct Injection Engine
Abstract
:1. Introduction
2. Experimental Setup
2.1. Experimental Engine and Fuel
2.2. Sample Points and Operating Conditions
2.3. Experimental Equipment and Procedure
3. Results and Discussion
3.1. Graphitization of Soot Particulate
3.1.1. Raman Spectrum Analysis
3.1.2. Graphitized Structure of Soot Particulate
3.2. Surface Functional Groups of Soot Particulate
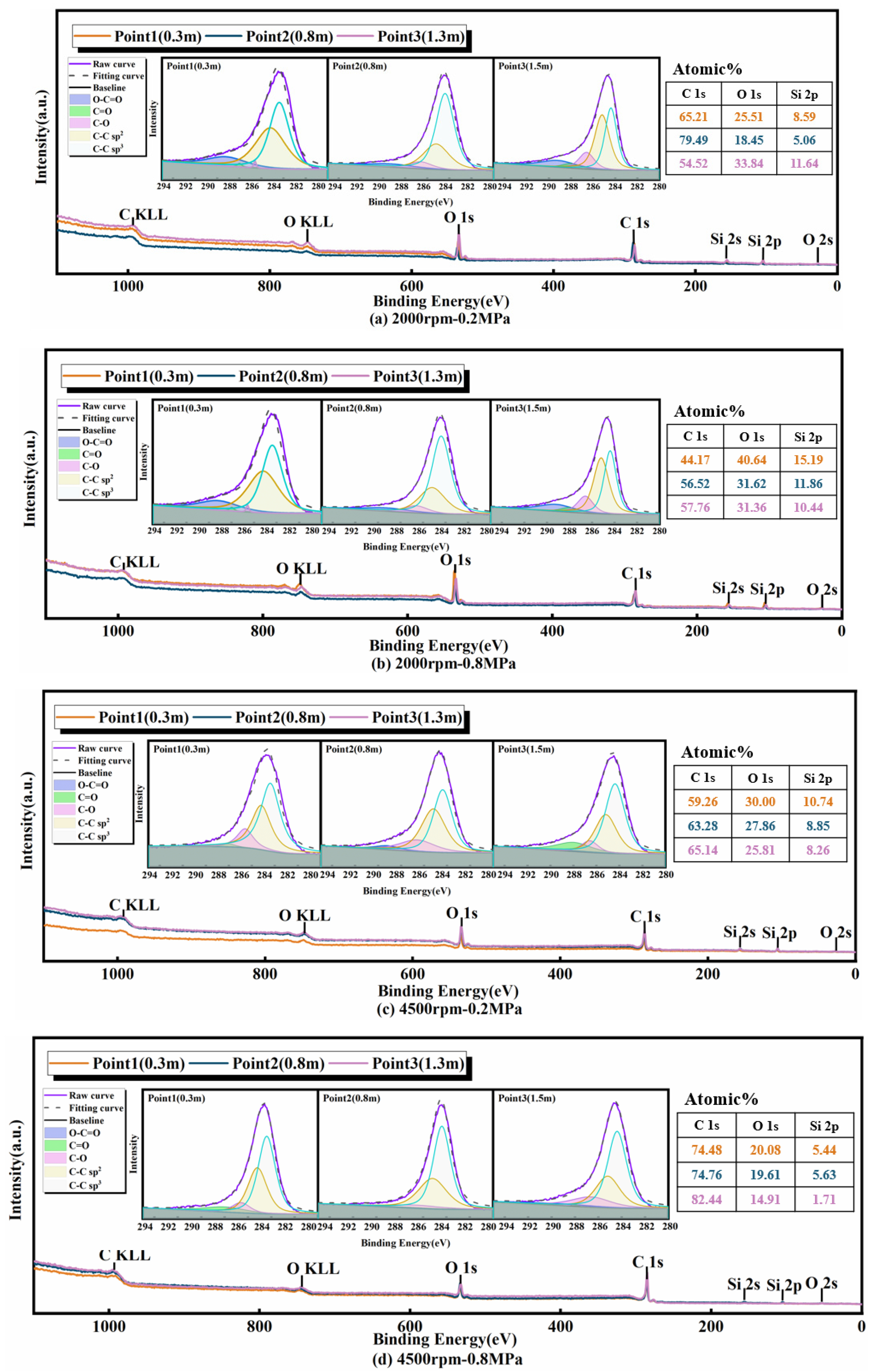
3.2.1. XPS Full-Spectrum Analysis
3.2.2. O/C Ratio
3.2.3. sp3/sp2 Ratio
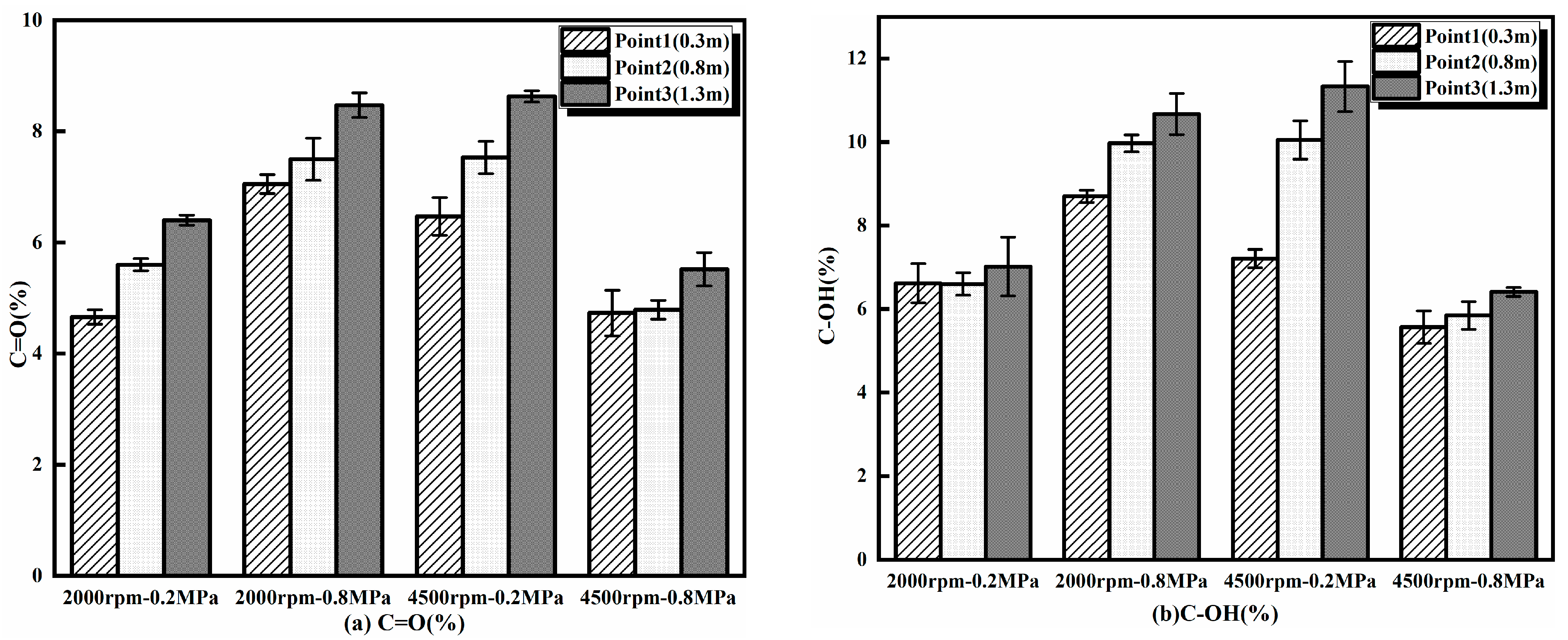
3.2.4. Surface Functional Groups
3.3. Oxidative Behavior of Soot Particulate
3.3.1. TG&DTG Analysis
3.3.2. Activation Energy of Soot Particulate
4. Conclusions
- The main oxidation temperature range of soot particulate from GDI engines ranges from 300 °C to 650 °C. The soot particulate generated at higher engine loads, or near the exhaust valve, has fewer structural defects in the base plane of the graphene layer, lower ratio of disordered graphite lattice, and higher degree of graphitization, exhibiting lower oxidation activity.
- The transportation distance along the exhaust pipe has a significant impact on the graphitization degree, oxygen-containing functional groups, and oxidation activity of soot particlute from a GDI engine. With an increase in the transportation distance, the degree of graphitization and activation energy of soot particulate decrease, and the sp3/sp2 ratio, O/C ratio, C=O% content, and C-OH% content increase, rendering the soot particulate easier to oxidize. At 2000 rpm-0.8 MPa, the sp3/sp2 ratio increased by 60.3% and the C=O% content increased from 8.70% to 10.67% after a 1 m increase in the exhaust transport distance. The activation energy decreased by 28.62 kJ/mol with increasing transport distance.
- The engine load has a significant impact on the graphitization, oxygen-containing functional groups, and oxidation behavior of soot particulate of a GDI engine. With an increase in the engine load, the graphitization degree and activation energy of soot particulate rise; the sp3/sp2 ratio, O/C ratio, C=O% content, and C-OH% content decrease, making the soot particulate more resistant to oxidation. Under a high engine load (4500 rpm-0.8 MPa), the activation energy increased by 22.37 kJ/mol, while the C=O% content decreased from 8.63% to 5.52% compared to the low engine load (4500 rpm-0.2 MPa).
- Engine speed has a certain impact on the graphitization, oxygen-containing functional groups, and oxidation behavior of soot particulate. With an increase in engine speed, there are no apparent changes in graphitization but a certain degree of reduction in the oxidation activity. In particular, the O/C ratio, sp3/sp2 ratio, C=O% content, and C-OH% content of soot particulate decrease with increasing engine speeds under high load but increase with an increase in engine speed under a light load. With the engine speed increase, the sp3/sp2 ratio decreased by 40.32%, and the C-OH% content decreased by 17.12%.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| Abbreviation | Description |
| GDI | Gasoline direct injection |
| XPS | X-ray photoelectron spectroscopy |
| TGA | Thermogravimetric analysis |
| PM | particulate mass |
| PN | particles number |
| PFI | port fuel injection |
| GPF | gasoline particulate filter |
| DPF | diesel particulate filter |
| TWC | three-way catalyst converter |
| EGR | exhaust gas recirculation |
| BMEP | brake mean effective pressure |
| 4L1G | Four-Layer One-Gaussian method |
| C=O% | the quality score of C=O |
| C-OH% | the quality score of C-OH |
| HC | hydrocarbons |
| SOF | soluble organic fraction |
| TG&DTG | Thermogravimetry & Derivative Thermogravimetry |
| Ea | The activation energy |
References
- Lu, L.; Pei, Y.; Qin, J. Experimental study on spatial distribution characteristics of cylinder-wall oil films under fuel spray impinging condition of GDI engine. Energy 2022, 254, 124381. [Google Scholar] [CrossRef]
- Gordon, T.D.; Presto, A.A.; May, A.A. Secondary organic aerosol formation exceeds primary particulate matter emissions for light-duty gasoline vehicles. Atmos. Chem. Phys. 2014, 14, 4661–4678. [Google Scholar]
- Gertler, A.W. Diesel vs. gasoline emissions: Does PM from diesel or gasoline vehicles dominate in the US. Atmos. Environ. 2005, 39, 2349–2355. [Google Scholar] [CrossRef]
- Jasiński, R.; Strzemiecka, B.; Koltsov, I. Physicochemical Analysis of the Particulate Matter Emitted from Road Vehicle Engines. Energies 2021, 14, 8556. [Google Scholar] [CrossRef]
- Joshi, A. Review of vehicle engine efficiency and emissions. SAE Int. J. Adv. Curr. Pract. Mobil. 2019, 1, 734–761. [Google Scholar] [CrossRef]
- Hu, Z.; Lu, Z.; Song, B.; Quan, Y. Impact of test cycle on mass, number and particle size distribution of particulates emitted form gasoline direct injection vehicles. Sci. Total Environ. 2021, 262, 143128. [Google Scholar] [CrossRef]
- Kontses, A.; Triantafyllopoulos, G.; Ntziachristos, L. Particle number (PN) emissions from gasoline, diesel, LPG, CNG and hybrid-electric light-duty vehicles under real-world driving conditions. Atmos. Environ. 2020, 222, 117–126. [Google Scholar] [CrossRef]
- Samaras, Z.C.; Andersson, J.; Bergmann, A. Measuring automotive exhaust particles down to 10 nm. SAE Int. J. Adv. Curr. Pract. Mobil. 2020, 3, 539–550. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, H.; Li, P. Low-content and highly effective zoned Rh and Pd three-way catalysts for gasoline particulate filter potentially meeting Euro 7. J. Rare Earths 2023, 41, 905–916. [Google Scholar] [CrossRef]
- Joshi, A.; Johnson, T.V. Gasoline Particulate Filters-a Review. Emiss. Control Sci. Technol. 2018, 4, 219–239. [Google Scholar] [CrossRef]
- McCaffery, C.; Zhu, H.; Li, C. On-road gaseous and particulate emissions from GDI vehicles with and without gasoline particulate filters (GPFs) using portable emissions measurement systems (PEMS). Sci. Total Environ. 2020, 710, 136366. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ou, Q.; Chang, C. Using aerosols to create Nano-scaled membranes that improve gasoline particulate filter performance and the development of Wafer-based membrane coated filter analysis (WMCFA) method. Sep. Purif. Technol. 2022, 284, 120310. [Google Scholar] [CrossRef]
- Nicolin, P.; Boger, T.; Dietrich, M.; Haft, G.; Bachurina, A. Soot Load Monitoring in Gasoline Particulate Filter Applications with RF-Sensors. SAE Tech. Pap. 2020. [Google Scholar] [CrossRef]
- Orihuela, M.P.; Gómez-Martín, A.; Miceli, P. Experimental measurement of the filtration efficiency and pressure drop of wall-flow diesel particulate filters (DPF) made of biomorphic Silicon Carbide using laboratory generated particles. Appl. Therm. Eng. 2018, 131, 41–53. [Google Scholar] [CrossRef]
- Adam, F.; Olfert, J.; Wong, K.; Kunert, S.; Richter, M.J. Effect of Engine-Out Soot Emissions and the Frequency of Regeneration on Gasoline Particulate Filter Efficiency. SAE Tech. Pap. 2020. [Google Scholar] [CrossRef]
- Li, Z.; Shen, B.; Zhang, Y. Simulation of deep-bed filtration of a gasoline particulate filter with inhomogeneous wall structure under different particle size distributions. Int. J. Engine Res. 2021, 7, 2107–2118. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Zhang, M. Exhaust non-volatile particle filtration characteristics of three-way catalyst and influencing factors in a gasoline direct injection engine compared to gasoline particulate filter. Fuel 2021, 290, 120065. [Google Scholar] [CrossRef]
- Guo, D.; Ge, Y.; Wang, X. Evaluating the filtration efficiency of close-coupled catalyzed gasoline particulate filter (cGPF) over the WLTC and simulated RDE cycles. Chemosphere 2022, 301, 134717. [Google Scholar] [CrossRef]
- Nossova, L.; Caraqvaggio, G. Effect of dopants on soot oxidation over doped Ag/ZrO2 catalysts for catalyzed gasoline particulate filter. Catal. Commun. 2023, 182, 106744. [Google Scholar] [CrossRef]
- Easter, J.E.; Fiano, A.; Bohac, S.; Premchand, K.; Hoard, J. Evaluation of low mileage GPF filtration and regeneration as influenced by soot morphology, reactivity, and GPF loading. SAE Tech. Pap. 2019. [Google Scholar] [CrossRef]
- Zinsmeister, J.; Storch, M.; Meder, J. Soot formation of renewable gasoline: From fuel chemistry to particulate emissions from engines. Fuel 2023, 348, 128109. [Google Scholar] [CrossRef]
- Duan, X.; Li, Y.; Liu, Y. Quantitative investigation the influences of the injection timing under single and double injection strategies on performance, combustion and emissions characteristics of a GDI SI engine fueled with gasoline/ethanol blend. Fuel 2020, 260, 116363. [Google Scholar] [CrossRef]
- Shi, L.; Ji, C.; Wang, S. Combustion and emissions characteristics of a S.I. engine fueled with gasoline-DME blends under different spark timings. Fuel 2018, 211, 11–17. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Z.; Zhang, H. Effect of working conditions on oxidation activity and surface functional group of particulate matter emitted from China Ⅵ GDI engine. Fuel 2022, 318, 123581. [Google Scholar] [CrossRef]
- Andy, T.; Harekrishna, Y.; Michael, S.; Leonid, T. Effect of Lubricant Formulation on Characteristics of Particle Emission from Engine Fed with a Hydrogen-Rich Fuel. SAE Tech. Pap. 2020. [Google Scholar] [CrossRef]
- Chaimanatsakun, A.; Sawatmongkhon, B.; Sittichompoo, S. Effects of reformed exhaust gas recirculation (REGR) of ethanol-gasoline fuel blends on the combustion and emissions of gasoline direct injection (GDI) engine. Fuel 2024, 355, 129506. [Google Scholar] [CrossRef]
- Fu, J.; Hu, Z.; Fang, L. An Experimental Study on Soot Particles Size Distribution and Nanostructure Evolution at Different Tailpipe Positions of a Dedicated Hybrid Engine. SAE Tech. Pap. 2023. [Google Scholar] [CrossRef]
- Wang, X.; Chen, W.; Huang, Y. Advances in soot particles from gasoline direct injection engines: A focus on physical and chemical characterization. Chemosphere 2023, 311, 137181. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Yu, Y. An experimental study on particle evolution in the exhaust gas of a direct injection SI engine. Appl. Energy 2020, 260, 114220. [Google Scholar] [CrossRef]
- Spiess, S.; Wong, K.F.; Joerg-Michael, R. Investigations of Emission Control Systems for Gasoline Direct Injection Engines with a Focus on Removal of Particulate Emissions. Top. Catal. 2013, 56, 434–439. [Google Scholar] [CrossRef]
- Wang, Y.K.; Zhang, K.M. Coupled turbulence and aerosol dynamics modeling of vehicle exhaust plumes using the CTAG model. Atmos. Environ. 2012, 59, 284–293. [Google Scholar] [CrossRef]
- Liu, H.; Yu, Y.; Wang, C. Brownian coagulation of particles in the gasoline engine exhaust system: Experimental measurement and Monte Carlo simulation. Fuel 2021, 303, 121340. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, X.; Wang, Y. Effects of Viscosity Index Improver on Morphology and Graphitization Degree of Diesel Particulate Matter. Energy Procedia 2017, 105, 4236–4241. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Knauer, M.; Schuster, M.E.; Su, D. Soot Structure and Reactivity Analysis by Raman Microspectroscopy, Temperature-Programmed Oxidation, and High-Resolution Transmission Electron Microscopy. J. Phys. Chem. A 2009, 113, 13871–13880. [Google Scholar] [CrossRef]
- Sorianoa, J.A.; Agudelob, J.R.; Lopezb, A.F. Oxidation reactivity and nanostructural characterization of the soot coming from farnesane—A novel diesel fuel derived from sugar cane. Carbon 2017, 125, 516–529. [Google Scholar] [CrossRef]
- Al-Qurashi, K.; Boehman, A.L. Impact of exhaust gas recirculation (EGR) on the oxidative reactivity of diesel engine soot. Combust. Flame 2008, 155, 675–695. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Bai, Y. Oxidation behaviors and nanostructure of particulate matter produced from a diesel engine fueled with n-pentanol and 2-ethylhexyl nitrate additives. Fuel 2021, 288, 119844. [Google Scholar] [CrossRef]
- Pfau, S.A.; La, R.A.; Haffner-Staton, E.; Rance, G.A.; Fay, M.W.; Brough, R.J. Comparative nanostructure analysis of gasoline turbocharged direct injection and diesel soot-in-oil with carbon black. Carbon 2018, 139, 342–352. [Google Scholar] [CrossRef]
- Mühlbauer, W.; Zöllner, C.; Lehmann, S.; Lorenz, S.; Brüggemann, D. Correlations between physicochemical properties of emitted diesel particulate matter and its reactivity. Combust. Flame 2016, 167, 39–51. [Google Scholar] [CrossRef]
- Nikhil, S.; Avinash, K.A. Macroscopic spray characteristics of a gasohol fueled GDI injector and impact on engine combustion and particulate morphology. Fuel 2021, 295, 120461. [Google Scholar] [CrossRef]
- Liu, Y.; Song, C.; Lv, G.; Cao, X.; Wang, L.; Qiao, Y.; Yang, X. Surface functional groups and sp3/sp2 hybridization ratios of in-cylinder soot from a diesel engine fueled with n-heptane and n-heptane/toluene. Fuel 2016, 179, 108–113. [Google Scholar] [CrossRef]
- E, J.; Xu, W.; Ma, Y. Soot formation mechanism of modern automobile engines and methods of reducing soot emissions: A review. Fuel Process. Technol. 2022, 235, 107373. [Google Scholar] [CrossRef]
- Piano, A.; Scalambro, A.; Millo, F. CFD-based methodology for the characterization of the combustion process of a passive pre-chamber gasoline engine. Transp. Eng. 2023, 13, 100200. [Google Scholar] [CrossRef]
- Awad, I.; Omar Ma, X.; Kamil, M. Particulate emissions from gasoline direct injection engines: A review of how current emission regulations are being met by automobile manufacturers. Sci. Total Environ. 2020, 718, 137302. [Google Scholar] [CrossRef]
- Fan, C.; Wei, J.; Huang, H. Chemical feature of the soot emissions from a diesel engine fueled with methanol-diesel blends. Fuel 2021, 297, 120739. [Google Scholar] [CrossRef]
- Russo, C.; Alfè, M.; Rouzaud, J.-N.; Stanzione, F.; Tregrossi, A.; Ciajolo, A. Probing structures of soot formed in premixed flames of methane, ethylene and benzene. Proc. Combust. Inst. 2013, 34, 1885–1892. [Google Scholar] [CrossRef]
- Qiu, C.; Jiang, L.; Gao, Y. Effects of oxygen-containing functional groups on carbon materials in supercapacitors: A review. Mater. Des. 2023, 230, 111952. [Google Scholar] [CrossRef]
- Wang, L.; Song, C.; Song, J.; Lv, G.; Pang, H.; Zhang, W. Aliphatic C–H and oxygenated surface functional groups of diesel in-cylinder soot: Characterizations and impact on soot oxidation behavior. Proc. Combust. Inst. 2013, 34, 3099–3106. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, J.; Oliva, F.; Vázquez, R.A. Characterization of the diesel soot oxidation process through an optimized thermogravimetric method. Energy Fuels 2011, 25, 2039–2048. [Google Scholar] [CrossRef]
- Bogarra, M.; Herreros, J.M.; Tsolakis, A. Gasoline direct injection engine soot oxidation: Fundamentals and determination of kinetic parameters. Combust. Flame 2018, 190, 177–187. [Google Scholar] [CrossRef]
- Piqueras, P.; Sanchis, E.J.; Herreros, J.M. Evaluating the oxidation kinetic parameters of gasoline direct injection soot from thermogravimetric analysis experiments. Chem. Eng. Sci. 2021, 234, 116437. [Google Scholar] [CrossRef]
- Easter, J.; Bohac, S.; Hoard, J.; Boehman, A. Influence of ash-soot interactions on the reactivity of soot from a gasoline direct injection engine. Aerosol Sci. Technol. 2020, 54, 1373–1385. [Google Scholar] [CrossRef] [PubMed]






| Parameter | Value |
|---|---|
| Displacement (L) | 1.4 |
| Bore × Storke (mm) | 74.5 × 80 |
| Rated power (kW) | 110 |
| Max torque (N·m) | 250 |
| Rated speed (rpm) | 6000 |
| Compression ratio | 10 |
| Parameter | Value | China 6 |
|---|---|---|
| Density (kg/m3) | 742.5 | 720–775 |
| Octane value (RON) | 92.1 | 91–95 |
| Sulphur content (mg/kg) | 6.4 | <10 |
| 10% evaporation temperature (°C) | 57.3 | <70 |
| 90% evaporation temperature (°C) | 157.5 | <190 |
| Aromatics content (volume fraction) (%) | 29.2 | <35 |
| Operating Condition | Speed/rpm | BMEP/MPa | Injection Timing/°CA | Injection Duration/ms | Air-to-Fuel Ratio (-) |
|---|---|---|---|---|---|
| A | 2000 | 0.2 | 272 | 0.81 | 14.93 |
| B | 2000 | 0.8 | 269 | 1.55 | 14.85 |
| C | 4500 | 0.2 | 313 | 0.87 | 15.13 |
| D | 4500 | 0.8 | 297 | 1.61 | 15.11 |
| Operating Condition | Sampling Location | Activation Energy/kJ/mol | R2 |
|---|---|---|---|
| 2000 rpm-0.2 MPa | Point 1 (0.3 m) | 128.26 | 0.91 |
| Point 2 (0.8 m) | 120.51 | 0.93 | |
| Point 3 (1.3 m) | 112.70 | 0.93 | |
| 2000 rpm-0.8 MPa | Point 1 (0.3 m) | 162.41 | 0.93 |
| Point 2 (0.8 m) | 157.32 | 0.98 | |
| Point 3 (1.3 m) | 133.79 | 0.96 | |
| 4500 rpm-0.2 MPa | Point 1 (0.3 m) | 119.88 | 0.91 |
| Point 2 (0.8 m) | 111.72 | 0.92 | |
| Point 3 (1.3 m) | 103.49 | 0.94 | |
| 4500 rpm-0.8 MPa | Point 1 (0.3 m) | 145.92 | 0.90 |
| Point 2 (0.8 m) | 138.71 | 0.97 | |
| Point 3 (1.3 m) | 125.86 | 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Yin, L.; Shen, J.; Lu, Z.; Tan, P.; Lou, D. Investigation on Graphitization, Surface Functional Groups, and Oxidation Behavior of Soot Particulate Along Exhaust Pipe of Gasoline Direct Injection Engine. Energies 2025, 18, 1684. https://doi.org/10.3390/en18071684
Hu Z, Yin L, Shen J, Lu Z, Tan P, Lou D. Investigation on Graphitization, Surface Functional Groups, and Oxidation Behavior of Soot Particulate Along Exhaust Pipe of Gasoline Direct Injection Engine. Energies. 2025; 18(7):1684. https://doi.org/10.3390/en18071684
Chicago/Turabian StyleHu, Zhiyuan, Li Yin, Jiayi Shen, Zhangying Lu, Piqiang Tan, and Diming Lou. 2025. "Investigation on Graphitization, Surface Functional Groups, and Oxidation Behavior of Soot Particulate Along Exhaust Pipe of Gasoline Direct Injection Engine" Energies 18, no. 7: 1684. https://doi.org/10.3390/en18071684
APA StyleHu, Z., Yin, L., Shen, J., Lu, Z., Tan, P., & Lou, D. (2025). Investigation on Graphitization, Surface Functional Groups, and Oxidation Behavior of Soot Particulate Along Exhaust Pipe of Gasoline Direct Injection Engine. Energies, 18(7), 1684. https://doi.org/10.3390/en18071684





