Role of -SF5 Groups in Modulating the Stability and Energy Characteristics of Fluorinated Molecules
Abstract
1. Introduction
2. Materials and Methods
- The equations developed by Politzer et al. [26], which include the molecular mass, the volume of the 0.001 electrons/bohr3 counter of the electronic density of a molecule, the degree of balance between positive potential and negative potential on the surface, and their sum;
- Methods implemented in ACD/ChemSketch based on the Van der Walls volumes molecular modeling program [24];
- The division of molecular weight by molar volume, obtained via B3LP/cc-pVTZ.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BEA | Cohesion |
| HOMO | The highest occupied molecular orbital |
| LUMO | The lowest unoccupied molecular orbital |
| Gap | HOMO–LUMO gap |
| CH | Chemical hardness |
| ELN | Electronegativity |
Appendix A
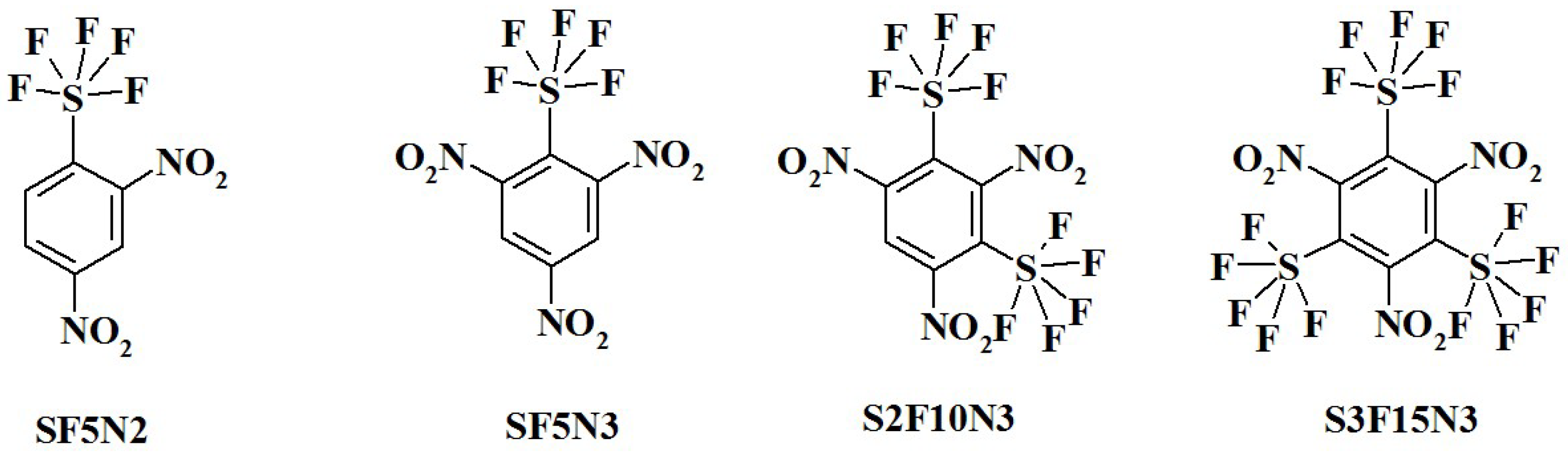
- Code (Assignation) and Chemical Name:
- SF5N2—2,4-dinitro-1-(pentafluoro-lambda⁶-sulfanyl)benzene;
- SF5N3—1,3,5-trinitro-2-(pentafluoro-lambda⁶-sulfanyl)benzene;
- S2F10N3—1,3,5-trinitro-2,4-bis(pentafluoro-lambda⁶-sulfanyl)benzene;
- S3F15N3—1,3,5-trinitro-2,4,6-tris(pentafluoro-lambda⁶-sulfanyl)benzene;
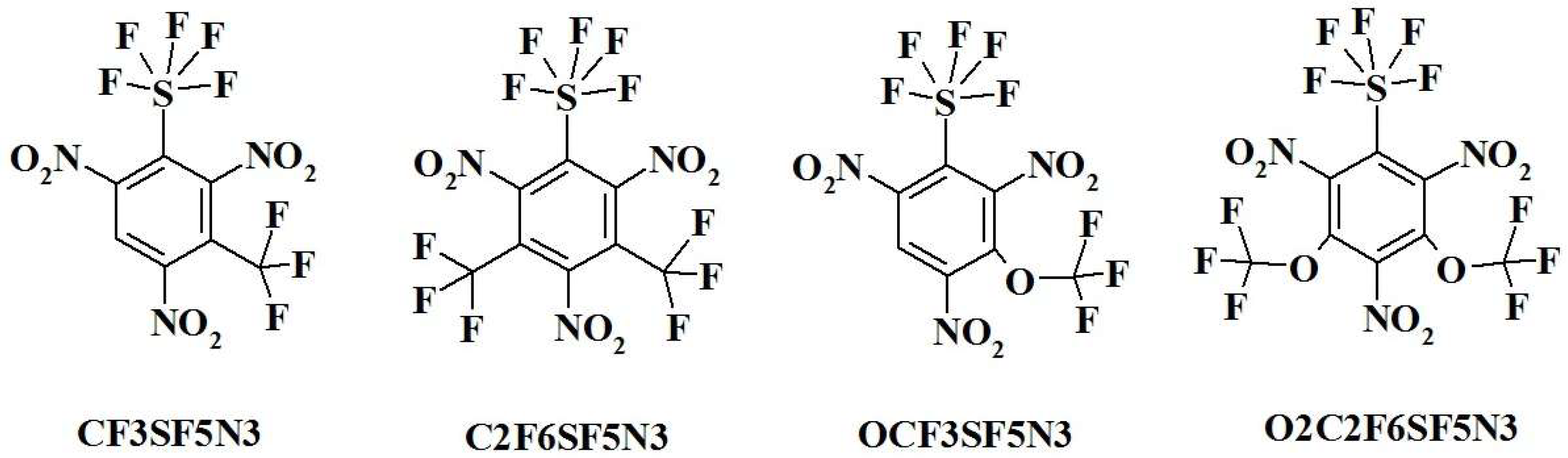
- Code (Assignation) and Chemical Name:
- CF3SF5N3—1,3,5-trinitro-2-(pentafluoro-lambda⁶-sulfanyl)-4-(trifluoromethyl)benzene;
- C2F6SF5N3—1,3,5-trinitro-2-(pentafluoro-lambda⁶-sulfanyl)-4,6-bis(trifluoromethyl)benzene;
- OCF3SF5N3—1,3,5-trinitro-2-(pentafluoro-lambda⁶-sulfanyl)-4-(trifluoromethoxy)benzene;
- O2C2F6SF5N3—1,3,5-trinitro-2-(pentafluoro-lambda⁶-sulfanyl)-4,6-bis(trifluoromethoxy)benzene;

- Code (Assignation) and Chemical Name:
- CF3N2—2,4-dinitro-1-(trifluoromethyl)benzene;
- CF3N3—1,3,5-trinitro-2-(trifluoromethyl)benzene;
- C2F6N3—1,3,5-trinitro-2,4-bis(trifluoromethyl)benzene;
- C3F9N3—1,3,5-trinitro-2,4,6-tris(trifluoromethyl)benzene;

- Code (Assignation) and Chemical Name:
- OCF3N2—2,4-dinitro-1-(trifluoromethoxy)benzene
- OCF3N3—1,3,5-trinitro-2-(trifluoromethoxy)benzene
- O2C2F6N3—1,3,5-trinitro-2,4-bis(trifluoromethoxy)benzene
- O3C3F9N3—1,3,5-trinitro-2,4,6-tris(trifluoromethoxy)benzene
Appendix B
| No. | Structural Formula | Mol. Formula | MW | Elemental Analysis Data (Calculated) | |||||
| C, % | H, % | N, % | O, % | S, % | F, % | ||||
|---|---|---|---|---|---|---|---|---|---|
| 1. | 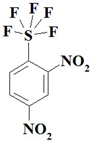 | C6H3F5N2O4S | 294.16 | 24.50 | 1.03 | 9.52 | 21.76 | 10.90 | 32.29 |
| 2. | 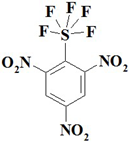 | C6H2F5N3O6S | 339.16 | 21.25 | 0.59 | 12.39 | 28.30 | 9.45 | 28.01 |
| 3. |  | C6HF10N3O6S2 | 465.20 | 15.49 | 0.22 | 40.84 | 9.03 | 13.78 | 40.84 |
| 4. |  | C6F15N3O6S3 | 591.25 | 12.19 | 0 | 7.11 | 16.24 | 16.27 | 48.20 |
| 5. | 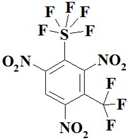 | C7HF8N3O6S | 407.15 | 20.65 | 0.25 | 10.32 | 23.58 | 7.88 | 37.33 |
| 6. | 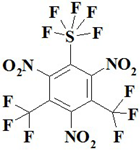 | C8F11N3O6S | 475.15 | 20.22 | 0 | 8.84 | 20.20 | 6.75 | 43.98 |
| 7. |  | C7HF8N3O7S | 423.15 | 19.87 | 0.24 | 9.93 | 26.47 | 7.58 | 35.92 |
| 8. |  | C8F11N3O8S | 507.15 | 18.95 | 0 | 8.29 | 25.24 | 6.32 | 41.21 |
| 9. |  | C6H3F3N2O4 | 236.11 | 35.61 | 1.28 | 11.86 | 27.11 | 0 | 24.14 |
| 10. | 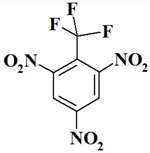 | C7H2F3N3O6 | 281.11 | 29.91 | 0.72 | 14.95 | 34.15 | 0 | 20.28 |
| 11. | 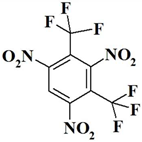 | C8HF6N3O6 | 349.10 | 27.52 | 0.29 | 12.04 | 27.50 | 0 | 32.65 |
| 12. | 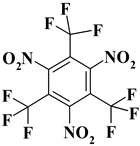 | C9F9N3O6 | 417.10 | 25.92 | 0 | 10.07 | 23.02 | 0 | 40.99 |
| 13. |  | C7H3F3N2O5 | 252.11 | 33.35 | 1.20 | 11.11 | 31.73 | 0 | 22.61 |
| 14. | 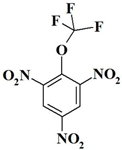 | C7H2F3N3O7 | 297.11 | 28.30 | 0.68 | 14.14 | 37.70 | 0 | 19.18 |
| 15. | 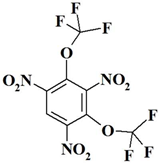 | C8HF6N3O8 | 381.11 | 25.21 | 0.26 | 11.03 | 33.59 | 0 | 29.91 |
| 16. |  | C9F9N3O9 | 465.10 | 23.24 | 0 | 9.03 | 30.96 | 0 | 36.76 |
References
- Lin, H.; Zhu, Q.; Huang, C.; Yang, D.D.; Lou, N.; Zhu, S.G.; Li, H.Z. Dinitromethyl, fluorodinitromethyl derivatives of RDX and HMX as high energy density materials: A computational study. Struct. Chem. 2019, 30, 2401–2408. [Google Scholar] [CrossRef]
- Yang, J.; Bai, T.; Guan, J.; Li, M.; Zhen, Z.; Dong, X.; Wang, Y.; Wang, Y. Novel fluorine-containing energetic materials: How potential are they? A computational study of detonation performance. J. Mol. Model. 2023, 29, 228. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, X.L.; Yu, Z.H.; Song, D.; An, C.W.; Li, F.S. A high density and low sensitivity carrier explosive promising to replace TNT: 3-Bromo-5-fluoro-2,4,6-trinitroanisole. Energetic Mater. Front. 2024, 5, 131–140. [Google Scholar] [CrossRef]
- Gao, H.; Ye, C.; Winter, R.W.; Gard, G.L.; Sitzmann, M.E.; Shreeve, J.N.M. Pentafluorosulfanyl (SF5) containing energetic salts. Eur. J. Inorg. Chem. 2006, 16, 3221–3226. [Google Scholar] [CrossRef]
- Martinez, H.; Zheng, Z.; Dolbier, W.R., Jr. Energetic materials containing fluorine. Design, synthesis and testing of furazan-containing energetic materials bearing a pentafluorosulfanyl group. J. Fluor. Chem. Y 2012, 143, 112–122. [Google Scholar] [CrossRef]
- Das, J.; Shem-Tov, D.; Zhang, S.; Gao, C.Z.; Zhang, L.; Yao, C.; Flaxer, E.; Stierstorfer, J.; Wurzenberger, M.; Rahinov, I.; et al. Power of sulfur–Chemistry, properties, laser ignition and theoretical studies of energetic perchlorate-free 1, 3, 4–thiadiazole nitramines. J. Chem. Eng. 2022, 443, 136246. [Google Scholar] [CrossRef]
- Kumar, P.; Ghule, V.D.; Dharavath, S. Advancing energetic chemistry: The first synthesis of sulfur-based C–C bonded thiadiazole-pyrazine compounds with a nitrimino moiety. Dalton Trans. 2024, 53, 19112–19115. [Google Scholar] [CrossRef]
- Tamuliene, J.; Sarlauskas, J. Polynitrobenzene derivatives, containing -CF3, -OCF3, and -O(CF2)nO- functional groups, as candidates for perspective fluorinated high-energy materials: Theoretical study. Energies 2024, 17, 6126. [Google Scholar] [CrossRef]
- Sitzmann, M.E.; Ornellas, D.L. The Effect of the Pentafluorothio (SF5S) Group on the Properties of Explosive Nitro Compounds: New SF5 Explosives. In Proceedings of the Ninth Symposium (International) on Detonation, Red Lion Inn, Columbia River Portland, OR, USA, 28 August–1 September 1989; Volume 2, pp. 1162–1169. Available online: https://archive.org/details/DTIC_ADA247996#:~:text=DTIC%20ADA247996%3A%20Proceedings%20of%20the%20International%20Symposium%20on,Free%20Download%2C%20Borrow%2C%20and%20Streaming%20%3A%20Internet%20Archive (accessed on 8 February 2025).
- Becke, A.D. Density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09 W Reference; Gaussian, Inc.: Wallingford, CT, USA, 2016; p. 139. Available online: https://gaussian.com/citation/ (accessed on 8 February 2025).
- Cardia, R.; Malloci, G.; Mattoni, A.; Cappellini, G. Effects of TIPS-Functionalization and Perhalogenation on the Electronic, Optical, and Transport Properties of Angular and Compact Dibenzochrysene. J. Phys. Chem. A 2014, 118, 5170–5177. [Google Scholar] [CrossRef] [PubMed]
- Cardia, R.; Malloci, G.; Rignanese, G.M.; Blasé, X.; Molteni, E.; Cappellini, G. Electronic and optical properties of hexathiapentacene in the gas and crystal phases. Phys. Rev. B 2016, 93, 235132. [Google Scholar] [CrossRef]
- Dardenne, N.; Cardia, R.; Li, J.; Malloci, G.; Cappellini, G.; Blasé, X.; Charlier, J.C.; Rignanese, G. Tuning Optical Properties of Dibenzochrysenes by Functionalization: A Many-Body Perturbation Theory Study. Phys. Chem. C 2017, 121, 24480–24488. [Google Scholar] [CrossRef]
- Antidormi, A.; Aprile, G.; Cappellini, G.; Cara, E.; Cardia, R.; Colombo, L.; Farris, R.; d’Ischia, M.; Mehrabanian, M.; Melis, C.; et al. Physical and Chemical Control of Interface Stability in Porous Si–Eumelanin Hybrids. J. Phys. Chem. C 2018, 122, 28405–28415. [Google Scholar] [CrossRef]
- Mocci, P.; Cardia, R.; Cappellini, G. Inclusions of Si-atoms in Graphene nanostructures: A computational study on the ground-state electronic properties of Coronene and Ovalene. J. Phys. Conf. Ser. 2018, 956, 012020. [Google Scholar] [CrossRef]
- Mocci, P.; Cardia, R.; Cappellini, G. Si-atoms substitutions effects on the electronic and optical properties of coronene and ovalene. New J. Phys. 2018, 20, 113008. [Google Scholar] [CrossRef]
- Kumar, A.; Cardia, R.; Cappellini, G. Electronic and optical properties of chromophores from bacterial cellulose. Cellulose 2018, 25, 2191–2203. [Google Scholar] [CrossRef]
- Szafran, M.; Koput, J. Ab initio and DFT calculations of structure and vibrational spectra of pyridine and its isotopomers. J. Mol. Struct. 2001, 565, 439–448. [Google Scholar] [CrossRef]
- Begue, D.; Carbonniere, P.; Pouchan, C. Calculations of Vibrational Energy Levels by Using a Hybrid ab Initio and DFT Quartic Force Field: Application to Acetonitrile. J. Phys. Chem. A. 2005, 109, 4611–4616. [Google Scholar] [CrossRef]
- Rajakumar, B.; Arathala, P.; Muthiah, B. Thermal Decomposition of 2-Methyltetrahydrofuran behind Reflected Shock Waves over the Temperature Range of 1179–1361 K. Phys. Chem. A. 2021, 125, 5406–5422. [Google Scholar] [CrossRef]
- Arathala, P.; Musah, R.A. Oxidation of Dipropyl Thiosulfinate Initiated by Cl Radicals in the Gas Phase: Implications for Atmospheric Chemistry. ACS Earth Space Chem. 2021, 5, 2878–2890. [Google Scholar] [CrossRef]
- Free Chemical Drawing Software. ChemSketch. Version 10.0. ACD/Labs. Available online: https://www.acdlabs.com/resources/free-chemistry-software-apps/chemsketch-freeware/ (accessed on 30 January 2023).
- Wen, L.; Wang, B.; Yu, T.; Lai, W.; Shi, J.; Liu, M.; Liu, Y. Accelerating the search of CHONF-containing highly energetic materials by combinatorial library design and high-throughput screening. Fuel 2022, 310, 122241. [Google Scholar] [CrossRef]
- Politzer, P.; Martinez, J.; Murray, J.S.; Concha, M.C.; Toro-Labbé, A. An electrostatic interaction correction for improved crystal density prediction. Mol. Phys. 2009, 107, 2095–2101. [Google Scholar] [CrossRef]
- Keshavarz, M.H.; Zamani, A. A simple and reliable method for predicting the detonation velocity of CHNOFCl and aluminized explosives. Cent. Eur. J. Energ. Mater. 2015, 12, 13–33. [Google Scholar]
- Keshavarz, M.H.; Pouretedal, H.R. An empirical method for predicting detonation pressure of CHNOFCl explosives. Thermochim. Acta 2004, 414, 203–208. [Google Scholar] [CrossRef]
- Singh, R.P.; Winter, R.W.; Gard, G.L.; Gao, Y.; Shreeve, J.N.M. Quaternary salts containing the pentafluorosulfanyl (SF5) group. Inorg. Chem. 2003, 42, 6142–6146. [Google Scholar]
- Ye, C.; Gard, G.L.; Winter, R.W.; Syvret, R.G.; Twamley, B.; Shreeve, J.M. Synthesis of pentafluorosulfanylpyrazole and pentafluorosulfanyl-1,2,3-triazole and their derivatives as energetic materials by click chemistry. Org. Lett. 2007, 9, 3841–3844. [Google Scholar] [CrossRef]
- Džingalašević, V.; Antić, G.; Mlađenović, D. Ratio of detonation pressure and critical pressure of high explosives with different compounds. Sci. Tech. Rev. 2004, 5, 72–76. Available online: http://www.vti.mod.gov.rs/ntp/rad2004/34-04/dzin/dzin.pdf (accessed on 8 February 2025).
- Garg, S.; Shreeve, J.M. Trifluoromethyl- or pentafluorosulfanyl- substituted poly-1, 2, 3-triazole compounds as dense stable energetic materials. J. Mater. Chem. 2011, 21, 4787–4795. [Google Scholar]
- Carlowitz, M.V.; Oberhammer, H.; Willner, H.; Boggs, J.E. Structural determination of a recalcitrant molecule (S2F4). J. Mol. Struct. 1983, 100, 161–177. [Google Scholar] [CrossRef]
- Chemical and Physical Properties of Sulfur Hexafluoride. Available online: https://www.chemicalbook.com/article/chemical-and-physical-properties-of-sulfur-hexafluoride.htm (accessed on 8 February 2025).
- Table of Chemical Bond Energies–CALCULLA. Available online: https://calculla.com/bond_energy (accessed on 8 February 2025).
- Chandler, J.; Ferguson, R.E.; Forbes, J.; Kuhl, A.L.; Oppenheim, A.K.; Spektor, R. Confined combustion of TNT explosion products in air. In Proceedings of the Conference: 8th International Colloquium on Dust Explosions, Schaumburg, IL, USA, 21–25 September 1998; Lawrence Livermore National Lab. (LLNL): Livermore, CA, USA. Available online: https://www.osti.gov/biblio/3648 (accessed on 8 February 2025).



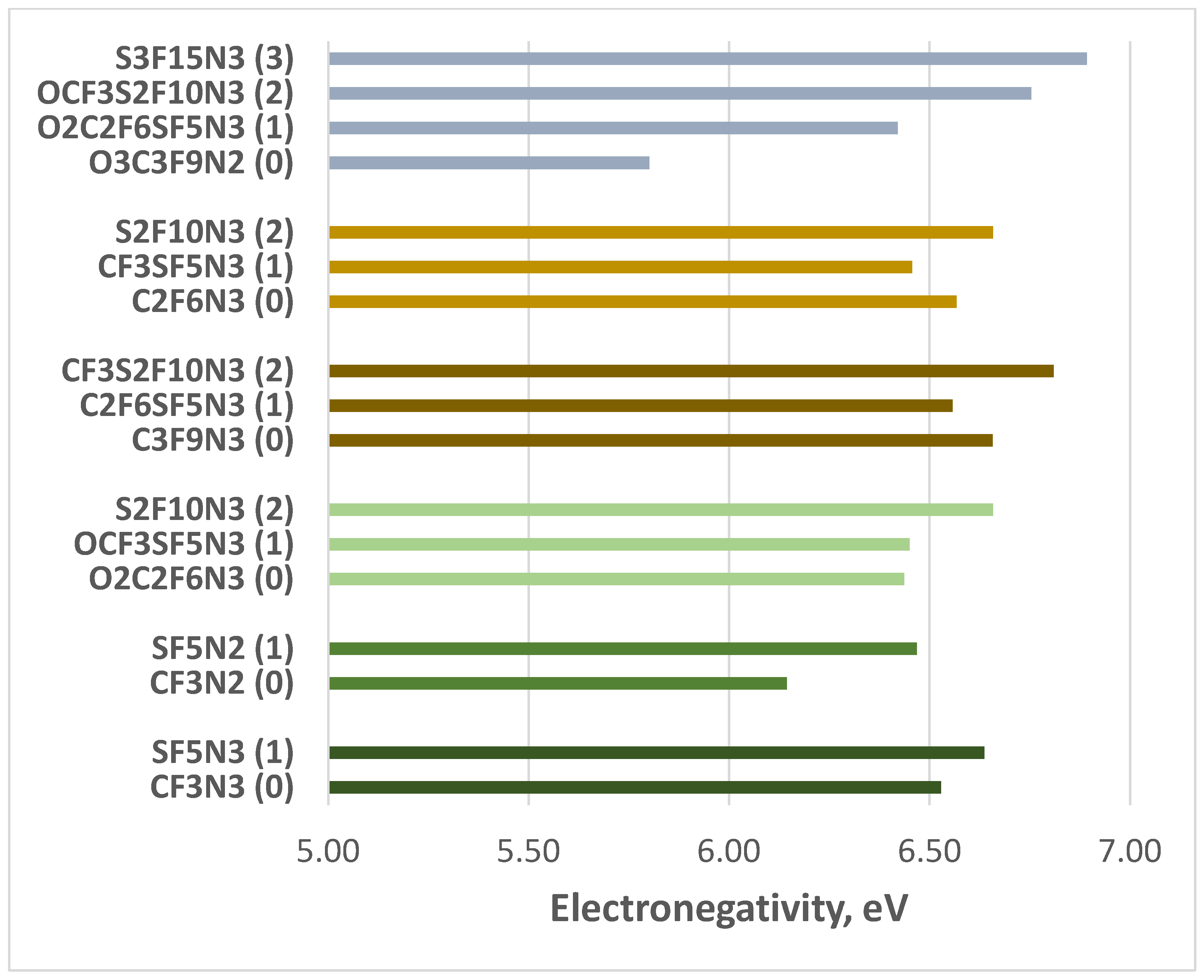
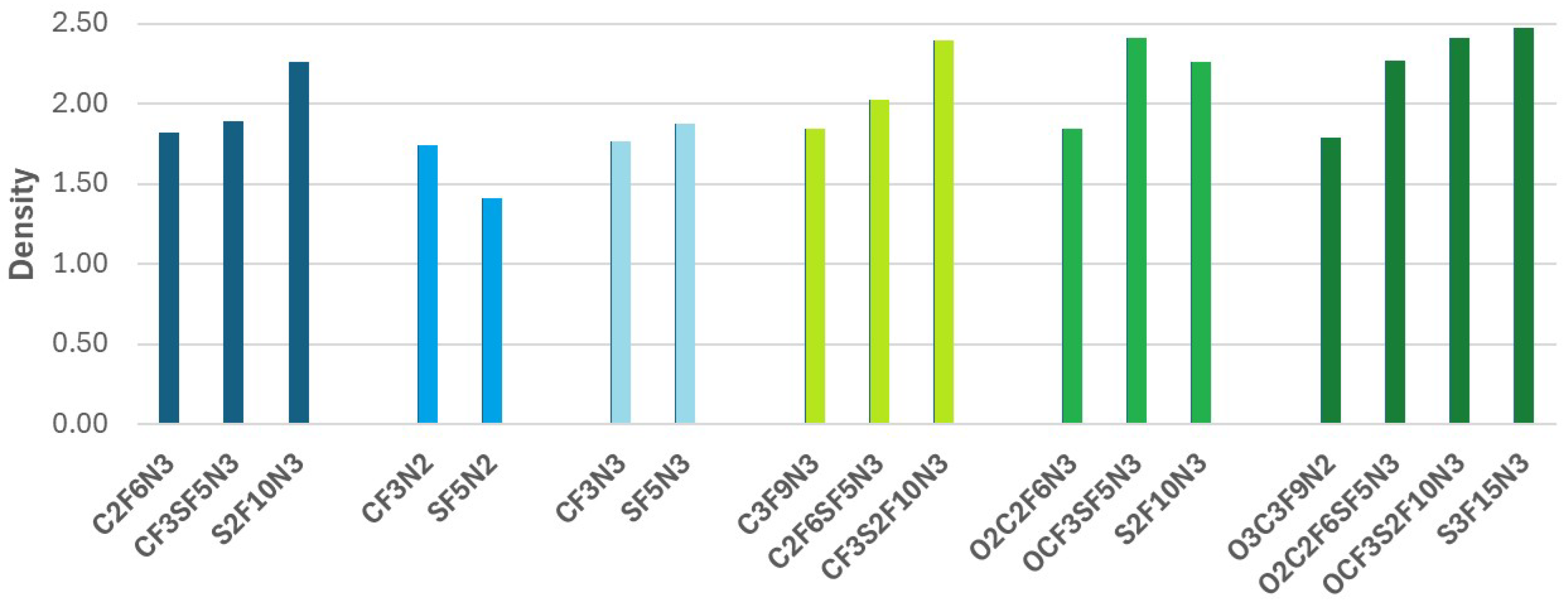

| Compounds | BEA, eV | Gap, eV | CH, eV | ELN, eV |
|---|---|---|---|---|
| C2F6N3 | 5.38 | 5.02 | 2.51 | 6.57 |
| CF3SF5N3 | 5.02 | 5.22 | 2.61 | 6.46 |
| S2F10N3 | 4.51 | 4.83 | 2.41 | 6.66 |
| CF3N2 | 5.47 | 4.94 | 2.47 | 6.14 |
| SF5N2 | 5.05 | 5.36 | 2.68 | 6.47 |
| CF3N3 | 5.43 | 4.73 | 2.36 | 6.53 |
| SF5N3 | 4.98 | 4.83 | 2.41 | 6.64 |
| C3F9N*/ | 5.39 | 5.01 | 2.50 | 6.66 |
| C2F6SF5N3 | 5.04 | 5.45 | 2.73 | 6.56 |
| CF3S2F10N3 | 4.65 | 4.87 | 2.43 | 6.81 |
| O2C2F6N3 | 5.00 | 4.96 | 2.48 | 6.44 |
| OCF3SF5N3 | 5.00 | 4.90 | 2.45 | 6.45 |
| S2F10N3 | 4.51 | 4.83 | 2.41 | 6.66 |
| O3C3F9N2 | 5.27 | 5.14 | 2.57 | 5.80 |
| O2C2F6SF5N3 | 5.00 | 5.07 | 2.54 | 6.42 |
| OCF3S2F10N3 | 4.65 | 4.75 | 2.38 | 6.75 |
| S3F15N3 | 4.31 | 4.72 | 2.36 | 6.89 |
| Compound | ρAC, g/cm3 | ρG, g/cm3 | ρ, g/cm3 |
|---|---|---|---|
| CF3N2 | 1.74 | 1.98 | 1.60 |
| C2F6N3 | 1.82 | 2.34 | 1.79 |
| CF3N3 | 1.77 | 2.07 | 1.83 |
| C3F9N2 | 1.85 | 2.09 | 2.02 |
| O3C3F9N2 | 1.79 | 2.11 | 1.80 |
| O3C3F9N3 | 1.89 | 2.05 | 1.95 |
| Compound | ρAC, g/cm3 | ρ, g/cm3 |
|---|---|---|
| CF3SF5N3 | 2.35 | 2.09 |
| SF5N2 | 2.19 | 1.41 |
| SF5N3 | 2.20 | 1.88 |
| C2F6SF5N3 | 2.26 | 2.03 |
| CF3S2F10N3 | 2.84 | 2.40 |
| CF3SF5N3 | 2.35 | 1.89 |
| OCF3SF5N3 | 2.41 | 1.76 |
| S2F10N3 | 2.26 | 1.60 |
| O2C2F6SF5N3 | 2.27 | 2.07 |
| OCF3S2F10N3 | 2.41 | 1.76 |
| S3F15N3 | 2.48 | 2.05 |
| Compound | P, kbar | D. km/s |
|---|---|---|
| CF3SF5N3 | 297.8 | 7.55 |
| SF5N2 | 160.4 | 6.41 |
| SF5N3 | 295.6 | 7.53 |
| C2F6SF5N3 | 325.2 | 7.73 |
| CF3S2F10N3 | 369.9 | 7.99 |
| OCF3SF5N3 | 335.2 | 7.79 |
| S2F10N3 | 222.9 | 6.97 |
| O2C2F6SF5N3 | 332.0 | 7.77 |
| OCF3S2F10N3 | 267.3 | 7.99 |
| S3F15N3 | 286.7 | 7.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamuliene, J.; Sarlauskas, J. Role of -SF5 Groups in Modulating the Stability and Energy Characteristics of Fluorinated Molecules. Energies 2025, 18, 1841. https://doi.org/10.3390/en18071841
Tamuliene J, Sarlauskas J. Role of -SF5 Groups in Modulating the Stability and Energy Characteristics of Fluorinated Molecules. Energies. 2025; 18(7):1841. https://doi.org/10.3390/en18071841
Chicago/Turabian StyleTamuliene, Jelena, and Jonas Sarlauskas. 2025. "Role of -SF5 Groups in Modulating the Stability and Energy Characteristics of Fluorinated Molecules" Energies 18, no. 7: 1841. https://doi.org/10.3390/en18071841
APA StyleTamuliene, J., & Sarlauskas, J. (2025). Role of -SF5 Groups in Modulating the Stability and Energy Characteristics of Fluorinated Molecules. Energies, 18(7), 1841. https://doi.org/10.3390/en18071841







