A Review of Ammonia Combustion Reaction Mechanism and Emission Reduction Strategies
Abstract
1. Introduction
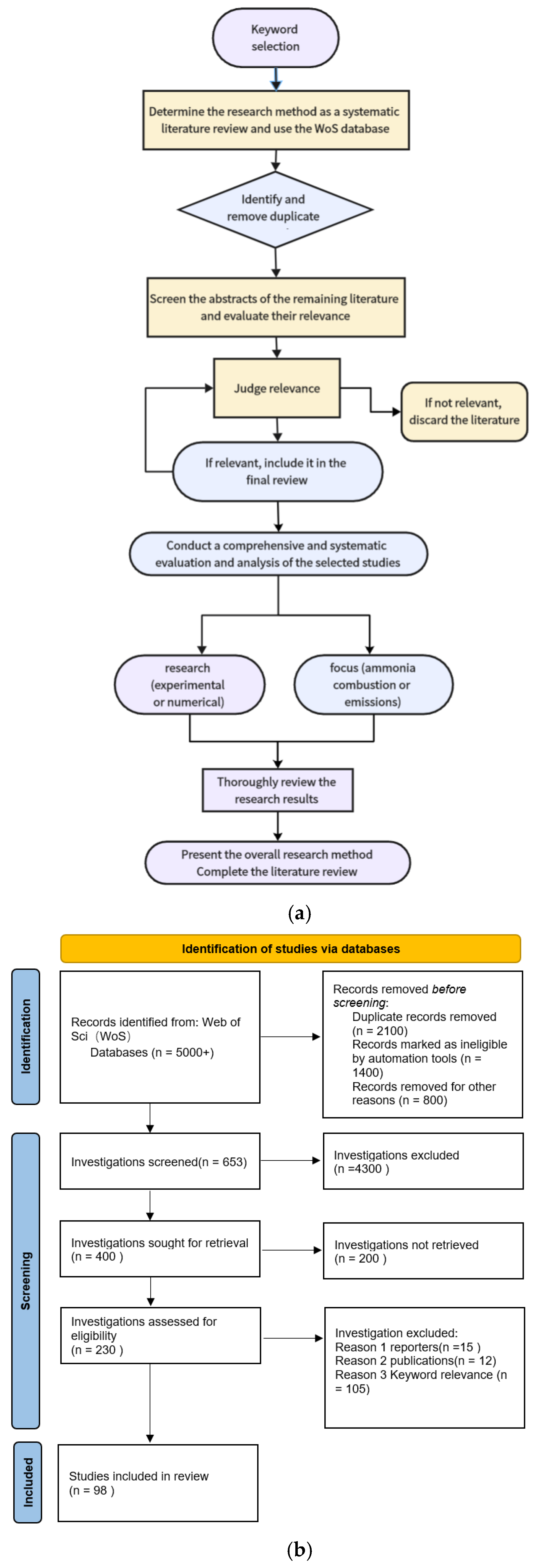
2. Methods
3. Basic Research on Ammonia (NH3) Combustion
3.1. Oxidation and Pyrolysis of NH3-Based Combustion Chemistry
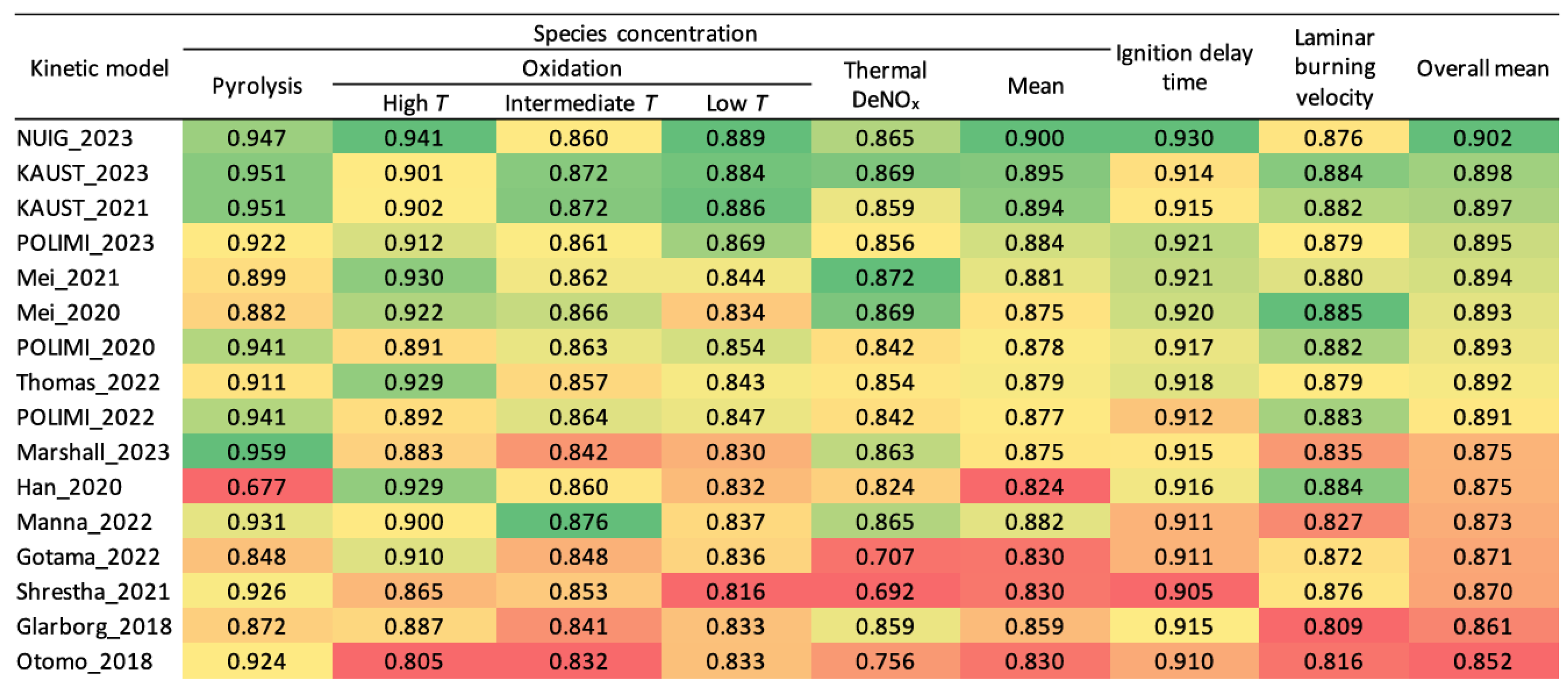
3.2. Chemical Kinetic Mechanisms
3.3. Construction of a Comprehensive Chemical Kinetic Model
4. Nitrogen Oxide Emission Reduction Strategies
4.1. Rich Combustion at High Inlet Pressure
4.2. Dual-Fuel Combustion
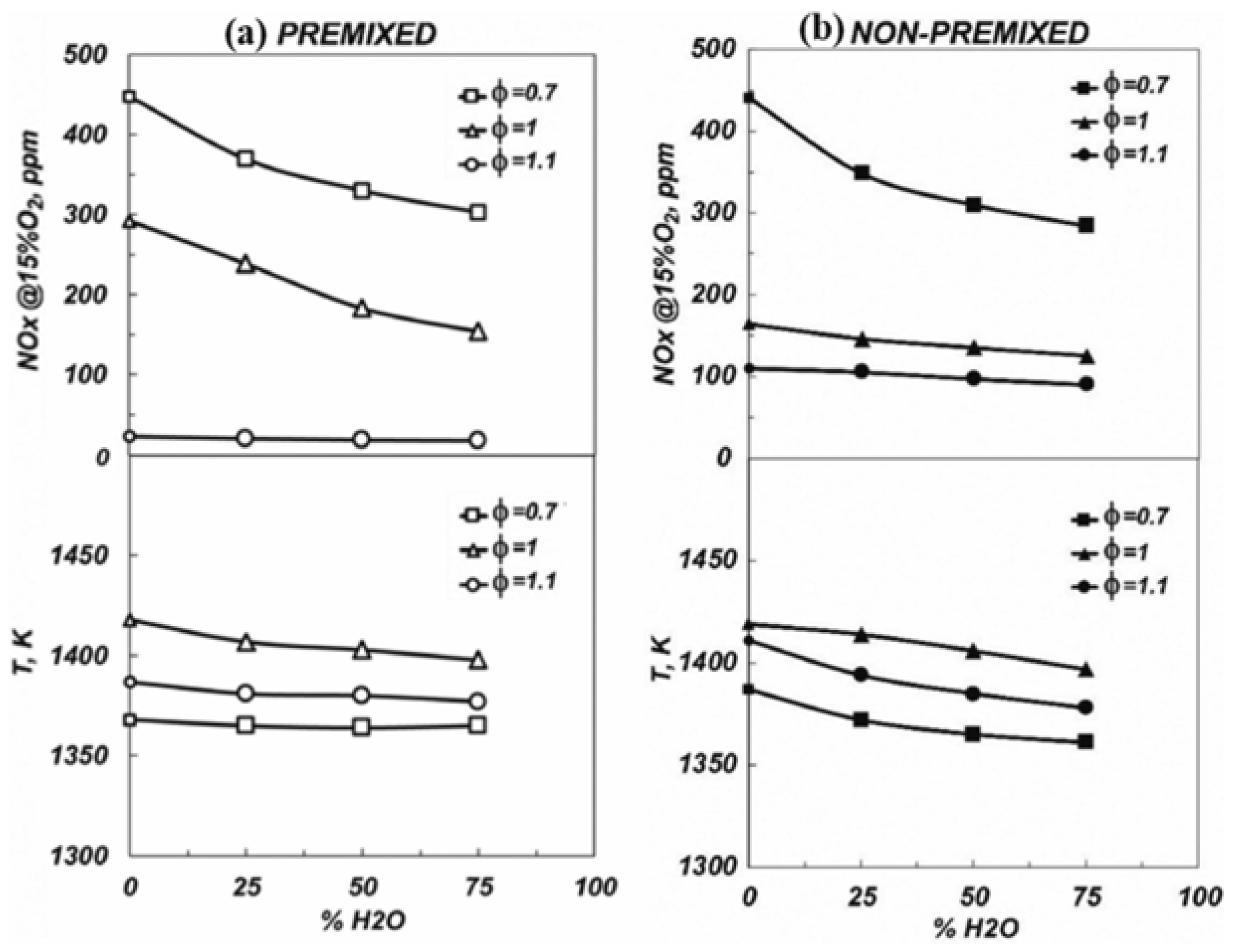
4.3. Reactant Humidity
4.4. Plasma-Assisted Combustion
4.5. Staged Combustion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cardoso, J.S.; Silva, V.; Rocha, R.C.; Hall, M.J.; Costa, M.; Eusébio, D. Ammonia as an energy vector: Current and future prospects for low-carbon fuel applications in internal combustion engines. J. Clean. Prod. 2021, 296, 126562. [Google Scholar] [CrossRef]
- Dimitriou, P.; Javaid, R. A review of ammonia as a compression ignition engine fuel. Int. J. Hydrogen Energy 2020, 45, 7098–7118. [Google Scholar] [CrossRef]
- Lin, S.D. Research on the Kinetic Mechanism of Ammonia/Highly Active Fuel Composite Combustion and the Optimization of Combustion Process; Jilin University: Changchun, China, 2024. [Google Scholar]
- Cai, T.; Zhao, D. Overview of Autoignition and Flame Propagation Properties for Ammonia Combustion. AIAA J. 2023, 61, 2754–2778. [Google Scholar]
- Oceanic, N. Trends in Atmospheric Carbon Dioxide. 2023. Available online: https://www.ntschools.org (accessed on 25 March 2025).
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar]
- Hasan, M.H.; Mahlia, T.M.I.; Mofijur, M.; Fattah, I.M.R.; Handayani, F.; Ong, H.C.; Silitonga, A.S. A Comprehensive Review on the Recent Development of Ammonia as a Renewable Energy Carrier. Energies 2021, 14, 3732. [Google Scholar] [CrossRef]
- Herbinet, O.; Bartocci, P.; Dana, A.G. On the use of ammonia as a fuel—A perspective. Fuel Commun. 2022, 11, 100064. [Google Scholar]
- Bicer, Y.; Dincer, I. Life cycle assessment of ammonia utilization in city transportation and power generation. J. Clean. Prod. 2018, 170, 1594–1601. [Google Scholar] [CrossRef]
- Matthews, H.D.; Tokarska, K.B.; Rogelj, J.; Smith, C.J.; MacDougall, A.H.; Haustein, K.; Mengis, N.; Sippel, S.; Forster, P.M.; Knutti, R. An integrated approach to quantifying uncertainties in the remaining carbon budget. Commun. Earth Environ. 2021, 2, 7. [Google Scholar] [CrossRef]
- Lee, H.; Lee, M.-J. Recent Advances in Ammonia Combustion Technology in Thermal Power Generation System for Carbon Emission Reduction. Energies 2021, 14, 5604. [Google Scholar] [CrossRef]
- Ghavam, S.; Vahdati, M.; Wilson, I.A.G.; Styring, P. Sustainable Ammonia Production Processes. Front. Energy Res. 2021, 9, 580808. [Google Scholar] [CrossRef]
- Tornatore, C.; Marchitto, L.; Sabia, P.; De Joannon, M. Ammonia as Green Fuel in Internal Combustion Engines: State-of-the-Art and Future Perspectives. Front. Mech. Eng. 2022, 8, 944201. [Google Scholar] [CrossRef]
- Kojima, Y.; Yamaguchi, M. Ammonia as a hydrogen energy carrier. Int. J. Hydrogen Energy 2022, 47, 22832–22839. [Google Scholar] [CrossRef]
- Yin, G.; Xiao, B.; Zhan, H.; Hu, E.; Huang, Z. Chemical kinetic study of ammonia with propane on combustion control and NO formation. Combust. Flame 2023, 249, 112617. [Google Scholar] [CrossRef]
- Vignat, G.; Zirwes, T.; Toro, E.R.; Younes, K.; Boigné, E.; Muhunthan, P.; Simitz, L.; Trimis, D.; Ihme, M. Experimental and numerical investigation of flame stabilization and pollutant formation in matrix stabilized ammonia-hydrogen combustion. Combust. Flame 2023, 250, 112642. [Google Scholar] [CrossRef]
- Stagni, A.; Cavallotti, C.; Arunthanayothin, S.; Song, Y.; Herbinet, O.; Battin-Leclerc, F.; Faravelli, T. An experimental, theoretical and kinetic-modeling study of the gas-phase oxidation of ammonia. React. Chem. Eng. 2020, 5, 696–711. [Google Scholar] [CrossRef]
- Yang, W.; Dinesh, K.R.; Luo, K.H.; Thevenin, D. Direct numerical simulation of turbulent premixed ammonia and ammonia-hydrogen combustion under engine-relevant conditions. Int. J. Hydrogen Energy 2022, 47, 11083–11100. [Google Scholar]
- Skreiberg, P.; Kilpinen, P. Glarborg, Ammonia chemistry below 1400 K under fuel-rich conditions in a flow reactor. Combust. Flame 2004, 136, 501–518. [Google Scholar] [CrossRef]
- Ariemma, G.B.; Sorrentino, G.; Ragucci, R.; de Joannon, M.; Sabia, P. Ammonia/Methane combustion: Stability and NOx emissions. Combust. Flame 2022, 241, 112071. [Google Scholar] [CrossRef]
- Jin, S.; Wu, B.; Zi, Z.; Yang, P.; Shi, T.; Zhang, J. Effects of fuel injection strategy and ammonia energy ratio on combustion and emissions of ammonia-diesel dual-fuel engine. Fuel 2023, 341, 127668. [Google Scholar] [CrossRef]
- Kurien, C.; Varma, P.S.; Mittal, M. Effect of ammonia energy fractions on combustion stability and engine characteristics of gaseous (ammonia/methane) fuelled spark ignition engine. Int. J. Hydrogen Energy 2023, 48, 1391–1400. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, S.; Xiong, Y.; Qin, J. Performance and emission characteristics of ammonia fueled scramjet engine. Energy Convers. Manag. 2023, 300, 117913. [Google Scholar] [CrossRef]
- Cai, T.; Zhao, D.; Karimi, N. Optimizing thermal performance and exergy efficiency in hydrogen-fueled meso-combustors by applying a bluff-body. J. Clean. Prod. 2021, 311, 127573. [Google Scholar] [CrossRef]
- Cai, T.; Tang, A.; Zhao, D.; Zhou, C.; Huang, Q. Flame dynamics and stability of premixed methane/air in micro-planar quartz combustors. Energy 2020, 193, 303–314. [Google Scholar] [CrossRef]
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Experimental and numerical study of the laminar burning velocity of CH4–NH3–air premixed flames. Combust. Flame 2018, 187, 185–198. [Google Scholar] [CrossRef]
- Mei, B.; Zhang, X.; Ma, S.; Cui, M.; Guo, H.; Cao, Z.; Li, Y. Experimental and kinetic modeling investigation on the laminar flame propagation of ammonia under oxygen enrichment and elevated pressure conditions. Combust. Flame 2019, 210, 236–246. [Google Scholar] [CrossRef]
- He, X.; Shu, B.; Nascimento, D.; Moshammer, K.; Costa, M.; Fernandes, R. Auto-ignition kinetics of ammonia and ammonia/hydrogen mixtures at intermediate temperatures and high pressures. Combust. Flame 2019, 206, 189–200. [Google Scholar] [CrossRef]
- Dai, L.; Gersen, S.; Glarborg, P.; Mokhov, A.; Levinsky, H. Autoignition studies of NH3/CH4 mixtures at high pressure. Combust. Flame 2020, 218, 19–26. [Google Scholar]
- Alnasif, A.; Mashruk, S.; Shi, H.; Alnajideen, M.; Wang, P.; Pugh, D.; Valera-Medina, A. Evolution of ammonia reaction mechanisms and modeling parameters: A review. Appl. Energy Combust. Sci. 2023, 15, 100175. [Google Scholar]
- Ramachandran, E.; Krishnaiah, R.; Venkatesan, E.P.; Medapati, S.R.; Sabarish, R.; Khan, S.A.; Asif, M.; Linul, E. Experimental Studies to Reduce Usage of Fossil Fuels and Improve Green Fuels by Adopting Hydrogen–Ammonia–Biodiesel as Trinary Fuel for RCCI Engine. ACS Omega 2024, 9, 741–752. [Google Scholar] [CrossRef]
- Colson, S.; Hayakawa, A.; Kudo, T.; Kobayashi, H. Extinction characteristics of ammonia/air counterflow premixed flames at various pressures. J. Therm. Sci. Technol. 2016, 11, JTST0048. [Google Scholar] [CrossRef]
- Ichimura, R.; Hadi, K.; Hashimoto, N.; Hayakawa, A.; Kobayashi, H.; Fujita, O. Extinction limits of an ammonia/air flame propagating in a turbulent field. Fuel 2019, 246, 178–186. [Google Scholar] [CrossRef]
- Brackmann, C.; Alekseev, V.A.; Zhou, B.; Nordström, E.; Bengtsson, P.-E.; Li, Z.; Aldén, M.; Konnov, A.A. Structure of premixed ammonia + air flames at atmospheric pressure: Laser diagnostics and kinetic modeling. Combust. Flame 2016, 163, 370–381. [Google Scholar] [CrossRef]
- Chiong, M.-C.; Chong, C.T.; Ng, J.-H.; Mashruk, S.; Chong, W.W.F.; Samiran, N.A.; Mong, G.R.; Valera-Medina, A. Advancements of combustion technologies in the ammonia-fuelled engines. Energy Convers. Manag. 2021, 244, 114460. [Google Scholar] [CrossRef]
- Pochet, M.; Dias, V.; Moreau, B.; Foucher, F.; Jeanmart, H.; Contino, F. Experimental and numerical study, under LTC conditions, of ammonia ignition delay with and without hydrogen addition. Proc. Combust. Inst. 2019, 37, 621–629. [Google Scholar]
- Xiao, H.; Lai, S.; Valera-Medina, A.; Li, J.; Liu, J.; Fu, H. Experimental and modeling study on ignition delay of ammonia/methane fuels. Int. J. Energy Res. 2020, 44, 6939–6949. [Google Scholar] [CrossRef]
- Issayev, G.; Giri, B.R.; Elbaz, A.M.; Shrestha, K.P.; Mauss, F.; Roberts, W.L.; Farooq, A. Ignition delay time and laminar flame speed measurements of ammonia blended with dimethyl ether: A promising low carbon fuel blend. Renew. Energy 2022, 181, 1353–1370. [Google Scholar] [CrossRef]
- Dai, L.; Hashemi, H.; Glarborg, P.; Gersen, S.; Marshall, P.; Mokhov, A.; Levinsky, H. Ignition delay times of NH3/DME blends at high pressure and low DME fraction: RCM experiments and simulations. Combust. Flame 2021, 227, 120–134. [Google Scholar]
- Dagaut, P.; Glarborg, P.; Alzueta, M.U. The oxidation of hydrogen cyanide and related chemistry. Prog. Energy Combust. Sci. 2008, 34, 1–46. [Google Scholar]
- Mathieu, O.; Petersen, E.L. Experimental and modeling study on the high-temperature oxidation of Ammonia and related NOx chemistry. Combust. Flame 2015, 162, 554–570. [Google Scholar] [CrossRef]
- Shu, B.; Vallabhuni, S.; He, X.; Issayev, G.; Moshammer, K.; Farooq, A.; Fernandes, R. A shock tube and modeling study on the autoignition properties of ammonia at intermediate temperatures. Proc. Combust. Inst. 2019, 37, 205–211. [Google Scholar] [CrossRef]
- Han, X.; Wang, Z.; Costa, M.; Sun, Z.; He, Y.; Cen, K. Experimental and kinetic modeling study of laminar burning velocities of NH3/air, NH3/H2/air, NH3/CO/ air and NH3/CH4/air premixed flames. Combust. Flame 2019, 206, 214–226. [Google Scholar] [CrossRef]
- Han, X.; Wang, Z.; He, Y.; Zhu, Y.; Cen, K. Experimental and kinetic modeling study of laminar burning velocities of NH3/syngas/air premixed flames. Combust. Flame 2020, 213, 1–13. [Google Scholar] [CrossRef]
- Mei, B.; Ma, S.; Zhang, Y.; Zhang, X.; Li, W.; Li, Y. Exploration on laminar flame propagation of ammonia and syngas mixtures up to 10 atm. Combust. Flame 2020, 220, 368–377. [Google Scholar] [CrossRef]
- Nakamura, H.; Hasegawa, S.; Tezuka, T. Kinetic modeling of ammonia/air weak flames in a micro flow reactor with a controlled temperature profile. Combust. Flame 2017, 185, 16–27. [Google Scholar] [CrossRef]
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Measurement and modelling of the laminar burning velocity of methane-ammonia-air flames at high pressures using a reduced reaction mechanism. Combust. Flame 2019, 204, 162–175. [Google Scholar] [CrossRef]
- Xiao, H.; Valera-Medina, A.; Bowen, P.J. Modeling Combustion of Ammonia/Hydrogen Fuel Blends under Gas Turbine Conditions. Energy Fuels 2017, 31, 8631–8642. [Google Scholar] [CrossRef]
- Xiao, H.; Howard, M.; Valera-Medina, A.; Dooley, S.; Bowen, P.J. Study on Reduced Chemical Mechanisms of Ammonia/Methane Combustion under Gas Turbine Conditions. Energy Fuels 2016, 30, 8701–8710. [Google Scholar] [CrossRef]
- Wang, Z.; Han, X.; He, Y.; Zhu, R.; Zhu, Y.; Zhou, Z.; Cen, K. Experimental and kinetic study on the laminar burning velocities of NH3 mixing with CH3OH and C2H5OH in premixed flames. Combust. Flame 2021, 229, 111392. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Elbaz, A.M.; Han, X.; He, Y.; Costa, M.; Konnov, A.A.; Roberts, W.L. Experimental study and kinetic analysis of the laminar burning velocity of NH3/syngas/air, NH3/CO/air and NH3/H2/air premixed flames at elevated pressures. Combust. Flame 2020, 221, 270–287. [Google Scholar] [CrossRef]
- Yin, G.; Li, J.; Zhou, M.; Li, J.; Wang, C.; Hu, E.; Huang, Z. Experimental and kinetic study on laminar flame speeds of ammonia/dimethyl ether/air under high temperature and elevated pressure. Combust. Flame 2022, 238, 111915. [Google Scholar] [CrossRef]
- da Rocha, R.C.; Costa, M.; Bai, X.-S. Chemical kinetic modelling of ammonia/hydrogen/air ignition, premixed flame propagation and NO emission. Fuel 2019, 246, 24–33. [Google Scholar]
- Kohse-Höinghaus, K. Combustion in the future: The importance of chemistry. Proc. Combust. Inst. 2020, 38, 1–56. [Google Scholar] [CrossRef]
- Basevich, V. Chemical kinetics in the combustion processes: A detailed kinetics mechanism and its implementation. Prog. Energy Combust. Sci. 1987, 13, 199–248. [Google Scholar] [CrossRef]
- Westbrook, C.K.; Dryer, F.L. Chemical kinetic modeling of hydrocarbon combustion. Prog. Energy Combust. Sci. 1984, 10, 1–57. [Google Scholar] [CrossRef]
- Lyon, R.K. Thermal DeNOx Controlling nitrogen oxides emissions by a noncatalytic process. Environ. Sci. Technol. 1987, 21, 231–236. [Google Scholar] [CrossRef]
- Miller, J.A.; Branch, M.; Kee, R.J. A chemical kinetic model for the selective reduction of nitric oxide by ammonia. Combust. Flame 1981, 43, 81–98. [Google Scholar] [CrossRef]
- Miller, J.A.; Glarborg, P. Modeling the thermal De-NOx process: Closing in on a final solution. Int. J. Chem. Kinet. 1999, 31, 757–765. [Google Scholar]
- Miller, J.A.; Smooke, M.D.; Green, R.M.; Kee, R.J. Kinetic Modeling of the Oxidation of Ammonia in Flames. Combust. Sci. Technol. 1983, 34, 149–176. [Google Scholar] [CrossRef]
- Miller, J.A.; Bowman, C.T. Mechanism and modeling of nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 1989, 15, 287–338. [Google Scholar] [CrossRef]
- Lindstedt, R.P.; Lockwood, F.C.; Selim, M.A. Detailed Kinetic Modelling of Chemistry and Temperature Effects on Ammonia Oxidation. Combust. Sci. Technol. 1994, 99, 253–276. [Google Scholar] [CrossRef]
- Lindstedt, R.P.; Lockwood, F.C.; Selim, M.A. A Detailed Kinetic Study of Ammonia Oxidation. Combust. Sci. Technol. 1995, 108, 231–254. [Google Scholar] [CrossRef]
- Klippenstein, S.J.; Harding, L.B.; Glarborg, P.; Miller, J.A. The role of NNH in NO formation and control. Combust. Flame 2011, 158, 774–789. [Google Scholar] [CrossRef]
- Duynslaegher, C.; Jeanmart, H.; Vandooren, J. Flame structure studies of premixed ammonia/hydrogen/oxygen/argon flames: Experimental and numerical investigation. Proc. Combust. Inst. 2009, 32, 1277–1284. [Google Scholar] [CrossRef]
- Duynslaegher, C.; Contino, F.; Vandooren, J.; Jeanmart, H. Modeling of ammonia combustion at low pressure. Combust. Flame 2012, 159, 2799–2805. [Google Scholar] [CrossRef]
- Konnov, A.A. Implementation of the NCN pathway of prompt-NO formation in the detailed reaction mechanism. Combust. Flame 2009, 156, 2093–2105. [Google Scholar] [CrossRef]
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar]
- Shrestha, K.P.; Seidel, L.; Zeuch, T.; Mauss, F. Detailed Kinetic Mechanism for the Oxidation of Ammonia Including the Formation and Reduction of Nitrogen Oxides. Energy Fuels 2018, 32, 10202–10217. [Google Scholar] [CrossRef]
- Ranzi, A.F.F. Kinetic modeling of the interactions between NO and hydrocarbons at high temperature. Combust. Flame 2003, 135, 97–112. [Google Scholar]
- Faravelli, T.; Frassoldati, A.; Ranzi, E. Kinetic modeling of the interactions between NO and hydrocarbons in the oxidation of hydrocarbons at low temperatures. Combust. Flame 2003, 132, 188–207. [Google Scholar]
- Song, Y.; Hashemi, H.; Christensen, J.M.; Zou, C.; Marshall, P.; Glarborg, P. Ammonia oxidation at high pressure and intermediate temperatures. Fuel 2016, 181, 358–365. [Google Scholar] [CrossRef]
- Jiang, Y.; Gruber, A.; Seshadri, K.; Williams, F. An updated short chemical-kinetic nitrogen mechanism for carbon-free combustion applications. Int. J. Energy Res. 2020, 44, 795–810. [Google Scholar] [CrossRef]
- Zhang, X.; Moosakutty, S.P.; Rajan, R.P.; Younes, M.; Sarathy, S.M. Combustion chemistry of ammonia/hydrogen mixtures: Jet-stirred reactor measurements and comprehensive kinetic modeling. Combust. Flame 2021, 234, 111653. [Google Scholar] [CrossRef]
- Zhang, X.; Yalamanchi, K.K.; Sarathy, S.M. Combustion chemistry of ammonia/C1 fuels: A comprehensive kinetic modeling study. Fuel 2023, 341, 127676. [Google Scholar] [CrossRef]
- Thomas, D.E.; Shrestha, K.P.; Mauss, F.; Northrop, W.F. Extinction and NO formation of ammonia-hydrogen and air non-premixed counterflow flames. Proc. Combust. Inst. 2023, 39, 1803–1812. [Google Scholar] [CrossRef]
- Glarborg, P. The NH3/NO2/O2 system: Constraining key steps in ammonia ignition and N2O formation. Combust. Flame 2023, 257, 112311. [Google Scholar]
- Stagni, A.; Arunthanayothin, S.; Dehue, M.; Herbinet, O.; Battin-Leclerc, F.; Bréquigny, P.; Mounaïm-Rousselle, C.; Faravelli, T. Low- and intermediate-temperature ammonia/hydrogen oxidation in a flow reactor: Experiments and a wide-range kinetic modeling. Chem. Eng. J. 2023, 471, 144577. [Google Scholar]
- Zhu, Y.; Curran, H.J.; Girhe, S.; Murakami, Y.; Pitsch, H.; Senecal, K.; Yang, L.; Zhou, C.-W. The combustion chemistry of ammonia and ammonia/hydrogen mixtures: A comprehensive chemical kinetic modeling study. Combust. Flame 2024, 260, 113239. [Google Scholar] [CrossRef]
- Ramalli, E.; Dinelli, T.; Nobili, A.; Stagni, A.; Pernici, B.; Faravelli, T. Automatic validation and analysis of predictive models by means of big data and data science. Chem. Eng. J. 2023, 454, 140149. [Google Scholar] [CrossRef]
- Girhe, S.; Snackers, A.; Lehmann, T.; Langer, R.; Loffredo, F.; Glaznev, R.; Beeckmann, J.; Pitsch, H. Ammonia and ammonia/hydrogen combustion: Comprehensive quantitative assessment of kinetic models and examination of critical parameters. Combust. Flame 2024, 267, 113560. [Google Scholar] [CrossRef]
- Pochet, M.; Jeanmart, H.; Contino, F. A 22:1 compression ratio ammonia- hydrogen HCCI engine: Combustion, load, and emission performances. Front. Mech. Eng. 2020, 6, 43. [Google Scholar]
- Li, S.; Zhang, S.; Zhou, H.; Ren, Z. Analysis of air-staged combustion of NH3/CH4 mixture with low NOx emission at gas turbine conditions in model combustors. Fuel 2019, 237, 50–59. [Google Scholar] [CrossRef]
- Rocha, R.C.; Zhong, S.; Xu, L.; Bai, X.-S.; Costa, M.; Cai, X.; Kim, H.; Brackmann, C.; Li, Z.; Aldén, M. Structure and Laminar Flame Speed of an Ammonia/Methane/Air Premixed Flame under Varying Pressure and Equivalence Ratio. Energy Fuels 2021, 35, 7179–7192. [Google Scholar] [CrossRef]
- Rocha, R.C.; Ramos, C.F.; Costa, M.; Bai, X.-S. Combustion of NH3/CH4/Air and NH3/H2/air mixtures in a porous burner: Experiments and kinetic modeling. Energy Fuels 2019, 33, 12767–12780. [Google Scholar] [CrossRef]
- Pugh, D.; Bowen, P.; Valera-Medina, A.; Giles, A.; Runyon, J.; Marsh, R. Influence of steam addition and elevated ambient conditions on NOx reduction in a staged premixed swirling NH3/H2 flame. Proc. Combust. Inst. 2019, 37, 5401–5409. [Google Scholar] [CrossRef]
- Choe, J.; Sun, W.; Ombrello, T.; Carter, C. Plasma assisted ammonia combustion: Simultaneous NOx reduction and flame enhancement. Combust. Flame 2021, 228, 430–432. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, Q.; Sun, J.; Ma, P.; Li, S. Reducing NOx Emission of Swirl-Stabilized Ammonia/Methane Tubular Flames through a Fuel-Oxidizer Mixing Strategy. Energy Fuels 2022, 36, 2277–2287. [Google Scholar] [CrossRef]
- Cai, T.; Zhao, D.; Wang, B.; Li, J.; Guan, Y. NO emission and thermal performances studies on premixed ammonia-oxygen combustion in a CO2-free micro-planar combustor. Fuel 2020, 280, 118554. [Google Scholar] [CrossRef]
- Ariemma, G.; Sabia, P.; Sorrentino, G.; Bozza, P.; de Joannon, M.; Ragucci, R. Influence of water addition on MILD ammonia combustion performances and emissions. Proc. Combust. Inst. 2021, 38, 5147–5154. [Google Scholar] [CrossRef]
- Tang, Y.; Xie, D.; Shi, B.; Wang, N.; Li, S. Flammability enhancement of swirling ammonia/air combustion using AC powered gliding arc discharges. Fuel 2022, 313, 122674. [Google Scholar] [CrossRef]
- Okafor, E.C.; Somarathne, K.K.A.; Ratthanan, R.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Tsujimura, T.; Furutani, H.; Kobayashi, H. Control of NOx and other emissions in micro gas turbine combustors fuelled with mixtures of methane and ammonia. Combust. Flame 2020, 211, 406–416. [Google Scholar] [CrossRef]


| Properties | NH3 | H2 | CH4 |
|---|---|---|---|
| Boiling temperature at 1 atm (°C) | −33.4 | −253 | −161 |
| Condensation pressure at 25 °C (atm) | 9.90 | N/A | N/A |
| Flammability limit (equivalence ratio) | 0.63–1.40 | 0.10–7.10 | 0.50–1.70 |
| Lower heating value (MJ/kg) | 18.6 | 120 | 50.0 |
| Adiabatic flame temperature (°C) | 1800 | 2110 | 1950 |
| Maximum laminar burning velocity (m/s) | 0.07 | 2.91 | 0.37 |
| Minimum auto ignition temperature (°C) | 650 | 520 | 630 |
| Strategies | Advantages | Disadvantages |
|---|---|---|
| Rich combustion at high inlet pressures | Easy to implement, with a wide range of applications | Incomplete combustion, ammonia leakage |
| Dual-fuel combustion | Easy to implement | Generate greenhouse gases |
| Reactant humidification | Low flame temperature | Emission problem |
| Plasma-assisted combustion | Ignition and flame enhancement | The scope of application is limited, making it difficult to apply on a large scale |
| Staged combustion | High fuel utilization rate | The related equipment is complex |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhao, S.; Zhang, Q.; Wang, Y.; Zhang, J. A Review of Ammonia Combustion Reaction Mechanism and Emission Reduction Strategies. Energies 2025, 18, 1707. https://doi.org/10.3390/en18071707
Zhang X, Zhao S, Zhang Q, Wang Y, Zhang J. A Review of Ammonia Combustion Reaction Mechanism and Emission Reduction Strategies. Energies. 2025; 18(7):1707. https://doi.org/10.3390/en18071707
Chicago/Turabian StyleZhang, Xiqing, Shiwei Zhao, Qisheng Zhang, Yaojie Wang, and Jian Zhang. 2025. "A Review of Ammonia Combustion Reaction Mechanism and Emission Reduction Strategies" Energies 18, no. 7: 1707. https://doi.org/10.3390/en18071707
APA StyleZhang, X., Zhao, S., Zhang, Q., Wang, Y., & Zhang, J. (2025). A Review of Ammonia Combustion Reaction Mechanism and Emission Reduction Strategies. Energies, 18(7), 1707. https://doi.org/10.3390/en18071707






