Sustainable Hydrogen Production with Negative Carbon Emission Through Thermochemical Conversion of Biogas/Biomethane
Abstract
:1. Introduction
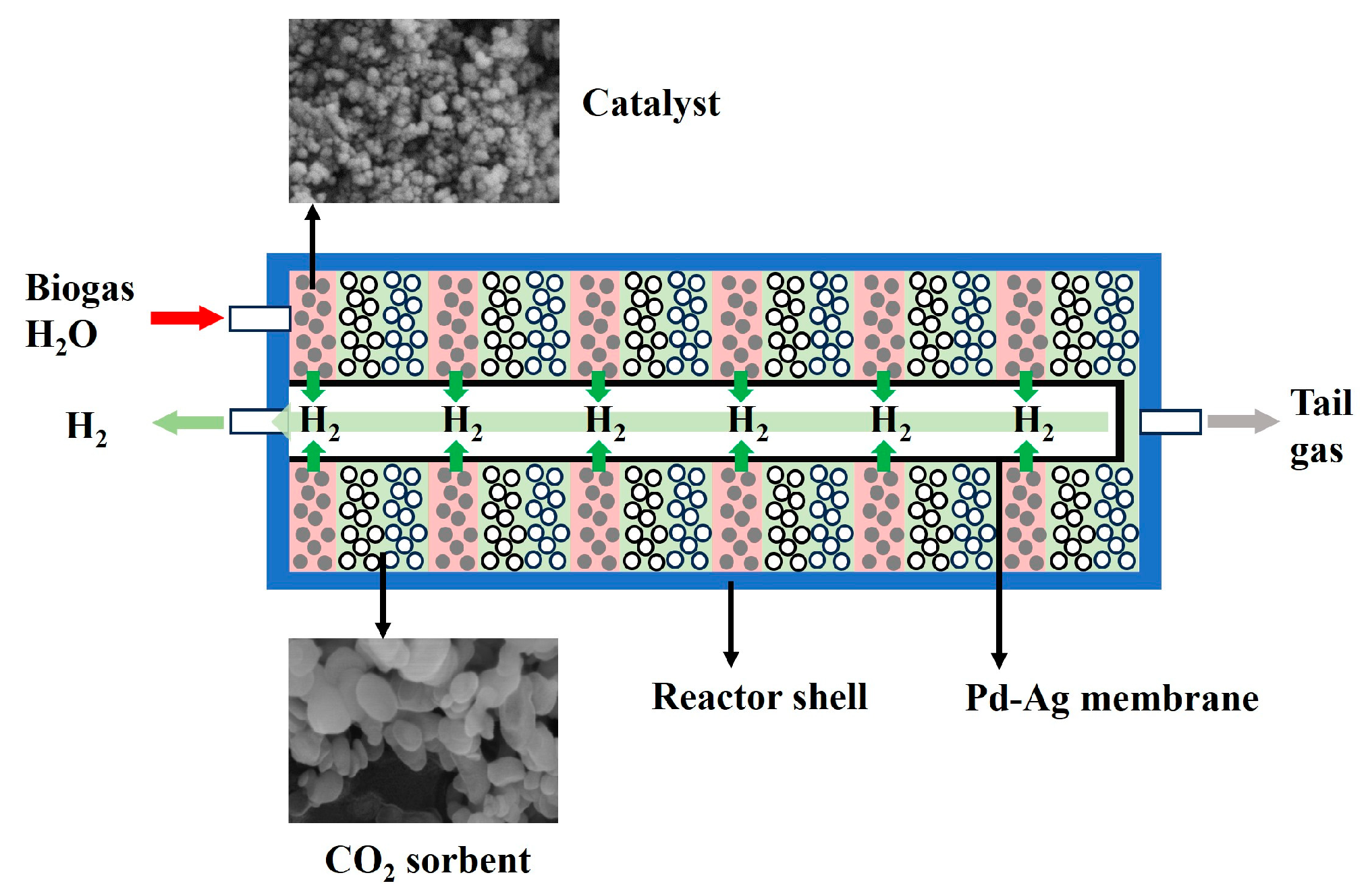
2. Experimental System
3. Evaluation Criteria
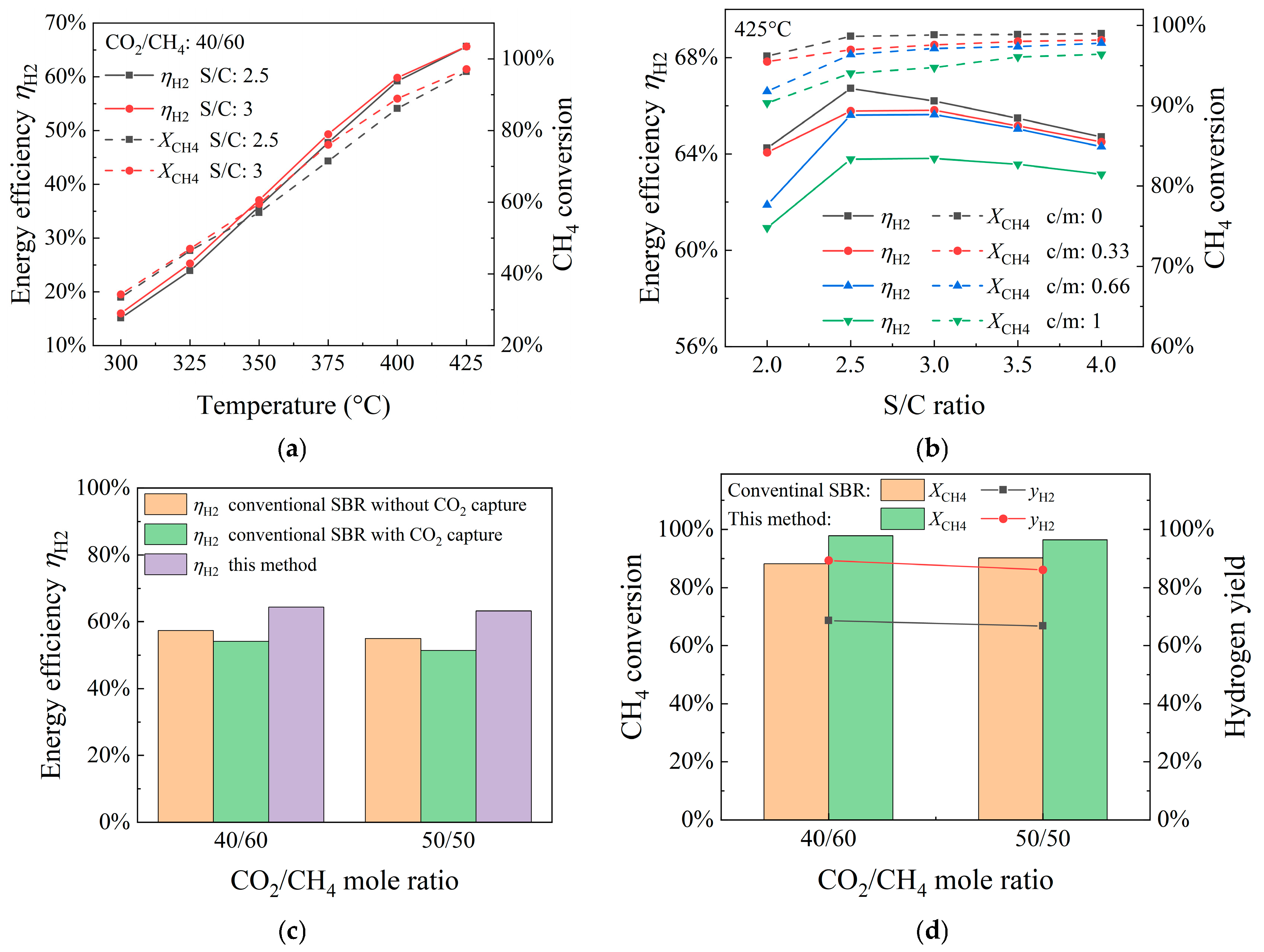
4. Results and Discussion
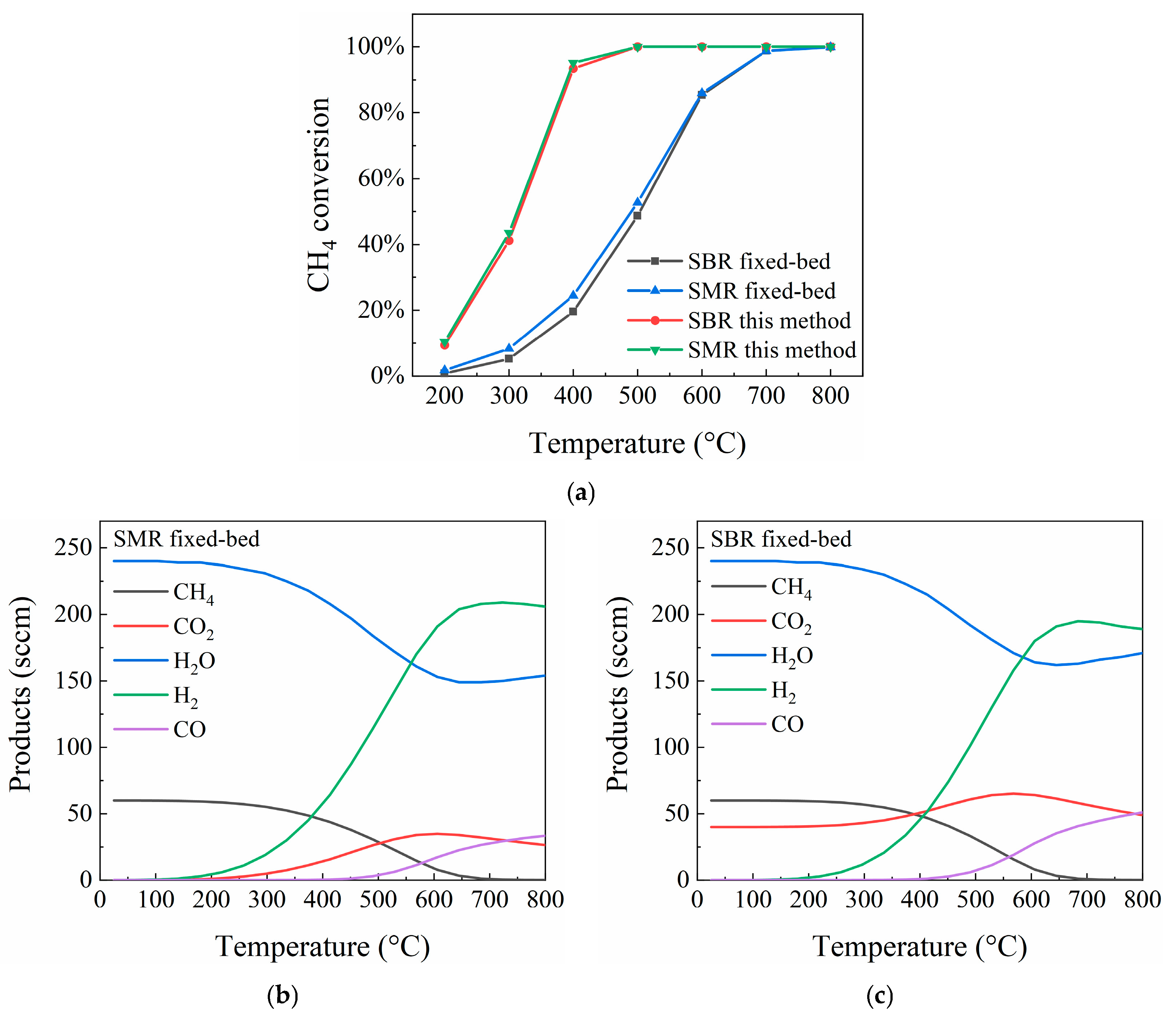
4.1. Influence of Temperature
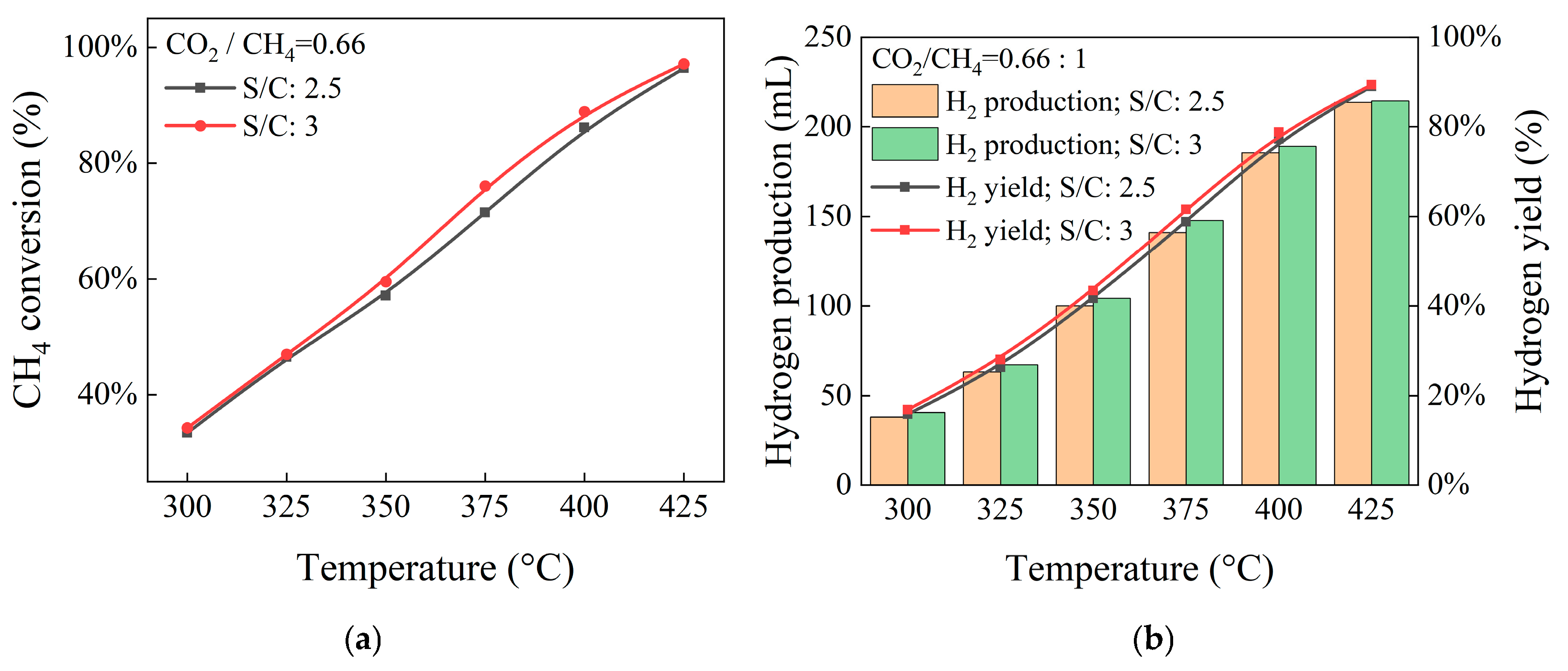
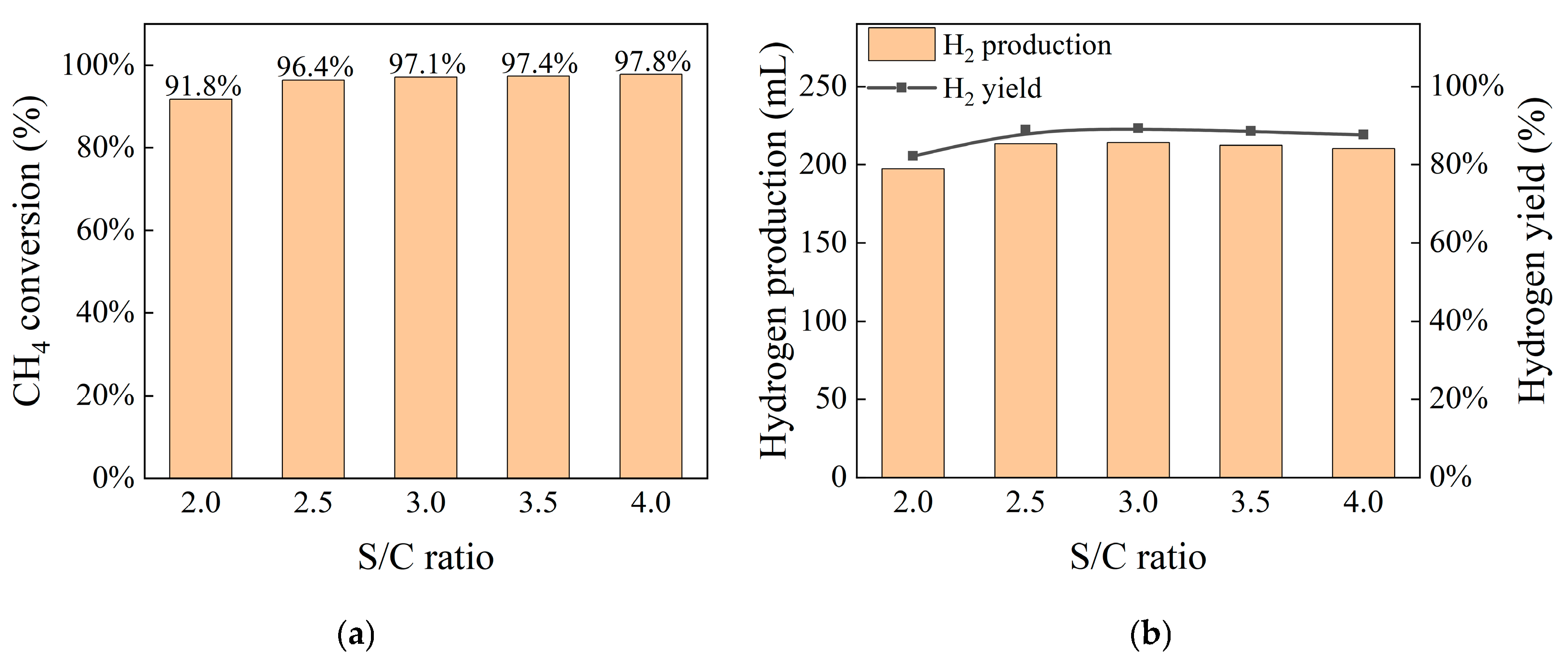
4.2. Influence of Steam-to-Methane Ratio
4.3. Influence of CO2/CH4 Ratio
4.4. Energy Efficiency
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Singh, S.B.; Hajare, P.; Konwar, R.J.; De, M. Physically and chemically modified zeolite templated nitrogenous carbons for enhanced hydrogen adsorption. FlatChem 2024, 48, 100767. [Google Scholar] [CrossRef]
- Singh, S.B.; De, M. Thermally exfoliated graphene oxide for hydrogen storage. Mater. Chem. Phys. 2020, 239, 122102. [Google Scholar]
- IEA. Global Hydrogen Review 2024; IEA: Paris, France, 2024. [Google Scholar]
- Wang, Z.; Gong, Z.; Turap, Y.; Wang, Y.; Zhang, Z.; Wang, W. Renewable hydrogen production from biogas using iron-based chemical looping technology. Chem. Eng. J. 2022, 429, 132192. [Google Scholar]
- Veiga, S.; Romero, M.; Faccio, R.; Segobia, D.; Apesteguía, C.; Pérez, A.L.; Dante Brondino, C.; Bussi, J. Biogas dry reforming over Ni-La-Ti catalysts for synthesis gas production: Effects of preparation method and biogas composition. Fuel 2023, 346, 128300. [Google Scholar]
- Bórawski, P.; Bełdycka-Bórawska, A.; Kapsdorferová, Z.; Rokicki, T.; Parzonko, A.; Holden, L. Perspectives of Electricity Production from Biogas in the European Union. Energies 2024, 17, 1169. [Google Scholar] [CrossRef]
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar]
- Alengebawy, A.; Mohamed, B.A.; Ghimire, N.; Jin, K.; Liu, T.; Samer, M.; Ai, P. Understanding the environmental impacts of biogas utilization for energy production through life cycle assessment: An action towards reducing emissions. Environ. Res. 2022, 213, 113632. [Google Scholar]
- Di Marcoberardino, G.; Foresti, S.; Binotti, M.; Manzolini, G. Potentiality of a biogas membrane reformer for decentralized hydrogen production. Chem. Eng. Process. Process Intensif. 2018, 129, 131–141. [Google Scholar]
- Madeira, J.G.F.; Oliveira, E.M.; Springer, M.V.; Cabral, H.L.; do Carmo Barbeito, D.F.; Souza, A.P.G.; da Silva Moura, D.A.; Delgado, A.R.S. Hydrogen production from swine manure biogas via steam reforming of methane (SRM) and water gas shift (WGS): A ecological, technical, and economic analysis. Int. J. Hydrogen Energy 2021, 46, 8961–8971. [Google Scholar]
- Corrente, M.V.; Manfro, R.L.; Souza, M.M.V.M. Hydrogen production by biogas reforming using Ni/MgO-Al2O3 catalysts. Fuel 2024, 368, 131561. [Google Scholar]
- Antonini, C.; Treyer, K.; Streb, A.; van der Spek, M.; Bauer, C.; Mazzotti, M. Hydrogen production from natural gas and biomethane with carbon capture and storage—A techno-environmental analysis. Sustain. Energy Fuels 2020, 4, 2967–2986. [Google Scholar]
- Cho, H.H.; Strezov, V.; Evans, T.J. A review on global warming potential, challenges and opportunities of renewable hydrogen production technologies. Sustain. Mater. Technol. 2023, 35, e00567. [Google Scholar]
- IEA. Global Hydrogen Review 2023; IEA: Paris, France, 2023. [Google Scholar]
- Ishaq, H.; Dincer, I.; Crawford, C. A review on hydrogen production and utilization: Challenges and opportunities. Int. J. Hydrogen Energy 2022, 47, 26238–26264. [Google Scholar]
- Farhana, K.; Mahamude, A.S.F.; Kadirgama, K. Comparing hydrogen fuel cost of production from various sources—A competitive analysis. Energy Convers. Manag. 2024, 302, 118088. [Google Scholar]
- Yue, M.; Lambert, H.; Pahon, E.; Roche, R.; Jemei, S.; Hissel, D. Hydrogen energy systems: A critical review of technologies, applications, trends and challenges. Renew. Sustain. Energy Rev. 2021, 146, 111180. [Google Scholar]
- Rosa, L.; Mazzotti, M. Potential for hydrogen production from sustainable biomass with carbon capture and storage. Renew. Sustain. Energy Rev. 2022, 157, 112123. [Google Scholar] [CrossRef]
- Gadirli, G.; Pilarska, A.A.; Dach, J.; Pilarski, K.; Kolasa-Więcek, A.; Borowiak, K. Fundamentals, Operation and Global Prospects for the Development of Biogas Plants—A Review. Energies 2024, 17, 568. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, A.; Pal, A. Overview of hydrogen production from biogas reforming: Technological advancement. Int. J. Hydrogen Energy 2022, 47, 34831–34855. [Google Scholar]
- Di Giuliano, A.; Gallucci, K. Sorption enhanced steam methane reforming based on nickel and calcium looping: A review. Chem. Eng. Process. Process Intensif. 2018, 130, 240–252. [Google Scholar]
- Peng, X.; Jin, Q. Molecular simulation of methane steam reforming reaction for hydrogen production. Int. J. Hydrogen Energy 2022, 47, 7569–7585. [Google Scholar]
- Usman, M.; Wan Daud, W.M.A.; Abbas, H.F. Dry reforming of methane: Influence of process parameters—A review. Renew. Sustain. Energy Rev. 2015, 45, 710–744. [Google Scholar] [CrossRef]
- Tuna, C.E.; Silveira, J.L.; da Silva, M.E.; Boloy, R.M.; Braga, L.B.; Pérez, N.P. Biogas steam reformer for hydrogen production: Evaluation of the reformer prototype and catalysts. Int. J. Hydrogen Energy 2018, 43, 2108–2120. [Google Scholar]
- Chouhan, K.; Sinha, S.; Kumar, S.; Kumar, S. Simulation of steam reforming of biogas in an industrial reformer for hydrogen production. Int. J. Hydrogen Energy 2021, 46, 26809–26824. [Google Scholar]
- Minh, D.P.; Siang, T.J.; Vo, D.-V.N.; Phan, T.S.; Ridart, C.; Nzihou, A.; Grouset, D. Chapter 4—Hydrogen Production From Biogas Reforming: An Overview of Steam Reforming, Dry Reforming, Dual Reforming, and Tri-Reforming of Methane. In Hydrogen Supply Chains; Azzaro-Pantel, C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 111–166. [Google Scholar]
- Phan, T.S.; Minh, D.P.; Espitalier, F.; Nzihou, A.; Grouset, D. Hydrogen production from biogas: Process optimization using ASPEN Plus®. Int. J. Hydrogen Energy 2022, 47, 42027–42039. [Google Scholar]
- Phromprasit, J.; Powell, J.; Wongsakulphasatch, S.; Kiatkittipong, W.; Bumroongsakulsawat, P.; Assabumrungrat, S. Activity and stability performance of multifunctional catalyst (Ni/CaO and Ni/Ca12Al14O33CaO) for bio-hydrogen production from sorption enhanced biogas steam reforming. Int. J. Hydrogen Energy 2016, 41, 7318–7331. [Google Scholar]
- Abanades, J.C.; Grasa, G. Production of a syngas and CaO by desorption-enhanced reverse water–gas shift of CaCO3 with H2. Chem. Eng. J. 2024, 493, 152191. [Google Scholar]
- Gapp, E.; Pfeifer, P. Membrane reactors for hydrogen production from renewable energy sources. Curr. Opin. Green Sustain. Chem. 2023, 41, 100800. [Google Scholar]
- Phromprasit, J.; Powell, J.; Wongsakulphasatch, S.; Kiatkittipong, W.; Bumroongsakulsawat, P.; Assabumrungrat, S. H2 production from sorption enhanced steam reforming of biogas using multifunctional catalysts of Ni over Zr-, Ce- and La-modified CaO sorbents. Chem. Eng. J. 2017, 313, 1415–1425. [Google Scholar] [CrossRef]
- Dang, C.; Xia, H.; Yuan, S.; Wei, X.; Cai, W. Green hydrogen production from sorption-enhanced steam reforming of biogas over a Pd/Ni–CaO-mayenite multifunctional catalyst. Renew. Energy 2022, 201, 314–322. [Google Scholar]
- Capa, A.; García, R.; Chen, D.; Rubiera, F.; Pevida, C.; Gil, M.V. On the effect of biogas composition on the H2 production by sorption enhanced steam reforming (SESR). Renew. Energy 2020, 160, 575–583. [Google Scholar]
- García, R.; Gil, M.V.; Rubiera, F.; Chen, D.; Pevida, C. Renewable hydrogen production from biogas by sorption enhanced steam reforming (SESR): A parametric study. Energy 2021, 218, 119491. [Google Scholar] [CrossRef]
- Ruales, H.B.T.; Italiano, C.; Vita, A.; Iulianelli, A. Low impact emissions H2 production via biogas steam reforming in a foam structured membrane reactor: Energy efficiency and exergy analyses, and H2 production cost assessment. Energy Convers. Manag. 2025, 326, 119504. [Google Scholar] [CrossRef]
- Ongis, M.; Di Marcoberardino, G.; Baiguini, M.; Gallucci, F.; Binotti, M. Optimization of Small-Scale Hydrogen Production with Membrane Reactors. Membranes 2023, 13, 331. [Google Scholar] [CrossRef] [PubMed]
- Ongis, M.; Di Marcoberardino, G.; Manzolini, G.; Gallucci, F.; Binotti, M. Membrane reactors for green hydrogen production from biogas and biomethane: A techno-economic assessment. Int. J. Hydrogen Energy 2023, 48, 19580–19595. [Google Scholar]
- Ongis, M.; Baiguini, M.; Di Marcoberardino, G.; Gallucci, F.; Binotti, M. Techno-economic analysis for the design of membrane reactors in a small-scale biogas-to-hydrogen plant. Int. J. Hydrogen Energy 2025, 101, 887–903. [Google Scholar] [CrossRef]
- Roy, P.S.; Park, C.S.; Raju, A.S.K.; Kim, K. Steam-biogas reforming over a metal-foam-coated (Pd–Rh)/(CeZrO2–Al2O3) catalyst compared with pellet type alumina-supported Ru and Ni catalysts. J. CO2 Util. 2015, 12, 12–20. [Google Scholar] [CrossRef]
- Saebea, D.; Authayanun, S.; Patcharavorachot, Y.; Arpornwichanop, A. Enhancement of Hydrogen Production for Steam Reforming of Biogas in Fluidized Bed Membrane Reactor. Chem. Eng. Trans. 2014, 39, 1177–1182. [Google Scholar]
- Ling, Y.; Wang, H.; Liu, M.; Wang, B.; Li, S.; Zhu, X.; Shi, Y.; Xia, H.; Guo, K.; Hao, Y.; et al. Sequential separation-driven solar methane reforming for H2 derivation under mild conditions. Energy Environ. Sci. 2022, 15, 1861–1871. [Google Scholar]
- Kumar, R.; Kumar, A. Recent advances of biogas reforming for hydrogen production: Methods, purification, utility and techno-economics analysis. Int. J. Hydrogen Energy 2024, 76, 108–140. [Google Scholar]
- van den Broek, M.; Berghout, N.; Rubin, E.S. The potential of renewables versus natural gas with CO2 capture and storage for power generation under CO2 constraints. Renew. Sustain. Energy Rev. 2015, 49, 1296–1322. [Google Scholar]
- Sultan, H.; Muhammad, H.A.; Bhatti, U.H.; Min, G.H.; Baek, I.H.; Baik, Y.-J.; Nam, S.C. Reducing the efficiency penalty of carbon dioxide capture and compression process in a natural gas combined cycle power plant by process modification and liquefied natural gas cold energy integration. Energy Convers. Manag. 2021, 244, 114495. [Google Scholar]
- Roine, A. HSC Chemistry 5.11; Outokumpu Research Oy: Pori, Finland, 2002. [Google Scholar]
- Wei, L.; Hu, X.; Yu, J.; Huang, Y. Aluminizing and oxidation treatments on the porous stainless steel substrate for preparation of H2-permeable composite palladium membranes. Int. J. Hydrogen Energy 2014, 39, 18618–18624. [Google Scholar]
- Pashchenko, D.; Makarov, I. Carbon deposition in steam methane reforming over a Ni-based catalyst: Experimental and thermodynamic analysis. Energy 2021, 222, 119993. [Google Scholar]
- Xu, J.; Yeung, C.M.Y.; Ni, J.; Meunier, F.; Acerbi, N.; Fowles, M.; Tsang, S.C. Methane steam reforming for hydrogen production using low water-ratios without carbon formation over ceria coated Ni catalysts. Appl. Catal. A Gen. 2008, 345, 119–127. [Google Scholar]
- Effendi, A.; Zhang, Z.-G.; Hellgardt, K.; Honda, K.; Yoshida, T. Steam reforming of a clean model biogas over Ni/Al2O3 in fluidized- and fixed-bed reactors. Catal. Today 2002, 77, 181–189. [Google Scholar]
- Park, H.; Lee, J.; Yun, S.; Kim, J.-K. Energy-efficient process design and optimization of the absorption-based CO2 capture process with a low-pressure flash column for the SMR-based hydrogen production plant. Energy Convers. Manag. 2025, 325, 119416. [Google Scholar]


| Methods | Biogas Composition | Reaction Temperature (°C) | Regeneration Temperature (°C) | CH4 Conversion | H2 Yield | CO2 Capture (kg-CO2/m3-Feed) | Ref. |
|---|---|---|---|---|---|---|---|
| Conventional SBR | 60% CH4 + 40% CO2 | 700 | * | 88% | 69% | No | [25] |
| Conventional SBR | 60% CH4 + 40% CO2 | 800 | * | 90% | 71% | No | [39] |
| Sorption-enhanced | 60% CH4 + 40% CO2 | 550 | - | 94% | 85% | 1.78 | [34] |
| Sorption-enhanced | 60% CH4 + 40% CO2 | 600 | 850 | 91% | 90% | 1.85 | [28] |
| Membrane | 60% CH4 + 40% CO2 | 500 | * | 74% | 55% | No | [35] |
| Membrane | 60% CH4 + 40% CO2 | 600 | * | 71% | 58% | No | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Shao, Y.; Yang, L.; Guo, K.; Li, X.; Sun, M.; Hao, Y. Sustainable Hydrogen Production with Negative Carbon Emission Through Thermochemical Conversion of Biogas/Biomethane. Energies 2025, 18, 1804. https://doi.org/10.3390/en18071804
Wang B, Shao Y, Yang L, Guo K, Li X, Sun M, Hao Y. Sustainable Hydrogen Production with Negative Carbon Emission Through Thermochemical Conversion of Biogas/Biomethane. Energies. 2025; 18(7):1804. https://doi.org/10.3390/en18071804
Chicago/Turabian StyleWang, Bin, Yu Shao, Lingzhi Yang, Ke Guo, Xiao Li, Mengzhu Sun, and Yong Hao. 2025. "Sustainable Hydrogen Production with Negative Carbon Emission Through Thermochemical Conversion of Biogas/Biomethane" Energies 18, no. 7: 1804. https://doi.org/10.3390/en18071804
APA StyleWang, B., Shao, Y., Yang, L., Guo, K., Li, X., Sun, M., & Hao, Y. (2025). Sustainable Hydrogen Production with Negative Carbon Emission Through Thermochemical Conversion of Biogas/Biomethane. Energies, 18(7), 1804. https://doi.org/10.3390/en18071804







